Abstract
Alpha‐momorcharin (α‐MMC), a member of the plant ribosomal inactivating proteins (RIPs) family, has been proven to exhibit important biological properties in animals, including antiviral, antimicrobial, and antitumour activities. However, the mechanism by which α‐MMC increases plant resistance to viral infections remains unclear. To study the effect of α‐MMC on plant viral defence and how α‐MMC increases plant resistance to viruses, recombinant DNA and transgenic technologies were employed to investigate the role of α‐MMC in Nicotiana benthamiana resistance to tobacco mosaic virus (TMV) infection. Treatment with α‐MMC produced through DNA recombinant technology or overexpression of α‐MMC mediated by transgenic technology alleviated TMV‐induced oxidative damage and reduced the accumulation of reactive oxygen species (ROS) during TMV‐green fluorescent protein infection of N. benthamiana. There was a significant decrease in TMV replication in the upper leaves following local α‐MMC treatment and in α‐MMC‐overexpressing plants relative to control plants. These results suggest that application or overexpression of α‐MMC in N. benthamiana increases resistance to TMV infection. Finally, our results showed that overexpression of α‐MMC up‐regulated the expression of ROS scavenging‐related genes. α‐MMC confers resistance to TMV infection by means of modulating ROS homeostasis through controlling the expression of antioxidant enzyme‐encoding genes. Overall, our study revealed a new crosstalk mechanism between α‐MMC and ROS during resistance to viral infection and provides a framework to understand the molecular mechanisms of α‐MMC in plant defence against viral pathogens.
Keywords: alpha‐momorcharin (α‐MMC), Nicotiana benthamiana, overexpression, reactive oxygen species (ROS), resistance, tobacco mosaic virus (TMV)
Application or overexpression of α‐MMC in Nicotiana benthamiana increased resistance to TMV infection by means of modulating ROS homeostasis through controlling the expression of antioxidant enzyme‐encoding genes.
1. INTRODUCTION
Plants are constantly exposed to multiple biotic stresses, such as bacteria, oomycetes, fungi, viruses, nematodes, and herbivores. To defend themselves, plants have evolved complex and efficient defence mechanisms and strategies (Katagiri, 2018; Saijo and Loo, 2019; Wang et al., 2020). For example, microbial (or pathogen)‐associated molecular patterns (MAMP/PAMP)‐triggered immunity (MTI/PTI), effector‐triggered immunity (ETI), systemic acquired resistance (SAR), and gene silencing are the major plant defence mechanisms (Spoel and Dong, 2012; Muthamilarasan and Prasad, 2013; Miller et al., 2017; Han, 2019; Nobori and Tsuda, 2019). In addition, some plants produce defensive chemicals (organic molecules and proteins) that are thought to play a key role in their defence mechanisms against pathogenic invaders (Calixto, 2000; Song et al., 2000; Wang et al., 2016). For instance, numerous studies have suggested that ribosomal inactivating proteins (RIPs) encoded by plant genes confer disease resistance and tolerance of environmental stresses in plants (Huang et al., 2008; Dowd et al., 2012).
RIPs were named due to their ability to inactivate ribosomes, thereby inhibiting protein synthesis (de Virgilio et al., 2010). They are N‐glycosidases that can damage ribosomes by irreversibly inhibiting protein synthesis by removing one or more adenine residues from ribosomal (r)RNA (Puri et al., 2012; Fabbrini et al., 2017). Many RIPs have been proven to exhibit unique bioactive properties, for instance antitumour activity, antibacterial activity, antifungal activity, broad‐spectrum antiviral activity, and insecticidal activity (Puri et al., 2009; Kaur et al., 2011; Zhu et al., 2018). Furthermore, RIPs enhance plant resistance against different biotic stresses (Zhu et al., 2013; Hamshou et al., 2016). The results of many studies have suggested that RIPs play a protective role in transgenic plants by inducing the overexpression of various foreign RIP genes (Fabbrini et al., 2017). Coexpressing foreign RIP genes in transgenic tobacco, tomato, potato, and rice plants enhanced their tolerance and resistance to various biotic stresses, including fungi, viruses, and insects. For example, overexpression of PhRIP I in transgenic potato plants significantly increased resistance against Botrytis cinerea and Rhizoctonia solani infection (Gonzales‐Salazar et al., 2017). Overexpression of alpha‐momorcharin (α‐MMC), an RIP isolated from Momordica charantia seeds, enhanced resistance to rice blast caused by Magnaporthe grisea in transgenic rice plants (Qian et al., 2014). The expression of type I or type II RIPs from apple in transgenic tobacco plants increased resistance against an insect pest, Spodoptera exigua (Hamshou et al., 2017). Overexpressing curcin 2 (an RIP) increased resistance to tobacco mosaic virus (TMV) infection in transgenic tobacco plants (Huang et al., 2008).
α‐MMC, a member of the RIP family, has been proven to exhibit important biological properties in animals including antiviral, antimicrobial, and antitumour activities (Fang et al., 2012; Pan et al., 2014). Zhu et al. (2013) demonstrated that foliar spraying of α‐MMC on tobacco plants increased their resistance to various phytopathogenic viruses, including chilli veinal mottle virus (ChiVMV), cucumber mosaic virus (CMV), TMV, and turnip mosaic virus (TuMV). Furthermore, application of α‐MMC in M. charantia led to a significant increase of jasmonic acid (JA), indicating that the antiviral activities of α‐MMC in M. charantia may be mediated through a JA‐related signalling pathway (Yang et al., 2016). Another study's results suggested that α‐MMC can enhance tobaccoNN plants’ genetic resistance against TMV by manipulating JA‐ and salicylic acid (SA)‐related signalling pathways (Yang et al., 2018). However, the mechanism by which α‐MMC increases plant resistance against viral infection remains unclear.
Reactive oxygen species (ROS) are continuously produced in plants as by‐products of various physiological and metabolic pathways, and were initially recognized as toxic molecules that cause oxidative damage to proteins, DNA, and lipids in plants (Apel and Hirt, 2004). The main types of ROS include free radicals such as superoxide radical () and hydroxyl radical (•OH), as well as nonradicals such as hydrogen peroxide (H2O2) and singlet oxygen (1O2). Increasing evidence indicates that ROS also function as important signalling molecules in plants and that they are involved in regulating a broad range of processes, such as growth, development, defence, and responses to various abiotic and biotic stresses (Radwan et al., 2010; Sharma et al., 2012; Bechtold et al., 2013; Baxter et al., 2014; Shi et al., 2014; Vuleta et al., 2016; Mignolet‐Spruyt et al., 2016; Xu et al., 2019). To use ROS as signalling molecules, ROS must be maintained at nontoxic levels by a delicate equilibrium between production and scavenging pathways (Baxter et al., 2014). Reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, encoded by respiratory burst oxidase homologs (RBOHs), play an important role in the network of ROS generation in plants (Suzuki et al., 2011). Plants have evolved complicated scavenging and regulation systems to monitor ROS redox homeostasis in order to avoid the excessive accumulation of ROS in plant cells (Foyer and Noctor, 2009; Das and Roychoudhury, 2014). Redox homeostasis in plants is maintained by an efficient antioxidative system composed of enzymatic and nonenzymatic antioxidants. The enzymatic components include catalase (CAT), superoxide dismutase (SOD), glutathione‐S‐transferase (GST), guaiacol peroxidase (GPX), and enzymes related to the ascorbate–glutathione cycle, such as monodehydroascorbate reductase (MDHAR), ascorbate peroxidase (APX), glutathione reductase (GR), and dehydroascorbate reductase (DHAR) (Gill and Tuteja, 2010; Miller et al., 2010; Das and Roychoudhury, 2014). Ascorbic acid (AA), α‐tocopherol, reduced glutathione (GSH), flavonoids, carotenoids, phenolics, and proline serve as potent nonenzymatic antioxidants (De Gara et al., 2003; Das and Roychoudhury, 2014).
In this study, we further investigated the antiviral mechanisms of α‐MMC in Nicotiana benthamiana. The α‐MMC‐overexpressing (OE) transgenic N. benthamiana plants exhibited enhanced systemic resistance to TMV infection. Our data indicated that α‐MMC regulates the systemic resistance responses to TMV infection by adjusting the redox homeostasis state through controlling the expression of antioxidant enzyme‐encoding genes. These findings indicate that α‐MMC is a crucial regulator in systemic resistance responses and has great potential for engineering crops with enhanced resistance against pathogen infections.
2. RESULTS
2.1. Prokaryotic expression and purification of α‐MMC and preparation of polyclonal antibodies
In our previous study, we focused on developing a prokaryotic expression system for producing the α‐MMC protein in Escherichia coli and the preparation of polyclonal antibodies that recognized it. As shown in Figure S1, a soluble recombinant His‐tagged α‐MMC protein with a molecular weight of approximately 29.7 kDa was successfully induced at 37°C and a final concentration of 1 mM isopropyl‐β‐D‐thiogalactopyranoside (IPTG). Furthermore, the α‐MMC recombinant protein was successfully purified by Ni–nitrilotriacetic acid (Ni‐NTA) resin affinity chromatography (Figure S2). Finally, the His‐tagged α‐MMC proteins were used to immunize rabbits and obtain serum antibodies. Western blotting results showed that the anti‐α‐MMC polyclonal antibodies had good specificity and sensitivity, and could be used to detect the expression of the α‐MMC protein in bitter melon (Figure S3).
2.2. Treatment with α‐MMC alleviated oxidative damage during TMV‐GFP infection of N. benthamiana plants
The soluble recombinant α‐MMC protein obtained from the E. coli prokaryotic expression system was used to spray N. benthamiana plants before TMV‐green fluorescent protein (GFP) infection. The cell membranes could be adversely affected by oxidative damage induced by pathogen infection. The lipid peroxidation, cell death, and penetrability of cell membranes can be analysed by malondialdehyde (MDA) accumulation and electrolyte leakage, used as oxidative stress parameters (Diaz‐Vivancos et al., 2008; Zhang et al., 2012). Therefore, MDA content and electrolyte leakage were investigated in α‐MMC‐treated N. benthamiana plants infected with TMV‐GFP at 3 days postinoculation (dpi) (the TMV‐GFP‐inoculated leaves were sampled) (Figure 1). No obvious differences of MDA content or electrolyte leakage were measured in α‐MMC‐ treated N. benthamiana plants and water‐treated plants (CK) without TMV‐GFP (Figure 1). However, the MDA content was significantly increased by TMV‐GFP infection in α‐MMC‐treated N. benthamiana plants and water‐treated plants (Figure 1a). Interestingly, α‐MMC‐treated N. benthamiana plants had less MDA formation than control plants after TMV‐GFP infection, indicating that α‐MMC‐treated N. benthamiana plants alleviated the lipid peroxidation of cell membranes under TMV‐GFP infection (Figure 1a). Furthermore, the level of leakage was also significantly reduced in α‐MMC‐treated plants compared with control plants after TMV‐GFP inoculation, implying that α‐MMC induced protection of the cell membranes during TMV‐GFP infection (Figure 1b). Overall, the results indicate that α‐MMC treatment alleviates TMV‐induced oxidative damage and that α‐MMC plays a positive role in N. benthamiana resistance to TMV infection.
Figure 1.
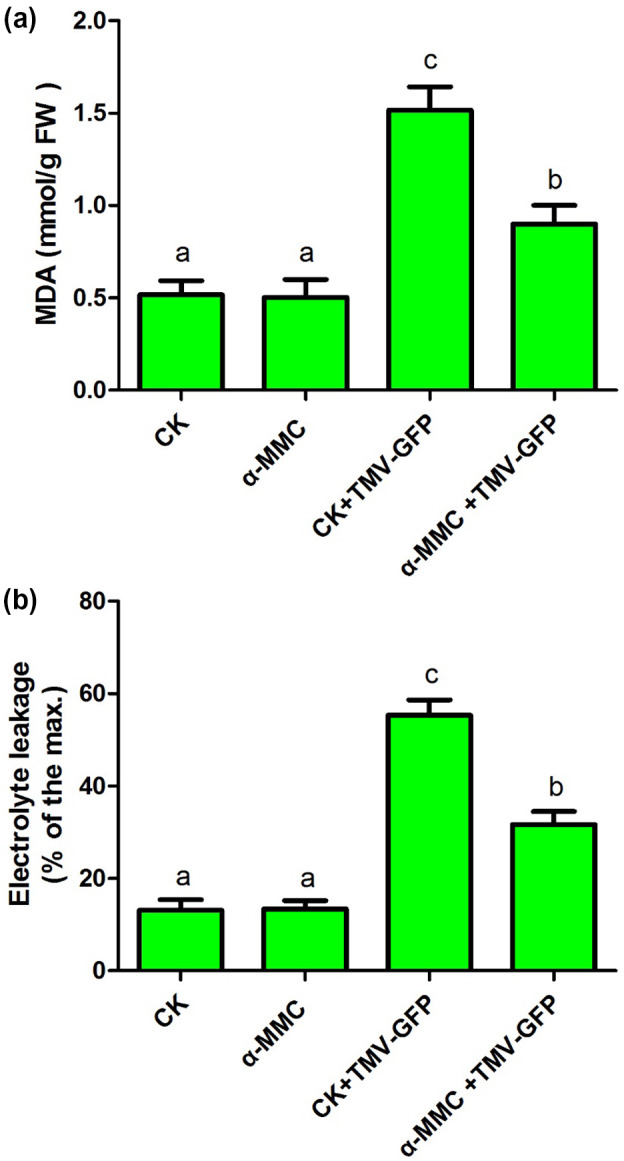
Malondialdehyde (MDA) content (a) and electrolyte leakage (b) were measured in α‐MMC‐treated Nicotiana benthamiana plants infected with TMV‐green fluorescent protein (GFP) (at 3 days postinoculation, dpi). The TMV‐GFP‐inoculated leaves were collected. CK, N. benthamiana plants pretreated with only water; α‐MMC, N. benthamiana plants pretreated with only 0.5 mg/ml α‐MMC obtained from the Escherichia coli prokaryotic expression system; CK + TMV‐GFP, N. benthamiana plants pretreated with water after TMV‐GFP infection (at 3 dpi); α‐MMC + TMV‐GFP, N. benthamiana plants pretreated with 0.5 mg/ml α‐MMC obtained from the E. coli prokaryotic expression system after TMV‐GFP infection (at 3 dpi). Bars represent mean and SD of values obtained from three biological replicates. Significant differences (p < .05) are denoted by different lowercase letters
2.3. Application of α‐MMC reduced the accumulation of ROS during TMV‐GFP infection of N. benthamiana plants
ROS production is correlated with plant cell death and increased susceptibility to pathogenic infection (Overmyer et al., 2003; Mittler et al., 2004; Zhu et al., 2015). Therefore, we first determined the levels of and H2O2 by nitroblue tetrazolium (NBT) and 3,3′‐diaminobenzidine (DAB) staining, respectively, of α‐MMC‐treated plants after TMV‐GFP infection (the TMV‐GFP‐inoculated leaves were sampled). Slight staining in areas of NBT and DAB occurred in α‐MMC‐treated plants and water‐treated plants (CK) in the absence of TMV‐GFP infection (Figure 2a). and H2O2 accumulation was significantly enhanced in α‐MMC‐treated plants and control plants during TMV‐GFP infection (Figure 2a). However, in the presence of TMV‐GFP, α‐MMC‐treated plants showed fewer stained areas and a less intense staining than control plants, indicating that α‐MMC‐treated N. benthamiana plants accumulated less ROS (Figure 2a). To investigate the H2O2 and contents more precisely, a sensitive quantitative Amplex red hydrogen peroxide/peroxidase assay kit was used to measure the H2O2 content, and the hydroxylamine oxygenation reaction method was used to examine the content. The results indicated that the H2O2 and contents were significantly reduced in α‐MMC‐treated plants compared with control plants after TMV‐GFP inoculation (Figure 2b,c). Taken together, these results suggest that application of α‐MMC on N. benthamiana plants reduces the accumulation of ROS during TMV‐GFP infection.
Figure 2.
The levels of reactive oxygen species (ROS) were measured in α‐MMC‐treated Nicotiana benthamiana plants infected with TMV‐green fluorescent protein (GFP) (at 3 days postinoculation, dpi). The TMV‐GFP‐inoculated leaves were collected. (a) The levels of and H2O2 were determined by nitroblue tetrazolium (NBT) and 3,3′‐diaminobenzidine (DAB) staining of α‐MMC‐treated plants after TMV‐GFP infection. (b) H2O2 content was measured using a sensitive quantitative Amplex red hydrogen peroxide/peroxidase assay kit. (c) content was examined by the hydroxylamine oxygenation reaction method. CK, N. benthamiana plants pretreated with only water; α‐MMC, N. benthamiana plants pretreated with only 0.5 mg/ml α‐MMC obtained from the Escherichia coli prokaryotic expression system; CK + TMV‐GFP, N. benthamiana plants pretreated with water after TMV‐GFP infection (at 3 dpi); α‐MMC + TMV‐GFP, N. benthamiana plants pretreated with 0.5 mg/ml α‐MMC obtained from an E. coli prokaryotic expression system after TMV‐GFP infection (at 3 dpi). Bars represent mean and SD of values obtained from three biological replicates. Significant differences (p <.05) are denoted by different lowercase letters
2.4. Application of α‐MMC on N. benthamiana plants enhanced systemic resistance to TMV
To further investigate the positive role of α‐MMC in N. benthamiana resistance to TMV infection, N. benthamiana plants were pretreated on their primary leaves with water and α‐MMC, and then inoculated with TMV‐GFP on the upper leaves (Figure 3). TMV accumulation was evaluated by direct observation of GFP fluorescence (Figure 3a), as well as by quantitative reverse transcription PCR (RT‐qPCR) and western blotting analysis of viral replication at 3 and 5 dpi. (Figure 3b,c). There was a significant decrease in GFP fluorescence in the upper leaves after α‐MMC treatment of the lower primary leaves relative to the control plants (CK) (Figure 3a). This conclusion is consistent with the RT‐qPCR and western blotting analysis of TMV accumulation. As shown in Figure 3b, the RT‐qPCR results suggested thata loweramount of TMV accumulation was detected in the leaves of the α‐MMC‐treated plants compared with control plants. Furthermore, western blotting analysis suggested that the levels of TMV movement protein were also significantly reduced in α‐MMC‐treated plants in comparison with controls (Figure 3c). These results indicate that the application of α‐MMC in N. benthamiana plants enhances systemic resistance to TMV infection.
Figure 3.
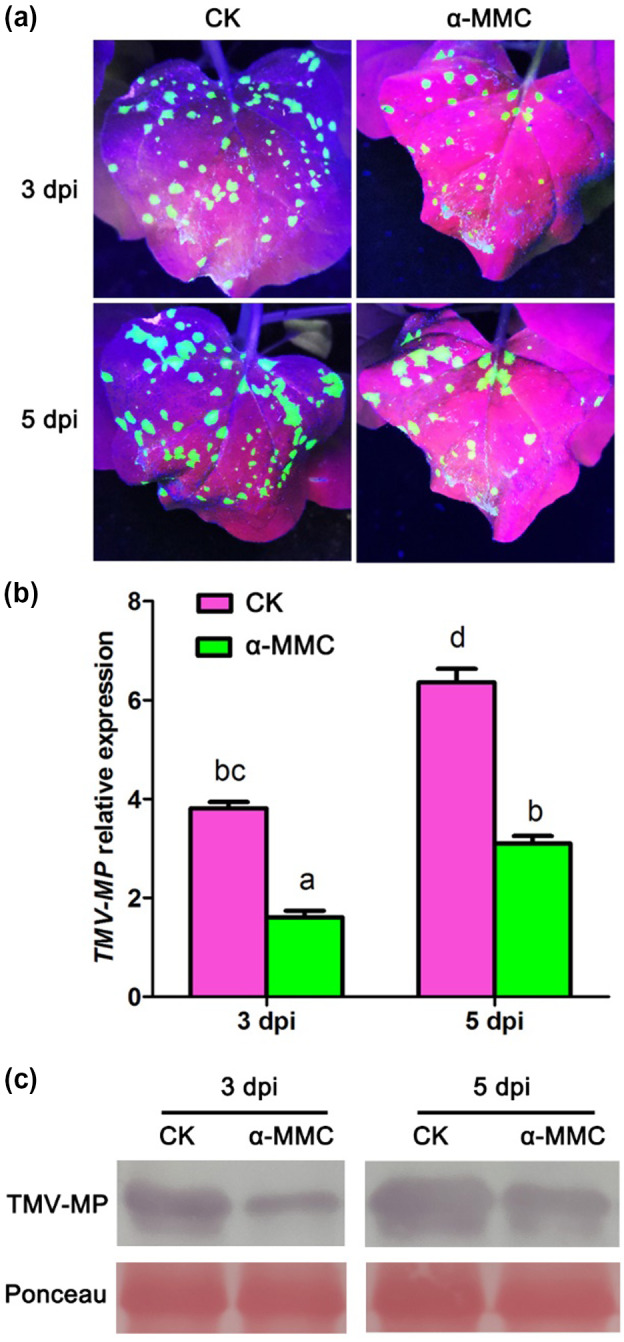
Application of α‐MMC in Nicotiana benthamiana plants enhanced systemic resistance to TMV‐green fluorescent protein (GFP) infection. Plants were pretreated on their primary leaves with water and α‐MMC, and 3 days later inoculated with TMV‐GFP on the upper leaves. (a) Analysis of GFP fluorescence in water‐ and α‐MMC‐treated N. benthamiana plants. GFP fluorescence was photographed from water‐ and α‐MMC‐treated N. benthamiana plants after TMV‐GFP infection at 3 and 5 days postinoculation (dpi). (b) Quantitative reverse transcription PCR analysis of TMV replication levels in water‐ and α‐MMC‐treated N. benthamiana plants after TMV‐GFP infection at 3 and 5 dpi. Bars represent mean and SD of values obtained from three biological replicates. Different lowercase letters indicate significant differences (p < .05). (c) Western blotting analysis of movement protein (MP) accumulation of TMV in water‐ and α‐MMC‐treated N. benthamiana plants after TMV‐GFP infection at 3 and 5 dpi. RuBisCO proteins were used as loading controls and were stained by Ponceau S. CK, N. benthamiana plants pretreated with water after TMV‐GFP infection at 3 and 5 dpi; α‐MMC, N. benthamiana plants pretreated with 0.5 mg/ml α‐MMC obtained from an Escherichia coli prokaryotic expression system after TMV‐GFP infection at 3 and 5 dpi
2.5. Identification of transgenic N. benthamiana lines
The gene encoding α‐MMC was cloned into the pCAMBIA1301 vector under the control of the ubiquitin (Ubi) promoter and used to transform N. benthamiana leaves. Kanamycin‐resistant and PCR‐positive transgenic N. benthamiana plants constitutively expressing α‐MMC were selected. Independent T2 transgenic lines grown in greenhouse conditions were screened by PCR analysis. Total RNA was isolated from positive lines as well as from the nontransformed wild type (WT). Semiquantitative RT‐PCR analysis indicated that the levels of α‐MMC were significantly increased in the OE‐2 and OE‐5 lines (Figure S4a). However, we did not detect the expression of α‐MMC in the nontransformed WT (Figure S4a). This conclusion is consistent with the RT‐qPCR and western blotting analyses of the levels of α‐MMC in the OE‐2 and OE‐5 lines. As shown in Figure S4b, RT‐qPCR results demonstrated that the expression of α‐MMC was significantly enhanced in the OE‐2 and OE‐5 lines. Western blotting analysis also suggested that the α‐MMC protein was detected in the OE‐2 and OE‐5 lines (Figure S4c). Therefore, the OE‐2 and OE‐5 lines were selected to further study the role of α‐MMC in resistance against TMV infection.
2.6. Overexpression of α‐MMC reduced oxidative damage during TMV‐GFP infection
Next, MDA content and electrolyte leakage were determined in the leaves of α‐MMC‐overexpressing plants (the OE‐2 and OE‐5 lines) and in WT plants inoculated with TMV‐GFP at 3 dpi (Figure 4). There was no obvious difference in MDA content or electrolyte leakage between the α‐MMC‐overexpressing plants (OE‐2 and OE‐5 lines) and the WT in the absence of TMV‐GFP infection (Figure 4). Interestingly, MDA content was significantly decreased in the α‐MMC‐overexpressing plants compared with the WT after TMV‐GFP infection (Figure 4a). These results indicated that overexpression of α‐MMC in the N. benthamiana plants reduced lipid peroxidation during TMV‐GFP infection. Furthermore, the α‐MMC‐overexpressing plants had a lower level of leakage than the WT after TMV‐GFP inoculation (Figure 4b). These results suggested that the cytomembranes of α‐MMC‐overexpressing plants suffered less oxidative damage after TMV‐GFP inoculation. Taken together, our results suggest that overexpression of α‐MMC in N. benthamiana plants alleviates TMV‐induced oxidative damage.
Figure 4.
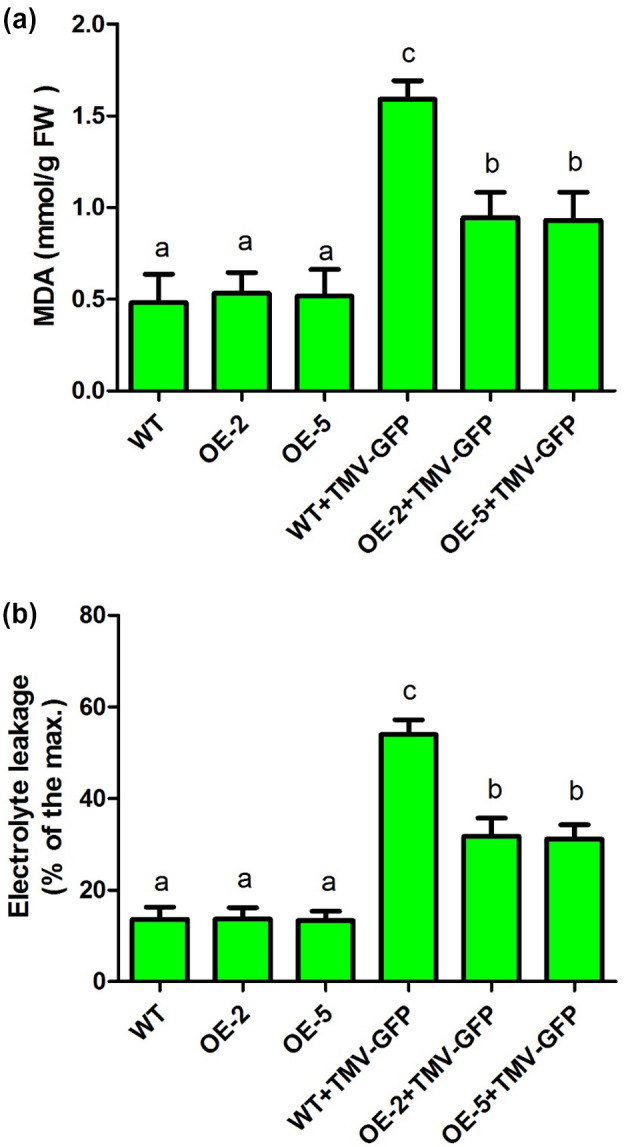
Malondialdehyde (MDA) content (a) and electrolyte leakage (b) were measured in leaves of wild‐type and the transgenic lines OE‐2 and OE‐5 inoculated with TMV‐green fluorescent protein (GFP) (at 3 days postinoculation, dpi). WT, nontransformed wild‐type Nicotiana benthamiana plants inoculated with 0.02 M phosphate‐buffered saline (PBS); OE‐2, α‐MMC‐transgenic N. benthamiana line 2 inoculated with 0.02 M PBS; OE‐5, α‐MMC‐transgenic N. benthamiana line 5 inoculated with 0.02 M PBS; WT + TMV‐GFP, leaves of nontransformed WT N. benthamiana plants inoculated with TMV‐GFP (at 3 dpi); OE‐2 + TMV‐GFP, leaves of α‐MMC‐transgenic N. benthamiana line 2 inoculated with TMV‐GFP (at 3 dpi); OE‐5 + TMV‐GFP, leaves of α‐MMC‐transgenic N. benthamiana line 5 inoculated with TMV‐GFP (at 3 dpi). Bars represent mean and SD of values obtained from three biological replicates. Significant differences (p < .05) are denoted by different lowercase letters
2.7. Overexpression of α‐MMC reduced the accumulation of ROS under TMV‐GFP infection
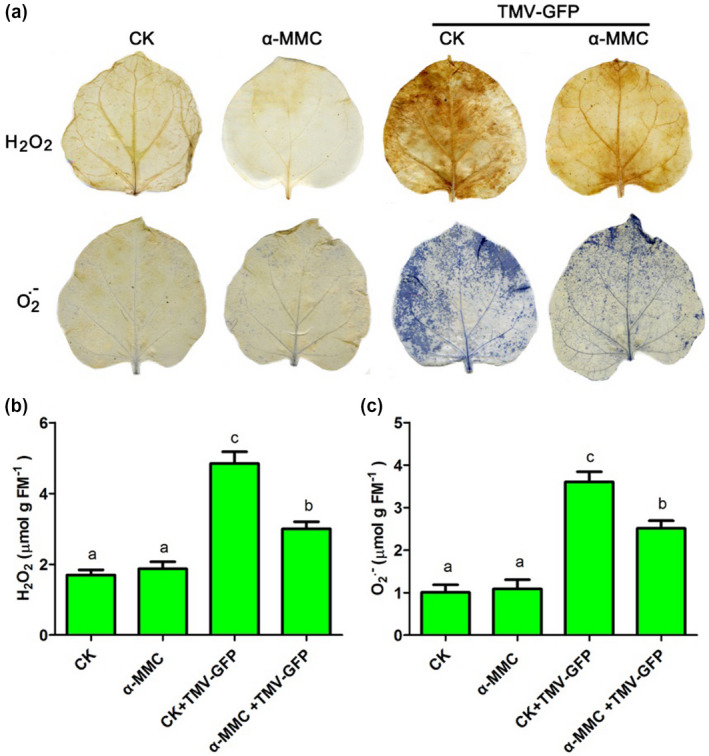
Next, the levels of and H2O2 were determined by histochemical staining with NBT and DAB, respectively, of leaves from α‐MMC‐overexpressing plants (OE‐2 and OE‐5 lines) and WT plants in the absence or the presence of TMV‐GFP infection. No obvious differences in the staining areas of NBT and DAB were observed in the α‐MMC‐overexpressing plants and WT without TMV‐GFP (Figure 5a). The levels of and H2O2 were significantly increased in the α‐MMC‐overexpressing plants and WT during TMV‐GFP infection (Figure 5a). However, the α‐MMC‐overexpressing plants showed fewer stained areas and less intense staining than the WT after TMV‐GFP infection (Figure 5a). These results indicate that α‐MMC‐overexpressing plants accumulate less ROS. Their H2O2 and contents were further analysed. Our results suggested that the levels of H2O2 and were significantly decreased in the α‐MMC‐overexpressing plants compared with that of WT plants after TMV‐GFP infection (Figure 5b,c). Overall, these results suggest that overexpression of α‐MMC in N. benthamiana plants significantly reduces the accumulation of ROS during TMV‐GFP infection.
Figure 5.
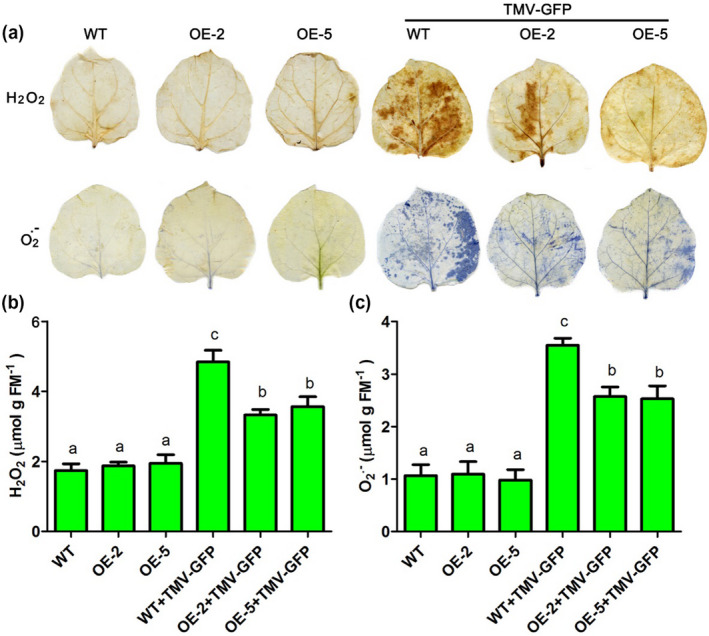
The levels of reactive oxygen species (ROS) were measured in leaves of wild‐type and transgenic lines OE‐2 and OE‐5 inoculated with TMV‐green fluorescent protein (GFP) (at 3 days postinoculation, dpi). (a) The levels of and H2O2 were determined by nitroblue tetrazolium (NBT) and 3,3′‐diaminobenzidine (DAB) staining of WT and transgenic lines OE‐2 and OE‐5 after TMV‐GFP infection. (b) H2O2 content was measured using a sensitive quantitative Amplex red hydrogen peroxide/peroxidase assay kit. (c) content was examined by the hydroxylamine oxygenation reaction method. WT, nontransformed wild‐type Nicotiana benthamiana plants inoculated with 0.02 M phosphate‐buffered saline (PBS); OE‐2, α‐MMC‐transgenic N. benthamiana line 2 inoculated with 0.02 M PBS; OE‐5, α‐MMC‐transgenic N. benthamiana line 5 inoculated with 0.02 M PBS; WT + TMV‐GFP, leaves of nontransformed WT N. benthamiana plants inoculated with TMV‐GFP (at 3 dpi); OE‐2 + TMV‐GFP, leaves of α‐MMC‐transgenic N. benthamiana line 2 inoculated with TMV‐GFP (at 3 dpi); OE‐5 + TMV‐GFP, leaves of α‐MMC‐transgenic N. benthamiana line 5 inoculated with TMV‐GFP (at 3 dpi). Bars represent mean and SD of values obtained from three biological replicates. Significant differences (p < .05) are denoted by different lowercase letters
2.8. N. benthamiana lines overexpressing α‐MMC showed enhanced resistance to TMV
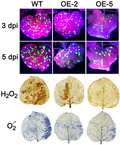
α‐MMC‐overexpressing plants (OE‐2 and OE‐5 lines) were inoculated with TMV‐GFP and monitored for viral replication and spread for at least 1 week. Our results indicated that there was a significant decline in GFP fluorescence in the α‐MMC‐overexpressing plants compared with WT (Figure 6a). Furthermore, the RT‐qPCR results suggested that the α‐MMC‐overexpressing plants had lower TMV accumulation than the WT did (Figure 6b). These results were also confirmed by western blotting analysis, indicating that the level of TMV movement protein accumulation was also significantly reduced in the α‐MMC‐overexpressing plants in comparison with WT (Figure 6c). Therefore, these results suggest that overexpression of α‐MMC in N. benthamiana plants increases resistance to TMV infection.
Figure 6.
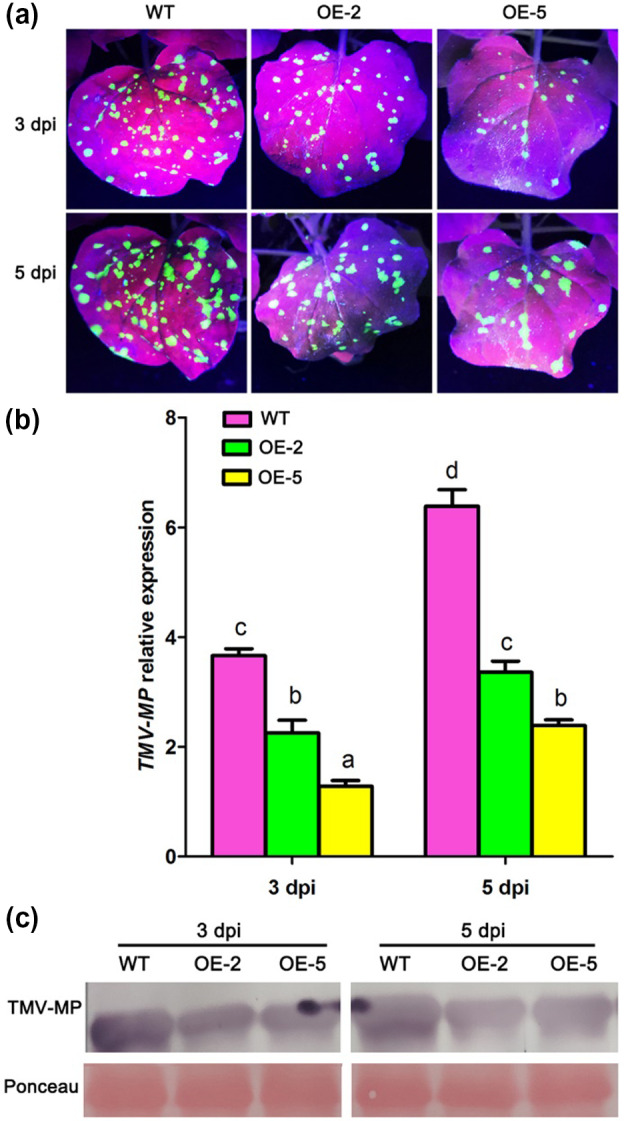
Overexpression of α‐MMC in Nicotiana benthamiana plants increased resistance to TMV infection. (a) Analysis of green fluorescent protein (GFP) fluorescence in leaves of TMV‐GFP‐inoculated wild‐type (WT) and transgenic lines OE‐2 and OE‐5. GFP fluorescence was photographed from WT and transgenic lines OE‐2 and OE‐5 after TMV‐GFP infection at 3 and 5 days postinoculation (dpi). (b) Quantitative reverse transcription PCR analysis of TMV replication levels in WT and transgenic lines OE‐2 and OE‐5 after TMV‐GFP infection at 3 and 5 dpi. Bars represent mean and SD of values obtained from three biological replicates. Different lowercase letters indicate significant differences (p <.05). (c) Western blotting analysis of movement protein accumulation of TMV in WT and transgenic lines OE‐2 and OE‐5 after TMV‐GFP infection at 3 and 5 dpi. RuBisCO proteins were used as loading controls and were stained by Ponceau S. WT, nontransformed WT N. benthamiana plants inoculated with TMV‐GFP at 3 and 5 dpi; OE‐2, α‐MMC‐transgenic N. benthamiana line 2 inoculated with TMV‐GFP at 3 and 5 dpi; OE‐5, α‐MMC‐transgenic N. benthamiana line 5 inoculated with TMV‐GFP at 3 and 5 dpi
2.9. The expression of ROS scavenging‐related genes was up‐regulated in N. benthamiana lines overexpressing α‐MMC
The results related to ROS accumulation and content suggested that α‐MMC may be involved in the regulation of ROS homeostasis. ROS‐scavenging enzymes play important roles in ROS cellular homeostasis under normal and stressful conditions. Increasing evidence indicates that catalase (CAT), superoxide dismutase (SOD), and ascorbate peroxidase (APX) are the three major types of ROS‐scavenging enzymes (Apel and Hirt, 2004; Das and Roychoudhury, 2014). Therefore, we investigated the expression levels of 13 ROS‐scavenging enzymes genes encoding CAT, SOD, or APX in the α‐MMC‐overexpressing plants (OE‐2 and OE‐5 lines) and WT using RT‐qPCR (Figures 7 and 8). The results indicated that the two genes encoding CAT, NbCAT1 and NbCAT2, were up‐regulated in the OE‐2 and OE‐5 lines compared with WT (Figure 7a,b). The expression level of NbCAT3 was also significantly increased in the OE‐5 line in comparison with the basal expression levels (Figure 7c). Next, the gene encoding SOD, NbFeSOD, was found to be significantly enhanced in the OE‐2 and OE‐5 lines compared with WT (Figure 7d). The expression levels of NbMnSOD and NbCu/Zn‐SOD were significantly increased in the OE‐2 and OE‐5 lines compared with WT, respectively (Figure 7e,f). Finally, the expression of the five genes encoding APX, NbAPX1, NbAPX3, NbAPX5, NbAPX6, and NbAPX7, were significantly up‐regulated in the OE‐2 and OE‐5 lines compared with WT (Figure 8a,c,e,f,g). The expression of NbAPX2 was statistically higher in OE‐5 plants than in WT or OE‐2 plants (Figure 8b). There were no obvious differences in the expression of NbAPX4 between the α‐MMC‐overexpressing plants and WT (Figure 8d). Taken together, these results suggest that α‐MMC may regulate the expression of a large number of ROS‐regulating genes, and overexpressing α‐MMC could trigger the induction of a series of ROS‐scavenging genes to cope positively with oxidative stress induced by viral infection.
Figure 7.
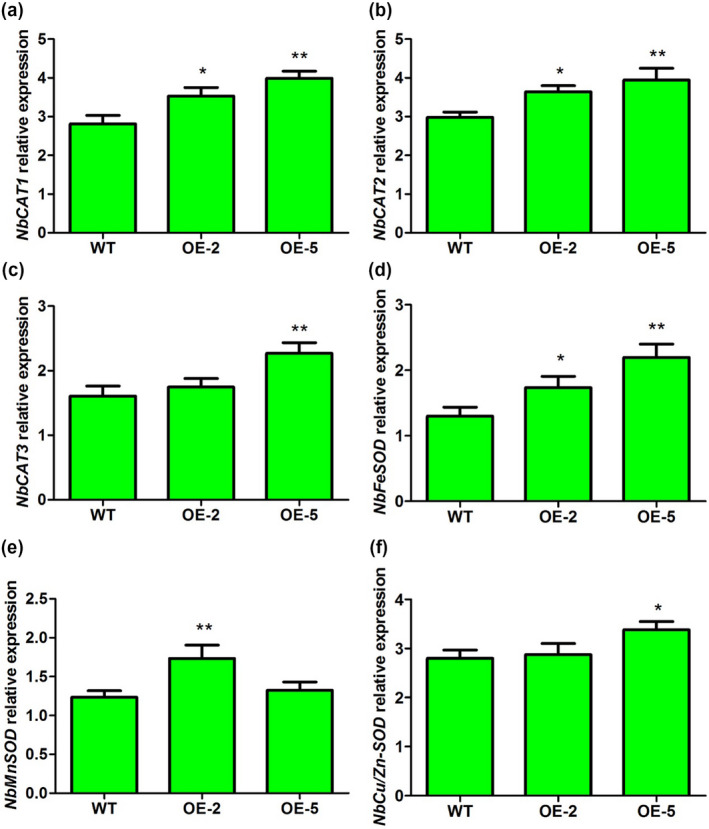
Quantitative reverse transcription PCR analysis of the expression of catalase (CAT) and superoxide dismutase (SOD) genes in wild‐type (WT) and transgenic lines OE‐2 and OE‐5. WT, nontransformed WT Nicotiana benthamiana plants; OE‐2 and OE‐5, α‐MMC‐transgenic N. benthamiana lines. Bars represent mean and SD of values obtained from three biological replicates. Asterisks represent significant difference determined by Student's t test (*p < .05; **p < .01)
Figure 8.
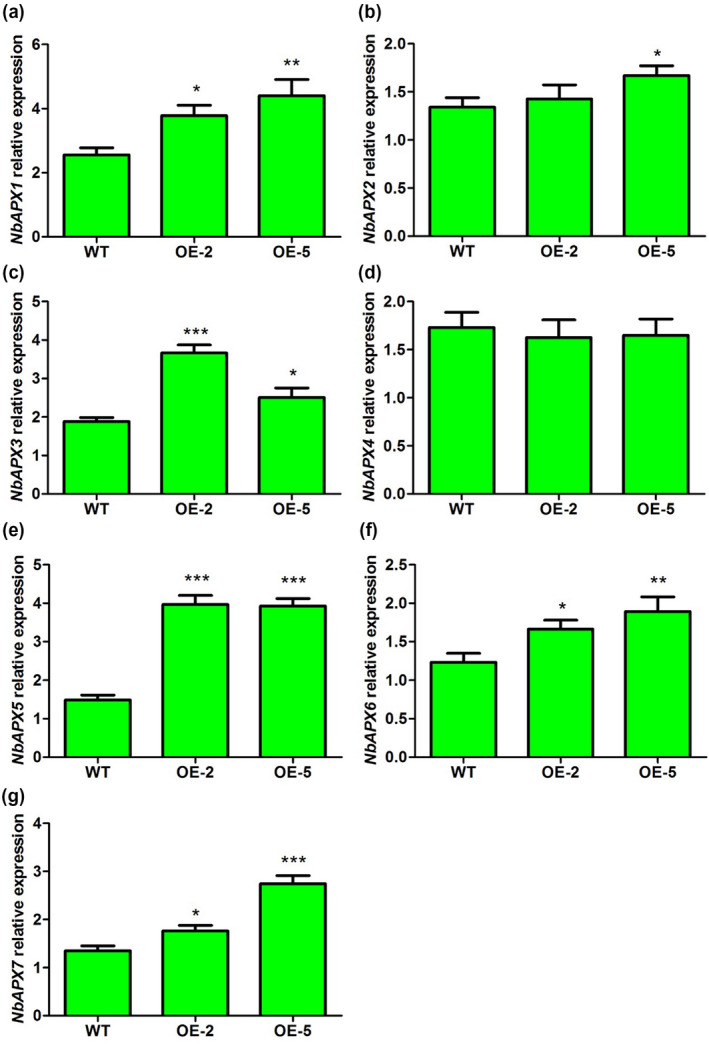
Quantitative reverse transcription PCR analysis of the expression of ascorbate peroxidase (APX) genes in wild‐type (WT) and transgenic lines OE‐2 and OE‐5. WT, nontransformed WT Nicotiana benthamiana plants; OE‐2 and OE‐5, α‐MMC‐transgenic N. benthamiana lines. Bars represent mean and SD of values obtained from three biological replicates. Asterisks represent significant differences determined by Student's t test (*p < .05; **p <.01; ***p < .001)
3. DISCUSSION
Ribosomal inactivating proteins (RIPs) have been proven to confer resistance against bacteria, fungi, viruses, and insects in vitro (Zhu et al., 2018). For example, an RIP isolated and purified from Mirabilis jalapa leaves showed significant antibacterial activity against Propionibacterium acnes and Staphylococcus epidermidis (Rumiyati et al., 2014). A soluble recombinant α‐MMC protein obtained from the E. coli prokaryotic expression system had significant antifungal activity against several fungal pathogens in vitro (Wang et al., 2012). Exogenous application of pokeweed antiviral protein (PAP), a type I RIP, to N. benthamiana plants enhanced their resistance to TMV infection (Zhu et al., 2016). Furthermore, an artificial diet supplemented with type I RIPs reduced the fecundity and survival of insect pests, such as Anticarsia gemmatalis and Spodoptera frugiperda (Bertholdo‐Vargas et al., 2009). In recent years, increasing evidence has suggested that RIPs play important roles in plant defences against bacteria, fungi, viruses, and insects in vivo (Kaur et al., 2011). Overexpression of curcin 2, a newly identified RIP, in transgenic tobacco plants increased their resistance against R. solani (Huang et al., 2008). Transgenic plants expressing barley antifungal gene chitinase and RIP demonstrated antifungal activity against corynespora leaf spot fungal disease (Chopra and Saini, 2014). Overexpression of cassin, a new RIP isolated from Cassia occidentalis, in tobacco plants enhanced their resistance to TMV infection (Ruan et al., 2007).
Transgenic technologies have also been used to investigate the role of α‐MMC in plant defences against fungal pathogens. For example, studies have shown that transgenic rice plants expressing α‐MMC display enhanced resistance to rice blast (Qian et al., 2014). However, studies of the role of α‐MMC in plant defence against virus infection using transgenic technologies have rarely been reported. Furthermore, the role of α‐MMC in plant defence against virus attack and the mechanisms involved remain unclear. Therefore, in this study, we first focused on developing a prokaryotic expression system for producing α‐MMC protein and investigated the role of α‐MMC protein obtained from the E. coli prokaryotic expression system in plant defence against TMV infection. Furthermore, we also used transgenic technologies by overexpressing α‐MMC in N. benthamiana to study the role of α‐MMC in plant defence against TMV and the mechanisms involved.
It is time‐consuming and laborious to purify α‐MMC protein directly from plants. A recombinant DNA technology was used to produce the recombinant proteins in a microbial system. The structure and function of proteins are often studied after using prokaryotic expression techniques. The usual prokaryotic expression host organism is E. coli and its modified strains, such as Rosetta, because it has lots of advantages, such as easy expression induction, a simple structure, and high protein yield (Chen, 2012; Rosano and Ceccarelli, 2014). Therefore, a soluble recombinant His‐tagged α‐MMC protein was successfully induced and purified in an E. coli prokaryotic expression system (Figures [Link], [Link], [Link]). Next, we investigated the role of the recombinant His‐tagged α‐MMC protein obtained from E. coli in plant defence against TMV infection. Oxidative damage induced by pathogen infection could adversely affect the cytomembrane. MDA content and electrolyte leakage have been considered as indicators for membrane lipid peroxidation and the penetrability of the cytomembrane (May et al., 1996; Zoeller et al., 2012; Zhu et al., 2016). Oxidative stress (i.e., the rapid and massive accumulation of ROS in infected tissues) is produced in some compatible virus–host plant interactions (Hernández et al., 2004, 2006, 2016; Diaz‐Vivancos et al., 2006). An accumulation of ROS was noticed in pea plants in response to plum pox virus (PPV) infection (Diaz‐Vivancos et al., 2008). Oxidative stress parameters such as protein oxidation, lipid peroxidation, and electrolyte leakage were increased in different PPV‐susceptible peach plants and apricot plants (Hernández et al., 2004, 2006; Diaz‐Vivancos et al., 2006; Clemente‐Moreno et al., 2015). Oxidative stress parameters (H2O2 content, lipid peroxidation, and protein oxidation) were also studied in the compatible hosts bell pepper and tomato during TMV and tomato mosaic virus (ToMV) infections (Madhusudhan et al., 2009; Hernández et al., 2016). Accumulation of higher amounts of H2O2 and, accordingly, higher levels of protein oxidation and lipid peroxidation occurred during these plant–virus interactions (Madhusudhan et al., 2009; Hernández et al., 2016). Our results showed that the level of leakage and MDA content were significantly reduced in α‐MMC‐treated plants compared with control plants after TMV‐GFP inoculation (Figure 1). Therefore, these results indicated that α‐MMC treatment alleviated TMV‐induced oxidative damage and that α‐MMC plays a positive role in N. benthamiana resistance to TMV infection. Furthermore, RT‐qPCR and western blotting results suggested that application of α‐MMC on N. benthamiana plants significantly reduced TMV replication (Figure 3). Overall, these results indicate that application of α‐MMC increases systemic resistance to TMV infection.
Plant responses to pathogen invasion are associated with the generation of ion fluxes, the induction of kinase cascades, nitric oxide (NO), accumulation of ROS, and increased expression of genes encoding pathogenesis‐related (PR) proteins (Kumar and Klessig, 2003; Baxter et al., 2014; Künstler et al., 2016). Various stresses may result in the overaccumulation of ROS, which can cause damage to plants (Mittler, 2002). For example, high levels of ROS in plants result in cell death/limitation of invading pathogens due to their high toxicity (Yoshioka et al., 2003; Choi et al., 2007; Hernández et al., 2016). ROS have been considered to be nothing but harmful by‐products for a long time. On the other hand, more recent studies have suggested that ROS also function as central signalling molecules during plant defence responses (Miller et al., 2009; Ge et al., 2015). In fact, low levels of ROS could increase tolerance during plant defence responses against various types of stresses (Orozco‐Cardenas et al., 2001; Baxter et al., 2014; Xu et al., 2014). To use ROS as signalling molecules, ROS must be maintained at nontoxic levels through a delicate balancing act between production, involving ROS‐producing enzymes and the unavoidable production of ROS during basic cellular processes, and ROS‐scavenging pathways (Mittler et al., 2004). ROS‐scavenging enzymes, including SOD, CAT, GST, GPX, and enzymes related to the ascorbate‐glutathione cycle, play important roles in normal cellular ROS homeostasis. Our previous studies indicated that PAP may improve plant resistance against TMV infection through regulating the levels of ROS (Zhu et al., 2016). A previous study suggested that α‐MMC could induce an ROS burst in response to CMV infection in M. charantia (Yang et al., 2016). The present data revealed that α‐MMC is a positive regulator of virus resistance and ROS scavenging processes in response to TMV infection in N. benthamiana plants. The ROS, electrolyte leakage, and MDA content that accumulated in the leaves of α‐MMC‐overexpressing plants and α‐MMC‐treated plants were significantly lower than that in the control plants (Figures 2 and 5). This suggests that the enhanced viral resistance of the α‐MMC‐overexpressing plants and α‐MMC‐treated plants may be due to a stronger capability of scavenging ROS during TMV infection to maintain a lower degree of membrane lipid peroxidation. ROS are considered to play an important role in plant disease resistance. Initial, very rapid, and transient increases in ROS levels can be detected in both incompatible and compatible plant–pathogen interactions (Hernández et al., 2016). Several studies have suggested that an early ROS burst, followed by suppression of ROS, is a requirement for successful resistance of plants to TMV and other viruses (Fodor et al., 1997; Abbink et al., 2002; Király et al., 2008; Balasubramaniam et al., 2014; Shang et al., 2019). In fact, overexpression of α‐MMC led to the up‐regulation of many ROS‐scavenging genes (Figures 7 and 8) in transgenic plants. Taken together, overexpressing α‐MMC can increase viral resistance by increasing the cell membrane stability and maintaining redox homeostasis. Therefore, it is proposed that α‐MMC plays a positive role in N. benthamiana resistance to TMV infection by controlling the expression of downstream genes involved in the ROS scavenging pathway. These antioxidant‐encoding genes may be promising candidates for genetic engineering to generate crops with enhanced resistance against virus infection.
In conclusion, α‐MMC confers resistance to virus infection by modulating ROS homeostasis through controlling the expression of ROS‐associated genes. Our findings thus reveal a new crosstalk mechanism between α‐MMC and ROS during virus infection and provide a framework to understand the molecular mechanisms involved in the role of α‐MMC in plant defence against virus pathogens.
4. EXPERIMENTAL PROCEDURES
4.1. Plant growth conditions
N. benthamiana was used as a transformation recipient in this study. The wild‐type N. benthamiana and α‐MMC‐transgenic N. benthamiana plants were planted in a greenhouse with a 16 hr light/8 hr dark cycle (100 μmol⋅m−2⋅s−1) at 25°C. Seedlings 6–7 weeks old were used in this study.
4.2. Prokaryotic expression and purification of α‐MMC
Prokaryotic expression and purification of the α‐MMC protein from E. coli were conducted as described previously (Ma et al., 2020). First, the α‐MMC gene was cloned from bitter gourd seeds by RT‐PCR. Then, the gene was ligated into the prokaryotic expression vector pET‐28a (+) and introduced into the E. coli Rosetta strain. A soluble recombinant protein with a molecular weight of approximately 29.7 kDa was successfully induced at 37°C and a final concentration of 1 mM IPTG. Finally, the α‐MMC recombinant protein was purified by Ni‐NTA resin affinity chromatography.
4.3. Preparation of polyclonal antibodies
New Zealand white rabbits were first immunized subcutaneously with 1 mg/ml purified α‐MMC proteins in the same volume of Freund's complete adjuvant. Further details of the antibody preparation have been described previously (Zhu et al., 2012; Ma et al., 2020).
4.4. Virus inoculation, GFP imaging, and α‐MMC treatments
TMV‐GFP was maintained in an aqueous suspension of 0.02 M phosphate‐buffered saline (PBS) at 4°C. The inoculation with TMV‐GFP was performed as described previously (Zhu et al., 2016). N. benthamiana plants were treated at one site on the primary (lower) leaves with water and 0.5 mg/ml α‐MMC for 3 days, then inoculated with TMV‐GFP on the secondary (upper) leaves. PBS rubbed onto the leaves was used as a control. GFP fluorescence was photographed under UV light using a B‐100AP longwave‐UV lamp (Ultra‐Violet Products) (Zhu et al., 2016).
4.5. Measurements of electrolyte leakage and malondialdehyde content
Electrolyte leakage and malondialdehyde (MDA) content measurements were performed as described previously (Zhu et al., 2016). N. benthamiana leaves were cut into approximately 1‐cm pieces and placed in deionized water at room temperature. First, the conductivity (C 1) was measured by a conductivity meter after 60 min at room temperature. Then, the N. benthamiana leaves were incubated in a boiling water bath for 15 min to achieve 100% electrolyte leakage (C 2). Finally, the percentage electrolyte leakage was calculated according to the formula (C 1/C 2) × 100. Next, the MDA content was determined by the thiobarbituric acid (TBA) reaction. The MDA content was measured using an MDA assay kit (Solarbio).
4.6. Histochemical detection and measurements of H2O2 and
The level of H2O2 was determined using DAB following the method reported previously (Zhu et al., 2013). In brief, N. benthamiana leaves were excised at the base with a razorblade and soaked in 2 mg/ml DAB solution for 8 hr under a vacuum. The leaves were then destained in boiling ethanol (95%) for 15 min. Endogenous concentrations of H2O2 were determined according to previous methods (Zhang et al., 2012; Zhu et al., 2015). The H2O2 production was measured using a hydrogen peroxide (H2O2) assay kit (Solarbio). The accumulation level of in the tobacco leaves was observed using NBT as described previously (Zhu et al., 2013). N. benthamiana leaves were excised at the base with a razorblade and soaked in NBT (0.5 mg/ml) solution for 2 hr under a vacuum. The leaves were then destained in boiling ethanol (95%) for 15 min. The content of in the N. benthamiana leaves was determined according to a method previously described by Wang and Luo (1990). The production was measured using a superoxide anion assay kit (Solarbio).
4.7. Plasmid construction and plant transformation
The plasmid pCAMBIA1301‐α‐MMC was kindly provided by Professor Yi Ding (Wuhan University, Wuhan, China). This vector is driven by the ubiquitin (Ubi) promoter and carries the kanamycin resistance (Kan) selectable marker in plants. The plasmid pCAMBIA1301‐α‐MMC was transformed into Agrobacterium tumefaciens EHA105 by electroporation. α‐MMC transgenic N. benthamiana plants were generated by leaf disc transformation through an agrobacterium‐mediated method, as previously described (Zhang et al., 2017). Transgenic plants were screened on Murashige & Skoog (MS) medium supplemented with 30 μg/ml kanamycin or hygromycin for 7–10 days. The survivors were transferred into the soil for propagation. Transgenic T0 plants were selected, transferred into a greenhouse, and maintained up to the T2 generation, which were used for further analysis.
4.8. Molecular analysis of α‐MMC‐transgenic N. benthamiana lines
Total RNA was isolated from positive lines as well as from the nontransformed WT N. benthamiana plants. Overexpression of α‐MMC in the transgenic plants was confirmed by RT‐PCR using specific primers (Table S1). Semiquantitative RT‐PCR was first performed to detect the expression levels of the α‐MMC gene using 1 μg of total RNA isolated from WT N. benthamiana and α‐MMC‐transgenic N. benthamiana plants (Zhu et al., 2014). First‐strand cDNA was carried out using M‐MLV reverse transcriptase (Takara). Equal loading for amplification of each cDNA was determined by the actin PCR product.
4.9. Quantitative real‐time PCR analysis
To further assay the expression levels of the α‐momorcharin gene, TMV‐MP, and the ROS‐scavenging genes in N. benthamiana plants, quantitative reverse transcription PCR analysis was performed. All of the RT‐qPCR primers are listed in Table S1. Relative quantitation of the target gene expression level was performed using the comparative C t (threshold cycle) method (Zhu et al., 2016). Three technical replicates were performed for each experiment. Amplification of the actin gene was used as an internal control.
4.10. Protein extraction and western blotting
Proteins were extracted from N. benthamiana leaves with extraction buffer (50 mM Tris‐Cl, pH 6.8, 5% mercaptoethanol, 10% glycerol, 4% SDS, 4 M urea) in an ice bath. Protein samples were quantified by the Bradford method (Bradford, 1976). Western blotting was carried out according to the protocol described by Zhu et al. (2013). In brief, protein samples were electrophoresed on 12% polyacrylamide gels and transferred onto nitrocellulose membranes (PALL Life Sciences). Then, the membranes were blocked with 5% skim milk followed by α‐MMC protein or TMV movement protein (TMV‐MP) bands being detected using a rabbit polyclonal antiserum raised against α‐MMC or TMV‐MP as the primary antibody, respectively, and an anti‐rabbit IgG conjugated to alkaline phosphatase (Sangon Biotech) as the secondary antibody. Immunoreactive proteins were visualized using 5‐bromo‐4‐chloro‐3‐indolyl phosphate/NBT.
4.11. Statistical analysis
The values are presented as mean ± SD of at least three replicates. Significant differences were analysed by two‐tailed Student's t test between two groups and by one‐way analysis of variance followed by Tukey test between multiple groups.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We are grateful to Professor Yi Ding (Wuhan University, Wuhan, China) for providing the plasmid pCAMBIA1301‐α‐MMC. We thank Professor David Baulcombe (University of Cambridge) for providing TMV‐GFP. This research was supported by the Natural Science Foundation of Jiangsu Province of China, the Qing Lan Project of Yangzhou University, and the earmarked fund for Modern Agro‐industry Technology Research System (CARS‐30‐3‐02).
Zhu F, Zhu P‐X, Xu F, Che Y‐P, Ma Y‐M, Ji Z‐L. Alpha‐momorcharin enhances Nicotiana benthamiana resistance to tobacco mosaic virus infection through modulation of reactive oxygen species. Molecular Plant Pathology. 2020;21:1212–1226. 10.1111/mpp.12974
Contributor Information
Feng Zhu, Email: zhufeng@yzu.edu.cn.
Zhao‐Lin Ji, Email: zhlji@yzu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abbink, T.E. , Peart, J.R. , Mos, T.N. , Baulcombe, D.C. , Bol, J.F. and Linthorst, H.J. (2002) Silencing of a gene encoding a protein component of the oxygen‐evolving complex of photosystem II enhances virus replication in plants. Virology, 295, 307–319. [DOI] [PubMed] [Google Scholar]
- Apel, K. and Hirt, H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55, 373–399. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam, M. , Kim, B.S. , Hutchens‐Williams, H.M. and Loesch‐Fries, L.S. (2014) The photosystem II oxygen‐evolving complex protein PsbP interacts with the coat protein of alfalfa mosaic virus and inhibits virus replication. Molecular Plant‐Microbe Interactions, 27, 1107–1118. [DOI] [PubMed] [Google Scholar]
- Baxter, A. , Mittler, R. and Suzuki, N. (2014) ROS as key players in plant stress signalling. Journal of Experimental Botany, 65, 1229–1240. [DOI] [PubMed] [Google Scholar]
- Bechtold, U. , Albihlal, W.S. , Lawson, T. , Fryer, M.J. , Sparrow, P.A. , Richard, F. et al (2013) Arabidopsis HEAT SHOCK TRANSCRIPTION FACTORA1b overexpression enhances water productivity, resistance to drought, and infection. Journal of Experimental Botany, 64, 3467–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholdo‐Vargas, L.R. , Martins, J.N. , Bordin, D. , Salvador, M. , Schafer, A.E. , Barros, N.M. et al (2009) Type 1 ribosome inactivating proteins entomotoxic, oxidative and genotoxic action on Anticarsia gemmatalis (Hübner) and Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Journal of Insect Physiology, 55, 51–58. [DOI] [PubMed] [Google Scholar]
- Bradford, M.N. (1976) A rapid and sensitive method for thequantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Analytical Biochemistry, 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Calixto, J.B. (2000) Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Brazilian Journal of Medical and Biological Research, 33, 179–189. [DOI] [PubMed] [Google Scholar]
- Chen, R. (2012) Bacterial expression systems for recombinant protein production: E. coli and beyond. Biotechnology Advances, 30, 1102–1107. [DOI] [PubMed] [Google Scholar]
- Choi, H.W. , Kim, Y.J. , Lee, S.C. , Hong, J.K. and Hwang, B.K. (2007) Hydrogen peroxide generation by the pepper extracellular peroxidase CaPO2 activates local and systemic cell death and defense response to bacterial pathogens. Plant Physiology, 145, 890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra, R. and Saini, R. (2014) Transformation of blackgram (Vigna mungo (L.) Hepper) by barley chitinase and ribosome‐inactivating protein genes towards improving resistance to corynespora leaf spot fungal disease. Applied Biochemistry and Biotechnology, 174, 2791–2800. [DOI] [PubMed] [Google Scholar]
- Clemente‐Moreno, M.J. , Hernandez, J.A. and Diaz‐Vivancos, P. (2015) Sharka: how do plants respond to plum pox virus infection? Journal of Experimental Botany, 66, 25–35. [DOI] [PubMed] [Google Scholar]
- Das, K. and Roychoudhury, A. (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS‐scavengers during environmental stress in plants. Frontiers in Environmental Science, 2, 53. [Google Scholar]
- De Gara, L. , de Pinto, M.C. and Tommasi, F. (2003) The antioxidant systems vis‐à‐vis reactive oxygen species during plant–pathogen interaction. Plant Physiology and Biochemistry, 41, 863–870. [Google Scholar]
- Diaz‐Vivancos, P. , Rubio, M. , Mesonero, V. , Periago, P.M. , Ros, B.A. , Martínez‐Gómez, P. et al (2006) The apoplastic antioxidant system in Prunus: response to plum pox virus. Journal of Experimental Botany, 57, 3813–3824. [DOI] [PubMed] [Google Scholar]
- Diaz‐Vivancos, P. , Clemente‐Moreno, M.J. , Rubio, M. , Olmos, E. , Garcia, J.A. , Martinez‐Gomez, P. et al (2008) Alteration in the chloroplastic metabolism leads to ROS accumulation in pea plants in response to plum pox virus. Journal of Experimental Botany, 59, 2147–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd, P.F. , Johnson, E.T. and Price, N.P. (2012) Enhanced pest resistance of maize leaves expressing monocot crop plant‐derived ribosome‐inactivating protein and agglutinin. Journal of Agricultural and Food Chemistry, 60, 10768–10775. [DOI] [PubMed] [Google Scholar]
- Fabbrini, M.S. , Katayama, M. , Nakase, I. and Vago, R. (2017) Plant ribosome‐inactivating proteins: progesses, challenges and biotechnological applications (and a few digressions). Toxins, 9, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, E.F. , Zhang, C.Z. , Ng, T.B. , Wong, J.H. , Pan, W.L. , Ye, X.J. et al (2012) Momordica charantia lectin, a type II ribosome inactivating protein, exhibits antitumor activity toward human nasopharyngeal carcinoma cells in vitro and in vivo. Cancer Prevention Research, 5, 109–121. [DOI] [PubMed] [Google Scholar]
- Fodor, J. , Gullner, G. , Adam, A.L. , Barna, B. , Komives, T. and Kiraly, Z. (1997) Local and systemic responses of antioxidants to tobacco mosaic virus infection and to salicylic acid in tobacco (role in systemic acquired resistance). Plant Physiology, 114, 1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer, C.H. and Noctor, G. (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxidants & Redox Signaling, 11, 861–905. [DOI] [PubMed] [Google Scholar]
- Ge, X.M. , Cai, H.L. , Lei, X. , Zhou, X. , Yue, M. and He, J.M. (2015) Heterotrimeric G protein mediates ethylene induced stomatal closure via hydrogen peroxide synthesis in Arabidopsis. Plant Journal, 82, 138–150. [DOI] [PubMed] [Google Scholar]
- Gill, S.S. and Tuteja, N. (2010) Reactive oxygen species and antioxidantmachinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48, 909–930. [DOI] [PubMed] [Google Scholar]
- Gonzales‐Salazar, R. , Cecere, B. , Ruocco, M. , Rao, R. and Corrado, G. (2017) A comparison between constitutive and inducible transgenic expression of the PhRIP I gene for broad‐spectrum resistance against phytopathogens in potato. Biotechnology Letters, 39, 1049–1058. [DOI] [PubMed] [Google Scholar]
- Hernández, J.A. , Rubio, M. , Olmos, E. , Ros‐Barceló, A. and Martínez‐Gómez, P. (2004) Oxidative stress induced by long‐term plum pox virus infection in peach (Prunus persica). Physiologia Plantarum, 122, 486–495. [Google Scholar]
- Hernández, J.A. , Diaz‐Vivancos, P. , Rubio, M. , Olmos, E. , Ros‐Barceló, A. and Martínez‐Gómez, P. (2006) Long‐term PPV infection produces an oxidative stress in a susceptible apricot cultivar but not in a resistant cultivar. Physiologia Plantarum, 126, 140–152. [Google Scholar]
- Hernández, J.A. , Gullner, G. , Clemente‐Moreno, M.J. , Künstler, A. , Juhász, C. , Díaz‐Vivancos, P. et al (2016) Oxidative stress and antioxidative responses in plant–virus interactions. Physiological and Molecular Plant Pathology, 94, 134–148. [Google Scholar]
- Hamshou, M. , Shang, C. , Smagghe, G. and Van Damme, E.J.M. (2016) Ribosome‐inactivating proteins from apple have strong aphicidal activity in artificial diet and in planta. Crop Proection, 87, 19–24. [Google Scholar]
- Hamshou, M. , Shang, C. , De Zaeytijd, J. , Van Damme, E.J.M. and Smagghe, G. (2017) Expression of ribosome‐inactivating proteins from apple in tobacco plants results in enhanced resistance to Spodoptera exigua . Journal of Asia‐Pacific Entomology, 20, 1–5. [Google Scholar]
- Han, G.Z. (2019) Origin and evolution of the plant immune system. New Phytologist, 222, 70–83. [DOI] [PubMed] [Google Scholar]
- Huang, M.X. , Hou, P. , Wei, Q. , Xu, Y. and Chen, F. (2008) A ribosome‐inactivating protein (curcin 2) induced from Jatropha curcas can reduce viral and fungal infection in transgenic tobacco. Plant Growth Regulation, 54, 115–123. [Google Scholar]
- Katagiri, F. (2018) Review: Plant immune signaling from a network perspective. Plant Science, 276, 14–21. [DOI] [PubMed] [Google Scholar]
- Kaur, I. , Gupta, R.C. and Puri, M. (2011) Ribosome inactivating proteins from plants inhibiting viruses. Virologica Sinica, 26, 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Király, L. , Hafez, Y.M. , Fodor, J. and Kiraly, Z. (2008) Suppression of tobacco mosaic virus‐induced hypersensitive‐type necrotization in tobacco at high temperature is associated with downregulation of NADPH oxidase and superoxide and stimulation of dehydroascorbate reductase. Journal of General Virology, 89, 799–808. [DOI] [PubMed] [Google Scholar]
- Kumar, D. and Klessig, D.F. (2003) High‐affinity salicylic acid binding protein 2 is required for plant innate immunity and has salicylic acid‐stimulated lipase activity. Proceedings of the National Academy of Sciences of the United States of America, 100, 16101–16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künstler, A. , Bacsó, R. , Gullner, G. , Hafez, Y.M. and Király, L. (2016) Staying alive – is cell death dispensable for plant disease resistance during the hypersensitive response? Physiological and Molecular Plant Pathology, 93, 75–84. [Google Scholar]
- Ma, Y.M. , Zhou, Y.K. , Zhu, P.X. , Che, Y.P. , Ji, Z.L. and Zhu, F. (2020) Prokaryotic expression of Alpha‐momorcharin and preparation of its polyclonal antibody. Fenzi Zhiwu Yuzhong [Molecular Plant Breeding]. [Google Scholar]
- Madhusudhan, K.N. , Srikanta, B.M. , Shylaja, M.D. , Prakash, H.S. and Shetty, H.S. (2009) Changes in antioxidant enzymes, hydrogen peroxide, salicylic acid and oxidative stress in compatible and incompatible host–tobamovirus interaction. Journal of Plant Interactions, 4, 157–166. [Google Scholar]
- May, M.J. , Hammond‐Kosack, K.E. and Jones, J.D.G. (1996) Involvement of reactive oxygen species, glutathione metabolism, and lipid peroxidation in the Cf‐gene‐dependent defense response of tomato cotyledons induced by race‐specific elicitors of Cladosporium fulvum . Plant Physiology, 110, 1367–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignolet‐Spruyt, L. , Xu, E. , Idanheimo, N. , Hoeberichts, F.A. , Muhlenbock, P. , Brosche, M. et al (2016) Spreading the news: subcellular and organellar reactive oxygen species production and signalling. Journal of Experimental Botany, 67, 3831–3844. [DOI] [PubMed] [Google Scholar]
- Miller, G. , Schlauch, K. , Tam, R. , Cortes, D. , Torres, M.A. , Shulaev, V. et al (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Science Signaling, 2, ra45. [DOI] [PubMed] [Google Scholar]
- Miller, G. , Suzuki, N. , Ciftci‐Yilmaz, S. and Mittler, R. (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell and Environment, 33, 453–467. [DOI] [PubMed] [Google Scholar]
- Miller, R.N. , Costa Alves, G.S. and Van Sluys, M.A. (2017) Plant immunity: unravelling the complexity of plant responses to biotic stresses. Annals of Botany, 119, 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler, R. (2002) Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7, 405–410. [DOI] [PubMed] [Google Scholar]
- Mittler, R. , Vanderauwera, S. , Gollery, M. and Van Breusegem, F. (2004) Reactive oxygen gene network of plants. Trends in Plant Science, 9, 490–498. [DOI] [PubMed] [Google Scholar]
- Muthamilarasan, M. and Prasad, M. (2013) Plant innate immunity: an updated insight into defense mechanism. Journal of Biosciences, 38, 433–449. [DOI] [PubMed] [Google Scholar]
- Nobori, T. and Tsuda, K. (2019) The plant immune system in heterogeneous environments. Current Opinion in Plant Biology, 50, 58–66. [DOI] [PubMed] [Google Scholar]
- Orozco‐Cardenas, M.L. , Narvaez‐Vasquez, J. and Ryan, C.A. (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. The Plant Cell, 13, 179–191. [PMC free article] [PubMed] [Google Scholar]
- Overmyer, K. , Brosche, M. and Kangasjarvi, J. (2003) Reactive oxygen species and hormonal control of cell death. Trends in Plant Science, 8, 335–342. [DOI] [PubMed] [Google Scholar]
- Pan, W.L. , Wong, J.H. , Fang, E.F. , Chan, Y.S. , Ng, T.B. and Cheung, R.C.F. (2014) Preferential cytotoxicity of the type I ribosome inactivating protein alpha‐momorcharin on human nasopharyngeal carcinoma cells under normoxia and hypoxia. Biochemical Pharmacology, 89, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri, M. , Kaur, I. , Kanwar, R.K. , Gupta, R.C. , Chauhan, A. and Kanwar, J.R. (2009) Ribosome inactivating proteins (RIPs) from Momordica charantia for antiviral therapy. Current Molecular Medicine, 9, 1080–1094. [DOI] [PubMed] [Google Scholar]
- Puri, M. , Kaur, I. , Perugini, M.A. and Gupta, R.C. (2012) Ribosome‐inactivating proteins: current status and biomedical applications. Drug Discovery Today, 17, 774–783. [DOI] [PubMed] [Google Scholar]
- Qian, Q. , Huang, L. , Yi, R. , Wang, S.Z. and Ding, Y. (2014) Enhanced resistance to blast fungus in rice (Oryza sativa L.) by expressing the ribosome‐inactivating protein alpha‐momorcharin. Plant Science, 217–218, 1–7. [DOI] [PubMed] [Google Scholar]
- Radwan, D.E.M. , Fayez, K.A. , Mahmoud, S.Y. and Lu, G.Q. (2010) Modifications of antioxidant activity and protein composition of bean leaf due to bean yellow mosaic virus infection and salicylic acid treatments. Acta Physiologiae Plantarum, 32, 891–904. [Google Scholar]
- Rosano, G.L. and Ceccarelli, E.A. (2014) Recombinant protein expression in Escherichia coli: advances and challenges. Frontiers in Microbiology, 5, 172–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan, X.L. , Liu, L.F. and Li, H.P. (2007) Transgenic tobacco plants with ribosome inactivating protein gene cassin from Cassia occidentalis and their resistance to tobacco mosaic virus. Journal of Plant Physiology and Molecular Biology, 33, 517–523. [PubMed] [Google Scholar]
- Rumiyati, N.A.W. , Sismindari‐Lukitaningsih, E. and Yuliati, T. (2014) Potential of ribosome‐inactivating proteins (RIPs) of Mirabilis jalapa L. as an antiacne: effect on proliferation of cultured sebocyte cells and its antibacterial activities against Propionibacterium acnes and Staphylococcus epidermidis . International Journal of Pharmaceutical Chemistry, 4, 130–133. [Google Scholar]
- Saijo, Y. and Loo, E.P. (2019) Plant immunity in signal integration between biotic and abiotic stress responses. New Phytologist, 225, 1–18. [DOI] [PubMed] [Google Scholar]
- Shang, J. , Zhang, L. , Jia, Q. , Tang, Z.Q. and Yuan, S. (2019) Early ROS accumulation in chloroplasts of Nicotiana glutinosa infected by cucumber mosaic virus. International Journal of Agriculture & Biology, 21, 149–154. [Google Scholar]
- Sharma, P. , Jha, A.B. , Dubey, R.S. and Pessarakli, M. (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany, 2012, 1–26. [Google Scholar]
- Shi, H. , Wang, X. , Ye, T. , Chen, F. , Deng, J. , Yang, P. et al (2014) The Cysteine2/Histidine2‐Type transcription factor ZINC FINGER OF ARABIDOPSIS THALIANA6 modulates biotic and abiotic stress responses by activating salicylic acid‐related genes and C‐REPEAT‐BINDING FACTOR genes in Arabidopsis. Plant Physiology, 165, 1367–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, S.K. , Choi, Y. , Moon, Y.H. , Kim, S.G. , Choi, Y.D. and Lee, J.S. (2000) Systemic induction of a Phytolacca insularis antiviral protein gene by mechanical wounding, jasmonic acid, and abscisic acid. Plant Molcular Biology, 43, 439–450. [DOI] [PubMed] [Google Scholar]
- Spoel, S.H. and Dong, X. (2012) How do plants achieve immunity? Defence without specialized immune cells. Nature Reviews Immunology, 12, 89–100. [DOI] [PubMed] [Google Scholar]
- Suzuki, N. , Miller, G. , Morales, J. , Shulaev, V. , Torres, M.A. and Mittler, R. (2011) Respiratory burst oxidases: the engines of ROS signaling. Current Opinion in Plant Biology, 14, 691–699. [DOI] [PubMed] [Google Scholar]
- de Virgilio, M. , Lombardi, A. , Caliandro, R. and Fabbrini, M.S. (2010) Ribosome inactivating proteins: from plant defense to tumor attack. Toxins, 2, 2699–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuleta, A. , Jovanović, S.M. and Tucić, B. (2016) Adaptive flexibility of enzymatic antioxidants SOD, APX and CAT to high light stress: the clonal perennial monocot Iris pumila as a study case. Plant Physiology and Biochemistry, 100, 166–173. [DOI] [PubMed] [Google Scholar]
- Wang, A.G. and Luo, G.H. (1990) Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiology Communications, 6, 55–57. [Google Scholar]
- Wang, S. , Zhang, Y. , Liu, H. , He, Y. , Yan, J. , Wu, Z. et al (2012) Molecular cloning and functional analysis of a recombinant ribosome‐inactivating protein (alpha‐momorcharin) from Momordica charantia . Applied Microbiology and Biotechnology, 96, 939–950. [DOI] [PubMed] [Google Scholar]
- Wang, S.S. , Zhang, H.Y. , Zheng, Y.Z. , Li, Z.L. , Xiang, F. , Ding, Y. et al (2016) Environmental factors and phytohormones enhancing expression of α‐momorcharin gene in Momordica charantia . Biologia, 71, 155–160. [Google Scholar]
- Wang, W. , Feng, B.M. , Zhou, J.M. and Tang, D.Z. (2020) Plant immune signaling: advancing on two frontiers. Journal of Integrative Plant Biology, 62, 2–24. [DOI] [PubMed] [Google Scholar]
- Xu, J. , Xie, J. , Yan, C.F. , Zou, X.Q. , Ren, D.T. and Zhang, S. (2014) A chemical genetic approach demonstrates that MPK3/MPK6 activation and NADPH oxidase‐mediated oxidative burst are two independent signaling events in plant immunity. Plant Journal, 77, 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y. , Magwanga, R.O. , Cai, X. , Zhou, Z. , Wang, X. , Wang, Y. et al (2019) Deep transcriptome analysis reveals reactive oxygen species (ROS) network evolution, response to abiotic stress and regulation of fiber development in cotton. International Journal of Molecular Sciences, 20, 1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, T. , Meng, Y. , Chen, L.J. , Lin, H.H. and Xi, D.H. (2016) The roles of alpha‐momorcharin and jasmonic acid in modulating the response of Momordica charantia to cucumber mosaic virus. Frontiers in Microbiology, 7, 1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, T. , Zhu, L.S. , Meng, Y. , Lv, R. , Zhou, Z. , Zhu, L. et al (2018) Alpha‐momorcharin enhances tobacco mosaic virus resistance in tobaccoNN by manipulating jasmonic acid‐salicylic acid crosstalk. Journal of Plant Physiology, 223, 116–126. [DOI] [PubMed] [Google Scholar]
- Yoshioka, H. , Numata, N. , Nakajima, K. , Katou, S. , Kawakita, K. , Rowland, O. et al (2003) Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans . The Plant Cell, 15, 706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Oh, Y. , Li, H. , Baldwin, I.T. and Galis, I. (2012) Alternative oxidase in resistance to biotic stresses: Nicotiana attenuata AOX contributes to resistance to a pathogen and a piercing‐sucking insect but not Manduca sexta larvae. Plant Physiology, 160, 1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K. , Zhang, Y. , Yang, M. , Liu, S. , Li, Z. , Wang, X. et al (2017) The barley stripe mosaic virus γb RNA silencing suppressor protein promotes chloroplast targeted replication by enhancing unwinding of RNA duplexes. PLoS Pathogens, 13, e1006319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, F. , Xu, M.Y. , Wang, S.D. , Jia, S.D. , Zhang, P. , Lin, H.H. et al (2012) Prokaryotic expression of pathogenesis related protein 1 gene from Nicotiana benthamiana: antifungal activity and preparation of its polyclonal antibody. Biotechnology Letters, 34, 919–924. [DOI] [PubMed] [Google Scholar]
- Zhu, F. , Zhang, P. , Meng, Y.F. , Xu, F. , Zhang, D.W. , Cheng, J. et al (2013) Alpha‐momorcharin, a RIP produced by bitter melon, enhances defense response in tobacco plants against diverse plant viruses and shows antifungal activity in vitro. Planta, 237, 77–88. [DOI] [PubMed] [Google Scholar]
- Zhu, F. , Xi, D.H. , Yuan, S. , Xu, F. , Zhang, D.W. and Lin, H.H. (2014) Salicylic acid and jasmonic acid are essential for systemic resistance against tobacco mosaic virus in Nicotiana benthamiana . Molecular Plant‐Microbe Interactions, 27, 567–577. [DOI] [PubMed] [Google Scholar]
- Zhu, F. , Deng, X.G. , Xu, F. , Jian, W. , Peng, X.J. , Zhu, T. et al (2015) Mitochondrial alternative oxidase is involved in both compatible and incompatible host‐virus combinations in Nicotiana benthamiana . Plant Science, 239, 26–35. [DOI] [PubMed] [Google Scholar]
- Zhu, F. , Yuan, S. , Zhang, Z.W. , Qian, K. , Feng, J.G. and Yang, Y.Z. (2016) Pokeweed antiviral protein (PAP) increases plant systemic resistance to tobacco mosaic virus infection in Nicotiana benthamiana . European Journal of Plant Pathology, 146, 541–549. [Google Scholar]
- Zhu, F. , Zhou, Y.K. , Ji, Z.L. and Chen, X.R. (2018) The plant ribosome‐inactivating proteins play important roles in defense against pathogens and insect pest attacks. Frontiers in Plant Science, 9, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller, M. , Stingl, N. , Krischke, M. , Fekete, A. , Waller, F. , Berger, S. et al (2012) Lipid profiling of the Arabidopsis hypersensitive response reveals specific lipid peroxidation and fragmentation processes: biogenesis of pimelic and azelaic acid. Plant Physiology, 160, 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


