Abstract
Background and Aim
Endoscopic duodenal stenting for patients with malignant gastric outlet obstruction (GOO) has been widespread; however, clinical trials evaluating the structures of duodenal stents are lacking. Thus, we aimed to investigate the clinical outcomes of a highly flexible duodenal stent for GOO patients.
Methods
A prospective study of duodenal stenting for GOO patients from five hospitals between August 2017 and August 2018 was performed. WallFlex Duodenal Soft were used in all procedures. The primary endpoint was clinical success, defined as an improvement in the GOO scoring system.
Results
The study enrolled 31 patients (12 women, 19 men) with GOO, with a median age of 70 (range 52–90) years. Primary diseases were pancreatic cancer, gastric cancer, biliary tract cancer, and others in 14, 10, 3, and 4 patients, respectively. The technical success rate was 97%, and the clinical success rate was 87%. Simultaneous biliary drainage was performed in 19% of patients. Adverse events occurred in three patients. Chemotherapy was given in 41% of clinically successful cases, and the median overall survival time after stent placement was 82 days (range, 30–341 days), and. Stent dysfunction occurred in 30% of clinically successful cases (stent ingrowth in seven and stent overgrowth in one patient). The median time to stent dysfunction was 157 days (range, 11–183 days). Six patients were treated with additional stent placement after dysfunction.
Conclusion
Placement of a highly flexible duodenal stent is an effective and safe treatment for patients with GOO (UMIN‐CTR 000028783).
Keywords: duodenal stent, endoscopy, gastric outlet obstruction
We evaluated the clinical outcome of a highly flexible duodenal stent for gastric outlet obstruction in this multicenter prospective study. Placement of WallFlex Duodenal Soft was an effective and safe procedure.
Introduction
Malignant gastric outlet obstruction (GOO) is a symptom caused by various tumors around the stomach or duodenum, significantly reducing the quality of life and nutritional status of patients due to vomiting and anorexia. Traditionally, GOO has been treated by surgical gastrojejunostomy1, 2; however, reports showing the effectiveness and safety of endoscopic duodenal stenting have been increasing, and it has been widely used as an alternative therapy for surgical treatment.3, 4, 5, 6, 7, 8 Endoscopic duodenal stenting for GOO has advantages: it is minimally invasive in patients with malignant tumors, allows short periods of time between meals, and can be repeatedly performed when stent dysfunction occurs.9, 10, 11
Although there are several types of stents used for endoscopic duodenal stenting, it is not clear which device is the most effective. A stent for GOO needs a strong radial force to expand the stricture of the gastrointestinal tract through which food passes, while also requiring flexibility to be placed at the duodenal bending site. It has been reported that less flexible stents can cause kinking at both ends or cause stent migration, which can lead to clinical failure or stent dysfunction.12, 13, 14
WallFlex Duodenal Soft (WFDS) (Boston Scientific Corporation, MA, USA) is a newly developed metal stent for GOO that has a reduced wire diameter to increase its flexibility. On the contrary, the cross‐wire structure maintains a sufficient radial force. These characteristics are expected to improve the passage of food while alleviating the direct stress on the duodenal wall, but no clinical trials on this topic have been reported. The purpose of this multicenter prospective study is to clarify the clinical outcome of WFDS for GOO patients.
Methods
Patients
Between August 2017 and August 2018, 31 patients with malignant GOO were enrolled in this study. Inclusion criteria were patients aged >20 years and with clinical symptoms of GOO. Exclusion criteria were patients from whom consent for duodenal stent deployment was not obtained and those with an Eastern Cooperative Oncology Group (ECOG) performance status of 4. Five hospitals in Kanagawa Prefecture (two university hospitals and three community hospitals) participated in this study. The institutional review board of each hospital approved the protocol of this study, and the study was registered by the University Hospital Medical Information Network Clinical Trials Registry (ID: 000028783). Written informed consent was obtained from all patients before study participation.
Device
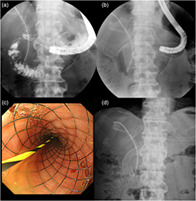
All patients were treated with a highly flexible nitinol uncovered self‐expandable metallic stent, WFDS (Fig. 1). This stent has a cross‐wire structure, and the diameter of each wire is 20% smaller than that of a conventional stent, WallFlex Duodenal stent, thereby improving flexibility by approximately 30%. In addition, it can be placed with scopes that have a working channel of 3.2 mm or more and easily inserted into narrow or winding stenosis because it is mounted in the 9‐Fr‐sized delivery system. It has variate size in a diameter of 18, 20, or 22 mm and a length of 6, 9, or 12 cm.
Figure 1.
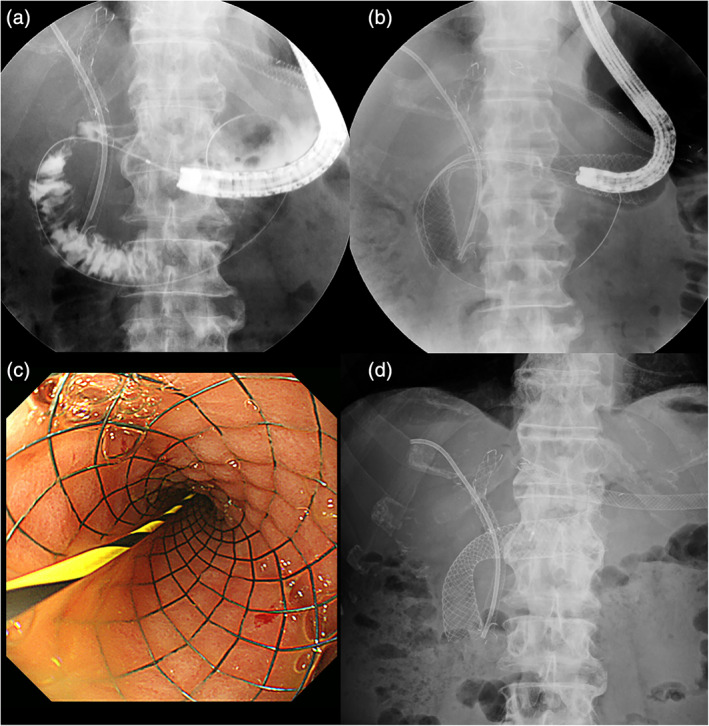
The patient was a 70‐year‐old man with gastric outlet obstruction due to external hepatic bile duct cancer. (a) Fluoroscopic image showing that duodenal stricture is located at the superior duodenal angle, and a biliary stent is previously placed. (b) A WallFlex Duodenal Soft stent, 22 mm in diameter and 12 cm length, is placed between the second part and stomach. (c) Optical image of the deployed duodenal stent. (d) On the second day after the procedure, the abdominal radiograph showed that the duodenal stent was sufficiently expanded and the best gastric outlet obstruction score improved to 2 points.
Procedure of duodenal stenting
All procedures were performed under conscious sedation using a front or lateral view video endoscope with at least 3.2‐mm working channels as follow: TJF 260V, JF 260V, GIF‐1T240, and PCF‐Q260 (Olympus Medical Systems, Tokyo, Japan). Each stenosis due to tumors was recognized by injecting a contrast medium, and a 0.025‐ or 0.035‐inch guide wire was inserted in the anal side with sufficient length. The length of the stenosis was measured, and a stent with a length exceeding the stenosis at both ends was selected. All stents were carefully placed while checking the position using fluoroscopy. If the position of either of the stents was wrong, it was restored and adjusted properly. Both ends of the stent were placed so as not to be located at the duodenal bending sites, such as the superior duodenal angle, inferior duodenal angle, and the Treitz ligament. After stent placement, its efficacy in dilating stenosis was evaluated by passage of the contrast medium. Oral intake was resumed on the next day while checking the degree of expansion of the stent by abdominal radiography.
Evaluation factor
The gastric outlet obstruction scoring system (GOOSS) was used to evaluate the severity of obstructive symptoms (GOOSS 0 = no oral intake, 1 = liquids, 2 = soft solids, 3 = low residue or full diet).15 The primary endpoint was set as the clinical success rate defined as a percentage of patients with at least 1 point improvement in GOOSS score. Secondary endpoints were technical success rate, stent‐related adverse event, survival time after stent deployment, and time to stent dysfunction. Technical success was defined as the placement of the stent in the area covering the stenosis. Stent‐related adverse events were evaluated by classifying them into early and late periods according to whether such events occurred before or after 30 days of stent placement. Stent dysfunction was diagnosed by clinical symptoms, including anorexia, nausea, and vomiting, and image evaluation. The time to stent dysfunction was defined as the time period between the day of stent placement and the occurrence of stent dysfunction. The patients were followed up at least for 3 months after stent deployment.
Statistical analysis
Continuous variables are presented as median values and ranges. Categorical variables are presented as counts and percentages. Survival time and time to stent dysfunction were determined using the Kaplan–Meier method. For statistical analysis, JMP Pro 12 (SAS Institute Inc., Cary, NC, USA) was used. All authors had access to the study data, and reviewed and approved the final manuscript.
Results
Patient's baseline characteristics
The baseline characteristics of the patients are summarized in Table 1. The median age of the patients (19 men and 12 women) was 70 (range: 52–90) years. Moreover, 21 and 10 patients were treated at university and community hospitals, respectively. The most common primary disease was pancreatic cancer with a rate of 45% (14/31), followed by gastric cancer with a rate of 32% (10/31). Other diseases included three cases of biliary tract cancer, three cases of colorectal cancer, and one case of urinary tract cancer. ECOG performance status was 0 in 19% (6/31), 1 in 58% (18/31), and 2 in 23% (7/31) of patients. Regarding comorbidities, 26% (8/31) of the patients had diabetes, but none of the patients had heart failure, cirrhosis, or renal failure requiring hemodialysis. In addition, 10% (3/31) of the patients had local bleeding and 35% (11/31) had ascites. Before stent placement, 39% (12/31) of the patients received chemotherapy and 35% (11/31) underwent biliary drainage for obstructive jaundice. Further, 39% (12/31) of the patients had nasogastric tube insertion just before duodenal stenting. The stenosis was found in the stomach, duodenum, and anastomotic area after Billroth I reconstruction in 26% (8/31), 71% (22/31), and 3% (1/31) of the patients, respectively. Baseline GOOSS scores were 0 and 1 in 61% (19/31) and 39% (12/31) of the patients, respectively, with a mean GOOSS score of 0.39 ± 0.50.
Table 1.
Baseline characteristics of patients (n = 31)
| Age, years (median, range) | 70 (52–90) |
| Sex (n, %) | |
| Male | 19 (61) |
| Female | 12 (39) |
| Tumor characteristics (n, %) | |
| Pancreatic cancer | 14 (45) |
| Gastric cancer | 10 (32) |
| Biliary tract cancer | 3 (10) |
| Others | 4 (13) |
| Performance status (n, %) | |
| 0 | 6 (19) |
| 1 | 18 (58) |
| 2 | 7 (23) |
| 3 | 0 |
| Comorbidities (n, %) | |
| Diabetes | 8 (26) |
| Heart failure | 0 |
| Liver cirrhosis | 0 |
| Renal failure (requiring hemodialysis) | 0 |
| Previous treatment (n, %) | |
| Chemotherapy | 13 (42) |
| Radiation | 0 |
| Biliary obstruction (n, %) | 11 (35) |
| Gastrointestinal bleeding (n, %) | 3 (10) |
| Ascites (n, %) | 11(35) |
| Previous nasogastric tube insertion (n, %) | 12 (39) |
| Prior gastrectomy (n, %) | 1 (3) |
| Primary site of stricture (n, %) | |
| Antrum of stomach | 8 (26) |
| First part of duodenum | 6 (19) |
| Second part of duodenum | 8 (26) |
| Third part of duodenum | 7 (23) |
| Fourth part of duodenum | 1 (3) |
| Anastomosis of Billroth I reconstruction | 1 (3) |
| GOOSS before procedure (n, %) | |
| Mean ± SD | 0.39 ± 0.50 |
| 0 (no oral intake) | 19 (61) |
| 1 (liquid diet) | 12 (39) |
| 2 (soft solid diet) | 0 |
| 3 (low residue or normal diet) | 0 |
Data are numbers; data in parentheses are percentages.
GOOSS, gastric outlet obstruction scoring system.
Procedure of duodenal stenting
The details of the duodenal stenting procedure are summarized in Table 2. The technical success rate was 97% (30/31). In only one case of technical failure, the length of the anal side was shortened because the range of stenosis was difficult to determine, and additional stent placement was performed on the next day. All procedures were performed with a single WFDS, with a diameter of 22 mm. Stents of lengths 6, 9, and 12 cm were inserted in 29% (9/31), 39% (12/31), and 32% (10/31) of patients, respectively. Stents were deployed at the duodenal bending site in 77% (24/31) of the patients, superior duodenal angle in 55% (17/31), inferior duodenal angle in 10% (3/31), and Treitz ligament in 16% (5/31). One patient had a stent placed on both the superior and the inferior duodenal angles. In 48% (15/31) of the patients, the stent was placed at oral side of papilla; in 29% (9/31), the stent was placed at anal side of the papilla; and in 23% (7/31), the stent was placed on the papilla. Biliary drainage was performed simultaneously in 19% (6/31) of the patients.
Table 2.
Details of stent deployment
| Number of SEMS (n, %) | |
| Single | 31 (100) |
| Multiple | 0 |
| Diameter of SEMS, mm (n, %) | |
| 18 | 0 |
| 20 | 0 |
| 22 | 31 (100) |
| Length of SEMS, cm (n, %) | |
| 6 | 9 (29) |
| 9 | 12 (39) |
| 12 | 10 (32) |
| Deployment on the duodenal bending site (n, %) | |
| Superior duodenal angle | 17 (55) |
| Inferior duodenal angle | 3 (10) |
| Treitz ligament | 5 (16) |
| Position relationship with papilla (n, %) | |
| Oral side of papilla | 15 (48) |
| On the papilla | 7 (23) |
| Anal side of papilla | 9 (29) |
| Simultaneous biliary drainage (n, %) | 6 (19) |
Data are numbers; data in parentheses are percentages.
SEMS, Self‐expandable metallic stent.
Clinical success
The best GOOSS score after stent placement is detailed in Table 2. GOOSS score improved in 87% (27/31) of the patients in this study, whereas four patients had no GOOSS score improvement. The cause of clinical failure was technical failure in one case, early stent migration in one case, and insufficient stent expansion in two cases. The cases with insufficient stent expansion, additional treatments were performed. In the first case, oral intake became possible after performing balloon dilation in the stent. However, in the second case, it was impossible to resume oral intake despite performing balloon dilation or additional stent placement; thus, surgical gastrojejunostomy was performed. Ascites was found in approximately one‐third of the cases before stent placement, but there were no cases in which food intake was not achieved due to cancerous peritonitis. The difference between the mean GOOSS score estimations before and after duodenal stent placement was 1.90 ± 0.94.
Stent‐related adverse event
The details of adverse events after stent placement are summarized in Table 3. Stent‐related adverse events occurred in three patients. Regarding the adverse events in the early period, stent migration occurred in a patient with peritoneal metastasis of colon cancer. Stenosis was found in the third part of the duodenum by endoscopy and fluoroscopy, and the stent was placed as usual; however, the stent migrated to the jejunum on the next day without any symptoms. The patient was carefully observed until five postprocedure days, and the stent was successfully removed using a transanal single‐balloon enteroscope. There was no sign of bleeding or perforation in this case. In the second adverse event, cholangitis occurred in a patient with pancreatic head cancer who was previously deployed biliary metallic stent. Endoscopic biliary drainage was performed via WFDS on the next day. Duodenal scope was easily inserted into second part of duodenum through the WFDS, however major papilla was invisible because of sludge and residue. Therefore, biliary cannulation was performed through the mesh according to biliary stent that was previously placed on fluoroscopic viewing. After placing additional biliary stent, jaundice improved promptly. In our study, a WFDS was placed across the papilla in 23% of patients, but cholangitis occurred in only one case, and none of the patients had pancreatitis. In the third adverse event, a patient with pancreatic head cancer had delayed gastric perforation, near the oral end of WFDS, at 130 days after stent deployment. This adverse event was probably caused by long‐term contact of the oral end of the stent. The patient recovered enough to restart oral intake by conservative treatment, but died due to the primary disease at 25 days after perforation.
Table 3.
Clinical outcomes in patients after stent deployment
| Technical success (n, %) | 30 (97) |
| Clinical success (n, %) | 27 (87) |
| GOOSS after procedure (n, %) | |
| Mean ± SD | 2.35 ± 0.91 |
| 0 (no oral intake) | 3 (10) |
| 1 (liquid diet) | 3 (10) |
| 2 (soft solid diet) | 7 (23) |
| 3 (low residue or normal diet) | 18 (58) |
| Improvement of GOOSS (mean ± SD) | 1.90 ± 0.94 |
| Procedure‐related adverse event | 3 (10) |
| Bleeding | 0 |
| Perforation | 1 (3) |
| Migration | 1 (3) |
| Cholangitis | 1 (3) |
| Pancreatitis | 0 |
| Additional chemotherapy | 11 (35) |
| Survival time, days (median, range) | 82 (30–341) |
| Stent dysfunction (total) (n, %) | 8 (30) |
| Stent in growth | 7 (26) |
| Stent overgrowth | 1 (4) |
| Stent migration | 0 |
| Stent kinking | 0 |
| Food impaction | 0 |
| Time to stent dysfunction (median, range) | 157 (11–183) |
| Additional stent deployment | 6 (75) |
Data are numbers; data in parentheses are percentages.
GOOSS, gastric outlet obstruction scoring system.
Survival time
The overall survival after duodenal stent placement is shown on the Kaplan–Meier curve (Fig. 2). In this study, 71% (22/31) of the patients died within 3 months after the procedures, and the median survival time was 81 (range, 30–341) days. The cause of death was progression of the primary disease in all patients. Chemotherapy was performed in 35% (11/31) of the patients.
Figure 2.
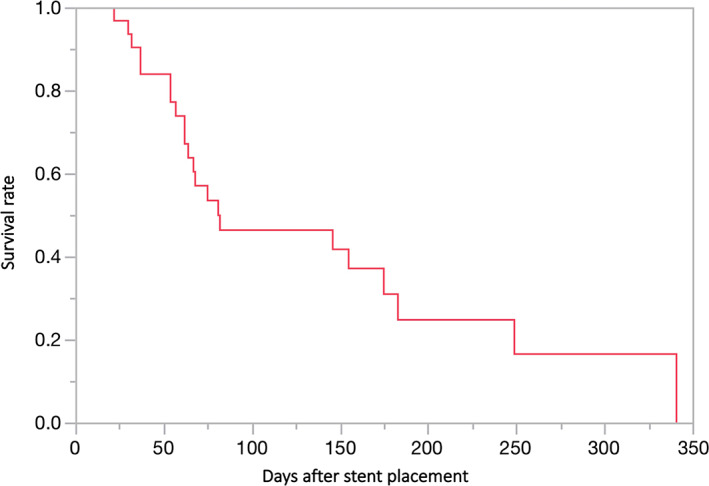
The Kaplan–Meier curve illustrates the overall survival from the duodenal stent placement.
Time to stent dysfunction
The time to stent dysfunction is shown on the Kaplan–Meier curve (Fig. 3). In this study, 30% (8/27) of patients had stent dysfunction, which was caused by tumor ingrowth in 88% (7/8) and tumor overgrowth in 12% (1/8) of patients. The median time to stent dysfunction was 157 (range, 11–183) days. After stent dysfunction, 75% (6/8) of patients were treated with additional stent placement. There was no kinking of the stent.
Figure 3.
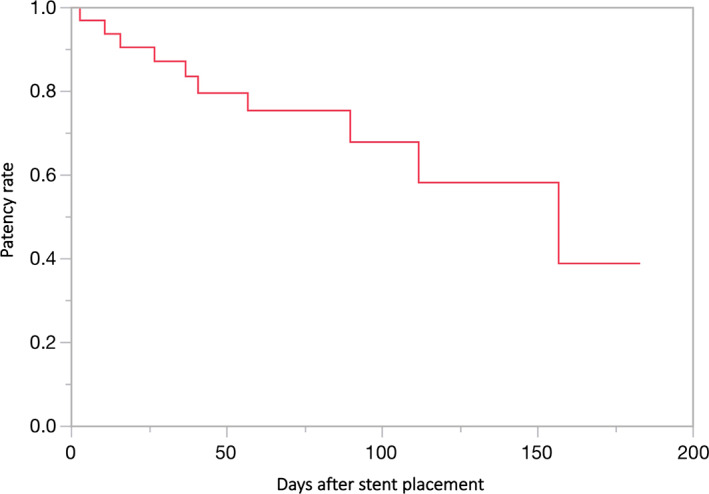
The Kaplan–Meier curve illustrates time to stent dysfunction.
Discussion
In this multicenter prospective study, deployment of WFDS was promising as an effective and feasible treatment for the patients with malignant GOO. The high technical success rate showed that the WFDS placement was an easy procedure. Furthermore, the clinical success rate was sufficiently high compared to that in previous reports (Table 4).8, 13, 16, 17, 18, 19, 20, 21 Regarding procedure‐related adverse events, two patients developed stent migration and cholangitis in the early period, and one case was complicated with delayed perforation.
Table 4.
Comparison of published prospective study
| Author | Year | n | Stent | Technical success (%) | Clinical success (%) | Adverse event (%) | Stent dysfunction (%) |
|---|---|---|---|---|---|---|---|
| van Hooft et al.16 | 2009 | 52 | WallFlex† | 98 | 84 | 6.0 | 18 |
| van Hooft et al.13 | 2011 | 52 | Niti‐S‡ | 96 | 85 | 3.8 | 21 |
| Costamagna et al.18 | 2012 | 202 | WallFlex† | 98 | 91 | 8.0 | 12 |
| van den Berg et al.20 | 2013 | 46 | Evolution§ | 89 | 72 | 8.7 | 30 |
| Tringali et al.8 | 2014 | 108 | Evolution§ | 99 | 91 | 5.0 | 13 |
| Okuwaki et al.12 | 2016 | 14 | WallFlex† | 100 | 93 | 29 | 64 |
| 17 | Niti‐S‡ | 100 | 84 | 24 | 24 | ||
| Current study | 31 | WallFlex soft¶ | 97 | 87 | 10 | 30 |
WallFlex Duodenal Stent (Boston Scientific).
Niti‐S Duodenal Stent (Taewoong Medical).
Evolution Duodenal Stent (Cook Medical).
WallFlex Duodenal Soft Stent (Boston Scientific).
The technical success rate of duodenal stent placement has been reported to exceed 95% in most studies.7, 8, 21, 22 In practice, placing the center of the stent in the stenosis is not difficult, but it is necessary not to put the end of the stent at the bending site of the duodenum to prevent stent kinking. A metallic stent braided with cross‐wires can be reinserted during the procedure, which allows adjusting the overall position of the stent to ensure its correct placement.
In previous prospective studies, high clinical success rates of duodenal stents for patients with GOO have been reported. Costamagna et al. reported that 91% of 202 patients had improved symptoms.18 In addition, Tringali et al. reported that the duodenal stent was placed in 108 patients with malignant GOO and the clinical success rate on the 14th day was 84.5% (82/97).8, 18 In our study, the clinical success rate, defined as an improvement of GOOSS score, was almost comparable to those of other prospective studies.12, 16, 20 Among the clinically successful cases, 41% (11/27) had received chemotherapy. Hence, our results show that WFDS deployment has clinical benefit not only for patients with a generally poor condition who need palliative therapy but also for patients with a good condition who are able to receive chemotherapy.
Okuwaki et al. reported the randomized control study focusing on the difference in axial force of duodenal stents.12 In that report, stent kinking occurred only in case with high axial force stent. In our study, there are no case with stent kinking during the observation period due to reducing axial force. Niti‐S pyloric/duodenal stent (Taewoong Medical, Gimpo‐si, Gyeonggi‐do, Korea) is a flexible duodenal stent consisted with hooked wire,13, 14 however the WFDS is stored into the 9 Fr small diameter delivery system and can be restored again during stent placement.
Adverse events related to the procedure occurred in three cases: two cases in the early period and one case in the late period. As early adverse events, stent migration and cholangitis occurred on the day after duodenal stent placement, which were treated with an endoscopic procedure. As a delayed adverse event, gastric perforation occurred near the oral end of the stent at 130 days after WFDS placement. The incidence of complications did not differ from that reported in previous studies.12, 16, 20
Although 35% (11/31) of cases received chemotherapy after WFDS deployment, the overall survival rate indicated that the patients in this study had a poor prognosis. On the contrary, the stent was well functioning until death in many cases; hence, WFDS deployment functioned favorably in patients with a poor prognosis. Furthermore, it was possible to place additional stents for most cases with stent dysfunction, as reported in previous studies.23, 24 WFDS is expected to be useful for additional treatments because it is less likely to cause kinking between both ends of the stent and the gastrointestinal lumen due to its high flexibility. Moreover, kinking at the middle part of the stent also did not occur, even though the stent was placed at the duodenal bending site in 77% of the patients.
There are several limitations to this study. First, the number of cases in this study is relatively low compared to that in previous studies; thus, other difficult cases and adverse events may occur as the number of cases increases. Second, this study was designed as a single group study. Randomized controlled trials with other types of stents are needed to ascertain which type of stent is the most effective and safe. Third, if the observation period had been prolonged, the incidence of stent dysfunction might have increased.
In our opinion, WFDS is indicated in patients with GOO due to unresectable malignant diseases, however it is contraindicated with poor general condition who cannot be safely treated with endoscope under sedation, as like respiratory or circulatory failure and severe bleeding tendency. Regarding the disadvantage of WFDS, the radial force has possibly decreased due to the reduced diameter of the wire.
In conclusion, the results of this multicenter prospective study showed that WFDS was sufficiently effective and safe for GOO patients. A high technical success rate was achieved owing to the ease of placement of this stent. Although many cases with a poor prognosis were included in this study, the clinical success rate and incidence of adverse events were not inferior to those in previous reports. Further, large‐scale trials are considered necessary to verify the usefulness of WFDS in cases of long‐term survival.
Declaration of conflict of interest: All authors declare that they have no conflict of interest.
References
- 1. Fujitani K, Ando M, Sakamaki K et al Multicentre observational study of quality of life after surgical palliation of malignant gastric outlet obstruction for gastric cancer. BJS Open. 2017; 1: 165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Minata MK, Bernardo WM, Rocha RS et al Stents and surgical interventions in the palliation of gastric outlet obstruction: a systematic review. Endosc. Int. Open. 2016; 4: E1158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soetikno RM, Lichtenstein DR, Vandervoort J et al Palliation of malignant gastric outlet obstruction using an endoscopically placed Wallstent. Gastrointest. Endosc. 1998; 47: 267–70. [DOI] [PubMed] [Google Scholar]
- 4. Dormann A, Meisner S, Verin N, Wenk LA. Self‐expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004; 36: 543–50. [DOI] [PubMed] [Google Scholar]
- 5. Holt AP, Patel M, Ahmed MM. Palliation of patients with malignant gastroduodenal obstruction with self‐expanding metallic stents: the treatment of choice? Gastrointest. Endosc. 2004; 60: 1010–17. [DOI] [PubMed] [Google Scholar]
- 6. van Hooft J, Mutignani M, Repici A, Messmann H, Neuhaus H, Fockens P. First data on the palliative treatment of patients with malignant gastric outlet obstruction using the WallFlex enteral stent: a retrospective multicenter study. Endoscopy. 2007; 39: 434–9. [DOI] [PubMed] [Google Scholar]
- 7. Sasaki T, Isayama H, Maetani I et al Japanese multicenter estimation of WallFlex duodenal stent for unresectable malignant gastric outlet obstruction. Dig. Endosc. 2013; 25: 1–6. [DOI] [PubMed] [Google Scholar]
- 8. Tringali A, Didden P, Repici A et al Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest. Endosc. 2014; 79: 66–75. [DOI] [PubMed] [Google Scholar]
- 9. Mehta S, Hindmarsh A, Cheong E et al Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg. Endosc. 2006; 20: 239–42. [DOI] [PubMed] [Google Scholar]
- 10. Yukimoto T, Morisaki T, Komukai S et al The palliative effect of endoscopic uncovered self‐expandable metallic stent placement versus gastrojejunostomy on malignant gastric outlet obstruction: a pilot study with a retrospective chart review in Saga, Japan. Intern. Med. 2018; 57: 1517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshida Y, Fukutomi A, Tanaka M et al Gastrojejunostomy versus duodenal stent placement for gastric outlet obstruction in patients with unresectable pancreatic cancer. Pancreatology. 2017; 17: 983–9. [DOI] [PubMed] [Google Scholar]
- 12. Okuwaki K, Kida M, Yamauchi H et al Randomized controlled exploratory study comparing the usefulness of two types of metallic stents with different axial forces for the management of duodenal obstruction caused by pancreatobiliary cancer. J. Hepatobiliary Pancreat. Sci. 2016; 23: 289–97. [DOI] [PubMed] [Google Scholar]
- 13. van Hooft JE, van Montfoort ML, Jeurnink SM et al Safety and efficacy of a new non‐foreshortening nitinol stent in malignant gastric outlet obstruction (DUONITI study): a prospective, multicenter study. Endoscopy. 2011; 43: 671–5. [DOI] [PubMed] [Google Scholar]
- 14. Kato H, Kawamoto H, Matsumoto K et al Outcome of self‐expandable metallic stent deployment in patients with malignant gastroduodenal outlet obstruction and Niti‐S and WallFlex comparison: a multicenter retrospective clinical study. J. Dig. Dis. 2016; 17: 518–25. [DOI] [PubMed] [Google Scholar]
- 15. Adler DG, Baron TH. Endoscopic palliation of malignant gastric outlet obstruction using self‐expanding metal stents: experience in 36 patients. Am. J. Gastroenterol. 2002; 97: 72–8. [DOI] [PubMed] [Google Scholar]
- 16. van Hooft JE, Uitdehaag MJ, Bruno MJ et al Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest. Endosc. 2009; 69: 1059–66. [DOI] [PubMed] [Google Scholar]
- 17. Kim YW, Choi CW, Kang DH et al A double‐layered (comvi) self‐expandable metal stent for malignant gastroduodenal obstruction: a prospective multicenter study. Dig. Dis. Sci. 2011; 56: 2030–6. [DOI] [PubMed] [Google Scholar]
- 18. Costamagna G, Tringali A, Spicak J et al Treatment of malignant gastroduodenal obstruction with a nitinol self‐expanding metal stent: an international prospective multicentre registry. Dig. Liver Dis. 2012; 44: 37–43. [DOI] [PubMed] [Google Scholar]
- 19. Moura EG, Ferreira FC, Cheng S, Moura DT, Sakai P, Zilberstain B. Duodenal stenting for malignant gastric outlet obstruction: prospective study. World J. Gastroenterol. 2012; 18: 938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van den Berg MW, Haijtink S, Fockens P et al First data on the Evolution duodenal stent for palliation of malignant gastric outlet obstruction (DUOLUTION study): a prospective multicenter study. Endoscopy. 2013; 45: 174–81. [DOI] [PubMed] [Google Scholar]
- 21. Sasaki R, Sakai Y, Tsuyuguchi T et al Endoscopic management of unresectable malignant gastroduodenal obstruction with a nitinol uncovered metal stent: a prospective Japanese multicenter study. World J. Gastroenterol. 2016; 22: 3837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanno Y, Ito K, Fujita N et al Efficacy and safety of a WallFlex enteral stent for malignant gastric obstruction. Dig. Endosc. 2013; 25: 386–91. [DOI] [PubMed] [Google Scholar]
- 23. Sasaki T, Isayama H, Nakai Y et al Clinical outcomes of secondary gastroduodenal self‐expandable metallic stent placement by stent‐in‐stent technique for malignant gastric outlet obstruction. Dig. Endosc. 2015; 27: 37–43. [DOI] [PubMed] [Google Scholar]
- 24. Sato T, Hara K, Mizuno N et al Gastroduodenal stenting with Niti‐S stent: long‐term benefits and additional stent intervention. Dig. Endosc. 2015; 27: 121–9. [DOI] [PubMed] [Google Scholar]


