Abstract
OBJECTIVE
We aimed to assess whether individuals with type 2 diabetes (T2D) have increased risk of vertebral fractures (VFs) and to estimate nonvertebral fracture and mortality risk among individuals with both prevalent T2D and VFs.
RESEARCH DESIGN AND METHODS
A systematic PubMed search was performed to identify studies that investigated the relationship between T2D and VFs. Cohorts providing individual participant data (IPD) were also included. Estimates from published summary data and IPD cohorts were pooled in a random-effects meta-analysis. Multivariate Cox regression models were used to estimate nonvertebral fracture and mortality risk among individuals with T2D and VFs.
RESULTS
Across 15 studies comprising 852,705 men and women, individuals with T2D had lower risk of prevalent (odds ratio [OR] 0.84 [95% CI 0.74–0.95]; I2 = 0.0%; Phet = 0.54) but increased risk of incident VFs (OR 1.35 [95% CI 1.27–1.44]; I2 = 0.6%; Phet = 0.43). In the IPD cohorts (N = 19,820), risk of nonvertebral fractures was higher in those with both T2D and VFs compared with those without T2D or VFs (hazard ratio [HR] 2.42 [95% CI 1.86–3.15]) or with VFs (HR 1.73 [95% CI 1.32–2.27]) or T2D (HR 1.94 [95% CI 1.46–2.59]) alone. Individuals with both T2D and VFs had increased mortality compared with individuals without T2D and VFs (HR 2.11 [95% CI 1.72–2.59]) or with VFs alone (HR 1.84 [95% CI 1.49–2.28]) and borderline increased compared with individuals with T2D alone (HR 1.23 [95% CI 0.99–1.52]).
CONCLUSIONS
Based on our findings, individuals with T2D should be systematically assessed for presence of VFs, and, as in individuals without T2D, their presence constitutes an indication to start osteoporosis treatment for the prevention of future fractures.
Introduction
Type 2 diabetes (T2D) is a chronic metabolic disease characterized by several complications such as cardiovascular disease, neuropathy, nephropathy, retinopathy, and mortality (1). Moreover, skeletal complications are also evident, as individuals with T2D present increased risk of hip and nonvertebral fractures compared with the general population, despite similar or higher levels of areal bone mineral density (BMD). This suggests that BMD underestimates risk of fracture in these individuals (2,3). Several mechanisms have been suggested to explain this increased fracture risk: increased frequency of falling, cortical porosity, and microvascular disease and increased levels of advanced glycation end products among others (2,4–7). Fracture risk assessment remains a low priority in clinical care, as compared with that of the extraskeletal comorbidities and complications affecting individuals with T2D, despite that fracture occurrence significantly increases disability (1). Vertebral fractures (VFs) are the most common type of osteoporotic fracture, and their identification is important not only for the diagnosis of osteoporosis but also for future fracture risk assessment and treatment decisions (8). They are very often asymptomatic, and there is evidence that they are greatly underdiagnosed worldwide (9). VFs and nonvertebral fractures differ in skeletal composition and biomechanical properties. Vertebrae are composed predominantly of trabecular bone, which is substantially more metabolically active and holds distinct biomechanical properties from cortical bone. Further, nonvertebral fractures are often preceded by a fall or some form of trauma, while this only applies to ∼10–15% of VFs (10). In contrast to the well-established increase in hip and nonvertebral fracture risk among individuals with T2D consistently found across numerous studies (2,3), evidence regarding the risk of VFs in T2D remains inconclusive. Five previously published meta-analyses reporting inconsistent findings (11–15) all hold several limitations and large underlying heterogeneity of effects of undocumented source.
We aimed to assess whether individuals with T2D have increased risk of VFs by bringing together a meta-analysis, published summary data, and individual participant data from prospective cohort studies. Moreover, in cohorts with individual participant data (IPD), we aimed to investigate the influence of T2D and VFs on the risk of nonvertebral fractures and mortality.
Research Design and Methods
Search Strategy and Selection Criteria
We performed a meta-analysis to ascertain the relationship between T2D and VFs in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines for reporting (16). Two systematic searches of the literature in PubMed were performed, and we included previously published studies in humans that reported estimates and 95% CIs concerning the association between T2D and VFs (Supplementary Fig. 1). A detailed description of the search strategy can be found in Supplementary Material S2.
IPD From Cohorts
We gathered individual-level data by contacting and inviting collaborators from seven different cohorts with participant information on prevalent T2D, prevalent or incident VFs, age, sex, BMI, BMD, corticosteroid use, and antiosteoporotic therapy. All contacted collaborators agreed to share the IPD, and all seven cohorts with IPD were available for analysis (Supplementary Figs. 1 and 3). Each study is approved by their relevant local or national ethics committees, and all participants have given informed consent to take part. Data shared was anonymized, and confidentiality agreements were signed from collaborating groups.
Prevalent T2D was defined at baseline across studies based either on general practitioner’s records or according to the current World Health Organization guidelines as a fasting blood glucose ≥7.0 mmol/L, a nonfasting blood glucose ≥11.1 mmol/L, or use of blood glucose–lowering medication. Presence of VFs was assessed on lateral radiographs or lateral DXA of the spine from the fourth thoracic to the fourth lumbar vertebrae. VFs were assessed at baseline (prevalent) and, where available, at the follow-up visit (incident) at, on average, 2.5–5 years after baseline across cohorts. Incident VFs were defined as new VFs occurring in a participant free of fracture across all vertebrae at baseline. Nonvertebral fracture events and death were reported either by general practitioners in a computerized system or extracted from hospital or research records. Follow-up time was calculated as time from baseline to first nonvertebral fracture, death, end of follow-up period, or loss to follow-up, whichever occurred first. Covariates were age, sex, BMI, femoral neck BMD (FN-BMD), corticosteroid use, and antiosteoporotic treatment. In a subset of the IPD cohorts, we also tested the association between prevalent T2D and FN-BMD measured at baseline and of T2D with lumbar spine BMD (LS-BMD) and trabecular bone score (TBS) (17) measured at follow-up (Supplementary Fig. 3).
Statistical Analysis
To address the absence of information regarding FN-BMD in 18.6% of all IPD cohorts, we imputed this information using the fully conditional specification-imputation method in SPSS. This is an iterative Markov chain Monte Carlo method that can be used to account for arbitrary missing data. Before performing the multiple imputation, FN-BMD values measured with a lunar DXA were transformed to hologic values through the formula: hologic FN-BMD = 0.939 × lunar FN-BMD − 0.023 (18). To impute missing FN-BMD values, we performed 20 imputations with 10 iterations each, in which predictors were age, height, weight, BMI, sex, study center, and FN-BMD. We have shown in this study results of the pooled estimates from all 20 imputations, after comparing it with the nonimputed data and finding no differences between the imputed and nonimputed data. The imputed values of FN-BMD were used when FN-BMD was a covariate in the analyses. When FN-BMD was an outcome or exposure in the exploratory analyses, only individuals with nonmissing values were included.
The published studies reported adjusted hazard ratios (HRs), ORs, relative risk, or standardized incidence ratios with 95% CIs, and we regarded them as ORs in the meta-analysis (Supplementary Table 4). For the meta-analysis across IPD cohorts, we ran separately for each cohort, a logistic regression model stratified by sex and adjusted for age, BMI, FN-BMD, corticosteroid use, and antiosteoporotic treatment. We then analyzed both published and unpublished data combined in a random-effects meta-analysis and have provided summary statistics for the random-effects model. The heterogeneity of the study estimates was assessed using the I2 statistic. When heterogeneity was present, we performed sensitivity analyses by excluding studies one by one from the meta-analysis. We explored potential sources of heterogeneity also by testing interactions between T2D and sex, age, and BMI in the IPD cohorts. The significance threshold for interaction terms was set to P < 0.1. Further, to estimate the risk of mortality among individuals with T2D and VF, we fitted a multivariate Cox regression model. The proportionality of hazard assumption for the Cox model fit was assessed based on Schoenfeld residuals. We found a significant relation among residuals of sex, BMI, and age with time (P value <0.001). Therefore, to fulfill the proportionality of hazard assumption, the Cox regression model was run separately for sex and BMI categories. The model was fitted adjusted for natural age spline with 5 df, corticosteroid use, antiosteoporotic treatment, cohort, and BMD T-score. We used a similar approach to assess the risk of nonvertebral fractures based on T2D and VF comorbidity at baseline. We found a significant relationship between residuals of age with time (P value <0.001), and the model was fitted and adjusted for natural age spline with 5 df, sex, BMI, corticosteroid use, antiosteoporotic treatment, cohort, and BMD. Based on FN-BMD T-score values, three clinical categories were created: normal (femoral neck BMD T-score >−1.0), osteopenia (FN-BMD T-score ≤−1.0 and >−2.5), and osteoporosis (FN-BMD T-score ≤−2.5). BMI categories were constructed based on World Health Organization guidelines into underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–30.0 kg/m2), and obese (>30.0 kg/m2). Analyses were performed in R with packages “meta” and “survival,” as well as SPSS software (IBM SPSS Statistics 24; Armonk, NY).
Results
Overall Characteristics of the Study Population
We identified 719 published studies through our literature search (Supplementary Material 1). Out of these, 711 were not eligible for inclusion, and 8 were included. The included studies were from the U.S. (19–21), Sweden (22), Spain (23), Germany (24), Israel (25), and Japan (26). The IPD cohorts were from Canada (27), U.K. (28), Switzerland (29), the Netherlands (30), and U.S. (31) (Supplementary Table 5). The published studies and IPD cohorts comprised 852,705 individuals, of which 58.6% were women. The prevalence of T2D and VFs, incidence of VFs, and follow-up time varied across studies (Supplementary Table 4). The association between T2D and VFs was adjusted across studies for multiple factors, including at least age and sex (Supplementary Table 4). Mean age ranged across studies from 45.6 to 79.5 years. There were 31,530 individuals in the IPD cohorts, of which 2,182 (6.9%) had a prevalent diagnosis of T2D (Table 1). Individuals with prevalent T2D were on average 1 year older (70.0 vs. 69.0; P < 0.001); had higher BMI (28.5 vs. 26.5 kg/m2; P < 0.001); had a higher FN-BMD T-score (−1.05 vs. −1.33; P < 0.001) reflected in lower prevalence of osteopenia (43.6 vs. 51.3%; P < 0.001) and osteoporosis (9.6 vs. 13.6%; P < 0.001); and had a higher prevalence of corticosteroid use (5.3 vs. 3.8%; P = 0.03) and antiosteoporotic treatment (10.3 vs. 8.9%; P = 0.03). Furthermore, in the 7,819 individuals from cohorts with TBS and BMD measurements, the 472 (6.0%) with prevalent T2D had higher LS-BMD (1.21 vs. 1.14 g/cm2; P < 0.001) and lower TBS (1.21 vs. 1.26; P < 0.001) than individuals without T2D (Table 1).
Table 1.
Comparison of baseline characteristics by T2D status across cohorts with IPD
| Without T2D (n = 29,348) | With T2D (n = 2,182) | P value | |
|---|---|---|---|
| Sex (% women) | 24,068 (82.0) | 1,608 (73.7) | <0.001 |
| Age years | 69.0 (9.1) | 70.0 (8.5) | <0.001 |
| Weight kg | 70.3 (13.2) | 76.5 (15.1) | <0.001 |
| Height cm | 162.6 (8.9) | 163.5 (9.7) | <0.001 |
| BMI kg/m2 | 26.5 (4.2) | 28.5 (4.7) | <0.001 |
| Underweight | 352 (1.2) | 11 (0.5) | <0.001 |
| Normal weight | 10,817 (36.8) | 478 (21.9) | |
| Overweight | 12,560 (42.8) | 919 (42.1) | |
| Obese | 5,619 (19.2) | 774 (35.5) | |
| FN-BMD T-score (continuous) | −1.33 (1.4) | −1.05 (1.7) | <0.001 |
| FN-BMD T-score clinical categories | |||
| Normal | 10,299 (35.1) | 1,021 (46.8) | <0.001 |
| Osteopenia | 15,067 (51.3) | 952 (43.6) | |
| Osteoporosis | 3,982 (13.6) | 209 (9.6) | |
| Corticosteroid use | 1,126 (3.8) | 115 (5.3) | 0.03 |
| Antiosteoporotic treatment | 2,622 (8.9) | 225 (10.3) | 0.03 |
| Prevalent VFs | 3,513 (11.9) | 228 (10.4) | 0.03 |
| LS-TBS* | 1.26 (0.12) | 1.21 (0.13) | <0.001 |
| LS-BMD, g/cm2* | 1.14 (0.21) | 1.21 (0.23) | <0.001 |
The P value corresponds to t test (continuous variables) or χ2 test (categorical variables) for difference between individuals with and without T2D at baseline.
LS-TBS, lumbar spine TBS.
Denotes that information was available only at follow up for 7,819 individuals (n = 7,347 without T2D and n = 472 with T2D).
Type 2 Diabetes and Prevalent VFs
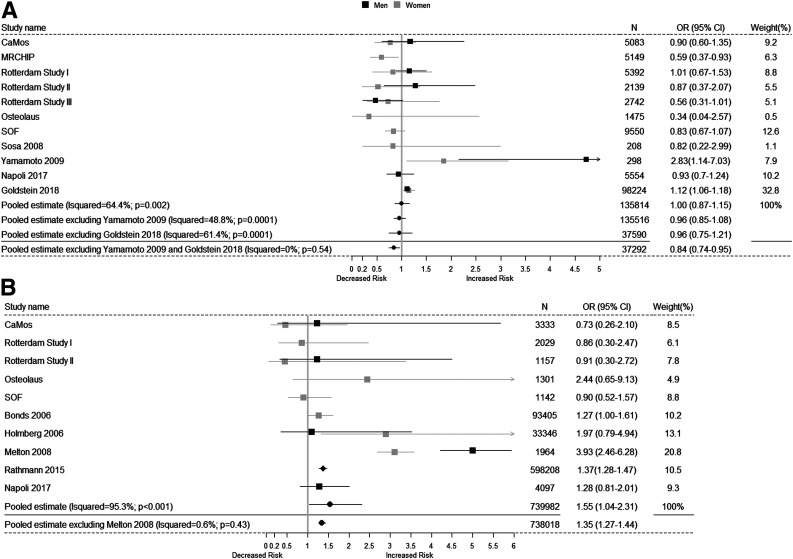
The meta-analysis concerning the risk of prevalent VFs among individuals with T2D included 124,631 individuals from four published studies and seven IPD cohorts. The combined meta-analysis of published studies and IPD cohorts showed no association between T2D and prevalent VFs (odds ratio [OR] 1.0 [95% CI 0.87–1.15]) but presented significant high heterogeneity (I2 = 64.4%; Phet = 0.0001). Sensitivity analyses of these data indicated that the main source of heterogeneity came from two published studies drawn in Japan (26) and Israel (25). Excluding those studies from the meta-analysis showed lower risk of VFs for individuals with T2D (OR 0.84 [95% CI 0.74–0.95]) without significant heterogeneity (I2 = 0.0%; Phet = 0.54) (Fig. 1A). In addition, sensitivity analysis (across age tertiles and BMI categories) in the cohorts with IPD showed that the lower risk of prevalent VFs in subjects with T2D was confined to individuals aged ≥74.0 years at baseline (OR 0.76 [95% CI 0.60–0.95]; P for interaction = 0.09) and particularly in obese individuals (OR 0.72 [95% CI 0.54–0.94]; P for interaction = 0.03) (Supplementary Table 6).
Figure 1.
Forest plot of the meta-analysis across studies concerning the association between T2D and risk of VFs. In A, it is shown the association between T2D and prevalent VFs and, in B, the association between T2D and incident VFs. Besides the pooled overall estimates, we also show in the figures the estimates after excluding studies that were introducing heterogeneity on the estimates.
Type 2 Diabetes and Incident VFs
In contrast to the meta-analysis of prevalent VFs, the meta-analysis of 738,018 individuals from 5 published studies and 6 IPD cohorts showed a higher risk of incident VFs among individuals with T2D (OR 1.55 [95% CI 1.04–2.31]), but displaying very high heterogeneity (I2 = 95.3%; Phet < 0.001). Sensitivity analyses, established that the heterogeneity was introduced by the study from Melton et al. (21). Excluding that study showed that individuals with T2D had an increased risk of incident VFs (OR 1.35 [95% CI 1.27–1.44]) without significant evidence for heterogeneity affecting the estimates (I2 = 0.6%; Phet = 0.43) (Fig. 1B).
Type 2 Diabetes and Bone Health Markers, BMD, and TBS
In all IPD cohorts, T2D was associated at baseline with higher FN-BMD both in men (B = 0.029; 95% CI 0.018–0.041) and women (B = 0.046; 95% CI 0.039–0.053). After adjusting for BMI, the association was attenuated but remained significant in both men (B = 0.013; 95% CI 0.01–0.025) and women (B = 0.022; 95% CI 0.015–0.029). In a subsample (n = 7,819) at follow-up, T2D was associated with higher BMD at the lumbar spine in both men (B = 0.040; 95% CI 0.013–0.066) and women (B = 0.065; 95% CI 0.039–0.091), but after adjustment for BMI this association was attenuated in both men (B = 0.016; 95% CI −0.010 to 0.043) and women (B = 0.020; 95% CI −0.004 to 0.045). In contrast, T2D was associated with decreased TBS in the fully adjusted model in both men (B = −0.019; 95% CI −0.035 to −0.004) and women (B = −0.041; 95% CI −0.055 to −0.026) (Supplementary Table 7).
Type 2 Diabetes With and Without VFs in Relation to Nonvertebral Fracture Risk and Mortality
Individuals with both type 2 diabetes and VFs had a higher risk of nonvertebral fractures as compared with individuals without either T2D or VFs (HR 2.42 [95% CI 1.86–3.15]), compared with individuals with T2D alone (HR 1.94 [95% CI 1.46–2.59]) or compared with individuals with VFs alone (HR 1.73 [95% CI 1.32–2.27]) after multivariate adjustments (Table 2). After stratification by FN-BMD category, we observed that individuals with both T2D and VFs had increased risk of nonvertebral fractures as compared with those without VFs and T2D, independent of BMD T-score category (HR 2.50 [95% CI 1.44–4.35] in those with normal BMD; HR 2.58 [95% CI 1.76–3.79] in those with osteopenia; and HR 2.06 [95% CI 1.25–3.38] in those with osteoporosis). When comparing individuals with both T2D and VFs to individuals with T2D alone, the increased risk of nonvertebral fractures was observed across all BMD T-score categories (being HR 2.04 [95% CI 1.14–3.65] in those with normal BMD; HR 2.0 [95% CI 1.33–3.03] in those with osteopenia; and HR 1.97 [95% CI 1.07–3.62] in those with osteoporosis). As compared with individuals with VFs alone, individuals with both T2D and VFs had a statistically nonsignificant increased risk of nonvertebral fractures independent of BMD levels (HR 1.55 [95% CI 0.86–2.78] in those with normal BMD; HR 1.82 [95% CI 1.23–2.70] in those with osteopenia; and HR 1.55 [95% CI 0.93–2.57] in those with osteoporosis). The mortality analysis based on VFs and T2D status at baseline was stratified by sex and BMI to meet the proportionality of hazards assumption; we show also the overall results but will interpret them cautiously. Overall, we found increased mortality risk among individuals with both VFs and T2D compared with individuals without either T2D or VFs (HR 2.05 [95% CI 1.73–2.43]), when compared with individuals with VFs alone (HR 1.71 [95% CI 1.43–2.03]) and with a statistically nonsignificant increase when compared with individuals with T2D alone (HR 1.21 [95% CI 0.97–1.49]). After stratification by sex and BMI category, we observed an increased risk of death among overweight men with both T2D and VFs compared with overweight men without T2D or VFs (HR 2.38 [95% CI 1.15–4.96]), compared with overweight men with VFs alone (HR 2.38 [95% CI 1.15–4.96]), with a nonsignificant increase as compared with individuals with T2D alone (HR 1.61 [95% CI 0.78–3.33]). Similarly, obese men with both T2D and VFs had increased risk of death compared with obese men without T2D or VFs (HR 3.11 [95% CI 1.50–6.46]), compared with obese men with T2D alone (HR 2.54 [95% CI 1.17–5.51]), and compared with individuals with VFs alone (HR 3.10 [95% CI 1.25–7.57]). Overweight women with both T2D and VFs had increased risk of mortality compared with overweight women without T2D or VFs (HR 2.32 [95% CI 1.73–3.11]) and with VFs alone (HR 1.86 [95% CI 1.38–2.56]) but not compared with overweight women with T2D alone (HR 1.13 [95% CI 0.85–1.55]). Obese women also had increased mortality if they had both T2D and VFs as compared with obese women without T2D or VFs (HR 1.92 [95% CI 1.37–2.68]) and with obese women with VFs alone (HR 1.65 [95% CI 1.16–2.34]), but not compared with obese women with T2D alone (HR 1.03 [95% CI 0.72–1.45]) (Table 3).
Table 2.
The association between T2D and/or prevalent VFs with incident nonvertebral fractures, overall and stratified by BMD category
| Outcome: incident nonvertebral fracture, HR (95% CI) | ||||
|---|---|---|---|---|
| Without T2D or VFs (reference) | 1.00 | 1.00 | 1.00 | 1.00 |
| With VFs alone | 1.39 (1.27–1.52) | 1.61 (1.29–2.00) | 1.41 (1.26–1.59) | 1.32 (1.13–1.55) |
| With T2D alone | 1.24 (1.09–1.40) | 1.22 (0.98–1.52) | 1.28 (1.08–1.52) | 1.04 (0.71–1.51) |
| With both T2D and VFs | 2.42 (1.86–3.15) | 2.50 (1.44–4.35) | 2.58 (1.76–3.79) | 2.06 (1.25–3.38) |
| With VFs alone (reference) | 1.00 | 1.00 | 1.00 | 1.00 |
| With both T2D and VFs | 1.73 (1.32–2.27) | 1.55 (0.86–2.78) | 1.82 (1.23–2.70) | 1.55 (0.93–2.57) |
| With T2D alone (reference) | 1.00 | 1.00 | 1.00 | 1.00 |
| With both T2D and VFs | 1.94 (1.46–2.59) | 2.04 (1.14–3.65) | 2.00 (1.33–3.03) | 1.97 (1.07–3.62) |
Data are results obrained from Cox regression model adjusted for age (natural splines with 6 df), BMI (natural splines with 5 df), sex, corticosteroid use, antiosteoporotic treatment, and study. The analysis without stratification was additionally adjusted for BMD measured at the femoral neck.
Table 3.
Mortality risk based on presence of T2D and VFs at baseline, stratified by sex and BMI category
| Outcome: death during follow-up, HR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| Overall (N = 19,822) | Men (N = 4,405) | Women (N = 15,417) | |||||
| Normal weight (N = 1,413) | Overweight (N = 2,323) | Obese (N = 643) | Normal weight (N = 5,747) | Overweight (N = 6,318) | Obese (N = 3,204) | ||
| Without T2D or VFs (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| With VFs alone | 1.20 (1.13– 1.27) | 1.09 (0.81–1.45) | 1.03 (0.81–1.31) | 1.08 (0.59–1.94) | 1.26 (1.15–1.39) | 1.24 (1.13–1.36) | 1.15 (1.01–1.32) |
| With T2D alone | 1.78 (1.65–1.91) | 1.84 (1.44–2.36) | 1.50 (1.21–1.84) | 1.22 (0.83–1.78) | 1.64 (1.39–1.95) | 2.04 (1.79–2.32) | 1.87 (1.62–2.15) |
| With both T2D and VFs | 2.05 (1.73–2.43) | 1.27 (0.46–3.48) | 2.42 (1.20– 4.88) | 3.11 (1.50–6.46) | 1.75 (1.23–2.47) | 2.32 (1.73–3.11) | 1.92 (1.37–2.68) |
| With T2D alone (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| With both T2D and VFs | 1.21 (0.97–1.49) | 0.68 (0.24–1.93) | 1.61 (0.78–3.33) | 2.54 (1.17–5.51) | 1.06 (0.72–1.55) | 1.13 (0.82–1.55) | 1.03 (0.72–1.45) |
| With VFs alone (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| With both T2D and VFs | 1.71 (1.43–2.03) | 1.15 (0.4–3.24) | 2.38 (1.15–4.96) | 3.1 (1.25–7.57) | 1.37 (0.97–1.95) | 1.86 (1.38–2.52) | 1.65 (1.16–2.34) |
Individuals were assigned at baseline to four groups: without T2D or VFs, with T2D only, with VFs only, and with both T2D and VFs. A Cox regression model adjusted for age (natural splines with 5 df), corticosteroid use, antiosteoporotic treatment, and cohort, stratified for sex and BMI, was fitted to estimate the survival of the individuals based on their T2D and VF status at baseline.
Conclusions
This meta-analysis of 15 published studies and cohorts showed that individuals with T2D are at higher risk of sustaining an incident VF compared with individuals without T2D. Furthermore, individuals with both T2D and VFs had almost double the risk of sustaining an incident nonvertebral fracture than individuals with either VFs or T2D alone. The increased risk for incident nonvertebral fractures among individuals with T2D and VFs compared with individuals with T2D or VFs alone was increased despite clinical categories of FN-BMD T-score. We also observed a decreased prevalence of VFs among individuals with T2D, and through sensitivity analysis, we could establish that such a protective effect was mostly present among the older or among obese individuals. In addition, we found that individuals with both T2D and VFs had increased mortality risk as compared with individuals without T2D or VFs and still significantly greater than the mortality observed in individuals with T2D or VFs alone. The increased mortality among individuals with T2D and VFs was particularly increased among overweight and obese individuals of both sexes.
Our findings give clarity to the conflicting findings observed across previous meta-analyses. Five previously published meta-analyses that have reported on the association between T2D and VFs (11–15) hold several limitations that could have influenced their findings. One meta-analysis in particular (11) had several limitations (pointed out in a letter to the editor [32]), one of which was misclassifying fracture location (major osteoporotic fracture vs. VFs) or using effect estimates that were different from those reported in the original study. Limitations of the other meta-analyses included claiming an effect on VFs when referring to nonvertebral fractures (13); not clearly explaining how point estimates were combined (14); or including studies looking at nonvertebral fractures instead of VFs (15). Moreover, none of these meta-analyses of T2D and VFs addressed the source of the heterogeneity encountered in their analyses and missed including some of the studies that were part of our present meta-analysis (11–15).
In the current meta-analysis, the studies by Yamamoto et al. (26), Goldshtein et al. (25), and Melton et al. (21) introduced heterogeneity across study estimates. The first study was in an Asian population, with a high prevalence of VFs among individuals with or without T2D. Although they mentioned that their population without T2D was chosen to be free of any skeletal disorders, an average BMD T-score of −2.0 among women and −2.4 among men with T2D suggests that this population likely had a high prevalence of osteoporosis and was at high risk of fracture. Further, in the Yamamoto et al. (26) study, individuals with T2D were recruited from tertiary centers, suggesting that they were severe cases of T2D. The heterogeneity introduced by the studies of Melton et al. (21) and Goldshtein et al. (25) might have been to a large extent, introduced by the type of estimation used to compare risk of fracture among individuals with T2D (i.e., standardized incidence ratio and standardized rate ratio, respectively). These types of estimations have made the assumption of expected numbers of VFs based on data from clinically diagnosed VFs, which do not include asymptomatic VFs. The latter might be more often incidentally diagnosed among individuals with T2D compared with those without, leading to an overestimation of the risk of VFs among individuals with T2D compared with those without. Although the meta-analysis regarding the incidence of VFs among individuals with T2D, after excluding the studies of Melton et al. (21) and Rathmann et al. (24), showed an increased VF incidence among individuals with T2D, it is important to note that among cohorts with IPD, we could not detect this increased incidence (Supplementary Table 8). We think this is mainly due to the fact that the IPD cohorts were population-based cohorts, all of which required participants to voluntarily visit the research centers to undergo radiographic examination in order for VFs to be diagnosed. Therefore, we may not have been able to determine when an incident VF occurred between visits among the participants who did not attend the research center the following visit. Moreover, individuals with both T2D and VFs might have not attended the following visit more often than those with VFs or T2D alone, based on our finding of increased mortality among individuals with both T2D and VFs. This finding might also explain the decreased prevalence of VFs among individuals with T2D and why the previously published meta-analyses have been inconclusive.
The increased VF risk among individuals with T2D is complicated to elucidate as multiple factors, and mechanisms might be involved directly and indirectly. Decreased bone strength might be a factor. Bone strength is a compound entity that includes bone mass, bone microarchitecture, bone turnover, and bone material properties. While BMD as assessed by DXA seems to be increased on average (33) among individuals with T2D compared with those without, lower TBS levels do suggest trabecular microarchitecture deterioration. In a recent meta-analysis, COOH-terminal cross-linked telopeptide, a bone resorption marker, was significantly lower in individuals with T2D as well as the bone formation markers procollagen type 1 amino terminal propeptide and osteocalcin (34). These findings suggest the presence of a low bone turnover state in individuals with T2D. On cortical bone, this might predispose to increased occurrence and accumulation of microcracks, resulting in increased nonvertebral fracture risk (35), but the effect of low bone turnover on trabecular bone and a potential deleterious effect leading to increased VF risk remains to be elucidated. In contrast, trabecular bone microstructure as measured by TBS has been found in other studies to be decreased in individuals with T2D (36,37), as well as in the subsample of our current study with TBS measurements. Individuals with T2D have been reported to have decreased muscle mass, increased immobility, and increased frailty compared with healthy subjects (38,39). The increased frailty among individuals with T2D could increase the risk of fragility fractures in this group (39). Similarly to our findings, a previous study found a 20% excess in all-cause mortality after a VF among individuals with T2D compared with individuals without T2D who acquired a VF (40).
Currently, clinical fracture risk estimation tools, such as the Fracture Risk Assessment Tool, underestimate the risk of fracture among individuals with T2D, due to the tendency of this group to fracture at a higher BMD T-score (33,41). This highlights the need for other bone strength markers that can increase accuracy in fracture risk prediction among individuals with T2D. VFs are often asymptomatic, and as such, they very often go undetected but even if asymptomatic, they are strongly associated with increased incidence of fractures not only in the spine but also elsewhere in the skeleton (42). Screening for VFs is recommended in high-risk groups such as postmenopausal women and older men with a height loss ≥4 cm, kyphosis, or long-term use of glucocorticoids (43). In the current study, the presence of prevalent VFs was associated with a 50% increased hazard of nonvertebral fractures, but individuals with T2D and VFs had almost twice the risk for incident nonvertebral fractures compared with individuals with T2D alone or VFs alone. The increased risk for nonvertebral fractures was similar across all FN-BMD T-score categories (normal, osteopenia, or osteoporosis). This, together with the elevated mortality risk observed, suggests that individuals with T2D might also be good candidates for systematic VF screening without the need of BMD assessment.
This study had several limitations. Although most of the studies included in the meta-analysis had their analyses adjusted for multiple confounders, including BMI, given the U-shaped association between BMI and fracture risk, it would have been appropriate stratifying the analysis for BMI categories. Furthermore, we did not have information on other comorbidities and cause-specific mortality; this information would have helped to better understand the mechanism by which T2D and VF comorbidity is associated with increased nonvertebral fractures and mortality. We did not have information regarding the type of treatment that individuals with T2D were receiving across studies and therefore could not explore potential mechanistic influences of antidiabetes medication in our findings. Moreover, in the cohorts with IPD, we found evidence of bias due to loss to follow-up that may have resulted in underestimation of the association between T2D and VF.
Currently, there are no specific guidelines for the assessment of fracture risk or treatment of osteoporosis in individuals with T2D. On average, individuals with T2D tend to fracture at a higher BMD T-score compared with healthy individuals without diabetes (33), and traditional clinical risk assessment tools underestimate their fracture risk (41). Based on our findings, we suggest that individuals with T2D should be systematically assessed for the presence of VF, measurement currently facilitated by the lateral VF assessment readily implemented in modern DXA devices. Similar to the management of individuals without T2D, presence of VFs among individuals with T2D should be an indication for starting osteoporosis treatment (independent of any given BMD T-score) in order to prevent future fracture. Notoriously, presence of VFs in patients with T2D also constitutes a call for attention to potentially frail individuals at higher risk of mortality than that expected from T2D alone.
Supplementary Material
Article Information
Acknowledgments. The authors thank the individuals of all cohorts for their cooperation, the dedicated research team that is conducting the studies, employees from Optasia Medical Ltd. who familiarized us with the use of the SpineAnalyzer software, the study individuals, and the staff from the Rotterdam Study (particularly Hannie van den Boogert for acquisition of the radiographs and DXA measurements) and the participating general practitioners and pharmacists. The authors also thank René Vermeren (Erasmus University Medical Center, Rotterdam, the Netherlands), Nano Suwarno (Department of Epidemiology, Erasmus University Medical Center, Rotterdam, the Netherlands), and Mart Rentmeester (Department of Radiology and Nuclear Medicine, Erasmus University Medical Center, Rotterdam, the Netherlands) for technical support and the team of radiographic readers for the tremendous efforts. The authors thank Marie Almudena Metzger (Bone and Joint Department, Center of Bone Diseases, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland), the principal research nurse of the OsteoLaus cohort, who made considerable contributions to the collection of the data, as well as our DXA technologists and Claudie Berger (CaMos National Coordinating Centre, McGill University, Montreal, Quebec, Canada) for the excellent statistical and data management work over this period of time.
Funding. The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, The Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (014-93-015, RIDE2), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (Directorate-General XII), and the Municipality of Rotterdam. F.K. and F.R. are funded by the Netherlands Scientific Organization and ZonMW project number NWO/ZONMW-VIDI-016-136-367. The Study of Osteoporotic Fractures is supported by National Institutes of Health funding. The National Institute on Aging provides support under the following grants: R01-AG-005407, R01-AR-35582, R01-AR-35583, R01-AR-35584, R01-AG-005394, R01-AG-027574, and R01-AG-027576. The OsteoLaus study was and is supported by research grants from Lausanne University Hospital (Strategic plan funds) and the Swiss National Science Foundation (grant 32473B_156978). The Canadian Multicentre Osteoporosis Study has been funded by the Canadian Institutes of Health Research and various other not-for-profit or pharmaceutical agencies from 1994 to 2017.
The funding sources had no role in the study design, data collection, analysis, and interpretation, writing of the report, or decision to submit the article for publication.
Duality of Interest. M.C.Z. has received in the past fee for lectures and/or advice from Amgen, Eli Lilly and Company, Merck, and UCB Pharma. G.P.K. performs consulting activities with Bracco Imaging and has received institutional research grants from Siemens Healthineers, GE Healthcare, and Bayer AG. D.H. is co-owner of the TBS patent and has corresponding ownership shares and a position at Medimaps. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. F.K. contributed to the literature search, data analysis, data interpretation, and prepared the first draft. F.K., L.O., E.S., K.T., J.S., T.M., O.H.F., M.A.I., M.C.Z., A.G.U., G.P.K., T.A., R.J., S.M.K., D.G., B.C.L., J.C.P., W.D.L., E.M., O.L., D.H., E.H.O., and F.R. contributed to data collection, study design, and data interpretation and critically reviewed the report. F.K. and F.R. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0925/-/DC1.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Oei L, Zillikens MC, Dehghan A, et al. . High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam Study. Diabetes Care 2013;36:1619–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan Y, Wei F, Lang Y, Liu Y. Diabetes mellitus and risk of hip fractures: a meta-analysis. Osteoporos Int 2016;27:219–228 [DOI] [PubMed] [Google Scholar]
- 4.Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology 2010;21:658–668 [DOI] [PubMed] [Google Scholar]
- 5.Schwartz AV, Hillier TA, Sellmeyer DE, et al. . Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002;25:1749–1754 [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 7.Shanbhogue VV, Hansen S, Frost M, Brixen K, Hermann AP. Bone disease in diabetes: another manifestation of microvascular disease? Lancet Diabetes Endocrinol 2017;5:827–838 [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) . European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 2013;24:23–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delmas PD, van de Langerijt L, Watts NB, et al.; IMPACT Study Group . Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res 2005;20:557–563 [DOI] [PubMed] [Google Scholar]
- 10.Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ III. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res 1992;7:221–227 [DOI] [PubMed] [Google Scholar]
- 11.Wang J, You W, Jing Z, Wang R, Fu Z, Wang Y. Increased risk of vertebral fracture in patients with diabetes: a meta-analysis of cohort studies. Int Orthop 2016;40:1299–1307 [DOI] [PubMed] [Google Scholar]
- 12.Dytfeld J, Michalak M. Type 2 diabetes and risk of low-energy fractures in postmenopausal women: meta-analysis of observational studies. Aging Clin Exp Res 2017;29:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia P, Bao L, Chen H, et al. . Risk of low-energy fracture in type 2 diabetes patients: a meta-analysis of observational studies. Osteoporos Int 2017;28:3113–3121 [DOI] [PubMed] [Google Scholar]
- 14.Moayeri A, Mohamadpour M, Mousavi SF, Shirzadpour E, Mohamadpour S, Amraei M. Fracture risk in patients with type 2 diabetes mellitus and possible risk factors: a systematic review and meta-analysis. Ther Clin Risk Manag 2017;13:455–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Ba Y, Xing Q, Du JL. Diabetes mellitus and the risk of fractures at specific sites: a meta-analysis. BMJ Open 2019;9:e024067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pothuaud L, Carceller P, Hans D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone 2008;42:775–787 [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Fuerst T, Hui S, Genant HK. Standardization of bone mineral density at femoral neck, trochanter and Ward’s triangle. Osteoporos Int 2001;12:438–444 [DOI] [PubMed] [Google Scholar]
- 19.Bonds DE, Larson JC, Schwartz AV, et al. . Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab 2006;91:3404–3410 [DOI] [PubMed] [Google Scholar]
- 20.Napoli N, Schwartz AV, Schafer AL, et al.; Osteoporotic Fractures in Men (MrOS) Study Research Group . Vertebral fracture risk in diabetic elderly men: the MrOS study. J Bone Miner Res 2018;33:63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melton LJ III, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. J Bone Miner Res 2008;23:1334–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmberg AH, Johnell O, Nilsson PM, Nilsson J, Berglund G, Akesson K. Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women. Osteoporos Int 2006;17:1065–1077 [DOI] [PubMed] [Google Scholar]
- 23.Sosa M, Saavedra P, Jódar E, et al.; GIUMO Study Group . Bone mineral density and risk of fractures in aging, obese post-menopausal women with type 2 diabetes. The GIUMO Study. Aging Clin Exp Res 2009;21:27–32 [DOI] [PubMed] [Google Scholar]
- 24.Rathmann W, Kostev K. Fracture risk in patients with newly diagnosed type 2 diabetes: a retrospective database analysis in primary care. J Diabetes Complications 2015;29:766–770 [DOI] [PubMed] [Google Scholar]
- 25.Goldshtein I, Nguyen AM, dePapp AE, et al. . Epidemiology and correlates of osteoporotic fractures among type 2 diabetic patients. Arch Osteoporos 2018;13:15. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res 2009;24:702–709 [DOI] [PubMed] [Google Scholar]
- 27.Kreiger N, Tenenhouse A, Joseph L, et al. . Research notes: the Canadian Multicentre Osteoporosis Study (CaMos): background, rationale, methods. Can J Aging 1999;18:376–387 [Google Scholar]
- 28.McCloskey EV, Beneton M, Charlesworth D, et al. . Clodronate reduces the incidence of fractures in community-dwelling elderly women unselected for osteoporosis: results of a double-blind, placebo-controlled randomized study. J Bone Miner Res 2007;22:135–141 [DOI] [PubMed] [Google Scholar]
- 29.Shevroja E, Marques-Vidal P, Aubry-Rozier B, et al. . Cohort profile: the OsteoLaus study. Int J Epidemiol 2019;48:1046–1047 [DOI] [PubMed] [Google Scholar]
- 30.Ikram MA, Brusselle GGO, Murad SD, et al. . The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol 2017;32:807–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cummings SR, Black DM, Nevitt MC, et al.; The Study of Osteoporotic Fractures Research Group . Appendicular bone density and age predict hip fracture in women. JAMA 1990;263:665–668 [PubMed] [Google Scholar]
- 32.Yu X, Zhao X, Yin H. Comment on Wang et al.: increased risk of vertebral fracture in patients with diabetes: a meta-analysis of cohort studies. Int Orthop 2016;40:1561–1562 [DOI] [PubMed] [Google Scholar]
- 33.Ma L, Oei L, Jiang L, et al. . Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol 2012;27:319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hygum K, Starup-Linde J, Harsløf T, Vestergaard P, Langdahl BL. Mechanisms in endocrinology: diabetes mellitus, a state of low bone turnover - a systematic review and meta-analysis. Eur J Endocrinol 2017;176:R137–R157 [DOI] [PubMed] [Google Scholar]
- 35.Farr JN, Khosla S. Determinants of bone strength and quality in diabetes mellitus in humans. Bone 2016;82:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leslie WD, Aubry-Rozier B, Lamy O, Hans D; Manitoba Bone Density Program . TBS (trabecular bone score) and diabetes-related fracture risk. J Clin Endocrinol Metab 2013;98:602–609 [DOI] [PubMed] [Google Scholar]
- 37.Kim JH, Choi HJ, Ku EJ, et al. . Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab 2015;100:475–482 [DOI] [PubMed] [Google Scholar]
- 38.Bianchi L, Volpato S. Muscle dysfunction in type 2 diabetes: a major threat to patient’s mobility and independence. Acta Diabetol 2016;53:879–889 [DOI] [PubMed] [Google Scholar]
- 39.Li G, Prior JC, Leslie WD, et al.; CaMos Research Group . Frailty and risk of fractures in patients with type 2 diabetes. Diabetes Care 2019;42:507–513 [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Laguna D, Nogues X, Abrahamsen B, et al. . Excess of all-cause mortality after a fracture in type 2 diabetic patients: a population-based cohort study. Osteoporos Int 2017;28:2573–2581 [DOI] [PubMed] [Google Scholar]
- 41.Giangregorio LM, Leslie WD, Lix LM, et al. . FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res 2012;27:301–308 [DOI] [PubMed] [Google Scholar]
- 42.Schousboe JT, Lix LM, Morin SN, et al. . Prevalent vertebral fracture on bone density lateral spine (VFA) images in routine clinical practice predict incident fractures. Bone 2019;121:72–79 [DOI] [PubMed] [Google Scholar]
- 43.Compston J, Cooper A, Cooper C, et al.; National Osteoporosis Guideline Group (NOGG) . UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 2017;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.