Abstract
OBJECTIVE
To evaluate the impact of once-weekly exenatide (EQW) on microvascular and cardiovascular (CV) outcomes by baseline renal function in the Exenatide Study of Cardiovascular Event Lowering (EXSCEL).
RESEARCH DESIGN AND METHODS
Least squares mean difference (LSMD) in estimated glomerular filtration rate (eGFR) from baseline between the EQW and placebo groups was calculated for 13,844 participants. Cox regression models were used to estimate effects by group on incident macroalbuminuria, retinopathy, and major adverse CV events (MACE). Interval-censored time-to-event models estimated effects on renal composite 1 (40% eGFR decline, renal replacement, or renal death) and renal composite 2 (composite 1 variables plus macroalbuminuria).
RESULTS
EQW did not change eGFR significantly (LSMD 0.21 mL/min/1.73 m2 [95% CI −0.27 to 0.70]). Macroalbuminuria occurred in 2.2% of patients in the EQW group and in 2.5% of those in the placebo group (hazard ratio [HR] 0.87 [95% CI 0.70–1.07]). Neither renal composite was reduced with EQW in unadjusted analyses, but renal composite 2 was reduced after adjustment (HR 0.85 [95% CI 0.74–0.98]). Retinopathy rates did not differ by treatment group or in the HbA1c-lowering or prior retinopathy subgroups. CV outcomes in those with eGFR <60 mL/min/1.73 m2 did not differ by group. Those with eGFR ≥60 mL/min/1.73 m2 had nominal risk reductions for MACE, all-cause mortality, and CV death, but interactions by renal function group were significant for only stroke (HR 0.74 [95% CI 0.58–0.93]; P for interaction = 0.035) and CV death (HR 1.08 [95% CI 0.85–1.38]; P for interaction = 0.031).
CONCLUSIONS
EQW had no impact on unadjusted retinopathy or renal outcomes. CV risk was modestly reduced only in those with eGFR ≥60 mL/min/1.73 m2 in analyses unadjusted for multiplicity.
Introduction
Patients with type 2 diabetes are at increased risk for microvascular complications, including retinopathy and nephropathy. The combination of chronic kidney disease (CKD) and diabetes augments the risk for macrovascular complications, making it higher than that with diabetes alone (1–3). While improved glycemic control reduces microvascular risk (4) and has a modest impact on macrovascular outcomes (5), recent evidence suggests that sodium–glucose cotransporter-2 (SGLT-2) inhibitors and some glucagon-like peptide 1 (GLP-1) receptor agonists (RAs) may exert beneficial effects independent of glucose lowering (6–8).
The Exenatide Study of Cardiovascular Event Lowering (EXSCEL) was a multinational, placebo-controlled, randomized cardiovascular (CV) outcome trial designed to assess the impact of the GLP-1 RA exenatide (2 mg taken once weekly; EQW) versus that of placebo when added to usual care in patients with type 2 diabetes who had a wide range of CV risk (9,10). The study randomized 14,752 participants from 35 countries and demonstrated, over a median 3.2-year follow-up, the noninferiority, but not superiority, of EQW compared with a placebo for the primary major adverse CV event (MACE) outcome—a composite of CV-related death, nonfatal myocardial infarction, or nonfatal stroke (hazard ratio [HR] 0.91 [95% CI 0.83–1.00]; P = 0.061)—and a reduced risk for all-cause mortality (HR 0.86 [95% CI 0.77–0.97]; P = 0.016) that was nominally significant because of the prespecified hierarchical testing paradigm (10). Although the study excluded participants with an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 at baseline, 21.7% had at least CKD stage 3 (eGFR <60 mL/min/1.73 m2). Here we report key primary and secondary CV outcomes, according to the degree of renal dysfunction, and microvascular outcomes measured among the overall population.
Research Design and Methods
Trial Design
The design and primary results of EXSCEL (clinical trial reg. no. NCT01144338, ClinicalTrials.gov) have been described (9,10). The trial was conducted jointly by the Duke Clinical Research Institute and the University of Oxford Diabetes Trials Unit in an academic collaboration with the sponsor, Amylin Pharmaceuticals, a wholly owned subsidiary of AstraZeneca. The protocol was approved by the ethics committee at each participating site, and all participants provided written informed consent for trial participation. Briefly, 14,752 adult participants with type 2 diabetes (HbA1c 6.5–10.0% [48–96 mmol/mol]) who had either had a prior CV event (n = 10,782 [73.1%]) or not had a prior CV event (n = 3,790 [26.9%]) were randomized 1:1 to receive EQW or placebo in addition to usual care. EXSCEL was a pragmatic trial in which laboratory data, measured per local clinical care guidelines in local laboratories, were collected opportunistically, with the only exceptions being serum creatinine, which was required to be measured annually to inform possible EQW dose changes, and calcitonin, which was measured annually by a central laboratory. Key exclusion criteria were a history of two or more episodes of severe hypoglycemia (defined as hypoglycemia for which a patient received third-party assistance) during the preceding 12 months, end-stage kidney disease or an eGFR <30 mL/min/1.73 m2 body surface area, or previous treatment with a GLP-1 RA. The primary outcome was time to the first occurrence of any component of the MACE composite (death from CV causes, nonfatal myocardial infarction, or nonfatal stroke). In time-to-event analyses, key secondary outcomes were death from any cause; death from a CV cause; and the first occurrence of nonfatal or fatal myocardial infarction, nonfatal or fatal stroke, hospitalization for acute coronary syndrome, or hospitalization for heart failure. Information was collected systematically for all events at 1 week; at 2, 6, and 12 months; and every 6 months thereafter. An independent clinical events classification committee blinded to treatment assignment adjudicated all components of the primary and secondary outcomes. Criteria for adjudication are defined in the Clinical Event Definitions section of the Supplementary Data.
Prespecified additional microvascular outcomes reported here comprise renal composite 1 (time to first event of a 40% decline in eGFR [11], renal replacement, or renal death), renal composite 2 (renal composite 1 variables plus incident macroalbuminuria), and incident retinopathy. Also analyzed were progression to a 30% or 40% decline in eGFR as well as progression to CKD stage 3, 4, or 5. Results for progression end points were not meaningfully different from those reflected in the renal composites and are not presented here. Laboratory values for eGFR were obtained from blood sampling during usual care, consistent with the pragmatic trial design. Progression to micro- or macroalbuminuria was classified as an expected diabetes complication and was assessed at each visit via a yes-or-no answer to the question, “Since the previous visit, did the patient experience any new or worsening occurrences of albuminuria?” An affirmative response indicated classification of the event as either micro- or macroalbuminuria. Additional clinical data (e.g., urine albumin-to-creatinine ratio) were neither collected nor adjudicated.
Retinopathy events were classified as an expected diabetes complication and subject to pragmatic prospective data collection at each visit via a yes-or-no answer to the following question: “Since the previous visit, did the patient experience any new or worsening occurrences of retinopathy?” Additional clinical data (e.g., retinal exam results) for this end point were neither collected nor adjudicated.
Statistical Analysis
The intention-to-treat population was used for all analyses. Baseline characteristics were summarized, using mean (±1 SD), median (25th, 75th percentile), or number (proportion), as appropriate, for continuous and categorical variables. The overall least squares mean difference (LSMD) in eGFR between the EQW and placebo treatment groups was calculated for participants with a baseline value and at least one follow-up value. Changes in HbA1c early after randomization were calculated for patients with baseline and follow-up values within the 1st year, for use in the retinopathy subgroup analyses; the value closest to 6 months after baseline (capped at 1 year) was chosen. Subgroups according to baseline renal function were eGFR <60 and ≥60 mL/min/1.73 m2, CKD stage 1 (eGFR ≥90 mL/min/1.73 m2), CKD stage 2 (eGFR 60–89 mL/min/1.73 m2), CKD stage 3a (eGFR 45–59 mL/min/1.73 m2), CKD stage 3b (eGFR 30–44 mL/min/1.73 m2), CKD stage 4 (eGFR 15–29 mL/min/1.73 m2), and CKD stage 5 (eGFR <15 mL/min/1.73 m2). Subgroups for CKD stages 4 (n = 14) and 5 (n = 0) were too small to allow robust analyses and have been excluded from this report. Although few of the individuals categorized as having CKD stage 1 or stage 2 had concomitant albuminuria, as classically defined (12,13), we use CKD staging nomenclature throughout for descriptive simplicity.
The impact of EQW on the two renal composite outcomes was estimated with interval-censored time-to-event models to account for clustering of eGFR collection dates around study visits. Unadjusted models and models adjusted for prespecified variables including age, sex, ethnicity, race, region, diabetes duration, history of CV event, diabetes therapy at baseline (including insulin use), baseline HbA1c, eGFR, and BMI are presented. Unadjusted and adjusted Cox regression models were used to estimate the impact of treatment on all other end points with continuous dates of events. Only unadjusted models are presented unless adjustment resulted in notably different changes.
Results
Baseline characteristics by CKD stage for the 14,691 participants included in the intention-to-treat analysis were well balanced between treatment groups (data not shown) and broadly demonstrate advancing age, increasing duration of diabetes, and increasing burden of comorbidities with advancing CKD (Table 1). To inform the retinopathy subgroup analysis, participants were divided into tertiles according to the degree of HbA1c change achieved during the first 6 months of study enrollment: 13.5% of the EQW group and 4.4% of the placebo group achieved an HbA1c reduction >2% (Supplementary Table 1).
Table 1.
Baseline characteristics of participants by CKD stage
| Stage 1 (n = 4,268) | Stage 2 (n = 7,246) | Stage 3a (n = 2,288) | Stage 3b (n = 889) | |
|---|---|---|---|---|
| Age (years) | 57.7 (9.3) | 62.3 (8.7) | 65.9 (8.4) | 68.0 (8.5) |
| <65 | 3,260/4,268 (76.4) | 4,278/7,246 (59.0) | 945/2,288 (41.3) | 300/889 (33.7) |
| ≥65 | 1,008/4,268 (23.6) | 2,968/7,246 (41.0) | 1,343/2,288 (58.7) | 589/889 (66.3) |
| ≥75 | 125/4,268 (2.9) | 547/7,246 (7.5) | 353/2,288 (15.4) | 215/889 (24.2) |
| Sex | ||||
| Male | 2,814/4,268 (65.9) | 4,487/7,246 (61.9) | 1,330/2,288 (58.1) | 485/889 (54.6) |
| Female | 1,454/4,268 (34.1) | 2,759/7,246 (38.1) | 958/2,288 (41.9) | 404/889 (45.4) |
| Race | ||||
| White | 3,093/4,267 (72.5) | 5,578/7,243 (77.0) | 1,777/2,287 (77.7) | 678/889 (76.3) |
| Asian | 493/4,267 (11.6) | 653/7,243 (9.0) | 211/2,287 (9.2) | 90/889 (10.1) |
| Black | 318/4,267 (7.5) | 398/7,243 (5.5) | 107/2,287 (4.7) | 51/889 (5.7) |
| Hispanic | 333/4,267 (7.8) | 554/7,243 (7.6) | 178/2,287 (7.8) | 66/889 (7.4) |
| Other | 30/4,267 (0.7) | 60/7,243 (0.8) | 14/2,287 (0.6) | 4/889 (0.5) |
| Region | ||||
| Europe | 2,172/4,268 (50.9) | 3,342/7,246 (46.1) | 923/2,288 (40.3) | 325/889 (36.6) |
| North America | 973/4,268 (22.8) | 1,753/7,246 (24.2) | 678/2,288 (29.6) | 288/889 (32.4) |
| Latin America | 628/4,268 (14.7) | 1,438/7,246 (19.8) | 473/2,288 (20.7) | 181/889 (20.4) |
| Asia Pacific | 495/4,268 (11.6) | 713/7,246 (9.8) | 214/2,288 (9.4) | 95/889 (10.7) |
| Duration of type 2 diabetes (years) | ||||
| Mean (SD) | 11.4 (7.2) | 13.0 (8.2) | 14.8 (8.9) | 17.4 (9.4) |
| Median (Q1, Q3) | 10.0 (6.0, 15.0) | 12.0 (7.0, 18.0) | 14.0 (8.0, 20.0) | 16.0 (11.0, 22.0) |
| <5 | 717/4,249 (16.9) | 1,001/7,231 (13.8) | 225/2,275 (9.9) | 57/883 (6.5) |
| ≥5 to <15 | 2,333/4,249 (54.9) | 3,571/7,231 (49.4) | 1,020/2,275 (44.8) | 312/883 (35.3) |
| ≥15 | 1,199/4,249 (28.2) | 2,659/7,231 (36.8) | 1,030/2,275 (45.3) | 514/883 (58.2) |
| BMI (kg/m2) | 32.8 (6.6) | 32.6 (6.3) | 32.8 (6.4) | 32.8 (6.7) |
| Prior CV event | 2,799/4,268 (65.6) | 5,364/7,246 (74.0) | 1,864/2,288 (81.5) | 763/889 (85.8) |
| Coronary artery disease | 1,969/4,268 (46.1) | 3,825/7,246 (52.8) | 1,365/2,288 (59.7) | 607/889 (68.3) |
| Cerebrovascular disease | 617/4,267 (14.5) | 1,202/7,246 (16.6) | 464/2,288 (20.3) | 218/888 (24.5) |
| Peripheral arterial disease | 685/4,267 (16.1) | 1,404/7,246 (19.4) | 499/2,288 (21.8) | 206/889 (23.2) |
| Prior congestive heart failure | ||||
| Yes | 558/4,268 (13.1) | 1,113/7,246 (15.4) | 477/2,288 (20.8) | 232/888 (26.1) |
| No | 3,710/4,268 (86.9) | 6,133/7,246 (84.6) | 1,811/2,288 (79.2) | 656/888 (73.9) |
| Cigarette smoking status | ||||
| Current | 691/4,266 (16.2) | 786/7,245 (10.8) | 188/2,287 (8.2) | 50/886 (5.6) |
| Former | 1,554/4,266 (36.4) | 2,904/7,245 (40.1) | 909/2,287 (39.7) | 396/886 (44.7) |
| Never | 2,021/4,266 (47.4) | 3,555/7,245 (49.1) | 1,190/2,287 (52.0) | 440/886 (49.7) |
| HbA1c | ||||
| % | 8.2 (1.0) | 8.1 (1.0) | 8.1 (1.0) | 8.1 (1.0) |
| mmol/mol | 65.6 (10.6) | 65.0 (10.4) | 65.0 (10.5) | 64.9 (10.4) |
| <8% (<63.93 mmol/mol) | 2,019/4,243 (47.6) | 3,557/7,208 (49.3) | 1,122/2,281 (49.2) | 442/886 (49.9) |
| ≥8% (≥63.93 mmol/mol) | 2,224/4,243 (52.4) | 3,651/7,208 (50.7) | 1,159/2,281 (50.8) | 444/886 (50.1) |
| eGFR (mL/min/1.73 m2) | 107.1 (18.5) | 74.4 (8.6) | 53.2 (4.2) | 38.8 (4.0) |
| Albuminuria | 558/3,120 (17.9) | 1,122/5,277 (21.3) | 445/1,680 (26.5) | 221/650 (34.0) |
| Microalbuminuria | 478/3,120 (15.3) | 931/5,277 (17.6) | 321/1,680 (19.1) | 135/650 (20.8) |
| Macroalbuminuria | 80/3,120 (2.6) | 191/5,277 (3.6) | 124/1,680 (7.4) | 86/650 (13.2) |
Unless otherwise indicated, data are the mean (SD) or number with the characteristic/Number in the column subgroup with nonmissing data (proportion), as appropriate for continuous and categorical variables.
Microvascular Outcomes by Treatment Group
Mean change in eGFR from baseline was similar with EQW treatment and placebo during follow-up in 13,844 patients (LSMD 0.21 mL/min/1.73 m2 [95% CI −0.27 to 0.70]; P = 0.39). Among 14,269 participants without macroalbuminuria at baseline, incident macroalbuminuria occurred in 2.2% of patients in the EQW group and 2.5% of those in the placebo group (HR 0.87 [95% CI 0.70–1.07]; P = 0.19) (Table 2). The hazard of experiencing the renal composite 1 end point, driven by eGFR decline events, was numerically but not statistically significantly reduced with EQW (Table 2). The hazard of experiencing the renal composite 2 end point, driven by eGFR decline and macroalbuminuria events, was significantly reduced with EQW in adjusted, but not unadjusted, models (Table 2). The impact of treatment was similar across all CKD stages, without evidence for interaction (Supplementary Table 2).
Table 2.
Microvascular outcomes by randomized treatment group
| EQW | Placebo | HR (95% CI) | P value | |
|---|---|---|---|---|
| New macroalbuminuria | 158/7,132 (2.2) | 180/7,137 (2.5) | 0.87 (0.70–1.07) | 0.19 |
| Adjusted HR* | 0.84 (0.67–1.04) | 0.11 | ||
| Renal composite 1 | 246/6,459 (3.8) | 273/6,466 (4.2) | 0.88 (0.74–1.05) | 0.16 |
| Adjusted HR* | 0.87 (0.73–1.04) | 0.13 | ||
| 40% decline in eGFR | 239 | 266 | ||
| Renal replacement | 7 | 7 | ||
| Renal death | 0 | 0 | ||
| Renal composite 2 | 366/6,259 (5.8) | 407/6,230 (6.5) | 0.88 (0.76–1.01) | 0.07 |
| Adjusted HR* | 0.85 (0.74–0.98) | 0.03 | ||
| 40% decline in eGFR | 216 | 228 | ||
| Renal replacement | 7 | 6 | ||
| Renal death | 0 | 0 | ||
| New macroalbuminuria | 143 | 173 | ||
| Postbaseline retinopathy | ||||
| First event | 214/7,356 (2.9) | 237/7,396 (3.2) | 0.89 (0.74–1.07) | 0.22 |
| Adjusted HR* | 0.89 (0.74–1.08) | 0.24 | ||
| All events | 244 | 275 | ||
| By HbA1c change (unadjusted) | 0.853 | |||
| Tertile 1 | 38/1,247 (3.1) | 82/2,783 (3.0) | 1.08 (0.74–1.59) | |
| Tertile 2 | 47/1,903 (2.5) | 49/1,966 (2.5) | 0.99 (0.67–1.48) | |
| Tertile 3 | 78/2,909 (2.7) | 36/1,264 (2.9) | 0.92 (0.62–1.37) | |
| By HbA1c decrease >2% | 0.614 | |||
| Yes | 25/814 (3.1) | 7/260 (2.7) | 1.15 (0.50–2.66) | |
| No | 138/5,245 (2.6) | 160/5,753 (2.8) | 0.93 (0.74–1.17) | |
| History of retinopathy at baseline (unadjusted) | 0.483** | |||
| Yes | 59/1,270 (4.6) | 71/1,246 (5.7) | 0.79 (0.56–1.11) | |
| No | 155/6,085 (2.5) | 166/6,150 (2.7) | 0.93 (0.75–1.16) |
Data are the number with the event/total population (%) unless otherwise indicated.
Analyses were adjusted for age, sex, ethnicity, race, region, duration of diabetes, history of CV event, insulin use, baseline HbA1c and eGFR, and BMI.
P value for interaction by HbA1c tertile.
EQW treatment did not increase the risk for retinopathy events among the overall population (HR 0.89 [95% CI 0.74–1.07]; P = 0.22) (Table 2). In particular, no significant impact of EQW was identified in subgroups defined by tertiles of initial HbA1c change from baseline to 6 months, in those whose HbA1c decreased by >2% from baseline to 6 months, or in those with a history of retinopathy.
CV Safety Outcomes by Baseline Renal Status
CV safety outcomes were calculated for those with a baseline eGFR ≥60 mL/min/1.73 m2 (n = 11,514) or <60 mL/min/1.73 m2 (n = 3,177) and for those with CKD stages 1, 2, 3a, and 3b (Fig. 1 and Supplementary Tables 2 and 3). In patients with eGFR <60 mL/min/1.73 m2 (CKD stage 3a or 3b), EQW had a neutral impact on CV outcomes. In univariate analyses unadjusted for multiplicity, risk was significantly reduced for MACE (HR 0.86 [95% CI 0.77–0.97]), all-cause mortality (HR 0.78 [95% CI 0.67–0.91]), CV-related death (HR 0.77 [95% CI 0.64–0.93]), and fatal or nonfatal stroke (HR 0.74 [95% CI 0.58–0.93]) in those with baseline eGFR ≥60 mL/min/1.73 m2 and treated with EQW. P values for interaction were significant only for fatal or nonfatal stroke (P for interaction = 0.035) and CV-related death (P for interaction = 0.031) (Fig. 1 and Supplementary Table 3). In analyses by CKD stage, risk reductions were nominally significant for MACE, fatal or nonfatal stroke, CV-related death, and all-cause mortality for CKD stage 2 and CKD stage 1; however, none of the P values for interaction for all event types by CKD stage were statistically significant, except for hospitalization for heart failure (P = 0.014), but risk was not significantly reduced in individual CKD stage subgroups (Supplementary Table 2).
Figure 1.
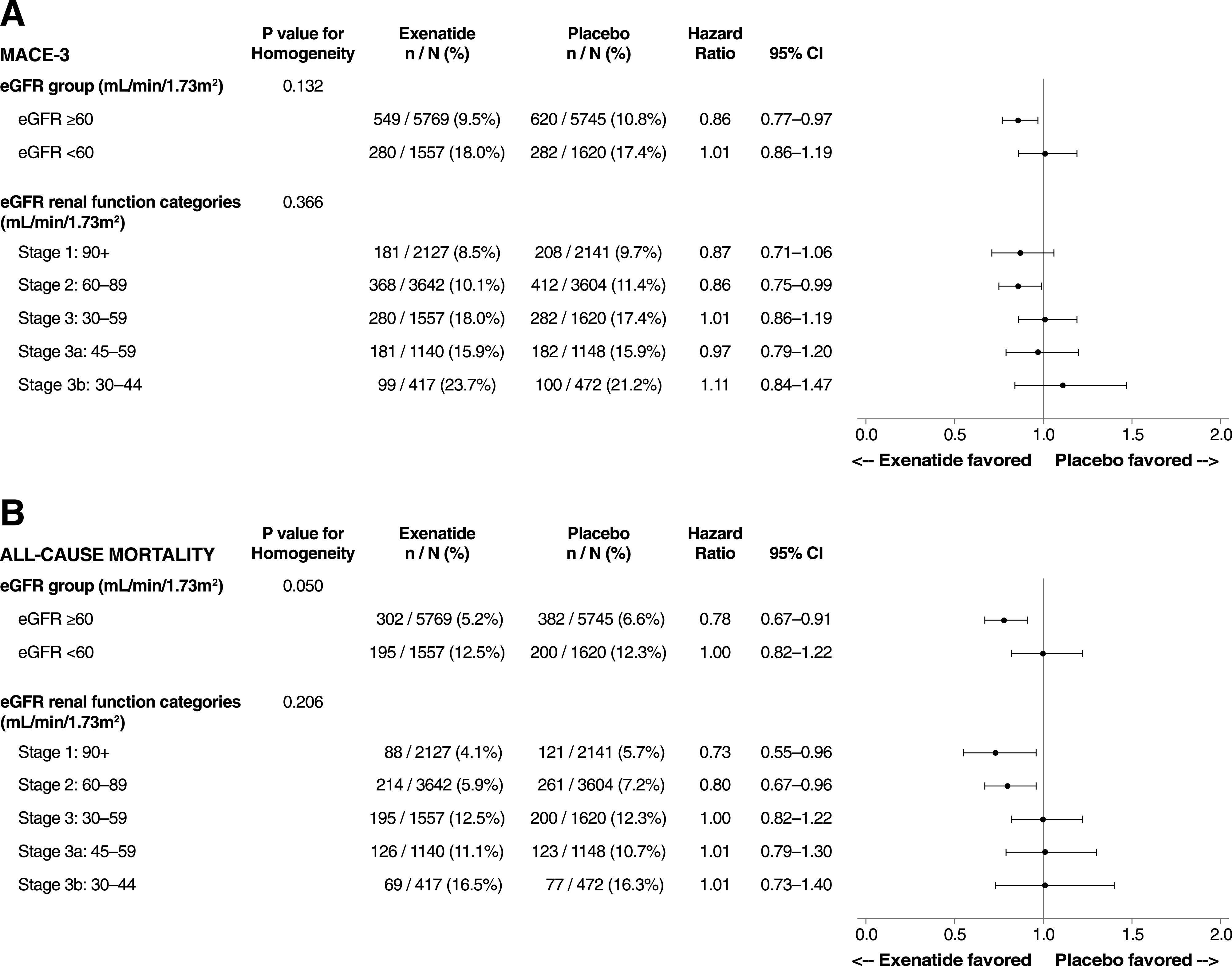
CV safety outcomes by baseline renal function: MACE (A) and all-cause mortality (B). MACE-3, three-item MACE composite.
Conclusions
Among patients with or without previous CV events who were receiving usual care for their type 2 diabetes, the addition of EQW was not associated with clinically meaningful change in eGFR and did not affect renal composite outcomes in unadjusted analyses. In analyses adjusted for demographic characteristics and disease severity, EQW was associated with a significant 15% reduction of relative risk in renal composite 2, driven mainly by a lower incidence of macroalbuminuria in the EQW group. EQW had no impact on the incidence of retinopathy overall or in any subgroup, and the CV safety of EQW was confirmed across a wide range of renal function.
Guarding against nephropathy in type 2 diabetes is a major tenant of therapy to prevent microvascular complications. SGLT-2 inhibitors—shown in several large outcomes trials (BI 10773 [Empagliflozin] Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients [EMPA-REG OUTCOME] [14], Canagliflozin Cardiovascular Assessment Study [CANVAS] [15], Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation [CREDENCE] [16], Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 [DECLARE-TIMI 58] [17]) to reduce the incidence of nephropathy by both reducing proteinuria and delaying decline in glomerular filtration—are considered second-line therapy (after metformin) for patients with diabetes and increased risk for progression of CKD (18). GLP-1 RAs, which affect the progression of proteinuria but have little effect on glomerular filtration (19), follow in the treatment algorithm for those who do not tolerate SGLT-2 inhibitors or in whom they are contraindicated. Both liraglutide (8,20) and semaglutide (21) have reduced the risk for nephropathy in CV outcomes trials, whereas EQW and albiglutide demonstrated renal safety (22). Although the analyses shown here demonstrate a reduction in a renal composite comprising a 40% eGFR decline, incident macroalbuminuria, renal replacement, or renal death, these results were adjusted for covariates and were not adjusted for multiplicity. Limitations to interpretation are introduced by the pragmatic data collection policy in EXSCEL. Data on eGFR were collected only as available from routine outpatient clinical surveillance, resulting in missing data; 93% of EXSCEL participants had both baseline and follow-up eGFR values recorded. Similarly, incomplete data exist regarding baseline albuminuria status, an important predictive variable for both CV and renal outcomes (23). Categorical data for baseline albuminuria status (micro-, macro-, or normoalbuminuria) were not collected for 27% of EXSCEL participants, and quantitative measures of albuminuria were not routinely collected.
Results from other CV outcomes trials have raised concerns about the impact of GLP-1 RAs on retinopathy. Both semaglutide, in the Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) (HR 1.76 [95% CI 1.11–2.78]) (21), and liraglutide, in the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial (HR 1.15 [95% CI 0.87–1.52]) (20), showed higher rates of retinopathy in groups treated with a GLP-1 RA than in those receiving the placebo. In both trials, the risk of retinopathy was highest in those with a history of retinopathy; this high risk may have been related to rapid HbA1c lowering early after randomization rather than to an independent adverse effect of the GLP-1 RA (24). Indeed, there are reasons to believe that GLP-1 RA therapies may be beneficial for patients with diabetic retinopathy. The GLP-1 receptor is expressed in the retina, and animal studies have suggested that GLP-1 RAs decrease apoptosis of retinal nerve cells and provide protection against damage to the blood-retinal barrier (25–28). It is encouraging that the Harmony Outcomes trial, evaluating albiglutide, did not demonstrate evidence of increased risk of retinopathy (22), and EXSCEL showed no statistically increased risk, regardless of the initial HbA1c change. However, there are limitations in ascertaining retinopathy events for all of these studies. Because none of the studies were designed or powered to investigate retinal outcomes, event numbers are small, and the results of fundoscopic exams and retinal images were not collected during follow-up. For EXSCEL, collection of retinopathy events was pragmatic, prospectively ascertained from a yes/no question; retinal imaging or other fundoscopic exam results were not collected.
GLP-1 RAs are effective treatments for type 2 diabetes, lowering glucose with minimal risk for hypoglycemia and often with accompanying weight loss (29). Agents in this class have consistently demonstrated CV safety (30), and some have shown CV benefit (20–22,31). Our analysis demonstrates a consistent CV safety profile for EQW over the spectrum of renal function studied (patients with eGFR <30 mL/min/1.73 m2 at baseline were excluded), without clear evidence of benefit. The suggestion of a greater impact on CV outcomes in patients with eGFR ≥60 mL/min/1.73 m2 is consistent with subgroup analyses performed in both SUSTAIN-6 with semaglutide and the Harmony Outcomes trial with albiglutide (but not in LEADER with liraglutide); however, these analyses were not adjusted for multiplicity (18–20). These findings support revised treatment guidelines advocating a broader use of GLP-1 RAs as the first injectable therapy for most patients (18).
With the increasing prominence of GLP-1 RAs in the treatment of type 2 diabetes, leveraging available long-term outcomes data to characterize the safety profile of drugs within the class can guide medication selection for individual patients. For EQW, the consistency of the CV and renal safety profiles across the range of renal function studied provides reassurance as the drug becomes used more widely, for example, in patients with established atherosclerotic CV disease or before the development of CV disease or CKD in patients who require glucose lowering but have a compelling need to minimize hypoglycemia or weight gain.
Supplementary Material
Article Information
Acknowledgments. Peter Hoffmann, an employee of the Duke Clinical Research Institute, provided editorial support. R.R.H. is an Emeritus National Institute for Health Research (NIHR) Senior Investigator.
Duality of Interest. EXSCEL was sponsored and funded by Amylin Pharmaceuticals, Inc., a wholly owned subsidiary of AstraZeneca. M.A.B. has received research support from Merck & Co. and AstraZeneca; has participated in advisory boards for Boehringer Ingelheim and Novo Nordisk; has received honoraria, personal fees, and other support from Merck, Novo Nordisk, AstraZeneca, and Sanofi; has received nonfinancial research support from Bayer and Merck Serono; and is an employee of Eli Lilly & Co. R.J.M. has received grants from Merck, AstraZeneca, and GlaxoSmithKline and personal fees from Merck, AstraZeneca, and Boehringer Ingelheim. J.B.B. has received contracted consulting fees paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Eli Lilly & Co., MannKind Corp., NovaTarg Therapeutics, Novo Nordisk, Senseonics, vTv Therapeutics, and Zafgen; has received grant support from Novo Nordisk, Sanofi, and vTv Therapeutics; is a consultant to Neurimmune AG; holds stock options in Mellitus Health, PhaseBio Pharmaceuticals, and Stability Health; and is supported by a grant from the National Institutes of Health (UL1TR002489). J.C.C. has received research grant support and/or honoraria for consultancy and/or lectures from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly & Co., GlaxoSmithKline, Merck Sharp & Dohme, Novo Nordisk, Pfizer, and Sanofi; all such proceeds have been donated to the Chinese University of Hong Kong to support research and education. The Chinese University of Hong Kong has received research grants and sponsorships from these companies. S.G.G. has received research grant support and/or personal fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly & Co., Fenix Group International, Ferring Pharmaceuticals, GlaxoSmithKline, Janssen/Johnson & Johnson, Matrizyme, Merck, Novartis, Pfizer, Regeneron, Sanofi, Laboratoires Servier, and Tenax Therapeutics. N.I. and B.K. are employees of AstraZeneca. N.J. has received support from Boehringer Ingelheim, Novo Nordisk, Sanofi, Eli Lilly & Co., and GlaxoSmithKline. Y.L. has received grants from Merck, AstraZeneca, and GlaxoSmithKline. R.D.L. has received research grants from Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Pfizer, and Sanofi and has received personal fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Pfizer, and Portola Pharmaceuticals. A.P.M. has received honoraria from Bayer, Novartis, Cardiorentis AG, and Fresenius Medical Care for participation in study committees. P.O. is a retired employee of AstraZeneca. T.T. has received consultancy honoraria and lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Merck Sharp & Dohme, Novo Nordisk, Sanofi, and Laboratoires Servier. G.L.B. has received research funding (paid to the University of Chicago) from Bayer, Janssen, AbbVie, and Vascular Dynamics; has received consulting fees from Merck, Vascular Dynamics, Relypsa, Boehringer Ingelheim, NxStage, Sanofi, AbbVie, Pfizer, Novo Nordisk, and AstraZeneca; and has served as an editor for the American Journal of Nephrology, Diabetes Care, Hypertension Research, Nephrology Dialysis and Transplantation, and UpToDate. A.F.H. has received research funding from Amgen, Amylin Pharmaceuticals, AstraZeneca, Daiichi Sankyo, Genentech, GlaxoSmithKline, Luitpold Pharmaceuticals, and Merck; and has received consulting fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Merck, MyoKardia, Novartis, and Pluristem Therapeutics. R.R.H. received grants from AstraZeneca during the conduct of the study and has received grants and personal fees from Bayer, Boehringer Ingelheim, and Merck; personal fees from Novartis, Amgen, and Laboratoires Servier; and other support from Elcelyx Therapeutics, GlaxoSmithKline, Janssen, and Takeda outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.A.B. designed the study; collected, analyzed, and interpreted the data; and wrote the manuscript. R.J.M., N.I., B.K., and P.O. designed the study and reviewed and edited the manuscript. P.M. performed the statistical analysis and reviewed and edited the manuscript. J.B.B., G.L.B., and R.R.H. designed the study, interpreted data, and reviewed and edited the manuscript. J.C.C., S.G.G., N.J., Y.L., R.D.L., A.P.M., T.T., and A.F.H. reviewed and edited the manuscript. M.A.B. and R.R.H. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This work was presented as a poster at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
M.A.B. is currently affiliated with Eli Lilly and Co., Indianapolis, IN.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-1065/-/DC1.
References
- 1.Ninomiya T, Perkovic V, de Galan BE, et al.; ADVANCE Collaborative Group . Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009;20:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.So WY, Kong AP, Ma RC, et al. Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care 2006;29:2046–2052 [DOI] [PubMed] [Google Scholar]
- 3.Perkovic V, Verdon C, Ninomiya T, et al. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PLoS Med 2008;5:e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet 1999;354:602] Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 5.Turnbull FM, Abraira C, Anderson RJ, et al.; Control Group . Intensive glucose control and macrovascular outcomes in type 2 diabetes [published correction appears in Diabetologia 2009;52:2470] Diabetologia 2009;52:2288–2298 [DOI] [PubMed] [Google Scholar]
- 6.Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 7.Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol 2018;6:691–704 [DOI] [PubMed] [Google Scholar]
- 8.Mann JFE, Ørsted DD, Brown-Frandsen K, et al.; LEADER Steering Committee and Investigators . Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017;377:839–848 [DOI] [PubMed] [Google Scholar]
- 9.Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J 2016;174:103–110 [DOI] [PubMed] [Google Scholar]
- 10.Holman RR, Bethel MA, Mentz RJ, et al.; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 2014;64:821–835 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80:17–28 [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Levin A, Kellum JA. Definition and classification of kidney diseases. Am J Kidney Dis 2013;61:686–688 [DOI] [PubMed] [Google Scholar]
- 14.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 15.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 16.Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 17.Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 18.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarafidis P, Ferro CJ, Morales E, et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol Dial Transplant 2019;34:208–230 [DOI] [PubMed] [Google Scholar]
- 20.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 22.Hernandez AF, Green JB, Janmohamed S, et al.; Harmony Outcomes Committees and Investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–1529 [DOI] [PubMed] [Google Scholar]
- 23.Stephen R, Jolly SE, Nally JV Jr, Navaneethan SD. Albuminuria: when urine predicts kidney and cardiovascular disease. Cleve Clin J Med 2014;81:41–50 [DOI] [PubMed] [Google Scholar]
- 24.Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab 2018;20:889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Wang Q, Zhang J, Lei X, Xu GT, Ye W. Protection of exendin-4 analogue in early experimental diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2009;247:699–706 [DOI] [PubMed] [Google Scholar]
- 26.Fan Y, Liu K, Wang Q, Ruan Y, Zhang Y, Ye W. Exendin-4 protects retinal cells from early diabetes in Goto-Kakizaki rats by increasing the Bcl-2/Bax and Bcl-xL/Bax ratios and reducing reactive gliosis. Mol Vis 2014;20:1557–1568 [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Zhang J, Wang Q, et al. Intravitreal injection of exendin-4 analogue protects retinal cells in early diabetic rats. Invest Ophthalmol Vis Sci 2011;52:278–285 [DOI] [PubMed] [Google Scholar]
- 28.Ma X, Lin W, Lin Z, et al. Liraglutide alleviates H2O2-induced retinal ganglion cells injury by inhibiting autophagy through mitochondrial pathways. Peptides 2017;92:1–8 [DOI] [PubMed] [Google Scholar]
- 29.Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab 2016;18:203–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bethel MA, Patel RA, Merrill P, et al.; EXSCEL Study Group . Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol 2018;6:105–113 [DOI] [PubMed] [Google Scholar]
- 31.Eli Lilly and Company Trulicity® (dulaglutide) demonstrates superiority in reduction of cardiovascular events for broad range of people with type 2 diabetes. [Internet], 2018. Available from https://www.multivu.com/players/English/8442751-lilly-trulicity-rewind-trial-type-2-diabetes/. Accessed 5 November 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


