Abstract
Background & Aims
Coronavirus disease 2019 (COVID-19) has placed a significant strain on national healthcare systems at a critical moment in the context of hepatitis elimination. Mathematical models can be used to evaluate the possible impact of programmatic delays on hepatitis disease burden. The objective of this analysis was to evaluate the incremental change in HCV liver-related deaths and liver cancer, following a 3-month, 6-month, or 1-year hiatus in hepatitis elimination programs.
Methods
Previously developed models were adapted for 110 countries to include a status quo or ‘no delay’ scenario and a ‘1-year delay’ scenario assuming significant disruption in interventions (screening, diagnosis, and treatment) in the year 2020. Annual country-level model outcomes were extracted, and weighted averages were used to calculate regional (WHO and World Bank Income Group) and global estimates from 2020 to 2030. The incremental annual change in outcomes was calculated by subtracting the ‘no-delay’ estimates from the ‘1-year delay’ estimates.
Results
The ‘1-year delay’ scenario resulted in 44,800 (95% uncertainty interval [UI]: 43,800–49,300) excess hepatocellular carcinoma cases and 72,300 (95% UI: 70,600–79,400) excess liver-related deaths, relative to the ‘no-delay’ scenario globally, from 2020 to 2030. Most missed treatments would be in lower-middle income countries, whereas most excess hepatocellular carcinoma and liver-related deaths would be among high-income countries.
Conclusions
The impact of COVID-19 extends beyond the direct morbidity and mortality associated with exposure and infection. To mitigate the impact on viral hepatitis programming and reduce excess mortality from delayed treatment, policy makers should prioritize hepatitis programs as soon as it becomes safe to do so.
Lay Summary
COVID-19 has resulted in many hepatitis elimination programs slowing or stopping altogether. A 1-year delay in hepatitis diagnosis and treatment could result in an additional 44,800 liver cancers and 72,300 deaths from HCV globally by 2030. Countries have committed to hepatitis elimination by 2030, so attention should shift back to hepatitis programming as soon as it becomes appropriate to do so.
Keywords: COVID-19, Hepatitis C, Viral hepatitis elimination, Mathematical modelling
Graphical abstract
Introduction
The emergence of the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in late 2019, which causes coronavirus disease 2019 (COVID-19) and was declared a global emergency by the World Health Organization (WHO), has resulted in an unprecedented global response and placed a significant strain on national healthcare systems.1 , 2 National responses to the COVID-19 pandemic vary, but disruptions in the supply chain and the necessary reallocation of healthcare resources and public health personnel are likely to have broad-reaching consequences for other disease areas. This event occurs at a critical moment in the context of hepatitis elimination, with only 10 years remaining to reach the Global Health Sector Strategy targets by 2030.3
Disruptions to hepatitis programming across the cascade of care have already been documented in Egypt and Italy, 2 countries with very different COVID-19 experiences, and are expected in many other countries. In February 2020, the Italian government enacted a law to conduct graduated birth cohort screening for hepatitis; however, as of May 2020, the implementation of these programs is still delayed. Meanwhile, in Egypt, all ongoing screening programs (including screening of children, pregnant women, foreigners living in Egypt, and prisoners) were halted in March 2020, and the number of Ministry of Health-affiliated HCV treatment and cirrhosis follow-up units operating regularly was reduced by >75% (unpublished data provided by Professor Imam Waked, Menoufia University, Egypt).
Although the full impact of delaying hepatitis elimination programs is yet to be seen, mathematical models can be used to evaluate the possible impact on hepatitis disease burden and mortality resulting from programmatic delays. The objective of this analysis was to evaluate the incremental change in HCV liver-related deaths and liver cancer at a regional and global level, following a 1-year hiatus in hepatitis elimination program progress. Secondary objectives for the analysis included evaluating the incremental change in HCV diagnosis, treatment, and new infections (indicators for hepatitis elimination) and to evaluate the impact of shorter delays (3 month or 6 month) on morbidity and mortality. This analysis can assist decision makers with the reprioritization of hepatitis programming and resources, once the pandemic has subsided.
Materials and methods
Summary
In this modelling study, we adapted previously developed models for 110 countries to include a scenario modelling a 1-year gap and delay in HCV intervention measures (screening, diagnosis, and treatment) in the year 2020. Annual country-level model outcomes, including HCV liver-related deaths, incident hepatocellular carcinoma (HCC), viraemic prevalent, and HCV incident cases, were extracted from 2015 to 2050 under ‘no-delay’ and ‘1-year delay’ scenarios, and were summarized for the years 2020–2030. Model outputs were extracted, and weighted averages were used to calculate regional and global estimates (see Section 1 in the supplemental information online). The incremental annual change in outcomes was calculated by subtracting the ‘no-delay’ estimates from the ‘1-year delay’ estimates.
HCV disease burden modelling
A Markov model developed in Excel® for Microsoft 365® (version 365; Microsoft Corporation, Redmond, WA, USA) was previously parameterized for 110 countries using national demographic data (population, all-cause mortality, births, and sex ratio at birth), HCV epidemiological data (anti-HCV prevalence, viraemic rate, age, and sex distributions), and annual HCV intervention coverage data (screening, diagnosis, antiviral treatment, and sustained virologic response [SVR]).4 The impact of HCV treatment as prevention was calculated in the model for horizontally and vertically acquired incident infections in future years. Horizontally acquired infections were calculated as a function of prevalence in future years, considering fibrosis restrictions for treatment. In countries without treatment or reimbursement restrictions by fibrosis stage (i.e. F0 on the METAVIR scale), future horizontal incident cases were assumed to change at the same rate as prevalence. However, in countries with restrictions (i.e. F1 or greater on the METAVIR scale), future horizontal incident cases were assumed to change at the same rate as modelled F0 prevalence. Vertically acquired infections were calculated considering fertility rates among women of childbearing age,5 the mother-to-child transmission rate of HCV,6 and the modelled age-specific chronic prevalence of HCV. The subsequent disease progression of HCV-infected infants was also tracked in the model. The models were run under 2 scenarios, as described below.
No-delay (2019 base) scenario
Where available, empirical national-level data regarding HCV screening, diagnosis, and treatment were included through 2019. After 2019, it was assumed that the number of screened patients would remain constant after the last year of available data; however, a constant screening paradigm generally resulted in fewer annual newly diagnosed cases as time progressed (Table 1 ). Additionally, in the absence of an extensive screening strategy, the number of patients initiated on treatment annually was assumed to drop 50% over 5 years from peak treatment, unless better data were available to inform a more accurate forecast.
Table 1.
Example scenario inputs under the ‘no-delay’ scenario and the ‘1-year delay’ scenario for fictitious country X.
| Year |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | 2026 | 2027 | 2028 | 2029 | 2030 | |
| No delaya | ||||||||||||
| Treated, n | 1,800 | 1,500 | 1,150 | 1,000 | 930 | 900 | 900 | 900 | 900 | 900 | 900 | 900 |
| Newly diagnosed, n | 1,750 | 1,700 | 1,650 | 1,600 | 1,550 | 1,500 | 1,450 | 1,400 | 1,350 | 1,300 | 1,250 | 1,200 |
| 1-year delayb | ||||||||||||
| Treated, n | 1,800 | 0 | 1,500 | 1,150 | 1,000 | 930 | 900 | 900 | 900 | 900 | 900 | 900 |
| Newly diagnosed, n | 1,750 | 0 | 1,700 | 1,650 | 1,600 | 1,550 | 1,500 | 1,450 | 1,400 | 1,350 | 1,300 | 1,250 |
In this fictitious country, 2019 is the year of peak treatment. After 2019, it takes about 5 years for annual treatments to decrease to 50% of peak. Similarly, the number of newly diagnosed decreases slightly over time as the undiagnosed fraction of the population decreases.
In the 1-year delay scenario, no patients are diagnosed or treated in 2020. The previous paradigm for 2020 is shifted 1 year to begin in 2021.
1-year delay scenario
To simulate the impact of delayed hepatitis programming, a scenario was developed in which no patients were diagnosed or initiated on treatment in the year 2020. Beginning in 2021, the original 2020 diagnosis and treatment paradigm resumed, assuming a 1-year offset (i.e. 2020 diagnosis and treatment would occur in 2021, 2021 diagnosis and treatment would occur in 2022, etc.). An example of the scenario inputs is included in Table 1.
Statistical analysis
For the years 2015–2050, outcome data were extracted from all country models under the ‘no-delay’ and ‘1-year delay’ scenarios. The number of deaths, HCC cases, viraemic cases, and incident cases from countries with models (n = 110) were used to calculate weighted regional averages (proportion or rate, as appropriate) by Global Burden of Disease region. These proportions or rates were applied in countries without models to ultimately calculate regional and global averages. The final results were summarized by WHO region, World Bank Income Group, and globally over the 2020–2030 timespan. The timespan was chosen because it corresponds with the year of program disruption (2020) plus the 10 years remaining to achieve the Global Health Sector Strategy targets for the elimination of hepatitis as a public health threat.3
After calculating the morbidity and mortality at the regional and global level under the ‘no-delay’ and ‘1-year delay’ scenarios, the outcomes were used to calculate the results of a shorter delay as follows. Incremental deaths and incremental treatments from 2020 to 2030 were used to estimate the number of deaths per missed treatment in each region. This was then applied to the number of treatments that would be expected with a 6-month delay (half the number of missed treatments in 2020, followed by the original ‘no-delay’ treatment paradigm beginning in 2021) or a 3-month delay (a quarter of the number of missed treatments in 2020, followed by the original ‘no-delay’ treatment paradigm in 2021).
Uncertainty analysis
Uncertainty intervals (UIs) and sensitivity analyses were completed with Crystal Ball, an Excel add-in by Oracle. For the 110 countries with models, a 1/0 switch was developed to include or exclude country data from the regional calculations. This switch was defined as an assumption for each country. Monte Carlo simulation (with 1,000 trials) was used to estimate 95% UIs for global prevalence under the ‘1-year delay’ scenario. A sensitivity analysis was run to identify countries that accounted for the greatest variation in the global prevalence through their inclusion in regional averages.
Results
Missed diagnoses
Under the ‘no-delay’ scenario, globally, ~1.1 million patients were expected to be newly diagnosed in 2020, with 10.5 million expected to be newly diagnosed from 2020 to 2030. Only the high-income group (HIC) was expected to meet the WHO target for diagnosis under the ‘no-delay’ scenario (a projected 91% of the 2015 population would be diagnosed by 2030). Under the ‘1-year delay’ scenario, no patients were modelled to be newly diagnosed in 2020, with 9.6 million expected to be newly diagnosed from 2020 to 2030 (a difference of 906,000 diagnosed from 2020 to 2030). Approximately 45% of missed diagnoses would be in the World Bank Income-designated upper-middle income group, followed by 35% of missed diagnoses in the lower-middle income (LMIC) group (Table 2 ). No regions were expected to meet the WHO target for diagnosis under the ‘1-year delay’ scenario (HIC projections would result in only 89% of the 2015 population diagnosed by 2030).
Table 2.
Incremental viraemic infections in 2030, missed diagnoses and treatments (2020–2030), and cumulative (2020–2030) excess incident HCV, HCC, and LRDs, by WHO region and World Bank Income Group under the 1-year delay scenario.
| Region | Incremental, 2030 |
Missed interventions, 2020–2030 |
Excess cases, 2020–2030 |
|||
|---|---|---|---|---|---|---|
| Viraemic infections | New diagnoses | Treatment starts | Incident HCV | Incident HCC | LRDs | |
| WHO region | ||||||
| African | 12,300 | –47,100 | –15,700 | 2,600 | 850 | 1,700 |
| Eastern Mediterranean | 217,000 | –222,000 | –242,000 | 47,900 | 9,800 | 15,800 |
| European | 96,900 | –142,000 | –130,000 | 15,800 | 8,700 | 13,800 |
| American | 68,300 | –105,000 | –103,000 | 4,500 | 10,200 | 14,800 |
| South-East Asia | 73,100 | –104,000 | –81,600 | 20,300 | 3,600 | 7,900 |
| Western Pacific | 155,000 | –285,000 | –174,000 | 30,000 | 11,700 | 18,200 |
| World Bank Income Group | ||||||
| High income | 150,000 | –131,000 | –209,000 | 18,100 | 20,000 | 29,900 |
| Upper-middle income | 174,000 | –406,000 | –196,000 | 33,700 | 10,200 | 15,400 |
| Lower-middle income | 285,000 | –317,000 | –322,000 | 66,200 | 13,700 | 25,100 |
| Low income | 14,400 | –51,400 | –18,300 | 3,200 | 920 | 1,800 |
| Global | 623,000 | –906,000 | –746,000 | 121,000 | 44,800 | 72,200 |
HCC, hepatocellular carcinoma; LRDs, liver-related deaths.
Missed treatments
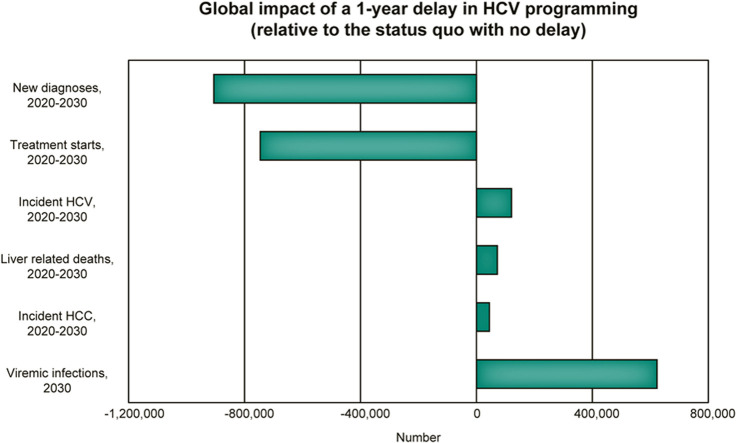
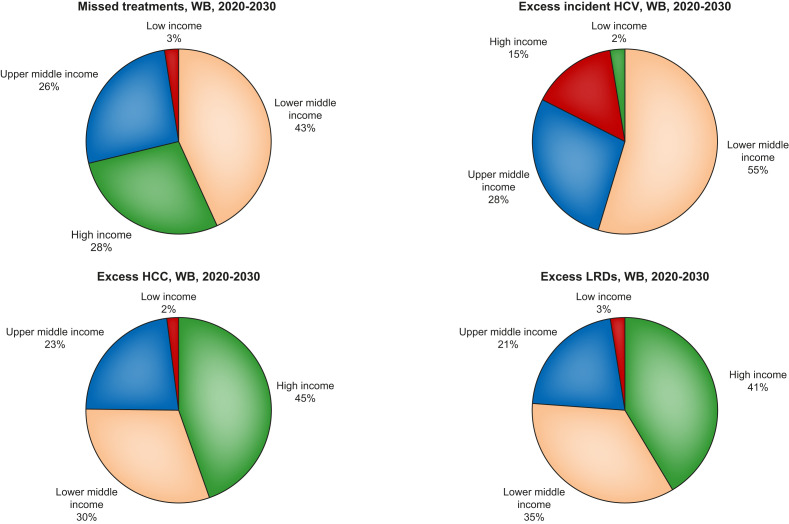
Under the ‘no-delay’ scenario, globally, approximately 1 million patients were expected to initiate treatment in 2020, with 9.1 million expected to initiate treatment from 2020 to 2030. Under the ‘1-year delay’ scenario, no patients were modelled to initiate treatment in 2020, with 8.4 million expected to initiate treatment from 2020 to 2030 (a difference of 746,000 treatment starts from 2020 to 2030). Approximately 43% of missed treatments would be the World Bank Income-designated LMIC group, with <3% of missed treatments in the low income group (Figure 1 ). No regions were projected to meet the WHO targets for treatment (80% of eligible patients initiated on treatment by 2030).
Fig. 1.
Proportion of missed treatments by World Bank Income Group, as well as cumulative (2020–2030) excess incident HCV, HCC and LRDs, by World Bank Income Group, under the 1-year delay scenario. HCC, hepatocellular carcinoma; LRDs, liver-related deaths; WB, World Bank.
1-year delay scenario
The ‘1-year delay’ scenario would result in 623,000 (95% UI: 609,000–685,000) more prevalent infections in 2030, relative to the ‘no-delay’ scenario, with 121,000 (95% UI: 118,000–133,000) excess incident infections, globally, from 2020 to 2030 (Table 2). Similarly, end-stage outcomes would increase, with 44,800 (95% UI: 43,800–49,300) excess HCC cases and 72,300 (95% UI: 70,600–79,400) excess liver-related deaths predicted over 2020–2030.
At the WHO regional level, the largest increase in incident HCV infections would be expected in the Eastern Mediterranean Region (47,900 excess incident cases from 2020 to 2030), with the most excess HCC and LRDs in the Western Pacific Region (11,700 excess incident HCC and 18,200 excess LRDs from 2020 to 2030) (Table 2). Considering World Bank Income Groups, most excess incident HCV infections would be in the LMIC group (66,200 excess incident HCV, 55% of all excess incident HCV infections). However, most excess HCC and LRDs would be among the HIC group (45% of excess HCC and 41% of excess LRDs) (Figs. 1 and 2 ).
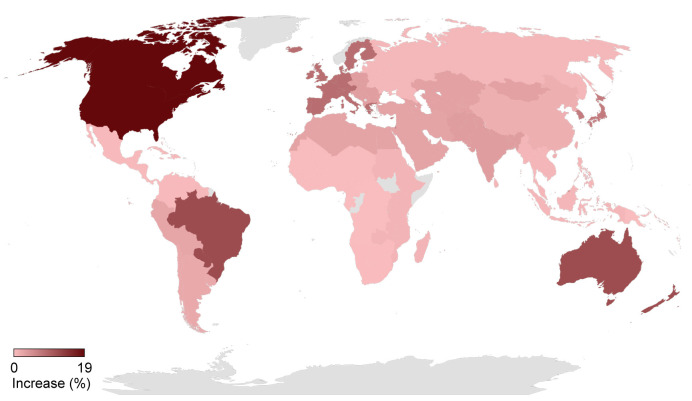
Fig. 2.
Impact of a 1-year delay on cumulative (2020–2030) liver-related deaths, by Global Burden of Disease region.
No regions were projected to meet the targets for incidence (90% reduction in new infections by 2030); and only the HIC group was projected to meet the target for mortality (65% reduction in liver-related deaths).
3- and 6-month delay scenarios
A shorter delay in hepatitis elimination programming would result in fewer LRDs, with only 50,600 excess deaths expected in the 6-month delay scenario and 25,300 excess deaths expected in the 3-month delay scenario.
Discussion
As of June 8, 2020, >400,000 deaths due to COVID-19 had been registered globally.7 For comparison, there were an estimated 400,000 deaths attributable to HCV in 2015 (1.34 million deaths were attributed to viral hepatitis in the same year).8 The full impact of COVID-19 is yet to be seen; however, in addition to the substantial morbidity and mortality directly attributed to infection, there are expected to be downstream consequences from delayed programming and care in other disease areas. This analysis suggests that a 1-year hiatus in HCV elimination programs could result in 72,300 (95% UI: 70,600–79,400) excess LRDs and 44,800 (95% UI: 43,800–49,300) excess liver cancers globally over the next 10 years.
For the past few years, HCV treatment starts have been declining, even in HIC (e.g. the USA was estimated to have treated 60% fewer patients in 2019 than in 2015).9 , 10 By 2020, 1 million patients were expected to initiate treatment globally, but recent estimates suggest that only 5 countries would be considered ‘on-track’ for HCV elimination (defined as a 65% reduction in LRDs, a 90% reduction in incident infections, and 80% diagnosed and 90% of diagnosed initiated on treatment).11 This means that even before COVID-19, many countries were playing ‘catch-up’ to reach the elimination targets. One example of a country that was previously on track for elimination but lost progress before the pandemic is Italy, where a 35% reduction in the annual number of patients initiated on treatment occurred in 2019, relative to 2018.12 Although the Government responded by enacting into law a screening program to begin in 2020 (see Section 2 in the supplemental information online), screening efforts have now been delayed due to COVID-19; and average weekly treatment starts have been reduced by >88% compared with 2018 (>80% reduction compared with 2019) (see Section 2 in the supplemental information online). This illustrates that further delays in elimination programming are likely to exacerbate already strained national and regional plans for hepatitis elimination. Most regions were not projected to reach any of the WHO targets under a ‘1-year delay’ scenario, with only the HIC group projected to achieve the mortality targets.
Currently, it is impossible to know how long treatment and programming delays will last, or if intermittent disruptions will become a ‘new normal’. Even after programs formally resume, patients might be reluctant to access healthcare services due to fear of contracting COVID-19 in those settings. To allow for even comparisons across regions, delays were assumed to occur uniformly within a scenario (i.e. in the 1-year delay scenario, we assumed that every country in the world simultaneously experienced a 1-year hiatus in programming). This analysis suggests that, if a delay in programming and treatment occurs globally, most missed treatments would be in LMIC. Although not all LMIC are experiencing or responding to the COVID-19 pandemic in the same way, access to treatment and care have still been impacted. For example, although Egypt has not imposed a strict lockdown, Ministry of Health-sponsored HCV management centres have experienced a 50% reduction in new patients and monthly visits, in addition to temporarily suspended screening programs (unpublished data provided by Professor Imam Waked). To account for differences in COVID-19 response and duration of response, data are presented by delay intervals (3-month, 6-month, and 1-year) and separately by the regional level (1-year delay) where outbreak responses are more likely to be similar. These provide decision makers with an array of options when considering how best to recover from delayed treatment.
Additionally, most excess incident HCV infections would occur in LMIC. This poses a significant challenge, because incident infections are unlikely to be diagnosed for decades, meaning that elimination efforts beyond 2030 might be necessary. One interesting finding was that the American Region is not expected to see a large number of incremental incident infections as a result of delayed treatment. These estimates are largely driven by treatment restrictions in the USA, which prevent access to treatment for people who inject drugs in many states.13 As a result, the disruption in diagnosis and treatment modelled here does not result in an increase in incident cases for the USA.
Limitations
A few limitations exist in our analysis and are described here. First, the number of excess incident HCV infections was calculated on the basis of delayed diagnosis and treatment programs and did not include the impact of increased risk behaviors (e.g. the impact of the economic crisis on drug or alcohol use) or delayed access to harm reduction. Including disruptions to harm reduction programs and changing patterns of substance use would likely increase the number of incremental incident infections seen in HIC. Additional studies are warranted to better understand the long-term impact of COVID-19 on HCV incidence.
A second limitation is that calculations for 3- and 6-month delays assumed that outcomes were evenly distributed across all calendar months. In reality, country-level treatment programs have shown that monthly distributions of treated patients vary based on local customs and holidays, as well as program rollouts. It is possible that countries experiencing only a 3-month delay in treatment programs might make up progress in the remaining months of the year, resulting in fewer excess incident cases and end-stage outcomes. However, it is also possible that countries experiencing a 3-month delay in formal programming might continue to experience decreased patient volume because of patient concerns over health and safety in healthcare settings.
The impact of COVID-19 extends beyond the direct morbidity and mortality associated with exposure and infection. To mitigate the impact on viral hepatitis programming and reduce excess mortality from delayed treatment, policy makers should prioritize hepatitis programs as soon as it becomes safe to do so.
Abbreviations
COVID-19, coronavirus disease 2019; HCC, hepatocellular carcinoma; HIC, high income countries; LMIC, lower-middle income countries; SVR, sustained virologic response; UI, uncertainty interval; WHO, World Health Organization.
Financial support
Financial support for this study was provided by the John C. Martin Foundation, United States of America, Grant number: G24. All authors contributed to the development of the publication and maintained control over the final content.
Authors' contributions
L.A.K., A.A., H.R. and A.C. were involved in developing the concept for the study; S.B., Z.C., E.D., C.E., I.G., S.M., D.R-S. and H.R. collected data; I.G. provided software; S.B. carried out the formal analysis and investigation, devised the methodology, and provided project administration, validation, and visualization; S.B. and H.R. wrote the original manuscript; H.R. acquired funding for and supervised the study; All authors were involved in writing and reviewing the final version of the manuscript.
Data availability
The regional and global data that support the findings of this study are available from the corresponding author (S.B.), upon reasonable request.
Conflicts of interest
S.B., Z.C., E.D., C.E., I.G., S.M. and D.R-S. are employees of the Center for Disease Analysis Foundation (CDAF). CDAF has received funding for this work from the John C. Martin Foundation. Over the past 3 years, CDAF has received research funding from Gilead, AbbVie, and Vaccine Impact Modeling Consortium. CDAF has also received grants from CDC Foundation, John Martin Foundation, ASTHO, Zeshan Foundation, and private donors. L.A.K. has received teaching grants from AbbVie and Gilead. A.A. has received research grants from AbbVie and Gilead, and has participated on advisory boards for MSD, AbbVie, Gilead, Mylan, Intercept, and Alfasigma. J.M.P. has received grants from Abbott, AbbVie, and Gilead. He has also received consulting fees from AbbVie, Gilead, Merck, GSK, and Siemens Healthcare. H.R. has been a member of advisory boards for Gilead, AbbVie, Merck, and VBI Vaccines. All proceeds are donated to CDAF. He is the managing director of Center for Disease Analysis (CDA) and CDAF. I.W. has been an investigator for AbbVie, Novartis, Marcyrl, Onexeo, and Pharco. He has been a speaker and advisory board member for MSD, Bayer, Astra-Zeneca, and Eva-Pharma. S.Z. has received lecture honoraria and consulting fees for AbbVie, Allergan, Gilead, Intercept, Janssen, and Merck/MSD. A.C. has been an advisor and speaker and has received research grants from AbbVie, Bayer, BMS, Gilead, Intercept, and MSD.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data associated with this article, can be found in the online version, at https://doi.org/10.1016/j.jhep.2020.07.042.
Supplementary data
References
- 1.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diaz A., Sarac B.A., Schoenbrunner A.R., Janis J.E., Pawlik T.M. Elective surgery in the time of COVID-19. Am J Surg. 2020;219:900–902. doi: 10.1016/j.amjsurg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . World Health Organization; Geneva: 2016. Global Health Sector Strategy on Viral Hepatitis 2016–2021: Towards Ending Viral Hepatitis. [Google Scholar]
- 4.Blach S., Zeuzem S., Manns M., Altraif I., Duberg A.-S., Muljono D.H., et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2016;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 5.United Nations . United Nations; New York: 2019. World Population Prospects 2019. [Google Scholar]
- 6.Benova L., Mohamoud Y.A., Calvert C., Abu-Raddad L.J. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765–773. doi: 10.1093/cid/ciu447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . World Health Organization; Geneva: 2020. Coronavirus Disease (COVID-19) Situation Report - 140. [Google Scholar]
- 8.World Health Organization . World Health Organization; Geneva: 2017. Global Hepatitis Report. [Google Scholar]
- 9.Gilead . Gilead; Foster City: 2019. Q4 2018 Earnings Results. [Google Scholar]
- 10.Gilead . Gilead; Foster City: 2020. Q4 2019 Earnings Results. [Google Scholar]
- 11.CDA Foundation Polaris Observatory 2020. 2020. https://cdafound.org/polaris/ Available from: Accessed September 28, 2020.
- 12.Agenzia Italiana del Farmaco . Agenzia Italiano del Farmace; Rome: 2020. Aggiornamento dati Registri AIFA DAAs - Epatite C cronica, 6 Gennaio 2020. [Google Scholar]
- 13.National Viral Hepatitis Roundtable . 2020. Center for Health Law and Policy Innovation. Hepatitis C: State of Medicaid Access.https://stateofhepc.org/ Available from: Accessed 30 September 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The regional and global data that support the findings of this study are available from the corresponding author (S.B.), upon reasonable request.