Abstract
Inflammatory bowel disease (IBD) is a chronic inflammatory condition with complex pathogenesis that currently has no cure. α7 nicotinic acetylcholine receptor (α7nAChR) is known to regulate multiple aspects of immune function. The present study aimed to evaluate the protective effects of PNU282987 and SHP099, which are a selective agonist of α7nAChR and an SHP2 inhibitor, respectively, in dextran sulfate sodium (DSS)-induced colitis in mice. Acute colitis was induced in mice using 3% DSS, and weight loss, colonic histology and cytokine production from colonic lamina propria were analyzed to evaluate disease severity. Bone marrow-derived macrophages were treated with lipopolysaccharide (LPS) to induce an inflammatory response. Cytokine expression and reactive oxygen species (ROS) levels were quantified. The α7nAChR agonist, PNU282987, and the SHP2 inhibitor, SHP099, were administered alone or in combination to LPS-induced macrophages or to colitic model mice to evaluate the inflammatory response and protective efficacy in colitis. α7nAChR protein levels were found to be markedly increased in the colon of DSS-induced colitic mice, and were found to co-localize with macrophages. Consistently, α7nAChR mRNA and protein levels were upregulated with colitis progression in DSS-induced colitic mice. Colonic inflammation was attenuated by PNU282987 treatment in DSS-induced mice, as evidenced by reduced weight loss and alleviated colonic epithelial cell disruption. These effects of PNU282987 on colitis were enhanced when it was combined with SHP099. Cytokine production and ROS levels induced by LPS in macrophages were decreased by a combination treatment of PNU282987 and SHP099. These findings identified α7nAChR as an essential element in the role of intestinal macrophages in colonic repair and demonstrated a synergistic effect of PNU282987 and SHP099, suggesting a new potential therapy for IBD.
Keywords: colitis, macrophages, PNU282987, SHP099
Introduction
Inflammatory bowel diseases (IBDs), such as Crohn's disease (CD) and ulcerative colitis (UC), are chronic inflammatory disorders of the gastrointestinal tract (1). Several factors have been identified that contribute to the disease pathogenesis, such as genetic factors, host immunity and environmental factors, including the intestinal microbiota (2). However, the precise etiology of IBD remains unclear, and treatments for IBD have limited efficacy; therefore, it is necessary to explore novel, effective and safe therapies to treat IBD.
Smoking is a controversial factor in colitis etiology that has been discussed for decades; although it has been shown to exacerbate CD, clinical studies have suggested it may reduce the severity of UC (3–5). Although the specific mechanisms underlying these effects remain unclear, a number of studies have proposed possible explanations for the effect of smoking on immune system reactivity and gut barrier integrity (6,7). As a potential beneficial compound, nicotine has anti-inflammatory effects through the activation of the nicotinic acetylcholine receptor (8,9), and several clinical studies suggest that nicotine could improve gut motility in smokers (10,11). The α7 subunit of the nicotinic acetylcholine receptor (α7nAChR) has been reported to play a crucial role in the responsiveness of macrophages, dendritic cells and other immune cells. Moreover, a specific agonist of α7nAChR shows anti-inflammatory effects in various experimental animal models (9,12).
According to research on the anti-inflammatory effects of α7nAChR in models of sepsis and acute inflammation, activation of α7nAChR in macrophages could be an effective pathway towards suppression of pro-inflammatory cytokine production (13,14). In addition, stimulation of α7nAChR induces STAT3 activation and subsequent protection of macrophages from endoplasmic reticulum stress-induced apoptosis, which affects the activity of the M2-type macrophage subset (15). Furthermore, Wazea et al (16) showed that the anti-atherogenic role of α7nAChR was due to attenuation of macrophage oxidative stress. In patients with CD, an inflammatory macrophage population present in the intestine produces large amounts of pro-inflammatory cytokines, including interleukin (IL)-23, tissue necrosis factor-α (TNF-α) and IL-6 (17). This work demonstrates that α7nAChR serves a crucial role in the regulation of macrophage activation and is suggestive of a potential role for macrophage α7nAChR in inflammatory diseases such as colitis. Indeed, some studies suggest that α7nAChR plays a negative role in the progression of colitis (18,19). However, treatment with pharmacological agonists of α7nAChR in colitis induced intolerable side effects in an experimental mouse model, and large doses of such agonists may have disruptive effects on colon integrity and induce anxiety-like behavior (20,21). So it was hypothesized that a reduced dosage of α7nAChR agonist, combined with other drugs, could reduce side effects and improve therapeutic effects.
SHP2, first identified as a proto-oncoprotein, is a tyrosine phosphatase family member. A number of previous studies have reported that SHP2 serves an important role in immune regulation. For example, upregulation of SHP2 in CD4+ T-cells causes Th17 cell loss in simian immunodeficiency virus infection (22). In addition, SHP2 activation is enhanced in Helicobacter pylori infection, which suppresses interferon-γ signaling (23). In dextran sulfate sodium (DSS)-induced colitic model mice, SHP2 phosphorylation levels are elevated, disrupting macrophage IL-10/STAT3 signaling and its negative effect on development of inflammation (24). Clinical data has revealed that macrophage-restricted SHP2 activation is associated with IBD (24). To develop a more potential therapy the activation of α7nAChR, the effects of treatment with PNU282987 (an α7nAChR agonist) on DSS-induced mouse colitis were evaluated. Furthermore, an optimized treatment using a combination of α7nAChR agonist PNU282987 and SHP2 inhibitor SHP099 is demonstrated in experimental colitis in mice.
Materials and methods
Mice
A total of 112 male C57BL/6 (B6) mice (age, 6–8 weeks; weight, 22–25 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd., Mice were maintained under specific pathogen-free conditions, all mice were provided free access to food and water, and conditions were a temperature of 23±2°C, humidity of 40–70% and a 12-h light/dark cycle. The procedures involving mice were carried out using protocols approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (Suzhou, China).
Antibodies and reagents
The following primary antibodies were used: Anti-CD68 (Bio-Rad Laboratories, Inc., cat. no. MCA1957T) and anti-α7nAChR (Sigma-Aldrich; Merck KGaA, cat. no. M220). PNU282987 was purchased from Sigma-Aldrich (Merck KGaA, cat. no. P6499), methyllycaconitine citrate (MLA) was purchased from Sigma-Aldrich (Merck KGaA, cat. no. M168) and SHP099 was from Selleck Chemicals (cat. no. S8278). All drugs were dissolved in PBS, and PBS was used as vehicle control. DSS (molecular mass, 36,000-50,000, cat. no. 9011-18-1) was purchased from MP Biomedicals, LLC and dissolved in the drinking water at 3% (w/v) concentration.
DSS-induced experimental colitis model
Mice were provided 3% (wt/vol) DSS dissolved in the drinking water for 7 days. Weight loss was monitored daily. At the end of treatment, mice were euthanized by 100% CO2 inhalation with 30% volume/minute flow rate; death was verified by cervical dislocation, colons were excised and colon length from the end of the cecum to the anus was recorded. In PNU282987-treated group, PNU282987 was dissolved in PBS and mice were intraperitoneal injection (i.p.) followed by DSS administration. For inhibiting a7nAChR, MLA was pre-administrated (i.p.) 1 day before PNU282987 treatment. PBS was used as vehicle control.
Histological analysis
Colons from mice treated with or without 3% (wt/vol) DSS were excised and fixed in 4% paraformaldehyde at 4°C overnight, then colons were dehydrated with graduated ethanol (70,80,85,90,95 and 100%) and embedded in paraffin. Paraffin-embedded colons were sectioned into 5 µM slices and stained with hematoxylin and eosin, as described previously (25). Histological score was assessed (25) based on leukocyte infiltration: (0, no infiltration; 1: Basolateral; 2: Infiltration to muscularis mucosae; 3: Infiltration to submucosa), epithelial cell disruption (0: No epithelium disruption; 1: Crypt hyperplasia; 2: Mild crypt disruption; 3: Loss of crypt in large area). The histological score was conducted blind by two of the authors. Immunofluorescence analysis. Mouse colon specimens were fixed in 4% paraformaldehyde at 4°C overnight, dehydrated with 30 and 20% sucrose solution, then embedded with O.C.T. compound (Sakura Finetek USA, Inc.). Frozen sections (7 µM) were blocked with 10% normal goat serum (Beyotime Institute of Biotechnology) diluted with 1X PBS (1 ml normal goat serum supplied with 9 ml PBS) for 1 h at room temperature, then incubated with primary antibody to α7nAChR (1:200) or CD68 antibody (1:200 dilution) at 4°C overnight, and fluorophore-coupled secondary antibodies (Mouse IgG2a Cross-Adsorbed Secondary Antibody Alexa Fluor 488 (cat no. A-21131; Thermo Fisher Scientific, Inc., 1:500); Rabbit anti-Rat IgG (H + L), Texas Red® (cat no. PA1-28571; Thermo Fisher Scientific, Inc., 1:500) for 1 h in the dark at room temperature. Sections were counterstained with 200 nM DAPI for 5 min and mounted in Fluorescent Mounting Medium. Fluorescent images were captured using a TCS SP8, laser-scanning confocal fluorescence microscope (Leica Microsystems GmbH).
Isolation of intestinal lamina propria macrophages
Briefly, mice were treated with 3% (wt/vol) DSS for 7 days to induce colitis, and the large intestines were removed and carefully cleaned of their mesentery and fat tissue. The large intestine was then opened longitudinally, washed of fecal contents, cut into 0.5 cm sections, and subjected to two sequential 20-min incubations at 37°C in Hanks balanced salt solution (HBSS) plus 5% FBS (Gibco; Thermo Fisher Scientific) and 5 mM EDTA at 37°C with gentle agitation to remove epithelial cells. After each incubation step, media containing epithelial cells and tissue debris was discarded. The remaining tissue was minced with ophthalmic scissors and incubated for 30 min in HBSS plus 5% FBS, 1 mg/ml collagenase VIII (Sigma-Aldrich; Merck KGaA) and 0.1 mg/ml DNase I (Sigma-Aldrich; Merck KGaA) at 37°C with gentle agitation. Cell suspensions were collected and filtered through a 70-µm strainer and pelleted by centrifugation at 300 × g at room temperature for 10 min. Cells were incubated with CD45-Percific Blue (diluted in PBS with 1:1,000), CD11b-FITC (diluted in PBS with 1:1,000) and F4/80-PE (diluted in PBS with 1:1,000) antibody at room temperature in dark for 30 min, after washing twice with PBS, cells were resuspended in PBS with 1×106 cells/ml. Colonic macrophages were sorted from CD45 positive cells, CD11b and F4/80 double positive gates by using flow cytometry (BD FACSAria™ III sorter). Purity of colonic macrophages was >85% analyzed with BD FACSCalibur™ with CD11b and F4/80 double positive gates. Purified colonic macrophages were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
Generation of bone marrow derived macrophages (BMDMs)
Mice were euthanized and their femurs and tibias were isolated and rinsed in 70% (vol/vol) ethanol. Bone marrow cells were flushed out with cold PBS and passed through 40 µM filters, then centrifuged at 2,500 × g for 5 min at room temperature. Pellets were suspended in 1 ml ACK red blood cell lysis buffer (Sangon Biotech Co., Ltd.) at room temperature for 1 min, then neutralized with 20 ml PBS. After centrifugation at 2,500 × g for 10 min at room temperature, the isolated bone marrow cells were resuspended in complete DMEM (supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin) containing 10 ng/ml macrophage colony stimulating factor (PeproTech, Inc.) and cultured at 37°C in a 5% CO2 incubator to allow for differentiation for 7 days.
RNA isolation and reverse transcription-quantitative PCR (RT-qPCR)
RNA was isolated from 30 mg of colon tissue. Biopsies were weighed and homogenized using liquid nitrogen and TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to isolate total RNA following the manufacturer's instructions. cDNA was reverse transcribed from 1 µg total RNA with the ReverTra Ace qPCR RT kit (Toyobo Life Science). qPCR was performed with SYBR® Green Realtime PCR Master Mix (Toyobo Life Science) and an ABI 7500 instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.), using the following primers were: α7nAChR, forward 5′-TCCTCATGTCAGCCCCAAAC-3′, reverse 5′-AGACCGACAGCTACAGGGAT-3′; IL-1β, forward 5′-AAGGGGACATTAGGCAGCAC-3′, reverse 5′-ATGAAAGACCTCAGTGCGGG-3′; IL-6, forward 5′-TGCCTTCTTGGGACTGATGC-3′, reverse 5′-TGAAGTCTCCTCTCCGGACT-3′; TNF-α, forward 5′-GCACCACCATCAAGGACTCA-3′, reverse 5′-TGTGAGGAAGGCTGTGCATT-3′; Il-12 p35, forward 5′-ATGATGACCCTGTGCCTTGG-3′, reverse 5′-CACCCTGTTGATGGTCACGA-3′; IL-23 p19, forward 5′-TGGAGCAACTTCACACCTCC-3′, reverse 5′-GGCAGCTATGGCCAAAAAGG-3′; IL-10, forward 5′-CCTCGTTTGTACCTCTCTCCG-3′, reverse 5′-CAAGTGTGGCCAGCCTTAGA-3′; GAPDH, forward 5′-CCTTCCGTGTTCCTACC-3′, reverse 5′-CAACCTGGTCCTCAGTGTA-3′. Gene transcripts were normalized against the loading control GAPDH, and relative expression levels were calculated using the 2−ΔΔCq method (26).
ELISA: Colon (0.5 cm) was excised from mice with sterile scissors, washed three times in cold PBS plus 100 µg/ml gentamycin, 100 U/ml penicillin and 100 µg/ml streptomycin. The colons slices were cultured with RPMI-1640 medium supplemented with 10% FBS and 100 µg/ml gentamycin at 37°C, 5% CO2 for 24 h. Supernatant was harvested for cytokine measurement. Cytokine production from colons was analyzed with Quantikine ELISA kits (R&D Systems; Cat. no. MLB00C, M6000B, MTA00B, M1270 and M1000B respectively), following the manufacturer's instructions. Cytokine production was normalized according to total colon tissue weight. Each treatment contained 3–4 mice.
Western blot analysis
Colon proteins were extracted as described previously (25). Briefly, RIPA buffer supplemented with protease inhibitors (Sangon Biotech Co., Ltd.) was used as lysis buffer. Colon proteins were separated by 10% SDS-PAGE, and the gels were then electro-transferred onto nitrocellulose filter membranes (Whatman; Cytiva). The membranes were incubated with antibodies against α7nAChR (Sigma-Aldrich; Merck KGaA, cat. no. M220, 1:1,000), phosphorylated (p-)SHP2 (Abcam; cat. no. ab62322, 1:500), SHP2 (Abcam, cat. no. ab131541, 1:500), p-STAT3 (Cell Signaling Technology, Inc.; cat. no. 9145, 1:1,000); STAT3 (Santa Cruz Biotechnology, Inc., cat. no. sc-482, 1:100), p-Jak2 (Abcam, cat. no. ab32101, 1:1,000); Jak2 (cat. no. ab108596, Abcam, 1:1,000) or GAPDH (Abcam, cat. no. ab181602, 1:2,000) overnight at 4°C. The membranes were then incubated with an IRDye 800CW-conjugated secondary antibody (Rockland Immunochemicals Inc. 1:20,000) for 1 h at room temperature. Images were acquired using an Odyssey infrared imaging system (Odyssey® CLx Imaging System, LI-COR Biosciences) and ImageJ software v1.52 (NIH) was used for analysis.
Intracellular ROS assay
Intracellular oxidative stress was measured using dichlorodihydrofluorescein diacetate oxidation. Macrophages were incubated with 100 µl of 1X DCFH-DA/media solution (Sangon Biotech Co., Ltd.) for 1 h at 37°C. After washed with DPBS three times, the fluorescence was read using a fluorometric plate reader (FlexStation 3, Molecular Devices, LLC) at 480 nm/530 nm.
Statistical analysis
Data are presented as the mean ± SD. All experiments were repeated three times. One-way ANOVA followed by Dunnett's multiple comparison post hoc test was performed when comparing all experimental groups with the control group, whereas Tukey's post hoc test was used for comparing the means of all treatments to the mean of every other treatment, using GraphPad software version 6.0 (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
α7nAChR expression in DSS-induced mouse colitis
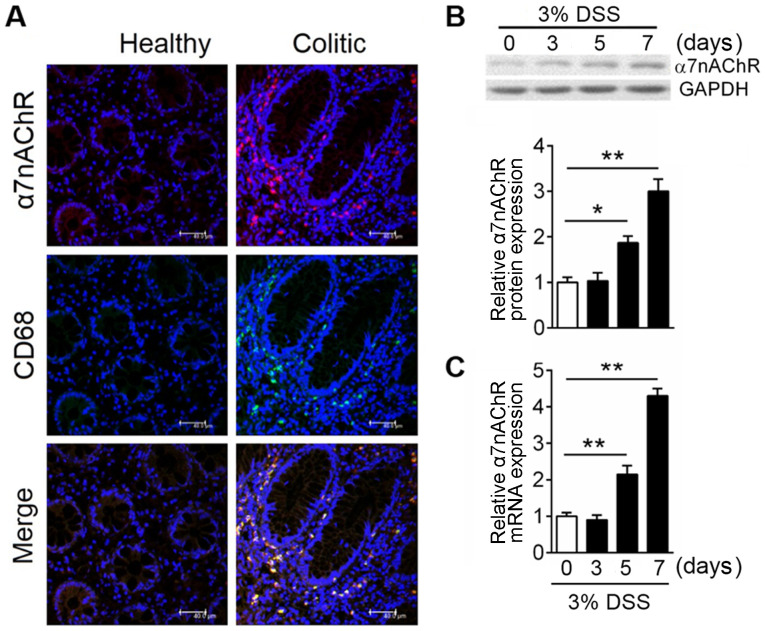
As a nicotinic acetyl cholinergic receptor, α7nAChR is expressed widely in the central and peripheral nervous systems; previous studies have shown that activation of α7nAChR could alleviate inflammation (12,27,28). However, the effects and associated mechanisms of receptor signaling in IBD is not completely understood. Immunofluorescence analysis was used to investigate the expression of α7nAChR in colonic tissues of mice, and the results demonstrated that α7nAChR expression was highly induced in inflammatory sites within the colon but was only sparsely expressed in healthy colon (Fig. 1A). Macrophages serve a crucial role in intestinal homeostasis (19), and expression of their specific surface marker, CD68, was more abundant in the inflamed compared with in the healthy colon (Fig. 1A); the merged panel demonstrates that these two proteins shared a degree of co-localization, which suggested that α7nAChR on macrophages may serve a crucial role in colitis. To clarify α7nAChR expression pattern after colitis onset, western blotting was used to analyze the protein expression levels of α7nAChR at day 0, 3, 5, 7 after DSS administration in mice. α7nAChR expression was significantly increased after 5 days of DSS treatment (Fig. 1B). To evaluate whether colitis-induced α7nAChR protein expression was due to reduced degradation or increased receptor synthesis, mRNA expression levels of α7nAChR in mice were assessed using RT-qPCR. The results showed α7nAChR mRNA levels have a similar expression pattern as protein levels in the mouse colon after DSS treatment (Fig. 1C). These data demonstrated that α7nAChR is highly expressed in the inflamed colon and suggested that the receptor on macrophages may play a critical role in colitis.
Figure 1.
Macrophages drive increased α7nAChR expression in IBD. In the experimental model, colitis was induced with 3% DSS in C57BL/6 mice, mice were sacrificed at the indicated time points and the colon was excised for protein and mRNA expression analysis. (A) Representative images of immunofluorescence analyses of colonic mucosal tissue in healthy and colitic mice. α7nAChR is in red; CD68 is in green expressions; DAPI is in blue. Co-localization of the α7nAChR and macrophage markers are displayed in merged images. Scale bar, 40 µM. Relative expression levels of α7nAChR (B) protein and (C) mRNA were analyzed by western blotting and reverse transcription-quantitative PCR, respectively, in DSS-induced colitis mice at days 0, 3, 5 and 7; GAPDH was used as internal reference for both. Data are expressed as the mean ± SD of three independent experiments; n=5–7 mice/group; *P<0.05, **P<0.01. α7nAChR, α7 nicotinic acetylcholine receptor; DSS, dextran sulfate sodium; IBD, inflammatory bowel disease.
Treatment with α7nAChR specific agonist PNU282987 ameliorates DSS-induced experimental colitis in mice
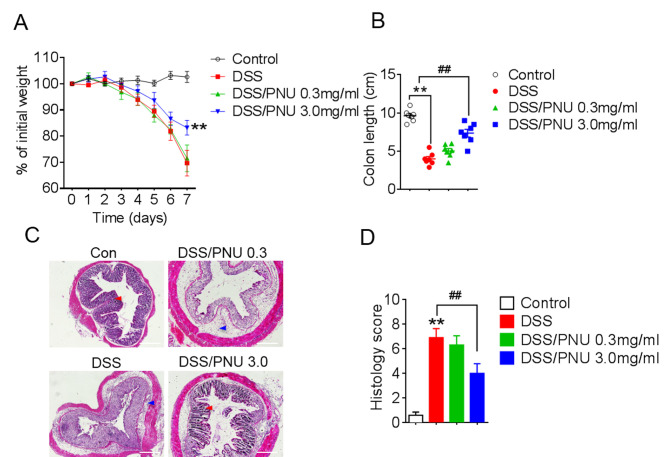
To investigate the role of α7nAChR in colitis, the α7nAChR specific agonist PNU282987 (0.3 or 3 mg/kg) was administered to mice by i.p. injection daily, following DSS administration. PNU282987 was dissolved in PBS and PBS was used as vehicle control. Pathological changes were observed in each group; progressive weight loss occurred in colitic mice treated with DSS, but the extent of weight loss was reduced with a 3 mg/kg of PNU282987 treatment in colitic mice compared with DSS+PBS-treated group (Fig. 2A). Moreover, the protective effects of PNU282987 were decreased by pre-treatment with MLA for 1 day, the antagonist of α7nAChR (Fig. S1). Colon shortening was lessened in colitic mice treated with PNU282987 at 3 mg/kg (Fig. 2B). Histological analysis of colons from colitic mice treated with the PBS control showed severe inflammation with loss of crypts (Fig. 2C, red arrow) and infiltration of leukocytes (Fig. 2C, blue arrow), whereas this damage was alleviated by treatment with PNU282987 (Fig. 2C). Histological scores were obtained based on colonic epithelial cell disruption and leukocyte infiltration, and the scores in the colitic mice were significantly lower after activation of α7nAChR by PNU282987 at 3 mg/kg (Fig. 2D). These results demonstrated that PNU282987 may exert a protective effect in DSS-induced mouse colitis.
Figure 2.
Activation of α7nAChR with PNU282987 exhibits a protective effect in DSS-induced colitis in mice. C57BL/6 mice received 3% DSS in drinking water ad libitum for 7 days, PNU282987 (0.3 mg/kg, n=10; 3 mg/kg, n=10) was intraperitoneally administered daily; normal drinking water was used as the negative control. (A) Body weight loss (presented as percentage of initial weight) was evaluated every day. **P<0.01 vs. DSS-treated group. (B) Colon length of mice in each group. **P<0.01 vs. control group, ##P<0.01 vs. DSS-treated group. (C) Representative images of hematoxylin and eosin staining of colon sections; scale bar = 20 µM. (D) Histological analysis scores of the images presented in (C), **P<0.01 vs. control group, ##P<0.01 vs. DSS-treated group. All data are expressed as the mean ± SD of three independent experiments. α7nAChR, α7 nicotinic acetylcholine receptor; DSS, dextran sulfate sodium; PNU, PNU282987.
Cytokine expression in colons of colitic mice treated with α7nAChR agonist PNU282987
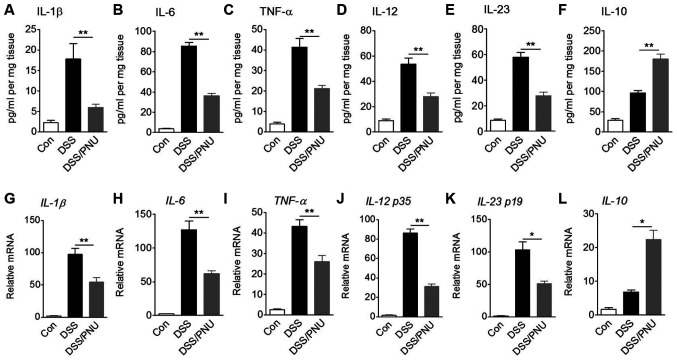
Cytokines serve a crucial role in the pathology of colitis (1,2); therefore, IL-1β, IL-6, TNF-α, IL-12, IL-23 and IL-10 levels in colonic tissue were analyzed by ELISA. Compared with untreated control colitic mice, colitic mice treated with 3 mg/kg PNU282987 exhibited a significant reduction in IL-1β, IL-6, TNF-α, IL-12 and IL-23 concentrations, whereas IL-10 levels were significantly increased after treatment with PNU282987 (Fig. 3A-F). In DSS alone-treated group, IL-10 level was also enhanced possibly due to the macrophage or a small amount of Treg cell activation. TGF-α and IL-22 production showed no significant differences between mice treated with PNU282987 or PBS; these two cytokines play a negative role in IBD development (data not shown).
Figure 3.
Effects of α7nAChR stimulation with PNU282987 on protein concentrations and mRNA expression levels of cytokines. C57BL/6 mice were treated with 3% DSS for 7 days, and intraperitoneally administered with PNU282987 (3 mg/kg; n=3-4/group) daily. Colonic samples were collected and cultured for 24 h. Supernatants of tissue culture were harvested and (A) IL-1β, (B) IL-6, (C) TNF-α, (D) IL-12, (E) IL-23 and (F) IL-10 protein concentrations were determined by ELISA. Colonic macrophages were sorted from colonic lamina propria cells of mice and mRNA expression levels of (G) IL-1β, (H) IL-6, (I) TNF-α, (J) IL-12 p35, (K) IL-23 p19 and (L) IL-10 were measured by reverse transcription-quantitative PCR. Data are displayed as the mean ± SD of three independent experiments. *P<0.05, **P<0.01. α7nAChR, α7 nicotinic acetylcholine receptor; DSS, dextran sulfate sodium; IL, interleukin; PNU, PNU282987; TNF-α, tissue necrosis factor-α.
One of the functions of macrophages is maintenance of intestinal homeostasis through cytokine production. In DSS-induced colitic mice treated with PNU282987 or with PBS, colonic macrophages were sorted >85% purity by flow cytometry using CD11b and F4/80 antibody staining (Fig. S2). IL-1β, IL-6, TNF-α, IL-12 p35, IL-23 p19 and IL-10 mRNA expression levels in macrophages were measured. In colitic mice treated with PNU282987, mRNA expression levels of IL-1β, IL-6, TNF-α, IL-12 p35 and IL-23 p19 were significantly decreased compared with colitic mice treated with PBS, whereas IL-10 mRNA expression was significantly raised in colonic macrophages of colitic mice treated with PNU282987 (Fig. 3G-L). Also due to the activation of macrophages in the colitic colon, IL-10 mRNA level was enhanced in the DSS-treated group compared with the control. These results are consistent with previous reports that suggest acute DSS-induced colitis development depends on macrophage activation (29,30).
SHP2 is activated in DSS-induced mouse colonic macrophages
SHP2 has previously been identified as a detrimental factor in the colonic immune system (24). To study SHP2 activation in colonic macrophages and the effect of treatment with PNU282987, mice were treated with DSS and colonic macrophages were isolated. JAK2 and STAT3 phosphorylation levels were notably upregulated in the colitic model mice, whereas a markedly decreased level was seen in the PNU282987-treated group (Fig. 4A). Moreover, SHP2 phosphorylation levels were also enhanced in the colitic group and were unchanged in the colitic group treated with PNU282987, indicating that PNU282987 has no direct effect on SHP2 activation (Fig. 4A). As STAT3 is involved in both α7nAChR and SHP2 pathways, activating α7nAChR or inhibiting SHP2 leads to upregulating the level of p-STAT3, which is beneficial for recovery in colitis (16,24). To investigate the possible role of SHP2 in PNU282987 effects on macrophages, SHP2 expression in BMDMs was knockdown by three siRNA, and the third siRNA show the highest efficacy on SHP2 knockdown (knockdown efficacy was verified by western blot; Fig. 4B). Cells were incubated with PNU282987 followed with LPS challenge which is a classical regent to inducing inflammation in macrophages. In BMDMs which had been challenged with LPS and treated with PNU282987, TNF-α and IL-6 cytokine production was significantly reduced in cells depleted of SHP2 or PNU282987 treatment compared with LPS only administration group. In addition, a combination of siRNA-SHP2 and PNU282987 decreased TNF-α and IL-6 cytokine protein level (Fig. 4C and D). These results indicated that inhibiting SHP2 activation may have a combined effect with PNU282987 in suppression of inflammatory macrophages.
Figure 4.
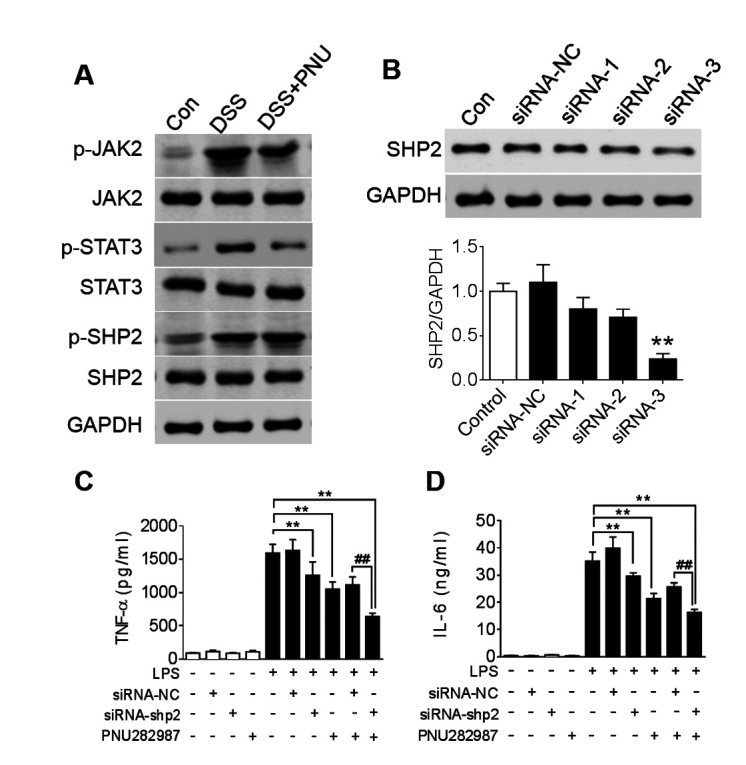
SHP2 is activated in DSS-induced colitis. (A) C57BL/6 mice were treated with 3% DSS for 7 days and intraperitoneally administered PNU282987 (3 mg/kg, n=3-4/group) daily; JAK2, STAT3 and SHP2 phosphorylation levels in the colon were analyzed after DSS treatment with or without PNU282987 treatment. (B) SHP2 protein level was analyzed with western blot to verify the efficacy of siRNA depletion. **P<0.01 vs. control group; Subsequently, BMDMs were treated with siRNA-3 to silence SHP2 expression, then treated with PNU282987 (10 µM) followed with LPS for 24 h, supernatant was harvested to determine the concentrations of (C) TNF-α and (D) IL-6 by ELISA. Data are presented as the mean ± SD of three independent experiments. **P<0.01 vs. LPS treatment; ##P<0.01 LPS+PNU282987+si-shp2 vs. LPS+PNU282987+si-NC. BMDMs, bone marrow derived macrophages; DSS, dextran sulfate sodium; IL-6, interleukin-6; LPS, lipopolysaccharide; PNU, PNU282987; TNF-α, tissue necrosis factor-α.
Inhibition of SHP2 with SHP099 and treatment with PNU282987 may synergistically suppress macrophages and colonic inflammation
SHP2 is a ubiquitously expressed tyrosine phosphatase and previous studies reported that it has an inhibitory effect on the JAK/STAT signaling pathway (24). To investigate whether inhibiting SHP2 has a synergistic effect in combination with PNU282987, BMDMs were treated with SHP099, a selective pharmacological inhibitor of SHP2, and PNU282987 simultaneously. Co-treatment with SHP099 and PNU282987 resulted in a significant decrease in TNF-α and IL-6 expression compared with PNU282987 only treatment in LPS-induced macrophages, whereas no significant decrease was identified in TNF-α and only a slight decrease in IL-6 production with SHP099 treatment alone (Fig. 5A). Excessive accumulation of reactive oxygen species (ROS) can induce further damage in inflamed colonic tissue (31). Consistent with the cytokine production data, ROS levels induced by LPS in macrophages were significantly decreased by a combination of SHP099 and PNU282987 treatments, whereas there were no differences identified between the SHP099-alone treatment group (Fig. 5B).
Figure 5.
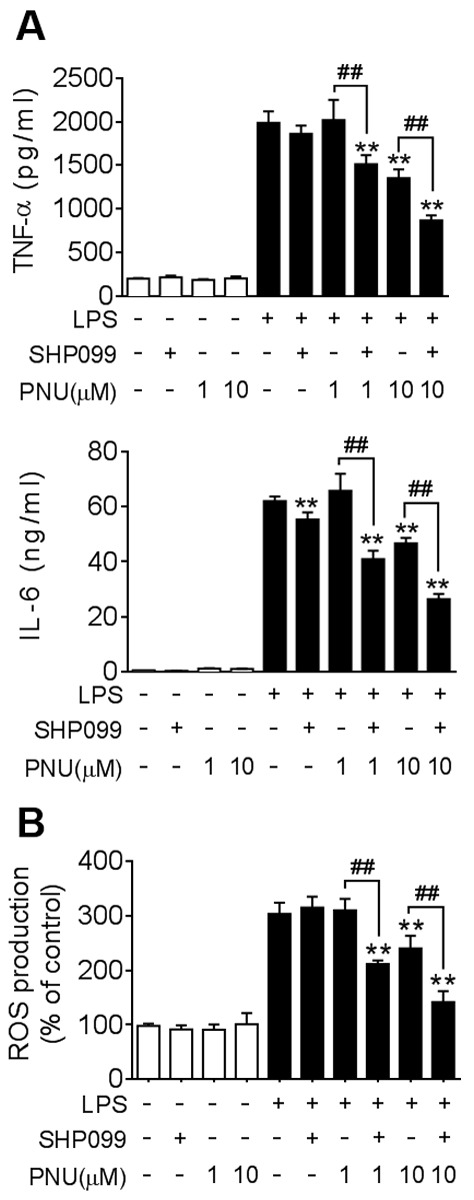
Co-treatment with SHP099 and PNU282987 synergistically suppresses cytokine production and ROS levels in macrophages. BMDMs were pre-treated with SHP099 (10 µM), PNU282987 (1 or 10 µM), or a combination of SHP099 and PNU282987 for 30 min, followed by LPS (100 ng/ml). (A) TNF-α and IL-6 protein levels in BMDMs, analyzed after LPS-treatment for 24 h. (B) ROS levels were quantified in BMDMs using an assay to detect fluorescent carboxyl DCFH-DA after LPS-treatment for 4 h. Data are displayed as the mean ± SD of three independent experiments. **P<0.01 vs. LPS treatment, ##P<0.01 LPS+PNU282987 vs. LPS + PNU282987+SHP099. IL-6, interleukin-6; LPS, lipopolysaccharide; PNU, PNU282987; ROS, reactive oxygen species; TNF-α, tissue necrosis factor-α.
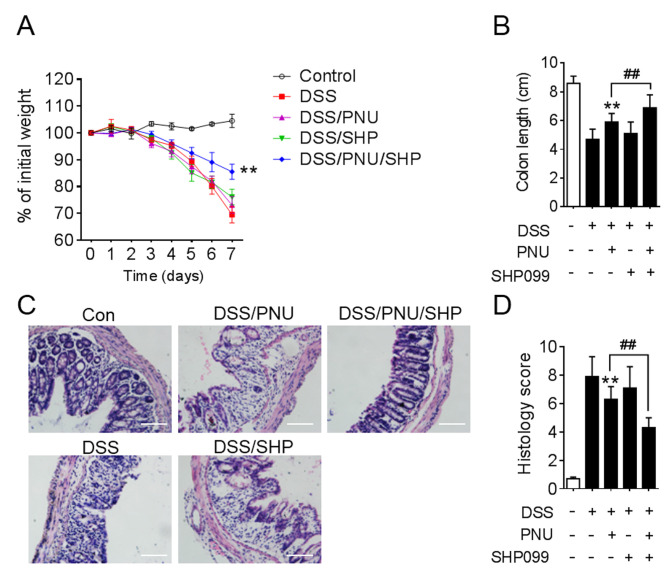
To further investigate the effect of SHP099 and PNU282987 in the experimental animal model, DSS-induced colitic mice were treated with PNU282987 combined with SHP099. Administration of DSS lead to weight loss, colon shortening and severe colonic disruption. The aim of the present study was to find a combined drug that could lower the dose of PNU282987 and protect mice from DSS-induced colitis, so 0.3 mg/kg PNU282987 or 1 mg/kg SHP099 treatment alone were chosen, which had no significant effect on colitis. However, when 0.3 mg/kg PNU282987 was combined with SHP099 administration, this resulted in attenuated weight loss and colon shortening (Fig. 6A and B). Histopathologically, in the PNU282987 and SHP099 co-treatment groups, mice displayed markedly mitigated mucosal damage and less leukocyte infiltration compared with PNU282987 treatment alone (Fig. 6C). Statistical analysis of histological scores supported these conclusions (Fig. 6D).
Figure 6.
Protective effects of PNU282987 on colitis is enhanced when combined with SHP099. C57BL/6 mice were administered 3% DSS to induce colitis, PNU282987 (0.3 mg/kg) and SHP099 (1 mg/kg) were given intraperitoneally, alone or in combination, daily. (A) Weight loss was monitored every day. (B) Colon length was analyzed at day 7. (C) Representative images of hematoxylin and eosin staining of colon sections; scale bar, 100 µM. (D) Histological analysis of images in (C). All data are expressed as the mean ± SD of three independent experiments. **P<0.01 vs. DSS treatment, ##P<0.01 LPS + PNU282987 vs. LPS+PNU282987+SHP099. DSS, dextran sulfate sodium; PNU, PNU282987; SHP, SHP099.
Discussion
Several studies have indicated that activating α7nAChR can alleviate colonic inflammation to a certain degree (18,19). The present study demonstrated that macrophage-specific expression of α7nAChR was induced in inflamed colons of DSS-induced colitis in mice. Intestinal integrity in the colitic mice was improved by a large dose of the α7nAChR agonist PNU282987, accompanied by side effects such as disrupted colon integrity and anxiety-like behavior (20,21). More importantly, inhibiting SHP2 with SHP099 was found to have a synergistic effect with PNU282987 on DSS-induced colitis. 0.3 mg/kg PNU282987 have no effect when treated colitic mice alone, while it has significantly protective effect combined with SHP099 administration.
Previous studies have also shown that α7nAChR activation is associated with alleviation of inflammation in LPS-induced endotoxin shock, accompanied by reduced pro-inflammatory cytokine production and leukocyte infiltration (9,16). Consistent with these studies, results from the present study demonstrated that stimulating α7nAChR with its selective agonist PNU282987 significantly attenuated DSS-induced acute colitis, which is accompanied by colonic shortening and weight loss in mice. Histological analysis of colon sections showed immune cell infiltration and tissue damage of the epithelial layer induced by DSS, which was markedly reduced after PNU282987 treatment compared with PBS-treated control mice. This evidence indicated that PNU282987 may have a protective effect, maintaining colonic homeostasis at large doses. In the present study, PNU282987 administration following DSS-induced colitis caused a marked drop in TNF-α, IL-1β, IL-6, IL-12 and IL-23 levels, whereas IL-10 was increased. IL-10 is a cytokine that modulates both innate and adaptive immunity, primarily by exerting anti-inflammatory effects. In inflammatory colon, IL-10 is produced by many cell types including both classical (M1) or alternative (M2) macrophages. In colitic colon of DSS-treated mice, macrophages exhibit M1 phenotype, while PNU282987 could alter macrophages into M2 phenotype (32). M2 macrophages produce more IL-10 than the M1 phenotype and this may be the reason why IL-10 levels were significantly increased after treatment with PNU282987 in colitic mice. By contrast, Tasaka et al (19) found that TNF-α and IL-1β levels were not changed after PNU282987 treatment in colitic mice. This disparity may have been due to differences in the measurement of cytokines from the colon (directly lysed colon vs. supernatant of colon culture). These cytokines are mainly produced by macrophages and are positively associated with the development of IBD, so a degree of manipulation of cytokine levels may help to improve IBD pathology. These findings have shown that the relative mRNA expression level of various cytokines in colonic macrophages was consistent with protein levels in colon culture.
In the present study, it was observed that combined use of PNU282987 and SHP099 presented an anti-inflammatory effect in LPS-induced macrophages. Abnormal macrophage activation is the major cause of tissue damage in colitis, and excessive ROS accumulation causes further disruption to tissue and increased pathology (1,2). Thus, a combination of these two drugs may have potential effect on protecting mice against DSS-induced colitis.
It was also found that treatment with SHP099 alone could not reverse the pathological deterioration in DSS-induced colitic mice, the possible reasons include that the treatment dosage used was not high enough in these DSS-induced colitic mice, thus inhibiting SHP2 alone may not represent a good method of treating colitis. However, SHP099 exhibits a promising synergistical effect with PNU282987 on the inflammatory response.
The present study demonstrated that IL-10 expression in colon cultures is significantly increased in DSS-induced colitis in mice treated with PNU282987. Based on other reports, STAT3 phosphorylation is crucial for IL-10 expression in macrophages induced by LPS, and STAT3 in innate cells has been shown to protect mice from colitis (33,34). A previous study identified SHP2 as an inhibitory molecule for STAT3 phosphorylation in macrophages and overexpression of SHP2 exacerbates colonic inflammation via disrupting the IL-10-STAT3 axis (20). Other findings indicate that SHP2 reduces JAK2/STAT3 signaling through promotion of JAK2 phosphorylation at Y570, resulting in amelioration of TGF-α induced fibroblast activation (35). Therefore, enhanced STAT3 activation may contribute to the combined potential therapy of a7nAChR activation and inhibiting SHP2 inhibition. Recently, SHP099 was developed as an allosteric inhibitor of SHP2 and showed an anti-proliferative effect in several cancers, such as non-small-cell lung cancer and hematologic malignancy (36). However, there are far fewer studies of SHP099 in inflammatory disease. The present study demonstrated that there was a synergistic effect between SHP099 and PNU282987 on DSS-induced colitis in mice. The observed colitis symptoms, including weight loss, colon shortening, colonic epithelium disruption and leukocyte infiltration were attenuated by co-treatment with SHP099 and PNU282987.
In conclusion, this study has described a combined effect between a selective agonist of α7nAChR, PNU282987, and a tyrosine phosphatase SHP2 inhibitor, SHP099, to protect mice from DSS-induced colitis. To the best of our knowledge, this data represents the first demonstration that treatment with these two compounds is effective in alleviating colonic inflammation, including leukocyte infiltration and inflammatory cytokine release. This evidence supports the notion that selectively stimulating α7nAChR while inhibiting SHP2 might represent a new and attractive therapy for colonic inflammation.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The Natural Science Foundation of Jiangsu Province (grant no. BK20170372), and Primary Research & Development Plan of Jiangsu Province (grant no. BE2018659).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JX and GZ performed most of the experiments and statistical analysis, SG performed cell experiments, JS, FH and ZH advised on IBD pathology and experimental design, JX, GZ and CX designed the study. JX wrote the manuscript and all authors reviewed and approved the final manuscript.
Ethics approval and consent to participate
The procedures involving mice were carried out using protocols approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (Suzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annual review of immunology. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 3.Opstelten JL, Plassais J, van Mil SW, Achouri E, Pichaud M, Siersema PD, Oldenburg B, Cervino AC. Gut microbial diversity is reduced in smokers with crohn's disease. Inflamm Bowel Dis. 2016;22:2070–2077. doi: 10.1097/MIB.0000000000000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav P, Ellinghaus D, Rémy G, Freitag-Wolf S, Cesaro A, Degenhardt F, Boucher G, Delacre M, International IBD GC, Peyrin-Biroulet L, et al. Genetic factors interact with tobacco smoke to modify risk for inflammatory bowel disease in humans and mice. Gastroenterology. 2017;153:550–565. doi: 10.1053/j.gastro.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueno A, Jijon H, Traves S, Chan R, Ford K, Beck PL, Iacucci M, Fort Gasia M, Barkema HW, Panaccione R, et al. Opposing effects of smoking in ulcerative colitis and Crohn's disease may be explained by differential effects on dendritic cells. Inflamm Bowel Dis. 2014;20:800–810. doi: 10.1097/MIB.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 6.Browning KN, Verheijden S, Boeckxstaens GE. The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology. 2017;152:730–744. doi: 10.1053/j.gastro.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingram JR, Rhodes J, Evans BK, Thomas GA. Preliminary observations of oral nicotine therapy for inflammatory bowel disease: An open-label phase I–II study of tolerance. Inflamm Bowel Dis. 2005;11:1092–1096. doi: 10.1002/ibd.3780111209. [DOI] [PubMed] [Google Scholar]
- 8.Galle-Treger L, Suzuki Y, Patel N, Sankaranarayanan I, Aron JL, Maazi H, Chen L, Akbari O. Nicotinic acetylcholine receptor agonist attenuates ILC2-dependent airway hyperreactivity. Nat Commun. 2016;7:13202. doi: 10.1038/ncomms13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H, Kim SR, Je J, Jeong K, Kim S, Kim HJ, Chang KC, Park SW. The proximal tubular α7 nicotinic acetylcholine receptor attenuates ischemic acute kidney injury through Akt/PKC signaling-mediated HO-1 induction. Exp Mol Med. 2018;50:40. doi: 10.1038/s12276-018-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2014;144:36–49. doi: 10.1053/j.gastro.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Severs M, Mangen MJ, van der Valk ME, Fidder HH, Dijkstra G, van der Have M, van Bodegraven AA, de Jong DJ, van der Woude CJ, Romberg-Camps MJ, et al. Smoking is associated with higher disease-related costs and lower health-related quality of life in inflammatory bowel disease. J Crohns Colitis. 2017;11:342–352. doi: 10.1093/ecco-jcc/jjw160. [DOI] [PubMed] [Google Scholar]
- 12.Kong W, Kang K, Gao Y, Liu H, Meng X, Cao Y, Yang S, Liu W, Zhang J, Yu K, Zhao M. GTS-21 protected against lps-induced sepsis myocardial injury in mice through α7nAChR. Inflammation. 2018;41:1073–1083. doi: 10.1007/s10753-018-0759-x. [DOI] [PubMed] [Google Scholar]
- 13.Li B, Alli R, Vogel P, Geiger TL. IL-10 modulates DSS-induced colitis through a macrophage-ROS-NO axis. Mucosal Immunol. 2014;7:869–878. doi: 10.1038/mi.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Y, Yang X, Yue W, Xu X, Li B, Zou L, He R. Chemerin aggravates DSS-induced colitis by suppressing M2 macrophage polarization. Cell Mol Immunol. 2014;11:355–366. doi: 10.1038/cmi.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi L, Lyn YJ, Peng C, Zhu RL, Bai SS, Liu L, Wang PX, Zhou H, Dong Y. Sinomenine inhibits fibroblast-like synoviocyte proliferation by regulating α7nAChR expression via ERK/Egr-1 pathway. Int Immunopharmacol. 2018;56:65–70. doi: 10.1016/j.intimp.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Wazea SA, Wadie W, Bahgat AK, El-Abhar HS. Galantamine anti-colitic effect: Role of alpha-7 nicotinic acetylcholine receptor in modulating Jak/STAT3, NF-κB/HMGB1/RAGE and p-AKT/Bcl-2 pathways. Sci Rep. 2018;8:5110. doi: 10.1038/s41598-018-23359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Zhou Y, Zhou Z, Liu Y, Bai Y, Xing X, Wang X. Nicotine induces the production of IL-1β and IL-8 via the α7 nAChR/NF-κB pathway in human periodontal ligament cells: An in vitro study. Cell Physiol Biochem. 2014;34:423–431. doi: 10.1159/000363011. [DOI] [PubMed] [Google Scholar]
- 18.Grandi A, Zini I, Flammini L, Cantoni AM, Vivo V, Ballabeni V, Barocelli E, Bertoni S. α7 nicotinic agonist AR-R17779 protects mice against 2,4,6-trinitrobenzene sulfonic acid-induced colitis in a spleen-dependent way. Front Pharmacol. 2017;8:809. doi: 10.3389/fphar.2017.00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tasaka Y, Yasunaga D, Kiyoi T, Tanaka M, Tanaka A, Suemaru K, Araki H. Involvement of stimulation of α7 nicotinic acetylcholine receptors in the suppressive effect of tropisetron on dextran sulfate sodium-induced colitis in mice. J Pharmacol Sci. 2015;127:275–283. doi: 10.1016/j.jphs.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Snoek SA, Verstege MI, van der Zanden EP, Deeks N, Bulmer DC, Skynner M, Lee K, Te Velde AA, Boeckxstaens GE, de Jonge WJ. Selective alpha7 nicotinic acetylcholine receptor agonists worsen disease in experimental colitis. Br J Pharmacol. 2010;160:322–333. doi: 10.1111/j.1476-5381.2010.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anshul AP, Jerrel LY. Activation of the α7 Nicotinic ACh Receptor Induces Anxiogenic Effects in Rats Which Is Blocked by a 5-HT1a Receptor Antagonist. Neuropharmacology. 2013;70:35–42. doi: 10.1016/j.neuropharm.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bixler SL, Sandler NG, Douek DC, Mattapallil JJ. Suppressed Th17 levels correlate with elevated PIAS3, SHP2, and SOCS3 expression in CD4 T cells during acute simian immunodeficiency virus infection. J Virol. 2013;87:7093–7101. doi: 10.1128/JVI.00600-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YC, Chen CL, Sheu BS, Yang YJ, Tseng PC, Hsieh CY, Lin CF. Helicobacter pylori infection activates Src homology-2 domain-containing phosphatase 2 to suppress IFN-γ signaling. J Immunol. 2014;193:4149–4158. doi: 10.4049/jimmunol.1400594. [DOI] [PubMed] [Google Scholar]
- 24.Xiao P, Zhang H, Zhang Y, Zheng M, Liu R, Zhao Y, Zhang X, Cheng H, Cao Q, Ke Y. Phosphatase Shp2 exacerbates intestinal inflammation by disrupting macrophage responsiveness to interleukin-10. J Exp Med. 2019;216:337–349. doi: 10.1084/jem.20181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao J, Shao L, Shen J, Jiang W, Feng Y, Zheng P, Liu F. Effects of ketanserin on experimental colitis in mice and macrophage function. Int J Mol Med. 2016;37:659–668. doi: 10.3892/ijmm.2016.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Harwani SC, Ratcliff J, Sutterwala FS, Ballas ZK, Meyerholz DK, Chapleau MW, Abboud FM. Nicotine mediates CD161a+ renal macrophage infiltration and premature hypertension in the spontaneously hypertensive rat. Circ Res. 2016;119:1101–1115. doi: 10.1161/CIRCRESAHA.116.309402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue T, Abe C, Sung SS, Moscalu S, Jankowski J, Huang L, Ye H, Rosin DL, Guyenet PG, Okusa MD. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J Clin Invest. 2016;126:1939–1952. doi: 10.1172/JCI83658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lv Z, Wang Z, Luo L, Chen Y, Han G, Wang R, Xiao H, Li X, Hou C, Feng J, et al. Spliceosome protein Eftud2 promotes colitis-associated tumorigenesis by modulating inflammatory response of macrophage. Mucosal Immunol. 2019;12:1164–1173. doi: 10.1038/s41385-019-0184-y. [DOI] [PubMed] [Google Scholar]
- 30.Xia Y, Tian LM, Liu Y, Guo KS, Lv M, Li QT, Hao SY, Ma CH, Chen YX, Tanaka M, et al. Low dose of cyanidin-3-O-glucoside alleviated dextran sulfate sodium-induced colitis, mediated by CD169+ macrophage pathway. Inflamm Bowel Dis. 2019;25:1510–1521. doi: 10.1093/ibd/izz090. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Li S, Cao Y, Tian X, Zeng R, Liao DF, Cao D. Oxidative stress and carbonyl lesions in ulcerative colitis and associated colorectal cancer. Oxid Med Cell Longev. 2016;2016:9875298. doi: 10.1155/2016/9875298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinheiro NM, Santana FPR, Almeida RR, Guerreiro M, Martins MA, Caperuto LC, Câmara NOS, Wensing LA, Prado VF, Tibério IFLC, et al. Acute lung injury is reduced by the α7nAChR agonist PNU-282987 through changes in the macrophage profile. FASEB J. 2017;31:320–332. doi: 10.1096/fj.201600431r. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Xu B, Shi F, Du M, Li Y, Yu T, Chen L. Protective effect of methane-rich saline on acetic acid-induced ulcerative colitis via blocking the TLR4/NF-κB/MAPK pathway and promoting IL-10/JAK1/STAT3-mediated anti-inflammatory response. Oxid Med Cell Longev. 2019;2019:7850324. doi: 10.1155/2019/7850324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian T, Hong J, Wang L, Wang Z, Lu Z, Li Y, Liu R, Chu Y. Regulation of CD11b by HIF-1α and the STAT3 signaling pathway contributes to the immunosuppressive function of B cells in inflammatory bowel disease. Mol Immunol. 2019;111:162–171. doi: 10.1016/j.molimm.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Zehender A, Huang J, Gyorfi AH, Matei AE, Trinh-Minh T, Xu X, Li YN, Chen CW, Lin J, Dees C, et al. The tyrosine phosphatase SHP2 controls TGFbeta-induced STAT3 signaling to regulate fibroblast activation and fibrosis. Nat Commun. 2018;9:3259. doi: 10.1038/s41467-018-05768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dardaei L, Wang HQ, Singh M, Fordjour P, Shaw KX, Yoda S, Kerr G, Yu K, Liang J, Cao Y, et al. SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. Nat Med. 2018;4:512–517. doi: 10.1038/nm.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.