Abstract
BACKGROUND AND PURPOSE:
Differential diagnosis of multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration from Parkinson disease on clinical grounds is often difficult. MR imaging biomarkers could assist in a more accurate diagnosis. We examined the utility of MR imaging surface measurements (MR imaging planimetry) in the differential diagnosis of patients with parkinsonism.
MATERIALS AND METHODS:
Fifty-two patients with Parkinson-plus (progressive supranuclear palsy, n = 24; corticobasal degeneration, n = 9; multiple system atrophy, n = 19), 18 patients with Parkinson disease, and 15 healthy controls were included. Corpus callosum, midbrain, and pons surfaces; relevant indices; and the Magnetic Resonance Parkinsonism Index were calculated. Corpus callosum subsection analysis was performed, and the corpus callosum posteroanterior gradient was introduced.
RESULTS:
A Magnetic Resonance Parkinsonism Index value of >12.6 discriminated progressive supranuclear palsy from other causes of parkinsonism with a 91% sensitivity and 95% specificity. No planimetry measurement could accurately discriminate those with multiple system atrophy with parkinsonism from patients with Parkinson disease. A corpus callosum posteroanterior gradient value of ≤191 was highly specific (97%) and moderately sensitive (75%) for the diagnosis of corticobasal degeneration versus all other groups. A midbrain-to-corpus callosum posteroanterior gradient ratio of ≤0.45 was highly indicative of progressive supranuclear palsy over corticobasal degeneration (sensitivity 86%, specificity 88%).
CONCLUSIONS:
MR imaging planimetry measurements are potent imaging markers of progressive supranuclear palsy and promising markers of corticobasal degeneration but do not seem to assist in the diagnosis of multiple system atrophy with parkinsonism.
Multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD) are neurodegenerative parkinsonian disorders that compose the Parkinson-plus syndromes. Despite the presence of distinct clinical features, differential diagnosis is often difficult.1 Diagnostic criteria for PSP and MSA lack sensitivity, particularly in atypical cases and at the early stages of the diseases.2,3 Recently established criteria of CBD, on the other hand, are considered to lack specificity, and misdiagnosis may reach up to 50%.4 Thus, objective diagnostic markers are needed to improve diagnostic accuracy in parkinsonian disorders.
PSP is characterized by relatively selective midbrain and superior cerebellar peduncle atrophy,5 as well as anterior corpus callosum thinning.6 Pons and middle cerebellar peduncle atrophy is pronounced in MSA.7 Patients with CBD present with asymmetric frontoparietal cortical atrophy as well as middle and posterior corpus callosum (CC) thinning.8,9 By means of MR imaging planimetry, the midbrain-to-pons surface ratio and the Magnetic Resonance Parkinsonism Index (MRPI), calculated by multiplying the pontine-to-midbrain area ratio by the middle cerebellar peduncle-to-superior cerebellar peduncle width ratio, have been suggested to assist in a more accurate and earlier diagnosis of PSP and, to a lesser extent, of MSA.10 To the best of our knowledge, no MR imaging planimetry study has incorporated midbrain, pons, and CC surface measurements in all 3 Parkinson-plus syndromes.
The aim of the present study was to examine the utility of already-suggested MR imaging brain stem surfaces and ratios in the differential diagnosis of patients with parkinsonism, including CBD, and to assess novel ones (incorporating the CC surface and CC subsections) in a well-characterized, prospectively diagnosed cohort.
Materials and Methods
Patients
Patients were consecutively and prospectively recruited (between 2011 and 2014) as part of the Parkinson-Plus Registry of the 1st Department of Neurology, National and Kapodistrian University of Athens. Detailed neurologic history was obtained, and a comprehensive neurologic examination was performed in all patients, with a follow-up of at least 2 years. Standard laboratory tests to exclude secondary causes of parkinsonism were performed in all patients, as appropriate.
All patients fulfilled the established the diagnostic criteria for probable PSP with a Richardson syndrome phenotype,11 probable corticobasal degeneration with a probable corticobasal syndrome phenotype,4 or multiple system atrophy.12 None of the included patients had a history of stroke or other focal lesions, in accordance with the exclusion criteria of the established diagnostic criteria.
The Unified Parkinson's Disease Rating Scale III was applied in all patients to measure the severity of parkinsonism. The bradykinesia and rigidity subscores were also included.
CSF β amyloid (Aβ42), τ protein (τΤ), and phosphorylated τ protein at threonine-181 (τP-181) were measured in all patients, as described elsewhere.13 Patients with a typical Alzheimer disease CSF biochemical profile were excluded (ie, decreased Aβ42, elevated τΤ and τP-181 according to cutoff values of our laboratory).13 Thus 5 patients fulfilling the criteria for probable CBD were excluded.
Finally, 52 patients with Parkinson-plus were included (PSP, n = 24; CBD, n = 9; MSA, n = 19). For comparison, a group of 18 patients with Parkinson disease (PD) diagnosed according to the UK Parkinson's Disease Society Brain Bank clinical diagnostic criteria were used.14 Furthermore, 15 otherwise healthy individuals, with no history of neurologic, psychiatric, or other major diseases and no signs of parkinsonism or cognitive dysfunction who were admitted to our department for nonrelevant issues (headache, dizziness, and so forth) served as a control group. Demographic data of our cohort are presented in Table 1.
Table 1:
Demographic and clinical characteristicsa
| Controls n = 15 | PSP n = 24 | CBD n = 9 | MSA n = 19 | PD n = 18 | P Value | |
|---|---|---|---|---|---|---|
| Demographic data | ||||||
| Sex (M/F) | 8:7 | 13:11 | 4:5 | 14:5 | 10:8 | .454b |
| Age (yr) | 62.6 (9.0) | 63.2 (6.8) | 67.9 (5.6) | 64.2 (7.1) | 64.4 (9.3) | .433c |
| Disease duration (yr) | NA | 3.3 (1.8) | 3.3 (1.8) | 3.1 (2.1) | 10.6 (6.1) | <.001c |
| Clinical data | ||||||
| UPDRS III | NA | 21.3 (9.8) | 25.2 (7.1) | 19.6 (19.1) | 32.3 (11.5) | .875c |
| UPDRSbrad | NA | 6.1 (4.7) | 12.5 (3.6) | 7.7 (9.3) | 14.5 (8.4) | .134c |
| UPDRSrig | NA | 4.1 (3.0) | 7.2 (4.4) | 3.9 (5.6) | 9.2 (6.5) | .266c |
| White matter lesion burden assessment | ||||||
| Fazekas PVH score | 0.38 (0.65) | 0.71 (0.75) | 0.70 (0.48) | 0.37 (0.50) | 0.47 (0.62) | .321c |
| Fazekas DWMH score | 0.77 (0.73) | 0.54 (0.66) | 0.40 (0.52) | 0.53 (061) | 0.53 (0.51) | .684c |
Note:—DWMH indicates deep white matter hyperintensity; UPDRS, Unified Parkinson Disease Rating Scale; UPDRSbrad, bradykinesia subscore of UPDRS; UPDRSrig, rigidity subscore of UPDRS; PVH, periventricular; NA, not applicable.
Data are presented as mean (SD).
χ2 test.
ANOVA.
Ethical Issues
All patients (or the next of kin caretaker in cases of compromised mental capacity) gave written informed consent for participation in the study, which was performed according to the ethics guidelines of the 1964 Declaration of Helsinki and had the approval of the Scientific and Ethics Committee of Eginition Hospital.
MR Imaging Acquisition
MR imaging was performed on a variety of high-field (1.5T to 3T) MR imaging units. The sequences included T1-weighted axial, sagittal, and coronal images or 3D T1-weighted turbo field echo sequences. MR imaging specifications were as follows: TR range, 500–650 ms; TE range, 10–15 ms; FOV range, 24–25 cm; matrix range, 192 × 256 to 320 × 320; section thickness, 1–5 mm; intersection spacing, 1 mm.
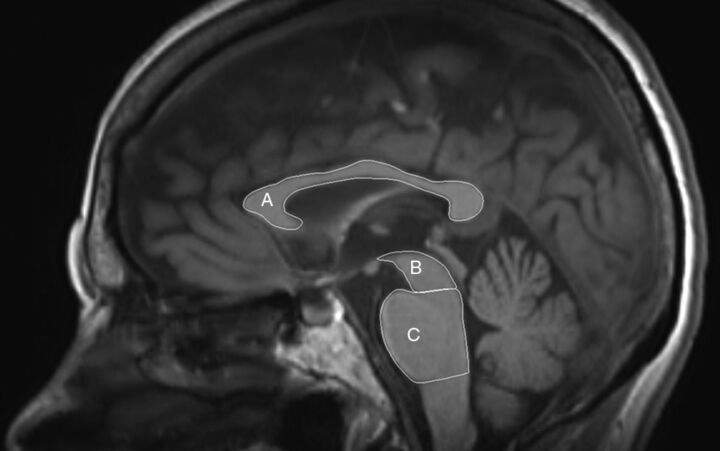
Midbrain, pons, and CC surfaces were measured at the midsagittal plane. Lines parallel to the mammillary–posterior commissural plane at the rostral and caudal pontine border were used to determine the midbrain and pons surfaces (Fig 1), according to the method of Kato et al.15 Middle cerebellar peduncle width was measured parasagittally, and superior cerebellar peduncle width was measured coronally (On-line Fig 1), according to the method of Longoni et al.16 The validity of the method has already been established as excellent, with low intra- and interrater variability of the measurements.16–18 All surfaces were manually traced by G.V. with the DICOM viewer R3.0-Sp3 (Philips Healthcare, Best, the Netherlands).
Fig 1.
MR imaging planimetry measurements. Midsagittal T1-weighted image depicts corpus callosum (A), midbrain tegmentum (B), and pons (C) surfaces.
CC subsections were determined according to the classification of Hofer and Frahm.19 According to this classification, the CC can be subdivided into 5 sections (CC1 to CC5), which represent, from anterior to posterior, the following brain regions: 1) prefrontal cortex, 2) premotor and supplementary motor cortex, 3) primary motor cortex, 4) primary sensory cortex, and 5) parietal, occipital, and temporal cortices (On-line Fig 2). To optimize diagnostic accuracy, we calculated and compared various indices based on CC subsections. Of these indices, the CC posteroanterior gradient (CCP-A grad) provided the greatest discriminative power and was therefore used. The CCP-A grad was calculated by subtracting the CC1 subsection from the remaining CC subsections (CCP-A grad = CC2 + CC3 + CC4 + CC5–CC1). The rationale behind the implementation of the CCP-A grad was the relatively selective (CC2 to CC4) atrophy in CBD, with a preserved CC1 surface (On-line Fig 3).
The Fazekas periventricular and deep white matter hyperintensity score was used to measure while matter lesion burden.20
Statistical Analysis
Numeric variables were checked for normality and homogeneity of variances by the Shapiro-Wilk and Levene tests, respectively. Analysis of covariance, using the diagnosis, sex, and magnetic field strength (1.5T versus 3T) as co-factors and age as a covariate, followed by post hoc Bonferroni correction for multiple comparisons or the Kruskal-Wallis test (followed by the Dunn post hoc test), was used as appropriate. Receiver operating characteristic curve analysis was applied for determination of the diagnostic value of each biomarker.
Two sets of analyses were performed. Initially, each diagnostic group (PSP, MSA, and CBD) was compared with all other groups, to examine the utility of planimetry MR imaging markers in the clinical scenario of a patient with parkinsonism of unknown etiology. The second analysis included pair-wise comparison of diagnoses that can be difficult to differentiate in clinical practice (ie, PSP versus multiple system atrophy with parkinsonism [MSA-P] versus CBD versus PD).
Analyses were performed by SPSS Statistics, Version 22.0.0.0 (IBM, Armonk, New York; 2013) and GraphPad Prism, Version 5.03 (GraphPad Software, San Diego, California; 2009).
Results
Clinical and Demographic Data
Groups did not differ among each other with respect to age and sex (Table 1). As expected, disease duration was significantly greater in those with PD compared with patients with Parkinson-plus. ANCOVA revealed significant effects by diagnostic group, but none of the cofactors and covariates affected the models significantly.
Planimetry Measurements
Patients with PSP exhibited significantly smaller midbrain surfaces compared with all other groups, resulting in smaller midbrain/CC and midbrain/pons ratios, as well as greater MRPI values (On-line Table). Furthermore, they presented with mild pons atrophy (numerically greater than MSA-P but lower than multiple system atrophy cerebellar type [MSA-C]).
Patients with MSA had smaller pons surfaces and pons-derived surface ratios as well as MRPI values. This difference came from patients with MSA-C because patients with MSA-P exhibited pons surfaces comparable with those of controls. Likewise, their midbrain and CC surfaces were like those of controls.
Patients with CBD had smaller CC surfaces compared with the other groups and numerically decreased midbrain surfaces (On-line Fig 4).
Comparison of Each Group versus All Other Groups
The MRPI provided excellent discriminative power for PSP versus all other groups, with a value of ≥12.6 providing 91% sensitivity and 95% specificity (Table 2 and On-line Fig 5). The MRPI was better compared with other indices for the differential diagnosis of MSA versus all other groups, with moderate sensitivity (74%) and excellent specificity (94%) for a cutoff point of ≤7.32.
Table 2:
ROC curve analysis of the discriminative power of morphometric measurements in patients with PSP, MSA, and CBD compared with all other groups
| AUC (SD) | P Value | Cutoff Value | Sensitivity (%) | Specificity (%) | Likelihood Ratio | |
|---|---|---|---|---|---|---|
| PSP vs all groups | ||||||
| Midbrain | 0.94 (0.03) | <.001 | ≤107 | 79 | 97 | 24.54 |
| Midbrain/pons | 0.93 (0.03) | <.001 | ≤0.22 | 88 | 84 | 5.42 |
| Midbrain/CC | 0.92 (0.04) | <.001 | ≤0.21 | 92 | 80 | 4.58 |
| MRPI | 0.98 (0.01) | <.001 | >12.6 | 91 | 95 | 18.48 |
| MSA (total) vs all groups | ||||||
| Pons | 0.74 (0.08) | .001 | ≤475 | 63 | 85 | 4.23 |
| Midbrain/pons | 0.86 (0.05) | <.001 | ≥0.29 | 74 | 90 | 7.05 |
| MRPI | 0.91 (0.04) | <.001 | ≤7.32 | 74 | 94 | 11.78 |
| CBD vs all groups | ||||||
| Corpus callosum | 0.79 (0.09) | .004 | ≤470 | 67 | 95 | 12.33 |
| CCP-A grad | 0.83 (0.10) | .002 | ≤191 | 75 | 97 | 25.12 |
| Pons/CCP-A grad | 0.81 (0.08) | .003 | ≥2.22 | 89 | 71 | 2.98 |
Note:—AUC indicates area under the curve.
The CCP-A grad with a cutoff point of ≤191 mm2 provided 97% specificity and 75% sensitivity for the diagnosis of CBD. A CC surface of ≤470 mm2 provided moderate sensitivity (67%) and excellent specificity (95%) for the diagnosis of CBD.
Pair-Wise Group Comparisons
The MRPI achieved very good discrimination between PSP and MSA-P with 100% specificity and sensitivity (Table 3). Likewise, the MRPI discriminated patients with PSP from those with PD with a sensitivity of 86% and a specificity of 100%. The midbrain/CCP-A grad ratio provided 86% sensitivity and 88% specificity in the differential diagnosis of PSP from CBD. No MR imaging measurement was clinically useful in differentiating patients with PD from those with MSA-P. The CCP-A grad provided moderate sensitivity and excellent specificity in the differential diagnosis of patients with CBD from those with PD (75% and 100%, respectively). Midbrain surface discriminated between patients with MSA-P and CBD with a sensitivity and specificity of 89%.
Table 3:
Pair-wise analysis of the discriminative power of morphometric measurements with ROC curve analysis
| MRPI | Midbrain (mm2) | Pons (mm2) | CC (mm2) | CCP-A grad (mm2) | Midbrain/Pons | Midbrain/CC | Midbrain/CCP-A grad | Pons/CC | |
|---|---|---|---|---|---|---|---|---|---|
| PSP vs MSA-P | |||||||||
| Cutoff | ≥11.0 | ≤129 | NS | NS | NS | ≤0.22 | ≤0.19 | ≤0.45 | NS |
| Sens (%) | 100 | 96 | 88 | 75 | 86 | ||||
| Spec (%) | 100 | 89 | 89 | 100 | 100 | ||||
| PSP vs PD | |||||||||
| Cutoff | ≥12.7 | ≤109 | ≤523 | NS | NS | ≤0.21 | ≤0.19 | ≤0.40 | NS |
| Sens (%) | 86 | 79 | 75 | 71 | 75 | 77 | |||
| Spec (%) | 100 | 94 | 78 | 100 | 100 | 94 | |||
| PSP vs CBD | |||||||||
| Cutoff | ≥13.7 | ≤100 | NS | ≥470 | ≥192 | ≤0.19 | ≤0.18 | ≤0.45 | ≥0.87 |
| Sens (%) | 73 | 71 | 92 | 91 | 67 | 71 | 86 | 46 | |
| Spec(%) | 100 | 100 | 67 | 75 | 89 | 100 | 88 | 100 | |
| MSA-P vs PD | |||||||||
| Cutoff | ≤8.0 | NS | NS | NS | NS | NS | NS | NS | NS |
| Sens (%) | 56 | ||||||||
| Spec (%) | 89 | ||||||||
| CBD vs PD | |||||||||
| Cutoff | NS | ≤146 | ≤563 | ≤479 | ≤193 | NS | NS | NS | NS |
| Sens (%) | 100 | 100 | 67 | 75 | |||||
| Spec (%) | 67 | 56 | 94 | 100 | |||||
| MSA-P vs CBD | |||||||||
| Cutoff | ≤11.0 | ≥129 | NS | ≥478 | ≥199 | NS | NS | NS | NS |
| Sens (%) | 100 | 89 | 89 | 100 | |||||
| Spec (%) | 63 | 89 | 67 | 75 |
Note:—NS indicates not significant; Sens, sensitivity; Spec, specificity; ROC, receiver operating characteristic.
Discussion
The present study aimed at examining the utility of MR imaging planimetry measurements as surrogate markers of midbrain, pons, and CC atrophy, in the differential diagnosis of patients with Parkinson-plus syndrome.
Patients with PSP in our cohort were characterized by severe midbrain and, to a lesser extent, pons atrophy. Of the patients with MSA, only those with MSA-C presented with pons atrophy. On the contrary, patients with MSA-P were not characterized by pons atrophy. Patients with CBD had predominantly CC atrophy and mild midbrain atrophy. Patients with PD did not differ from control subjects.
Most studies support midbrain and superior cerebellar peduncle atrophy as predominant features in patients with PSP.21,22 This translates into a significantly higher MRPI and lower midbrain-to-pons surface ratio values in patients with PSP.10,23,24 The MRPI was indeed the most potent imaging marker for the differential diagnosis of PSP from all other groups in our cohort. This also applied to the differential diagnosis of PSP from MSA-P or PD. It has been reported that these imaging findings are present early in the disease course of patients with PSP, often before the complete clinical phenotype of Richardson syndrome is evident.21,22,25 This finding renders the MRPI and midbrain-to-pons surface ratio useful in the early differentiation of patients with PSP from those with MSA-P and PD.
Low MRPI values have been suggested to further aid in the differential diagnosis of MSA from other causes of parkinsonism.17,26–28 In our cohort, low MRPI values were moderately sensitive (74%) and highly specific (94%) in the differentiation of patients with MSA from all other groups. This however was due to the inclusion of patients with MSA-C in the MSA group. These patients have particularly low MRPI values, due to pronounced pons and middle cerebellar peduncle atrophy. After we excluded patients with MSA-C, the MRPI was not clinically useful in the differential diagnosis of patients with MSA-P from those with PD or CBD. Thus, we could not establish the utility of the MRPI in the discrimination of patients with MSA-P from other patients with parkinsonism, except for PSP. Considering that patients with MSA-C are rarely confused with other patients with Parkinson-plus, due to their predominant cerebellar symptoms, low MRPI values may not provide clinically relevant assistance in most cases.
Patients with CBD in our cohort had decreased CC surfaces compared with other groups. A low (<470 mm2) corpus callosum surface was highly specific (95%) for CBD but lacked sensitivity (67%). This finding is in agreement with a single planimetry study that demonstrated CC atrophy in patients with CBD compared with PSP, with substantial between-group overlap however.29 CC subsection analysis indicated a posteroanterior CC atrophy gradient in CBD, with relative preservation of the CC1 segment (prefrontal cortex). PSP, on the other hand, had more pronounced anterior CC atrophy. This generated the CCP-A grad, which provided improved sensitivity (75%) and specificity (97%) in the diagnosis of CBD versus all other groups. The CCP-A grad was superior to all other indices in the differentiation of CBD from PD, with excellent specificity (100%) and moderate sensitivity (75%). Furthermore, the midbrain/CCP-A grad ratio was superior to the MRPI in the differential diagnosis of PSP from CBD (sensitivity 86%, specificity 88%).
Most planimetry studies of Parkinson-plus syndromes in the field lack pathologic confirmation, as is the case with our study. To compensate for the problem, we exclusively included prospectively diagnosed patients who fulfilled the “probable” diagnostic criteria. To keep the possibility of misdiagnosis as low as possible, all patients were followed up for at least 2 years. None of the patients presented with any atypical clinical features during this period. Furthermore, we used CSF biochemical profile analysis to exclude patients with an underlying Alzheimer disease pathology.
The sample size of our cohort is moderate, as is expected for diseases as rare as Parkinson-plus syndromes, but comparable with most studies on the subject. Intra- and interrater agreement of MR imaging surface measurements was not tested in the present study because it has already been proved excellent in previous studies.17,22,25,28
Further studies of larger cohorts of patients with Parkinson-plus syndrome, incorporating clinical, imaging, and pathologic data, would assist in further elucidating the complex interaction among underlying pathology, atrophy profile, and clinical phenotype. These studies could examine the utility of more focused planimetry markers, which take into account the topographic selectivity of atrophy (such as the CCP-A grad).
Conclusions
MR imaging planimetry can facilitate the differential diagnosis of patients with parkinsonism. Midbrain surface and relevant indices (such as the MRPI) are already established markers of PSP. Likewise, corpus callosum surface indices are promising markers of CBD. MR imaging planimetry, however, does not assist in the differential diagnosis of MSA-P from PD.
ABBREVIATIONS:
- CBD
corticobasal degeneration
- CC
corpus callosum
- CCP-A grad
corpus callosum posteroanterior gradient
- MRPI
Magnetic Resonance Parkinsonism Index
- MSA
multiple system atrophy
- MSA-C
multiple system atrophy cerebellar type
- MSA-P
multiple system atrophy with parkinsonism
- PD
Parkinson disease
- PSP
progressive supranuclear palsy
REFERENCES
- 1. Rolland Y, Vérin M, Payan C, et al. ; NNIPPS Study Group. A new MRI rating scale for progressive supranuclear palsy and multiple system atrophy: validity and reliability. J Neurol Neurosurg Psychiatry 2011;82:1025–32 10.1136/jnnp.2010.214890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Respondek G, Roeber S, Kretzschmar H, et al. . Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Mov Disord 2013;28:504–09 10.1002/mds.25327 [DOI] [PubMed] [Google Scholar]
- 3. Osaki Y, Ben-Shlomo Y, Lees AJ, et al. . A validation exercise on the new consensus criteria for multiple system atrophy. Mov Disord 2009;24:2272–76 10.1002/mds.22826 [DOI] [PubMed] [Google Scholar]
- 4. Armstrong MJ, Litvan I, Lang AE, et al. . Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503 10.1212/WNL.0b013e31827f0fd1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hauw JJ, Daniel SE, Dickson D, et al. . Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 1994;44:2015–19 10.1212/WNL.44.11.2015 [DOI] [PubMed] [Google Scholar]
- 6. Yamauchi H, Fukuyama H, Nagahama Y, et al. . Atrophy of the corpus callosum, cognitive impairment, and cortical hypometabolism in progressive supranuclear palsy. Ann Neurol 1997;41:606–14 10.1002/ana.410410509 [DOI] [PubMed] [Google Scholar]
- 7. Wenning GK, Tison F, Ben Shlomo Y, et al. . Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord 1997;12:133–47 10.1002/mds.870120203 [DOI] [PubMed] [Google Scholar]
- 8. Dickson DW, Bergeron C, Chin SS, et al. ; Office of Rare Diseases of the National Institutes of Health. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 2002;61:935–46 10.1093/jnen/61.11.935 [DOI] [PubMed] [Google Scholar]
- 9. Yamauchi H, Fukuyama H, Nagahama Y, et al. . Atrophy of the corpus callosum, cortical hypometabolism, and cognitive impairment in corticobasal degeneration. Arch Neurol 1998;55:609–14 10.1001/archneur.55.5.609 [DOI] [PubMed] [Google Scholar]
- 10. Quattrone A, Nicoletti G, Messina D, et al. . MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology 2008;246:214–21 10.1148/radiol.2453061703 [DOI] [PubMed] [Google Scholar]
- 11. Litvan I, Agid Y, Calne D, et al. . Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996;47:1–9 10.1212/WNL.47.1.1 [DOI] [PubMed] [Google Scholar]
- 12. Gilman S, Wenning GK, Low PA, et al. . Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–76 10.1212/01.wnl.0000324625.00404.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paraskevas GP, Kasselimis D, Kourtidou E, et al. . Cerebrospinal fluid biomarkers as a diagnostic tool of the underlying pathology of primary progressive aphasia. J Alzheimers Dis 2017;55:1453–61 10.3233/JAD-160494 [DOI] [PubMed] [Google Scholar]
- 14. Hughes AJ, Daniel SE, Kilford L, et al. . Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–84 10.1136/jnnp.55.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kato N, Arai K, Hattori T. Study of the rostral midbrain atrophy in progressive supranuclear palsy. J Neurol Sci 2003;210:57–60 10.1016/S0022-510X(03)00014-5 [DOI] [PubMed] [Google Scholar]
- 16. Longoni G, Agosta F, Kostić VS, et al. . MRI measurements of brainstem structures in patients with Richardson's syndrome, progressive supranuclear palsy-parkinsonism, and Parkinson's disease. Mov Disord 2011;26:247–55 10.1002/mds.23293 [DOI] [PubMed] [Google Scholar]
- 17. Cosottini M, Ceravolo R, Faggioni L, et al. . Assessment of midbrain atrophy in patients with progressive supranuclear palsy with routine magnetic resonance imaging. Acta Neurol Scand 2007;116:37–42 10.1111/j.1600-0404.2006.00767.x [DOI] [PubMed] [Google Scholar]
- 18. Morelli M, Arabia G, Salsone M, et al. . Accuracy of magnetic resonance parkinsonism index for differentiation of progressive supranuclear palsy from probable or possible Parkinson disease. Mov Disord 2011;26:527–33 10.1002/mds.23529 [DOI] [PubMed] [Google Scholar]
- 19. Hofer S, Frahm J. Topography of the human corpus callosum revisited; comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 2006;32:989–94 10.1016/j.neuroimage.2006.05.044 [DOI] [PubMed] [Google Scholar]
- 20. Fazekas F, Chawluk JB, Alavi A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;8:421–26 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 21. Aiba I, Hashizume Y, Yoshida M, et al. . Relationship between brainstem MRI and pathological findings in progressive supranuclear palsy: study in autopsy cases. J Neurol Sci 1997;152:210–17 10.1016/S0022-510X(97)00166-4 [DOI] [PubMed] [Google Scholar]
- 22. Slowinski J, Imamura A, Uitti RJ, et al. . MR imaging of brainstem atrophy in progressive supranuclear palsy. J Neurol 2008;255:37–44 10.1007/s00415-007-0656-y [DOI] [PubMed] [Google Scholar]
- 23. Hussl A, Mahlknecht P, Scherfler C. Diagnostic accuracy of the magnetic resonance Parkinsonism index and the midbrain-to-pontine area ratio to differentiate progressive supranuclear palsy from Parkinson's disease and the Parkinson variant of multiple system atrophy. Mov Disord 2010;25:2444–49 10.1002/mds.23351 [DOI] [PubMed] [Google Scholar]
- 24. Kim YH, Ma HI, Kim YJ. Utility of the midbrain tegmentum diameter in the differential diagnosis of progressive supranuclear palsy from idiopathic Parkinson's disease. J Clin Neurol 2015;11:268–74 10.3988/jcn.2015.11.3.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morelli M, Arabia G, Novellino F, et al. . MRI measurements predict PSP in unclassifiable parkinsonisms: a cohort study. Neurology 2011;77:1042–47 10.1212/WNL.0b013e31822e55d0 [DOI] [PubMed] [Google Scholar]
- 26. Massey LA, Jäger HR, Paviour DC, et al. . The midbrain to pons ratio: a simple and specific MRI sign of progressive supranuclear palsy. Neurology 2013;80:1856–61 10.1212/WNL.0b013e318292a2d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaasinen V, Kangassalo N, Gardberg M, et al. . Midbrain-to-pons ratio in autopsy-confirmed progressive supranuclear palsy: replication in an independent cohort. Neurol Sci 2015;36:1251–53 10.1007/s10072-015-2184-3 [DOI] [PubMed] [Google Scholar]
- 28. Oba H, Yagishita A, Terada H, et al. . New and reliable MRI diagnosis for progressive supranuclear palsy. Neurology 2005;64:2050–55 10.1212/01.WNL.0000165960.04422.D0 [DOI] [PubMed] [Google Scholar]
- 29. Gröschel K, Hauser T-K, Luft A, et al. . Magnetic resonance imaging-based volumetry differentiates progressive supranuclear palsy from corticobasal degeneration. Neuroimage 2004;21:714–24 10.1016/j.neuroimage.2003.09.070 [DOI] [PubMed] [Google Scholar]