Abstract
Objective
To better understand the potential risks of Nipah virus emergence in Cambodia by studying different components of the interface between humans and bats.
Methods
From 2012 to 2016, we conducted a study at two sites in Kandal and Battambang provinces where fruit bats (Pteropus lylei) roost. We combined research on: bat ecology (reproductive phenology, population dynamics and diet); human practices and perceptions (ethnographic research and a knowledge, attitude and practice study); and Nipah virus circulation in bat and human populations (virus monitoring in bat urine and anti-Nipah-virus antibody detection in human serum).
Findings
Our results confirmed circulation of Nipah virus in fruit bats (28 of 3930 urine samples positive by polymerase chain reaction testing). We identified clear potential routes for virus transmission to humans through local practices, including fruit consumed by bats and harvested by humans when Nipah virus is circulating, and palm juice production. Nevertheless, in the serological survey of 418 potentially exposed people, none of them were seropositive to Nipah virus. Differences in agricultural practices among the regions where Nipah virus has emerged may explain the situation in Cambodia and point to actions to limit the risks of virus transmission to humans.
Conclusion
Human practices are key to understanding transmission risks associated with emerging infectious diseases. Social science disciplines such as anthropology need to be integrated in health programmes targeting emerging infectious diseases. As bats are hosts of major zoonotic pathogens, such integrated studies would likely also help to reduce the risk of emergence of other bat-borne diseases.
Résumé
Objectif
Mieux comprendre les risques potentiels d'émergence du virus Nipah au Cambodge en étudiant différents composants de l'interface entre humains et chauves-souris.
Méthodes
De 2012 à 2016, nous avons réalisé une étude à deux endroits dans les provinces de Kandal et Battambang, où nichent les chauves-souris frugivores (Pteropus lylei). Nous avons associé plusieurs domaines de recherche: écologie des chauves-souris (phénologie reproductive, dynamique démographique et régime alimentaire); perceptions et pratiques humaines (recherches ethnographiques et étude des connaissances, attitudes et pratiques); et enfin, circulation du virus Nipah au sein des populations de chauves-souris et d'humains (dépistage du virus dans l'urine de chauve-souris et détection des anticorps contre le virus Nipah dans le sérum humain).
Résultats
Nos résultats ont confirmé la circulation du virus Nipah chez les chauves-souris frugivores (28 échantillons d'urine sur 3930 se sont révélés positifs lors des tests de réaction en chaîne par polymérase). Nous avons clairement identifié plusieurs vecteurs de transmission potentiels aux humains liés aux pratiques locales, dont les fruits consommés par les chauves-souris et récoltés par les humains lorsque le virus Nipah circule, ainsi que la production de jus de palme. Néanmoins, une enquête sérologique menée auprès de 418 personnes potentiellement exposées a montré qu'aucune d'entre elles n'était séropositive au virus Nipah. Les différences au niveau des pratiques agricoles dans les régions où le virus Nipah est apparu pourrait expliquer la situation au Cambodge, et conduire à des actions qui permettront de limiter les risques de transmission du virus aux humains.
Conclusion
Les pratiques humaines sont la clé d'une meilleure compréhension des risques de transmission associés aux maladies infectieuses émergentes. Certaines disciplines de sciences sociales telles que l'anthropologie doivent être intégrées dans les programmes de santé qui ciblent les maladies infectieuses émergentes. Étant donné que les chauves-souris sont porteuses d'agents pathogènes zoonotiques majeurs, des études intégrées de ce type pourraient contribuer à réduire le risque d'émergence d'autres maladies propagées par les chauves-souris.
Resumen
Objetivo
Entender mejor los riesgos potenciales de la aparición del virus Nipah en Camboya mediante el estudio de los diversos componentes de la interfaz entre los seres humanos y los murciélagos.
Métodos
De 2012 a 2016, se realizó un estudio en dos lugares de las provincias de Kandal y Battambang en donde habitan los murciélagos fruteros (Pteropus lylei). Se combinaron investigaciones sobre: la ecología de los murciélagos (fenología reproductiva, dinámica de la población y alimentación); las prácticas y las percepciones humanas (investigación etnográfica y un estudio de conocimientos, actitudes y prácticas); y la circulación del virus Nipah en las poblaciones de los murciélagos y de los seres humanos (monitoreo del virus en la orina de los murciélagos y detección de anticuerpos contra el virus Nipah en el suero humano).
Resultados
Los resultados confirmaron la circulación del virus Nipah en murciélagos fruteros (28 de 3930 muestras de orina positivas en la prueba de reacción en cadena de la polimerasa). Se identificaron las rutas claras de transmisión del virus a los seres humanos a través de las prácticas locales, incluidas las frutas que consumen los murciélagos y que los seres humanos recolectan cuando el virus Nipah está en circulación, y la producción de zumo de palma. Sin embargo, según el estudio serológico de 418 personas potencialmente expuestas, ninguna de ellas fue seropositiva al virus Nipah. Es posible que las diferencias en las prácticas agrícolas entre las regiones en donde el virus Nipah se ha presentado expliquen la situación en Camboya y señalen las medidas para limitar los riesgos de transmisión del virus a los seres humanos.
Conclusión
Las prácticas de los seres humanos son esenciales para entender los riesgos de transmisión asociados a las enfermedades infecciosas emergentes. Las disciplinas de las ciencias sociales, como la antropología, se deben integrar en los programas de salud que se ocupan de esas enfermedades. Teniendo en cuenta que los murciélagos son huéspedes de los principales patógenos zoonóticos, es probable que esos estudios integrados también ayuden a reducir el riesgo de aparición de otras enfermedades que se transmiten por los murciélagos.
ملخص
الغرض تحقيق فهم أفضل للمخاطر المحتملة لظهور فيروس نيباه في كمبوديا عن طريق دراسة المكونات المختلفة للتفاعل بين البشر والخفافيش.
الطريقة من عام 2012 حتى عام 2016، قمنا بإجراء دراسة في موقعين في مقاطعتي كاندال وباتامبانج حيث تستوطن خفافيش الفاكهة ( Pteropus lylei ). قمنا بدمج الأبحاث عن: علم بيئة الخفافيش (علم الظواهر التناسلية، وآليات الكثافة السكنية، والنظام الغذائي)؛ والممارسات والتصورات البشرية (الأبحاث الإثنوغرافية، ودراسة المعرفة والموقف والممارسة)؛ وانتشار فيروس نيباه في الخفافيش والتجمعات السكان (رصد الفيروس في بول الخفافيش واكتشاف الأجسام المضادة لفيروس نيباه في مصل الإنسان).
النتائج أكدت نتائجنا انتشار فيروس نيباه في خفافيش الفاكهة (28 من 3930 عينة بول، كانت إيجابية عن طريق اختبار تفاعل سلسلة بوليميريز). قمنا بتحديد مسارات محتملة واضحة لانتقال الفيروس إلى البشر من خلال الممارسات المحلية، بما في ذلك الفاكهة التي تستهلكها الخفافيش ويحصدها البشر عندما ينتشر فيروس نيباه، وكذلك إنتاج عصير من منتجات النخيل. وبالرغم من ذلك، فإن المسح المصلي لعدد 418 شخصًا من المحتمل تعرضهم للإصابة، أوضح أنه لم يكن مصل أي منهم إيجابيًا لفيروس نيباه. إن الاختلافات في الممارسات الزراعية بين المناطق التي ظهر فيها فيروس نيباه، قد تفسر الموقف في كمبوديا، وقد تشير إلى إجراءات للحد من مخاطر انتقال الفيروس إلى البشر.
الاستنتاج إن الممارسات البشرية هي الأساس لاستيعاب مخاطر الانتقال المرتبطة بالأمراض المعدية الناشئة. إن تخصصات العلوم الاجتماعية، مثل الأنثروبولوجيا، بحاجة إلى الدمج البرامج الصحية التي تستهدف الأمراض المعدية الناشئة. نظراً لأن الخفافيش هي العائل للمسببات الأساسية للأمراض حيوانية المنشأ، فمن المرجح أن تساعد هذه الدراسات المتكاملة أيضًا على الحد من خطر ظهور أمراض أخرى تنقلها الخفافيش.
摘要
目的
旨在通过研究人与蝙蝠之间相互接触的不同部分,更好地了解柬埔寨境内的尼帕病毒潜在风险。
方法
从 2012 年至 2016 年,我们在坎达尔和马德望省的两个地点进行了研究,这些地方都栖息着果蝠(莱氏狐蝠)。我们综合了以下方面的研究:蝙蝠生态学(生殖物候、种群动态和饮食);人类的实践和观念(人种学研究以及知识、态度和实践研究);以及尼帕病毒在蝙蝠和人群中的传播(蝙蝠尿液中的病毒监测和人血清中抗尼帕病毒抗体的检测)。
结果
我们的结果证实了尼帕病毒在果蝠中的传播(通过聚合酶链反应测试发现 3930 个尿液样本中有 28 个呈阳性)。我们通过当地实践确定了将病毒传播给人类的明确潜在途径,包括传播尼帕病毒时蝙蝠食用的和人类收获的果实,以及棕榈汁的生产。尽管如此,在对 418 名潜在接触者进行的血清学调查中,没有人对尼帕病毒呈血清反应阳性。已经出现尼帕病毒的地区中在农业实践方面的差异可能说明了柬埔寨的情况以及限制病毒传播给人类的风险的行动指向。
结论
人类实践是了解与新发传染病相关的传播风险的关键。需要将人类学等社会科学学科纳入针对新发传染病的卫生方案。由于蝙蝠是主要的人畜共患病病原体的宿主,因此此类综合研究还可能有助于降低其他蝙蝠传播疾病出现的风险。
Резюме
Цель
Добиться лучшего понимания потенциальных рисков появления вируса Нипах в Камбодже за счет изучения разного рода компонентов взаимодействия людей и летучих мышей.
Методы
В период с 2012 по 2016 год авторы провели исследование в двух районах провинций Кандаль и Баттамбанг со значительными колониями крыланов (Pteropus lylei). Авторы использовали исследования по экологии летучих мышей (репродуктивная фенология, динамика популяций и питание), жизнедеятельности и представлениям людей (этнографические исследования, исследование знаний, отношения и практик) и циркуляции вируса Нипах в популяциях людей и летучих мышей (мониторинг вируса в моче летучих мышей и обнаружение антител к вирусу Нипах в сыворотке крови людей).
Результаты
Было подтверждено, что в организмах крыланов циркулирует вирус Нипах (28 из 3930 образцов мочи были положительными по результатам тестирования методом полимеразной цепной реакции). Авторы выявили четкие потенциальные пути передачи вируса людям через принятые в этих местностях практикуемые виды деятельности, включая сбор людьми фруктов, которыми питаются крыланы, во время циркуляции вируса Нипах и добычу пальмового сока. Тем не менее в серологическом исследовании 418 потенциально зараженных людей ни один человек не продемонстрировал положительного результата при серологической диагностике вируса Нипах. Различия в сельскохозяйственных практиках, существующие между регионами Камбоджи, где отмечается появление вируса Нипах, могут объяснять сложившуюся ситуацию и указывать на необходимые мероприятия с целью ограничения риска передачи вируса людям.
Вывод
Практикуемые людьми виды деятельности являются ключом к пониманию рисков, связанных с передачей новых инфекционных заболеваний. Социальные научные дисциплины, такие как антропология, должны быть включены в программы медицинского обслуживания, направленные на борьбу с новыми инфекционными заболеваниями. Поскольку летучие мыши являются переносчиками серьезных зоонозных патогенов, такие объединенные исследования могут способствовать снижению риска появления других заболеваний, передающихся через летучих мышей.
Introduction
Infectious diseases remain an important threat to global health, as demonstrated by the recent emergence of coronavirus disease-2019 (COVID-19).1 The risk of such an emergence was known and in its 2018 list of priority diseases the World Health Organization (WHO) recognized that a serious international epidemic could be caused by a pathogen then unknown.2 Emerging infectious diseases related to known pathogens also pose a serious threat. For example, Nipah virus has caused recurrent disease outbreaks in Bangladesh and western India3 and the most recent outbreak in Kerala in 2018 was the first recorded in southern India.4 Some of these outbreaks involved human-to-human transmission, demonstrating potential for wider spread of this virus causing highly lethal encephalitis.5 Most emerging infectious diseases are zoonotic and originate in wildlife, and the epidemiology of the disease is often complex.6 Since multiple factors including environmental and anthropological factors are often involved in the processes of disease emergence,7 surveillance and control requires integrated (transdisciplinary and trans-sectoral) approaches in health management.8
Fruit bats of the Pteropus genus, also known as flying foxes, are the main reservoir for the Nipah virus, which makes understanding of their ecology central to any research on Nipah virus emergence.9,10 Agricultural practices have also been key factors in the emergence of the disease in humans. For instance, in Malaysia, where the first reported Nipah outbreak led to 265 human cases and 105 deaths, intensification of pig farming and cultivation of fruit trees in the same locations was an important determinant of the outbreak. The outbreak was ultimately curtailed by the culling of over 1 million pigs.11,12 In Bangladesh and India, consumption of raw palm sap is the main route of transmission of the virus to humans.3,13 Although simple prevention measures such as use of bamboo skirts on trees to prevent bats from accessing the palm sap have been suggested, the adoption of these skirts by local communities depends on their perceptions of transmission risks and diseases in general.14 As a consequence, understanding communities’ perceptions towards bats and diseases is critical to the success of any prevention plan.
Little is known about the circulation of bat-borne diseases in general and of Nipah virus in particular in Cambodia. Though Nipah virus was isolated in 2000 from a Pteropus lylei roost in western Cambodia, this finding was never replicated and no human case has been reported in the country.15 This situation may be similar to Thailand, where Nipah virus circulates in P. lylei populations, but where human and domestic animal cases have never been reported.16 Over a dozen P. lylei roosts are known in Cambodia and most of these are located in villages or cities, which suggests clear interfaces with humans and potential for direct or indirect contact.17 Studies of Nipah virus circulation in bats, coupled with research on agricultural practices and risk perceptions within local communities, would shed light on the potential risk for Nipah virus emergence in Cambodia, either through the transmission routes observed elsewhere or due to country-specific factors. Better knowledge would also help to identify human populations at risk who could then be targeted for future surveillance and prevention programmes.
The overall objective of our study was to better understand the potential risks of Nipah virus emergence in Cambodia. We combined research on bat ecology (reproductive phenology, population dynamics and diet); human practices and perceptions (ethnographic research and knowledge, attitude and practice studies); and Nipah virus circulation studies in bat and human populations (virus monitoring in bat urine and anti-Nipah virus antibody detection in human serum). We aimed to: (i) confirm the presence of Nipah virus in bat populations and understand circulation patterns; (ii) determine potential transmission routes for the virus from bats to humans; and (iii) identify potential unreported virus transmission from fruit bats to humans (spillover) and the associated risk factors.
Methods
Our study was undertaken at two sites in rural areas of Kandal and Battambang provinces in Cambodia, where large P. lylei populations have been reported (Fig. 1). More details of the location and laboratory methods are in the authors’ data repository.18
Fig. 1.
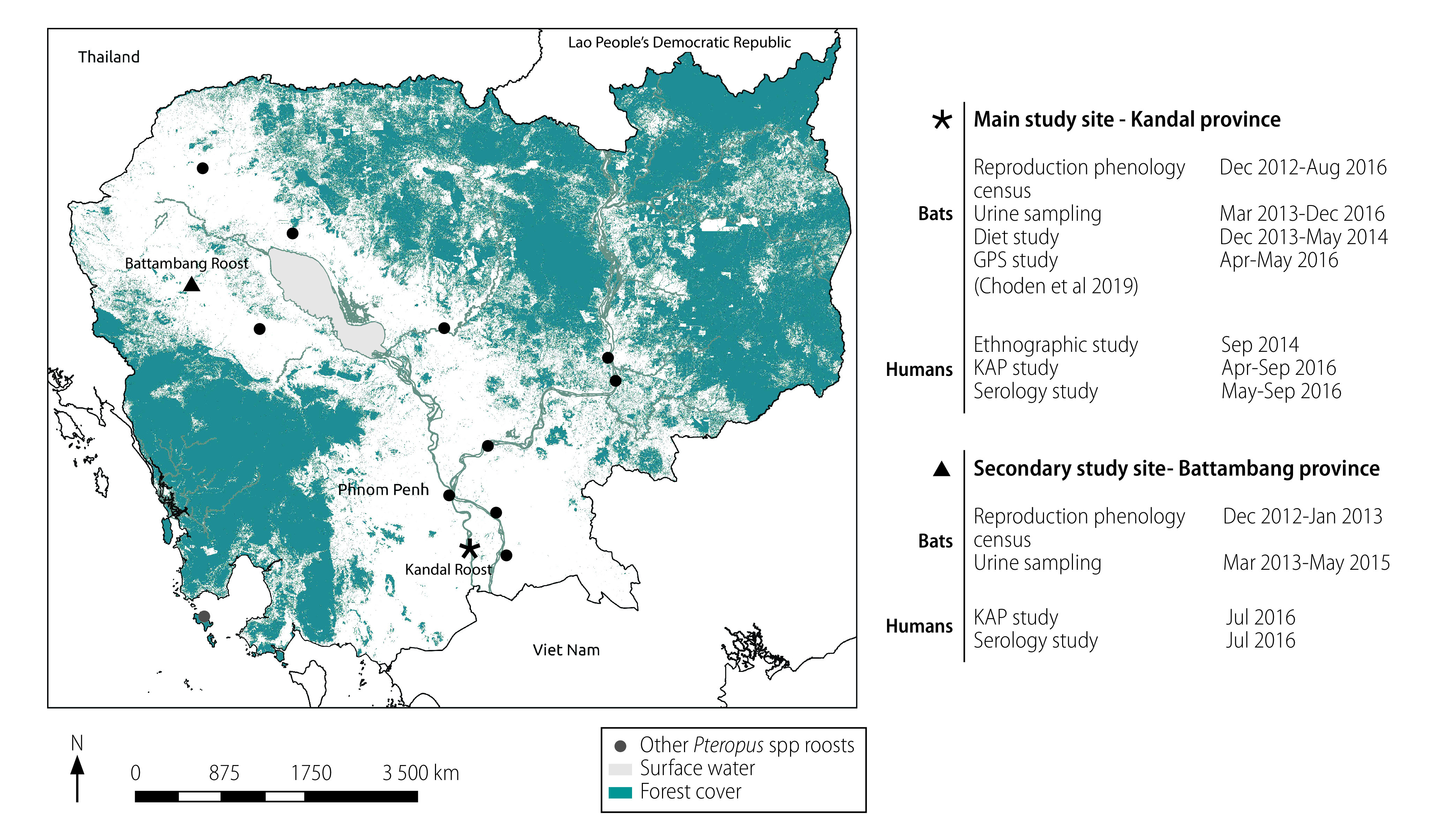
Location of study sites in Cambodia and studies undertaken at each site, 2013–2016
GPS: global positioning system; KAP: knowledge, attitude and practice.
Census, diet and reproductive cycle
We conducted monthly censuses from March 2013 to August 2016 to estimate the size of the P. lylei population at the primary study site in Kandal province. An exit census was undertaken on two consecutive evenings every month when the bats emerged from the roost at dusk. During the exit censuses, we used hand-held tally counters to count the bats on the two main routes they used to disperse from the roost each evening.19 The bats were also observed directly with binoculars during the day every month to identify specific phases of their annual reproductive cycle, including mating, parturition and weaning. A study of the diet of P. lylei based on the analysis of faecal samples was also implemented from December 2013 to May 2014 at the primary study site.18
Urine sampling and testing
We undertook research on Nipah virus circulation in P. lylei by testing bat urine samples collected at the primary study site from 2013 to 2016. We performed preliminary sampling each month from March to July 2013 to assess seasonality in the occurrence of Nipah virus, as suggested by a previous study on P. lylei in Thailand.20 We subsequently conducted longitudinal sampling at the same site from March 2014 to September 2016, which comprised 36 sampling sessions separated by intervals of 3 weeks to 2 months. We also collected urine samples at the secondary study site in Battambang province each month from March to June 2013, and in May 2014, and April and May 2015. The timing of the latter sampling was again chosen to reflect the seasonality of Nipah virus observed in Thailand.20 In each sampling session, we collected a target of 100 urine samples with micropipettes over one or two consecutive mornings from plastic sheets laid under the trees where the bats roost. A volume of 0.5 mL of urine was collected per sample, which was transferred to cryotubes and immediately stored in liquid nitrogen.
We screened all samples for Nipah virus ribonucleic acid detection using a SYBR® Green reverse transcription polymerase chain reaction (RT–PCR) assay21 and suspect samples were tested by a duplex nested RT–PCR method.18,22
Ethnographic study
We conducted an ethnographic study at our primary study site in Kandal province to understand local uses and perceptions related to fruit bats, with a specific emphasis on health issues and potential risks for disease transmission from bats to humans. A Khmer-speaking French anthropologist undertook semi-structured interviews with villagers representative of the people potentially exposed to fruit bats. Individual and collective interviews were conducted with the following participants: a group of monks and elders involved in the social and religious life of the Buddhist pagoda where the P. lylei roost is located; the head of the pagoda; a fruit-bat hunter; two palm-juice collectors; two mango and sapodilla fruit plantation owners; two fruit sellers at the local market; the owner of a commercial pig farm; and the owner of a pig-breeding farm who also owned a mango plantation. All of the data obtained were transcribed and analysed in a qualitative manner.
Knowledge, attitude and practice study
Using the results of our ethnographic study we developed a questionnaire comprehensible to our respondents. The questionnaire was developed in Khmer by our anthropologist and team of epidemiologists and ecologists to obtain quantitative information on the uses and perceptions related to fruit bats in the study area.
We interviewed residents in the primary study area in 2016 at the same time as a P. lylei global positioning system (GPS) telemetry study.23 Additional residents were selected based on the results of the GPS telemetry in areas where clear interactions between fruit bats and humans were observed, most notably as a result of fruit farming, fruit collection and pig farming.23 In addition, a group of residents living within a 10 km radius of the P. lylei roost in Battambang province were also interviewed and asked to participate in the serological study.
Serological survey
To identify potentially undetected spillovers of Nipah virus from P. lylei to humans in the past, we implemented a serological survey of people with the highest exposure risk to fruit bats at our study sites. The study was first presented to the village authorities and eligible subjects were then selected based on their occupation and the results of the GPS telemetry study.23 Palm juice collectors, fruit farmers and collectors, fruit-bat hunters and people living or working under the roost were specifically targeted. We tested sera for Nipah virus immunoglobulin G antibodies by an in-house enzyme-linked immunosorbent assay.18
Ethics
The study was approved by the Cambodian National Ethics Committee for Human Research (approval #087NECHR/2011). We obtained written informed consent from the participants, or their parent or guardian, for inclusion in the study and for blood sampling.
Results
Population dynamics of P. lylei
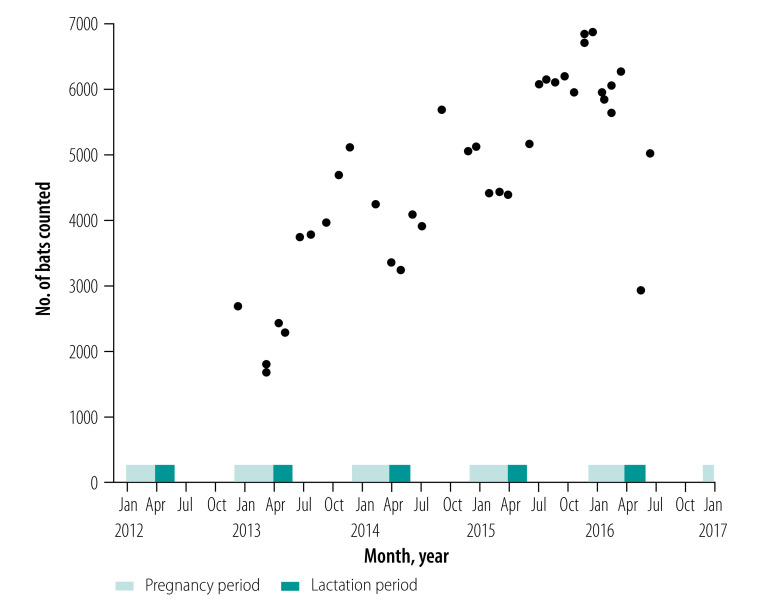
The monthly estimated population of P. lylei at our primary study site ranged from about 2000 to 7000 individuals and increased during the study period (Fig. 2). Mating, parturition and weaning of fruit bats occurred at the same time each year, with mating in November, parturition in April and weaning in June. Pregnancy occurred from December to March, while lactation took place in April and May (Fig. 2). As anticipated, the bat population increased each year after weaning, when annual cohorts of juvenile bats started flying and were consequently recorded in our exit censuses.
Fig. 2.
Population dynamics and reproductive phenology of Pteropus lylei in Kandal province, Cambodia, 2013–2016
Diet of P. lylei
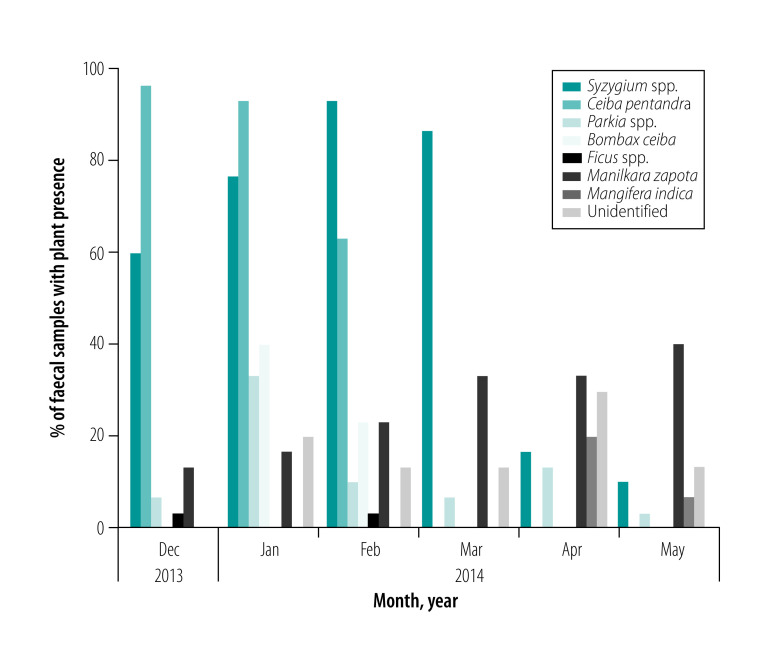
We identified seven plant species from the 210 faecal samples collected during the study. These included pollen from flowers of the Malay apple (Syzygium spp.), kapok (Ceiba pentandra), petai (Parkia spp.) and cotton tree (Bombax ceiba), seeds from fig trees (Ficus spp.) and fibres and pulp of sapodilla (Manilkara zapota) and mango (Mangifera indica). Pollen belonging to two additional unidentified species were also present in our samples. Pollen from flowers (mainly Syzygium spp. and Ceiba pentandra) were identified in most samples collected from December 2013 to March 2014, whereas fruit (mainly sapodilla) were most abundant in samples collected in April and May 2014 (Fig. 3).
Fig. 3.
Percentage of plant species in faecal samples from Pteropus lylei in Kandal province, Cambodia, 2014
Note: We analysed a total of 210 faecal samples collected from December 2013 to May 2014.
Nipah virus circulation in P. lylei
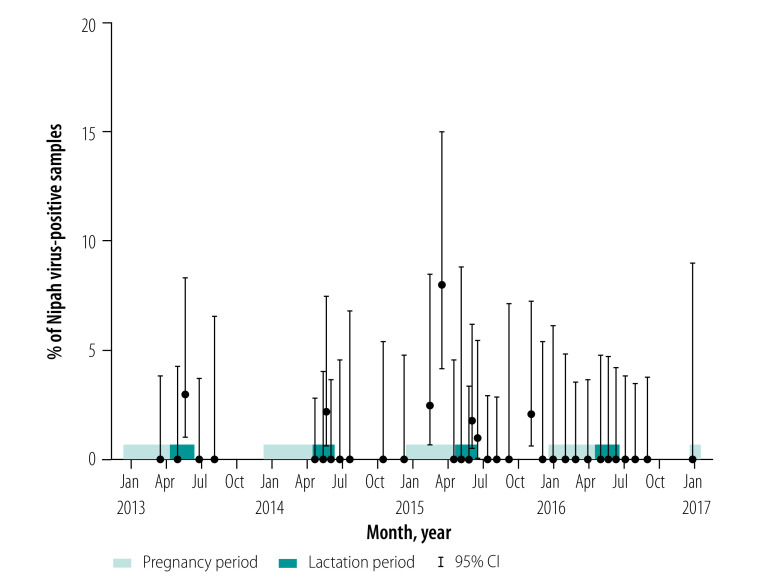
We collected a total of 3157 urine samples during 36 sampling sessions at the primary study site in Kandal province. Among these, 20 samples (0.6%) were positive for Nipah virus genome detection. The proportions of positive samples in individual sessions ranged from 0% to 8%, with no clear seasonal pattern of Nipah virus circulation (Fig. 4; data repository).18 Nonetheless, Nipah virus was detected in female bats during lactation in 2013, 2014 and 2015, and during pregnancy in 2015. Outside of these periods, the virus was only detected in one sampling session in October 2015 and was not detected in 2016, despite 10 sampling sessions undertaken in nine different months. As a result, detection rates of Nipah virus were higher during the reproductive stages of pregnancy and lactation compared with the rest of the year, with 18 out of 1962 (0.9%) and 2 out of 1213 (0.2%) samples positive, respectively (logistic regression, adjusted odds ratio, OR: 5.61; 95% confidence interval, CI: 1.61–35.3).
Fig. 4.
Percentage of Nipah virus-positive samples from individual sampling sessions of Pteropus lylei urine in Kandal province, Cambodia, 2013–2016
CI: confidence interval.
Note: We collected a total of 3157 urine sample (range: 39 to 156 samples per session).
A total of 773 urine samples were collected during seven sampling sessions at the Battambang roost site. Most of these sampling sessions occurred during the lactation period of female bats with only one session during pregnancy and one following lactation in 2013 (data repository).18 Overall, eight samples (1.0%) were confirmed positive for Nipah virus and the proportions of positive samples from individual sessions ranged from 0 to 4.5% (data repository).18
Ethnographic study
Farmers reported that fruit bats ate cultivated fruits, but that these nocturnal visits did not bother them because the bats consumed ripe fruit, whereas mango and sapodilla fruit, the most commonly cultivated fruit, are collected before they ripen. As a result, the impact of bats on fruit production was considered negligible by respondents. However, longan (Dimocarpus longan), another fruit consumed by bats, was an exception because it is allowed to ripen before harvesting. Farmers consequently reported using nets to protect these fruits from bats.
Fruit bats can drink the juice which flows from palm flowers into collecting containers (typically bamboo vessels or plastic bottles). Due to the small diameter of the upper portion of these containers, however, bats can presumably only access nectar within them when they are full or almost full (data repository).18
Study participants reported that fruit bats were not considered a potential source of disease for humans but that it was considered disgusting to eat a fruit partially consumed by an animal. More generally, animals were not considered by participants to be a potential source of diseases for humans, with the exception of a few diseases including rabies, avian influenza and malaria.
According to study participants, the cosmological world is often implicated in human illness. In Cambodia, the dead (khmoch), including ancestors (daun ta), evil spirits (preay), tutelary spirits linked to a particular place (kru batjeay) and household gods (neak ta), can all make a person ill. As a consequence, all health care can potentially involve the invisible world in addition to modern medicines, which are also used. For instance, a medical injection may sometimes work by providing an offering to a tutelary spirit who, if offended, might conversely prevent it from working.
Knowledge, attitude and practice study
We interviewed 41 people from 36 households during the GPS telemetry study in Kandal province using the questionnaire.18 Following the telemetry study, we interviewed and took blood samples from 164 individuals from 41 additional households in Kandal, bringing the total number of households surveyed in the province to 77. In Battambang, we interviewed and sampled 254 people from 103 households. Due to the time required to inform participants about the study, we completed only the three first sections of the questionnaire for participants who also agreed to give blood samples.
The primary difference in the main occupation of the participants between the two sites was the presence of palm-juice collectors only in Kandal (14; 7.0%) and a greater proportion of bat-guano collectors in Battambang (27 people; 10.6%) than in Kandal (1 person; 0.6%). Numbers of participants raising pigs were similar at both sites and only 16 households reported owning from one to 11 pigs. Table 1 summarizes the key results for practices and perceptions related to potential Nipah virus transmission at the two study sites (data repository).18
Table 1. Main results of the knowledge, attitude and practice study in two provinces, Cambodia, 2016.
| Variable | No. (%) of respondents |
|
|---|---|---|
| Battambang province (n = 254 from 103 households) | Kandal province (n = 205 from 77 households) | |
| Frequency of consuming palm juice | ||
| Never | 118 (46.4) | 56 (27.3) |
| Occasionally | 117 (46.1) | 114 (55.6) |
| Often | 15 (5.9) | 19 (9.3) |
| Very often | 4 (1.6) | 16 (7.8) |
| Type of palm juice consumed | ||
| Raw | 131 (51.6) | 84 (41.0) |
| Processed | 14 (5.5) | 67 (32.7) |
| Don’t know | 109 (42.9) | 43 (21.0)a |
| Frequency of hunting fruit bats | ||
| Never | 210 (82.7) | 186 (90.7) |
| Occasionally | 34 (13.4) | 17 (8.3) |
| Often | 8 (3.1) | 2 (1.0) |
| Very often | 2 (0.8) | 0 (0.0) |
| Frequency of consuming fruit bats | ||
| Never | 227 (89.4) | 173 (84.4) |
| Occasionally | 22 (8.6) | 29 (14.1) |
| Often | 2 (0.8) | 2 (1.0) |
| Very often | 3 (1.2) | 1 (0.5) |
| Use of partially eaten fruit by householdsb | ||
| Eat fruit | 11 (10.7) | 5 (6.5) |
| Feed animals | 9 (8.7) | 15 (19.5) |
| Cook | 0 (0.0) | 12 (15.6) |
| Nothing or thrown away | 76 (73.8) | 37 (48.1) |
| Other | 7 (6.8) | 8 (10.3) |
| Can fruit bats transmit diseases to humans?c | ||
| Yes | NA | 3 (7.3) |
| No | NA | 13 (31.7) |
| Don’t know | NA | 25 (61.0) |
NA: not applicable.
a Only 194 respondents replied to this question.
b Only one respondent per household replied to this question.
c Only 41 respondents from Kandal province replied to this question.
Notes: Surveys were conducted in Kandal in April to September 2016 and in Battambang in July 2016. Palm juice was most commonly consumed in raw form. While most respondents disposed of partially eaten fruits, practices including consumption or feeding them to animals were still relatively common.18
Serological survey among exposed villagers
Most heads of families were farmers owning fruit trees. Participants who were considered particularly at risk of potential contact with fruit bats and Nipah virus transmission included 14 palm-juice collectors and one palm-juice seller, as well as four fruit-bat hunters. All sera tested (164 in Kandal and 254 in Battambang) were negative for Nipah virus antibodies (data repository).18
Discussion
Our integrated research allowed us to consistently collect information on different factors that could be involved in Nipah virus disease emergence among humans in our study areas. First, by combining the results of our study of reproductive phenology and virus monitoring, we confirmed the presence of Nipah virus in bat urine at both study sites. While Nipah virus circulation appears to be intermittent with no clear seasonal pattern, specific periods, notably pregnancy (December to March) and parturition (April–May), coincided with increased virus detection rates in bats. Second, by combining the results of our diet study, virus monitoring in bat urine and our ethnographic and knowledge, attitude and practice studies, we identified potential routes for virus transmission from bats to humans through fruit, such as mango and sapodilla. These two fruits are consumed by bats and harvested by humans when Nipah virus is potentially circulating in the bats (April and May). Palm nectar could also act as a transmission route because it is commonly consumed raw by humans and was also reported to be consumed by bats. Furthermore, these practices are mostly conducted in the absence of any perception of zoonotic risks by participants. Finally, the lack of a confirmed Nipah virus spillover based on our serology study of people residing in areas with close bat–human contact enables us to consider the likelihood of Nipah virus emergence in our study areas.
The absence of confirmed antibodies against Nipah virus among study participants could be due to two reasons: an actual absence of previous spillovers or a failure to detect these. These hypotheses are discussed in more detail in the data repository.18 Nevertheless, our results suggest that Nipah virus spillover would be a rare event in our study sites. Even in Bangladesh, where most Nipah virus outbreaks have occurred to date, Nipah virus infection is rare in humans and suggests that spillover may require temporal alignment of a complex combination of factors that rarely occur. Palm sap, and not palm juice, is collected in Bangladesh using large clay pots. The ease of access to the pots may facilitate contamination by bats. Consumption of palm sap also appears to be more popular in Bangladesh, whereas in our study areas palm juice is preferred, but only occasionally consumed.24 Thus, greater exposure related to these practices in Bangladesh may explain why intermittent shedding of Nipah virus by bats results in recurrent outbreaks in Bangladesh, but not in Cambodia.
Our results must be interpreted with caution because we only monitored one fruit-bat colony for 4 years and another one for several months over 2 years. Furthermore, the absence of detection of Nipah virus in 2016 suggests inter-annual variation could also occur, as suggested by serological monitoring in Malaysia.25 We are still modelling our data set to test different factors that might contribute to this apparent seasonality. Possible factors include a sharp increase in numbers of susceptible juvenile bats or a revival of virus outbreaks in pregnant and lactating female bats.26,27 We also aim to explain circulation patterns involving fluctuating herd immunity over several years.28 The apparent increase in the number of bats in our study colony over the study period is another factor that could be considered in such a model. However, in the absence of population data from nearby roosts, differentiating between the natural growth in numbers and immigration to herds could prove difficult.
Notwithstanding these limitations, our study suggests certain surveillance and control measures would be helpful in Cambodia. For example, Nipah virus should be considered as a potential cause of acute encephalitis in Cambodia and encephalitis patients should be tested for Nipah virus, particularly in the case of severe infection. In bats, surveillance could also be targeted based on our results, which suggest increased Nipah virus circulation occurs when females are pregnant and lactating. As our sampling method did not allow us to collect individual urine samples, we could not estimate the prevalence of the virus in the bat population. However, the increased circulation of Nipah virus during bat pregnancy and lactation suggested by our finding is consistent with observations in Malaysia and Thailand.20,25 Thus, pending available resources, Nipah virus surveillance in bats could be intensified in the March to May period. Allied to this, GPS telemetry at our main study site demonstrated that the bats moved relatively frequently to several other roosts located up to 105 km away. This result suggests a global population structure rather than a meta-population structure,23 in which case Nipah virus surveillance at a few roost sites might be sufficient to detect virus circulation in the country.
Our results also allow us to propose interventions to limit the exposure of local communities to bats. However, these would involve changes in practices that can be hindered by people’s perception of a low risk of disease. Our ethnographic and subsequent knowledge, attitude and practice study showed that people in our study areas did not perceive risks related to bats and more generally to animals. As in many other Asian countries, health beliefs in Cambodia combine modern medicine and mixing of several religious beliefs and practices, including animistic representations of diseases.29,30 This viewpoint was also the case in Bangladesh, where communities impacted by Nipah virus outbreaks developed causal explanations involving supernatural powers.31 This belief may in turn explain why costly interventions are needed to change human practices despite the availability of affordable solutions, such as bamboo skirts on trees that prevent bats from accessing and contaminating palm sap.14 Similarly, intensive actions may be required in Cambodia because our ethnographic study showed interviewees were only aware of two zoonotic diseases, including avian influenza, for which massive national awareness campaigns have been implemented. As such, our study suggests efforts to improve zoonotic risk awareness in general may be needed before specific communication programmes are planned. Our findings also suggest that interventions related to the risk of Nipah emergence should focus on hunting and consumption of fruit bats, consumption of fruit partially eaten by animals, palm-nectar collection practices and dissociation of pig farming and fruit farming.
In conclusion, our integrated approach allowed us to gather information on different components of a complex socio-ecosystem where Nipah virus could emerge. Although each study provides insufficient information to draw any conclusions, when considered together, recommendations can be provided to interrupt potential transmission routes and limit the risk of disease emergence.
As bats are hosts of major zoonotic pathogens, such studies would likely also help to reduce the risk of emergence of other bat-borne diseases, including diseases similar to severe acute respiratory syndrome or COVID-19.32,33 While the precise chain of events leading to the emergence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and its precise origin are still unknown, studies suggest the virus originated in bats.1,34 Integrated approaches are necessary to better understand the factors driving these emergences and implement efficient and socially acceptable surveillance and control measures at the interfaces between bats and humans. In this context, the emergence of SARS-CoV-2 should act as a reminder that stringent efforts will be required to reduce the risk of future pandemics.
Acknowledgements
We thank all the respondents of Kandal and Battambang provinces and Dr Keo Omaliss. JC was affiliated to Institut Pasteur du Cambodge during the study. NF is also affiliated to Harrison Institute, Bowerwood House, Kent, England. SP is also affiliated to the Institut de Recherches Asiatiques (CNRS UMR 7306), Marseille, France.
Funding:
This study was supported by the European Commission Innovate program (ComAcross project, grant no. DCI-ASIE/2013/315–047).
Competing interests:
PB is an employee of GSK Vaccines.
References
- 1.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 Mar;579(7798):270–3. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2018 annual review of diseases prioritized under the Research and Development Blueprint. Informal consultation. 6–7 February 2018, Geneva, Switzerland [internet]. Geneva: World Health Organization; 2018. Available from: https://www.who.int/emergencies/diseases/2018prioritization-report.pdf?ua=1 [cited 2019 Jul 31]
- 3.Luby SP, Hossain MJ, Gurley ES, Ahmed B-N, Banu S, Khan SU, et al. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001–2007. Emerg Infect Dis. 2009 Aug;15(8):1229–35. doi: 10.3201/eid1508.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arunkumar G, Chandni R, Mourya DT, Singh SK, Sadanandan R, Sudan P, et al. Nipah Investigators People and Health Study Group Outbreak investigation of nipah virus disease in Kerala, India, 2018. J Infect Dis. 2019 May 24;219(12):1867–78. doi: 10.1093/infdis/jiy612. [DOI] [PubMed] [Google Scholar]
- 5.Gurley ES, Montgomery JM, Hossain MJ, Bell M, Azad AK, Islam MR, et al. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg Infect Dis. 2007 Jul;13(7):1031–7. doi: 10.3201/eid1307.061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008 Feb 21;451(7181):990–3. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morse SS. Factors in the emergence of infectious diseases. Emerg Infect Dis. 1995 Jan-Mar;1(1):7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daszak P, Zambrana-Torrelio C, Bogich TL, Fernandez M, Epstein JH, Murray KA, et al. Interdisciplinary approaches to understanding disease emergence: the past, present, and future drivers of Nipah virus emergence. Proc Natl Acad Sci USA. 2013 Feb 26;110(Suppl 1):3681–8. doi: 10.1073/pnas.1201243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn MB, Epstein JH, Gurley ES, Islam MS, Luby SP, Daszak P, et al. Roosting behaviour and habitat selection of Pteropus giganteus reveals potential links to Nipah virus epidemiology. J Appl Ecol. 2014 Apr 1;51(2):376–87. doi: 10.1111/1365-2664.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plowright RK, Eby P, Hudson PJ, Smith IL, Westcott D, Bryden WL, et al. Ecological dynamics of emerging bat virus spillover. Proc Biol Sci. 2015 Jan 7;282(1798):20142124. doi: 10.1098/rspb.2014.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, et al. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000 May 26;288(5470):1432–5. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 12.Pulliam JRC, Epstein JH, Dushoff J, Rahman SA, Bunning M, Jamaluddin AA, et al. Henipavirus Ecology Research Group (HERG) Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J R Soc Interface. 2012 Jan 7;9(66):89–101. doi: 10.1098/rsif.2011.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman MA, Hossain MJ, Sultana S, Homaira N, Khan SU, Rahman M, et al. Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector Borne Zoonotic Dis. 2012 Jan;12(1):65–72. doi: 10.1089/vbz.2011.0656. [DOI] [PubMed] [Google Scholar]
- 14.Nahar N, Mondal UK, Hossain MJ, Khan MSU, Sultana R, Gurley ES, et al. Piloting the promotion of bamboo skirt barriers to prevent Nipah virus transmission through date palm sap in Bangladesh. Glob Health Promot Educ. 2014 Dec;21(4):7–15. doi: 10.1177/1757975914528249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynes JM, Counor D, Ong S, Faure C, Seng V, Molia S, et al. Nipah virus in Lyle’s flying foxes, Cambodia. Emerg Infect Dis. 2005 Jul;11(7):1042–7. doi: 10.3201/eid1107.041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wacharapluesadee S, Lumlertdacha B, Boongird K, Wanghongsa S, Chanhome L, Rollin P, et al. Bat Nipah virus, Thailand. Emerg Infect Dis. 2005 Dec;11(12):1949–51. doi: 10.3201/eid1112.050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravon S, Furey NM, Hul V, Cappelle J.A rapid assessment of flying fox (Pteropus spp.) colonies in Cambodia. Cambodian J Nat Hist 2014114–8.Available fromhttps://cms.fauna-flora.org/wp-content/uploads/2017/11/FFI_201408_Cambodian-Journal-of-Natural-History.pdf.pdf[cited 2020 Jun 9]. [Google Scholar]
- 18.Cappelle J, Hoem T, Hul V, Furey N, Nguon K, Prigent S, et al. Supplementary webappendix: supplementary material, figures and tables [data repository]. 2020. 10.6084/m9.figshare.12448958.v1 10.6084/m9.figshare.12448958.v1 [DOI]
- 19.Kunz TH, Thomas DW, Richards GR, Tideman ED, Racey PA, editors.Observational techniques for bats: measuring and monitoring biological diversity. Washington, DC: Smithsonian Institution Press; 1996. pp. 105–14. [Google Scholar]
- 20.Wacharapluesadee S, Boongird K, Wanghongsa S, Ratanasetyuth N, Supavonwong P, Saengsen D, et al. A longitudinal study of the prevalence of Nipah virus in Pteropus lylei bats in Thailand: evidence for seasonal preference in disease transmission. Vector Borne Zoonotic Dis. 2010 Mar;10(2):183–90. doi: 10.1089/vbz.2008.0105. [DOI] [PubMed] [Google Scholar]
- 21.Feldman KS, Foord A, Heine HG, Smith IL, Boyd V, Marsh GA, et al. Design and evaluation of consensus PCR assays for henipaviruses. J Virol Methods. 2009 Oct;161(1):52–7. doi: 10.1016/j.jviromet.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Wacharapluesadee S, Hemachudha T. Duplex nested RT-PCR for detection of Nipah virus RNA from urine specimens of bats. J Virol Methods. 2007 Apr;141(1):97–101. doi: 10.1016/j.jviromet.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Choden K, Ravon S, Epstein JH, Hoem T, Furey N, Gely M, et al. Pteropus lylei primarily forages in residential areas in Kandal, Cambodia. Ecol Evol. 2019 Mar 13;9(7):4181–91. doi: 10.1002/ece3.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nahar N, Paul RC, Sultana R, Gurley ES, Garcia F, Abedin J, et al. Raw sap consumption habits and its association with knowledge of Nipah virus in two endemic districts in Bangladesh. PLoS One. 2015 Nov 9;10(11):e0142292. doi: 10.1371/journal.pone.0142292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman SA, Hassan L, Epstein JH, Mamat ZC, Yatim AM, Hassan SS, et al. Henipavirus Ecology Research Group Risk factors for Nipah virus infection among pteropid bats, Peninsular Malaysia. Emerg Infect Dis. 2013 Jan;19(1):51–60. doi: 10.3201/eid1901.120221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sohayati AR, Hassan L, Sharifah SH, Lazarus K, Zaini CM, Epstein JH, et al. Henipavirus Ecology Research Group Evidence for Nipah virus recrudescence and serological patterns of captive Pteropus vampyrus. Epidemiol Infect. 2011 Oct;139(10):1570–9. doi: 10.1017/S0950268811000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amman BR, Carroll SA, Reed ZD, Sealy TK, Balinandi S, Swanepoel R, et al. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathog. 2012;8(10):e1002877. doi: 10.1371/journal.ppat.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plowright RK, Peel AJ, Streicker DG, Gilbert AT, McCallum H, Wood J, et al. Transmission or within-host dynamics driving pulses of zoonotic viruses in reservoir-host populations. PLoS Negl Trop Dis. 2016 Aug 4;10(8):e0004796. doi: 10.1371/journal.pntd.0004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baer HA, Singer M, Susser I.editors. Medical anthropology and the world system: critical perspectives. 3rd ed. Santa Barbara: ABC-CLIO; 2013. p. 534. [Google Scholar]
- 30.Husson L. La santé : miroir des sociétés d’Asie du Sud-Est. Moussons No. 15. [internet]. Marseille: University of Provence Publications and Research Institute for South-East Asia; 2010. Available from: https://amades.hypotheses.org/2606 [cited 2019 Aug 6].
- 31.Blum LS, Khan R, Nahar N, Breiman RF. In-depth assessment of an outbreak of Nipah encephalitis with person-to-person transmission in Bangladesh: implications for prevention and control strategies. Am J Trop Med Hyg. 2009 Jan;80(1):96–102. doi: 10.4269/ajtmh.2009.80.96. [DOI] [PubMed] [Google Scholar]
- 32.Drexler JF, Corman VM, Müller MA, Maganga GD, Vallo P, Binger T, et al. Bats host major mammalian paramyxoviruses. Nat Commun. 2012 Apr 24;3(1):796. doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olival KJ, Hosseini PR, Zambrana-Torrelio C, Ross N, Bogich TL, Daszak P. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017 Jun 29;546(7660):646–50. doi: 10.1038/nature22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020 Apr;26(4):450–2. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]