Abstract
Myocardial fibrosis is a major complication of diabetic cardiomyopathy (DCM) that is primarily caused by cardiac fibroblasts that are highly activated by persistent hyperglycemic stimulation, resulting in excessive collagen deposition. Calcium sensing receptor (CaSR) is a member of the G protein-coupled receptor superfamily and regulates intracellular calcium concentrations, which are associated with numerous diseases, including myocardial infarction, tumors and pulmonary hypertension. However, whether CaSR participates in the pathological process of myocardial fibrosis in DCM remains unknown. The present study aimed to investigate the mechanism via which CaSR regulates high glucose (HG)-induced cardiac fibrosis in vitro. HG treated-cardiac fibroblast (CFs) were used and western blotting, immunoprecipitation, Cell Counting Kit-8 assay, ELISA and transfection technology were performed to examine the role of CaSR. In the HG group, treatment with HG increased CaSR, α-smooth muscle actin, collagen I/III and matrix metalloproteinase 2/9 expression and enhanced autophagosome generation and CF proliferation. Furthermore, CaSR activation upregulated the expression of Smad ubiquitin regulatory factor 2 (Smurf2), which led to increased intracellular Ca2+ concentrations, increased ubiquitination levels of SKI like proto-oncogene and Smad7 and autophagy activation. Furthermore, the CaSR agonist (R568) or the CaSR inhibitor (Calhex231) and Smurf2-small interfering RNA promoted or inhibited HG-induced alterations, including the enhanced and weakened effects, respectively. Taken together, the results from the present study suggested that increased CaSR expression in CFs activated the Smurf2-ubiquitin proteasome and autophagy, causing excessive CF proliferation and extensive collagen deposition, which resulted in HG-induced myocardial fibrosis. These findings indicated a novel pathogenesis of DCM and may provide a novel strategy for the diagnosis and treatment of DCM.
Keywords: diabetic cardiomyopathy, myocardial fibrosis, calcium sensitive receptor, fibroblasts, smad ubiquitin regulatory factor 2, autophagy
Introduction
Diabetes is a chronic metabolic disease and has become a major global health issue, which is attributed to diet, smoking, lifestyle and environmental changes (1). According to statistics, there are an increasing number of cases of diabetes worldwide, and it is predicted that the global diabetes population will increase to 591.9 million individuals by 2035 (2). Diabetes can lead to the damage of multiple organs including eyes, heart and kidney, and diabetic cardiomyopathy (DCM) is a primary complication of the disease (3). Currently, drug treatment is still the main therapeutic strategies, however, diet control, regular exercise, hyperbaric oxygen therapy and stem cell therapy can also reduce the diabetic complications (1).
Myocardial fibrosis is a major pathological process of DCM and the characteristic is an imbalance of extracellular matrix leading to excessive deposition of collagens, which alters the cardiac structure and impairs the systolic and diastolic function of the heart, eventually leading to heart failure (4). A recent study demonstrated that continuous hyperglycemia leads to high activation of cardiac fibroblasts (CFs) and induces CF differentiation into myofibroblasts, resulting in cardiac extracellular matrix unbalance and myocardial fibrosis in the heart tissue (5–7). However, the underlying mechanisms of myocardial fibrosis in DCM remain unclear.
Calcium sensitive receptor (CaSR) is a member of the C family of the G protein-coupled receptor superfamily, which is composed of 1,078 amino acids and consists of four major regions (8–10). CaSR is widely expressed in prokaryotic and eukaryotic cells, where it regulates the release of parathyroid hormone and maintains the homeostasis of calcium and other metal ions (11,12).
The ubiquitin proteasome signaling pathway is a predominant protein degradation pathway (13), and its activation is regulated by numerous proteins, such as the Smad ubiquitin regulatory factor 2 (Smurf2) that regulates the transforming growth factor (TGF)-β1/Smads signaling pathway (14). Autophagy is the primary metabolic process of eukaryotic organisms (15). During autophagy, substances in the cytoplasm are phagocytized by autophagosomes, which are spherical structures with bilayer membranes, and are transported to lysosomes for degradation. After binding to endosomes or lysosomes, autophagosomes and their contents are degraded (16) and can therefore provide energy for the synthesis of macromolecules. Numerous studies have indicated that autophagy can improve heart diseases. For example, TGF-β1 treatment causes fibrogenesis and increases autophagy in human atrial fibroblasts (17). Furthermore, exogenous H2S treatment promotes the clearance of ubiquitin aggregates via autophagy in a type 2 diabetes model mice (18).
Previous studies have indicated that CaSR is associated with a variety of heart and lung diseases (19,20). The preliminary results indicated that high glucose (HG)-treated CFs displayed significantly upregulated CaSR expression and an increased number of autophagosomes. To explore the functional role and underlying mechanisms of CaSR in HG-induced myocardial fibrosis, a CaSR agonist and inhibitor were used in the present study.
Materials and methods
Isolation and culture of neonatal rat CFs
Primary CFs were isolated from neonatal Wistar rat (3 days of age). All experiments were approved by the Mudanjiang Medical University Medical Science Ethics Committee. As previously described (8,21,22), neonatal rats were sacrificed via 2% isoflurane inhalation and subsequent cervical dislocation, the heart was rapidly excised, cut into pieces (0.5 mm3) in PBS solution and digested using trypsin for 8 min under sterile conditions at 37.5°C. Dulbecco's modified Εagles medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) was added to terminate the digestion process. The aforementioned step was repeated 8 times. Cells were obtained by centrifugation for 10 min at 800 × g at 4°C. Following incubation for 2 h, the unattached cells were discarded, the attached cells (CFs) were plated in a petri dish with DMEM containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.) and placed in an incubator at 37°C with 5% CO2. CFs were passaged every 3 days and CFs at passage 3 were treated with HG (40 mM), a CaSR agonist (R568; 5 µM; cat. no. sc-361302; Santa Cruz Biotechnology, Inc.), a CaSR inhibitor (Calhex231; 3 µM; cat. no. sc-207394; Santa Cruz Biotechnology, Inc.) for 24 or 48 h according to different experiments. CFs were transfected with Smurf2-small interfering (si)RNA (cat. no. sc-41676; Santa Cruz Biotechnology, Inc.) for 12 h. CFs cultured with DMEM containing 5.5 mM glucose composed the control group.
Western blotting
Total proteins were extracted from CFs by RIPA buffer (Beyotime Institute of Biotechnology) at 4°C and quantified using the BCA assay kit (Beyotime Institute of Biotechnology). Proteins (20 µg) were separated by SDS-PAGE (10 or 14%) and transferred onto PVDF membranes. After blocking with 5% non-fat dried milk for 2 h at room temperature, membranes were incubated overnight at 4°C with primary antibodies (1:1,000) against CaSR, collagen I/III (Col-I/III), p62, Beclin1, microtubule associated protein 1 light chain 3 α-I/II (LC3-I/II), SKI like proto-oncogene (SnoN), α-smooth muscle actin (α-SMA), β-actin (cat. nos. sc-47741, 59772/271249, 28359, 48341, 398822, 136958, 130617 and 47778, respectively; Santa Cruz Biotechnology, Inc.), Smurf2, smad2, phosphorylated (p)-smad2, smad3, p-smad3, transforming growth factor (TGF-β1), MMP9 (cat. nos. 12024, 5339, 18338, 9513, 9520, 3709 and 3852, respectively; Cell Signaling Technology, Inc.), MMP2 and smad7 (cat. nos. 10373-2-AP and 25840-1-AP, respectively; ProteinTech Group, Inc.). Membranes were then incubated with the secondary antibodies anti-rat or anti-rabbit immunoglobulin G (1:10,000; cat. nos. bs-0295M-HRP or bs-0296R-HRP; BIOSS) for 1 h at room temperature. Bands were detected using ECL Western Blotting Substrate kit (Beyotime Institute of Biotechnology). Relative expression levels were normalized to endogenous control β-actin using Gel-Pro-Analyser Software Version 6.3 (Media Cybernetics, Inc.).
Immunoprecipitation
CFs were seeded into 35-mm culture dishes (1×106/dish) and cultured at 37°C for 48 h. After treatments, CFs were harvested and treated with lysed in lysis buffer plus 1% PMSF (Roche Diagnostics) for 30 min at 4°C. Cells were centrifuged at 4°C at 3,000 × g for 25 min. Subsequently, cells were incubated with ubiquitin specific antibody (1:1,000; cat. no. sc-8017; Santa Cruz Biotechnology, Inc.) overnight at 4°C. Protein A/G Magnetic Beads (Selleck Chemicals) were used for binding according to the manufacturer's protocol. Following incubation for 2 h, the beads were eluted, centrifuged and the supernatant was collected. Subsequently, western blotting was performed as aforementioned to detect the level of protein ubiquitination.
Cell proliferation detection by EdU and cell cycle analysis
Cell proliferation was detected using an EdU kit (Guangzhou RiboBio Co., Ltd.). Briefly, CFs were seeded into 96-well plates (5×103/well) and treated with HG (40 mM), R568 (5 µM) or Calhex231 (3 µM) for 24 h. Subsequently, cells were stained using the EdU kit according to the manufacturers' instructions and the nucleus was stained with Hoechst at 37°C for 10 min. Stained cells were observed using an EVOS M50000 fluorescence microscope (magnification, ×200; Thermo Fisher Scientific, Inc.).
For cell cycle analysis, CFs were seeded into 6-well plates (1×105 cells per well) and treated with HG (40 mM), R568 (5 µM) or Calhex231 (3 µM) for 48 h. Subsequently, CFs were washed three times with cold PBS, fixed with 70% ethanol at 4°C overnight and centrifuged at 800 × g at room temperature for 3 min. Cells were then treated with staining buffer, suspended and incubated with RNaseA (cat. no. C1052; Beyotime Institute of Biotechnology). CFs were stained with PI (50 µg/ml; Beyotime Institute of Biotechnology) for 30 min at 37°C. Cell cycle phase analysis was detected by flow cytometry using a BD C6 flow cytometer (BD Biosciences) and data analysis of the cytometric files was performed using BD ModFit LT version 2.0 (Verity Software House).
Detection of cell viability by the cell counting kit (CCK)-8 assay
CFs were seeded (2×103 cells/well) into 96-well plates and treated with HG (40 mM), R568 (5 µM) or Calhex231 (3 µM). Cell viability was detected at 0, 12, 24, 36, 48, 60 or 72 h using a CCK-8 assay kit (cat. no. AR1199; Wuhan Boster Biological Technology, Ltd.) according to the manufacturer's protocol. The absorbance was read at 450 nm on a microplate reader.
Transmission electron microscopy (TEM)
To observe autophagosomes of CFs, ultrastructural analysis was performed as previously described (23,24). Briefly, CFs were fixed with 2.5% glutaraldehyde at 4°C overnight, and with 1% osmium tetroxide for 2 h at room temperature. Subsequently, CFs were dehydrated using a graded ethanol series (50, 70, 90 and 100%), embedded in epoxy resin, stained with 3% uranyl acetate and 3% lead citrate for 15 min at room temperature and observed using a H-7650 electron microscopy (magnification, ×10,000). The number of autophagosomes in each field was calculated using the Adobe Photoshop CS5 (version 12.0.3; Adobe Systems, Inc.) and ImageJ (version 1.46r; National Institutes of Health) software (25,26).
Cell autophagy analysis and transfection with Smurf2-siRNA
CFs were seeded in a confocal dish (3×105 cells/well; Thermo Fisher Scientific, Inc.). Cell autophagy was determined using a CYTO-ID autophagy detection kit (Enzo Life Sciences, Inc.) according to the manufacturer's protocol. LC3II-positive punctate pattern was observed using a BX61 fluorescence microscope (magnification, ×200; Olympus Corporation). The number of autophagosomes was quantified using ImageJ software (version 1.48u; National Institutes of Health).
For the transfection, CFs were seeded into a 35-mm dish (2×105 per dish) and maintained for 24 h in culture medium without antibiotics before transfection. After being washed 3 times with PBS, transfection was performed using Lipofectamine® 3000 transfection reagent (Thermo Fisher Scientific, Inc.) in Opti-MEM medium and control siRNA (5′-CCCTTAAAGTTCATTCGGG-3′) or Smurf2-siRNA (5′-GCGTTTGATGCGAGGCATA-3′) at a final concentration of 300 nM (Santa Cruz Biotechnology, Inc.) for 12 h. After transfection, CFs were cultured in regular medium for 24 h and then the cells were subjected to further analysis.
Measurement of intracellular calcium
CFs were treated with HG (40 mM), HG + R568 (40 mM + 5 µM) or HG + Calhex231 (40 mM + 3 µM). Subsequently, Fluo-3 AM (5 mM; cat. no. ab145254) was added to each group in the dark for 30 min at 37°C. CFs were washed with Ca2+ free Tyrode's solution and subsequently observed using a BX61 fluorescence microscope (magnification, ×200; Olympus Corporation).
ELISA
CF culture media was collected to detect Col-I/III and TGF-β1 levels using ELISA detection kits (cat. nos. EK0411, EK0424 and EK0513, respectively; Boster Biological Technology) according to the manufacturer's protocols.
Statistical analysis
All experiments were performed at least 3 times independently. Data were presented as the means ± standard error of the mean. Statistical analysis was performed by a two-tailed Student's t-test or one-way ANOVA followed by the Bonferroni multiple comparisons test using SPSS 18.0 software (SPSS, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of CaSR on Col-I/III and MMPs expression in CFs and on CF proliferation
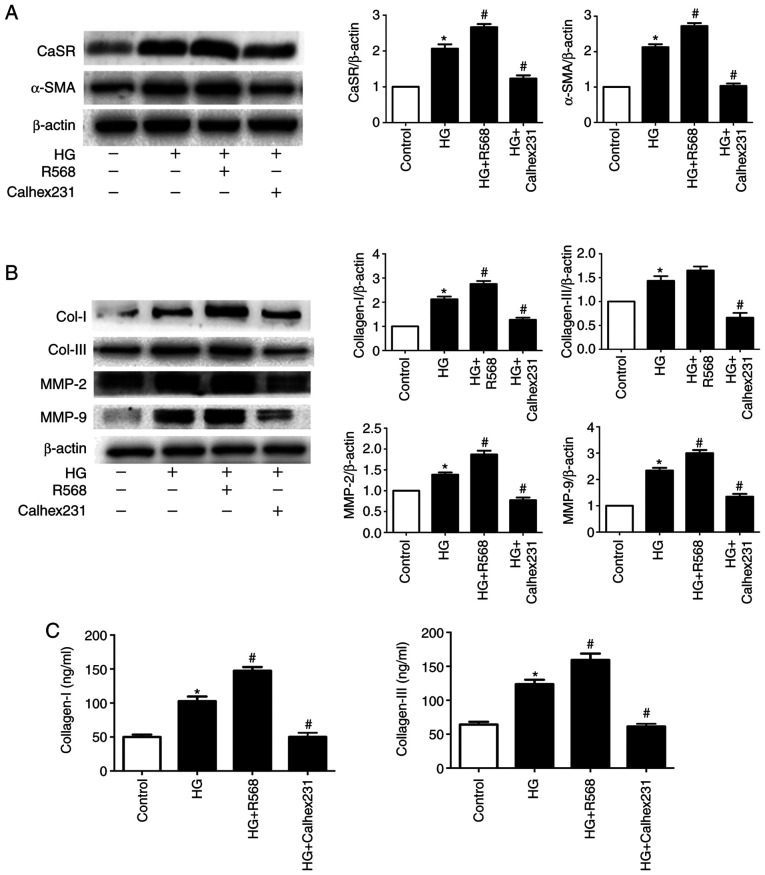
Following 48 h culture, the expression of CaSR, α-SMA, Col-I, Col-III, MMP2 and MMP9 was significantly increased in the HG group compared with control group. Furthermore, treatment with R568 and Calhex231 enhanced or attenuated the effects of HG, respectively (Fig. 1A and B). In CF supernatant, the concentration of Col-I/III was significantly higher in the HG and HG + R568 groups, but significantly lower in the HG + Calhex231 group (Fig. 1C).
Figure 1.
Effects of CaSR on Col-I/II and MMPs in CFs treated with HG. CFs in the control group were cultured with 5.5 mM glucose and CFs in the HG group were cultured with 40 mM glucose with or without 5 µM R568 or 3 µM Calhex231. (A and B) CaSR, α-SMA, Col-I, Col-III, MMP2 and MMP9 expression was determined by western blotting. (C) Concentration of Col-I and Col-III in the CFs supernatant determined by ELISA. *P<0.05 vs. control; #P<0.05 vs. HG. n≥3. CaSR, calcium sensing receptor; CFs, cardiac fibroblasts; HG, high glucose; α-SMA, α-smooth muscle actin; Col-I, collagen I; Col-III, collagen III; MMP2, matrix metalloproteinase 2; MMP9, matrix metalloproteinase 9.
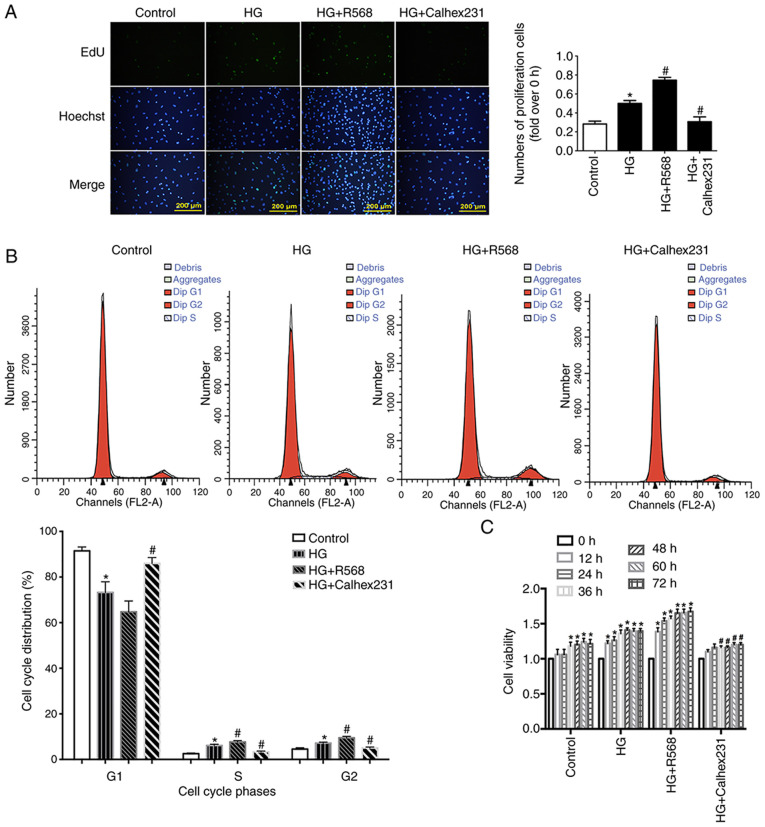
To detect cell proliferation, EdU assay was performed at the 24 h time point. Cell proliferation was significantly higher in the HG and HG + R568 groups compared with the control group. However, cell proliferation was significantly lower in the HG + Calhex231 group compared with the HG group (Fig. 2A).
Figure 2.
Effects of CaSR on the proliferation of HG-treated CFs. After different treatments (HG 40 mM, R568 5 µM, Calhex231 3 µM), (A) CFs proliferation was detected via EdU kit (n=16). (B) Cell cycle distribution of CFs was determined by flow cytometry *P<0.05 vs. control; #P<0.05 vs. HG. n≥3. (C) Cell viability of CFs detected with the Cell Counting Kit-8. *P<0.05 vs. control; #P<0.05 vs. HG. n≥3. CaSR, calcium sensing receptor; CFs, cardiac fibroblasts; HG, high glucose.
Cell proliferation is closely related to the cell cycle, including the transition from the G1 phase to the S and G2 phases (27). Compared with the control group, the results from cell cycle analysis demonstrated that the S and G2 phase distribution of CFs was significantly increased in the HG and HG + R568 groups, and that the G1 phase distribution was significantly decreased in the HG group (Fig. 2B). In addition, CF viability at different time points (0, 12, 24, 36, 48, 60 and 72 h) was detected with the CCK-8 assay. CF viability in the HG and HG + R568 groups was significantly increased compared with the control group. However, compared with the HG group, cell viability was significantly lower in the HG + Calhex231 group at each time point (Fig. 2C).
CaSR activation enhances HG-induced autophagy
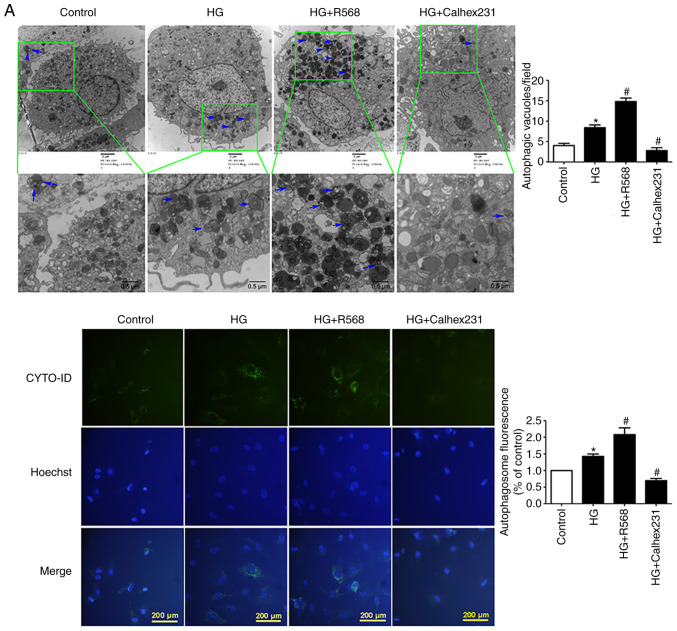
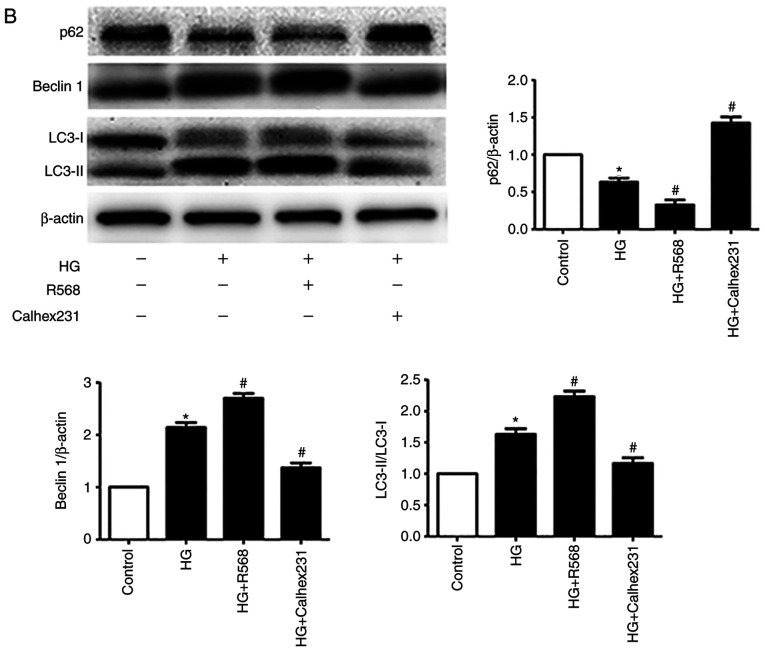
CFs were treated with HG, R568 or Calhex231 for 48 h. A significant increase in the number of autophagosomes was observed in the HG and HG + R568 groups via TEM and the CYTO-ID autophagy detection kit; however, Calhex231 significantly decreased the number of autophagosomes (Fig. 3A). The results from western blotting demonstrated that p62 protein expression was significantly downregulated, whereas Beclin1 and LC3-II expression was significantly upregulated in the HG and HG + R568 groups. Compared with the HG group, opposite results were observed in the Calhex231 group (Fig. 3B).
Figure 3.
CaSR activation enhanced HG-induced autophagy in CFs. Cells were treated with HG (40 mM), R568 (5 µM) and Calhex231 (3 µM) for 48 h. (A) Cells were subjected to transmission electron microscopy and the presence of double-membrane autophagosomes was evaluated. The autophagy of cells was detected by a CYTO-ID autophagy detection kit. (B) Protein expression of p62, Beclin1 and LC3-II was assessed by western blotting. *P<0.05 vs. control; #P<0.05 vs. HG. n≥3. CaSR, calcium sensing receptor; HG, high glucose.
CaSR regulates intracellular Ca2+ and the TGF-β1/Smads signaling pathway
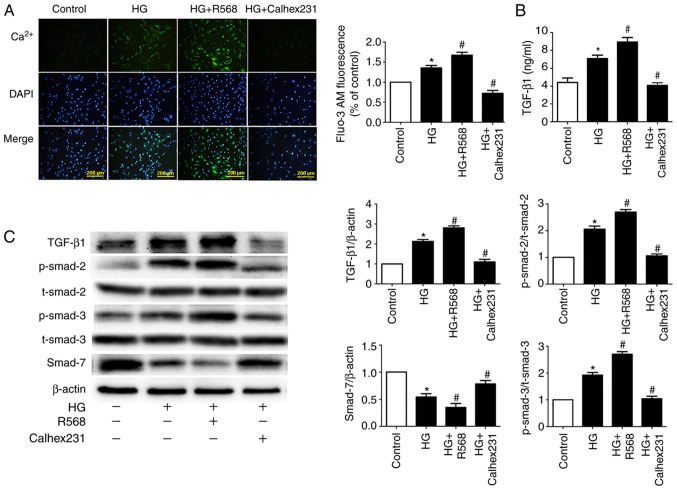
To further investigate the underlying mechanism of myocardial fibrosis, Fluo-3/AM fluorescent dyes were used to detect the fluorescence intensity of cytosolic Ca2+. Compared with the control group, the results indicated that fluorescence intensity was higher in the HG and HG + R568 groups. Compared with the HG group, fluorescence intensity was lower in the HG + Calhex231 group (Fig. 4A). To detect CF TGF-β1 secretion, the ELISA assay was performed. The concentration of TGF-β1 was significantly higher in the HG and HG + R568 groups compared with the control group. Conversely, the concentration of TGF-β1 was significantly lower in the HG + Calhex231 group compared with the HG group (Fig. 4B).
Figure 4.
Measurement of intracellular Ca2+ in CFs and signal pathway related to myocardial fibrosis. (A) Intracellular Ca2+ was stained with Fluo-3/AM and estimated by fluorescence intensity. n=16. (B) Concentration of TGF-β1 in CF supernatants was detected using ELISA. (C) Western blotting of TGF-β1, Smad7, p-Smad2 and p-Smad3 in CFs. *P<0.05 vs. control; #P<0.05 vs. HG. n≥3. HG, high glucose; TGF-β1, transforming growth factor-β1. CaSR, calcium sensing receptor; CFs, cardiac fibroblasts.
Activation of the TGF-β1/Smads signaling pathway can lead to myocardial fibrosis (28); therefore, western blotting was performed to detect the expression of primary proteins. The expression of TGF-β1 and p-Smad2/3 proteins was significantly increased, whereas Smad7 expression was significantly decreased in the HG and HG + R568 groups compared with the control group. As expected, the HG + Calhex231 group displayed the opposite results compared with the HG group (Fig. 4C).
Smurf2-ubiquitin proteasome pathway and autophagy are involved in HG-induced myocardial fibrosis
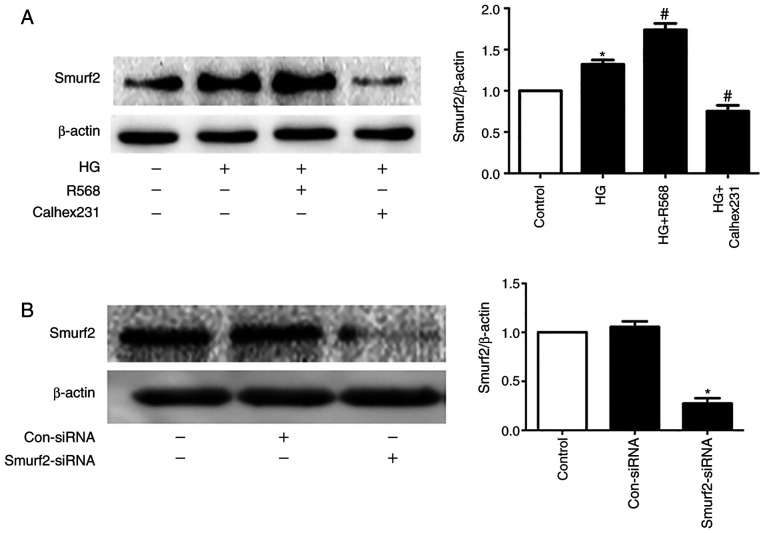
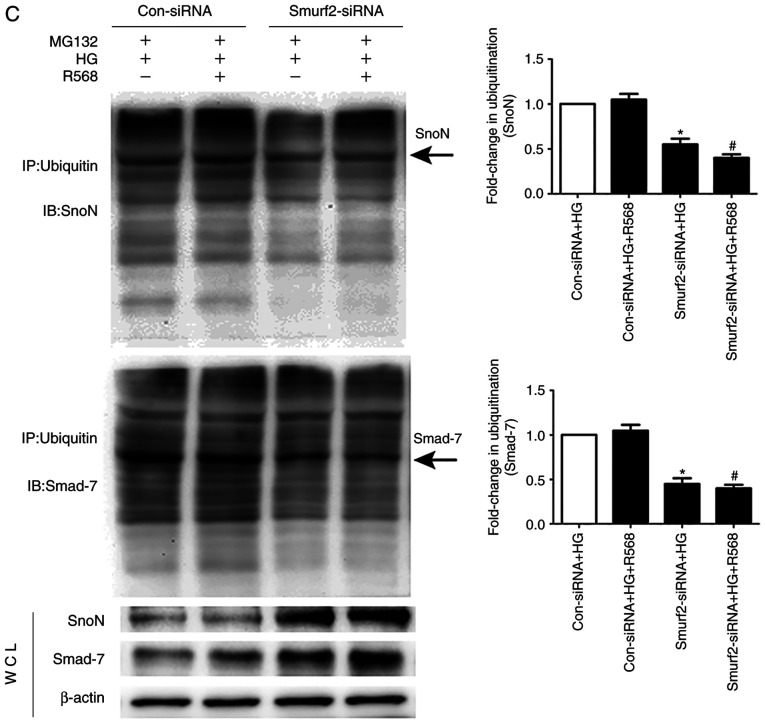
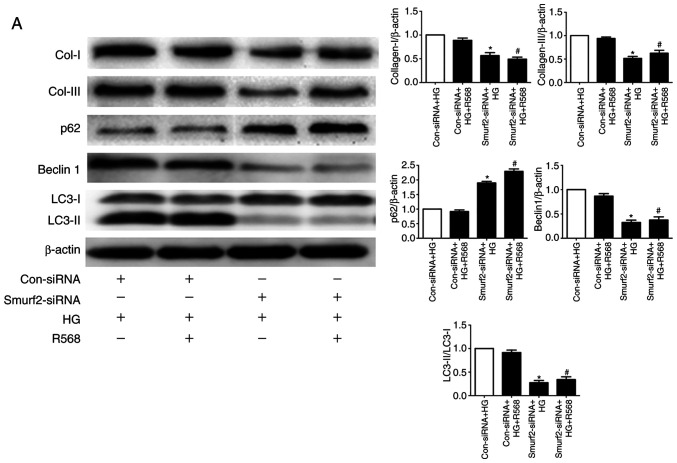
The results from western blotting demonstrated that Smurf2 expression was significantly increased in the HG and R568 groups compared with the control group, but Calhex231 displayed the opposite effect compared with the HG group, suggesting CaSR activation increased Smurf2 expression (Fig. 5A). Furthermore, Smurf2 expression was significantly inhibited following Smurf2-siRNA transfection compared with transfection with control siRNA (Fig. 5B). Although CFs were treated with HG and R658, the detection of ubiquitination levels demonstrated that the expression levels of SnoN and Smad7 were significantly suppressed by Smurf2-siRNA. The results also suggested that Smurf2-siRNA decreased the enhancing effect of HG and R658 (Fig. 5C). Furthermore, compared with the Con-siRNA + HG and Con-siRNA + HG + R568 groups, Smurf2-siRNA significantly decreased the expression levels of Col-I/III, Beclin1 and LC3-II, while p62 expression was significantly increased in transfected CFs of Smurf2-siRNA + HG and Smurf2-siRNA + HG + R568 groups (Fig. 6A).
Figure 5.
Activation of CaSR enhanced the degradation of SnoN and Smurf2 by HG-Smurf2-ubiquitin proteasome pathway. CFs were treated with HG (40 mM) and 5 µM R568 or 3 µM Calhex231. (A) Western blotting of Smurf2 expression in CFs. (B) CFs transfected with Smurf2-siRNA or Con-siRNA and Smurf2 expression was detected by western blotting. (C) Immunoprecipitation analysis of the ubiquitination level of SnoN and Smad7 in CFs following transfected with Smurf2-siRNA or Con-siRNA *P<0.05 vs. control/Con-siRNA /Con-siRNA + HG; #P<0.05 vs. HG/Con-siRNA+HG+R568. n≥3. CaSR, calcium sensing receptor; CFs, cardiac fibroblasts; HG, high glucose; Con, control; si, small interfering.
Figure 6.
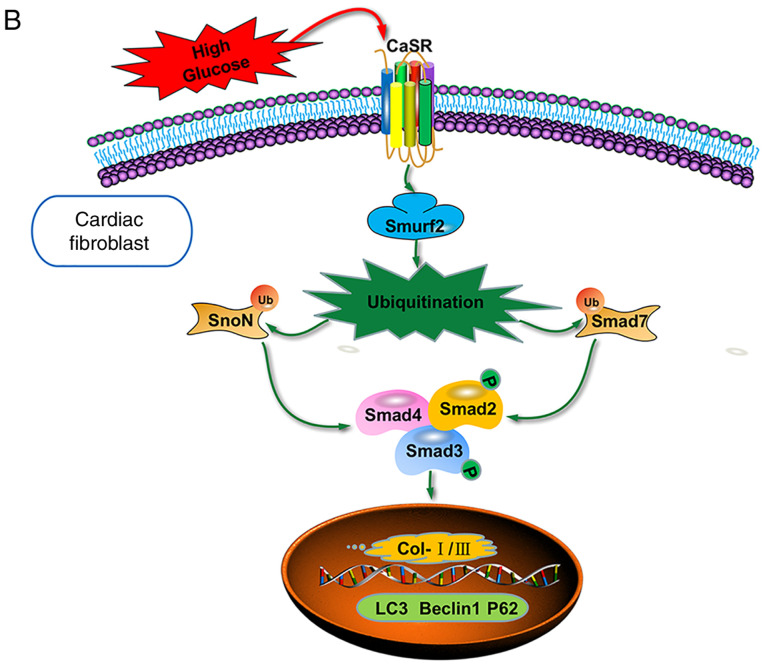
Role of autophagy in diabetic myocardial fibrosis. CFs were treated with HG (40 mM) or R568 (5 µM) for 48 h, and transfected with Smurf2-siRNA or Con-siRNA (A) Western blotting of Col-I, Col-III, p62, Beclin1 and LC3-II. *P<0.05 vs. Con-siRNA + HG; #P<0.05 vs. Con-siRNA + HG + R568 (n≥3). (B) Schematic diagram showing the role of CaSR through activating Smurf2-ubiquitin proteasome pathway and autophagy in CFs. CaSR, calcium sensing receptor; CFs, cardiac fibroblasts; HG, high glucose; Con, control; si, small interfering.
Discussion
Diabetes mellitus is a metabolic syndrome characterized by hyperglycemia that is caused by insufficient insulin secretion or resistance, which can eventually lead to DCM (29). Myocardial fibrosis is a process of cardiac remodeling and inflammatory gradual infiltration, which causes numerous conditions, including DCM, myocardial infarct (30). According to previous data, CFs serve a key role in the pathological process of myocardial fibrosis (31) by maintaining homeostasis and remodelling the extracellular matrix (32). Previous studies have confirmed that durative hyperglycemia can induce CF proliferation, boost myofibroblast trans-differentiation and increase the secretion of extracellular matrix proteins; however, the underlying mechanisms remain unclear (33–35).
Collagen secretion (Col-I/III) and increased extracellular matrix accumulation that occurs during myocardial fibrosis can lead to the proliferation and activation of CFs (36). A previous study indicated that CaSR is expressed in CFs (37); however, no study has investigated the relationship between CaSR expression and myocardial fibrosis in DCM. R568 is a positive allosteric modulator (calcimimetics) and can activate CaSR expression to increase intracellular Ca2+concentration. Calhex231 is an inhibitor of the CaSR via negative allosteric modulation and can reduce intracellular calcium (38,39). In the present study, when CFs were treated with HG, the protein expression of CaSR, α-SMA, Col-I, Col-III, MMP2 and MMP9 was significantly increased. EdU staining, CCK-8 assays and the cell cycle analysis indicated that HG enhanced CF proliferation and promoted the secretion of Col-I/III into the supernatant. In addition, the CaSR agonist (R568) and the CaSR inhibitor (Calhex231) promoted and inhibited HG-induced alterations, respectively. The results indicated that CaSR was closely associated with HG-induced myocardial fibrosis in DCM.
Autophagy is a highly conservative catabolic process that is associated with several cardiac pathologies (40). Autophagy can also stabilize and maintain the metabolic process of intracellular environmental balance. In injured cells, damaged substances and organelles are phagocytosed by autophagosomes of spherical double layer membranes, and then transported to lysosomes via specific mechanisms to degradation and reuse (41). Previous studies have confirmed that autophagy participates in the process of fibrosis (42). The present study investigated therefore the role of CaSR in CF autophagy. In CFs treated with HG and R568, a large number of autophagosomes were observed, and the expression of Beclin1 and LC3-II was increased, whereas p62 expression was decreased. These results suggested that CaSR activation induced fibroblast proliferation and phenotype conversion, which may be associated with the autophagy pathway.
The TGF-β1/Smads signaling pathway is the primary route of tissue fibrosis, which occurs during autophagy. p62, LC3-II and Beclin1 are the key proteins associated with autophagy (28). Smad7 can degrade Smad2/3, preventing nuclear translocation and restraining activation of the TGF-β1/Smads signaling pathway (43). SnoN is a member of the SKI proto-oncogene family, which can inhibit Smad2/3 and Smad4 to form a combined complex, resulting in fibrosis inhibition (44). The present study further indicated that HG and CaSR agonists remarkably enhanced the expression of TGF-β1 and p-Smad2/3, and degraded Smad7. However, CaSR antagonists had the opposite effect.
As a primary secondary messenger, intracellular Ca2+ concentrations regulate numerous physiological functions such as electrophysiology of cardiac myocytes, the contraction of smooth muscle cells and immune cell response (45–47). Previous studies have indicated that CaSR could increase intracellular calcium via the G protein-phospholipase C-inositol triphosphate signaling pathway (20,48). However, why the activation of CaSR can enhance the effects of HG on myocardial fibrosis has not been reported. It was speculated that increased intracellular calcium could induce Smurf2 expression, which could degrade SnoN and Smad7 proteins via the ubiquitin proteasome signaling pathway (49,50). This hypothesis was investigated in the present study by detecting the ubiquitination level of SnoN and Smad7. Smurf2-siRNA significantly reduced the ubiquitination level of SNoN and Smad7, which prevented the stimulating effects of HG and R568. Col-I/III are essential components of the extracellular matrix; therefore, Col-I/III content increases can account for myocardial fibrosis. The present study detected the relationship between autophagy and alterations to Col-I/III expression in CFs. Smurf2-siRNA significantly decreased the expression of Col-I/III, Beclin1 and LC3-II in CFs. However, the expression of p62 was significantly increased. Taken together, these results indicated that downregulation of autophagy could inhibit collagen secretion by CFs.
In summary, it has been hypothesized that the continuous stimulation of hyperglycemia could upregulate CaSR expression in CFs to increase intracellular Ca2+ (51) and activate Smurf2-ubiquitin proteasome and autophagy. The present study demonstrated that proliferative and activated CFs promoted collagen deposition, which may be a cause of myocardial fibrosis (Fig. 6B), but this requires further investigation. However, the present study only investigated the functional role of CaSR in HG-treated CFs. To identify the underlying mechanisms of CaSR-mediated autophagy in HG-induced cardiac fibrosis, further investigation is required in vivo, which may help the discovery of novel strategies for the prevention and treatment of DCM.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- MF
myocardial fibrosis
- CaSR
calcium sensing receptor
- DCM
diabetic cardiomyopathy
- MMP2/9
matrix metalloproteinase 2/9
- α-SMA
α-smooth muscle actin
- TGF-β1
transforming growth factor-β1
- CFs
cardiac fibroblasts
- SnoN
ski-related novel gene
- Smurf2
smad ubiquitin regulatory factor 2
Funding
This research was supported by the Research Projects of Basic Scientific Research Business Expenses in Higher Institutions of Heilongjiang Province (grant no. 2018-KYYWF MY-0070).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HY and ZW conceived and supervised the study. HY, JX, YZ and LL designed experiments. QW, YY, BZ and YL performed experiments. XX and HY analyzed data. HY wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Ethics approval and consent to participate
The animal raising and handling procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Mudanjiang Medical University Medical Science Ethics Committee (Mudanjiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sun L, Yu M, Zhou T, Zhang S, He G, Wang G, Gang X. Current advances in the study of diabetic cardiomyopathy: From clinicopathological features to molecular therapeutics (Review) Mol Med Rep. 2019;20:2051–2062. doi: 10.3892/mmr.2019.10473. [DOI] [PubMed] [Google Scholar]
- 2.Xue H, Tao Y, Deng Y, Yu J, Sun Y, Jiang G. Metformin accelerates wound healing in type 2 diabetic db/db mice. Mol Med Rep. 2017;16:8691–8698. doi: 10.3892/mmr.2017.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, Taylor R. Periodontitis and diabetes: A two-way relationship. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westermeier F, Riquelme JA, Pavez M, Garrido V, Díaz A, Verdejo HE, Castro PF, García L, Lavandero S. New molecular insights of insulin in diabetic cardiomyopathy. Front Physiol. 2016;7:125. doi: 10.3389/fphys.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowlkes V, Clark J, Fix C, Law BA, Morales MO, Qiao X, Ako-Asare K, Goldsmith JG, Carver W, Murray DB, Goldsmith EC. Type II diabetes promotes a myofibroblast phenotype in cardiac fibroblasts. Life Sci. 2013;92:669–676. doi: 10.1016/j.lfs.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavalera M, Wang J, Frangogiannis NG. Obesity, metabolic dysfunction, and cardiac fibrosis: Pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl Res. 2014;164:323–335. doi: 10.1016/j.trsl.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchinson KR, Lord CK, West TA, Stewart JA., Jr Cardiac fibroblast-dependent extracellular matrix accumulation is associated with diastolic stiffness in type 2 diabetes. PLoS One. 2013;8:e72080. doi: 10.1371/journal.pone.0072080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Gao P, Wei C, Li H, Zhang L, Zhao Y, Wu B, Tian Y, Zhang W, Wu L, et al. Calcium sensing receptor protects high glucose-induced energy metabolism disorder via blocking gp78-ubiquitin proteasome pathway. Cell Death Dis. 2017;8:e2799. doi: 10.1038/cddis.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Miller CL, Brown EM, Yang JJ. The calcium sensing receptor: From calcium sensing to signaling. Sci China Life Sci. 2015;58:14–27. doi: 10.1007/s11427-014-4779-y. [DOI] [PubMed] [Google Scholar]
- 10.Tennakoon S, Aggarwal A, Kállay E. The calcium-sensing receptor and the hallmarks of cancer. Biochim Biophys Acta. 2016;1863:1398–1407. doi: 10.1016/j.bbamcr.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Tharmalingam S, Hampson DR. The calcium-sensing receptor and integrins in cellular differentiation and migration. Front Physiol. 2016;7:190. doi: 10.3389/fphys.2016.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendy GN, Lucie C. Calcium-sensing receptor gene: Regulation of expression. Front Physiol. 2016;7:394. doi: 10.3389/fphys.2016.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goru SK, Pandey A, Gaikwad AB. E3 ubiquitin ligases as novel targets for inflammatory diseases. Pharmacol Res. 2016;106:1–9. doi: 10.1016/j.phrs.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Bizet AA, Trankhanh N, Saksena A, Liu K, Buschmann MD, Philip A. CD109-mediated degradation of TGF-β receptors and inhibition of TGF-β responses involve regulation of SMAD7 and Smurf2 localization and function. J Cell Biochem. 2011;113:238–246. doi: 10.1002/jcb.23349. [DOI] [PubMed] [Google Scholar]
- 15.Yuan H, Guo SX, Zhang JM. Effect of autophagy in traumatic brain injury. Chin J Pathophysiol. 2011;27:1652–1656. [Google Scholar]
- 16.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghavami S, Cunnington RH, Gupta S, Yeganeh B, Filomeno KL, Freed DH, Chen S, Klonisch T, Halayko AJ, Ambrose E, et al. Autophagy is a regulator of TGF-β1-induced fibrogenesis in primary human atrial myofibroblasts. Cell Death Dis. 2015;6:e1696. doi: 10.1038/cddis.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Tian Z, Sun Y, Lu C, Liu N, Gao Z, Zhang L, Dong S, Yang F, Zhong X, et al. Exogenous H2S facilitating ubiquitin aggregates clearance via autophagy attenuates type 2 diabetes-induced cardiomyopathy. Cell Death Dis. 2017;8:e2992. doi: 10.1038/cddis.2017.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng X, Li HX, Shao HJ, Li GW, Sun J, Xi YH, Li HZ, Wang XY, Wang LN, Bai SZ, et al. Involvement of calcium-sensing receptors in hypoxia-induced vascular remodeling and pulmonary hypertension by promoting phenotypic modulation of small pulmonary arteries. Mol Cell Biochem. 2014;396:87–98. doi: 10.1007/s11010-014-2145-9. [DOI] [PubMed] [Google Scholar]
- 20.Xu C, Zhang W, Jiang C, Sun Y, Wang R. Involvement of calcium sensing receptor in myocardial ischemia/reperfusion injury and apoptosis. J Mol Cell Cardiol. 2007;42:S80–S81. doi: 10.1016/j.yjmcc.2007.03.741. [DOI] [Google Scholar]
- 21.Pan YL, Han ZY, He SF, Yang W, Cheng J, Zhang Y, Chen ZW. miR133b5p contributes to hypoxic preconditioningmediated cardioprotection by inhibiting the activation of caspase8 and caspase-3 in cardiomyocytes. Mol Med Rep. 2018;17:7097–7104. doi: 10.3892/mmr.2018.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Hua J, Cai W, Zhan Q, Lai W, Zeng Q, Ren H, Xu D. N-terminal truncated peroxisome proliferator-activated receptor-γ coactivator-1α alleviates phenylephrine-induced mitochondrial dysfunction and decreases lipid droplet accumulation in neonatal rat cardiomyocytes. Mol Med Rep. 2018;18:2142–2152. doi: 10.3892/mmr.2018.9158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S, Chen S, Li M, Zhang B, Shen P, Liu P, Zheng D, Chen Y, Jiang J. Autophagy activation attenuates angiotensin II-induced cardiac fibrosis. Arch Biochem Biophys. 2016;590:37–47. doi: 10.1016/j.abb.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Tran QG, Yoon HR, Cho K, Lee SJ, Crespo JL, Ramanan R, Kim HS. Dynamic interactions between autophagosomes and lipid droplets in chlamydomonas reinhardtii. Cells. 2019;8:992. doi: 10.3390/cells8090992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Z, Wang Y, Ji S, Wang K, Zhao Y. Computer-aided analysis with Image J for quantitatively assessing psoriatic lesion area. Skin Res Technol. 2015;21:437–443. doi: 10.1111/srt.12211. [DOI] [PubMed] [Google Scholar]
- 26.Mo Y, Lou Y, Zhang A, Zhang J, Zhu C, Zheng B, Li D, Zhang M, Jin W, Zhang L, Wang J. PICK1 deficiency induces autophagy dysfunction via lysosomal impairment and amplifies sepsis-induced acute lung injury. Mediators Inflamm. 2018;2018:6757368. doi: 10.1155/2018/6757368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estévez-García IO, Cordoba-Gonzalez V, Lara-Padilla E, Fuentes-Toledo A, Falfán-Valencia R, Campos-Rodríguez R, Abarca-Rojano E. Glucose and glutamine metabolism control by APC and SCF during the G1-to-S phase transition of the cell cycle. J Physiol Biochem. 2014;70:569–581. doi: 10.1007/s13105-014-0328-1. [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Zhang Q, Mo W, Feng J, Li S, Li J, Liu T, Xu S, Wang W, Lu X, et al. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-β1/Smads and PI3K/Akt pathways. Sci Rep. 2017;7:9289. doi: 10.1038/s41598-017-09673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groop L, Pociot F. Genetics of diabetes-are we missing the genes or the disease? Mol Cell Endocrinol. 2014;382:726–739. doi: 10.1016/j.mce.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Adeghate E. Molecular and cellular basis of the aetiology and management of diabetic cardiomyopathy: A short review. Mol Cell Biochem. 2004;261:187–191. doi: 10.1023/B:MCBI.0000028755.86521.11. [DOI] [PubMed] [Google Scholar]
- 31.Lam S, Verhagen NAM, Strutz F, van der Pijl JW, Daha MR, van Kooten C. Glucose-induced fibronectin and collagen type III expression in renal fibroblasts can occur independent of TGF-beta1. Kidney Int. 2003;63:878–888. doi: 10.1046/j.1523-1755.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 32.Kehlet SN, Willumsen N, Armbrecht G, Dietzel R, Brix S, Henriksen K, Karsdal MA. Age-related collagen turnover of the interstitial matrix and basement membrane: Implications of age- and sex-dependent remodeling of the extracellular matrix. PLoS One. 2018;13:e0194458. doi: 10.1371/journal.pone.0194458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2016;90:84–93. doi: 10.1016/j.yjmcc.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loboda A, Sobczak M, Jozkowicz A, Dulak J. TGF-β1/Smads and miR-21 in renal fibrosis and inflammation. Mediators Inflamm. 2016;2016:8319283. doi: 10.1155/2016/8319283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao M, Wang X, Wang X, Zhang T, Chi Y, Gao F. The Notch pathway mediates the angiotensin II-induced synthesis of extracellular matrix components in podocytes. Int J Mol Med. 2015;36:294–300. doi: 10.3892/ijmm.2015.2193. [DOI] [PubMed] [Google Scholar]
- 36.Yasuda J, Fukui K, Okada M, Yamawaki H. T3 peptide, a fragment of tumstatin, stimulates proliferation and migration of cardiac fibroblasts through activation of Akt signaling pathway. Naunyn Schmiedebergs Arch Pharmacol. 2017;390:1135–1144. doi: 10.1007/s00210-017-1413-0. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Zhang T, Wu J, Yu X, Zheng D, Yang F, Li T, Wang L, Zhao Y, Dong S, et al. Calcium sensing receptor promotes cardiac fibroblast proliferation and extracellular matrix secretion. Cell Physiol Biochem. 2014;33:557. doi: 10.1159/000358634. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura A, Hotsubo T, Kobayashi K, Mochizuki H, Ishizu K, Tajima T. Loss-of-function and gain-of-function mutations of calcium-sensing receptor: Functional analysis and the effect of allosteric modulators NPS R-568 and NPS 2143. J Clin Endocrinol Metab. 2013;98:E1692–E1701. doi: 10.1210/jc.2013-1974. [DOI] [PubMed] [Google Scholar]
- 39.Petrel C, Kessler A, Dauban P, Dodd RH, Rognan D, Ruat M. Positive and negative allosteric modulators of the Ca2+-sensing receptor interact within overlapping but not identical binding sites in the transmembrane domain. J Biol Chem. 279:18990–18997. doi: 10.1074/jbc.M400724200. [DOI] [PubMed] [Google Scholar]
- 40.Gottlieb RA, Mentzer RM., Jr Autophagy: An affair of the heart. Heart Fail Rev. 2013;18:575–584. doi: 10.1007/s10741-012-9367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chi J, Wang L, Zhang X, Fu Y, Liu Y, Chen W, Liu W, Shi Z, Yin X. Activation of calcium-sensing receptor-mediated autophagy in AngiotensinII-induced cardiac fibrosis in vitro. Biochem Biophys Res Commun. 2018;497:571–576. doi: 10.1016/j.bbrc.2018.02.098. [DOI] [PubMed] [Google Scholar]
- 42.Liu L, Wang C, Sun D, Jiang S, Li H, Zhang W, Zhao Y, Xi Y, Shi S, Lu F, et al. Calhex231 ameliorates cardiac hypertrophy by inhibiting cellular autophagy in vivo and in vitro. Cell Physiol Biochem. 2015;36:1597–1612. doi: 10.1159/000430322. [DOI] [PubMed] [Google Scholar]
- 43.Yao LH. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7:1056–1067. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deheuninck J, Luo K. Ski and SnoN, potent negative regulators of TGF-beta signaling. Cell Res. 2004;14:65–70. doi: 10.1038/cr.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith GL, Eisner DA. Calcium buffering in the heart in health and disease. Circulation. 2019;139:2358–2371. doi: 10.1161/CIRCULATIONAHA.118.039329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iino M. Spatiotemporal dynamics of Ca2+ signaling and its physiological roles. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:244–256. doi: 10.2183/pjab.86.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baba Y, Kurosaki T. Physiological function and molecular basis of STIM1-mediated calcium entry in immune cells. Immunol Rev. 2009;231:174–188. doi: 10.1111/j.1600-065X.2009.00813.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhang WH, Fu SB, Lu FH, Wu B, Gong DM, Pan ZW, Lv YJ, Zhao YJ, Li QF, Wang R, et al. Involvement of calcium-sensing receptor in ischemia/reperfusion-induced apoptosis in rat cardiomyocytes. Biochem Biophys Res Commun. 2006;347:872–881. doi: 10.1016/j.bbrc.2006.06.176. [DOI] [PubMed] [Google Scholar]
- 49.Lönn P, Vanlandewijck M, Raja E, Kowanetz M, Watanabe Y, Kowanetz K, Vasilaki E, Heldin CH, Moustakas A. Transcriptional induction of salt-inducible kinase 1 by transforming growth factor β leads to negative regulation of type I receptor signaling in cooperation with the Smurf2 ubiquitin ligase. J Biol Chem. 2012;287:12867–12878. doi: 10.1074/jbc.M111.307249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai Y, Shen XZ, Zhou CH, Wang JY. Abnormal expression of Smurf2 during the process of rat liver fibrosis. Chin J Dig Dis. 2010;7:237–245. doi: 10.1111/j.1443-9573.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- 51.Duran J, Troncoso M, Lagos D, Ramos S, Marin G, Estrada M. GDF11 modulates Ca2+-dependent Smad2/3 signaling to prevent cardiomyocyte hypertrophy. Int J Mol Sci. 2018;19:1508. doi: 10.3390/ijms19051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.