Abstract
Immunity to fungal infections is mediated by cells of the innate and adaptive immune system including Th17 cells. Ca2+ influx in immune cells is regulated by stromal interaction molecule 1 (STIM1) and its activation of the Ca2+ channel ORAI1. We here identify patients with a novel mutation in STIM1 (p.L374P) that abolished Ca2+ influx and resulted in increased susceptibility to fungal and other infections. In mice, deletion of STIM1 in all immune cells enhanced susceptibility to mucosal C. albicans infection, whereas T cell‐specific deletion of STIM1 impaired immunity to systemic C. albicans infection. STIM1 deletion impaired the production of Th17 cytokines essential for antifungal immunity and compromised the expression of genes in several metabolic pathways including Foxo and HIF1α signaling that regulate glycolysis and oxidative phosphorylation (OXPHOS). Our study further revealed distinct roles of STIM1 in regulating transcription and metabolic programs in non‐pathogenic Th17 cells compared to pathogenic, proinflammatory Th17 cells, a finding that may potentially be exploited for the treatment of Th17 cell‐mediated inflammatory diseases.
Keywords: Ca2+ channel, Candida albicans, immunodeficiency, STIM1, Th17 cells
Subject Categories: Immunology; Microbiology, Virology & Host Pathogen Interaction
Pathogenic Th17 cells have been implicated in autoimmune diseases, while non‐pathogenic Th17 cells provide immunity to fungal pathogens. Patients with mutations in ORAI1 or STIM1 have impaired Ca2+ signaling in immune cells and are more susceptible to infections with fungal pathogens.
The paper explained.
Problem
Calcium signals are critical for the function of cells of the innate and adaptive immune system and their ability to mediate protective immune responses to infection. Calcium signals in immune cells are mediated by CRAC channels that are formed by ORAI1 and STIM1 proteins. We had previously reported that defects in this pathway render patients and mice susceptible to viral infections. The role of CRAC channels for immunity to infection with fungal pathogens has not been studied and the mechanisms by which calcium signals regulate antifungal immunity are largely unexplored.
Results
We here describe patients with an inherited novel loss‐of‐function mutation in the STIM1 gene that abolishes calcium influx through CRAC channels and therefore the function of immune cells. These patients, like others with mutations in the same pathway described before, are more susceptible to fungal infections with C. albicans, A. fumigatus, P. jirovecii, and potentially other fungal pathogens. In this study, we describe the molecular mechanisms by which the mutation abolishes the ability of STIM1 to activate CRAC channels and show that lack of calcium influx in the patients’ T cells suppresses several metabolic pathways that are required for normal T‐cell function. To understand the mechanisms by which CRAC channels control immunity to fungal infections, we used mice with genetic deletion of STIM1 and its homologue STIM2 to abolish calcium influx in all immune cells or more selectively only in T cells. Mice lacking STIM1 or both STIM1 and STIM2 in all immune cells showed increased susceptibility to oral C. albicans infection, which was associated with defective neutrophil function. Deletion of STIM1 only in T cells, by contrast, had little effect on immunity to oral C. albicans infection but rendered mice susceptible to systemic fungal infection. A subset of CD4+ T cells, T helper (Th) 17 cells, are important mediators of antifungal immunity. Deletion of STIM1 in Th17 cells impaired not only the expression of several Th17 cytokines but also that of many genes which regulate the metabolic function of Th17 cells. This included genes controlling the utilization of glucose by aerobic glycolysis and the generation of ATP in mitochondria by oxidative phosphorylation (OXPHOS). In contrast to Th17 cells that mediate antifungal immunity, a related subset of Th17 cells that cause inflammation in the context of many autoimmune diseases required CRAC channel function only to regulate OXPHOS but not glycolysis.
Impact
Our study offers new insights into the role of calcium influx through CRAC channels in cells of the innate and adaptive immune system and how this signaling pathway provides immunity to fungal pathogens. Furthermore, we describe distinct roles of CRAC channels in regulating the metabolic function of Th17 cell subsets that contribute to antifungal immunity and those that mediate inflammation in autoimmune diseases like multiple sclerosis, Crohn's disease, and rheumatoid arthritis. We propose that the latter finding may potentially be exploited for the treatment of Th17 cell‐mediated autoimmune diseases.
Introduction
Over 150 million people worldwide are estimated to suffer from fungal diseases, with the severity ranging from asymptomatic‐mild to life‐threatening systemic infections resulting in ~1.6 million deaths associated with fungal disease each year (Bongomin et al, 2017). Aspergillus, Candida, Cryptococcus species, and Pneumocystis jirovecii are the main fungal pathogens responsible for the majority of serious fungal disease cases. Candida species are part of the normal human microflora of the gastrointestinal and reproductive tracts in 50–80% of healthy individuals, but can become pathogenic in immune compromised hosts (Brown et al, 2012). Common causes of increased susceptibility to Candida infections include HIV/AIDS, immunosuppressive therapies, antibiotic use, and inherited immunodeficiencies (Lanternier et al, 2013; Bongomin et al, 2017; Mengesha & Conti, 2017). Infections with Candida (C.) albicans manifest as mucosal or mucocutaneous candidiasis, onychomycosis or systemic fungal infection. Systemic C. albicans infection can occur after dissemination of local fungal infections or as nosocomial, often catheter‐associated, infections in patients receiving critical care (Villar & Dongari‐Bagtzoglou, 2008; Lanternier et al, 2013). Immunity to C. albicans infections involves innate and adaptive immune responses (Hernandez‐Santos & Gaffen, 2012; Conti & Gaffen, 2015; Netea et al, 2015; Sparber & LeibundGut‐Landmann, 2015). C. albicans is initially recognized by cells of the innate immune system including dendritic cells, macrophages, and neutrophils. At skin and mucosal surfaces, C. albicans hyphae may enter epithelial cells resulting in their activation and production of IL‐1β, TNF‐α, and IL‐6, which activate neutrophils and other innate immune cells. The recruitment and activation of neutrophils also depend on TNF‐α, IFN‐γ, and IL‐17A produced by Th1, Th17 cells, type 3 innate lymphoid cells (ILC3) as well as NK cells and γδ T cells (Bar et al, 2014; Conti & Gaffen, 2015; Netea et al, 2015). Neutrophils are required for clearing fungal pathogens, and C. albicans is among the most frequently isolated pathogens in neutropenic patients with nosocomial systemic candidiasis (Delaloye & Calandra, 2014).
On the adaptive side of the immune system, non‐pathogenic Th17 cells are critical for antifungal immunity as shown by studies in mice and human patients with inherited defects in Th17 cell differentiation and/or function (Mengesha & Conti, 2017). Individuals with mutations in IL‐17A, IL‐17 receptor A (IL‐17RA), or IL‐17RC (Puel et al, 2011; Ling et al, 2015; Levy et al, 2016) are susceptible to chronic mucocutaneous candidiasis (CMC) as are patients with dominant‐negative mutations in the transcription factor signal transducer and activator of T cells (STAT) 3 (Milner et al, 2008) and gain‐of‐function (GOF) mutations in STAT1 (Toubiana et al, 2016), which result in defects of Th17 cell differentiation. Furthermore, neutralizing autoantibodies to IL‐17A, IL‐17F, and IL‐22 are associated with an increased susceptibility to C. albicans infections in patients with autoimmune polyglandular syndrome type 1 (APS1) due to mutations in autoimmune regulator (AIRE) (Kisand et al, 2010). In mice, deletion of IL‐23p19, which is required for the differentiation of Th17 cells, or the IL‐17A receptor (IL‐17RA) causes severe oropharyngeal candidiasis (OPC; Conti et al, 2009). By contrast, Il‐12 −/− mice lacking Th1 cells do not develop OPC but fail to prevent Candida dissemination to the kidney. Besides immunity to local candidiasis, non‐pathogenic Th17 cells are also crucial for immunity to systemic Candida infection (Huang et al, 2004). Mice lacking IL‐17RA had a C. albicans dose‐dependent survival defect after systemic infection (Huang et al, 2004). Intestinal colonization of mice with C. albicans was recently shown to mediate Th17 cell differentiation and expansion, which provides immunity to systemic Candida infection (Shao et al, 2019). Collectively, these studies show that IL‐17 signaling is critical for immunity to local Candida infection and identify Th17 cells as critical during systemic candidiasis.
The function of T cells depends on calcium (Ca2+) influx and signaling (Feske, 2007). The main mode of Ca2+ influx in T cells is store‐operated Ca2+ entry (SOCE) that is mediated by Ca2+ release‐activated Ca2+ (CRAC) channels. CRAC channels are hexamers of ORAI1 proteins located in the plasma membrane that are activated by STIM1 and its homologue STIM2. STIM1 and STIM2 are single‐pass membrane proteins located in the endoplasmic reticulum (ER) membrane. T‐cell receptor stimulation results in the production of inositol (1,4,5) trisphosphate (IP3) and opening of IP3 receptors in the ER membrane, followed by Ca2+ release from the ER. This triggers a conformational change of STIM proteins and their binding to ORAI1 in the plasma membrane, resulting in CRAC channel opening and SOCE (Deng et al, 2009; Maus et al, 2015). Loss‐of‐function (LOF) mutations in ORAI1 or STIM1 genes (OMIM 610277 and 605921) abolish SOCE and cause CRAC channelopathy, which is characterized by combined immunodeficiency (CID), humoral autoimmunity, and ectodermal dysplasia (Lacruz & Feske, 2015; Concepcion et al, 2016). CID typically presents in early infancy and can be severe resulting in death of patients from viral and bacterial infections (Feske et al, 2006, 2005; McCarl et al, 2009; Lacruz & Feske, 2015). A common feature of CRAC channelopathy are fungal infections with a variety of pathogens including, most commonly, C. albicans, Aspergillus fumigatus, and Pneumocystis jirovecii (Table 1).
Table 1.
Synopsis of patients with CRAC channelopathy due to loss‐of‐function mutations in ORAI1 or STIM1 and associated fungal infections
| Gene | Mutation | Fungal infections | References | |
|---|---|---|---|---|
| Local | Systemic | |||
| ORAI1 | p.V181SfsX8 | Pneumocystis jirovecii pneumonia | n.r. | Lian et al (2018) |
| p.L194P | n.r. | C. albicans sepsis | Lian et al (2018) | |
| p.G98R | Aspergillus fumigatus, Candida albicans | n.r. | Lian et al (2018) | |
| p.R91W | Oral candidiasis, gastrointestinal candidiasis | n.r. | Feske et al (1996), Feske et al (2000), McCarl et al (2009) | |
| p.A88SfsX25 | n.r. | n.r. | Partiseti et al (1994), McCarl et al (2009) | |
| p.A103E/p.L194P | n.r. | n.r. | Le Deist et al (1995), McCarl et al (2009) | |
| p.H165PfsX1 | n.r. | n.r. | Chou et al (2015) | |
| p.R270X | Pneumocystis jirovecii pneumonia | Badran et al (2016) | ||
| p.I148S | Pneumocystis jirovecii pneumonia, oral thrush, diaper rash | Klemann et al (2017) | ||
| STIM1 | p.E128RfsX9 | n.r. | n.r. | Picard et al (2009) |
| C1538‐1 G>A | n.r. | n.r. | Sahin et al (2010), Byun et al (2010) | |
| p.R429C | n.r. | n.r. | Fuchs et al (2012), Maus et al (2015) | |
| p.R426C | n.r. | n.r. | Wang et al (2014) | |
| p.P165Q | n.r. | n.r. | Schaballie et al (2015) | |
| p.L74P | n.r. | n.r. | Parry et al (2016) | |
| p.L374P | Diaper rash, Onychomycosisa | n.r. | This study | |
n.r., not reported.
Present prior to, but aggravated by, treatment with infliximab and inhalative corticosteroids.
We previously reported that the function of pathogenic Th17 cells, which orchestrate inflammation in many autoimmune diseases, depends on CRAC channels. Mice with T cell‐specific deletion of ORAI1, STIM1, or both STIM1 and STIM2 had profound defects in Th17 cell function resulting in partial or complete protection from experimental autoimmune encephalomyelitis (EAE), a Th17 cell‐dependent murine model of multiple sclerosis (MS; Ma et al, 2010; Schuhmann et al, 2010; Kaufmann et al, 2016). Furthermore, pathogenic Th17 cells that differentiate under the influence of a hyperactive form of STAT3 require STIM1 for their function and ability to cause multiorgan inflammation (Kaufmann et al, 2019). By contrast, the role of CRAC channels in non‐pathogenic Th17 cells and their ability to mediate immunity to infection with bacterial and fungal pathogens is unknown. In addition, the cause of impaired antifungal immunity in CRAC channel‐deficient patients remains elusive.
In this study, we report patients with a novel LOF mutation in STIM1 (p.L374P) that abolishes STIM1 function and SOCE. Like many other SOCE‐deficient patients, they suffer from chronic fungal infections. Using mice with conditional deletion of STIM1, we demonstrate that SOCE in non‐pathogenic Th17 cells is essential for antifungal immunity to systemic infection with C. albicans. Mechanistically, the lack of functional STIM1 and SOCE in non‐pathogenic Th17 cells resulted in impaired production of IL‐17A and other cytokines, but did not impair the expression of Th17 signature molecules including RORγt. An unbiased transcriptome analysis of non‐pathogenic Th17 cells revealed that STIM1 regulates two key metabolic pathways, aerobic glycolysis, and mitochondrial oxidative phosphorylation (OXPHOS), whose function was impaired in STIM1‐deficient non‐pathogenic Th17 cells. In pathogenic Th17 cells, by contrast, STIM1 only controlled OXPHOS but not glycolysis. The greater reliance of pathogenic Th17 cells on STIM1 and OXPHOS may be exploitable for the treatment of autoimmune diseases in which pathogenic Th17 cells play an important role without affecting the antimicrobial function of non‐pathogenic Th17 cells.
Results
Mutation of p.L374 in the C terminus of STIM1 abolishes SOCE and causes CRAC channelopathy
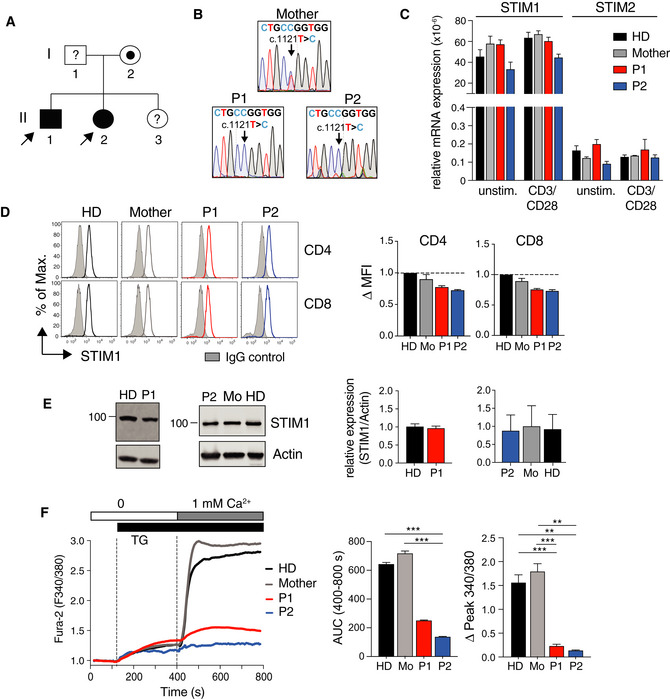
We here report two patients (P1, P2) born to parents who are first‐cousins‐once‐removed. The patients presented with combined immune deficiency (CID) and recurrent infections since childhood with varicella zoster virus (VZV), pneumonias caused by atypical mycobacteria, onychomycosis, and skin infections with C. albicans species (Fig 1A, Table EV1). Patients also had non‐immunological symptoms including congenital muscular hypotonia and anhidrotic ectodermal dysplasia, which are typical of CRAC channelopathy due to LOF mutations in STIM1 and ORAI1 (Lacruz & Feske, 2015). Detailed case reports are provided in the Appendix. Laboratory analyses demonstrated overall normal immune cell populations in both patients. P1 had a profound defect in T‐cell proliferation after T‐cell receptor (TCR) stimulation with several viruses and C. albicans whereas proliferative responses to mitogens (PHA, PWA) were normal (Appendix Table S1). Sequencing of genomic DNA (gDNA) of P1, P2, and their asymptomatic mother revealed a homozygous missense mutation (c.1121T>C) within exon 8 of STIM1 in both patients, whereas the mother was heterozygous for the same mutation (Fig 1B). gDNA of the father and a sister of P1 and P2, who are both asymptomatic, was not available for analysis. The STIM1 c.1121T>C mutation is predicted to cause a single amino acid substitution (p.L374P) in the second coiled‐coil domain (CC2, aa 345–391) within the C terminus of STIM1. Analysis of 71,702 genomes from unrelated individuals using the gnomAD v3 database (GRCh38) (https://gnomad.broadinstitute.org) demonstrated that the p.L374P mutation of STIM1 is extremely rare with an allele frequency of 6.98e‐6 (1 allele out of 143,330). As CRAC channelopathy due to mutations in ORAI1 or STIM1 genes follows an autosomal recessive inheritance, the homozygous STIM1 c.1121T>C mutation detected in both patients is compatible with their disease. The scaled CADD score of the STIM1 c.1121T>C, p.L374P mutation is 28.5, indicating that it is within the top 0.1% of the most deleterious variants in the human genome.
Figure 1. p.L374P mutation in the C terminus of STIM1 abolishes SOCE and causes CRAC channelopathy.
-
APedigree of the patients P1 (II‐1) and P2 (II‐2) presenting with CRAC channelopathy. Filled circles (females) and squares (males) indicate homozygous patients; dotted symbols indicate heterozygous asymptomatic carriers; symbols with “?” indicate asymptomatic family members not available for DNA sequencing.
-
BSanger sequencing of genomic DNA isolated from PBMC of the mother (I‐2), P1, and P2.
-
CSTIM1 and STIM2 mRNA expression in PBMC of P1, P2, mother, and a healthy donor (HD) that were left unstimulated or stimulated with anti‐CD3/CD28 for 24 h. mRNA levels of STIM1/2 normalized to 18S rRNA. Graph shows the mean ± SEM of duplicates from one experiment.
-
DSTIM1 protein expression in CD4+ and CD8+ T cells from P1, P2, the mother, and a HD analyzed by flow cytometry. Shaded histograms: polyclonal rabbit IgG control antibody; open histograms: polyclonal anti‐STIM1 antibody. Bar graphs show the delta MFI calculated as MFISTIM1 – MFIIgG control that was normalized to HD T cells. Bar graphs are mean ± SEM of two independent repeat experiments.
-
ESTIM1 protein expression in expanded human T cells analyzed by immunoblotting. One representative Western blot of 3 is shown. Bar graphs are the mean ± SEM of STIM1 expression normalized to actin from three independent experiments.
-
FCa2+ influx in Fura‐2 loaded PBMC of P1, P2, the mother, and a HD. T cells were stimulated with 1 μM thapsigargin (TG) in the absence of extracellular Ca2+ followed by addition of 1 mM extracellular Ca2+. Bar graphs show the integrated Ca2+ influx response (AUC, area under the curve) from 400 to 800 s and the peak Ca2+ influx normalized to baseline Ca2+ levels (F340/380) at 400 s. Data represent the mean ± SEM from 2 experiments. Statistical analysis by unpaired Student's t‐test. **P < 0.01, ***P < 0.001.
Source data are available online for this figure.
The c.1121T>C mutation does not interfere with STIM1 mRNA expression as transcript levels were comparable in PBMC from both patients, their mother, and a healthy donor (HD) control either before and after stimulation with anti‐CD3/CD28 (Fig 1C). No compensatory upregulation of STIM2 was observed in P1 and P2. Intracellular staining of CD4+ and CD8+ T cells from P1, P2, their mother and a HD also showed comparable STIM1 protein expression (Fig 1D). The specificity of STIM1 staining was verified using fibroblasts of a patient homozygous for a STIM1 p.E128RfsX9 frameshift mutation that abolishes STIM1 protein expression (Appendix Fig S1A) (Picard et al, 2009). Similar STIM1 protein expression in T cells of P1, P2, their mother, and two HD controls was confirmed by Western blot analysis (Fig 1E). Despite normal STIM1 mRNA and protein expression, SOCE in PBMC isolated from P1 and P2 was severely impaired after stimulation with the sarcoplasmic/endoplasmic Ca2+ ATPase inhibitor thapsigargin (TG) compared to PBMC from the patients’ mother and a HD control (Fig 1F). Abolished SOCE was also observed in T cells from P1 and P2 cultured in vitro compared to those of a HD (Appendix Fig S1B). SOCE in the mother's T cells was reduced, likely because she is a heterozygous carrier of the STIM1 p.L374P mutation.
STIM1 p.L374P mutation abolishes STIM1 puncta formation and co‐localization with ORAI1
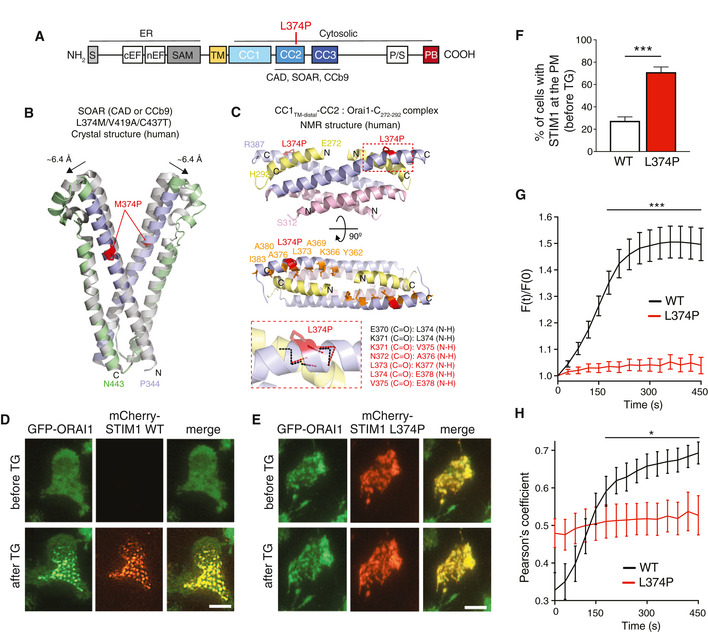
Because SOCE is strongly impaired but STIM1 protein expression is normal, we hypothesized that the p.L374P mutation affects STIM1 protein function. Leucine 374 is located in the second coiled‐coil (CC2) domain of the cytoplasmic C‐terminal segment of STIM1. CC2 is part of a functional domain that is necessary and sufficient for STIM1 binding to ORAI1 and that is alternatively referred to as CRAC activation domain (CAD, aa 342–448) (Park et al, 2009), STIM‐ORAI activation region (SOAR, 344–442) (Yuan et al, 2009), or CC fragment b9 (CCb9, 339–444) (Kawasaki et al, 2009; Fig 2A). We modeled the impact of the p.L374P mutation on the structure of the STIM1 CC2 and CC3 domains using available crystal structures of CAD/SOAR/CCb9 and NMR structures of C‐terminal STIM1 fragments (Yang et al, 2012; Stathopulos et al, 2013). Based on the L374M/V419A/C437T triple mutant crystal structure (Yang et al, 2012), the human CAD/SOAR/CCb9 domain forms a V‐shaped dimer. Our homology model of the dimer places the M374P mutation (red) at the base of each apex formed by CC2 (light blue ribbon) and CC3 (light green ribbon) (Fig 2B). Backbone atom alignment of residues 344–370 with the template structure (gray ribbon) reveals an ~6.4 Å movement of each mutant apex position, which is predicted to perturb STIM1 interaction with ORAI1. Furthermore, we created a homology model of the p.L374P mutation in the context of the solution NMR structure of a human STIM1‐ORAI1 dimer complex (Stathopulos et al, 2013). The dimer is formed by two STIM1 peptides (aa 312–387) containing the most carboxyl (C) terminal part of CC1 (light pink ribbon) and the entire CC2 (light blue ribbon) in complex with two ORAI1 peptides (aa 272–292) corresponding to the C‐terminal CC domain of ORAI1 (Orai1‐C272–292, yellow ribbons) (Fig 2C). In this model, the p.L374P mutation (red) is located in a critical area of the STIM‐ORAI association pocket (SOAP), which confers a hydrophobic surface for each STIM1‐ORAI1‐C272–292 interaction. The loss of a backbone amide hydrogen (NH) caused by the p.L374P mutation results in an altered hydrogen bond network near the mutation, which is predicted to interfere with STIM1 helix stability, folding, and thus binding to ORAI1. Note that both the crystal and NMR conformations could similarly experience a helical bend due to the rigid nature of the Pro peptide bond and helical destabilization due to the absence of a backbone NH in Pro.
Figure 2. L374P mutation abolishes STIM1 puncta formation and co‐localization with ORAI1.
-
ADomain architecture of STIM1. Abbreviations: CAD: CRAC activation domain; CC1‐3: coiled‐coil domains 1–3; CCb9, coiled‐coil fragment b9; cEF, canonical EF‐hand; nEF, non‐canonical EF‐hand; PB, polybasic domain; P/S, proline/serine‐rich region; SAM: sterile alpha motif; S, signal peptide; SOAR: STIM1‐ORAI1 activation region; TM: transmembrane domain.
-
B, CHomology models of the STIM1 p.L374P mutation based on published crystal (Yang et al, 2012) and NMR (Stathopulos et al, 2013) structures of STIM1. (B) Location of the M374P mutation (red sticks) in the context of the triple mutant (L374M/V419A/C437T) human CAD/SOAR/CCb9 dimer structure relative to CC2 (light blue ribbon) and CC3 (light green ribbon). The template structure without the M374P mutation (gray ribbon) and the movement of the apex positions of both subunits are indicated. (C) Location of the L374P mutation in the context of the NMR structure of a bimolecular complex of two STIM1 CC1TM‐distal‐CC2 peptides (CC1TM‐distal region shown as light pink ribbon, and CC2 shown as light blue ribbon) and two ORAI1 C‐terminal (aa 272–292) peptides (yellow ribbons) (Stathopulos et al, 2013). The loss of the backbone amide hydrogen (NH) due to the L374P mutation results in an altered hydrogen bond network near the mutation (small red dashed box). The zoomed view of this area (large red dashed box) shows the backbone hydrogen bond network for the L374P protein (red dashed lines) compared to the WT protein (black dashed lines). The maintained backbone atom hydrogen bonds in this region for the L374P mutant compared to the WT protein are listed in red text while the lost hydrogen bonds are listed in black text.
-
D–HGFP‐ORAI1 expressing HEK293 cells were transfected with mCherry‐STIM1 (WT or L374P) and left unstimulated or stimulated with 1 μM TG in Ca2+‐free Ringer's solution. (D,E) Representative TIRF microscopy images from one of two repeat experiments. Scale bars, 10 μm. (F) Percentages of WT or mutant mCherry‐STIM1 present at the plasma membrane (i.e., visible in the TIRFM evanescent field) before TG stimulation. Data are the mean ± SEM calculated from 550 (WT) and 830 (L374P) cells from two independent experiments. (G) Change of mCherry‐STIM1 (WT or L374P mutant) fluorescence [F(t)/F(0)] in the TIRFM evanescence field. Fluorescence normalized to values at the time of TG addition in Ca2+‐free Ringer's buffer at 0 s. Traces show mean ± SEM of 40–50 cells from two independent experiments. (H) Co‐localization of mCherry‐STIM1 (WT or L374P mutant) and GFP‐ORAI1 from the time of TG addition at 0 s. Co‐localization measured using Pearson's coefficient for GFP and mCherry. Traces show mean ± SEM of 27 cells from two independent experiments.
To test whether the p.L374P mutation interferes with STIM1 function and its ability to bind to ORAI1, we investigated its co‐localization with ORAI1. We first analyzed the formation of STIM1 puncta by total internal reflection fluorescence microscopy (TIRFM) using HEK293 cells stably expressing GFP‐ORAI1 and co‐transfected with either wild‐type (WT) mCherry‐STIM1 or mutant mCherry‐STIM1 p.L374P. In non‐stimulated cells with replete ER Ca2+ stores, WT STIM1 was localized in the ER and outside the TIRFM evanescence field as expected (Fig 2D). Following TG‐induced depletion of ER stores WT STIM1 became visible in the TIRFM layer and redistributed into discrete puncta consistent with its localization in ER‐PM junctions as reported earlier (Figs 2D, F–G and EV1A–C) (Liou et al, 2005; Wu et al, 2014). By contrast, the mutant STIM1 p.L374P was already localized near the PM in the majority of cells prior to TG stimulation (70.1 ± 4.9% of cells) but without forming discrete puncta (Figs 2E–G and EV1B and C). After store depletion, STIM1 p.L374P localization at the PM did not further increase and it failed to form puncta. Importantly, WT STIM1 and ORAI1 strongly colocalized in puncta after TG stimulation (Figs 2D and H, and EV1B and C). By contrast, no increase in co‐localization was observed between mutant STIM1 p.L374P and ORAI1 above levels seen in cells before ER store depletion (Figs 2E and H, and EV1B and C).
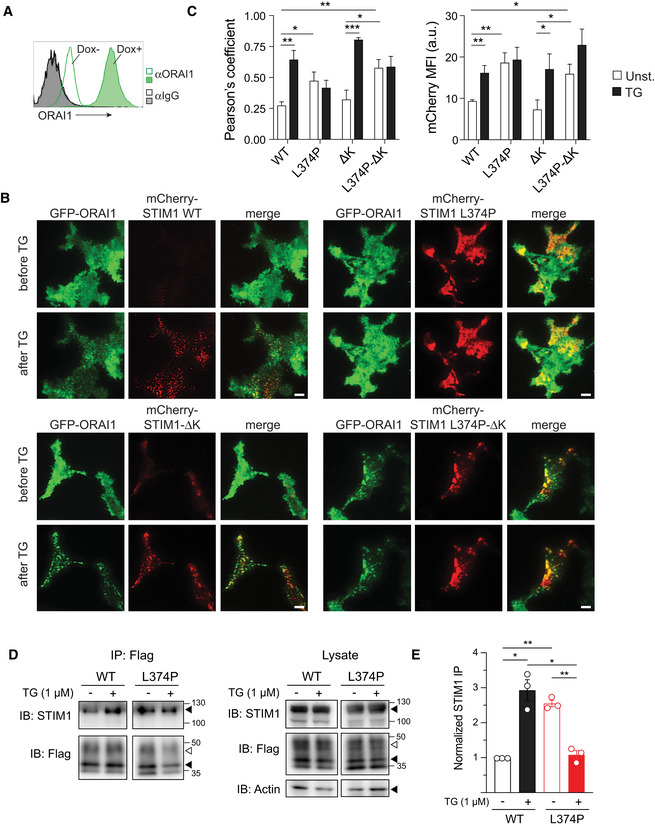
Figure 3. Plasma membrane localization of STIM1 p.L374P mutant is independent STIM1 polybasic domain and ORAI1 expression.
-
ARepresentative flow cytometry plots of ORAI1 expression on HEK293 cells after doxycycline (Dox)‐induced overexpression of ORAI1 for 24 h. ORAI1 was detected using a mouse anti‐human monoclonal antibody (29A2) recognizing the second extracellular loop of ORAI1. Isotype control: mouse IgG1. Open histograms (no Dox) represent controls.
-
BTIRFM images of HEK293 cells overexpressing GFP‐ORAI1 and mCherry‐STIM1 (either WT, L374P, ΔK, or L374P‐ΔK). Cells were analyzed before or after stimulation with 1 μM TG in Ca2+‐free Ringer's buffer for 10 min. Representative images from one of 3–4 repeat experiments. Scale bars, 10 μm.
-
C(left panel) Co‐localization of mCherry‐STIM1 (WT or mutants) and GFP‐ORAI1 before and 10 min after treatment with TG. Co‐localization was measured using Pearson's coefficient for GFP and mCherry. (right panel) Mean fluorescence intensity (MFI) of WT or mutant mCherry‐STIM1 in the TIRFM evanescent field before and after TG stimulation as in (B). Data are the mean ± SEM from 9 to 14 cells from 3 to 4 independent experiments per condition.
-
DCo‐immunoprecipitation of mCherry‐STIM1 (WT or L374P) and Flag‐ORAI1 coexpressed in HEK293 cells in resting condition or after TG stimulation (1 μM). STIM1 and ORAI1 were detected by IB using anti‐STIM1 (3917) and anti‐Flag (M2) antibodies. Shown is one representative experiment of three. Filled triangles indicate bands for mCherry‐STIM1, Flag‐ORAI1 and actin; open triangles indicate bands of glycosylated Flag‐ORAI1.
-
EThe immunoprecipitated (IP) mCherry‐STIM1 signal was normalized to total protein content (actin), total mCherry‐STIM1 expression, and IP efficiency (Flag‐ORAI1). Shown is the mean ± SEM of three independent experiments.
These findings suggested that the STIM1 p.L374P mutant has a partially active configuration that allows it to translocate to the PM without activating ORAI1 channels and SOCE. Recruitment of STIM1 to ER‐PM junctions is known to depend on the release of a lysine (K)‐rich polybasic domain (PB) in its far C terminus that promotes STIM1 binding to membrane phospholipids (Fig 2A; Liou et al, 2007; Park et al, 2009). To test if the localization of STIM1 p.L374P near the PM is dependent on the PB, we transfected cells expressing GFP‐ORAI1 with WT or mutant m‐Cherry‐STIM1 lacking the PB (ΔK) and analyzed the presence of STIM1 at the PM and its co‐localization with ORAI1 before and after stimulation with TG by TIRFM. Deletion of the PB in WT STIM1‐ΔK had no effect on its TG‐inducible recruitment to the PM and ability to form puncta (Fig EV1B and C) consistent with previous findings in cells overexpressing both STIM1‐ΔK and ORAI1 (Park et al, 2009). Removal of the PB in STIM1 p.L374P‐ΔK had no effect on its localization near the PM before or after TG stimulation. An alternative explanation for the constitutive presence of STIM1 p.L374P near the PM is its binding to ORAI1, and we therefore overexpressed WT or mutant STIM1 in HEK293 cells in which ORAI1 had been deleted by CRISPR/Cas9 gene targeting. However, despite the lack of ORAI1, STIM1 p.L374P was detected at the PM and additional deletion of the PB had no effect on this localization (A.R.C., S.F. unpublished observation), suggesting that ORAI1 is not required for the recruitment of mutant STIM1 to the PM. This finding and the fact that STIM1 p.L374P partially colocalized with ORAI1 (Figs 2E and H, and EV1B and C) prompted us to test whether mutant STIM1 can bind to ORAI1. As expected, we found an increased binding of WT STIM1 to ORAI1 after TG stimulation in co‐immunoprecipitation experiments (Fig EV1D and E). By contrast, STIM1 p.L374P and ORAI1 already co‐immunoprecipitated in unstimulated cells, suggesting that mutant STIM1 retains its ability to bind to ORAI1. Stimulation with TG disrupted this interaction, potentially due to TG‐induced activation of endogenous STIM1 or STIM2, which may outcompete the weaker binding of mutant STIM1 to ORAI1. Together, our results indicate that STIM1 p.L374P has a partially active conformation that promotes its recruitment to ER‐PM junctions. Although this recruitment is not dependent on ORAI1, mutant STIM1 is able to bind to ORAI1 but fails to activate its channel function and SOCE.
STIM1 p.L374P mutation impairs T‐cell proliferation and cytokine production
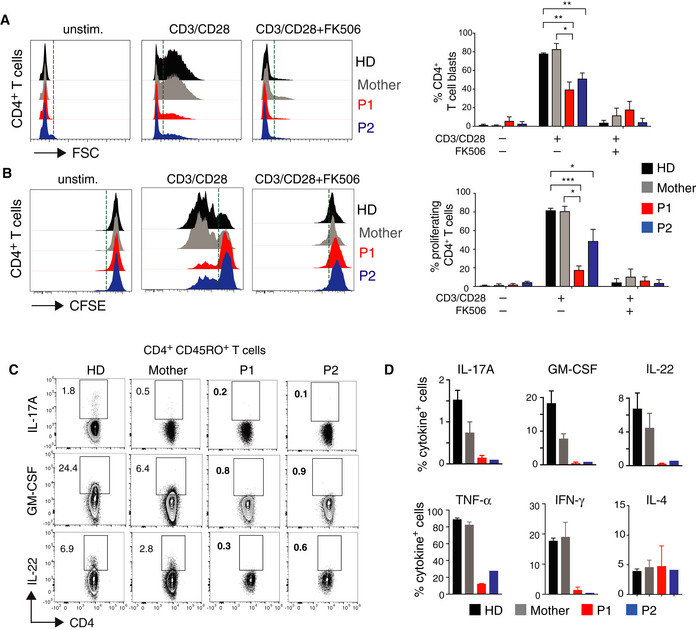
As patients with STIM1 p.L374P mutation suffered from CID, we analyzed the composition of their immune cells. Most immune cell populations were only marginally altered (Appendix Table S1). The number of naïve CD4+CD45RO–CCR7+ T cells was strongly decreased in P1 and P2 compared to their mother and a HD control, whereas the frequencies of CD4+CD45RO+CCR7– effector memory T cells were increased (S.K., S.F. unpublished observations), which was most likely a consequence of the patients’ chronic and recurrent infections (McCarl et al, 2009; Lian et al, 2018). To analyze whether the STIM1 p.L374P mutation impairs lymphocyte function, we first assessed the response of T cells isolated from the patients’ PBMC to stimulation by anti‐CD3 and anti‐CD28 crosslinking. Stimulation of HD and maternal CD4+ T cells resulted in the formation of T‐cell blasts, whereas no increase in cell size was observed in T cells of P1 and P2 (Fig 3A), consistent with data previously reported for P1 (Vaeth et al, 2017a). This defect was mimicked by stimulation of HD and maternal T cells in the presence of FK506 (tacrolimus), which inhibits the Ca2+‐dependent phosphatase calcineurin. Stimulation of CD4+ and CD8+ T cells by anti‐CD3/CD28 crosslinking resulted in robust proliferation of the mother's and HD's cells, whereas the majority of T cells of P1 and P2 did not divide (Fig 3B). A similar or even more pronounced proliferation defect was observed in T cells of P1, P2, mother, and HD after treatment with FK506. It is noteworthy that no proliferation defect is observed when T cells of P1 and P2 are first stimulated with PHA and irradiated allogeneic cells and restimulated with anti‐CD3/CD28 (Appendix Fig S2), suggesting that extensive costimulation can bypass the TCR and SOCE dependency of T‐cell proliferation. This is consistent with our previous report that SOCE is critical for the metabolic reprogramming of naïve T cells and cell cycle entry after initial TCR stimulation and the ability of IL‐2 and IL‐7 to bypass this SOCE requirement (Vaeth et al, 2017a). We next measured cytokine expression by CD4+CD45RO+ memory T cells within PBMC isolated from P1, P2, their mother, and a HD after stimulation with PMA and ionomycin in vitro. The production of IFN‐γ, TNF‐α, GM‐CSF, and the Th17 cytokines IL‐17A and IL‐22 was strongly reduced in T cells of P1 and P2 compared to maternal and HD T cells (Fig 3C and D). By contrast, IL‐4 production was comparable in patients and controls. Expression levels of IL‐2, IFN‐γ, and IL‐17A cytokines were also impaired in CD4+ and CD8+ T cells of P1 and P2 that had been cultured in vitro (Appendix Fig S3A and B). Collectively these findings demonstrate that STIM1 function and SOCE are required for the growth, proliferation, and cytokine production of T cells.
Figure 3. STIM1 p.L374P mutation causes defect in T‐cell proliferation and cytokine production.
-
A, BCell size (A) and proliferation (B) of CD4+ T cells from P1 (red), P2 (blue), their mother (gray), and a HD (black) stimulated with anti‐CD3 (5 μg/ml) and anti‐CD28 (10 μg/ml) in the presence or absence of 1 μM FK506 for 24 h. (A) Representative histograms of FSC (left panel) and percentages of T‐cell blasts (defined as cells to the right of the dotted vertical line) analyzed by flow cytometry (right panel). (B) Representative histograms of CFSE dilution (left panel) and percentages of proliferating cells (defined as cells to the left of the dotted vertical line) (right panel). Bar graphs in A and B are the mean ± SEM from two independent experiments.
-
C, DCytokine production by PBMC from P1, P2, the mother, and an unrelated HD after stimulation with PMA (40 ng/ml) and ionomycin (500 ng/ml) for 4 h. Cytokines were analyzed by flow cytometry following surface staining with antibodies against CD3, CD4, and CD45RO, permeabilization and intracellular cytokine staining for GM‐CSF, IL‐22, and IL‐17A. Representative flow cytometry plots (C) and quantification of Th17 (GM‐CSF, IL‐22, IL‐17A), Th1 (TNF‐α, IFN‐γ), and Th2 (IL‐4) cytokines (D). Data represent the mean ± SEM from two independent experiments.
STIM1‐deficient human Th17 cells primed with Candida albicans fail to produce IL‐17A
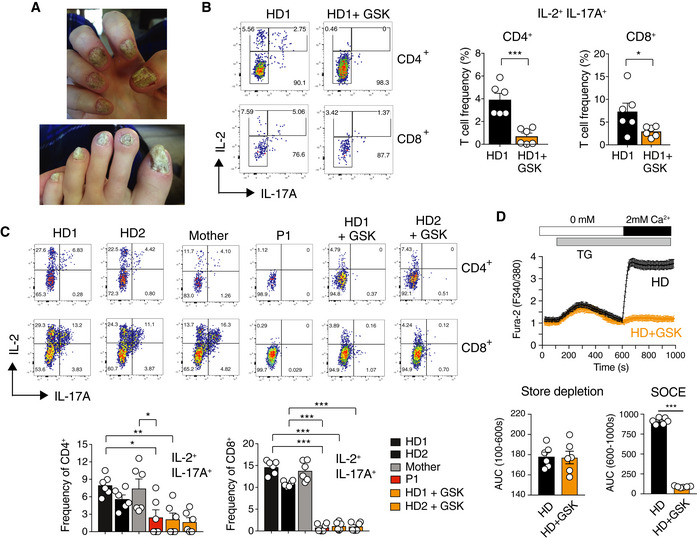
Combined immune deficiency in P1 and P2 was associated with fungal infections including severe onychomycosis in P2 and to lesser degree in P1 (Fig 4A, Table EV1). Local and systemic fungal infections were also reported in other patients with CRAC channelopathy (Table 1), but the immunological mechanisms by which SOCE controls antifungal immunity remain poorly understood. To test how loss of SOCE affects the function of human C. albicans‐specific Th17 cells, we cultured PBMC of a HD for 6 days in the presence of C. albicans and restimulated cells in the presence of the CRAC channel inhibitor GSK‐7975A or DMSO (Rice et al, 2013). CD4+ and CD8+ T cells treated with GSK‐7975A showed a significant defect in IL‐17A production compared to untreated cells (Fig 4B). We next tested if T cells from patients with the STIM1 p.L374P mutation have a similar defect in cytokine production. T cells of a HD or the patients’ mother that were cultured in the presence of C. albicans for several weeks produced robust amounts of IL‐17A, whereas T cells of P1 and GSK‐7975A‐treated T cells from 2 HDs failed to do so (Fig 4C). The extent of SOCE inhibition in T cells of P1 and HD T cells treated with the CRAC channel inhibitor was comparable (Fig 4D). T cells of P1 cultured in the presence of C. albicans also failed to produce TNF‐α and IFN‐γ upon restimulation (Appendix Fig S3C and D). It is noteworthy that IL‐22 production was not detectable under these culture conditions. Our findings demonstrate that SOCE is required for the function of Th17 cells in response to C. albicans infection.
Figure 4. STIM1 and SOCE mediate IL‐17A production of T cells primed with C. albicans .
-
ASevere onychomycosis in P2 (STIM1 p.L374P).
-
BPBMC of a healthy donor (HD1) cultured for 6 days in the presence of 6.5 mg/ml C. albicans protein. At day 6, PBMC were restimulated with PMA and ionomycin in the presence of 10 μM GSK‐7975A or DMSO for 6 h and CD4+ and CD8+ T cells were analyzed for cytokine production by flow cytometry. Bar graphs represent the mean ± SEM from two independent experiments.
-
C, DHuman T cells from two healthy donors, P1, and his mother were expanded and cultured in the presence of C. albicans and IL‐2 for 2–4 weeks. (C) T cells were restimulated with PMA and ionomycin for 6 h in the presence or absence of 10 μM GSK‐7975A and analyzed for cytokine production by flow cytometry. Bar graphs represent the mean ± SEM from two independent experiments. (D) Fura‐2 loaded T cells were stimulated with thapsigargin (TG) in Ca2+‐free buffer followed by readdition of 2 mM Ca2+ to induce SOCE. Bar graphs represent the mean ± SEM from two independent experiments.
Immunity to mucosal Candida albicans infection requires SOCE mediated by STIM1 and STIM2
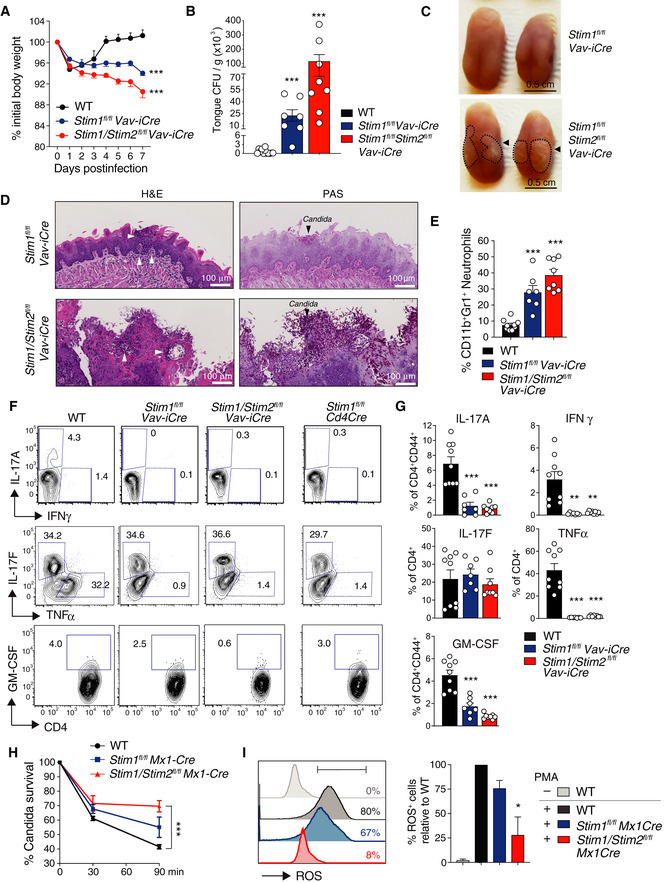
Immunity to mucocutaneous Candida infection is dependent on cells of the innate and adaptive immune system such as neutrophils, macrophages, NK cells, mast cells, γδ T cells, and αβ T cells (Netea et al, 2015). We tested how complete lack of SOCE by genetic deletion of STIM1 and its homologue STIM2 in all immune cells affects immunity to mucosal C. albicans infection. Stim1 fl/fl Vav‐iCre and Stim1/Stim2 fl/fl Vav‐iCre mice, which lack SOCE in lymphoid and myeloid cells, as well as WT control mice were infected sublingually with C. albicans. STIM‐deficient mice showed a significant loss of body weight and dramatic increase in fungal burdens in their tongues 7 days post‐infection (p.i.) compared to WT controls (Fig 5A and B). The tongues of STIM1 and STIM1/2‐deficient mice were covered with white plaques indicative of high fungal burdens and histologically showed severe inflammation with neutrophil infiltration and large epithelial lesions (Fig 5C and D, left). Periodic acid–Schiff (PAS) staining revealed “nests” of C. albicans in the lamina propria of Stim1 fl/fl Vav‐iCre tongues, whereas the tongues of Stim1/Stim2 fl/fl Vav‐iCre mice were completely infiltrated with large numbers of C. albicans hyphae, resulting in the destruction of the normal tongue epithelial architecture (Fig 5C and D, right). The cellular analysis of tongue‐infiltrating immune cells demonstrated higher frequencies of neutrophils in Stim1 fl/fl Vav‐iCre and Stim1/Stim2 fl/fl Vav‐iCre mice 7 days p.i. compared to WT controls (Fig 5E). By contrast, the numbers of CD4+ T cells producing TNF‐α, IL‐17A, GM‐CSF, and IFN‐γ in the draining LNs of Stim1 fl/fl Vav‐iCre and Stim1/Stim2 fl/fl Vav‐iCre mice were reduced compared to controls (Fig 5F and G). Although the numbers of neutrophils were increased in Stim1 fl/fl Vav‐iCre and Stim1/Stim2 fl/fl Vav‐iCre mice 7 days p.i. (Fig 5E), STIM1 and STIM1/STIM2‐deficient neutrophils failed to kill ingested C. albicans (Fig 5H) and had reduced ROS production upon stimulation in vitro (Fig 5I). Together, these data demonstrate that abrogation of SOCE in all immune cells strongly impairs immunity to mucosal C. albicans infection.
Figure 5. STIM1 and STIM2 in immune cells are essential for immunity to mucosal C. albicans infection.
-
A–GSublingual infection of WT, Stim1 fl/fl Vav‐iCre, and Stim1 fl/fl Stim2 fl/fl Vav‐iCre mice with 2 × 107 CFU of C. albicans. (A) Body weight (BW) measured for 7 days and normalized to BW at day 0 of infection. Data are mean ± SEM measured from 4 repeat experiments and 13 WT, 7 Stim1 fl/fl Vav‐iCre, 8 Stim1 fl/fl Stim2 fl/fl Vav‐iCre mice. (B) Quantification of CFU of C. albicans per gram tongue tissue of the indicated mice at day 7 p.i. Data are mean ± SEM from 4 repeat experiments and 12 WT, 7 Stim1 fl/fl Vav‐iCre, 8 Stim1 fl/fl Stim2 fl/fl Vav‐iCre mice. (C) Macroscopic images of the C. albicans‐infected tongues of mice at day 7 p.i. Arrows and dashed lines indicate C. albicans infiltrated areas. (D) Histological images of H&E (left)‐ and PAS (right)‐stained tongue tissues at day 7 p.i. Clusters of polymorphonuclear (PMN) cells (neutrophils, white arrows) and Candida (black arrows) are indicated. (E) Frequencies of Cd11b+Gr1+ neutrophils in the tongues from the indicated mice at day 7 p.i. Bar graphs represent the mean ± SEM from three experiments and 9 WT, 7 Stim1 fl/fl Vav‐iCre, 8 Stim1 fl/fl Stim2 fl/fl Vav‐iCre mice. (F,G) Cytokine production by CD4+ T cells isolated from submandibular lymph nodes of WT, Stim1 fl/fl Vav‐iCre, Stim1 fl/fl Stim2 fl/fl Vav‐iCre, and Stim1 fl/fl Cd4Cre mice 7 days after sublingual infection with 2 × 107 CFU C. albicans. Representative contour plots of cytokine production (F) and percentages of CD4+ T cells producing TNF‐α, IFN‐γ, IL‐17A, IL‐17F, and GM‐CSF (G). Data are the mean ± SEM from two independent experiments and 9 WT, 7 Stim1 fl/fl Vav‐iCre, 8 Stim1 fl/fl Stim2 fl/fl Vav‐iCre mice.
-
H, ICandicidal function and ROS production of neutrophils depend on SOCE. (H) Coculture of Cd11b+Gr‐1+ neutrophils from poly(I:C) treated WT, Stim1 fl/fl Mx1Cre, and Stim1 fl/fl Stim2 fl/fl Mx1Cre mice with C. albicans in vitro for 0, 30, 60, and 90 min (MOI 0.5). Percentages of live C. albicans isolated from neutrophils that were lysed at the indicated time points. Data are the mean ± SEM from 6 mice per genotype and three independent repeat experiments. (I) ROS production by Cd11b+Gr‐1+ neutrophils from poly(I:C) treated WT, Stim1 fl/fl Mx1Cre, and Stim1 fl/fl Stim2 fl/fl Mx1Cre mice was measured after loading of cells with dihydrorhodamine 123 and stimulation with 10 nM PMA for 30 min. Representative histogram plots and bar graphs indicating the percentages of ROS+ neutrophils normalized to WT neutrophils stimulated with PMA. Data are the mean ± SEM from two mice per genotype and two independent repeat experiments.
STIM1 in T cells is required for immunity to systemic Candida albicans infection
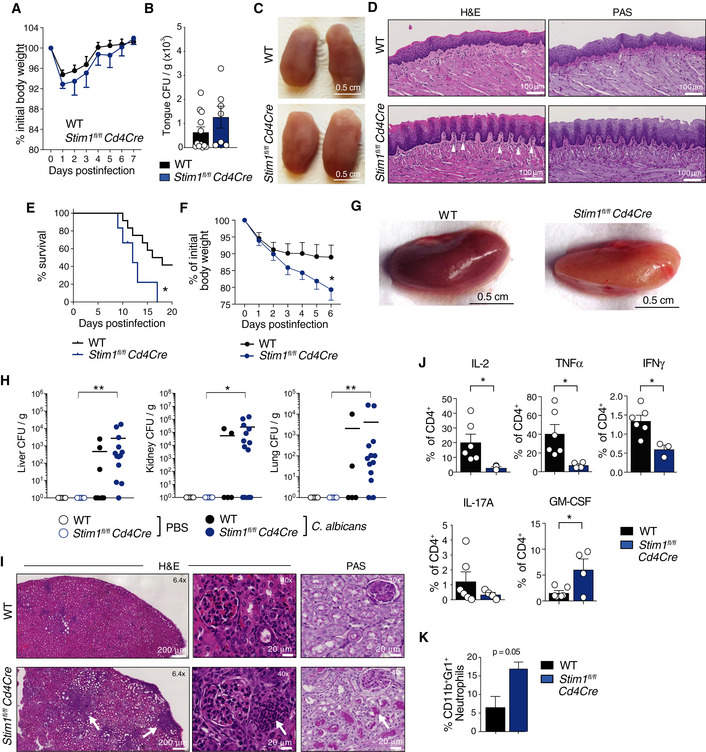
Because of the defect in Th17 cytokine production in P1 and P2 and the known role of Th17 cells in antifungal immunity (Mengesha & Conti, 2017), we analyzed mucosal and systemic antifungal immune responses of mice with conditional STIM1 deletion in T cells, which strongly reduces SOCE (Oh‐Hora et al, 2008; Desvignes et al, 2015). Sublingual infection of Stim1 fl/fl Cd4Cre mice with 2 × 107 CFU of C. albicans did not result in a sustained loss of body weight (Fig 6A) or a significant increase in C. albicans burdens in the tongue 7 days p.i. (Fig 6B) compared to littermate controls. Macroscopically the tongues of Stim1 fl/fl Cd4Cre mice appeared normal without observable signs of C. albicans infection (Fig 6C) and histological analysis by H&E and PAS staining 7 days p.i. showed only moderately more neutrophil infiltration in the lamina propria, small epithelial lesions, and no signs of fungal infiltration compared to tongues of WT mice (Fig 6D). The lack of a more pronounced defect in adaptive immunity to mucosal C. albicans infection occurs despite severely impaired cytokine production by T cells in the absence of STIM1 (Fig 5F), which is consistent with reports that T cell‐deficient Rag1 −/− mice are only moderately more susceptible to oropharyngeal C. albicans infection (Gladiator et al, 2013; Conti et al, 2014).
Figure 6. STIM1 is required for T cell‐mediated immunity to systemic fungal infection.
-
A–DSublingual C. albicans infection of WT and Stim1 fl/fl Cd4Cre mice as described in Fig 5. (A) Body weight (BW) change measured for 7 days p.i. relative to BW at the day of infection. Data are mean ± SEM from 4 repeat experiments and 13 WT and 7 Stim1 fl/fl Cd4Cre mice. (B) Quantification of CFU of C. albicans isolated from the tongues of mice at day 7 post‐infection (p.i.). Data are mean ± SEM from 4 repeat experiments and 7–13 mice per genotype. (C) Macroscopic images of the C. albicans‐infected tongues of mice at day 7 p.i. (D) Histological images of H&E (left) and PAS‐stained (right) tongue tissues at day 7 p.i. Scale bars 100 μm. Arrows point to clusters of polymorphonuclear (PMN) cell (neutrophil) infiltrates.
-
E–KSystemic C. albicans infection. WT and Stim1 fl/fl Cd4Cre mice were injected i.v. with 2 × 105 CFU C. albicans or PBS as control. (E) Kaplan–Meier plot showing survival of mice. Data from 5 Stim1 fl/fl Cd4Cre mice and 12 WT mice is shown. (F) Body weight (BW) of mice normalized to BW at the day of infection. Data are the mean ± SEM from 4 independent experiments and 8 WT and 7 Stim1 fl/fl Cd4Cre mice. (G) Representative macroscopic images of kidneys at day 6 p.i. (H) CFUs of C. albicans per gram tissue isolated from the liver, the right kidney, and the lung of mice at day 6 p.i. CFU values where transform to CFU+1 to plot titers on log10 scale. Each dot represents one mouse. Horizontal lines represent mean CFU values. (I) Representative histological images of H&E‐ and PAS‐stained kidneys of mice at day 6 p.i. Magnification 6.4× (left) and 40× (right). White arrows define areas infiltrated with leukocytes (H&E) and C. albicans (PAS). (J) Frequencies of CD4+ T cells isolated from the spleens of mice at day 6 p.i. producing the indicated cytokines after restimulation with 20 nM PMA and 1 μM ionomycin for 6 h and analyzed by flow cytometry. (K) Frequencies of CD45+Cd11b+Gr‐1+ neutrophils in the blood of mice at day 6 post‐infection analyzed by flow cytometry. Data in J are the mean ± SEM from two independent experiments and 6 WT and 4 Stim1 fl/fl Cd4Cre mice; data in K are the mean ± SEM from one experiment and 2 WT and 3 Stim1 fl/fl Cd4Cre mice.
At least one patient with CRAC channelopathy developed C. albicans sepsis (Table 1; Lian et al, 2018), suggesting that SOCE may be required for immunity to systemic fungal infection. We therefore challenged WT and Stim1 fl/fl Cd4Cre mice with a single i.v. injection of C. albicans (or PBS as control). The survival rate of WT mice at 20 days p.i. with C. albicans was ~ 40% whereas all infected Stim1 fl/fl Cd4Cre mice had died by day 18 (Fig 6E). Infected Stim1 fl/fl Cd4Cre mice lost ~ 20% of their body weight at 6 days p.i. compared to ~ 10% in WT littermates (Fig 6F). To assess fungal burdens of different organs during systemic candidiasis, we harvested kidney, liver, and lung of mice 6 days p.i. The kidneys of Stim1 fl/fl Cd4Cre mice appeared paler than those of WT controls consistent with C. albicans dissemination (Fig 6G). The fungal burdens detected in tissue homogenates of kidneys, livers, and lungs from Stim1 fl/fl Cd4Cre mice were markedly higher at 6 days p.i. compared to WT mice (Fig 6H). H&E staining of kidneys from C. albicans‐infected Stim1 fl/fl Cd4Cre mice showed a strong focal infiltration of immune cells that was markedly more pronounced than in WT mice, and PAS staining revealed higher fungal burdens in Stim1 fl/fl Cd4Cre kidneys (Fig 6I). To analyze T‐cell function after systemic C. albicans infection, we isolated splenocytes from WT and Stim1 fl/fl Cd4Cre mice at 6 days p.i. and restimulated them ex vivo. The frequencies of STIM1‐deficient CD4+ T cells producing IL‐2, TNF‐α, IFN‐γ, and IL‐17A were significantly reduced compared to WT controls, whereas the percentage of GM‐CSF‐secreting CD4+ T cells was increased (Fig 6J). GM‐CSF functions as a chemoattractant for neutrophils in vivo (Khajah et al, 2011). Consistent with the enhanced frequencies of GM‐CSF+ T cells in Stim1 fl/fl Cd4Cre mice, the frequencies of neutrophils in the blood of C. albicans‐infected Stim1 fl/fl Cd4Cre mice were markedly increased 7 days p.i. compared to WT controls (Fig 6K), whereas neutrophil frequencies in the bone marrow of infected WT and Stim1 fl/fl Cd4Cre mice were similar (Appendix Fig S4). Together, these data demonstrate that STIM1 in T cells is critical for immunity to systemic fungal infection.
STIM1 differentially regulates gene expression in pathogenic and non‐pathogenic Th17 cells
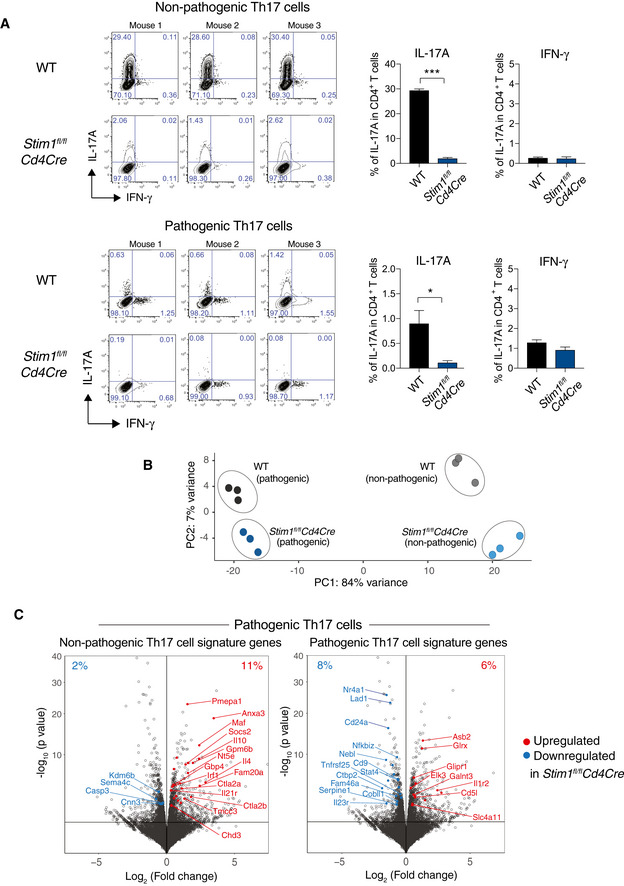
Non‐pathogenic Th17 cells play an important role in antifungal immunity (Netea et al, 2015), and our data show that STIM1 controls the production of cytokines promoting antifungal immunity including IL‐17A, TNF‐α, and IFN‐γ. Importantly, non‐pathogenic Th17 cells differ in their gene expression (and thus likely their function) from pathogenic Th17 cells that promote inflammation in a variety of autoimmune diseases (Lee et al, 2012; Gaublomme et al, 2015). We therefore investigated how STIM1 and SOCE affect gene expression in non‐pathogenic and pathogenic Th17 cells. We isolated CD4+ T cells from Stim1 fl/fl Cd4Cre mice and WT controls and differentiated them in vitro into non‐pathogenic Th17 cells (with IL‐6, TGF‐β) or pathogenic Th17 cells (IL‐23, IL‐6, IL‐1β) for 2 days. IL‐17A production was strongly impaired in pathogenic and non‐pathogenic Th17 cells from Stim1 fl/fl Cd4Cre mice (Fig EV2A). Transcriptome analysis by RNA sequencing and a subsequent principal component analysis (PCA) showed that non‐pathogenic and pathogenic Th17 cells of WT origin were markedly distinct from one another in PC1, with additional variances between WT and Stim1 fl/fl Cd4Cre Th17 cells found in PC2 (Fig EV2B). The total number of differentially expressed genes (DEG) in WT and STIM1‐deficient non‐pathogenic Th17 cells was 2,880, whereas in pathogenic Th17 cells this number was 622 (Fig 7A). 387 DEG were shared between non‐pathogenic and pathogenic Th17 cells in the absence of STIM1, which included genes like Foxp3 and Maf that were upregulated in non‐pathogenic and pathogenic Stim1 fl/fl Cd4Cre Th17 cells.
Figure 7. RNA sequencing of non‐pathogenic and pathogenic Th17 cells.
-
AIL‐17A and IFN‐γ expression of CD4+ T cells isolated from WT and Stim1 fl/fl Cd4Cre mice, differentiated into non‐pathogenic (np) and pathogenic (p) Th17 cells for 2 days and then analyzed by RNA sequencing. Data are the mean ± SEM from 3 mice per genotype.
-
BPrincipal component analysis (PCA) of CD4+ T cells from three WT and three Stim1 fl/fl Cd4Cre mice differentiated into non‐pathogenic npTh17 and pTh17 cells in vitro. Each dot represents one biological replicate (donor mouse).
-
CVolcano plots of differentially expressed genes (DEGs) in pathogenic Th17 cells. Highlighted in red (upregulated in Stim1 fl/fl Cd4Cre) and blue (downregulated in Stim1 fl/fl Cd4Cre) are DEGs that belong to a gene expression signature of npTh17 cells (left plot) and pTh17 cells (right plot) defined previously (Lee et al, 2012; Gaublomme et al, 2015). The corresponding DEG analysis of npTh17 cells is shown in Fig 7B and C.
Figure 7. STIM1 differentially regulates expression of genes associated with pathogenic and non‐pathogenic Th17 cells.
-
ADifferentially expressed genes (DEG) in WT and STIM1‐deficient npTh17 and pTh17 cells. P adj. < 0.10.
- B
-
CNormalized expression of Th17 cell‐associated cytokines and receptors in npTh17 cells derived from WT and Stim1 fl/fl Cd4Cre mice. Bar graphs represent the mean ± SEM from 3 mice per group. Statistical analysis by unpaired Student's t‐test with the following significance levels: *P < 0.05, ***P < 0.001.
-
DSummary of KEGG pathway analysis of npTh17 and pTh17 cells. Venn diagram shows pathways that are dysregulated in one or both Th17 subsets. P adj. < 0.05 for all pathways. Some unique and shared pathways are indicated.
-
EKEGG network analysis of npTh17 cells. P adj. < 0.05 for all pathways.
-
FHeat map of DEGs (P adj. < 0.1) within the KEGG pathways “Foxo signaling” and “HIF‐1 signaling” that are specifically dysregulated in npTh17 cells. Numerical values are relative gene expression.
-
GGene set enrichment analysis (GSEA) of DEGs in WT and STIM1‐deficient npTh17 cells. Normalized enrichment score (NES) and P‐values are as indicated for the following genesets: Myc targets: “Menssen_myc_targets”; Glycolysis: “Humancyc_mm_glycolysis_i”; Mitochondrial function: “Wong_mitochondria_gene_module”; TCA cycle: “Kegg_mm_citrate_cycle”.
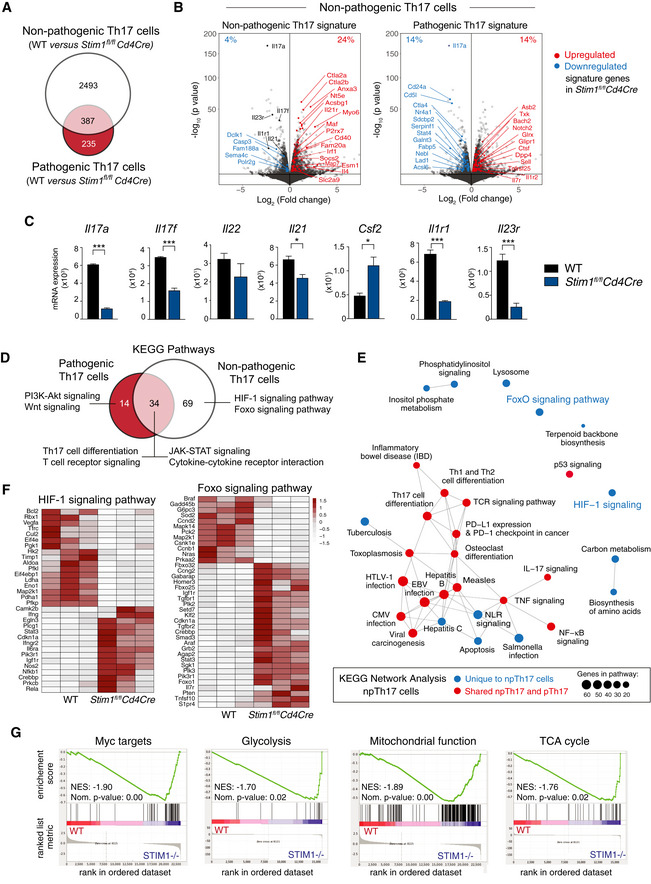
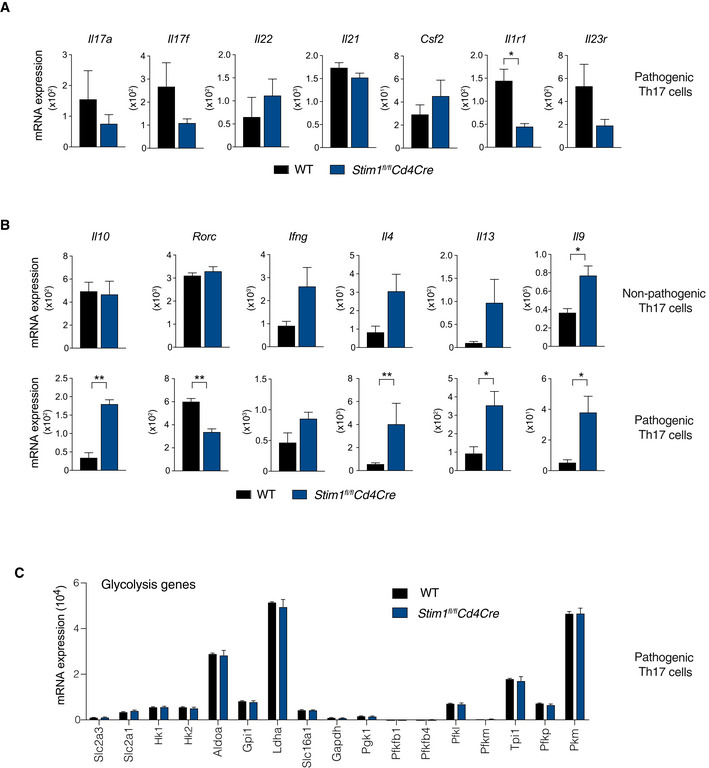
We next compared DEG in non‐pathogenic Th17 cells to published gene expression signatures of non‐pathogenic and pathogenic Th17 cells (Fig 7B; Lee et al, 2012; Gaublomme et al, 2015). Surprisingly, most non‐pathogenic Th17 cell signature genes were upregulated in non‐pathogenic Stim1 fl/fl Cd4Cre Th17 cells, whereas pathogenic Th17 cell signature genes were equally up‐ and downregulated (Fig 7B), resulting in an overall bias toward a non‐pathogenic Th17 gene expression signature in the absence of STIM1. Some genes, however, that are essential for Th17 cell differentiation, such as the cytokine receptors Il1r1 and Il23r, and effector functions, including the cytokines Il17a, Il17f, and Il21, were significantly downregulated in the absence of STIM1 (Fig 7C). An exception was Csf2 whose upregulation in STIM1‐deficient non‐pathogenic Th17 cells is consistent with the increased GM‐CSF expression in CD4+ T cells isolated from Stim1 fl/fl Cd4Cre mice after systemic C. albicans infection (Fig 6J). Together, these findings demonstrate that deletion of STIM1 results in a specific reduction of Th17‐associated cytokines and cytokine receptors, possibly explaining the impaired antifungal immunity of Stim1 fl/fl Cd4Cre mice, whereas most non‐pathogenic Th17 signature genes (including Rorc encoding RORγt) were not impaired or upregulated indicating that the overall identity of non‐pathogenic Th17 cells is intact in the absence of STIM1. We also analyzed how deletion of STIM1 affects gene expression in pathogenic Th17 cells. We observed a downregulation of many published pathogenic Th17 signature genes in the absence of STIM1 (Lee et al, 2012; Gaublomme et al, 2015), whereas non‐pathogenic Th17 signature genes were upregulated compared to WT (Fig EV2C). The expression of Th17 cytokines and cytokine receptors was reduced in STIM1‐deficient pathogenic Th17 cells, albeit to a lesser degree than in non‐pathogenic Th17 cells (Fig EV3A). It is noteworthy that several non‐Th17 cytokines were expressed at higher levels in both pathogenic and non‐pathogenic Th17 cells in the absence of STIM1 including Il4, Il13, and Il9 (Fig EV3B). These data demonstrate that deletion of STIM1 in pathogenic Th17 cells results in a shift toward a non‐pathogenic Th17 gene signature and partial loss of pathogenic Th17 cell identity.
Figure 8. Expression of cytokines, cytokine receptors, and glycolysis‐associated genes in STIM1‐deficient Th17 cells.
-
A, BAnalysis of mRNA expression of the indicated cytokines, cytokine receptors, and transcription factors in CD4+ T cells from WT and Stim1 fl/fl Cd4Cre mice that were differentiated into pathogenic (A) or pathogenic and non‐pathogenic (B) Th17 cells in vitro for 2 days and then analyzed by RNA sequencing.
-
CAnalysis of mRNA expression of glycolytic enzymes in CD4+ T cells from WT and Stim1 fl/fl Cd4Cre mice that were differentiated into pathogenic Th17 cells in vitro for 2 days and then analyzed by RNA sequencing.
STIM1 controls several metabolic pathways in non‐pathogenic Th17 cells
Because STIM1 deletion only impaired the production of Th17 cytokines but did not interfere with the transcriptional identity of non‐pathogenic Th17 cells, we further analyzed STIM1 regulated pathways in non‐pathogenic Th17 cells that could explain the impaired antifungal immunity of Stim1 fl/fl Cd4Cre mice. KEGG pathway analyses revealed 34 pathways that were dysregulated in both STIM1‐deficient pathogenic and non‐pathogenic Th17 cells, which included Th17 cell differentiation, TCR signaling and JAK‐STAT signaling pathways (Fig 7D). In addition, we identified 69 pathways that were uniquely dysregulated in STIM1‐deficient non‐pathogenic Th17 cells. These included the HIF‐1, Foxo1 and phosphatidylinositol signaling pathways, carbon metabolism, and biosynthesis of amino acids (Fig 7D and E). A KEGG network analysis showed that two of these pathways, HIF‐1 and Foxo1 signaling, were unique in the sense that they did not share genes with any of the other dysregulated pathways in non‐pathogenic Th17 cells (Fig 7E). Within the Foxo signaling pathway most genes, including Foxo1 itself, were upregulated in STIM1‐deficient non‐pathogenic Th17 cells, whereas genes in the HIF‐1 signaling pathway were equally up‐ and downregulated (Fig 7F). HIF1‐α positively regulates expression of genes encoding key glycolytic enzymes, whereas Foxo1 negatively regulates the metabolic fitness of CD4+ T cells through downregulation of myc (Buck et al, 2017; Newton et al, 2018). Gene set enrichment analysis (GSEA) further demonstrated the depletion of myc target genes and genes regulating glycolysis in STIM1‐deficient non‐pathogenic Th17 cells (Fig 7G). Other metabolic pathways were also downregulated in the absence of STIM1, including genes associated with mitochondrial function and the TCA cycle (Fig 7G). Together these findings indicate that STIM1 controls several metabolic pathways in non‐pathogenic Th17 cells including glycolysis through the Foxo‐ and HIF1α‐dependent regulation of myc, as well as TCA cycle function and mitochondrial respiration.
STIM1 regulates aerobic glycolysis and oxidative phosphorylation in non‐pathogenic Th17 cells
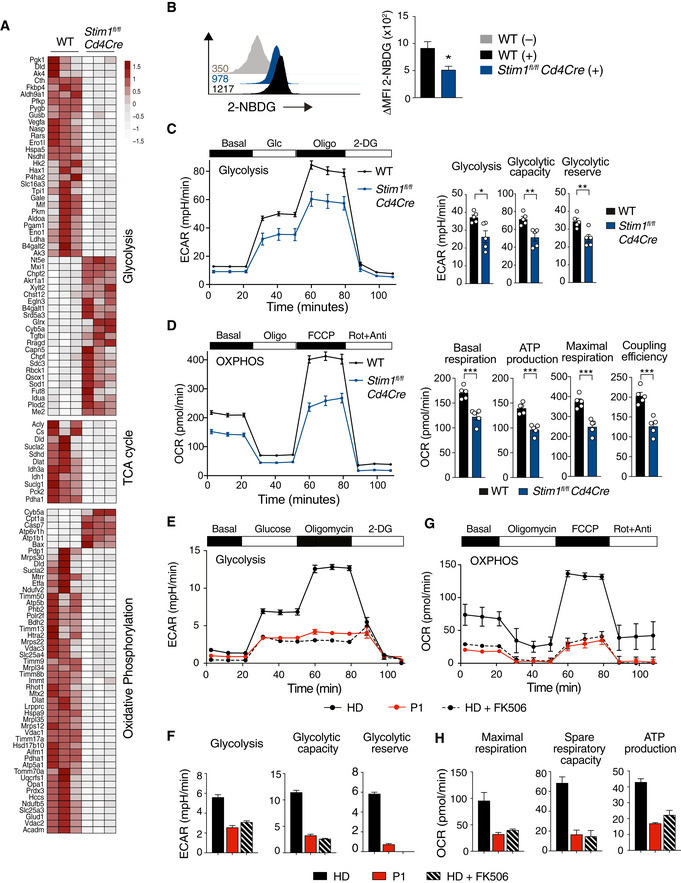
Given the dysregulation of several metabolic pathways in non‐pathogenic Th17 cells lacking STIM1 and our previous reports that SOCE controls glycolytic gene expression in T cells (Vaeth et al, 2017a) and mitochondrial function in pathogenic Th17 cells (Kaufmann et al, 2019), we investigated the role of STIM1 in the metabolism of non‐pathogenic Th17 cells in more detail. We found significantly reduced mRNA levels of genes encoding glycolytic enzymes in non‐pathogenic Th17 cells of Stim1 fl/fl Cd4Cre compared to WT mice, which included hexokinase 2 (Hk2), phosphofructokinase (Pfkp), aldolase A (Aldoa), phosphoglycerate kinase 1 (Pgk1), enolase (Eno1), pyruvate kinase (Pkm), and lactate dehydrogenase A (ldha) (Fig 8A). Apart from glycolysis, numerous genes encoding enzymes of the TCA cycle (including the rate‐limiting isocitrate dehydrogenase homologues Idh1 and Idh3a) and factors promoting mitochondrial respiration such as components of the electron transport chain (ETC) were markedly reduced in STIM1‐deficient non‐pathogenic Th17 cells compared to WT cells (Fig 8A). We next evaluated the role of STIM1 in the glycolytic function of non‐pathogenic Th17 cells. The uptake of the fluorescent glucose analog 2‐NBDG (2‐(N‐(7‐nitrobenz‐2‐oxa‐1,3‐diazol‐4‐yl)amino)‐2‐deoxyglucose) was significantly reduced in STIM1‐deficient non‐pathogenic Th17 cells (Fig 8B). To measure the glycolytic activity, we analyzed the extracellular acidification rate (ECAR) in the supernatant of WT and STIM1‐deficient non‐pathogenic Th17 cells. The basal glycolytic rate, glycolytic capacity, and glycolytic reserve were significantly reduced in Stim1 fl/fl Cd4Cre non‐pathogenic Th17 cells (Fig 8C), consistent with impaired expression of glycolytic enzymes and glucose uptake (Fig 8A and B). It is important to note that deletion of STIM1 in pathogenic Th17 cells had no effect on the mRNA expression of glucose transporters and glycolytic enzymes (Fig EV3C), which is consistent with our previous findings in a different model of pathogenic Th17 cells (Kaufmann et al, 2019) and indicates a crucial difference between the role of SOCE in regulating metabolism in pathogenic and non‐pathogenic Th17 cells. Since our GSEA also showed strongly dysregulated expression of genes involved in the TCA cycle and mitochondrial function, we analyzed mitochondrial respiration and OXPHOS by measuring the oxygen consumption rate (OCR) of non‐pathogenic Th17 cells. The basal respiration of STIM1‐deficient non‐pathogenic Th17 cells as well as their maximal respiration and spare respiratory capacity (SRC) measured after uncoupling of mitochondria with FCCP was significantly reduced (Fig 8D). Collectively, our findings demonstrate that STIM1 controls the function of several metabolic pathways in non‐pathogenic Th17 cells including aerobic glycolysis and oxidative phosphorylation, which is in contrast to pathogenic Th17 cells in which STIM1 controls OXPHOS but not glycolysis.
Figure 8. STIM1 controls aerobic glycolysis and oxidative phosphorylation in non‐pathogenic murine Th17 cells and human T cells.
-
AHeat map of metabolism‐associated DEGs (p (adj.) < 0.10) in npTh17 cells of WT and Stim1 fl/fl Cd4Cre mice determined by RNA‐Seq as described in Fig 7. Heatmap shows relative minimum and maximum values per gene (row min/max). Numerical values are relative gene expression. TCA, tricarboxylic acid.
-
B–DGlucose and mitochondrial metabolism in npTh17 cells from WT (gray or black) and Stim1 fl/fl Cd4Cre (blue)mice differentiated for 3 days in vitro. (B) Glucose uptake by npTh17 cells was measured using 2‐NBDG (+) or not (−) and analyzed by flow cytometry 90 min later. Bar graphs show the delta MFI of 2‐NBDG fluorescence normalized to unlabeled cells. Data represent the mean ± SEM from three independent experiments. (C) Extracellular acidification rate (ECAR) of npTh17 cells before and after addition of 25 mM glucose, 5 μM oligomycin, and 100 mM 2‐deoxy‐d‐glucose (2‐DG). Seahorse graphs and bar graphs depicting glycolysis, glycolytic capacity and glycolytic reserve represent the mean ± SEM from 2 mice per genotype in technical replicates from one representative out of three independent experiments with a total of 6 mice per genotype. (D) Oxygen consumption rate (OCR) of npTh17 cells before and after addition of 1 μM oligomycin, 1.5 μM FCCP, and 100 nM rotenone/1 μM antimycin. Seahorse graphs and bar graphs depicting basal and maximal respiration; coupling efficiency and ATP production represent the mean ± SEM from 2 mice per genotype in technical replicates from one representative out of three independent experiments with a total of 6 mice per genotype.
-
E–HGlucose and mitochondrial metabolism in human CD4+ T cells isolated from PBMC of a HD and P1 and stimulated with 5 μg/ml anti‐CD3 and 10 μg/ml anti‐CD28 for 24 h before analysis. Some HD T cells were stimulated in the presence of 1 μM FK506. (E,F) ECAR before and after addition of 25 mM glucose, 5 μM oligomycin, and 100 mM 2‐DG. Seahorse graphs (E) and bar graphs (F) represent the mean ± SEM of 3 (HD), 4 (P1), and 2 (HD + FK506) technical replicates from isolated T cells from one experiment. (G,H) OCR before and after addition of 1 μM oligomycin, 1.5 μM FCCP, and 100 nM rotenone/1 μM antimycin. Seahorse graphs (G) and bar graphs (H) depicting basal and maximal respiration, SRC and ATP production represent the mean ± SEM of 2 technical replicates from isolated T cells from one experiment.
STIM1 regulates glycolysis, OXPHOS, and mTORC1 signaling in human T cells
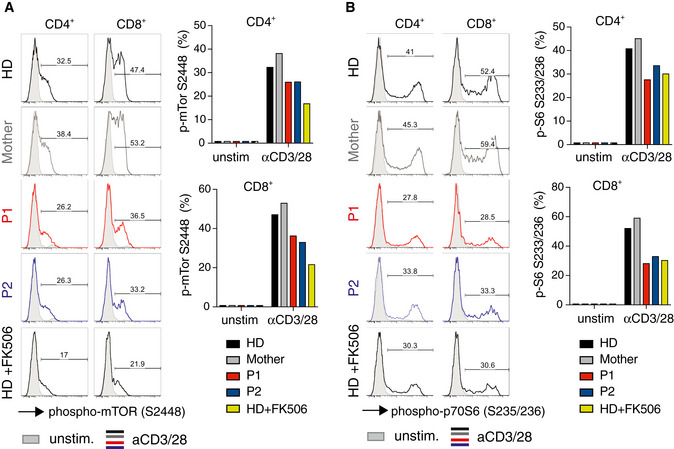
Given the important role of STIM1 for T‐cell metabolism and function in mice, we hypothesized that STIM1 may also regulate the metabolism of human T cells and could thereby be required for their ability to mediate antifungal immunity. To test this hypothesis, we analyzed the glycolytic function and OXPHOS of CD4+ T cells isolated from PBMC of P1 and a HD control that were stimulated with anti‐CD3/CD28 in the presence or absence of the calcineurin inhibitor FK506 for 24 h. STIM1‐deficient human CD4+ T cells had significantly reduced glycolysis and an all but abolished glycolytic reserve after ATP synthase inhibition with oligomycin (Fig 8E and F). Similar defects were observed in HD CD4+ T cells treated with FK506, suggesting that the STIM1 effects on glycolysis are dependent on calcineurin function. Besides impaired glycolysis, freshly isolated CD4+ T cells of the STIM1‐deficient P1 had a severe defect in mitochondrial function. Their basal respiration as well as their spare respiratory capacity, ATP production, and coupling efficiency were strongly reduced compared to CD4+ T cells from a HD (Fig 8G and H). A similar defect in OCR was observed when HD CD4+ T cells were treated with FK506 indicating that STIM1 regulates mitochondrial function in a calcineurin‐dependent manner. It is noteworthy that similar metabolic defects in glycolysis and OXPHOS were not observed in anti‐CD3/CD28 stimulated T cells of P1 and P2 after they had been cultured in vitro for several weeks (Appendix Fig S5A–D), which is consistent with their normal proliferation (Appendix Fig S2). These findings suggest that nutrient‐rich and cytokine‐supplemented cell culture conditions can overcome the metabolic defect of SOCE‐deficient T cells or that SOCE is critical specifically for the metabolic adaptation of naive T cells after initial TCR stimulation. We had recently reported that combined deletion of STIM1 and STIM2 in murine T cells results in impaired activation of the PI3K‐AKT‐mTOR nutrient‐sensing pathway (Vaeth et al, 2017a) accounting, at least partially, for their impaired glycolytic function. To investigate mTOR signaling in STIM1‐deficient T cells, we stimulated PBMC of P1, P2, their mother, and a HD with anti‐CD3/CD28 for 24 h and measured phosphorylation of mTOR (on S2448) and the ribosomal protein the p70S6 (on S235/236). CD4+ and CD8+ T cells of P1 and P2 had a marked defect in mTOR and p70S6 phosphorylation compared to T cells from their mother and a HD (Fig EV4). A comparable decrease in mTOR and p70S6 phosphorylation was observed after FK506 treatment of HD T cells, suggesting that mTOR signaling is regulated by SOCE and calcineurin signaling in ex vivo isolated T cells. Consistent with their normal proliferation and metabolic function, in vitro cultured T cells of P1 and P2 had no defect in mTOR and p70S6 phosphorylation (Appendix Fig S5E and F). Taken together, our data demonstrate that STIM1 plays a conserved role in the glycolytic function and mitochondrial respiration of human and mouse T cells.
Figure 9. Reduced mTOR and S6 phosphorylation in T cells of STIM1‐deficient patients isolated ex vivo .
-
A, BAnalysis of mTOR and p70S6 phosphorylation in CD4+ and CD8+ T cells freshly isolated from PBMC of P1 (red), P2 (blue), their mother (gray), and a HD (black) and stimulated with anti‐CD3/CD28 for 24 h or left unstimulated. HD T cells were stimulated in the presence or absence of 1 μM FK506. Histograms represent intracellular staining of cells with anti‐phospho‐mTOR (Ser2448) (A) and anti‐phospho‐p70S6 (Ser235/236) (B) antibodies. Filled gray histograms represent unstimulated cells. Numbers above gates in histograms and in bar graphs indicate the frequencies of CD4+ and CD8+ T cells positive for phospho‐mTOR and phospho‐p70S6 staining.
Discussion
We here report a novel LOF missense mutation in STIM1 that abolishes SOCE by interfering with STIM1 function, but not its expression. The mutant STIM1 p.L374P protein is constitutively located near the PM and partially colocalizes with ORAI1 even before depletion of ER Ca2+ stores, suggesting it is in a partially active state. The localization of mutant STIM1 at the PM does not depend on its PB or expression of ORAI1, although it is able to bind to ORAI1 as detected by co‐immunoprecipitation. Following store depletion, mutant STIM1 does not form puncta and its co‐localization with ORAI1 does not increase unlike that of WT STIM1. Together these findings indicate that mutant STIM1 p.L374P protein retains its ability to bind to ORAI1 but fails to activate it, resulting in impaired SOCE. One explanation for the impaired activation of ORAI1 channels is that this process requires the formation of STIM1 clusters (Luik et al, 2008). Although mutant STIM1 is located near the PM, where it partially colocalizes with and binds to ORAI1, it fails to form puncta after store depletion, suggesting that the L374P mutation interferes with proper oligomerization of STIM1, which is required for the formation of macromolecular complexes with the correct STIM1:ORAI1 stoichiometry needed for CRAC channel activation (Yen & Lewis, 2019). An additional explanation for impaired SOCE could be that the L374P mutation results in conformational changes that do not abrogate STIM1 binding to ORAI1 but specifically interfere with its ability to gate the CRAC channel. This interpretation is supported by the finding that the binding of mutant STIM1 to ORAI1 observed in co‐IP experiments is lost after cell stimulation, likely because it is replaced by active endogenous WT STIM1 that has a higher affinity for ORAI1. This changed or weaker binding implies that the interaction of mutant STIM1 with ORAI1 may be different in a fundamental way and prevents proper ORAI1 gating.
This behavior of STIM1 p.L374P is similar to that of another LOF mutation, STIM1 p.R429C, we had reported earlier (Maus et al, 2015). L374 and R429 are located in the second (CC2) and third (CC3) coiled‐coil domains, respectively, within the cytosolic region of STIM1, which together form the cytoplasmic CAD (SOAR or CCb9) domain of STIM1 that is required for ORAI1 binding and CRAC channel activation (Kawasaki et al, 2009; Park et al, 2009; Yuan et al, 2009). Different models of STIM1‐ORAI1 interaction exist based on available crystal and NMR structures (Yang et al, 2012; Stathopulos et al, 2013), and there are several potential explanations why the STIM1 p.L374P mutation interferes with SOCE. The NMR structure of a STIM1 (aa 312–387) – ORAI1 (aa 272–292) dimer complex (Stathopulos et al, 2013) shows that L374 is centrally located near each STIM‐ORAI interaction site with hydrophobic residues that directly contact ORAI1‐C272–292 located N‐ and C‐terminally to L374. Our NMR‐based model suggests that loss of the free backbone NH due to the L374P substitution results in an altered hydrogen bond network near the mutation, which perturbs the structure and side‐chain orientations in the immediate vicinity of L374P that are important for STIM1‐ORAI1 interaction.
The crystal structure of the human STIM1 CAD dimer has a “V”‐shaped structure (Yang et al, 2012) with two apex regions critical for interactions with ORAI1 (Yang et al, 2012; Zhou et al, 2015). Our homology model of the M374P mutation in the context of the CAD crystal structure shows a movement of each apex position by ~6.4 Å compared to the template dimer (note that the CAD template is a L374M/V419A/C437T triple mutant engineered to promote crystallization and yield a high‐resolution structure (Yang et al, 2012)). This apex movement is caused by the perturbation of the backbone hydrogen bond network and a limited backbone rotation around proline. These structural changes increase the distance between the apex regions within dimers (a ~5.0 Å increase in the F394‐F394′ intermolecular distance), which is predicted to interfere with STIM1 binding to ORAI1 in a proposed bimolecular STIM‐ORAI interaction model in which each STIM1 dimer subunit interacts with one ORAI1 subunit of the same hexameric CRAC channel complex (Stathopulos et al, 2013). Interestingly, M374P in CC2 is located very near the intramolecular CC3 interface in the CAD crystal structure (Yang et al, 2012). Given the altered hydrogen bonding around M374P, the position of residue L373 is likely perturbed due to the L374P mutation, potentially triggering a conformational extension in the cytosolic CC domains of STIM1, which is consistent with the finding that a L373S mutation causes a similar conformational extension of STIM1 (Frischauf et al, 2009; Zhang et al, 2013). Together, our data support a model in which L374 is required to keep STIM1 in a resting state and regulate its proper binding to ORAI1.
Increased susceptibility to fungal infections in humans is caused by a wide range of defects in innate and adaptive immunity. SCID and CID due to defects in T‐cell development and function, respectively, are associated with fungal infections including chronic mucocutaneous candidiasis (CMC) and Pneumocystis jirovecii pneumonia (Lanternier et al, 2013). Defects in IL‐17 signaling and Th17 cell function cause CMC, which may result from inherited defects in IL‐17RA, IL‐17RC, and IL‐17F genes, LOF mutations in STAT3, or GOF mutations in STAT1 (Hernandez‐Santos & Gaffen, 2012). Mutations in AIRE resulting in autoimmune polyglandular syndrome type 1 (APS1) are associated with the formation of autoantibodies against IL‐17A, IL‐17F, and IL‐22 and CMC (Lanternier et al, 2013; Mengesha & Conti, 2017). Patients with CRAC channelopathy due to LOF mutations in ORAI1 and STIM1 suffer from life‐threatening viral and bacterial infections early in life (Feske, 2010; Vaeth & Feske, 2018). Several patients have been reported to also suffer from fungal infections (McCarl et al, 2009; Badran et al, 2016; Klemann et al, 2017; Lian et al, 2018). Most of them had mucosal or cutaneous C. albicans infections, and at least one had gastrointestinal candidiasis, whereas others suffered from infections with Aspergillus fumigatus or Pneumocystis jirovecii. The most severe form of fungal infection associated with CRAC channelopathy reported to date was a systemic C. albicans infection in a patient with ORAI1 p.L194P LOF mutation (Lian et al, 2018). The patients with STIM1 p.L374P mutation reported here presented with severe onychomycosis. Since most fungal infections in CRAC channel‐deficient patients are not life‐threatening, it is possible that their incidence is underreported. The underlying causes of increased susceptibility to fungal infection in these patients likely involve a combination of defects in innate and adaptive immunity. We here show that SOCE is required for immunity to mucosal infection with C. albicans infection as deletion of STIM1 or both STIM1 and STIM2 in all immune cells (including T cells, ILCs, neutrophils, and macrophages) strongly exacerbated the severity of mucosal candidiasis. Abolishing SOCE compromised the ability of neutrophils to kill ingested C. albicans and to produce ROS upon PKC stimulation, likely contributing to impaired immunity to mucosal fungal infection.
By contrast, T cell‐specific deletion of STIM1 did not result in increased susceptibility to mucosal Candida infection. This finding is in line with the observation that complete deletion of T and B cells in Rag1 −/− mice results in only moderately increased C. albicans burdens at day 7 p.i. (Gladiator et al, 2013; Conti et al, 2014). In humans, by contrast, T cells play an important role in adaptive immunity to mucosal candidiasis as evidenced by the high susceptibility of AIDS and T cell‐deficient patients to C. albicans infections. We here show that SOCE is essential for the function of Candida‐primed human T cells. T cells of HDs cultured in the presence of C. albicans produced robust amounts of IFN‐γ, TNF‐α, and IL‐17A, cytokines that are important for immunity to fungal infection (Conti & Gaffen, 2015; Netea et al, 2015). Cytokine expression was abolished, however, in HD T cells treated with a CRAC channel inhibitor and in T cells of patients with the STIM1 p.L374P mutation. The limited role of murine T cells in immunity to mucosal candidiasis may be due to the fact that mice housed under SPF conditions are immunologically naive to C. albicans and infection causes an acute immune response (Conti et al, 2014). Nevertheless, we found that SOCE in murine T cells is critical for immunity to systemic fungal infection. T cell‐specific deletion of STIM1 resulted in increased weight loss, fungal dissemination, and mortality after systemic C. albicans infection of Stim1 fl/fl Cd4Cre mice compared to WT littermates, which was associated with reduced production of IFN‐γ, TNF‐α, and IL‐17A in the absence of STIM1. These findings are consistent with the important role of Th1 and Th17 cells for immunity to systemic fungal infection (Netea et al, 2003; Huang et al, 2004; van de Veerdonk et al, 2010; Shao et al, 2019). Furthermore, the increased kidney inflammation of Stim1 fl/fl Cd4Cre mice we observed after systemic C. albicans infection is consistent with a similar kidney pathology due to conditional deletion of IL‐17RA in renal tubular epithelial cells (RTEC) (Ramani et al, 2018), indicating that IL‐17 signaling is critical to prevent kidney inflammation and damage after systemic C. albicans infection.
Th17 cells are essential for immunity to fungal pathogens and extracellular bacteria (Curtis & Way, 2009). Pathogenic Th17 cells, on the other hand, promote inflammation in a variety of autoimmune disorders such as MS, colitis, rheumatoid arthritis (RA), and psoriasis (Stockinger & Omenetti, 2017) emphasizing the dual role of Th17 cells in immunity. We previously showed that SOCE is essential for pathogenic Th17 cell function as mice with T cell‐specific deletion of ORAI1, STIM1 or STIM2 were protected in Th17 cell‐dependent animal models of autoimmune diseases such as EAE and colitis (Ma et al, 2010; McCarl et al, 2010; Schuhmann et al, 2010; Kaufmann et al, 2016). In another model of pathogenic Th17 cell‐driven disease, caused by expression of a hyperactive form of STAT3, we demonstrated that STIM1 and SOCE are required to cause pulmonary and skin inflammation (Kaufmann et al, 2019). At the molecular level, STIM1 was found to regulate the expression of many genes required for mitochondrial function, especially OXPHOS.
We here show that SOCE is also critical for the function of non‐pathogenic Th17 cells during C. albicans infection and that SOCE has markedly different effects on gene expression and the molecular function of non‐pathogenic and pathogenic Th17 cells. In the absence of STIM1, the majority of genes associated with a non‐pathogenic Th17 cell signature (Lee et al, 2012; Gaublomme et al, 2015) were upregulated in Th17 cells of Stim1 fl/fl Cd4Cre mice. By contrast, genes associated with a pathogenic Th17 cell signature were equally up‐ and downregulated in the absence of STIM1. These findings suggest that STIM1‐deficient non‐pathogenic Th17 cells largely maintain their molecular identity with the exception of cytokine production (IL‐17A, IL‐17F), whereas deletion of STIM1 results in a greater loss of genes previously shown to define pathogenic Th17 cells.
We furthermore found that lack of STIM1 has differential effects on key metabolic pathways in non‐pathogenic and pathogenic Th17 cells. STIM1‐deficient non‐pathogenic Th17 cells had impaired expression of many glycolysis‐associated genes, which was not observed in pathogenic Th17 cells. A likely mechanistic explanation for this finding is that many genes associated with HIF‐1α and myc signaling were downregulated in STIM1‐deficient non‐pathogenic Th17 cells, whereas most genes in the Foxo signaling pathway including Foxo1 itself were upregulated. HIF‐1α and myc positively regulate several key glycolytic enzymes, whereas Foxo1 negatively regulates myc expression (Buck et al, 2017; Newton et al, 2018). Accordingly, we observed significantly reduced glucose uptake and glycolysis in STIM1‐deficient non‐pathogenic Th17 cells. These findings are consistent with the critical role of SOCE in regulating the expression of glucose transporters and glycolytic enzymes by controlling HIF‐1α, myc, and IRF4 levels in naive T cells after TCR stimulation (Vaeth et al, 2017a). Importantly, the dependence of glycolysis on STIM1 was specific to non‐pathogenic Th17 cells and not observed in pathogenic Th17 cells. Normal glycolysis in STIM1‐deficient pathogenic Th17 cells is in line with our previous study of STAT3‐dependent pathogenic Th17 cells (Kaufmann et al, 2019). Besides glycolysis, STIM1 also regulates the expression of TCA cycle enzymes and mitochondrial ETC complexes in non‐pathogenic Th17 cells. Accordingly, OXPHOS was markedly reduced in these cells. The role of SOCE in mitochondrial function is shared between non‐pathogenic and pathogenic Th17 cells. This finding is consistent with the fact that deletion of STIM1 in STAT3‐dependent pathogenic Th17 cells abolishes expression of many mitochondrial ETC genes and impairs OXPHOS (Kaufmann et al, 2019). Collectively our data demonstrate that SOCE is essential for mitochondrial function and OXPHOS in both pathogenic and non‐pathogenic Th17 cells, whereas its role in glycolysis is specific to non‐pathogenic Th17 cells. Similar defects in glycolysis and mitochondrial respiration were observed in T cells of the STIM1 p.L374P mutant patients.
Taken together, we here show that STIM1 is a critical regulator of antifungal immunity by regulating the expression of cytokines and metabolic pathways in non‐pathogenic Th17 cells, which may explain the increased susceptibility of patients with STIM1 and ORAI1 mutations to fungal infections. It is intriguing to speculate that the different roles of SOCE in the metabolic regulation of non‐pathogenic Th17 cells (OXPHOS and glycolysis) and pathogenic Th17 cells (OXPHOS, but not glycolysis) may potentially be exploited for the treatment of Th17‐cell‐mediated autoimmune diseases such as MS or rheumatoid arthritis without compromising antifungal immunity.
Materials and Methods
Patients
Detailed case reports of P1 and P2 are provided in Supplemental Information. Written informed consent was obtained from both patients, in accordance with research ethics board policies at the University of British Columbia and Oregon Health & Science University. Experiments using deidentified cell samples of patients were conducted with Institutional Review Board approval at the New York University School of Medicine.
Mice
The generation of Stim1 fl/fl and Stim1 fl/fl Stim2 fl/fl has been described before (Oh‐Hora et al, 2008). These mice were further crossed to Cd4‐Cre mice (B6.Cg‐Tg(Cd4‐cre)1Cwi/BfluJ, JAX strain 22071), Vav‐iCre mice (B6.Cg‐Commd10Tg(Vav1‐icre)A2Kio/J, JAX strain 008610), and Mx1Cre mice (B6.Cg‐Tg(Mx1‐cre)1Cgn/J, JAX strain 003556) as described (Vaeth et al, 2015; Saint Fleur‐Lominy et al, 2018). To induce deletion of Stim1 and Stim2 genes in hematopoietic lineage cells, Stim1 fl/fl Mx1Cre and Stim1 fl/fl Stim2 fl/fl Mx1Cre mice were injected i.p. with 10 μg/g body weight of poly(I:C) (Sigma‐Aldrich) every other day for 1 week followed by 1 month without treatment. Mice from both sexes were evenly used for all experiments and were between 8 and 14 weeks old at the time of analysis. All mice are on the C57BL/6 genetic background. Animals were maintained under SPF conditions in accordance with institutional guidelines for animal welfare approved by the IACUC at NYU School of Medicine.
Cell lines
Flip‐in T‐Rex HEK293 cells with inducible GFP‐ORAI1 expression were a gift of T. Shuttleworth (University of Rochester, Rochester, NY). Cells were maintained in DMEM (Fisher) supplemented with 10% FCS, 2 mM l‐glutamine, 100 U/ml, and 0.1 mg/ml streptomycin (Sigma‐Aldrich) at 37°C, 10% CO2. GFP‐ORAI1 expression was induced by addition of 1 μg/ml doxycycline (Sigma‐Aldrich) for 24 h as described previously (Thompson & Shuttleworth, 2012; Maus et al, 2015).
DNA sequencing analysis
Genomic DNA was extracted from PBMC using the Flexigene DNA Kit (QIAGEN). PCR was conducted using primers flanking all 12 exons of the STIM1 gene. Primers used to amplify and sequence exon 8 were as follows: fwd5′ AAAGCAGATAAGAAGTCTGAGTTCTG and rev5′ ACCACCAGGATATCTCTTCAC; PCR products were separated on 1.5% agarose gel, excised using the QIAGEN gel extraction kit and sequenced directly (Macrogen Inc., NY) using the same PCR primers.
Genetic analysis
Variant analysis was performed using the Genome Aggregation Database (gnomAD) https://gnomad.broadinstitute.org. To predict the deleteriousness of the p.L374P mutation, the Combined Annotation‐Dependent Depletion (CADD) score was obtained using https://cadd.gs.washington.edu/snv and the CADD model GRCh37‐v1.4. The genomic position of the mutation was determined using https://mutalyzer.nl and human genome assembly GRCh37 (hg19).
Homology modeling of STIM1 p.L374P mutant
Twenty homology models of M374P CAD/SOAR/CCb9 were generated using 3TEQ.pdb (which harbors the L374M/V419A/C437T triple mutation) as the template structure in MODELLER (version 9.16) (Webb and Sali 2014) to model the structural changes caused by the human p.L374P mutation. Similarly, 20 homology models of the human p.L374P CC1TM‐distal‐CC2:ORAI1‐C272‐292 complex were generated using the lowest energy structure of 2MAK.pdb as the template structure in MODELLER. The models with the lowest DOPE score were taken for visualization and structural analyses. These models orient the mutant side chains in positions that are homologous to the template residues. All structure figures, distance measurements, and backbone hydrogen bond identifications were done using PyMOL (Version 1.7.4, Schrödinger, LLC).
Intracellular Ca2+ measurements
Fibroblasts, T cells, and PBMC were labeled with 2 μM Fura‐2 AM (Life Technologies) for 30 min in RPMI 1640 medium supplemented with 10% FCS, 1% l‐glutamine, and 1% penicillin/streptomycin. Cells were attached for 10 min to 96‐well glass‐bottom plates (Fisher) that had been precoated with 0.01% poly‐L‐lysine (w/v) (Sigma‐Aldrich) for 2 h. Intracellular Ca2+ measurements were performed using a Flexstation 3 plate reader (Molecular Devices). Cells were stimulated with 1 μM thapsigargin (TG) in Ca2+‐free Ringer solution (155 mM NaCl, 4.5 mM KCl, 3 mM MgCl2, 10 mM d‐glucose, 5 mM Na HEPES), followed by addition of 2 mM Ca2+ Ringer solution (final [Ca2+]o 1 mM) to induce SOCE. Fura‐2 emission ratios (F340/380) were acquired at 510 nm following excitation at 340 and 380 nm every 5 s. F340/380 ratios were quantified by analyzing the integrated Ca2+ signal (area under the curve, AUC) after TG stimulation or readdition of extracellular Ca2+ and by analyzing the peak F340/380 response after Ca2+ readdition (normalized to the baseline F340/380 ratio before Ca2+ readdition) using GraphPad Prism 8.0 software.
Total internal reflection microscopy
Flip‐in T‐Rex HEK293 cells stably transfected with doxycycline‐inducible GFP‐ORAI1 were plated onto UV‐sterilized coverslips and transfected with mCherry‐STIM1 (WT or the following mutants: p.L374P, ΔK lacking the C‐terminal PB, p.L374P‐ΔK). One day after STIM1 transfection, GFP‐ORAI1 expression was induced by 1 μg/ml doxycycline. ORAI1 expression on the surface of HEK293 cells was confirmed by flow cytometry using a mouse anti‐human ORAI1 monoclonal IgG1 antibody (1:200), clone 29A2, that recognizes an epitope (amino acids 196–234) in the second extracellular loop of ORAI1 (Vaeth et al, 2017b). A mouse IgG1 was used as isotype control and a goat anti‐mouse IgG‐Alexa Fluor 647 as secondary antibody (Life Technologies, 1:500). TIRFM images for Fig 2 were acquired using a Nikon Eclipse Ti inverted microscope, an Apo TIRF 100×/NA1.49 oil‐immersion DIC objective, and an Andor iXon DU897 electron‐multiplier charge‐coupled device (EMCCD); two laser lines (488 and 568 nm; Coherent) were used to acquire fluorescent images. TIRFM images for Fig EV1 were acquired using an Olympus IX81 inverted epifluorescence microscope, a 60×/NA1.45 oil‐immersion objective, and EMCCD (Hamamatsu); two laser lines (488 and 543 nm; Melles Griot) were used sequentially to acquire fluorescent images. Experiments were performed at 37°C (Fig 2) or room temperature (Fig EV1). Image analysis was conducted using NIH ImageJ Fiji. Recruitment of mCherry‐STIM1 to ER–PM junctions in TIRFM images was evaluated by calculating the mCherry fluorescence over time [F(t)] after ER store depletion. The mCherry signal was either normalized to baseline mCherry fluorescence (F0) image for each individual cell using NIH ImageJ Fiji or subtracted as mean fluorescent intensity (MFI) using NIH ImageJ Fiji. The co‐localization of GFP‐ORAI1 with mCherry‐STIM1 in the TIRFM evanescent field was measured by using the co‐localization analysis plug‐in from NIH ImageJ Fiji. Pearson's coefficients were calculated for each time point and cell individually using NIH ImageJ Fiji.
Western blotting
Expanded human T cells were lysed in RIPA buffer (10 mM Tris–HCl, pH 7.5, with 150 mM NaCl, 1% Triton X‐100, 1% Nonidet P‐40, 2 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride, and protease inhibitor mixture), and total protein extracts (30–50 μg) were resolved by 4–20% SDS–PAGE. Proteins were transferred onto nitrocellulose membranes (Bio‐Rad), incubated with a polyclonal rabbit anti‐human antibody to STIM1 (1:1,000) described earlier (Picard et al, 2009) or a polyclonal antibody to β‐actin (Santa Cruz,1:500). Fluorescently labeled secondary antibodies (LICOR) were used for protein detection using a Li‐Cor Odyssey Fc Image system (Li‐Cor).
Immunoprecipitation
HEK293 cells were co‐transfected with Flag‐ORAI1 and mCherry‐STIM1 (WT or mutant p.L374P). One day after transfection, cells were treated with either 2 mM Ca2+ Ringer solution or 1 μM Thapsigargin in Ca2+ free Ringer solution. Cells were lysed in RIPA buffer as described above. Cells lysates were incubated with anti‐Flag (clone M2, Sigma‐Aldrich, 5 μg/ml) overnight at 4°C. Protein complexes were purified on protein A/G‐agarose beads (Pierce) and eluted by competition with Flag peptide (0.4 μg/μl) for 1 h at 4°C. Immunoprecipitated proteins and 10% of total protein extract (input control) were separated by 4–20% SDS–PAGE. Proteins were detected by immunoblotting using either HRP coupled anti‐Flag antibody (Sigma‐Aldrich, 1:2,000), or a polyclonal rabbit‐anti‐human antibody to STIM1 (1:1,000) described previously (Picard et al, 2009) or a monoclonal mouse anti‐human antibody to β‐actin (Proteintech, 1:5,000) followed by an HRP coupled secondary antibody (Sigma‐Aldrich, 1:7,500). Signals were detected using Western Lightning Plus‐ECL (PerkinElmer) with the Amersham Imager 680 (GE Healthcare). Image analysis was performed by densitometry using NIH ImageJ software.
PBMC isolation and human T‐cell culture
PBMCs were isolated from whole blood samples by density centrifugation using Ficoll‐Paque plus (GE Amersham). Human CD4+ T cells isolation was performed using CD4 MicroBeads (Miltenyi Biotec). PBMCs or purified CD4+ T cells were stimulated with 5 μg/ml plate‐bound anti‐CD3 (clone OKT3) and 10 μg/ml soluble anti‐CD28 (clone CD28.2, both eBioscience) monoclonal antibodies in the presence or absence of 1 μM FK506 (Sigma‐Aldrich) in RPMI 1640 medium supplemented with 10% FCS, 1% l‐glutamine, and 1% penicillin/streptomycin (Mediatech Inc., VA). For the expansion of human CD4+ T cells, PBMC were stimulated with 1 μg/ml PHA (Thermo Fisher Scientific), irradiated buffy coat cells, and irradiated EBV‐transformed B cells. Cells were cultured in RPMI 1640 medium supplemented with 2% human serum and 10% FCS, 1% l‐glutamine, 1% penicillin/streptomycin, 25 mM HEPES, and 50 U/ml recombinant human IL‐2.
Murine CD4+ T‐cell isolation and Th17 cell differentiation
CD4+ T cells were isolated from the spleen and submandibular, axillar, inguinal, and mesenterial lymph nodes of mice by negative enrichment using a MagniSort mouse CD4+ T‐cell enrichment kit (eBioscience) and stimulated with 0.25 μg/ml plate‐bound anti‐CD3 (clone 2C11) and 1 μg/ml anti‐CD28 (clone 37.51; both Bio X Cell) antibodies in the presence of 2 μg/ml anti‐IL‐4 (clone 11B11) and 2 μg/ml anti‐IFN‐γ (clone XMG1.2; both eBioscience). For the differentiation into non‐pathogenic Th17 cells, CD4+ T cells were cultured in the presence of 20 ng/ml IL‐6 (Peprotech) and 0.5 ng/ ml hTGFβ1 (PeproTech) for 2 or 3 days. For the differentiation into pathogenic Th17 cells, CD4+ T cells were cultured in the presence of 20 ng/ml IL‐6 (Peprotech), 20 ng/ml IL‐1β (Peprotech), and 20 ng/ml IL‐23 (eBioscience) for 2 or 3 days.
T‐cell proliferation
PBMC or expanded human T cells were loaded with 2.5 μM CFSE (Molecular Probes) and stimulated with anti‐CD3 and anti‐CD28 antibodies in the presence or absence of 1 μM FK506. After 96 h, cells were stained for CD3, CD4, and CD8 and analyzed by flow cytometry for surface markers, cell size (FSC), and CFSE dilution using a LSRII flow cytometer (BD Biosciences) and FlowJo v.8.7 software (Tree Star).
cDNA synthesis and quantitative real‐time PCR
Total RNA was extracted from human PBMC using Trizol Reagent (Invitrogen, Carlsbad, California) as described before (McCarl et al, 2009 ). cDNA was synthesized using Superscript II™ RT (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. PCRs were performed using the iCycler system (Bio‐Rad Laboratories) and SYBR green PCR kit (Applied Biosystems, Foster City, CA). Primers used: STIM1 (forward: ACACAGGGGCTTGTCAATTC; reverse: GTCACAGTGAGAAGGCGACA) and STIM2 (forward: GCATGGTGGACTCAGTGACA; reverse: ACTGGCTCTGCCGCAACT).
Intracellular cytokine staining by flow cytometry
For intracellular cytokine measurements, total splenocytes (systemic infection) or total lymph node cells (sublingual infection) were isolated and restimulated with 20 nM phorbol 12‐myristate 13‐acetate (PMA, Calbiochem) and 1 μM ionomycin (Invitrogen) in the presence of 5 μM brefeldin A for 4–6 h followed by washing of the cells in PBS containing 2% FBS and incubation with anti‐FcγRII/FcγRIII antibodies (2.4G2, eBioscience) for 20 min. Cell surface staining with fluorescently labeled antibodies was performed at room temperature for 30 min in the dark followed by permeabilization and fixation by incubation of the cells with IC Fix Buffer (eBioscience) for 30 min. Thereafter, cells were washed and stained with antibodies against cytokines. A complete list of anti‐human and anti‐mouse antibodies used in this study can be found in Appendix Table S2. For all flow cytometry experiments, cells were analyzed using a LSRII flow cytometer (BD Biosciences) and FlowJo v.8.7 software (Tree Star).
Analysis of mTOR signaling and STIM1 protein expression by flow cytometry
PBMC were stimulated for 24 h with anti‐CD3/CD28 in the presence or absence of 1 μM FK506. After antibody staining of CD4 and CD8 at the cell surface, fixation, and permeabilization, the phosphorylation of proteins in the mTOR signaling pathway was detected by using anti‐phospho‐mTOR Ser2448 (MRRBY) and anti‐phospho‐S6 Ser235/236 (cupk43k, all eBioscience) antibodies. For the analysis of STIM1 protein expression, expanded human T cells were fixed and permeabilized with IC Fix Buffer (eBioscience) followed by intracellular staining with an unconjugated, affinity‐purified, polyclonal rabbit‐anti‐human antibody directed against a C‐terminal STIM1 epitope that has been described earlier (Picard et al, 2009). Antibody binding to STIM1 was visualized using a secondary, Alexa Fluor 647‐conjugated mouse anti‐rabbit antibody. Expanded human T cells incubated with serum from non‐immunized rabbits followed by treatment with secondary antibody served as negative control.
Candida albicans culture and CFU counts
1 × 107 cells of the C. albicans strain UC 820 (ATCC, MYA‐3573) were added to YPD medium (Sigma‐Aldrich), placed in a shaking incubator at 28°C for 24 h, centrifuged at 1,000 rpm for 5 min, and resuspended in fresh 15 ml YPD media followed by culture of C. albicans on YPD agar plates (Sigma‐Aldrich) for another 24 h to determine CFUs before systemic or oral infection of mice.
Sublingual and systemic candida infection
Mice were anesthetized with a mixture of ketamine and xylazine before C. albicans infections. For systemic infection, 2 × 105 CFUs of C. albicans were injected i.v. into the retrobulbar venous plexus and the body weight monitored for the duration of the experiment. Mice were sacrificed at day 6 p.i. to analyze fungal burdens and tissue inflammation and to isolate cells. For sublingual C. albicans infection, mice were infected by placing cotton‐tipped applicators saturated with 2 × 107 CFUs of C. albicans under the tongue for 90 min. Mice were sacrificed at day 7 p.i. to collect organs, which were homogenized using a Power Gen 125 tissue homogenizer (Fisher Scientific). Fungal burdens were measured by culturing tissue homogenates at different dilutions on YPD agar plates for 24 h at 28°C followed by determination of CFUs.
Histology
Tongues and kidneys of mice infected with C. albicans sublingually or i.v., respectively, were formalin‐fixed and paraffin‐embedded (FFPE), cut into 5‐μm slices, and stained with hematoxylin and eosin (H&E) or periodic acid–Schiff (PAS) in 95% ethanol using standard protocols. Images were acquired using a SCN400 slide scanner (Leica) and viewed with Slidepath Digital Image Hub (Leica). Leukocytes and fungal burdens were evaluated in H&E and PAS‐stained slides, respectively.
Candida killing assay
CD11b+Gr1+ neutrophils from poly(I:C) treated WT, Stim1 fl/fl Mx1Cre, and Stim1 fl/fl Stim2 fl/fl Mx1Cre mice were cocultured with C. albicans in vitro for 0, 30, 60, and 90 min at a MOI 0.5 before neutrophils were lysed. Cell lysates were plated on YPD agar plates for 24 h to determine CFUs of live C. albicans.
ROS production by neutrophils
CD11b+Gr1+ neutrophils isolated from poly(I:C) treated WT, Stim1 fl/fl Mx1Cre, and Stim1 fl/fl Stim2 fl/fl Mx1Cre mice were incubated with 50 μM dihydrorhodamine 123 (DHR 123) for 10 min at 37°C. The non‐fluorescent DHR 123 is oxidized to rhodamine 123 (R 123) in the presence of H2O2. After neutrophil stimulation with 10 nM PMA for 30 min, DHR 123 fluorescence was measured by flow cytometry.
Metabolic analyses
Oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) were measured using an XFe24 Extracellular Flux Analyzer (Seahorse Bioscience). Before analysis, human CD4+ T cells enriched from PBMC and murine Th17 cells differentiated from naive CD4+ T cells were counted. OCR was measured using the mitochondria stress test protocol. Cells were resuspended in XF media (Seahorse Biosciences) supplemented with 10 mM glucose (Sigma‐Aldrich), 1 mM GlutaMAX (GIBCO), and 1 mM sodium pyruvate (Corning). OCR was measured under basal conditions and upon treatment with the following reagents: the ATP synthase inhibitor oligomycin (1 μM); the protonophore carbonyl cyanide‐4‐(trifluoromethoxy)phenylhydrazone (FCCP) (1.5 μM) to uncouple mitochondria; the mitochondrial complex I inhibitor rotenone (100 nM); and the mitochondrial complex III inhibitor antimycin A (1 μM). For ECAR measurements, cells were resuspended in XF media (Seahorse Biosciences) supplemented with 1 mM GlutaMAX (GIBCO) and 1 mM sodium pyruvate (Corning). ECAR was measured under basal conditions and upon addition of the following reagents: glucose (25 mM); oligomycin (5 μM); and 2‐DG (100 mM) to block glycolysis. Different OXPHOS and glycolysis parameters were calculated as described previously (Vaeth et al, 2017a).
Glucose uptake
Glucose uptake was measured by loading murine non‐pathogenic Th17 cells with the fluorescent glucose analog 2‐NBDG (Thermo Fisher Scientific). Stimulated and unstimulated Th17 cells were incubated in glucose‐free RPMI medium containing 100 μM 2‐NBDG for 90 min at 37°C, and the amount of 2‐NBDG taken up by cells was measured by flow cytometry after surface staining for CD4 for 15 min at RT.
RNA sequencing and data processing
Total RNA was extracted from 1 × 106 non‐pathogenic and pathogenic Th17 cells using the RNeasy Micro RNA Isolation Kit (Qiagen). The RNA quality and quantity were analyzed on a Bioanalyzer 2100 (Agilent) using a PICO chip and samples with an RNA integrity number (RIN) of > 9 were used for library preparation. RNA‐seq libraries were prepared using the TruSeq RNA sample prep v2 kit (Illumina), starting from 100 ng of DNAse I (Qiagen)‐treated total RNA, following the manufacturer's protocol with 15 PCR cycles. The amplified libraries were purified using AMPure beads (Beckman Coulter), quantified by Qubit 2.0 fluorometer (Life Technologies), and visualized using an Agilent Tapestation 2200. The libraries were pooled equimolarly and loaded on the HiSeq 2500 Sequencing System (Illumina) and run as single 50‐nucleotides reads generating about 30 million reads per sample. Sequencing reads were mapped to the mouse reference genome (GRCm38.85/mm10) using the STAR aligner (v2.5.0c; Dobin et al, 2013). Alignments were guided by a Gene Transfer Format file (GTF GRCm38.85). The mean read insert sizes and their standard deviations were calculated using Picard tools (v.1.126) (http://broadinstitute.github.io/picard). The read count tables were generated using HTSeq (v0.6.0) (Anders et al, 2015), normalized based on their library size factors using DEseq2(Love et al, 2014), and differential expression analysis was performed. The Read Per Million (RPM) normalized BigWig files were generated using BEDTools (v2.17.0) (Quinlan & Hall, 2010) and bedGraphToBigWig tool (v4). KEGG pathway analysis and Gene Ontology (GO) analysis were performed using the clusterProfiler R package (v3.0.0) (Yu et al, 2012) and DAVID. To compare the level of similarity among the samples and their replicates, we used two methods: principal component analysis and Euclidean distance‐based sample clustering. Gene set enrichment analysis was performed using GSEA (v3.0; Subramanian et al, 2005). All the downstream statistical analyses and generating plots were performed in R environment (v3.1.1; https://www.r-project.org/).
Statistical analysis
Animal experiments were conducted unblinded. For certain readouts (e.g., determining C. albicans CFUs within organs), investigators were blinded. If not indicated otherwise, data are represented as mean ± SEM to provide an estimate of variation. No randomization was used. Results were analyzed with Prism version 8.0 (GraphPad software, Inc.). Statistical significance between groups was determined by using the two‐tailed unpaired Student's t‐test when a normal distribution was assumed or otherwise by Mann–Whitney U‐test. Where indicated, data were analyzed by one‐way ANOVA followed by Tukey's honestly significant difference (HSD) post hoc test for multiple comparisons. P values < 0.05 were considered significant; different levels of significance are indicated as follows: *P < 0.05; **P < 0.01; ***P < 0.001.
Author contributions
SK and SF designed the research and wrote the manuscript. SK, UK, ARC, LN, MV, LK, JY, JM, MM, PP, and PS conducted experiments. SK, UK, ARC, LN, MV, LK, JY, JM, MM, AK‐J, DR, ZS, PP, PBS and PS, DU and SF analyzed data and interpreted the results. CF, SBC, and SET provided patient materials and data. SF supervised the study. All authors read and approved the final version of the manuscript.
Conflict of interest
S.F. is a scientific cofounder of Calcimedica. The other authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Table EV1
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Acknowledgements
We thank the patients and their families for consenting to participate in this study; Gilad Evrony for help with genetic analyses; Thomas Scambler for help with T cell culture and calcium measurements and Michael Cammer for providing the ImageJ Script for analyzing ORAI1 and STIM1 co‐localization. 29A2 anti‐ORAI1 antibody was kindly provided by Dr. I. Kacskovics (ImmunoGenes). This study was funded by NIH grants AI097302 and AI137004 (S.F.) and AI065303 (D.U.), postdoctoral fellowships by the German Research Foundation (DFG) to S.K. (KA 4514/1‐1), M.V. (VA 882/1‐1), and U.K. (KA 4083/2‐1), and a postdoctoral fellowship by the National Multiple Sclerosis Society to U.K. (FG‐1608‐25544). The Microscopy Laboratory at NYUSOM is partially supported by the Cancer Center Support Grant P30CA016087 of the Laura and Isaac Perlmutter Cancer Center; the Seahorse XFe24 analyzer was purchased with funding by NIH SIG grant 1S10OD016304‐01.
EMBO Mol Med (2020) 12: e11592
Data availability
The datasets produced in this study are available in the following databases. RNA‐Seq data: Gene Expression Omnibus GSE149162 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE149162).
References
- Badran YR, Massaad MJ, Bainter W, Cangemi B, Naseem SUR, Javad H, Al‐Tamemi S, Geha RS, Chou J (2016) Combined immunodeficiency due to a homozygous mutation in ORAI1 that deletes the C‐terminus that interacts with STIM 1. Clin Immunol 166: 100–102 [DOI] [PubMed] [Google Scholar]
- Bar E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut‐Landmann S (2014) IL‐17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 40: 117–127 [DOI] [PubMed] [Google Scholar]
- Bongomin F, Gago S, Oladele RO, Denning DW (2017) Global and multi‐national prevalence of fungal diseases‐estimate precision. J Fungi (Basel) 3: 57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC (2012) Hidden killers: human fungal infections. Sci Transl Med 4: 165rv13 [DOI] [PubMed] [Google Scholar]
- Buck MD, Sowell RT, Kaech SM, Pearce EL (2017) Metabolic instruction of immunity. Cell 169: 570–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun M, Abhyankar A, Lelarge V, Plancoulaine S, Palanduz A, Telhan L, Boisson B, Picard C, Dewell S, Zhao C et al (2010) Whole‐exome sequencing‐based discovery of STIM1 deficiency in a child with fatal classic Kaposi sarcoma. J Exp Med 207: 2307–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Badran YR, Yee CSK, Bainter W, Ohsumi TK, Al‐Hammadi S, Pai S‐Y, Feske S, Geha RS (2015) A novel mutation in ORAI1 presenting with combined immunodeficiency and residual T cell function. J Allergy Clin Immunol 36: 479–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concepcion AR, Vaeth M, Wagner LE, Eckstein M, Hecht L, Yang J, Crottes D, Seidl M, Shin HP, Weidinger C et al (2016) Store‐operated Ca2+ entry regulates Ca2+‐activated chloride channels and eccrine sweat gland function. J Clin Invest 126: 4303–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW et al (2009) Th17 cells and IL‐17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206: 299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Peterson AC, Brane L, Huppler AR, Hernandez‐Santos N, Whibley N, Garg AV, Simpson‐Abelson MR, Gibson GA, Mamo AJ et al (2014) Oral‐resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J Exp Med 211: 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Gaffen SL (2015) IL‐17‐mediated immunity to the opportunistic fungal pathogen Candida albicans . J Immunol 195: 780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MM, Way SS (2009) Interleukin‐17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 126: 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaloye J, Calandra T (2014) Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 5: 161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Wang Y, Zhou Y, Soboloff J, Gill D (2009) STIM and Orai ‐ dynamic intermembrane coupling to control cellular calcium signals. J Biol Chem284: 22501–22505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvignes L, Weidinger C, Shaw P, Vaeth M, Ribierre T, Liu M, Fergus T, Kozhaya L, McVoy L, Unutmaz D et al (2015) STIM1 controls T cell‐mediated immune regulation and inflammation in chronic infection. J Clin Invest 125: 2347–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA‐seq aligner. Bioinformatics 29: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Draeger R, Peter HH, Rao A (2000) Impaired NFAT regulation and its role in a severe combined immunodeficiency. Immunobiology 202: 134–150 [DOI] [PubMed] [Google Scholar]
- Feske S, Muller JM, Graf D, Kroczek RA, Drager R, Niemeyer C, Baeuerle PA, Peter HH, Schlesier M (1996) Severe combined immunodeficiency due to defective binding of the nuclear factor of activated T cells in T lymphocytes of two male siblings. Eur J Immunol 26: 2119–2126 [DOI] [PubMed] [Google Scholar]
- Feske S, Prakriya M, Rao A, Lewis RS (2005) A severe defect in CRAC Ca2+ channel activation and altered K+ channel gating in T cells from immunodeficient patients. J Exp Med 202: 651–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185 [DOI] [PubMed] [Google Scholar]
- Feske S (2007) Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol 7: 690–702 [DOI] [PubMed] [Google Scholar]
- Feske S (2010) CRAC channelopathies. Pflugers Arch 460: 417–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf I, Muik M, Derler I, Bergsmann J, Fahrner M, Schindl R, Groschner K, Romanin C (2009) Molecular determinants of the coupling between STIM1 and Orai channels: differential activation of Orai1‐3 channels by a STIM1 coiled‐coil mutant. J Biol Chem 284: 21696–21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S, Rensing‐Ehl A, Speckmann C, Bengsch B, Schmitt‐Graeff A, Bondzio I, Maul‐Pavicic A, Bass T, Vraetz T, Strahm B et al (2012) Antiviral and regulatory T cell immunity in a patient with stromal interaction molecule 1 deficiency. J Immunol 188: 1523–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaublomme JT, Yosef N, Lee Y, Gertner RS, Yang LV, Wu C, Pandolfi PP, Mak T, Satija R, Shalek AK et al (2015) Single‐cell genomics unveils critical regulators of Th17 Cell pathogenicity. Cell 163: 1400–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladiator A, Wangler N, Trautwein‐Weidner K, LeibundGut‐Landmann S (2013) Cutting edge: IL‐17‐secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol 190: 521–525 [DOI] [PubMed] [Google Scholar]
- Hernandez‐Santos N, Gaffen SL (2012) Th17 cells in immunity to Candida albicans . Cell Host Microbe 11: 425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Na L, Fidel PL, Schwarzenberger P (2004) Requirement of interleukin‐17A for systemic anti‐Candida albicans host defense in mice. J Infect Dis 190: 624–631 [DOI] [PubMed] [Google Scholar]
- Kaufmann U, Shaw PJ, Kozhaya L, Subramanian R, Gaida K, Unutmaz D, McBride HJ, Feske S (2016) Selective ORAI1 inhibition ameliorates autoimmune central nervous system inflammation by suppressing effector but not regulatory T cell function. J Immunol 196: 573–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann U, Kahlfuss S, Yang J, Ivanova E, Koralov SB, Feske S (2019) Calcium signaling controls pathogenic Th17 cell‐mediated inflammation by regulating mitochondrial function. Cell Metab 29: 1104–1118.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Lange I, Feske S (2009) A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem Biophys Res Commun 385: 49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khajah M, Millen B, Cara DC, Waterhouse C, McCafferty DM (2011) Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF): a chemoattractive agent for murine leukocytes in vivo . J Leukoc Biol 89: 945–953 [DOI] [PubMed] [Google Scholar]
- Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N et al (2010) Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17‐associated cytokines. J Exp Med 207: 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemann C, Ammann S, Heizmann M, Fuchs S, Bode SF, Heeg M, Fuchs H, Lehmberg K, Zur Stadt U, Roll C et al (2017) Hemophagocytic lymphohistiocytosis as presenting manifestation of profound combined immunodeficiency due to an ORAI1 mutation. J Allergy Clin Immunol 140: 1721–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Feske S (2015) Diseases caused by mutations in ORAI1 and STIM1. Ann N Y Acad Sci 1356: 45–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL, Puel A (2013) Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr 25: 736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA et al (2012) Induction and molecular signature of pathogenic TH17 cells. Nat Immunol 13: 991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Deist F, Hivroz C, Partiseti M, Thomas C, Buc HA, Oleastro M, Belohradsky B, Choquet D, Fischer A (1995) A primary T‐cell immunodeficiency associated with defective transmembrane calcium influx. Blood 85: 1053–1062 [PubMed] [Google Scholar]
- Levy R, Okada S, Beziat V, Moriya K, Liu C, Chai LY, Migaud M, Hauck F, Al Ali A, Cyrus C et al (2016) Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL‐17RA deficiency. Proc Natl Acad Sci USA 113: E8277–E8285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J, Cuk M, Kahlfuss S, Kozhaya L, Vaeth M, Rieux‐Laucat F, Picard C, Benson MJ, Jakovcevic A, Bilic K et al (2018) ORAI1 mutations abolishing store‐operated Ca(2 + ) entry cause anhidrotic ectodermal dysplasia with immunodeficiency. J Allergy Clin Immunol 142: 1297–1310.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y, Cypowyj S, Aytekin C, Galicchio M, Camcioglu Y, Nepesov S, Ikinciogullari A, Dogu F, Belkadi A, Levy R et al (2015) Inherited IL‐17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med 212: 619–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T (2005) STIM is a Ca2+ sensor essential for Ca2+‐store‐depletion‐triggered Ca2+ influx. Curr Biol 15: 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Fivaz M, Inoue T, Meyer T (2007) Live‐cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA 104: 9301–9306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol 15: 550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS (2008) Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 454: 538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, McCarl CA, Khalil S, Luthy K, Feske S (2010) T‐cell specific deletion of STIM1 and STIM2 protects mice from EAE by impairing the effector functions of Th1 and Th17 cells. Eur J Immunol 40: 3028–3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus M, Jairaman A, Stathopulos PB, Muik M, Fahrner M, Weidinger C, Benson M, Fuchs S, Ehl S, Romanin C et al (2015) Missense mutation in immunodeficient patients shows the multifunctional roles of coiled‐coil domain 3 (CC3) in STIM1 activation. Proc Natl Acad Sci USA 112: 6206–6211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarl CA, Picard C, Khalil S, Kawasaki T, Rother J, Papolos A, Kutok J, Hivroz C, Ledeist F, Plogmann K et al (2009) ORAI1 deficiency and lack of store‐operated Ca2 + entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J Allergy Clin Immunol 124: 1311–1318.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarl CA, Khalil S, Ma J, Oh‐hora M, Yamashita M, Roether J, Kawasaki T, Jairaman A, Sasaki Y, Prakriya M et al (2010) Store‐operated Ca2+ entry through ORAI1 is critical for T cell‐mediated autoimmunity and allograft rejection. J Immunol 185: 5845–5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengesha BG, Conti HR (2017) The role of IL‐17 in protection against mucosal candida infections. J Fungi (Basel) 3: 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML et al (2008) Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper‐IgE syndrome. Nature 452: 773–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Vonk AG, van den Hoven M, Verschueren I, Joosten LA, van Krieken JH, van den Berg WB, Van der Meer JW, Kullberg BJ (2003) Differential role of IL‐18 and IL‐12 in the host defense against disseminated Candida albicans infection. Eur J Immunol 33: 3409–3417 [DOI] [PubMed] [Google Scholar]
- Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL (2015) Immune defence against Candida fungal infections. Nat Rev Immunol 15: 630–642 [DOI] [PubMed] [Google Scholar]
- Newton RH, Shrestha S, Sullivan JM, Yates KB, Compeer EB, Ron‐Harel N, Blazar BR, Bensinger SJ, Haining WN, Dustin ML et al (2018) Maintenance of CD4 T cell fitness through regulation of Foxo1. Nat Immunol 19: 838–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh‐Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A (2008) Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol 9: 432–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS (2009) STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136: 876–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry DA, Holmes TD, Gamper N, El‐Sayed W, Hettiarachchi NT, Ahmed M, Cook GP, Logan CV, Johnson CA , Joss s et al (2016) A homozygous STIM1 mutation impairs store‐operated calcium entry and natural killer cell effector function without clinical immunodeficiency. J Allergy Clin Immunol 137: 955–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partiseti M, Le Deist F, Hivroz C, Fischer A, Korn H, Choquet D (1994) The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem 269: 3237–32335 [PubMed] [Google Scholar]
- Picard C, McCarl CA, Papolos A, Khalil S, Luthy K, Hivroz C, LeDeist F, Rieux‐Laucat F, Rechavi G, Rao A et al (2009) STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med 360: 1971–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M et al (2011) Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin‐17 immunity. Science 332: 65–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani K, Jawale CV, Verma AH, Coleman BM, Kolls JK, Biswas PS (2018) Unexpected kidney‐restricted role for IL‐17 receptor signaling in defense against systemic Candida albicans infection. JCI Insight 3: e98241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice LV, Bax HJ, Russell LJ, Barrett VJ, Walton SE, Deakin AM, Thomson SA, Lucas F, Solari R, House D et al (2013) Characterization of selective Calcium‐Release Activated Calcium channel blockers in mast cells and T‐cells from human, rat, mouse and guinea‐pig preparations. Eur J Pharmacol 704: 49–57 [DOI] [PubMed] [Google Scholar]
- Sahin G, Palanduz A, Aydogan G, Cassar O, Ertem AU, Telhan L, Canpolat N, Jouanguy E, Picard C , Gessain A et al (2010) Classic Kaposi sarcoma in 3 unrelated Turkish children born to consanguineous kindreds. Pediatrics 125: e704‐8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint Fleur‐Lominy S, Maus M, Vaeth M, Lange I, Zee I, Suh D, Liu C, Wu X, Tikhonova A, Aifantis I et al (2018) STIM1 and STIM2 mediate cancer‐induced inflammation in T cell acute lymphoblastic leukemia. Cell Rep 24: 3045–3060.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaballie H, Rodriguez R, Martin E, Moens L, Frans G, Lenoir C, Dutre J, Canioni D, Bossuyt X , Fischer A et al (2015) A novel hypomorphic mutation in STIM1 results in a late‐onset immunodeficiency. J Allergy Clin Immunol 136: 816–819 [DOI] [PubMed] [Google Scholar]
- Schuhmann MK, Stegner D, Berna‐Erro A, Bittner S, Braun A, Kleinschnitz C, Stoll G, Wiendl H, Meuth SG, Nieswandt B (2010) Stromal interaction molecules 1 and 2 are key regulators of autoreactive T cell activation in murine autoimmune central nervous system inflammation. J Immunol 184: 1536–1542 [DOI] [PubMed] [Google Scholar]
- Shao TY, Ang WXG, Jiang TT, Huang FS, Andersen H, Kinder JM, Pham G, Burg AR, Ruff B, Gonzalez T et al (2019) Commensal Candida albicans positively calibrates systemic Th17 immunological responses. Cell Host Microbe 25: 404–417.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparber F, LeibundGut‐Landmann S (2015) Interleukin 17‐mediated host defense against Candida albicans . Pathogens 4: 606–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopulos PB, Schindl R, Fahrner M, Zheng L, Gasmi‐Seabrook GM, Muik M, Romanin C, Ikura M (2013) STIM1/Orai1 coiled‐coil interplay in the regulation of store‐operated calcium entry. Nat Commun 4: 2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Omenetti S (2017) The dichotomous nature of T helper 17 cells. Nat Rev Immunol 17: 535–544 [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES et al (2005) Gene set enrichment analysis: a knowledge‐based approach for interpreting genome‐wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JL, Shuttleworth TJ (2012) A plasma membrane‐targeted cytosolic domain of STIM1 selectively activates ARC channels, an arachidonate‐regulated store‐independent Orai channel. Channels (Austin) 6: 370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, Ouachee‐Chardin M, Fouyssac F, Girisha KM, Etzioni A et al (2016) Heterozygous STAT1 gain‐of‐function mutations underlie an unexpectedly broad clinical phenotype. Blood 127: 3154–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaeth M, Zee I, Concepcion AR, Maus M, Shaw P, Portal‐Celhay C, Zahra A, Kozhaya L, Weidinger C, Philips J et al (2015) Ca2+ signaling but not store‐operated Ca2+ entry is required for the function of macrophages and dendritic cells. J Immunol 195: 1202–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaeth M, Maus M, Klein‐Hessling S, Freinkman E, Yang J, Eckstein M, Cameron S, Turvey SE, Serfling E, Berberich‐Siebelt F et al (2017a) Store‐operated Ca(2 + ) entry controls clonal expansion of T cells through metabolic reprogramming. Immunity 47: 664–679.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaeth M, Yang J, Yamashita M, Zee I, Eckstein M, Knosp C, Kaufmann U, Karoly Jani P, Lacruz RS, Flockerzi V et al (2017b) ORAI2 modulates store‐operated calcium entry and T cell‐mediated immunity. Nat Commun 8: 14714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaeth M, Feske S (2018) Ion channelopathies of the immune system. Curr Opin Immunol 52: 39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Kullberg BJ, Netea MG (2010) Pathogenesis of invasive candidiasis. Curr Opin Crit Care 16: 453–459 [DOI] [PubMed] [Google Scholar]
- Villar CC, Dongari‐Bagtzoglou A (2008) Immune defence mechanisms and immunoenhancement strategies in oropharyngeal candidiasis. Expert Rev Mol Med 10: e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Choi M, Richardson AS, Reid BM, Seymen F, Yildirim M, Tuna E, Gncay K, Simmer JP , Hu JC (2014) STIM1 and SLC24A4 Are Critical for Enamel Maturation. J Dent Res 93: 94S–100S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Covington ED, Lewis RS (2014) Single‐molecule analysis of diffusion and trapping of STIM1 and Orai1 at endoplasmic reticulum‐plasma membrane junctions. Mol Biol Cell 25: 3672–3685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Jin H, Cai X, Li S, Shen Y (2012) Structural and mechanistic insights into the activation of Stromal interaction molecule 1 (STIM1). Proc Natl Acad Sci USA 109: 5657–5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M, Lewis RS (2019) Numbers count: how STIM and Orai stoichiometry affect store‐operated calcium entry. Cell Calcium 79: 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang LG, Han Y, He QY (2012) clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16: 284–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S (2009) SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol 11: 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gonzalez‐Cobos JC, Schindl R, Muik M, Ruhle B, Motiani RK, Bisaillon JM, Zhang W, Fahrner M, Barroso M et al (2013) Mechanisms of STIM1 activation of store‐independent leukotriene C4‐regulated Ca2 + channels. Mol Cell Biol 33: 3715–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang X, Wang X, Loktionova NA, Cai X, Nwokonko RM, Vrana E, Wang Y, Rothberg BS, Gill DL (2015) STIM1 dimers undergo unimolecular coupling to activate Orai1 channels. Nat Commun 6: 8395 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Table EV1
Source Data for Expanded View
Review Process File
Source Data for Figure 1
Data Availability Statement
The datasets produced in this study are available in the following databases. RNA‐Seq data: Gene Expression Omnibus GSE149162 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE149162).


