Abstract
Leaf chlorosis, severe defoliation and wilt associated with root rot were observed on mature olive trees cv. Nera di Gonnos in an experimental orchard at Mirto Crosia (Calabria, southern Italy). An oomycete was consistently isolated from rotten roots of symptomatic olive trees. It was identified as Phytophthora bilorbang by morphological characters and sequencing of Internal Transcribed Spacer (ITS) regions of ribosomal DNA (rDNA). Pathogenicity was verified by inoculating potted two-month-old rooted cuttings of Olea europaea var. Nera di Gonnos in a soil infestation trial. P. bilorbang was re-isolated from roots of symptomatic, artificially inoculated olive cuttings to fulfill Koch’s postulates. This is the first report of P. bilorbang on O. europaea L. and on a species of the Oleaceae family worldwide.
Keywords: root rot, Phytophthora, olive, leaf chlorosis, defoliation, wilt, molecular identification, morphological identification, ITS region
1. Introduction
Olive (Olea europaea L., family Oleaceae) originated in the Near East and spread westward in the Mediterranean basin where it is widely cultivated as a fruit tree [1,2]. Based on data from the International Olive Oil Council (IOOC), more than 10 million hectares are cultivated with olive globally and 95% of them are in the Mediterranean basin [3]. With ca. 1.1 million ha of olive groves, Italy is the second largest olive growing country in the world, with Apulia, Calabria, and Sicily regions of southern Italy accounting for about 70% of the production [4]. As a typical Mediterranean plant, olive has been traditionally cultivated in arid lands. However, during the last decades in many olive growing countries, including Italy, the olive cultivation has been extended to different types of soil and irrigation has become a common practice in olive orchards. An emerging phytopathological problem of olive trees growing in wet or waterlogged soils is root rot caused by Phytophthora spp. [5]. The genus Phytophthora (Pythiaceae, Peronosporales, Oomycota, and Chromista) comprises more than 180 described taxa [6]. With the advent of DNA sequencing the systematics of the genus evolved from morphological criteria to molecular phylogeny. Therefore, the species of Phytophthora were identified using molecular markers and grouped into 12 phylogenetic clades some of which have subclades [7,8,9,10]. Several Phytophthora species of different phylogenetic clades have been reported as causative agents of leaf chlorosis, wilting, defoliation, and twig dieback, as a consequence of root rot and basal stem cankers on olive worldwide, including Phytophthora acerina, Phytophthora cactorum, Phytophthora cinnamomi, Phytophthora citricola sensu lato, Phytophthora cryptogea, Phytophthora drechsleri, Phytophthora inundata, Phytophthora megasperma, Phytophthora nicotianae, Phytophthora oleae, Phytophthora palmivora, Phytophthora pini, and Phytophthora plurivora [5,11,12,13,14,15,16,17,18,19,20,21]. These species differ in aggressiveness, temperature requirements for growth, geographical distribution, and ecology. Phytophthora palmivora, alone or in association with Verticillium dahliae, was reported as causal agent of rot of fine roots and wilt of young olive trees in nurseries and new plantings in Italy [12,22]. Phytophthora oleae was recovered from soil and roots of wild olive trees in protected natural areas in Spain and Sicily (southern Italy) and is widespread in soil of commercial olive orchards in Calabria (southern Italy) [20,23,24]. Phytophthora inundata is responsible for root rot and wilt of olive trees in clay soils after flooding, acting as an opportunistic albeit aggressive root pathogen [15,18,25]. In moist environments, some soil-inhabiting species, like P. nicotianae and P. oleae have occasionally adapted to an aerial lifestyle and may infect aboveground parts of olive trees such as drupes, leaves, and twigs causing fruit rot, leaf drying, filloptosis, and twig dieback [26,27,28].
In autumn 2019, symptoms of defoliation, wilt, and root rot were observed on 15-year-old olive trees in an experimental orchard at Mirto Crosia, in Calabria (Figure 1a). Trees were watered in summer using a drip irrigation system. About 40% of the trees of the cv. Nera di Gonnos, originating from Sardinia (southern Italy), were affected. Symptoms were indicative of Phytophthora root rot (PRR). The main aim of the present study was to identify the causative agent of this disease.
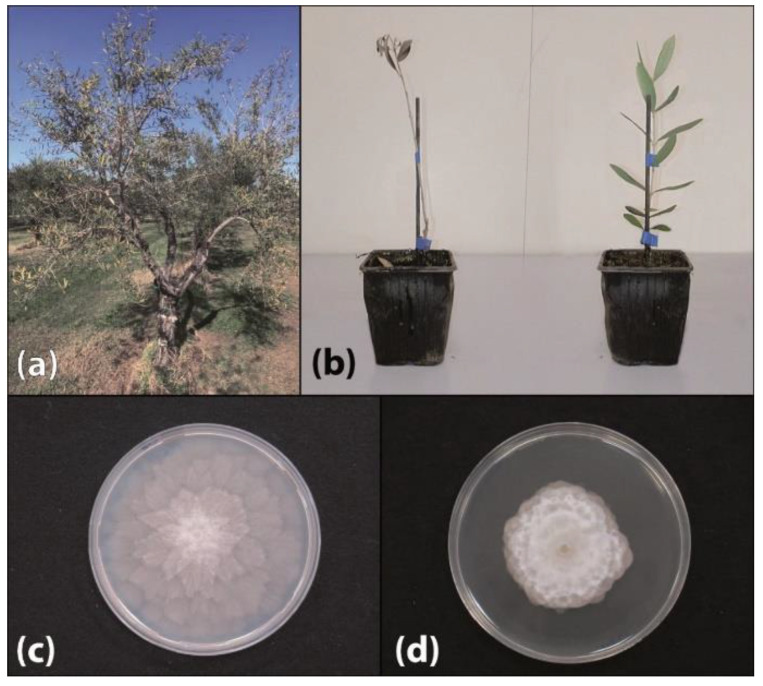
Figure 1.
(a) Decline symptoms on a tree of olive (Olea europaea) cv. Nera di Gonnos incited by Phytophthora bilorbang in Calabria. (b) Wilt of a potted rooted cutting of olive cv. Nera di Gonnos (on the left) artificially inoculated with P. bilorbang through the soil, 6 weeks after transplanting into infested soil, and control non-inoculated cutting (on the right). (c) Morphology of 6-day-old colonies of P. bilorbang grown on V8 juice-agar and (d) on potato-dextrose-agar at 25 °C in the dark.
2. Results
2.1. Species Identification and Morphological Features of Isolates
Isolations from rotten roots of symptomatic olive trees sampled in the experimental orchard of Mirto Crosia found consistently a homothallic Phytophthora taxon with a notable colony morphology (Figure 1c,d). Eighteen single-hypha isolates of this Phytophthora taxon, obtained from three independent trees (six from each tree), were characterized. They formed stellate to petaloid colonies on V8 juice-agar (V8A) and dense-felty, chrysanthemum-like and dome shaped in the center colonies on potato-dextrose-agar (PDA). Extreme temperatures for growth were 4 (minimum) and 32 °C (maximum), with an optimum at 25 °C. Sporangia formed on V8A were persistent, non-papillate, limoniform to ellipsoid and internally proliferating. Their dimensions were 45.0 to 55.0 × 25.2 to 30.2 μm, with a mean length to breadth ratio of 1.8. Globose oogonia (diameter ranging from 27.3 to 32.0 μm), paragynous antheridia and plerotic oospores (diameter ranging from 28.2 to 35.3 μm) with a thick wall (2.5 to 3.0 μm) were also observed in single cultures on V8A.
2.2. Molecular Identification
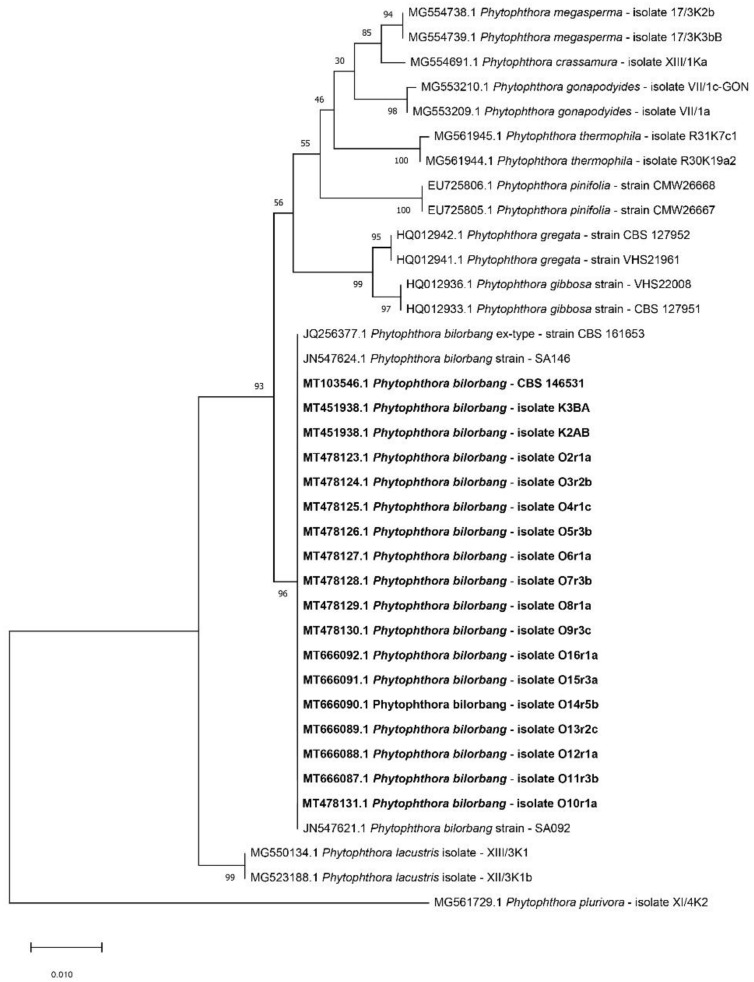
Amplification and sequencing of Internal Transcribed Spacer (ITS) regions of ribosomal DNA (rDNA) of all 18 isolates revealed 100% identity with the sequence of Phytophthora bilorbang ex-type CBS 161653 (GenBank Accession Number JQ256377 [29]). The phylogenetic analysis of the combined data set of sequences from ITS region of 10 out of 18 isolates recovered from olive at Mirto Crosia along with reference sequences of Phytophthora species within the ITS clade 6 produced a phylogenetic tree with a similar topology and high concordance with the one reported in the original description of P. bilorbang [29]. All isolates from olive clustered (bootstrap values of 1000 replicate) with the ex-type isolate of this species (Figure 2). The ITS sequences of all isolates from olive were deposited in GenBank (the respective GenBank accession numbers are given in Figure 2).
Figure 2.
Phylogenetic tree for the ITS loci obtained by the maximum likelihood method, based on the Tamura–Nei model. Relationships between the 18 Phytophthora bilorbang isolates from olive (in bold), the ex-type isolate of P. bilorbang from European raspberry and other Phytophthora species within the ITS Clade 6. P. plurivora was used as outgroup taxon. The bootstrap consensus tree inferred from 1000 replicates is taken to represent the evolutionary history of the taxa analyzed. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test are shown next to the branches. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site (below the branches).
2.3. Pathogenicity
The P. bilorbang isolate CBS146531 from olive proved to be pathogenic on olive cuttings cv. Nera di Gonnos. All 10 rooted cuttings transplanted into pots filled with infested soil developed severe symptoms of root rot, leaf chlorosis, defoliation, wilt, and final death of the whole cutting within six weeks after the transplant (Figure 1b). Mean severity of symptoms (±S.D.) in inoculated cuttings as evaluated according to Engelbrecht et al. [30] was 4.3 ± 1.16. Conversely, control cuttings grown in non-infested potting mixture showed no aerial symptoms and no necrotic fine roots. Difference between mean severity of symptoms of inoculated and control cuttings was significant for p = 0.01. Phytophthora bilorbang was re-isolated only from roots of symptomatic cuttings; thus, fulfilling Koch’s postulates. The identity of isolates obtained from necrotic roots of symptomatic, artificially inoculated cuttings, was determined by the colony morphology, microscopy observations, and ITS rDNA sequencing.
3. Discussion
Phytophthora bilorbang was described in 2012 in Western Australia as a new species in ITS clade 6 sub-clade II, and as a pathogen of European raspberry (Rubus anglocandicans) [29]. Its role as the main causative agent of “raspberry decline” syndrome in Australia was further confirmed in a later study [31]. The ITS sequences of P. bilorbang are identical to the corresponding sequences of several isolates deposited as Phytophthora taxon oaksoil and an isolate deposited as Phytophthora taxon riversoil, whose provisional names refer to their origin, i.e., soil of oak forests and riparian ecosystems, respectively [29,32,33]. The relationship between P. bilorbang, Phytophthora taxon oaksoil, and Phytophthora taxon riversoil is still debated [32]. However, as the most distinctive character separating P. bilorbang from the other two taxa is homothallism [32], the isolates obtained from olive in Calabria were confidently referred to this species. In general, Phytophthora species of clade 6 have been found in forests and riparian ecosystems and, with some exceptions such as Phytophthora asparagi, Phytophthora crassamura, P. megasperma and, Phytophthora rosacearum, showing only a limited association with agriculture [34,35,36,37,38,39,40,41,42]. Additionally, Phytophthora species of clade 6 are predominantly sterile or homothallic in culture and appear functionally adapted to survive and thrive in aquatic environments and inundated soils [35,38]. The function of most of these species within the ecosystems is not yet fully understood. It has been hypothesized they have a prevalently saprotrophic lifestyle and their common presence and even dominance in environmental water surveys have been assumed as evidence for this hypothesis [36]. However, some members of ITS Clade 6, such as Phytophthora pinifolia, P. inundata, Phytophthora taxon Pgchlamydo, and Phytophthora gonapodyides, can be opportunistic and sometimes aggressive tree pathogens [36,37,38,39,40]. Results of pathogenicity tests on olive cuttings confirm that P. bilorbang, which has a prevalently aquatic lifestyle and is frequently recovered from streams and irrigation reservoirs, can be included in this group of opportunistic aggressive pathogens. It is hypothesized that in the experimental plot of Mirto Crosia soil waterlogging and asphyxiation as a consequence of flooding events or excessive irrigation predisposed the olive trees to P. bilorbang infections. In an extensive survey of European nurseries, P. bilorbang was found in rhizosphere soil of potted plants suggesting this species, like other soil-inhabitant Phytophthora species, can be spread worldwide through the trade of nursery plants [43].
Phytophthora bilorbang, like most other soil-borne Phytophthora species, is a polyphagous pathogen whose host-range comprises, besides Rubus anglocandicans (Rosaceae), plants of different families including Alnus glutinosa (Betulaceae), Juniperus phoenicea (Cupressaceae), and Pistacia lentiscus (Anacardiaceae) [29,40,44]. In Italy, it was recovered from rhizosphere soil and plants of the Mediterranean maquis in Sardinia (southern Italy) [40,44]. However, to the best of our knowledge, this is the first report of P. bilorbang as a pathogen of olive and other plants in the Oleaceae family worldwide.
4. Materials and Methods
4.1. Isolation and Morphological Identification of Isolates
In November 2019 necrotic fine roots were sampled from three distinct symptomatic olive trees cv. Nera di Gonnos in the experimental orchard at Mirto Crosia characterized by a soil with silty loam texture (Geographic Coordinates (DATUM WGS 84) 39°61′59.0″ N, 16°76′11.4″ E, Cosenza, Calabria, southern Italy). Roots were thoroughly washed in tap water, superficially disinfected in 1% NaClO for 2 min, then immersed in 70% EtOH for 30 s, rinsed in sterile distilled water, dipped dry, and plated on selective PARPNH V8-agar [45]. After an incubation period of 24–48 h in the dark at 25° C, pure cultures were obtained by transferring outgrowing single hyphae onto V8-juice agar (V8A) [11]. Purified cultures were finally obtained by single hyphal culture on V8-agar. Colony morphology and morphological features of isolates, including the morphology and dimensions of reproductive structures, were determined on colonies grown on V8A at 20–22 °C in the dark according with standard procedures [11]. Sporangia production was stimulated following the method described by Jung et al. [46]. Small fragments (size 2 mm) were cut from the growing edge of 5 to 7-d-old cultures grown in Petri dishes (15 mm diam.) on V8A at 20 °C in the dark, they were placed in a 5 cm diameter Petri dish and flooded with non-sterile soil extract water (200 g soil suspended in 1 L of de-ionized water for 24 h at room temperature and then filtered). After incubation at 20 °C in the dark for 24–72 h, dimensions, and morphological features of 50 mature sporangia of each isolate were determined at ×400 magnification.
4.2. Molecular Identification of Isolates
Species were molecularly identified by the amplification and analysis of Internal Transcribed Spacer (ITS) of ribosomal DNA (rDNA). To this aim, total DNA was extracted from 7-d-old cultures grown on V8-agar at 20 °C by using the PowerPlant® Pro DNA isolation Kit (MO BIO Laboratories, Inc., Carlsbad, CA, USA), following the manufacturer’s instructions. The PCR amplification was performed by using the primer pairs ITS6 (5′-GAAGGTGAAGTCGTAACAAGG-3′) [47] and ITS4 (5′- TCCTCCGCTTATTGATATGC-3′) [48] in a 25 µL reaction mix containing PCR Buffer (1X), dNTP mix (0.2 mM), MgCl2 (1.5 mM), forward and reverse primers (0.5 µM each), Taq DNA Polymerase (1 U), and 100 ng of DNA. The thermocycler conditions were as follows: 94 °C for 3 min; followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; and then 72 °C for 10 min.
Amplicons were detected in 1% agarose gel and sequenced in both directions by an external service (Macrogen, Amsterdam, The Netherlands). All the sequences were analyzed by using FinchTV v.1.4.0 [49]. Species identification was performed by blast searches in GenBank [50] and in a local database containing sequences of ex-type or key isolates from published studies. Isolates were assigned to a species when the respective consensus sequence was between 99 and 100% identical to that of a reference isolate.
Validated sequences representative of Phytophthora species identified within the ITS clade 6 were phylogenetically analyzed. Sequences from ex-type or authentic culture were included in the analysis as a reference [29]. Phylogenetic analysis was conducted for the ITS sequences by the maximum likelihood method, based on the Tamura–Nei model (the software MEGA-X was used).
4.3. Pathogenicity Test
Pathogenicity of P. bilorbang, isolated from roots of O. europaea, was tested using a soil infestation method according to Jung et al. (2017) [7]. The isolate CBS 146531 (GenBank accession number MT103546), sourced from symptomatic olive trees cv. Nera di Gonnos in an experimental orchard at Mirto Crosia, was used in pathogenicity tests. Ten potted 2-month-old rooted cuttings of olive cv. Nera di Gonnos were transplanted into free-draining pots containing a mixture of autoclaved universal potting soil (©Cifo Srl, Giorgio di Piano, Bologna, Italy) and inoculum (20 cm3 of inoculum per 1000 cm3 of potting mixture). Inoculum consisted of a 21-day-old culture of the isolate CBS 146531 grown in the dark at 25 °C in a 750 mL jar containing a sterilized medium made of 50 mL of wheat seeds and 50 mL V8-juice broth. Ten control plants were transplanted in free-draining pots containing non-infested potting mixture. After transplanting, all plants were maintained in saturated soil for 48 h and then transferred to a growth chamber at 23 °C, 80% relative humidity, and a photoperiod of 16 h of light and 8 h of dark. The trial was considered concluded when inoculated plants showed severe symptoms of decay (6 weeks post inoculation). At the end of the test, P. bilorbang was re-isolated from necrotic roots using the selective PARPNH V8-agar and sequenced.
Symptoms were assessed visually in accordance with Engelbrecht et al. [30]. The wilting categories reported were (1) normal (no signs of wilting or drought stress), (2) slightly wilted (slight leaf angle changes but no folding, rolling, or changes in leaf surface structure), (3) wilted (strong leaf angle change but no cell death), (4) severely wilted (very strong change of leaf angle or protrusion of veins on the leaf surface with beginning necrosis), (5) nearly dead (most leaves necrotic, some young leaves still green near the midrib, leaf angles mostly near 0°), and (6) dead (all above-ground parts dead). Data from pathogenicity test were analyzed by a two sample t-test performed by using the software R for p = 0.01 [51].
5. Conclusions
The report of P. bilorbang on olive in southern Italy widens the range of Phytophthora species involved in PRR of this typical Mediterranean crop. PRR may be a concern in recently established olive orchards and it would be useful to understand the factors fostering its worldwide and local emergence. Probably, they include the involvement of an aggressive pathogen species as well as local environmental and agro-ecological conditions, as in the case of anthracnose, a better-known emergent disease of olive [52,53,54]. Other factors fostering the emergence of PRR of olive may include climate change effects, the host range expansion of endemic polyphagous Phytophthora species, or the global spread of Phytophthora and the introduction of exotic species through the nursery plant trade [43,55].
Acknowledgments
The Authors are grateful to Ann Davies for the English revision of the text.
Author Contributions
Conceptualization, S.O.C., F.L.P., and M.R.; methodology, A.P., E.S., F.L.S., M.R., and S.O.C.; analysis of results, F.L.S., M.R., and S.O.C.; resources, S.O.C., A.P., and E.S.; original—draft preparation, M.R., F.L.S., and S.O.C.; editing, S.O.C., M.R., and F.L.S.; and supervision, S.O.C. and E.S. All authors have read and agreed to publish this version of the manuscript.
Funding
This research was funded by “MIUR-FFABR 2017” of S.O.C., grant number 5A725192051; University of Catania, “project DIFESA” of S.O.C. and A.P., grant number 5A722192134; “MIPAAF-D.M. n. 0033437 del 21/12/2017” Project 2018–2022 “Salvaguardia e valorizzazione del patrimonio olivicolo italiano con azioni di ricerca nel settore della difesa fitosanitaria”—SALVAOLIVI”; M.R. has been granted a fellowship by CREA “OFA” (Rende), this study is part of his activity as PhD, Doctorate “Agricultural, Food, and Forestry Science”, University Mediterranea of Reggio Calabria, XXXV cycle.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bartolini G., Petrucelli R. Classification, Origin, Diffusion and History of the Olive. Food and Agriculture Organization of the United Nations; Rome, Italy: 2002. pp. 13–19. [Google Scholar]
- 2.Krishnamurthy K.V. Textbook of Biodiversity. Science Publishers; Enfield, NH, USA: 2003. pp. 39–51. [Google Scholar]
- 3.Russo C., Cappelletti G.M., Nicoletti G.M., Di Noia A.E., Michalopoulos G. Comparison of European olive production systems. Sustainability. 2016;8:825. doi: 10.3390/su8080825. [DOI] [Google Scholar]
- 4.ISTAT. [(accessed on 23 June 2020)]; Available online: http://www.istat.it.
- 5.Cacciola S.O., Faedda R., Pane A., Scarito G. Root and crown rot of olive caused by Phytophthora spp. In: Schena L., Agosteo G.E., Cacciola S.O., editors. Olive Diseases and Disorders. Transworld Research Network; Trivandrum, Kerala, India: 2011. pp. 305–327. [Google Scholar]
- 6.Riddell C.E., Frederickson-Matika D., Armstrong A.C., Elliot M., Forster J., Hedley P.E., Morris J., Thorpe P., Cooke D.E.L., Pritchard L., et al. Metabarcoding reveals a high diversity of woody host-associated Phytophthora spp. in soils at public gardens and amenity woodlands in Britain. Peer J. 2019;7:e6931. doi: 10.7717/peerj.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung T., Horta Jung M., Cacciola S.O., Cech T., Bakonyi J., Seress D., Mosca S., Schena L., Seddaiu S., Pane A., et al. Multiple new cryptic pathogenic Phytophthora species from Fagaceae forests in Austria, Italy and Portugal. IMA Fungus. 2017;8:219–244. doi: 10.5598/imafungus.2017.08.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X., Tyler B., Hong C. An expanded phylogeny for the genus Phytophthora. IMA Fungus. 2017;8:355–384. doi: 10.5598/imafungus.2017.08.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X., Hong C. Differential usefulness of nine commonly used genetic markers for identifying Phytphthora species. Front. Microbiol. 2018;9:2334. doi: 10.3389/fmicb.2018.02334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung T., Horta Jung M., Scanu B., Seress D., Kovács G.M., Maia C., Pérez-Sierra A., Chang T.-T., Chandelier A., Heungens K., et al. Six new Phytophthora species from ITS Clade 7a including two sexually functional heterothallic hybrid species detected in natural ecosystems in Taiwan. Pers. Mol. Phylogeny Evol. Fungi. 2017;38:100–135. doi: 10.3767/003158517X693615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erwin D.C., Ribeiro O.K. Phytophthora Diseases Worldwide. APS Press—The American Phytopathological Society; St. Paul, MN, USA: 1996. pp. 84–95, 96–144, 456–463. [Google Scholar]
- 12.Cacciola S.O., Agosteo G.E., Pane A. First report of Phytophthora palmivora as a pathogen of olive in Italy. Plant Dis. 2000;84:1153. doi: 10.1094/PDIS.2000.84.10.1153A. [DOI] [PubMed] [Google Scholar]
- 13.Cacciola S.O., Agosteo G.E., Magnano di San Lio G. Collar and root rot of olive trees caused by Phytophthora megasperma in Sicily. Plant Dis. 2001;85:96. doi: 10.1094/PDIS.2001.85.1.96A. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez M.E., Muñoz-Garcia M., Brasier C.M. Identity and pathogenicity of two Phytophthora taxa associated with a new root disease of olive trees. Plant Dis. 2001;85:411–416. doi: 10.1094/PDIS.2001.85.4.411. [DOI] [PubMed] [Google Scholar]
- 15.Brasier C.M., Sánchez-Hernandez E., Kirk S.A. Phytophthora inundata sp. nov., a part heterothallic pathogen of trees and shrubs in wet or flooded soils. Mycol. Res. 2003;107:477–484. doi: 10.1017/S0953756203007548. [DOI] [PubMed] [Google Scholar]
- 16.Lucero G., Vettraino A.M., Pizzuolo P., Di Stefano C., Vannini A. First report of Phytophthora palmivora on olive trees in Argentina. Plant Pathol. 2007;56:728. doi: 10.1111/j.1365-3059.2007.01588.x. [DOI] [PubMed] [Google Scholar]
- 17.Taheri M., Mirabolfathy M., Rahnama K. Phytophthora root and crown rot of several field and orchard crops in Gorgan area, Iran. J. Plant Pathol. 2012;48:85–88. [Google Scholar]
- 18.Kurbetli I., Sulu G., TaŞtekİn E., Polat I. First report of Phytophthora inundata causing olive tree decline in Turkey. Can. J. Plant Pathol. 2016;38:254–257. doi: 10.1080/07060661.2016.1157832. [DOI] [Google Scholar]
- 19.González M., Pérez-Sierra A., Serrano M.S., Sanchez M.E. Two Phytophthora species causing decline of wild olive (Olea europaea subsp. europaea var. sylvestris) Plant Pathol. 2017;66:941–948. [Google Scholar]
- 20.González M., Pérez-Sierra A., Sánchez M.E. Phytophthora oleae, a new root pathogen of wild olives. Plant Pathol. 2019;68:901–907. doi: 10.1111/ppa.13024. [DOI] [Google Scholar]
- 21.Linaldeddu B.T., Bregant C., Montecchio L., Favaron F., Sella L. First report of Phytophthora acerina, P. pini, and P. plurivora causing root rot and sudden death of olive trees in Italy. Plant Dis. 2020;104:996. doi: 10.1094/PDIS-10-19-2080-PDN. [DOI] [Google Scholar]
- 22.Lo Giudice V., Raudino F., Magnano di San Lio R., Cacciola S.O., Faedda R., Pane A. First report of a decline and wilt of young olive trees caused by simultaneous infections of Verticillium dahliae and Phytophthora palmivora in Sicily. Plant Dis. 2010;94:1372. doi: 10.1094/PDIS-07-10-0480. [DOI] [PubMed] [Google Scholar]
- 23.Riolo M., Evoli M., Schena L., Aloi F., Santilli E., Ruano-Rosa D., Agosteo G.E., Pane A., La Spada F., Cacciola S.O. Distribution of Phytophthora oleae in southern Italy. In Abstracts of Presentations at the XXV Congress of the Italian Phytopathological Society (SIPaV) J. Plant Pathol. 2019;101:811–847. [Google Scholar]
- 24.Riolo M., Schena L., Aloi F., Santilli E., Ruano-Rosa D., Agosteo G.E., Pane A., La Spada F., Cacciola S.O. Phytophthora oleae a widespread species in soil of olive (Olea europaea) orchards in Southern Italy; Proceedings of the Phytophthora in Forests and Natural Ecosystems Proceeding 9th Meeting—IUFRO—Working Party 7.02.09; La Maddalena, Sardinia, Italy. 17–26 October 2019; p. 35. [Google Scholar]
- 25.Cacciola S.O., Scarito G., Salamone A., Fodale A.S., Mulé R., Pirajno G., Sammarco G. Phytophthora species associated to root rot of olive in Sicily. In: Kalaitzaki A., editor. Proceedings of the IOBC/WPRS, Working Group “Integrated Protection of Olive Crops”; Florence, Italy. 26–28 October 2005; Dijon, France: International Organization for Biological and Integrated Control of Noxious Animals and Plants (OIBC/OILB), West Palaearctic Regional Section (WPRS/SROP); 2007. p. 251. Bull.OILB/SROP. [Google Scholar]
- 26.Nigro F., Ippolito A. Occurrence of new rots of olive drupes in Apulia. Acta Hort. 2002;586:777–780. doi: 10.17660/ActaHortic.2002.586.168. [DOI] [Google Scholar]
- 27.Vettraino A.M., Lucero G., Pizzuolo P., Franceschini S., Vannini A. First report of root rot and twigs wilting of olive trees in Argentina caused by Phytophthora nicotianae. Plant Dis. 2009;93:765. doi: 10.1094/PDIS-93-7-0765B. [DOI] [PubMed] [Google Scholar]
- 28.Ruano-Rosa D., Schena L., Agosteo G.E., Magnano di San Lio G., Cacciola S.O. Phytophthora oleae sp. nov. causing fruit rot of olive in southern Italy. Plant Pathol. 2018;67:1362–1373. doi: 10.1111/ppa.12836. [DOI] [Google Scholar]
- 29.Aghighi S., Hardy G.E.S.J., Scott J.K., Burgess T.I. Phytophthora bilorbang sp. nov., a new species associated with the decline of Rubus anglocandicans (European blackberry) in Western Australia. Eur. J. Plant Pathol. 2012;133:841–855. doi: 10.1007/s10658-012-0006-5. [DOI] [Google Scholar]
- 30.Engelbrecht B.M.J., Tyree M.T., Kursar T.A. Visual assessment of wilting as a measure of leaf water potential and seedling drought survival. J. Trop. Ecol. 2007;23:497–500. doi: 10.1017/S026646740700421X. [DOI] [Google Scholar]
- 31.Aghighi S., Burgess T.I., Scott J.K., Calver M., Hardy G.E.S.J. Isolation and pathogenicity of Phytophthora species from declining Rubus anglocandicans. Plant Pathol. 2016;65:451–461. doi: 10.1111/ppa.12436. [DOI] [Google Scholar]
- 32.Brasier C., Grunwald N., Hansen E., Reeser P., Sims L., Sutton W. What is a species?—You be the judge; Proceedings of the Phytophthora in Forests and Natural Ecosystems Proceeeding 7th Meeting—IUFRO—Working Party 7.02.09; Esquel, Argentina. 10–14 November 2014; pp. 109–111. [Google Scholar]
- 33.Kroon L.P.N.M., Brouwer H., De Cock A.W.A.M., Govers F. The genus Phytophthora anno 2012. Phytopathology. 2012;102:348–364. doi: 10.1094/PHYTO-01-11-0025. [DOI] [PubMed] [Google Scholar]
- 34.Mora-Sala B., Gramaje D., Abad-Campos P., Berbegal M. Diversity of Phytophthora species associated with Quercus ilex L. in three Spanish regions evaluated by NGS. Forests. 2019;10:979. doi: 10.3390/f10110979. [DOI] [Google Scholar]
- 35.Brasier C.M., Cooke D.E.L., Duncan J.M., Hansen E.M. Multiple new phenotypic taxa from trees and riparian ecosystems in Phytophthora gonapodyides-P. megasperma ITS Clade 6, which tend to be high-temperature tolerant and either inbreeding or sterile. Mycol. Res. 2003;107:277–290. doi: 10.1017/S095375620300738X. [DOI] [PubMed] [Google Scholar]
- 36.Jung T., Stukely M.J.C., Hardy G.E.S.J., White D., Paap T., Dunstan W.A., Burgess T.I. Multiple new Phytophthora species from ITS Clade 6 associated with natural ecosystems in Australia: Evolutionary and ecological implications. Persoonia. 2011;26:13–39. doi: 10.3767/003158511X557577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X., Copes W.E., Hong C. Phytophthora mississippiae sp. nov., a new species recovered from irrigation reservoirs at a plant nursery in Mississippi. J. Plant Pathol. Microb. 2013;4 doi: 10.4172/2157-7471.1000180. [DOI] [PubMed] [Google Scholar]
- 38.Nechwatal J., Bakonyi J., Cacciola S.O., Cooke D.E.L., Jung T., Nagy Z.Á., Vannini A., Vettraino A.M., Brasier C.M. The morphology, behaviour and molecular phylogeny of Phytophthora taxon Salixsoil and its redesignation as Phytophthora lacustris sp. nov. Plant Pathol. 2013;62:355–369. doi: 10.1111/j.1365-3059.2012.02638.x. [DOI] [Google Scholar]
- 39.Sims L.L., Sutton W., Reeser P., Hansen E.M. The Phytophthora species assemblage and diversity in riparian alder ecosystems of western Oregon, USA. Mycologia. 2015;107:889–902. doi: 10.3852/14-255. [DOI] [PubMed] [Google Scholar]
- 40.Scanu B., Linaldeddu B.T., Deidda A., Jung T. Diversity of Phytophthora species from declining Mediterranean maquis vegetation, including two new species, Phytophthora crassamura and P. ornamentata sp. nov. PLoS ONE. 2015;10:e0143234. doi: 10.1371/journal.pone.0143234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung T., La Spada F., Pane A., Aloi F., Evoli M., Horta Jung M., Scanu B., Faedda R., Rizza C., Puglisi I., et al. Diversity and distribution of Phytophthora species in protected natural areas in Sicily. Forests. 2019;10:259. doi: 10.3390/f10030259. [DOI] [Google Scholar]
- 42.Oliva J., Redondo M.A., Stenlid J. Functional ecology of forest disease. Annu. Rev. Phytopathol. 2020;58:8.1–8.19. doi: 10.1146/annurev-phyto-080417-050028. [DOI] [PubMed] [Google Scholar]
- 43.Jung T., Orlikowski L., Henricot B., Abad-Campos P., Aday A.G., Aguin Casal O., Bakonyi J., Cacciola S.O., Cech T., Chavarriaga D., et al. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2016;46:134–163. doi: 10.1111/efp.12239. [DOI] [Google Scholar]
- 44.Scanu B., Linaldeddu B.T., Peréz Sierra A., Deidda A., Franceschini A. Phytophthora ilicis as a leaf and stem pathogen of Ilex aquifolium in Mediterranean islands. Phytopathol. Mediterr. 2014;53:480–490. [Google Scholar]
- 45.Jung T., Blaschke H., Neumann P. Isolation, identification and pathogenicity of Phytophthora species from declining oak stands. Eur. J. For. Pathol. 1996;26:253–272. doi: 10.1111/j.1439-0329.1996.tb00846.x. [DOI] [Google Scholar]
- 46.Jung T., Cooke D.E.L., Blaschke H., Duncan J.M., Oßwald W. Phytophthora quercina sp. nov., causing root rot of European oaks. Mycol. Res. 1999;103:785–798. doi: 10.1017/S0953756298007734. [DOI] [Google Scholar]
- 47.Cooke D.E.L., Drenth A., Duncan J.M., Wagels G., Brasier C.M. A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet. Biol. 2000;30:17–32. doi: 10.1006/fgbi.2000.1202. [DOI] [PubMed] [Google Scholar]
- 48.White T.J., Bruns T., Lee S., Taylor J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Volume 18. Academic Press, Inc.; San Diego, CA, USA: 1990. pp. 315–322. [Google Scholar]
- 49.FinchTV v.1.4.0. [(accessed on 18 May 2020)]; Available online: https://digitalworldbiology.com/FinchTV.
- 50.BLAST Searches. [(accessed on 18 May 2020)]; Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi.
- 51.R: The R Project for Statistical Computing. [(accessed on 18 May 2020)]; Available online: https://www.r-project.org/
- 52.Cacciola S.A., Faedda R., Sinatra F., Agosteo G.E., Schena L., Frisullo S., Magnano di San Lio G. Olive anthracnose. J. Plant Pathol. 2012;94:29–44. [Google Scholar]
- 53.Talhinhas P., Loureiro A., Oliveira H. Olive anthracnose: A yield- and oil quality-degrading disease caused by several species of Colletotrichum that differ in virulence, host preference and geographical distribution. Mol. Plant Pathol. 2018;19:1797–1807. doi: 10.1111/mpp.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Materatski P., Varanda C., Carvalho T., Dias A.B., Campos M.D., Gomes L., Nobre T., Rei F., Felix M. Effect of long-term fungicide applications on virulence and diversity of Colletotrichum spp. associated to olive anthracnose. Plants. 2019;8:311. doi: 10.3390/plants8090311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cacciola S.O., Gullino M.L. Emerging and re-emerging fungus and oomycete soil-borne plant diseases in Italy. Phytopathol. Mediterr. 2019;58:451–472. [Google Scholar]