Abstract
The aim of this study was to investigate the effect of serial amnioinfusion therapy (SAT) for pulmonary hypoplasia in lower urinary tract obstruction (LUTO) or congenital renal anomalies (CRAs), introduce patient selection criteria, and present a case of SAT in bilateral renal agenesis. We conducted a search of the MEDLINE, EMBASE, Web of Science, and Scopus databases for articles published from database inception to November 10, 2017. Eight studies with 17 patients (7 LUTO, 8 CRA, and 2 LUTO + CRA) were included in the study. The median age of the mothers was 31 years (N=9; interquartile range [IQR], 29-33.5 years), the number of amnioinfusions was 7 (N=17; IQR, 4.5-21), gestational age at first amnioinfusion was 23 weeks and 4 days (N=17; IQR, 21-24.07), gestational age at delivery was 32 weeks and 2 days (N=17; IQR, 30 weeks to 35 weeks and 6.5 days), birthweight of newborns was 3.7 kg (N= 9; IQR, 2.7-3.7 kg), Apgar score at 1 minute was 2.5 (N=8; IQR, 1-6.5), and Apgar score at 5 minutes was 5.5 (N=8; IQR, 0-7.75). In conclusion, SAT may provide fetal pulmonary palliation by reducing the risk of newborn pulmonary compromise secondary to oligohydramnios. Multidisciplinary research efforts are required to further inform treatment and counseling guidelines. We propose a multidisciplinary approach to prenatal classification of fetuses with LUTO to inform patient selection.
Abbreviations and Acronyms: AF, amniotic fluid; AFI, AF index; AFV, AF volume; BRA, bilateral renal agenesis; CRA, congenital renal anomaly; DOL, day of life; GA, gestational age; IQR, interquartile range; LUTO, lower urinary tract obstruction; MVP, maximal vertical pocket; PD, peritoneal dialysis; PPROM, preterm premature rupture of membranes; SAT, serial amnioinfusion therapy; WHO, World Health Organization
Article Highlights.
-
•
Severe oligohydramnios secondary to fetal renal anomalies and lower urinary tract obstruction leads to poor neonatal outcomes.
-
•
Available evidence suggests that fetal serial amnioinfusion is a regenerative therapy that may provide pulmonary palliation and reduce the risk of newborn mortality from pulmonary compromise.
-
•
Multidisciplinary prenatal counseling and management is necessary prior to offering this fetal intervention as substantial resources are involved in serial amnioinfusion therapy with the newborn ultimately relying on peritoneal dialysis and requiring renal transplant in the future.
-
•
Given the lack of strong evidence supporting this fetal intervention, a clinical trial would be necessary to further assess the long term outcomes of this intervention.
Oligohydramnios secondary to fetal lower urinay tract obstruction (LUTO) or congenital renal anomalies (CRAs) is associated with poor neonatal outcomes.1,2 Before the advent of fetal intervention, termination of pregnancy or expectant management were the only available approaches. Adequate amniotic fluid volume (AFV) is crucial in fetal development and growth. It promotes lung and skeletal development, serves to cushion the fetus, and prevents umbilical cord compression and death. In cases of LUTO or CRA, oligohydramnios results due to a disruption in the production and/or flow of fetal urine, which serves as a major source of amniotic fluid (AF) during later gestation. The lack of AF then results in fetal compression, which limits breathing movements and volume to be maintained into fetal lungs, thus inhibiting pulmonary development and impeding necessary canalicularization at 17 to 26 gestational weeks.2,3 Oligohydramnios presenting at an earlier gestational age (GA) correlates with a potentially more severe degree of resulting pulmonary hypoplasia. Although oligohydramnios with severe pulmonary hypoplasia was once uniformly fatal, today there is hope that a regenerative therapy for pulmonary hypoplasia, termed serial amnioinfusion therapy (SAT), may provide promise of better outcomes.2
LUTO consists of a heterogeneous group of pathologies, which most commonly includes posterior urethral valves, and has an incidence of 1 in 10,000.4, 5, 6, 7 Vesicoamniotic shunting and fetal cystoscopy are current fetal interventions available in LUTO.6,8, 9, 10, 11 Functional renal impairment occurs in over 70% of LUTO. In 1986, even in cases of LUTO following vesicoamniotic shunting, the most common cause of death was pulmonary hypoplasia. Physicians during this time noted that with such disappointing results, therapeutic amnioinfusion should be considered in cases of LUTO with severe renal impairment.12, 13, 14, 15, 16
Congenital renal anomalies include a variety of pathologies and severities, including bilateral renal agenesis (BRA), dysplastic kidneys, or severe polycystic disease. BRA is rare, with an incidence of 1 in 3000 pregnancies.2 Typically, termination of pregnancy is recommended given the expected mortality resulting from severe oligohydramnios and fatal pulmonary hypoplasia.17
Without therapeutic amnioinfusion, a diagnosis of LUTO or CRA may be incompatible with newborn survival. The objective of this study was to utilize the available literature to investigate the effect of SAT as a method of temporarily correcting oligohydramnios associated with LUTO and CRA, and thereby reducing mortality secondary to pulmonary hypoplasia. We report neonatal survival outcomes that may inform future direction in research and the clinical application of SAT, as well as highlight the need for a prospective clinical trial. We propose a classification system stratified by disease severity in fetuses with LUTO that can be used for SAT patient selection and present an introductory case report of SAT for fetal BRA.
Report of a Case
A 26-year-old woman, gravida 1, para 0, with a pregnancy complicated by fetal BRA was admitted to the antepartum service at our institution for preterm premature rupture of membranes (PPROM) at a GA of 30 weeks and 6 days in the setting of anhydramnios. Before presenting to our institution, the patient had SAT initiated at 27 weeks’ GA at an outside institution to palliate pulmonary hypoplasia. All amnioinfusion procedures took place with ultrasound under color flow Doppler, sterile procedure technique, 1% lidocaine without epinephrine analgesic, 22-gauge 5-inch spinal needle, and warm saline. At the first amnioinfusion at 27 weeks and 5 days’ GA, the patient’s body mass index (calculated as weight in kilograms divided by height in meters squared) was 26.95 kg/m2, and her only current medication was prenatal vitamins. The results of chorionic villus sampling were normal, with a male chromosome detected. After infusion of 550 mL, the postprocedure AF index (AFI) was 15.9 cm and the maximal vertical pocket (MVP) was 6.0 cm. No complications occurred, and 3 mL of fluid was withdrawn for Gram stain and culture, which produced negative results. At the second amnioinfusion at 29 weeks and 4 days’ GA with a 600-mL infusion, the postprocedure AFI was 13.1 cm, MVP was 6.4 cm, and there were no complications. At the third amnioinfusion at 30 weeks and 3 days’ GA with a 650-mL infusion, the postprocedure AFI was 18.1 cm, MVP was 5.0 cm, and fetal heart rate deceleration to 58 beats/min was detected, which resolved postprocedure to 158 beats/min. The fourth amnioinfusion at 30 weeks and 6 days consisted of 700 mL warm saline and 5 mL indigo carmine infusion, and 5 mL of AF was aspirated for Gram stain and culture. The postprocedure AFI was 17.7 cm, MVP was 5.4 cm, and there were no complications. Approximately 7½ hours after the fourth amnioinfusion, the patient presented to the outside institution with PPROM. The patient felt a gush of fluid with blue dye noted. At the time of presentation, ultrasonography revealed an AFI of 0.7 cm and MVP of 0.7 cm, classifying her as having severe oligohydramnios. The placenta was noted to be anterior, the biophysical profile was 6/8 for decreased AF, and the cervix was 3.1 cm in length and closed. During the hospitalization, the patient received betamethasone, nifedipine, terbutaline, and antibiotics. After discharge on hospital day 4, she took a commercial flight to our institution.
She presented to our institution after PPROM at 30 weeks and 6 days’ GA to have her delivery closer to her family. She had serious social issues that complicated her pregnancy, including the death of her fiancé very early in the pregnancy. She was counseled by a multidisciplinary team on the potential negative outcomes of the pregnancy, including that her fetus was not expected to survive with great confidence given the fatal risk of pulmonary hypoplasia in BRA. After being counseled extensively about the labor process and likely outcomes of fetal resuscitation, she was offered an induction of labor at 34 weeks unless there were clinical indications to recommend an induction sooner and monitored labor if she were to go into spontaneous labor before 34 weeks. . She was offered a trial of resuscitation consisting of intubation and mechanical ventilation of the newborn. The patient clearly communicated to the team that she desired full cares for her child after delivery.
The patient was diagnosed as having gestational hypertension at 32 weeks and 4 days’ GA, and she remained asymptomatic. At 34 weeks and 6 days’ GA, the patient was admitted to the labor and delivery service for induction of labor in the setting of PPROM. Induction was started with dinoprostone. Labor augmentation included misoprostol and oxytocin. The patient received epidural anesthesia before delivery, and the labor progressed without complications. The patient had a normal spontaneous vaginal delivery of a liveborn male at 35 weeks’ GA. The estimated blood loss was 350 mL, and the placenta was delivered intact with a histologically normal 3-vessel umbilical cord. The mother remained normotensive and had an uncomplicated postpartum course.
The newborn male was intubated at birth and given surfactant because of signs of hypotonia and lack of adequate respiratory effort. Apgar scores at 1, 5, and 10 minutes were 2, 6, and 8. Initial chest radiography revealed right pneumothorax, but the team opted not to intervene because of reassuring oxygen status. The family was aware of the serious concerns for pulmonary hypoplasia and requested a chaplain at the bedside. The team briefly paused cares to allow for baptism by the chaplain per the mother’s request.
After transfer to the neonatal intensive care unit, the patient was extubated at 5 hours of life to low-flow nasal cannula at 50 mL/min. The neonatal intensive care unit team continued as the primary service for the child with consultation from multiple specialties during the hospital stay including pediatric surgery, pediatric nephrology, infectious diseases, respiratory therapy, pediatric palliative care team, and ethicists. Weight on day of life (DOL) 1 had increased by 20 g from newborn weight, and the infant became critically ill, requiring vasopressive support for hypotension. Blood cultures obtained returned negative. On DOL 2, he was taken to the operating room for placement of a peritoneal dialysis (PD) catheter and G (gastrostomy) tube, as well as repair of an incidentally found inguinal hernia. The operation was complicated by an accidental colotomy with minimal meconium spillage that was repaired.
Enteral feeding was initiated on DOL 3 with breast milk via the G tube. The absence of renal tissue was confirmed with Doppler ultrasonography and no bladder was visualized (Figure 1). On DOL 4, electrolyte abnormalities were detected, and PD was initiated. Initially, the infant was unable to tolerate PD because of catheter leakage. A purse-string suture was surgically placed, but because the infant had tolerable laboratory findings, he did not undergo PD for several days. Peritoneal dialysis was then reinitiated on DOL 10 at minimal volumes. The infant required vasopressive support during dialysis. Unfortunately, he continued to struggle during his hospitalization with leakage from the PD catheter site, and bacterial peritonitis developed. The final culture growth included Klebsiella, and placement of his hemodialysis catheter was delayed during antibiotic treatment. Specimens from his central venous line grew coagulase-negative cocci. After culture results were negative for 24 hours, the team proceeded with hemodialysis catheter placement.
Figure 1.
Retroperitoneal transverse midline ultrasonogram supporting a prenatal diagnosis of bilateral renal agenesis with no renal tissue visible within the abdomen.
On DOL 43, multiple procedures were performed related to catheter and central line placement, laryngoscopy, and bronchoscopy. Continuous renal replacement therapy was initiated. The patient had periods of hemodynamic instability that required frequent replacement of electrolytes, multiple units of blood products, and vasopressors. He advanced to feeding goals via the G tube though remained intubated and sedated until DOL 55.
Continuous renal replacement therapy was continued until DOL 68, when it was switched to PD. The infant underwent low-volume PD that had to be titrated because of catheter leakage. Complications that ensued during the remainder of his hospital stay included infiltration of the peripherally inserted central catheter line used for dialysis resulting in right brachial plexus injury. The patient then exhibited symptoms of increased tone on the left side and decreased tone of the right arm prompting magnetic resonance imaging that revealed multiple small cerebellar infarcts and a deep venous thrombosis in his right arm. The patient continued to have labile blood pressure and had a bradycardic arrest requiring resuscitation at 3 months and 28 days of age, which was thought to be due to excessive vagal responses to light stimulation during nursing cares. The patient had tracheostomy placement at 6 months of age, and a completely thrombosed inferior vena cava was noted below the liver with only collateral supply to the lower limbs, for which he underwent recanalization with interventional radiology. At 7 months of age, he weighed 5.1 kg, which placed him at less than 1 percentile (z score, −4.34) based on the World Health Organization (WHO) weight for age data on males 0 to 2 years old. His length was 49 cm, which placed him at less than 1 percentile (z score, −9.29) based on WHO length for weight data on males 0 to 2 years old. His head circumference was 38.8 cm, which placed him at less than 1 percentile (z score, −8.10) based on WHO head circumference for age data. During his seventh month of age, he had had multiple periods of hemodynamic instability and required multiple units of blood products for blood dyscrasias. He was receiving vasopressors on and off for several days and experienced bradycardic arrests with several resuscitations. Given that the infant’s long-term goal was renal transplant, the family agreed to proceed with palliative care since transplant was no longer an option due to poor growth. Brain magnetic resonance imaging revealed global cerebral loss and encephalomalacia resulting in laminar necrosis in the left parieto- occipital region (Figure 2). Electroencephalography revealed no brain wave activity. A brain death examination by the pediatric neurology service revealed no brain stem function or any higher cortical function. A cerebral blood flow study detected no flow through his cerebral vessels. The infant was pronounced dead at 7 months, 2 weeks, and 4 days of age after withdrawal of ventilatory and medication support.
Figure 2.

Brain magnetic resonance image showing generalized parenchymal volume loss, ex vacuo ventricular dilatation, diffuse thinning of the corpus callosum, and encephalomalacia involving left parieto-occipital lobes with laminar necrosis.
Literature Review
Literature Search
A search was conducted in collaboration with an expert librarian for studies with SAT indicated for fetal LUTO or CRA. Inclusion required clear data on the SAT procedure, features of each patient who underwent SAT, and outcomes of each fetus diagnosed with LUTO or CRA. A search on MEDLINE, EMBASE, Web of Science, and Scopus was completed for articles published since the date of database inception to November 10, 2017. Key search terms included serial amnioinfusion therapy and lower urinary tract obstruction or renal anomalies or renal agenesis. Language was a criterion for exclusion. Only articles in English or that could be translated to English in text were used. The detailed search strategy is provided in the Supplemental Appendix, available online at http://www.mcpiqojournal.org.
Eligibility Criteria and Study Selection
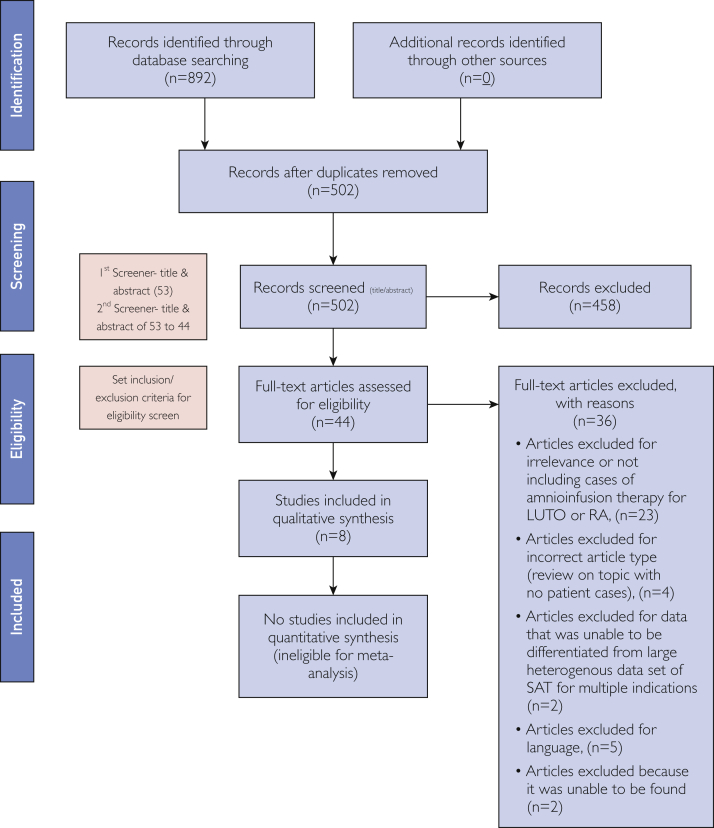
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines created by the National Institutes of Health were utilized throughout the review process (Figure 3). After the identification phase, duplicate removal was performed. Two reviewers screened all abstracts for relevant studies and assessed full-text articles for eligibility before inclusion. Studies reporting on SAT for LUTO or CRA with clear procedure details and fetal outcomes were included in the review. Studies in which individual patient data could not be extracted from a larger heterogeneous cohort of procedures done for other indications, such as PPROM or diagnostics, were not included. Two reviewers screened articles based on title and abstract relevance.
Figure 3.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses, 2009) flow diagram. LUTO = lower urinary tract obstruction; RA = renal anomaly; SAT = serial amnioinfusion therapy.
Data Abstraction
A standardized form was used for data abstraction that included authors, year of publication, country of origin, type of study, fetal indication for amnioinfusion therapy, maternal age, number of amnioinfusion procedures, GA at time of amnioinfusion in weeks, procedure technique, GA at delivery in weeks, method of delivery, complications during pregnancy or delivery, infant sex, newborn respiratory status, birth weight, Apgar scores at 1 and 5 minutes, newborn complications, and postnatal survival including status of dialysis or renal transplant. The main text, abstract, tables, and figures were used for data extraction. Prognostic variables and outcomes were reported in a descriptive manner for each study. Authors made descriptive conclusions, and no statistical analyses were conducted.
Because the studies consisted mostly of case reports and observational studies, there was considerable study-to-study heterogeneity that could not to be assessed due to large variability in the details reported for each patient. Pooled analysis was not feasible because of the heterogeneity, and all studies presented descriptive conclusions rather than quantitative conclusions with statistical analyses.
Data Analysis
A descriptive statistical analysis was completed using data amalgamation. Risk of bias in individual or across studies was not assessed. The descriptive analysis of important features including maternal age, number of amnioinfusions, GA at first amnioinfusion, GA at delivery, birth weight, and Apgar scores at 1 and 5 minutes using sample size, median, interquartile range (IQR), and range are presented in Table 1. The number of LUTO cases, cesarean sections, male infants, intubation in first 24 hours of life, 1-week survival, 1-month survival that did not require intubation at birth, 1-month survival, PD, renal transplant, and lost to follow-up after successful PD at discharge are reported in Table 2 using numbers and percentages.
Table 1.
Descriptive Statistical Analysis of Features Reported and Sample Sizes
| Feature | Sample size | Median (IQR, range) |
|---|---|---|
| Maternal age (y) | 9 | 31 (29-33.5, 21-36) |
| No, of amnioinfusions | 17 | 7 (4.5-21, 2-22) |
| Gestational age (wk+d) at first amnioinfusion | 17 | 23+4 (21-24.07, 16+2-29) |
| Gestational age (wk+d) at delivery | 17 | 32+2 (30-35+6.5, 28-41) |
| Birth weight (kg) | 9 | 3.7 (2.7-3.7, 1.47-3.70) |
| Apgar score at 1 min | 8 | 2.5 (1-6.5, 0-7) |
| Apgar score at 5 min | 8 | 5.5 (0-7.75, 0-8) |
IQR = interquartile range.
Table 2.
Sample Size of Features Reported in Articles Eligible for Systematic Review
| Feature | Sample size | No. (%) of patients |
|---|---|---|
| Fetuses with lower urinary tract obstruction | 17 | 9 (52.9) |
| Cesarean section | 7 | 4 (57.1) |
| Male infant | 12 | 11 (91.7) |
| Intubated within first 24 h of life | 11 | 6 (54.5) |
| Survival | ||
| 1 wk | 17 | 11 (64.7) |
| 1 mo | 11 | 10 (90.9) |
| Peritoneal dialysis | 16 | 9 (56.2) |
| Renal transplant | 9 | 4 (44.4) |
| Lost to follow-up after successful discharge on peritoneal dialysis | 9 | 2 (22.2) |
| Did not require intubation at time of birth and survived to 1 mo | 8 | 4 (50.0) |
Results
A total of 892 records were identified through the database search. There were 502 articles remaining after duplicate removal, and 44 articles remained after title and abstract screening. Articles were assessed in full text and eligibility criteria by 2 individuals. A total of 36 articles were excluded based on set criteria after full-text article assessment. Study publication dates ranged between 1991 and 2016. Eventually, 8 studies with data on 17 patients who underwent SAT for LUTO, CRA, or both were eligible.1,2,18, 19, 20, 21, 22, 23 Eleven patients were from the United States, 3 were from Taiwan, and 1 each were from Canada, Germany, and the United Kingdom.
Among the 17 eligible studies, there were 7 cases diagnosed with LUTO, 8 with CRA, and 2 with LUTO + CRA. All of the studies provided information about the fetal diagnosis, number of amnioinfusions, procedure technique (serial percutaneous infusions vs transabdominal amnioport), GA at delivery, and postnatal survival. The studies differed with respect to the degree of detail that was reported. There was variable reporting of maternal age, mode of delivery, neonatal birth weight, Apgar scores at 1 and 5 minutes, infant sex, pulmonary status at birth, and length of postnatal follow-up. The details reported for each patient are available in Table 3 and Table 4.
Table 3.
Summary of Systematic Review Data on Patient Demographic Characteristics, Amnioinfusion Procedure, and Mode of Delivery
| Patient | Reference, year | Study type | Fetal diagnosis | Maternal age (y) | No. of amnioin-fusions | Gestational age at instillations (wk+d) | Technique (fluid) | Total fluid infused per procedure (mL) | Delivery GA (wk+d) | Type of delivery | Pregnancy or delivery complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Fisk et al,18 1991 | Observational | Lower urinary tract obstruction | Not reported | 6 | 22-29 | Serial percutaneous instillations | Not reported | 30 | Cesarean | Chorioamnionitis |
| 2 | Hansmann et al,19 1991 | Observational | Lower urinary tract obstruction | 36 | 2 | First infusion at 20 | Serial percutaneous instillations | Average 86.3 | 41 | Vaginal | Not reported |
| 3 | Cameron et al,20 1994 | Case report | Bilateral renal agenesis, club feet | 21 | 10 | 16+2, 19, 21, 24, remaining at 1- to 2-wk intervals, last procedure at 33+4 | Serial percutaneous instillations | #1, 205; #2, 250; #3, 400; #4, 250; 6 additional instillations to subjective assessment of normal. The amniotic fluid volume max was 1400 | 33+6 | Vaginal | Presented at 33 wk + 6 d with painful abdominal cramps. Tocolysis initiated, then discontinued due to suspected chorioamnionitis. Administered intravenous antibiotics and labor progressed. Severe oligohydramnios noted at delivery. Tight double nuchal cord noted. |
| 4 | Hsu et al,21 2007 | Observational | Bilateral renal dysgenesis | 20-37 | 6 | First infusion at 21 | Serial percutaneous instillations | 250-600; rate, 10-15 mL/min 250-600, rate 10-15 mL/min |
29 | Not reported | Not reported |
| 5 | Hsu et al,21 2007 | Observational | Right renal agenesis, left renal dysgenesis | 20-38 | 2 | First infusion at 22 | Serial percutaneous instillations | 250-600; rate, 10-15 mL/min | 28 | Not reported | Not reported |
| 6 | Hsu et al,21 2007 | Care report | Autosomal recessive polycystic kidney disease | 20-39 | 2 | First infusion at 24 | Serial percutaneous instillations | 250-600; rate, 10-15/min | 30 | Not reported | Not reported |
| 7 | Bienstock et al,2 2014 | Case report | Bilateral renal agenesis, absent bladder | 34 | 5 | 24+1, 25+1, 26+1, 27+1, 28+1 | Serial percutaneous instillations | ~15 mL per week of gestational age, goal amniotic fluid index ~10 cm | 28+5 | Vaginal | Presented at 28 wk + 1 d with preterm contractions. Given betamethasone & terbutaline. Presented at 28 wk + 4 d with preterm contractions. Given magnesium sulfate. Delivery next day with 10% placental abruption |
| 8 | Haeri,22 2015 | Case report | Lower urinary tract obstruction and renal dysplasia | 30 | 4 | 21, 24, 26, 28 | Serial percutaneous instillations | Average 10-15 mL per week of gestational age, need assessed by weekly amniotic fluid volume measurements | 33+3 | Cesarean | None |
| 9 | Haeri,22 2015 | Case report | Lower urinary tract obstruction and renal dysplasia | 33 | 5 | 21, 24, 26, 28, 29 | Serial percutaneous instillations | Average 10-15 mL per week of gestational age, need assessed by weekly amniotic fluid volume measurements | 37 | Cesarean | Fetal growth restriction |
| 10 | Whittaker & Leonardi,23 2016 | Case report | Bilateral renal agenesis | 28 | 7 | 24, 25, 26, 27, 28, 29, 30 | Serial percutaneous instillations | Not reported | 30 | Cesarean | Persistent fetal bradycardia diagnosed at time of 30 wk amnioinfusion led to emergent cesarean delivery |
| 11 | Polzin et al,1 2017 | Observational | Lower urinary tract obstruction | Not reported | 10 | Amnioport placed at 26+6, dislodged & replaced at 28+2, infusions started at 29 | Transabdominal amnioport | Average 457 | 32 | Not reported | Not reported |
| 12 | Polzin et al,1 2017 | Observational | Lower urinary tract obstruction (partial obstruction) | Not reported | 9 | Amnioport placed at 27, infusions started at 28+1 | Transabdominal amnioport | Average 298 | 37+1 | Not reported | Not reported |
| 13 | Polzin et al,1 2017 | Observational | Lower urinary tract obstruction | Not reported | 21 | Amnioport placed at 23+2, infusions started 26 | Transabdominal amnioport | Average 437 | 37 | Not reported | Not reported |
| 14 | Polzin et al,1 2017 | Observational | Lower urinary tract obstruction (partial obstruction) | Not reported | 22 | Amnioport placed at 22+4, infusions started at 23+4 | Transabdominal amnioport | Average 431 | 31+5 | Not reported | Not reported |
| 15 | Polzin et al,1 2017 | Observational | Lower urinary tract obstruction | Not reported | 13 | Amnioport placed at 23+4, infusions started at 23+5 | Transabdominal amnioport | Average 470 | 32+2 | Not reported | Not reported |
| 16 | Polzin et al,1 2017 | Observational | Dysplastic horseshoe kidneys | Not reported | 11 | Amnioport placed at 22+3, infusions started at 23+5 | 4 Transabdominal amnioport , 7 serial percutaneous instillations | Average 311 | 35+4 | Not reported | Not reported |
| 17 | Polzin et al,1 2017 | Observational | Bilateral cystic dysplasia | Not reported | 21 | Amnioport placed at 22, infusions started at 22 | Transabdominal amnioport | Average 352 | 34+6 | Not reported | Not reported |
Table 4.
Summary of Systematic Review Data on Neonatal Demographic Characteristics and Outcomes
| Patient | Reference, year | Indication | Infant sex | Respiratory status at birth | Birth weight (g) | Apgar score at 1 min | Apgar score at5 min | Infant birth complications | Postnatal survival | Dialysis | Transplant | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Fisk et al,181991 | LUTO | NR | Clinical pulmonary hypoplasia, ventilated | NR | NR | NR | Respiratory compromise | Death at 8 h | No | NR | NR |
| 2 | Hansmann et al,191991 | LUTO | NR | NR | 3700 | NR | NR | Renal failure | Survived neonatal period | Yes | NR | NR |
| 3 | Cameron et al,201994 | RA | M | Initial PPV with bag mask, at 30 min of age intubated and ventilated for respiratory distress, extubated to room air at day 1 of life | 1965 | 4 | 8 | Respiratory compromise, chorioamnionitis at delivery requiring 5 d of antibiotic therapy | Palliative care at 23 wk, neonatal death | Tenckhoff peritoneal dialysis , 50 mL hourly cycles heparinized dialysis solution, day 4 leakage and removal, reinstituted on day 8, then leakage and reinstituted on day 9, creatinine increased, dialysis discontinued with parent consent | NR | Autopsy revealed absent kidneys and ureters, small bladder, discoid adrenal glands, patchy lung atelectasis and mild hypoplasia, extensive cavitating lesions in the brain with retrograde neuronal loss and gliosis in thalamus, intracranial abnormalities were not noted antenatally and suggest ischemic insult antenatally |
| 4 | Hsu et al,21 2007 | RA | NR | NR | 1100 | 0 | 0 | NR | No survival | No | NR | NR |
| 5 | Hsu et al,21 2007 | RA | NR | NR | 1160 | 1 | 0 | NR | No survival | No | NR | NR |
| 6 | Hsu et al,21 2007 | RA | NR | NR | 1450 | 1 | 0 | NR | No survival | No | NR | NR |
| 7 | Bienstock et al,2 2014 | RA | F | Spontaneous cry at birth, nasal continuous positive airway pressure, excellent respiratory effort | 1230 | 7 | 8 | RDS with appropriate lung volumes | Obstruction of PD catheter complicated by peritonitis, hospitalized at 3 wks of life for 10 wks. Alive at 9 mo, meeting GA milestones and growing appropriately | PD, started at 36 hours of life | Evaluate at 12-24 mo | Lost to follow-up |
| 8 | Haeri,22 2015 | LUTO and RA | M | Initial nasal continuous positive airway pressure, subsequently to room air | 2900 | 5 | 7 | Suprapubic catheter postnatally, dialysis started | Discharged at 2 mo. At time of publication, undergoing PD and doing well | PD | NR | Posterior urethral valves treated with ablation |
| 9 | Haeri,22 2015 | LUTO and RA | M | NR | 2405 | 7 | 7 | Dialysis started due to anuria | Day 30, developed necrotizing enterocolitis requiring bowel rest and 3 wk of TPN/IV antibiotics. Discharged on day of life 87 to home on automated PD with gastrostomy tube and on room air | PD | NR | Stable at time of publication |
| 10 | Whittaker & Leonardi,23 2016 | RA | NR | Intubation within 5 min of life | 1470 | 1 | 4 | Pulmonary compromise | 5 mo of peritoneal dialysis, then died secondary to peritonitis from mucous fistula perforation. | PD | NR | Died at 5 mo from peritonitis secondary to perforation of a mucous fistula |
| 11 | Polzin et al,1 2017 | LUTO | M | Ventilated and surfactant given, extubated on day of life 2 | NR | NR | NR | NR | Neonatal survival, 4 years old (32 wk at birth) with living related transplant at 22 months of age without rejection | NR | At 22 mo, living related transplant without rejection | NR |
| 12 | Polzin et al,1 2017 | LUTO | M | Ventilated, bronchial plug and barotrauma, extubated day of life 18 | NR | NR | NR | NR | Neonatal survival, now 4 years old with living related transplant at 2 years of age without rejection | Yes | At 2 y, living related transplant without rejection | NR |
| 13 | Polzin et al,1 2017 | LUTO | M | No support required | NR | NR | NR | NR | Neonatal survival, 4 years old (32 wks at birth) with living related transplant at 19 months of age without rejection | Yes | At 19 mo, living related transplant without rejection | NR |
| 14 | Polzin et al,1 2017 | LUTO | M | Lethal pulmonary hypoplasia | NR | NR | NR | NR | No neonatal survival | No | NR | NR |
| 15 | Polzin et al,1 2017 | LUTO | M | No support required | NR | NR | NR | NR | Bowel obstruction, renal failure, died day of life 3 | No | NR | NR |
| 16 | Polzin et al,1 2017 | RA | M | No support required | NR | NR | NR | NR | Renal failure, hypotension, died day of life 9 | No | NR | NR |
| 17 | Polzin et al,1 2017 | RA | M | No support required | NR | NR | NR | NR | Neonatal survival, died day of life 94 from peritonitis | No | NR | NR |
F = female; GFR = glomerular filtration rate; IV = intravenous; LUTO = lower urinary tract obstruction; M = male; NR = not reported; PD = peritoneal dialysis; PPV = positive pressure ventilation; RA = renal agenesis; RDS = respiratory distress syndrome; TPN = total parenteral nutrition.
Descriptive analysis using sample size, median, IQR, and ranges of important features are available in Table 1. The median age of women who underwent SAT and had a fetal diagnosis of LUTO and/or CRA was 31 years (N=9; IQR, 29-33.5 years). The median number of amnioinfusions was 7 (N=17; IQR, 4.5-21). The median GA at first amnioinfusion was 23 weeks and 4 days (N=17; IQR, 21-24.07). The median GA of patients at delivery was 32 weeks and 2 days (N=17; IQR, 30 weeks to 35 weeks and 6.5 days). All of the patients who underwent SAT had preterm deliveries. The median birth weight of newborns was 3.7 kg (N= 9; IQR, 2.7-3.7 kg). The median Apgar score at 1 minute was 2.5 (N=8; IQR, 1-6.5). The median Apgar score at 5 minutes was 5.5 (N=8; IQR, 0-7.75).
The number of LUTO cases, cesarean deliveries, male infants, intubations in the first 24 hours of life, 1-week survivals, 1-month survivals, peritoneal dialyses, renal transplants, patients lost to follow-up after successful PD before discharge, and patients who did not require intubation at time of birth with 1-month postnatal survival are reported in Table 2 using sample size and numbers and percentages. There were 9 of 17 LUTO cases, 4 of 7 cesarean deliveries, 11 of 12 male infants, 6 of 11 intubations within the first 24 hours of life, 11 of 17 cases of postnatal survival to 1 week, 10 of 11 cases of postnatal survival to 1 month, 9 of 16 cases of PDs to renal transplant, 2 of 9 cases on successful PD were lost to follow-up after initial hospital discharge, and 4 of 8 cases with 1-month neonatal survival and reported newborn pulmonary status did not require intubation at time of birth.
Discussion
To our knowledge, this is the first systematic review on SAT for fetuses with intrauterine renal failure or severe renal anomalies. This study comprehensively reviewed the evidence of SAT outcomes in cases of LUTO and CRA. The current literature includes descriptive data and inconsistently reported on SAT technique, prenatal features, and postnatal outcomes. The amalgamation of outcomes indicates that SAT in cases of oligohydramnios diagnosed at early gestation secondary to fetal LUTO or CRA can mitigate the risk of pulmonary compromise in newborns.
After the inception of amnioinfusion, diagnostic amnioinfusion became widely accepted to improve fetal ultrasound visibility in the setting of oligohydramnios. The adequate visualization rate improved from 51% to 77% after diagnostic amnioinfusion was used in unexplained second-trimester oligohydramnios.24 Amnioinfusion as a therapeutic intervention was first described as active intrapartum management of pregnancies complicated by low AFV in 1983 by Miyazaki and Taylor.25 Intrapartum amnioinfusion led to suppression of variable decelerations during labor and improved newborn outcomes. SAT can also prevent pulmonary hypoplasia in patients with severe oligohydramnios attributable to PPROM.2,26, 27, 28 More recently, in animal models and humans with obstructive uropathy, fetal LUTO decompression and restoration of AFV by amnioinfusion has been reported to decrease the degree of pulmonary hypoplasia and renal dysplasia in newborns.7,29,30 Cases of fetal LUTO and CRA managed with SAT are rare, and therefore, minimal literature and research are available to thoroughly describe its effectiveness in improving fetal outcomes.
The studies reviewed for the purposes of this systematic review were conducted globally, including in the United States, Taiwan, Germany, United Kingdom, and Canada. Given the observational nature of these studies and inconsistent reporting on data features, a comparative and quantitative statistical analysis was not feasible. Of the 17 total patients who underwent SAT, there were 9 cases of newborns who successfully started PD (3 with renal agenesis, 4 with LUTO, 2 with LUTO with reported renal agenesis). Of these 9 patients who underwent PD, 1 renal agenesis and 3 LUTO cases had successful renal transplant between the ages of 19 months and 3 years from a related donor without rejection. All 4 of these successful renal transplant cases took place in the United States after 2014. These cases may reflect medical practice advances specific to the region and time the studies were conducted, which likely contributed to the successful outcomes. A limitation of this study was the lack of consistent patient selection criteria that hindered our ability to perform a statistical outcomes analysis given the widely varying severities of disease. The philosophy and criteria guiding which patients were selected for SAT were not fully described in the studies, and therefore, we could not account for selection bias or potential confounding factors when reviewing survival outcomes.
Evidence suggests that SAT provides clinically important pulmonary palliation and reduces the severity of pulmonary hypoplasia at birth in cases of oligohydramnios. When discussed with patients, SAT should be presented as one therapeutic option on a continuum of management choices for fetuses with LUTO or CRA.2 Selection criteria are imperative to direct how we counsel patients with varying degrees of abnormal fetal renal imaging, urinalyses, or oligohydramnios or in cases of other failed fetal interventions. Our team consisting of specialists from maternal and fetal medicine, neonatology, pediatric nephrology, pediatric surgery, and transplant surgery suggests the use of a classification system (Table 5) that defines patient groups by fetal renal disease severity. This multidisciplinary classification system, which is a modified version of the proposed classification of fetal LUTO according to severity by Ruano et al15 in 2016, is useful when considering which patients would be appropriate for SAT. Fetal urinary biochemistry after 18 weeks’ GA and ultrasound characteristics of fetal kidneys can be used to best estimate the fetal renal function.31, 32, 33, 34, 35 The primary imaging modality for prenatal diagnosis of the fetal urogenital tract is fetal ultrasonography. Additionally, magnetic resonance imaging can provide further information in the case of inconclusive findings by ultrasonography.17 Based on our observations, the postnatal outcomes of a fetal intervention are dependent on the severity of renal disease present prior to the intervention and can be categorized into 1 of 4 groups as described in Table 5.
Table 5.
Proposed Classification for Fetal Renal Disease in Lower Urinary Tract Obstruction According to Severity
| Variable | Stage I | Stage II | Stage III | Stage IV |
|---|---|---|---|---|
| Description | Mild lower urinary tract obstruction | Severe lower urinary tract obstruction, with prenatal findings suggestive of preserved fetal renal function | Severe lower urinary tract obstruction, with prenatal findings suggestive of fetal abnormal renal function | Lower urinary tract obstruction with intrauterine fetal renal failure |
| Amount of amniotic fluid | Normal | Oligohydramnios or anhydramnios | Oligohydramnios, but usually anhydramnios | Anhydramnios |
| Echogenicity of fetal kidneys | Normal | Hyperechogenic | Hyperechogenic | Hyperechogenic or absent |
| Renal cortical cysts | Absent | Absent | Can be present | Can be present |
| Renal dysplasia | Absent | Absent | Can be present | Can be present |
| Fetal urinary biochemistry | Favorable | Favorable within 3 consecutive evaluations | Not favorable after 3 consecutive evaluations | Not favorable after 3 consecutive evaluations |
| Fetal intervention | Not indicated | Indicated to prevent pulmonary hypoplasia and severe renal impairment | May be indicated to prevent pulmonary hypoplasia but not postnatal renal impairment; further studies are necessary | May be indicated to prevent pulmonary hypoplasia but not postnatal renal impairment; further studies are necessary |
Data from references 13 through 15.
Further clinical trials to evaluate feasibility, safety, and efficacy of SAT in mothers with fetuses diagnosed as having severe LUTO (stage III and IV) and CRA need to be completed in order to better determine the advantages and risks of SAT.6,12,14,15 Optimal procedure protocol, possible prognostic factors, and detailed quality-of-life outcomes should be published before SAT can be adopted as routine practice. To further evaluate SAT in fetuses with BRA, a multicenter study is under way at Mayo Clinic in Rochester, Minnesota, Stanford University in Pal Alto, California and Johns Hopkins Hospital in Baltimore, Maryland, supported by the North American Fetal Therapy Network (ClinicalTrials.gov Identifiers: NCT03723564 and NCT03101891). The study was designed after an extensive multidisciplinary national ethics conference, and the main goals are to assess the safety, feasibility, and efficacy of SAT to treat BRA diagnosed before 26 weeks’ GA.
Our data did not support a correlation between starting SAT at earlier GA and improved postnatal outcomes, likely because the earlier the onset of severe oligohydramnios, the greater the severity of renal disease and thus worse outcomes. Lung development occurs between 17 and 23 weeks’ GA, yet whether SAT should begin immediately after the diagnosis and cease after the canalicular phase of lung development ends has not been determined.36 There have been hypotheses reported in the past, such as Fisk et al18 suggesting 17 to 23 weeks as the appropriate GA, as supported by animal models.7,30 Fisk et al37 have also suggested that an increase in amniotic pressure may implicate complications such as PPROM and thus advocate for pressure monitoring during amnioinfusion. In 2005, Stefos et al38 noted that in hopes of necessitating fewer percutaneous procedures, physicians tend to overdistend the amnion with each therapy. Although there have been successful reports of AFV restoration with serial percutaneous infusion, Hsu et al21 described an amnioport technique, similar to the Tchirikov modification published in 2010,39 that decreased the risk of PPROM overall. With an amnioport placement, the amnion is not punctured with each subsequent amnioinfusion procedure. This technique provides a more gradual increase in volume that could allow pregnancy to reach later GA and decrease PPROM risk.1 Hsu et al also determined that patients who cannot preserve AFV after the first amnioinfusion are not suitable for SAT. In 2000, Freedman et al40 reported that creatinine may be used as an appropriate marker of renal injury and possibly serve as a strong prognostic indicator. Fetuses with a creatinine nadir more than 1.0 mg/dL progressed to end-stage renal failure, while 75% of a creatinine level of less than 0.8 mg/dL maintained normal renal function for 54 months. Research efforts toward development of urinary markers to assist in proper selection of fetuses with salvageable renal function must be pursued.
SAT is generally accepted as a fetal intervention to improve short-term outcomes by palliating the most common cause of death in severe oligohydramnios, pulmonary compromise.2 However, before offering this short-term success of decreased pulmonary hypoplasia risk, it is necessary to assess the long-term outcomes in cases with complicated renal profiles so that informed counseling is possible. Beneficence should always remain a guiding principle for physicians. Even though scientific advancement requires stepping into experimental territory and pushing the envelope of current available therapies, it should never be done without thorough, evidence-based, informed counseling and research to support it. Even with the best of intentions, medicine remains imperfect, and newly designed therapies can harm patients. Patients may not fully understand the implications of caring for children with renal failure requiring PD and renal transplant, and this is especially difficult to fully grasp if evidence on long-term outcomes is unavailable.
Before the advent of SAT, there was no obligation to begin or continue a fetal intervention because the diagnosis of many cases of LUTO and CRA were incompatible with life. In other words, nontreatment did not pose an ethical dilemma.41 More recently, given the improved pulmonary outcomes in newborns of mothers who undergo SAT published in the literature and presented in the media, fetuses with LUTO or CRA and poor renal profiles have begun to be viewed similarly to newborns in whom terminal renal failure develops within the first few weeks of life. Some argue for the rights of an unborn child who will need lifelong management, such as renal transplant immunosuppression and high medical costs, as implicated by the choices of their parents to pursue SAT. Additionally, the US Food and Drug Administration approval for SAT is lacking, and there are risks associated with placement of a foreign body in a woman’s abdomen with this technique. Extensive counseling by multidisciplinary teams should be required by institutions that choose to offer SAT. These teams should consist, at minimum, of specialists in maternal and fetal medicine, neonatal intensive care medicine, pediatric nephrology, transplant surgery, pediatric surgery, palliative care, social work, and ethical oversight.
The amnioinfusion procedure itself is associated with complications such as chorioamnionitis, placental abruption, and a predisposition to preterm delivery for the mother.20,42 Dialysis is notably challenging in newborns and therefore typically is only offered as a bridge to renal transplant. A number of investigators have reported success with long-term PD,43,44 although there are substantial hurdles including growth failure, developmental delay, and infections that complicate survival to renal transplant and offer a low quality of life for what may be the only few years of the infant’s life. Other complications of PD can include poor drainage, leakage, or perforation of intra-abdominal organs.45 Case series have estimated short-term survival on PD for 1 to 5 years ranging from 52% to 89%.46, 47, 48 In a Canadian cohort of infants with renal replacement therapy initiated before age 2 years, 65.5% underwent successful renal transplant with a mean age of 2.7 years and 26.4% died; 95.7% of those deaths occurred in patients who did not survive on PD to renal transplant.46 In this 2012 study, mortality was highest in neonates with renal replacement initiated before 3 months of age, which may be consistent with a higher degree of renal disease in this cohort.
Overall, this fetal intervention involves multiple invasive procedures to mothers and leads inevitably to newborns who will need PD and renal transplant in the future. Choosing to pursue SAT may be associated with pain, psychological trauma, and high costs with no guarantee of quality of life. This intervention presents a challenging course that may not be desired by many parents or families.
The inception of SAT and its association with short-term success has created hope that with less pulmonary morbidity overshadowing, care teams can focus on genitourinary problems and improve outcomes even further. There have been multiple cases of newborns without pulmonary hypoplasia who have been successfully placed on PD with an ultimate goal of renal transplant.2 In 1978, there was a 76% overall patient survival rate to 4 years of age in a cohort of 21 cases in which dialysis began in the neonatal period with transplantation occurring at a mean age of 3 years.49, 50, 51 Of note, caution should be utilized when extrapolating outcomes data of neonates with successful placement on PD. The majority of studies include full-term neonates rather than neonates born at a preterm gestation, as would be the more likely patient population in the setting of SAT. Children with successful renal transplants after PD are at risk of growth complications and immunosuppressant-related infections. Fetuses with other anomalies in addition to LUTO or CRA, such as ureteric agenesis or an underdeveloped bladder, present even more complex urologic problems for a transplanted kidney.20 In renal agenesis, the absence of a bladder or ureters necessitates an eventual nonbladder conduit for drainage of urine.52,53
The patient case report in this article highlights the necessary multidisciplinary team and substantial resources required to care for an infant with BRA after antenatal SAT to palliate pulmonary hypoplasia. The infant in the case report unfortunately endured multiple complications during his 7-month hospital stay, and due to his insufficient growth during PD, he was unable to receive a kidney transplant. The possibility of such outcomes necessitates thorough and informed patient consent from the family choosing to undergo fetal SAT.
Conclusion
Evidence suggests that SAT may provide regenerative therapy resulting in pulmonary palliation and reduce the risk of newborn mortality due to pulmonary compromise. However, before SAT can be adopted into widespread practice, further research is needed, and SAT should only be undertaken in the context of a clinical trial to inform treatment and counseling guidelines. The present study proposes a multidisciplinary prenatal management classification system based on fetal disease severity that can be used as a guideline to inform patient selection in clinical trials evaluating SAT for mothers of fetuses with LUTO. With strong evidence lacking for this fetal intervention, a clinical trial is justified to further assess the long-term outcomes of this intervention in cases of severe fetal renal dysfunction.t
Footnotes
Potential Competing Interests: The authors report no competing interests.
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Polzin W.J., Lim F.Y., Habli M. Use of an amnioport to maintain amniotic fluid volume in fetuses with oligohydramnios secondary to lower urinary tract obstruction or fetal renal anomalies [published correction appears in Fetal Diagn Ther. 2017;42(1):80] Fetal Diagn Ther. 2017;41(1):51–57. doi: 10.1159/000445946. [DOI] [PubMed] [Google Scholar]
- 2.Bienstock J.L., Birsner M.L., Coleman F., Hueppchen N.A. Successful in utero intervention for bilateral renal agenesis. Obstet Gynecol. 2014;124(2, pt 2, suppl 1):413–415. doi: 10.1097/AOG.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 3.Harding R., Hooper S.B., Dickson K.A. A mechanism leading to reduced lung expansion and lung hypoplasia in fetal sheep during oligohydramnios. Am J Obstet Gynecol. 1990;163(6, pt 1):1904–1913. doi: 10.1016/0002-9378(90)90772-y. [DOI] [PubMed] [Google Scholar]
- 4.Lissauer D., Morris R.K., Kilby M.D. Fetal lower urinary tract obstruction. Semin Fetal Neonatal Med. 2007;12(6):464–470. doi: 10.1016/j.siny.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Denny E., Quinlan-Jones E., Bibila S., Kilby M. The experience of pregnant women with a diagnosis of fetal lower urinary tract obstruction (LUTO) Midwifery. 2014;30(6):636–642. doi: 10.1016/j.midw.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Ruano R., Sananes N., Sangi-Haghpeykar H. Fetal intervention for severe lower urinary tract obstruction: a multicenter case-control study comparing fetal cystoscopy with vesicoamniotic shunting. Ultrasound Obstet Gynecol. 2015;45(4):452–458. doi: 10.1002/uog.14652. [DOI] [PubMed] [Google Scholar]
- 7.Harrison M.R., Ross N., Noall R., de Lorimier A.A. Correction of congenital hydronephrosis in utero. I. The model: fetal urethral obstruction produces hydronephrosis and pulmonary hypoplasia in fetal lambs. J Pediatr Surg. 1983;18(3):247–256. doi: 10.1016/s0022-3468(83)80094-3. [DOI] [PubMed] [Google Scholar]
- 8.Ruano R., Yoshizaki C.T., Giron A.M., Srougi M., Zugaib M. Cystoscopic placement of transurethral stent in a fetus with urethral stenosis. Ultrasound Obstet Gynecol. 2014;44(2):238–240. doi: 10.1002/uog.13293. [DOI] [PubMed] [Google Scholar]
- 9.Morris R.K., Ruano R., Kilby M.D. Effectiveness of fetal cystoscopy as a diagnostic and therapeutic intervention for lower urinary tract obstruction: a systematic review. Ultrasound Obstet Gynecol. 2011;37(6):629–637. doi: 10.1002/uog.8981. [DOI] [PubMed] [Google Scholar]
- 10.Ruano R., Yoshisaki C.T., Salustiano E.M., Giron A.M., Srougi M., Zugaib M. Early fetal cystoscopy for first-trimester severe megacystis. Ultrasound Obstet Gynecol. 2011;37(6):696–701. doi: 10.1002/uog.8963. [DOI] [PubMed] [Google Scholar]
- 11.Ruano R., Duarte S., Bunduki V., Giron A.M., Srougi M., Zugaib M. Fetal cystoscopy for severe lower urinary tract obstruction—initial experience of a single center. Prenat Diagn. 2010;30(1):30–39. doi: 10.1002/pd.2418. [DOI] [PubMed] [Google Scholar]
- 12.Enninga E.A., Ruano R. Fetal surgery for lower urinary tract obstruction: the importance of staging prior to intervention. Minerva Pediatr. 2018;70(3):263–269. doi: 10.23736/S0026-4946.17.05105-2. [DOI] [PubMed] [Google Scholar]
- 13.Ruano R., Dunn T., Braun M.C., Angelo J.R., Safdar A. Lower urinary tract obstruction: fetal intervention based on prenatal staging. Pediatr Nephrol. 2017;32(10):1871–1878. doi: 10.1007/s00467-017-3593-8. [DOI] [PubMed] [Google Scholar]
- 14.Ruano R., Safdar A., Au J. Defining and predicting 'intrauterine fetal renal failure' in congenital lower urinary tract obstruction. Pediatr Nephrol. 2016;31(4):605–612. doi: 10.1007/s00467-015-3246-8. [DOI] [PubMed] [Google Scholar]
- 15.Ruano R., Sananes N., Wilson C. Fetal lower urinary tract obstruction: proposal for standardized multidisciplinary prenatal management based on disease severity. Ultrasound Obstet Gynecol. 2016;48(4):476–482. doi: 10.1002/uog.15844. [DOI] [PubMed] [Google Scholar]
- 16.Manning F.A., Harrison M.R., Rodeck C. Catheter shunts for fetal hydronephrosis and hydrocephalus: report of the International Fetal Surgery Registry. N Engl J Med. 1986;315(5):336–340. doi: 10.1056/NEJM198607313150532. [DOI] [PubMed] [Google Scholar]
- 17.Huber C., Shazly S.A., Blumenfeld Y.J., Jelin E., Ruano R. Update on the prenatal diagnosis and outcomes of fetal bilateral renal agenesis. Obstet Gynecol Surv. 2019;74(5):298–302. doi: 10.1097/OGX.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 18.Fisk N.M., Ronderos-Dumit D., Soliani A., Nicolini U., Vaughan J., Rodeck C.H. Diagnostic and therapeutic transabdominal amnioinfusion in oligohydramnios. Obstet Gynecol. 1991;78(2):270–278. [PubMed] [Google Scholar]
- 19.Hansmann M., Chatterjee M.S., Schuh S., Gembruch U., Bald R. Multiple antepartum amnioinfusions in selected cases of oligohydramnios. J Reprod Med. 1991;36(12):847–851. [PubMed] [Google Scholar]
- 20.Cameron D., Lupton B.A., Farquharson D., Hiruki T. Amnioinfusions in renal agenesis. Obstet Gynecol. 1994;83(5, pt 2):872–876. [PubMed] [Google Scholar]
- 21.Hsu T.L., Hsu T.Y., Tsai C.C., Ou C.Y. The experience of amnioinfusion for oligohydramnios during the early second trimester. Taiwan J Obstet Gynecol. 2007;46(4):395–398. doi: 10.1016/S1028-4559(08)60009-1. [DOI] [PubMed] [Google Scholar]
- 22.Haeri S. Fetal lower urinary tract obstruction (LUTO): a practical review for providers. Matern Health Neonatol Perinatol. 2015;1:26. doi: 10.1186/s40748-015-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whittaker N., Leonardi M. Five-month survival of neonate after serial amnioinfusions for fetal bilateral renal agenesis [abstract] Obstet Gynecol. 2016;127(suppl 1):39S. [Google Scholar]
- 24.Pryde P.G., Hallak M., Lauria M.R. Severe oligohydramnios with intact membranes: an indication for diagnostic amnioinfusion. Fetal Diagn Ther. 2000;15(1):46–49. doi: 10.1159/000020974. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki F.S., Taylor N.A. Saline amnioinfusion for relief of variable or prolonged decelerations: a preliminary report. Am J Obstet Gynecol. 1983;146(6):670–678. doi: 10.1016/0002-9378(83)91010-4. [DOI] [PubMed] [Google Scholar]
- 26.Ogunyemi D., Thompson W. A case controlled study of serial transabdominal amnioinfusions in the management of second trimester oligohydramnios due to premature rupture of membranes. Eur J Obstet Gynecol Reprod Biol. 2002;102(2):167–172. doi: 10.1016/s0301-2115(01)00612-1. [DOI] [PubMed] [Google Scholar]
- 27.Garzetti G.G., Ciavattini A., De Cristofaro F., La Marca N., Arduini D. Prophylactic transabdominal amnioinfusion in oligohydramnios for preterm premature rupture of membranes: increase of amniotic fluid index during latency period. Gynecol Obstet Invest. 1997;44(4):249–254. doi: 10.1159/000291538. [DOI] [PubMed] [Google Scholar]
- 28.Tranquilli A.L., Giannubilo S.R., Bezzeccheri V., Scagnoli C. Transabdominal amnioinfusion in preterm premature rupture of membranes: a randomised controlled trial. BJOG. 2005;112(6):759–763. doi: 10.1111/j.1471-0528.2005.00544.x. [DOI] [PubMed] [Google Scholar]
- 29.Crombleholme T.M., Harrison M.R., Golbus M.S. Fetal intervention in obstructive uropathy: prognostic indicators and efficacy of intervention. Am J Obstet Gynecol. 1990;162(5):1239–1244. doi: 10.1016/0002-9378(90)90026-4. [DOI] [PubMed] [Google Scholar]
- 30.Harrison M.R., Nakayama D.K., Noall R., de Lorimier A.A. Correction of congenital hydronephrosis in utero II. Decompression reverses the effects of obstruction on the fetal lung and urinary tract. J Pediatr Surg. 1982;17(6):965–974. doi: 10.1016/s0022-3468(82)80476-4. [DOI] [PubMed] [Google Scholar]
- 31.Morris R.K., Quinlan-Jones E., Kilby M.D., Khan K.S. Systematic review of accuracy of fetal urine analysis to predict poor postnatal renal function in cases of congenital urinary tract obstruction. Prenat Diagn. 2007;27(10):900–911. doi: 10.1002/pd.1810. [DOI] [PubMed] [Google Scholar]
- 32.Nicolini U., Spelzini F. Invasive assessment of fetal renal abnormalities: urinalysis, fetal blood sampling and biopsy. Prenat Diagn. 2001;21(11):964–969. doi: 10.1002/pd.212. [DOI] [PubMed] [Google Scholar]
- 33.Lipitz S., Ryan G., Samuell C. Fetal urine analysis for the assessment of renal function in obstructive uropathy. Am J Obstet Gynecol. 1993;168(1, pt 1):174–179. doi: 10.1016/s0002-9378(12)90909-6. [DOI] [PubMed] [Google Scholar]
- 34.Morris R.K., Malin G.L., Khan K.S., Kilby M.D. Antenatal ultrasound to predict postnatal renal function in congenital lower urinary tract obstruction: systematic review of test accuracy. BJOG. 2009;116(10):1290–1299. doi: 10.1111/j.1471-0528.2009.02194.x. [DOI] [PubMed] [Google Scholar]
- 35.Dreux S., Rosenblatt J., Moussy-Durandy A. Urine biochemistry to predict long-term outcomes in fetuses with posterior urethral valves. Prenat Diagn. 2018;38(12):964–970. doi: 10.1002/pd.5359. [DOI] [PubMed] [Google Scholar]
- 36.Wigglesworth J.S., Desai R. Use of DNA estimation for growth assessment in normal and hypoplastic fetal lungs. Arch Dis Child. 1981;56(8):601–605. doi: 10.1136/adc.56.8.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisk N.M., Tannirandorn Y., Nicolini U., Talbert D.G., Rodeck C.H. Amniotic pressure in disorders of amniotic fluid volume. Obstet Gynecol. 1990;76(2):210–214. [PubMed] [Google Scholar]
- 38.Stefos T., Staikos I., Plachouras N., Dousias V. Serial saline amnioinfusion from 16th week of gestation resulted in successful outcome of pregnancy: report of two cases. Eur J Obstet Gynecol Reprod Biol. 2005;122(2):250–251. doi: 10.1016/j.ejogrb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Tchirikov M., Steetskamp J., Hohmann M., Koelbl H. Long-term amnioinfusion through a subcutaneously implanted amniotic fluid replacement port system for treatment of PPROM in humans. Eur J Obstet Gynecol Reprod Biol. 2010;152(1):30–33. doi: 10.1016/j.ejogrb.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Freedman A.L., Johnson M.P., Gonzalez R. Fetal therapy for obstructive uropathy: past, present…..future? Pediatr Nephrol. 2000;14(2):167–176. doi: 10.1007/s004670050035. [DOI] [PubMed] [Google Scholar]
- 41.Paris J.J., Crone R.K., Reardon F.E. Ethical context for physician refusal of requested treatment. J Perinatol. 1991;11(3):273–275. [PubMed] [Google Scholar]
- 42.Tan L.K., Kumar S., Jolly M., Gleeson C., Johnson P., Fisk N.M. Test amnioinfusion to determine suitability for serial therapeutic amnioinfusion in midtrimester premature rupture of membranes. Fetal Diagn Ther. 2003;18(3):183–189. doi: 10.1159/000069375. [DOI] [PubMed] [Google Scholar]
- 43.Warady B.A., Kriley M., Lovell H., Farrell S.E., Hellerstein S. Growth and development of infants with end-stage renal disease receiving long-term peritoneal dialysis. J Pediatr. 1988;112(5):714–719. doi: 10.1016/s0022-3476(88)80687-5. [DOI] [PubMed] [Google Scholar]
- 44.Kohaut E.C., Whelchel J., Waldo F.B., Diethelm A.G. Aggressive therapy of infants with renal failure. Pediatr Nephrol. 1987;1(2):150–153. doi: 10.1007/BF00849286. [DOI] [PubMed] [Google Scholar]
- 45.Steele B.T., Vigneux A., Blatz S., Flavin M., Paes B. Acute peritoneal dialysis in infants weighing less than 1500 g. J Pediatr. 1987;110(1):126–129. doi: 10.1016/s0022-3476(87)80306-2. [DOI] [PubMed] [Google Scholar]
- 46.Alexander R.T., Foster B.J., Tonelli M.A. Pediatric Renal Outcomes Group Canada. Survival and transplantation outcomes of children less than 2 years of age with end-stage renal disease. Pediatr Nephrol. 2012;27(10):1975–1983. doi: 10.1007/s00467-012-2195-8. [DOI] [PubMed] [Google Scholar]
- 47.Genc G., Bicakci U., Gunaydin M. Temporary peritoneal dialysis in newborns and children: a single-center experience over five years. Ren Fail. 2012;34(9):1058–1061. doi: 10.3109/0886022X.2012.715574. [DOI] [PubMed] [Google Scholar]
- 48.Vidal E., Edefonti A., Murer L. Italian Registry of Paediatric Chronic Dialysis. Peritoneal dialysis in infants: the experience of the Italian Registry of Paediatric Chronic Dialysis. Nephrol Dial Transplant. 2012;27(1):388–395. doi: 10.1093/ndt/gfr322. [DOI] [PubMed] [Google Scholar]
- 49.Lantos J.D., Warady B.A. The evolving ethics of infant dialysis. Pediatr Nephrol. 2013;28(10):1943–1947. doi: 10.1007/s00467-012-2351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ledermann S.E., Scanes M.E., Fernando O.N., Duffy P.G., Madden S.J., Trompeter R.S. Long-term outcome of peritoneal dialysis in infants. J Pediatr. 2000;136(1):24–29. doi: 10.1016/s0022-3476(00)90044-1. [DOI] [PubMed] [Google Scholar]
- 51.Carey W.A., Talley L.I., Sehring S.A., Jaskula J.M., Mathias R.S. Outcomes of dialysis initiated during the neonatal period for treatment of end-stage renal disease: a North American Pediatric Renal Trials and Collaborative Studies special analysis. Pediatrics. 2007;119(2):e468–e473. doi: 10.1542/peds.2006-1754. [DOI] [PubMed] [Google Scholar]
- 52.Askenazi D., Ingram D., White S. Smaller circuits for smaller patients: improving renal support therapy with Aquadex™. Pediatr Nephrol. 2016;31(5):853–860. doi: 10.1007/s00467-015-3259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishimi S., Ishikawa K., Sasaki M., Furukawa H., Takada A., Chida S. Ability of a novel system for neonatal extracorporeal renal replacement therapy with an ultra-small volume circuit to remove solutes in vitro. Pediatr Nephrol. 2016;31(3):493–500. doi: 10.1007/s00467-015-3233-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.