Abstract
The demand for recombinant proteins in terms of quality, quantity, and diversity is increasing steadily, which is attracting global attention for the development of new recombinant protein production technologies and the engineering of conventional established expression systems based on bacteria or mammalian cell cultures. Since the advancements of plant genetic engineering in the 1980s, plants have been used for the production of economically valuable, biologically active non-native proteins or biopharmaceuticals, the concept termed as plant molecular farming (PMF). PMF is considered as a cost-effective technology that has grown and advanced tremendously over the past two decades. The development and improvement of the transient expression system has significantly reduced the protein production timeline and greatly improved the protein yield in plants. The major factors that drive the plant-based platform towards potential competitors for the conventional expression system are cost-effectiveness, scalability, flexibility, versatility, and robustness of the system. Many biopharmaceuticals including recombinant vaccine antigens, monoclonal antibodies, and other commercially viable proteins are produced in plants, some of which are in the pre-clinical and clinical pipeline. In this review, we consider the importance of a plant- based production system for recombinant protein production, and its potential to produce biopharmaceuticals is discussed.
Keywords: biopharmaceuticals, molecular farming, Nicotiana, plant production system, plant-derived protein, recombinant protein, transient expression
1. Introduction
Recombinant proteins are complex exogenous (“foreign”) proteins that are produced in expression hosts, and mainly used as medical diagnostic reagents and in human healthcare as vaccines, drugs, or monoclonal antibodies [1]. The prominent role and increasing market demand for high-value recombinant proteins in novel drug discovery creates an opportunity for the development of various protein expression hosts to manufacture proteins by following the existing rigid standards laid down for veterinary and human applications. The industry is focusing mainly on already established production platforms using prokaryotic and eukaryotic expression host systems such as Escherichia coli, a selection of yeast, insect, and mammalian cell cultures, due to their well-defined processes in-line with current good manufacturing practice (cGMP) [2]. Moreover, industrially established mammalian and other cell cultures have stringent regulatory approval in place, which hinders the industrial acceptance of the new technology or production system. Bacterial expression systems offer rapid production with high product yield, whereas Saccharomyces cerevisiae and Pichia pastoris (yeast) offer post-translational modifications (PTMs) which are essential for functional activity of the recombinant proteins [3]. The majority of the approved recombinant biopharmaceuticals are produced in mammalian cell lines [4]. However, all the production systems have their own merits and setbacks such as production time, high operating costs, protein yield, chances of contamination with pathogenic microorganisms, limited post-translational modifications, and regulatory approval. In order to compete with the established platform, the new expression system must have unique advantages that can overcome the limitations of the existing ones. Plants offer several potential benefits over conventional expression platforms and prove the reliability of the system for the production of highly valuable proteins. Advancements in plant molecular farming approaches in the recent decade have made plants an attractive manufacturing system that can even achieve commercially relevant production levels in a short period [1,5,6]. As the progress is continuously being made in this ever-growing field, here in this review, we summarize the importance and prospects of plant expression systems for the cost-effective production of recombinant proteins. Potential vaccine candidates, monoclonal antibodies, and industrial enzymes expressed in plants are also described.
2. Plant Expression Platform
Plants were utilized for the expression of recombinant proteins from the late 1980s [7]. Since then, the plant expression platform has faced several hurdles, until recently the first plant-based product “Elelyso” was commercialized by Protalix Biotherapeutics for the treatment of Gaucher’s disease in 2012 [8]. The practice of using plants for high-value recombinant protein production ranging from pharmaceutical therapeutics to non-pharmaceutical products such as antibodies, vaccine antigens, enzymes, growth factors, research or diagnostic reagents, and cosmetic ingredients [9] has improved over time and advanced significantly in recent decades, which in turn has led to a major paradigm shift in the pharmaceutical sector. The technology has rapidly developed, and the potential drawbacks associated with plant molecular farming during their early stages of development, including the need for a high protein expression level and efficient downstream processing, have now been achieved. The advantages of plant expression platforms are cited in several earlier reports showing head-to-head comparisons with other existing platforms (Table 1) [10,11,12,13,14,15,16,17,18]. The key advantages of all plant-based systems are easy cultivation, low expenses, low or no pathogen load, rapid mass production of recombinant proteins, and the ability of the plants to assemble complex proteins with eukaryotic-like post-translational modifications (PTMs) [19].
Table 1.
Available expression platforms for recombinant protein production with potential advantages and disadvantages (adapted from Shanmugaraj et al., 2020) [25].
| Expression System | Advantages | Disadvantages |
|---|---|---|
| Bacteria | Easy to manipulate Low cost High expression Ease of scale up Short turnaround time Established regulatory procedures and approval |
Improper folding Lack of post-translational modifications which may affect the protein function Endotoxin accumulation |
| Mammalian Cells | Proper folding and authentic post-translational modifications Existing regulatory approval |
High production cost Expensive media and culture condition requirements |
| Yeast | Rapid growth and scalable Easy to manipulate Simple and inexpensive media requirements and culture conditions Post-translational modifications of recombinant proteins |
Difficulty in cell disruption due to the thick and hard cell walls Hyperglycosylation of proteins Limited glycosylation capacity |
| Insect cells | High expression levels Ability to produce complex proteins including secreted, membrane, and intracellular proteins Proper folding and post-translational modifications |
High cost and time consuming Expensive media and culture condition requirements |
| Plant | Rapid and affordable Optimized growth conditions Free from pathogen and bacterial toxin contaminants Economical Post-translational modification somewhat similar like mammalian system |
Regulatory compliance Limited glycosylation capacity |
Protein folding is highly essential to retain the biological activity of the recombinant therapeutic proteins. Due to the lack of the protein processing complex and limited capacity for PTMs, proper protein folding cannot be achieved in the prokaryotic expression system [20]. Plants have the capacity to assemble and perform PTMs of large multimeric proteins required for their functional biological activity. However, plants lack the authentic human N-glycosylation processing mechanism which has been overcome by the glycoengineering approaches towards the synthesis of targeted human and non-human structures to increase product homogeneity, quality, and quantity [21,22].
The Nicotiana genus is often used for genetic transformation studies due to its growth rate and easy genetic manipulation. Most of the recombinant proteins such as pharmaceuticals, vaccines, hormones, cytokines, growth regulators, and industrial products are produced in tobacco, which is considered as a molecular biology workhouse of the plant world. Nicotiana benthamiana and N. tabacum are two common species used for the stable and transient expression of recombinant proteins. Further, several cereal crops, fruits, and vegetables such as rice, maize, lettuce, tomato, potato, and alfalfa were also evaluated for their applicability in plant molecular farming (PMF) depending on the protein target and application [1]. Many plant-produced therapeutic proteins are in pre-clinical and clinical trials and are close to commercialization [23,24]. The strength and bottlenecks of the commercial potential of the plant expression system was critically reviewed and summarized by Schillberg et al. (2019) [2].
3. Plant-Derived Recombinant Proteins
The concept of using plants for the production of foreign proteins including pharmaceutical and non-pharmaceutical proteins has been well explored and documented. Many reports proved the ability of in vivo and in vitro plant systems to produce vaccine candidates both for veterinary and human applications and showed that plant-produced antigens elicit potential immune responses in animal models and even confer protection in animal challenge experiments. Examples of the variety of pharmaceutical and non-pharmaceutical proteins expressed in plant systems are illustrated in Table 2 and Table 3.
Table 2.
Selected list of vaccine candidates and antibodies expressed in plants against various diseases.
| Vaccine Candidates | |||||
|---|---|---|---|---|---|
| Recombinant Protein | Pathogen/Disease | Expression System | Transformation Method | Expression Level | Reference |
| Hepatitis B surface antigen | Hepatitis B virus | Tobacco (Nicotiana tabacum) |
Agrobacterium mediated (Stable expression/Nucleus) |
66 ng/mg of soluble protein | [38] |
| Structural protein VP60 | Rabbit hemorrhagic disease virus (RHDV) | Potato (Solanum tuberosum) |
Agrobacterium mediated (Stable expression/Nucleus) |
0.3% of total soluble protein | [39] |
| Spike (S) protein of transmissible gastroenteritis virus | Transmissible gastroenteritis virus (TGEV) | Tobacco (Nicotiana tabacum) |
Agrobacterium mediated (Stable expression/Nucleus) |
0.1–0.2% of total soluble protein | [40] |
| Hemagglutinin protein of rinderpest virus | Rinderpest virus (RPV) | Peanut (Arachis hypogea L.) |
Agrobacterium mediated (Stable expression/Nucleus) |
0.2–1.3% of total soluble protein | [41] |
| Glycoprotein D (gD) of bovine herpes virus | Bovine herpes virus | Tobacco (Nicotiana benthamiana) | Mechanical inoculation (Stable expression/Nucleus) | 20 μg/g fresh weight (FW) | [42] |
| L1 major capsid protein | Human papillomavirus | Tobacco (Nicotiana tabacum) |
Agrobacterium mediated (Stable expression/Nucleus) |
2–4 µg/kg FW | [43] |
| Spike (S) protein of transmissible gastroenteritis virus | Transmissible gastroenteritis virus (TGEV) | Corn (Zea mays) |
Agrobacterium mediated (Stable expression/Nucleus) |
13 mg/kg FW | [44] |
| Spike (S) protein of infectious bronchitis virus | Infectious bronchitis virus (IBV) | Potato (Solanum tuberosum) |
Agrobacterium mediated (Stable expression/Nucleus) |
2.39–2.53 µg/g FW | [26] |
| Anthrax protective antigen (PA) | Anthrax | Tobacco (Nicotiana tabacum) | Biolistic method (Stable expression/Chloroplast) | 14.2% of total soluble protein | [45] |
| Hepatitis B virus surface antigen | Hepatitis B virus (HBV) | Potato (Solanum tuberosum) |
Agrobacterium mediated (Stable expression/Nucleus) |
8.5 μg/g FW | [46] |
| Fusion (F) protein of Newcastle disease virus | Newcastle disease virus (NDV) | Corn (Zea mays L.) | Biolistic method (Stable expression/Chloroplast) | 3.0% of total soluble protein | [47] |
| F4 fimbrial adhesin FaeG | Enterotoxigenic E. coli | Alfalfa (Medicago sativa L.) |
Agrobacterium mediated (Stable expression/Chloroplast) |
1.0% of total soluble protein | [48] |
| L1 capsid protein gene | Cottontail rabbit papillomavirus | Tobacco (Nicotiana tabacum) |
Agrobacterium mediated (Stable expression/Nucleus) |
0.4–1 mg/kg of total leaf mass | [49] |
| Structural protein VP2 | Infectious bursal disease virus (IBDV) | Rice |
Agrobacterium mediated (Stable expression/Nucleus) |
40.21 µg/g FW | [50] |
| Hepatitis B virus surface antigen | Hepatitis B virus (HBV) | Tobacco (Nicotiana benthamiana) |
Agrobacterium mediated (Transient expression) |
295 µg/g FW | [51] |
| Haemagglutinin (HA) | H5N1 (avian influenza virus) & H1N1 (human) influenza strains | Tobacco (Nicotiana benthamiana) |
Agrobacterium mediated (Transient expression) |
50 mg/kg FW | [52] |
| Heat-labile toxin B subunit (LTB) | Enterotoxigenic E. coli | Carrot (Daucus carota) |
Agrobacterium mediated (Stable expression/Nucleus) |
0.3% of total soluble protein | [53] |
| Norwalk virus capsid protein | Norwalk virus | Tobacco (Nicotiana benthamiana) |
Agrobacterium mediated (Transient expression) |
0.8 mg/g FW | [54] |
| Structural protein VP1 | Foot-and-mouth disease virus (FMDV) | Legume (Stylosanthes guianensis) |
Agrobacterium mediated (Stable expression/Nucleus) |
0.1–0.5% of total soluble protein | [55] |
| HIV-1 Pr55gag Polyprotein | Human immunodeficiency virus type 1 (HIV) | Tobacco (Nicotiana tabacum) | Biolistic method (Stable expression/Chloroplast) | 312–363 mg/kg FW | [56] |
| Japanese encephalitis virus (JEV) envelope protein (E) | Japanese encephalitis virus | Japonica rice (Nipponbare) |
Agrobacterium mediated (Stable expression/Nucleus) |
1.1–1.9 μg/mg of total soluble protein | [57] |
| Hemagglutinin (HA) | Avian influenza (H5N1) | Tobacco (Nicotiana benthamiana) |
Agrobacterium mediated (Transient expression) |
0.3 g/kg FW | [58] |
| Haemagglutinin (HA) | Influenza virus | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 400–1300 mg/kg FW | [59] |
| Haemagglutinin (HA) | Avian influenza A (H7N7) | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 0.2 g/kg FW | [60] |
| Structural protein VP2 | Infectious bursal disease virus (IBDV) | Tobacco (Nicotiana benthamiana) |
Agrobacterium mediated (Transient expression) |
1.0% of total soluble protein | [61] |
| Structural protein E2 | Bovine viral diarrhea virus (BVDV) | Alfalfa (Medicago sativa L.) |
Agrobacterium mediated (Stable expression/Nucleus) |
1 μg/g FW | [62] |
| Bluetongue virus-like particles | Bluetongue virus | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 70 mg/kg FW | [63] |
| Narita 104 virus virus-like particles | Narita 104 virus | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 0.3 mg/g FW | [64] |
| Glycoprotein (GP) of PRRSV | Porcine reproductive and respiratory syndrome virus (PRRSV) | Arabidopsis thaliana |
Agrobacterium mediated (Stable expression/Nucleus) |
2.74% of total soluble protein | [65] |
| Matrix protein 2 ectodomain (M2e) | Avian influenza (H5N1) | Duckweed (Lemna minor) |
Agrobacterium mediated (Stable expression/Nucleus) |
90–970 mg/kg FW | [66] |
| Matrix protein 2 ectodomain (M2e) | Avian influenza (H5N1) | Tobacco (Nicotiana benthamiana) |
Agrobacterium mediated (Transient expression) |
125–205 mg/kg FW | [67] |
| Consensus domain III of dengue virus E glycoprotein (cEDIII) | Dengue virus | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 5.2 mg/g FW | [68] |
| Dengue envelop protein domain III (EDIII) | Dengue virus | Tobacco (Nicotiana tabacum) | Biolistic method (Stable expression/Chloroplast) | 0.8–1.6% of total soluble protein | [69] |
| PV3 VLPs | Poliovirus (PV) | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 60 mg/kg FW | [28] |
| E. maxima gametocyte antigen (Gam82) | Eimeria maxima | Tobacco (Nicotiana tabacum) | Agrobacterium mediated (Transient expression) | 20 mg/kg FW | [70] |
| CHIKV E1 and E2 | Chikungunya virus | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 8–13 mg/kg of fresh leaf weight | [71] |
| ZIKV envelope (E) protein | Zika virus (ZIKV) | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 160 μg/g FW | [72] |
| Porcine circovirus type 2 (PCV-2) capsid protein | Porcine circovirus type 2 | Tobacco (Nicotiana benthamiana) |
Agrobacterium mediated (Transient expression) |
6.5 mg/kg leaf wet weight | [73] |
| HIV Env gp140 | Human immunodeficiency virus (HIV) | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 5–6 mg/kg FW | [74] |
| H6 subtype haemagglutinin (HA) | Influenza A virus (H6N2) | Tobacco (Nicotiana benthamiana) |
Agrobacterium mediated (Transient expression) |
95 mg/kg FW | [31] |
| Antibodies | |||||
| cT84.66 | Cancer (tumor marker) | Tobacco (Nicotiana tabacum) | Agrobacterium mediated (Transient expression) | 1 mg/kg FW | [75] |
| scFvT84.66 | Cancer (tumor marker) | Tobacco (Nicotiana tabacum) | Agrobacterium mediated (Transient expression) | 5 mg/kg FW | [75] |
| scFvT84.66 | Cancer (tumor marker) | Rice (Oryza sativa) | Biolistic method (Stable expression/Nucleus) | 3.8 μg/g FW | [76] |
| scFvT84.66 | Cancer (tumor marker) | Cereal crops (wheat and rice) | Biolistic method (Stable expression/Nucleus) | 30 μg/g FW | [77] |
| BR55-2 | Human colorectal cancer | Tobacco (Nicotiana tabacum) | Agrobacterium mediated (Stable expression/Nucleus) | 30 mg/kg FW | [78] |
| 2F5 | HIV | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Stable expression/Nucleus) | 0.01% of total soluble protein | [79] |
| 2G12 | HIV | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 0.3 g/kg FW | [80] |
| 2G12 | HIV | Tobacco (Nicotiana tabacum) | Agrobacterium mediated (Stable expression/Nucleus) | 8 mg/L culture medium | [81] |
| 6D8 | Ebola virus | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 0.5 mg/g FW | [82] |
| 6D8 | Ebola virus | Lettuce (L. sativa) |
Agrobacterium mediated (Transient expression) | 0.23–0.27 mg/g FW | [83] |
| CO17-1AK | Human colorectal cancer | Tobacco (Nicotiana tabacum) | Agrobacterium mediated (Stable expression/Nucleus) | 0.25 mg/kg FW | [84] |
| Palivizumab-N | Respiratory syncytial virus | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 180 mg/kg FW | [85] |
| E559 | Rabies | Tobacco (Nicotiana tabacum) | Agrobacterium mediated (Stable expression/Nucleus) | 1.8 mg/kg FW (0.04% of total soluble protein) | [86] |
| pE16 | West Nile virus | Tobacco (Nicotiana benthamiana ∆XF) | Agrobacterium mediated (Transient expression) | 0.74 mg/g FW | [87] |
| pE16scFv-CH | West Nile virus | Tobacco (Nicotiana benthamiana ∆XF) | Agrobacterium mediated (Transient expression) | 0.77 mg/g FW | [87] |
| E60 | Dengue virus | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 120 μg/g FW | [88] |
| 2G12 | HIV | Rice (Oryza sativa) | Biolistic method (Stable expression/Nucleus) | 46.4 μg/g dry seed weight | [89] |
| 8B10 and 5F10 | Chikungunya virus | Tobacco (Nicotiana benthamiana) |
Agrobacterium mediated (Transient expression) |
20–30 mg/kg FW | [71] |
| SO57 | Rabies virus | Tobacco (Nicotiana tabacum) | Agrobacterium mediated (Transient expression) | 0.014–0.019% of total soluble protein | [90] |
| cD5 | Enterovirus 71 | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 50 μg/g FW | [91] |
| PD1 | Cancer | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 140 μg/g FW | [92] |
| c2A10G6 | Zika virus | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 1.47 mg/g FW | [93] |
| 6D8 | Ebola | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 1.21 mg/g FW | [93] |
| HSV8 | Herpes simplex virus | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 1.42 mg/g FW | [93] |
| CHKV mab | Chikungunya virus | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Transient expression) | 100 μg/g FW | [94] |
Table 3.
Selected list of various non-pharmaceutical proteins produced in plants.
| Recombinant Proteins | Expression System | Transformation Method | Expression Level | Reference |
|---|---|---|---|---|
| Human serum albumin | Potato (Solanum tuberosum) | Agrobacterium mediated (Stable expression/Nucleus) | 0.25 μg/mg (0.02% of total soluble protein) | [95] |
| Erythropoietin | Tobacco (Nicotiana tabacum) | Agrobacterium mediated (Stable expression/Nucleus) | 26 pg/mg total protein | [96] |
| α1-antitrypsin | Rice (Japonica rice) | Biolistic method (Stable expression/Nucleus) | 4.6–5.7 mg/g dry cell | [97] |
| Aprotinin | Corn | Biolistic method (Stable expression/Nucleus) | 0.069% of total extractable seed protein | [98] |
| Human-secreted alkaline phosphatase | Tobacco (Nicotiana tabacum) | Agrobacterium mediated (Stable expression/Nucleus) | 1.1 μg/g FW (3% of total soluble protein) | [99] |
| Collagen | Tobacco (Nicotiana tabacum) |
Agrobacterium mediated (Stable expression/Nucleus) | 0.03 g/kg powdered plants | [100] |
| Human somatotropin (hST) | Tobacco | Biolistic method (Stable expression/Chloroplast) |
>7% of total soluble protein | [101] |
| Bacillus thuringiensis (Bt) cry2Aa2 | Tobacco | Biolistic method (Stable expression/Chloroplast) |
5 mg/g FW (45.3–46.1% of total soluble protein) | [102] |
| Human serum albumin | Tobacco (Nicotiana tabacum) | Biolistic method (Stable expression/Chloroplast) | 11.1% of total protein | [103] |
| Human epidermal growth factor (hEGF) | Tobacco (Nicotiana tabacum) | Agrobacterium mediated (Stable expression/Nucleus) | 34.2 µg/g FW | [104] |
| Human basic fibroblast growth factor (bFGF) | Soybean (Glycine max) | Cotyledonary node explant method (Stable expression/Nucleus) | 2.3% of total soluble protein | [105] |
| Type I interferon (IFNα2b) | Tobacco (Nicotiana tabacum) | Biolistic method (Stable expression/Chloroplast) | 3 mg/g FW (20% of total soluble protein) | [106] |
| Human growth hormone (hGH) | Rice (Oryza sativa) | Biolistic method (Stable expression/Nucleus) | 57 mg/L culture medium | [107] |
| PlyGBS lysin | Tobacco (Nicotiana tabacum) |
Biolistic method (Stable expression/Chloroplast) | >70% of the total soluble protein | [108] |
| Human growth hormone (hGH) | Tobacco BY-2 cells | Agrobacterium mediated (Stable expression/Nucleus) | 35 mg/L culture medium (2-4% of total soluble protein) | [109] |
| Human basic fibroblast growth factor (bFGF) | Rice (Oryza sativa) | Agrobacterium mediated (Stable expression/Nucleus) | 185.66 mg/kg | [110] |
| Lumbrokinase | Sunflower (Helianthus annuus L.) | Agrobacterium mediated (Stable expression/Nucleus) | 5.1 g/kg seeds | [111] |
| Human acidic fibroblast growth factor 1 (FGF-1) | Salvia miltiorrhiza | Agrobacterium mediated (Stable expression/Nucleus) | 272 ng/g FW | [112] |
| Glucocerebrosidase (GCase) | Tobacco (Nicotiana benthamiana) | Agrobacterium mediated (Stable expression/Nucleus) | 68 μg/g FW (1.45% of total soluble protein) | [113] |
| Human acid alpha glucosidase | Tobacco (Nicotiana tabacum) |
Biolistic method (Stable expression/Chloroplast) | 6.38 μg/g FW | [114] |
| Human basic fibroblast growth factor (bFGF) | Tobacco (Nicotiana tabacum) |
Biolistic method (Stable expression/Chloroplast) | 0.1% of total soluble protein | [115] |
| Endo-β-1,4-xylanase | Tobacco (Nicotiana tabacum) |
Biolistic method (Stable expression/Chloroplast) | 35.7% of total soluble protein | [116] |
| β-Glucosidase | Tobacco (Nicotiana tabacum) |
Biolistic method (Stable expression/Chloroplast) | >75% of total soluble protein | [116] |
| Osteopontin (OPN) | Tobacco (Nicotiana benthamiana) |
Agrobacterium mediated (Transient expression) |
100 ng/g FW | [117] |
| Dentin matrix protein-1 (DMP1) | Tobacco (Nicotiana benthamiana) |
Agrobacterium mediated (Transient expression) |
0.3 µg/g FW | [118] |
Tobacco has been engineered to express a variety of antigens in the nucleus and chloroplast including, but not limited to, chikungunya, dengue, Ebola, influenza, and Zika. The transformation protocols for recombinant protein production are also established for fruits and vegetables such as tomatoes and potatoes. Transgenic potatoes expressing the S1 glycoprotein of the infectious bronchitis virus confers protection to chickens upon virus challenge [26]. Leafy crops such as lettuce, alfalfa, and clover have been investigated for molecular farming to obtain the oral delivery of vaccine antigens eliminating purification and injections. The lettuce chloroplast-derived booster vaccine using lyophilized plant cells expressing the poliovirus capsid protein induced neutralization antibodies in mice primed with inactivated poliovirus vaccine (IPV) and conferred protection against all polio serotypes [27]. Plant systems have also been evaluated for the expression of virus-like particles (VLP) of many viruses including norovirus, poliovirus, foot-and-mouth disease virus, influenza, [28,29,30,31], and the potential for plant-derived VLPs to be used as candidate vaccines and reagents has been reviewed in detail elsewhere [18,32,33]. Apart from expressing antigens for human diseases, several antigens for veterinary applications and non-pharmaceutical proteins have also been well tested for expression in plants, and are particularly gaining attention due to the fact that these products can quickly reach the market due to lower regulatory burden [9]. This was clearly evidenced by the commercialization of avidin [34], β-glucuronidase [35], and trypsin [36] by the US-based biotechnology company ProdiGene, Inc. The vaccine against Newcastle disease virus (NDV) was the first plant-based poultry vaccine (Dow Agrosciences) that obtained regulatory approval from the United States Department of Agriculture in 2006, opening a new avenue for the commercialization of plant-derived vaccines. Currently, many plant-derived non-pharmaceutical and pharmaceutical proteins are in clinical development.
Although proof-of-concept and efficacy of many vaccine candidates proved the feasibility and scalability of the robust plant system, it is high time to compete with the established expression systems. Now the plant-based good manufacturing practices (GMP) complaint production facilities such as Fraunhofer (Germany), Kentucky BioProcessing (USA), Medicago (Canada), and Protalix Biotherapeutics (Israel) are available to manufacture GMP materials for human clinical trials. Fraunhofer IME received a GMP license for the production of neutralizing anti-HIV antibody 2G12 in tobacco for phase I clinical testing [37]. The plant molecular farming research community continuously thrives to set up a regulatory framework for plant-derived products.
4. Strategies Used for Recombinant Protein Production in Plants
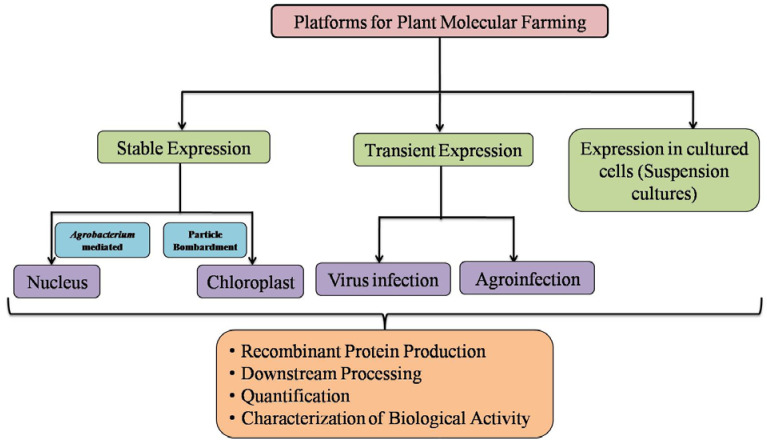
The expression methods used for the recombinant protein production in plants can be either stable or transient expression. PMF relies on following approaches for the expression of vaccine candidates, i.e., stable nuclear transformation, stable chloroplast transformation, or transient expression, by using plant viral vectors and stable transformation of hydroponically grown plants in which recombinant proteins are recovered from the medium [119] (Figure 1).
Figure 1.
Overview of plant transformation approaches employed for the production of recombinant pharmaceutical and non-pharmaceutical proteins in plants.
Stable nuclear transformation is the traditional strategy of genetic manipulation in plants for recombinant protein production. The transgene in the plant expression vector can be introduced into the in vitro grown plantlets either with Agrobacterium tumefaciens-mediated transformation or particle bombardment, and stable transgenic lines can be developed. The best transgenic line for protein production will be subsequently screened from the pool of transgenic lines. By this method, recombinant proteins can be produced in successive generations, as the transgene has been stably integrated into the plant genome. The model plants such as Arabidopsis thaliana and tobacco were more commonly used during the early stages of genetic transformation to develop stable transformants [79]. Stable transformation in plants requires substantial time and is a labor-intensive process, and the protein expression is insufficient to meet the industrial-level protein production. However, the antigen expression in stable transgenic line could be used for developing oral vaccines that could reduce the cost associated with protein purification [120,121].
Alternatively, transient expression based on agroinfiltration or virus-based vectors have been developed to complement transgenic plants that offer rapid and high-level protein expression within a few days. The drawbacks and challenges associated with stable expression, such as insufficient protein expression, time, and consistency, have been overcome by the development of novel strategies involving deconstructed viral vector systems such as MagnICON® technology, geminiviral, and pEAQ, which allows rapid accumulation of recombinant proteins in a short time [12]. Hence, it is considered as a suitable convenient platform, especially for the production of vaccine antigens or monoclonal antibodies against infectious diseases (Figure 2). Gleba et al. (2007) summarized the application of plant viral vectors for the transient expression of heterologous proteins in plants [122]. Plant transient expression holds tremendous potential to produce rapid-response proteins, emergency vaccines, or biologics, which was impressively shown during the Ebola outbreak in 2014. Mapp Biopharmaceutical Inc., USA produced an experimental drug ZMapp, an anti-Ebola antibody cocktail of three chimeric monoclonal antibodies manufactured in tobacco plants (Nicotiana benthamiana) to treat humans during the recent Ebola outbreak [123]. During a pandemic situation, in order to cope with a rapidly spreading infectious disease, production methods should meet the demand for production targets of strategic vaccines to control the disease. One of the recent examples is the pandemic, corona virus disease (COVID-19). The virus has spread rapidly, and millions of people have been affected across 6 continents in few months, posing a constant threat to global health. This infection has created a massive demand for diagnostic reagents, vaccines, and therapeutic development. Given the speed advantages, and proven viability of the plant production platform, the transient expression system in particular could be employed to produce recombinant proteins at high levels to meet the sudden demand for production of viral antigens or antiviral proteins that could be used as research reagents, emergency vaccines (SARS-CoV-2 subunit and virus-like particle vaccines), or other biopharmaceuticals to fight against COVID-19 [25,124]. The neutralizing monoclonal antibodies against SARS-CoV-2 could also be produced in plants with minimal investment, which could be used for passive immunotherapy [125]. Recently, Medicago (Quebec, Canada), Kentucky BioProcessing (Owensboro, KT, USA), and iBio (Bryan, TX, USA) joined the global race for developing potential plant-based vaccines for COVID-19 [126]. By using the transient expression platform, recombinant protein production in plants could be scaled up rapidly, and milligram quantities of proteins could be produced in a timeframe of less than 4 weeks after receiving the corresponding gene construct [5,59,127].
Figure 2.
Schematic representation depicting the application of transient expression system for the production of various recombinant proteins.
Alternately, chloroplast expression focuses on expressing the transgenes in chloroplast by the precise insertion of foreign DNA by homologous recombination into the chloroplast genome. Much progress has been made in chloroplast engineering in recent years. The transformation of the chloroplast genome has many advantages over nuclear transformation which includes higher protein production, lack of gene silencing and position effect, polycistronic mRNA expression, and prevention of transmission of foreign DNA through pollen by uniparental plastid gene inheritance (maternal inheritance) in crop plants [128,129,130,131].
Similar to bacterial and mammalian cells, heterologous protein production can be achieved by using individual suspension of plant cells rather than whole plants. The cell suspension derived from undifferentiated callus grown in liquid medium can be scaled up in bioreactors for large-scale protein production under an aseptic environment. The first USDA-approved poultry vaccine and the first FDA-approved recombinant plant-produced pharmaceutical protein “Elelyso” were produced in tobacco and carrot cell suspension cultures, respectively, which proved the importance and competitiveness of plant suspension culture in high-value protein production in the biopharmaceutical industry [121,132,133,134]. Hairy root cultures are also being explored as an alternative recombinant protein production system due to their ease in protein recovery and low costs. The recombinant proteins are secreted from the transgenic plant roots into the culture medium viz., rhizosecretion; hence, this allows continuous protein production and recovery from the culture medium without the requirement of cell lysis during extraction. Moreover, recombinant proteins produced from root cultures attribute to the improved protein quality and quantity without complex downstream processing that could eventually reduce production costs as well [135]. A recent review on the applications of hairy root cultures for protein production has been extensively discussed by Gutierrez–Valdes et al. (2020) [136].
5. Perspectives
Although plants are attractive with several unique advantages, they are unable to compete with the existing microbial and mammalian systems, as both are well established and characterized, especially in terms of GMP manufacturing and regulatory approval in an industrial setting. Even after many years of research, which has shown the proof-of-concept of expressing many therapeutic proteins in plants, the process of producing therapeutic proteins from the lab bench to commercialization is slow. Hence, in order to move forward, the commercial potential and economic sustainability of technology needs to be exploited by developing veterinary vaccines, non-pharmaceutical diagnostic, cosmetic products, and industrial enzymes in plants, as they have a low regulatory burden compared to therapeutic proteins [2,9]. This technology can also be employed to reproduce rapid response vaccines or diagnostic reagents against emerging infections. For the past few years, extensive research has been carried out to combat the several emerging diseases including Zika, chikungunya, Nipah, SARS-CoV, MERS-CoV, and more recently SARS-CoV-2. Even though several efforts have been made for many years to develop effective vaccine candidates for many of those emerging and zoonotic diseases, still, there are no vaccine candidates or therapeutic measures available commercially. Even if a successful vaccine or drug developed against such diseases, it is unlikely that it would have a significant impact on developing and under-developed countries, due to the high cost associated with it, and scalability concern. In such a scenario, a plant-derived vaccine or diagnostic reagent would be a feasible approach to rapidly respond to the demand and need for recombinant proteins. However, harnessing the full potential of this plant molecular farming technology for cost-effective vaccines or drug development will be evident in the upcoming years.
6. Conclusions
Plants have both economic and technical advantages over conventional expression systems for the production of pharmaceutical and non-pharmaceutical products. The different PMF technologies such as nuclear, chloroplast expression, viral transfection, and transient expression systems have their unique features, enabling them to address a production of diversified product “targets” with less production constraints in a short time. Many scientific and technical challenges associated with the plant platform were met in recent years. However, the regulatory burden associated with therapeutic protein production is a major barrier that hinders the widespread acceptance of the plant system. Considering the low costs and greater scalability of plant production systems, the commercialization of non-pharmaceutical proteins is straightforward and faster due to lower regulatory challenges. Hence, the universal acceptance of the technology will be strongly influenced by the regulatory framework and restrictions applied to plant-derived products worldwide. The demand for industrially or pharmaceutically useful recombinant proteins, together with demonstrated production capability and economic feasibility of the plant system, suggests a bright future for the plant-made biologics.
Acknowledgments
The authors would like to acknowledge Scholarship Program for Asean Countries (C.J.I.B.) and Second Century Fund-C2F (B.S.), Chulalongkorn University for financial support.
Abbreviations
| cGMP | Current good manufacturing practice |
| COVID-19 | Coronavirus disease |
| FW | Fresh weight |
| mAb | Monoclonal antibody |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| PMF | Plant molecular farming |
| PTM | Post-translational modification |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
Author Contributions
Conceptualization, B.S. and W.P.; literature review, B.S. and C.J.I.B.; writing—original draft preparation, B.S.; writing—review and editing, B.S., C.J.I.B. and W.P.; supervision, W.P.; funding acquisition, W.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Burnett M.J.B., Burnett A.C. Therapeutic recombinant protein production in plants: Challenges and opportunities. Plants People Planet. 2020;2:121–132. doi: 10.1002/ppp3.10073. [DOI] [Google Scholar]
- 2.Schillberg S., Raven N., Spiegel H., Rasche S., Buntru M. Critical analysis of the commercial potential of plants for the production of recombinant proteins. Front. Plant Sci. 2019;10:720. doi: 10.3389/fpls.2019.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vieira Gomes A.M., Souza Carmo T., Silva Carvalho L., Mendonca Bahia F., Parachin N.S. Comparison of yeasts as hosts for recombinant protein production. Microorganisms. 2018;6:38. doi: 10.3390/microorganisms6020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lalonde M.E., Durocher Y. Therapeutic glycoprotein production in mammalian cells. J. Biotechnol. 2017;251:128–140. doi: 10.1016/j.jbiotec.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Sainsbury F., Lomonossoff G.P. Transient expressions of synthetic biology in plants. Curr. Opin. Plant Biol. 2014;19:1–7. doi: 10.1016/j.pbi.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanmugaraj B., Ramalingam S. Plant expression platform for the production of recombinant pharmaceutical proteins. Austin J. Biotechnol. Bioeng. 2014;1:4. [Google Scholar]
- 7.Hiatt A., Cafferkey R., Bowdish K. Production of antibodies in transgenic plants. Nature. 1989;342:76–78. doi: 10.1038/342076a0. [DOI] [PubMed] [Google Scholar]
- 8.Fox J.L. First plant-made biologic approved. Nat. Biotechnol. 2012;30:472. doi: 10.1038/nbt0612-472. [DOI] [Google Scholar]
- 9.Tschofen M., Knopp D., Hood E., Stöger E. Plant molecular farming: Much more than medicines. Annu. Rev. Anal. Chem. 2016;9:271–294. doi: 10.1146/annurev-anchem-071015-041706. [DOI] [PubMed] [Google Scholar]
- 10.Daniell H., Singh N.D., Mason H., Streatfield S.J. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009;14:669–679. doi: 10.1016/j.tplants.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lomonossoff G.P., D’Aoust M.A. Plant-produced biopharmaceuticals: A case of technical developments driving clinical deployment. Science. 2016;353:1237–1240. doi: 10.1126/science.aaf6638. [DOI] [PubMed] [Google Scholar]
- 12.Park K.Y., Wi S.J. Potential of plants to produce recombinant protein products. J. Plant Biol. 2016;59:559–568. doi: 10.1007/s12374-016-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topp E., Irwin R., McAllister T., Lessard M., Joensuu J.J., Kolotilin I., Conrad U., Stoger E., Mor T., Warzecha H., et al. The case for plant-made veterinary immunotherapeutics. Biotechnol. Adv. 2016;34:597–604. doi: 10.1016/j.biotechadv.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Sohrab S.S., Suhail M., Kamal M.A., Husen A., Azhar E.I. Recent development and future prospects of plant-based vaccines. Curr. Drug Metab. 2017;18:831–841. doi: 10.2174/1389200218666170711121810. [DOI] [PubMed] [Google Scholar]
- 15.Wong-Arce A., Gonzalez-Ortega O., Rosales-Mendoza S. Plant-made vaccines in the fight against cancer. Trends Biotechnol. 2017;35:241–256. doi: 10.1016/j.tibtech.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Taunt H.N., Stoffels L., Purton S. Green biologics: The algal chloroplast as a platform for making biopharmaceuticals. Bioengineered. 2018;9:48–54. doi: 10.1080/21655979.2017.1377867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donini M., Marusic C. Current state-of-the-art in plant-based antibody production systems. Biotechnol. Lett. 2019;41:335–346. doi: 10.1007/s10529-019-02651-z. [DOI] [PubMed] [Google Scholar]
- 18.Rybicki E.P. Plant molecular farming of virus-like nanoparticles as vaccines and reagents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020;12:e1587. doi: 10.1002/wnan.1587. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q., Davis K.R. The potential of plants as a system for the development and production of human biologics. F1000Research. 2016;5 doi: 10.12688/f1000research.8010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahdev S., Khattar S.K., Saini K.S. Production of active eukaryotic proteins through bacterial expression systems: A review of the existing biotechnology strategies. Mol. Cell. Biochem. 2008;307:249–264. doi: 10.1007/s11010-007-9603-6. [DOI] [PubMed] [Google Scholar]
- 21.Strasser R., Stadlmann J., Schahs M., Stiegler G., Quendler H., Mach L., Glossl J., Weterings K., Pabst M., Steinkellner H. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol. J. 2008;6:392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 22.Montero-Morales L., Steinkellner H. Advanced plant-based glycan engineering. Front. Bioeng. Biotechnol. 2018;6:81. doi: 10.3389/fbioe.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul M., Ma J.K. Plant-made pharmaceuticals: Leading products and production platforms. Biotechnol. Appl. Biochem. 2011;58:58–67. doi: 10.1002/bab.6. [DOI] [PubMed] [Google Scholar]
- 24.Yao J., Weng Y., Dickey A., Wang K.Y. Plants as factories for human pharmaceuticals: Applications and challenges. Int. J. Mol. Sci. 2015;16:28549–28565. doi: 10.3390/ijms161226122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanmugaraj B., Malla A., Phoolcharoen W. Emergence of novel coronavirus 2019-nCoV: Need for rapid vaccine and biologics development. Pathogens. 2020;9:148. doi: 10.3390/pathogens9020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J.Y., Cheng L.Q., Zheng X.J., Wu J.X., Shang S.B., Wang J.Y., Chen J.G. Generation of the transgenic potato expressing full-length spike protein of infectious bronchitis virus. J. Biotechnol. 2004;111:121–130. doi: 10.1016/j.jbiotec.2004.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniell H., Rai V., Xiao Y. Cold chain and virus-free oral polio booster vaccine made in lettuce chloroplasts confers protection against all three poliovirus serotypes. Plant Biotechnol. J. 2019;17:1357–1368. doi: 10.1111/pbi.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsian J., Fox H., Bahar M.W., Kotecha A., Fry E.E., Stuart D.I., Macadam A.J., Rowlands D.J., Lomonossoff G.P. Plant-made polio type 3 stabilized VLPs-a candidate synthetic polio vaccine. Nat. Commun. 2017;8:245. doi: 10.1038/s41467-017-00090-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diamos A.G., Mason H.S. High-level expression and enrichment of norovirus virus-like particles in plants using modified geminiviral vectors. Protein Expr. Purif. 2018;151:86–92. doi: 10.1016/j.pep.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Veerapen V.P., van Zyl A.R., Wigdorovitz A., Rybicki E.P., Meyers A.E. Novel expression of immunogenic foot-and-mouth disease virus-like particles in Nicotiana benthamiana. Virus Res. 2018;244:213–217. doi: 10.1016/j.virusres.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 31.Smith T., O’Kennedy M.M., Wandrag D.B.R., Adeyemi M., Abolnik C. Efficacy of a plant-produced virus-like particle vaccine in chickens challenged with Influenza A H6N2 virus. Plant Biotechnol. J. 2020;18:502–512. doi: 10.1111/pbi.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scotti N., Rybicki E.P. Virus-like particles produced in plants as potential vaccines. Expert Rev. Vaccines. 2013;12:211–224. doi: 10.1586/erv.12.147. [DOI] [PubMed] [Google Scholar]
- 33.Rybicki E.P. Plant-based vaccines against viruses. Virol. J. 2014;11:205. doi: 10.1186/s12985-014-0205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hood E.E., Witcher D.R., Maddock S., Meyer T., Baszczynski C., Bailey M., Flynn P., Register J., Marshall L., Bond D., et al. Commercial production of avidin from transgenic maize: Characterization of transformant, production, processing, extraction and purification. Mol. Breed. 1997;3:291–306. doi: 10.1023/A:1009676322162. [DOI] [Google Scholar]
- 35.Witcher D.R., Hood E.E., Peterson D., Bailey M., Bond D., Kusnadi A., Evangelista R., Nikolov Z., Wooge C., Mehigh R., et al. Commercial production of β-glucuronidase (GUS): A model system for the production of proteins in plants. Mol. Breed. 1998;4:301–312. doi: 10.1023/A:1009622429758. [DOI] [Google Scholar]
- 36.Woodard S.L., Mayor J.M., Bailey M.R., Barker D.K., Love R.T., Lane J.R., Delaney D.E., McComas-Wagner J.M., Mallubhotla H.D., Hood E.E., et al. Maize (Zea mays)-derived bovine trypsin: Characterization of the first large-scale, commercial protein product from transgenic plants. Biotechnol. Appl. Biochem. 2003;38:123–130. doi: 10.1042/BA20030026. [DOI] [PubMed] [Google Scholar]
- 37.Ma J.K.-C., Drossard J., Lewis D., Altmann F., Boyle J., Christou P., Cole T., Dale P., van Dolleweerd C.J., Isitt V., et al. Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol. J. 2015;13:1106–1120. doi: 10.1111/pbi.12416. [DOI] [PubMed] [Google Scholar]
- 38.Mason H.S., Lam D.M., Arntzen C.J. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA. 1992;89:11745. doi: 10.1073/pnas.89.24.11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castanon S., Marin M.S., Martin-Alonso J.M., Boga J.A., Casais R., Humara J.M., Ordas R.J., Parra F. Immunization with potato plants expressing VP60 protein protects against rabbit hemorrhagic disease virus. J. Virol. 1999;73:4452–4455. doi: 10.1128/JVI.73.5.4452-4455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuboly T., Yu W., Bailey A., Degrandis S., Du S., Erickson L., Nagy E. Immunogenicity of porcine transmissible gastroenteritis virus spike protein expressed in plants. Vaccine. 2000;18:2023–2028. doi: 10.1016/S0264-410X(99)00525-3. [DOI] [PubMed] [Google Scholar]
- 41.Khandelwal A., Lakshmi Sita G., Shaila M.S. Oral immunization of cattle with hemagglutinin protein of rinderpest virus expressed in transgenic peanut induces specific immune responses. Vaccine. 2003;21:3282–3289. doi: 10.1016/S0264-410X(03)00192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pérez Filgueira D.M., Zamorano P.I., Domínguez M.G., Taboga O., Del Médico Zajac M.P., Puntel M., Romera S.A., Morris T.J., Borca M.V., Sadir A.M. Bovine herpes virus gD protein produced in plants using a recombinant tobacco mosaic virus (TMV) vector possesses authentic antigenicity. Vaccine. 2003;21:4201–4209. doi: 10.1016/S0264-410X(03)00495-X. [DOI] [PubMed] [Google Scholar]
- 43.Varsani A., Williamson A.L., Rose R.C., Jaffer M., Rybicki E.P. Expression of Human papillomavirus type 16 major capsid protein in transgenic Nicotiana tabacum cv. Xanthi. Arch. Virol. 2003;148:1771–1786. doi: 10.1007/s00705-003-0119-4. [DOI] [PubMed] [Google Scholar]
- 44.Lamphear B.J., Jilka J.M., Kesl L., Welter M., Howard J.A., Streatfield S.J. A corn-based delivery system for animal vaccines: An oral transmissible gastroenteritis virus vaccine boosts lactogenic immunity in swine. Vaccine. 2004;22:2420–2424. doi: 10.1016/j.vaccine.2003.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koya V., Moayeri M., Leppla S.H., Daniell H. Plant-based vaccine: Mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect. Immun. 2005;73:8266–8274. doi: 10.1128/IAI.73.12.8266-8274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thanavala Y., Mahoney M., Pal S., Scott A., Richter L., Natarajan N., Goodwin P., Arntzen C.J., Mason H.S. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc. Natl. Acad. Sci. USA. 2005;102:3378–3382. doi: 10.1073/pnas.0409899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guerrero-Andrade O., Loza-Rubio E., Olivera-Flores T., Fehervari-Bone T., Gomez-Lim M.A. Expression of the Newcastle disease virus fusion protein in transgenic maize and immunological studies. Transgenic Res. 2006;15:455–463. doi: 10.1007/s11248-006-0017-0. [DOI] [PubMed] [Google Scholar]
- 48.Joensuu J.J., Verdonck F., Ehrstrom A., Peltola M., Siljander-Rasi H., Nuutila A.M., Oksman-Caldentey K.M., Teeri T.H., Cox E., Goddeeris B.M., et al. F4 (K88) fimbrial adhesin FaeG expressed in alfalfa reduces F4+ enterotoxigenic Escherichia coli excretion in weaned piglets. Vaccine. 2006;24:2387–2394. doi: 10.1016/j.vaccine.2005.11.056. [DOI] [PubMed] [Google Scholar]
- 49.Kohl T., Hitzeroth I.I., Stewart D., Varsani A., Govan V.A., Christensen N.D., Williamson A.L., Rybicki E.P. Plant-produced cottontail rabbit papillomavirus L1 protein protects against tumor challenge: A proof-of-concept study. Clin. Vaccine Immunol. 2006;13:845–853. doi: 10.1128/CVI.00072-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J., Yu L., Li L., Hu J., Zhou J., Zhou X. Oral immunization with transgenic rice seeds expressing VP2 protein of infectious bursal disease virus induces protective immune responses in chickens. Plant Biotechnol. J. 2007;5:570–578. doi: 10.1111/j.1467-7652.2007.00270.x. [DOI] [PubMed] [Google Scholar]
- 51.Huang Z., LePore K., Elkin G., Thanavala Y., Mason H.S. High-yield rapid production of hepatitis B surface antigen in plant leaf by a viral expression system. Plant Biotechnol. J. 2008;6:202–209. doi: 10.1111/j.1467-7652.2007.00316.x. [DOI] [Google Scholar]
- 52.D’Aoust M.-A., Lavoie P.-O., Couture M.M.-J., Trépanier S., Guay J.-M., Dargis M., Mongrand S., Landry N., Ward B.J., Vézina L.-P. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol. J. 2008;6:930–940. doi: 10.1111/j.1467-7652.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- 53.Rosales-Mendoza S., Soria-Guerra R.E., Lopez-Revilla R., Moreno-Fierros L., Alpuche-Solis A.G. Ingestion of transgenic carrots expressing the Escherichia coli heat-labile enterotoxin B subunit protects mice against cholera toxin challenge. Plant Cell Rep. 2008;27:79–84. doi: 10.1007/s00299-007-0439-z. [DOI] [PubMed] [Google Scholar]
- 54.Santi L., Batchelor L., Huang Z., Hjelm B., Kilbourne J., Arntzen C.J., Chen Q., Mason H.S. An efficient plant viral expression system generating orally immunogenic Norwalk virus-like particles. Vaccine. 2008;26:1846–1854. doi: 10.1016/j.vaccine.2008.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang D.M., Zhu J.B., Peng M., Zhou P. Induction of a protective antibody response to FMDV in mice following oral immunization with transgenic Stylosanthes spp. as a feedstuff additive. Transgenic Res. 2008;17:1163–1170. doi: 10.1007/s11248-008-9188-1. [DOI] [PubMed] [Google Scholar]
- 56.Scotti N., Alagna F., Ferraiolo E., Formisano G., Sannino L., Buonaguro L., De Stradis A., Vitale A., Monti L., Grillo S., et al. High-level expression of the HIV-1 Pr55gag polyprotein in transgenic tobacco chloroplasts. Planta. 2009;229:1109–1122. doi: 10.1007/s00425-009-0898-2. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y., Deng H., Zhang X., Xiao H., Jiang Y., Song Y., Fang L., Xiao S., Zhen Y., Chen H. Generation and immunogenicity of Japanese encephalitis virus envelope protein expressed in transgenic rice. Biochem. Biophys. Res. Commun. 2009;380:292–297. doi: 10.1016/j.bbrc.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 58.Kalthoff D., Giritch A., Geisler K., Bettmann U., Klimyuk V., Hehnen H.R., Gleba Y., Beer M. Immunization with plant-expressed hemagglutinin protects chickens from lethal highly pathogenic avian influenza virus H5N1 challenge infection. J. Virol. 2010;84:12002–12010. doi: 10.1128/JVI.00940-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shoji Y., Farrance C.E., Bautista J., Bi H., Musiychuk K., Horsey A., Park H., Jaje J., Green B.J., Shamloul M., et al. A plant-based system for rapid production of influenza vaccine antigens. Influenza Other Respir. Viruses. 2012;6:204–210. doi: 10.1111/j.1750-2659.2011.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanagarajan S., Tolf C., Lundgren A., Waldenstrom J., Brodelius P.E. Transient expression of hemagglutinin antigen from low pathogenic avian influenza A (H7N7) in Nicotiana benthamiana. PLoS ONE. 2012;7:e33010. doi: 10.1371/journal.pone.0033010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gómez E., Lucero M.S., Chimeno Zoth S., Carballeda J.M., Gravisaco M.J., Berinstein A. Transient expression of VP2 in Nicotiana benthamiana and its use as a plant-based vaccine against Infectious Bursal Disease Virus. Vaccine. 2013;31:2623–2627. doi: 10.1016/j.vaccine.2013.03.064. [DOI] [PubMed] [Google Scholar]
- 62.Perez Aguirreburualde M.S., Gomez M.C., Ostachuk A., Wolman F., Albanesi G., Pecora A., Odeon A., Ardila F., Escribano J.M., Dus Santos M.J., et al. Efficacy of a BVDV subunit vaccine produced in alfalfa transgenic plants. Vet. Immunol. Immunopathol. 2013;151:315–324. doi: 10.1016/j.vetimm.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Thuenemann E.C., Meyers A.E., Verwey J., Rybicki E.P., Lomonossoff G.P. A method for rapid production of heteromultimeric protein complexes in plants: Assembly of protective bluetongue virus-like particles. Plant Biotechnol. J. 2013;11:839–846. doi: 10.1111/pbi.12076. [DOI] [PubMed] [Google Scholar]
- 64.Mathew L.G., Herbst-Kralovetz M.M., Mason H.S. Norovirus Narita 104 virus-like particles expressed in Nicotiana benthamiana induce serum and mucosal immune responses. Biomed. Res. Int. 2014;2014:807539. doi: 10.1155/2014/807539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piron R., De Koker S., De Paepe A., Goossens J., Grooten J., Nauwynck H., Depicker A. Boosting in planta production of antigens derived from the porcine reproductive and respiratory syndrome virus (PRRSV) and subsequent evaluation of their immunogenicity. PLoS ONE. 2014;9:e91386. doi: 10.1371/journal.pone.0091386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Firsov A., Tarasenko I., Mitiouchkina T., Ismailova N., Shaloiko L., Vainstein A., Dolgov S. High-yield expression of M2e peptide of Avian Influenza virus H5N1 in transgenic duckweed plants. Mol. Biotechnol. 2015;57:653–661. doi: 10.1007/s12033-015-9855-4. [DOI] [PubMed] [Google Scholar]
- 67.Mbewana S., Mortimer E., Pera F.F., Hitzeroth I.I., Rybicki E.P. Production of H5N1 influenza virus matrix protein 2 ectodomain protein bodies in tobacco plants and in insect cells as a candidate universal influenza vaccine. Front. Bioeng. Biotechnol. 2015;3:197. doi: 10.3389/fbioe.2015.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim M.-Y., Jang Y.-S., Yang M.-S., Kim T.-G. High expression of consensus dengue virus envelope glycoprotein domain III using a viral expression system in tobacco. Plant Cell Tissue Organ Cult. 2015;122:445–451. doi: 10.1007/s11240-015-0781-8. [DOI] [Google Scholar]
- 69.Gottschamel J., Lössl A., Ruf S., Wang Y., Skaugen M., Bock R., Clarke J.L. Production of dengue virus envelope protein domain III-based antigens in tobacco chloroplasts using inducible and constitutive expression systems. Plant Mol. Biol. 2016;91:497–512. doi: 10.1007/s11103-016-0484-5. [DOI] [PubMed] [Google Scholar]
- 70.Kota S., Subramanian M., Shanmugaraj B., Challa H., Ponanna N.M. Subunit vaccine based on plant expressed recombinant Eimeria gametocyte antigen Gam82 elicit protective immune response against chicken coccidiosis. J. Vaccines Vaccin. 2017;8 doi: 10.4172/2157-7560.1000374. [DOI] [Google Scholar]
- 71.Iyappan G., Shanmugaraj B.M., Inchakalody V., Ma J.K.C., Ramalingam S. Potential of plant biologics to tackle the epidemic like situations—Case studies involving viral and bacterial candidates. Int. J. Infect. Dis. 2018;73:363. doi: 10.1016/j.ijid.2018.04.4236. [DOI] [Google Scholar]
- 72.Yang M., Sun H., Lai H., Hurtado J., Chen Q. Plant-produced Zika virus envelope protein elicits neutralizing immune responses that correlate with protective immunity against Zika virus in mice. Plant Biotechnol. J. 2018;16:572–580. doi: 10.1111/pbi.12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gunter C.J., Regnard G.L., Rybicki E.P., Hitzeroth I.I. Immunogenicity of plant-produced porcine circovirus-like particles in mice. Plant Biotechnol. J. 2019;17:1751–1759. doi: 10.1111/pbi.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Margolin E., Chapman R., Meyers A.E., van Diepen M.T., Ximba P., Hermanus T., Crowther C., Weber B., Morris L., Williamson A.L., et al. Production and immunogenicity of soluble plant-produced HIV-1 subtype C envelope gp140 immunogens. Front. Plant Sci. 2019;10:1378. doi: 10.3389/fpls.2019.01378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vaquero C., Sack M., Chandler J., Drossard J., Schuster F., Monecke M., Schillberg S., Fischer R. Transient expression of a tumor-specific single-chain fragment and a chimeric antibody in tobacco leaves. Proc. Natl. Acad. Sci. USA. 1999;96:11128–11133. doi: 10.1073/pnas.96.20.11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torres E., Vaquero C., Nicholson L., Sack M., Stöger E., Drossard J., Christou P., Fischer R., Perrin Y. Rice cell culture as an alternative production system for functional diagnostic and therapeutic antibodies. Transgenic Res. 1999;8:441–449. doi: 10.1023/A:1008969031219. [DOI] [PubMed] [Google Scholar]
- 77.Stöger E., Vaquero C., Torres E., Sack M., Nicholson L., Drossard J., Williams S., Keen D., Perrin Y., Christou P., et al. Cereal crops as viable production and storage systems for pharmaceutical scFv antibodies. Plant Mol. Biol. 2000;42:583–590. doi: 10.1023/A:1006301519427. [DOI] [PubMed] [Google Scholar]
- 78.Brodzik R., Glogowska M., Bandurska K., Okulicz M., Deka D., Ko K., van der Linden J., Leusen J.H., Pogrebnyak N., Golovkin M., et al. Plant-derived anti-Lewis Y mAb exhibits biological activities for efficient immunotherapy against human cancer cells. Proc. Natl. Acad. Sci. USA. 2006;103:8804–8809. doi: 10.1073/pnas.0603043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Floss D.M., Sack M., Stadlmann J., Rademacher T., Scheller J., Stöger E., Fischer R., Conrad U. Biochemical and functional characterization of anti-HIV antibody–ELP fusion proteins from transgenic plants. Plant Biotechnol. J. 2008;6:379–391. doi: 10.1111/j.1467-7652.2008.00326.x. [DOI] [PubMed] [Google Scholar]
- 80.Sainsbury F., Lomonossoff G.P. Extremely high-level and rapid transient protein production in plants without the use of viral replication. Plant Physiol. 2008;148:1212–1218. doi: 10.1104/pp.108.126284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holland T., Sack M., Rademacher T., Schmale K., Altmann F., Stadlmann J., Fischer R., Hellwig S. Optimal nitrogen supply as a key to increased and sustained production of a monoclonal full-size antibody in BY-2 suspension culture. Biotechnol. Bioeng. 2010;107:278–289. doi: 10.1002/bit.22800. [DOI] [PubMed] [Google Scholar]
- 82.Huang Z., Phoolcharoen W., Lai H., Piensook K., Cardineau G., Zeitlin L., Whaley K.J., Arntzen C.J., Mason H.S., Chen Q. High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnol. Bioeng. 2010;106:9–17. doi: 10.1002/bit.22652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lai H., He J., Engle M., Diamond M.S., Chen Q. Robust production of virus-like particles and monoclonal antibodies with geminiviral replicon vectors in lettuce. Plant Biotechnol. J. 2012;10:95–104. doi: 10.1111/j.1467-7652.2011.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.So Y., Lee K.-J., Kim D.-S., Lee J.-H., Oh D.-B., Hwang K.-A., Ko K., Choo Y.-K., Ko K. Glycomodification and characterization of anti-colorectal cancer immunotherapeutic monoclonal antibodies in transgenic tobacco. Plant Cell Tissue Organ Cult. 2013;113:41–49. doi: 10.1007/s11240-012-0249-z. [DOI] [Google Scholar]
- 85.Zeitlin L., Bohorov O., Bohorova N., Hiatt A., Kim D.H., Pauly M.H., Velasco J., Whaley K.J., Barnard D.L., Bates J.T., et al. Prophylactic and therapeutic testing of Nicotiana-derived RSV-neutralizing human monoclonal antibodies in the cotton rat model. MAbs. 2013;5:263–269. doi: 10.4161/mabs.23281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Dolleweerd C.J., Teh A.Y., Banyard A.C., Both L., Lotter-Stark H.C., Tsekoa T., Phahladira B., Shumba W., Chakauya E., Sabeta C.T., et al. Engineering, expression in transgenic plants and characterisation of E559, a rabies virus-neutralising monoclonal antibody. J. Infect. Dis. 2014;210:200–208. doi: 10.1093/infdis/jiu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai H., He J., Hurtado J., Stahnke J., Fuchs A., Mehlhop E., Gorlatov S., Loos A., Diamond M.S., Chen Q. Structural and functional characterization of an anti-West Nile virus monoclonal antibody and its single-chain variant produced in glycoengineered plants. Plant Biotechnol. J. 2014;12:1098–1107. doi: 10.1111/pbi.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dent M., Hurtado J., Paul A.M., Sun H., Lai H., Yang M., Esqueda A., Bai F., Steinkellner H., Chen Q. Plant-produced anti-dengue virus monoclonal antibodies exhibit reduced antibody-dependent enhancement of infection activity. J. Gen. Virol. 2016;97:3280–3290. doi: 10.1099/jgv.0.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vamvaka E., Twyman R.M., Murad A.M., Melnik S., Teh A.Y.-H., Arcalis E., Altmann F., Stoger E., Rech E., Ma J.K.C., et al. Rice endosperm produces an underglycosylated and potent form of the HIV-neutralizing monoclonal antibody 2G12. Plant Biotechnol. J. 2016;14:97–108. doi: 10.1111/pbi.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shafaghi M., Maktoobian S., Rasouli R., Howaizi N., Ofoghi H., Ehsani P. Transient expression of biologically active anti-rabies virus monoclonal antibody in tobacco leaves. Iran. J. Biotechnol. 2018;16:e1774. doi: 10.21859/ijb.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rattanapisit K., Chao Z., Siriwattananon K., Huang Z., Phoolcharoen W. Plant-produced anti-enterovirus 71 (EV71) monoclonal antibody efficiently protects mice against EV71 infection. Plants. 2019;8:560. doi: 10.3390/plants8120560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rattanapisit K., Phakham T., Buranapraditkun S., Siriwattananon K., Boonkrai C., Pisitkun T., Hirankarn N., Strasser R., Abe Y., Phoolcharoen W. Structural and in vitro functional analyses of novel plant-produced anti-human PD1 antibody. Sci. Rep. 2019;9:15205. doi: 10.1038/s41598-019-51656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Diamos A.G., Hunter J.G.L., Pardhe M.D., Rosenthal S.H., Sun H., Foster B.C., DiPalma M.P., Chen Q., Mason H.S. High level production of monoclonal antibodies using an optimized plant expression system. Front. Bioeng. Biotechnol. 2019;7:472. doi: 10.3389/fbioe.2019.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hurtado J., Acharya D., Lai H., Sun H., Kallolimath S., Steinkellner H., Bai F., Chen Q. In vitro and in vivo efficacy of anti-chikungunya virus monoclonal antibodies produced in wild-type and glycoengineered Nicotiana benthamiana plants. Plant Biotechnol. J. 2020;18:266–273. doi: 10.1111/pbi.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sijmons P.C., Dekker B.M., Schrammeijer B., Verwoerd T.C., van den Elzen P.J., Hoekema A. Production of correctly processed human serum albumin in transgenic plants. Biotechnology. 1990;8:217–221. doi: 10.1038/nbt0390-217. [DOI] [PubMed] [Google Scholar]
- 96.Matsumoto S., Ikura K., Ueda M., Sasaki R. Characterization of a human glycoprotein (erythropoietin) produced in cultured tobacco cells. Plant Mol. Biol. 1995;27:1163–1172. doi: 10.1007/BF00020889. [DOI] [PubMed] [Google Scholar]
- 97.Terashima M., Murai Y., Kawamura M., Nakanishi S., Stoltz T., Chen L., Drohan W., Rodriguez R.L., Katoh S. Production of functional human α1-antitrypsin by plant cell culture. Appl. Microbiol. Biotechnol. 1999;52:516–523. doi: 10.1007/s002530051554. [DOI] [PubMed] [Google Scholar]
- 98.Zhong G.-Y., Peterson D., Delaney D.E., Bailey M., Witcher D.R., Register Iii J.C., Bond D., Li C.-P., Marshall L., Kulisek E., et al. Commercial production of aprotinin in transgenic maize seeds. Mol. Breed. 1999;5:345–356. doi: 10.1023/A:1009677809492. [DOI] [Google Scholar]
- 99.Komarnytsky S., Borisjuk N.V., Borisjuk L.G., Alam M.Z., Raskin I. Production of recombinant proteins in tobacco guttation fluid. Plant Physiol. 2000;124:927–934. doi: 10.1104/pp.124.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ruggiero F., Exposito J.Y., Bournat P., Gruber V., Perret S., Comte J., Olagnier B., Garrone R., Theisen M. Triple helix assembly and processing of human collagen produced in transgenic tobacco plants. FEBS Lett. 2000;469:132–136. doi: 10.1016/S0014-5793(00)01259-X. [DOI] [PubMed] [Google Scholar]
- 101.Staub J.M., Garcia B., Graves J., Hajdukiewicz P.T., Hunter P., Nehra N., Paradkar V., Schlittler M., Carroll J.A., Spatola L., et al. High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat. Biotechnol. 2000;18:333–338. doi: 10.1038/73796. [DOI] [PubMed] [Google Scholar]
- 102.De Cosa B., Moar W., Lee S.B., Miller M., Daniell H. Overexpression of the Bt cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat. Biotechnol. 2001;19:71–74. doi: 10.1038/83559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fernandez-San Millan A., Mingo-Castel A., Miller M., Daniell H. A chloroplast transgenic approach to hyper-express and purify Human Serum Albumin, a protein highly susceptible to proteolytic degradation. Plant Biotechnol. J. 2003;1:71–79. doi: 10.1046/j.1467-7652.2003.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wirth S., Calamante G., Mentaberry A., Bussmann L., Lattanzi M., Barañao L., Bravo-Almonacid F. Expression of active human epidermal growth factor (hEGF) in tobacco plants by integrative and non-integrative systems. Mol. Breed. 2004;13:23–35. doi: 10.1023/B:MOLB.0000012329.74067.ca. [DOI] [Google Scholar]
- 105.Ding S.H., Huang L.Y., Wang Y.D., Sun H.C., Xiang Z.H. High-level expression of basic fibroblast growth factor in transgenic soybean seeds and characterization of its biological activity. Biotechnol. Lett. 2006;28:869–875. doi: 10.1007/s10529-006-9018-6. [DOI] [PubMed] [Google Scholar]
- 106.Arlen P.A., Falconer R., Cherukumilli S., Cole A., Cole A.M., Oishi K.K., Daniell H. Field production and functional evaluation of chloroplast-derived interferon-alpha2b. Plant Biotechnol. J. 2007;5:511–525. doi: 10.1111/j.1467-7652.2007.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim T.G., Baek M.Y., Lee E.K., Kwon T.H., Yang M.S. Expression of human growth hormone in transgenic rice cell suspension culture. Plant Cell Rep. 2008;27:885–891. doi: 10.1007/s00299-008-0514-0. [DOI] [PubMed] [Google Scholar]
- 108.Oey M., Lohse M., Kreikemeyer B., Bock R. Exhaustion of the chloroplast protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J. 2009;57:436–445. doi: 10.1111/j.1365-313X.2008.03702.x. [DOI] [PubMed] [Google Scholar]
- 109.Xu J., Okada S., Tan L., Goodrum K.J., Kopchick J.J., Kieliszewski M.J. Human growth hormone expressed in tobacco cells as an arabinogalactan-protein fusion glycoprotein has a prolonged serum life. Transgenic Res. 2010;19:849–867. doi: 10.1007/s11248-010-9367-8. [DOI] [PubMed] [Google Scholar]
- 110.An N., Ou J., Jiang D., Zhang L., Liu J., Fu K., Dai Y., Yang D. Expression of a functional recombinant human basic fibroblast growth factor from transgenic rice seeds. Int. J. Mol. Sci. 2013;14:3556–3567. doi: 10.3390/ijms14023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guan C., Du X., Wang G., Ji J., Jin C., Li X. Expression of biologically active anti-thrombosis protein lumbrokinase in edible sunflower seed kernel. J. Plant Biochem. Biotechnol. 2014;23:257–265. doi: 10.1007/s13562-013-0209-7. [DOI] [Google Scholar]
- 112.Tan Y., Wang K.Y., Wang N., Li G., Liu D. Ectopic expression of human acidic fibroblast growth factor 1 in the medicinal plant, Salvia miltiorrhiza, accelerates the healing of burn wounds. BMC Biotechnol. 2014;14:74. doi: 10.1186/1472-6750-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Limkul J., Misaki R., Kato K., Fujiyama K. The combination of plant translational enhancers and terminator increase the expression of human glucocerebrosidase in Nicotiana benthamiana plants. Plant Sci. 2015;240:41–49. doi: 10.1016/j.plantsci.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 114.Su J., Sherman A., Doerfler P.A., Byrne B.J., Herzog R.W., Daniell H. Oral delivery of Acid Alpha Glucosidase epitopes expressed in plant chloroplasts suppresses antibody formation in treatment of Pompe mice. Plant Biotechnol. J. 2015;13:1023–1032. doi: 10.1111/pbi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y.P., Wei Z.Y., Zhong X.F., Lin C.J., Cai Y.H., Ma J., Zhang Y.Y., Liu Y.Z., Xing S.C. Stable expression of basic fibroblast growth factor in chloroplasts of tobacco. Int. J. Mol. Sci. 2016;17:19. doi: 10.3390/ijms17010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Castiglia D., Sannino L., Marcolongo L., Ionata E., Tamburino R., De Stradis A., Cobucci-Ponzano B., Moracci M., La Cara F., Scotti N. High-level expression of thermostable cellulolytic enzymes in tobacco transplastomic plants and their use in hydrolysis of an industrially pretreated Arundo donax L. biomass. Biotechnol. Biofuels. 2016;9:154. doi: 10.1186/s13068-016-0569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rattanapisit K., Abdulheem S., Chaikeawkaew D., Kubera A., Mason H.S., Ma J.K., Pavasant P., Phoolcharoen W. Recombinant human osteopontin expressed in Nicotiana benthamiana stimulates osteogenesis related genes in human periodontal ligament cells. Sci. Rep. 2017;7:17358. doi: 10.1038/s41598-017-17666-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ahmad A.R., Kaewpungsup P., Khorattanakulchai N., Rattanapisit K., Pavasant P., Phoolcharoen W. Recombinant human dentin matrix protein 1 (hDMP1) expressed in Nicotiana benthamiana potentially induces osteogenic differentiation. Plants. 2019;8:566. doi: 10.3390/plants8120566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Menary J., Hobbs M., Mesquita de Albuquerque S., Pacho A., Drake P.M.W., Prendiville A., Ma J.K., Fuller S.S. Shotguns vs Lasers: Identifying barriers and facilitators to scaling-up plant molecular farming for high-value health products. PLoS ONE. 2020;15:e0229952. doi: 10.1371/journal.pone.0229952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Twyman R.M., Stoger E., Schillberg S., Christou P., Fischer R. Molecular farming in plants: Host systems and expression technology. Trends Biotechnol. 2003;21:570–578. doi: 10.1016/j.tibtech.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 121.Yusibov V., Rabindran S. Recent progress in the development of plant derived vaccines. Expert Rev. Vaccines. 2008;7:1173–1183. doi: 10.1586/14760584.7.8.1173. [DOI] [PubMed] [Google Scholar]
- 122.Gleba Y., Klimyuk V., Marillonnet S. Viral vectors for the expression of proteins in plants. Curr. Opin. Biotechnol. 2007;18:134–141. doi: 10.1016/j.copbio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 123.Qiu X., Wong G., Audet J., Bello A., Fernando L., Alimonti J.B., Fausther-Bovendo H., Wei H., Aviles J., Hiatt E., et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosales-Mendoza S. Will plant-made biopharmaceuticals play a role in the fight against COVID-19? Expert Opin. Biol. Ther. 2020;20:545–548. doi: 10.1080/14712598.2020.1752177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shanmugaraj B., Siriwattananon K., Wangkanont K., Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pac. J. Allergy Immunol. 2020;38:10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 126.Capell T., Twyman R.M., Armario-Najera V., Ma J.K.C., Schillberg S., Christou P. Potential applications of plant biotechnology against SARS-CoV-2. Trends Plant Sci. 2020;25:635–643. doi: 10.1016/j.tplants.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Buyel J.F. Plant molecular farming—Integration and exploitation of side streams to achieve sustainable biomanufacturing. Front. Plant Sci. 2018;9:1893. doi: 10.3389/fpls.2018.01893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maliga P. Engineering the plastid genome of higher plants. Curr. Opin. Plant Biol. 2002;5:164–172. doi: 10.1016/S1369-5266(02)00248-0. [DOI] [PubMed] [Google Scholar]
- 129.Bock R. Plastid biotechnology: Prospects for herbicide and insect resistance, metabolic engineering and molecular farming. Curr. Opin. Biotechnol. 2007;18:100–106. doi: 10.1016/j.copbio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 130.Jin S., Daniell H. The engineered chloroplast genome just got smarter. Trends Plant Sci. 2015;20:622–640. doi: 10.1016/j.tplants.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang B., Shanmugaraj B., Daniell H. Expression and functional evaluation of biopharmaceuticals made in plant chloroplasts. Curr. Opin. Chem. Biol. 2017;38:17–23. doi: 10.1016/j.cbpa.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu J., Zhang N. On the way to commercializing plant cell culture platform for biopharmaceuticals: Present status and prospect. Pharm. Bioprocess. 2014;2:499–518. doi: 10.4155/pbp.14.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tekoah Y., Shulman A., Kizhner T., Ruderfer I., Fux L., Nataf Y., Bartfeld D., Ariel T., Gingis–Velitski S., Hanania U., et al. Large-scale production of pharmaceutical proteins in plant cell culture—The protalix experience. Plant Biotechnol. J. 2015;13:1199–1208. doi: 10.1111/pbi.12428. [DOI] [PubMed] [Google Scholar]
- 134.Santos R.B., Abranches R., Fischer R., Sack M., Holland T. Putting the spotlight back on plant suspension cultures. Front. Plant Sci. 2016;7:297. doi: 10.3389/fpls.2016.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gurusamy P.D., Schafer H., Ramamoorthy S., Wink M. Biologically active recombinant human erythropoietin expressed in hairy root cultures and regenerated plantlets of Nicotiana tabacum L. PLoS ONE. 2017;12:e0182367. doi: 10.1371/journal.pone.0182367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gutierrez-Valdes N., Häkkinen S.T., Lemasson C., Guillet M., Oksman-Caldentey K.-M., Ritala A., Cardon F. Hairy root cultures—A versatile tool with multiple applications. Front. Plant Sci. 2020;11:33. doi: 10.3389/fpls.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]