Abstract
Background
Structural disorders of the hypopharynx can lead to dysphagia‐related morbidity. Endoscopic therapy in this area, for example, myotomy for Zenker's diverticulum (ZD), has traditionally been performed under general anesthesia (GA). We have developed a two‐stage sedation process, which is used along with high‐flow nasal oxygen therapy (HFNOT) to facilitate endoscopic hypopharyngeal procedures.
Methods
In this prospective, single‐center study, patients undergoing endoscopic procedures between June 2016 and March 2018 were included. All endoscopies were performed with propofol and/or remifentanil and supported with HFNOT. In patients with ZD, the diverticulum and stomach were cleared of debris under conscious sedation to reduce the risk of aspiration, before sedation was deepened to facilitate myotomy. Sedation‐related adverse events were recorded.
Results
A total of 50 patients were included for analysis (mean age of 71.1, range 31–93; 58% male); 48% were categorized as American Society of Anesthesiologists (ASA) Grade III and 6% as Grade IV. The median procedure time was 20 min. Of patients, 83% were sedated with both propofol and remifentanil using a target‐controlled infusion under specialist anesthetic supervision. Sedation‐related adverse events included transient hypotension (38%), bradycardia (8%), and hypoxia (8%). No procedures were abandoned due to complications, and no patients required conversion to GA. Patients achieved full postprocedure recovery from sedation after a median duration of 5 min.
Conclusions
HFNOT is a useful adjunct to two‐stage sedation, which can enable high‐risk patients to safely undergo deep sedation during hypopharyngeal endoscopic procedures.
Keywords: endoscopy, high‐flow nasal oxygen therapy, optiflow, sedation
![]()
First prospective study of usefulness of high‐flow nasal oxygen therapy (HFNOT) adjunct to two‐stage sedation, which can enable high‐risk patients to safely undergo deep sedation during hypopharyngeal endoscopic procedures.
Introduction
Structural disorders of the hypopharynx and upper esophagus are associated with significant patient morbidity due to complications of dysphagia, regurgitation malnutrition, and recurrent aspiration. Therapy in this area remains challenging due to its rich nervous innervation and confined working space. Over the last two decades, endoscopic techniques have been developed and refined for hypopharyngeal conditions such as Zenker's diverticulum (ZD), 1 cricopharyngeal hypertrophy, and upper esophageal disorders including strictures and webs. 2
With its favorable efficacy and safety profiles, endoscopic therapy has largely superseded surgical alternatives, particularly in the case of ZD. 1 Such cases are generally performed under general anesthesia (GA) or deep sedation and may require invasive ventilation. However, the incidence of hypopharyngeal and upper esophageal disorders increases with age, with a peak preponderance in the 70s and 80s age groups, where patients are particularly susceptible to medical comorbidities, which may be an unacceptably high risk for GA. Thus, it is desirable to develop a safe approach that avoids general anaesthsia to facilitate endoscopic therapy in this group of potentially frail patients.
In most centers, deep sedation is used to minimize patient discomfort and keep the patient still to enable endoscopic therapy. 3 This patient group is at risk of aspiration of gastric and pouch contents due to the combination of sedation and the pathology of the condition. 4 Therefore, to improve patient safety, we developed a novel two‐stage approach to sedation, with an initial conscious phase, followed by a deep phase. The conscious phase enabled suctioning of the pharyngeal pouch and stomach, while the deep phase facilitated the myotomy. The sedative drugs are associated with the risk of hypoxia, hypotension, and bradycardia. 5 We therefore introduced an approach to further improve patient safety, especially during the deep phase of sedation.
The advent of high‐flow nasal oxygen therapy (HFNOT) has enabled procedures requiring deep sedation to be performed without the need for endotracheal intubation 6 , 7 and reduces the risk of hypoxia during the deep phase. However, there is paucity of evidence documenting its use, particularly for endoscopic interventional procedures of the hypopharynx.
In this study, we aimed to assess the efficacy and safety of HFNOT during a two‐stage approach to sedation as an adjunct to endoscopic pharyngeal and upper esophageal procedures with regard to the safety and successful completion of procedures and limiting sedation‐related adverse events.
Methods
Study design
This was a prospective observational single‐center study that assessed the efficacy and safety of HFNOT in patients undergoing flexible endoscopic therapy for hypopharyngeal and upper esophageal disorders. The study was conducted at Russells Hall Hospital, Dudley, a national referral center for patients with ZD, including patients who had been deemed unsuitable for surgery under a general anesthetic due to it being too high risk or those with recurring ZD following previous therapy.
Study approval
This study was carried out according to the principles of the Declaration of Helsinki of Good Clinical Practice. Study approval was formally granted by the Research and Development department of the Dudley Group of Hospitals Foundation Trust. All patients provided written informed consent to participate.
Patients
Participants included any patient scheduled for a hypopharyngeal procedure (endoscopic treatment of ZD, botox injection for cricopharyngeal hypertrophy) or esophageal dilatation between June 2016 and March 2018. There were no exclusion criteria.
Procedures
Patients were instructed to fast for 12 h prior to their procedure and were assessed by an anesthetist prior to entering the endoscopy suite. An intravenous cannula was inserted, and monitoring was carried out. This included a pulse oximeter, three‐lead electrocardiography (ECG), and noninvasive blood pressure and respiratory rate monitoring. Capnography monitoring could not be used alongside HFNOT. Blood pressure readings were taken every 5 min. HFNOT (Optiflow™, Fisher and Paykel Healthcare Limited, Auckland, New Zealand) was applied to all patients, and the throat was sprayed with 1% lidocaine. All patients were placed in the left lateral position. All patients received intravenous paracetamol. A clinical assistant collected the data intraoperatively.
An experienced consultant anesthetist administered the sedation and monitored the patients. Patients with ZD who were deemed at high risk of aspiration underwent conscious sedation with a remifentanil target‐controlled infusion (TCI) in order to allow the endoscopist to suction both the pharyngeal pouch and the stomach. Once the aspiration risk was optimized, deeper sedation was commenced either with higher doses of remifentanil or the addition of a propofol TCI. In accordance with guidance of the American Society of Anesthesiologists (ASA), 8 deep sedation was defined as “a drug‐induced depression of consciousness during which patients cannot be easily aroused but respond purposefully following repeated or painful stimulation.” All patients were spontaneously breathing.
All procedures were performed by an experienced interventional endoscopist. For ZD, a nasogastric tube was placed after emptying the pouch and aspiration of gastric fluid. This helped to protect the anterior esophageal wall. ZD repair was performed using a 9.8‐mm endoscope (Pentax EG‐2990i, Pentax, Tokyo, Japan), using a transparent cap at the tip. The scope was carefully introduced to identify the cricopharyngeus muscle (CP) muscle and pouch. A single incision along the midline of the septum (CP) was carried out to dissect mucosa and fibers of the CP muscle using SB knife junior scissor knife (Sumitomo Bakelite, Japan). One to three clips (HX‐610‐090L; Olympus, Tokyo, Japan) were then placed at the bottom of the incision to prevent mucosal dissection from underlying muscle and perforation (Fig. 1). The patients were discharged home the same day unless they were from outside the region. Patients were allowed to have liquids on day two and soft diet from day three onward. At least 2 L of intravenous fluids were administered prior to discharge. For cricopharyngeal hypertrophy, botulinum toxin (Botox) injections of the CP muscle consisted of identifying the CP muscle and injecting it with diluted 100 IU Botox in two to three sites followed by dilatation over a guidewire if required. For esophageal dilatations alone, a guidewire was placed over the stricture and then dilated with bouginage.
Figure 1.
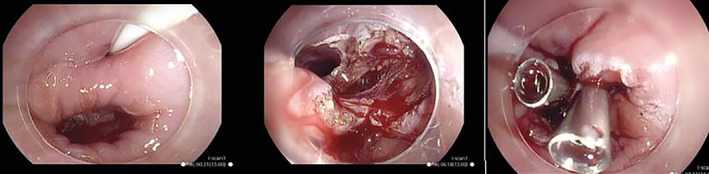
Endoscopic septum division using the SB knife. From left to right: Septum (cricopharyngeus muscle) is identified and nasogastric tube placed to delineate the esophageal entrance; the SB knife is used to perform the midline incision on the septum to dissect the mucosa and muscle fibers to fully divide the septum; two clips are positioned at the bottom of the incision.
Outcomes
The primary outcome studied was the incidence of sedation‐related adverse events. These comprised hypotension (mean arterial pressure < 70 mmHg), hypoxia (saturation < 90% for more than 10 s), and bradycardia (heart rate < 50 beats per minute). Episodes of bradycardia and hypotension were treated with glycopyrrolate, vasopressors, and intravenous fluids. Hypoxia was treated at the discretion of the anesthetist according to the cause, such as airway support or removal of the endoscope temporarily.
Secondary outcomes included the total doses of propofol and remifentanil and the total time taken for completion of the procedure. Time to recovery was also recorded (defined as recovery of protective airway reflexes) every 5 min from the time of endoscope extubation.
Study covariates
For each patient, the ASA score and comorbidities were systematically recorded. At the end of the procedure, the endoscopist graded the difficulty of the procedure on the following scale: I‐ satisfactory, II‐ difficult, or III‐ extremely difficult.
Statistical analyses
All continuous variables were subjected to normality testing. Data were expressed as frequency (percentage) for categorical variables and mean (± standard deviation [SD]) for parametric data and median (interquartile range) for nonparametric data. Patients were classified into binary categories of those who experienced complications (hypoxia, bradycardia, or MAP<70 mmHg) and those who did not. Comparisons were made between the two groups using chi‐square or Fisher's exact test for categorical data and student t‐test or Mann–Whitney U test for parametric or nonparametric continuous variables, respectively. P < 0.05 was considered statistically significant. Data were analyzed using SPSS Statistics V21.0.
Results
A total of 50 consecutive patients underwent hypopharyngeal or upper esophageal endoscopic therapy between June 2016 and March 2018. Mean age was 71.1 years (range 31–93); 58% of patients were male. ASA status comprised: Grade 1–8%, Grade 2–42%, Grade 3–38%, and Grade 4–6%. Endotherapy comprised: ZD (70%), upper esophageal dilatation (20%), and botulinum toxin injection (10%).
Of patients, 85.4% were sedated with both propofol and remifentanil TCI, 8.3% with propofol only, and 6.3% with remifentanil only. The mean (±SD) total dose of propofol and remifentanil used was 126.7 ± 93.35 mg (median[interquartile range 25%–75%]:103[57–192]) and 188.5 ± 115.81 mcg (median[interquartile range 25%–75%]:167[110–256]), respectively. The median procedure time was 20 min (range 10–30 min).
HFNOT was used for all patients with flow rates of between 30 and 70 L per minute. Most procedures were classified as difficulty II (32%) or III (58%), whereas only 6% were classified as difficulty I. Median (interquartile range 25–75%) dose of propofol for procedures of difficulty I/II and III was 90.5 (59–185.5) mg (mean[±SD]: 131.2 ± 110.6) and 105 (49.75–187) mg (mean[±SD]: 119.7 ± 83.6), respectively (p = 0.79). Median (interquartile range 25–75%) dose of remifentanil for procedures of difficulty I/II and III was 130.5 (99.75–213.5) mcg (mean[±SD]: 152.8 ± 78.2) and 175.5 (118.25–265.25) mcg (mean[±SD]: 200.4 ± 120.1), respectively (p = 0.14).
Table 1 presents patients and procedural factors associated with sedation‐related complications, including hypotension, bradycardia, and hypoxemia. There was no significant association found between patient characteristics and sedation‐related adverse events, but rates of hypoxia and bradycardia were associated with procedural difficulty.
Table 1.
Univariate analyses of patient and procedural factors associated with sedation‐related complications
| Hypoxic events | Bradycardic events | Hypotensive events | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hypoxia | No hypoxia | P value | Bradycardia | No bradycardia | P value | Hypotension | No hypotension | P value | |
| Age (years) † | 70.5 ± 11.7 (58–85) | 72.5 ± 11 (43–93) | 0.71 | 74.3 ± 4.8 (72–80) | 71.8 ± 11.3 (43–93) | 0.63 | 70.1 ± 10.2 (45–84) | 73.2 ± 11.4 (43–93) | 0.46 |
| Gender | 0.63 | 1 | 0.52 | ||||||
| Male | 1 (5%) ‡ | 19 (95%) | 2 (10%) | 18 (90%) | 9 (45%) | 11 (55%) | |||
| Female | 3 (10.7%) | 25 (89.3%) | 2 (7.1%) | 26 (92.9%) | 10 (35.7%) | 18 (64.3%) | |||
| BMI (kg/m2) † | 30.5 ± 3.4 (27–36) | 26 ± 6.4 (15–48) | 0.07 | 26 ± 2.6 (23–27) | 26.6 ± 6.8 (15–48) | 0.89 | 29.2 ± 8.4 (15–48) | 24.6 ± 4.2 (17–34) | 0.04 |
| ASA classification | 0.51 | 0.49 | 0.89 | ||||||
| I | 0 (0) | 4 (100) | 0 (0) | 4 (100) | 1 (25) | 3 (75) | |||
| II | 2 (10) | 18 (90) | 1 (5) | 19 (95) | 7 (35) | 13 (65) | |||
| III | 1 (5.3) | 18 (94.7) | 2 (10.5) | 17 (89.5) | 9 (47.4) | 10 (52.6) | |||
| IV | 0 (0) | 2 (100) | 0 (0) | 2 (100) | 1 (50) | 1 (50) | |||
| Procedure | 0.66 | 0.59 | 0.47 | ||||||
| Zenker's diverticulotomy | 4 (11.4%) | 31 (88.6%) | 2 (5.7%) | 33 (94.3%) | 15 (42.9%) | 20 (57.1%) | |||
| Dilatation | 0 (0%) | 6 (100%) | 1 (16.7%) | 5 (83.3%) | 3 (50%) | 3 (50%) | |||
| Botox injection | 0 (0%) | 5 (100%) | 1 (20%) | 4 (80%) | 1 (20%) | 4 (80%) | |||
| Dilatation and Botox | 0 (0%) | 2 (100%) | 0 (0%) | 2 (100%) | 0 (0%) | 2 (100%) | |||
| Duration of Endoscopy (min) † | 17.5 ± 5 (10–20) | 19.5 ± 6.7 (10–30) | 0.79 | 14 ± 2.7 (10–16) | 19.8 ± 6.6 (10–30) | 0.08 | 19.4 ± 5.7 (10–30) | 19.3 ± 7.7 (10–30) | 0.86 |
| Recovery time (min) † | 4.8 ± 2.5 (2–8) | 5.1 ± 3.2 (1–10) | 0.83 | 4.5 ± 2.1 (2–5) | 5.1 ± 3.2 (1–10) | 0.78 | 5.4 ± 2.4 (2–10) | 4.8 ± 3.5 (1–8) | 0.30 |
| Propofol dose (mg) † | 94.8 ± 49.1 (41–158) | 129.6 ± 96.2 (0–445) | 0.58 | 101.5 ± 88 (47–66) | 129 ± 94.5 (0–445) | 0.48 | 129.1 ± 81.4 (41–316) | 125.1 ± 101.8 (0–445) | 0.68 |
| Remifentanil dose (mcg) † | 155.5 ± 34.3 (109–186) | 191.6 ± 120.4 (0–421) | 0.72 | 135 ± 21.7 (110–148) | 192.2 ± 118.8 (0–421) | 0.42 | 208 ± 128.7 (0–337) | 175.4 ± 106.6 (0–421) | 0.35 |
| Procedure difficulty score | 0.017 | 0.041 | 0.072 | ||||||
| I | 0 (0%) | 3 (100%) | 0 (0%) | 3 (100%) | 0 (0%) | 3 (100%) | |||
| II | 0 (0%) | 15 (100%) | 1 (6.7%) | 14 (93.3%) | 9 (60% | 6 (40%) | |||
| III | 4 (13.8%) | 25 (86.2%) | 3 (10.3%) | 26 (89.7%) | 9 (31%) | 20 (69%) | |||
Data presented as mean ± standard deviation.
The significane represents frequency (percent).
The most common sedation‐related adverse event was hypotension (Table 2). Just over one third of patients experienced at least one episode of hypotension, whereas only 8% of patients experienced at least one episode of either bradycardia or hypoxia. Boluses of metaraminol were required for one patient to treat hypotension. No procedures were abandoned.
Table 2.
Sedation‐related adverse events based on definitions described under Methods
| Adverse event | 1 episode | 2 episodes | 3 episodes | 4 episodes | 5 episodes | Total |
|---|---|---|---|---|---|---|
| Hypotension | 9 (18%) | 7 (14%) | 2 (4%) | 0 | 1 (2%) | 19 (38%) |
| Hypoxia | 3 (6%) | 0 | 0 | 1 (2%) | 0 | 4 (8%) |
| Bradycardia | 3 (6%) | 1 (2%) | 0 | 0 | 0 | 4 (8%) |
Postprocedure, the median recovery time was 5 min; 30‐day mortality was zero. Minor adverse events were noted in four patients (8%), with three patients complaining of neck pain and one developing pyrexia. These patients were admitted for overnight observation and monitoring, with no radiological evidence of perforation or pneumonia. No patients required readmission due to postprocedure complications.
Discussion
This single‐center prospective observational study demonstrates that supplementary oxygenation with HFNOT is a feasible and safe adjunct to use alongside a two‐stage sedation technique during therapeutic endoscopy within the hypopharynx. Rates of sedation‐related complications were low, with no patients requiring conversion to endotracheal intubation, despite the age and high rates of ASA grade 3/4 patients. These results suggest that our technique is a feasible and safe alternative to GA and invasive ventilation, which remains the mainstay airway modality in other ZD centers.
Patients with ZD are at a high risk of aspiration due to the potential for food residue within the diverticulum. Anesthetic techniques have traditionally relied on endotracheal intubation to provide definitive airway protection. In this study, the use of HFNOT in combination with suctioning of the pharyngeal pouch and stomach prior to deep sedation provided optimal operating conditions while minimizing the aspiration risk and maintaining oxygenation. The low rate of hypoxia is reassuring and may be attributable to the use of HFNOT, which delivers 100% oxygen up to a rate of 60 L/min.
Optiflow™ (Fisher and Paykel Healthcare) is a system that delivers heated (up to 37°C), humidified oxygen at flow rates up to 60 L/min via wide bore nasal cannulae. It provides up to 100% Fio2 and can deliver low levels of positive pressure, preventing alveolar collapse and atelectasis. Using higher gas flow rates, higher airway pressures can be generated with the mouth closed. 9 It has been used to treat respiratory failure in intensive care and emergency departments 10 and in anesthesia for preoxygenation and for procedures requiring apnoeic oxygenation. It is thought that its ability to flush out the anatomical dead space and aid gaseous mixing through continuous gas insufflation and positive airway pressures facilitates both oxgyenation and carbon dioxide clearance. 11 These effects are particularly beneficial during deep sedation to keep patients still for flexible endoscopic septal division (FESD), when ventilatory efforts may be somewhat suppressed. It has also been shown to improve Pao2 levels both during and after procedures. 7
Endotherapy within the hypopharynx can be challenging due to the limited operating space. Although FESD may be performed without GA, patients suffering from ZD are often elderly with multiple comorbidities. Procedures requiring FESD require deep sedation, which can pose significant anesthetic challenges. A meta‐analysis on the efficacy and safety of FESD included 20 studies with anesthetic techniques comprising GA, deep sedation without tracheal intubation, and moderate (conscious) sedation. 12 Procedures performed under GA had relatively higher complication rates of bleeding, fever, and perforation, 13 , 14 , 15 whereas studies involving conscious sedation appeared to report a higher incidence of subcutaneous emphysema, bleeding, pneumonia, and perforation with mediastinitis and sepsis. 16 , 17 , 18 , 19 It is possible that the observed differences in GA complication profiles may be due to endotracheal intubation, which may limit the view and operating space during hypophayngeal procedures. Conversely, under conscious sedation, the patient may move unexpectedly in response to noxious stimuli, thus predisposing him or her to procedure‐related complications. The use of deep sedation with additional HFNOT combines the advantages of both techniques.
There is ongoing debate on the balance of safety and feasibility of propofol sedation and who should administer sedation to these patients. 20 In the United Kingdom, propofol sedation is undertaken by anesthetists. Sedative agents work synergistically, with a narrow therapeutic window. One study of 799 patients investigating sedation‐related complications with propofol for advanced endoscopic procedures showed that rates of hypoxaemia can be as high as 12.8%, with 0.6% of procedures being terminated due to adverse effects. 5 Another study comparing midazolam to propofol sedation in Endoscopic Retrograde Cholangiopancreatography (ERCP) showed more patients desaturated, and one required bag mask ventilation in the propofol group. 21 Unlike this study, others that investigate deep sedation may exclude high‐risk patients (ASA III or IV), which is associated with significantly higher rates of hypoxia and hypotension. 22 The Joint Royal Colleges of Anaesthetists (RCoA) and British Society of Gastroenterologists (BSG) Working Party advocates the involvement of an anesthetist for complex upper gastrointestinal procedures. 20
Our study and approach had several limitations, principally the lack of a comparison arm. Due to the frailty of some participants, they would not have been eligible for randomization into a GA arm, owing to selection bias. As such, our study provides real‐world data for a higher‐risk population. Next, procedural difficulties owing to HFNOT should be mentioned. There were difficulties in positioning the nasal prongs, while the patient was in the left lateral position. We were also unable to provide capnography to monitor the respiratory rate, airway patency, and end tidal CO2 measurements. Instead, we had to use transthoracic impedance to record the respiratory rate and clinical vigilance to monitor airway patency. Finally, this was a single operator study involving a small sample size. However, ZD is rare with prevalence of 0.01–0.11%, and at present, our center is the only center in the United Kingdom to use HFNOT with FESD.
In conclusion, our study demonstrates that a two‐stage sedation technique, along with the use of HFNOT for ZD repair using a flexible endoscope, is both safe and effective. Anesthetic complications were transient, predictable, and responded to first‐line treatment. This HFNOT technique allows deep sedation without the need for invasive ventilation, which may enable therapy in patients who would otherwise be unsuitable for other techniques. Moreover, use of HFNOT can preserve the operating space within the hypopharynx, which has the potential to mitigate the risk of postprocedural complications as seen in other studies. The key to the success of this technique is the team work of an experienced anesthetist working closely with the endoscopist to allow optimal operating conditions and improve patient outcomes.
Declaration of conflict of interest: No conflicts of interest declared.
Author contribution: Z.R. was the anesthetic assistant during procedures and contributed to data collection, data analysis, writing up first draft, and editing subsequent drafts of paper. N.P. and K.S. contributed to writing up the discussion and editing later drafts of paper. A.G. was the anesthetist during procedures and contributed to study design, data collection, and editing drafts of paper. A.D. was the anesthetist during procedures and contributed to data collection and editing drafts of paper. C.M. contributed to study design, critical review, and editing draft paper. K.S. contributed to editing draft, critical review, and help with statistics. S.I. contributed to study design, data collection, and editing drafts of paper and was the endoscopist for procedures. H.M. was the statistician.
REFERENCES
- 1. Ishaq S, Sultan H, Siau K, Kuwai T, Mulder CJ, Neumann H. New and emerging techniques for endoscopic treatment of Zenker's diverticulum: state‐of‐the‐art review. Dig. Endosc. 2018; 30: 449–60. [DOI] [PubMed] [Google Scholar]
- 2. Ashman A, Dale OT, Baldwin DL. Management of isolated cricopharyngeal dysfunction: systematic review. J. Laryngol. Otol. 2016; 130: 611–5. [DOI] [PubMed] [Google Scholar]
- 3. Horiuchi A, Nakayama Y, Hidaka N, Ichise Y, Kajiyama M, Tanaka N. Low‐dose propofol sedation for diagnostic esophagogastroduodenoscopy: results in 10,662 adults. Am. J. Gastroenterol. 2009; 104: 1650–5. [DOI] [PubMed] [Google Scholar]
- 4. Kuwata NY, Gotoda T, Suzuki S, Mukai S, Itoi T, Moriyasu F. Reasonable decision of anesthesia methods in patients who underwent endoscopic submucosal dissection for superficial esophageal carcinoma: A retrospective analysis in a single Japanese institution. Turk. J. Gastroenterol. 2016; 27: 91–6. [DOI] [PubMed] [Google Scholar]
- 5. Coté GA, Hovis RM, Ansstas MA et al Incidence of sedation‐related complications with propofol use during advanced endoscopic procedures. Clin. Gastroenterol. Hepatol. 2010; 8: 137–42. [DOI] [PubMed] [Google Scholar]
- 6. Tam K, Jeffery C, Sung CK. Surgical management of supraglottic stenosis using intubationless optiflow. Ann. Otol. Rhinol. Laryngol. 2017; 126: 669–72. [DOI] [PubMed] [Google Scholar]
- 7. Lucangelo U, Vassallo FG, Marras E et al High‐flow nasal interface improves oxygenation in patients undergoing bronchoscopy. Crit. Care Res. Pract. 2012; 2012: 506382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blayney MR. Procedural sedation for adult patients: an overview. Contin. Educ. Anaesth. Crit. Care Pain. 2012; 12: 176–80. [Google Scholar]
- 9. Ritchie JE, Williams AB, Gerard C, Hockey H. Evaluation of a humidified high‐flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth. Intensive Care. 2011; 39: 1103–10. [DOI] [PubMed] [Google Scholar]
- 10. Parke RL, McGuiness SP, Eccleston ML. A preliminary randomized controlled trial to assess effectiveness of nasal high‐flow oxygen in intensive care patients. Respir. Care. 2011; 56: 265–70. [DOI] [PubMed] [Google Scholar]
- 11. Patel A, Nouraei SAR. Transnasal Humidified Rapid Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015; 70: 323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishaq S, Hassan C, Antonello A et al Flexible endoscopic treatment for Zenker's diverticulum: a systematic review and meta‐analysis. Gastrointest. Endosc. 2016; 83: 1076–89.e5. [DOI] [PubMed] [Google Scholar]
- 13. Laquiere A, Grandval P, Arpurt JP et al Interest of submucosal dissection knife for endoscopic treatment of Zenker's diverticulum. Surg. Endosc. 2015; 29: 2802–10. [DOI] [PubMed] [Google Scholar]
- 14. Costamagna G, Iacopini F, Bizzotto A et al Prognostic variables for the clinical success of flexible endoscopic septotomy of Zenker's diverticulum. Gastrointest. Endosc. 2016; 83: 765–73. [DOI] [PubMed] [Google Scholar]
- 15. Pescarus R, Shlomovitz E, Sharata AM et al Trans‐oral cricomyotomy using a flexible endoscope: technique and clinical outcomes. Surg. Endosc. 2016; 30: 1784–9. [DOI] [PubMed] [Google Scholar]
- 16. Ishioka S, Sakai P, Maluf Filho F, Melo JM. Endoscopic incision of Zenker's diverticula. Endoscopy. 1995; 27: 433–7. [DOI] [PubMed] [Google Scholar]
- 17. Hashiba K, de Paula AL, da Silva JGN et al Endoscopic treatment of Zenker's diverticulum. Gastrointest. Endosc. 1999; 49: 93–7. [DOI] [PubMed] [Google Scholar]
- 18. Christiaens P, De Roock W, Van Olmen A, Moons V, D'Haens G. Treatment of Zenker's diverticulum through a flexible endoscope with a transparent oblique‐end hood attached to the tip and a monopolar forceps. Endoscopy. 2007; 39: 137–40. [DOI] [PubMed] [Google Scholar]
- 19. Rabenstein T, May A, Michel J et al Argon plasma coagulation for flexible endoscopic Zenker's diverticulotomy. Endoscopy. 2007; 39: 141–5. [DOI] [PubMed] [Google Scholar]
- 20. Sidhu R, Turnbull D, Newton M et al Deep sedation and anaesthesia in complex gastrointestinal endoscopy: a joint position statement endorsed by the British Society of Gastroenterology (BSG), Joint Advisory Group (JAG) and Royal College of Anaesthetists (RCoA). Frontline Gastroenterol. 2019; 10: 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wehrmann T, Kokabpick S, Lembcke B, Caspary WF, Seifert H. Efficacy and safety of intravenous propofol sedation during routine ERCP: a prospective, controlled study. Gastrointest. Endosc. 1999; 49: 677–83. [DOI] [PubMed] [Google Scholar]
- 22. Schilling D, Rosenbaum A, Schweizer S, Richter H, Rumstadt B. Sedation with propofol for interventional endoscopy by trained nurses in high‐risk octogenarians: a prospective, randomized, controlled study. Endoscopy. 2009; 41: 295–8. [DOI] [PubMed] [Google Scholar]


