Abstract
OBJECTIVE
To determine if the International Hypoglycaemia Study Group (IHSG) level 2 low glucose definition can identify clinically relevant hypoglycemia in clinical trials and offer value as an end point for future trials.
RESEARCH DESIGN AND METHODS
A post hoc analysis was performed of the SWITCH (SWITCH 1: n = 501, type 1 diabetes; SWITCH 2: n = 721, type 2 diabetes) and DEVOTE (n = 7,637, type 2 diabetes) trials utilizing the IHSG low glucose definitions. Patients in all trials were randomized to either insulin degludec or insulin glargine 100 units/mL. In the main analysis, the following definitions were compared: 1) American Diabetes Association (ADA) 2005 (plasma glucose [PG] confirmed ≤3.9 mmol/L with symptoms); and 2) IHSG level 2 (PG confirmed <3.0 mmol/L, independent of symptoms).
RESULTS
In SWITCH 2, the estimated rate ratios of hypoglycemic events indicated increasing differences between treatments with decreasing PG levels until 3.0 mmol/L, following which no additional treatment differences were observed. Similar results were observed for the SWITCH 1 trial. In SWITCH 2, the IHSG level 2 definition produced a rate ratio that was lower than the ADA 2005 definition.
CONCLUSIONS
The IHSG level 2 definition was validated in a series of clinical trials, demonstrating its ability to discriminate between basal insulins. This definition is therefore recommended to be uniformly adopted by regulatory bodies and used in future clinical trials.
Introduction
Currently, the definition of hypoglycemia differs between regulatory bodies as well as across clinical trials (1–7). For example, hypoglycemia can be defined with or without a plasma glucose (PG) value, with or without the presence of symptoms, with a requirement for medical assistance (e.g., emergency medical services or hospitalization), or by different levels of cognitive dysfunction. This variation in hypoglycemia definitions has limited the diabetes community’s ability to compare the safety and tolerability of therapies across clinical trials, despite some studies investigating a range of hypoglycemia definitions, such as the EDITION trials (7).
As a result, the International Hypoglycaemia Study Group (IHSG), a group of clinicians formed to promote greater understanding of the clinical impact of hypoglycemia, recently proposed a revised classification of low glucose in diabetes (8). The IHSG proposed three definition levels be adopted universally: level 1, glucose ≤3.9 mmol/L; level 2, glucose <3.0 mmol/L; and level 3, severe events requiring third-party intervention independent of a defined glucose value (9). Of these three, the IHSG proposed that the level 2 definition should be added to current classifications as a level sufficiently low to indicate clinically significant hypoglycemia. This proposal has subsequently been accepted and adopted by the American Diabetes Association (ADA), European Association for the Study of Diabetes, and other organizations including, importantly, the European Medicines Agency (8,10,11). The IHSG level 2 definition was based on evidence derived from experimental pathophysiological studies and small clinical trials, but has not been applied to larger-scale clinical trial data involving patients with type 2 diabetes (8). The aim of the current study was to validate the IHSG level 2 definition by applying it to a series of clinical trials in patients with type 1 or type 2 diabetes, investigate the clinical relevance of hypoglycemia defined with a low glucose cutoff, and demonstrate the utility of the IHSG level 2 definition in the conduct of future clinical trials of basal insulins.
Research Design and Methods
To apply the IHSG low glucose definitions, data from three double-blind, basal insulin comparator trials (SWITCH trials [ClinicalTrials.gov numbers NCT02030600 (SWITCH 2, type 2 diabetes) and NCT02034513 (SWITCH 1, type 1 diabetes) (12,13)]; and DEVOTE cardiovascular outcomes trial [ClinicalTrials.gov number NCT01959529 (type 2 diabetes) (14,15)]) were used. These trials were all conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice Guidelines (16,17), and detailed accounts of their trial designs, methods, and primary results have been published previously (12–15). A summary of these trials is included in Supplementary Table 1.
The purpose of this study was to apply the IHSG low glucose definitions to the SWITCH and DEVOTE trials. A summary of the definitions used in this analysis can be found in Supplementary Table 2. The IHSG considered glucose concentrations detected by self-/laboratory-measured PG or continuous glucose monitoring. In this study, we use the IHSG terminology of “glucose” whether from capillary, venous, arterial, or interstitial fluid sample measurements. Briefly, the IHSG level 2 (confirmed events <3.0 mmol/L [8]) and IHSG level 3 (events requiring third-party assistance [8,9]) definitions were applied to the trial data. For additional comparison, the ADA 2005 definition (a PG of ≤3.9 mmol/L with symptoms [18]) and the Novo Nordisk definition (a PG of <3.1 mmol/L with symptoms plus severe events [events requiring third-party assistance] [12,13]) were also included. Due to the DEVOTE trial design, only the IHSG level 3 hypoglycemia definition was assessed in this trial.
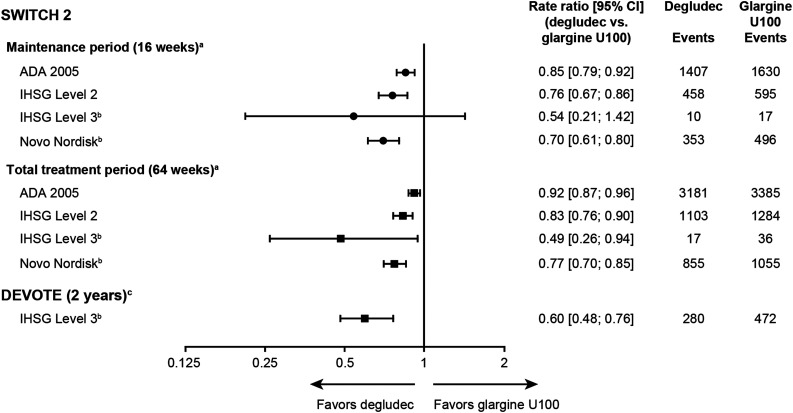
In view of the double-blind, crossover design of the SWITCH trials and increasing attention on hypoglycemia as an important consideration in type 2 diabetes management, the focus of this analysis was on the SWITCH 2 (type 2 diabetes) trial results. Results from the SWITCH 1 (type 1 diabetes) trial can be found in the Supplementary Data, and the severe hypoglycemia (IHSG level 3) results from DEVOTE are included in Fig. 4.
Figure 4.
Hypoglycemic events in SWITCH 2 and DEVOTE by treatment group. aThe total trial duration was 64 weeks; this included 32 weeks’ treatment with once-daily degludec or glargine U100 followed by crossover to glargine U100 or degludec, respectively, for a further 32 weeks. Each 32-week treatment period consisted of a 16-week titration period and a 16-week maintenance period. bPrespecified hypoglycemia definition as used during the original SWITCH 2 and DEVOTE trials. cThe median observation time was 1.99 years, and the median exposure time was 1.83 years. ADA 2005: PG ≤3.9 mmol/L with symptoms; IHSG level 2: glucose <3.0 mmol/L; IHSG level 3: severe events requiring third-party intervention independent of a defined glucose value; Novo Nordisk: PG <3.1 mmol/L with symptoms plus severe events. Glargine U100, insulin glargine 100 units/mL.
Statistical Analyses
Due to the crossover design of the SWITCH trials, hypoglycemia was analyzed with a Poisson model with patients as a random effect comparing insulin degludec (degludec) with insulin glargine 100 units/mL (glargine U100). The DEVOTE trial analyzed hypoglycemia using a negative binomial-regression model comparing degludec with glargine U100.
The frequency of hypoglycemic events was assessed by PG levels within the range of 2.0–3.9 mmol/L on a pooled randomized treatment data set. The hypoglycemia rate ratios (degludec vs. glargine U100) were assessed by glucose levels within the same range. This range was selected to encompass the full extent of the ADA 2005 and IHSG level 2 definitions while taking into consideration the low number of hypoglycemic events that occur below 2.0 mmol/L.
Data and Resource Availability
The data sets analyzed during the current study are available from the corresponding author on reasonable request.
Results
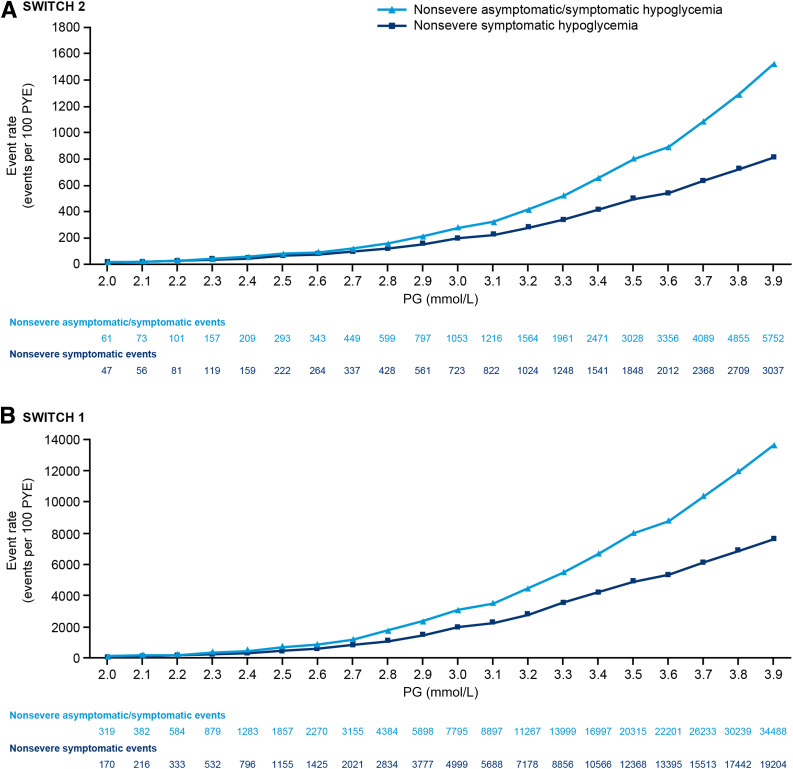
In SWITCH 1 and 2, the rate of nonsevere hypoglycemic events (both symptomatic and combined asymptomatic/symptomatic events) increased from 3.0 to 3.9 mmol/L, with a greater number of events at PG levels approaching 3.9 mmol/L versus PG levels approaching 3.0 mmol/L (Fig. 1). Similar rates of hypoglycemic events were seen for the total treatment period and after 16 weeks of titration (the maintenance period) (Fig. 1 and Supplementary Fig. 1).
Figure 1.
Nonsevere hypoglycemic events (total and symptomatic) in the maintenance period of SWITCH 2 (A) and 1 (B) at different PG levels in a pooled randomized treatment data set. The event rates in the pooled randomized treatment data set (degludec and glargine U100) are plotted at a given PG level or lower. PYE, patient year of exposure.
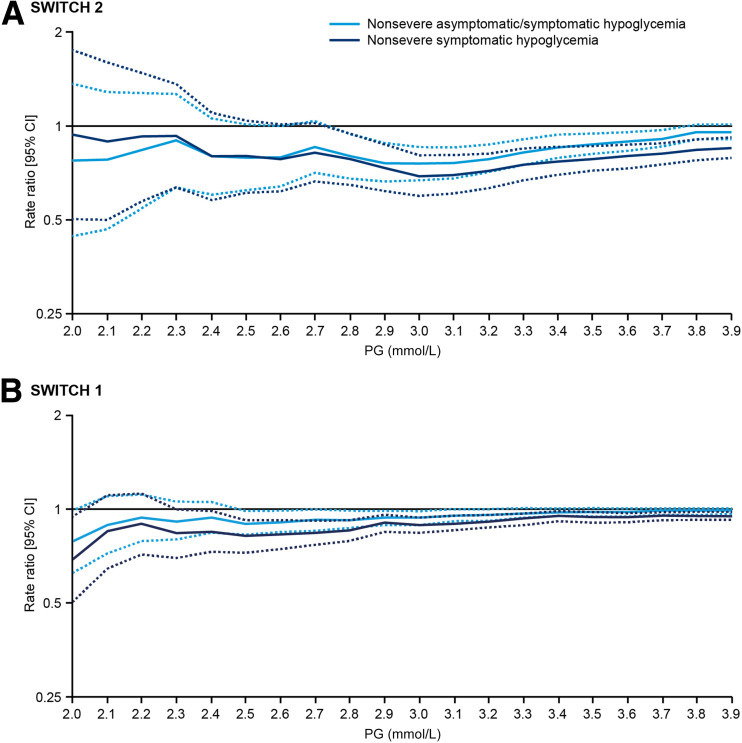
In SWITCH 1 and 2, the estimated rate ratios of hypoglycemic events comparing two basal insulins decreased with decreasing glucose cutoff levels until 3.0 mmol/L, indicating greater divergence between the basal insulins with respect to hypoglycemic risk (Fig. 2). Thereafter, a plateau was reached, and the differences between the two insulins did not increase any further. A similar result was seen for both the maintenance and total treatment periods (Fig. 2 and Supplementary Fig. 2). It is important to note that the CIs widen at the lower glucose levels due to the fewer number of events as illustrated in Fig. 1 and Supplementary Fig. 1. This is reflected by the upper CIs, which in SWITCH 2 cross the rate ratio of 1 at ∼2.7 mmol/L and in SWITCH 1 at ∼2.5 mmol/L (Fig. 2). In addition, the differences between treatments in terms of reduction in hypoglycemia increased, represented by the lower estimated rate ratios at each glucose level, when asymptomatic events were excluded (Fig. 2). Lastly, minimal differences in the rate ratios were observed between the 3.0 mmol/L and the 3.1 mmol/L cutoffs.
Figure 2.
Estimated rate ratios of nonsevere hypoglycemic events (total and symptomatic; degludec vs. glargine U100) in the maintenance period of SWITCH 2 (A) and 1 (B) at different PG levels. The solid lines represent the estimated rate ratio (degludec vs. glargine U100) at different PG levels. The dashed lines represent the upper and lower 95% CIs.
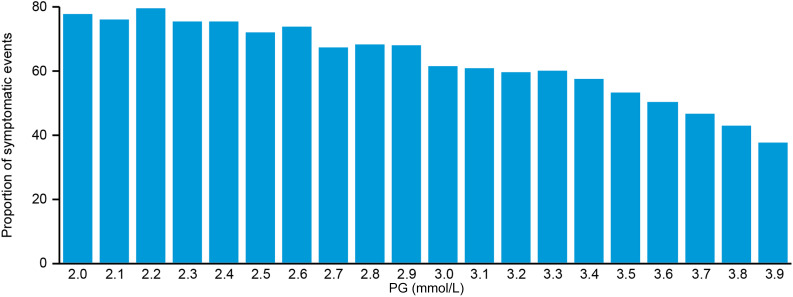
Figure 3 shows that symptoms were reported for 70–80% of nonsevere hypoglycemic events with glucose levels <3.0 mmol/L while decreasing to ∼40% for those events with glucose levels at 3.9 mmol/L.
Figure 3.
Proportion of nonsevere hypoglycemic events that were symptomatic in SWITCH 2 at different PG levels.
In SWITCH 2, the IHSG level 2 definition produced a rate ratio of 0.76 (95% CI 0.67; 0.86) for degludec versus glargine U100, which is in line with the point estimates using the prespecified Novo Nordisk hypoglycemia definition (Fig. 4). Compared with the ADA 2005 definition, the differences between the insulins increased with the IHSG level 2 and Novo Nordisk definitions although the rate ratios for the Novo Nordisk definition were lower, as this definition included confirmed symptomatic events only (Fig. 4). Comparable results across the definitions were observed for the maintenance and total treatment periods.
In terms of severe hypoglycemia (IHSG level 3), there was a similarly lower rate ratio of hypoglycemia with degludec versus glargine U100 for both treatment periods and also for the larger and longer trial, DEVOTE, in which the CIs were narrower due to the larger number of events (Fig. 4). The rate ratios of hypoglycemic events for the SWITCH 1 trial can be seen in Supplementary Fig. 3.
Conclusions
The results from this analysis provide external validation for the IHSG level 2 definition by demonstrating that this definition could identify greater differences between two different basal insulins in patients with type 1 or type 2 diabetes compared with the ADA 2005 definition. The limited power to identify differences between two basal insulins recorded at blood glucose levels above 3.0 mmol/L supports the IHSG’s recommendations that events between 3.0 and 3.9 mmol/L (level 1), while serving as an alert value, do not necessarily need to be reported as the primary outcome in clinical trials. However, while the discriminatory power between treatments at glucose levels between 3.1 and 3.9 mmol/L is limited and, arguably, less clinically relevant, it may be pertinent for these events still to be recorded, perhaps as secondary outcomes, given the importance to patients of needing to deal with such episodes. This would ensure that events most critical for the ongoing health of patients (<3.0 mmol/L) are recorded and allow for accurate comparisons between treatments, while still recording those events at higher glucose levels (≥3.0 mmol/L). It seems likely that our understanding of the consequences of particular glucose levels will evolve as more trials begin to include continuous glucose monitoring that can assess the full range of glucose levels over 24 h.
An additional observation from this analysis was that the rate ratios of hypoglycemia change across a range of PG values. This highlights the need for the same glucose definitions to be used across clinical trials, as different results can be obtained depending on the definition. Furthermore, applying the IHSG level 2 definition did not change the previous conclusions from the SWITCH trials. Therefore, we acknowledge that while the differences between 3.0 and 3.1 mmol/L are negligible, as might be expected, our study supports the importance of the IHSG level 2 definition (glucose <3.0 mmol/L) for consistency across clinical trials.
Lastly, the requirement to identify symptoms associated with a hypoglycemic event is not essential to be able to differentiate between treatments, particularly since thresholds for symptoms can vary between individuals as well as within the same individual on different occasions. In the present analysis, the power to differentiate between basal insulins increased when only symptomatic events were included (Novo Nordisk definition). This is pertinent to the U.S. Food and Drug Administration definition of hypoglycemia that currently stipulates that an event should be associated with symptoms and have a PG level <3.9 mmol/L (19). However, asymptomatic events should not be overlooked; arguably, they are more clinically relevant, as they lead to cognitive impairment and reduced hypoglycemia awareness and are associated with cardiac arrhythmias (18,20–22). Therefore, we would argue that in future clinical trials, both events with and without symptoms are recorded, but with the knowledge that excluding asymptomatic events might further highlight the differences between interventions, at least in the case of basal insulins.
Removing the confounding element of different hypoglycemia definitions will add power to future analyses of treatments across clinical trials. By also focusing on those events with a lower glucose, trials can highlight clinically important events while excluding events at higher glucose levels that may be less clinically relevant. The ADA, European Association for the Study of Diabetes, and European Medicines Agency have already taken the step to incorporate the IHSG definitions in their guidance documents (8,10,11). To ensure that clinicians are aligned globally, we hope that the Food and Drug Administration and other regulatory bodies will consider incorporating these definitions in an updated guidance document. It will also be important for pharmaceutical companies to take steps to align the definitions of hypoglycemia in their clinical trials. Furthermore, it could be pertinent for companies to analyze their data retrospectively with the IHSG level 2 definition so that new meta-analyses across trials can be conducted.
The current analysis has a number of limitations. DEVOTE only collected severe hypoglycemic events (IHSG level 3), thereby limiting comparisons with the other definitions used in this analysis. However, the point estimate for the relative risk was comparable to SWITCH 2 with narrower CIs, emphasizing the increased statistical power of a trial with a larger number of events.
As shown in Fig. 2, there was a smaller number of events at lower PG levels, reflected by the widening CIs, thereby limiting the interpretation of these events. Thus, the choice of a clinically relevant hypoglycemic level to be used in clinical trials is a compromise between one that highlights the difference between two insulins while retaining sufficient power to demonstrate statistical significance. This is clearly shown in SWITCH 2 (Fig. 2), in which the rate ratios fall to a minimum at PG levels of 2.9–3.0 mmol/L. At PG levels below this, the CIs widen, so that at 2.7 mmol/L, the rate ratio no longer achieves statistical significance. We would argue this justifies the choice of a glucose level below 3.0 mmol/L to be used in clinical trials. The differences observed between the two basal insulins in SWITCH 1 are less clear-cut, presumably due to the addition of bolus insulin that contributed to the total number of hypoglycemic events, but a PG level below 3.0 mmol/L appears to be a reasonable compromise.
Our analysis only extends to clinical trials investigating basal insulins, as that was the focus of the comparisons in the trials analyzed. Therefore, the applicability of these findings to trials comparing other interventions is unknown. Lastly, these trials did not include data from continuous glucose monitoring, which is being increasingly adopted as a gold standard for hypoglycemia detection with its own recommendations for reporting (23).
This analysis also has a number of strengths, including the double-blind trial design of the SWITCH and DEVOTE trials and the crossover design of the SWITCH trials. Furthermore, in the SWITCH and DEVOTE trials, individuals with a prior history of multiple hypoglycemic events or severe hypoglycemia were not excluded, arguably making these trials and our analysis more relevant to patients seen in routine clinical practice. In addition, this analysis included a large number of patients across the SWITCH and DEVOTE trials as well as a high number of independently adjudicated severe hypoglycemic events (level 3; SWITCH and DEVOTE), emphasizing the robustness of this analysis.
In conclusion, as the glucose threshold defining hypoglycemic events is decreased, fewer events occur. These events are more symptomatic, and the discriminatory power between two basal insulins increases. Therefore, the results from this analysis provide empirical support, based on clinical trials with a double-blind design, for the adoption of the IHSG level 2 definition by regulatory bodies and future clinical trials.
Supplementary Material
Article Information
Acknowledgments. Medical writing assistance and editorial/submission support were provided by Francesca Hemingway and Richard McDonald of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc (Dublin, Ireland), funded by Novo Nordisk A/S. Novo Nordisk was involved in the design of this secondary analysis, provided logistical support, and obtained the data, which were evaluated jointly by the authors and the sponsor.
Funding. J.B.B. is supported by grants from the National Institutes of Health (UL1-TR-002489, U01-DK-098246, UC4-DK-108612, and U54-DK-118612), Patient-Centered Outcomes Research Institute, and ADA.
Duality of Interest. These trials and this secondary analysis were funded by Novo Nordisk. S.R.H. has served on speaker panels for Eli Lilly and Company and Novo Nordisk, for which he has received remuneration, and has served on advisory panels or as a consultant for Zeeland, UNEEG Medical, Boehringer Ingelheim, Novo Nordisk, Eli Lilly and Company, Sanofi Aventis, and Takeda Pharmaceutical Company, for which his institution has received remuneration. J.B.B. has received contracted consulting fees paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm Inc., Eli Lilly and Company, MannKind Corporation, NovaTarg Therapeutics, Novo Nordisk, Senseonics, vTv Therapeutics, and Zafgen and grant support from Novo Nordisk, Sanofi, and vTv Therapeutics; is a consultant to Cirius Therapeutics, CSL Behring, Neurimmune Holding AG, and Whole Biome; and holds stock options in Mellitus Health, PhaseBio Pharmaceuticals, Inc., Stability Health, and Whole Biome. R.R. has served as a consultant to Novo Nordisk, Merck, Dexcom, Intarcia Therapeutics, and Virta Health. E.S. has served as a consultant for Eli Lilly and Company, Zucara Therapeutics, Sanofi, MannKind Corporation, WebMD, and 360 Consulting and received grant funding from Eli Lilly and Company and Locemia Solutions that went to her institution. L.B., C.T.H., and D.T. are full-time employees of and hold stock in Novo Nordisk A/S. A.C.M. was a full-time employee of Novo Nordisk until 30 June 2018 and currently serves as an independent consultant and holds shares in Novo Nordisk A/S.
Author Contributions. S.R.H., J.B.B., and A.C.M. conceived and designed the study, acquired, analyzed, and interpreted the data, and drafted and revised the manuscript. R.R., E.S., C.T.H., and D.T. analyzed and interpreted the data and drafted and revised the manuscript. L.B. conceived and designed the study, acquired, analyzed, and interpreted the data, performed statistical analyses, and drafted and revised the manuscript. S.R.H. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-2361/-/DC1.
A.C.M. is currently an independent consultant in Portsmouth, NH.
See accompanying article, p. 272.
References
- 1.American Diabetes Association Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(Suppl. 1):S1–S13527979885 [Google Scholar]
- 2.Zoungas S, Patel A, Chalmers J, et al.; ADVANCE Collaborative Group . Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 3.Green JB, Bethel MA, Armstrong PW, et al.; TECOS Study Group . Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–242 [DOI] [PubMed] [Google Scholar]
- 4.Bonds DE, Miller ME, Bergenstal RM, et al. . The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerstein HC, Bosch J, Dagenais GR, et al.; ORIGIN Trial Investigators . Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 7.Ritzel R, Roussel R, Giaccari A, Vora J, Brulle-Wohlhueter C, Yki-Järvinen H. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab 2018;20:541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Hypoglycaemia Study Group Glucose concentrations of less than 3.0 mmol/l (54 mg/dl) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017;40:155–157 [DOI] [PubMed] [Google Scholar]
- 9.Seaquist ER, Anderson J, Childs B, et al. . Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S1–S15929222369 [Google Scholar]
- 11.European Medicines Agency Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. CPMP/EWP/1080/00 Rev. 2 [Internet], 2018. Available from https://www.ema.europa.eu/documents/scientific-guideline/draft-guideline-clinical-investigation-medicinal-products-treatment-prevention-diabetes-mellitus_en.pdf. Accessed 15 October 2019
- 12.Wysham C, Bhargava A, Chaykin L, et al. . Effect of Insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 2 diabetes: the SWITCH 2 randomized clinical trial. JAMA 2017;318:45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane W, Bailey TS, Gerety G, et al.; Group Information; SWITCH 1 . Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 1 diabetes: the SWITCH 1 randomized clinical trial. JAMA 2017;318:33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marso SP, McGuire DK, Zinman B, et al. . Design of DEVOTE (trial comparing cardiovascular safety of insulin degludec vs insulin glargine in patients with type 2 diabetes at high risk of cardiovascular events) - DEVOTE 1. Am Heart J 2016;179:175–183 [DOI] [PubMed] [Google Scholar]
- 15.Marso SP, McGuire DK, Zinman B, et al.; DEVOTE Study Group . Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med 2017;377:723–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–2194 [DOI] [PubMed] [Google Scholar]
- 17.International Council for Harmonisation ICH Harmonised Tripartite Guideline: guideline for good clinical practice. J Postgrad Med 2001;47:199–203 [PubMed] [Google Scholar]
- 18.Workgroup on Hypoglycemia, American Diabetes Association Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245–1249 [DOI] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Guidance for Industry - Diabetes Mellitus: Developing Drugs and Therapeutic Biologics for Treatment and Prevention (Draft Guidance) [Internet], 2008. Available from https://www.fda.gov/media/71289/download. Accessed 15 October 2019
- 20.Chow E, Bernjak A, Walkinshaw E, et al. . Cardiac autonomic regulation and repolarization during acute experimental hypoglycemia in type 2 diabetes. Diabetes 2017;66:1322–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson RT, Harris ND, Ireland RH, Lee S, Newman C, Heller SR. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycemia. Diabetes 2003;52:1469–1474 [DOI] [PubMed] [Google Scholar]
- 22.Veneman T, Mitrakou A, Mokan M, Cryer P, Gerich J. Induction of hypoglycemia unawareness by asymptomatic nocturnal hypoglycemia. Diabetes 1993;42:1233–1237 [DOI] [PubMed] [Google Scholar]
- 23.Schnell O, Barnard K, Bergenstal R, et al. . Role of continuous glucose monitoring in clinical trials: recommendations on reporting. Diabetes Technol Ther 2017;19:391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.