Abstract
The aim of the present study was to explore the dynamic relationship between Notch and non-alcoholic fatty liver disease (NAFLD), both in vitro and in vivo. The LX2, Huh7 and MIHA hepatic cell lines were used to establish a cell steatosis model induced by palmitic acid (PA) at different concentrations (0.1, 0.25 and 0.5 mM). Cell proliferation and migration were assessed using a 5-bromo-2′-deoxyuridine kit and a wound healing assay. The dosage of 0.25 mM PA for 36–48 h treatment was chosen for subsequent experiments. Steatotic cells were identified by Oil Red O staining. Feeding mice a methionine-choline-deficient (MCD) diet is known induce a model of NAFLD, compared with a methionine-choline-sufficient (MCS) diet. Therefore, Notch family mRNA expression was evaluated in the liver of MCD-fed mice at varying time points (days 5, 10, 21 and 70) using reverse transcription-quantitative PCR. Notch expression levels were also assessed in cell lines at 12, 24, 36 and 48 h after PA treatment. Notch signaling molecules changed in the PA or MCD model over time. In vitro, the mRNA levels of Notch1, −2 and −4 increased in all cell lines after 12-h PA treatment. At 24 h, these genes were upregulated only in LX2 cells, while showing a ‘down-up’ pattern in MIHA cells (i.e. these genes were downregulated at 24 h but upregulated at 36 h). However, expression of Notch1, −2, −3 and −4 mRNA rose significantly in the early stage (day 10) of NAFLD. At week 3, the levels of Notch1 and −2 were higher in the MCD group than in the MCS group, while the reverse was observed for Notch3 and −4. Expression of these four genes increased again in the late stage (day 70) of NAFLD. Therefore, these results indicated that Notch family members Notch1-4 were involved in the development of NAFLD and played an important role in steatosis in this model.
Keywords: Notch signaling genes, palmitic acid, non-alcoholic fatty liver disease, mice
Introduction
Non-alcoholic fatty liver disease (NAFLD) refers to a spectrum of liver pathologies with hepatic fat accumulation, which are collectively the leading cause of chronic liver disease (1–3). The pathogenesis of NAFLD is not fully understood. The widely accepted ‘multiple hits’ hypothesis suggests that excessive oxidative metabolites and lipid peroxidation occur as a result of insulin resistance (IR). This causes oxidative stress (OS) and continuously damages the mitochondria within hepatocytes, while producing inflammatory mediators and cytokines (1,3). Hepatocytic inflammation and necrosis with steatosis is referred to as non-alcoholic steatohepatitis (NASH). Without intervention, NASH can exacerbate IR and inflammation, causing fatty liver accumulation and increasing the risk of liver cirrhosis and cancer (1,3). Currently, effective NAFLD treatment is still lacking (4).
The Notch family was initially discovered in Drosophila and is expressed in a number of species. Notch molecules serve an important role in the embryonic development of organisms, as well as the occurrence of malignant tumors (5,6). Previous studies predominantly focused on the role of Notch genes in the regulation of cell differentiation, proliferation and apoptosis (7,8). However, it has also been demonstrated that Notch genes are involved in cell metabolism. Indeed, changes in Notch gene expression leads to dysfunction in glucose and lipid metabolism, which can lead to IR, lipid deposition and obesity (9,10). Therefore, the relationship between Notch genes and metabolic diseases has gradually become a research focus.
NAFLD is the hepatic manifestation of metabolic syndrome. With recent changes in living standards and lifestyle, the incidence of NAFLD is rising rapidly, along with those of type 2 diabetes mellitus (T2DM) and overweight/obesity (11,12). Nevertheless, the association of the Notch family with NAFLD is rarely reported (13). Therefore, the aim of the present study was to examine the dynamic association between Notch and NAFLD, both in vitro and in vivo. The time course of Notch gene expression was assessed in liver cell lines treated with palmitic acid (PA), as well as a murine NAFLD model induced by a methionine-choline-deficient (MCD) diet.
Materials and methods
Cell culture and treatments
The following hepatic cell lines were obtained from The Beijing Union Medical College Resource Center and cultured in DMEM: i) Hepatic stellate cell line LX2; ii) hepatocellular carcinoma (HCC) cell line Huh7; and iii) human immortalized hepatocytes MIHA. MIHA, LX2 and Huh7 cell lines were chosen so that different stages of NAFLD (hepatocytes steatosis, fibrosis and carcinoma, respectively), would be represented. PA (MedChemExpress) was dissolved in DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 1% BSA (Sigma-Aldrich; Merck KGaA) and filtered through a 0.22-µm filter, then added to the cells and incubated at 37°C to 80% confluency in DMEM with 10% FBS. Filter-sterilized complete DMEM with 1% BSA without PA was used as a control. The concentrations of PA used were 0.1, 0.25 or 0.5 mM, and the incubation times were 12–72 h.
Cell proliferation and migration assay
Cell proliferation under different PA concentrations was assessed using a 5-bromo-2′-deoxyuridine assay kit (Sigma-Aldrich; Merck KGaA), following the manufacturer's instructions. Cell migration was evaluated using a wound healing assay. A total of three cell lines were treated with or without PA. A thick black line was drawn under each plate as a base line, and shown in every field under a Leica DC 300F optical microscope (magnification, ×400). The plate was scratched with the head of a pipette to disrupt the cellular growth, creating a break in the cells that simulates an injury. The scratch is vertical to the black line under the plate. The wound width (WW) was measured in three microscope fields. For every condition, the WW to initial WW ratio was recorded and averaged. The lower the ratio, the faster the cell migration. ImageJ software version 1.44p (National Institutes of Health) was used for imaging.
Oil Red O (ORO) staining
All three cell lines were counted and seeded into 24-well tissue culture plates in DMEM with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37°C to 80% confluency, then exposed to 0.25 mM PA. Cells were observed 12–72 h after PA treatment. Cells were washed with PBS, fixed with 4% paraformaldehyde for 10 min, washed by 60% isopropanol for 1 min, and stained with fresh ORO dissolved in 60% isopropanol (Sigma-Aldrich; Merck KGaA) for 30 min, as previously described (14). Then, cells were washed with ddH2O, counterstained with hematoxylin for 1 min, and mounted by Permount solution (Thermo Fisher Scientific, Inc.). Finally, the cells were examined under a light microscope (magnification, ×400) (Leica Microsystems GmbH) to observe lipid droplets and identify steatotic cells.
Expression of Notch signaling pathway genes
Total RNA was extracted from cell lines and primary mouse liver tissues using an RNeasy Mini kit (Qiagen GmbH). Primers and probes for qRT-PCR were purchased from Applied Biosystems (Thermo Fisher Scientific, Inc.). Sequences of primers are listed in Table SI. RNAase inhibitor, dNTP and Oligo (dT) were from Toyobo Life Science. RNA was then used for cDNA synthesis (42°C for 60 min, 70°C for 15 min, and 4°C) by reverse transcription according to the manufacturer's instructions. Reverse transcription-quantitative PCR (RT-qPCR) was performed using a SYBR Green kit (Takara Biotechnology Co., Ltd.) in an ABI 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The standard conditions for qPCR were: 50°C for 10 min and 95°C for 15 and 45 sec annealing/elongation at 58°C or 60°C. GAPDH was used as an internal control. Relative genes expressions were calculated using the 2−ΔΔCq method. qRT-PCR experiments were repeated three times (15).
Animals and in vivo assay
A total of 80, 4–6-week-old, male C57BL/6 nude mice (weight, 13–20 g) were obtained from The Institute of Laboratory Animal Science (Chinese Academy of Medical Sciences). All animals were housed in a controlled room with 22.0±2.0°C temperature and 65±5% humidity under a 12-h dark-light cycle. Mice were randomized into 2 groups (n=40 in each group) receiving different diets. The MCD group was used as the NAFLD model, and the methionine-choline-sufficient (MCS) group served as a negative control. Both groups were examined daily, then divided into four subgroups (n=10 in each subgroup) euthanized at different time points (days 5, 10, 21 and 70) (16,17). All mice were subjected to overnight fasting, anesthetized by intraperitoneal injection of 400 mg/kg chloral hydrate, then euthanized by exsanguination performed by cardiac puncture. The liver tissues were harvested only after the mice were confirmed to lose the vital signs. No animal died accidently during the experiment. All experiments were approved by the Ethics Committee of Xin Hua Hospital (Shanghai Jiao Tong University School of Medicine).
Harvested livers were frozen at −70°C for subsequent examination of the expression levels of Notch1-4, hairy and enhancer of split-1 (Hes1) and Hes-related family bHLH transcription factor with YRPW motif 1 (Hey1) using RT-qPCR.
Statistical analysis
A total of three independent experiments were performed. Data are presented as the mean ± SD. Multi-group comparisons were carried out using one-way ANOVA followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference. All data were analyzed with SPSS 13.0 statistical package (SPSS, Inc.).
Results
Effect of PA on proliferation and migration of hepatic cells
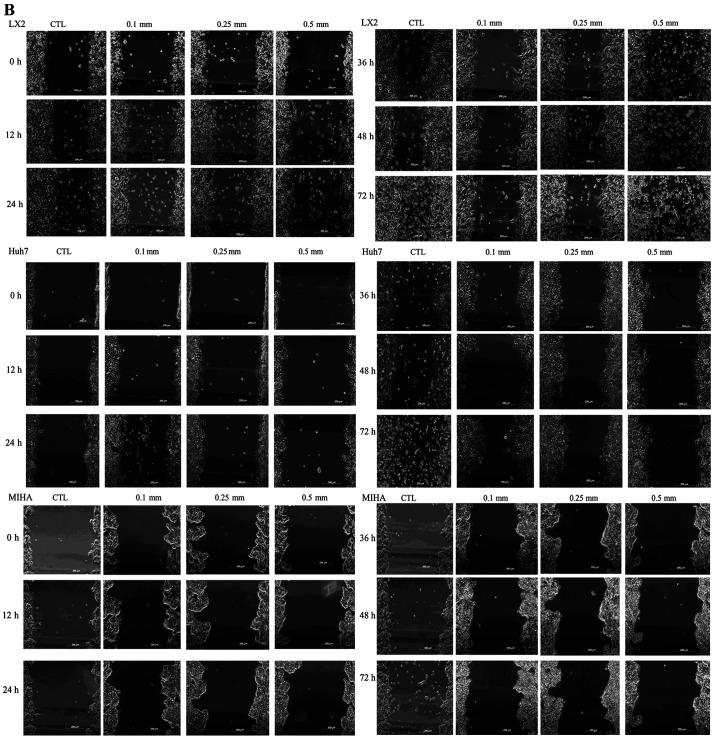
To determine the effects of PA exposure on proliferation and migration of hepatic cells, Huh7, MIHA and LX2 cells were treated with 0.10, 0.25 or 0.50 mM PA for different incubation times. After 12 h of exposure to PA, cell proliferation increased in Huh7, MIHA and LX2 cells, compared with their respective untreated controls. Proliferation reached a maximal value at 36 h for LX2 and MIHA, and at 48 h Huh7 cells. Peak proliferation was observed with 0.1 mM PA in the Huh7 and MIHA cell line, as well as 0.25 mM for LX2. MIHA proliferation ratio seemed close at certain timepoint whatever PA concentrations (Fig. 1A).
Figure 1.
LX2, Huh7 and MIHA cell lines were treated with filter-sterilized complete DMEM with 1% BSA without PA (CTL) or various concentrations of PA (0.1, 0.25 or 0.5 mM) over time. (A) Proliferation rates of hepatic cell lines following PA treatment (ratio to CTL). (B) Microscopic images of cell migration in hepatic cell lines following PA treatment (magnification, ×400). (C) Quantification of cell migration. Data are presented as the mean ± SD. *P<0.05. PA, palmitic acid; CTL, control.
In addition, a wound healing assay indicated more suspended (dead) LX2 cells in the medium at PA 0.25 mM after 48 h (WW, 0.70±0.03), and 0.5 mM from 24 h (WW, 0.78±0.03) onward (Fig. 1B; Table SII). Notable growth was observed between 12 and 72 h following treatment with 0.25 mM PA (P<0.05). The Huh7 cell line displayed increased growth rates between 12 and 72 h at all concentrations of PA (Fig. 1B and C). The WW at 0.1 and 0.25 mM PA were lower than that at 0.5 mM (Table SII). However, PA did not affect the MIHA growth rate at any concentration (P>0.05). Therefore, a dosage of 0.25 mM PA was used for subsequent experiments. It should be noted that the shape of the suspended LX2 cells was different from that of the adherent cells, and were excluded when WW was calculated (Table SII).
PA induces steatosis in hepatic cells
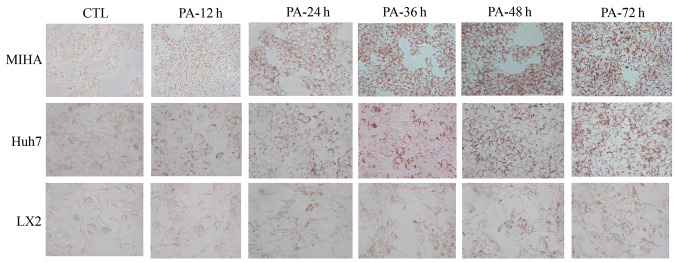
ORO stains intracellular neutral lipids (18). Hepatic cells were stained with ORO in the presence or absence of PA (Fig. 2). Following exposure to PA, notably more lipid droplets were observed in three microscope fields, compared with the control. In addition, a time-dependent accumulation of lipid appeared in LX2, Huh7 and MIHA cells treated with 0.25 mM PA. These results suggested that PA could enhance lipid synthesis and steatosis in hepatic cell lines.
Figure 2.
ORO staining of hepatic cells (magnification, ×400). MIHA, Huh7 and LX2 cells were treated without PA (CTL) or with 0.25 mM PA for 12, 34, 36, 48 or 72 h. Representative images of ORO staining after various durations of treatment are shown. PA, palmitic acid; CTL, control; ORO, Oil Red O.
Notch family gene expression in vitro
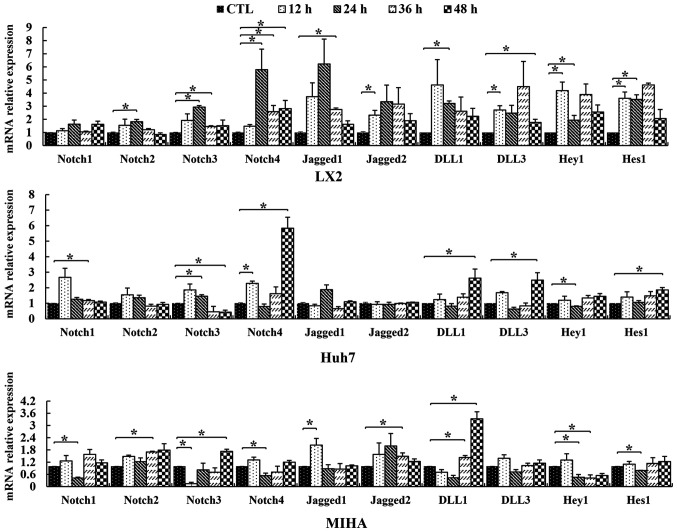
RT-qPCR assays were carried out to examine the expression of Notch pathway genes in LX2, Huh7 and MIHA cells following 0.25 mM PA exposure. Notch pathway members Notch1, −2, −3, −4, Jagged1, Jagged2, Δ-like canonical Notch ligand (DLL) 1 and DLL3, Hey1 and Hes1 were affected by PA exposure. The expression of these signaling molecules followed varying trends over the course of the experiment (Fig. 3). After 12 h PA treatment, the expression of Notch4 was significantly upregulated in Huh7 lines, compared with control. The expression of Notch3 was similar in LX2 and Huh7, but significantly downregulated in MIHA cells at 12 h. At the 24-h time point, Notch2, −3 and −4 genes were upregulated only in the hepatic stellate cell line LX2, compared with the control. At this time, point, Notch1 and −4 were significantly downregulated in MIHA cells, while, Notch3 was upregulated in Huh7 cells. However, the expression of these four Notch genes partly recovered slowly with continuous PA exposure at the endpoint of the experiment [CTL vs. 36 h: Notch1 (Huh7), Notch2 (MIHA), Notch3 (LX2), Notch4 (LX2), P<0.05] [CTL vs. 48 h: Notch3 (Huh7, MIHA), Notch4 (LX2, Huh7), P<0.05]. For Jagged1/2, DLL1/3, Hey1 and Hes1, there were no regular trend changes found in the three cell lines, although their expression was statistically different at some time points.
Figure 3.
Relative expression levels of Notch genes in hepatic cell lines. LX2, Huh7 and MIHA cells were treated without PA (CTL) or 0.25 mM palmitic acid, and RT-qPCR was performed to assess expression levels of Notch1, −2, −3, −4, Jagged1, Jagged2, DLL1, DLL3, Hey1 and Hes1 following various durations of treatment (12, 24, 36 or 48 h). Data are presented as the mean ± SD. *P<0.05. CTL, control; DLL, delta-like ligand; Hey1, hes-related family bHLH transcription factor with YRPW motif 1; Hes1, hairy and enhancer of split-1.
Abnormal expression of Notch signaling pathway genes during NAFLD in vivo
C57BL/6 mice were fed an MCD diet (Fig. 4). The MCD model is mainly characterized by steatosis and inflammatory responses of hepatocytes at the third week, while liver fibrosis begins at week 8 (16,17). Notch1, −2, −3 and −4 mRNA decreased significantly in MCD-fed mice at the early stage of NAFLD, compared with MCS mice (day 5; P<0.05). This trend changed from day 10, at which point, the expression levels of Notch1 and −3 were significantly upregulated (P<0.05). At the final time point, the expression of all Notch genes was significantly higher in MCD-fed mice, compared with MCS controls (day 70; P<0.05).
Figure 4.
Expression levels of Notch genes in liver tissues of MCD-/MCS-fed mice at days 5, 10, 21 and 70. Data are presented as the mean ± SD. *P<0.05. MCD, methionine-choline-deficient; MCS, methionine-choline-sufficient; Hey1, hes-related family bHLH transcription factor with YRPW motif 1; Hes1, hairy and enhancer of split-1.
Discussion
NAFLD is the main cause of chronic liver disease and cirrhosis worldwide, irrespective of age (19). The spectrum of NAFLD includes steatohepatitis, NASH and associated cirrhosis, and HCC (1,2). Although steatosis presents minimal clinical manifestations, NASH with lobular inflammation is regarded as a driving force in the progression of NAFLD. The ‘multi-hit’ theory is the most widely accepted theory accounting for the complex pathophysiology of NAFLD (12,13,16). The onset of NAFLD is characterized by the production of reactive oxygen species, reduced levels of β-oxidation and increased lipogenesis, followed by lipid accumulation in hepatocytes along with cellular imflammation (20). In addition, adaptive immunity, dysfunction of Notch signaling, vitamin D deficiency and sleep deprivation have been reported as additional factors promoting liver inflammation or injury during NAFLD process (11,13,21–23). In the present study, an NAFLD cell line was established and verified using ORO staining. In order to examine the PA dosage and treatment duration required in this model, cell proliferation and migration were assessed in liver cells treated with PA over time. Peak proliferation was observed at 36 h (LX2, MIHA) or 48 h (Huh7) with 0.1 and 0.25 mM PA, respectively. In addition, increased cell death was observed in LX2 cells when PA was 0.25 mM (≥48 h) and 0.5 mM (from 24 h onward). Thus, a concentration of 0.25 mM PA was used in subsequent experiments, with a 36 to 48-h treatment time.
The Notch signaling pathway regulates downstream genes and plays important roles in the physiological and pathological processes of cell differentiation, proliferation, apoptosis, embryonic development and tumor formation (24,25). Previous studies suggested that the effects of Notch signaling vary between different types of tumor, or within the same tumor during different periods. In the liver, the Notch family is associated with the onset and development of a number of liver diseases (26,27). Aimaiti et al (28) demonstrated that LY450139, an inhibitor of the Notch pathway, could decrease the expression of Notch1 and myofibroblast markers in hepatic stellate cells via transforming growth factor-β1, providing novel insight into stellate cell activation by Notch signalling (28).
To investigate the role played by Notch signaling in the liver, hepatic cell lines LX2, Huh7 and MIHA were used in the present study to assess the expression of Notch genes. Changes in the expression of Notch signaling genes occurred at different time points in these cell lines. The levels of all Notch mRNA was altered in all cell lines starting at 24 h of PA treatment. Notch4 was then upregulated at the latest time point in Huh7 cells. Expression of Notch3 increased gradually in MIHA cells with PA exposure time. In LX2 cells, Notch3 was upregulated at 12–24 h and downregulated from 36 h; in Huh7 cells, it was upregulated at 12 h but downregulated after 24 h. In the present animal experiment, the expression levels of these genes increased at days 10 and 70. According to the progress of NAFLD, the early stage primarily comprises inflammation of hepatocytes and steatosis, whereas the later stage involves liver fibrosis and/or cirrhosis (1,4,15). Therefore, it is generally considered that 12–24 h in vitro corresponds to days 5 and 10 in vivo, and 48 h in vitro corresponds to day 70 in vivo. It may be hypothesized that Notch1, Notch2 and Notch4 primarily participated in inflammatory responses (early stage) and fibrosis (late stage) during NALFD, while Notch3 might be primarily involved in steatosis and inflammatory lesions.
Several studies have investigated the associations between Notch signaling, liver fibrosis, cirrhosis and carcinoma, both in humans and animal models. For instance, Zhang et al (29) demonstrated that ring finger protein 187 (RNF187) and Notch1 promoted invasion and metastasis in HCC, and prognosis was poorer for patients who exhibited Notch1-RNF187 activation. In patients with NASH, Notch activity in hepatocytes was associated with severity and responsiveness to treatment (30). In Notch loss-of-function mouse models, hepatocyte-specific liver inflammation and fibrosis are reduced, suggesting maladaptive hepatocytic Notch response in NASH-associated liver fibrosis (30). In the present study, the dynamic effect of Notch genes during NAFLD development in vivo was evaluated. Similar results were obtained in both MCD mice and cell experiments. At the onset of NAFLD (day 10), expression of Notch1, Notch2, Notch3 and Notch4 mRNA increased. In the NASH period (week 3), Notch1 and Notch2 levels were higher in the MCD group than in the MCS group, although these were not significant. In a previous study, aggravation of fatty degeneration and OS, liver fibrosis appeared in week 10 (17), and expression of these four genes' mRNA rose again significantly. Thus, changes in Notch gene expression may be related to the development of NAFLD. Notch genes, and the associated upstream and downstream pathways, could represent therapeutic targets for patients with NAFLD.
However, the present study has some limitations. The MCD dietary model differed from the high-fat dietary (HFD) model, and also from the human NASH microenvironment. MCD was not used to simulate metabolic syndrome or IR, which often occur in the HFD animal model and human patients with NAFLD. Future experiments should focus on clinical samples or HFD animal models to verify the conclusion that Notch expression levels change dynamically in NAFLD and are differentially distributed across disease stages. Moreover, while the same dynamic between Notch genes and NAFLD was observed both in vitro and in vivo, the underlying mechanism and potential associations between different Notch molecules require extensive in-depth studies. Future studies should focus on these aspects.
In conclusion, the present study demonstrated that PA or MCD regulated the levels of Notch signaling genes during NAFLD. The Notch family participated in the development of NAFLD. These findings might provide a direction for molecular-targeted therapy and early detection of NAFLD. However, additional studies are needed to assess these possibilities.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The National Natural Science Foundation of China (grant nos. 81400610, 81400799 and 81470840), the Cross-Institute Research Fund of Shanghai Jiao Tong University (grant no. YG2016MS72), The Project Sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (grant no. 20144802) and The Shanghai Municipal Commission of Health and Family Planning for Youth (grant no. 20134Y043).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
WJD performed the experiments. JGF and LQ conceptualized and designed the study. WJD, YWC and HBC performed data analysis and interpretation. WJD and WJW drafted the manuscript. WJW generated and revised the figures. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experiments were approved by the Ethics Committee of Xin Hua Hospital affiliated to Shanghai Jiao Tong University School of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, Cummings O, Yeh M, Gill R, Chalasani N, et al. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open. 2019;2:e1912565. doi: 10.1001/jamanetworkopen.2019.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhee EJ. Nonalcoholic fatty liver disease and diabetes: An epidemiological perspective. Endocrinol Metab (Seoul) 2019;34:226–233. doi: 10.3803/EnM.2019.34.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomaraschi M, Fracanzani AL, Dongiovanni P, Pavanello C, Giorgio E, Da Dalt L, Norata GD, Calabresi L, Consonni D, Lombardi R, et al. Lipid accumulation impairs lysosomal acid lipase activity in hepatocytes: Evidence in NAFLD patients and cell cultures. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:158523. doi: 10.1016/j.bbalip.2019.158523. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Wong GL, He FP, Sun J, Chan AW, Yang J, Shu SS, Liang X, Tse YK, Fan XT, et al. Quantifying and monitoring fibrosis in non-alcoholic fatty liver disease using dual-photon microscopy. Gut. 2020;69:1116–1126. doi: 10.1136/gutjnl-2019-318841. [DOI] [PubMed] [Google Scholar]
- 5.Yang MH, Chang KJ, Li B, Chen WS. Arsenic trioxide suppresses tumor growth through antiangiogenesis via notch signaling blockade in small-cell lung cancer. Biomed Res Int. 2019;2019:4647252. doi: 10.1155/2019/4647252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto S, Schulze KL, Bellen HJ. Introduction to Notch signaling. Methods Mol Biol. 2014;1187:1–14. doi: 10.1007/978-1-4939-1139-4_1. [DOI] [PubMed] [Google Scholar]
- 7.Xiao W, Chen X, He M. Inhibition of the Jagged/Notch pathway inhibits retinoblastoma cell proliferation via suppressing the PI3K/Akt, Src, p38MAPK and Wnt/β-catenin signaling pathways. Mol Med Rep. 2014;10:453–458. doi: 10.3892/mmr.2014.2213. [DOI] [PubMed] [Google Scholar]
- 8.Aithal MGS, Rajeswari N. Bacoside a induced Sub-G0 arrest and early apoptosis in human glioblastoma cell line U-87 MG through Notch signaling pathway. Brain Tumor Res Treat. 2019;7:25–32. doi: 10.14791/btrt.2019.7.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen CH, Kosmina R, Rydén M, Baun C, Hvidsten S, Andersen MS, Christensen LL, Gastaldelli A, Marraccini P, Arner P, et al. The imprinted gene Delta like non-canonical notch ligand 1 (Dlk1) associates with obesity and triggers insulin resistance through inhibition of skeletal muscle glucose uptake. EBioMedicine. 2019;46:368–380. doi: 10.1016/j.ebiom.2019.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang KC, Chuang PY, Yang TY, Huang TW, Chang SF. Hyperglycemia inhibits osteoblastogenesis of rat bone marrow stromal cells via activation of the Notch2 signaling pathway. Int J Med Sci. 2019;16:696–703. doi: 10.7150/ijms.32707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trovato FM, Martines GF, Brischetto D, Catalano D, Musumeci G, Trovato GM. Fatty liver disease and lifestyle in youngsters: Diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int. 2016;36:427–433. doi: 10.1111/liv.12957. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Zhu Z, Mao Y, Xu Y, Du J, Tang X, Cao H. HbA1c may contribute to the development of non-alcoholic fatty liver disease even at normal-range levels. Biosci Rep. 2020;40:BSR20193996. doi: 10.1042/BSR20193996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romeo S. Notch and Nonalcoholic fatty liver and fibrosis. N Engl J Med. 2019;380:681–683. doi: 10.1056/NEJMcibr1815636. [DOI] [PubMed] [Google Scholar]
- 14.Niture S, Gyamfi MA, Kedir H, Arthur E, Ressom H, Deep G, Kumar D. Serotonin induced hepatic steatosis is associated with modulation of autophagy and notch signaling pathway. Cell Commun Signal. 2018;16:78. doi: 10.1186/s12964-018-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Yamada T, Obata A, Kashiwagi Y, Rokugawa T, Matsushima S, Hamada T, Watabe H, Abe K. Gd-EOB-DTPA-enhanced-MR imaging in the inflammation stage of nonalcoholic steatohepatitis (NASH) in mice. Magn Reson Imaging. 2016;34:724–729. doi: 10.1016/j.mri.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Larter CZ, Yeh MM, Williams J, Bell-Anderson KS, Farrell GC. MCD-induced steatohepatitis is associated with hepatic adiponectin resistance and adipogenic transformation of hepatocytes. J Hepatol. 2008;49:407–416. doi: 10.1016/j.jhep.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 18.Gu LY, Qiu LW, Chen XF, Lü L, Mei ZC. Oleic acid induced hepatic steatosis is coupled with downregulation of aquaporin 3 and upregulation of aquaporin 9 via activation of p38 signaling. Horm Metab Res. 2014;13:125–129. doi: 10.1055/s-0034-1384569. [DOI] [PubMed] [Google Scholar]
- 19.Shakir AK, Suneja U, Short KR, Palle S. Overview of pediatric nonalcoholic fatty liver disease: A guide for general practitioners. J Okla State Med Assoc. 2018;111:806–811. [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Park JS, Roh YS. Molecular insights into the role of mitochondria in non-alcoholic fatty liver disease. Arch Pharm Res. 2019;42:935–946. doi: 10.1007/s12272-019-01178-1. [DOI] [PubMed] [Google Scholar]
- 21.Sutti S, Albano E. Adaptive immunity: An emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol. 2020;17:81–92. doi: 10.1038/s41575-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Thorne JL, Moore JB. Vitamin D and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2019;22:449–458. doi: 10.1097/MCO.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 23.Trovato FM, Castrogiovanni P, Szychlinska MA, Purrello F, Musumeci G. Early effects of high-fat diet, extra-virgin olive oil and vitamin D in a sedentary rat model of non-alcoholic fatty liver disease. Histol Histopathol. 2018;33:1201–1213. doi: 10.14670/HH-18-008. [DOI] [PubMed] [Google Scholar]
- 24.Aleĭnik AN, Kondakova IV. The Notch signaling systemand oncogenesis. Vopr Onkol. 2012;58:593–597. (In Russian) [PubMed] [Google Scholar]
- 25.Dang TP. Notch, apoptosis and cancer. Adv Exp Med Biol. 2012;727:199–209. doi: 10.1007/978-1-4614-0899-4_15. [DOI] [PubMed] [Google Scholar]
- 26.Geisler F, Strazzabosco M. Emerging roles of Notch signaling in liver disease. Hepatology. 2015;61:382–392. doi: 10.1002/hep.27268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun L, Sun G, Yu Y, Coy DH. Is Notch signaling a specific target in hepatocellular carcinoma? Anticancer Agents Med Chem. 2015;15:809–815. doi: 10.2174/1871520615666150202102809. [DOI] [PubMed] [Google Scholar]
- 28.Aimaiti Y, Yusufukadier M, Li W, Tuerhongjiang T, Shadike A, Meiheriayi A, Gulisitan, Abudusalamu A, Wang H, Tuerganaili A, et al. TGF-β1 signaling activates hepatic stellate cells through Notch pathway. Cytotechnology. 2019;71:881–891. doi: 10.1007/s10616-019-00329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Chen J, Yong J, Qiao L, Xu L, Liu C. An essential role of RNF187 in Notch1 mediated metastasis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:384. doi: 10.1186/s13046-019-1382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu C, Kim K, Wang X, Bartolome A, Salomao M, Dongiovanni P, Meroni M, Graham MJ, Yates KP, Diehl AM, et al. Hepatocyte Notch activation induces liver fibrosis in nonalcoholic steatohepatitis. Sci Transl Med. 2018;10:eaat0344. doi: 10.1126/scitranslmed.aat0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.