Abstract
Introduction
Tofacitinib is an oral Janus kinase inhibitor for the treatment of psoriatic arthritis (PsA). We report the interim safety, tolerability, and efficacy of tofacitinib in PsA patients in OPAL Balance, a 3-year, open-label, long-term extension study (data cut-off: August 2017; database not locked, data may change).
Methods
Eligible patients from two phase (P) 3 (P3) tofacitinib PsA studies (OPAL Broaden, NCT01877668; OPAL Beyond, NCT01882439) entered OPAL Balance ≤ 3 months after completing the P3 study or discontinuing for reasons other than study-drug-related adverse events (AEs). Patients received open-label tofacitinib 5 mg twice daily (BID), with adjustments to 10 mg BID permitted post-month (M) 1. Certain concomitant conventional synthetic disease-modifying antirheumatic drugs were allowed. Primary endpoints were incidence/severity of AEs and laboratory abnormalities, and changes from baseline in laboratory parameters (reported up to M36 and M30, respectively). Efficacy (clinical/patient-reported outcomes) was reported through M30.
Results
A total of 686 patients were treated; at data cut-off, 68.2% remained in the study. Mean (range) treatment duration was 641 (1–1032) days; total treatment duration was 1153.2 patient-years. By M36, 79.6, 13.8, and 8.6% of patients reported AEs, serious AEs, and discontinuations due to AEs, respectively. Five deaths occurred; one within the risk period (incidence rate [IR; patients with events/100 patient-years] 0.1). IRs for AEs of special interest were: all (non-serious and serious) herpes zoster, 1.7; serious infections, 0.9; opportunistic infections, 0.3 (all disseminated/multi-dermatomal herpes zoster); malignancies excluding non-melanoma skin cancer (NMSC), 0.8; NMSC, 1.0; major adverse cardiovascular events, 0.3; pulmonary embolisms, 0.1; and arterial thromboembolisms, 0.4. No patients had deep vein thrombosis. Alanine aminotransferase and aspartate aminotransferase levels were elevated ≥ 3-fold the upper limit of normal in 4.0 and 2.2% of patients, respectively. Changes in laboratory parameters were generally stable over time, although lymphocyte counts decreased slightly. Efficacy was maintained through M30.
Conclusions
In this interim analysis of OPAL Balance, tofacitinib safety and efficacy in patients with PsA appeared to be consistent with those of the P3 studies. Efficacy was maintained over time.
Trial Registration
ClinicalTrials.gov identifier: NCT01976364.
Electronic Supplementary Material
The online version of this article (10.1007/s40744-020-00209-4) contains supplementary material, which is available to authorized users.
Keywords: Long-term extension, Psoriatic arthritis, Tofacitinib
Plain Language Summary
In many countries, tofacitinib is an approved medicine that can be used to treat psoriatic arthritis (PsA). In the study reported here (OPAL Balance), adult patients with PsA took tofacitinib for up to 3 years. We report a planned interim analysis, i.e., an analysis of information collected before the study finished. This early information suggests that the safety of tofacitinib, and how well it improved symptoms and quality of life (efficacy), was similar in this long study as in shorter studies. Information from the finished study will be reported later. OPAL Balance started on 17 February 2014. This interim analysis includes information collected by 31 August 2017. Before joining, patients had finished a 6-month or 12-month tofacitinib study (OPAL Broaden or OPAL Beyond). Patients in OPAL Balance took a 5 mg tofacitinib pill twice a day, but if PsA symptoms did not improve after 1 month, they could take a 10 mg pill twice a day. They could also take other medicines (including methotrexate or corticosteroids). Of the 686 patients who took tofacitinib, 546 (80%) experienced side effects over 3 years. These were considered serious for 95 patients (14%) and caused 59 patients (9%) to leave the study. Five patients died from causes not related to tofacitinib. Known tofacitinib side effects, including shingles (herpes zoster), serious infections (needing hospitalization), infections in patients with weakened immune systems, cancer, heart (cardiovascular) problems, and vein blockages (embolisms), were each reported by fewer than 20 patients. Most blood test results and tofacitinib efficacy were stable over 2.5 years.
Electronic Supplementary Material
The online version of this article (10.1007/s40744-020-00209-4) contains supplementary material, which is available to authorized users.
Key Summary Points
| Why carry out this study? |
| Psoriatic arthritis (PsA) is a chronic disease that requires long-term treatment. |
| Tofacitinib is an oral Janus kinase inhibitor for the treatment of PsA. |
| This interim analysis of the OPAL Balance long-term extension study reports the long-term safety (up to month 36) and efficacy (up to month 30) of open-label tofacitinib in patients with PsA. |
| What was learned from this study? |
| The long-term safety and efficacy of tofacitinib in patients with PsA appeared to be consistent with those of prior PsA phase 3 studies, and efficacy was maintained over time. |
Introduction
Psoriatic arthritis (PsA) is a chronic, immune-mediated, inflammatory, musculoskeletal disease, manifesting as peripheral arthritis, enthesitis, dactylitis, spondylitis, and skin and nail psoriasis [1, 2]. Current pharmacologic therapies for PsA include conventional synthetic disease-modifying antirheumatic drugs (csDMARDs; e.g., methotrexate [MTX]), biologic DMARDs (i.e., biologic inhibitors of tumor necrosis factor [TNFi], interleukin-12/23 and interleukin-17, and cytotoxic T-lymphocyte-associated antigen 4-immunoglobulin), and targeted synthetic DMARDs (e.g., apremilast and Janus kinase [JAK] inhibitors) [3–5].
Tofacitinib is an oral JAK inhibitor for the treatment of PsA. The efficacy and safety of tofacitinib 5 and 10 mg twice daily (BID) in patients with PsA have been reported in two phase 3 randomized controlled trials: OPAL Broaden (ClinicalTrials.gov identifier: NCT01877668), a 12-month study in patients with PsA who were naïve to TNFi therapy and had experienced an inadequate response to ≥ 1 csDMARD (422 patients randomized and treated), and OPAL Beyond (ClinicalTrials.gov identifier: NCT01882439), a 6-month study in patients with PsA who had experienced an inadequate response to ≥ 1 TNFi therapy (395 patients randomized, 394 treated) [6, 7].
In both OPAL Broaden and OPAL Beyond, tofacitinib efficacy was superior to placebo at month 3 (end of the placebo-controlled period), as demonstrated by American College of Rheumatology (ACR) 20 response rates and mean changes from baseline in the Health Assessment Questionnaire-Disability Index (HAQ-DI). Rates of adverse events (AEs) were numerically more frequent with tofacitinib than with placebo up to month 3 [6, 7].
Patients from OPAL Broaden and OPAL Beyond were eligible to enter OPAL Balance (ClinicalTrials.gov identifier: NCT01976364), a long-term extension (LTE) study designed to assess the long-term safety, tolerability, and efficacy of tofacitinib treatment in PsA. Two previous interim analyses of OPAL Balance have shown that the long-term safety profile of tofacitinib in patients with PsA was generally similar to that of OPAL Broaden and OPAL Beyond. Efficacy was maintained over time [8, 9].
In this third interim analysis of the OPAL Balance study (data cut-off: 31 August 2017), we report results on the long-term safety (up to month 36) and efficacy (up to month 30) of tofacitinib in adult patients with PsA.
Methods
Study Design and Treatment
OPAL Balance is a phase 3, open-label LTE study conducted in 124 centers across 16 countries. It was designed to evaluate the safety, tolerability, and efficacy of tofacitinib over 36 months in patients with PsA who had previously participated in the qualifying phase 3 studies OPAL Broaden [6] and OPAL Beyond [7].
Patients in OPAL Broaden and OPAL Beyond were randomized to receive tofacitinib 5 mg BID, tofacitinib 10 mg BID, placebo advancing to tofacitinib 5 or 10 mg BID after 3 months, or adalimumab 40 mg subcutaneously every 2 weeks (OPAL Broaden only). Upon entry into OPAL Balance, all patients received open-label tofacitinib 5 mg BID, regardless of previous treatment in the qualifying studies (Electronic Supplementary Material [ESM] Fig. S1). Patients entering OPAL Balance from OPAL Broaden received the first dose of open-label tofacitinib ≥ 1 week after the last injection of placebo or adalimumab. At the month 1 visit and at additional visits, the tofacitinib dose could be increased to 10 mg BID for reasons of inadequate control of PsA symptoms; the dose could subsequently be reduced back to 5 mg BID at any time due to safety events, at the investigator’s discretion. Concomitant csDMARD treatment according to local standard-of-care, stable doses of concomitant oral corticosteroids (≤ 10 mg prednisone daily or equivalent) or nonsteroidal anti-inflammatory drugs, or cyclo-oxygenase 2 inhibitors were allowed, but not required.
All data retrieved up to and including 31 August 2017 (study start date: 17 February 2014) were included in this interim analysis. Data included in this analysis are not from a locked database; therefore, some values may change with the final, locked study database.
The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the principles of the Declaration of Helsinki, and applicable local regulatory requirements and laws. The study protocol was approved by the Institutional Review Boards and/or Independent Ethics Committee at each study center (see ESM). An independent Data Safety Monitoring Board external to the study sponsor reviewed safety data on a cumulative basis, and Safety Endpoint Adjudication Committees provided standardized safety assessment for selected events. All patients provided written, informed consent.
Patients
Full patient inclusion and exclusion criteria for the qualifying studies have been reported previously [6, 7]. In brief, patients eligible to enter OPAL Broaden and OPAL Beyond were ≥ 18 years of age (≥ 20 years in Taiwan), had been diagnosed with PsA for ≥ 6 months, met the ClASsification criteria for Psoriatic ARthritis (CASPAR) [10], and had active arthritis and plaque psoriasis at screening. Patients from these two studies were eligible to enter OPAL Balance ≤ 3 months after completing the qualifying study or discontinuing for reasons other than study-drug-related AEs. Patients who enrolled into OPAL Balance > 14 days after the end of the study visit of the qualifying study must have had sufficient evidence of PsA disease activity to warrant the use of tofacitinib, discontinued all disallowed concomitant medications, and had no evidence of active, latent, or inadequately treated infection with Mycobacterium tuberculosis. Additional exclusion criteria for these patients included the following laboratory assessments at the LTE screening visit or ≤ 3 months prior to the LTE baseline visit: evidence of hematopoietic disorders or evidence of hemoglobin levels < 10 g/dL; absolute white blood cell count of < 3.0 × 103/mm3; absolute neutrophil count (ANC) of ≤ 1.5 × 103/mm3; absolute lymphocyte count (ALC) of < 1.0 × 103/mm3; and platelet count of < 100 × 103/mm3. The following laboratory assessments at the LTE screening visit only were also considered as exclusion criteria for patients who enrolled in OPAL Balance > 14 days after the end of the qualifying study: estimated creatinine clearance < 40 mL/min (Cockcroft–Gault equation); and total bilirubin, alanine aminotransferase (ALT), or aspartate aminotransferase (AST) > 1.5-fold the upper limit of normal (ULN).
Trial Objectives and Interim Analysis Endpoints
The primary objective of OPAL Balance was to evaluate the long-term safety and tolerability of tofacitinib in adult patients with PsA. Evaluating the long-term efficacy of tofacitinib was a secondary objective.
The primary endpoints included in this interim analysis were the incidence and severity of AEs, the incidence of laboratory abnormalities, and changes from baseline in laboratory parameters during treatment. AEs were reported up to month 36, including serious AEs (SAEs), severe AEs (AEs that interfere significantly with a patient’s usual function), discontinuations and dose reductions due to AEs, deaths, and AEs of special interest (herpes zoster, serious infections, tuberculosis [TB], opportunistic infections, gastrointestinal perforations, interstitial lung disease, inflammatory bowel disease, malignancies [all malignancies, malignancies excluding non-melanoma skin cancer (NMSC), and NMSC], major adverse cardiovascular events [MACE], venous thromboembolism [VTE] events [pulmonary embolism (PE) and deep vein thrombosis (DVT)], and arterial thromboembolism (ATE) events]. PE, DVT, and ATE events were based on selected events in a narrow Standardized Medical Dictionary for Regulatory Activities (MedDRA) Query (SMQ) for embolic and thrombotic events. Laboratory abnormalities (elevations in ALT and AST, and the proportions of patients meeting discontinuation criteria) were reported up to month 36. Changes from baseline in laboratory parameters were reported up to month 30 and included ALT, AST, hemoglobin, ALC, ANC, creatinine, and creatine kinase assessed at months 1 and 3, and every 3 months thereafter. Percentage changes from baseline in absolute CD16+56 cells (a subset of lymphocytes) were assessed at month 3, and every 3 months thereafter until month 30. Percentage changes from baseline in high-density lipoprotein cholesterol (HDL-cholesterol), low-density lipoprotein cholesterol (LDL-cholesterol), total cholesterol, and triglycerides were assessed in patients at months 3 and 6, and every 6 months thereafter until month 30, during visits requiring fasting lipid panels. Absolute mean values for these laboratory parameters were also reported up to month 30 (ESM Fig. S2).
Secondary and other endpoints reported in this interim analysis included the efficacy data listed here, and these were reported up to month 30 (as sample sizes were too small [N ≤ 50] for meaningful analysis beyond this point). Rheumatologic and dermatologic endpoints included: response rates of ACR20, ACR50, and ACR70 (the percentages of patients reporting a ≥ 20%, ≥ 50%, and ≥ 70% improvement, respectively, in ACR criteria); Psoriatic Arthritis Response Criteria (PsARC; improvement in two of the following four criteria, one of which must be joint pain or swelling, without worsening in any measure: ≥ 30% improvement in tender joint counts [68 joints] or swollen joint counts [66 joints], and ≥ 20% improvement in Patient or Physician Global Assessment of Arthritis) [11]; Psoriasis Area and Severity Index 75% improvement (PASI75; the percentage of patients reporting a ≥ 75% improvement in PASI, calculated in patients with plaque psoriasis affecting ≥ 3% of body surface area at baseline and with a baseline PASI score > 0); and changes from baseline in Physician Global Assessment of Psoriasis (PGA-PsO; range: 0 [clear] to 4 [severe]; assessed in patients with baseline PGA-PsO > 0), Leeds Enthesitis Index (LEI; range 0–6; higher scores indicate more affected sites; assessed in patients with baseline LEI > 0), and Dactylitis Severity Score (DSS; range 0–60; higher scores indicate greater severity; assessed in patients with baseline DSS > 0). Composite endpoints included: rates of achievement of minimal disease activity (MDA; defined as a patient meeting at least five of the seven MDA criteria) [12]; and changes from baseline in Disease Activity in Psoriatic Arthritis (DAPSA; higher scores indicate higher disease activity), Composite Psoriasis Disease Activity Index (CPDAI; range 0–15; higher scores indicate more severe disease activity; calculated in patients with plaque psoriasis affecting ≥ 3% of body surface area at baseline), and Psoriatic ArthritiS Disease Activity Score (PASDAS; higher scores indicate higher disease activity). The following patient-reported outcomes (PROs) were also assessed: rates of HAQ-DI response (decrease from baseline in HAQ-DI ≥ 0.35; assessed in patients with baseline HAQ-DI ≥ 0.35); and changes from baseline in HAQ-DI (range 0–3; higher scores indicate greater disability), Patient Assessment of Arthritis Pain (Pain; Visual Analog Scale [VAS] 0–100 mm; higher scores indicate more severe arthritis pain), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) total score (range 0–52; lower scores indicate more fatigue), Dermatology Life Quality Index (DLQI; range 0–30; higher values indicate a greater impact on quality of life), Short Form-36 Health Survey Version 2 (SF-36v2) Physical Component Summary (PCS) and Mental Component Summary (MCS) scores and eight norm-based domain scores (physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health; lower scores indicate a worse health outcome), and EuroQol five dimensions, three levels (EQ-5D-3L) dimension scores (mobility, self-care, usual activities, pain/discomfort, anxiety/depression; higher scores indicate greater impact on dimension) and EQ-VAS (“Your own health state today” measured using a VAS 0–100 mm; lower scores indicate a worse health state).
Statistical Analysis
This was an open-label study with no formal hypothesis testing. Safety and efficacy endpoints were summarized using a single tofacitinib group (all tofacitinib group), which included all patients who received at least one dose of tofacitinib in OPAL Balance. This was the main analysis group, since patients were able to switch tofacitinib doses between 5 and 10 mg BID per the investigator’s discretion, as permitted by the study protocol. For all efficacy and safety endpoints, only observed values were used, and no imputation was applied to missing values.
To provide information on potential dose-related effects, post-hoc analyses, including average total daily dose (ATDD; i.e., sum of total daily doses of tofacitinib received divided by the number of days of treatment) and constant daily dose were also performed based on treatment assignment algorithms. The average tofacitinib 5 mg BID and average tofacitinib 10 mg BID groups included all patients who received an ATDD of < 15 mg and ≥ 15 mg, respectively, from day 1 through the data cut-off in OPAL Balance. The constant tofacitinib 5 mg BID group included all patients in the study who started on tofacitinib 5 mg BID and stayed on tofacitinib 5 mg BID (as per sponsor’s smoothing algorithm), until switching to tofacitinib 10 mg BID or discontinuing the study. Exposure and events captured after a dose switch were excluded from the analysis. Therefore, unlike the all tofacitinib group, which included all exposure and events regardless of whether patients switched between tofacitinib 5 and 10 mg BID during OPAL Balance, the constant tofacitinib 5 mg BID group represented a group of patients who only received tofacitinib 5 mg BID during their participation in OPAL Balance.
Patient demographics, baseline disease characteristics, and baseline values for all efficacy or health outcome endpoints came from the baseline of the qualifying study (OPAL Broaden or OPAL Beyond), disregarding the enrollment gap. For safety endpoints, baseline values from the qualifying study were used for patients who enrolled ≤ 14 days after the final visit of the qualifying study, whereas baseline values from OPAL Balance were used for patients who enrolled > 14 days after the final visit of the qualifying study.
Incidence rates (IRs) were calculated for SAEs, discontinuations due to AEs, deaths, and selected AEs of special interest. These were expressed as the number of patients with events per 100 patient-years (PY) and were estimated by dividing the number of patients with events during their risk periods by the sum of exposure times of patients during the risk period, then multiplying it by 100 (exposure times being the time from exposure to treatment to the first event for patients with event, or to the end of the risk period for patients without event, in PY). The risk period was defined as the time from exposure to treatment to the patient’s last dose plus 28 days or to the date of last observation, whichever was earlier. This approach provided exposure-adjusted IRs that accounted for differing follow-up times between patients and quantified the risk of developing an event during the treatment and immediate post-treatment (up to 28 days) periods. Inclusion of all events (numerator) without regard to elapsed time may inflate IR estimations because the exposure time (denominator) is not similarly increased. The 95% confidence intervals (CIs) of IRs were calculated based on Exact Poisson distribution. Any events that occurred outside the risk period, although not included in the calculation of IRs, were summarized for completeness.
Results
Patients
In total, 686 patients were treated in OPAL Balance (363 patients from OPAL Broaden, 323 from OPAL Beyond). At the data cut-off for this interim analysis (31 August 2017), 468 (68.2%) patients remained in the study, 190 (27.7%) patients had discontinued, and 28 (4.1%) patients had completed 36 months of treatment (ESM Fig. S3); the mean (range) duration of tofacitinib treatment for the all tofacitinib group was 614 (1–1032) days. The total duration of tofacitinib treatment was 1153.2 PY for the all tofacitinib group (Table 1). Six patients did not receive tofacitinib 5 mg BID at study entry due to protocol deviations; therefore, 680 patients were included in the constant tofacitinib 5 mg BID group, and the total duration of tofacitinib treatment (constant tofacitinib 5 mg BID) was 686.9 PY in this group. Overall, the most common reasons for patients discontinuing from the study included that they were no longer willing to participate (58/190 patients [30.5%]), had insufficient clinical response (37/190 patients [19.5%]), had an AE related to the study drug (33/190 patients [17.4%]), or had an AE not related to the study drug (21/190 [11.1%]). Additional reasons for discontinuation included patients that were lost to follow-up (9/190 [4.7%]), had protocol violations (8/190 [4.2%]), died (4/190 [2.1%]), withdrew due to pregnancy (4/190 [2.1%]), no longer met eligibility criteria (2/190 [1.1%]), had a medication error without associated AE (1/190 [0.5%]), or discontinued due to ‘other’ reasons (13/190 [6.8%]).
Table 1.
Patient demographics and baseline disease characteristics
| Patient demographics and baseline disease characteristicsa | All tofacitinib doses (N = 686) | Average tofacitinib 5 mg BIDb (N = 407) | Average tofacitinib 10 mg BIDb (N = 279) | Constant tofacitinib 5 mg BIDc (N = 680) |
|---|---|---|---|---|
| Total duration,d PY | 1153.2 | 664.2 | 489.1 | 686.9 |
| Demographics | ||||
| Age (years), mean (SD) | 48.8 (11.8) | 49.4 (11.8) | 48.0 (11.7) | 48.8 (11.8) |
| Female, n (%) | 370 (53.9) | 224 (55.0) | 146 (52.3) | 367 (54.0) |
| White, n (%) | 646 (94.2) | 384 (94.3) | 262 (93.9) | 641 (94.3) |
| BMI (kg/m2), mean (SD) | 29.6 (6.1) | 29.6 (6.2) | 29.7 (5.9) | 29.7 (6.1) |
| Smoking history, n (%) | ||||
| Never smoked | 431 (62.8) | 258 (63.4) | 173 (62.0) | 429 (63.1) |
| Smoker | 121 (17.6) | 82 (20.1) | 39 (14.0) | 118 (17.4) |
| Ex-smoker | 134 (19.5) | 67 (16.5) | 67 (24.0) | 133 (19.6) |
| Baseline disease characteristics | ||||
| Duration of PsA (years), mean (SD) | 7.6 (7.2) | 7.7 (7.4) | 7.5 (6.8) | 7.6 (7.2) |
| Tender joint count (68), mean (SD) | 20.8 (14.4) | 19.5 (13.5) | 22.8 (15.3) | 20.8 (14.4) |
| Swollen joint count (66), mean (SD) | 11.9 (9.6) | 10.9 (8.7) | 13.2 (10.7) | 11.9 (9.6) |
| Plaque psoriasis affecting body surface area ≥ 3%, n (%) | 475 (69.2) | 286 (70.3) | 189 (67.7) | 472 (69.4) |
| PASI,e median (range) [N1] | 7.3 (0.3–66.0) [474] | 6.3 (0.3–66.0) [285] | 8.8 (0.9–46.0) [189] | 7.2 (0.3–66.0) [471] |
| Presence of enthesitis (LEI > 0), n (%) | 458 (66.8) | 264 (64.9) | 194 (69.5) | 453 (66.6) |
| LEI,f mean (SD) [N2] | 2.8 (1.6) [458] | 2.5 (1.4) [264] | 3.3 (1.7) [194] | 2.8 (1.6) [453] |
| Presence of dactylitis (DSS > 0), n (%) | 366 (53.4) | 206 (50.6) | 160 (57.3) | 363 (53.4) |
| DSS,g mean (SD) [N3] | 8.4 (8.2) [366] | 8.1 (8.0) [206] | 8.9 (8.4) [160] | 8.4 (8.2) [363] |
| HAQ-DI, mean (SD) | 1.2 (0.7) | 1.2 (0.7) | 1.2 (0.7) | 1.2 (0.7) |
| CRP > 2.87 mg/L, n (%) | 416 (60.6) | 241 (59.2) | 175 (62.7) | 413 (60.7) |
| CRP, mean (SD) | 11.9 (20.5) | 11.2 (18.9) | 12.8 (22.6) | 11.9 (20.6) |
| Concomitant oral corticosteroid use, n (%) | 132 (19.2) | 88 (21.6) | 44 (15.8) | 130 (19.1) |
| Oral corticosteroid dose (mg/day),h mean (SD) [N4] | 6.4 (2.7) [131] | 6.2 (2.5) [87] | 6.7 (3.1) [44] | 6.4 (2.7) [129] |
| Concomitant csDMARD use, n (%) | 675 (98.4) | 398 (97.8) | 277 (99.3) | 669 (98.4) |
| Methotrexate | 548 (79.9) | 330 (81.1) | 218 (78.1) | 543 (79.9) |
| Methotrexate dose (mg/week),h mean (SD) [N4] | 18.5 (7.0) [481] | 18.7 (7.0) [296] | 18.1 (6.8) [185] | 18.5 (7.0) [475] |
| Otheri,j | 128 (18.7) | 68 (16.7) | 60 (21.5) | 127 (18.7) |
| Baseline diabetes mellitus, n (%) | 88 (12.8) | 54 (13.3) | 34 (12.2) | 87 (12.8) |
| Baseline hypertension, n (%) | 255 (37.2) | 158 (38.8) | 97 (34.8) | 254 (37.4) |
| Baseline metabolic syndrome,k n (%) | 279 (40.7) | 170 (41.8) | 109 (39.1) | 277 (40.7) |
ATDD average total daily dose, BID twice daily, BMI body mass index, CRP C-reactive protein, csDMARD conventional synthetic disease-modifying antirheumatic drug, DSS Dactylitis Severity Score, HAQ-DI Health Assessment Questionnaire-Disability Index, HDL high-density lipoprotein, LEI Leeds Enthesitis Index, PASI Psoriasis Area and Severity Index, PsA psoriatic arthritis, PY patient-years, SD standard deviation
aBaseline demographics and disease characteristics were taken from the baseline of the qualifying study (OPAL Beyond or OPAL Broaden). Concomitant medications were those taken on day 1 of OPAL Balance. N is the number of patients in each treatment group
bAverage dosing was based on an ATDD of tofacitinib: patients who received an ATDD < 15 mg/day were assigned to the average tofacitinib 5 mg BID group; patients who received an ATDD ≥ 15 mg/day were assigned to the average tofacitinib 10 mg BID group
cConstant tofacitinib 5 mg BID included all patients who started OPAL Balance on tofacitinib 5 mg BID and stayed on tofacitinib 5 mg BID (as per sponsor’s smoothing algorithm) until switching to tofacitinib 10 mg BID or discontinuing the study. Assessments (including exposure and adverse events) after a dose switch to tofacitinib 10 mg BID were excluded from the analysis
dTotal duration (in PY) is calculated as the sum of durations of treatment in days across all patients in a group divided by 365.25. Duration of treatment (in days) is defined as date of last dose − date of first dose + 1
eAmong patients with plaque psoriasis affecting body surface area ≥ 3% and PASI > 0 at baseline, denoted as N1
fAmong patients with LEI > 0 at baseline, denoted as N2
gAmong patients with DSS > 0 at baseline, denoted as N3
hAmong patients evaluable for the dosing summary, denoted as N4
i‘Other’ concomitant csDMARDs used on day 1 were chloroquine, hydroxychloroquine, leflunomide, and sulfasalazine
jIn total, 128 patients used ‘other’ csDMARDs on day 1, with one patient taking two different ‘other’ csDMARDs
kMeets metabolic syndrome definition by meeting ≥ 3 of 5 criteria: abdominal obesity based on elevated waist circumference; triglyceride ≥ 150 mg/dL and/or concomitant lipid-lowering agent taken; HDL-cholesterol < 40 mg/dL for males and < 50 mg/dL for females; systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg and/or concomitant anti-hypertensive drug treatment taken; and glucose ≥ 100 mg/dL and/or concomitant anti-diabetic drug treatment taken
Patient demographics and baseline disease characteristics (from the qualifying studies) are shown in Table 1. In the all tofacitinib group, the mean duration of PsA was 7.6 years. A slightly lower proportion of patients in the average tofacitinib 10 mg BID group (15.8%) received oral corticosteroids on day 1 of OPAL Balance, compared with the average tofacitinib 5 mg BID (21.6%) and constant tofacitinib 5 mg BID groups (19.1%). Most patients (675 [98.4%]) were receiving concomitant csDMARDs on day 1 of OPAL Balance (most commonly MTX); 86 (12.7%) later discontinued and did not restart csDMARD treatment. Mean doses of concomitant oral corticosteroids and MTX were 6.4 mg/day and 18.5 mg/week, respectively, for the all tofacitinib group; doses were similar across all treatment groups. Of the 686 patients in OPAL Balance, 21 (3.1%) enrolled > 14 days after the end of study visit of the qualifying study; therefore, baseline values for safety endpoints for these patients were taken from the LTE screening visit.
Safety
Treatment-Emergent AEs and SAEs
In the all tofacitinib group, 2189 all-causality AEs were reported in 546 (79.6%) patients up to month 36; 95 (13.8%) patients reported SAEs, 73 (10.6%) experienced severe AEs, 59 (8.6%) discontinued due to AEs, and 212 (30.9%) reduced their dose or temporarily discontinued tofacitinib due to AEs. The IR (95% CI) for SAEs occurring within the risk period was 7.8 (6.2–9.6) per 100 PY for the all tofacitinib group (Table 2); IRs were generally similar across the other treatment groups, with overlapping 95% CIs. The IR (95% CI) for discontinuations due to AEs during the risk period was 3.8 (2.7–5.1) per 100 PY for the all tofacitinib group; IRs (95% CI) per 100 PY were numerically lower in the average tofacitinib 10 mg BID group (2.0 [1.0–3.7]) than in the average tofacitinib 5 mg BID group (5.1 [3.5–7.1]), with 95% CIs marginally overlapping (Table 2). AEs that occurred in ≥ 5% of all tofacitinib patients included upper respiratory tract infection, nasopharyngitis, urinary tract infection, bronchitis, and hypertension (ESM Table S1).
Table 2.
Incidence rates for all-causality serious adverse events, discontinuations due to adverse events, deaths, and select adverse events of special interest occurring within the risk period up to month 36
| Safety outcomesa | All tofacitinib doses (N = 686) | Average tofacitinib 5 mg BIDb (N = 407) | Average tofacitinib 10 mg BIDb (N = 279) | Constant tofacitinib 5 mg BIDc (N = 680) |
|---|---|---|---|---|
| SAEsd | ||||
| n (%) | 85 (12.4) | 51 (12.5) | 34 (12.2) | 48 (7.1) |
| Total PY exposure, years | 1093.3 | 631.5 | 461.8 | 659.7 |
| IR (95% CI), per 100 PY | 7.8 (6.2–9.6) | 8.1 (6.0–10.6) | 7.4 (5.1–10.3) | 7.3 (5.4–9.7) |
| n1 (%) | 9 (1.3) | 6 (1.5) | 3 (1.1) | 5 (0.7) |
| Discontinuations due to AEse | ||||
| n (%) | 44 (6.4) | 34 (8.4) | 10 (3.6) | 29 (4.3) |
| Total PY exposure, years | 1167.1 | 671.9 | 495.2 | 693.9 |
| IR (95% CI), per 100 PY | 3.8 (2.7–5.1) | 5.1 (3.5–7.1) | 2.0 (1.0–3.7) | 4.2 (2.8–6.0) |
| n1 (%) | 15 (2.2) | 10 (2.5) | 5 (1.8) | 8 (1.2) |
| Deaths | ||||
| n (%) | 1 (0.1) | 1 (0.2) | 0 | 1 (0.1) |
| Total PY exposure, years | 1169.6 | 673.9 | 495.7 | 695.5 |
| IR (95% CI), per 100 PY | 0.1 (0.0–0.5) | 0.2 (0.0–0.8) | 0.0 (0.0–0.7) | 0.1 (0.0–0.8) |
| n1 (%) | 4 (0.6) | 4 (1.0) | 0 | 3 (0.4) |
| Herpes zoster (non-serious and serious) | ||||
| n (%) | 19 (2.8) | 10 (2.5) | 9 (3.2) | 9 (1.3) |
| Total PY exposure, years | 1149.0 | 663.3 | 485.7 | 687.8 |
| IR (95% CI), per 100 PY | 1.7 (1.0–2.6) | 1.5 (0.7–2.8) | 1.9 (0.9–3.5) | 1.3 (0.6–2.5) |
| n1 (%) | 1 (0.1) | 1 (0.2) | 0 | 1 (0.1) |
| Serious infections | ||||
| n (%) | 11 (1.6) | 7 (1.7) | 4 (1.4) | 6 (0.9) |
| Total PY exposure, years | 1167.7 | 672.9 | 494.8 | 694.6 |
| IR (95% CI), per 100 PY | 0.9 (0.5–1.7) | 1.0 (0.4–2.1) | 0.8 (0.2–2.1) | 0.9 (0.3–1.9) |
| n1 (%) | 1 (0.1) | 0 | 1 (0.4) | 0 |
| Adjudicated opportunistic infections | ||||
| n (%) | 4 (0.6) | 1 (0.2) | 3 (1.1) | 1 (0.1) |
| Total PY exposure, years | 1166.3 | 672.2 | 494.1 | 693.8 |
| IR (95% CI), per 100 PY | 0.3 (0.1–0.9) | 0.2 (0.0–0.8) | 0.6 (0.1–1.8) | 0.1 (0.0–0.8) |
| n1 (%) | 0 | 0 | 0 | 0 |
| Adjudicated malignancies (excluding NMSC) | ||||
| n (%) | 9 (1.3) | 8 (2.0) | 1 (0.4) | 6 (0.9) |
| Total PY exposure, years | 1168.7 | 673.3 | 495.4 | 695.2 |
| IR (95% CI), per 100 PY | 0.8 (0.4–1.5) | 1.2 (0.5–2.3) | 0.2 (0.0–1.1) | 0.9 (0.3–1.9) |
| n1 (%) | 3 (0.4) | 2 (0.5) | 1 (0.4) | 1 (0.1) |
| Adjudicated NMSC | ||||
| n (%) | 11 (1.6) | 7 (1.7) | 4 (1.4) | 7 (1.0) |
| Total PY exposure, years | 1157.9 | 665.0 | 492.9 | 687.3 |
| IR (95% CI), per 100 PY | 1.0 (0.5–1.7) | 1.1 (0.4–2.2) | 0.8 (0.2–2.1) | 1.0 (0.4–2.1) |
| n1 (%) | 1 (0.1) | 1 (0.2) | 0 | 1 (0.1) |
| Adjudicated MACE | ||||
| n (%) | 3 (0.4) | 2 (0.5) | 1 (0.4) | 2 (0.3) |
| Total PY exposure, years | 1167.6 | 672.2 | 495.4 | 694.5 |
| IR (95% CI), per 100 PY | 0.3 (0.1–0.8) | 0.3 (0.0–1.1) | 0.2 (0.0–1.1) | 0.3 (0.0–1.0) |
| n1 (%) | 2 (0.3) | 2 (0.5) | 0 | 1 (0.1) |
| PEf | ||||
| n (%) | 1 (0.1) | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Total PY exposure, years | 1168.7 | 673.0 | 495.7 | 694.7 |
| IR (95% CI), per 100 PY | 0.1 (0.0–0.5) | 0.2 (0.0–0.8) | 0.0 (0.0–0.7) | 0.1 (0.0–0.8) |
| n1 (%) | 1 (0.1) | 1 (0.2) | 0 | 1 (0.1) |
| DVTf | ||||
| n (%) | 0 | 0 | 0 | 0 |
| Total PY exposure, years | 1169.6 | 673.9 | 495.7 | 695.5 |
| IR (P95% CI), per 100 PY | 0.0 (0.0–0.3) | 0.0 (0.0–0.6) | 0.0 (0.0–0.7) | 0.0 (0.0–0.5) |
| n1 (%) | 0 | 0 | 0 | 0 |
| ATEf | ||||
| n (%) | 5 (0.7) | 2 (0.5) | 3 (1.1) | 2 (0.3) |
| Total PY exposure, years | 1163.3 | 671.3 | 492.0 | 693.9 |
| IR (95% CI), per 100 PY | 0.4 (0.1–1.0) | 0.3 (0.0–1.1) | 0.6 (0.1–1.8) | 0.3 (0.0–1.0) |
| n1 (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.4) |
AE adverse event, ATDD average total daily dose, ATE arterial thromboembolism, BID twice daily, CI confidence interval, DVT deep vein thrombosis, IR incidence rate, MACE major adverse cardiovascular events, MedDRA Medical Dictionary for Regulatory Activities, NMSC non-melanoma skin cancer, PE pulmonary embolism, PY patient-years, SAE serious adverse event, SMQ Standardized MedDRA Query
aCrude IRs (number of patients with events per 100 PY) were calculated by the inclusion of events occurring within the risk period, defined as time from first dose to 28 days after the last tofacitinib dose or the last observation date, whichever was shorter. The total PY exposure was the denominator for the IR calculation. n is the number of patients with an event within the risk period; these patients are included in the IR calculations. n1 is the number of patients with an event outside the risk period; these patients are not included in the IR calculations. N is the number of patients in each treatment group
bAverage dosing was based on an ATDD of tofacitinib: patients who received an ATDD < 15 mg/day were assigned to the average tofacitinib 5 mg BID group; patients who received an ATDD ≥ 15 mg/day were assigned to the average tofacitinib 10 mg BID group
cConstant tofacitinib 5 mg BID included all patients who started OPAL Balance on tofacitinib 5 mg BID and stayed on tofacitinib 5 mg BID (as per sponsor’s smoothing algorithm) until switching to tofacitinib 10 mg BID or discontinuing the study. Assessments (including exposure and adverse events) after dose switch to tofacitinib 10 mg BID were excluded from the analysis
dOne male patient reported that his partner experienced a spontaneous abortion; this SAE has not been included in this table because it was not reported in a treated patient
eIncludes four patients who withdrew due to maternal exposure and three patients who died; excludes one patient who had an AE that was not considered as treatment emergent and one patient who had missing data
fThe events were based on selected events in narrow SMQ for embolic and thrombotic events
Deaths
There were five deaths reported up to month 36 in the all tofacitinib group (Table 2); four of the five deaths occurred after the 28-day risk period (one death due to chronic obstructive pulmonary disease [COPD] occurred within the risk period). The IR (95% CI) for deaths occurring within the risk period in the all tofacitinib group was 0.1 (0.0–0.5) per 100 PY (Table 2). The causes of death were acute cardiac failure secondary to hypertensive heart disease, cardiovascular insufficiency leading to sudden cardiac death, COPD, pancreatic adenocarcinoma, and PE (all n = 1; ESM Table S2). The patients who died from acute cardiac failure and cardiovascular insufficiency both had a history of hypercholesterolemia and hypertension; the patient with cardiovascular insufficiency also had diabetes mellitus. The patient who died from COPD discontinued tofacitinib upon exacerbation of COPD. Of the patients who died outside the 28-day risk period, only one patient discontinued tofacitinib because of the condition that caused their death (pancreatic adenocarcinoma). The patient who died from acute cardiac failure (following post-elective surgery for myocardial infarction) had discontinued tofacitinib due to an infection, while the patient with a PE was reported to have discontinued tofacitinib due to non-serious AEs of viral upper respiratory tract infection and lower respiratory tract infection. The reason for discontinuation of tofacitinib for the patient who died from cardiovascular insufficiency is unknown.
AEs of Special Interest
The IRs for select AEs of special interest occurring within the risk period up to month 36 are reported in Table 2.
The IR (95% CI) for all (non-serious and serious) herpes zoster was 1.7 (1.0–2.6) per 100 PY for the all tofacitinib group, which was generally similar across the other treatment groups (overlapping 95% CI). Of the 19 patients in the all tofacitinib group who reported herpes zoster within the risk period, one case of facial herpes zoster was an SAE. This event occurred on day 83 in a 67-year-old white male patient who had previously completed 11 months of treatment with tofacitinib 5 mg BID in OPAL Broaden and was receiving the same dose in OPAL Balance; tofacitinib treatment was permanently discontinued, and the event subsequently resolved. One severe event of herpes zoster occurred in a 61-year-old white female patient who had previously completed 5 months of treatment with tofacitinib 5 mg BID in OPAL Beyond and was receiving the same dose in OPAL Balance at the onset of the event on day 43; tofacitinib was temporarily discontinued, and the event resolved. Four patients with herpes zoster, including three patients with multi-dermatomal herpes zoster and one with disseminated herpes zoster, were adjudicated as having opportunistic infections (IR 0.3, 95% CI 0.1–0.9); these were the only opportunistic infections reported in the study. Five patients with herpes zoster were receiving concomitant glucocorticoids; none of these patients had adjudicated opportunistic infections. In the all tofacitinib group, 11 patients reported serious infections within the risk period, with an IR (95% CI) of 0.9 (0.5–1.7) per 100 PY; this was generally similar across the other treatment groups (overlapping 95% CI). Four patients (one of whom was living in Russia, a country with a high burden of TB [13]), reported AEs of latent TB where a previously negative QuantiFERON response became positive; no cases of active TB were reported.
In the all tofacitinib group, the IR (95% CI) for adjudicated malignancies (excluding NMSC) was 0.8 (0.4–1.5) per 100 PY, and the IR (95% CI) for NMSC was 1.0 (0.5–1.7) per 100 PY. These were generally similar across the other treatment groups (95% CI overlapping), with the exception that the IR (95% CI) per 100 PY for malignancies (excluding NMSC) for the average tofacitinib 10 mg BID group (0.2 [0.0–1.1]) was numerically lower than that for the average tofacitinib 5 mg BID group (1.2 [0.5–2.3]), with 95% CI marginally overlapping. These malignancies (20 in total, including NMSC, in the all tofacitinib group within the risk period) included one case of pancreatic adenocarcinoma (diagnosed on day 85 of tofacitinib treatment in OPAL Balance, after 12 months of adalimumab treatment in the OPAL Broaden qualifying study; the patient received concomitant MTX during OPAL Balance) and one case of non-Hodgkin’s B-cell lymphoma (day 585 of treatment in OPAL Balance, after 11 months of tofacitinib 5 mg BID treatment in OPAL Broaden; the patient also received MTX in OPAL Broaden but switched to sulfasalazine due to lack of efficacy, and the patient later discontinued sulfasalazine in OPAL Broaden due to improvement).
The IR (95% CI) of adjudicated MACE was 0.3 (0.1–0.8) per 100 PY in the all tofacitinib group, which was generally similar across the other treatment groups (overlapping 95% CI). In the all tofacitinib group, three non-fatal MACE occurred within the risk period; these were myocardial infarction, ischemic stroke, and stress cardiomyopathy. Two fatal cases of MACE occurred outside the risk period; these were acute cardiac failure and cardiovascular insufficiency.
In the all tofacitinib group, the IR (95% CI) of PE was 0.1 (0.0–0.5) per 100 PY, with one patient experiencing a non-fatal PE within the risk period. This patient, a 66-year-old white male, was also included in the average tofacitinib 5 mg BID and constant tofacitinib 5 mg BID groups. The patient had previously received tofacitinib 10 mg BID in OPAL Broaden. He experienced a PE on day 439, was hospitalized, and temporarily discontinued tofacitinib 5 mg BID; once he had recovered (day 448), the patient was discharged from the hospital and resumed tofacitinib treatment. It was noted that two of the patient’s brothers had died from PE, but investigations for coagulopathy disorders had not been carried out in either the patient or his brothers. One fatal PE occurred outside the risk period in a 45-year-old female patient in the average tofacitinib 5 mg BID and constant tofacitinib 5 mg BID groups. The patient had previously received tofacitinib 5 mg BID in OPAL Beyond. The patient had developed non-serious AEs of viral upper respiratory tract infection and lower respiratory tract infection, which reportedly led to the temporary discontinuation of tofacitinib 5 mg BID on day 170; 29 days after discontinuing tofacitinib, the patient experienced the PE (considered to be the result of deep vein embolism) and died. The IR (95% CI) for ATE events was 0.4 (0.1–1.0) for the all tofacitinib group; of the total of five patients with ATE events, two were in the average tofacitinib 5 mg BID group and three in the average tofacitinib 10 mg BID group.
Finally, there were no reports of DVT, gastrointestinal perforations, interstitial lung disease, or inflammatory bowel disease.
Laboratory Parameters
In the all tofacitinib, average tofacitinib 5 mg BID, average tofacitinib 10 mg BID, and constant tofacitinib 5 mg BID groups, ALT was elevated ≥ 3-fold the ULN in 27 (4.0%), 19 (4.7%), 8 (2.9%), and 14 (2.4%) patients, respectively, while AST was elevated ≥ 3-fold the ULN in 15 (2.2%), 10 (2.5%), 5 (1.8%), and 9 (1.5%) patients, respectively (ESM Table S3).
Up to month 36, in the all tofacitinib group, seven (1.0%) patients met any protocol criteria for discontinuing the study due to changes in laboratory parameters: one patient due to two sequential hemoglobin values of < 8.0 g/dL or decreases of > 30% from baseline value; one patient due to two sequential platelet counts < 75 × 103/mm3; one patient due to two sequential increases in serum creatinine > 50% and an increase > 0.5 mg/dL over the average of screening and baseline; one patient due to two sequential ALT or AST elevations ≥ 5-fold the ULN regardless of total bilirubin or accompanying signs or symptoms; two patients due to a confirmed positive urine pregnancy test; and one patient due to multiple criteria, including two sequential ALT or AST elevations ≥ 5-fold the ULN regardless of total bilirubin or accompanying signs or symptoms, two sequential ALT or AST elevations ≥ 3-fold the ULN with at least one total bilirubin value > 2-fold the ULN, and two sequential ALT or AST elevations ≥ 3-fold the ULN, accompanied by signs or symptoms consistent with hepatic injury. Additionally, 17 (2.5%) patients met the monitoring criterion of a creatine kinase elevation > 5-fold the ULN; however, no patients met the discontinuation criterion of two sequential creatine kinase elevations > 10-fold the ULN. Furthermore, no patients met the criteria for Hy’s law.
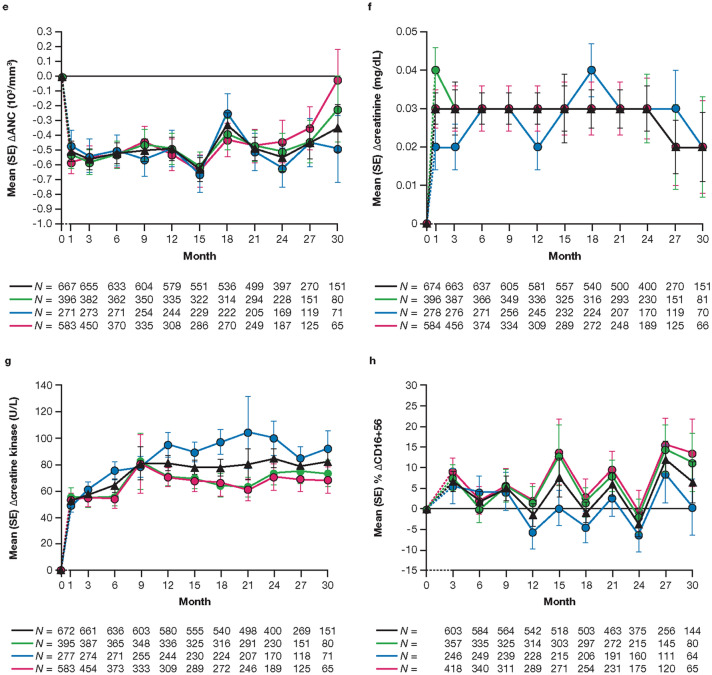
ALT and AST showed a slight increase from baseline through month 27 in all treatment groups, with values decreasing slightly at month 30 (Fig. 1a, b). Changes from baseline in hemoglobin remained generally stable through month 24, then increased up to month 30, in patients in the all tofacitinib group. A similar trend was observed in patients in the average tofacitinib 5 mg BID and constant tofacitinib 5 mg BID groups, whereas in the average tofacitinib 10 mg BID group, hemoglobin declined slightly up to month 21 and then increased and returned to baseline by month 30 (Fig. 1c). In all treatment groups, ALC declined from baseline and stabilized by month 24 (Fig. 1d), while ANC appeared relatively stable up to month 30 after an initial decline at month 1 (Fig. 1e). Up to month 36, no patient in the all tofacitinib group met the discontinuation criterion of confirmed (i.e., two or more consecutive measurements) ALC < 0.5 × 103/mm3, and ANC < 0.5 × 103/mm3. In all treatment groups, changes from baseline in creatinine remained relatively stable until month 24 (Fig. 1f). Changes from baseline in creatine kinase showed an increase until month 9 in the all tofacitinib group, after which the values remained generally stable until month 30 (Fig. 1g). The trend was similar in the average tofacitinib 5 mg BID and constant tofacitinib 5 mg BID groups, whereas values continued to increase slightly to month 21 in the average tofacitinib 10 mg BID group, after which levels decreased to be similar to those in the other treatment groups. Levels of CD16+56 cells appeared to fluctuate throughout the study (Fig. 1h). HDL-cholesterol, LDL-cholesterol, total cholesterol, and triglyceride levels increased at month 3, then appeared to be generally stable up to month 30 (Fig. 1i–l). Absolute mean values for these laboratory parameters are reported in ESM Fig. S2.
Fig. 1.
Mean (standard error [SE]) change from baseline in alanine aminotransferase (a), aspartate aminotransferase (b), hemoglobin (c), absolute lymphocyte count (d), absolute neutrophil count (e), creatinine (f), and creatine kinase (g), and mean (SE) percentage change from baseline in absolute CD16+56 (h), high-density lipoprotein-cholesterol (i), low-density lipoprotein-cholesterol (j), total cholesterol (k), and triglycerides (l), up to month 30. The dashed line represents the time between the baseline (month 0) and month 1 or month 3, as baseline refers to the baseline visit of the qualifying study for patients who enrolled within the 14-day window from the last visit of the qualifying study, or the baseline visit of this long-term extension (LTE) study for patients who enrolled outside of the 14-day window from the last visit of the qualifying study. Data are presented to month 30 because sample sizes were too small (N ≤ 50) for meaningful analysis beyond this point. N is the number of patients evaluable at each time point. Only evaluable patients at a visit of interest were included in the analysis and missing values were not imputed. aAverage dosing was based on an average total daily dose (ATDD) of tofacitinib: patients who received an ATDD < 15 mg/day were assigned to the average tofacitinib 5 mg twice daily (BID) group; patients who received an ATDD ≥ 15 mg/day were assigned to the average tofacitinib 10 mg BID group. bThe constant tofacitinib 5 mg BID group included all patients who started OPAL Balance on tofacitinib 5 mg BID and stayed on tofacitinib 5 mg BID (as per sponsor’s smoothing algorithm) until switching to tofacitinib 10 mg BID or discontinuing the study. Assessments (including exposure and adverse events) after dose switch to tofacitinib 10 mg BID were excluded from the analysis. Δ change from baseline, ALC absolute lymphocyte count, ALT alanine aminotransferase, ANC absolute neutrophil count, AST aspartate aminotransferase, ATDD average total daily dose, BID twice daily, HDL high-density lipoprotein, LDL low-density lipoprotein
Efficacy
Rheumatologic and Dermatologic Outcomes
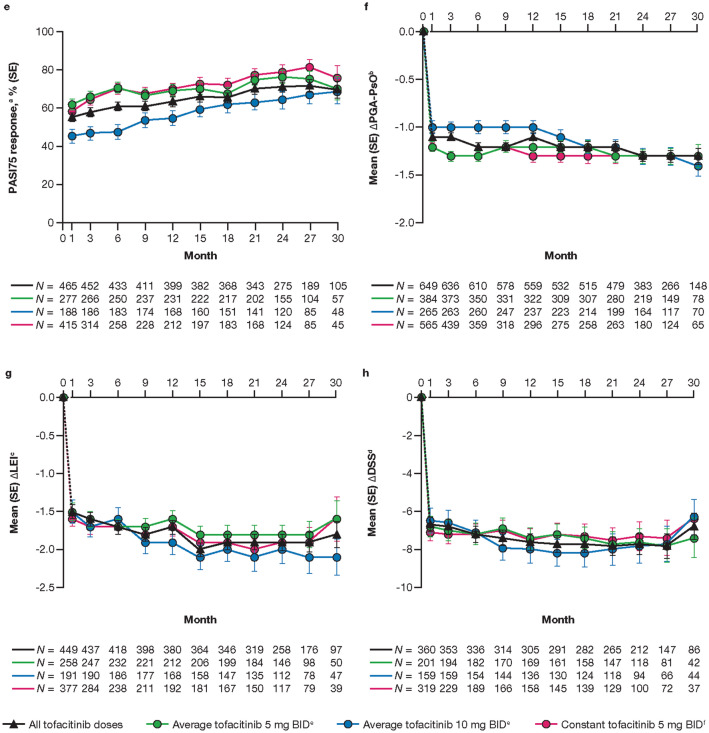
Across all treatment groups, the proportions of patients who had an ACR20, ACR50, ACR70, and PsARC response were maintained up to month 30 (Fig. 2a–d). These proportions were generally higher in the average tofacitinib 5 mg BID and constant tofacitinib 5 mg BID groups versus the average tofacitinib 10 mg BID group up to month 21; however, it must be noted that patient numbers were smaller in the latter group. Improvements in skin outcomes (i.e., PASI75 response rates and changes from baseline in PGA-PsO; Fig. 2e, f) were also maintained over time, as were improvements from baseline in LEI and DSS (Fig. 2g, h).
Fig. 2.
Proportion (SE) of patients reporting ACR20 (a), ACR50 (b), ACR70 (c), PsARC (d), and PASI75a response (e), and mean (SE) changes from baseline in PGA-PsOb (f), LEIc (g), and DSSd (h), up to month 30. The dashed line represents the time between the baseline (month 0) and month 1, as baseline refers to the baseline of the qualifying study for all patients regardless of their enrollment gaps between the qualifying studies and this study. Data are presented to month 30 because sample sizes were too small (N ≤ 50) for analysis of efficacy outcomes beyond this point. N is the number of patients evaluable at each time point. Only evaluable patients at a visit of interest were included in the analysis and missing values were not imputed. aAmong patients with plaque psoriasis affecting body surface area ≥ 3% and PASI > 0 at baseline. bAmong patients with PGA-PsO > 0 at baseline. cAmong patients with LEI > 0 at baseline. dAmong patients with DSS > 0 at baseline. eAverage dosing was based on an ATDD of tofacitinib: patients who received an ATDD < 15 mg/day were assigned to the average tofacitinib 5 mg BID group; patients who received an ATDD ≥ 15 mg/day were assigned to the average tofacitinib 10 mg BID group. fThe constant tofacitinib 5 mg BID group included all patients who started OPAL Balance on tofacitinib 5 mg BID and stayed on tofacitinib 5 mg BID (as per sponsor’s smoothing algorithm) until switching to tofacitinib 10 mg BID or discontinuing the study. Assessments (including exposure and adverse events) after dose switch to tofacitinib 10 mg BID were excluded from the analysis. Δ Change from baseline, ACR20/50/70 American College of Rheumatology ≥ 20%, ≥ 50%, or ≥ 70% response criteria, DSS Dactylitis Severity Score, LEI Leeds Enthesitis Index, PASI Psoriasis Area and Severity Index, PASI75 ≥ 75% PASI improvement from baseline, PGA-PsO Physician Global Assessment of Psoriasis, PsARC Psoriatic Arthritis Response Criteria
Composite Outcomes
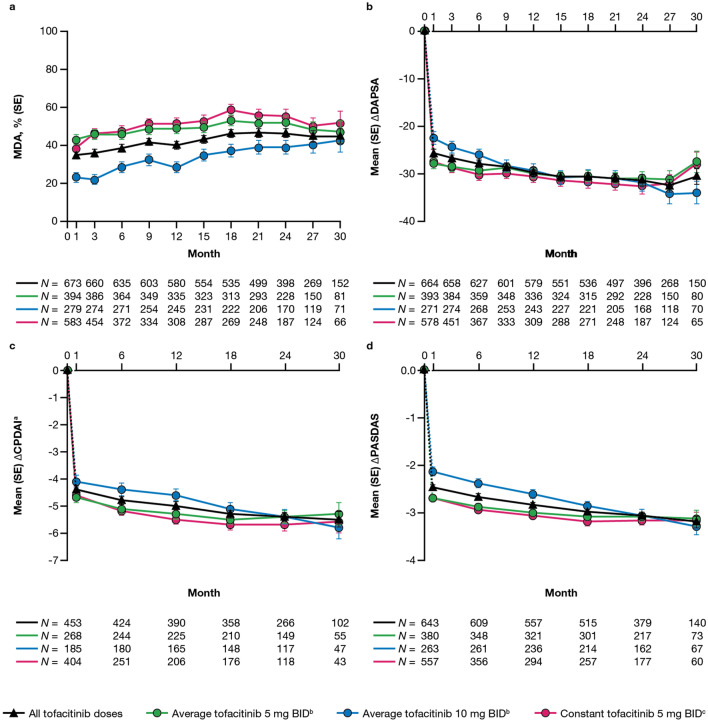
The proportions of patients who achieved MDA were maintained up to month 30; these were also generally higher in the average tofacitinib 5 mg BID and constant tofacitinib 5 mg BID groups versus the average tofacitinib 10 mg BID group (Fig. 3a). Improvements from baseline in DAPSA were also generally maintained up to month 30 (Fig. 3b), while improvements in CPDAI and PASDAS continued over time (Fig. 3c, d).
Fig. 3.
Proportion of patients (SE) reporting MDA (a), and mean (SE) changes from baseline in DAPSA (b), CPDAIa (c), and PASDAS (d), up to month 30. The dashed line represents the time between the baseline (month 0) and month 1, as baseline refers to the baseline of the qualifying study for all patients regardless of their enrollment gaps between the qualifying studies and this study. Data are presented to month 30 because sample sizes were too small (N ≤ 50) for analysis of efficacy outcomes beyond this point. N is the number of patients evaluable at each time point. Only evaluable patients at a visit of interest were included in the analysis and missing values were not imputed. aAmong patients with plaque psoriasis affecting ≥ 3% of body surface area at baseline. bAverage dosing was based on an ATDD of tofacitinib: patients who received an ATDD < 15 mg/day were assigned to the average tofacitinib 5 mg BID group; patients who received an ATDD ≥ 15 mg/day were assigned to the average tofacitinib 10 mg BID group. cThe constant tofacitinib 5 mg BID group included all patients who started OPAL Balance on tofacitinib 5 mg BID and stayed on tofacitinib 5 mg BID (as per sponsor’s smoothing algorithm) until switching to tofacitinib 10 mg BID or discontinuing the study. Assessments (including exposure and adverse events) after dose switch to tofacitinib 10 mg BID were excluded from the analysis. Δ Change from baseline, CPDAI Composite Psoriasis Disease Activity Index, DAPSA Disease Activity in PSoriatic Arthritis, MDA minimal disease activity, PASDAS Psoriatic Arthritis Disease Activity Score
Patient-Reported Outcomes
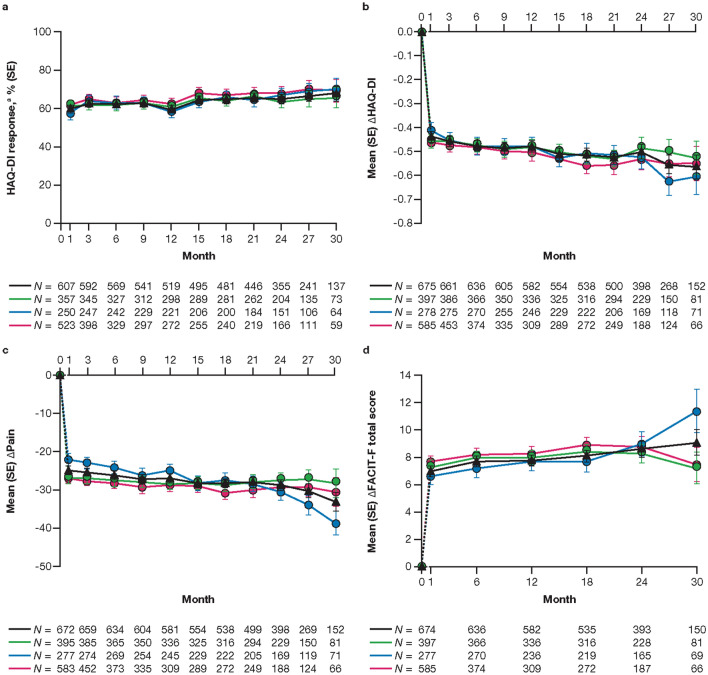
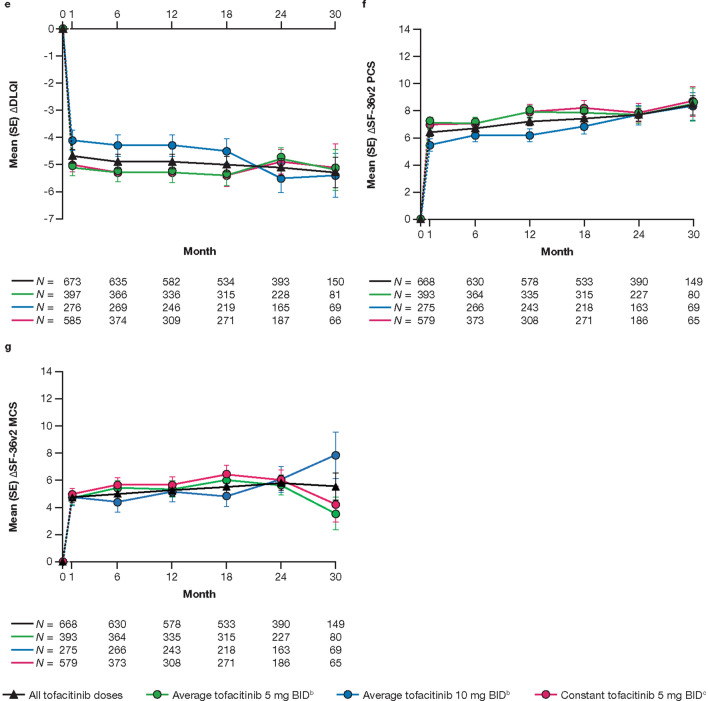
The proportions of patients who achieved HAQ-DI response (decrease from baseline in HAQ-DI ≥ 0.35) were generally constant up to month 30 (Fig. 4a), and improvements from baseline were maintained over time in HAQ-DI, Pain, FACIT-F total score, and DLQI (Fig. 4b–e). Improvements from baseline in SF-36v2 PCS, MCS (Fig. 4f, g), and eight norm-based domain scores (ESM Fig. S4), as well as all EQ-5D-3L dimensions and EQ-VAS (ESM Fig. S5), were also generally maintained over time.
Fig. 4.
Proportion (SE) of patients achieving HAQ-DI responsea (a), and mean (SE) changes from baseline in HAQ-DI (b), Pain (Patient Assessment of Arthritis Pain; c), FACIT-F total score (d), DLQI (e), SF-36v2 PCS (f), and SF-36v2 MCS (g), up to month 30. The dashed line represents the time between the baseline (month 0) and month 1, as baseline refers to the baseline of the qualifying study for all patients regardless of their enrollment gaps between the qualifying studies and this study. Data are presented to month 30 because sample sizes were too small (N ≤ 50) for analysis of efficacy outcomes beyond this point. N is the number of patients evaluable at each time point. Only evaluable patients at a visit of interest were included in the analysis and missing values were not imputed. aDecrease from baseline HAQ-DI ≥ 0.35, among patients with HAQ-DI ≥ 0.35 at baseline. bAverage dosing was based on an ATDD of tofacitinib: patients who received an ATDD < 15 mg/day were assigned to the average tofacitinib 5 mg BID group; patients who received an ATDD ≥ 15 mg/day were assigned to the average tofacitinib 10 mg BID group. cThe constant tofacitinib 5 mg BID group included all patients who started OPAL Balance on tofacitinib 5 mg BID and stayed on tofacitinib 5 mg BID (as per sponsor’s smoothing algorithm) until switching to tofacitinib 10 mg BID or discontinuing the study. Assessments (including exposure and adverse events) after dose switch to tofacitinib 10 mg BID were excluded from the analysis. Δ Change from baseline, DLQI Dermatology Life Quality Index, FACIT-F Functional Assessment of Chronic Illness Therapy-Fatigue, HAQ-DI Health Assessment Questionnaire-Disability Index, MCS Mental Component Summary, PCS Physical Component Summary, SF-36v2 Short Form-36 Health Survey Version 2
Discussion
This interim analysis of the LTE study OPAL Balance provides safety and efficacy data up to month 36 and 30, respectively, for tofacitinib in > 650 patients with PsA.
Overall, the safety profile of tofacitinib in this study (AEs and laboratory abnormalities assessed up to month 36, and changes from baseline in laboratory parameters assessed up to month 30) was similar to that reported in the qualifying phase 3 studies (6 and 12 months in duration) [6, 7], with efficacy maintained up to month 30. The safety profile in OPAL Balance was consistent with that reported in OPAL Broaden and OPAL Beyond [6, 7], and with the known long-term safety profile of tofacitinib in patients with rheumatoid arthritis (RA) [14, 15].
Up to month 36, 79.6% of all tofacitinib patients in OPAL Balance reported AEs. IRs for SAEs were similar for all tofacitinib patients (7.8 per 100 PY) and the three treatment groups (8.1, 7.4, and 7.3 per 100 PY for the average tofacitinib 5 mg BID, average tofacitinib 10 mg BID, and constant tofacitinib 5 mg BID groups, respectively). The IR for discontinuations due to AEs for all tofacitinib patients was 3.8 per 100 PY; this was numerically lower in patients in the average tofacitinib 10 mg BID group compared with the average tofacitinib 5 mg BID group (2.0 and 5.1 per 100 PY, respectively). It should be noted that while the proportions of patients receiving concomitant csDMARDs were similar across treatment groups, the proportions of patients receiving concomitant oral corticosteroids were slightly lower in the average tofacitinib 10 mg BID group (15.8%) versus the average tofacitinib 5 mg BID (21.6%) and constant tofacitinib 5 mg BID (19.1%) groups, which may have impacted the rates of AEs. In general, IRs for SAEs and discontinuations due to AEs appeared to be lower in patients with PsA than in patients with RA treated with tofacitinib, with IRs for the all tofacitinib group reported as 9.0–9.4 per 100 PY for SAEs and 6.8–7.5 per 100 PY for discontinuations due to AEs [14, 15].
The most common AEs in OPAL Balance were similar to those reported in the two phase 3 PsA tofacitinib studies (OPAL Broaden and OPAL Beyond) and in ORAL Sequel, the global LTE study of tofacitinib in patients with RA for up to 9.5 years [6, 7, 15]. Upper respiratory tract infection, nasopharyngitis, urinary tract infection, and bronchitis were the most common AEs in this interim analysis of OPAL Balance (PsA) and in ORAL Sequel (RA), occurring in ≥ 5% of patients in the all tofacitinib groups [15].
Of the select AEs of special interest, IRs were highest for all herpes zoster events (non-serious and serious; 1.7 per 100 PY in the all tofacitinib group), with 19 patients experiencing herpes zoster up to month 36, within the risk period. Of these, one was an SAE (facial herpes zoster) and four were adjudicated as opportunistic infections (including three cases of multi-dermatomal herpes zoster, and one case of disseminated herpes zoster). IRs for herpes zoster, as well as for serious infections (0.9 per 100 PY in the all tofacitinib group), were similar across treatment groups. IRs for these short-latency events appeared to be lower in patients with PsA than in patients with RA, reported as 3.4–4.4 per 100 PY for herpes zoster and 2.4–2.7 per 100 PY for serious infections [14–16]. Long-latency events, such as malignancies (excluding NMSC), NMSC, and MACE, had IRs of 0.8, 1.0, and 0.3 per 100 PY, respectively, in the all tofacitinib group; these were generally similar across treatment groups (with the exception of malignancies [excluding NMSC], which were numerically lower for the average tofacitinib 10 mg BID group vs. the average tofacitinib 5 mg BID group) and between patients with PsA and RA (IRs for RA have been reported as 0.8–0.9, 0.6–0.7, and 0.4–0.6 per 100 PY, respectively) [14, 15, 17]. The IR for PE was 0.1 per 100 PY, with one patient experiencing a PE during the risk period; this was similar to the IR reported for patients with RA (0.1 per 100 PY) [15]. The IR for ATE was 0.4 per 100 PY in the all tofacitinib group.
The long-term safety profile of tofacitinib in patients with PsA appears to be generally comparable with that of biologic treatments for PsA; however, comparisons must be made with caution, due to differences in study designs, patient populations, disease activities and comorbidities, and treatment exposure. Adalimumab has been assessed in a 120-week open-label study (with data reported for 2 years of exposure) [18] and in a pooled analysis of clinical trials for up to 3.5 years [19]; secukinumab has been assessed in a 2-year follow-up of a randomized study [20]; and certolizumab pegol has been assessed in a pooled analysis of randomized and open-label studies across several indications (including PsA) for up to 7.8 years [21]. Proportions of patients with PsA experiencing AEs, SAEs, and discontinuations due to AEs, respectively, were 91.6, 16.8, and 6.7% for adalimumab (2-year open-label treatment) [18], 84.5, 14.1, and 4.6% for secukinumab [20], and 93.4, 25.4, and 12.7% for certolizumab pegol [21]. Considering AEs of special interest, the IR for herpes zoster appeared to be higher for tofacitinib than that previously reported for certolizumab pegol (0.1 events per 100 PY) [21]; no cases of herpes zoster were reported with adalimumab or secukinumab [18–20]. The following IRs (events per 100 PY) have been previously reported for serious infections: 2.4 for adalimumab (2-year open-label treatment) [18], 2.8 for adalimumab (pooled clinical trial data for up to 3.5 years) [19], and 1.6 for certolizumab pegol [21]. IRs (events per 100 PY) of opportunistic infections (excluding TB) were reported as 0.6 and 0.1 for adalimumab and certolizumab pegol, respectively [18, 21]. IRs (events per 100 PY) of malignancies were reported as 0.1–0.3 for adalimumab (excluding lymphoma and NMSC) and 0.5 for certolizumab pegol (excluding NMSC) [18, 19, 21]. IRs of NMSC ranged from 0 to 0.3 events per 100 PY for adalimumab; no events were reported for certolizumab pegol [18, 19, 21]. Considering MACE, three patients who received secukinumab for up to 2 years reported myocardial infarctions (IR 0.3 per 100 PY), and four strokes occurred (IR 0.4 per 100 PY) [20]. Four patients reported VTE (IR 0.3 events per 100 PY), and three patients experienced PEs (IR 0.2 events per 100 PY) with certolizumab pegol [21].
Laboratory parameters observed in OPAL Balance were generally similar to those previously observed in OPAL Broaden and OPAL Beyond [6, 7]. Mean changes from baseline in ALT and AST showed a slight and generally stable increase up to month 27, with values decreasing slightly at month 30. Changes from baseline in hemoglobin appeared to be minimal. Similar to the trend observed in OPAL Broaden [6], patients who received average tofacitinib 5 mg BID showed a small increase in hemoglobin levels, whereas those who received average tofacitinib 10 mg BID showed an initial decrease in hemoglobin levels; this decrease lasted until month 21, with levels returning to baseline by month 30. Changes from baseline in ALC showed an increase at month 1 followed by a decrease over time, stabilizing by month 24. This trend was similar to that observed in long-term analyses of tofacitinib-treated patients with RA, which showed an initial increase in ALC followed by a decline to stable levels by month 48 [22]. In OPAL Balance, one patient was diagnosed with a non-serious infection (pneumonia) prior to a measurement of ALC < 0.5 × 103/mm3, and was being treated with antibiotics at the time of the visit; repeat testing 6 days later reported ALC at 0.6 × 103/mm3. No patients reported at least two consecutive measurements of ALC < 0.5 × 103/mm3 and, therefore, these patients were not required by the protocol to be discontinued from the study. This protocol requirement is in line with the tofacitinib prescribing information, which recommends the discontinuation of tofacitinib if ALC is < 0.5 × 103/mm3 (due to an increased risk of serious infections [14, 22]), dose reduction or interruption of tofacitinib if ALC is 0.5–0.75 × 103/mm3, and dose maintenance if ALC is > 0.75 × 103/mm3 [23, 24]. Changes from baseline in ANC, creatinine and creatine kinase (both reported only in OPAL Beyond), and percentage changes from baseline in HDL-cholesterol, LDL-cholesterol, total cholesterol, and triglyceride levels appeared generally similar to values observed up to month 12 in OPAL Broaden and up to month 6 in OPAL Beyond [6, 7]. Levels of natural killer cells, measured by CD16+56 cells, fluctuated in OPAL Balance. In an analysis of the effect of tofacitinib on lymphocytes in patients with RA, short- and mid-term (up to 22 months) decreases in natural killer cells (CD3−/CD16+/CD56+) were observed; however, these were observed to have increased at month 50 in the long-term study ORAL Sequel [22].
The efficacy of tofacitinib in patients with PsA was maintained up to month 30, which was a continuation of the improvements observed in OPAL Broaden and OPAL Beyond [6, 7], although it should be noted that these data reflect only those patients who remained in the study. Some patients discontinued from the study, and others switched to a MTX withdrawal sub-study from month 24; these patients were therefore not followed beyond this time point. Although it appeared that a greater proportion of patients in the average tofacitinib 5 mg BID and constant tofacitinib 5 mg BID groups achieved ACR20, ACR50, ACR70, PASI75 responses, and MDA compared with patients in the average tofacitinib 10 mg BID group, this may be due to the comparatively low number of patients in the latter group.
This interim analysis of OPAL Balance has a number of limitations. Patients were eligible for enrollment into the LTE study if they had completed the phase 3 qualifying studies or had discontinued these studies due to non-study-drug-related AEs. The study population therefore represents patients who were known to respond to, and tolerate, tofacitinib (or adalimumab, for those who received adalimumab in OPAL Broaden). The lack of a placebo group prevented the direct determination of the impact of tofacitinib on safety outcomes. Only observed values were used in the analyses, without imputation for missing values. Further, sample sizes for laboratory parameters and efficacy outcomes were low beyond month 30, as many patients had not yet reached this time point; this resulted in a large standard error in these values after this time point. The treatment algorithms used to assess potential dose-related effects also had their own limitations. An important limitation of the ATDD method is that a patient is assigned to the same category throughout their experience in the program, and thus events may be attributed to a dose category that is different from the actual dose received at the time of the event. Additionally, this approach narrows the differences between the point estimates for both doses, bringing them closer to each other and confounding the ability to evaluate differences between them. As the constant daily dose method excluded exposure and events that occurred after a dose switch, it resulted in a shorter overall exposure to tofacitinib and the risk of confounding by the reasons for discontinuation or dose change. Because of the exposure differences among defined treatment groups, percentages for events of interest are presented for descriptive purposes only and should not be used to compare treatment groups. Finally, as this is an interim analysis only, full details of the safety and efficacy of long-term tofacitinib use in patients with PsA are yet to be elucidated. Comparisons between this analysis and the prior RA analyses must be made with caution because of the differences in PY of exposure to tofacitinib, as patients with PsA have been observed for up to 36 months, and patients with RA were observed for up to 9.5 years. Additionally, the sample size of OPAL Balance (686 patients in the all tofacitinib group) was smaller than that of the RA LTE study, ORAL Sequel (4481 patients in the all tofacitinib group) [15].
Conclusions
The results of this third interim analysis of the LTE study OPAL Balance support the long-term safety and efficacy of tofacitinib in patients with active PsA. The safety and efficacy of tofacitinib 5 and 10 mg BID in patients with PsA appeared to be consistent with that observed in the qualifying studies, OPAL Broaden and OPAL Beyond. Laboratory parameters generally remained consistent over time. Efficacy across various PsA disease domains and PROs was also maintained over time in those patients remaining in the study.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the patients and investigators involved in OPAL Balance.
Funding
This study and the Rapid Service Fee were sponsored by Pfizer Inc.
Medical Writing Assistance
Medical writing support, under the guidance of the authors, was provided by Mark Bennett, PhD, and Christina Viegelmann, PhD, CMC Connect, McCann Health Medical Communications and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–4).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
PN, AJK, PJM, JAC-C, DF, CW, JW, M-AH, SM, and KSK contributed to the conception/design of the study, acquisition of the data, and data analysis. All authors contributed to the interpretation of the data, critically revised each draft of the manuscript for intellectual content, provided final approval of the version submitted for publication, and accept accountability for the accuracy and integrity of the work.
Disclosures
Peter Nash has received research grants from, is a consultant for, and is a member of the speakers’ bureau for AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sanofi, and UCB. Laura C. Coates has received research grants from AbbVie, Celgene, Eli Lilly, Janssen, Novartis, and Pfizer Inc, and has received consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Biogen, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer Inc, and UCB. Laura C. Coates is also a member of the journal’s Editorial Board. Alan J. Kivitz has received consulting fees from Gilead, Pfizer Inc, Regeneron, Sanofi, and Sun Pharma, is a member of the speakers’ bureau for AbbVie, Celgene, Eli Lilly, Flexion, GSK, Horizon, Merck, Novartis, Pfizer Inc, Regeneron, and Sanofi, is an advisory committee member for AbbVie, Boehringer Ingelheim, Flexion, Genzyme, Janssen, Pfizer Inc, Regeneron, Sanofi, and UCB, and is a shareholder of Novartis and Pfizer Inc. Philip J. Mease has received research grants and consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Novartis, Pfizer Inc, Sun Pharma, and UCB, and is a member of the speakers’ bureau for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Genentech, Janssen, Novartis, Pfizer Inc, and UCB. Dafna D. Gladman has received research grants and/or consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer Inc, and UCB. José A. Covarrubias-Cobos has received research grants from Bristol-Myers Squibb, Eli Lilly, Janssen, and Pfizer Inc. Oliver FitzGerald has received research grants and/or consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer Inc, and UCB. Dona Fleishaker, Cunshan Wang, Joseph Wu, Ming-Ann Hsu, Sujatha Menon, Lara Fallon, and Keith S. Kanik are employees and shareholders of Pfizer Inc. Ana Belén Romero was an employee and shareholder of Pfizer Inc at the time of this analysis, and is currently an employee of LEO Pharma.
Compliance with Ethics Guidelines
The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the principles of the Declaration of Helsinki, and applicable local regulatory requirements and laws. The study protocol was approved by the Institutional Review Boards and/or Independent Ethics Committee at each study center (see ESM). All patients provided written, informed consent.
Data Availability
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the US and/or EU, or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Footnotes
Ana Belén Romero was affiliated with Pfizer Inc at the time of analysis.
Digital Features
To view digital features for this article go to: 10.6084/m9.figshare.12163389.
References
- 1.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64:ii14–ii17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376:957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 3.Singh JA, Guyatt G, Ogdie A, et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res (Hoboken) 2019;71:2–29. doi: 10.1002/acr.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- 5.Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 6.Mease P, Hall S, FitzGerald O, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377:1537–1550. doi: 10.1056/NEJMoa1615975. [DOI] [PubMed] [Google Scholar]
- 7.Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med. 2017;377:1525–1536. doi: 10.1056/NEJMoa1615977. [DOI] [PubMed] [Google Scholar]
- 8.Nash P, Coates LC, Kivitz AJ, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, up to 24 months in patients with active psoriatic arthritis: interim data from OPAL Balance, an open-label, long-term extension study. Ann Rheum Dis. 2017;76(Suppl 2):682. [Google Scholar]
- 9.Nash P, Coates LC, Kivitz AJ, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, up to 36 months in patients with active psoriatic arthritis: data from the second interim analysis of OPAL Balance, an open-label, long-term extension study. Arthritis Rheumatol. 2017;69(Suppl 10):840–841. [Google Scholar]
- 10.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 11.Clegg DO, Reda DJ, Mejias E, et al. Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis. A Department of Veterans Affairs Cooperative Study. Arthritis Rheum. 1996;39:2013–2020. doi: 10.1002/art.1780391210. [DOI] [PubMed] [Google Scholar]
- 12.Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis. 2010;69:48–53. doi: 10.1136/ard.2008.102053. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Global tuberculosis report 2019. 2019. https://www.who.int/tb/publications/global_report/en/. Accessed 6 Nov 2019.
- 14.Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis. 2017;76:1253–1262. doi: 10.1136/annrheumdis-2016-210457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wollenhaupt J, Lee EB, Curtis JR, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019;21:89. doi: 10.1186/s13075-019-1866-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winthrop KL, Yamanaka H, Valdez H, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2675–2684. doi: 10.1002/art.38745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charles-Schoeman C, Wicker P, Gonzalez-Gay MA, et al. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum. 2016;46:261–271. doi: 10.1016/j.semarthrit.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Mease PJ, Ory P, Sharp JT, et al. Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT) Ann Rheum Dis. 2009;68:702–709. doi: 10.1136/ard.2008.092767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burmester GR, Mease P, Dijkmans BA, et al. Adalimumab safety and mortality rates from global clinical trials of six immune-mediated inflammatory diseases. Ann Rheum Dis. 2009;68:1863–1869. doi: 10.1136/ard.2008.102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a Phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken) 2017;69:347–355. doi: 10.1002/acr.23111. [DOI] [PubMed] [Google Scholar]
- 21.Curtis JR, Mariette X, Gaujoux-Viala C, et al. Long-term safety of certolizumab pegol in rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis and Crohn's disease: a pooled analysis of 11 317 patients across clinical trials. RMD Open. 2019;5:e000942. doi: 10.1136/rmdopen-2019-000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Vollenhoven R, Lee EB, Strengholt S, et al. Evaluation of the short-, mid-, and long-term effects of tofacitinib on lymphocytes in patients with rheumatoid arthritis. Arthritis Rheumatol. 2019;71:685–695. doi: 10.1002/art.40780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Food and Drug Administration. XELJANZ® (tofacitinib): highlights of prescribing information. 2019. https://labeling.pfizer.com/ShowLabeling.aspx?id=959. Accessed 08 Apr 2020.
- 24.European Medicines Agency. Xeljanz (tofacitinib)—summary of product characteristics. 2019. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf. Accessed 29 Oct 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the US and/or EU, or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.