Abstract
Osteosarcoma is the most common primary malignant bone tumor among children and young people and is associated with poor prognosis. Punicalagin is an antioxidant ellagitannin found in pomegranate juice with known antiproliferation and anti-angiogenesis properties. However, the antitumor effect of punicalagin on osteosarcoma requires further investigation. In the present study, the inhibitory effect of punicalagin on proliferation and invasion was evaluated in one human osteoblast cell line (hFOB1.19) and three human osteosarcoma cell lines (U2OS, MG63 and SaOS2). The cancer cell apoptosis ratio was determined using flow cytometry. NF-κB signaling in these cells was also evaluated using western blotting analysis. A subcutaneous tumor xenograft model was initiated to study the efficacy of punicalagin on osteosarcoma development and angiogenesis in vivo. Punicalagin treatment significantly decreased osteosarcoma cell proliferation and increased apoptosis. In addition, the invasion potential of these cells in a transwell assay was also dramatically suppressed in osteosarcoma cells. Punicalagin not only induced the degradation of IκBα but also the nuclear translocation of p65, suggesting the attenuation of NF-κB signaling pathway following treatment. Moreover, punicalagin markedly downregulated interleukin (IL)-6 and IL-8 levels, which was consistent with the inhibition of NF-κB signaling. An NF-κB activator could reverse these effects. Using a tumor xenograft mouse model, it was demonstrated that punicalagin exposure inhibited osteosarcoma growth and angiogenesis in vivo. These observations confirmed the suppressive effect of punicalagin against osteosarcoma malignancies. The underlying molecular mechanisms may include inhibition of the NF-κB signaling pathway.
Keywords: osteosarcomas, punicalagin, NF-κB pathway, malignancy
Introduction
Osteosarcoma accounts <1% of malignancies overall, with an incidence of ~5 cases per million in individuals <19 years of age in the USA. However, it is the most widely diagnosed primary malignant bone tumor, particularly among children and young people. Males are prone to the onset of osteosarcoma, and the ratio of male to female incidence is ~3:2 (1). Osteosarcoma is thought to arise from mesenchymal primitive bone-forming cells, and is characterized by the sustainable production of malignant osteogenesis. In addition, the production of pro-angiogenic factors in the malignant development of osteosarcoma has also been suggested by a previous study, which concluded that osteosarcoma had a strong tendency to metastasize early and was associated with poor prognosis (2). Thus, the degree of osteosarcoma malignancy is extremely high, and increased tumor invasiveness and vascularity are associated with metastatic potential and poor prognosis.
The main current treatment strategy for patients with newly diagnosed osteosarcoma includes neoadjuvant chemotherapy, followed by surgical removal of the primary tumor and all metastatic lesions with clinical manifestations, as well as postoperative adjuvant chemotherapy (3). The three-drug chemotherapy regimen of cisplatin, doxorubicin and methotrexate constitutes the primary option for backbone treatment, and the overall 5-year survival rate in America for osteosarcoma has increased to 60–70% in patients receiving the three-drug regimen (4). Recently, biologic agents, such as muramyl tripeptide and IFN-α-2b, and additional cytotoxic chemotherapy, such as ifosfamide, have been introduced into clinical trials. However, these have failed to significantly improve the survival of young patients with osteosarcoma (5,6). Therefore, an improved understanding of the underlying mechanisms of tumor progression and angiogenesis in osteosarcoma is required in order to identify and develop more effective therapies.
Dysregulation in nuclear factor-κB (NF-κB) signaling is associated with excessive cellular proliferation and developmental signals during tumorigenesis. Indeed, this pathway has been reported to be involved in inflammatory proliferation and differentiation of osteosarcoma cells (7). NF-κB can also regulate the generation of proinflammatory and proangiogenic cytokines around cancer cells (8). Thus, it was previously suggested that NF-κB could serve a causative role in osteosarcoma progression (9). Punicalagin is an antioxidant ellagitannin found in pomegranate juice with known anti-proliferation or anti-angiogenesis properties against many cancer cell lines, including leukemia, glioma and prostate cancer cells (10). The present study aimed to examine the detailed function of the NF-κB pathway in osteosarcoma, and to determine whether punicalagin can inhibit the NF-κB pathway to suppress inflammation and osteosarcoma tumorigenicity. Combined treatment targeting the NF-κB pathway may represent a novel and promising strategy to significantly enhance the therapeutic activity of routine anticancer drugs against osteosarcoma.
Materials and methods
Reagents and cell lines
A 50 mM stock solution consisting of 5 mg punicalagin (Sigma-Aldrich; Merck KGaA) in 1 ml DMSO were prepared. The stock was diluted to the desired concentrations with culture medium to give a water-soluble fraction, in which DMSO concentration did not exceed 0.2% in the highest punicalagin concentrations applied. The three human osteosarcoma cell lines (U2OS, MG63 and SaOS2) and one normal osteoblast cell line (hFOB1.19) were purchased from American Type Culture Collection and cultured according to the instructions. All of the cell lines were grown in Dulbecco's modified Eagle medium supplemented with 10% FBS (both from Invitrogen; Thermo Fisher Scientific, Inc.) in a humidified atmosphere with 5% CO2 at 37°C. Following treatment, culture medium was prepared serum-free and collected in 24-h cultures. Phorbol myristate acetate (PMA; Sigma-Aldrich; Merck KGaA), an activator of the NF-κB pathway, was added to cells together with punicalagin and incubated for 45 min in in a humidified atmosphere with 5% CO2 at 37°C.
Cell proliferation assay
Cell proliferation was examined using Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.). Cells were seeded at a density of 1×103 cells per well in 96-well plates, then treated with different concentrations of punicalagin or DMSO as control. Following 1–3 days of culture, 10 µl CCK-8 reagent was added to each well and cells were cultured for 1 h. A microplate reader (Bio-Rad Laboratories, Inc.) was used to measure the absorbance at 450 nm.
Cell invasion assay
Transwell migration assays were performed with 8.0-µm pore polycarbonate filter inserts (Corning, Inc.) coated with Matrigel™ (BD Biosciences) at room temperature for 1 h before use. Briefly, osteosarcoma cells in 100 µM punicalagin contained or vehicle medium supplemented with 1% FBS were placed in the top chamber at a density of 1×104 cells/well. In the bottom chamber, complete medium with 10% FBS was used as a positive control. After 48 h of incubation, the migrated cells were fixed with 4% paraformaldehyde for 20 min and stained with 1% crystal violet for 10 min at room temperature. Images were captured with a light microscope at ×400 magnification and the migrated cells were counted manually and averaged from 5 high-power fields.
Cell apoptosis analysis
To investigate early and late apoptotic cells, annexin V-FITC and propidium iodide (PI) double staining was performed. The APOAF annexin V apoptosis kit (Sigma-Aldrich; Merck KGaA) was used for annexin V staining, according to the manufacturer's protocol. All samples were quantified using a Canto II flow cytometer (BD Biosciences) and analyzed with FlowJo version 7.6 software (TreeStar). Early apoptotic cells were defined as FITChighPIlow cells and late apoptotic cells were defined as FITChighPIhigh. Additionally, FITClowPIlow represented healthy cells and FITClowPIhigh accounted for cells debris that was eliminated.
Western blotting assay
The cells or tissues were homogenized in RIPA buffer (Beyotime Institute of Biotechnology). A BCA protein assay kit (Thermo Fisher Scientific, Inc.) was used to determine the protein concentration in lysates and conditioned medium. Equal amounts of protein (15 µg) were loaded per lane and separated via SDS-PAGE (10% gel), then transferred to a PVDF membrane (Bio-Rad Laboratories, Inc.). The PVDF membrane was blocked with 5% skimmed milk in TBS + 0.1% Tween®−20 buffer on a shaker for 1 h at room temperature. The membrane was then incubated in 4°C with the following primary antibodies overnight: Anti-phosphorylated (phosphor)-inhibitor of κBα (IκBα; Ser32; cat. no. 2859), anti-IκBα (cat. no. 9242), anti-phospho-mammalian target of rapamycin (mTOR; Ser2448, cat. no. 2971), anti-mTOR (cat. no. 2983), anti-p65 (cat. no. 8242), anti-histone 2A family member X (H2AX, cat. no. 7631) (all 1:1,000; all from Cell Signaling Technology Biological Reagents Co., Ltd.), anti-β-actin (cat. no. sc-130656; 1:2,000), anti-interleukin (IL)-6 (cat. no. sc-130326; 1:500), and anti-IL-8 (cat. no. sc-8427; 1:500; all purchased from Santa Cruz Biotechnology, Inc.). After washing, the membrane was incubated with horseradish peroxide-conjugated secondary antibody (cat. no 7074; 1:1,000 Cell Signaling Technology Biological Reagents Co., Ltd.) for 1 h on the shaker at room temperature. The membrane was then incubated with chemiluminescence reagent (GE Healthcare Life) for 5 min at room temperature. The relative quantity of the protein was measured using ImageJ software v1.51 (National Institutes of Health).
Tumor xenografts
The in vivo experiment protocol was approved by the Institutional Animal Care and Use Committee at The Second Affiliated Hospital of Air Force Medical University and followed the Chinese national standards: Laboratory animal welfare ethics review guidelines for the humane and customary care and use of experimental animals. A total of 20 female 6–8-week-old, 18 g, Balb/c nude mice (n=5 per group) were purchased from Model Animal Research Center of Nanjing University. Mice were housed at 20–24°C with an average humidity of 40% and a 12-h light/dark cycle and received food and water ad libitum. Mice were then injected with osteosarcoma cells near the back of the neck at a density of 2×107 cells in 200 µl PBS. Mice were anesthetized by inhalation using 2.0–2.5% sevoflurane during injection and measurement. After 1 week of tumor cell inoculation, 5 mg/kg punicalagin in saline or an equal volume (300 µl) saline as vehicle (control) was injected intraperitoneally once a week for a total of 7 weeks, and the mouse health and behavior were monitored daily for 8 weeks. No death was observed prior to sacrifice. The tumor size was measured with a sliding caliper twice a week, and the tumor volume was calculated using the formula: Size, mm3 =[tumor length × (tumor width)2]/2. When volume was >500 mm3, the experiment was stopped and the mice were sacrificed using CO2 asphyxiation with a flow rate ≤50% of the chamber volume per min, followed by cervical dislocation. Tumors were then harvested, weighed and snap-frozen in liquid nitrogen and stored at −80°C for subsequent use immunohistochemistry assays.
Immunohistochemistry assay
Solid tumors were fixed with 10% formaldehyde for 48 h at room temperature and embedded in paraffin. Tissue slides were blocked with 1% BSA (Beyotime Institute of Biotechnology) in PBS for blocking for 1 h at room temperature. To identify infiltrating blood vessels, immunohistochemistry was carried out on 5-µm deparaffinized sections using an anti-CD31 antibody (cat. no. 77699; 1:150; Cell Signaling Technology Biological Reagents Co., Ltd.) at 4°C overnight, and then peroxide-conjugated secondary antibodies (cat. no. ab6721; 1:500; Abcam) for 1 h at room temperature with ABC Staining kits (Thermo Fisher Scientific, Inc.) were applied for generating chromogenic signals. Images were captured with a light microscope at magnification, ×100.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM), unless otherwise stated. Analysis of two independent groups was performed using unpaired Student's t-test. One-way ANOVA followed by Bonferroni correction was used for multiple comparisons between groups. Statistical analysis was carried out using the GraphPad software v5.0 (GraphPad, Inc.). Each experiment was performed in triplicate. P<0.05 was considered to indicate a statistically significant difference.
Results
Punicalagin treatment inhibits the proliferation of human osteosarcoma cell lines
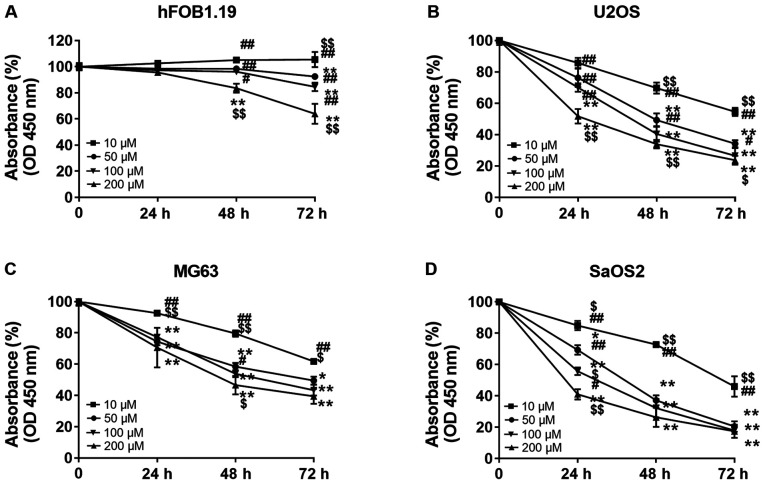
In order to investigate whether punicalagin treatment could affect the viability malignant cells, hFOB1.19 osteoblast cells and U2OS, MG63 and SaOS2 osteosarcoma cells were treated with increasing concentrations of punicalagin (10, 50, 100 and 200 µM) for 24–72 h. Cell viability was then evaluated using CCK-8 assays (Fig. 1). Cell proliferation was suspended after 24 h but the viability of osteoblast cells did not significantly decrease at punicalagin concentrations <100 µM or incubation time <48 h. Overall, the viability of human osteosarcoma cell lines was decreased in a concentration- and time-dependent manner. In 2 of the 3 (MG2 and SaOS2) osteosarcoma cell lines, the decrease in cell viability was not further exacerbated following prolonged treatment >48 h and concentrations >100 µM. Thus, a concentration of 100 µM was selected for subsequent experimentation.
Figure 1.
Effect of punicalagin on the viability of human osteoblast and osteosarcoma cell lines. Cells were treated with different concentrations of punicalagin for 24–72 h. Absorbance measured using a CCK-8 assay relative to the untreated subgroup in (A) hFOB1.19, (B) U2OS, (C) MG63, and (D) SaOS2. Data are presented as the mean ± SEM of three independent experiments. *P<0.05 and **P<0.01 vs. 10 µM punicalagin; $P<0.05 and $$P<0.01, vs. 50 µM punicalagin; #P<0.05 and ##P<0.01, vs. 200 µM punicalagin. OD, optical density.
Punicalagin increases apoptosis of human osteosarcoma cell lines
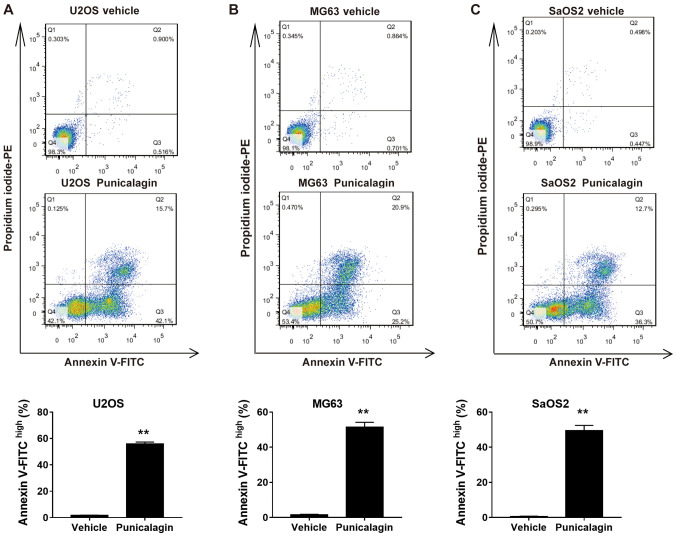
In order to determine whether the decrease in cell viability following treatment with a moderate concentration of punicalagin was due to increased apoptosis, annexin V-FITC and PI double staining was used to assess the frequency of early (FITChighPIlow) and late (FITChighPIhigh) apoptotic cells in U2OS, MG63 and SaOS2 cell lines. Treatment with 100 µM punicalagin for 48 h increased the cumulative percentage of early and late apoptotic tumor cells (Fig. 2).
Figure 2.
Effect of punicalagin on the apoptosis of human osteosarcoma cell lines. Cells were treated with 100 µM punicalagin for 48 h, then analyzed using annexin V and propidium iodide double staining. Punicalagin treatment induced consistent changes seen as increased frequencies of both early (FITChighPIlow) and late (FITChighPIhigh) apoptotic cells in (A) U2OS, (B) MG63 and (C) SaOS2 cells. The representative diagrams of flow cytometry analyses were displayed. Data are presented as the mean ± SEM of three independent experiments. **P<0.01, vs. control group. PE, phycoerythrin; FITC, Fluorescein isothiocyanate; Ctrl, control.
Punicalagin suppresses the invasion of human osteosarcoma cells
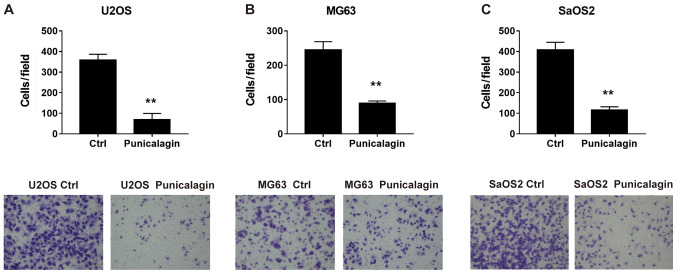
To determine whether punicalagin could suppress the invasiveness of osteosarcoma cells, a Transwell Matrigel™ invasion assay was performed using U2OS, MG63 and SaOS2 cells in the presence or absence of punicalagin. Following treatment with punicalagin for 24 h, fewer migrated tumor cells were detected, suggesting that cell migration was inhibited in the presence of punicalagin (Fig. 3).
Figure 3.
Effect of punicalagin on the invasion of human osteosarcoma cell lines (A) U2OS, (B) MG63 (B), and (C) SaOS2. Cells were treated with punicalagin for 24 h, then analyzed using a Transwell invasion assay. Data are expressed as the mean ± SEM of three independent experiments. **P<0.01, vs. control group. Ctrl, control.
Punicalagin downregulates the NF-κB, but not mTOR signaling pathway in osteosarcoma cell lines
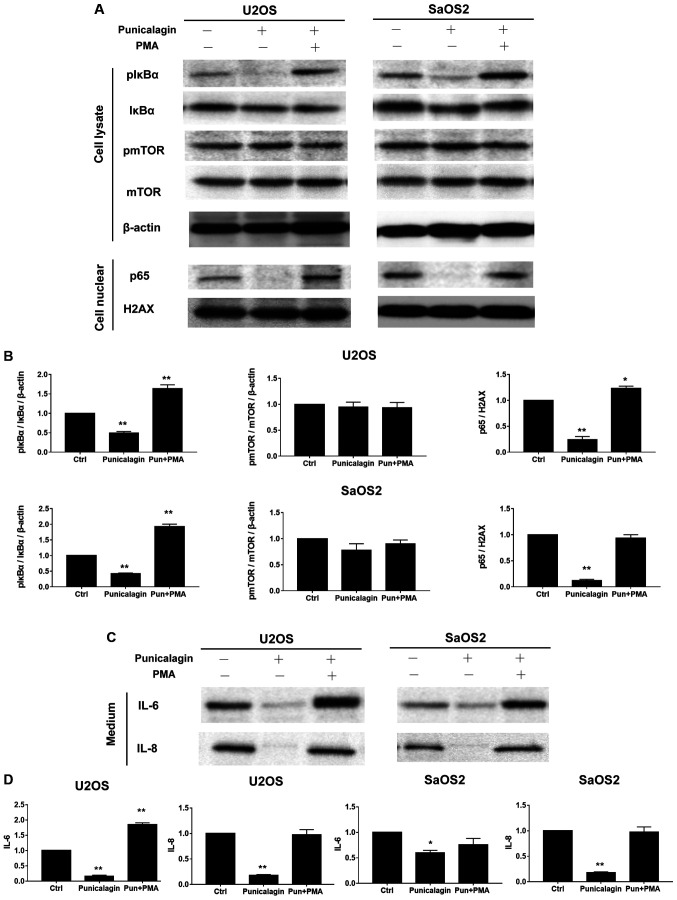
AKT signaling regulates cell survival. As one of the main downstream mediators, mTOR signaling is essential for cell proliferation, and suppressed mTOR signaling is associated with apoptosis induction by various stimuli, delayed cell cycle progression and cell proliferation (11). The levels of phospho-mTOR/mTOR expression in osteosarcoma cells lysates were not altered by punicalagin treatment, but compared with those of the control group the levels of pIκBα/IκBα, p65, IL-6, IL-8 were significantly reduced by punicalagin treatment (Fig. 4).
Figure 4.
Punicalagin regulates the NF-κB pathway in human osteosarcoma cells. (A) Total cell extracts or nuclear fractions were collected and subjected to western blot analysis using anti-mTOR, pmTOR (Ser2448), IκBα, pIκBα (Ser32), β-actin, nuclear p65 and H2AX. (B) Quantification of the results shown in (A). (C) U2OS and SaOS2 cells were also treated with punicalagin solution. The conditioned medium with or without NF-κB signaling activator, PMA and punicalagin treatment were used to determine the levels of IL-6 and IL-8. (D) Quantification of the results shown in (C). *P<0.05 and **P<0.01 vs. control group. Ctrl, control; p, phosphorylated; IL, interleukin; mTOR, mechanistic target of rapamycin kinase; NFκB; nuclear factor κB; IκBα, inhibitor of κBα, H2AX, histone 2A family member X; PMA, phorbol myristate acetate; Pun, punicalagin.
NF-κB is a key regulator of inflammatory immune responses, including cytokine production (12). During tumorigenesis, these cytokine-associated chemotactic effects are required for the initiation of tumor-associated inflammation and neovascularization (13). Osteosarcoma cells were treated with saline (vehicle) or 100 µM punicalagin alone for 48 h, following which, changes in the activated levels of NF-κB represented by pIκBα/IκBα and p65, and its downstream inflammatory factors, including IL-6, IL-8 were examined (Fig. 4). Punicalagin affected the stable expression of NF-κB in U2OS and SaOS2 cells. The expression levels of phosphor-IκBα, nuclear p65 and IL-6, IL-8 significantly decreased in U2OS and SaOS2 cells compared with untreated cells. To further evaluate the effect of punicalagin on the NF-κB pathway, 200 nM PMA, an activator of the NF-κB pathway, was added and cells were incubated for 45 min. Following the addition of PMA, the downregulation of IL-6 and IL-8 levels observed in punicalagin pre-treated osteosarcoma cells was reversed.
Punicalagin attenuates proliferation and angiogenesis of osteosarcoma cells in a murine tumor xenograft model
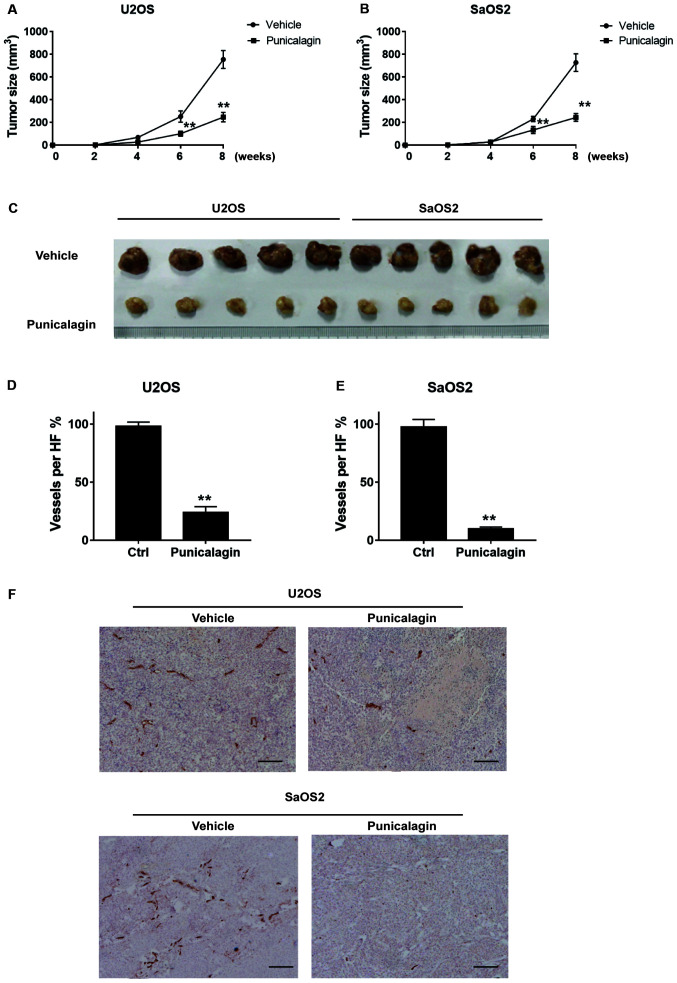
Proliferation and migration of osteosarcoma cells were significantly attenuated in a xenograft model in mice. Tumors typically had a length of 11–18 mm and a width of 8–12 mm in the vehicle group. By contrast, punicalagin treatment significantly decreased tumor growth, to a length of 7–11 mm and width of 6–8 mm (Fig. 5A-C). Thus, punicalagin injection resulted in slower malignant growth of human osteosarcoma cells in the mouse model in vivo. Staining of blood vessels with CD31 antibodies was then used to evaluate angiogenesis in malignant tissues of xenograft mice. CD31 staining represented the wall of the blood vessel of osteosarcoma core sections that were used in a rat subcutaneous model, as described in a previous study (14). The mean density of blood vessels in the punicalagin-treated was significantly reduced, compared with that in the vehicle group, which suggested that that punicalagin could inhibit tumor angiogenesis (Fig. 5D-F).
Figure 5.
Punicalagin attenuates the malignant growth of osteosarcoma cells in mice. (A and B) Xenografted osteosarcoma growth curves of (A) U2OS and (B) SaOS2 were performed according to the xenograft tumor volume. (C) The representative osteosarcoma tissues after resection in mice xenograft models were captured. (D and E) Quantification of the angiogenesis results in xenografted osteosarcoma of (D) U2OS and (E) SaOS2 cells. (F) Punicalagin inhibits tumor angiogenesis represented by decreased blood vessel staining. **P<0.01 vs. vehicle group. Blood vessels are indicated by CD31 expression stained brown. Scale bar, 10 µm. Ctrl, control.
Discussion
The present study suggested that punicalagin treatment in osteosarcoma cells significantly decreased tumor cell viability and induced cell apoptosis. Punicalagin can inhibit proliferation and survival of osteosarcoma cells in a concentration- and time-dependent manner. These results were also replicated in a xenograft model, in which impaired angiogenesis was also observed following injection of punicalagin. The molecular mechanisms were further investigated using biological methods, which demonstrated that the therapeutic effects of punicalagin were associated with downregulation of NF-κB but not mTOR signaling.
Osteosarcoma is the most commonly diagnosed primary solid bone malignancy. Metastasis is the main cause of death in patients with osteosarcoma, and treatment options remain unsatisfactory. The incidence of osteosarcoma in the general population is 2–3 per million per year. However, annual incidence is around 1.2–7.6 per million per year in people younger than 24 years of age worldwide (15). Although major efforts have been made to establish the potential pathognomonic driver mutations in young patients, only sporadic mutations have detected in great majority of cases. Similar to the majority of common types of human cancer, osteosarcoma exhibits a high degree of mutational diversity. This diversity is driven by complex rearrangement processes, chromothripsis and chromothripsic amplification that predominate in osteosarcoma, although the underlying causes remain unknown (16). Currently, early detection, quick confirmation or targeted therapeutic strategies by molecular biology are not available in clinical practice. Therefore, the present study aimed to investigate the efficacy of a potential anti-cancer compound, punicalagin, in osteosarcoma cells.
Although they represent the main available treatment in osteosarcoma, chemotherapeutics are also toxic to normal tissue, and can lead to myelosuppression, opportunistic infection, heart damage and other adverse reactions, thereby decreasing the patient survival rate and quality of life (17). Therefore, new agents with fewer side effects and improved therapeutic advantages are required. Block et al (18) suggested that a phytochemical-rich diet, which includes compounds such as polyphenols, salicylates, phytosterols, saponins, glucosinolates, protease inhibitors, monoterpenes, terpenes, lectins, was associated with decreased risk of cancer. Punicalagin is one of the most abundant polyphenols in pomegranate. In addition, increasing evidence suggests that punicalagin inhibits tumor invasion and metastasis of various types of cancer, such as cervical (19), ovarian (20), colon (21) and lung cancer (22) as well as antioxidants in chronic inflammation (23). To the best of our knowledge, the present study was the first to identify that punicalagin could significantly inhibit osteosarcoma cell proliferation and invasion, induce apoptosis, and decrease angiogenesis. Thus, the results of the present study may provide insight into future therapeutic strategies against osteosarcoma.
However, as previously shown in both rats and humans, the poor bioavailability of punicalagin represents a considerable limitation to pharmaceutical research on its potential therapeutic effects in vivo (24). The low bioavailability of ellagic acid generated from punicalagin is due to its hydrophilic structure and large molecular weight, which limits its absorption by simple diffusion, including oral administration (25). In addition, extremely low lipid solubility further restricts its permeability through the lipophilic layer of the gastrointestinal tract (26). Furthermore, punicalagin can be metabolized into the bioavailable but relatively poor antioxidant, hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora in healthy humans (27). Based on animal studies, the serum concentration after absorption of punicalagin in rodents was ~30 µM (28), which is lower than the concentration used in vitro in the present study. Thus, the multifaceted therapeutic benefits of punicalagin observed in the present study may be difficult to fully replicate in patients. Furthermore, a relatively high concentration of punicalagin may result in non-specific effects due to the biological differences between osteoblasts and osteosarcoma cells. However, the development of novel punicalagin derivatives, compound preparation and administration methods may overcome these limitations in the future.
The precise mechanisms through which punicalagin inhibits osteosarcoma invasion and angiogenesis, as well as its regulation, are not well understood. Several previous studies demonstrated that multiple signaling pathways, including the MAPK (29), β-catenin (19), TGF-β1 (30), AKT, and JNK (31) pathways, were modulated by punicalagin administration. Furthermore, Adams et al (32) suggested that punicalagin decreased phosphorylation of the p65 subunit and binding of NF-κB about 3.6-fold in colon cancer. In nerve cells, chronic neuroinflammation and oxidative stress were dramatically diminished by punicalagin via NF-κB inhibition (33). Furthermore, vascular endothelial growth factor, an NF-κB transcriptional target gene, was downregulated by punicalagin, thereby decreasing angiogenesis in the tumor environment (34,35). The in vitro and in vivo results of the present study were consistent with previous reports, and demonstrated the therapeutic potential of punicalagin against mesodermal illness likes osteosarcoma, through modulation of NF-κB activity.
In general, NF-κB signaling controls many cellular processes, including immune responses, immune cell proliferation and viability, lymphogenesis and B cell maturation (36). The NF-κB pathway is also involved in the regulation of skeletal muscle cell differentiation (37). Recently, activation of NF-κB was demonstrated to increase glucose uptake and glycolytic flux in sarcoma cells, which suggested that NF-κB played a crucial role in the development of osteosarcoma malignancies (38). Consistently, Gong et al (39) found at least 75% osteosarcoma tissues from patients showed positive stain of activated NF-κB pathways and patients whose osteosarcoma with active NF-κB had short median overall survival time as compared with patients whose osteosarcoma had inactive NF-κB. Expression of metastasis-associated proteins, angiogenesis, cell invasion and metastasis have also been linked to NF-ĸB activation in osteosarcoma (8). Liao et al (40) used short hairpin RNA to knockdown NF-ĸB expression, which abolished cell invasion and metastasis in osteosarcoma. In another previous study, the NF-ĸB inhibitor QNZ suppressed NF-ĸB activation, which resulted in downregulation of proteins associated with metastasis, cell migration and cell invasion in osteosarcoma cells (41). The present study further demonstrated that NF-κB is an important transcription factor during pathogenesis of osteosarcoma, and that punicalagin was involved in modulating the expression of molecules downstream of NF-κ B, such as IL-6, and IL-8.
A previous study demonstrated that IL-6 and IL-8 genes were directly regulated by the NF-κB pathway (42) and that IL-6 and IL-8 levels increased with NF-κB overexpression during chronic inflammation in bone and joint tissues (43). IL-6 and IL-8 activation promotes an inflammatory microenvironment during malignant progression (44) and these cytokine-associated chemotactic effects are required for the initiation of tumor-associated inflammation and neovascularization (45). The present study indicated that punicalagin decreased IL-6 as well as IL-8 production by osteosarcoma cells, which was consistent with angiogenesis inhibition in xenograft models. Thus, these findings further elucidate the mechanisms underlying the preventive and therapeutic potential of punicalagin against osteosarcoma.
Although a previous study suggested that pomegranate extract, including a large amount of active punicalagin, had a strong anti-aging effect through the mTOR pathway (46), the present study failed to confirm this finding. Thus, the present results highlight the significance of punicalagin as a promising tumor suppressor in osteosarcoma by targeting NF-κB, but not mTOR pathway. Further characterization of this compound will provide a new insight into punicalagin-mediated suppression of osteosarcoma genesis and development.
In conclusion, punicalagin treatment inhibited osteosarcoma growth, including proliferation, invasion and angiogenesis through NF-κB suppression. Further in-depth and long-term studies are required in order to establish the therapeutic target of the NF-κB signaling pathway in punicalagin-induced cell survival and inhibition of invasive abilities, as well as excessive angiogenesis. Nonetheless, the results of our present study provide preliminary evidence to support punicalagin, a phytochemical used in herbal medicine, as a novel and effective candidate for the systemic treatment and/or chemoenhancement of osteosarcoma.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- NF-κB
nuclear factor-κB
- PMA
phorbol myristate acetate
- mTOR
mammalian target of rapamycin
- Ctrl
control
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
HW designed and directed the study, and analyzed and interpreted the data. TH performed the experiments and wrote the manuscript. XZ performed the literature search, analyzed the data and designed the figures. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by The Animal Ethics Committee of The Second Affiliated Hospital of Air Force Medical University (approval no. 201904-11).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: National cancer data base report. Clin Orthop Relat Res. 2007;459:40–47. doi: 10.1097/BLO.0b013e318059b8c9. [DOI] [PubMed] [Google Scholar]
- 2.Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47:283–292. doi: 10.1016/j.ocl.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Geller DS, Gorlick R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–718. [PubMed] [Google Scholar]
- 4.The epidemiology of osteosarcoma (M)// In: Ottaviani G, Jaffe N, editors; Jaffe N, Bruland OS, Bielack S, editors. Pediatric and adolescent osteosarcoma. Boston, MA: Springer US; 2010. pp. 3–13. [Google Scholar]
- 5.Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33:3029–3035. doi: 10.1200/JCO.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM, Krailo MD, Gebhardt M, Pápai Z, Meyer J, et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon Alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: First results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol. 2015;33:2279–2287. doi: 10.1200/JCO.2014.60.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach HP, Wang C. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avnet S, Di Pompo G, Chano T, Errani C, Ibrahim-Hashim A, Gillie RJ, Donati DM, Baldini N. Cancer-associated mesenchymal stroma fosters the stemness of osteosarcoma cells in response to intratumoral acidosis via NF-κB activation. Int J Cancer. 2017;140:1331–1345. doi: 10.1002/ijc.30540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mongre RK, Sodhi SS, Ghosh M, Kim JH, Kim N, Sharma N, Jeong DK. A new paradigm to mitigate osteosarcoma by regulation of MicroRNAs and suppression of the NF-κB signaling cascade. Dev Reprod. 2014;18:197–212. doi: 10.12717/DR.2014.18.4.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang QL, Xie XB, Wang J, Chen Q, Han AJ, Zou CY, Yin JQ, Liu DW, Liang Y, Zhao ZQ, et al. Glycogen synthase kinase-3β, NF-κB signaling, and tumorigenesis of human osteosarcoma. J Natl Cancer Inst. 2012;104:749–763. doi: 10.1093/jnci/djs210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene. 2005;24:7391–7393. doi: 10.1038/sj.onc.1209100. [DOI] [PubMed] [Google Scholar]
- 12.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43:374–379. doi: 10.1016/j.cyto.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Peng N, Gao S, Guo X, Wang G, Cheng C, Li M, Liu K. Silencing of VEGF inhibits human osteosarcoma angiogenesis and promotes cell apoptosis via VEGF/PI3K/AKT signaling pathway. Am J Transl Res. 2016;8:1005. [PMC free article] [PubMed] [Google Scholar]
- 15.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behjati S, Tarpey PS, Haase K, Ye H, Young MD, Alexandrov LB, Farndon SJ, Collord G, Wedge DC, Martincorena I, et al. Recurrent mutation of IGF signalling genes and distinct patterns of genomic rearrangement in osteosarcoma. Nat Commun. 2017;8:15936. doi: 10.1038/ncomms15936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B, Zhang Y, Li R, Li J, Lu X, Zhang Y. The efficacy and safety comparison of first-line chemotherapeutic agents (high-dose methotrexate, doxorubicin, cisplatin, and ifosfamide) for osteosarcoma: A network meta-analysis. J Orthop Surg Res. 2020;15:51. doi: 10.1186/s13018-020-1576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 19.Tang J, Li B, Hong S, Liu C, Min J, Hu M, Li Y, Liu Y, Hong L. Punicalagin suppresses the proliferation and invasion of cervical cancer cells through inhibition of the β-catenin pathway. Mol Med Rep. 2017;16:1439–1444. doi: 10.3892/mmr.2017.6687. [DOI] [PubMed] [Google Scholar]
- 20.Tang JM, Min J, Li BS, Hong SS, Liu C, Hu M, Li Y, Yang J, Hong L. Therapeutic effects of punicalagin against ovarian carcinoma cells in association with β-Catenin signaling inhibition. Int J Gynecol Cancer. 2016;26:1557–1563. doi: 10.1097/IGC.0000000000000805. [DOI] [PubMed] [Google Scholar]
- 21.Omar U, Aloqbi A, Yousr M, Howell NK. Effect of punicalagin on human colon cancer caco-cells. Malaysian J Nutri. 2016;22:125–136. [Google Scholar]
- 22.Li Y, Yang F, Zheng W, Hu M, Wang J, Ma S, Deng Y, Luo Y, Ye T, Yin W. Punica granatum (pomegranate) leaves extract induces apoptosis through mitochondrial intrinsic pathway and inhibits migration and invasion in non-small cell lung cancer in vitro. Biomed Pharmacother. 2016;80:227–235. doi: 10.1016/j.biopha.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Aloqbi A, Omar U, Yousr M, Grace M, Lila MA, Howell N. Antioxidant activity of pomegranate juice and punicalagin. Nat Sci. 2016;8:235–246. [Google Scholar]
- 24.Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, Hingorani L, Derendorf H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J Agric Food Chem. 2006;54:8956–8961. doi: 10.1021/jf061674h. [DOI] [PubMed] [Google Scholar]
- 25.Vora A, Londhe V, Pandita N. Herbosomes enhance the in vivo antioxidant activity and bioavailability of punicalagins from standardized pomegranate extract. J Funct Foods. 2015;12:540–548. doi: 10.1016/j.jff.2014.12.017. [DOI] [Google Scholar]
- 26.Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin Chim Acta. 2004;348:63–68. doi: 10.1016/j.cccn.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 27.Cerdá B, Espín JC, Parra S, Martínez P, Tomás-Barberán FA. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur J Nutr. 2004;43:205–220. doi: 10.1007/s00394-004-0461-7. [DOI] [PubMed] [Google Scholar]
- 28.Cerdá B, Llorach R, Cerón JJ, Espín JC, Tomás-Barberán FA. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr. 2003;42:18–28. doi: 10.1007/s00394-003-0396-4. [DOI] [PubMed] [Google Scholar]
- 29.Chu G, Zhang W, Chen M, Yang H, Yuan Z. Punicalagin inhibits RANKL-induced osteoclastogenesis by suppressing NF-κB and MAPK signaling pathways. Int J Clin Exp Med. 2018;11:6571–6582. [Google Scholar]
- 30.Tang J, Liu C, Min J, Hu M, Li Y, Hong L. Potential therapeutic role of punicalagin against mechanical-trauma-induced stress urinary incontinence via upregulation of Nrf2 and TGF-β1 signaling. Int Urogynecol J. 2017;28:947–955. doi: 10.1007/s00192-017-3283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwatake M, Okamoto K, Tanaka T, Tsukuba T. Punicalagin attenuates osteoclast differentiation by impairing NFATc1 expression and blocking Akt-and JNK-dependent pathways. Mol Cell Biochem. 2015;407:161–172. doi: 10.1007/s11010-015-2466-3. [DOI] [PubMed] [Google Scholar]
- 32.Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54:980–985. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- 33.Kim YE, Hwang CJ, Lee HP, Kim CS, Son DJ, Ham YW, Hellström M, Han SB, Kim HS, Park EK, Hong JT. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology. 2017;117:21–32. doi: 10.1016/j.neuropharm.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 34.Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toi M, Bando H, Ramachandran C, Melnick SJ, Imai A, Fife RS, Carr RE, Oikawa T, Lansky EP. Preliminary studies on the anti-angiogenic potential of pomegranate fractions in vitro and in vivo. Angiogenesis. 2003;6:121–128. doi: 10.1023/B:AGEN.0000011802.81320.e4. [DOI] [PubMed] [Google Scholar]
- 36.Bakkar N, Guttridge DC. NF-kappaB signaling: A tale of two pathways in skeletal myogenesis. Physiol Rev. 2010;90:495–511. doi: 10.1152/physrev.00040.2009. [DOI] [PubMed] [Google Scholar]
- 37.Bakkar N, Wang J, Ladner KJ, Wang H, Dahlman JM, Carathers M, Acharyya S, Rudnicki MA, Hollenbach AD, Guttridge DC. IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol. 2008;180:787–802. doi: 10.1083/jcb.200707179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Londhe P, Yu PY, Ijiri Y, Ladner KJ, Fenger JM, London C, Houghton PJ, Guttridge DC. Classical NF-κB metabolically reprograms sarcoma cells through regulation of hexokinase 2. Front Oncol. 2018;8:104. doi: 10.3389/fonc.2018.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong T, Su X, Xia Q, Wang J, Kan S. Expression of NF-κB and PTEN in osteosarcoma and its clinical significance. Oncol Lett. 2017;14:6744–6748. doi: 10.3892/ol.2017.6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao CL, Lin JH, Lien JC, Hsu SC, Chueh FS, Yu CC, Wu PP, Huang YP, Lin JG, Chung JG. The crude extract of Corni Fructus inhibits the migration and invasion of U-2 OS human osteosarcoma cells through the inhibition of matrix metalloproteinase-2/-9 by MAPK signaling. Environ Toxicol. 2015;30:53–63. doi: 10.1002/tox.21894. [DOI] [PubMed] [Google Scholar]
- 41.Pan PJ, Tsai JJ, Liu YC. Amentoflavone inhibits metastatic potential through suppression of ERK/NF-κB activation in osteosarcoma U2OS cells. Anticancer Res. 2017;37:4911–4918. doi: 10.21873/anticanres.11900. [DOI] [PubMed] [Google Scholar]
- 42.Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lv F, Song LJ, Wang XH, Qiu F, Li XF. The role of Act1, a NF-κB-activating protein, in IL-6 and IL-8 levels induced by IL-17 stimulation in SW982 cells. Pharm Biol. 2013;51:1444–1450. doi: 10.3109/13880209.2013.798668. [DOI] [PubMed] [Google Scholar]
- 44.Karin M. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 46.Syed DN, Chamcheu JC, Adhami VM, Mukhtar H. Pomegranate extracts and cancer prevention: Molecular and cellular activities. Anticancer Agents Med Chem. 2013;13:1149–1161. doi: 10.2174/1871520611313080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.