Abstract
Background
The benefit of integrated care management was unknown in frail atrial fibrillation (AF) patients. This study evaluated whether compliance with the atrial fibrillation Better Care (ABC) pathway for integrated care management would improve clinical outcomes in frail AF patients.
Methods
From the Korea National Health Insurance Service database, 262,987 nonvalvular AF patients were enrolled between 1 January 2005 and 31 December 2015. For each patient, the Hospital Frailty Risk Score and category were calculated retrospectively using all available ICD‐10 diagnostic codes. Patients were divided into three frailty‐based risk categories: low (<5 points, n = 221,542), intermediate (5‐15 points, n = 37,341), and high risk (>15 points, n = 4,104).
Results
Over a mean follow‐up of 5.9 (interquartile range 3.2, 9.4) years, in high frailty risk patients, the ABC group had lower rates of all‐cause death (6.5 vs 17.5 per 100 person‐years, P < .001; hazard ratio [HR] 0.74; 95% confidence interval [CI] 0.56‐0.97) but was nonsignificant for the composite outcome (10.5 vs 26.0 per 100 person‐years, P = .101; HR 0.79; 95% CI 0.59‐1.05) compared with the Non‐ABC group. When the three frailty categories were compared, the greatest benefit on mortality was seen in the high frailty group (pint < 0.001), but for the composite outcome, there was no statistical interaction for the three frailty categories (pint = 0.063).
Conclusions
Compliance with the simple ABC pathway is associated with improved outcomes in AF patients with high frailty risk. Given the high healthcare burden associated with frail AF patients, integrated AF management should be implemented to improve outcomes in these patients.
Keywords: atrial fibrillation, frailty, integrated management, mortality
Compliance with the simple ABC pathway is associated with improved outcomes in AF patients with high frailty risk.
![]()
1. INTRODUCTION
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia among elderly individuals, 1 , 2 , 3 and it has enormous socioeconomic implications given the risk of mortality and morbidity resulting from stroke, congestive heart failure, dementia, and impaired quality of life. 1 , 2 , 3 , 4 , 5 Frailty is also associated with more adverse clinical outcomes in elderly individuals admitted to the hospital. AF may be a marker of frailty in elderly individuals and may be related to a loss of independence in performing activities of daily living. 6 AF could worsen the state of frailty, and patients with AF could have 4‐fold increased odds of being classified as frail, compared with patients without AF. 7
Recent trials involving AF 8 , 9 revealed a high (4.6% per year) rate of all‐cause death in patients with AF, with only one out of 10 deaths related to stroke and approximately five or six out of 10 deaths related to cardiovascular causes. Therefore, a more integrated and holistic approach beyond anticoagulation therapy for patients with AF has been advocated in guidelines to reduce mortality and adverse outcomes in AF. 10 , 11 , 12 One way is to streamline management approaches that would be applicable across the entire AF patient pathway, starting with primary care and linking with secondary care (even for cardiologists and noncardiologists), and be understandable for patients with AF. The ABC (atrial fibrillation better care) pathway has been proposed as a simple, integrated approach. 13 This pathway streamlines the care pathway as follows: “A” Avoid stroke with Anticoagulation; “B” Better symptom management (ie, patient‐centered, symptom‐directed decisions on rate vs rhythm control); and “C” Cardiovascular and comorbidity management, including lifestyle factors. 13
Application of the simple ABC pathway was associated with a lower risk of adverse outcomes in patients with AF in a post hoc analysis of a clinical trial cohort as well as other AF cohorts. 14 , 15 , 16 However, the population‐based benefit on clinical outcomes owing to a compliance with the ABC pathway has not been previously evaluated in patients with AF at a high frailty risk. Give the close association between AF and frailty, this study aimed to evaluate whether compliance with the ABC pathway would improve population‐based clinical outcomes in patients with AF belonging to different frailty risk categories, using a nationwide AF cohort.
2. METHODS
2.1. Data source
We performed a retrospective analysis of data from the national health claims database (NHIS‐2016‐4‐026) established by the National Health Insurance Service (NHIS) of Korea. 1 , 2 , 3 The NHIS is a single insurer managed by the Korean government, and the majority (97.1%) of the Korean population are mandatory subscribers, with the remaining 3% of the population being medical aid subjects. Since 2006, information regarding Medical Aid beneficiaries has been incorporated into a single NHIS database. Therefore, the data extracted from the NHIS claims database are based on the entire Korean population. The NHIS claims database includes diagnoses, procedures, biochemical test results, prescription records, and demographic information. The database is open to researchers whose study protocols are approved by official review committees. This study was approved by the Institutional Review Board of Yonsei University Health System (4‐2016‐0179).
2.2. Study cohort
From the Korean NHIS database, a total of 955 111 patients with prevalent AF who were aged 18 years or older were identified from 1 January 2005 to 31 December 2015. Patients with valvular AF, such as those with any mechanical or bioprosthetic heart valves, mitral valve repair, or rheumatic mitral stenosis (n = 59 189); those without baseline health check‐up data up to 1 year before enrolment (n = 571 585); and those who had ischemic stroke (n = 61 350) were excluded. Finally, a total of 262,987 patients with nonvalvular AF were enrolled in the study to evaluate the impact of the ABC pathway on the long‐term clinical outcomes of these patients (Figure 1).
FIGURE 1.
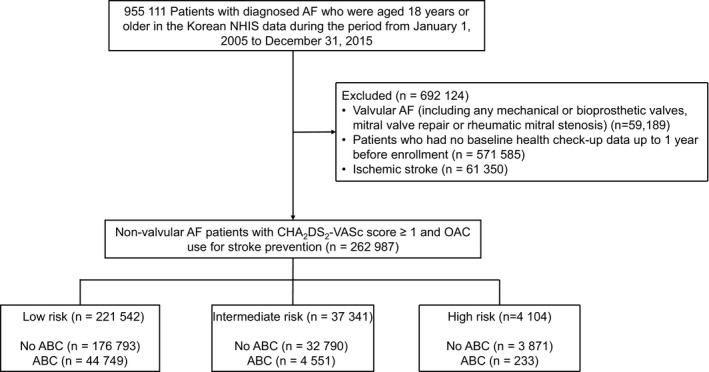
Flowchart of study population enrolment and analyses. AF, atrial fibrillation; OAC, oral anticoagulant; NHIS, National Health Insurance Service
For each patient, the Hospital Frailty Risk Score was calculated retrospectively using all available ICD‐10 diagnostic codes that were documented for the particular admission, as recommended by Gilbert et al. 17 The score is an aggregate of 109 ICD‐10 diagnostic codes found to be associated with frailty‐based risk (Supplementary Table S1). Each diagnostic code was assigned a specific value proportional to how strongly it predicted frailty. According to the aggregate score, patients were divided into three frailty‐based risk categories: low risk (<5 points, n = 221 542), intermediate risk (5‐15 points, n = 37 341), and high risk (>15 points, n = 4104). 17
2.3. Definition of the ABC pathway‐compliant group
The integrated care group (ABC group) was defined according to the criteria summarized in Supplementary Figure S1. “A” was defined as the use of oral anticoagulants, in accordance with guidelines, with high adherence (prescription covering ≥80% of days); “B” was defined in relation to visits requiring medical contact with outpatient clinics (<5 visits per year during the follow‐up period); “C” was defined as optimal management of the main cardiovascular comorbidities (hypertension, heart failure, myocardial infarction, peripheral artery disease, stroke/transient ischemic attack [TIA], diabetes mellitus, and obesity). Optimal management of hypertension was defined as baseline blood pressure values <140/90 mmHg. For obesity, body mass index less than 30 kg/m2 was considered optimal management. For other comorbidities, appropriate use of cardiovascular prevention medications according to current guidelines was considered optimal management. Patients who fulfilled all criteria were defined as the “ABC” group, and those who did not fulfill all criteria were defined as the “non‐ABC” group.
2.4. Comorbidities and endpoints
AF was identified using International Classification of Disease, Tenth Revision (ICD‐10) codes: I48. To ensure accuracy, the diagnosis was established based on more than one or two outpatient records of ICD‐10 codes in the database. The diagnosis of AF has previously been validated in the NHIS database, with a positive predictive value of 94.1%. 1 , 4 , 18 , 19 Comorbidities were identified from ICD‐10 codes and prescription, as in previous studies (Supplementary Table S2). 18
The primary clinical outcomes of this study were all‐cause death, ischemic stroke, heart failure admission, acute myocardial infarction, major bleeding, and a composite outcome of these five outcomes. Any diagnosis of ischemic stroke in the emergency room or inpatient clinic with concomitant brain imaging studies, including computed tomography or magnetic resonance imaging, was defined as incident ischemic stroke. The accuracy of the diagnosis of ischemic stroke in the NHIS claims data has been previously validated. 4 The other definitions of clinical outcomes are presented in Supplementary Table S2. Patients were followed from the index date until the study outcomes occurred or up to the end of follow‐up, whichever occurred first.
2.5. Statistical analysis
Categorical data are reported as proportions, while continuous data are reported as medians with interquartile ranges (IQRs). The categorical variables were compared using Fisher's exact test or Pearson chi‐square test, and continuous variables were compared using Student's t test. The main analyses compared the clinical outcomes between the ABC (ie, integrated care) and non‐ABC groups. Incidence rates were defined as events per 100 person‐years at risk but expressed as annualized percentage rates for comprehensiveness. The relationships between the total number of ABC criteria fulfilled and the clinical outcomes were also investigated.
The cumulative incidences of adverse outcomes were presented using Kaplan–Meier curves and compared across the groups using the log‐rank test. Using Cox proportional hazard regression model, the hazard ratios (HRs) for adverse outcomes according to the use of integrated care (ABC) were analyzed. Clinical variables including age, gender, heart failure, hypertension, diabetes mellitus, previous myocardial infarction, peripheral artery disease, economic status, CHA2DS2‐VASc, and HAS‐BLED score were adjusted for HR. P‐values < 05 were considered significant. Statistical analyses were conducted using SAS version 9.3 (SAS Institute) and R version 3.3.2 (The R Foundation, www.R‐project.org).
3. RESULTS
3.1. Baseline characteristics
Comparisons between the ABC and non‐ABC groups are presented in Supplementary Table S3. Compared with the non‐ABC group, patients in the ABC group were less likely to be female (39.4% vs 38.6%, P = .001) and had a lower mean age (65.0 [IQR 56.0, 72.0] vs 50.0 [IQR 41.0, 58.0], P < .001). Compared with patients in the non‐ABC group, those in the ABC group had a lower prevalence of comorbidities, such as hypertension, heart failure, diabetes mellitus, stroke/TIA, hemorrhage stroke, vascular disease, chronic kidney disease, and dyslipidemia. In addition, patients in the ABC group had a lower mean CHA2DS2‐VASc score than that of patients in the non‐ABC group.
The baseline characteristics between patients with AF with different hospital frailty risk categories who were compliant and noncompliant with the ABC pathway are compared in Table 1. Multivariable analysis showed that in patients with high frailty risk, factors including age ≥65 years and female gender were independently associated with a likelihood of being compliant with the ABC pathway. In contrast, high CHA2DS2‐VASc score, hypertension, liver disease, or history of intracranial bleeding were related to noncompliance with the ABC pathway. In patients with intermediate and low frailty risk, heart failure, and vascular disease were also related to compliance with the ABC pathway (Table 2).
TABLE 1.
Comparison of baseline characteristics between patients with atrial fibrillation and different hospital frailty risks who were compliant with and not compliant with the ABC pathway
| Characteristics | Low frailty risk | Intermediate frailty risk | High frailty risk | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
No‐ABC (N = 176 793) |
ABC (N = 44,749) |
P‐value |
No‐ABC (N = 32 790) |
ABC (N = 4,551) |
P‐value |
No‐ABC (N = 3871) |
ABC (N = 233) |
P‐value | |
| Female | 38.1% | 38.3% | .522 | 45.2% | 41.3% | <.001 | 48.5% | 42.5% | .088 |
| Age, y | 65 (50, 71) | 50 (41, 57) | <.001 | 69 (60, 75) | 53 (44, 60) | <.001 | 73 (63, 79) | 58 (49, 64) | <.001 |
| Age ≥ 65 | 50.2% | 5.9% | <.001 | 65.1% | 12.5% | <.001 | 72.8% | 23.6% | <.001 |
| Age ≥ 75 | 14.5% | 1.6% | <.001 | 28.0% | 4.4% | <.001 | 41.6% | 10.3% | <.001 |
| Economic status | 12 (5, 17) | 13 (6, 17) | <.001 | 13 (5, 17) | 12 (6,16) | <.001 | 12 (5,17) | 9 (3,15) | <.001 |
| CHA2DS2‐VASc score | 2 (1, 3) | 0 (0, 1) | <.001 | 3 (2, 4) | 1 (0, 1) | <.001 | 4 (2, 5) | 1 (0, 2) | <.001 |
| mHAS‐BLED score† | 2 (1, 3) | 0 (0, 1) | <.001 | 2 (1, 3) | 0 (0, 1) | <.001 | 3 (2, 3) | 1 (0, 1) | <.001 |
| Hospital Frailty Risk Score | 0 (0, 1.8) | 0 (0, 1.6) | <.001 | 7.4 (6, 9.7) | 6.9 (5.7, 8.9) | <.001 | 18 (16, 22) | 18 (16, 20) | <.001 |
| Charlson comorbidity index | 2 (1, 4) | 1 (0, 2) | <.001 | 4 (2, 6) | 2 (1, 3) | <.001 | 6 (4, 9) | 3 (2, 6) | <.001 |
| Hypertension | 64.2% | 6.5% | <.001 | 70.9% | 11.2% | <.001 | 78.4% | 20.2% | <.001 |
| Heart failure | 22.6% | 1.6% | <.001 | 29.4% | 2.5% | <.001 | 38.3% | 5.6% | <.001 |
| Diabetes mellitus | 17.9% | 2.1% | <.001 | 29.8% | 4.7% | <.001 | 42.8% | 10.3% | <.001 |
| Previous ischemic stroke/ TIA | 0.0% | 0.0% | — | 0.0% | 0.0% | — | 0.0% | 0.0% | — |
| Previous MI | 6.2% | 0.4% | <.001 | 11.4% | 0.9% | <.001 | 16.2% | 1.7% | <.001 |
| PAOD | 8.5% | 0.7% | <.001 | 12.8% | 1.6% | <.001 | 17.1% | 1.3% | <.001 |
| Vascular disease | 13.8% | 1.0% | <.001 | 21.9% | 2.3% | <.001 | 29.3% | 2.6% | <.001 |
| Hypertrophic cardiomyopathy | 1.2% | 0.6% | <.001 | 1.0% | 0.5% | <.001 | 0.7% | 0.9% | <.001 |
| Chronic kidney disease | 2.3% | 0.7% | <.001 | 7.7% | 1.8% | <.001 | 15.6% | 5.2% | <.001 |
| Liver disease | 36.1% | 28.0% | <.001 | 48.9% | 38.8% | <.001 | 60.2% | 50.6% | .005 |
| Malignant neoplasm | 17.4% | 14.0% | <.001 | 31.8% | 22.5% | <.001 | 41.5% | 33.0% | .014 |
| Hyperthyroidism | 8.2% | 7.3% | <.001 | 10.6% | 8.4% | <.001 | 12.8% | 7.3% | .019 |
| Hypothyroidism | 6.8% | 5.5% | <.001 | 10.1% | 6.9% | <.001 | 13.3% | 12.0% | .643 |
| Venous thromboembolism | 2.4% | 1.8% | <.001 | 5.9% | 4.0% | <.001 | 11.4% | 9.4% | .413 |
| COPD | 11.1% | 3.6% | <.001 | 22.1% | 7.1% | <.001 | 31.5% | 15.5% | <.001 |
| Previous intracranial bleeding | 0.4% | 0.2% | <.001 | 2.6% | 1.8% | .002 | 9.3% | 2.6% | .001 |
| History of any bleeding | 3.9% | 2.5% | <.001 | 20.0% | 13.7% | <.001 | 40.3% | 27.5% | <.001 |
| Coagulation/ platelet defect | 2.2% | 1.5% | <.001 | 8.1% | 5.4% | <.001 | 16.4% | 14.2% | .424 |
| Osteoporosis | 22.8% | 10.1% | <.001 | 41.5% | 19.1% | <.001 | 55.7% | 34.8% | <.001 |
| Medications | |||||||||
| OAC, baseline | 4.0% | 3.3% | <.001 | 4.7% | 6.1% | <.001 | 6.1% | 18.1% | <.001 |
| OAC, follow‐up | 30.9% | 22.1% | <.001 | 26.8% | 35.5% | <.001 | 21.1% | 58.5% | <.001 |
| Antiplatelet, baseline | 53.1% | 14.2% | <.001 | 58.5% | 22.9% | <.001 | 65.1% | 38.3% | <.001 |
| Antiplatelet, follow‐up | 68.3% | 31.6% | <.001 | 58.1% | 25.7% | <.001 | 48.3% | 31.8% | <.001 |
| Statin | 25.2% | 7.2% | <.001 | 34.0% | 16.4% | <.001 | 41.6% | 33.1% | <.001 |
| Beta blocker | 34.2% | 7.2% | <.001 | 39.9% | 13.0% | <.001 | 47.3% | 25.7% | <.001 |
| ACE inhibitor/ARB | 38.4% | 5.1% | <.001 | 47.9% | 12.6% | <.001 | 58.3% | 28.6% | <.001 |
| Diuretics | 38.2% | 5.2% | <.001 | 49.0% | 13.3% | <.001 | 59.1% | 30.7% | <.001 |
| Digoxin | 8.5% | 1.3% | <.001 | 6.9% | 1.2% | <.001 | 7.3% | 2.1% | <.001 |
Values are presented as median (Q1, Q3, quartiles [25th and 75th percentiles]) or %. †Modified HAS‐BLED = hypertension, 1 point: >65 years old, 1 point: stroke history, 1 point: bleeding history or predisposition, 1 point: liable international normalized ratio, not assessed: ethanol or drug abuse, 1 point: drug predisposing to bleeding, 1 point.
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; OAC, oral anticoagulant; PAOD, peripheral artery occlusive disease; TIA, transient ischemic attack.
TABLE 2.
Factors associated with compliance to the ABC pathway in different frailty categories
| Low frailty risk | Intermediate frailty risk | High frailty risk | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Age ≥ 65 y | 1.07 (1.05‐1.09) | <.001 | — | — | 1.06 (1.04‐1.08) | <.001 |
| Age ≥ 75 y | 1.21 (1.19‐1.23) | <.001 | 1.12 (1.11‐1.13) | <.001 | — | — |
| Female | 1.17 (1.15 ‐1.19) | <.001 | 1.09 (1.08‐1.10) | <.001 | 1.05 (1.04‐1.07) | <.001 |
| Economic status | 1.00 (1.00‐1.00) | <.001 | 1.00 (1.00‐1.00) | <.001 | — | — |
| CHA2DS2‐VASc score | 0.93 (0.91‐0.95) | <.001 | 0.97 (0.96‐0.97) | <.001 | 0.98 (0.97‐0.99) | <.001 |
| HAS‐BLED score | — | — | 1.02 (1.01‐1.02) | <.001 | — | — |
| Hospital Frailty Risk Score | 1.00 (1.00‐1.00) | <.001 | — | — | — | — |
| Charlson comorbidity Index | 1.00 (1.00‐1.00) | .005 | — | — | — | — |
| Heart failure | 1.05 (1.03‐1.07) | <.001 | 1.02 (1.01‐1.03) | <.001 | — | — |
| Hypertension | 0.85 (0.84‐0.87) | <.001 | 0.88 (0.87‐0.89) | <.001 | 0.94 (0.92‐0.96) | <.001 |
| Diabetes | 1.03 (1.02‐1.05) | <.001 | — | — | — | — |
| Previous MI | 1.03 (1.02‐1.05) | <.001 | — | — | — | — |
| Vascular disease | 1.05 (1.03‐1.07) | <.001 | 1.02 (1.01‐1.04) | <.001 | ‐ | ‐ |
| Liver disease | 0.99 (0.98‐0.99) | <.001 | 0.97 (0.96‐0.97) | <.001 | 0.97 (0.96‐0.99) | .002 |
| Malignant neoplasm | 1.02 (1.01‐1.02) | <.001 | — | — | — | — |
| Hyperthyroidism | 0.99 (0.98‐0.99) | <.001 | 0.99 (0.98‐1.00) | .017 | — | — |
| Venous thromboembolism | 1.01 (1.00‐1.02) | .031 | — | — | — | — |
| Intracranial bleeding | 0.97 (0.95‐0.99) | .006 | 0.97 (0.95‐0.99) | <.001 | 0.96 (0.94‐0.99) | .003 |
| History of bleeding | — | — | 0.99 (0.98‐1.00) | .004 | 0.98 (0.97‐1.00) | .013 |
| COPD | 1.01 (1.00‐1.01) | .008 | — | — | — | — |
| Osteoporosis | 0.99 (0.99‐1.00) | <.001 | — | — | — | — |
| OAC use | 1.10 (1.09‐1.11) | <.001 | 1.08 (1.06‐1.10) | <.001 | 1.05 (1.02‐1.09) | .005 |
| NOAC use | — | — | 1.37 (1.01‐1.87) | .044 | — | — |
| Antiplatelet use | 1.04 (1.03‐1.05) | <.001 | 1.03 (1.01‐1.04) | <.001 | — | — |
| Statin use | 1.01 (1.00‐1.01) | .005 | — | — | — | — |
| ACE inhibitor/ARB use | 1.02 (1.01‐1.02) | <.001 | 1.01 (1.00‐1.02) | .005 | — | — |
| Diuretics use | 1.02 (1.02‐1.03) | <.001 | 1.02 (1.01‐1.02) | <.001 | — | — |
| Digoxin use | 1.01 (1.01‐1.02) | <.001 | — | — | — | — |
Abbreviations: CI, confidence interval; HR, hazard ratio; NOAC nonvitamin K antagonist oral anticoagulant. Other abbreviations are same as in Table 1.
3.2. Death and composite outcomes
Patients with all three types of frailty risk in the ABC group had significantly lower cumulative incidences of all‐cause death (Figure 2A). During the mean follow‐up period of 5.9 (IQR 3.2, 9.4) years, compared with the non‐ABC group, the ABC group had lower rates of all‐cause death in the overall cohort (0.9 vs 3.3 per 100 person‐years, P < .001), and in the low (0.7 vs 2.6 per 100 person‐years, P < .001), intermediate (2.8 vs 7.2 per 100 person‐years, P < .001), and high frailty risk groups (6.5 vs 17.5 per 100 person‐years, P < .001) (Figure 3). After adjusting for clinical variables, compared with the non‐ABC group, the ABC group had a significantly lower risk of all‐cause death in the overall cohort (adjusted HR 0.93; 95% CI 0.90‐0.97, P < .001), and in the low (adjusted HR 0.95; 95% CI 0.91‐0.99, P = .031), intermediate (adjusted HR 0.89; 95% CI 0.82‐0.97, P = .008), and high frailty risk groups (adjusted HR 0.74; 95% CI 0.56‐0.97, P = .031) (Figure 3). There was a significant statistical interaction for the three frailty categories, with the greatest benefit observed in the high frailty group (P int < 0.001) (Figure 3).
FIGURE 2.
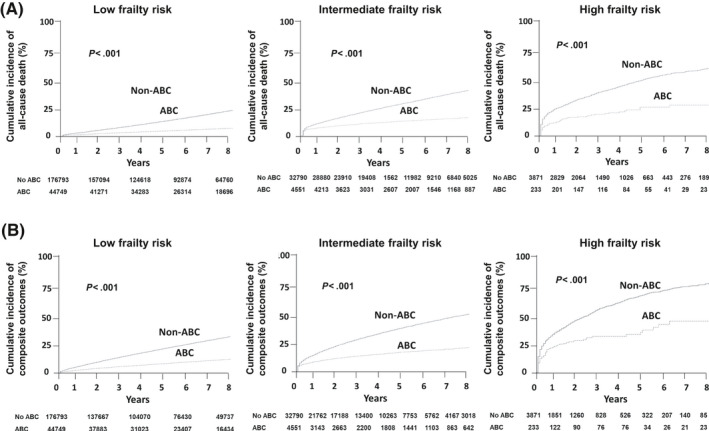
Cumulative incidences of all‐cause death (A) and composite outcomes (B) according to the use of integrated care (ABC) in patients with low (left panels), intermediate (mid panels), and high frailty risk (right panels)
FIGURE 3.
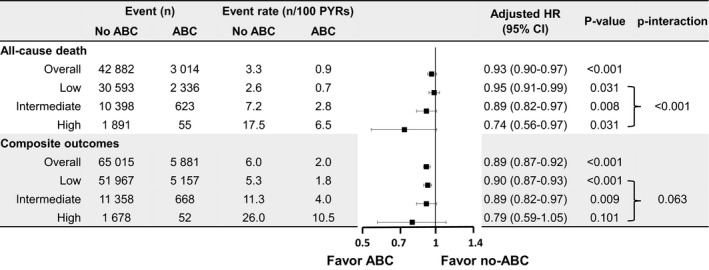
Events, event rates, risk of all‐cause death, and composite outcomes according to the use of integrated care (ABC) in patients with different frailty risks. CI, confidence interval; HR, hazard ratio; PYRs, person‐years
The ABC group had significantly lower cumulative incidences of composite outcomes in all three frailty risk groups (Figure 2B). During follow‐up, the ABC group had lower rates of composite outcomes in the overall cohort (2.0 vs 6.0 per 100 person‐years, P < .001; adjusted HR 0.89; 95% CI 0.87‐0.92, P < .001), and in the low (1.8 vs 5.3 per 100 person‐years, P < .001; adjusted HR 0.90; 95% CI 0.87‐0.93, P < .001) and intermediate (4.0 vs 11.3 per 100 person‐years, P = .009; adjusted HR 0.89; 95% CI 0.82‐0.97, P = .009) frailty risk groups (Figure 3). With regard to the composite outcomes in the high frailty group, there was no significant difference between the ABC and non‐ABC groups (10.5 vs 26.0 per 100 person‐years, P = .101; HR 0.79; 95% CI 0.59‐1.05, P = .101), and there was no statistically significant interaction for the three frailty categories (pint = 0.063) (Figure 3).
3.3. Other outcomes
The Kaplan–Meier cumulative incidence curves for other outcomes are presented in Figure 4. Among AF patients with low and intermediate frailty risk, lower cumulative incidences of ischemic stroke, heart failure admission, acute myocardial infarction, and major bleeding were observed in the ABC group compared with the non‐ABC group (all log‐rank P < .001). Among AF patients with high frailty risk, the lower cumulative incidence of clinical outcomes was also observed in the ABC group than in the non‐ABC group, but statistical significance was on the border.
FIGURE 4.
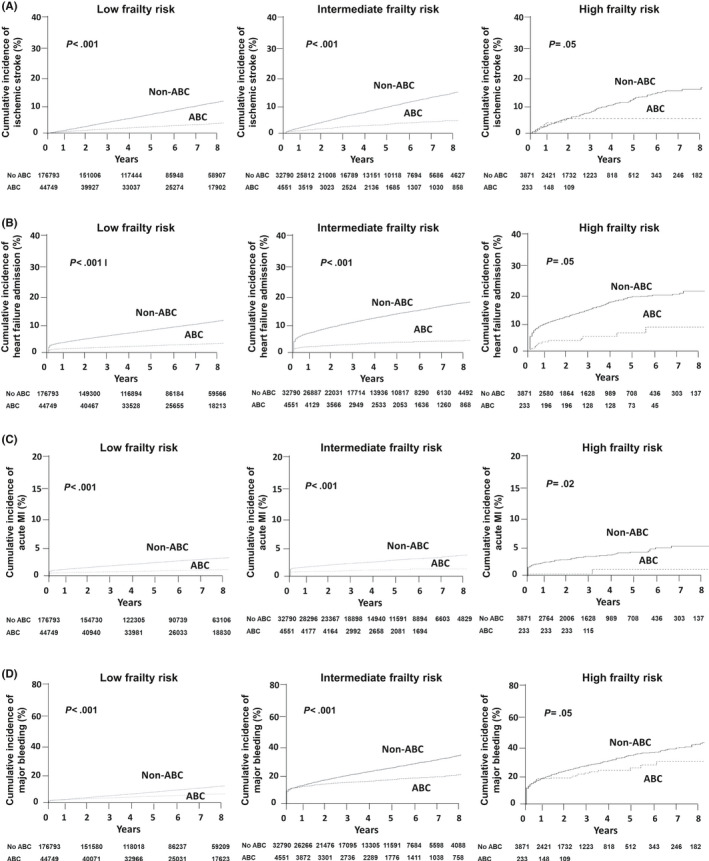
Cumulative incidences of ischemic stroke (A), heart failure admission (B), acute myocardial infarction (C), and major bleeding (D) according to use of integrated care (ABC) in patients with low (left panels), intermediate (mid panels), and high frailty risk (right panels). MI, myocardial infarction
The event rates and risks for other outcomes according to the ABC and non‐ABC groups are presented in Figure 5. Compared with the non‐ABC group, the ABC group had lower event rates and risk of ischemic stroke, heart failure admission, and acute myocardial infarction in overall, low and intermediate frailty risk groups (all P < .05). But among AF patients with high frailty risk group, there was no significant difference in risks of ischemic stroke, heart failure admission, and acute myocardial infarction between ABC and non‐ABC groups. Compared with the non‐ABC group, the ABC group had lower event rates and risk of major bleeding among patients with intermediate and high frailty risk, with a statistically significant interaction (P < .001). But among AF patients with overall and low frailty risk group, there was no significant difference in the risk of major bleeding between ABC and non‐ABC groups.
FIGURE 5.
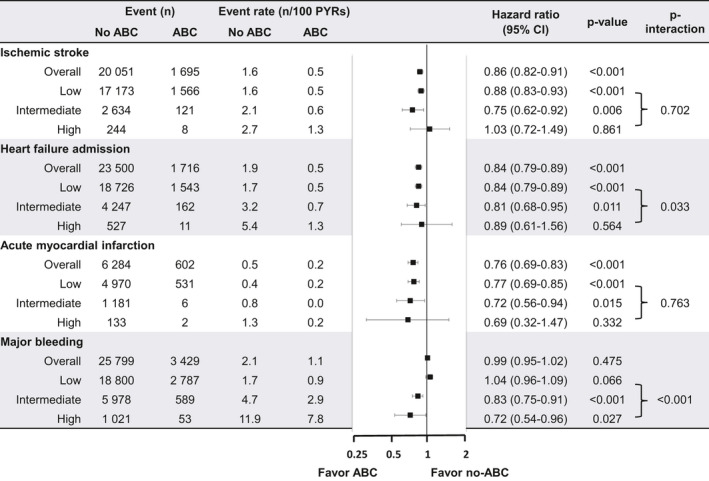
Events, event rates, and risks of other adverse outcomes according to the use of integrated care (ABC) in patients with different frailty risks. CI, confidence interval; HR, hazard ratio; PYRs, person‐years
4. DISCUSSION
In this largest nationwide analysis of patients with AF according to frailty risk, the event rates and risks of the all‐cause death, ischemic stroke, heart failure admission, acute myocardial infarction, major bleeding, and composite of these outcomes were significantly lower in the ABC group than in the non‐ABC group. In addition, among patients with high frailty risk, compared with patients in the non‐ABC group, those in the ABC group had lower rates of all‐cause death; however, the composite outcome was nonsignificantly lower in the ABC group. When the three frailty categories were compared, the greatest benefit on mortality was observed in the high frailty group; however, with regard to the composite outcome, there was no statistical interaction for the three frailty categories. Given the close association between AF and frailty and the high healthcare burden associated with AF, a streamlined holistic approach to the management of AF would improve outcomes in such patients.
4.1. Mortality and outcomes according to frailty risk
The use of an integrated care approach to AF management has been associated with reduced cardiovascular hospitalization and all‐cause mortality. 20 Nevertheless, approaches for providing integrated care have varying complexity. 11 There is a need to have a simple, practical, and easily operational method to streamline the decision‐making management approaches to allow uniform applicability across the entire AF patient pathway, linking primary care and secondary care (including cardiologist and noncardiologists), and to be understandable for patients with AF, facilitating their engagement.
The ABC pathway was proposed to streamline the interventions and decision‐making, and to optimize the patient management pathway, providing simple guidance for the main components of integrated care. 13 Recent AF management guidelines have incorporated the ABC pathway. 12 , 21 Nevertheless, there are limited data on the value of the ABC pathway in high‐risk patient groups. In this study, we show that the ABC pathway was related to reduced mortality and composite outcomes in frail patients with AF. The strong impact of the ABC pathway on overall mortality substantiates and strengthens the concept that a holistic approach for integrated management is associated with a significant clinical benefit for patients with AF. Indeed, compliance to the ABC pathway was also associated with a lower risk of ischemic stroke, heart failure admission, and acute myocardial infarction, as well as major bleeding, in patients with AF. A greater benefit in terms of major bleeding was observed in patients with AF and high frailty risk.
Although the risk of thromboembolic events is high, the rate of adequate oral anticoagulation is lower in frail patients with AF compared to nonfrail patients. 8 , 9 , 10 However, specific suggestions on oral anticoagulant (OAC) use have been based on advanced age and/or the presence of various comorbid disease states (eg, age > 75, renal impairment, prior history of bleeding), but not on frailty. 11 , 12 It may be appropriate to use no anticoagulation to avoid bleeding events in severe frail patients with AF. A recent US cohort study has shown that rivaroxaban but not apixaban or dabigatran was associated with reduced stroke versus warfarin in frail patients with nonvalvular AF while no significant difference in bleeding versus warfarin. 22 Therefore, “A” (Avoid stroke with Anticoagulation) as an integrated approach in patients with AF at a high frailty risk may not be always associated with good outcomes.
4.2. Study limitations
This retrospective population‐based observational study was performed using nationwide cohort data and should be interpreted in the context of the following limitations. First, the baseline diagnoses of AF, comorbidities, and clinical outcomes were dependent on the diagnostic codes registered by the physicians; therefore, these could be inaccurate, although the method for diagnosis has been validated in previous studies, and our internal validation found a high correlation with the actual diagnosis of AF. 4 Second, patients included in the non‐ABC group appeared more complex from a clinical perspective, with multiple comorbidities; however, compared with the non‐ABC group, the ABC group had adjusted lower risk of all‐cause death and composite outcome in all subgroups regardless of age, gender, and comorbidities. Conversely, given the high prevalence of comorbidities in the non‐ABC group, we can speculate that full implementation of the ABC pathway may reduce the risk even further. Third, in this study based on claims data, we used adherence (≥80%) as a surrogate of OAC optimization use, but fully recognize the limitations of lack of time in therapeutic range (TTR) data in patients with vitamin K antagonist use, or the availability of label‐adherence prescribing. Fourth, this study defined the low frequency of medical contact as “B” (better symptom management). However, a symptom is just one of the factors to decide the frequency of medical contact. Our definition using the frequency of medical contact alone may not have sufficiently reflected “B” (better symptom management) of AF patients. Fifth, this study included some patients who have undergone catheter ablation for eliminating AF. In Castle AF trial, ablation for AF in patients with heart failure improved all‐caused death. Hence, ablation may influence the improvement of outcomes of patients. Finally, in this study, we enrolled only East Asian patients, and therefore, whether the results can be extrapolated to other ethnic populations remains uncertain. Despite these limitations, to the best of our knowledge, this study presents the largest nationwide population dataset available in the literature to investigate the relationship between frailty and cardiovascular outcomes in patients with AF.
5. CONCLUSIONS
Compliance with the simple ABC pathway is associated with improved outcomes in patients with AF who have a high frailty risk. Given the high healthcare burden associated with AF, such a streamlined holistic approach to the management of AF should be implemented to improve outcomes in such patients.
CONFLICT OF INTEREST
GYHL: Consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi‐Sankyo, and speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi‐Sankyo. No fees were directly received personally. BJ: Speaker for Bayer, BMS/Pfizer, Medtronic, and Daiichi‐Sankyo. Research fund from Medtronic and Abbott. None of the other authors have anything to disclose.
AUTHOR CONTRIBUTIONS
PSY, JHS, and BJ designed the study, conducted the data analysis, and wrote the manuscript; GYHL designed the study and revised the manuscript. EJ participated in data analysis; HTY and THK participated in data collection. All authors have reviewed and approved the final version of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The National Health Information Database was provided by the National Health Insurance Service of Korea. We thank the National Health Insurance Service for its cooperation.
Yang P‐S, Sung J‐H, Jang E, et al. Application of the simple atrial fibrillation better care pathway for integrated care management in frail patients with atrial fibrillation: A nationwide cohort study. J Arrhythmia. 2020;36:668–677. 10.1002/joa3.12364
Pil‐Sung Yang and Jung‐Hoon Sung contributed equally to this work.
Gregory Y. H. Lip and Boyoung Joung are joint senior authors.
Funding information
This work was supported by a research grant from the Korean Healthcare Technology R&D project funded by the Ministry of Health and Welfare, Republic of Korea (HI15C1200).
Contributor Information
Gregory Y. H. Lip, Email: gregory.lip@liverpool.ac.uk.
Boyoung Joung, Email: cby6908@yuhs.ac.
REFERENCES
- 1. Kim D, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. 10‐year nationwide trends of the incidence, prevalence, and adverse outcomes of non‐valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am Heart J. 2018;202:20–6. [DOI] [PubMed] [Google Scholar]
- 2. Kim D, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. Increasing trends in hospital care burden of atrial fibrillation in Korea, 2006 through 2015. Heart. 2018;104:2010–7. [DOI] [PubMed] [Google Scholar]
- 3. Lee H, Kim TH, Baek YS, Uhm JS, Pak HN, Lee MH, et al. The trends of atrial fibrillation‐related hospital visit and cost, treatment pattern and mortality in korea: 10‐year nationwide sample cohort data. Korean Circ J. 2017;47:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim TH, Yang PS, Uhm JS, Kim JY, Pak HN, Lee MH, et al. CHA2DS2‐VASc score (congestive heart failure, hypertension, age >/=75 [doubled], diabetes mellitus, prior stroke or transient ischemic attack [doubled], vascular disease, age 65–74, female) for stroke in Asian patients with atrial fibrillation: a Korean Nationwide Sample Cohort Study. Stroke. 2017;48:1524–30. [DOI] [PubMed] [Google Scholar]
- 5. Kim D, Yang PS, Yu HT, Kim TH, Jang E, Sung JH, et al. Risk of dementia in stroke‐free patients diagnosed with atrial fibrillation: data from a population‐based cohort. Eur Heart J. 2019;40:2313–23. [DOI] [PubMed] [Google Scholar]
- 6. Marzona I, O'Donnell M, Teo K, Gao P, Anderson C, Bosch J, et al. Increased risk of cognitive and functional decline in patients with atrial fibrillation: results of the ONTARGET and TRANSCEND studies. CMAJ. 2012;184:E329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polidoro A, Stefanelli F, Ciacciarelli M, Pacelli A, Di Sanzo D, Alessandri C. Frailty in patients affected by atrial fibrillation. Arch Gerontol Geriatr. 2013;57:325–7. [DOI] [PubMed] [Google Scholar]
- 8. Gomez‐Outes A, Lagunar‐Ruiz J, Terleira‐Fernandez AI, Calvo‐Rojas G, Suarez‐Gea ML, Vargas‐Castrillon E. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508–21. [DOI] [PubMed] [Google Scholar]
- 9. Fauchier L, Villejoubert O, Clementy N, Bernard A, Pierre B, Angoulvant D, et al. Causes of death and influencing factors in patients with atrial fibrillation. Am J Med. 2016;129:1278–87. [DOI] [PubMed] [Google Scholar]
- 10. Kotecha D, Breithardt G, Camm AJ, Lip GYH, Schotten U, Ahlsson A, et al. Integrating new approaches to atrial fibrillation management: the 6th AFNET/EHRA Consensus Conference. Europace. 2018;20:395–407. [DOI] [PubMed] [Google Scholar]
- 11. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- 12. Joung B, Lee JM, Lee KH, Kim TH, Choi EK, Lim WH, et al. 2018 Korean Guideline of Atrial Fibrillation Management. Korean Circ J. 2018;48:1033–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14:627–8. [DOI] [PubMed] [Google Scholar]
- 14. Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Improved outcomes by integrated care of anticoagulated patients with atrial fibrillation using the simple ABC (atrial fibrillation better care) pathway. Am J Med. 2018;131(1359–1366):e1356. [DOI] [PubMed] [Google Scholar]
- 15. Pastori D, Pignatelli P, Menichelli D, Violi F, Lip GYH. integrated care management of patients with atrial fibrillation and risk of cardiovascular events: The ABC (atrial fibrillation better care) pathway in the ATHERO‐AF study cohort. Mayo Clin Proc. 2019;94:1261–7. [DOI] [PubMed] [Google Scholar]
- 16. Yoon M, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. Improved population‐based clinical outcomes of patients with atrial fibrillation by compliance with the simple ABC (atrial fibrillation better care) pathway for integrated care management: a nationwide cohort study. Thromb Haemost. 2019;19:1695–703. [DOI] [PubMed] [Google Scholar]
- 17. Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391:1775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim D, Yang PS, Kim TH, Jang E, Shin H, Kim HY, et al. Ideal blood pressure in patients with atrial fibrillation. J Am Coll Cardiol. 2018;72:1233–45. [DOI] [PubMed] [Google Scholar]
- 19. Kim TH, Yang PS, Kim D, Yu HT, Uhm JS, Kim JY, et al. CHA2DS2‐VASc score for identifying truly low‐risk atrial fibrillation for stroke: A Korean Nationwide Cohort Study. Stroke. 2017;48:2984–90. [DOI] [PubMed] [Google Scholar]
- 20. Gallagher C, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, Mahajan R, et al. Integrated care in atrial fibrillation: a systematic review and meta‐analysis. Heart. 2017;103:1947–53. [DOI] [PubMed] [Google Scholar]
- 21. Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154:1121–201. [DOI] [PubMed] [Google Scholar]
- 22. Martinez BK, Sood NA, Bunz TJ, Coleman CI. Effectiveness and safety of apixaban, dabigatran, and rivaroxaban versus warfarin in frail patients with nonvalvular atrial fibrillation. J Am Heart Assoc . 2018;7 10.1161/JAHA.118.008643 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


