Abstract
Background
Controversies existed surrounding the use of hematocrit to guide early fluid therapy in acute pancreatitis (AP). The association between hematocrit, early fluid therapy, and clinical outcomes in ward AP patients needs to be investigated.
Methods
Data from prospectively maintained AP database and retrospectively collected details of fluid therapy were analyzed. Patients were stratified into three groups: Group 1, hematocrit < 44% both at admission and at 24 h thereafter; Group 2: regardless of admission level, hematocrit increased and >44% at 24 h; Group 3: hematocrit >44% on admission and decreased thereafter during first 24 h. “Early” means first 24 h after admission. Baseline characteristics, early fluid rates, and clinical outcomes of the three groups were compared.
Results
Among the 628 patients, Group 3 had a higher hematocrit level, greater baseline predicted severity, faster fluid rate, and more fluid volume in the first 24 h compared with Group 1 or 2. Group 3 had an increased risk for persistent organ failure (POF; odds ratio 2, 95% confidence interval [1.1–3.8], P = 0.03) compared with Group 1 after adjusting for difference in baseline clinical severity scores, there was no difference between Group 2 and Group 3 or Group 1. Multivariate regression analyses revealed that hemoconcentration and early faster fluid rate were risk factors for POF and mortality (both P < 0.05).
Conclusions
Hemoconcentration is associated with faster fluid rate and POF in ward AP patients. Randomized trials comparing standardized early fast and slow fluid management is warranted.
Keywords: acute necrotic collection, acute pancreatitis, fluid therapy, hemoconcentration, mortality, persistent organ failure
In the present study, we investigated the relationship between hemoconcentration, early fluid therapy, and clinical outcomes in ward acute pancreatitis (AP) patients. We found that hemoconcentration is associated with faster fluid rate and POF in ward AP patients. Randomized trials comparing standardized early fast and slow fluid therapy is warranted.
Introduction
Acute pancreatitis (AP) is one of the most common digestive diseases affecting 34 cases per 100 000 person/year.1, 2 Its severe form, defined as developing persistent organ failure (POF) or multiple organ failure (MOF), carries a mortality of 36–50%.3 Currently, there is no effective pharmacological therapy for AP4 and Practice Guidelines support fluid therapy using crystalloids as the mainstay treatment for its early management.5, 6
Recent studies related to early fluid therapy in AP focused on the population who admitted primarily in Emergency Department (ED) without any fluid therapy before and did not progress to organ failure on admission,7, 8, 9 or those who directly admitted to Intensive Care Unit (ICU). However, the population who have completed emergency care, but do not merit the criteria of ICU monitoring actually represent the majority of hospitalized patients. As far as we know, the fluid characterization of these patients (i.e. patients who admitted in ward after initial fluid management) has been rarely studied.
Hypovolemia caused by AP is the origin of the increase in hematocrit. Early studies have linked increased admission serum hematocrit level and its subsequent rise with acute necrotic collection (ANC) and organ failure.10, 11, 12 In a recent prospective multicenter study, admission hematocrit more than 44% has been shown to outperform other biomarkers in predicting POF and ANC.13 Further, hematocrit is one of the mostly widely used, routinely obtained and simple laboratory biomarkers to guide early fluid resuscitation for AP patients but there is still controversy.6, 14
In the study, we investigated the feature of early fluid management of AP patients from the largest AP tertiary center in China.15, 16 The association between hemoconcentration, early fluid therapy, and outcomes in general ward AP patients were delineated (Fig. 1a). We hypothesized that hemoconcentration would be associated with early faster fluid rate and increased risk of POF in ward AP patients, with the aim to promote randomized trials comparing standardized early fast and slow fluid therapy strategies.
Figure 1.
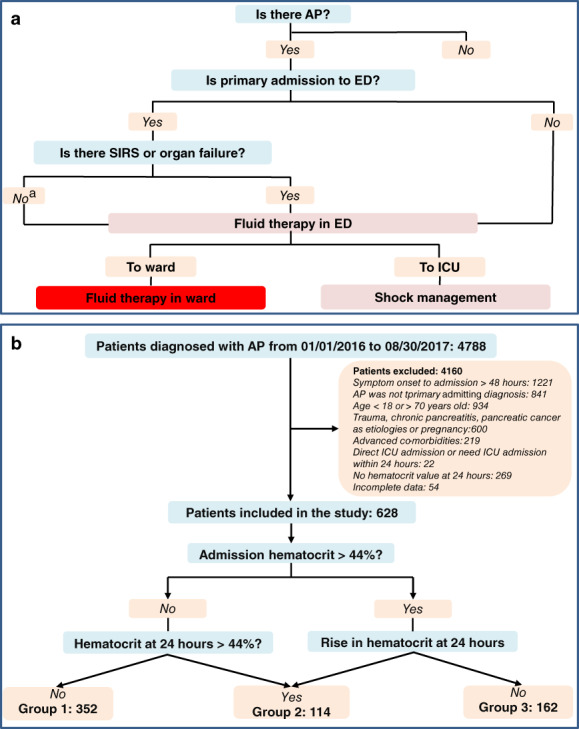
Fluid management and patient inclusion flow chart. (a) Fluid management course of acute pancreatitis in West China hospital. (b) Patients inclusion flow chart. AP, acute pancreatitis; ED, emergency department; ICU, intensive care unit; SIRS, systemic inflammatory response syndrome.
Materials and methods
Study design, ethics, and patients
This was a single‐center study following the STROBE guidelines for observational studies in West China Hospital of Sichuan University. Our hospital is a 4900‐bed national center (180 ICU beds) for the diagnosis and treatment of complex and critical diseases in Western China. The study protocol was approved by the Institutional Review Board (No. 247) of our hospital, informed consent was waived due anonymized use of data. All AP patients were consecutively admitted to the Department of Integrated Traditional Chinese and Western Medicine (Sichuan Provincial Pancreatitis Centre) of our institution between first January 2016 and 31th August 2017 and relevant data were maintained in our prospective AP database. Data of fluid management details were retrospectively collected.
Inclusion and exclusion criteria
AP was diagnosed as per the revised Atlanta classification3 and patients were included if they had less than 48 h of symptom, regardless of whether they were from a primary or referral status. Exclusion criteria were in Supplementary Materials and Methods.
Fluid management
Patients were first assessed in the ED to confirm the diagnosis of AP, regardless of whether they were primarily admitted or referred. They were routinely given normal saline fluid therapy. When transferred to designated general wards, patients were continued on intravenous crystalloids (normal saline or Ringer's lactate solution) at 1–5 mL/kg/h for the first 24 h according to severity of patients and hydration status. Patients routinely receive maintenance fluid rates unless shock was present (bolus fluid [10–20 mL/kg/h was given within 30–45 min]) when shock happened.17
Indications for admission to High Dependency Unit (HDU) included the need for noninvasive ventilation. Indications for admission to ICU included invasive ventilation or renal replacement therapy.
Definitions and patient groups
“Early” refers to the first 24 h after general ward admission and starting fluid therapy. Hematocrit was measured upon ward admission and around the next 24 h. Hemoconcentration was defined as a serum hematocrit >44%.10, 12, 13 The patients were stratified into three groups based on their hematocrit profiles within the first 24 h since ward admission: Group 1: hematocrit <44% both on admission and at 24 h thereafter; Group 2: regardless of inclusion level, hematocrit increased and > 44% at 24 h; Group 3: hematocrit >44% on admission and decreased thereafter during first 24 h. The primary clinical outcome was development of POF.18 Other outcomes included MOF, HDU/ICU admission, respiratory support, local complications, and overall mortality. Definitions of other variables are listed in Supplementary Materials and Methods.
Statistical analysis
Categorical data are expressed as number and percentage and compared by χ2 test (or Fisher's exact test) or proportional trend test (ordered multiple groups). Continuous data are presented as median and interquartile range (IQR) and compared using Mann–Whitney U test (two groups) or Kruskal–Wallis H test (three groups) because of the skewed distribution. Baseline variables with or without clinical severity score on admission are compared between designated groups using univariate analysis, these covariates with P value < 0.2 are further fitted into multivariate logistic regression (MLR) analyses for categorical clinical outcomes and expressed as odds ratio (OR) with 95% confidence interval (CI). A two‐sided P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 21.0 (IBM, Armonk, New York, USA).
Results
Baseline characteristics of overall included patients
Patient selection flow chart is shown in Figure 1b. Six hundred and twenty eight patients were included: median age 45 years and 68% males. Hypertriglyceridemia (40.0%) was the leading etiology, followed by biliary (23.2%) and heavy alcohol use (7.8%). The baseline characteristics and clinical outcomes stratified by Revised Atlanta Classification3 are summarized (Table S1) and there were 223 (35.5%), 274 (43.6%), and 131 (20.9%) patients graded as mild, moderately severe, and severe category, respectively.
The clinical outcomes are demonstrated (Table S1). The median symptom onset time was 24 h. The proportion of patients that developed persistent OF on day 1, 2, 3 and ≥4 was 64.2%, 29.8%, 2%, and 5% patients respectively (Fig. S1). There were 124 (19.7%) respiratory, 14 (2.2%) circulatory and 22 (3.5%) renal persistent OF,23 (3.7%) patients developed MOF. The number of patients that required HDU/ICU admission was 133 (21.2%). Local complications were diagnosed in 403 patients, 265 (42.2%) with acute peripancreatic fluid collection, and 138 (21.9%) with ANC. Necrosectomy was done in 32 (5.1%) patients. Twenty‐four patients died, all of them were from severe AP group (24/131, 18.3%), leading to an overall mortality rate of 3.8%.
Stratification by hematocrit
Baseline parameters
Patients in Group 3 (n = 162) or Group 2 (n = 114) were significantly younger (median 43 or 44 versus 46 years, both P < 0.05), had more males (82.1% versus 92.1% versus 52.8%, all P < 0.05), higher body mass index (26.0 or 26.1 versus 24.4 kg/m2, both P < 0.001) compared with those in Group 1 (n = 352). Group 3 had severe admission clinical severity scores compared with those in Group 2 or Group 1 (systemic inflammatory response syndrome, Glasgow, Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment scores, both P < 0.001; Table 1).
Table 1.
Baseline characteristics in patients stratified by hematocrit status during first 24 h of general ward admission
| Parameters | Group 1 (n = 352) | Group 2 (n = 114) | Group 3 (n = 162) | P value* |
|---|---|---|---|---|
| Age, years† | 46 (40, 53) | 44 (35, 51) | 43 (36, 49) | 0.020‡ |
| Gender, male, % | 186 (52.8) | 104 (91.2) | 133 (82.1) | 0.032§ |
| Body mass index, kg/m2, † | 24.4 (22.3, 27.0) | 26.1 (24.0, 28.6) | 26.0 (23.8, 28.0) | <0.001‡ |
| Charlson comorbidity index† | 1 (0, 2) | 2 (0, 2) | 1 (0, 2) | 0.108 |
| Etiology | ||||
| Biliary | 90 (25.6) | 25 (21.9) | 31 (19.1) | <0.001¶ |
| Hypertriglyceridaemia | 131 (37.2) | 58 (50.9) | 62 (38.3) | 0.038†† |
| Heavy alcohol use | 26 (7.4) | 7 (6.1) | 16 (9.9) | <0.001‡‡ |
| Others | 105 (29.8) | 24 (21.1) | 53 (32.7) | <0.001‡‡ |
| Time of ED to ward admission, hours† | 6.5 (5.5, 8.0) | 7.0 (5.5, 8.5) | 6.0 (5.0, 9.0) | 0.326 |
| SIRS† | 2 (1, 2) | 1 (1, 3) | 2 (1, 3) | <0.001§§ |
| Glasgow† | 1 (0, 2) | 1 (0, 2) | 2 (1, 3) | <0.001§§ |
| APACHE II† | 4 (2, 6) | 4 (2, 6) | 6 (3, 8) | <0.001§§ |
| SOFA† | 0 (0, 1) | 0 (0, 1) | 0 (0, 2) | 0.001§§ |
Indicates χ 2 (or Fisher's exact test) for qualitive data and Kruskal–Wallis H test for quantitative data.
Values are median (IQR).
P < 0.05, Group 1 versus Group 2 or Group 3.
P < 0.05, between any two groups.
P < 0.05, Group1 versus Group 2.
P < 0.05, Group 2 versus Group 1 or Group 3.
P < 0.05, Group 2 versus Group 3.
P < 0.05, Group 3 versus Group 1 or Group 2.
Group 1: hematocrit <44% on admission and < 44% at 24 h; Group 2: hematocrit increased and > 44% at 24 h; Group 3: hematocrit >44% on admission and decreased thereafter during first 24 h.
APACHE II, acute physiology and chronic health evaluation II; ED, emergency department; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment.
Clinical outcomes
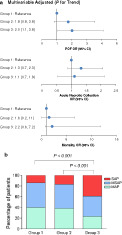
After adjusting for both baseline variables and admission clinical severity scores, Group 3 showed an increased risk of developing POF (OR 2, 95% CI [1.1–3.8], P = 0.03), while the incidence of mortality and ANC were similar when compared with Group 1 (both P > 0.1); there were no significant differences in clinical outcomes between Group 2 and Group 3 or 1 (all P > 0.15; Fig. 2a). As a result, Group 3 had more actual severe cases than Group 2 or 1 (both P < 0.001), while there was no significant difference between Group 2 and Group 1 (Fig. 2b).
Figure 2.
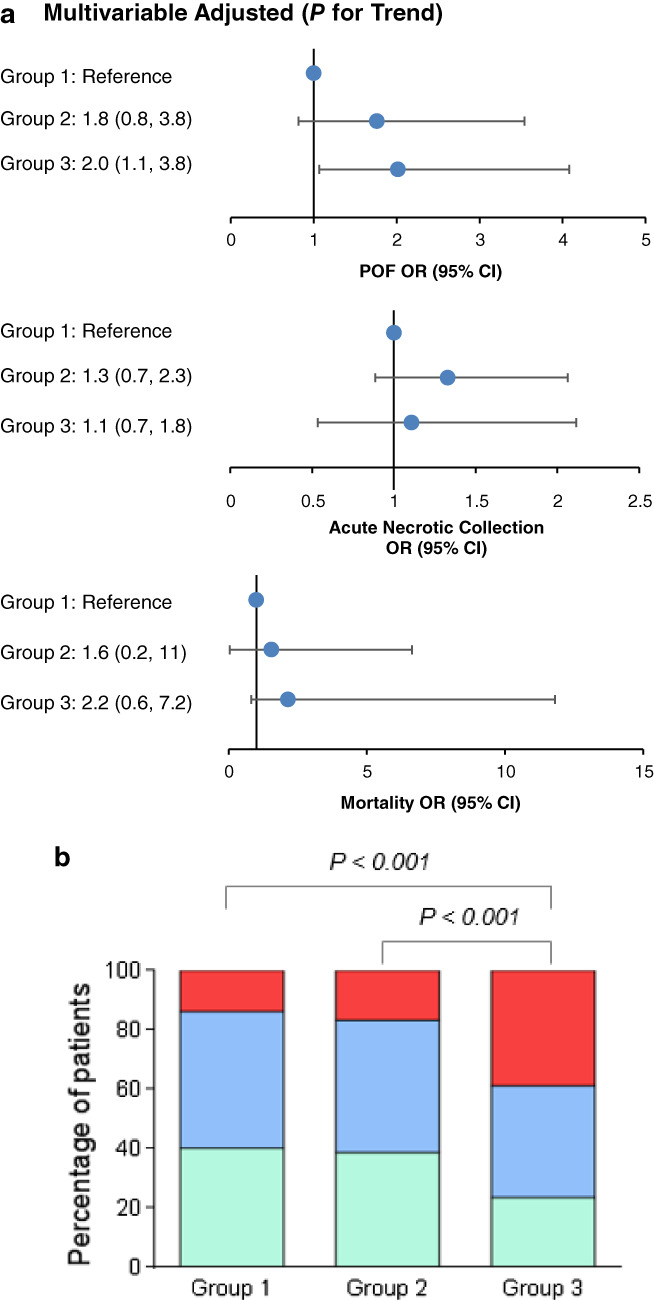
Features of patients stratified by hematocrit status during the first 24 h of admission to general awards. (a) Trend analysis for clinical outcomes after adjusting for both baseline variables and admission clinical severity scores. (b) Severity classification. Group 1: Hematocrit < 44% both on admission and at 24 h; group 2: Regardless of admission level, hematocrit increased and > 44% at 24 h; group 3: Hematocrit > 44% on admission and decreased thereafter during first 24 h. CI, confidence interval;  MAP, mild acute pancreatitis;
MAP, mild acute pancreatitis;  MSAP, moderately severe acute pancreatitis; OR, odds ratio; POF, persistent organ failure;
MSAP, moderately severe acute pancreatitis; OR, odds ratio; POF, persistent organ failure;  SAP, severe acute pancreatitis.
SAP, severe acute pancreatitis.
MLR and sensitivity analyses
In the MLR analyses, 7 (age, Charlson comorbidity index, time to admission, body mass index, referral, hemoconcentration, fluid rates of the first 24 h) of 9 independent covariables included in the model were significantly associated with POF, 3 (body mass index, referral, fluid rates of the first 24 h) of 9 independent covariables associated with ANC, 5 (age, Charlson comorbidity index, body mass index, hemoconcentration, fluid rates of the first 24 h) of 9 independent covariables associated with mortality (Table 2). Hemoconcentration on admission of general wards was significantly associated with POF (OR 1.88, 95% CI [1.12–3.15], P = 0.016) and mortality (4.02 [1.46–11.1], P = 0.007), but not ANC. The fluid rates of the first 24 h was associated with POF (5.19 [3.55–7.59], P < 0.001), ANC (1.73 [1.34–2.23], P < 0.001) and mortality (2.51 [1.68–3.73], P < 0.001).
Table 2.
Multivariate analyses for persistent organ failure, acute necrotic collection, and mortality in all patients
| Persistent organ failure | Acute necrotic collection | Mortality | ||||
|---|---|---|---|---|---|---|
| Confounders | Estimate | P value | Estimate | P value | Estimate | P value |
| Age, year† | 1.03 (1.01, 1.06) | 0.008 | 1.00 (0.98, 1.02) | 0.745 | 1.05 (1.00, 1.10) | 0.042 |
| Gender | 0.84 (0.51, 1.41) | 0.514 | 1.07 (0.68, 1.70) | 0.771 | 0.49 (0.18, 1.33) | 0.161 |
| Charlson comorbidity index† | 0.85 (0.70, 1.02) | 0.084 | 0.91 (0.77, 1.07) | 0.253 | 0.58 (0.37, 0.91) | 0.016 |
| Time to admission, hour† | 1.03 (1.01, 1.05) | 0.008 | 1.00 (0.98, 1.02) | 0.962 | 1.03 (0.99, 1.07) | 0.179 |
| Body mass index, kg/m2, † | 1.09 (1.04, 1.17) | 0.002 | 1.04 (0.98, 1.09) | 0.198 | 1.19 (1.06, 1.33) | 0.002 |
| Etiology | 0.85 (0.71, 1.03) | 0.096 | 0.88 (0.75, 1.04) | 0.138 | 0.99 (0.69, 1.43) | 0.962 |
| Referral | 3.64 (2.15, 6.16) | <0.001 | 2.65 (1.70, 4.12) | <0.001 | 2.81 (0.88, 9.00) | 0.082 |
| Hemoconcentration | 2.49 (1.57, 3.96) | <0.001 | 1.41 (0.93, 2.14) | 0.101 | 4.36 (1.68, 11.4) | 0.003 |
| Fluid rate of first the 24 h | 3.12 (1.99, 4.90) | <0.001 | 1.35 (0.90, 2.04) | 0.152 | 3.27 (1.32, 8.11) | 0.010 |
Continuous variable.
Age, gender (male versus female), Charlson comorbidity index, time to admission, body mass index, etiology (hypertriglyceridemia, biliary, alcoholic, others), referral (yes versus no), hemoconcentration (yes, hematocrit >44% versus no, ≤44%), fluid rate of the first 24 h were included in multivariate logistic regression analysis.
When the analysis was restricted to patients (n = 271) admitted ≤24 h after symptoms onset, the results were unchanged from the overall patients except that the fluid rates of the first 24 h became just a trend toward increased ANC (1.48 [1.00–2.18], P = 0.05) (Table S2). Mortality was removed from this subgroup analysis because there were only seven deaths.
When the analysis was restricted to patients (n = 292) who admitted primarily (i.e. tertiary admissions removed), the results were unchanged from the overall patients (Table S3). Mortality was removed from this subgroup analysis because there were only four deaths.
Fluid therapy rate and volume
The hematocrit level at admission was the highest in Group 3 followed by Group 2 and Group 1 (median 48% versus 45% versus 40%, all P < 0.001), while at 24 h the hematocrit in the Group 2 was the highest and followed by Group 3 and Group 1 (48% versus 43% versus 38%, all P < 0.001; Fig. 3a). The fluid therapy rate in the first 24 h was significantly faster in Group 3 compared with Group 2 (median 2.3 versus 2.1 mL/kg/h, P < 0.05) and Group 1 (median 2.3 versus 2.0 mL/kg/h, P < 0.05; Fig. 3b). Patients in Group 3 also received a greater fluid volume over the first 24 h compared with Group 2 or Group 1 (median 3875 versus 3450 versus 3200 mL, all P < 0.001; Fig. 3c).
Figure 3.
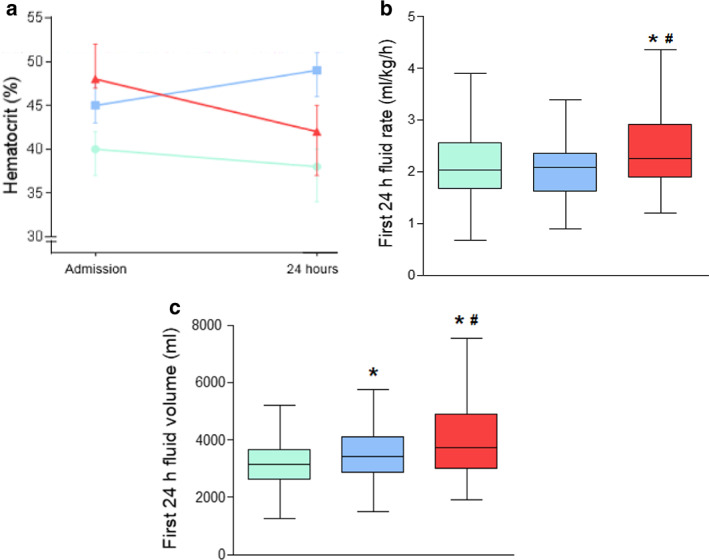
Fluid rates and volume of patients stratified by hematocrit status during the first 24 h of admission to ward. (a) Hematocrit change on admission and 24 h. (b) Comparison of fluid rates during the first 24 h between 3 groups. (c) Comparison of fluid volume during the first 24 h between three groups.  Group 1: Hematocrit < 44% both on admission and at 24 h;
Group 1: Hematocrit < 44% both on admission and at 24 h;  group 2: Regardless of admission level, hematocrit increased and > 44% at 24 h;
group 2: Regardless of admission level, hematocrit increased and > 44% at 24 h;  group 3: Hematocrit > 44% on admission and decreased thereafter during first 24 h. *P < 0.05 versus group 1; #
P < 0.05 versus group 2.
group 3: Hematocrit > 44% on admission and decreased thereafter during first 24 h. *P < 0.05 versus group 1; #
P < 0.05 versus group 2.
Discussion
This study investigated the role of hemoconcentration in early fluid therapy and clinical outcomes of AP patients in ward. The considerable number of patients, especially the largest number of severe AP patients of fluid therapy studies in this field so far, are the strong points of our work. The key finding was that hemoconcentrated AP patients were more ill on admission, and clinicians tended to give them faster fluid rates and more fluid volume in the first 24 h. Then these patients had increased risk of POF even when after adjusting both baseline parameters and admission clinical severity scores (predicted severity) than other patients. MLR analyses confirmed that hemoconcentration on admission and faster fluid rates in the first 24 h after admission were significantly associated with POF and mortality, while only the latter one was significantly associated with ANC. Given the fact that early fluid rate was normally directly caused by severity of AP and hematocrit level, our findings highlight the necessity of conducting randomized trials to compare standardized early fast and slow fluid therapy approaches for AP.
The rationale for fast fluid therapy in hemoconcentrated patients is straightforward. Because hemoconcentration increases viscosity and flow resistance,19 it is thought that hemodilution by fast fluid therapy will improve flow and tissue oxygenation and therefore clinical outcomes of AP.10, 12 However, the oxygen carrying capacity of the blood is reduced during hemodilution, although there are compensatory mechanisms to ensure normal oxygen delivery, including an increase in cardiac output and tissue oxygen extraction.19 There is a limit to these compensatory mechanisms in extreme hemodilution.19 Furthermore, experimental studies suggest that rapid hemodilution can worsen outcomes by decreasing microcirculatory perfusion and increasing expression of inflammation and endothelial activation.20 Therefore, both early hemoconcentration and rapid hemodilution are likely to contribute to more severe disease.
There have been no previous studies that have explored the association of hemoconcentration of general ward admission and early fluid therapy with clinical outcomes in AP patients. When stratified according to the changes of hematocrit during the first 24 h, patients in Group 3 had the highest hematocrit level and severe admission clinical severity scores than Group 2 and Group 1, so faster fluid rate and more fluid volume in the first 24 h were given. Then, there was a significantly increased risk of POF in Group 3 compared with those in Group 1 while there was no significant difference between Group 2 and Group 3 and Group 1. The phenomenon still existed when after adjusting both baseline parameters and admission clinical severity scores. Due to retrospective nature of data for fluid management, it cannot be confirmed whether the worse clinical outcomes of Group 3 was natural process of disease itself, or early fast fluid therapy might exacerbate the process.
In our study, 64% of patients with severe AP had POF onset during the first 24 h of admission to general wards (about 6.5 h since ED admission). This finding was consistent with a recently published study that investigated the timing of POF and found that its onset occurred early during the disease course in most patients.16 Respiratory failure is the most common POF in patients with severe AP.16 While rapid and high‐volume fluid therapy will increase the risk of pulmonary edema and exacerbate the respiratory failure.8, 21, 22 Thus, when AP has already combined with respiratory dysfunction, it has to consider a restricted fluid therapy regimen,23, 24 and the statement “not all patients require or benefit from “aggressive” fluid resuscitation, (and) calls into question previously held belief that “more is better” may be the ideology.25
MLR analyses confirmed hemoconcentration and early faster fluid rate were both significantly associated with POF, and the latter was significantly associated with ANC. The tendency of the cognition that early fast fluid therapy may be associated with worse clinical outcomes is consistent with the prospective study by Mao et al.26, 27 in which rapid expansion and hemodilution was accompanied by increased incidence of sepsis and death in patients with predicted severe AP who were directly admitted to ICU. However, the cognition is contradictory with other recent prospective studies in which primarily admitted patients who were without systemic inflammatory response syndrome and organ failure and were directly managed in the ED.7, 9, 28 These controversies highlight that the effect of early fast fluid therapy would be different in patients with different severity and in different disease course. Our study raised an important question for fluid therapy in AP that is how the timing of “early” is defined? Our recent work29, 30 has shown that patients (admitted ≤36 h after symptom onset) may benefit from early fast fluid therapy within the first 2 h of recruitment, but 6–8 h after recruitment can increase the risk of POF and mortality, indicating a narrow window for “very early” aggressive fluid therapy, and early rapid fluid therapy is associated with increased rate of noninvasive positive‐pressure ventilation in hemoconcentrated patients with severe AP. It is advisable for future studies to use timing of pain onset to enrolment to define “very early” and “early” for fluid therapy in AP, but the clear cut‐off for these two time frames needs to be developed.
Our study has several limitations. Practice variability is evaluated in a single center; the results may not therefore be representative. We lacked data on pre‐ward fluid rate and volume. One of our findings are indicative of a contribution of early faster fluid rate, after adjusting for both baseline variables and predicted severity, but owing to the retrospective observational design of the study, it is difficult to gain any conclusions about whether early fast fluid therapy is harmful or beneficial in these AP patients. Therefore, the original intention of the study is to highlight the necessity of conducting randomized trials to compare standardized early fast and slow fluid therapy strategies for AP. Finally, our cohort contained high proportion of hyperlipidemia etiology (40%), much higher than Western population but consistent with our recent publications15, 16 and national trend.31
Conclusions
In conclusion, this study found that in AP patients who are admitted in general wards after initial fluid therapy, hemoconcentration is associated with faster fluid rate and an increased risk of POF. However, whether early fluid therapy is harmful or beneficial to these patients needs to be confirmed in randomized trials that compare standardized early fast and slow fluid therapy.
Supporting information
Appendix S1 Supporting Information
Acknowledgments
These authors thank all the staff from pancreas multidisciplinary team at West China Hospital of Sichuan University. Dr. Xia and Dr. Windsor's institutions received joint funding “NZ‐China Strategic Research Alliance 2016 Award” from Ministry of Science and Technology (No. 2016YFE0101800); Xia Q, Jin T, Huang W, Yang X, Deng L and Guo J and Health Research Council (Philips A and Windsor JA), respectively. Dr. Sutton received funding from National Institute for Health Research Senior Investigator Award.
Tao Jin and Lan Li contributed equally to this work.
Declaration of conflict of interest: All authors declare that they have no conflicts of interest.
Author contribution: TJ and LL are co‐joint first authors. Concept and design: WH, QX, JAW and TJ, acquisition of data: LL, TJ, SW, RZ, NS, ZL, KJ, JG, TL, LD and XY; statistical analysis: LL, PZ; drafting: LL, TJ and WH; revision: AP, RS, WH, QX and JAW; obtaining funding: WH, QX and JAW.
Contributor Information
Wei Huang, Email: dr_wei_huang@163.com.
Qing Xia, Email: xiaqing@medmail.com.cn.
References
- 1. Xiao AY, Tan MLY, Wu LM et al Global incidence and mortality of pancreatic diseases: a systematic review, meta‐analysis, and meta‐regression of population‐based cohort studies. Lancet Gastroenterol. Hepatol. 2016; 1: 45–55. [DOI] [PubMed] [Google Scholar]
- 2. Peery AF, Crockett SD, Murphy CC et al Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019; 156: 254–72 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banks PA, Bollen TL, Dervenis C et al Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62: 102–11. [DOI] [PubMed] [Google Scholar]
- 4. Moggia E, Koti R, Belgaumkar AP et al Pharmacological interventions for acute pancreatitis. Cochrane Database Syst. Rev. 2017; 4: CD011384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tenner S, Baillie J, DeWitt J, Vege SS, American College of Gastroenterology . American College of Gastroenterology guideline: management of acute pancreatitis. Am. J. Gastroenterol. 2013; 108: 1400–16. [DOI] [PubMed] [Google Scholar]
- 6. Working Group IAPAPAAPG . IAP/APA evidence‐based guidelines for the management of acute pancreatitis. Pancreatology. 2013; 13: e1–15. [DOI] [PubMed] [Google Scholar]
- 7. Buxbaum JL, Quezada M, Da B et al Early aggressive hydration hastens clinical improvement in mild acute pancreatitis. Am. J. Gastroenterol. 2017; 112: 797–803. [DOI] [PubMed] [Google Scholar]
- 8. de‐Madaria E, Soler‐Sala G, Sanchez‐Paya J et al Influence of fluid therapy on the prognosis of acute pancreatitis: a prospective cohort study. Am. J. Gastroenterol. 2011; 106: 1843–50. [DOI] [PubMed] [Google Scholar]
- 9. Warndorf MG, Kurtzman JT, Bartel MJ et al Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin. Gastroenterol. Hepatol. 2011; 9: 705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown A, Orav J, Banks PA. Hemoconcentration is an early marker for organ failure and necrotizing pancreatitis. Pancreas. 2000; 20: 367–72. [DOI] [PubMed] [Google Scholar]
- 11. Wu BU, Johannes RS, Conwell DL, Banks PA. Early hemoconcentration predicts increased mortality only among transferred patients with acute pancreatitis. Pancreatology. 2009; 9: 639–43. [DOI] [PubMed] [Google Scholar]
- 12. Brown A, Baillargeon JD, Hughes MD, Banks PA. Can fluid resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology. 2002; 2: 104–7. [DOI] [PubMed] [Google Scholar]
- 13. Koutroumpakis E, Wu BU, Bakker OJ et al Admission hematocrit and rise in blood urea nitrogen at 24 h outperform other laboratory markers in predicting persistent organ failure and pancreatic necrosis in acute pancreatitis: a post hoc analysis of three large prospective databases. Am. J. Gastroenterol. 2015; 110: 1707–16. [DOI] [PubMed] [Google Scholar]
- 14. Haydock MD, Mittal A, van den Heever M et al National survey of fluid therapy in acute pancreatitis: current practice lacks a sound evidence base. World J. Surg. 2013; 37: 2428–35. [DOI] [PubMed] [Google Scholar]
- 15. Zhang R, Deng L, Jin T et al Hypertriglyceridaemia‐associated acute pancreatitis: diagnosis and impact on severity. HPB (Oxford). 2019; 21: 1240–9. [DOI] [PubMed] [Google Scholar]
- 16. Shi N, Liu T, de la Iglesia D et al Duration of organ failure impacts mortality in acute pancreatitis. Gut. 2019. 10.1136/gutjnl-2019-318241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cecconi M, De Backer D, Antonelli M et al Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014; 40: 1795–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dellinger EP, Forsmark CE, Layer P et al Determinant‐based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann. Surg. 2012; 256: 875–80. [DOI] [PubMed] [Google Scholar]
- 19. Schumacker PT, Cain SM. The concept of a critical oxygen delivery. Intensive Care Med. 1987; 13: 223–9. [DOI] [PubMed] [Google Scholar]
- 20. Morariu AM, Maathuis MH, Asgeirsdottir SA et al Acute isovolemic hemodilution triggers proinflammatory and procoagulatory endothelial activation in vital organs: role of erythrocyte aggregation. Microcirculation. 2006; 13: 397–409. [DOI] [PubMed] [Google Scholar]
- 21. National Heart Lung, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network , Wiedemann HP, Wheeler AP et al Comparison of two fluid‐management strategies in acute lung injury. N. Engl. J. Med. 2006; 354: 2564–75. [DOI] [PubMed] [Google Scholar]
- 22. Grissom CK, Hirshberg EL, Dickerson JB et al Fluid management with a simplified conservative protocol for the acute respiratory distress syndrome. Crit. Care Med. 2015; 43: 288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beitler JR, Schoenfeld DA, Thompson BT. Preventing ARDS: progress, promise, and pitfalls. Chest. 2014; 146: 1102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hashimoto S, Sanui M, Egi M et al The clinical practice guideline for the management of ARDS in Japan. J. Intensive Care. 2017; 5: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Finfer S, Myburgh J, Bellomo R. Intravenous fluid therapy in critically ill adults. Nat. Rev. Nephrol. 2018; 14: 541–57. [DOI] [PubMed] [Google Scholar]
- 26. Mao EQ, Tang YQ, Fei J et al Fluid therapy for severe acute pancreatitis in acute response stage. Chin Med J (Engl). 2009; 122: 169–73. [PubMed] [Google Scholar]
- 27. Mao EQ, Fei J, Peng YB, Huang J, Tang YQ, Zhang SD. Rapid hemodilution is associated with increased sepsis and mortality among patients with severe acute pancreatitis. Chin Med J (Engl). 2010; 123: 1639–44. [PubMed] [Google Scholar]
- 28. Singh VK, Gardner TB, Papachristou GI et al An international multicenter study of early intravenous fluid administration and outcome in acute pancreatitis. United European Gastroenterol. J. 2017; 5: 491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jin T, Jiang K, Deng L et al Response and outcome from fluid resuscitation in acute pancreatitis: a prospective cohort study. HPB (Oxford). 2018; 20: 1082–91. [DOI] [PubMed] [Google Scholar]
- 30. Li L, Jin T, Wen S et al Early Rapid Fluid Therapy Is Associated with Increased Rate of Noninvasive Positive‐Pressure Ventilation in Hemoconcentrated Patients with Severe Acute Pancreatitis. Dig. Dis. Sci. 2020; 2: 937. PMID: 31912265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mukherjee R, Nunes Q, Huang W, Sutton R. Precision medicine for acute pancreatitis: current status and future opportunities. Prec. Clin. Med. 2019; 2: 81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information


