Abstract
Background and Aim
Surgical resection is the standard local therapy for patients with colorectal liver metastases (CRLM). However, elderly and vulnerable patients sometimes have various organ dysfunctions. We have to conduct nonsurgical local therapies for those patients who might not tolerate surgery or systemic chemotherapy.
Methods
We retrospectively reviewed medical records of 254 patients who underwent local therapies, including surgery, radiofrequency ablation (RFA), and stereotactic body radiation therapy (SBRT), for CRLM from January 2010 to December 2016, at seven tertiary‐care institutions in Japan. This study was designed to include elderly, vulnerable patients who received local therapy for CRLM. For those undergoing liver resection, only those having one or more points of the Charlson comorbidity index (CCI) were enrolled.
Results
Of the total 169 enrolled patients, 122 patients underwent surgery, 42 RFA, and 5 SBRT as the first local therapy for CRLM. Median overall survival from the first local therapy was 5.9 years for the surgery group, 2.7 years for the RFA group, and 3.8 years for the SBRT group. The proportion of the patients with CCI ≧3 was significantly higher in the group of RFA/SBRT than surgery (P < 0.0001). In selected patients with CCI ≧3, there was no difference of the median survival time between the surgery group and the RFA group.
Conclusions
We could have other treatment options to provide nonsurgical local therapies (RFA/SBRT) for elderly, vulnerable CRLM patients who have risks for surgery.
Keywords: colorectal liver metastases, elderly patients, radiofrequency ablation, stereotactic body radiation therapy, vulnerable patients
The number of elderly patients with colorectal liver metastasis (CRLM) is increasing. This article indicates the possibility of other treatment options to provide nonsurgical local therapies (radiofrequency ablation/stereotactic body radiation therapy) for elderly, vulnerable CRLM patients who have risks for surgery.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in males and the second in females, with over 1.2 million newly diagnosed cases and 608 700 deaths estimated to have occurred in 2008.1 The liver is the most common metastatic site of CRC. Surgical resection is the standard therapy to cure patients with colorectal liver metastasis (CRLM) if the tumors can be removed completely.
There is a worldwide increase in the elderly population. The number of elderly patients with CRLM is also increasing.2 Elderly patients may have various risk factors for surgery under general anesthesia. Liver surgery is a technically demanding operation with high risk of morbidity and even mortality, because of the anatomical complexity with meticulous vascular/biliary system, presence of abundant blood flow, and possibility of massive intraoperative blood loss. Postoperative morbidities may include infectious complications and failure of cardiopulmonary, renal, and/or hepatic function. Thus, for elderly patients having medical comorbidities, surgical indication should be decided carefully.
Systemic chemotherapy can be selected for those elderly CRLM patients with risks for surgical procedures. However, it is sometimes even difficult to provide systemic chemotherapy for elderly patients with interstitial lung disease, renal dysfunction, or hepatic dysfunction. In those patients whose CRLM is localized and without evidence of metastatic CRC to other organs, local therapies including radiofrequency ablation (RFA) or radiation therapy (RT) may play an alternative role to treat CRLM. RFA demonstrated clear survival benefits for patients who were deemed inoperable and/or did not respond to systemic chemotherapy.3 Stereotactic body radiation therapy (SBRT) was also shown to be effective for local control in patients with CRLM in a recent study.4 Therefore, RFA or SBRT could be used for vulnerable patients with CRLM who were not absolutely suitable for surgical resection or systemic chemotherapy.
It is assumed to be difficult to conduct a randomized clinical trial to test the benefits of these nonsurgical local therapies over surgical resection for those elderly, vulnerable patients with medical comorbidities. Patients with CRLM may require multiple therapeutic modalities as the disease progresses in their clinical course. Therefore, in this study, we collected clinicopathological data of elderly, vulnerable patients with CRLM who underwent surgical or nonsurgical local therapy at multiple institutions, and evaluated their survival outcomes for every opportunity of the local therapy.
Methods
Patients and clinical collection
We retrospectively reviewed medical records of 254 patients who underwent local therapies, including liver resection, RFA, and SBRT, for CRLM from January 2010 to December 2016, at seven tertiary‐care institutions in Japan. The study was designed to include elderly, vulnerable patients who received local therapy for CRLM. The inclusion criteria of this study were those patients older than 64 years who underwent local therapy for CRLM with or without systemic chemotherapy. For those undergoing liver resection, only those having one or more points of the Charlson comorbidity index (CCI) were included.5 There were a total of 169 patients undergoing local therapy for CRLM meeting these inclusion criteria, while 85 patients were excluded (Fig. 1).
Figure 1.
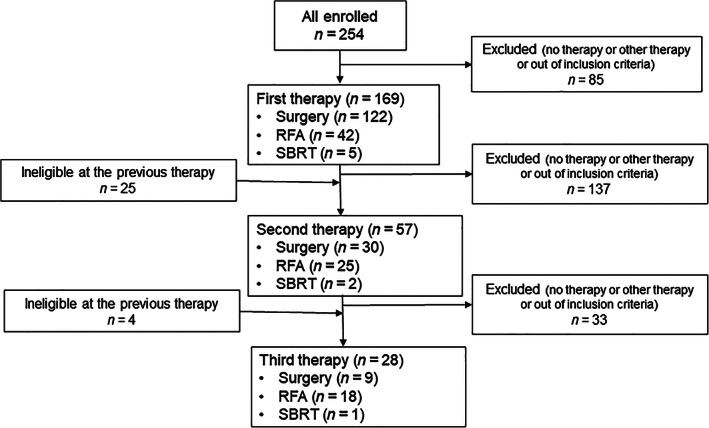
Patient flow diagram. RFA, radiofrequency ablation; SBRT, stereotactic body radiation therapy.
The treatment strategy was similarly decided through multidisciplinary conference at each institution. Liver resection was indicated for CRLM patients usually as the first choice if technically resectable on imaging. Systemic chemotherapy was used at medical oncologist's discretion before and/or after surgery adjuvantly. RFA or SBRT was selectively used for the patients who preferred it to surgery or who were not deemed to have sufficient organ function for surgical resection under general anesthesia or high‐intensity systemic chemotherapy. RFA was performed only with percutaneous approach.
Since there were a considerable number of patients undergoing local therapy for CRLM several times, the enrolled patients were classified based on the timeline of the local therapy. Then, we compared clinicopathological data, posttherapeutic complications, and survival outcomes between the patients who underwent surgical resection and nonsurgical local therapy, including RFA or SBRT.
This study was approved by the Institutional Review Board of each institution and conducted in accordance with the mandates of the Helsinki Declaration.
Statistical analysis
All clinicopathological data of each institution were centralized and analyzed. Categorical variables were evaluated using chi‐square test and are presented as the numbers and percentages. Continuous variables were evaluated using Wilcoxon test and are presented as the median and range. All P values were based on two‐sided statistical tests and <0.05 was considered significant. Overall survival was estimated using the Kaplan–Meier method. The starting date of the assessment was the starting day of each local therapy and the ending date was the day of death or recurrence of the case or the day of finishing the follow‐up of the case as outpatient. All calculations were performed using the SAS software (release 9.4) (SAS Institute Inc., Cary, North Carolina, USA) and Microsoft Excel 2013 (Microsoft Corporation, Redmond, Washington, USA).
Results
Local therapies provided for the patients
Treatment course of the enrolled patients is shown in Figure 1. In total of the 169 patients, 122 patients underwent surgical resection, 42 RFA, and 5 SBRT as the first local therapy for CRLM. Next, 57 patients underwent the second local therapy for recurrence of CRLM: 30 surgical resection, 25 RFA, and 2 SBRT. Then, 28 patients underwent the third local therapy for recurrence of CRLM: 9 surgical resection, 18 RFA, and 1 SBRT.
Comparison of clinicopathological characteristics between each treatment timeline
Clinicopathological characteristics of the enrolled patients according to the treatment timelines are shown in Table 1. The proportion of the patients with CCI ≧3 was significantly higher at the second therapy or third therapy than at the first therapy (P = 0.0122 and 0.0021, respectively). Posttherapeutic complications occurred more frequently at the second therapy than the first therapy (P = 0.0116).
Table 1.
Patient characteristics
| Variables | First treatment (n = 169) | Second treatment (n = 57) | Third treatment (n = 28) | P |
|---|---|---|---|---|
| Age (median, range) | 71 (65–94) | 71 (65–90) | 72 (65–92) |
First vs second: 0.9906 Second vs Third: 0.4040 third vs first: 0.4743 |
| ECOG performance status (0 or 1) (%) | 159 (94.1) | 56 (98.2) | 27 (96.4) |
First vs second: 0.2066 Second vs Third: 0.6035 third vs first: 0.6166 |
| Charlson comorbidity index ≧3 (%) | 52 (30.8) | 28 (49.1) | 17 (60.7) |
First vs second: 0.0122 Second vs Third: 0.3143 third vs first: 0.0021 |
| BMI (median, range) | 22.5 (14.6–33.3) | 22.5 (15.1–31.7) | 23.6 (16.6–28.3) |
First vs second: 0.5365 Second vs third: 0.4515 Third vs first: 0.6347 |
| Child‐Pugh score (B or C) (%) | 2 (1.2) | 1 (1.8) | 0 |
First vs second: 0.7448 Second vs third: 0.4848 Third vs first: 0.5664 |
| Number of liver metastasis (single) (%) | 93 (55.0) | 33 (57.9) | 18 (64.3) |
First vs second: 0.6687 Second vs third: 0.3589 Third vs first: 0.8748 |
| Maximum diameter of liver metastasis (mm) (median, range) | 23 (2–120) | 22 (5–67) | 29.5 (9–60) |
First vs second: 0.4441 Second vs third: 0.0437 Third vs first: 0.1044 |
| Other distant metastatic site than liver at the therapy (yes) (%) | 29 (17.2) | 8 (14.0) | 5 (17.9) |
First vs second: 0.5814 Second vs third: 0.6454 Third vs first: 0.9279 |
| Uncontrolled other metastatic site than liver (yes) (%) | 25 (14.8) | 6 (10.5) | 3 (10.7) |
First vs second: 0.4181 Second vs third: 0.9789 Third vs first: 0.5670 |
| Chemotherapy before or after the therapy (yes) (%) | 98 (58.0) | 29 (50.9) | 14 (50.0) |
First vs second: 0.3494 Second vs third: 0.9394 Third vs first: 0.4293 |
| Primary lesion (rectum) (%) | 53 (31.4) | 18 (31.6) | 7 (25.0) |
First vs second: 0.9755 Second vs third: 0.5315 Third vs first: 0.4982 |
| Histology (tubular) (%) | 163 (96.4) | 52 (91.2) | 26 (92.9) |
First vs second: 0.1131 Second vs third: 0.7973 Third vs first: 0.3724 |
| Lymph node metastasis of primary lesion (n1) (%) | 98 (58.0) | 35 (61.4) | 20 (71.4) |
First vs second: 0.6505 Second vs third: 0.3633 Third vs first: 0.1789 |
| Complication after the therapy (yes) (%) | 9 (5.3) | 9 (15.8) | 3 (10.7) |
First vs second: 0.0116 Second vs third: 0.5277 Third vs first: 0.2695 |
| Extent of complication (Clavien–Dindo classification grade ≧3A) (%) | 6 (3.6) | 5 (8.8) | 2 (7.1) |
First vs second: 0.1131 Second vs third: 0.7973 Third vs first: 0.3724 |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group.
Comparison of clinicopathological characteristics between local therapies at the first local therapy
We compared clinicopathological variables of the patients with RFA or SBRT versus surgical resection at the time of the first local therapy (Table 2). The proportion of the patients with CCI ≧3 was significantly higher in the group of RFA/SBRT than surgical resection (P < 0.0001). The proportion of the presence of other distant metastatic sites than liver and uncontrolled other metastatic sites than liver were also significantly higher in the group of RFA/SBRT than surgical resection (P < 0.0001 and P = 0.0066, respectively). The proportion of rectal cancer as a primary lesion was significantly lower in the group of RFA/SBRT than surgical resection (P < 0.0157). There was no significant difference in the incidence of posttherapeutic complications between RFA/SBRT and surgical resection, but the incidence was zero in the group of RFA/SBRT.
Table 2.
Comparison of clinicopathological characteristics at the first local therapy
| First treatment for liver metastasis | |||
|---|---|---|---|
| Variables | Surgery (n = 122) | RFA/SBRT (n = 47) | P |
| Age (median, range) | 71 (65–85) | 72 (65–94) | 0.5371 |
| ECOG performance status (0 or 1) (%) | 115 (94.3) | 44 (93.6) | 1.0000 |
| Charlson comorbidity index (CCI) (≧3) (%) | 15 (12.3) | 37 (78.7) | <0.0001 |
| BMI (median, range) | 22.3 (14.6–31.9) | 23.5 (15.1–33.3) | 0.4212 |
| Child‐Pugh score (B or C) (%) | 1 (0.8) | 1 (2.1) | 0.4610 |
| Number of liver metastasis (single) (%) | 66 (54.1) | 27 (57.4) | 0.8202 |
| Maximum diameter of liver metastasis (mm) (median, range) | 23 (2–110) | 24 (7–120) | 0.8925 |
| Other distant metastatic site than liver at the therapy (yes) (%) | 9 (7.4) | 20 (42.6) | <0.0001 |
| Uncontrolled other metastatic site than liver (yes) (%) | 12 (9.8) | 13 (27.7) | 0.0066 |
| Chemotherapy before or after the therapy (yes) (%) | 66 (54.1) | 32 (68.1) | 0.1185 |
| Primary lesion (rectum) (%) | 45 (36.9) | 8 (17.0) | 0.0157 |
| Histology (tubular) (%) | 118 (96.7) | 45 (95.7) | 0.6707 |
| Lymph node metastasis of primary lesion (n1) (%) | 70 (57.4) | 28 (59.6) | 0.8628 |
| Complication after the therapy (yes) (%) | 9 (7.4) | 0 | 0.0636 |
| Extent of complication (Clavien–Dindo classification grade ≧3A) (%) | 6 (4.9) | 0 | 0.1878 |
BMI, body mass index; CCI, Charlson comorbidity index; ECOG, Eastern Cooperative Oncology Group; RFA, radiofrequency ablation; SBRT, stereotactic body radiation therapy.
Survival analyses
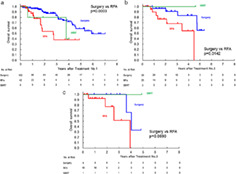
Median overall survival from the first local therapy was 5.9 years for the surgery group, 2.7 years for the RFA group, and 3.8 years for the SBRT group, as shown in Figure 2a (surgery vs RFA: P = 0.0003). Survival data from the second or third local therapy are also shown in Figure 2b (surgery vs RFA: P = 0.0142) or Figure 2c (surgery vs RFA: P = 0.0590), respectively. In selected patients with CCI ≧3, the median survival time of the surgery group was shorter than all patients with surgery. There was no difference of the median survival time compared with the RFA group (Fig. 3). As shown in Figure 4, in selected patients without other distant metastatic sites than the liver, survival curves for both the RFA group and the surgery group seems to be similar within 1 year after the first therapy.
Figure 2.
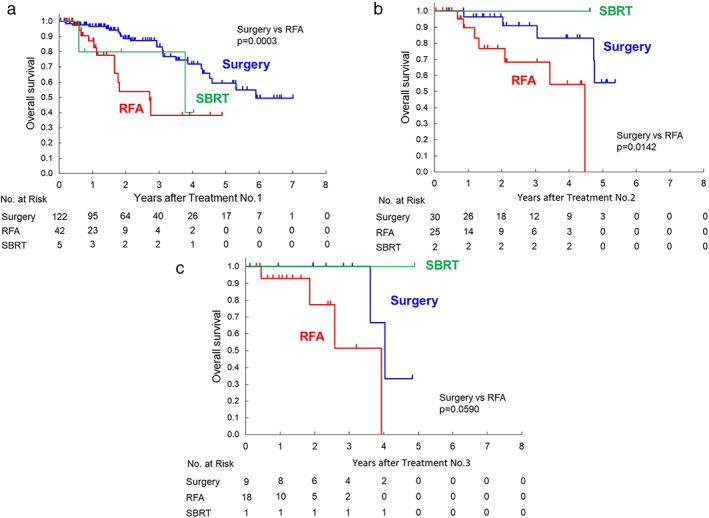
(a) Kaplan–Meier survival curves of 169 colorectal liver metastasis (CRLM) patients undergoing surgery or radiofrequency ablation (RFA) or stereotactic body radiation therapy (SBRT) from the first time of each therapy. (b) Kaplan–Meier survival curves of 57 CRLM patients undergoing surgery or RFA or SBRT from the second time of each therapy. (c) Kaplan–Meier survival curves of 28 CRLM patients undergoing surgery or RFA or SBRT from the third time of each therapy.
Figure 3.
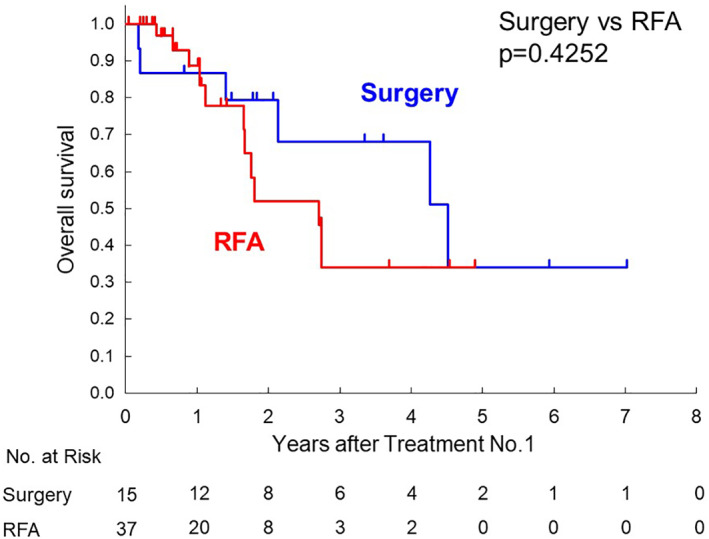
Kaplan–Meier survival curves of 52 colorectal liver metastasis patients with Charlson comorbidity index ≧3 undergoing surgery or radiofrequency ablation (RFA) from the first time of both therapies.
Figure 4.
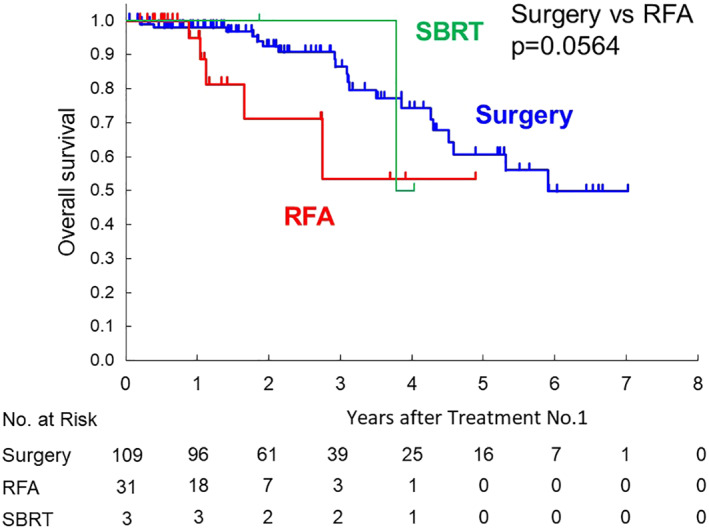
Kaplan–Meier survival curves of 143 colorectal liver metastasis patients undergoing surgery or radiofrequency ablation (RFA) or stereotactic body radiation therapy (SBRT) from the first time of each therapy (excluding patients with other metastases at the therapy).
Discussion
There were a lot of reports and systematic reviews regarding the outcome after RFA in patients with CRLM.6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Surgical resection is still the gold standard in the treatment of resectable CRLM.17 The National Comprehensive Cancer Network guidelines also provide recommendations of surgical resection or systemic chemotherapy for patients with CRLM.18, 19 Five‐year survival rates were reportedly 23–66% after surgical resection and 21–49% after RFA.7, 8, 9, 10, 11, 12, 13 Those studies demonstrated that survival outcomes were better after surgical resection than RFA for CRLM patients. However, the difference of survival outcomes among the therapeutic modalities could be explained in some points. In the majority of previous studies of which study designs were retrospective, surgical resection was used for those patients with resectable CRLM, while RFA was used for those with unresectable CRLM; therefore, selection bias for local treatment cannot be denied in those studies. In the present study, compared with those patients with surgical resection, those with RFA/SBRT had other distant metastatic sites or uncontrolled other metastatic sites than the liver at the therapy more frequently (Table 2).
Furthermore, the prevalence of comorbidity among patients with CRC reportedly increased from 1995 to 2010.20 Aging contributes to more comorbid diseases. Although over 60% of the total incidence of cancer occurs in the elderly (more than 65 years) population, there is a general belief that elderly patients may not be able to tolerate high‐intensity cancer treatments, which may result in this patient population being excluded from prospective clinical trials. The proportion of elderly patients included in clinical trials is significantly lower than the actual proportion of elderly patients suffering from cancer.21 The results from clinical trials in a younger generation of patients may not directly be applicable to the treatment for the elderly. Therefore, in clinical situation, it is often difficult to decide the suitable treatment for elderly, vulnerable patients. We need to consider an alternative to the standard therapy especially for elderly, vulnerable patients. As the number of elderly patients with CRLM increases more, the alternative treatment for those patients will be more required in the near future.
Although survival outcomes were better after surgical resection than RFA for CRLM patients, short posttreatment outcomes should also be evaluated for elderly, vulnerable patients. The RFA‐related morbidity was reportedly 4%–8.1%.22, 23, 24, 25 In a meta‐analysis comparing the short‐ and long‐term outcomes after RFA and surgical resection,26 morbidity was shown to be 9.98% in RFA and 24.1% in surgical resection. Morbidity was higher in the surgical resection group and the relative risk was 2.495 (95% confidence interval, 1.881–3.308). In our study complication occurred in 7.4% after surgical resection but none after RFA/SBRT (Table 2). RFA/SBRT seems less invasive and safer to patients than surgical resection. Therefore, RFA or SBRT can be selected for patients who cannot tolerate surgery due to poor physical condition or inadequate organ function.
RT is also a less invasive local therapy. Recently, SBRT has emerged as a more effective technique of RT than ordinary RT. SBRT is a high‐precision conformal external‐beam radiation technique that ablates targets at extracranial sites by delivering hypofractionated high‐dose radiation while sparing the normal surrounding tissue. SBRT was shown to provide high rates of local control for patients with stage I non‐small cell lung cancer27 and 91% of the 3‐year local control rate for patients with hepatocellular carcinoma.28 There were some reports showing clinical outcomes after SBRT for patients with metastatic liver tumor. Kirichenko et al. reported the local control rate of 93.8% (3/48 failures, 2 for CRLM and 1 for breast cancer liver metastases).29 Takeda et al. studied the effect of SBRT in patients with hepatic or pulmonary oligometastases from CRC, and reported that the local control rate and overall survival were both 100% at 2 years, especially in 12 patients with CRLM.4 In our study, 8 CRLM patients with SBRT developed no local recurrences during the study period.
It is worthwhile evaluating whether elderly patients with organ dysfunction can tolerate high‐intensity therapy or not. The CCI was developed as a scoring system to rank comorbidity into specific risk classification by assigning type and severity scores to a range of specific illnesses.5 Although the CCI was not designed to predict perioperative mortality in surgical cohorts, some reports showed the correlation between the CCI and perioperative death in elderly patients. Laor et al. analyzed postoperative morbidity and mortality in geriatric patients (≧75 years of age) undergoing general surgery and demonstrated that the score of CCI of early nonsurvivors (death within 30 days after surgery) was significantly higher than that of survivors. The mean CCI score of early nonsurvivors was over 3 points.30 In a national cohort study by Doat et al., the CCI ≧ 3 was shown to be associated with the risk of death in patients with metastatic CRC. In our study, the proportion of patients with the CCI ≧ 3 having undergone surgical resection was significantly lower than those having other local therapies (12.3% vs 78.7%) (Table 2). Surgical candidates should have been selected carefully through preoperative evaluation, especially in elderly, vulnerable patients. Only in patients with CCI ≥3, there was no significant difference in overall survival after surgical resection and RFA in this study (Fig. 3).
Systemic chemotherapy can be considered as another option for patients who are not fit for surgery. However, as systemic chemotherapy can cause adverse events or organ injury, it may be difficult to provide high‐intensity chemotherapy for patients with poor physiological condition. Doat et al. reported that elderly patients had a smaller chance of receiving chemotherapy in the advanced setting (48% of patients ≥75 years vs 85% of patients <75 years; P < 0.0001).2 Therefore, noninvasive local therapy can be considered for elderly, vulnerable patients who might not tolerate surgical resection or systemic chemotherapy.
There are some limitations in this study. First, this study is retrospective. The selection bias of the patients for different therapeutic modalities might exist. Second, patients receiving various regimens of systemic chemotherapies were included. Some patients had systemic chemotherapy before and/or after the local therapy. We counted only the number of times of local therapies but not for systemic chemotherapy. We did not analyze the effect of systemic chemotherapy on survival outcomes. Finally, it was difficult to evaluate the survival outcomes after SBRT alone from our data because the number of patients with SBRT was too small. As shown in a few previous reports, it could be expected that SBRT provided good local control for patients with CRLM. Prospective studies to evaluate efficacy of SBRT for CRLM patients should be conducted in the near future.
In conclusion, we could have other treatment options to provide nonsurgical local therapies (RFA/SBRT) for elderly, vulnerable CRLM patients who have risks for surgical resection or systemic chemotherapy and otherwise might be indicated palliative care only.
Acknowledgments
We thank Drs Seiji Natsume, Takuya Shimizuguchi, Yuichiro Tsurugai, Masaaki Ito, and Asao Ogawa for their various support. This study was supported by a grant‐in‐aid for Cancer Research from the Ministry of Health, Welfare, and Labor of Japan (28‐A‐8).
Declaration of conflict of interest: There are no conflicts of interest to declare, from any of the authors, in relation to this research.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J. Clin. 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Doat S, Thiebaut A, Samson S, Ricordeau P, Guillemot D, Mitry E. Elderly patients with colorectal cancer: treatment modalities and survival in France. National data from the ThInDiT cohort study. Eur. J. Cancer. 2014; 50: 1276–83. [DOI] [PubMed] [Google Scholar]
- 3. Siperstein AE, Berber E, Ballem N, Parikh RT. Survival after radiofrequency ablation of colorectal liver metastases: 10‐year experience. Ann. Surg. 2007; 246: 559–65. [DOI] [PubMed] [Google Scholar]
- 4. Takeda A, Sanuki N, Tsurugai Y, Oku Y, Aoki Y. Stereotactic body radiotherapy for patients with oligometastases from colorectal cancer: risk‐adapted dose prescription with a maximum dose of 83‐100 Gy in five fractions. J. Radiat. Res. 2016; 57: 400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 6. Stang A, Fischbach R, Teichmann W, Bokemeyer C, Braumann D. A systematic review on the clinical benefit and role of radiofrequency ablation as treatment of colorectal liver metastases. Eur. J. Cancer. 2009; 45: 1748–56. [DOI] [PubMed] [Google Scholar]
- 7. Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br. J. Surg. 2003; 90: 1240–3. [DOI] [PubMed] [Google Scholar]
- 8. Abdalla EK, Vauthey JN, Ellis LM et al Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann. Surg. 2004; 239: 818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. White RR, Avital I, Sofocleous CT et al Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J. Gastrointest. Surg. 2007; 11: 256–63. [DOI] [PubMed] [Google Scholar]
- 10. Gleisner AL, Choti MA, Assumpcao L, Nathan H, Schulick RD, Pawlik TM. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection‐radiofrequency ablation. Arch. Surg. 2008; 143: 1204–12. [DOI] [PubMed] [Google Scholar]
- 11. Lee WS, Yun SH, Chun HK et al Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J. Clin. Gastroenterol. 2008; 42: 945–9. [DOI] [PubMed] [Google Scholar]
- 12. Berber E, Tsinberg M, Tellioglu G, Simpfendorfer CH, Siperstein AE. Resection versus laparoscopic radiofrequency thermal ablation of solitary colorectal liver metastasis. J. Gastrointest. Surg. 2008; 12: 1967–72. [DOI] [PubMed] [Google Scholar]
- 13. Hur H, Ko YT, Min BS et al Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am. J. Surg. 2009; 197: 728–36. [DOI] [PubMed] [Google Scholar]
- 14. Reuter NP, Woodall CE, Scoggins CR, McMasters KM, Martin RC. Radiofrequency ablation vs. resection for hepatic colorectal metastasis: therapeutically equivalent? J. Gastrointest. Surg. 2009; 13: 486–91. [DOI] [PubMed] [Google Scholar]
- 15. McKay A, Fradette K, Lipschitz J. Long‐term outcomes following hepatic resection and radiofrequency ablation of colorectal liver metastases. HPB Surg. 2009; 2009: 346863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim KH, Yoon YS, Yu CS et al Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J. Korean Surg. Soc. 2011; 81: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann. Surg. Oncol. 2006; 13: 1271–80. [DOI] [PubMed] [Google Scholar]
- 18. Benson AB, Venook AP, Al‐Hawary MM et al NCCN guidelines insights: colon cancer, version 2.2018. J. Natl. Compr. Canc. Netw. 2018; 16: 359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benson AB 3rd, Venook AP, Al‐Hawary MM et al Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2018; 16: 874–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Leersum NJ, Janssen‐Heijnen ML, Wouters MW et al Increasing prevalence of comorbidity in patients with colorectal cancer in the South of The Netherlands 1995‐2010. Int. J. Cancer. 2013; 132: 2157–63. [DOI] [PubMed] [Google Scholar]
- 21. Aapro MS, Kohne CH, Cohen HJ, Extermann M. Never too old? Age should not be a barrier to enrollment in cancer clinical trials. Oncologist. 2005; 10: 198–204. [DOI] [PubMed] [Google Scholar]
- 22. Jakobs TF, Hoffmann RT, Trumm C, Reiser MF, Helmberger TK. Radiofrequency ablation of colorectal liver metastases: mid‐term results in 68 patients. Anticancer Res. 2006; 26: 671–80. [PubMed] [Google Scholar]
- 23. Sorensen SM, Mortensen FV, Nielsen DT. Radiofrequency ablation of colorectal liver metastases: long‐term survival. Acta Radiol. 2007; 48: 253–8. [DOI] [PubMed] [Google Scholar]
- 24. Abitabile P, Hartl U, Lange J, Maurer CA. Radiofrequency ablation permits an effective treatment for colorectal liver metastasis. Eur. J. Surg. Oncol. 2007; 33: 67–71. [DOI] [PubMed] [Google Scholar]
- 25. Veltri A, Sacchetto P, Tosetti I, Pagano E, Fava C, Gandini G. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc. Intervent. Radiol. 2008; 31: 948–56. [DOI] [PubMed] [Google Scholar]
- 26. Weng M, Zhang Y, Zhou D et al Radiofrequency ablation versus resection for colorectal cancer liver metastases: a meta‐analysis. PLoS One. 2012; 7: e45493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Timmerman R, Paulus R, Galvin J et al Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010; 303: 1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanuki N, Takeda A, Oku Y et al Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol. 2014; 53: 399–404. [DOI] [PubMed] [Google Scholar]
- 29. Kirichenko A, Gayou O, Parda D et al Stereotactic body radiotherapy (SBRT) with or without surgery for primary and metastatic liver tumors. HPB. 2016; 18: 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laor A, Tal S, Guller V, Zbar AP, Mavor E. The Charlson Comorbidity Index (CCI) as a Mortality Predictor after Surgery in Elderly Patients. Am. Surg. 2016; 82: 22–7. [PubMed] [Google Scholar]


