Abstract
BACKGROUND AND PURPOSE:
Delayed cerebral ischemia is a severe complication of aneurysmal SAH and is associated with a high case morbidity and fatality. The total blood volume and the presence of intraventricular blood on CT after aneurysmal SAH are associated with delayed cerebral ischemia. Whether quantified location-specific (cisternal, intraventricular, parenchymal, and subdural) blood volumes are associated with delayed cerebral ischemia has been infrequently researched. This study aimed to associate quantified location-specific blood volumes with delayed cerebral ischemia.
MATERIALS AND METHODS:
Clinical and radiologic data were collected retrospectively from consecutive patients with aneurysmal SAH with available CT scans within 24 hours after ictus admitted to 2 academic centers between January 2009 and December 2011. Total blood volume was quantified using an automatic hemorrhage-segmentation algorithm. Segmented blood was manually classified as cisternal, intraventricular, intraparenchymal, or subdural. Adjusted ORs with 95% confidence intervals for delayed cerebral ischemia per milliliter of location-specific blood were calculated using multivariable logistic regression analysis.
RESULTS:
We included 282 patients. Per milliliter increase in blood volume, the adjusted OR for delayed cerebral ischemia was 1.02 (95% CI, 1.01–1.04) for cisternal, 1.02 (95% CI, 1.00–1.04) for intraventricular, 0.99 (95% CI, 0.97–1.02) for intraparenchymal, and 0.96 (95% CI, 0.86–1.07) for subdural blood.
CONCLUSIONS:
Our findings suggest that in patients with aneurysmal subarachnoid hemorrhage, the cisternal blood volume has a stronger relation with delayed cerebral ischemia than the blood volumes at other locations in the brain.
Although case fatality rates have been declining during the past years, aneurysmal subarachnoid hemorrhage (aSAH) is still a devastating disease with a case fatality of approximately 30%.1 Delayed cerebral ischemia (DCI) is a severe complication that occurs in approximately 20%–30% of patients and is associated with high morbidity and mortality.2 One of the strongest predictors of DCI is the amount of blood on the admission CT scan.3–6
Apart from the amount of extravasated blood, the breakthrough of blood from the subarachnoid cisterns into the ventricle system is also associated with the occurrence of DCI. Various studies have shown that the presence of an intraventricular hemorrhage (IVH) in patients with aSAH is an independent risk factor for DCI.5,7–11 However, conflicting results regarding the association between the presence of intraparenchymal hemorrhage (IPH) and DCI in patients with SAH are found.9,11–14 The occurrence of subdural hemorrhage (SDH) is relatively rare after SAH, and its association with DCI is currently unknown.15
Although various studies determined the association between the presence of IVH or IPH and DCI, only a few studies associated estimates of these volumes with DCI. One study found an association between IVH volume and DCI.6 However, a more recent study did not confirm this finding.16 The only study that determined IPH volume in patients with aSAH found no association with DCI.5 These studies used radiologic scales such as the Hijdra sum score to estimate the cisternal blood volume, the IVH score for IVH volume, and the ABC/2 score for IPH volume.6,17,18 These radiologic scales are not very accurate and are shown to have poor-to-moderate interobserver agreement, limiting their discriminative power.16,19 Moreover, the ABC/2 score has been shown to overestimate the IPH volume.20
Quantitative measures have the promise of more precisely assessing blood volumes. Previous studies have found a strong relation between the quantified amount of total blood volume and DCI.9,21 However, in these studies, the volumes of blood in the separate compartments of the brain were not analyzed separately. It is therefore currently unknown whether taking the blood volumes at these locations into account improves the prediction of DCI. In this study, we took the first step in addressing this issue by quantifying the cisternal, intraventricular, intraparenchymal, and subdural blood volumes separately and determining their independent associations with DCI.
Materials and Methods
Population
We included patients from a retrospectively collected cohort consisting of all patients with aSAH admitted to the Academic Medical Center Amsterdam and University Medical Center Utrecht, the Netherlands, between January 2009 and December 2011.9 Inclusion criteria were the following: 1) SAH proved on noncontrast CT; 2) aneurysm proved on either CTA, MRA, or DSA; and 3) NCCT performed within 24 hours after ictus and available for review. Patients who did not survive the first 3 days after SAH onset were excluded. Furthermore, patients with NCCTs whom we could not use for hemorrhage quantification, for instance due to movement and/or metal artifacts from previous treatment, were excluded.22
Clinical Data
Collected demographic and clinical variables were the following: age, sex, history of hypertension, neurologic condition on admission (according to the World Federation of Neurosurgical Societies [WFNS] scale),23 time between ictus and admission, location of the aneurysm (anterior or posterior circulation) and treatment technique (no aneurysm treatment, clipping, or coiling), rebleeding, and the occurrence of clinical DCI during admission. Aneurysms of the posterior communicating artery were allocated to the posterior circulation.
Delayed Cerebral Ischemia
DCI was defined as the occurrence of new focal neurologic impairment or a decrease on the Glasgow Coma Scale score that could not be explained by any other cause. A CT scan of the brain was performed to rule out hydrocephalus, and blood was sampled to exclude a metabolic encephalopathy, such as infection or electrolyte disturbances, to exclude other causes of neurologic deterioration. An electroencephalogram was obtained in case of suspicion of seizures.24 DCI was diagnosed by the treating physician who could be either a neurologist, neurosurgeon, or intensivist. A new cerebral infarct on follow-up CT, which could not be attributed to surgical clipping, endovascular treatment, or drain placement, was supportive but not required for the diagnosis of DCI (On-line Figure). The standard care to prevent DCI was similar in both participating centers. All patients received nimodipine (6 times daily, 60 mg orally) and intravenous fluids aiming at normovolemia. The mean arterial pressure was kept above 65 mm Hg. Furthermore, if the patient used antihypertensive medication, it was stopped at admission.
Image Analysis
The first scan after ictus was used for the analysis. However, if rebleeding occurred within 24 hours, the scan after rebleeding was used. All scans were reviewed for the presence of cisternal blood, IVH, IPH, and SDH by 2 observers (I.A.Z. and W.E.v.d.S.). The hemorrhage was segmented on admission NCCT using an automatic hemorrhage-segmentation algorithm.22 All segmentations were checked and, if needed, manually corrected using ITK-SNAP, Version 3.4.0 (www.itksnap.org) by a trained observer (W.E.v.d.S.) who was blinded to outcome.25 From this segmentation, the total blood volume was calculated in milliliters. Subsequently, segmented blood was classified as cisternal, intraparenchymal, intraventricular, or subdural by manually outlining the ventricular, intraparenchymal, and/or subdural part of the segmented total hemorrhage by a trained observer (W.E.v.d.S.) (Fig 1). If part of the SAH was in proximity of the Sylvian fissure and not clearly located inside of the fissure on NCCT, the CTA was evaluated to differentiate the cisternal and intraparenchymal part of the hematoma.26 If contrast-enhanced vessels were present in the hematoma on CTA, this part was classified as cisternal. If no vessels were detected, it was classified as IPH. The classifications were checked by a second observer, an experienced radiologist (I.A.Z.). After we classified the blood as cisternal, intraparenchymal, intraventricular, or subdural, the location-dependent volumes could be calculated by multiplying the number of classified voxels by its voxel size.
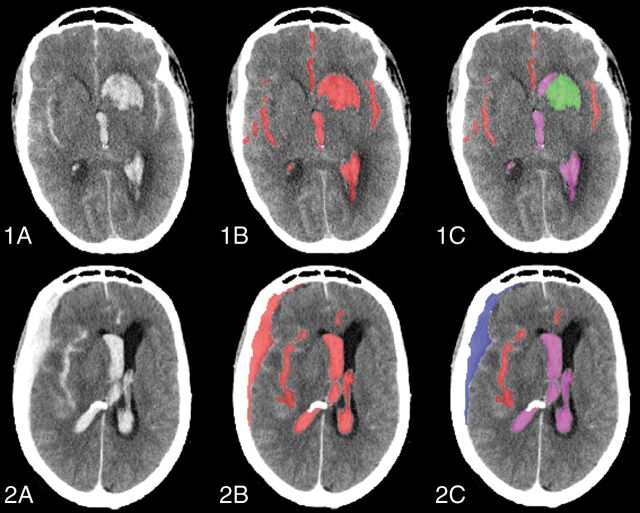
Fig 1.
Classification of the total blood volume as cisternal, intraventricular, intraparenchymal, and subdural. 1A, Axial slice of a noncontrast CT of a patient with aSAH with concomitant IVH and IPH. 1B, Quantified total blood volume. 1C, Classified cisternal (red), intraventricular (magenta), and intraparenchymal (green) blood. 2A, Axial slice of a noncontrast CT scan of a patient with aSAH with concomitant IVH and SDH. 2B. Quantified total blood volume. 2C, Classified cisternal (red), intraventricular (magenta), and subdural (blue) blood.
Statistical Analysis
Baseline variables were compared between patients with and without DCI using the Fisher exact test for dichotomous and categoric variables, the independent samples t test for normally distributed continuous variables, and the Mann-Whitney U test for non-normally distributed continuous variables. Variables were checked for normality using the Shapiro-Wilk test (W > 0.9 was considered normally distributed).
Correlations between the cisternal blood volume and IVH, IPH, and SDH volume were calculated using the Spearman rank correlation coefficient.
Univariable logistic regression analysis of the total blood; cisternal blood; and IVH, IPH, and SDH volume with DCI was performed to determine ORs with 95% confidence intervals for DCI per milliliter of blood.
Associative models for the cisternal, IVH, IPH, SDH, and total blood volume were created by calculating adjusted odds ratios (aORs) by separately adding potential confounders to the univariable model. Variables that changed the OR of the univariable model by ≥10% were considered confounders and were included in the final associative model. Potential confounders were age, sex, neurologic condition on admission (WFNS grade), treatment technique (no treatment, clipping, or coiling), rebleeding, and hypertension in accordance with previous literature.9,27 For the 4 associative models of cisternal, IVH, IPH, and SDH volume, the remaining 3 volumes were also included in the confounding analysis. In the associative model of the total volume, the location-dependent volumes were not added as confounders because the total volume consisted of these volumes.
A P value < .05 was considered statistically significant. Statistical analyses were performed using SPSS, Version 23.0.0.2 (IBM, Armonk, New York).
Results
Of the 458 patients who were evaluated for study inclusion, 176 patients were excluded because of the following reasons: NCCT not available (n = 33); NCCT not suitable for assessment because of movement and/or metal artifacts (n = 13) or other technical reasons (n = 16); the first NCCT not performed within 24 hours (n = 38); no aneurysm found on CTA, MRA or DSA (n = 29); and death within 3 days after SAH onset (n = 47).9
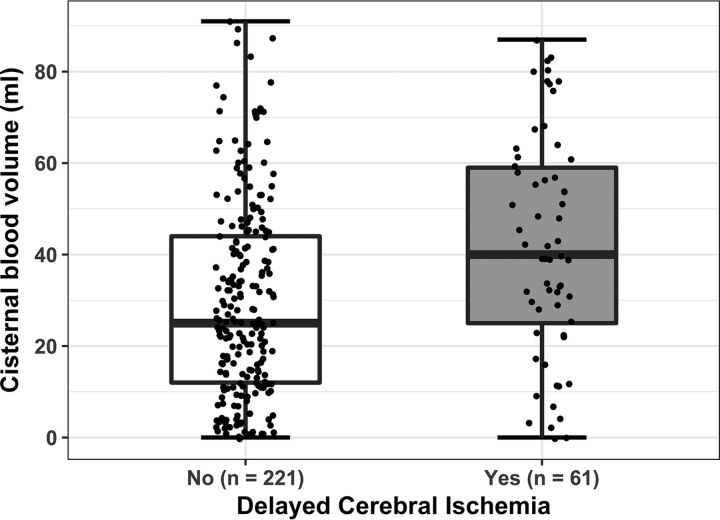
Characteristics of the 282 included patients are shown in Table 1 for the total study population and stratified by DCI group. The mean age was 55.8 ± 12.0 years, and 73% were female. In this cohort, 61 (22%) patients developed DCI. Patients with DCI had larger total (P = .01) and cisternal (P < .001) blood volumes (Fig 2), more frequently had an IVH (P = .003), and had a larger IVH volume (P = .01).
Table 1:
Patient characteristics
| Parameter | All (n = 282) | No DCI (n = 221) | DCI (n = 61) | P Value |
|---|---|---|---|---|
| Age (mean) (±SD) | 55.8 (12.0) | 55.9 (11.8) | 55.4 (13.0) | .80 |
| Female sex (No.) | 205 (73%) | 163 (74%) | 42 (69%) | .52 |
| History of hypertension (No.) | 79 (28%) | 58 (26%) | 21 (34%) | .26 |
| Aneurysm location (No.) | .62 | |||
| Anterior | 212 (75%) | 168 (76%) | 44 (72%) | |
| Posterior | 70 (25%) | 53 (24%) | 17 (28%) | |
| WFNS grade (No.) | .08 | |||
| I | 79 (28%) | 68 (31%) | 11 (18%) | |
| II | 67 (24%) | 50 (23%) | 17 (28%) | |
| III | 8 (3%) | 6 (3%) | 2 (3%) | |
| IV | 68 (24%) | 56 (25%) | 12 (20%) | |
| V | 59 (21%) | 40 (18%) | 19 (31%) | |
| Treatment modality (No.) | .23 | |||
| No treatment | 14 (5%) | 13 (6%) | 1 (2%) | |
| Clipping | 138 (49%) | 111 (50%) | 27 (44%) | |
| Coiling | 130 (46%) | 97 (44%) | 33 (54%) | |
| Rebleeding (No.) | 34 (12%) | 26 (12%) | 8 (13%) | .83 |
| Total blood volume (median) (IQR) | 41.2 (23.6–62.5) | 37.7 (21.8–57.9) | 52.1 (34.5–78.1) | .01 |
| Cisternal blood volume (median) (IQR) | 29.8 (14.0–47.0) | 25.2 (12.4–44.1) | 39.7 (24.2–60.1) | <.001 |
| IVH presence (No.) | 187 (66%) | 137 (62%) | 50 (82%) | .003 |
| IVH volume (median) (IQR) | 0.7 (0.0–2.8) | 0.5 (0.0–2.2) | 1.2 (0.3–5.0) | .01 |
| IPH presence (No.) | 85 (30%) | 70 (32%) | 15 (25%) | .35 |
| IPH volume (median) (IQR) | 0.0 (0.0–3.3) | 0.0 (0.0–3.6) | 0.0 (0.0–0.8) | .29 |
| SDH presence (No.) | 16 (6%) | 12 (5%) | 4 (7%) | .76 |
| SDH volume (median) (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | .74 |
Note:—IQR indicates interquartile range.
Fig 2.
Dot boxplot of quantified cisternal blood volume in patients with and without DCI.
There was a weak positive correlation between cisternal and IVH blood volumes (Spearman ρ = 0.15, P = .01), a weak negative correlation between cisternal and IPH blood volumes (Spearman ρ = −0.16, P = .01), and no correlation between cisternal and SDH blood volumes.
In the univariable analysis, both the total blood volume (OR = 1.02; 95% CI, 1.01–1.03) per milliliter increase in volume and the cisternal blood volume (OR = 1.02; 95% CI, 1.01–1.04) were significantly associated with DCI. After correction for confounders, the total (aOR = 1.02; 95% CI, 1.01–1.03) and cisternal (aOR = 1.02; 95% CI, 1.01–1.04) blood volumes remained significantly associated with DCI. In both the univariable and multivariable analysis, no statistically significant associations were found among the IVH volume (aOR = 1.02; 95% CI, 1.00–1.04), IPH volume (aOR = 0.99; 95% CI, 0.97–1.02), SDH volume (aOR = 0.96; 95% CI, 0.86–1.07), and DCI (Table 2).
Table 2:
Association of blood volumes and DCI per milliliter increase in volume
| Variable | OR (95% CI) | aOR (95% CI) |
|---|---|---|
| Total blood volume | 1.02 (1.01–1.03)a | 1.02 (1.01–1.03)a,b |
| Cisternal blood volume | 1.02 (1.01–1.04)a | 1.02 (1.01–1.04)a,c |
| IVH volume | 1.02 (1.00–1.04) | 1.02 (1.00–1.04)d |
| IPH volume | 0.99 (0.97–1.01) | 0.99 (0.97–1.02)e |
| SDH volume | 0.96 (0.85–1.08) | 0.96 (0.86–1.07)f |
Statistically significant.
Confounders: none.
Confounders: none.
Confounders: WFNS, cisternal blood volume.
Confounders: WFNS, cisternal blood volume, and IVH volume.
Confounders: WFNS, treatment, cisternal blood volume, IVH volume, and IPH volume.
Discussion
In this study, we associated location-specific blood volumes with the occurrence of DCI. In our population, increasing cisternal blood volume was associated with a higher risk of DCI. This relation was not found with intraventricular, intraparenchymal, and subdural blood volume.
Our results confirm that larger amounts of blood in the subarachnoid space are associated with a higher chance of DCI.3,5,9,21,28 The IVH volume was not significantly associated with DCI, though the point estimate of the aOR was like that of the cisternal volume, which may indicate a lack of power to show a statistically significant effect. The only other study that quantified IVH volume showed a higher median IVH volume in patients with DCI compared with patients without DCI.28 In our study, patients with DCI also had a higher median IVH volume. Two other studies that used qualitative scores to assess IVH volume in patients with SAH found that a higher ventricular clot volume was independently associated with a higher risk of DCI.5,6 Another study that included only patients with aSAH with concomitant IVH showed no association between IVH volume and DCI.16 Our findings neither support nor reject the hypothesis that IVH volume is related to the occurrence of DCI.
We found no association between IPH volume and DCI, similar to findings of the only other study that assessed IPH volume.5 Recently, 2 studies found an association between the presence of an IPH and DCI.12,13 However, in both studies, a new ischemic lesion was used as the end point instead of clinical DCI. Not all patients with clinical DCI will develop a cerebral infarct, making the results of these studies difficult to compare with those of our study.29 Moreover, the presence rather than the volume of IPH was considered in these studies. The SDH volume has not been previously associated with the occurrence of DCI, to our knowledge.
Our results show a difference between cisternal and IVH volumes on the one hand and IPH and SDH volumes on the other in relation to DCI. A possible explanation might be that the ventricles are an overflow compartment of the cisterns. A larger volume of blood in the ventricles may actually result from a larger cisternal blood volume that has either been directly released into the ventricles or has been redistributed via the foramina of Luschka and possibly via the interpeduncular cistern.30 However, in our data, only a weak correlation between the cisternal blood volume and the IVH volume was found. Intraparenchymal and subdural hematomas, on the other hand, are a more direct extension of blood from the aneurysm without an interstitial subarachnoid compartment. The underlying mechanism causing DCI (cerebral vasospasm, microthrombosis, microvascular spasm, inflammation, and/or cortical spreading ischemia) has been thought to be related to both rupture of an intracranial aneurysm and to blood being released into the subarachnoid space containing CSF.31 The latter may not apply to the intraparenchymal compartment. This possibility may explain the absence of a relation between the IPH and SDH volumes and the occurrence of DCI. Furthermore, a complicating factor in the assessment of clinical DCI is caused by the presence of IPH because such a hematoma can already cause a focal neurologic deficit itself. Moreover, in general, it is difficult to score DCI when the condition of a patient deteriorates shortly after the aneurysm treatment, while the new hypodensity surrounding the initial hematoma on a CT scan can also be caused by edema or infarction due to the hematoma itself or the aneurysm treatment. This combination of factors could lead to underscoring of DCI in this time period.
An important strength of this study is the computer-assisted quantification of the volume of blood in all different compartments of the brain. With computer-assisted quantification, even a very small layering of blood could be delineated, adding to the total volume of intracranial blood. This delineation allowed a calculation of risk per milliliter of blood and a more quantitative means to assess the association with DCI as opposed to the very coarse qualitative grading scales. A remaining limitation of the computer-assisted technique up to this moment is that even though the total blood volume could be segmented automatically, the ventricular, intraparenchymal, and subdural outlines were manually drawn. This feature may lead to some observer-dependent variation. We tried to limit this by inspection of the segmentations by an experienced radiologist; however, it has been proved difficult to accurately differentiate IPH and cisternal hematoma, especially in patients with ruptured middle cerebral artery aneurysms. We tried to overcome this problem by combining the NCCT with the CTA to allow differentiation between these 2 compartments and to assess the IPH volumes.26 Nevertheless, even with the use of CTA, some misclassification of IPH may have occurred.
A weakness of this study is its retrospective design, which may have resulted in suboptimal analysis of the clinical data. However, by including all consecutive patients in a limited time span, we have tried to minimize this bias because all patient data were analyzed in the same way.
Our results suggest that patients with high cisternal blood volume have a high risk of DCI. Thus, these patients could be a target for intensive monitoring and new prophylactic treatment strategies.32 However, even though our study shows associations between location-specific blood volumes and DCI, the question remains as to whether these volumes improve the existing prediction models, including, for instance, the modified Fisher score.10 If this is the case, these volumes may be of clinical value. This will have to be confirmed by the development and validation of prediction models for DCI, including the location-specific volumes. Furthermore, in this study, patients were not routinely followed up after the admission period. Thus, the correlation between the location-specific volumes and clinical outcome could not be reliably determined. Future prospective studies are warranted to answer this important remaining question. Manual selection of the IVH, IPH, or SDH region is too cumbersome to use in daily practice. Automatic region-detection techniques should be developed before this can be used as a clinical tool.
Conclusions
In our population, increasing cisternal blood volume was associated with a higher risk of DCI. This relation was not found with intraventricular, intraparenchymal, and subdural blood volume. Our findings suggest that in patients with an aSAH, the cisternal blood volume has a stronger relation to DCI than the blood volumes at other locations in the brain.
ABBREVIATIONS:
- aOR
adjusted odds ratio
- aSAH
aneurysmal subarachnoid hemorrhage
- DCI
delayed cerebral ischemia
- IPH
intraparenchymal hemorrhage
- IVH
intraventricular hemorrhage
- SDH
subdural hemorrhage
- WFNS
World Federation of Neurosurgical Societies
Footnotes
Disclosures: René van den Berg—UNRELATED: Consultancy: Codman Neuro-DePuy Synthes Neurovascular. Charles B.L.M. Majoie—UNRELATED: Consultancy: Stryker, Comments: Consultations*; Grants/Grants Pending: TWIN Foundation, Dutch Heart Foundation.* Henk A. Marquering—OTHER RELATIONSHIPS: founder and shareholder of Nico-lab. *Money paid to the institution.
Henk A. Marquering received a research grant from Fonds NutsOhra (1403-023) to study the prognostic value of quantified blood in patients with aneurysmal subarachnoid hemorrhage.
Paper previously presented as an abstract at: European Stroke Organisation Conference, May 16–18 2017; Prague, Czech Republic.
REFERENCES
- 1. Vergouwen MD, Jong-Tjien-Fa AV, Algra A, et al. . Time trends in causes of death after aneurysmal subarachnoid hemorrhage: a hospital-based study. Neurology 2016;86:59–63 10.1212/WNL.0000000000002239 [DOI] [PubMed] [Google Scholar]
- 2. Hijdra A, Braakman R, van Gijn J, et al. . Aneurysmal subarachnoid hemorrhage: complications and outcome in a hospital population. Stroke 1987;18:1061–67 10.1161/01.STR.18.6.1061 [DOI] [PubMed] [Google Scholar]
- 3. Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980;6:1–9 10.1227/00006123-198001000-00001 [DOI] [PubMed] [Google Scholar]
- 4. Frontera JA, Claassen J, Schmidt JM, et al. . Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery 2006;59:21–27; discussion 21–27 10.1227/01.NEU.0000218821.34014.1B [DOI] [PubMed] [Google Scholar]
- 5. Claassen J, Bernardini GL, Kreiter K, et al. . Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke 2001;32:2012–20 10.1161/hs0901.095677 [DOI] [PubMed] [Google Scholar]
- 6. Hijdra A, van Gijn J, Nagelkerke NJ, et al. . Prediction of delayed cerebral ischemia, rebleeding, and outcome after aneurysmal subarachnoid hemorrhage. Stroke 1988;19:1250–56 10.1161/01.STR.19.10.1250 [DOI] [PubMed] [Google Scholar]
- 7. Rosen DS, Macdonald RL, Huo D, et al. . Intraventricular hemorrhage from ruptured aneurysm: clinical characteristics, complications, and outcomes in a large, prospective, multicenter study population. J Neurosurg 2007;107:261–65 10.3171/JNS-07/08/0261 [DOI] [PubMed] [Google Scholar]
- 8. De Rooij NK, Greving JP, Rinkel GJ, et al. . Early prediction of delayed cerebral ischemia after subarachnoid hemorrhage: development and validation of a practical risk chart. Stroke 2013;44:1288–94 10.1161/STROKEAHA.113.001125 [DOI] [PubMed] [Google Scholar]
- 9. Zijlstra IA, Gathier CS, Boers AM, et al. . Association of automatically quantified blood volume after aneurysmal subarachnoid hemorrhage with delayed cerebral ischemia. AJNR Am J Neuroradiol 2016;37:1588–93 10.3174/ajnr.A4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Oliveira Manoel AL, Jaja BN, Germans MR, et al. . The VASOGRADE: a simple grading scale for prediction of delayed cerebral ischemia after subarachnoid hemorrhage. Stroke 2015;46:1826–31 10.1161/STROKEAHA.115.008728 [DOI] [PubMed] [Google Scholar]
- 11. Macdonald RL, Rosengart A, Huo D, et al. . Factors associated with the development of vasospasm after planned surgical treatment of aneurysmal subarachnoid hemorrhage. J Neurosurg 2003;99:644–52 10.3171/jns.2003.99.4.0644 [DOI] [PubMed] [Google Scholar]
- 12. Wan A, Jaja BN, Schweizer TA, et al. . Clinical characteristics and outcome of aneurysmal subarachnoid hemorrhage with intracerebral hematoma. J Neurosurg 2016;125:1344–51 10.3171/2015.10.JNS151036 [DOI] [PubMed] [Google Scholar]
- 13. Platz J, Güresir E, Wagner M, et al. . Increased risk of delayed cerebral ischemia in subarachnoid hemorrhage patients with additional intracerebral hematoma. J Neurosurg 2017;126:504–10 10.3171/2015.12.JNS151563 [DOI] [PubMed] [Google Scholar]
- 14. Crobeddu E, Mittal MK, Dupont S, et al. . Predicting the lack of development of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke 2012;43:697–701 10.1161/STROKEAHA.111.638403 [DOI] [PubMed] [Google Scholar]
- 15. Schuss P, Konczalla J, Platz J, et al. . Aneurysm-related subarachnoid hemorrhage and acute subdural hematoma: single-center series and systematic review. J Neurosurg 2013;118:984–90 10.3171/2012.11.JNS121435 [DOI] [PubMed] [Google Scholar]
- 16. Kramer AH, Mikolaenko I, Deis N, et al. . Intraventricular hemorrhage volume predicts poor outcomes but not delayed ischemic neurological deficits among patients with ruptured cerebral aneurysms. Neurosurgery 2010;67:1044–52; discussion 1052–53 10.1227/NEU.0b013e3181ed1379 [DOI] [PubMed] [Google Scholar]
- 17. Hallevi H, Dar NS, Barreto AD, et al. . The IVH score: a novel tool for estimating intraventricular hemorrhage volume: clinical and research implications. Crit Care Med 2009;37:969–74, e1 10.1097/CCM.0b013e318198683a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Broderick JP, Brott TG, Duldner JE, et al. . Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–93 10.1161/01.STR.24.7.987 [DOI] [PubMed] [Google Scholar]
- 19. van der Jagt M, Hasan D, Bijvoet HW, et al. . Interobserver variability of cisternal blood on CT after aneurysmal subarachnoid hemorrhage. Neurology 2000;54:2156–58 10.1212/WNL.54.11.2156 [DOI] [PubMed] [Google Scholar]
- 20. Scherer M, Cordes J, Younsi A, et al. . Development and validation of an automatic segmentation algorithm for quantification of intracerebral hemorrhage. Stroke 2016;47:2776–82 10.1161/STROKEAHA.116.013779 [DOI] [PubMed] [Google Scholar]
- 21. Friedman JA, Goerss SJ, Meyer FB, et al. . Volumetric quantification of Fisher grade 3 aneurysmal subarachnoid hemorrhage: a novel method to predict symptomatic vasospasm on admission computerized tomography scans. J Neurosurg 2002;97:401–07 10.3171/jns.2002.97.2.0401 [DOI] [PubMed] [Google Scholar]
- 22. Boers AM, Zijlstra IA, Gathier CS, et al. . Automatic quantification after subarachnoid hemorrhage on noncontrast CT. AJNR Am J Neuroradiol 2014;35:2279–86 10.3174/ajnr.A4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teasdale GM, Drake CG, Hunt W, et al. . A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry 1988;51:1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vergouwen MD, Vermeulen M, van Gijn J, et al. . Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010;41:2391–95 10.1161/STROKEAHA.110.589275 [DOI] [PubMed] [Google Scholar]
- 25. Yushkevich PA, Piven J, Hazlett HC, et al. . User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006;31:1116–28 10.1016/j.neuroimage.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 26. van der Zande JJ, Hendrikse J, Rinkel GJ. CT angiography for differentiation between intracerebral and intra-sylvian hematoma in patients with ruptured middle cerebral artery aneurysms. AJNR Am J Neuroradiol 2011;32:271–75 10.3174/ajnr.A2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Rooij NK, Rinkel GJ, Dankbaar JW, et al. . Delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review of clinical, laboratory, and radiological predictors. Stroke 2013;44:43–54 10.1161/STROKEAHA.112.674291 [DOI] [PubMed] [Google Scholar]
- 28. Ko SB, Choi HA, Carpenter AM, et al. . Quantitative analysis of hemorrhage volume for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Stroke 2011;42:669–74 10.1161/STROKEAHA.110.600775 [DOI] [PubMed] [Google Scholar]
- 29. Rabinstein AA, Friedman JA, Weigand SD, et al. . Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke 2004;35:1862–66 10.1161/01.STR.0000133132.76983.8e [DOI] [PubMed] [Google Scholar]
- 30. Bedussi B, van der Wel NN, de Vos J, et al. . Paravascular channels, cisterns, and the subarachnoid space in the rat brain: a single compartment with preferential pathways. J Cereb Blood Flow Metab 2017;37:1374–85 10.1177/0271678X16655550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vergouwen MD, Vermeulen M, Coert BA, et al. . Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab 2008;28:1761–70 10.1038/jcbfm.2008.74 [DOI] [PubMed] [Google Scholar]
- 32. Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 2014;10:44–58 10.1038/nrneurol.2013.246 [DOI] [PubMed] [Google Scholar]