Abstract
Feces from 184 sheep from Dakahlia governorate, Egypt were tested for Eimeria species oocysts by using the standard floatation technique; oocysts were detected in 126 (68.4%). The prevalence was significantly higher in young sheep than adults. Eleven Eimeria species were identified: Eimeria ahsata, Eimeria bakuensis, Eimeria crandallis, Eimeria faurei, Eimeria granulosa, Eimeria intricata, Eimeria marsica, Eimeria ovinoidalis, Eimeria pallida, Eimeria parva and Eimeria webybridgensis. Oocysts of the most pathogenic ovine species, E. ovinoidalis, were detected in 27 (14.6%) sheep. This is the first report of E. webybridgensis in sheep from Egypt, possibly due to close similarity of their oocysts to those of E. crandallis which stated in the earlier reports. Worldwide reports on epidemiology of Eimeria spp. infections in sheep are tabulated.
Keywords: Eimeria, Eimeria ovinoidalis, Coccidiosis, Prevalence, Sheep, Egypt
Introduction
Ovine coccidiosis can be a serious disease with economic consequences (Chartiera and Paraud 2012). Sheep are infected with 1 or more Eimeria spp; of them, E. ovinoidalis and E. crandallis are the most pathogenic species (Gregory et al. 1989). Until 3 decades ago, Eimeria spp. in sheep and goats were considered common. Cross transmission trials revealed that Eimeria in sheep and goats are species specific (McDougald 1979).
In Egypt, the estimated sheep population is 5.5 million (FAO 2015); 25% of them are reared in the Nile Delta region (Thomson et al. 2000). However, no reports on sheep coccidiosis from Dakahlia governorate, the largest agricultural governorate in the Delta region, are available. Additionally, Chartiera and Paraud (2012) reviewed different epidemiological, clinical and control aspects of small ruminants’ coccidiosis; however, no data on the prevalence or the revealed Eimeria species worldwide were included.
Here, we aimed to determine the prevalence of different Eimeria spp. in sheep from Dakahlia governorate, Egypt, and to review reports concerned with the prevalence of this common parasite in sheep worldwide.
Materials and methods
Fresh feces collected from 184 sheep of various ages and genders during August 2015 to July 2016, were tested for oocysts of Eimeria spp. using the standard flotation technique (Duszynski and Wilber 1997). Sheep in this region are raised in small flocks (4–15 animals/flock) kept in households in rural areas of Dakahlia governorate (31° 50′ N, 31° 00′ E), Egypt. Flocks are reared in a nomadic pastoralism system where animals move within the agricultural lands and graze on residues of the harvested crops. Mixing of flocks from different households during grazing is common. Flocks are not treated with any anticoccidial. Sheep were divided into 3 age groups; young (< 1 year old), yearlings (1–2 years) and adults (> 2 years). Eimeria oocysts from positive samples were sporulated at room temperature using 2.5% potassium dichromate. Morphological observations and micrographs of sporulated and non-sporulated oocysts were performed using a binocular microscope coupled to Amscope® camera (Carl Zeiss, Oberkochen, Germany); oocysts’ sizes were measured using a calibrated ocular micrometer. Different Eimeria spp. were identified per Eckert et al. (1995). Results were statistically analyzed using a chi-square test. The 95% confidence intervals of a proportion including continuity correction and odds ratios were calculated using www.vassarstats.net.
Results
Eimeria spp. oocysts were detected in 126 (68.4%) of 184 feces. The prevalence varied with age; the highest prevalence was in young sheep (59/63; 93.6%) followed by yearlings (37/53; 69.8%; OR = 6.38; P = 0.00071) and at least in adults (30/68; 44.1%; OR = 18.68; P ≤ 0.0001). Prevalence was higher in females (97/135; 71.8%) than males (29/49; 59.1%; OR = 1.76; P = 0.102). The highest prevalence was in Autumn (26/33; 78.7%) followed by Spring (22/39; 56.4%; OR = 2.87; P = 045), Summer (23/34; 67.6%; OR = 1.78; P = 0.303), and Winter (55/78; 70.5%; OR = 1.55; P = 0.37).
Eleven Eimeria spp. were identified; of them, E. crandallis/E. webybridgensis (40.2%) and E. bakuensis (33.7%) were the most prevalent. Eimeria ovinoidalis were detected in 14.6% (27/184). The other identified species were E. granulosa, E. ahsata, E. parva, E. intricata, E. pallida, E. faurei and E. marsica. Mixed infections (73.0%: dual 31.7%, triple 30.1%, quadruple 10.3% and quintuple 0.8%) were common than single infections (26.9%), Table 1.
Table 1.
Prevalence of different Eimeria spp. in feces of 184 examined sheep from Dakahlia governorate, Egypt
| Eimeria species | No. positive (%) | Single infection | Mixed infection |
|---|---|---|---|
| No. positive (%) | No. positive (%) | ||
| E. ahsata | 26 (14.1) | 1 (0.8) | 25 (19.8) |
| E. bakuensis | 62 (33.7) | 9 (7.1) | 53 (42.1) |
| E. crandallis/E. webybridgensis | 74 (40.2) | 19 (15.1) | 55 (43.6) |
| E. faurei | 12 (6.5) | 0.0 | 12 (9.5) |
| E. granulosa | 27 (14.6) | 3 (2.4) | 24 (19.1) |
| E. ovinoidalis | 27 (14.6) | 1 (0.8) | 26 (20.6) |
| E. intricata | 17 (9.2) | 0.0 | 17 (13.5) |
| E. marsica | 5 (2.7) | 0.0 | 5 (3.9) |
| E. pallida | 14 (7.6) | 1 (0.8) | 13 (10.3) |
| E. parva | 21 (11.4) | 0.0 | 21 (16.6) |
| Total | 126 (68.4) | 34 (26.9) | 92 (73.0) |
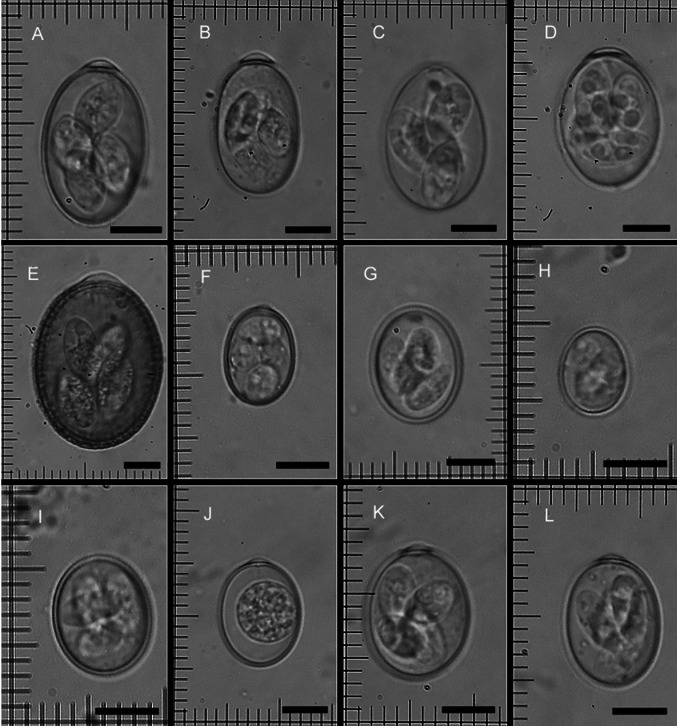
Morphological characteristics of different Eimeria spp. oocysts revealed in the present study (Fig. 1) were like the description of these species in literature (Norton et al. 1974; Eckert et al. 1995).
Fig. 1.
Oocysts of Eimeria spp. in feces of sheep from Dakahlia governorate, Egypt. From a to i: Sporulated oocysts of E. ahsata (a), E. bakuensis (b), E. faurei (c), E. granulosa (d), E. intricata (e), E. marsica (f), E. ovinoidalis (g), E. pallida (h), E. parva (i). Non-sporulated oocyst of E. crandallis/E. webybridgensis (j). Sporulated oocyst of E. crandallis (k). Sporulated oocyst of E. webybridgensis (l). Unstained. Scale bars = 10 µm
Discussion
The present study is the first report of different Eimeria spp. in sheep from Dakahlia governorate, Egypt; 126 (68.4%) of 184 were infected. Earlier reports from other Egyptian governorates together with the worldwide reports are summarized in Table 2.
Table 2.
Prevalence of Eimeria species in sheep worldwide
| Country/region | Age range | No. examined | No. positive (%) | No. of Eimeria spp. | Eimeria species and remarks | References |
|---|---|---|---|---|---|---|
| Australia | ||||||
| South regions | 3–13 months | 136 | 109 (80.0) | 11 | E. ahs 31%, E. bak 55%, E. cran/E, weyb 76%, E. fau 24%, E. intr 37%, E. gran 49%, E. ovin 54%, E. par/E, pal 44%, E. pun 1% | O’Callaghan et al. (1987) |
| Different | Variable | 3412 | 616 (18.1) | 5 |
118 positive samples were genotyped (PCR/Sequencing) E. ahs 28%, E. cran 48.3%, E. ovin 10.1%, E. weyb 10.1%, E. cylindrica 4.2% No mixed infection |
Yang et al. (2014) |
| Austria | ||||||
| Styria | Ewes, lambs | 60, 126 | NS | 8 |
E. ahs 19.1%, E. bak 18.4%, E. cran/E. weyb 27.3%, E. fau 4.8%, E. gran 1.0%, E. ovin 28.3%, E. pal 10.9%, E. par 14.0% in adults Lambs were infected with same species except E. gran |
Platzer et al. (2005) |
| Bangladesh | ||||||
| Different region | NS | 136 | 42 (30.8) | NS | NS | Islam and Taimur (2008) |
| Brazil | ||||||
| Sao Paolo | 2–32 weeks | 25 | NS | 8 | E. ahs, E. bak, E. cran, E. intr, E. ovin, E. pal, E. par, E. weyb | Amarante and Barbosa (1992) |
| Sobral, Ceara | Lambs, dams | 30,10 | NS | 9 | E. ahs, E. bak, E. caprovina, E. cran, E. fau, E. gran, E. intr, E. ovin, E. par | Vieira et al. (1999) |
| Mostardas | NS | 100 | 59 (59.0) | 9 | E. ahs 23.72%, E. bak 6.77%, E. cran 5.08%, E. fau 1.69%, E. gran 20.33%, E. ovin 11.86%, E. pal 1.69%, E. par 37.28%, E. pun 23.72% | da Silva et al. (2008) |
| Rio Grande do Norte | 1–90 days | 27 | 17–100% | 8 | E. ahs 43.3%, E. bak 43.3%, E. cran 65.7%, E. fau 29.0%, E. gran 53.7%, E. intr 3%, E. ovin 48.8%, E. par 54.7% | Silva et al. (2011) |
| Santa Ines | NS | 100 | 63 (63.0) | 6 | E. arloingi 6%, E. Fau 38%, E. intr 4%, E. ninakohlyakimovae 32%, E. pal 2%, E. par 12%, | Martins et al. (2011) |
| Lajes | 4–8 months | 64 | 36 (56.2) | NS | NS | Souza et al. (2012) |
| Colinas | Lambs, adults | 255 | 50 (19,6) | 8 | E. ahs, E. bak, E. cran, E. fau, E. Intr, E. ovin, E. pal, E. par | Almeida (2013) |
| Parana | 1–8 months | 210 | 147 (70.0) | 9 | E. ahs 8.1%, E. baka 0.6%, E. cran 50%, E. fau 8.1%, E. gran 2.7%, E. intr 5.4%, E. ovinaa 1.3%, E. ovin 2%, E. par 21.6% | Lopes et al. (2013) |
| Garanhuns | 12 months | 408 | 270 (66.1) | 8 | E. ahs 51.8%, E. bak 54.8%, E. cran 58.9%, E. fau 22.9%, E. gran 56.3%, E. ovin 72.6%, E. pal 14.4%, E. par 64.8%, | de Macedo et al. (2019) |
| Canada | ||||||
| Alberta | NS | 211 | 211 (100.0) | 10 | E. ahs 86%, E. arloingi 82%, E. cran 88%, E. fau 52%, E. gran 7%, E. intr 14%, E. ninakohlyakimovae 69%, E. pal 6%, E. par 53%, E. pun 4% | Mahrt (1969) |
| Western region | NS | 510 | 461 (90.0) | 8 | E. ahs 33%, E. bak 56%, E. cran 34%, E. fau 6%, E. gran 1%, E intr 5%, E. ninakohlyakimovae 19%, E. par 35% | Uhazy et al. (1971) |
| Croatia | ||||||
| North Dalmatia | Lambs | 49 | 37 (75.5) | 9 | E. bak, E. cran, E. fau, E. gran, E. intr, E. mar, E. ovin, E. pal, E. par | Šarić et al. (2015) |
| China | Wang et al. (2010) | |||||
| Heilongjiang | Adults, lambs | 309 | 287 (92.9) | 8 | E. ahs 67.2%, E. bak 44.3%, E. cran 11.2%, E. fau 17.1%, E. gran 12.9%, E. intr 12.5%, E. pal 3.8%,E. par 59.9% | |
| Columbia | ||||||
| Tennessee | 15 months | 23 | 9 (39.0) | 5 | E. ahs, E. bak, E. fau, E. gilruthi, E. ovin | Ammar et al. (2019) |
| Encino, Duitama, and Belen | < 12 to > 24 months | 97 | 30 (30.9) | NS | NS | León et al. (2019) |
| Czech Republic | ||||||
| Sokolov | Ewes | 348 | 188 (54.0) | 4 | E. cran/weyb, E. intr, E. ovin, E. par | Kyriánová et al. (2017) |
| Lambs | 333 | 252 (75.7) | E. ovin was the most prevalent (84% in ewes and 85% in lambs) | |||
| Egypt | ||||||
| Kalubia | 6–9 months | 18 | 13 (72.2) | 2 | E. cran, E. ovin. 13 lambs had bloody diarrhea | Ghanem and Abd El-Raof (2005) |
| Sinai | Different | 240 | 16 (6.7) | NS | NS | Abouzeid et al. (2010) |
| Matrouh | 1–60 days | 185 | 98 (52.9) | 9 | E. ahs 14.6%, E. bak 21.1%, E. cran 49.2%, E. fau 14.1%, E. gran 11.3%, E. intr 8.4%, E. mar 4.8%, E. ovin 36.7%, E. par 30.2% | Abou-El-Naga (2010) |
| Kafrelsheikh | NS | 224 | 37 (16.5) | NS | NS | Sultan et al. (2016) |
| Suez | > 6 months | 142 | 82 (57.7) | 10 | E. ahs 30.5%, E. arloingi, E. bak 26.8%, E. cran 30.5%, E. fau 18.3%, E. gran 14.6%, E. intr 7.3%, E. ovin 12.2%, E. pal 13.4%, E. par 18.3% | Mohamaden et al. (2018) |
| Dakahlia | Different | 184 | 126 (68.4) | 11 | E. ahs 14.1%, E. bak 33.7%, E. cran/E. weyb 40.2%, E. fau 6.5%, E. gran 14.6%, E. intr 9.2%, E. mar 2.7%, E. ovin 14.6%, E. pal 7.6%, E. par 11.4% | Present study |
| Estonia | ||||||
| Vormsi, Hiiumaa, and Saaremaa | NS | 92 herds | 87 (94.6) | 11 | E. ahs 23%, E. bak 50.6%, E. cran 14.9%, E. faur 28.7%, E. gran 26.4%, E. intr 4.6%, E. pal 31.0%, E. par 37.9%, E. mar 2.3%, E. ovin 93.1%, E. weyb 33.3% | Lassen et al. (2013) |
| Ethiopia | ||||||
| Elfora export abattoir | < 1 to 2 years | 262 | 175 (66.8) | 12 | The most prevalent species were E. cran 30%, E. pal 13.8%, E. par 30.8%, | Ayana et al. (2009) |
| Bishoftu (Oromia) | < 1 to > 1 years | 157 | 78 (49.7) | NS | NS | Bersissa et al. (2011) |
| Gechi District | < 2 to > 2 years | 255 | 31 (12.2) | NS | NS | Emiru et al. (2013) |
| Gemechis and Boke Districts | Different | 384 | 121 (31.5) | NS | NS | Daniel et al. (2014) |
| Germany | ||||||
| Northwest regions | Different | 69 | 100% in < 10 wk | 10 | E. ahs, E. bak, E. cran /weyb, E. fau, E. gran, E. intr, E. ovin, E. pal, E. par | Barutzki et al. (1990) |
| All regions | NS | 374 | 155 (41.4) | NS | NS | Raue et al. (2017) |
| Ghana | ||||||
| Ayeduase | Different | 110 | 57 (51.8) | NS | Lambs had higher prevalence (87.5%) | Owusu et al. (2016) |
| Coastal Savannah | Different | 502 | 387 (77.1) | NS | Result from sheep and goats are combined together | Squire et al. (2019) |
| Iceland | ||||||
| Fjárborgir | Lambs | NS | NS | 10 | E. ahs, E. bak, E. cran, E. fau, E. gran, E. intr, E. ovin, E. pal, E. par, E. weyb | Reginsson and Richter (1997) |
| Fossárdalur | Ewes and lambs | NS | NS | 10 | E. ahs 5.6%, E. bak 18.9%, E. cran 1.4%, E. fau 4.2%, E. gran 8.2%, E. intr 1.6%, E. ovin 40.7%, E. pal 1.6%, E. par 6.7%, E. weyb 11.1% | Skirnisson (2007) |
| Iran | ||||||
| Tabriz | < 6 months to > 1 years | 240 | 40 (16.7) | 6 | E. ahs 8%, E. bak 18%, E. fau 18%, E. par 13%, E. pal 8%, E. intr 35% | Yakhchali and Zarei (2008) |
| Sanandaj | Different | 240 | 46 (19.2) | 6 | E. ahs 10%, E. bak 10%, E. fau 29%, E. intr 10%, E. ovin 31%, E. par 10% | Yakhchali and Golami (2008) |
| Malayer | Different | 250 | 40 (16.67) | 7 | E. ahs 6%, E. bak 16%, E. fau 16%, E. intr 39%, E. ovin 4%, E. pal 7%, E. par 12% | Yakhchali and Rezaei (2010) |
| Kabodan | NS | 41 | 33 (80.4) | 4 | E. ahs 6.5%, E. fau 6.5%, E. ovin 9.7%, E. par 32.3%. wild sheep | Tavassoli and Khoshvaghti (2010) |
| Kermanshah and Ilam | Adult, lambs | 410 | 375 (91.5) | 10 | E. ahs 81.8%, E. bak 56.2%, E. cran 33.06%, E. fau 24.8%, E. gran 2.93%, E. intr 15.2%, E. ovin 41.6%, E. pal 58.4%, E. par 67.4%, E. weyb 5.06%, | Hashemnia et al. (2014) |
| Rudsar | Different | 270 | 170 (63.0) | 5 | E. ahs, E. bak, E. cran, E. ovin, E. par | Nourollahi-Fard et al. (2016) |
| Zabol | Different | 420 | 84 (20.0) | 6 | E. ahs 8.3%, E. intr 0.9%, E.ovin 3.5%, E. pal 2.8%, E. par 7.3%, E. weyb 2.1%, | Mirzaei et al. (2016) |
| India | ||||||
| Karnataka | NS | 300 | 120 (40.0) | NS | NS | Mamatha and D'Souza (2007) |
| Mathura | Different | 596 | 208 (34.9) | 5 | E. bak 27.6%, E. fau 11.24%, E. intr 0.11%, E. ovin 11.1%, E. par 15.4%, | Om et al. (2010) |
| Rajasthan | NS | 3964 | 2010 (50.7) | NS | NS | Swarnkar et al. (2010) |
| Maharashtra | NS | 2462 | 594 (24.1) | 10 | E. ajantai 5.7%, E. ahs 9.2%, E. bak 6.9%, E. balloonii 4.3%, E. beedatus 3.7%, E. cran 18.2%, E. intr 10.2%, E. ninakohlyakimovae 12.6%, E. par 15.1%, E. weyb 13.8% | More et al. (2011) |
| Kashmir | 12–89 months | 500 | 49 (9.8) | NS | NS | Bhat et al. (2012) |
| Andhra Pradesh | NS | 150 | 7 (4.6) | 2 | E. granulose, E. parva | Murthy and Rao (2014) |
| Omerga | NS | 127 | 92 (72.4) | NS | NS | Sontakke et al. (2015) |
| Karnataka | 6–9 months | 47 | 42 (89.3) | 8 | E. ahs, E. arloingi, E. bak, E. fau, E. gran, E. intr, E. ovin, E. par. Outbreak with bloody diarrhea and mortalities in lambs | Adeppa et al. (2016) |
| Jalpaiguri | < 1 to > 3 years | 1350 | 431 (31.9) | NS | NS | Molla and Bandyopadhyay (2016) |
| Iraq | ||||||
| Baghdad | NS | 306 | 230 (75.1) | 9 | E. bak, E.cran, E.fau, E. gran, E. mar, E. ovin, E. pal, E. par, E. weyb | Fadl et al. (2011) |
| Mosul | Different | 500 | 318 (63.6) | 9 | E. ahs 65.4%, E. bak 86.7%, E. cran 30.5%, E. fau 19.8%, E. gran10%, E. intr 11%, E. ovin 73.5%, E. pal 38.9%, E. par 56.6% | Hasan and Abed (2012) |
| Baghdad | NS | 280 | 195 (69.6) | NS | E. bak (18.4%) was the highest and the lowest was E. arloingi (1.5%) | Kalef et al. (2013) |
| Diyala | Different | 143 | 124 (86.7) | 8 | E. ahs 22.6%, E. cran 18.8%, E. fau 3.77%, E. gran 16.1%, E. intr 8.5%, E. ovin 6.6%, E. pal 10.3%, E. par 13.2% | Minnat (2014) |
| Sulaimaniya | Different | 150 | 108 (72.0) | 11 | E. ahs 23.1%, E. bak 33.3%, E. cran 25%, E. fau 23.1%, E. gran 14.81%, E. intr 32.4%, E. mar 25.9%, E. ovin 35.2%, E. pal 50.9%, E. par 53.7%, E. weyb 26.8%, | Kareem and Yücel (2015) |
| Wasite | NS | 120 | 69 (57.5) | 10 | E. ahs 22.5%, E. bak 20%, E. cran 11.6%, E. fau 12.5%, E. gran 3.3%, E. intr 21.6%, E. ovin 15%, E.pal 7.5%, E.par 18.3%. E.weyb 2.5% | Al-Rubaie and Al-Saadoon (2018) |
| Wasite | 0–36 months | 120 | 60 (50.0) | 7 | E. ahs 7.5%, E.bak 24.1%, E. cran 9.1%, E. intr 0.8%, E. ovin 18.3%, E.par 20.8%, E. weyb 5.8% | Al-Saadoon and Al-Rubaie (2018) |
| Kirkuk | < 1 to > 2 years | 160 | 23 (27) | 8 | E. bak 26%, E. cran 21.7%, E. fau 39%, E. gran 56.5%, E. intr 56.5%, E. ovin 60.8%, E.pal 73.9%, E. par 47.8% | Al-Robaiee et al. (2019) |
| Italy | ||||||
| Rome | – | 20 | – | 5 | E. ahs, E. bak, E. intr, E. ovin, E. parva | Battelli and Poglayen (1980) |
| Jordan | ||||||
| Sekhra | Ewes | 61 | 39 (63.4) | NS | NS | Jawasreh et al. (2013) |
| Kenya | ||||||
| Different districts | < 1 to > 1 years | 50 | NS | 10 | E. ahs, E. bak 43%, E. cran, E. fau, E. gran, E. intr, E. ovin 16.5%, E. pal, E. par | Kanyari (1993) |
| Nyandarua | Different | 575 | 253 (44.0) | 8 | E. ahs 15.2%, E. bak 43.6%, E. fau 2.8%, E. gran 4.8%, E. intr 8.27%, E. ovin 23.6%, E. pal 0.67%, E. par 1.06% | Maingi and Munyua (1994) |
| Kuwait | Different | 17 | 3 (17.5) | 3 | E. bak, E. cran, E. ovin | Majeed et al. (2015) |
| Malaysia | ||||||
| Perak | NS | 175 | 162 (92.5) | NS | NS. 175 animals were examined including 150 goats and 25 sheep | Zainalabidin et al. (2015) |
| Mexico | ||||||
| Huixquilucan | Ewes, lambs | 62 | NS | 9 | E. ahs, E. bak, E. cran, E. fau, E. gran, E. intr, E. ovin, E. pal, E. par | Gonzalez et al. (1990) |
| Southeast region I | 2 months to 2 years | 412 | NS | 11 | E. ahs, E. bak, E. cran, E. fau, E. gran, E. intr, E. mar, E. ovin, E. pal, E. par, E. weyb | Trejo-Huitrón et al. (2020) |
| Nigeria | ||||||
| Ibadan | NS | 1040 | 832 (80.0) | 7 | E. arloingi 10%, E. fau 31%, E. gran 3%, E. intr 6%, E. Ninakohlyakimovae 22%, E. pal 19%, E. par 8% | Majaro and Dipeolu (1981) |
| Gwagwalada | Adult, young | 44 | 1 (2.3) | 1 | E. fau | Jegede et al. (2015) |
| Sri Lanka | ||||||
| Jaffna | Adults, lambs | 100 | 76 (76.0) | 4 | E. bak, E. intr, E. ovin, E. par | Kandasamy et al. (2011) |
| Sudan | ||||||
| Khartoum | Adult, lambs | NS | 58.8% | 11 | E. ahs 42%, E. bak 60%, E. cran 33%, E. fau 28.9%, E. gran 7.7%, E. intr 9.7%, E. mar 12.7%, E. ovin 47%, E. pal 11%, E. par 27%, E. pun 0.9% | Elamin et al. (2004) |
| Pakistan | ||||||
| Punjab | More or less than 6 months | 486 | 209 (43.0) | 5 | E. ahs 45.4%, E. fau 19.1%, E. intr 28.7%, E. ovin 48.3%, E. par 24.4% | Khan et al. (2011) |
| Papua New Guinea | ||||||
| University farms | < 1 to > 3 years | 75 | 67 (89.0) | 8 | E. ahs 45%, E. bak 72%, E. cran 39%, E. fau 28%, E. intr 24%, E. gran 4%, E. ovin 48%, E. par 58% | Varghese and Yayabu (1985) |
| Different regions | NS | 110 | 19 (17.3) | NS | NS | Koinari et al. (2013) |
| Poland | ||||||
| Various regions | Adult | 400 | 136 (34.1) | NS | NS | Gorski et al. (2004) |
| Western Pomerania | NS | 20 | 20 (100) | NS | NS | Juszczak et al. (2019) |
| Saudi Arabia | ||||||
| Jeddah | Different | 100 | 41 (41.0) | 4 | E. arloingi 22%, E. bak 17.1%, E. intr 26.8%, E. par 31.7% | Toulah (2007) |
| Al-Baha | Different | 487 | 227 (46.6) | 8 | E. ahs 12.3%, E. bak 27.9%, E. cran 29.8%, E. fau 7.6%, E. intr 9.9%, E. pal 2.9%, E. par 4.7%, E. weyb 23.4%, | Ibrahim and Afsa (2013) |
| Scotland | ||||||
| Hirta, St Kilda | Lambs, adults | Different | Different | 11 | E. ahs 27. 5%, E. bak 33.7%, E. cran 22.1%, E. fau 24%, E. gran 13.3%, E. intr 28.2%, E. mar 19.8%, E. ovin 19.8%, E. pal 9.5%, E. par 27.1%, E. weyb 18.7% | Craig et al. (2007) |
| Senegal | ||||||
| Sahelian zone | 6 months-4 years | 2234 | 2204 (94.0) | 8 | E. ahs 28%, E. bak 69.6%, E. cran 62%, E. fau 23%, E. intr 15%, E. ovin 75.6%, E. pal 18%, E. par 25% | Vercruysse (1982) |
| Slovakia | ||||||
| Various regions | Adults, lambs | 445 | 445 (100.0) | 5 |
E. par (in lambs 42%, in adults 37%), E. bak, E. cran, E. fau, E. ovin 2% of oocysts in lambs and 5% in adults could not be identified |
Vasilková et al. (2004) |
| South Africa | ||||||
| North-West | < 1 year | NS | NS | 6 | E. ahs 40%, E. bak 100%, E. cran 100%, E. intr 20%, E. ovin 20%, E. weyb 60% | Bakunzi et al. (2010) |
| Spain | ||||||
| Galicia | Different | 1882 | 1393 (74.0) | 9 | E. ahs 71%, E. bak 59%, E. cran/E. weyb 64%, E. fau 59%, %, E. gran 18%, E. intr 15%, E. mar 3%, E. ovin 74%, E. par 36% | Díaz et al. (2010) |
| Cartagena | < 1 to > 1 year | 396 | 396 (100.0) | 11 | E. ahs 75.8%, E. bak 48.5%, E. cran 89.4%, E. fau 62.1%, E. gran 74.2%, E. intr 18.2%, E. mar 43.9%, E. ovin 97%, E. par/E.pal 97%, E. weyb 90.9% | Carrau et al. (2018) |
| Tanzania | ||||||
| Vingunguti | < 2 years | 43 | 40 (93.0) | 7 | E. ahs 21%, E. bak 29%, E. cran 96%, E. fau 29%, E. gran 8%, E. ovin 29%, E. par 92% | Kusiluka et al. (1996) |
| Morogoro | > 1 year | 121 | 118 (97.5) | NS | NS | Kambarage et al. (1996) |
| Turkey | ||||||
| Elaziğ | 2–4 months | 155 | 147 (94.8) | 9 | E. ahs, E. bak, E. cran, E. ninakohlyakimovae, E. fau, E. gran, E. intr, E. pal, E. par, | Güler et al. (1990) |
| Different regions | NS | 444 | 434 (97.7) | 9 | E. ahs, 29.9%, E. bak 39.4%., E. cran 3.9%, E. fau 1.1%, E. gran 41.9%, E. intr 19.3, E. ninakohlyakimovae 16.3%, E. pal 0.4%, E. par 6.6% | Demir (1995) |
| Kars | Different | 592 | 556 (93.9) | 10 | E. ahs 23.4%, E. bak 46.6%, E. cran 13.7%, E. fau 15.1%, E. gran 27.7%, E. intr 13.9%, E. ovin 47.7%, E. pal 23.2%, E. par 37.1%, E. pun 2.3% | Arslan et al. (1999) |
| Van | NS | 350 | 349 (99.9) | 9 | E. ahs 39.4%, E. bak 39.1%, E. cran 38.8%, E. fau 15.4%, E. gran 16.5%, E. intr 11.4%, E. ovin 43.1%, E. pal 33.1%, E. par 46.5% | Gül and Değer (2002) |
| Antakya | Lambs | 248 | 248 (100.0) | 10 | E. ahs 11.3%, E. bak 38.7%, E. cran 64.9%, E. fau 11.3%, E. intr 9.3%, E. mar 16.9%, E. ovin 55.2%, E. pal 3.6%, E. par 13.3%, E. weyb 30.2% | Kaya (2004) |
| Bitlis | NS | 241 | 215 (89.2) | 9 | E. ahs 46.1%, E. bak 49.4%, E. cran 35.2%, E. fau 10.7%, E. gran 12.8%, E. intr 8.7%, E. ovin 43.5%, E. pal 30.3%, E. par 45.6%, | Gül (2007) |
| Van | 1–60 days | 132 | 80 (60.6) | NS | NS | Ozdal et al. (2009) |
| USA | ||||||
| Alabama | Lambs | NS | NS | 2 | E. ahs, E. cran | Smith and Davis (1961) |
| Illinois | NS | 153 | 105 (69.0) | 10 | E. arloingi 53%, E. ahs 24%, E. cran 24%, E. fau 6%, E. gran 4%, E. intr 7%, E. ninakohlyakimovae 1%, E. pal 6%, E. par 5%, E. pun 1% | Shah (1963) |
| Louisiana | ewes | 109 | 94 (86.2) | 10 | E. ahs 41.3%, E. bak 48.6%, E. cran 36.7%, E. fau 43.1%, E. gran 28.4%, E. intr 17.4%, E. ovin 59.6%, E. pal 4.6%, E. par 45.9%, E. pun 1.8% | da Silva and Miller (1991) |
| Wales | NS | 60 | 57 (95.0) | 9 | E. ahs, E. arloingi, E. ninaekohlyakimovae, E. cran were the most common | Michael and Probert (1970) |
| England and Wales | NS | 639 | NS | 11 | E ahs 42.1%, E bak 81.7%, E cran 71.4%, E fau 57.7%, E gran 1.7%, E intr 14.9%, E mar 14.2%,E ovin 64.8%, E pal 13.9%, E par 59.5%, E weyb 43.8%, | Macrelli et al. (2019) |
| Zimbabwe | ||||||
| Harare | – | 497 | 414 (83.3) | 11 | E. ahs 91%, E. bak 95%, E. caprovina 26.2%, E. christenseni 12.3%, E. cran 69.7%, E. fau 61.4%, E. gran 53.2%, E. intr 23.8%, E. ovin 100%, E. pal 24.6%, E. par 98.3% | Chhabra and Pandey (1992) |
E. ahs, E. ahsata; E. bak, E. bakuensis; E. cran, E. crandallis; E. fau, E. faurei; E. gran, E. granulosa; E. intr, E. intricata; E. mar, E. marsica; E. ovin, E. ovinoidalis; E. pal, E. pallida; E. par, E. parva; E. pun, E. punctata; E. weyb, E. weybridgensis; NS, not stated. All E. ovina recorded in the table as E. bakuensis
Bold species are considered invalid
aBoth are same species but recorded with different infection rates
Eleven Eimeria spp. were detected in the present study: E. ahsata, E. bakuensis, E. crandallis, E. faurei, E. granulosa, E. intricata, E. marsica, E. ovinoidalis, E. pallida, E. parva and E. webybridgensis. These species were previously reported in sheep from Egypt (Ghanem and Abd El-Raof 2005; Abou-El-Naga 2010; Mohamaden et al. 2018); however, this is the first report of E. weybridgensis in sheep from Egypt, probably due to close similarity of their oocysts to those of E. crandallis (Norton et al. 1974). Oocysts of both species are similar shape and size. Sporocysts morphology is variable (broad ovoid 10–12 × 7–8 µm in E. crandallis and elongate ovoid 14–15 × 7–8 µm in E. weybridgensis), but not enough to easily distinguish both species (Fig. 1). Thus, both species were grouped together in our results.
In the present study, oocysts of the most pathogenic species in sheep (E. ovinoidalis) were detected in 14.6% (27/184) and all sheep were subclinical. Earlier in Egypt, subclinical E. ovinoidalis infections were also reported in 12.2% of 142 sheep from Suez governorate (Mohamaden et al. 2018). However, E. ovinoidalis and E. crandallis were detected in 13 lambs with bloody diarrhea in Kalubiya governorate (Ghanem and Abd El-Raof 2005), and respectively in 68 and 91 of 185 lambs suffered from diarrhea in Matrouh governorate (Abou-El-Naga 2010).
There is a debate concerning the validity of species of Eimeria in sheep because the endogenous stages are known only in a few of them. Some authors consider 15 Eimeria species in sheep as valid (Kaufmann 1996). Of them, 13 were reported worldwide: E. ahsata, E. bakuensis, E. crandallis, E. faurei, Eimeria gilruthi, E. granulosa, E. intricata, E. marsica, E. ovinoidalis, E. pallida, E. parva, Eimeria punctata and E. weybridgensis. In addition to Eimeria gonzalezi (Bazalar and Guerrero 1970) in sheep from South America and Eimeria dalli in Dall sheep (Ovis dalli) from Alaska, USA (Clark and Colwell 1974). Other species are considered invalid because of inadequate description or lack of archived specimens, for example Eimeria ajantai, Eimeria balloonii and Eimeria beedatus in sheep from India (More et al. 2011). Eimeria macusaniensis (camelid species) was reported in 2 sheep herds from Argentina grazed with guanaco (a closely related species to lama) on the same pasture (Vázquez et al. 2014), notable in this report, the oocyst per gram (OPG) in sheep was low (1420) in one herd; however, it exceeds 29,000 OPG in the other herd. Enteric developmental stages of E. macusaniensis were not investigated in intestinal samples of sheep. Furthermore, E. cylindrica (bovine species) infection was molecularly identified in sheep from Australia (Yang et al. 2014).
Global reports on Eimeria species infecting sheep indicating high parasite diversity in small or large scale surveys even at the level of small size populations, which revolutionize our understanding of this parasite. Subsequently, more studies are needed to clarify the transmission dynamics depending on the multilocus genetic analysis of different Eimeria species infecting sheep and other ruminant animals.
Acknowledgements
This study was conducted as a part of the master of the first author, and did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We thank Dr. Ragab Fereig (South Valley University) for helping in statistical analysis.
Author’s contribution
IA, YA, MA and SA designed and coordinated the study and shared in parasite identification. EE collected and examined samples. JPD, IA and EE collected and analyzed the data, wrote and revised the manuscript.
Data availability
All data generated or analyzed during this study are included in this manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Written informed consents were taken from owners of the sheep involved in this study prior to collection of samples.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abou-El-Naga TR (2010) Prevalence of some parasitic diseases causing diarrhoea in small ruminants in northwest coastal area. In: Proceedings of the 3rd animal wealth research conference in the Middle East and North Africa, pp 14–24
- Abouzeid NZ, Selim AM, El-Hady KM. Prevalence of gastrointestinal parasites infections in sheep in the Zoo garden and Sinai district and study the efficacy of anthelmintic drugs in the treatment of these parasites. J Am Sci. 2010;6:544–551. [Google Scholar]
- Adeppa J, Javaregowda AK, Krishnamurthy CM. An outbreak of coccidiosis in a stall fed sheep farm and its treatment in Shimoga Region, Karnataka. Indian Vet J. 2016;93:17–19. [Google Scholar]
- Almeida JDM. Infection due to Eimeria spp. in sheep in the municipality of Colinas, state of Tocantins. Med Vet (UFRPE) 2013;7:33–36. [Google Scholar]
- Al-Robaiee I, Sabah Z, Ahmed K, Salih SA. Diagnostic study of ovine gastrointestinal parasites in kirkuk city, Iraq. Adv Anim Vet Sci. 2019;7(9):727–731. doi: 10.17582/journal.aavs/2019/7.9.727.731. [DOI] [Google Scholar]
- Al-Rubaie HM, Al-Saadoon ZM. Detection of Eimeria spp. of sheep in Wasit province-Iraq. J Entomol Zool Stud. 2018;6:943–947. [Google Scholar]
- Al-Saadoon MZ, Al-Rubaie HMA. Traditional and molecular study for prevalence of coccidiosis in sheep in Wasit-Iraq. Indian J Nat Sci. 2018;8:14394–14401. [Google Scholar]
- Amarante AFT, Barbosa MA. Species of coccidia occurring in lambs in Sao Paulo State, Brazil. Vet Parasitol. 1992;41:189–193. doi: 10.1016/0304-4017(92)90078-n. [DOI] [PubMed] [Google Scholar]
- Ammar SI, Watson AM, Craig LE, Cope ER, Schaefer JJ, Mulliniks JT, Gerhold RW. Eimeria gilruthi–associated abomasitis in a group of ewes. J Vet Diagn Investig. 2019;31:128–132. doi: 10.1177/1040638718814109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan MO, Umur S, Kara M. The prevalence of coccidian species in sheep in Kars province of Turkey. Trop Anim Health Prod. 1999;31:161–165. doi: 10.1023/a:1005186624978. [DOI] [PubMed] [Google Scholar]
- Ayana D, Tilahun G, Wossene A. Study on Eimeria and Cryptosporidium infections in sheep and goats at Elfora export abattoir, Debre-zeit, Ethiopia. Turk J Vet Anim Sci. 2009;33:367–371. [Google Scholar]
- Bakunzi FR, Thwane SN, Motsei LE, Dzoma BM. Diversity and seasonal occurrence of Eimeria species in a mixed flock of communally reared sheep and goats in Mafikeng in the North West Province, South Africa. J S Afr Vet Assoc. 2010;81:148–150. doi: 10.4102/jsava.v81i3.137. [DOI] [PubMed] [Google Scholar]
- Barutzki D, Marquardt S, Gothe R. Eimeria infections of sheep in northwest Germany. Vet Parasitol. 1990;37:79–82. doi: 10.1016/0304-4017(90)90027-9. [DOI] [PubMed] [Google Scholar]
- Battelli G, Poglayen G. Eimeri ahsata Honess from domestic sheep (Ovis aries) in Italy. J Protozool. 1980;27:151–152. doi: 10.1111/j.1550-7408.1980.tb04670.x. [DOI] [PubMed] [Google Scholar]
- Bazalar H, Guerrero CA. Coccidias en ovinos domesticos de altura com una descripcion de Eimeria gonzalezi n.sp. Revista de la Facultad de Medicina Veterinarya Lima. 1970;22:172–180. [Google Scholar]
- Bersissa K, Tigist T, Teshale S, Reta D, Bedru H. Helminths of sheep and goats in central Oromia (Ethiopia) during the dry season. J Anim Vet Adv. 2011;10:1845–1849. [Google Scholar]
- Bhat SA, Mir MUR, Qadir S, Allaie IM, Khan HM, Husain I, Sheikh BA. Prevalence of gastro-intestinal parasitic infections in Sheep of Kashmir valley of India. Vet World. 2012;5:667–671. [Google Scholar]
- Carrau T, Silva LMR, Pérez D, Failing K, Martínez-Carrasco C, Macías J, Taubert A, Hermosilla C, de Ybáñez RR. Associated risk factors influencing ovine Eimeria infections in southern Spain. Vet Parasitol. 2018;263:54–58. doi: 10.1016/j.vetpar.2018.10.004. [DOI] [PubMed] [Google Scholar]
- Chartier C, Paraud C. Coccidiosis due to Eimeria in sheep and goats, a review. Small Rumin Res. 2012;103:84–92. doi: 10.1016/j.smallrumres.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra RC, Pandey VS. Prevalence of coccidia in sheep in Zimbabwe. Small Rumin Res. 1992;8:257–264. [Google Scholar]
- Clark GW, Colwell DA. Eimeria dalli sp. n. (Protozoa: Eimeriidae) from dall sheep Ovis dalli. J Protozool. 1974;21:197–199. doi: 10.1111/j.1550-7408.1974.tb03640.x. [DOI] [PubMed] [Google Scholar]
- Craig BH, Pilkington JG, Kruuk LEB, Pemberton JM. Epidemiology of parasitic protozoan infections in Soay sheep (Ovis aries L.) on St Kilda. Parasitology. 2007;134:9–21. doi: 10.1017/S0031182006001144. [DOI] [PubMed] [Google Scholar]
- da Silva NRS, Miller JE. Survey of Eimeria spp. oocysts in feces from Louisiana State University ewes. Vet Parasitol. 1991;40:147–150. doi: 10.1016/0304-4017(91)90091-9. [DOI] [PubMed] [Google Scholar]
- da Silva FRC, de Souza JD, Fialho CG, Escopeli KS, de Araújo FAP. Identification of Eimeria species in sheep in Mostardas, southern Brazil. Vet Em Foco. 2008;6:16–20. [Google Scholar]
- Daniel A, Deneke Y, Ibrahim N. Gastrointestinal parasites in sheep in Gemechis and Boke Districts, West Harerghe Zone, Ethiopia. Acta Parasitol Glob. 2014;5:120–124. [Google Scholar]
- de Macedo LO, Santos MAB, da Silva NMM, do Rêgo Barros GMM, Alves LC, Giannelli A, Ramos RAN, de Carvalho GA. Morphological and epidemiological data on Eimeria species infecting small ruminants in Brazil. Small Rumin Res. 2019;171:37–41. [Google Scholar]
- Demir S. Eimeria species in sheep slaughtered in Bursa meat and fish plant. Turk Parazitol Derg. 1995;19:132–139. [Google Scholar]
- Díaz P, Painceira A, Dacal V, Vázquez L, Cienfuegos S, Arias MS, Pato FJ, Paz-Silva A, Panadero R, Sánchez-Andrade R, López C. Eimeria infections in wild (Capreolus capreolus) and extensive-reared domestic ruminants from Galicia (NW Spain) Revista Ibero-latinoamericana de parasitología. 2010;69:83–89. [Google Scholar]
- Duszynski DW, Wilber PG. A guideline for the preparation of species descriptions in the Eimeriidae. J Parasitol. 1997;83:333–336. [PubMed] [Google Scholar]
- Eckert J, Braun R, Shirley MW, Coudert P (1995) Guidelines on techniques in coccidiosis research. COST 89/820. European Commission, DGXII, pp 103–117
- Elamin EA, Osman AY, Osman HM. Coccidian infection of sheep in Khartoum-Sudan. J Anim Vet Adv. 2004;3:648–651. [Google Scholar]
- Emiru B, Amede Y, Tigre W, Feyera T, Deressa B. Epidemiology of gastrointestinal parasites of small ruminants in Gechi District, Southwest Ethiopia. Adv Biol Res. 2013;7:169–174. [Google Scholar]
- Fadl SR, Kalef DA, Abbas SM. Prevalence of parasitic infection in sheep from different regions in Baghdad. Iraqi J Vet Sci. 2011;35:204–209. [Google Scholar]
- Food and Agriculture Organization (2015) Africa sustainable livestock 2050 report, Country brief Egypt. www.fao.org/3/a-i7312e/pdf. Accessed Sept 2019
- Ghanem MM, Abd El-Raof YM. Clinical and Haemato-Biochemical studies on lamb coccidiosis and changes following amprolium and sulphadimthoxine therapy. Benha Vet Med J. 2005;16:286–299. [Google Scholar]
- Gonzalez M, Sanchez A, Vazquez P (1990) Presence and dynamics of oocysts of some species of Eimeria in ewes and lambs during the perinatal period in Huixquilucan, Mexico. In: Memoria III Congreso Nacional de Producción Ovina, Tlaxcala, pp 225–228
- Gorski P, Niznikowski R, Strzelec E, Popielarczyk D, Gajewska A, Wedrychowicz H. Prevalence of protozoan and helminth internal parasite infections in goat and sheep flocks in Poland. Arch Fur Tierzucht. 2004;47:43–49. [Google Scholar]
- Gregory MW, Catchpole J, Nolan A, Hebert CN. Ovine coccidiosis: studies on the pathogenicity of Eimeria ovinoidalis and E. crandallis in conventionally-reared lambs, including possible effects of passive immunity. Dtsch Tierarztl Wochenschr. 1989;96:287–292. [PubMed] [Google Scholar]
- Gül A. Prevalence of Eimeria species in sheep in the Bitlis province. Turk Parazitol Derg. 2007;31(1):20–24. [PubMed] [Google Scholar]
- Gül A, Değer S. The prevalance and distribution of Eimeria species found in sheep in Van. Turk J Vet Anim Sci. 2002;26:859–864. [Google Scholar]
- Güler S, Dumanl N, Özer E, Erdoğmus Z, KöroĞlu E. Investigations on the incidence of Eimeria species found in lambs and kids in the Elaziğ region of Turkey. Doğa- Turk J Vet Anim Sci. 1990;14:295–300. [Google Scholar]
- Hasan MH, Abed HM. A study of Eimeria species in sheep in Mosul city. Iraqi J Vet Sci. 2012;26:45–53. [Google Scholar]
- Hashemnia M, Rezaei F, Chalechale A, Kakaei S, Gheichivand S. Prevalence and intensity of Eimeria infection in sheep in Western Iran. Int J Lives Res. 2014;4:107–112. [Google Scholar]
- Ibrahim MM, Afsa AAS. Natural co-infection and species composition of Eimeria in sheep in Al-Baha area, Saudi Arabia. Egypt Acad J Biol Sci. 2013;5:49–58. [Google Scholar]
- Islam KBMS, Taimur MJFA. Helminthic and protozoan internal parasitic infections in free ranging small ruminants of Bangladesh. Slov Vet Res. 2008;45:67–72. [Google Scholar]
- Jawasreh KI, Mukbel RM, Qader AA, Mayyas MA. Coccidiosis in Awassi, Romanov, Charollais and Suffolk sheep breeds during the winter and summer seasons in Jordan. Int J Appl Sci Technol. 2013;3:10–15. [Google Scholar]
- Jegede OC, Adejoh AA, Obeta SS, Olayemi OD. Gastrointestinal parasites of sheep and goats in Gwagwalada area council, federal capital territory, Abuja, Nigeria; with a special reference to sex, breed and age. Alex J Vet Sci. 2015;46:170–176. [Google Scholar]
- Juszczak M, Sadowska N, Udała J. Parasites of the digestive tract of sheep and goats from organic farms in Western Pomerania. Poland Ann Parasitol. 2019;65(3):245–250. doi: 10.17420/ap6503.206. [DOI] [PubMed] [Google Scholar]
- Kalef DA, Fadl SR, Abbas SM. Occurence of Eimeria infection of sheep from different regions of Baghdad city. Diyala Agric Sci J. 2013;5:32–37. [Google Scholar]
- Kambarage DM, Kimera SI, Kusiluka LJM, Mtambo MMA. Prevalence of Eimeria and Cryptosporidium oocysts in cattle, sheep and goats in Morogoro Region, Tanzania. J Appl Anim Res. 1996;9:73–78. [Google Scholar]
- Kandasamy G, Rajapakse RPV, Rajakaruna RS (2011) Gastrointestinal and blood parasites of sheep in Kaithady farm in Jaffna district. In: Proceedings of the Peradeniya University research sessions, Sri Lanka, 16, p 145
- Kanyari PW. The relationship between coccidal and helminth infections in sheep and goats in Kenya. Vet Parasitol. 1993;51:137–141. doi: 10.1016/0304-4017(93)90204-z. [DOI] [PubMed] [Google Scholar]
- Kareem SI, Yücel ŞY. Prevalence of Eimeria species in sheep in Sulaimaniya province, Iraq. J Entomol Zool Stud. 2015;3:317–322. [Google Scholar]
- Kaufmann J. Parasitic infections of domestic animals: a diagnostic manual. Basel: Birkhäuser; 1996. p. 423. [Google Scholar]
- Kaya G. Prevalence of Eimeria species in lambs in Antakya Province. Turk J Vet Anim Sci. 2004;28:687–692. [Google Scholar]
- Khan MN, Rehman T, Iqbal Z, Sajid MS, Ahmad M, Riaz M. Prevalence and associated risk factors of Eimeria in sheep of Punjab, Pakistan. World Acad Sci Eng Technol. 2011;5:334–338. [Google Scholar]
- Koinari M, Karl S, Ryan U, Lymbery AJ. Infection levels of gastrointestinal parasites in sheep and goats in Papua New Guinea. J Helminthol. 2013;87:409–415. doi: 10.1017/S0022149X12000594. [DOI] [PubMed] [Google Scholar]
- Kusiluka LJM, Kambarage DM, Matthewman RW, Harrison LJS, Daborn CJ. Coccidiosis of small ruminants in Tanzania. Small Rumin Res. 1996;21:127–131. [Google Scholar]
- Kyriánová IA, Vadlejch J, Langrová I. Eimeriosis seasonal dynamics patterns at an organic sheep farm in the Czech Republic. Sci Agric Bohem. 2017;48:70–75. [Google Scholar]
- Lassen B, Järvis T, Mägi E. Gastrointestinal parasites of sheep on Estonian Islands. Agraarteadus J Agric Sci. 2013;24:7–14. [Google Scholar]
- León JCP, Delgado NU, Florez AA. Prevalence of gastrointestinal parasites in cattle and sheep in three municipalities in the Colombian Northeastern Mountain. Vet World. 2019;12(1):48–54. doi: 10.14202/vetworld.2019.48-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes WDZ, Borges FDA, Faiolla TDP, Antunes LT, Borges DGL, Rodriguez FDS, Ferraro G, Teixeira WF, Maciel WG, Felippelli G, Costa AJD. Eimeria species in young and adult sheep raised under intensive and/or semi-intensive systems of a herd from Umuarama city, Parana State, Brazil. Ciência Rural. 2013;43:2031–2036. [Google Scholar]
- Macrelli M, Dunnett L, Mitchell S, Carson A of the APHA Small Ruminant Species Expert Group Coccidiosis in sheep. Vet Rec. 2019;184:549–550. doi: 10.1136/vr.l2019. [DOI] [PubMed] [Google Scholar]
- Mahrt JJ. Prevalence of coccidia in domestic sheep in central Alberta. Can Vet J. 1969;10:176–178. [PMC free article] [PubMed] [Google Scholar]
- Maingi N, Munyua WK. The prevalence and intensity of infection with Eimeria species in sheep in Nyandarua district of Kenya. Vet Res Commun. 1994;18:19–25. doi: 10.1007/BF01839257. [DOI] [PubMed] [Google Scholar]
- Majaro OM, Dipeolu OO. The seasonal incidence of coccidia infections in trade cattle, sheep, and goats in Nigeria. Vet Q. 1981;3:85–90. doi: 10.1080/01652176.1981.9693802. [DOI] [PubMed] [Google Scholar]
- Majeed QA, Alazemi MS, Henedi AA, Tahrani L. Study on parasites from farm animals in Kuwait. J Egypt Soc Parasitol. 2015;45:71–74. doi: 10.12816/0010851. [DOI] [PubMed] [Google Scholar]
- Mamatha GS, D'Souza PE. Gastrointestinal parasitism in sheep and goats from different districts of Karnataka. Intas Polivet. 2007;8:112–114. [Google Scholar]
- Martins GF, Moura MS, Cabral DD, de Souza RR (2011) Frequência de oocisto de Eimeria spp. emovinos de propriedadesrurais do Município de Uberlândia-MG. Pubvet, Londrina 5, Art-1038
- McDougald LR. Attempted cross-transmission of coccidia between sheep and goats and description of Eimeria ovinoidalis sp. n. J Protozool. 1979;26:109–113. doi: 10.1111/j.1550-7408.1979.tb02741.x. [DOI] [PubMed] [Google Scholar]
- Michael E, Probert AJ. The prevalance of coccidia in faecal samples from sheep in North Wales. Res Vet Sci. 1970;11:402–403. [PubMed] [Google Scholar]
- Minnat TR. Detection of gastrointestinal parasite infection of sheep and goats in Diyala Province-Iraq. AL-Qadisiyah J Vet Med Sci. 2014;13:118–123. [Google Scholar]
- Mirzaei M, Dahmardeh E, Sharifi H. The prevalence of Eimeria species in sheep in Zabol city, Iran. Sci Res Iran Vet J. 2016;11:98–105. [Google Scholar]
- Mohamaden WI, Sallam NH, Abouelhassan EM. Prevalence of Eimeria species among sheep and goats in Suez Governorate. Egypt Int J Vet Sci Med. 2018;6:65–72. doi: 10.1016/j.ijvsm.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla SH, Bandyopadhyay PK. Prevalence of gastro-intestinal parasites in economically important Bonpala sheep in India. IOSR J Agric Vet Sci. 2016;30:87–93. [Google Scholar]
- More BV, Nikam SV, Bhamare NDS, Jaid EL. Percentage prevalence of eimerian species composition of sheep and goats from beed district, Maharashtra. Recent Res Sci Technol. 2011;3:24–26. [Google Scholar]
- Murthy GSS, Rao PV. Prevalence of gastro-intestinal parasites in ruminants and poultry in Telangana region of Andhra Pradesh. J Parasit Dis. 2014;38:190–192. doi: 10.1007/s12639-012-0218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton CC, Joyner LP, Catchpole J. Eimeria weybridgensis sp. nov. and Eimeria ovina from the domestic sheep. Parasitology. 1974;69:87–95. doi: 10.1017/s0031182000046205. [DOI] [PubMed] [Google Scholar]
- Nourollahi-Fard SR, Khedri J, Ghashghaei O, Mohammadyari N, Sharifi H. The prevalence of ovine Eimeria infection in Rudsar, North of Iran, (2011–2012) J Parasit Dis. 2016;40:954–957. doi: 10.1007/s12639-014-0613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan MG, O'Donoghue PJ, Moore E. Coccidia in sheep in South Australia. Vet Parasitol. 1987;24:175–183. doi: 10.1016/0304-4017(87)90038-0. [DOI] [PubMed] [Google Scholar]
- Om H, Kumar S, Singh P. Prevalence of coccidia in Mathura region of Uttarpradesh. Vet World. 2010;3:503–505. [Google Scholar]
- Owusu M, Sekyere JO, Adzitey F. Prevalence and burden of gastrointestinal parasites of Djallonke sheep in Ayeduase, Kumasi, Ghana. Vet World. 2016;9:361–364. doi: 10.14202/vetworld.2016.361-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdal N, Tanritanir P, Goz Y, Deger S, Kozat S. Parasitic protozoans (Eimeria, Giardia, and Cryptosporidium) in lambs with diarrhoea in the Van province (Turkey) Bull Vet Inst Pulawy. 2009;53:47–51. [Google Scholar]
- Platzer B, Prosl H, Cieslicki M, Joachim A. Epidemiology of Eimeria infections in an Austrian milking sheep flock and control with diclazuril. Vet Parasitol. 2005;129:1–9. doi: 10.1016/j.vetpar.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Raue K, Heuer L, Böhm C, Wolken S, Epe C, Strube C. 10-year parasitological examination results (2003 to 2012) of faecal samples from horses, ruminants, pigs, dogs, cats, rabbits and hedgehogs. Parasitol Res. 2017;116(12):3315–3330. doi: 10.1007/s00436-017-5646-0. [DOI] [PubMed] [Google Scholar]
- Reginsson K, Richter SH. Coccidia of the genus Eimeria in sheep in Iceland. Icel Agric Sci. 1997;11:99–106. [Google Scholar]
- Šarić T, Beck R, Bosnić S, Župan I, Šaran T, Aćimov D, Tkalčić S (2015) Species identification and prevalence of Eimeria spp. coccidial parasites in sheep from the North Dalmatia (Croatia). In: Proceedings of XXII international congress of mediterranean federation for health and production of ruminants, pp 351–354
- Shah HL. Coccidia (Protozoa: Eimeriidae) of domestic sheep in the United States, with descriptions of the sporulated oocysts of six species. J Parasitol. 1963;49:799–807. [PubMed] [Google Scholar]
- Silva RMD, Facury-Filho EJ, Souza MF, Ribeiro MFB. Natural infection by Eimeria spp. in a cohort of lambs raised extensively in Northeast Brazil. Rev Brasil Parasitol Vet. 2011;20:134–139. doi: 10.1590/s1984-29612011000200008. [DOI] [PubMed] [Google Scholar]
- Skirnisson KARL. Eimeria spp. (Coccidia, Protozoa) infections in a flock of sheep in Iceland: species composition and seasonal abundance. Icel Agric Sci. 2007;20:73–80. [Google Scholar]
- Smith WN, Davis LR. Two species of sheep coccidia new to Alabama. Proc Helminthol Soc Wash. 1961;28:95–96. [Google Scholar]
- Sontakke TA, Kanse VS, Bansode VK, Lokahnde SC, Nikam SV. Occurrence of coccidian parasites in sheep in Omerga region. Int J Life Sci. 2015;A3:92–94. [Google Scholar]
- Souza MDFD, Pimentel-Neto M, Silva RMD, Farias ACB, Guimarães MP. Gastrointestinal parasites of sheep, municipality of Lajes, Rio Grande do Norte, Brazil. Rev Brasil Parasitol Vet. 2012;21:71–73. doi: 10.1590/s1984-29612012000100015. [DOI] [PubMed] [Google Scholar]
- Squire SA, Robertson ID, Yang R, Ayi I, Ryan U. Prevalence and risk factors associated with gastrointestinal parasites in ruminant livestock in the Coastal Savannah zone of Ghana. Acta Trop. 2019;199:105126. doi: 10.1016/j.actatropica.2019.105126. [DOI] [PubMed] [Google Scholar]
- Sultan K, Elmonir W, Hegazy Y. Gastrointestinal parasites of sheep in Kafrelsheikh governorate, Egypt: prevalence, control and public health implications. Beni-Suef Univ J Basic Appl Sci. 2016;5:79–84. [Google Scholar]
- Swarnkar CP, Singh D, Solanki VK. Prevalence of gastrointestinal parasites of sheep in semi-arid Rajasthan: a field study. Indian J Small Rumin. 2010;16:221–227. [Google Scholar]
- Tavassoli M, Khoshvaghti H. Helminthes and coccidia infection of wild sheep (Ovis ammon Orintalis) in Kabodan Island of National Park of Urmia Lake. Iran Vet Res Forum. 2010;1:26–29. [Google Scholar]
- Thomson EF, von Kaufmann R, Li Pun H, Treacher T, van Houten H (2000) Global agenda for livestock research. In: Proceedings of a consultation on setting livestock research priorities in West Asia and North Africa (WANA) region, ICARDA, Aleppo, Syria, 12–16 November 1997. Nairobi (Kenya): ILRI/Aleppo (Syria): ICARDA, p 172
- Toulah FH. Prevalence and comparative morphological study of four Eimeria sp. of sheep in Jeddah area. Saudi Arabia J Biol Sci. 2007;7:413–416. [Google Scholar]
- Trejo-Huitrón G, Bautista-Gómez LG, Martínez-Castañeda JS, Romero-Núñez C, Trejo-Castro L, Espinosa-Ayala E. Morphological characterization and first molecular identification of the eleven Eimeria species that infect sheep from Mexico. Parasitol Res. 2020;119:115–122. doi: 10.1007/s00436-019-06477-6. [DOI] [PubMed] [Google Scholar]
- Uhazy LS, Mahrt JL, Holmes JC. Coccidia of rocky mountain bighorn sheep in western Canada. Can J Zool. 1971;49:1461–1464. doi: 10.1139/z71-215. [DOI] [PubMed] [Google Scholar]
- Varghese T, Yayabu R. Ovine coccidia in Papua New Guinea. Vet Parasitol. 1985;17:181–191. doi: 10.1016/0304-4017(85)90030-5. [DOI] [PubMed] [Google Scholar]
- Vasilková Z, Krupicer I, Legáth J, Kovalkovicova N, Petko B. Coccidiosis of small ruminants in various regions of Slovakia. Acta Parasitol. 2004;49:272–275. [Google Scholar]
- Vázquez MB, Genzelis M, Mijalenko S, Beltramino JB. Survey of Eimeria spp. in sheep: first notice of Eimeria macusaniensis in the region of Governor Gregores, Santa Cruz. Argent Revista de Salud Anim. 2014;36:62–64. [Google Scholar]
- Vercruysse J. The coccidia of sheep and goats in Senegal. Vet Parasitol. 1982;10:297–306. doi: 10.1016/0304-4017(82)90080-2. [DOI] [PubMed] [Google Scholar]
- Vieira LDS, Cavalcante ACR, Ximenes LJF. Infection with Eimeria species in hair sheep reared in Sobral, Ceará State. Brazil Rév Méd Vét. 1999;6:547–550. [Google Scholar]
- Wang CR, Xiao JY, Chen AH, Chen J, Wang Y, Gao JF, Zhu XQ. Prevalence of coccidial infection in sheep and goats in northeastern China. Vet Parasitol. 2010;174:213–217. doi: 10.1016/j.vetpar.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Yakhchali M, Golami E. Eimeria infection (Coccidia: Eimeriidae) in sheep of different age groups in Sanandaj city, Iran. Veterinarski arhiv. 2008;78:57–64. [Google Scholar]
- Yakhchali M, Rezaei AA. The prevalence and intensity of Eimeria spp. infection in sheep of Malayer suburb, Iran. Arch Razi Inst. 2010;65:27–32. [Google Scholar]
- Yakhchali M, Zarei MR. Prevalence of Eimeria infection in sheep of Tabriz suburb, Iran. Iran J Vet Res. 2008;9:277–280. [Google Scholar]
- Yang R, Jacobson C, Gardner G, Carmichael I, Campbell AJ, Ryan U. Longitudinal prevalence, oocyst shedding and molecular characterisation of Eimeria species in sheep across four states in Australia. Exp Parasitol. 2014;145:14–21. doi: 10.1016/j.exppara.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Zainalabidin FA, Raimy N, Yaacob MH, Musbah A, Bathmanaban P, Ismail EA, Mamat ZC, Zahari Z, Ismail MI, Panchadcharam C. The prevalence of parasitic infestation of small ruminant farms in Perak, Malaysia. Trop Life Sci Res. 2015;26:1–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.