Abstract
Osteoarthritis (OA) is a chronic disease that is mainly characterized by chondrocyte degeneration. Inflammatory mediators participate in the development of OA, leading to chondrocyte apoptosis and destruction of the cartilage. Genistein is the major active component of isoflavone, with a chemical composition and a biological effect that is similar to that of estrogens, which prevents the degradation of cartilage; however, its underlying mechanisms of action remain unknown. The aim of the present study was to investigate the anti-apoptotic effects of genistein on chondrocytes for the treatment of inflammation-induced OA. Interleukin (IL)-1β was used to establish a chondrocyte OA model. After treatment with different concentrations of genistein, western blotting identified that expression levels of collagen II and aggrecan were increased in a concentration-dependent manner, while caspase 3 expression gradually decreased after genistein application. Moreover, flow cytometry and ELISA results demonstrated that genistein could decrease chondrocyte apoptosis and reduce the levels of tumor necrosis factor (TNF)-α in a dose-dependent manner. Furthermore, the in vitro data were evaluated in an OA rat model. Genistein increased the collagen and acid glycosaminoglycan content, as well as decreased the levels of TNF-α and IL-1β. Genistein also promoted the expression levels of collagen II and aggrecan in the articular cartilage, and decreased the expression of caspase 3, thus alleviating cartilage degradation. In conclusion, the results indicated that genistein mediated inflammation and had an anti-apoptotic role in treating OA. Therefore, genistein may serve as an alternative treatment for OA.
Keywords: chondrocyte, apoptosis, genistein, cartilage degeneration, inflammation
Introduction
Osteoarthritis (OA) is a chronic disease characterized by chondrocyte degeneration and cartilage matrix structure degradation (1). The pathological characteristics of OA include abnormal chondrocyte metabolism, damage to the articular cartilage and excessive degradation of collagen (2). Furthermore, the leading causes of OA may be associated with inflammation, oxidative stress, cellular damage and apoptosis of chondrocytes and synoviocytes (3). With aging populations and an accelerated pace of life, the incidence of OA has not only significantly increased in those aged >50 years (4), but even in the younger population. Thus, the prevention and treatment of OA has attracted increased attention worldwide.
In a healthy state, the extracellular matrix of articular cartilage has a metabolic balance between synthesis and degradation. However, when cartilage degeneration occurs, matrix metalloproteinases (MMPs) degrade collagen and proteoglycan in the cartilage by destroying their structures and disrupting the dynamic balance between degradation and synthesis of the extracellular matrix (5). The inflammatory reaction serves a vital role in the pathogenesis underlying OA. For instance, inflammatory mediators, such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6, inducible nitric oxide synthase and prostaglandin E2, participate in the development of OA and lead to chondrocytes apoptosis and cartilage destruction (6,7).
Isoflavone is a phytoestrogen found in soybeans that has a chemical composition and biological effect similar to that of estrogen (8). Genistein, which is the major active component of isoflavone, alleviates osteoporosis caused by decreases in estrogen levels in postmenopausal women, as well as reduces fracture occurrence and cartilage degeneration caused by the lack of estrogens (9). Previous studies have reported that genistein not only restores the histomorphological structures of cartilage but also corrects the abnormal synthesis of cartilage matrix caused by estrogen deficiencies (10,11). Despite these findings, the underlying mechanisms of action of genistein on chondrocytes are yet to be elucidated. Previous studies have revealed that genistein can be used to treat bladder cancer and laryngeal cancer by regulating apoptosis (12,13). Therefore, the aim of this present study was to investigate the efficacy and mechanisms of action of genistein for the treatment of inflammation induced OA by regulating chondrocyte apoptosis in vitro and in vivo.
Materials and methods
Culturing and identification of human chondrocytes
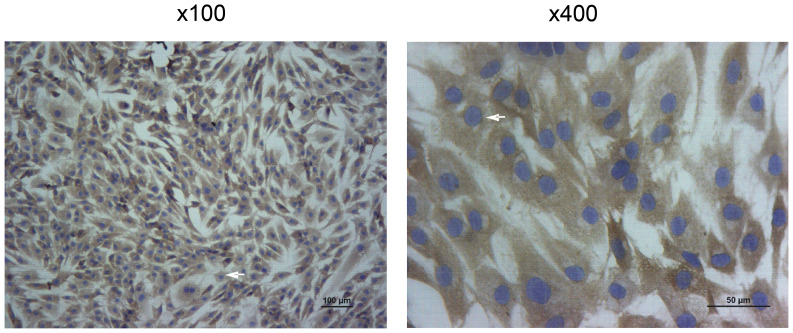
Human chondrocytes, CHON-001 (cat. no. ATCC®CRL-2846TM), used in the present study were from American Type Culture Collection. Cells were cultured at a concentration of 5×105 cells in 25-cm2 flasks with DMEM/F12 (cat. no. 1662222, Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (cat. no. 10099-141, Gibco; Thermo Fisher Scientific, Inc.) and antibiotics (10% penicillin/streptomycin solution) in an incubator at 37°C with 5% CO2. Chondrocytes, which were identified using collagen II immunohistochemical detection, were inoculated in a culture dishes pre-coated with a coverslip and collected after 4 h, then washed with PBS. The cells were fixed using 4% paraformaldehyde at room temperature for 20 min, embedded with neutral resin at room temperature for 20 min and sectioned at 18 µm. After being washed three times with PBS for 5 min each time and incubated with 0.5% Triton X-100 at room temperature for 20 min, chondrocytes were treated with 3% H2O2 at room temperature for 15 min and blocked in 5% BSA (cat. no. mu30432, Bio-Swamp) at room temperature for 20 min after dehydration. Subsequently, chondrocytes were incubated with anti-collagen II antibodies (rabbit; 1:100; cat. no. AF0135; Affinity Biosciences) at 4°C overnight. The following day, sections were washed with PBS and incubated with the secondary antibody [goat anti-rabbit horseradish peroxidase (HRP); 1:50; cat. no. A0208; Beyotime Institute of Biotechnology) for 50 min at 4°C. The sections were then washed three times with PBS, with each wash lasting for 5 min, and were stained with 100 µl 3,3-diaminobenzidine (cat. no. P0203; Beyotime Institute of Biotechnology) at room temperature for 8 min, then counter-stained with hematoxylin (cat. no. H9627, Sigma-Aldrich; Merck KGaA) at room temperature for 25 sec. Images were captured from each section at magnification ×100 and ×400, using an IX51 light microscope (Olympus Corporation).
OA cell model induced with IL-1β
Human chondrocytes were divided into normal chondrocytes and those from the OA cell model. The OA cell model was induced using IL-1β (cat. no. SRP6169; Sigma-Aldrich; Merck KGaA) at the recommended concentration of 10 ng/ml (14). Chondrocytes were treated with IL-1β at room temperature for 24 h.
Effect of genistein on chondrocytes in the OA model
Human chondrocytes in the present study were divided into six groups as follows: i) Group A, normal chondrocytes; ii) group B, OA cell model (chondrocytes + IL-1β); iii) group C, OA cell model (chondrocytes + IL-1β) + genistein (cat. no. 1288816, Sigma-Aldrich; Merck KGaA) (25 µg/ml); iv) group D, OA cell model (chondrocytes + IL-1β) + genistein (50 µg/ml); v) group E, OA cell model (chondrocytes + IL-1β) + genistein (100 µg/ml); vi) and group F, OA cell model (chondrocytes + IL-1β) + estradiol valerate (cat. no. 1252003, Sigma-Aldrich; Merck KGaA) (100 µg/ml). After treatment with IL-1β at room temperature for 24 h, chondrocytes in groups B, C, D, E and F were incubated with different concentrations of genistein and estradiol valerate as aforementioned at room temperature for 72 h.
Detection of chondrocytes apoptosis using flow cytometry
Chondrocyte apoptosis was assessed using flow cytometry (FACSCalibur, BD Biosciences). The apoptotic rate was calculated as the percentage of early + late apoptotic cells. A total of 2×105 cells were seeded in 6-well plates. Briefly, a cell apoptosis detection kit (cat. no. KGA108, Nanjing KeyGen Biotech Co. Ltd.) was used: 500 µl binding buffer mixed with 5 µl Annexin V-FITC and 5 µl propidium iodide (PI) was added to each well. Cells were then incubated at 25°C in darkness for 15 min. In the flow cytometry assay, green fluorescence indicated Annexin V-FITC and red fluorescence indicated PI. The excitation wavelength of FITC was 488 nm and was detected in the FL1 channel. Red fluorescence was detected in the FL2 channel. The staining profile was analyzed using Cell Quest Pro software, version 5.1 (BD Biosciences). In the two-color dot plot, the x-axis represented FL1 and y-axis represented FL2. In total, 10,000 events were collected for each sample.
Western blot analysis for chondrocytes
Protein from chondrocytes was extracted using a lysis buffer [containing 20 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1% TritonX-100, 1.5 M NaCl, 0.1% SDS and 1% phenylmethanesulfonyl fluoride; cat. no. ST505; Beyotime Institute of Biotechnology] and was preserved at −80°C after centrifugation at 16,000 × g for 10 min at 4°C. The protein concentration was determined using the Bio-Rad DC protein assay(ChemiDoc XRS + system, Bio-Rad Laboratories, Inc.). A total of 30 µg protein from each group was analyzed using electrophoresis on 10% SDS-PAGE with a constant voltage initially set at 80 V for 30 min, and then 120 V for 120 min. The proteins were transferred onto PVDF membranes in the Bio-Rad TransBlot apparatus (Bio-Rad Laboratories, Inc.) at 100 V for 120 min. Subsequently, the membrane was blocked with 5% non-fat dry milk, which was dissolved in 0.15 M NaCl containing Tris-buffered saline-0.2% Tween-20 (TBST) and 10 mM Tris-HCl, for 2 h at room temperature. The membrane was incubated overnight at 4°C with the following primary antibodies: Aggrecan (rabbit; 1:1,000; cat. no. DF7561; Affinity Biosciences), caspase 3 (rabbit; 1:1,000; cat. no. AF6311; Affinity Biosciences), collagen II (rabbit; 1:1,000; cat. no. AF0135; Affinity Biosciences), estrogen receptor α (ERα; rabbit; 1:500; cat. no. AF6058; Affinity Biosciences) and GAPDH (rabbit; 1:1,000; cat. no. AP0063; Bioworld Technology, Inc.). The following day, the membrane was washed with TBST three times for 5 min each time at room temperature and was then incubated with secondary antibodies (goat anti-rabbit HRP; 1:50; cat. no. A0208; Beyotime Institute of Biotechnology) at room temperature for 2 h. Subsequently, the membrane was incubated with ECL chemiluminescence reagents (cat. no. NCI5079, Thermo Fisher Scientific, Inc.) and exposed to Kodak X-OMAT films for detection with ImageJ v1.8.0 (National Institutes of Health).
TNF-α levels in chondrocytes analyzed using ELISA
To analyze the expression levels of TNF-α in chondrocytes of each group, a TNF-α ELISA kit (cat. no. EHJ-10039; Xiamen Huijia Biotechnology Co., Ltd.) was used according to the manufacturer's protocol. Optical density values were determined using a microplate reader at 450 nm and the concentration of TNF-α was calculated using the linear regression equation (y=0.004×-0.089, R2=0.9874).
OA model in Sprague-Dawley rats
All 40 pathogen-free male juvenile Sprague-Dawley rats (age, 4 weeks; weight, 180–200 g) were provided by the Shanghai Experimental Animal Center of the Chinese Academy of Science [animal certificate no. SCXK (Shanghai 2007-0005)] and housed at a constant temperature of 23±1°C, humidity of 40–70% and under a 12-h light-dark cycle, with free access to water and food. All animal studies, including the mice euthanasia procedure, were performed in compliance with the regulations and guidelines of the Zhejiang Chinese Medical University Institutional Animal Care and Conducted according to the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) (15) and the Institutional Animal Care and Use Committee (IACUC) (16) guidelines. The present study was approved by Zhejiang Chinese Medical University Institutional Animal Care.
The OA rat model used in this study was performed based on a previous report (17). Rats were anesthetized with 2.25% pentobarbital sodium (45 mg/kg, intraperitoneally) and the medial knee joint of one of the limbs of each rat was incised to expose an area of skin ~1×2 cm, thus opening the knee joint cavity. Then, the medial and anterior crucial ligaments were exposed and resected. Subsequently, the medial meniscus was removed and sutured. On days 1–3 post-surgery, rats were treated with an intramuscular injection of 1 ml penicillin (40,000 U/ml). All 40 rats were randomly divided into a normal group, OA group, genistein group and estradiol valerate group (n=10 for each group). Rats in the OA, genistein and estradiol valerate groups underwent OA model surgery as aforementioned and were intragastrically administrated normal saline (2 ml), genistein (20 mg/kg) (18) or estradiol valerate (0.8 mg/kg) (19), respectively, each day for 6 weeks. Rats in the normal group were intragastrically administrated normal saline (2 ml) each day for 6 weeks and housed under the same conditions.
Paraffin-cut section of cartilage in OA rats model
After 6 weeks post-surgery, all the rats were sacrificed by dislocation of the neck and the cartilage of rats in each group was removed to create paraffin-cut sections. Cartilage tissues were fixed with formaldehyde-acetic acid-ethanol fixative (90 ml 70% ethanol, 5 ml acetic acid and 5 ml formaldehyde) at room temperature for 48 h, embedded in paraffin, and sectioned coronally to 18 µm. Sections were dewaxed using xylene (cat. no. 10023418; Sinopharm Chemical Reagent Co., Ltd.) after drying and placed in 100, 95, 85 and 75% ethanol respectively, each for 5 min at room temperature, and finally soaked in distilled water. After staining with Masson trichrome (cat. no. DC0032; Beijing Leagene Biotech Co., Ltd.) and toluidine blue (cat. no. G1032; Servicebio, Inc.) at room temperature for 20 min, the histopathological changes of cartilage were viewed under an optical microscope at ×400 magnification.
Levels of TNF-α and IL-1β in synovial liquid analyzed using ELISA. Synovial liquid (1 ml) was collected from each rat and the concentration of TNF-α and IL-1β in the synovial liquid was calculated using ELISA kits (cat. no. EHJ-10039 and EHJ-10293, respectively; Xiamen Huijia Biotechnology Co., Ltd.) according to the manufacturer's protocol.
Western blot analysis for articular cartilages
The cartilage of rats in each group was removed with a small scalpel and scissors for western blot analysis. Protein from the articular cartilage was extracted using SDS lysis buffer (cat. no. 10014118, Sinopharm Chemical Reagent Co., Ltd.), and the protein expression levels of collagen II, aggrecan, caspase 3 and ERα were detected using the aforementioned protocol.
Statistical analysis
All data were analyzed using SPSS 19.0 software (IBM Corp.). Each experiment was repeated ≥3 times. Comparisons between experimental groups were performed using one-way ANOVA and Tukey's test. Data are presented as the mean ± SD. P<0.05 was considered to indicate a statistically significant difference.
Results
Collagen II immunohistochemical identification of human chondrocytes
The collagen II immunohistochemical identification of human chondrocytes is presented in Fig. 1. Collagen II is specifically secreted by chondrocytes and can be accurately used to localize chondrocytes (20).
Figure 1.
Immunohistochemical identification of collagen II in human chondrocytes. Magnification, ×100 and ×400, as indicated. Arrow indicates positive cells.
After being counterstained with hematoxylin, collagen II was labelled brown and the cell nucleus was stained blue-purple. Collagen II immunohistochemical staining identified the heterochromatism of cultured chondrocytes, which were confirmed to be human chondrocytes (Fig. 1).
Expression levels of collagen II, aggrecan, caspase 3 and ERα of chondrocytes in the OA cell model
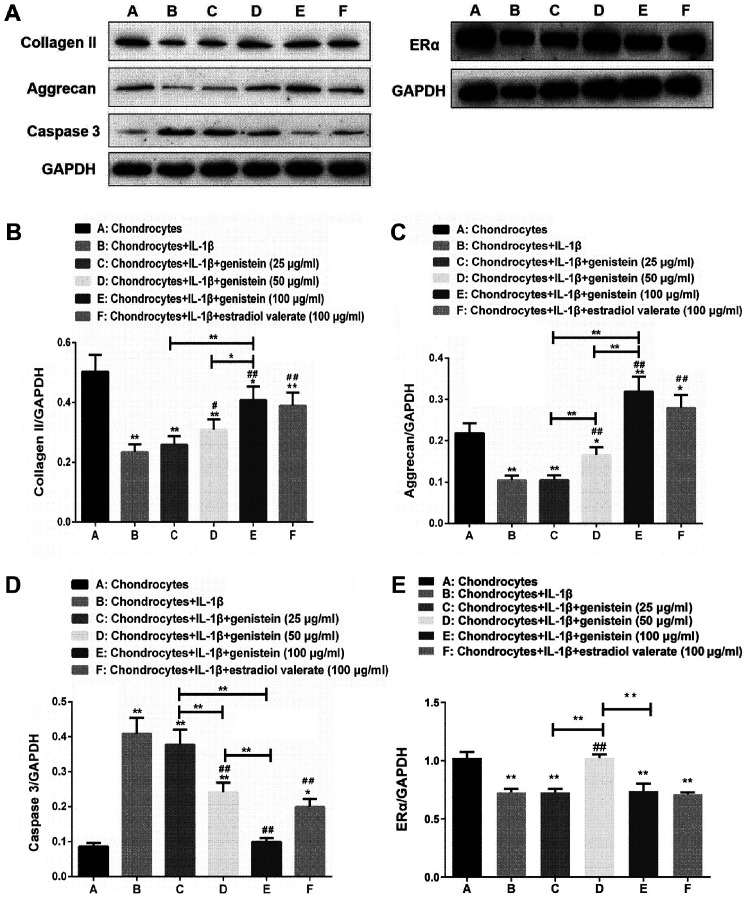
The protein expression levels of collagen II, aggrecan, caspase 3 and ERα of chondrocytes in the OA model treated with varying concentrations of genistein and estradiol valerate were detected using western blotting (Fig. 2A). Compared with normal chondrocytes in group A, the expression levels of collagen II (Fig. 2B), aggrecan (Fig. 2C) and ERα (Fig. 2E) decreased in group B (all P<0.01), while those of caspase 3 increased (Fig. 2D; P<0.01). Compared with group B, the expression levels of collagen II and aggrecan increased in groups D and E (P<0.01 and P<0.05), while the expression of caspase 3 decreased in a dose-dependent manner (both P<0.01). Furthermore, compared with group B, the expression of ERα increased in group D (P<0.01). Compared with group E, the expression levels of collagen II and aggrecan decreased in groups C and D (P<0.01 and P<0.05), while the expression of caspase 3 increased in groups C and D (P<0.01), and the expression of ERα increased in group D (P<0.01).
Figure 2.
Protein expression levels of collagen II and aggrecan increase with the added concentration of genistein, while caspase 3 protein levels gradually decrease in each group. (A) Western blot analysis of samples from the experimental groups were tested with the indicated antibodies. (B) Semi-quantitative analyses of collagen II/GAPDH, (C) aggrecan/GAPDH, (D) caspase 3/GAPDH and (E) ERα/GAPDH ratios. Data are presented as the mean ± SD (n=10). *P<0.05, **P<0.01 vs. group A or as indicated; #P<0.05, ##P<0.01 vs. group B. ERα, estrogen receptor α.
Effect of different concentrations of genistein on chondrocyte apoptosis
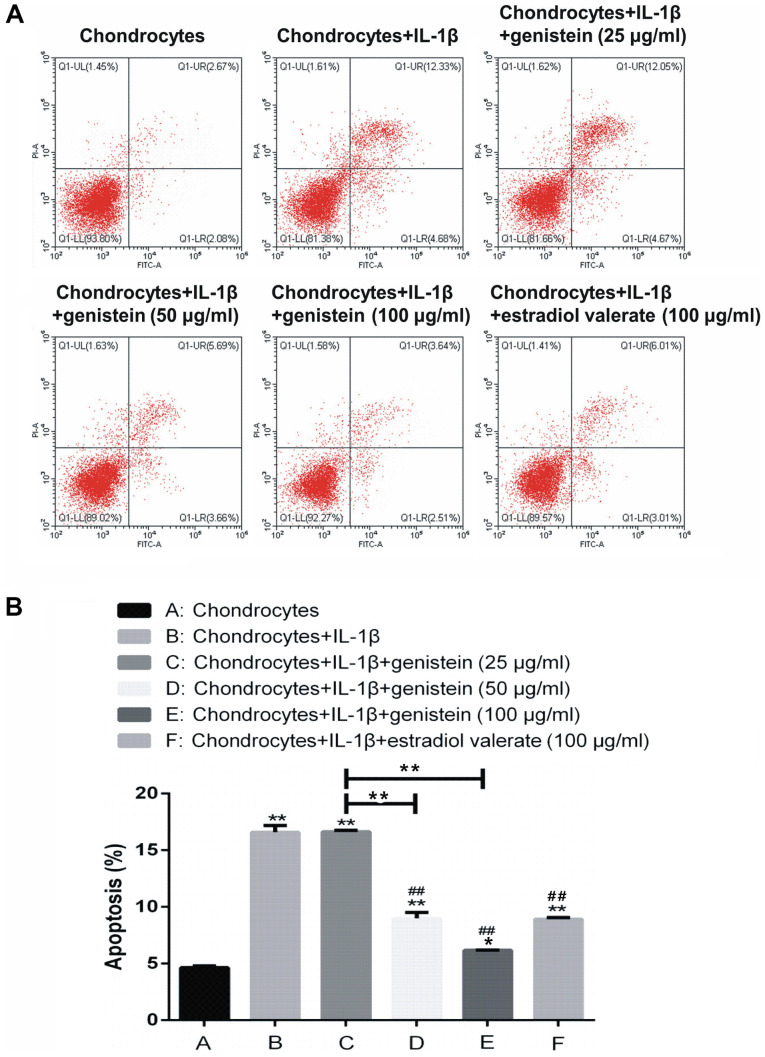
The extent of chondrocytes apoptosis was detected using flow cytometry (Fig. 3). Compared with group A, the rate of apoptosis in groups B, C, D, E and F was significantly increased (P<0.01 and P<0.05). In addition, compared with group B, there was no significant difference in the apoptotic rate of group C with 25 µg/ml genistein (P>0.05); while in groups D, E and F, the rate of apoptosis was significantly decreased (P<0.01). Thus, the results indicated that IL-1β promoted chondrocyte apoptosis, but genistein reversed this effect in a dose-dependent manner.
Figure 3.
Genistein reduces chondrocyte apoptosis induced by IL-1β. With increasing concentration of genistein, the level of chondrocyte apoptosis decreased significantly. (A) Chondrocyte apoptosis scatter plots and the (B) statistical analysis from the flow cytometry assays. Data are presented as the mean ± SD (n=10). *P<0.05, **P<0.01 vs. group A or as indicated; ##P<0.01 vs. group B. IL, interleukin.
Effect of various concentrations of genistein on the level of TNF-α in chondrocytes
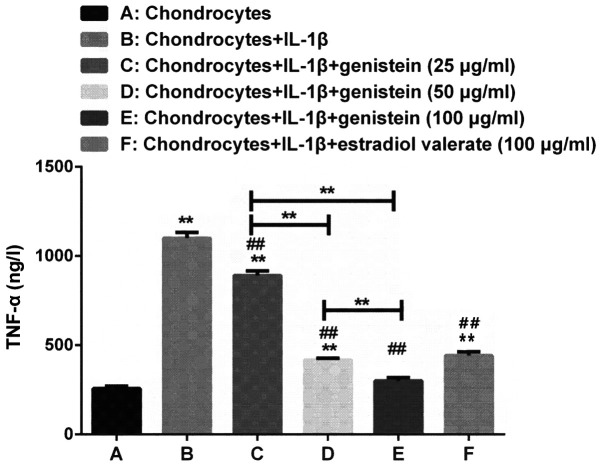
TNF-α plays an important role in the development of OA (21). The levels of TNF-α in chondrocytes of each group were measured using ELISA. Compared with group A, the levels of TNF-α were higher in groups B, C, D and F (P<0.01; Fig. 4). However, the levels of TNF-α in groups C, D, E and F, with different concentrations of genistein, were significantly decreased compared with group B (P<0.01). Compared with group C, the levels of TNF-α significantly decreased in group D and E (P<0.01).
Figure 4.
As the concentration of genistein increases, the levels of TNF-α in the chondrocytes of the OA model gradually decrease. Compared with group A, the levels of TNF-α were higher in groups B, C, D and F, as detected using ELISA. However, the expression levels of TNF-α in groups C, D, E and F, treated with various concentrations of genistein and estradiol valerate, significantly decreased compared with group B. Data are presented as the mean ± SD (n=10). **P<0.01 vs. group A or as indicated; ##P<0.01 vs. B. OA, osteoarthritis; TNF, tumor necrosis factor; IL, interleukin.
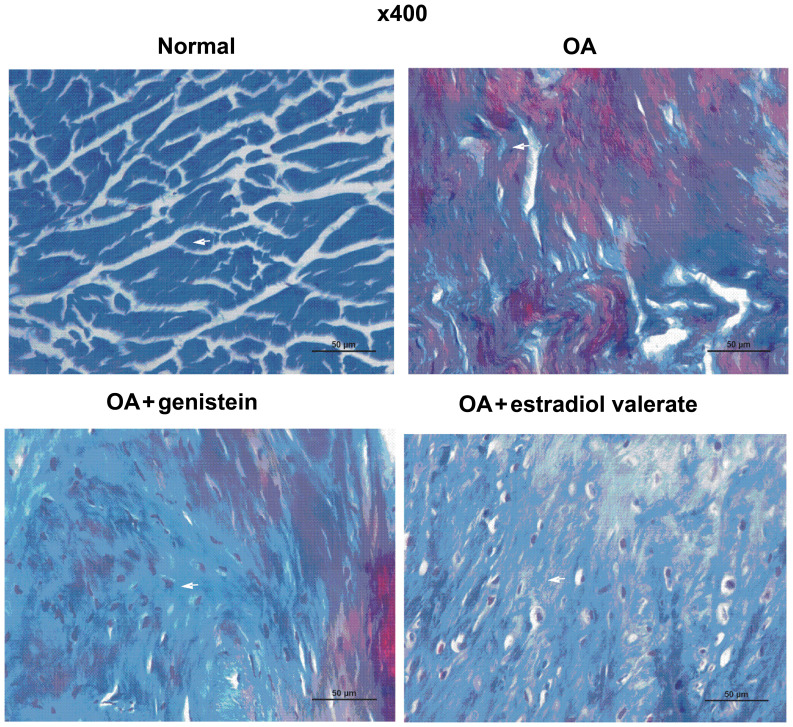
Effect of genistein on the collagen content in cartilage of OA model rats
The Masson staining of the articular cartilage in each group of the OA model rats is presented in Fig. 5. Collagenous fibers were closely arranged and were stained blue in the normal group. Compared with the normal group, collagenous fibers in the OA group were disordered and locally broken, while the articular cartilage was degraded with a decreased collagen content. Furthermore, compared with the OA group, collagenous fibers in the genistein group were more closely arranged and had an increased collagen content, which reduced the cartilage degradation. In the estradiol valerate group, the staining results were similar to the genistein group, although the collagenous fibers were more closely arranged and collagen content was increased compared with the OA group.
Figure 5.
Masson staining of articular cartilage indicated that genistein increases the collagen content in the OA rats. Compared with the normal group, the arrangement of collagenous fibers in the OA group was disordered and locally broken, while the articular cartilage was degraded in line with the decrease in the collagen content. Furthermore, compared with the OA group, collagenous fibers in the genistein group were more closely arranged, with an increased collagen content, which prevented the cartilage degradation. Scale bar, 50 µm. Arrow indicates the blue stained collagenous fibers. OA, osteoarthritis.
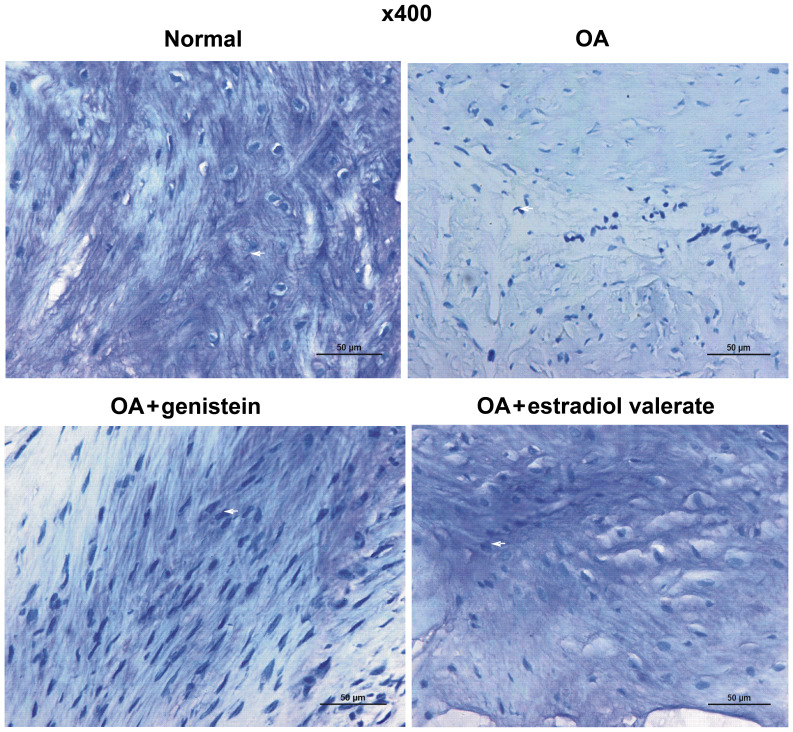
Effect of genistein on the acid glycosaminoglycan content in the cartilage of the OA model rats
The toluidine blue staining of articular cartilage from each group of the OA model rats is presented in Fig. 6. Toluidine blue is a basic dye, which turns blue after combining with acid glycosaminoglycan (22). Acid glycosaminoglycans were abundantly identified in the cartilage matrix of the normal group rats, demonstrating metachromasia. Furthermore, the acid glycosaminoglycan content in the cartilage matrix was markedly decreased in the OA group but notably increased in the genistein group. The acid glycosaminoglycan content in the estradiol valerate group rats increased compared with the OA group but was lower compared with the normal group.
Figure 6.
Toluidine blue staining of articular cartilage in OA rats identified that genistein upregulates acid glycosaminoglycan content. The content of acid glycosaminoglycan in the cartilage matrix was significantly decreased in the OA group and increased in the genistein group. Scale bar, 50 µm. Arrow indicates the blue stained acid glycosaminoglycan. OA, osteoarthritis.
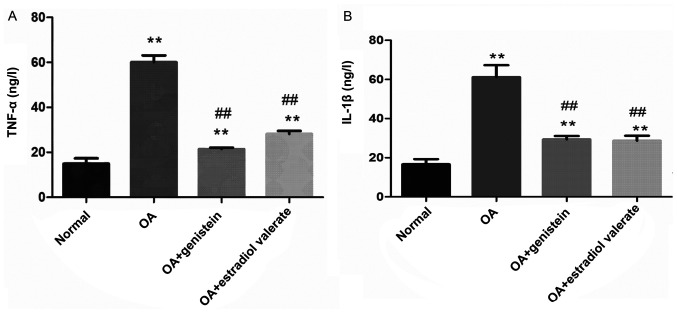
Effect of genistein on the levels of TNF-α and IL-1β in the synovial fluid of the OA model rats
The levels of TNF-α and IL-1β in the synovial fluid of each group were detected using ELISA (Fig. 7). Compared with the normal group, levels of TNF-α and IL-1β in the OA group were significantly increased (both P<0.01). However, in the genistein and estradiol valerate treated groups, TNF-α and IL-1β levels were significantly decreased compared with the OA group (all P<0.01).
Figure 7.
Genistein decreases the levels of TNF-α and IL-1β in the synovial fluid of OA rat model. Compared with the normal group, levels of (A) TNF-α and (B) IL-1β in the OA group were significantly increased, as measured using ELISA. In the genistein group, TNF-α and IL-1β were decreased compared with the OA group. Data are presented as the mean ± SD (n=10). **P<0.01 vs. the normal group; ##P<0.01 vs. the OA group. IL, interleukin; OA, osteoarthritis; TNF, tumor necrosis factor.
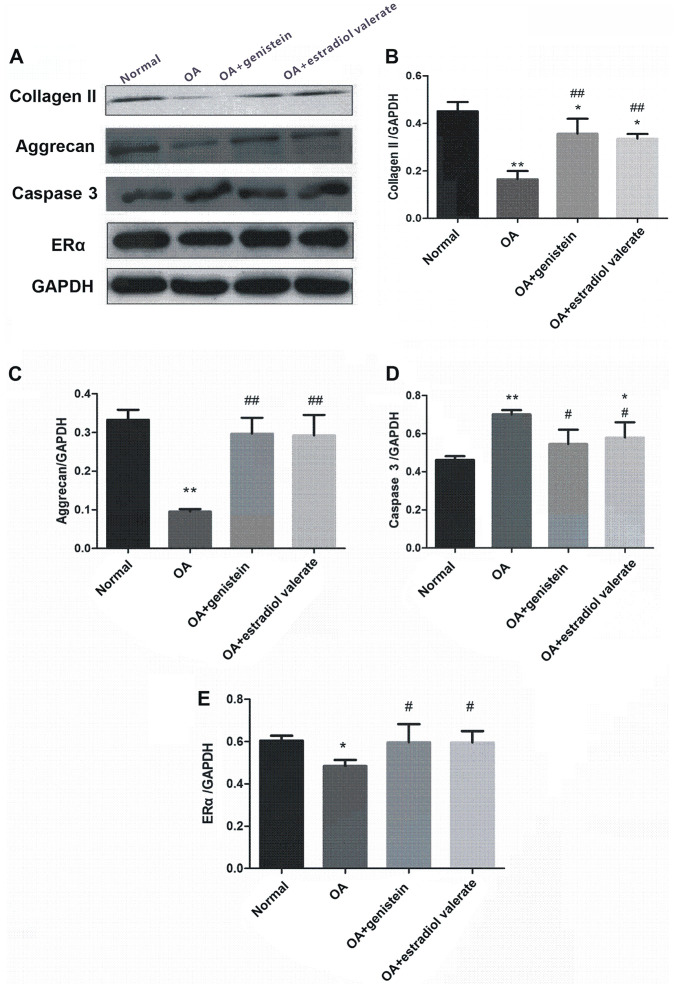
Expression levels of collagen II, aggrecan, caspase 3 and ERα in the articular cartilage in the OA model rats
The expression levels of collagen II, aggrecan, caspase 3 and ERα from the articular cartilage in the OA model rats treated with genistein and estradiol valerate were measured using western blotting (Fig. 8A). Compared with the normal group, protein expression levels of collagen II (Fig. 8B; P<0.01), aggrecan (Fig. 8C; P<0.01) and ERα (Fig. 8E; P<0.05) were decreased in the OA group, while caspase 3 expression was increased (Fig. 8D; P<0.01). Moreover, compared with the OA group, the expression levels of collagen II (P<0.01), aggrecan (P<0.01) and ERα (P<0.05) were increased in the genistein and estradiol valerate groups, but the expression of caspase 3 was decreased (P<0.05).
Figure 8.
Genistein treatment upregulates the protein expression levels of collagen II, aggrecan and ERα, and downregulates caspase 3 expression. (A) Western blotting of samples from the experimental groups using the indicated antibodies. Semi-quantitative analyses of the (B) collagen II/GAPDH, (C) aggrecan/GAPDH, (D) caspase 3/GAPDH and (E) ERα/GAPDH ratios. Data are presented as the mean ± SD (n=10). *P<0.05, **P<0.01 vs. the normal group; #P<0.05, ##P<0.01 vs. the OA group. ERα, estrogen receptor; OA, osteoarthritis.
Discussion
Currently, the repair and treatment of cartilage degeneration in OA remains challenging and requires further investigation. In the early and mid-term stages of OA, cartilage repair promoting drugs are the most extensively used treatment in the clinic (23). Chondroitin sulfate, hyaluronic acid and glucosamine polysaccharides, which are most commonly used, function under the basic treatment principle of promoting the formation of cartilage matrix components and slowing collagen frame disintegration, thus inhibiting the local inflammatory response of the joint and constructing a molecular barrier that has a chemical protective role to reduce chondrocyte apoptosis (24–26). However, it has been reported that these drugs can only temporarily relieve pain, while the local site is mainly composed of fibrocartilage and the outcome remains degeneration and necrosis in the local cartilage (27).
Related biological proteins, such as serum adiponectin, leptin and resistin, released from the articular cartilage and synovium can trigger an intra-articular inflammatory reaction, which is closely related to cartilage degeneration in OA (28,29). Chondrocytes are the main target of inflammatory mediators, particularly IL-1β and TNF-α (30). Inflammatory mediators activate the p38 mitogen-activated protein kinase, ERK1/2, JNK and NF-κB signaling pathways in chondrocytes by binding to receptors on the superficial surface of the articular cartilage and inducing the release of MMPs to degrade collagen and proteoglycan in cartilage (31,32). Furthermore, inflammatory mediators, such as IL-1β and TNF-α, promote gene transcription of Fas, Fas ligand and TNF receptor 1, thus accelerating the release of cytochrome c from mitochondria and activating the caspase gene to induce upregulation of apoptosis in chondrocyte (33).
Phytoestrogens, which are extracted from plants, are highly safe and reliable. Previous studies have reported that the chemical structure and pharmacological activity of phytoestrogens are similar to that of estrogens (34–36). The estrogen-like effects of phytoestrogens in human and mammalian cells are generated from the combination between phytoestrogens and ERs (37). Isoflavones, natural organic compounds found in traditional Chinese herbal medicine, are a type of phytoestrogens that have a notable effect on the regulation of ER (38). The chondrocyte is a target cell of estrogen and there are ERs on the surface of chondrocytes (39). Moreover, estrogen regulates chondrocyte metabolism in the treatment of OA by inhibiting the release of MMPs and promoting cartilage matrix formation (40). Genistein, which is the main component of isoflavone extracted from soybean, has antitumor, neuroprotective, bone metabolism-regulating, anti-osteoporosis, anti-inflammatory and antioxidant effects (41–43).
In the present study, it was found that genistein significantly inhibited chondrocyte apoptosis induced by IL-1β, decreased the levels of TNF-α and promoted the expression levels of collagen II and aggrecan in chondrocytes, in a dose-dependent manner. In addition, these data were further demonstrated in the OA rat model. Genistein increased the collagen and acid glycosaminoglycan content, promoted the expression levels of collagen II and aggrecan, decreased the expression of caspase 3 in cartilage and downgraded the levels of TNF-α and IL-1β, thus alleviating cartilage degradation. Therefore, these data indicated that genistein reduced the release of TNF-α and IL-1β in OA, downregulated the apoptotic-related protein expression and decreased chondrocyte apoptosis, thus reducing cartilage degradation.
ERs are expressed in articular cartilage, and participate in cartilage growth and absorption (44). ERs are also associated with the occurrence and development of OA and include three subtypes, ERα, ERβ and ERγ (45,46). According to previous research, ERα serves an important role in cartilage formation and is vital for the maintenance of cartilage homeostasis (47). Levels of estrogen affect the expression and function of ERs in the articular cartilage. For instance, decreased levels of estrogen downregulate the expression levels of the ER gene, while estrogen supplementation can delay the development of OA (48). Estrogen replacement therapy also relieves cartilage injury, inhibits the catabolic activity of proteases within the chondrocyte extracellular matrix and can treat OA (49). In the present in vitro study, the expression of ERα increased significantly in group D following treatment with 50 µg/ml genistein. In addition, the expression of ERα in the genistein and estradiol valerate groups were significantly enhanced compared with the OA group in vivo study. These preliminary results suggested that genistein upregulated the expression levels of ER in chondrocytes when treating cells for OA. However, the sex of the experimental animals and the polymorphism presented in the ER genotype may affect the expression and function of ER (50). Therefore, additional studies investigating the regulation of ER by genistein for the treatment of OA should be performed. Moreover, a novel clinical treatment for OA aiming to increase the expression of ER levels would be beneficial.
In conclusion, the present study demonstrated that genistein decreased the release of TNF-α and IL-1β in the OA model, thus reducing chondrocyte apoptosis and slowing cartilage degeneration. The current findings also suggested that genistein could be used as a suitable drug to treat OA by preventing cartilage degeneration. Moreover, it was indicated that the underlying mechanism of action was related to inflammatory mediators that reduced chondrocyte apoptosis. However, further studies should be performed to investigate the underlying mechanism of action for genistein in treating OA.
Acknowledgements
The authors would like to thank Professor Changxing Wang and Dr Weidong Wang from The Second Affiliated Hospital of Zhejiang Chinese Medical University for their technical assistance.
Funding
The present study was supported by the Natural Science Foundation of China (grant no. 81803876), Science and Technology Program of Zhejiang province (grant no. 2018C37107) and Medical Science and Technology Project of Zhejiang province (grant no. 2016KYA153).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JH designed the study. YZ, QML, PTG, YH and ZCY performed the experiments. YZ and QML reviewed and edited the manuscript. All authors wrote, read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The present study was approved by Zhejiang Chinese Medical University Institutional Animal Care and conducted according to the AAALAC and the IACUC guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Guilak F, Nims RJ, Dicks A, Wu CL, Meulenbelt I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol 71–72. 2018:40–50. doi: 10.1016/j.matbio.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Guan Y, Tian S, Wang Y, Sun K, Chen Q. Mechanical and IL-1β responsive miR-365 contributes to osteoarthritis development by targeting histone deacetylase 4. Int J Mol Sci. 2016;17:436. doi: 10.3390/ijms17040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitworth DJ, Banks TA. Stem cell therapies for treating osteoarthritis: Prescient or premature? Vet J. 2014;202:416–424. doi: 10.1016/j.tvjl.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Singer SP, Dammerer D, Krismer M, Liebensteiner MC. Maximum lifetime body mass index is the appropriate predictor of knee and hip osteoarthritis. Arch Orthop Trauma Surg. 2018;138:99–103. doi: 10.1007/s00402-017-2825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker M, Brook BS, Owen MR. Mathematical modelling of cytokines, MMPs and fibronectin fragments in osteoarthritic cartilage. J Math Biol. 2017;75:985–1024. doi: 10.1007/s00285-017-1104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaneva MK, Kerrigan MJ, Grieco P, Curley GP, Locke IC, Getting SJ. Chondroprotective and anti-inflammatory role of melanocortin peptides in TNF-α activated human C-20/A4 chondrocytes. Br J Pharmacol. 2012;167:67–79. doi: 10.1111/j.1476-5381.2012.01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao ZZ, Hu AX, Liu XS. DUSP19 regulates IL-1β-induced apoptosis and MMPs expression in rat chondrocytes through JAK2/STAT3 signaling pathway. Biomed Pharmacother. 2017;96:1209–1215. doi: 10.1016/j.biopha.2017.11.097. [DOI] [PubMed] [Google Scholar]
- 8.Johnson KA, Vemuri S, Alsahafi S, Castillo R, Cheriyath V. Glycone-rich Soy Isoflavone Extracts Promote Estrogen Receptor Positive Breast Cancer Cell Growth. Nutr Cancer. 2016;68:622–633. doi: 10.1080/01635581.2016.1154578. [DOI] [PubMed] [Google Scholar]
- 9.Liu FC, Wang CC, Lu JW, Lee CH, Chen SC, Ho YJ, Peng YJ. Chondroprotective effects of genistein against osteoarthritis induced joint inflammation. Nutrients. 2019;11:E1180. doi: 10.3390/nu11051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliviero F, Scanu A, Zamudio-Cuevas Y, Punzi L, Spinella P. Anti-inflammatory effects of polyphenols in arthritis. J Sci Food Agric. 2018;98:1653–1659. doi: 10.1002/jsfa.8664. [DOI] [PubMed] [Google Scholar]
- 11.Pie JE, Park JH, Park YH, Ryu YM, Kim KN, Suh SW, Becker KG, Cho-Chung YS, Kim MK. Effect of genistein on the expression of bone metabolism genes in ovariectomized mice using a cDNA microarray. J Nutr Biochem. 2006;17:157–164. doi: 10.1016/j.jnutbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Wang H, Zhang W, Shao C, Xu P, Shi CH, Shi JG, Li YM, Fu Q, Xue W, et al. Genistein sensitizes bladder cancer cells to HCPT treatment in vitro and in vivo via ATM/NF-κB/IKK pathway-induced apoptosis. PLoS One. 2013;8:e50175. doi: 10.1371/journal.pone.0050175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma CH, Zhang YX, Tang LH, Yang XJ, Cui WM, Han CC, Ji WY. MicroRNA-1469, a p53-responsive microRNA promotes Genistein induced apoptosis by targeting Mcl1 in human laryngeal cancer cells. Biomed Pharmacother. 2018;106:665–671. doi: 10.1016/j.biopha.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Du G, Song Y, Wei L, Li L, Wang X, Xu Q, Zhan H, Cao Y, Zheng Y, Ding D. Osthole inhibits proliferation and induces catabolism in rat chondrocytes and cartilage tissue. Cell Physiol Biochem. 2015;36:2480–2493. doi: 10.1159/000430208. [DOI] [PubMed] [Google Scholar]
- 15.Newcomer CE. The evolution and adoption of standards used by AAALAC. J Am Assoc Lab Anim Sci. 2012;51:293–297. [PMC free article] [PubMed] [Google Scholar]
- 16.Couto M, Cates C. Laboratory Guidelines for Animal Care. Methods Mol Biol. 2019;1920:407–430. doi: 10.1007/978-1-4939-9009-2_25. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Zhang L, Guo X, Liu G, Wang G, Fu S. A macaca fascicularis knee osteoarthritis model developed by modified hulth combined with joint scratches. Med Sci Monit. 2018;24:3393–3404. doi: 10.12659/MSM.906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King TJ, Shandala T, Lee AM, Foster BK, Chen KM, Howe PR, Xian CJ. Potential effects of phytoestrogen genistein in modulating acute methotrexate chemotherapy-induced osteoclastogenesis and bone damage in rats. Int J Mol Sci. 2015;16:18293–18311. doi: 10.3390/ijms160818293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Cui G, Jin B, Wang K, Chen X, Sun Y, Qin L, Bai W. Estradiol Valerate and Remifemin ameliorate ovariectomy-induced decrease in a serotonin dorsal raphe-preoptic hypothalamus pathway in rats. Ann Anat. 2016;208:31–39. doi: 10.1016/j.aanat.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Rejtarová O, Hejna P, Soukup T, Kuchar M. Age and sexually dimorphic changes in costal cartilages. A preliminary microscopic study. Forensic Sci Int. 2009;193:72–78. doi: 10.1016/j.forsciint.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Li ZC, Han N, Li X, Li G, Liu YZ, Sun GX, Wang Y, Chen GT, Li GF. Decreased expression of microRNA-130a correlates with TNF-α in the development of osteoarthritis. Int J Clin Exp Pathol. 2015;8:2555–2564. [PMC free article] [PubMed] [Google Scholar]
- 22.Bergholt NL, Lysdahl H, Lind M, Foldager CB. A standardized method of applying toluidine blue metachromatic staining for assessment of chondrogenesis. Cartilage. 2019;10:370–374. doi: 10.1177/1947603518764262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Ouyang H, Dass CR, Xu J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040. doi: 10.1038/boneres.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang XX, Cai L. Expression level of proteoglycan, collagen and type II collagen in osteoarthritis rat model is promoted and degradation of cartilage is prevented by glucosamine methyl ester. Eur Rev Med Pharmacol Sci. 2018;22:3609–3616. doi: 10.26355/eurrev_201806_15188. [DOI] [PubMed] [Google Scholar]
- 25.Bishnoi M, Jain A, Hurkat P, Jain SK. Chondroitin sulphate: A focus on osteoarthritis. Glycoconj J. 2016;33:693–705. doi: 10.1007/s10719-016-9665-3. [DOI] [PubMed] [Google Scholar]
- 26.Barreto RB, Sadigursky D, de Rezende MU, Hernandez AJ. Effect of hyaluronic acid on chondrocyte apoptosis. Acta Ortop Bras. 2015;23:90–93. doi: 10.1590/1413-785220152302144341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani V, Maccari F, Volpi N. Chondroitin sulfate and glucosamine as disease modifying anti- osteoarthritis drugs (DMOADs) Curr Med Chem. 2016;23:1139–1151. doi: 10.2174/0929867323666160316123749. [DOI] [PubMed] [Google Scholar]
- 28.Bonnet CS, Williams AS, Gilbert SJ, Harvey AK, Evans BA, Mason DJ. AMPA/kainate glutamate receptors contribute to inflammation, degeneration and pain related behaviour in inflammatory stages of arthritis. Ann Rheum Dis. 2015;74:242–251. doi: 10.1136/annrheumdis-2013-203670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Boer TN, van Spil WE, Huisman AM, Polak AA, Bijlsma JW, Lafeber FP, Mastbergen SC. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage. 2012;20:846–853. doi: 10.1016/j.joca.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 31.Santiago B, Baleux F, Palao G, Gutiérrez-Cañas I, Ramírez JC, Arenzana-Seisdedos F, Pablos JL. CXCL12 is displayed by rheumatoid endothelial cells through its basic amino-terminal motif on heparan sulfate proteoglycans. Arthritis Res Ther. 2006;8:R43. doi: 10.1186/ar1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu YC, Yang RS, Hsieh KH, Fong YC, Way TD, Lee TS, Wu HC, Fu WM, Tang CH. Stromal cell-derived factor-1 induces matrix metalloprotease-13 expression in human chondrocytes. Mol Pharmacol. 2007;72:695–703. doi: 10.1124/mol.107.036541. [DOI] [PubMed] [Google Scholar]
- 33.Dondelinger Y, Darding M, Bertrand MJ, Walczak H. Poly-ubiquitination in TNFR1-mediated necroptosis. Cell Mol Life Sci. 2016;73:2165–2176. doi: 10.1007/s00018-016-2191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet. 2013;38:15–25. doi: 10.1007/s13318-012-0112-y. [DOI] [PubMed] [Google Scholar]
- 35.Basu P, Maier C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed Pharmacother. 2018;107:1648–1666. doi: 10.1016/j.biopha.2018.08.100. [DOI] [PubMed] [Google Scholar]
- 36.Landete JM, Arqués J, Medina M, Gaya P, de Las Rivas B, Muñoz R. Bioactivation of Phytoestrogens: Intestinal Bacteria and Health. Crit Rev Food Sci Nutr. 2016;56:1826–1843. doi: 10.1080/10408398.2013.789823. [DOI] [PubMed] [Google Scholar]
- 37.Nanashima N, Horie K, Maeda H. Phytoestrogenic activity of blackcurrant anthocyanins is partially mediated through estrogen receptor beta. Molecules. 2017;23:E74. doi: 10.3390/molecules23010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chinigarzadeh A, Karim K, Muniandy S, Salleh N. Isoflavone genistein inhibits estrogen-induced chloride and bicarbonate secretory mechanisms in the uterus in rats. J Biochem Mol Toxicol. 2017;31:e21878. doi: 10.1002/jbt.21878. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz N, Verma A, Bivens CB, Schwartz Z, Boyan BD. Rapid steroid hormone actions via membrane receptors. Biochim Biophys Acta. 2016;1863:2289–2298. doi: 10.1016/j.bbamcr.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Parikka V, Lehenkari P, Sassi ML, Halleen J, Risteli J, Härkönen P, Väänänen HK. Estrogen reduces the depth of resorption pits by disturbing the organic bone matrix degradation activity of mature osteoclasts. Endocrinology. 2001;142:5371–5378. doi: 10.1210/endo.142.12.8533. [DOI] [PubMed] [Google Scholar]
- 41.Wang ZL, Sun JY, Wang DN, Xie YH, Wang SW, Zhao WM. Pharmacological studies of the large-scaled purified genistein from Huaijiao (Sophora japonica-Leguminosae) on anti-osteoporosis. Phytomedicine. 2006;13:718–723. doi: 10.1016/j.phymed.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Ji G, Yang Q, Hao J, Guo L, Chen X, Hu J, Leng L, Jiang Z. Anti-inflammatory effect of genistein on non-alcoholic steatohepatitis rats induced by high fat diet and its potential mechanisms. Int Immunopharmacol. 2011;11:762–768. doi: 10.1016/j.intimp.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 43.Honndorf VS, Wiehr S, Rolle AM, Schmitt J, Kreft L, Quintanilla-Martinez L, Kohlhofer U, Reischl G, Maurer A, Boldt K, et al. Preclinical evaluation of the anti-tumor effects of the natural isoflavone genistein in two xenograft mouse models monitored by [18F]FDG, [18F]FLT, and [64Cu]NODAGA-cetuximab small animal PET. Oncotarget. 2016;7:28247–28261. doi: 10.18632/oncotarget.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu K, Sha Y, Wang S, Chi Q, Liu Y, Wang C, Yang L. Effects of Bakuchiol on chondrocyte proliferation via the PI3K-Akt and ERK1/2 pathways mediated by the estrogen receptor for promotion of the regeneration of knee articular cartilage defects. Cell Prolif. 2019;52:e12666. doi: 10.1111/cpr.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fytili P, Giannatou E, Papanikolaou V, Stripeli F, Karachalios T, Malizos K, Tsezou A. Association of repeat polymorphisms in the estrogen receptors alpha, beta, and androgen receptor genes with knee osteoarthritis. Clin Genet. 2005;68:268–277. doi: 10.1111/j.1399-0004.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- 46.Son YO, Chun JS. Estrogen-related receptor γ is a novel catabolic regulator of osteoarthritis pathogenesis. BMB Rep. 2018;51:165–166. doi: 10.5483/BMBRep.2018.51.4.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian L, Su Z, Ma X, Wang F, Guo Y. Inhibition of miR-203 ameliorates osteoarthritis cartilage degradation in the postmenopausal rat model: Involvement of Estrogen Receptor α. Hum Gene Ther Clin Dev. 2019;30:160–168. doi: 10.1089/humc.2019.101. [DOI] [PubMed] [Google Scholar]
- 48.Xu X, Li X, Liang Y, Ou Y, Huang J, Xiong J, Duan L, Wang D. Estrogen modulates cartilage and subchondral bone remodeling in an ovariectomized rat model of postmenopausal osteoarthritis. Med Sci Monit. 2019;25:3146–3153. doi: 10.12659/MSM.916254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang Y, Duan L, Xiong J, Zhu W, Liu Q, Wang D, Liu W, Li Z, Wang D. E2 regulates MMP-13 via targeting miR-140 in IL-1β-induced extracellular matrix degradation in human chondrocytes. Arthritis Res Ther. 2016;18:105. doi: 10.1186/s13075-016-0997-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Q, Yan XB, Sun QQ, Hu AM, Liu HL, Yin YW. Genetic polymorphism of the estrogen receptor alpha gene and susceptibility to osteoarthritis: Evidence based on 15,022 subjects. Curr Med Res Opin. 2015;31:1047–1055. doi: 10.1185/03007995.2015.1037727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.