Abstract
Background: Amyotrophic lateral sclerosis (ALS) is a neurodegenerative condition characterized by the progressive loss of motor neurons. Patients usually die 3–5 years after diagnosis from respiratory failure. Several studies investigated the role of vitamin D as a biomarker or a therapeutic option for ALS patients. To clarify the scientific evidence, we performed a systematic review and different meta-analyses regarding the potential role of vitamin D in ALS.
Methods: We performed a systematic review of clinical trials, cohorts, and case–control studies retrieved from PubMed, EMBASE, and Cochrane databases reporting vitamin D levels as a putative biomarker for ALS diagnosis or prognosis or the effect of vitamin D supplementation in ALS patients. Whenever possible, data were pooled using a random-effects model, with an assessment of heterogeneity.
Results: Out of 2,996 articles retrieved, we finally included 13 research articles, 12 observational studies (50% prospective), and 1 clinical trial. We found that ALS patients had slightly lower levels of vitamin D than controls (mean difference −6 ng/ml, 95% CI [−10.8; −1.3]), but important confounding factors were not considered in the studies analyzed. We found no relationship between vitamin D levels and ALS functional rate score—revised (ALSFRS-R), with highly heterogeneous results. Discordant results were reported in three studies regarding survival. Finally, five studies reported the effects of vitamin D supplementation with discordant results. Two of them showed a small improvement, whereas two others showed a deleterious effect on ALSFRS-R. One very small clinical trial with important methodological limitations showed some improvement in ALSFRS-R with high doses of vitamin D compared with normal doses.
Conclusions: Our review did not find evidence to support the role of vitamin D on ALS diagnosis, prognosis, or treatment. Most studies had important limitations, mostly regarding the risk of bias for not considering confounding factors. Vitamin D supplementation should be offered to ALS patients to avoid other health issues related to vitamin D deficiency, but there is not enough evidence to support the use of vitamin D as a therapy for ALS.
Keywords: amyotrophic lateral sclerosis, vitamin D, meta-analysis, systematic review, biomarker
Introduction
Vitamin D deficiency has been associated with diverse pathologies, from cardiovascular diseases to cancer. However, an umbrella review performed in 2014 could not draw any conclusion about the relationship between vitamin D levels or vitamin D supplementation and various conditions, such as skeletal, cardiovascular, autoimmune, metabolic diseases, or even cognitive disorders like Alzheimer's disease (1). Interestingly, amyotrophic lateral sclerosis (ALS) was not included in the list of conditions examined by this study. Since then, several studies have been investigating the possible relationship between vitamin D and ALS, its role ranging from biomarker to therapy.
ALS is a neurodegenerative disease characterized by the progressive loss of motor neurons that leads to paralysis and respiratory failure 3–5 years after the onset of symptoms (2). Only two drugs are approved for ALS treatment, with only moderate efficacy on delaying disease progression or increasing survival, despite the large number of new and old drugs evaluated in clinical trials (3, 4). These successive failures could be related to the fact that ALS is diagnosed 1 year after the onset of symptoms, a critical period for initiation of treatments that could prevent motor neuron degeneration. This delay is partly due to the absence of reliable biomarkers for the diagnosis of ALS, even if several candidates have been proposed (5–9). Regarding the role of vitamin D in ALS, studies reported very contradictory results, going from protective (10–14), to detrimental (15, 16).
To determine if there is scientific evidence supporting some role for vitamin D in ALS patients, we performed a systematic review of studies reporting data about any potential relationship between vitamin D and ALS. We aimed to investigate if vitamin D levels could be either used as a biomarker for ALS diagnosis or correlated to disease prognosis or progression and if vitamin D supplementation could improve clinical outcomes in ALS patients.
Methods
Search Strategy and Studies Selection
One reviewer (DL) searched PubMed, Embase, and Cochrane databases using the algorithm presented in Table 1, for studies published until September 2019. The search was limited to humans but without other limitations (for age or sex or language, for example). After screening on title, abstract, and types of studies, the full text of eligible studies published in English, Spanish, French, and Portuguese were retrieved for further analysis. Titles and abstracts of the retrieved studies were screened to select those that reported some relation between ALS and vitamin D; then, a second reviewer (JG) checked the titles and abstracts to confirm the selection. We considered studies that reported data about the role of vitamin D levels as a biomarker for ALS diagnosis, for vitamin D levels as a prognostic factor for disease or vitamin D supplementation effect on clinical outcomes. To address these relations, we considered studies that were clinical trials, cohort studies, or case–control studies and were reported in research articles or abstracts from specialized congresses. Although case reports and series of individual case reports were initially considered, we finally decided to exclude them because no solid conclusions could be drawn out of them either way. A full-text article was then examined to further confirm eligibility of studies. Any discrepancies were resolved after discussion with a third reviewer (TBA). The protocol was registered in the International Prospective Register of Systematic Reviews (preregister number: 154572) and is available on-demand to the corresponding author of this study. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist is included as supplementary material (17) (Supplementary File 2).
Table 1.
Combination of terms used in our search strategy for the PubMed database that was adapted for the other two databases.
| Vitamin D domain | Vitamin d/ or 25-OHD.mp. or 25 hydroxyvitamin D.mp. or cholecalciferol/or colecalciferol.mp. or hydroxycholecalciferols/or hydroxycolecalciferols.mp. or calcifediol/or dihydroxycholecalciferols/ or dihydroxycolecalciferols.mp. or calcitriol/ or 24,25-dihydroxyvitamin d 3/ or 24,25-OH2 D3.mp. or ergocalciferols/ or dihydrotachysterol/ or 25-hydroxyvitamin d 2/ or 25- OHD2.mp. or 1,25-dihydroxyvitamin d.mp. or 1,25-OH2 D.mp. or 1,25- dihydroxyvitamin d2.mp. or 1,25-dihydroxyergocalciferol.mp. or 1,25-OH2 D2.mp. or 1,25-dihydroxyvitamin d3.mp. or 1,25-OH2 D3.mp. or ergocalciferols/ or vitamin D2.mp. or vitamin D 2.mp. or vitamin D3.mp. or vitamin D 3.mp |
| AND | |
| ALS domain | Amyotrophic lateral sclerosis or ALS or motor neuron disease |
Quality Assessment (Risk of Bias)
We used different tools to analyze the risk of bias in the retrieved studies in accordance with the type of study analyzed. RoB 2 tool was used to evaluate a randomized clinical trial (RCT) (18), whereas ROBINS-I was used to evaluate the risk of bias in the non-randomized trials (19). For the studies analyzing the prevalence of vitamin D deficiency in ALS patients vs. control subjects, the risk of bias was assessed according to Hoy et al. (20). Finally, we conducted the Quality in Prognosis Studies (QUIPS) analysis to evaluate the risk of bias in studies that evaluated the prognostic role of vitamin D (21). The overall risk of bias was considered low if all domains were at low risk or with only one at a moderate level; high if at least one domain was at high risk or >3 classified as moderate; unknown if no judgment could be made; and moderate otherwise (22). Each domain described for each tool was classified according to the risk of bias by one researcher (DL) and revised by a second researcher (JG). TBA was consulted for a final decision when a disagreement on classifications occurred.
Data Extraction
For each study, the following data were collected: first author, year of publication, journal of publication, data regarding study design, data about study population characteristics, and data about vitamin D dosage or supplementation. To make a quantitative synthesis, the following data were collected: (1) numerical values for vitamin D levels for ALS patients and controls and any informed statistical index; (2) statistical index of the relationship between vitamin D levels and functional score and its confidence interval (CI) and/or the p-value; (3) statistical index of the relationship between vitamin D levels and patient survival (hazard ratio, HR) and its CI and/or the p-value; and (4) the statistical index of the correlation between vitamin D levels in patients receiving supplementation and the functional score. Data were extracted by one author (DL) and then checked for accuracy by another author (JG).
Endpoints and Comparisons
We considered three types of comparisons: (1) vitamin D levels in patients with ALS vs. patients without ALS/healthy controls; (2) the relation between vitamin D levels and either survival of patients or their functional disability as expressed by ALS Functional Rate Score—Revised (ALSFRS-R); (3) survival or ALSFRS in patients with and without vitamin D supplementation. Outcomes considered were ALS diagnosis, survival, and ALSFRS-R.
Statistical Analysis
For each study, we collected either aggregate, raw data or statistics used to describe the levels of vitamin D in ALS patients compared with those in control subjects or the relationship between vitamin D and prognosis (survival or ALSFRS). Whenever we could have sufficient information (at least three studies reporting similar aggregate data or statistics), we used an inverse variance method to pool the data. We chose to perform a random-effects model, as we anticipated high heterogeneity, as we had already shown in a previous systematic review and meta-analysis (9). We assessed heterogeneity by using Cochrane's Q test and I2; heterogeneity was considered significant when p < 0.10, as major if I2 > 70%, important if I2 > 50%, or moderate if I2 <50%. Publication bias was explored by funnel plot and asymmetry tests if 10 or more studies could be included. Meta-analyses were performed using RStudio (23) package meta (24).
Results
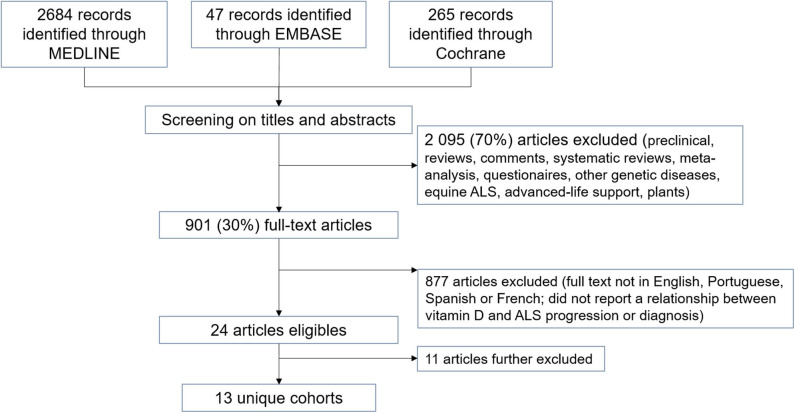
Our search strategy allowed us to retrieve 2,996 studies. After the screening of titles, abstracts, and types of study, we excluded 2.095 studies (70%). After full-text evaluation of the remaining studies, 24 studies reported some relationship between vitamin D levels or supplementation and ALS diagnosis or progression (Figure 1).
Figure 1.
Flow diagram of literature search results for our systematic review.
Articles Further Excluded
After evaluation, seven studies were further excluded from the analysis. Two were case reports: Moling et al. (25) reported a 39-year-old man with ALS and hepatosplenic schistosomiasis, high IL-17 levels, and low vitamin D (14 ng/ml); Shanmugarajah and Archer (26) reported a 69-year-old man bearing the C9orf72 expansion and normal vitamin D levels. Another study reported the mean vitamin D levels of three ALS patients in comparison with other neurological conditions, such as stroke and multiple sclerosis (27). Yanagihara et al. (28) analyzed vitamin D levels in the Chamorros population in the Guam territory. The other two studies evaluated nutritional intake as a source of vitamin D but did not report quantifiable results; moreover, although Gallo et al. (29) said that vitamin D intake was protective against ALS, Dawczynski et al. (30) reported no difference regarding vitamin D and disease progression. One retrieved paper (31) was later retracted (32) (due to “concerns about data integrity and scientific misconduct”) and, therefore, was not included in the analysis.
Besides, four of the retained abstracts and one paper were all published by the group of Camu and co-workers and presented results from the same cohort of ALS patients, so we only included the paper (13) and the more recent abstract (14). Another group published an abstract and a paper based on the same cohort (33), so we only included the paper.
Summary of Included Studies
Included studies reported (a) the levels of vitamin D in ALS patients compared with those in control subjects (4 studies: 163 ALS and 276 control patients); (b) correlations of levels of vitamin D with clinical markers of prognosis (as ALSFRS-S or survival; 9 studies: 986 ALS patients); or c) effect of supplementation with vitamin D on clinical parameters of progression (3 studies: 77 vitamin D-treated ALS patients, 41 non-treated ALS patients). Of the retained studies, six were retrospective (13, 16, 33–36) and seven prospective (14, 15, 37–41). Four studies were only available as abstracts (14, 34, 35, 38). Twelve were observational studies, and one was described as an RCT (41). No study reported the inclusion (or exclusion) of familial ALS cases, except Cortese et al. (38), who reported the inclusion of only sporadic cases in the evaluated cohort. The characteristics of ALS patients from the retained studies are presented in Table 2. Overall, studies sorted patients according to their levels of vitamin D, and in the majority of cases, ALS patients fell into the categories “insufficient” or “low” levels of vitamin D (Supplementary File 1).
Table 2.
Description of cohorts retained after the systematic review and included in the meta-analysis.
| Study | Number of patients | Age (years) | Age of onset (years) | Men (%) | Bulbar form (%) | ALSFRS | BMI (kg/m2) | Vitamin D levels (ng/ml) | Relation vitamin D and ALS |
|---|---|---|---|---|---|---|---|---|---|
| Gennings et al. (34) | 302 | 63.2 | 56.2 ± 11.8 | 59 | NR | 37 | 26 | NR | Prognosis |
| Cortese et al. (38) | 71 | NR | NR | NR | NR | NR | NR | 11.0 (4.0–38.0) | Diagnosis Prognosis |
| Bretón et al. (35) | 213 | 61.9 ± 13.3 | BO 67.4 ± 12 SO 59.4 ± 13.2 |
57 | 31.5 | NR | 25.6 ± 4.2 | <20 | Prognosis (BO x SO) |
| Trojsi et al. (41)** | 50.000: 10 75.000: 12 100.000: 11 |
57.6 (11.69) 62.17 (10.05) 54 (14) |
56.5 (11.75) 61.17 (10.49) 53.27 (14.1) |
20 58 81 |
NR | 37.6 (6.32) 35.67 (7.83) 38.45 (6.86) |
25.35 (2.63) 26.48 (3.29) 23.73 (2.92) |
16.7 (6.9) 14.3 (6.5) 14.9 (7.6) |
Therapy |
| Camu et al. (13) | 74 | 66.8* | 64.3 | 70 | 31 | 0.92 (ASS)*** | NR | 21.2 | Prognosis |
| Karam et al. (33) | 37 | 55 | vitD 47.2 (37.3–61) no vitD 60 (47.4–63.3) |
57 | NR | vitD 29 (16.5–39) no vitD 29 (22–38) | NR | 22.3 (13.5–32) | Therapy |
| Libonati et al. (36) | 57 | 63.0 ± 9.9 | NR | 82.4 | NR | NR | NR | 18.8 ± 12.1 ng/dl | Diagnosis Prognosis Therapy |
| Elf et al. (37) | 24 | 58 ± 12 | 57.6* | 58.3 | NR | 31 | 25.6 (18.1–43.6) | 23.6 ± 10.4 | Diagnosis Prognosis |
| Yang et al. (16) | 100 | 56.3* | 53.5 (45–63) | 59 | 31 | 40 (36–43) | 22.3 (20.5–24.7) | 13.7 ± 8.9 | Prognosis |
| Paganoni et al. (40) | 106 | 58.0 ± 9.8 | 56.2 ± 9.1* | 58.5 | 26.4 | 33.2 ± 8.4 | 27.2 ± 5.2 | NR | Prognosis |
| Blasco et al. (15) | 125 | 65 (63–67) | 62.4* | 50.4 | 34.2 | 34.4 (33.0–35.7) | 24.2 (23.5–24.8) | 20.3 (19.8–22.4) | Prognosis |
| Crick et al. (39) | 11 | 64.5 ± 10.8 | NR | 63.6 | NR | NR | NR | 5.2 (6.37) | Diagnosis |
| Pageot et al. (14) | 127 | NR | NR | NR | NR | NR | NR | NR | Prognosis |
| % Data | 100 | 69.2 | 46.1 | 84.6 | 38.5 | 61.5 | 53.8 | 76.9 | |
| Average | 98.5 | 55.7 ± 8.5 | 57.9 ± 10.7 | 59.6 | 30.8 | 31.4 ± 5.9 | 25.2 ± 4.3 | 14.5 ± 8.8 |
Data reported as mean ± S.D, except for Gennings, Karam, Yang, and Blasco who presented data as median.
NR, not reported; BO, bulbar onset; SO, spinal onset.
As estimated from the age of the cohort and disease duration or from age at diagnosis and disease duration.
Vitamin D supplementation groups (IU/month).
ASS: ALSFRS severity score (mean points lost per month).
Prevalence of Vitamin D Deficiency in Amyotrophic Lateral Sclerosis and Control Individuals
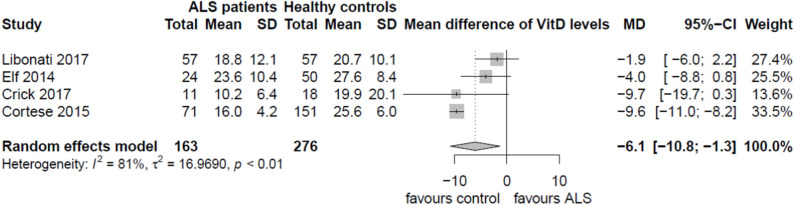
Four observational studies reported levels of vitamin D in ALS patients and in control subjects (healthy controls in three studies and other neurological conditions in the fourth) (36–39). Overall, only Cortese et al. (38) reported a significant difference in vitamin D levels between ALS patients and controls. The data available from these four studies (Table 3) were pooled to estimate the mean vitamin D difference between ALS and controls (Figure 2).
Table 3.
Median (range) of vitamin D levels in ALS and control cases.
| Study | Number of patients Type of controls | Vit D levels in ALS patients | Vit D levels in controls | p value |
|---|---|---|---|---|
| Cortese et al. (38) | 71 ALS 151 healthy controls |
11 ng/ml | 22.3 ng/ml (5–52.8) | <0.0001 |
| Libonati et al. (36) | 57 ALS 57 healthy controls |
18.8 ± 12.1 ng/dl |
20.7 ± 10.1 ng/dl |
0.369 |
| Elf et al. (37) | 24 MND 50 healthy controls |
23.64 ± 10.4 ng/ml | 27.64 ± 8.41 ng/ml | 0.15 |
| Crick et al. (39) | 11 ALS 18 controls* |
10.15 ± 6.37 ng/ml | 19.94 ± 20.08 ng/ml | ns |
Neurological symptoms but no diagnosis at time of sampling.
ns, non-significant; MND, motor neuron disease.
Figure 2.
Vitamin D levels in ALS and controls.
Overall, ALS patients presented lower levels of vitamin D compared with the normal range (>30 ng/ml; 41). In a random-effects model, vitamin D levels in ALS patients were slightly lower than those in control subjects by a mean difference of −6.1 ng/ml; 95% CI (−10.8; −1.3) but with major heterogeneity (I2 = 81%; p < 0.01) introduced mainly by the study of Cortese et al. (38). Heterogeneity could not be explained by any particular characteristic of the study of Cortese and co-workers—apart from its presentation as only an abstract. However, the analysis of bias from retrieved studies raised concerns, as two studies (36, 38) displayed a moderate risk of bias, one study was rated with high risk of bias (39), and only one study (37) presented low risk of bias (Supplementary File 3).
Vitamin D Levels and Amyotrophic Lateral Sclerosis Prognosis
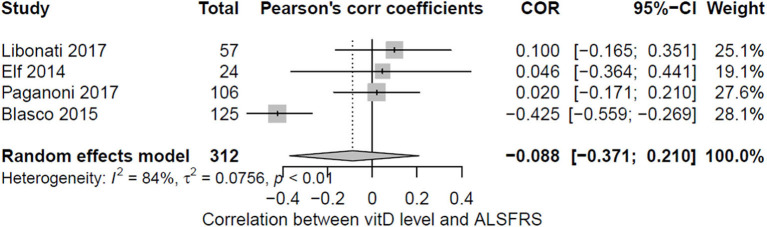
Nine observational studies correlated the levels of vitamin D with a clinical marker used for the evaluation of ALS prognosis (as ALSFRS-R, forced vital capacity, or survival) (13–16, 34, 36–38, 40). Out of these studies, only four (44%) reported sufficient information about the correlation (Pearson's coefficient) between vitamin D levels and ALSFRS and could be pooled (15, 36, 37, 40) (Figure 3). Two other studies reported statistical data about the correlation of ALSFRS and vitamin D levels, but for different reasons, they could not be included in the meta-analysis: Camu et al. (13) reported a correlation between vitamin D levels and a severity score (calculated as the points of ALSFRS-R lost per month), and Cortese et al. performed a Spearman correlation. Interestingly, both studies reported contradictory results: Camu et al. showed a negative correlation, meaning the lower the levels of vitamin D, the higher the severity score; meanwhile, Cortese et al. reported a positive one, meaning the higher the vitamin D levels, the higher the severity score. Overall, no significant correlation between vitamin D and motor dysfunction measured by ALSFRS-R was apparent when pooling the data (−0.088; 95% CI: −0.371; 0.210), with major heterogeneity being found (I2 = 84%; p < 0.01). This discordance in results was also visible when pooling the four studies, with no correlation found in the three studies, whereas one found a significant but negative correlation.
Figure 3.
Pooled correlation between vitamin D levels and ALSFRS.
Three studies performed correlations between vitamin D levels and survival, but due to high heterogeneity in reported data, it was not possible to pool them together (Table 4). Studies reported again very conflicting results: whereas Camu et al. reported a positive association between vitamin D levels and survival, Blasco et al. reported a negative effect of vitamin D on survival. On the other hand, Yang reported no significant association between vitamin D levels and survival. The overall quality of studies evaluating the prognostic role of vitamin D (QUIPS tool) was judged to be at high risk of bias, mostly because of the lack of consideration of confounding factors (Table 5). Moreover, three reports of a link between vitamin D levels and prognosis in ALS patients were published as abstracts only (14, 34, 38).
Table 4.
Correlation of vitamin D levels and survival.
| Study | VitD Categories | N | KM log-rank test | Univariate | Multivariate* |
|---|---|---|---|---|---|
| Camu et al. (13) | <25 vs. >75 nmol/l | 74 | p = 0.002 | Not performed | 5.9 (1.4–24.3); p = 0.01 |
| Blasco et al. (15) | Per nmol/l Per categories of BMI | 125 | BMI < median: P = 0.014 BMI > median: P = 0.022 |
6.28 (1.88–19.42); p = 0.003 | BMI < median: 6.6 (1.03–35.8); p = 0.047 BMI > median: 6.5 (1.56–28.18); p=0.0098 |
| Yang et al. (16) | <10 vs. >10 ng/ml | 100 | P = 0.204 | 0.63 (0.31–1.31); p = 0.221 | 1.09 (0.466–2.547); p = 0.842 |
Table 5.
Risk of bias assessment using the Quality in Prognosis Studies (QUIPS) tool for studies reporting vitamin D as prognostic in ALS.
| Study | Participation | Attrition | Prognostic factor measurement | Outcome measurement | Confusion factors | Statistical analysis | Overall risk of bias |
|---|---|---|---|---|---|---|---|
| Elf et al. (37) | Moderate | Low | Moderate | Low | High | High | High |
| Libonati et al. (36) | High | Low | Moderate | Moderate | High | Moderate | High |
| Camu et al. (13) | Moderate | Low | Moderate | Moderate | High | Moderate | High |
| Blasco et al. (15) | Moderate | Low | Moderate | Moderate | Moderate | Moderate | High |
| Yang et al. (16) | Moderate | Low | Moderate | Low | Moderate | Moderate | High |
| Paganoni et al. (40) | Low | Low | Moderate | Moderate | Low | Moderate | Moderate |
| Pageot et al. (14) | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| Gennings et al. (34) | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| Cortese et al. (38) | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
Vitamin D in Bulbar-Onset and Spinal-Onset Amyotrophic Lateral Sclerosis Patients
Some studies evaluated vitamin D levels in spinal-onset and bulbar-onset ALS. Bretón et al. (35) reported vitamin D deficiency (<20 ng/ml) in 61% of spinal-onset patients (89 from 146) and 37% of bulbar-onset (25 from 67), with no statistical differences between both groups. Libonati et al. (36) also did not find any differences in vitamin D levels between spinal- or bulbar-onset ALS (p = 0.9). On the other hand, Cortese et al. (38) reported a statistical difference in vitamin D levels between spinal- and bulbar-onset patients (12.45 vs. 18.50 ng/ml, respectively; p = 0.01), and only in spinal-onset patients' vitamin D was positively correlated with ALSFRS (rs = 0.37, p = 0.004) and negatively with disease duration (rs = −0.33, p = 0.013). Blasco et al. (15) also reported a significant higher level of vitamin D in bulbar-onset than that in spinal-onset ALS patients (spinal: 19.7 [17.6–21.6]; bulbar: 25 [22.2–27.8]; p = 0.003).
Supplementation With Vitamin D and Amyotrophic Lateral Sclerosis Progression
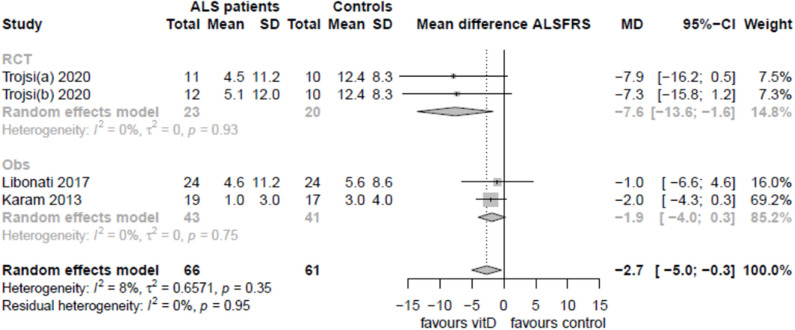
Five studies reported information regarding the effect of vitamin D supplementation on ALS progression (15, 33, 36, 40, 41), but only three (60%) reported data that could be pooled: one RCT (41) and two observational, retrospective studies (33, 36) (Figure 4). After 6 months of treatment, no statistical differences were observed regarding the ALSFRS-R progression rate, although serum 25(OH)D levels were increased in treated groups. Whereas, Libonati et al. and Karam et al. compared treated with untreated patients, Trojsi et al. compared groups of patients treated with three different doses of vitamin D. There was no significant effect of vitamin D supplementation in observational studies (mean difference −1.9 [95% CI: −4.0; 0.3]), whereas a higher dose of vitamin D in the only randomized trial slightly improved ALSFRS (mean difference −2.7 [95% CI: −5.0, −0.3]). Analysis of risk of bias for these studies suggests that these results should be considered carefully. A critical risk of bias was found for Libonati et al., serious concerns for Karam et al. (ROBINS-I tool), and some concerns for Trojsi et al. (RoB, 2v tool; Supplementary File 4). An important source of bias in these studies is that ALS patients with vitamin D deficiency were supplemented and then compared with normal vitamin D, non-supplemented ALS patients. Only Trojsi et al. randomized different deficiency levels into all groups of treatment but with non-untreated ALS control, and therefore, results should be considered very carefully.
Figure 4.
Pooled correlation between vitamin D supplementation and ALSFRS.
Importantly, some authors reported deleterious effects of vitamin D supplementation. Paganoni et al. (40) (Caucasian sample, season-averaged vitamin D levels) reported that vitamin D supplementation was associated with a faster decline in the ALSFRS-R by 0.5 points/month (95% CI −0.9 to −0.1 points/month, p = 0.018), even after adjusting for covariates. On the other hand, Blasco et al. (15) reported that ALS patients receiving supplementation presented a significantly lower ALSFRS-R (p = 0.007).
Discussion
In this systematic review, we analyzed all studies reporting any relationship between vitamin D and ALS. Thirteen different cohorts totaling 1,280 ALS patients were reported in 12 observational studies and one RCT. We observed a small tendency toward a 6-ng/ml lower vitamin D level in ALS patients compared mainly with that in healthy controls, but data were not adjusted for mean vitamin D level of the general population, and pooled data were highly heterogeneous. The results regarding the relationship between vitamin D levels and ALSFRS-R could be pooled only for 44% of retrieved studies and were highly heterogeneous and inconclusive; similarly, discordant results were reported in three studies regarding survival. Therefore, we do not consider that a vitamin D level could be a biomarker for disease diagnosis or disease severity. Finally, supplementation of ALS patients with vitamin D had no beneficial effect on ALSFRS-R in two observational studies (subject to important confounding either taken into account or residual) and deleterious effects when compared with that in non-treated ALS patients in two other observational studies. Furthermore, one clinical trial reported no statistical benefit of very high doses compared with normal supplementation—but should be very carefully considered because of insufficiency in design and very small sample size.
Heterogeneous results regarding the prognostic and therapeutic role of vitamin D in ALS patients could reflect the diverse potential mechanisms of action of vitamin D in in vitro/in vivo studies. Its role in preventing ALS progression in in vitro studies was demonstrated by different research groups (10, 42). However, in in vivo studies, different results were reported. Although vitamin D supplementation seems to improve motor function in a well-known ALS mouse model (SOD1-G93A) (43), and vitamin D deficiency can exacerbate disease pathology (44), it can also promote deleterious effects in motor function and disease severity (45, 46).
Only two studies reported positive effects of vitamin D in ALS patients. Camu et al. reported that patients with severe deficiency of vitamin D have higher severity scores (ALSFRS units lost per month), and patients with normal levels of vitamin D have increased survival. Karam et al. investigated the possible effects of vitamin D supplementation, and although no beneficial effect was reported at month 6 after starting supplementation, they found a positive effect of vitamin D on ALSFRS only after 9 months of supplementation. From the three retained studies, which investigated the effect of vitamin D supplementation in improving motor symptoms, the other two (36, 41) established the final outcome at month 6.
Several important points need to be considered when analyzing vitamin D levels in patients with neurodegenerative diseases, as these patients may suffer from low vitamin D because they lack sunlight exposure and physical activity (especially the ones with more restricted movement, as it is the case for ALS patients) (41). In the study of Camu et al. (13), however, the authors excluded patients who could not walk without assistance, as this handicap could induce a lower sun exposure and, thus, lower vitamin D levels. Interestingly, this study found a positive correlation between vitamin D and survival, whereas low vitamin D was correlated with faster disease progression measured by the ALSFRS severity score (13). However, the Camu et al. study is not representative of all French ALS patients, as another study from Blasco et al. (15) showed no protective effect of vitamin D in another cohort of French ALS patients.
In a Swedish cohort (37), the motor neuron disease group presented a tendency of higher vitamin D levels in the summer, although not significant (p = 0.057). This study included seasoned-averaged vitamin D levels, as did Paganoni et al. (40)—both studies did not find positive correlations between vitamin D levels and ALSFRS-R score. Variations in the levels of vitamin D regarding different seasons should be considered, especially in populations where daytime sunlight changes substantially between seasons. Another consideration is that the vitamin D levels considered as “normal” can change from country to country and in the function of ethnicity. This variability is taken into account in the study of Yang et al. (16), who reported the mean of vitamin D levels for the Korean population as being 19.6 ± 6.9 ng/ml (47)—a number considered low in comparison with that in other countries and ethnicities (48). Furthermore, a systematic review reported the lack of available worldwide data for vitamin D levels in the different populations, and when data were available, they report vitamin D deficiency as a global public health problem in all age groups (49).
Other important factors of variability in the levels of vitamin D, such as bone density, calcium metabolism, and kidney function, were not considered in the majority of analyzed studies. Renal function was assessed in the study of Cortese et al. who stated that in both control and ALS patients, kidney function was normal. In addition, Yang et al. investigated bone mineral density and calcium metabolism in ALS patients, and although they reported very low levels of vitamin D in the ALS cohort analyzed, vitamin D was not a predictor of survival.
As low levels of vitamin D seems to be a global public health problem regardless of age, vitamin D supplementation should be combined with increased sun exposure with the purpose of increasing overall health and also to avoid bone fractures in the elderly but not with the aim to treat the symptoms of neurodegenerative diseases.
Conclusion
Our analyses suggest that there is a tendency for ALS patients to have lower levels of vitamin D than controls, but most of the studies did not adjust for the mean vitamin D of the country population and failed to report important factors of variability for vitamin D levels (for example, lack of sun exposure). When studies presented enough data to be analyzed, our meta-analyses did not find strong evidence for a relationship between vitamin D levels and prognosis, nor did vitamin D supplementation induce an improvement in motor function. Our study does not support any particular positive link between vitamin D and ALS. Furthermore, the risk of bias analysis raised some concerns about the quality of these studies. ALS patients should receive vitamin D supplementation for other health issues related to vitamin D deficiency, but there is not enough evidence to support vitamin D as a therapy for ALS.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
DL, TB-A, and HB contributed to the conception and design of the study. DL and JG organized the database. DL and TB-A performed the statistical analysis. DL wrote the first draft of the manuscript. TB-A, JG, HB, and PC wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Mrs. Catherine Weill from the Bibliothèque Interuniversitaire de Santé, Paris Descartes University, for her help with the Embase search strategy.
Footnotes
Funding. This work was supported by the program ARD2020 Biomédicaments and the French Ministry of Higher Education and Research as part of the Investissements d'Avenir program: LabEx MAbImprove ANR-10-LABX-53-01.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00697/full#supplementary-material
References
- 1.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. (2014) 348:g2035. 10.1136/bmj.g2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primers. (2017) 3:17085 10.1038/nrdp.2017.71 [DOI] [PubMed] [Google Scholar]
- 3.Hergesheimer R, Lanznaster D, Vourc'h P, Andres CR, Bakkouche SE, Beltran S, et al. Advances in disease-modifying pharmacotherapies for the treatment of amyotrophic lateral sclerosis. Exp Opin Pharmachother. (2020) 21:1103–10. 10.1080/14656566.2020.1746270 [DOI] [PubMed] [Google Scholar]
- 4.Wobst HJ, Mack KL, Brown DG, Brandon NJ, Shorter J. The clinical trial landscape in amyotrophic lateral sclerosis-Past, present, and future. Med Res Rev. (2020) 40. 10.1002/med.21661. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasco H, Nadal-Desbarats L, Pradat PF, Gordon PH, Madji Hounoum B, Patin F, et al. Biomarkers in amyotrophic lateral sclerosis: combining metabolomic and clinical parameters to define disease progression. Eur J Neurol. (2016) 23:346–53. 10.1111/ene.12851 [DOI] [PubMed] [Google Scholar]
- 6.Blasco H, Patin F, Madji Hounoum B, Gordon PH, Vourc'h P, Andres CR, et al. Metabolomics in amyotrophic lateral sclerosis: how far can it take us? Eur J Neurol. (2016) 23:447–54. 10.1111/ene.12956 [DOI] [PubMed] [Google Scholar]
- 7.Blasco H, Vourc'h P, Pradat PF, Gordon PH, Andres CR, Corcia P. Further development of biomarkers in amyotrophic lateral sclerosis. Exp Rev Mol Diagn. (2016) 16:853–68. 10.1080/14737159.2016.1199277 [DOI] [PubMed] [Google Scholar]
- 8.Lanznaster D, de Assis DR, Corcia P, Pradat PF, Blasco H. Metabolomics biomarkers: a strategy toward therapeutics improvement in ALS. Front Neurol. (2018) 9:1126. 10.3389/fneur.2018.01126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanznaster D, Bejan-Angoulvant T, Patin F, Andres CR, Vourc'h P, Corcia P, et al. Plasma creatinine and amyotrophic lateral sclerosis prognosis: a systematic review and meta-analysis. Amyotroph Lateral Scler Frontotemporal Degener. (2019) 20:199–206. 10.1080/21678421.2019.1572192 [DOI] [PubMed] [Google Scholar]
- 10.Karam C, Scelsa SN. Can vitamin D delay the progression of ALS? Med Hypotheses. (2011) 76:643–5. 10.1016/j.mehy.2011.01.021 [DOI] [PubMed] [Google Scholar]
- 11.Pageot N, Juntas-Morales R, Raoul C, Tremblier B, Scamps F, Vincent T, et al. Severe vitamin D deficiency correlates with worse ALS prognosis. Amyotrophic Lateral Scler Front Degeneration. (2012) 13:35. [Google Scholar]
- 12.Camu W, Tremblier B, Plassot C, Alphandery S, Salsac C, Pageot N, et al. Vitamin d is a prognostic factor of amyotrophic lateral sclerosis and confers protection to motoneurons in vitro. Neurology. (2014) 82(Suppl 10). [DOI] [PubMed] [Google Scholar]
- 13.Camu W, Tremblier B, Plassot C, Alphandery S, Salsac C, Pageot N, et al. Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis. Neurobiol Aging. (2014) 35:1198–205. 10.1016/j.neurobiolaging.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 14.Pageot N, Juntas-Morales R, Marin G, Alphandery S, Picot MC, Camu W. Blood vitamin D levels correlate with amyotrophic lateral sclerosis severity: a prospective study. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration. (2017) 18:65. [Google Scholar]
- 15.Blasco H, Madji Hounoum B, Dufour-Rainfray D, Patin F, Maillot F, Beltran S, et al. Vitamin D is Not a Protective Factor in ALS. CNS Neurosci Ther. (2015) 21:651–6. 10.1111/cns.12423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Park JS, Oh KW, Oh SI, Park HM, Kim SH. Vitamin D levels are not predictors of survival in a clinic population of patients with ALS. J Neurol Sci. (2016) 367:83–8. 10.1016/j.jns.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. (2009) 62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 18.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 19.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. (2012) 65:934–9. 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 21.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 22.Grooten WJA, Tseli E, Ang BO, Boersma K, Stalnacke BM, Gerdle B, et al. Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using QUIPS-aspects of interrater agreement. Diagn Progn Res. (2019) 3:5. 10.1186/s41512-019-0050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.RStudio T. RStudio: Integrated Development for R. Boston, MA: RStudio, Inc; (2019). [Google Scholar]
- 24.Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moling O, Di Summa A, Capone L, Stuefer J, Piccin A, Porzia A, et al. Increased IL-17, a pathogenic link between hepatosplenic schistosomiasis and amyotrophic lateral sclerosis: a hypothesis. Case Reports Immunol. (2014) 2014:804761. 10.1155/2014/804761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanmugarajah PD, Archer JOS. A case of hexanucleotide repeat expansion causing amyotrophic lateral sclerosis. J Neuromuscular Dis. (2018) 5:S364. [Google Scholar]
- 27.Gutierrez M, Rodriguez GE, Garcea D, Rey R, Lepera S, Monica E, et al. Relationship between bone mass and muscle weakness in chronic neurological diseases. Neurology. (2014) 82:10. [Google Scholar]
- 28.Yanagihara R, Garruto RM, Gajdusek DC, Tomita A, Uchikawa T, Konagaya Y, et al. Calcium and vitamin D metabolism in Guamanian Chamorros with amyotrophic lateral sclerosis and parkinsonism-dementia. Ann Neurol. (1984) 15:42–8. 10.1002/ana.410150108 [DOI] [PubMed] [Google Scholar]
- 29.Gallo V, Vineis P, Riboli E, Middleton L. The large-scale European prospective investigation into cancer and nutrition. (EPIC) unravels risk factors for ALS. Eur J Neurol. (2009) 16:55–334. [Google Scholar]
- 30.Dawczynski C, Ringer TM, Prell T, Stubendorff B, Witte OW, Lorkowski S, et al. Evaluating the potential of diet and food components as disease modifiers in amyotrophic lateral sclerosis. (ALS). Amyotrophic Lateral Scler Front Degeneration. (2016) 288. [Google Scholar]
- 31.Sato Y, Honda Y, Asoh T, Kikuyama M, Oizumi K. Hypovitaminosis D and decreased bone mineral density in amyotrophic lateral sclerosis. Eur Neurol. (1997) 37:225–9. 10.1159/000117447 [DOI] [PubMed] [Google Scholar]
- 32.Retraction statement. Eur Neurol. (2018) 79:229 10.1159/000488922 [DOI] [PubMed] [Google Scholar]
- 33.Karam C, Barrett MJ, Imperato T, MacGowan DJ, Scelsa S. Vitamin D deficiency and its supplementation in patients with amyotrophic lateral sclerosis. J Clin Neurosci. (2013) 20:1550–3. 10.1016/j.jocn.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 34.Gennings C, Nieves J, Factor-Litvak P, Hupf J, Singleton J, Sharf V, et al. Nutrition and functional assessment in ALS patients. Amyotrophic Lateral Scler Front Degeneration. (2014) 15:47. [Google Scholar]
- 35.Bretón I, Gutiérrez G, Velasco C, Cuerda C, Arhip L, Maíz M, et al. Nutritional and micronutrient status in bulbar and spinal onsetamyotrophic lateral sclerosis patients. Clinical Nutrition. (2016) 35:S84 10.1016/S0261-5614(16)30450-2 [DOI] [Google Scholar]
- 36.Libonati L, Onesti E, Gori MC, Ceccanti M, Cambieri C, Fabbri A, et al. Vitamin D in amyotrophic lateral sclerosis. Funct Neurol. (2017) 32:35–40. 10.11138/FNeur/2017.32.1.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elf K, Askmark H, Nygren I, Punga AR. Vitamin D deficiency in patients with primary immune-mediated peripheral neuropathies. J Neurol Sci. (2014) 345:184–8. 10.1016/j.jns.2014.07.040 [DOI] [PubMed] [Google Scholar]
- 38.Cortese R, D'Errico E, Introna A, Schirosi G, Scarafino A, Distaso E, et al. Vitamin D levels in serum of amyotrophic lateral sclerosis patients. Neurology. (2015) 84(Suppl 14). [Google Scholar]
- 39.Crick PJ, Griffiths WJ, Zhang J, Beibel M, Abdel-Khalik J, Kuhle J, et al. Reduced plasma levels of 25-hydroxycholesterol and increased cerebrospinal fluid levels of bile acid precursors in multiple sclerosis patients. Mol Neurobiol. (2017) 54:8009–20. 10.1007/s12035-016-0281-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paganoni S, Macklin EA, Karam C, Yu H, Gonterman F, Fetterman KA, et al. Vitamin D levels are associated with gross motor function in amyotrophic lateral sclerosis. Muscle Nerve. (2017) 56:726–31. 10.1002/mus.25555 [DOI] [PubMed] [Google Scholar]
- 41.Trojsi F, Siciliano M, Passaniti C, Bisecco A, Russo A, Lavorgna L, et al. Vitamin D supplementation has no effects on progression of motor dysfunction in amyotrophic lateral sclerosis. (ALS). Eur J Clin Nutr. (2020) 74:167–75. 10.1038/s41430-019-0448-3 [DOI] [PubMed] [Google Scholar]
- 42.Long K, Nguyen LT. Roles of vitamin D in amyotrophic lateral sclerosis: possible genetic and cellular signaling mechanisms. Mol Brain. (2013) 6:16. 10.1186/1756-6606-6-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gianforcaro A, Hamadeh MJ. Dietary vitamin D3 supplementation at 10x the adequate intake improves functional capacity in the G93A transgenic mouse model of ALS, a pilot study. CNS Neurosci Ther. (2012) 18:547–57. 10.1111/j.1755-5949.2012.00316.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moghimi E, Solomon JA, Gianforcaro A, Hamadeh MJ. Dietary vitamin D3 restriction exacerbates disease pathophysiology in the spinal cord of the G93A mouse model of amyotrophic lateral sclerosis. PLoS ONE. (2015) 10:e0126355. 10.1371/journal.pone.0126355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solomon JA, Gianforcaro A, Hamadeh MJ. Vitamin D3 deficiency differentially affects functional and disease outcomes in the G93A mouse model of amyotrophic lateral sclerosis. PLoS One. (2011) 6:e29354. 10.1371/journal.pone.0029354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gianforcaro A, Solomon JA, Hamadeh MJ. Vitamin D(3) at 50x AI attenuates the decline in paw grip endurance, but not disease outcomes, in the G93A mouse model of ALS, and is toxic in females. PLoS ONE. (2013) 8:e30243. 10.1371/journal.pone.0030243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang YC, Ahn HY, Jeong IK, Ahn KJ, Chung HY. Optimal serum concentration of 25-hydroxyvitamin D for bone health in older Korean adults. Calcif Tissue Int. (2013) 92:68–74. 10.1007/s00223-012-9669-3 [DOI] [PubMed] [Google Scholar]
- 48.Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. (2011) 14:938–9. 10.1017/S1368980011000565 [DOI] [PubMed] [Google Scholar]
- 49.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. (2013) 144 Pt A:138–45. 10.1016/j.jsbmb.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.