Abstract
BACKGROUND AND PURPOSE:
CHARGE syndrome is a multisystemic congenital disorder, most commonly including coloboma, heart malformations, choanal atresia, developmental delay, and genital and ear anomalies. The diagnostic criteria for CHARGE syndrome have been refined with time. However, limited reports describe skull base and craniocervical junction abnormalities. Recently, a coronal clival cleft has been identified in association with CHARGE syndrome. The aim of our study was to assess the prevalence of clival pathology in CHARGE syndrome.
MATERIALS AND METHODS:
In this retrospective study, the CT/MR imaging data base at a single academic children's hospital was queried for the phrase “CHARGE syndrome” during a 17-year period (2001–2017). Electronic medical records were reviewed to confirm the diagnosis. Images were assessed for skull base anomalies, specifically clival hypoplasia and dysplasia.
RESULTS:
The search yielded 42 examinations (21 CTs and 21 MRIs) from 15 distinct patients (mean age, 4.1 ± 5.6 years; range, 2 days to 19 years). CHARGE syndrome diagnosis was confirmed either by clinical and genetic testing (n = 6) or by clinical diagnosis only (n = 9). A coronal clival cleft was identified in 87% of patients (37 examinations, n = 13 patients), either partial (53%) or complete (33%). Clival hypoplasia without clefting was present in all 5 examinations from the remaining 2 patients.
CONCLUSIONS:
Clival pathology is universal in CHARGE syndrome. Coronal clival clefts are extremely common, representing a useful additional diagnostic finding. Detection of a clival cleft should alert the radiologist to examine the palate, choana, eyes, ears, and olfactory centers for other signs of CHARGE syndrome.
CHARGE syndrome is a rare genetic disorder with widespread malformations. The acronym CHARGE includes coloboma, heart malformation, choanal atresia, retardation of growth and/or development, genital anomalies, and ear anomalies.1 Across time, there has been refinement of the diagnostic criteria to 3 major (the classic 3C's: choanal atresia, coloboma, semicircular canal hypoplasia) and 5 minor features.2,3 The prevalence of CHARGE syndrome was estimated to be 1/8500 live births by the Canadian Pediatric Surveillance Program.4 The discovery of a CHARGE syndrome–associated gene, CHD7 (chromodomain helicase DNA-binding protein 7, MIM 608892), has greatly assisted syndromic prevalence estimations due to the phenotypic diversity of the disease.5 Mutations of the CHD7 gene are present in two-thirds of patients with CHARGE syndrome. Phenotype and genotype correlation are required to better assess this syndrome.6 The skull base and craniocervical junction abnormalities are often underrecognized in CHARGE syndrome. Detection of these osseous abnormalities should alert the radiologist to examine the palate, choana, eyes, ears, and olfactory centers for other signs of CHARGE syndrome. Recently, a coronal clival cleft has been identified in association with CHARGE syndrome.7 The aim of our study was to assess the prevalence of clival pathology including clival clefts in patients with CHARGE syndrome.
Materials and Methods
Subjects
A retrospective study was performed after institutional review board approval with Health Insurance Portability and Accountability Act compliance. Requirement for informed consent was waived. The imaging data base at a single academic children's hospital was queried for the term “CHARGE syndrome” provided by the history or by the suspected radiologic findings after filtering by technique (CT and MR imaging) during a 17-year period (2001–2017). Review of the electronic medical records of all patients was performed to confirm the diagnosis and symptomatology. Images were assessed for skull base anomalies, specifically clival hypoplasia/dysplasia and coronal clival cleft.
Imaging Characteristics
All examinations were of diagnostic quality. MR imaging studies were performed on either 1.5T or 3T scanners (Discovery MR 750 and 450; GE Healthcare, Milwaukee, Wisconsin). Sagittal T1WI, axial T2WI, axial T2 fluid-attenuated inversion recovery imaging, coronal fat-saturated T2WI, and axial DTI with 7 noncollinear directions of encoding were reviewed. Axial T1WI and coronal fat-saturated T1WI of the brain were also performed in 5 MRIs after IV gadolinium administration in 5 different patients (0.1 mmol/kg of either gadoterate meglumine [n = 2] or gadopentetate dimeglumine [n = 3]). CT images of the head, temporal bone, facial bone, sinuses, and/or cervical spine were acquired on a 16–detector row scanner (GE Healthcare). Studies were reviewed in a blinded manner by a qualified pediatric neuroradiologist (M.T.W.) with >5 years of experience. Imaging studies were qualitatively examined for all visible abnormalities involving the brain, ocular and olfactory systems, temporal bones, cleft lip/palate, and the presence of clival abnormalities.
Results
Characteristics of the Cohort
The search yielded 17 patients with a history or suspected radiologic findings of CHARGE syndrome. Two patients lacking diagnostic confirmation were excluded. The final cohort comprised 42 examinations (21 CTs and 21 MRIs) from 15 patients (mean age, 4.1 ± 5.6 years; range, 2 days to 19 years); 3 patients were older than 11 years at the time of imaging.
Diagnosis of CHARGE syndrome was confirmed by either clinical and genetic testing (n = 6) or clinical diagnosis only (n = 9).2,3 Fifty-three percent of the patients were male (n = 8).
Clival Imaging Findings
A coronal clival cleft was identified in 87% of patients (37 examinations, n = 13 patients), either complete (33%, n = 5) (Figs 1 and 2) or partial (53%, n = 8) (Fig 3). Clival hypoplasia without clefting was present in all 5 examinations from the remaining 2 patients (On-line Table).8 Those with a partial cleft were further characterized as having either a unilateral cleft in 25% (n = 2) (Fig 3) or bilateral cleft with minimal central fusion in 75% (n = 6).
Fig 1.
Midline sagittal spoiled gradient-recalled acquisition T1WI (1.5T MR imaging; TR/TE = 11/5.1 ms; TI = 500 ms; slice thickness = 1.5 mm) from a 3-month-old girl with CHARGE syndrome shows a distinctive complete coronally oriented clival cleft in the basiocciput (large arrow). Also note mild thinning of the corpus callosum (small arrow), cerebellar vermian hypoplasia, and brain stem volume loss.
Fig 2.
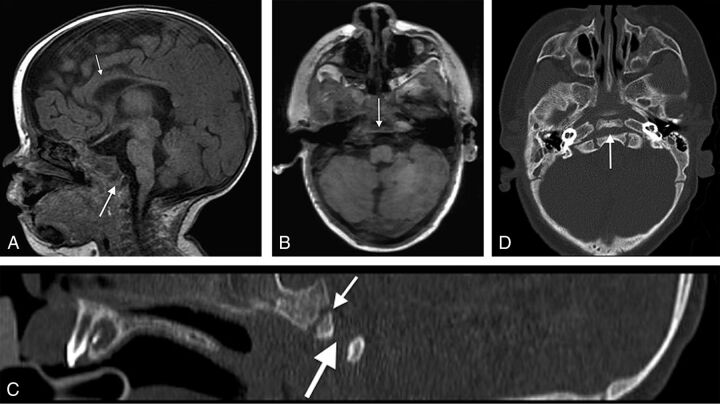
A, Midline sagittal spoiled gradient-recalled acquisition T1WI (1.5T MR imaging; TR/TE = 12/5 ms; TI = 500 ms; slice thickness = 2 mm) from a 4-month-old girl with CHARGE syndrome shows a complete coronally oriented clival cleft in the basiocciput (large arrow). Also note thinning of the corpus callosum (small arrow), cerebellar vermian hypoplasia, and brain stem volume loss. B, Axial spoiled gradient-recalled acquisition of the same patient shows a coronally oriented cleft traversing the clivus (arrow). Corresponding sagittal (C) and axial (D) CT images demonstrate the complete cleft (large arrow) as distinct from the normal spheno-occipital synchondrosis (small arrow in C).
Fig 3.
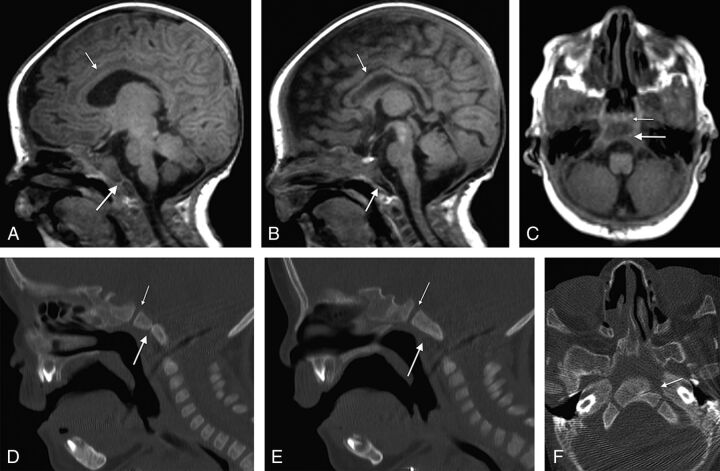
Spoiled gradient-recalled acquisition T1WI (3T MR imaging; TR/TE = 6.6/2.5 ms; TI = 700 ms; slice thickness = 0.63 mm) from a 34-day-old boy with CHARGE syndrome shows a partial (unilateral left side) coronally oriented clival cleft in the basiocciput. A, Left parasagittal unilateral clival cleft (large arrow), mild thinning of the corpus callosum (small arrow), cerebellar vermian hypoplasia, and brain stem volume loss are illustrated. B, Right parasagittal image shows a normal appearance of the clivus without clefting, a thin corpus callosum (small arrow), and vermian and brain stem hypoplasia. C, Axial T1WI of the same patient with a left-sided cleft (large arrow) and normal spheno-occipital synchondrosis (small arrow). Corresponding sagittal and axial CT images (D and F) further demonstrate the unilateral left clival cleft (large arrow, D and F) and absence of clival clefting on the contralateral side (E). Note normal spheno-occipital synchondrosis (small arrow, D and E).
Clival Clefting/Hypoplasia and CHD7 Mutation
The clival findings were further stratified according to the CHD7 mutation. Those with CHD7 mutation (n = 6) had an equal number of complete and partial clival clefts (50%, n = 3) of each type. Those with a non-CHD7-confirmed clinical diagnosis (n = 9) were variable (partial cleft, n = 5; complete cleft, n = 2; and clival hypoplasia, n = 2).
Other imaging features pertinent to the diagnosis of CHARGE syndrome affecting the eye, olfactory apparatus, ears, and brain are classified in the On-line Table.
Discussion
CHARGE syndrome is a systemic disorder with multiorgan involvement. Specific organ abnormalities include cardiac (75%–80%, including atrial septal defect, ventricular septal defect, patent ductus arteriosus, tetralogy of Fallot, atrioventricular canal defect, and double outlet right ventricle),9 genitourinary (including hypospadias; penile agenesis; bifid scrotum; cryptorchidism; atresia of the vagina, cervix, and uterus; renal hypoplasia; solitary kidney; hydronephrosis; vesicoureteral reflux; and duplex kidneys),9 ocular (80%, including coloboma of the iris, retina-choroid, and/or disc; and microphthalmos),9 ear (80%–100%, including external ear dysmorphology; incus hypoplasia; decreased number of cochlear turns; and absent semicircular canals),9,10 olfactory (100%, including unilateral or bilateral absence or hypoplasia of the olfactory bulbs or sulci),9,11 choanal atresia (50%–60%),12 orofacial (15%–20%, including cleft lip/palate),9 brain (including corpus callosum agenesis; posterior fossa anomalies; cranial nerve dysfunction; and cerebellar vermis hypoplasia),12,13 and osseous anomalies (skull base and craniocervical junction).9,8,14 Prenatal diagnosis of CHARGE syndrome has been reported through the finding of CHD7 mutations on chorionic villus sampling and ultrasonographic findings of hydramnios, choanal atresia, tracheoesophageal fistula, and semicircular canal agenesis.15,16
Craniocervical junction abnormalities are common in CHARGE syndrome. Fujita et al8 demonstrated a high prevalence of basioccipital hypoplasia and basilar invagination in CHARGE syndrome and recommended routine assessment of these anomalies to exclude a life-threatening basilar invagination. Natung et al14 reported a case of basilar invagination, short clivus, fused cervical vertebrae, and occipitalization of the atlas. A recent case report of a coronal clival cleft has been identified in association with CHARGE syndrome.7 Recently, Hoch et al17 described a dorsally angulated clivus with posterior displacement of an ossific density in 7 of 10 patients with CHARGE syndrome. The dominant findings we have described herein, namely partial and complete coronal clival clefting, correspond to the description and imaging depictions that those authors provided and further emphasize the importance of craniocervical junction anomalies in CHARGE syndrome.
The pathogenesis of these anomalies in CHARGE syndrome is not clearly understood. They likely originate in the first trimester, concurrent with other CHARGE malformations.18 The CHD7 gene encoding chromodomain helicase DNA-binding protein 7 (CHD7 protein) may play a role through the assumption of the interaction between the neural crest and somite cells, which are responsible for formation of the basiocciput during development.19,20
The clivus is formed from 2 main components, the upper basisphenoid and the lower basiocciput. The 2 are separated by the normal spheno-occipital synchondrosis. The basiocciput is formed from 4 occipital sclerotomes.19 The clivus reaches the full developmental length by 11 years of age.21 Clival clefts occur caudal to and distinct from the spheno-occipital synchondrosis, which usually fuses between 12 and 18 years of age.19 The presence of normal anatomic variants such as clival canal/foramen, fossa navicularis magna, or basilar transverse fissure (Sauser fissure) should not be confused with a clival cleft.22–24Although the etiology of coronal clival clefts is unknown, these clefts could be related to incomplete fusion of clival ossification centers, enlarged clival canals (fossa navicularis), or persistence of basilar transverse fissure.19,22–24
This study has some limitations by being retrospective and having a relatively small sample size. Despite these limitations, to date, it is the largest study to describe and illustrate clival pathology and clefting in CHARGE syndrome.
Conclusions
Clival pathology is universal in CHARGE syndrome. Coronal clival clefts are extremely common, representing a useful diagnostic feature and should be considered an additional finding in CHARGE syndrome. The skull base should be scrutinized for these characteristic anomalies. Detection of a clival cleft should alert the radiologist to examine the palate, choana, eyes, ears, and olfactory centers for other signs of CHARGE syndrome.
ABBREVIATIONS:
- CHARGE
Coloboma of the eye, Heart defects, Atresia of the choanae, Retardation of growth and/or development, Genital and/or urinary abnormalities, and Ear abnormalities and deafness
- CHD7
chromodomian helicase DNA-binding protein 7
REFERENCES
- 1. Pagon RA, Graham JM Jr, Zonana J, et al. . Coloboma, congenital heart disease, and choanal atresia with multiple anomalies: CHARGE association. J Pediatr 1981;99:223–27 10.1016/S0022-3476(81)80454-4 [DOI] [PubMed] [Google Scholar]
- 2. Verloes A. Updated diagnostic criteria for CHARGE syndrome: a proposal. Am J Med Genet A 2005;133A:306–08 10.1002/ajmg.a.30559 [DOI] [PubMed] [Google Scholar]
- 3. Blake KD, Davenport SL, Hall BD, et al. . CHARGE association: an update and review for the primary pediatrician. Clin Pediatr (Phila) 1998;37:159–73 10.1177/000992289803700302 [DOI] [PubMed] [Google Scholar]
- 4. Issekutz KA, Graham JM Jr, Prasad C, et al. . An epidemiological analysis of CHARGE syndrome: preliminary results from a Canadian study. Am J Med Genet A 2005;133A:309–17 10.1002/ajmg.a.30560 [DOI] [PubMed] [Google Scholar]
- 5. Vissers LE, van Ravenswaaij CM, Admiraal R, et al. . Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet 2004;36:955–57 10.1038/ng1407 [DOI] [PubMed] [Google Scholar]
- 6. Sanlaville D, Verloes A. CHARGE syndrome: an update. Eur J Hum Genet 2007;15:389–99 10.1038/sj.ejhg.5201778 [DOI] [PubMed] [Google Scholar]
- 7. Mahdi E, Whitehead MT. Coronal clival cleft in CHARGE syndrome. Neuroradiol J 2017;30:574–77 10.1177/1971400916678248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujita K, Aida N, Asakura Y, et al. . Abnormal basiocciput development in CHARGE syndrome. AJNR Am J Neuroradiol 2009;30:629–34 10.3174/ajnr.A1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blake KD, Prasad C. CHARGE syndrome. Orphanet J Rare Dis 2006;1:34 10.1186/1750-1172-1-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morimoto AK, Wiggins RH 3rd, Hudgins PA, et al. . Absent semicircular canals in CHARGE syndrome: radiologic spectrum of findings. AJNR Am J Neuroradiol 2006;27:1663–71 [PMC free article] [PubMed] [Google Scholar]
- 11. Blustajn J, Kirsch CF, Panigrahy A, et al. . Olfactory anomalies in CHARGE syndrome: imaging findings of a potential major diagnostic criterion. AJNR Am J Neuroradiol 2008;29:1266–69 10.3174/ajnr.A1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lalani SR, Hefner MA, Belmont JW, et al. . CHARGE syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews. Seattle: University of Washington; 1993–2018. [Google Scholar]
- 13. Yu T, Meiners LC, Danielsen K, et al. . Deregulated FGF and homeotic gene expression underlies cerebellar vermis hypoplasia in CHARGE syndrome. Elife 2013;2:e01305 10.7554/eLife.01305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Natung T, Goyal A, Handique A, et al. . Symmetrical chorioretinal colobomata with craniovertebral junction anomalies in CHARGE syndrome: a case report with review of literature. J Clin Imaging Sci 2014;4:5 10.4103/2156-7514.126046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Becker R, Stiemer B, Neumann L, et al. . Mild ventriculomegaly, mild cerebellar hypoplasia and dysplastic choroid plexus as early prenatal signs of CHARGE association. Fetal Diagn Ther 2001;16:280–83 10.1159/000053928 [DOI] [PubMed] [Google Scholar]
- 16. Hertzberg BS, Kliewer MA, Lile RL. Antenatal ultrasonographic findings in the CHARGE association. J Ultrasound Med 1994;13:238–42 10.7863/jum.1994.13.3.238 [DOI] [PubMed] [Google Scholar]
- 17. Hoch MJ, Patel SH, Jethanamest D, et al. . Head and neck MRI findings in CHARGE syndrome. AJNR Am J Neuroradiol 2017;38:2357–63 10.3174/ajnr.A5297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanlaville D, Etchevers HC, Gonzales M, et al. . Phenotypic spectrum of CHARGE syndrome in fetuses with CHD7 truncating mutations correlates with expression during human development. J Med Genet 2006;43:211–17 10.1136/jmg.2005.036160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smoker WR. Craniovertebral junction: normal anatomy, craniometry, and congenital anomalies. Radiographics 1994;14:255–77 10.1148/radiographics.14.2.8190952 [DOI] [PubMed] [Google Scholar]
- 20. Nie X. Cranial base in craniofacial development: developmental features, influence on facial growth, anomaly, and molecular basis. Acta Odontol Scand 2005;63:127–35 10.1080/00016350510019847 [DOI] [PubMed] [Google Scholar]
- 21. Krmpotić-Nemanić J, Vinter I, Kelovizć Z, et al. . Postnatal changes of the clivus. Ann Anat 2005;187:277–80 10.1016/j.aanat.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 22. Inal M, Muluk NB, Ozveren MF, et al. . The presence of clival foramen through multidetector computed tomography of the skull base. J Craniofac Surg 2015;26:e580–82 10.1097/SCS.0000000000002129 [DOI] [PubMed] [Google Scholar]
- 23. Beltramello A, Puppini G, El-Dalati G, et al. . Fossa navicularis magna. AJNR Am J Neuroradiol 1998;19:1796–98 [PMC free article] [PubMed] [Google Scholar]
- 24. Hofmann E, Prescher A. The clivus: anatomy, normal variants and imaging pathology. Clin Neuroradiol 2012;22:123–39 10.1007/s00062-011-0083-4 [DOI] [PubMed] [Google Scholar]