Abstract
Sulfur centered radicals are widely employed in chemical synthesis, in particular for alkene and alkyne hydrothiolation towards thioether bioconjugates. The steadfast radical chain process that enables efficient hydrothiolation has been explored in the context of cascade reactions to furnish complex molecular architectures. The use of thiyl radicals offers a much cheaper and less toxic alternative to the archetypal organotin-based radical methods. This review outlines the development of thiyl radicals as reactive intermediates for initiating carbocyclization cascades. Key developments in cascade cyclization methodology are presented and applications for natural product synthesis are discussed. The review provides a chronological account of the field, beginning in the early seventies up to very recent examples; a span of almost 50 years.
Keywords: radical, cyclisation, thiyl radicals, sulfur, carbocyclization
1. Introduction
Since the turn of the millennium, organosulfur compounds have garnered substantial interest in the fields of medicinal chemistry, chemical biology and material science [1,2,3,4,5,6,7]. Of the diverse biochemical processes involving free radicals, many are thiyl radical mediated, highlighting the chemoselectivity, efficiency and biocompatibility of these reactive intermediates [8]. Sulfur-centered radicals have been broadly utilized in a wide range of synthetic applications over the past three decades. While their general utility has been previously reviewed [9,10,11,12,13,14,15,16,17], this account seeks to update the reader specifically on the application of thiyl radicals (RS•), in the carbocyclizations of unsaturated moieties, and the scope and limitations of these reactions in natural product synthesis.
Sulfur, in its −2 oxidation state, can act as either a nucleophile or as a radical. The S-H bond is generally considered relatively weak, with a bond dissociation energy (BDE) of 365 kJ/mol [18], and as such, both alkyl thiols and thiocarboxylic acids (thioacids) are precursors to thiyl/acyl thiyl radicals through facile homolytic cleavage. Thiyl radicals may be generated by homolytic bond breakage, and by one-electron redox processes. In the former process, a carbon centered radical (for example, from the radical initiator azobisisobutyronitrile (AIBN)) will abstract a hydrogen atom from the thiol. Rate constants for this homolytic breakdown are of the magnitude 108 M−1 s−1, which is below the diffusion limit for this reaction [19]. In the case of redox processes, single electron oxidants such as Mn (III) complexes or other metal sources can be used to accomplish radical formation [20,21,22]. Both radiolysis and direct photolysis of the S-H bond under UV irradiation are also valuable pathways to thiyl radical formation [23,24].
Thiyl radicals are well known to react efficiently at sites of unsaturation, through the thiol-ene and thiol-yne reactions. A full analysis of the utility of these reactions is beyond the scope of this review, and readers are directed to the reviews already present in the literature [6,24,25,26,27,28,29]. This account will focus exclusively on the intramolecular trapping of the nascent carbon radical formed via thiyl radical addition, and how these radicals form rings and products in cascade reactions.
2. Dienes and Diynes
2.1. Origin
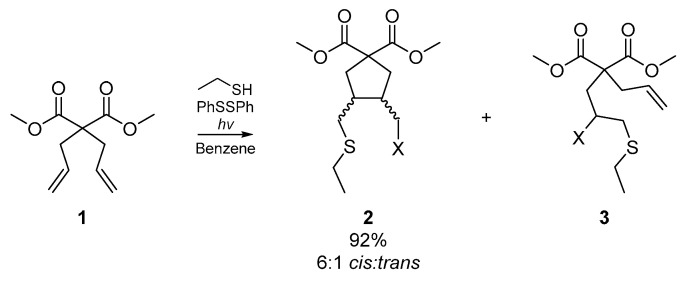
For the purpose of this review, ‘homogenous’ infers the same degree of unsaturation for all reactive sites in the substrate or substrates; i.e., dienes, diynes, trienes, etc. Possibly the earliest example of a cyclization cascade on a homogenous substrate was published by Kuehne et al. in 1977 [30]. The account details the cyclization of selected dienes with a range of thiyl radical precursors, relying upon radical initiation via photolysis of diphenyl disulfide or thermolysis of benzoyl peroxide. Kuehne and co-workers sought to compare the cyclizations effected by a range of representative thiyl radical species, and thus experimented with the initiator itself, and thermal versus photoinitiation. While they obtained a number of acyclic adducts and complex mixtures of stereoisomers, a number of cyclized products were also identified. The representative example in Scheme 1 shows the cyclization of dimethyl diallylmalonate 1 under a number of reaction conditions, the best of which employed the photolysis of diphenylsulfide in benzene to generate the thiyl radical of ethanethiol. These conditions resulted in the formation of over 90% cyclized product 2, and six minor acyclic adducts. However, the formation of any six-membered cyclization products was ruled out systematically.
Scheme 1.
Cyclization of dimethyl diallylmalonate by ethanethiyl radical. X = H or SCH2CH3.
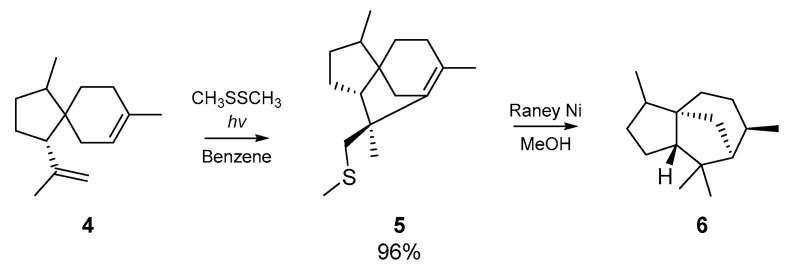
Kuehne and co-workers applied these findings to the analogous cyclization of α-acoradiene 4, which proceeded almost quantitatively. Desulfurization with Raney nickel yielded dihydrocedrene 6, the hydrogenated form of a sesquiterpene found in the essential oil of cedar (Scheme 2). This approach was later applied to the cyclization of geranyl acetate, but the acyclic monoadducts of geranyl acetate and the thiyl radical source were mostly obtained.
Scheme 2.
Cyclization of α-Acoradiene 4 by Kuehne et al.
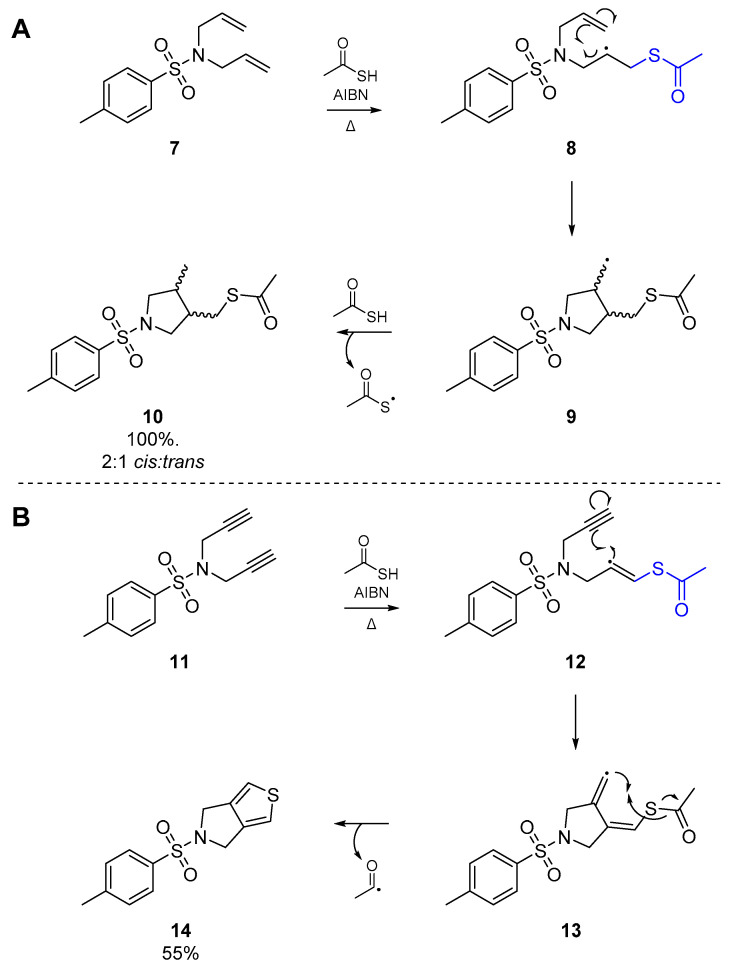
These results were greatly expanded upon in work published by Padwa et al. in 1985 [31]. Using thioacetic acid and AIBN, Padwa and co-workers were able to cyclize a relatively simple tosylamine 7 with two allyl substituents, in a 5-exo-trig process. Addition of the thiyl radical, formed indirectly by thermolysis of AIBN, and intramolecular cyclization of the nascent carbon-centered radical 8 immediately proved the utility of this reaction as a viable route to heterocycles (Scheme 3A). It may be expected that the trans isomer would dominate in this process, whether for steric reasons or otherwise, but in fact a general preference for the cis isomer is observed. Hyperconjugative mixing of the half-filled p orbital and the C-H σ and σ* orbitals produce a semioccupied delocalized orbital which is of matching symmetry to the alkene π* orbital. Therefore in the transition state, the cis-isomer exhibits a second interaction between the carbon-centered radical and remaining olefin, offsetting the nonbonded repulsion between the vicinal substituents of the newly formed pyrrolidine [32,33,34]. Simple substitution at the alkene terminus does not affect the cyclization, and the process continues to follow a 5-exo-trig pathway.
Scheme 3.
Cyclization of diene (A) and diyne (B) systems via thiyl radical cascade by Padwa et al.
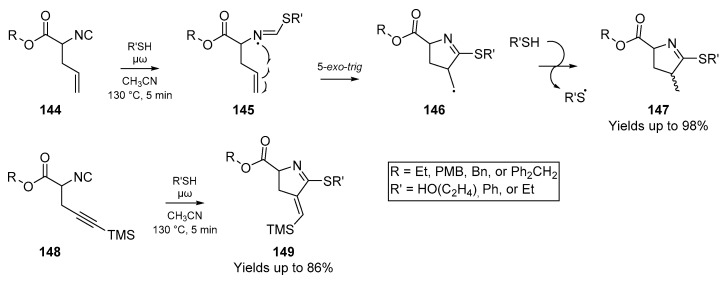
Padwa et al. also reported the addition of an acetylthiyl radical onto another homogenously unsaturated substrate; the corresponding N,N-dipropargylsulfonamide 11 (Scheme 3B). In this case however, a crystalline solid was obtained from the reaction as the major product, which was later identified as the thienopyrrole 14. Following addition of the thiyl radical to the alkyne, the nascent carbon-centered radical cyclizes as shown in Scheme 3. However, this results in formation of a highly unstable vinyl radical intermediate 13, which through an intramolecular homolytic substitution (SHi) extrudes an acyl radical to afford the observed product 14. Indeed, this is one of the few examples of a diyne cyclization via thiyl radicals that one can find in the literature. A second account of diyne reactivity under thiyl radical addition appeared three decades later, in which Agrawal et al. optimized a number of substrates with malonate-type backbones for cyclization to the respective thiophene [35]. This approach relies on thermolysis of AIBN in toluene with thioacetic acid as the acyl thiyl radical source, and furnished a number of the bicyclic thiophenes, in good yield.
2.2. Early Applications in Total Synthesis
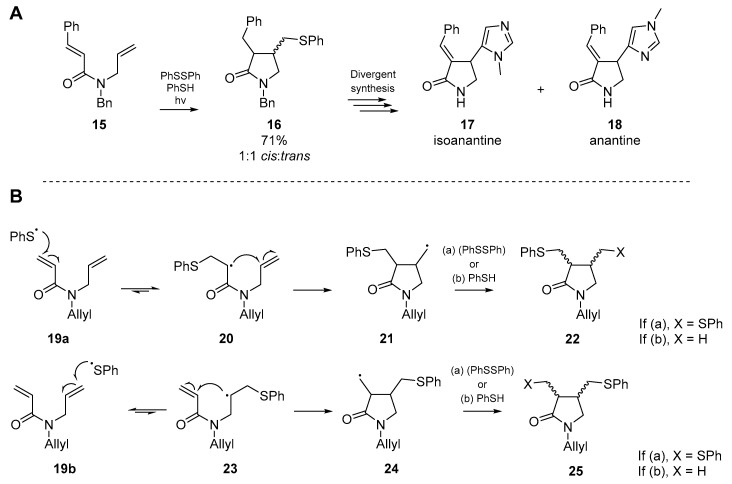
Shortly thereafter, a significant advancement in the application of thiyl radical initiated cascades was published by Naito et al. [36]. In a publication focused on the total synthesis of two imidazole alkaloids, Naito and co-workers employed the thiyl radical addition/cyclization cascade of an N-allyl-N-benzylcinnamamide 15 with diphenyl disulfide and thiophenol in equimolar quantities, which under photochemical irradiation cyclized to the desired 3,4-disubstituted pyrrolidinone 16, albeit as a racemic mixture. This afforded the necessary building block to complete their synthesis of isoanatine 17 and anantine 18 (Scheme 4A) [37]. Just two years later, Naito et al. expanded upon the utility of this reaction, in part one of a series on radical cyclization in heterocycle synthesis [38]. Through application of the methodology developed for their previous alkaloid synthesis, Naito and co-workers developed a new synthetic method which furnished nitrogen-containing heterocycles from simple dienylamide starting materials. This addition generates either one or two carbon-sulfur bonds, and the desired carbon-carbon bond. The authors sought to establish the regiochemistry of thiyl radical addition, the cyclization mode (i.e., 5-exo-trig vs. 6-endo-trig) and finally, whether any stereochemical control was exerted by the process. By using a substrate with two different types of olefins, an insight into the chemoselectivity was also recorded.
Scheme 4.
Dienylamide cyclizations published by Naito et al. in 1993 (A) and 1995 (B).
It was postulated that the cyclization occurred through addition to the acrylamide terminus, followed by cyclization onto the alkene of the neighboring allyl substituent, through a 5-exo-trig pathway (Scheme 4B). The phenylthiyl radical generated in this reaction displays reactivity somewhere between a nucleophilic and electrophilic radical [39], therefore the SOMO should react more favorably with the lower energy LUMO of an electron deficient alkene—in this case the acrylamide substituent. Furthermore, as the thiol-ene reaction is reversible [40,41] the concomitant radical formed via addition to the acrylamide terminus 20 would be further stabilized by its adjacent carbonyl group. In the case of thiyl radical addition to the allylic terminus 23, this stabilization is absent and as such the equilibrium of the thiol-ene reaction favors the starting amide. Naito and co-workers went on to examine the substituent effects, and found that those substrates with no additional functionality at the β-position of the acrylamide group primarily furnished lactams. The presence of a substituent α to the unsaturated amide appeared to have no effect on cyclization dynamics. However, in the case where both olefin termini are substituted, even with a methyl group, no cyclization was observed.
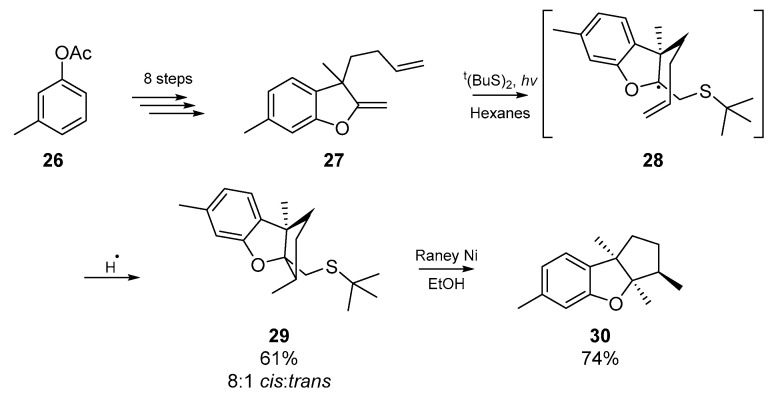
Shortly before the turn of the millennium, another example highlighting the efficacy of the thiyl radical addition/cyclization process emerged from Harrowven and collaborators [42]. In the course of their work in the terpenoid field, the authors took advantage of a sulfur mediated radical cyclization strategy to complete the total syntheses of aplysin and debromoaplysin. Through clever retrosynthetic analysis, Harrowven et al. identified that a 5-exo-trig cyclization through a chair-like transition state would simultaneously furnish both the sterically demanding tricyclic backbone of aplysin, and generate the relative stereochemical configuration for three neighboring stereogenic centers. Employing a Raney nickel desulfurization strategy, Harrowven et al. obtained the necessary skeleton 29 in a diastereomeric ratio of 8:1 (Scheme 5). Similar to the previously discussed strategy employed by Kuehne et al., the use of thiyl radicals offers a much cheaper and less toxic alternative to the archetypal organotin based radical methods.
Scheme 5.
Thiyl radical mediated cyclization and desulfurization gives 30, the precursor to all the aplysins.
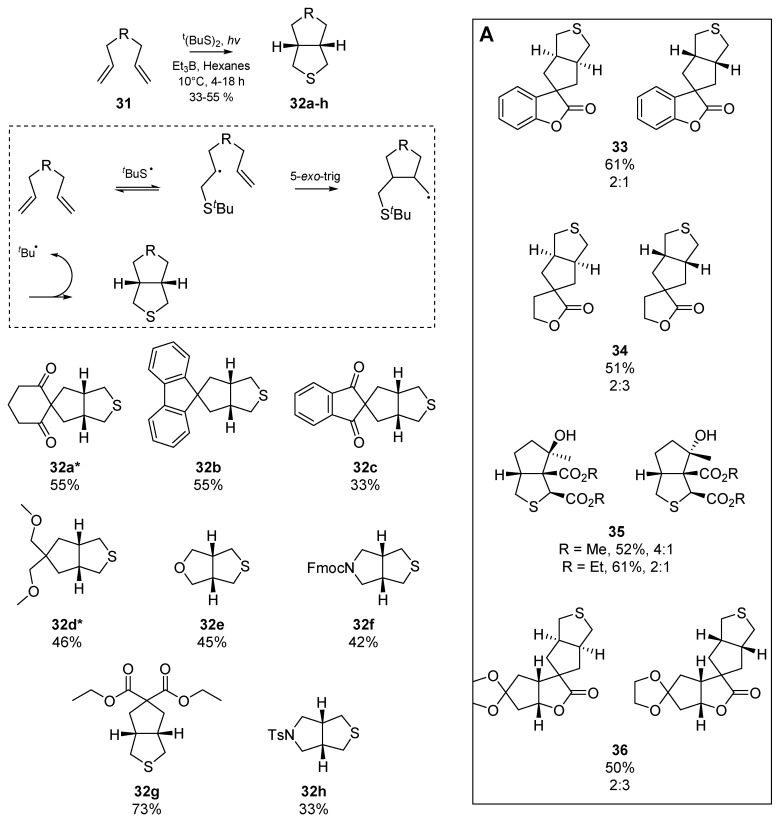
From this point, Harrowven and co-workers delved deeper into the dynamics of the thiyl mediated cyclization and exploited the reaction for the cyclization of 1,6-dienes. A further account published by Harrowven et al. [43] supersedes the earlier aplysin work, and a diverse library of thiabicyclo[3.3.0]octanes were synthesised through the co-cyclization of 1,6-dienes with concomitant sulfur atom transfer. The authors proposed that if the rules provided by Beckwith for ring closure [44,45] were obeyed, a second cyclization (following initial diene cyclization), of a radical intermediate predisposed to homolytic substitution at sulfur would be observed. In their screening of reaction conditions, Harrowven et al. identified that photolysis of a solution of the diene and di-tert-butyl disulfide in hexanes provided the desired tetrahydrothiophene; reactions only reached an appreciable efficiency when a quartz photochemical cell was employed (instead of a Pyrex cell), and addition of triethylborane greatly accelerated the rate. Up to 31% starting material was recovered in some cases, yet the tolerance of the thiyl radical addition/cyclization is evident in the examples shown below (Scheme 6). Insert A shows some of the more exotic examples furnished by Harrowven and co-workers. In a follow up letter Harrowven et al. expanded the cyclization to a solid support [46] using a carbodiimide coupling to a Wang-type resin [47], noteworthy in that very few tin-free radical cyclizations have been conducted on solid supported substrates [48,49,50]. Relying instead on thermolysis of AIBN for initiation, the authors demonstrated conservation of both yield and diastereoselectivity with respect to their preceding solution phase results. Further utility of the reaction in terpenoid synthesis by Barrero et al. [51] involved a short synthesis of (±)-dehydroiridomyrmecin, a natural iridoid. Again using two equivalents of thiophenol and one equivalent of AIBN in refluxing benzene afforded the backbone for this biologically interesting iridane, and comprised the first total synthesis of this substrate.
Scheme 6.
The 1,6-diene co-cyclization by Harrowven et al. * Did not require Et3B as an additive. Insert A: Examples by Harrowven et al.
2.3. Dienes as Precursors to Heterocycles.
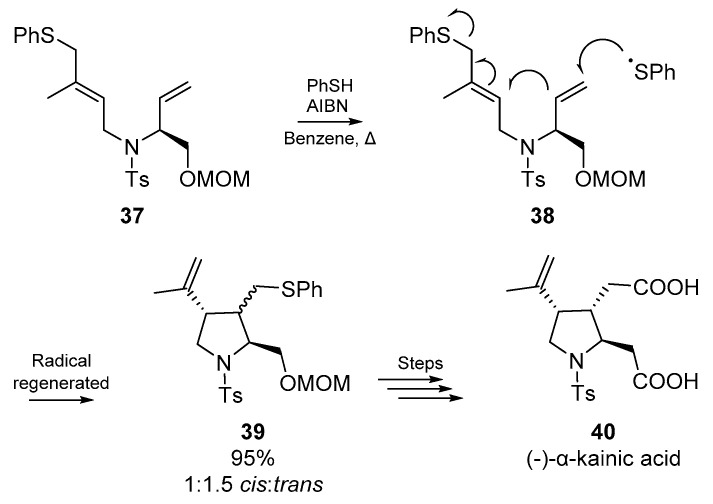
Miyata et al. published two brief accounts on the application of thiyl radical addition/cyclization; these accounts comprise parts ten and eleven in a series on heterocycle synthesis via radical cyclization from the Naito group [52]. The first of these accounts employs a familiar cyclization strategy, but includes a clever elimination step (Scheme 7) which permits the use of a catalytic amount of the thiol (thiophenol). Miyata and co-workers designed a sequential formation of two bond and one bond cleavage, which furnished a trisubstitutued pyrrolidine ring 39. This methodology was implemented by the authors in the total synthesis of the marine product (−)-α-kainic acid 40, a potent neuroexcitatory amino acid agonist with central nervous system activity [53]. Good yields were obtained using low loading of equimolar thiophenol and AIBN.
Scheme 7.
Strategy employed by Miyata et al. in the total synthesis of (−)-α-kainic acid 40.
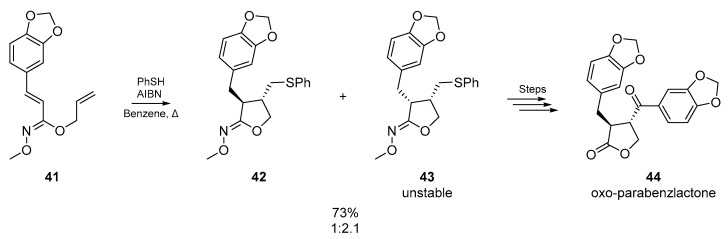
A later example by Miyata et al. [52] features a variation on previous approaches, many of which involve backbones composed mainly of carbon (Scheme 8). Using dienes connected via hydroximates 41, the authors reported a convenient conversion of cyclic hydroximates formed from thiyl radical addition/cyclization, into the corresponding α,β-disubstituted-γ-lactones. The hydrolysis of the cyclic hydroximates gave the desired cis- and trans-lactones in excellent yield, and the methodology was employed in the synthesis of (±)-oxo-parabenzlactone. Interestingly, the unstable cis-isomer 43 formed via cyclization could be irreversibly resolved into the synthetically more valuable trans-isomer 42 by treatment with sodium ethoxide.
Scheme 8.
Miyata et al. apply the thiyl radical cyclization, and resolve the stereoisomers.
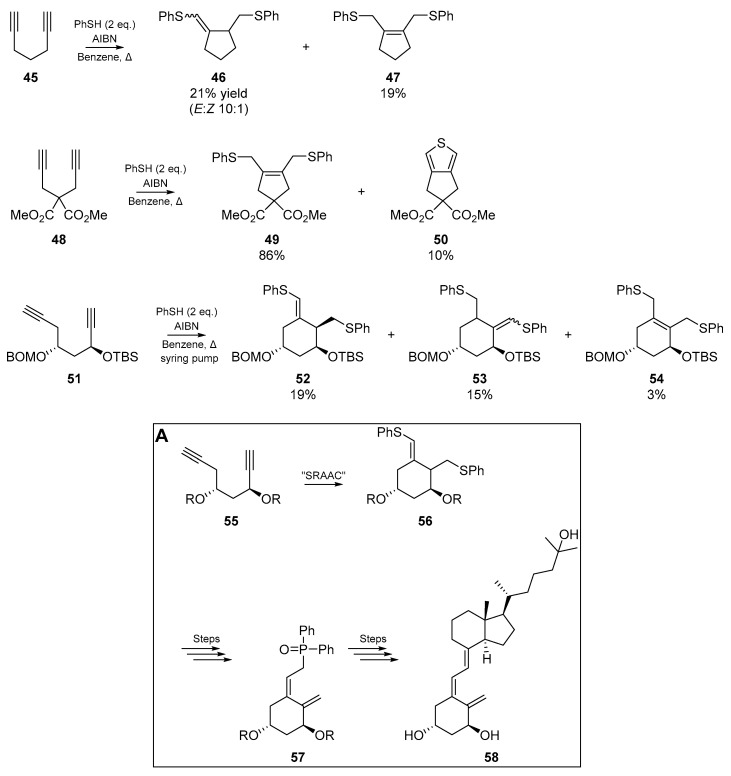
Miyata et al. [54] also published a wealth of cyclizable diyne systems, in their “sulfanyl radical addition-addition-cyclization” (SRAAC) strategy. The unbranched diynes discussed herein yield exo-olefins upon completion of the cascade, and those diynes possessing quaternary carbons furnished cyclized endo-olefins (Scheme 9, vide infra). The authors applied this chemistry to the synthesis of a small fragment of 1α,25-dihydroxy vitamin D3. In agreement with results published by their contemporaries, the 1,8-diyne species failed to cyclize under a variety of conditions.
Scheme 9.
Diyne cyclizations published by Miyata et al. Insert A: Synthesis of Vitamin D3 derivative.
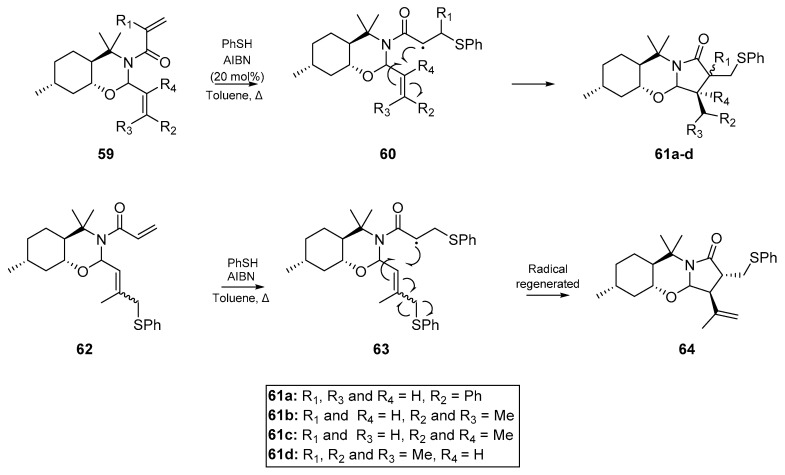
Pedrosa et al. [52] shortly thereafter reported on the carbocyclizations of perhydro-1,3-benzoxazines, furnishing 3,4-disubtitued pyrrolidinone derivatives with good diastereoselectivity and complete regioselectivity. A wide array of substituted products were synthesized, and substitution at both olefin termini was well tolerated. The author’s results further supported the findings of Miyata et al. [55], in that a phenyl thioether 62 suitably disposed to radical elimination can generate secondary olefins 64 as a result of the carbocyclization process (Scheme 10). The preference of the thiyl radical SOMO to interact with electron deficient alkenes was also exploited, with the nascent phenythiyl radical attacking the acrylic olefin before cyclizing through the expected 5-exo-trig pathway onto the substituted alkene.
Scheme 10.
The 3,4-disubstituted pyrrolidinones by Pedrosa et al.
2.4. Moving Forward
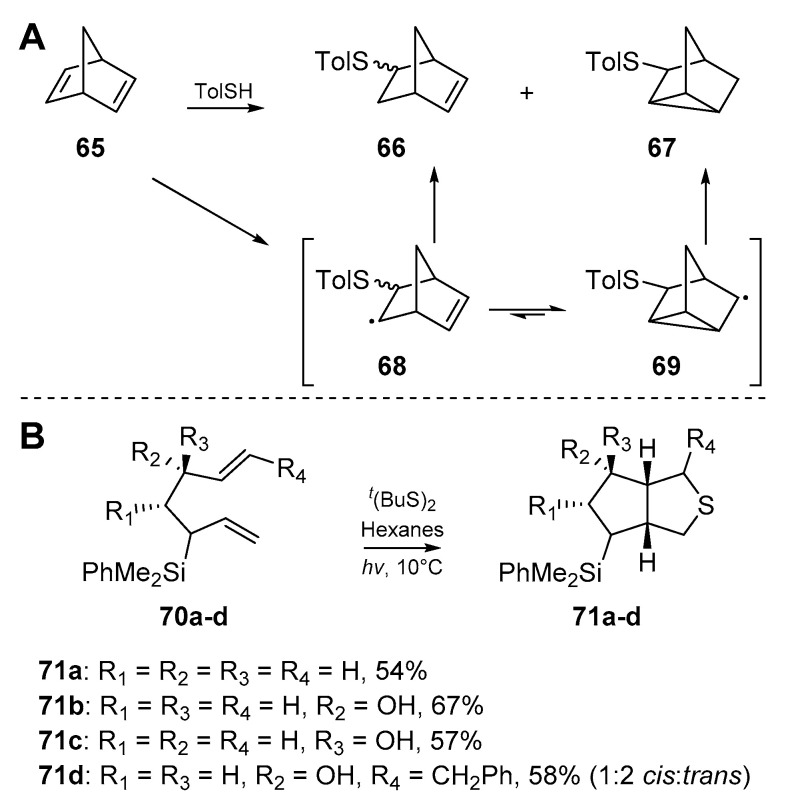
At this juncture, the literature is composed of further accounts on the tolerance of the thiyl radical addition/cyclization process to varying functional groups (FGs), and steric demands. Hodgson et al. [56] demonstrated the utility of this process to access sterically challenging substrates, and synthesized a number of 7-thio-substituted norbornenes from norbornadienes (Scheme 11A). The diene cyclization product 67 appeared to dominate over radical quenching from solvent or otherwise, with a ratio of 1:6 of 66:67 obtained. This work also constitutes one of the first examples in this field of 3-exo-trig cyclization, further highlighting the potential of thiyl radicals to access sterically demanding substrates. A communication by James et al. [57] investigated the tolerance of the cascade in question to 3-silylheptanyldiene systems (Scheme 11B). While sulfur-mediated cyclizations only composed a short portion of this work, a number of substituted dienes were successfully cyclized, providing the familiar thiabicyclo[3.3.0] skeleton in one step due to the SHi mechanism previously discussed.
Scheme 11.
(A) Work by Hodgson et al. (B) Cyclization work by James et al.
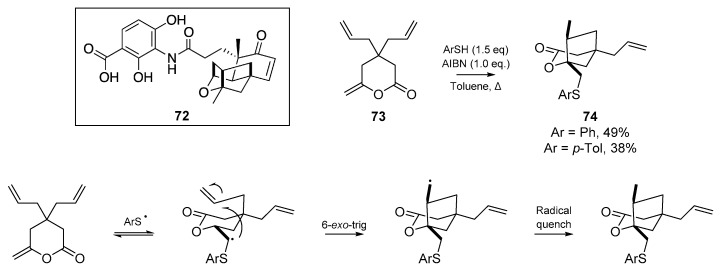
Kamimura and co-workers published an account [58] on the application of radical cascades for the synthesis of the oxa-tricyclic core of platensimycin 72, an antibiotic that inhibits two enzymes in fatty acid biosynthesis (Scheme 12). In a concise account from 2015, the authors describe a short thiyl radical triggered cyclization in their attempts to form the multicyclic core, and while their cyclization of diallymethylene-δ-lactone 73 proceeded in a highly stereoselective manner, the incorrect stereochemistry was obtained.
Scheme 12.
Synthetic attempts at accessing the multicyclic core of platensimycin 72, with mechanism.
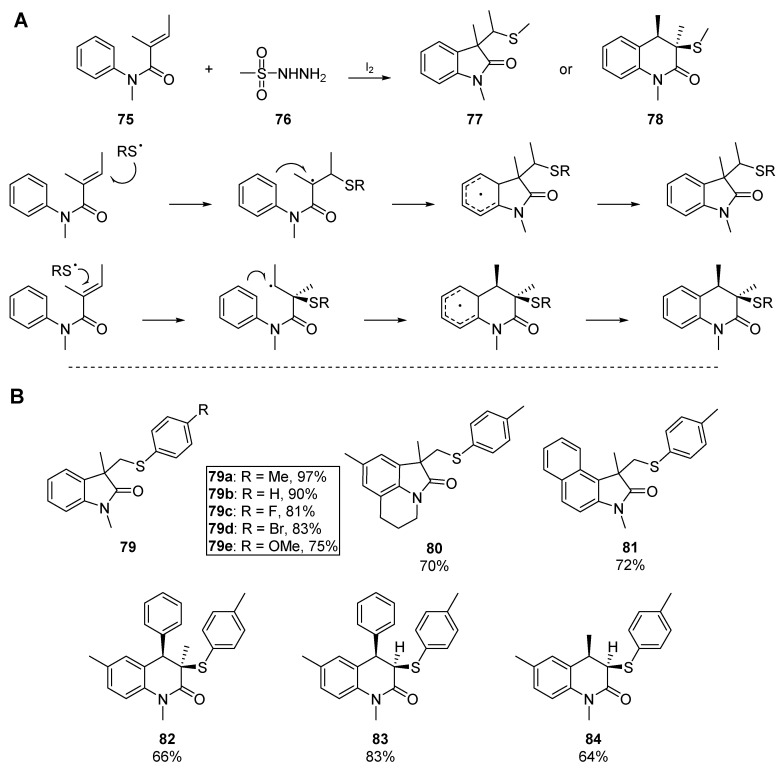
Wang and colleagues have also been active in this area, and published a tandem sulfenylation/cyclization of N-arylacrylamides through a 5-exo-trig mechanism, which boasted excellent yields and control of regioselectivity for certain substrates (Scheme 13) [59]. This methodology furnished a number of 3-(sulfenymethyl)oxoindoles 77 and 3-sulfenyl-3,4-dihydroquinolin-2(1H)-ones 78. Up to this point in the literature, the vast majority of thiyl radical precursors have been the respective thiols, thioacids or disulfides; this account offers an unconventional precursor to aryl thiyl radicals. The authors propose a mechanism in which iodine functions as an oxidant, reductant, and radical initiator, to generate aryl thiyl radicals from the corresponding sulfonyl hydrazides 76. This offers an accessible route to thiyl radical addition/cyclization as sulfonyl hydrazides are generally moisture-compatible solids, lack the expected odour and are amenable to handling by organic chemists outside traditional ‘sulfur labs’. This methodology permits the cyclization of α,β-disubstituted acrylamides, substrates which have previously been found recalcitrant to cyclization. It is noteworthy that the α,β-unsubstituted acrylamides failed to cyclize, instead furnishing bisthioethers.
Scheme 13.
The cyclization mechanism (A) and select examples (B) by Wang and co-workers.
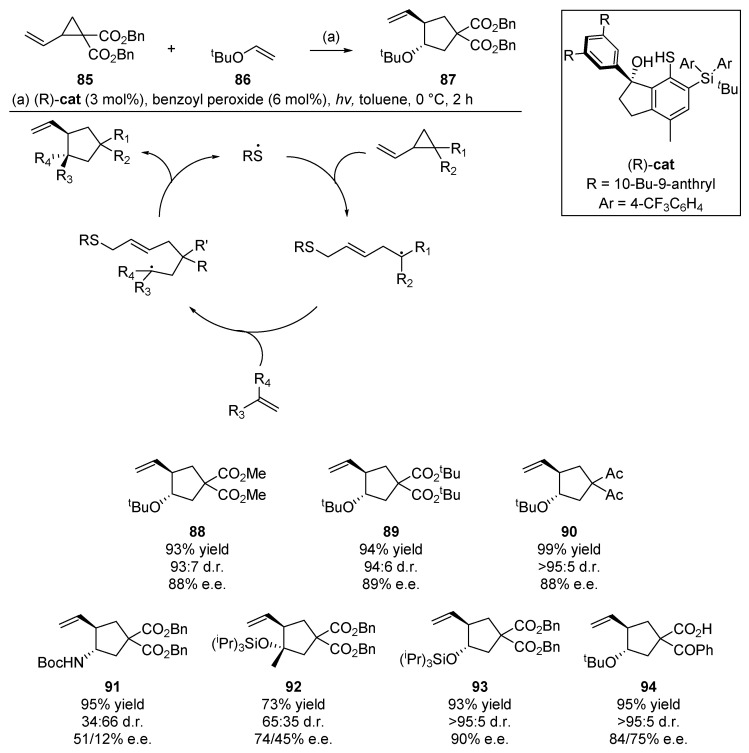
An example which highlights the significant potential of the thiyl radical addition/cyclization in diene cascade reactions comes from Hashimoto et al. [60]. This publication from the Maruoka group details an organic thiyl radical catalyst for enantioselective cyclization (Scheme 14). Through the careful design of a chiral pocket around the chiral thiol precatalyst, the authors were able to exact an enantio- and diastereoselective C-C bond forming cyclization with concomitant extrusion of the thiyl radical at the end of the cascade. With 3 mol% loading of a binaphthyl-modified catalyst and benzoyl peroxide to generate the thiyl radical, a wide array of substrates were successfully cyclized by this catalyst, in yields up to 99% and diastereomeric rations of up to 95:5. Indeed the catalyst loading could be scaled as low as 1 mol% at larger scales (1 mmol) [61]. In their studies to optimize catalyst structure, Hashimoto et al. were able to attain yields of up to 95%, with a diastereomeric ratio (d.r.) of 95:5 and enantiomeric excess (e.e.) of 86% using sunlight as the photolysis source. Hashimoto et al. continued to develop this methodology, and in 2016 published a communication on the use of bulky thiyl radical catalysts for [3 + 2] cyclizations, this time with N-tosylvinylaziridines and alkenes [62].
Scheme 14.
Organocatalytic work published by Hashimoto et al., with a selection of substrate scope.
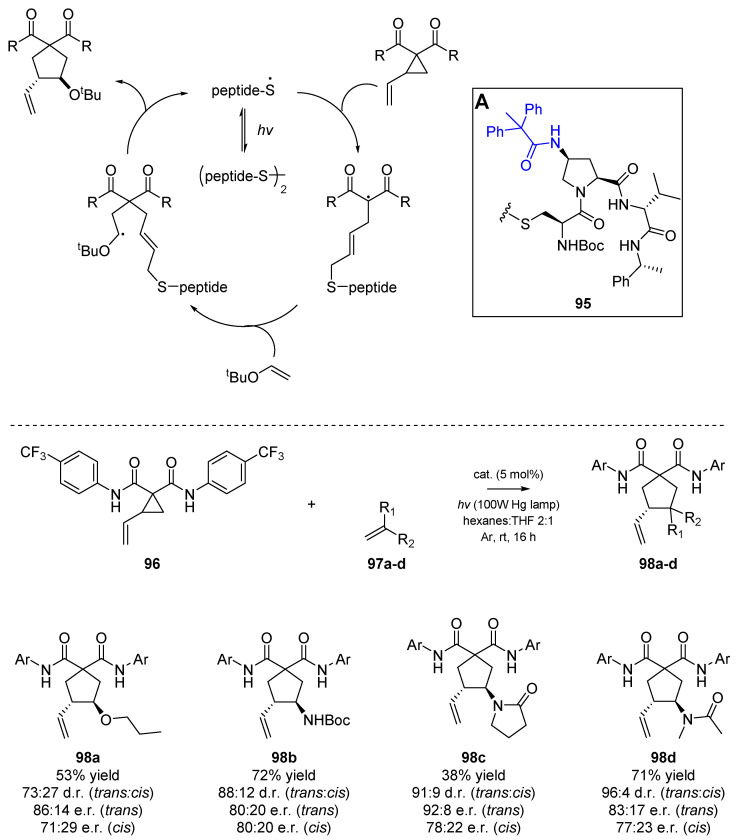
In a further example of the application of thiyl radicals within organocatalytic systems, work analogous to that of Hashimoto et al. was published by Ryss et al. (Scheme 15) [63]. This study detailed the use of disulfide-bridged peptides 95 to mediate enantioselective ring-opening and cycloaddition of vinylcyclopropanes. Using photolysis to cleave the disulfide bridge of a suitable cysteine-based dimeric peptide, two equivalents of the thiyl radical per equivalent of catalyst were generated in situ. The authors provided an extensive analysis of potential backbone interactions between their malonic acid scaffold and the catalyst, and found that while the malonic esters provided mostly racemic products, the corresponding amide-substituted vinylcyclopropanes 96 were more successful in the discussed transformation. This was presumed by Ryss et al. to originate from hydrogen bonding interaction between the peptide catalyst and the substrate. In their design of the catalyst the appendage attached to the proline backbone was altered; one example (in blue) is shown in Insert A below.
Scheme 15.
Enantioselective work by Rhyss et al., employing a peptide-based thiyl catalyst (Insert A, 95).
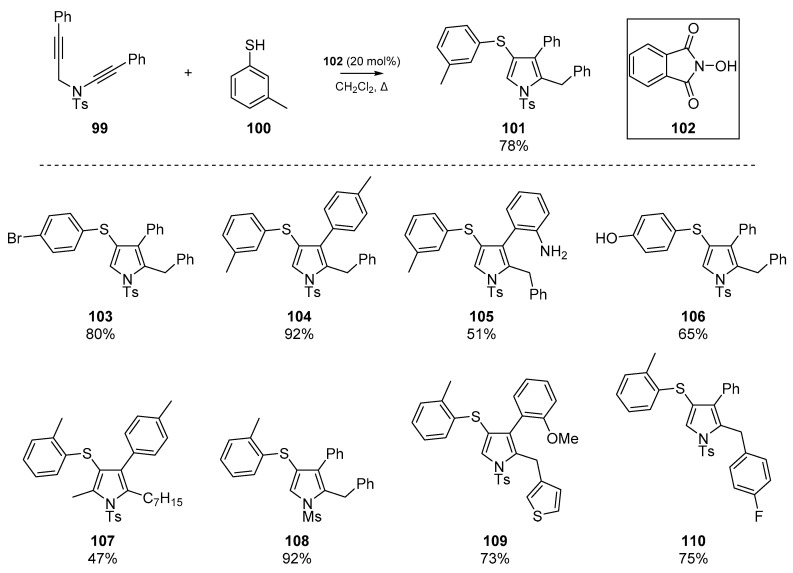
The use of thiyl radical addition/cyclization has been previously described as a useful route to thiophenes, through clever use of homolytic substitution reactions. Possibly the most recent cyclization of a diyne substrate was reported by Dutta et al., in which a number of pyrroles were prepared (Scheme 16) [64]. This account describes the use of ynamides and alkynes for cyclization; the authors report a ‘serendipitous observation’ that the reactivity of simple alkynes was found to exceed the traditionally more reactive ynamides moieties for cyclization. This led to the development of a methodology in which an alkyne 99 is first attacked by the aryl sulfenyl radical formed from 100, which then cyclizes in a 5-exo-dig pathway onto the ynamides to generate a number of novel 4-thioaryl pyrroles 101, 103–110. The methodology tolerates an array of functional groups and N-protecting groups, and forbearance to modification of the ynamides and propargylic terminus was also recorded.
Scheme 16.
Work by Dutta et al. 102 operates as a radical initiator, and scope examples are shown.
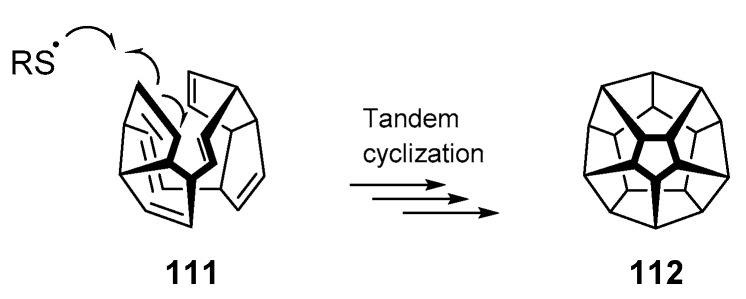
Thus far, this review has shown numerous examples that have employed the thiyl radical addition/cyclization cascade for a variety of substrates. But what are the limitations of the process? A 2017 publication [65] by Wang and colleagues poses an interesting question—is it possible that radical polycyclization could yield dodecahedrane 112, one of the top synthetic targets sought by organic chemists for decades [66]? Woodward failed in synthesizing this Platonic hydrocarbon [66,67], but the Paquette group succeeded the first synthesis in the early 1980s [68]. The account by Wang et al. assesses the viability of thiyl radicals as mediators of polycyclizations to construct complex molecular architectures (Scheme 17). Using density functional theory (DFT) calculations, the authors concluded that the process is energetically viable in both a kinetic and thermodynamic sense. While the reversibility of the thiol-ene and potential quenching by hydrogen atom transfer were highlighted, the quantum chemical calculations indicate the possibility that one day, these complex geometries may be obtained using simple thiyl radical cascades.
Scheme 17.
Multiple 5-exo-trig cyclizations to give dodecahedrane 112 are estimated to be possible.
3. Heterogeneous Substrates: Enynes and Other Mixed Systems
3.1. Origin
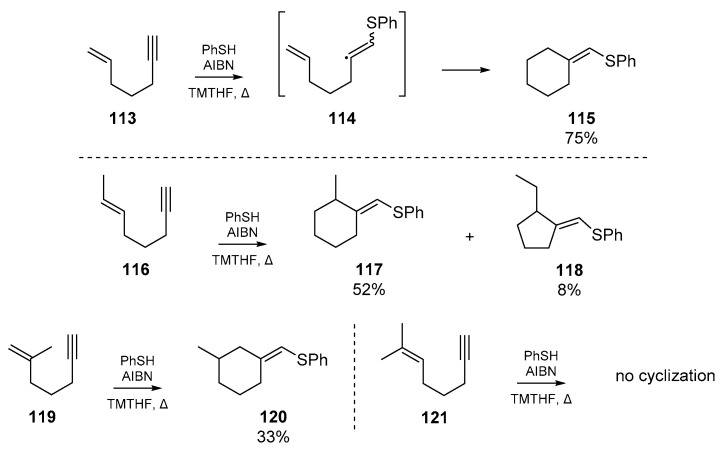
The two earliest examples of thiyl radical cascades orchestrated on heterogeneous or ‘mixed’ systems were both reported in 1987 (Scheme 18). The first of these sulfur-triggered cyclizations, reported by Broka et al. [69], in which nascent vinyl radicals 114 formed via thiyl addition to enynes 113, regioselectively cyclized to furnish the respective cyclohexylidine 115. The authors identified that under ionic conditions (in this case, refluxing thiophenol with the enyne in benzene), very little cyclization takes place as the rate of hydrogen abstraction by the vinyl radical outcompetes the cascade reaction. By switching to a procedure with AIBN, and slow addition of the thiol, the authors were able to favor cyclization over quenching of the radical cascade. This gave 115 as the cyclized product, in a 75% yield when 2,2,5,5-tetramethyltetrahydrofuran (TMTHF) was used as the solvent. Broka et al. proposed that equilibration of the cyclopentylmethyl radical, formed via 5-exo-trig cyclization of 114, to the more stable cyclohexyl isomer prior to hydrogen abstraction facilitates the generation of 115. A number of other systems were tested to examine the generality of enyne cyclization, and it was found that as the substitution of the olefin increased, the success of cyclization severely diminished; for example, enyne 119 gave only 33% cyclized products, and substrate 121 gave none.
Scheme 18.
Initial work by Broka et al. on the generality of enyne cyclization.
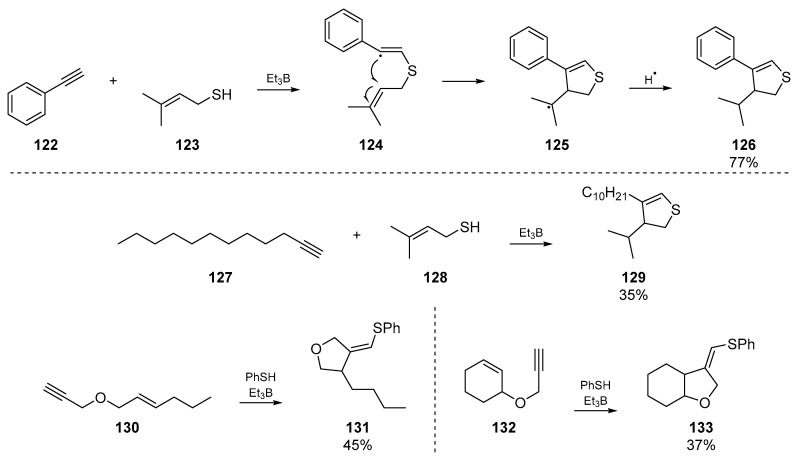
Ichinose et al. reported an account of triethylborane based radical initiation for addition to acetylenic compounds. The authors explored addition of thiyl groups to terminal acetylene compounds [70], which proceeded regioselectively but not stereoselectively (Scheme 19). The three examples of interest to this review are that of an intermolecular dihydrothiophene synthesis 129, and the vinyl radical cyclization of two acetylenic olefins 130 and 132. Analogous to the work carried out by Broka et al. in the same year, a clear trend in the substitution and steric demands around the olefin was identified.
Scheme 19.
Work published by Ichinose et al.
3.2. Broadening the Scope
Broadening the scope of the review to consider ‘mixed’ unsaturated systems, the inclusion of unsaturation at heteroatomic moieties must also be considered. A very early example form El Kaim et al. [71] offers 5- and 6-exo-trig cyclizations of oxime ethers and hydrazones. By generating thiyl radicals from thiophenol, a number of ethylenic and acetylenic hydrazones were cyclized under thermal initiation with AIBN in refluxing cyclohexane (Scheme 20). Analogous to early work by Padwa et al. previously discussed, the cis isomer predominates in the cyclization, despite the inclusion of heteroatoms. Furthermore, no trace of uncyclized product was detected for the conditions reported herein. The methodology published in this account widened the scope for cyclizations on the C=N bond, and general examples from this work are detailed below to show the range of functional group tolerance. Despite this clear forbearance to varying functionality, example 138 did not cyclize.
Scheme 20.
Scope explored by El Kaim et al. Insert: Abbreviations used in the original work.
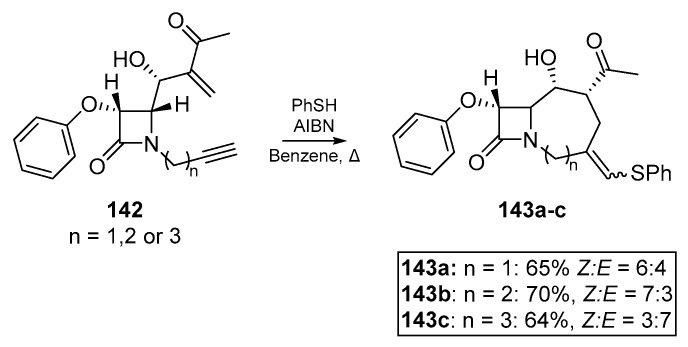
In an account by Alcaide et al., an early medicinal chemistry application of the thiyl radical addition/cyclization cascade is described [72]. Highly functionalized rings fused to a common pharmacophore, a β-lactam, were synthesized via radical cascade chemistry on a number of Baylis-Hillman adducts, providing the basis for this stereocontrolled and chemoselective approach shown in Scheme 21. Several phenylthiovinyl derivatives 143a–c of the yne-ene based β-lactams 142 were obtained, and subject to further transformation. It should be noted that in the case of n = 4 i.e., the 5-hexynyl conjugated β-lactam, neither the thiyl radical addition nor classical methods such as organotin chemistry could cyclize the substrate. It is also noteworthy that it was possible for the authors to initiate thiyl-yne conjugation prior to the Michael addition, which one would expect to compete for the thiol via thia-Michael addition.
Scheme 21.
The key radical step in an account published by Alcaide et al.
With each publication that bolsters the robustness of thiyl radical utility in organic synthesis, further technologies such as flow chemistry and microwave assisted synthesis become available to the sulfur chemist. In one such paper on the use of microwave assisted radical cyclizations, Lamberto et al. [73] discuss the use of a wide variety of thiols to generate pyrrolines and pyroglutamates from alkenyl and alkynyl isocyanides (Scheme 22). This use of microwave ‘flash-heating’ technology boasts the advantage of significantly faster reaction times, and increased yields. It is worth noting that in the case of alkynyl isocyanides, protection of the alkyne terminus as a silyl ether was necessary, as unprotected substrates suffered from severely diminished cyclization yield. In a further publication [73] the authors demonstrated the applicability of this methodology to solid phase synthesis, and furnished pyroglutamates and 2-mercaptopyrrolines from three polymer-supported isocyanides using commercially available resins.
Scheme 22.
General scheme of work published by Lamberto et al.
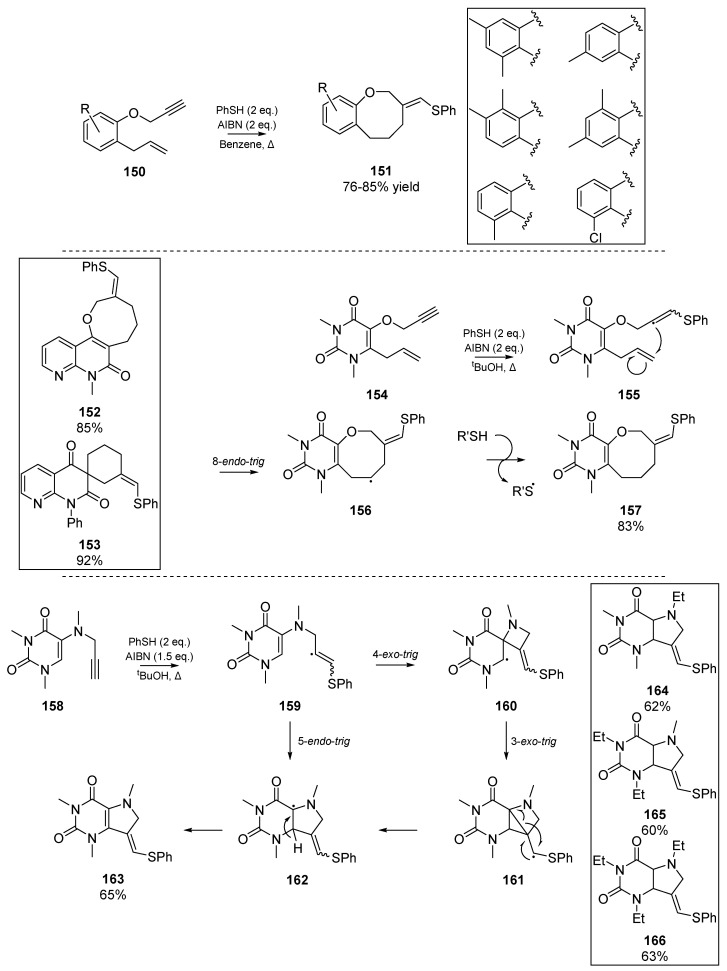
In the mid-2000s, a relative ‘renaissance’ in the use of thiyl radicals as instigators of carbocyclizations was observed. While the present review is far from exhaustive, a description of the main outcomes from key research groups will be discussed. Majumdar and co-workers have been particularly active in this area, publishing heterogeneous cyclizations for a range of heterocycles, including cyclic ethers 157, pyrimidine-fused azocine derivatives [74], furo- and pyranocoumarin species [75], a range of pyrrolopyrimidines as 9-deazaxanthine analogs 163 [76], as well as benzoxocine 151 [77] and oxepin derivatives [78]. In several publications on the topic of heterogeneous carbocyclizations between 2007 and 2010, Majumdar et al. have reported a number of difficult annulations, and broadened the role of thiyl radicals to catalyzing Claisen rearrangement, and undergoing 8-endo cyclizations for accessing sesquiterpenes. In the interest of space constraints, these accounts will not be described in full detail herein and instead a selection of key achievements is shown in Scheme 23.
Scheme 23.
Selected works by Majumdar et al. Inserts: Representative examples from each paper.
Montevecchi et al. detailed the addition of thiyl radicals to alkynyl azides [79] as well as attempted cyclizations onto carbonyls and cyano groups [80]. The group continued to explore these processes, and also published on the relative reaction rates of thiyl radical addition and hydrogen atom abstraction [81]. In their investigation of alkynyl azides as suitable substrates for thiyl radical mediated cyclization, the authors identified two suitable 2-sulfanylvinyl radicals which underwent 5-exo cyclization onto the azido moiety, preventing intramolecular addition to the sulfanyl aromatic ring (Scheme 24). In the course of this project, Montevecchi and co-workers rationalized the ability of these two radicals to cyclize by considering the resonance stabilization of the nascent triazenyl radicals, caused by delocalization into the indole ring. They also reasoned that the failure of other radicals to undergo cyclization may be explained by unfavorable polar effects in the transitions state between the more electrophilic vinyl radicals and the aliphatic azido moiety. Note that while benzyl mercaptan is shown as the thiol agent in Scheme 24, similar success was observed with thiophenol as the thiyl radical precursor.
Scheme 24.
Alkynyl azide cyclizations reported by Montevecchi et al. Insert A: Uncyclizable radicals.
Indeed, even if cyclization attempts fail and do not yield any desired product, these results are nonetheless valuable. In a follow up publication to their success with alkynyl azides, Montevecchi et al. identified several sulfanylvinyl radicals which did not undergo any 5-membered cyclization to esteric or thioesteric carbonyl moieties, nor did they obtain any evidence of successful toluenesulfanyl radicals addition to a number of alkynyl nitriles. Montevecchi and co-workers did, however, identify two radical intermediates which were successful in cyclizing onto aromatic cyano groups, which furnished a useful synthetic route to indenones.
3.3. Cyclizations onto Nitrogenous Moieties, and Further SHi Chemistry
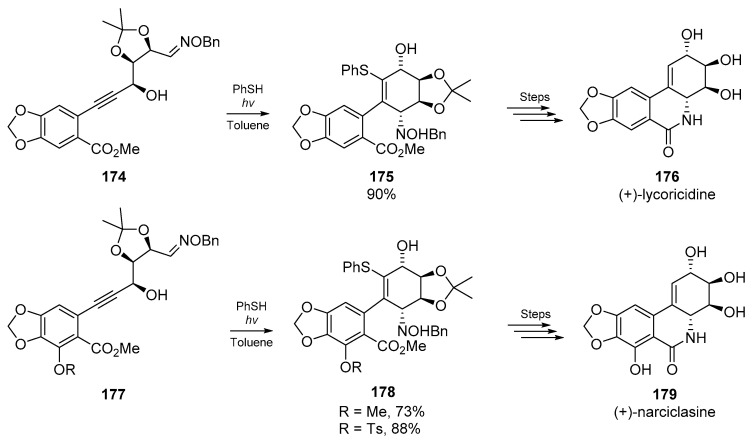
An excellent example of the convenience of thiyl radical addition/cyclization cascades with heterogeneous substrates comes from Keck et al. who employed 6-exo cyclizations of substituted thiovinyl radicals with oxime ethers for the synthesis of high value alkaloids. In their total synthesis of (+)-lycoricidine 176 and (+)-narciclasine 179, a nascent vinyl radical is generated via addition of a phenylthiyl radical to a disubstituted alkyne. Thiyl addition to the alkyne, and subsequent addition to an oxime ether furnishes the corresponding ring system 175 or 178, which was then further transformed to the target alkaloid. In the course of these studies, the authors noted that no formal synthesis of narciclasine had been accomplished, and thus applied the same methodology with some modification to the substrate backbone (Scheme 25). Keck and co-workers also identified the reverse regiochemistry of stannyl and phenylthiyl radicals to the same alkyne in this account.
Scheme 25.
Total syntheses of lycoricidine and narciclasine by Keck et al., involving radical cyclization.
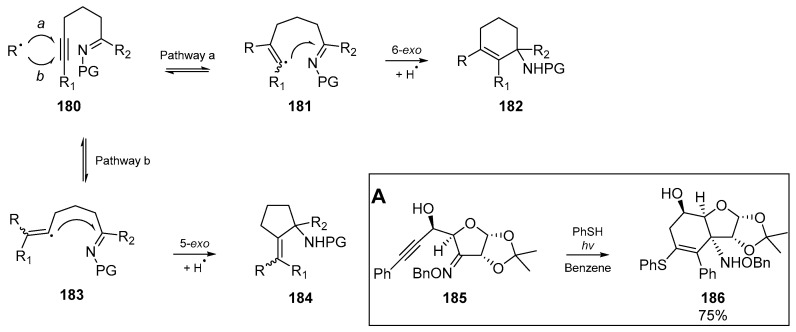
Fernández et al. published a one-step cyclization of ketimines into cyclized allylamine derivatives in the mid-2000s, which again relies upon a tandem radical addition/cyclization strategy (Scheme 26) [82]. Using a substrate inspired by the research group’s interest in tetrodotoxin, a number of thiyl radical mediated cyclizations were attempted. The authors sought to exert control over cyclization through the 6-endo versus the 5-exo mode. Indeed by substitution the alkyne terminus with a phenyl group 185 restored some control over the cyclization mode, and through using the traditional PhSH/AIBN couple, a number of conditions were explored. Interestingly, the use of a medium pressure mercury lamp as the irradiation source permitted the use of just 1.05 equivalents of thiophenol and no AIBN was required; these conditions gave the highest cyclization yield of 75%. However this success was reliant on the alkynyl substituent being a phenyl group, as esters and ethers did not promote formation of the desired cyclohexeynlamine 186.
Scheme 26.
Possible ketimine cyclization pathways. Insert A: Optimized cyclization by Fernández et al.
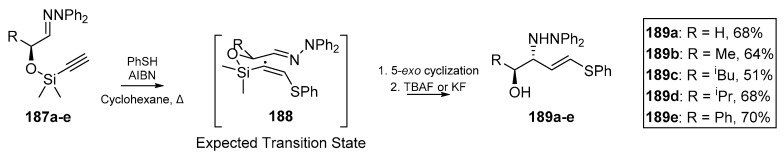
Friestad et al. published an account of chiral hydrazone cyclization, using silicon tethered alkynes (Scheme 27) [83]. A one pot process involving thiyl addition to the ethynyl terminus and a desilylation with standard fluoride sources furnished (E)-vinylsulfides 189a–e. The authors report this cascade as the synthetic equivalent of an acetaldehyde Mannich reaction. While the intermediate heterocycle was not isolated or explicitly discussed in this publication, it is implied that the 5-exo mode of cyclization predominates with the nascent thiovinyl radical. Collapse of the intermediate cyclized product following fluoride-mediated cleave provides the desired product in good yield. Friestad and Messari had also developed a diastereoselective vinyl addition to chiral hydrazones in an earlier account [84], and proceeded to develop a similar chiral hydrazone cyclization with silicon-tethered alkenes analogous to 187a–e [85]. In the first of these accounts, vinyl addition to α,β-dihydroxyhydrazones was also investigated, with excellent extension of the scope of their methodology and without extensive protection strategies or hydroxyl group differentiation. The methodology employed by Friestad et al. hinges on the exploitation of temporary silicon-tethers, which permits facile and stereocontrolled vinyl addition to C=N bonds to furnish valuable synthetic scaffolds, such as acyclic amino alcohols [84].
Scheme 27.
(E)-vinylsulfide work by Friestad et al.
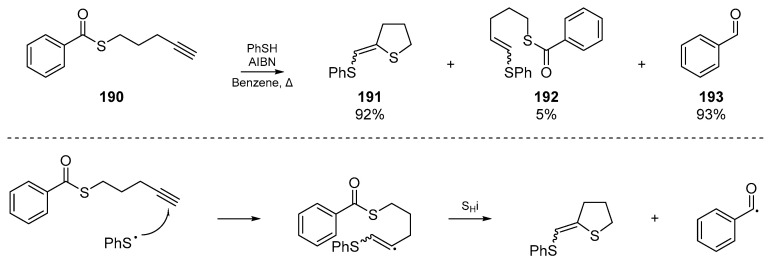
Previously discussed work by Padwa et al. has shown that a sulfur atom suitably disposed to undergo SHi can extrude an acyl radical under the appropriate conditions. Almost twenty years after this account, Benati and co-workers exploited this methodology for the production of aldehydes under tin and silicon free conditions (Scheme 28) [86]. Benati’s radical cascade reaction between thiophenol and pentynylthiol esters 190 provides both aromatic and aliphatic aldehydes, and permits a formal reduction of esters to aldehydes even with substituents which would normally be very sensitive to any reducing conditions. The formation of concomitant dihydrothiophenes 191 frames this account as relevant to the present review, and offers a useful synthetic route to this sulfur-based heterocycle; albeit with a lack of diastereoselectivity.
Scheme 28.
Radical production of aldehydes, by Benati et al.
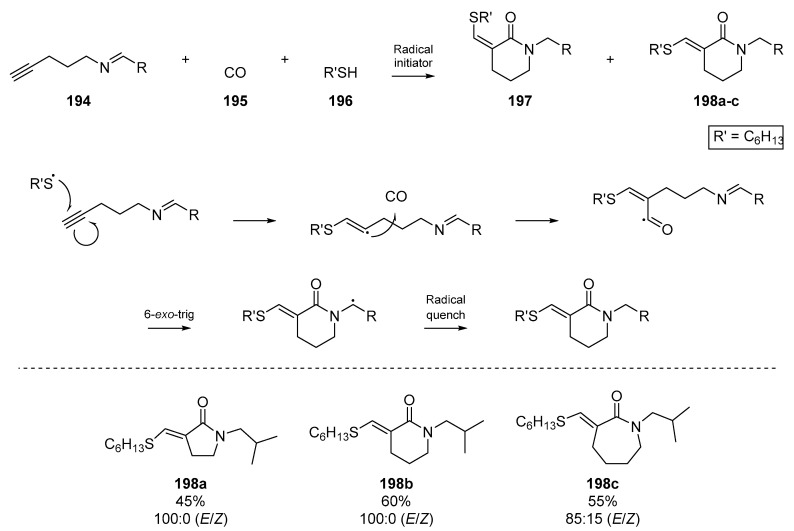
In a more exotic example of radical annulation, a powerful method to access lactams was described by Tojino et al., which proceeded with incorporation of carbon monoxide 195 into the incipient heterocycle (Scheme 29) [87]. This cyclizative radical carbonylation of suitable azaenyne substrates 194 generated a number of nitrogenous heterocycles, with a comparison between silicon and thiol based radical precursors. E/Z selectivity was observed for the thiol and silane mediated cyclizations in which 100% E-selectivity was observed in many cases (e.g., 198a and 198b); this is in contrast to classical tin-based methods, which mostly initiate a Z-selective process [88].
Scheme 29.
Work by Tojino et al., displaying excellent E/Z selectivity. The proposed mechanism is shown.
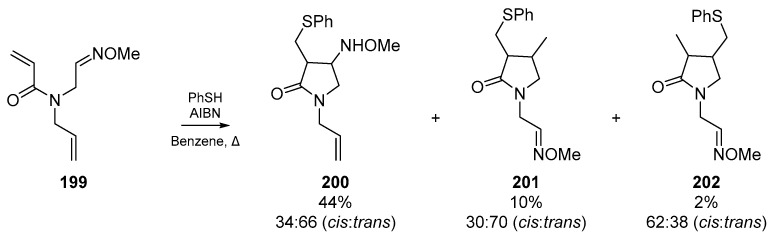
Okiko Miyata and co-workers published an account in their series of radical cyclizations for heterocycle synthesis, in which selectivity for cyclization onto oxime ethers was reported (Scheme 30). This account details some of the previously discussed selectivity for radical addition to electron deficient alkenes, and the key advancement of this work is the generation of 5-membered lactams with alkyloxyamino moieties 200, which is the result of preferential addition of an α-carbonyl radical to the oxime ether 199. Analogous to the explanation in Section 2.2., the regioselectivity of this process can be explained by SOMO/HOMO interactions, and the stability of the radical intermediate [89].
Scheme 30.
Oxime ether work by Miyata et al.
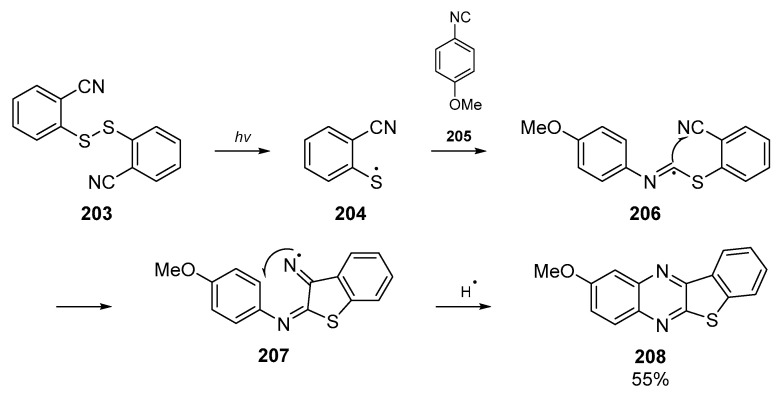
A short communication from Nanni et al. on the regioselectivity of imidoyl radical cyclizations features aryl thiyl radicals, generated from photolytic cleavage of a disulfide (Scheme 31) [90]. This paper, published in the 2000s, expands upon original work conducted in the late 90s by the authors who could not unambiguously assign the identity of the products at the time [91]. This reaction between thiyl radicals and isocyanides comprises a cascade reaction in which radical 204 attacks the isocyanide group of 205. Concomitant nitrogen-centered radical formation allows the cascade to continue through cyclization into the aromatic backbone 207. The authors postulated that the fast, reversible cyclization of the imidoyl radical onto the sulfur center is offset by the slow but essentially irreversible radical addition to the cyano group. Two reaction pathways are proposed to explain the products observed, and the one to yield the major product 208 is shown below in Scheme 31.
Scheme 31.
Reaction pathway to major product isolated by Nanni et al.
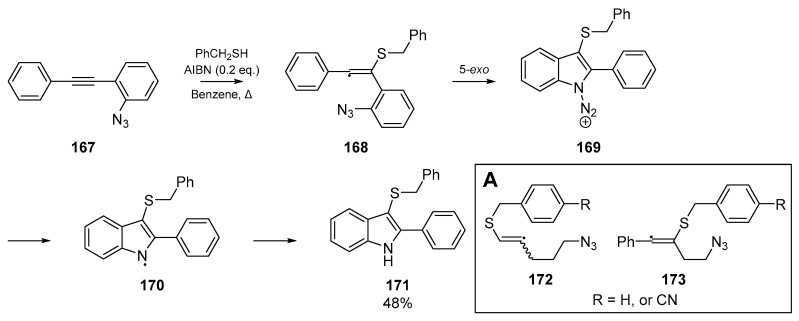
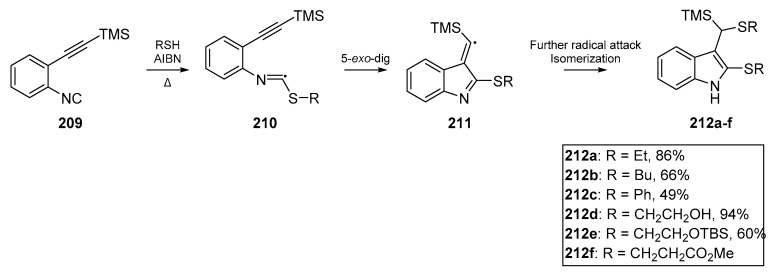
In the same year, Rainer and Kennedy reported a cascade to dithioindoles 212a–f from arylisonitriles bearing alkynes as ortho substituents 209 [92]. A number of indoles were successfully synthesized, with the 5-exo-dig process appearing to outcompete other cyclization pathways (Scheme 32). A number of thiyl radical precursors were tested using a model substrate, and successful cyclization yields ranged from ca. 50 to 95%
Scheme 32.
Results published by Rainer et al. on arylisonitrile cyclization methodology.
A similar approach was published by Mitamura et al., relying on cyclization of the thioimidoyl radical onto an ortho alkene [93]. Radical cyclization of ortho-vinyl and ortho-allyl phenylisocyanides furnished bisthiolated indole and quinoline derivatives in moderate yields. This approach employed a photoinduced thiotelluration, and while tellurium is absent from the bisthiolated product, it appears to play a crucial role in the reaction mechanism; this is analogous to systems employing the diphenyl disulfide and diphenyl diselenide pair. It is probable that the tellurium/sulfur combination affords regioselectivity due to the high reactivity of the phenylthiyl radical [94], and the high carbon radical capturing ability of the phenyltelluride species. It is noteworthy to consider that the relative rate of dichalcogenides for capturing carbon-entered radicals is of the order kPhSSPh:kPhSeSePh:kPhTeTePh = 1:160:630 [95,96,97].
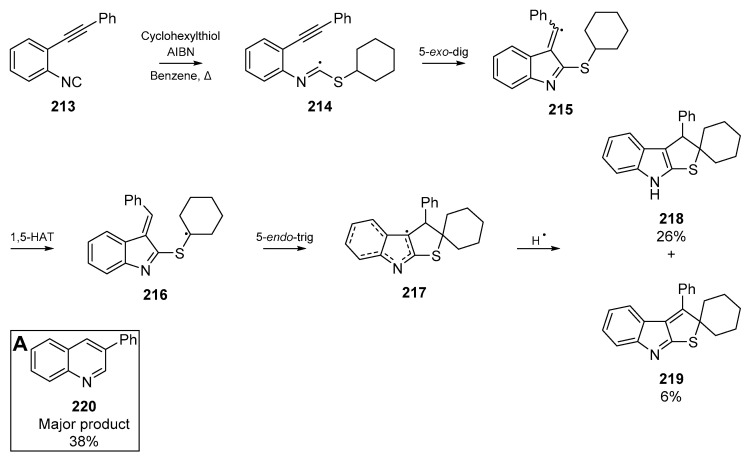
Analogous to those results by Rainer and co-workers, Minozzi et al. published a similar radical cyclization of an alkynyl arylisonitrile 213 [98]. Interestingly, this proceeded as a multi-step cascade which resulted in the formation of quinoline 220 (Scheme 33, Insert A), as well as formation of spirocycles 218 and 219. The authors propose that the thioimidoyl radical 214 formed from thiyl addition to the isonitrile can cyclize onto the alkyne as previously shown, but a 1,5-hydorgen migration and cyclization then furnishes the observed spirocyclic compounds. A proposed mechanism is detailed in Scheme 33.
Scheme 33.
Spirocycle formation by Minozzi et al. Major product 220 formed through separate pathway.
3.4. Modern Approaches
Each new report that demonstrates the usefulness of thiyl radicals for cascade chemistry further broadens the array of substrates that can be successfully cyclized. In the following subsection, recent results from the last decade are discussed, with the intent of outlining the current state-of-the-art for heterogeneous cyclizations.
An excellent account of enyne cyclization by Taniguchi et al. builds upon work published forty years previous by Wang [99], in which the disulfide bond of diphenyl disulfide was cleaved by dimethylaniline to yield polymers via reaction with a suitable nitrile species. This original reaction was postulated to initiate by single-electron transfer (SET) from the amine. Taniguchi and co-workers then published a hydrothiolation of alkynes and subsequent cascade cyclization, with initiation of the process by tripropylamine (Scheme 34) [100]. This methodology offers an inexpensive approach to cyclization, and the annulated products can be separated by evaporation of the alkyl amine and standard chromatography. A mixture of 5-exo and 6-endo products were obtained, and the account provides an interesting example of aqueous thiyl radical addition, and non-classical initiation. A number of examples, with a range of olefinic substituents and varying heteroatoms bridging the alkyne and alkene were reported.
Scheme 34.
Cyclization reaction by Taniguchi et al. Insert A: Examples of scope by Taniguchi et al.
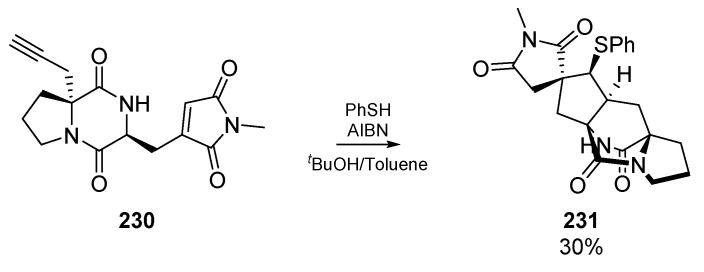
Access to the pentacyclic core of the fungal-derived asperparalines was explored by Crick et al., who designed a clever radical cascade sequence comprised of 1,6-hydrogen atom transfer, followed by two cyclization steps (Scheme 35) [101]. These valuable alkaloids have been shown to act as strong selective antagonists of nicotinic acetylcholine receptors [102] and possess a bridged bicyclic core which is the backbone of many natural products. While this account describes multiple cyclized products generated during the optimization of cyclization methodology, Crick and co-workers were able use diketopiperazine 230 to isolate a single diastereomer product 231 with the desired configuration for the asperparaline core, albeit in modest yield.
Scheme 35.
Key cyclization step employed by Crick and co-workers.
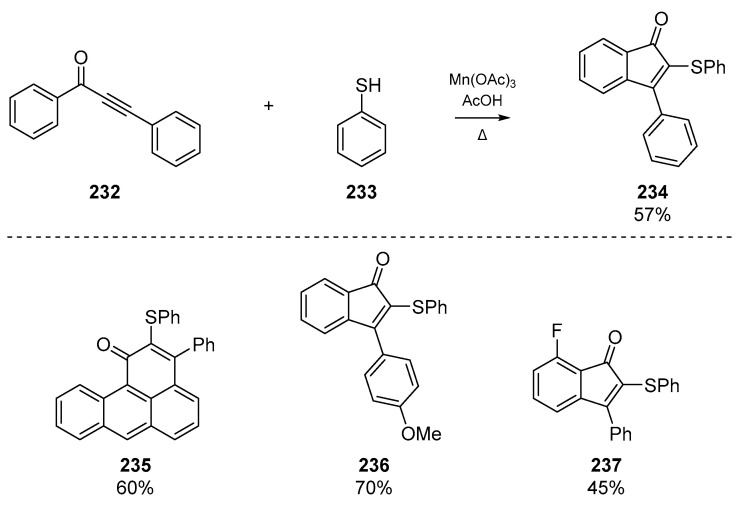
Shortly thereafter, Zhou and colleagues published a manganese (III) mediated addition of thiyl radicals to 1,3-diarylpropynones to yield thiolated indenones (Scheme 36). The account describes the use of Mn(OAc)3-induced formation of thiophenoxyl radical which undergo regioselective addition to an array of substrates, which can be further functionalized. The authors note that this offers an alternative to the heavy-metal catalysis (e.g., Pd or Rh complexes) employed to form 2,3-disubstituted indenones 234 from alkynes 232, and permits mild conditions and moderate scope.
Scheme 36.
Indenone synthesis by Zhou et al.
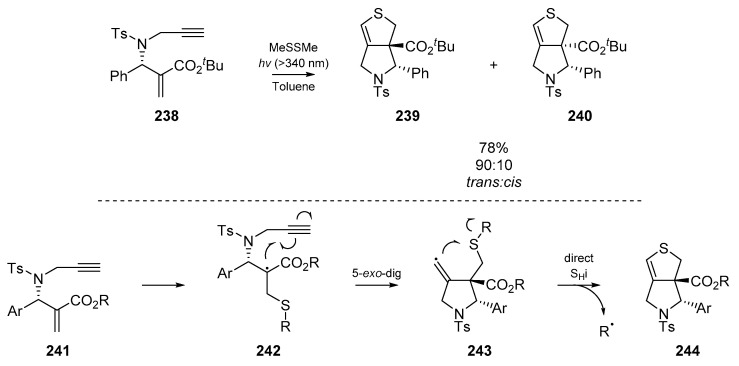
Previously we have seen the use of SHi chemistry to synthesize thiophenes from suitable diyne precursors. These are limited to substrates bearing homogenous unsaturation, and thus Kamimura et al. published a detail account [103] on the use of radical cascades to prepare bicyclic dihydrothiophenes (Scheme 37). Once again, the translocated radical 243 undergoes intermolecular addition at the sulfur atom, resulting in extrusion of an alkyl radical. A reaction pathway proceeding through 5-exo-dig cyclization and either stepwise or direct homolytic substitution was proposed. Overall, chiral enynes were converted to bicyclic pyrolidinodihydrothiophenes 244 in a process where a thiyl radical serves as a sulfur biradical surrogate. Improvements in stereoselectively were observed by Kamimura et al. when large excesses of the thiyl radical precursor were employed in a photoinitiated reaction. An assortment of alkyl disulfides were tested as thiyl radical precursors, and dimethyl disulfide was determined as the best precursor, with 78% yield and a 90:10 trans:cis ratio attained with 20 equivalents of disulfide. The authors also varied the substitution of the tertiary amine backbone, and furnished a number of novel compounds, with fair to good stereoselectivity.
Scheme 37.
Cyclization by Kamimura et al., with proposed mechanism.
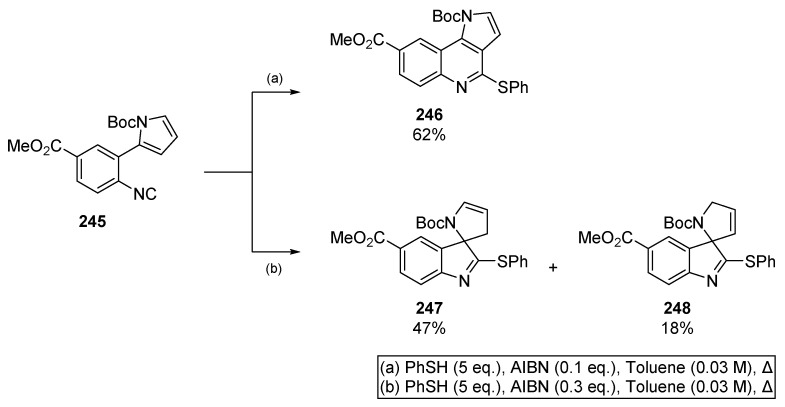
Patel and co-workers published an extensive methodology for the synthesis of tricyclic and spirocyclic heterocycles based on a thiyl radical/isocyanide couple (Scheme 38) [104]. This alternative to tin-based methods provides access to pyrroloquinolines 246, and spirocycles 247 and 248 from a common precursor, aiding the investigation of different heterocyclic motifs. Interestingly, the authors were able to select for 6-endo or 5-exo cyclization by altering the equivalents of radical initiator. Generally, one observes a trend of 5-exo favoured cyclization when concentration is low (ca 0.05 M) and the number of equivalents of AIBN is high (ca. 1.5 equivalents). In contrast, high concentration (0.1 to 0.2 M) and low loadings of initiator (ca. 0.3 equivalents) seem to favour 6-endo cyclization. As discussed by the authors, the kinetic 5-exo spirocycle predominates with increased quantities of initiator, indicating the nascent heterocycle is trapped and that the reaction is reversible. It follows that the thermodynamic 6-membered heterocycle predominates when the cyclization is slow, such as in the case where AIBN loading is reduced.
Scheme 38.
Cyclizations by Patel et al., in which control over cyclization mode can be tuned.
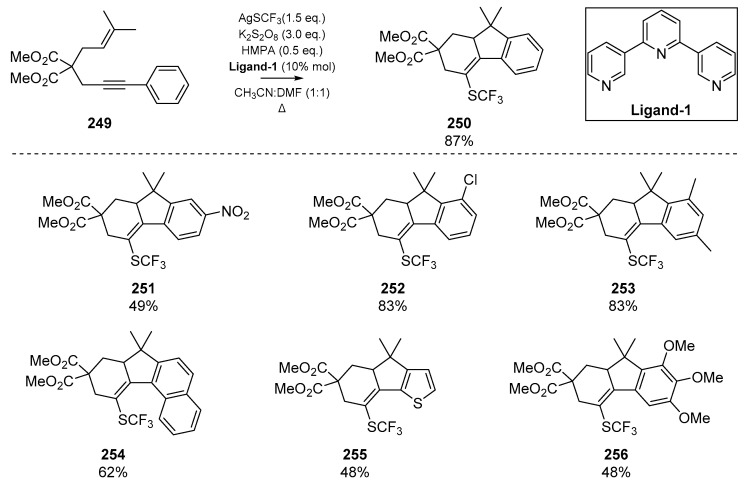
Many of the cyclization reactions of enynes discussed herein utilize thiophenol as the thiyl radical precursor; while this reagent has enjoyed a special place in sulfur radical addition/cyclizations, a number of diverse thiyl precursors have demonstrated utility in this area. One such example that boasts desirable biological properties such as lipophilicity and resistance to metabolic processes is the trifluoromethylthio group (SCF3). Qiu et al. recently published a trifluoromethylthiolation cyclization between 1,6-enynes and an aromatic substituent [105], which permits the construction of a number of polycyclic fluorene systems (Scheme 39). Improved yield and reaction control necessitated the addition of a terpyridine ligand Ligand-1, which was postulated to coordinate to the HMPA additive and reduce the redox potential of the silver trifluoromethylthiol; this likely reduces oxidative decomposition. A number of annulated products were synthesized, and representative examples of this methodology are shown in Scheme 39.
Scheme 39.
Trifluoromethylthiolation work by Qiu et al. Substitution on the aryl ring was well tolerated.
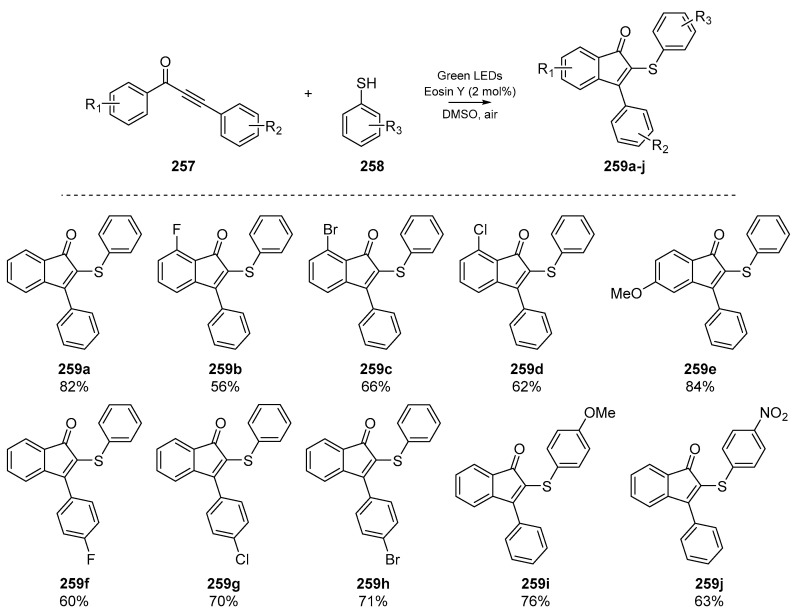
One account of particular interest was published by Chen and co-workers in early 2019, in which the authors used visible light (in the form of a green LED) and Eosin Y to synthesize 2-sulfenylindenones from the corresponding 1,3-diarylpropynones (Scheme 40) [106]. While this approach boasts environmental compatibility and cheap reagents, admittedly the reaction time is quite long (up to 36 h) and yields varied widely based on reaction solvent. However, a number of indenone species 259a–j were prepared and a wide substrate scope was explored, showing the forbearance of the methodology to differing functional groups on both the aromatic backbone and phenylthiyl species.
Scheme 40.
Substrate scope examined by Chen and co-workers.
3.5. State of the Art
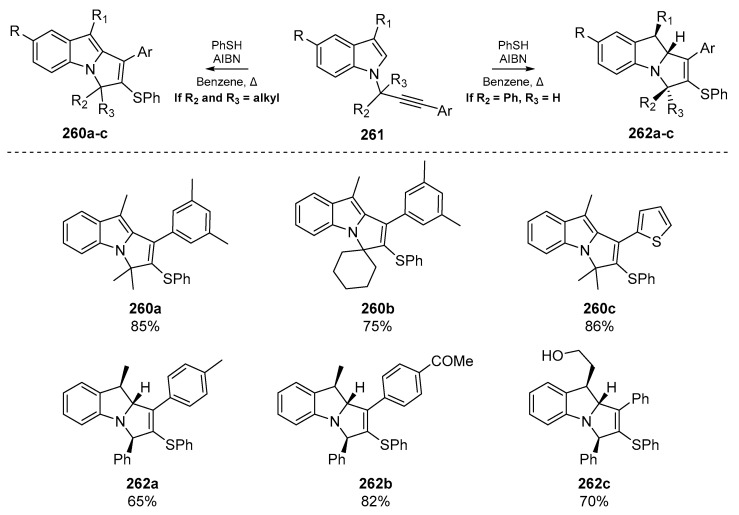
Thiyl radical mediated cyclization has been employed in the synthesis of a number of privileged scaffolds and complex substrates. One such example by Gharpure et al. [107], reports the synthesis of pyrrolo[1,2-a]indole derivatives. This motif appears in a number of important pharmaceutical compounds such as those possessing antimalarial properties like Flinderole C [108], and mitomycins which act as antitumor agents that can cross-link DNA [109]. This account by Gharpure and co-workers describes the use of enyne cyclization through a 5-exo-trig mechanism to generate a number of N-fused indolines 262a–c and indoles 260a–c from the corresponding N-propargylindones 261 (Scheme 41). The preference for indole vs. indolines was dictated by the identity of the propargylic substituents; indoline derivatives were furnished when R2 = Ph and R3 = H, and indoles were generated when these substituents were replaced by alkyl moieties such as a gem-dimethyl group. The authors propose that the bulkier aryl ring prefers to occupy the less sterically crowded face of the transition state. As the reduction of the radical (formed via 5-exo-trig cyclization onto the heterocyclic backbone) proceeds through a late transition state, the R1 substituent also prefers to occupy the less sterically incumbent face of the transition state, which permits delivery of a hydrogen atom to furnish the stereochemistry observed in 262a–c. To explain the generation of 260a–c, Gharpure et al. propose that the presence of an alkyl substituent on both faces of the transition state significantly slows down this hydrogen atom delivery, and thus when R2 and R3 = alkyl, a competing hydrogen abstraction provides the indole structures observed in 260a–c with concomitant aromatization as the driving force.
Scheme 41.
Representative indole and indolines examples published by Gharpure et al.
The products obtained through this methodology were elaborated to the multicyclic core of yuremamine, a natural product featuring five contiguous stereocenters [110]. Shortly thereafter, Shibata and co-workers described the thiyl radical mediated cyclization of ω-alkynyl O-silyloximes with an odourless thiyl precursor, 4-tert-butylbenzenethiol [111]. This approach furnished a number of cyclic O-silylhydroxylamines in good yields. Removal of the silyl group yielded a number of hydroxylamines which could be further transformed to nitrones, a motif which has demonstrated potential in neuroprotective and antiaging drugs [112].
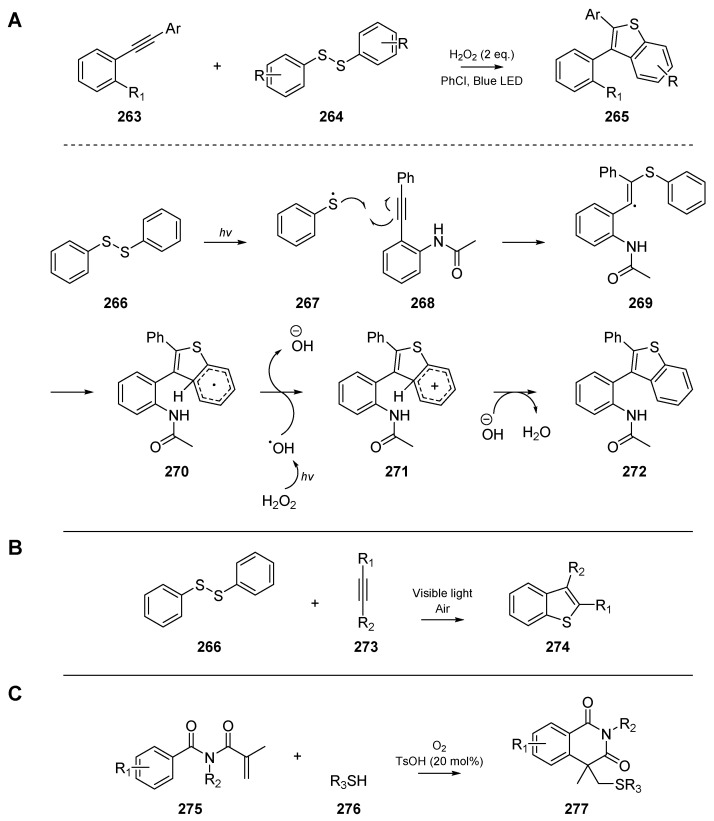
While many of the examples discussed herein have employed thermal initiation in boiling benzene, or photolysis with high intensity ultraviolet lamps, there are a number of visible-light induced cascades reported in the literature. Xie et al. developed a reliable route to benzothiophenes (Scheme 42A) through the visible-light induced cyclization of 2-alkynylanilines 263 at room temperature [113]. Good yields and overall forbearance to substitution on the alkyne terminus are reported, and a mechanism in which hydrogen peroxide oxidizes cyclized intermediate 270 to reform aromaticity is proposed. Analogous to the control experiments by their contemporaries, the authors employed a ‘TEMPO test’, in which one equivalent of TEMPO is added to the reaction, and the quenched radical intermediates identified by mass spectrometry. A similar approach was published by Ye et al. [114], which again relied on visible light to cyclize alkynes 273 and aryldisulfides 266 to benzothiophenes 274 (Scheme 42B). Interestingly, the authors found that oxygen could be used as the sole oxidant, and irradiation with sunlight could initiate the reaction efficiently. Ye and co-workers identified the optimal concentration of O2 to be around 15% and explained that although the reaction also occurs under argon, this is likely due to oxygen seeping through the septum. Yuan et al. achieved similar findings (Scheme 42C) to Ye et al. and Xie et al., while exploring the use of N-methacryloylbenzamides 275 as substrates to generate isoquinoline-type compounds 277 [115]. The contributions from the three aforementioned authors are summarized in below, and the putative mechanism proposed by Xie et al. is included.
Scheme 42.
Cyclizations reported by Xie et al. (A), Ye et al. (B), and Yuan et al. (C) respectively.
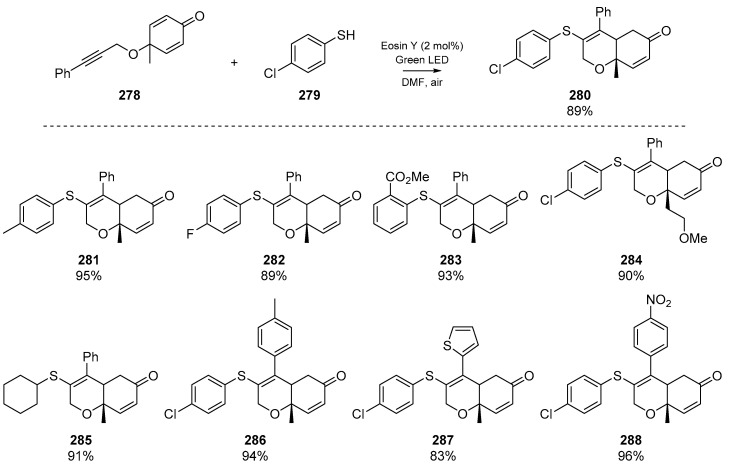
Shortly thereafter, Nair et al. described a diastereoselective visible-light mediated cascade of cyclohexadienones 278 with alkyne tethers (Scheme 43) [116]. Good yields were reported, as was good functional group tolerance. This account relies on Eosin Y as the organic photocatalyst, and boasts an array of thiophenols/thiols as radical precursors, with electron donating and electron withdrawing groups in para- and ortho- positions tolerated for the aromatic thiols. The authors were also able to demonstrate the applicability to gram scale synthesis, and reported favourable green chemistry metrics (atom economy, and efficiency). A publication [117] by Mallick et al. reported comparable results, employing the same reactions conditions previously developed in the Sahoo group (Section 2.4, Dutta et al.). Mallick and co-worker’s approach employed a N-hydroxyphthalimide-mediated radical cyclization of yne-dienones, yielding 3-thioaryl-[6,6]-dihydrochromenone derivatives. Altering the substituents of the aryl thiol, or the functional groups presented by the yne-dienone were again well tolerated in the cascade.
Scheme 43.
Cyclization reported by Nair et al. Select examples that demonstrate FG tolerance are shown.
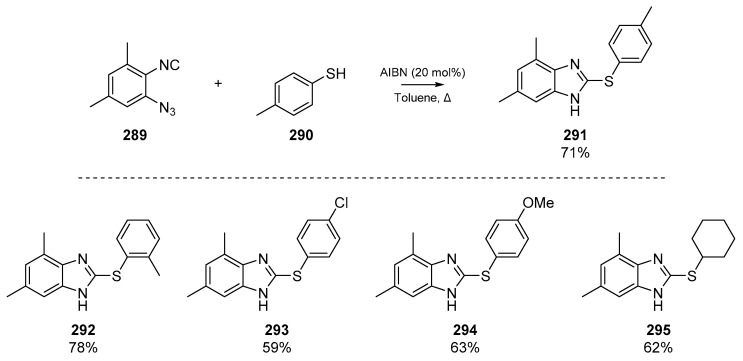
Further examples of the use of isocyano groups as radical acceptors was published by Li et al. in 2019, in which a denitrogenative annulation of 1-azido-2-isocyanarenes 289 furnished an array of 2-thiolated benzimidazoles 291 (Scheme 44) [118]. The authors report both a ‘promoter-free’ pathway and one involving AIBN, both of which are heated in toluene in an inert atmosphere. While the AIBN pathway offers higher yields in almost every example, a catalyst-free pathway in which the only by-product is the extruded nitrogen gas from cyclization onto the ortho-azide is appealing. Li et al. argue that that the thiyl radicals could be initiated by trace oxygen in the reaction vessel, although it has previously been reported that certain thiols can react successfully via a radical mechanism without catalysts or initiators [119], and indeed thiols may react with isocyanides at elevated temperatures [120]. The authors report this one pot method as having high functional group tolerance, and a number of prepared examples are shown in Scheme 44.
Scheme 44.
AIBN-initiated pathway to benzimidazoles described by Li et al.
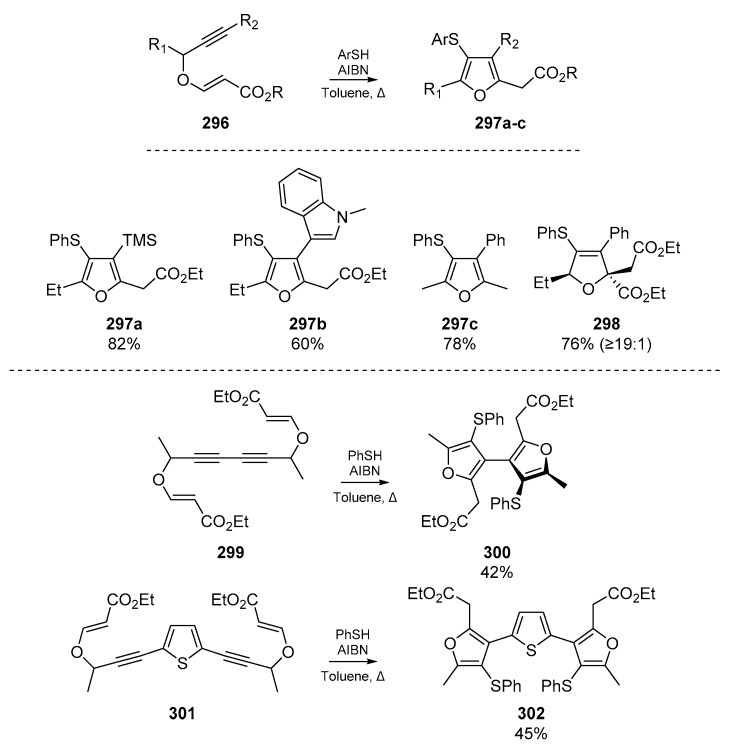
Research from Gharpure et al. elaborates on the pyrrole synthesis previously developed by Dutta et al., but instead offers tetrasubstituted furans 297a–c from alkynyl vinylogous carbonates 296 (Scheme 45) [121]. The authors note that substitution at the α-position of the alkynyl vinylogous carbonate affects diastereoselectivity of the cascade, and propose a mechanism in which the thiyl radical attacks the alkyne first. The nascent carbon-centered radical then cyclizes onto the remaining olefin, and the cascade continues with propagation of the radical chain. It is noteworthy that in this account, thiophenol can act not only as a source of thiyl radicals but as an oxidant with AIBN to transform dihydrofurans to furans.
Scheme 45.
Cyclizations and polyheterocycle syntheses reported by Gharpure et al.
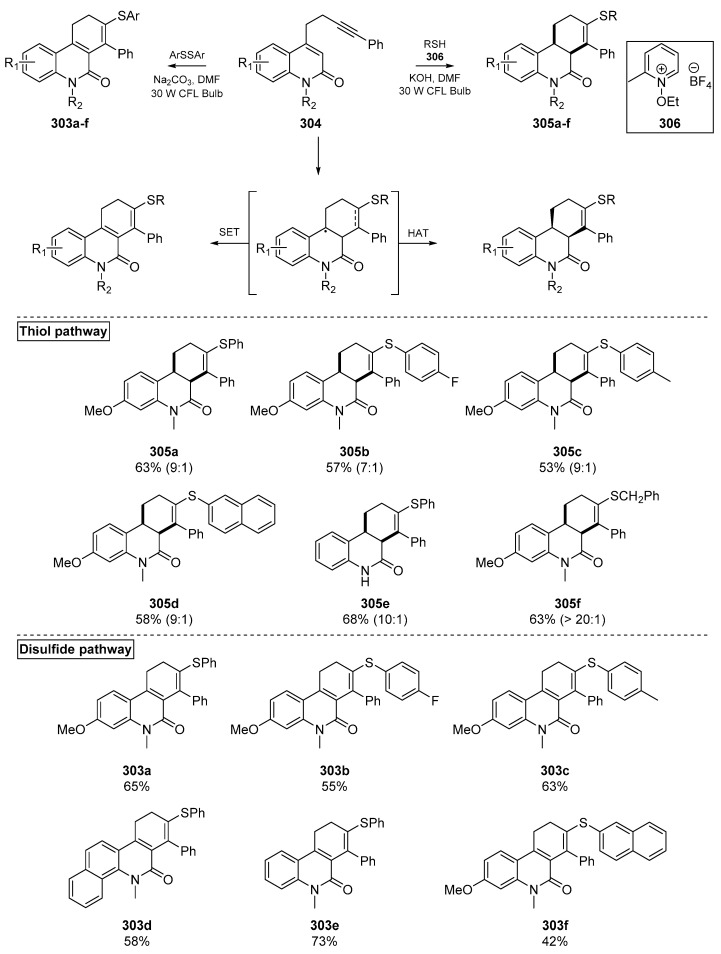
The Hong group reported a breakthrough in mid-2019, in which the substrate and photosensitizer were combined in a single molecule (Scheme 46). This account by Kim et al. examined the photochemical activity of quinolinone-based substrates 304 which could reach an excited state and initiate thiyl radical formation, while the ground state acted as a radical accepting substrate. The products of this ground state substrate are dictated by whether a hydrogen atom transfer (HAT) or single electron transfer (SET) step occurs, which is determined by the coupling reagents used [122]. The authors report a diverse assemblage of dihydro- and tetrahydrophenanthridin-6(5H)-ones prepared in a metal-free and essentially photocatalyst-free cascade. A number of bioactive thiols such as cysteine, captopril and gemfibrozil were successfully applied in this methodology, which exerts a 6-endo-trig cyclization following thiyl radical addition. The tolerance of this methodology to bioactive thiols indicates that this method may be applied to generating compound libraries for combinatorial chemistry (or otherwise), or modifying existing bioactive thiols. A selection of examples from both the disulfide and thiol pathways are shown in Scheme 46.
Scheme 46.
Selected examples from Kim et al. Insert: 306 serves as an additive in the thiol pathway.
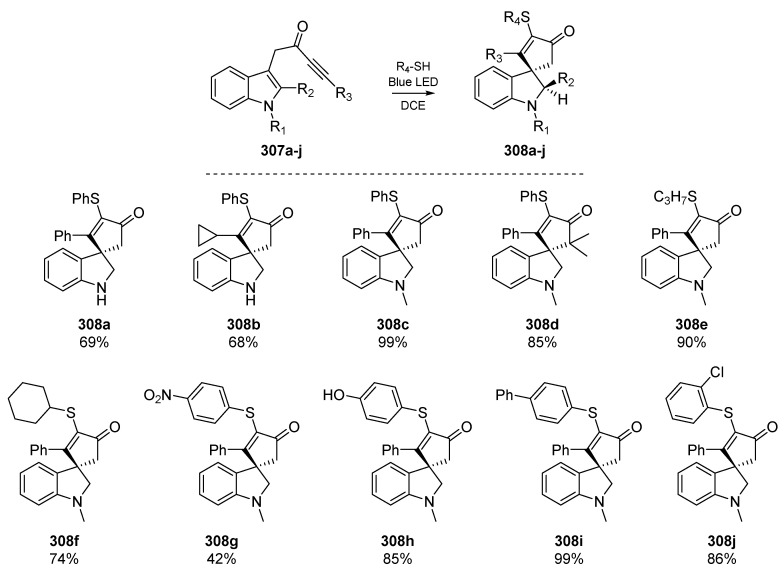
Somewhat analogous to the work reported by the Hong group, a recent report by Ho et al. describes an electron-donor-acceptor (EDA) complex which undergoes visible light-induced charge transfer, and facilitates the radical spirocyclization of a number of indole-tethered ynones 307a–j (Scheme 47) [123]. The EDA complex promotes thiyl radical generation and initiates a cascade that features a dearomatizing spirocyclization with concomitant carbon-sulfur bond formation, to yield a number of indolines 308a–j. The authors highlight that this is only the second reported use of intramolecular EDA complexes in the literature [124], and admit that this rare form of radical activation was uncovered serendipitously. Regioselective addition of the somewhat electrophilic thiyl radicals to ynones initiates the process, followed by attack of incipient carbon-centered radical on the tethered aromatic group.
Scheme 47.
Cascade developed by Ho et al., with selected examples. Ynone/thiol scope was well tolerated.
4. Conclusions
Thiyl and acyl thiyl radicals have found broad application in organic synthesis, from heterocycle formation to sterically recalcitrant carbocyclizations. They boast mild generation and remarkable reactivity; their utility provides access to radical chemistry to those chemists beyond the archetypal sulfur laboratory. The addition of these radicals to homogenous systems facilitates the synthesis of alicycles and thiophenes, while their addition to heterogeneous systems gives rise to numerous heterocycles and fused ring systems. Their application to intramolecular cascade reactions has been widely explored, and continues to be developed for organocatalytic and enantioselective systems. This review highlights the vast array of complex architectures rendered accessible by the thiol-ene and thiol-yne reactions in particular, and hopes to demonstrate the versatility of thiyl and acyl thiyl radicals as a whole.
Acknowledgments
In this section you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Author Contributions
Writing—original draft preparation, D.M.L.; writing—review and editing, E.M.S.; funding acquisition, E.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Science Foundation Ireland, grant number 15/CDA/3310.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Banerjee M., Poddar A., Mitra G., Surolia A., Owa T., Bhattacharyya B. Sulfonamide Drugs Binding to the Colchicine Site of Tubulin: Thermodynamic Analysis of the Drug-Tubulin Interactions by Isothermal Titration Calorimetry. J. Med. Chem. 2005;48:547–555. doi: 10.1021/jm0494974. [DOI] [PubMed] [Google Scholar]
- 2.Boyd D.A. Sulfur and Its Role In Modern Materials Science. Angew. Chem. Int. Ed. 2016;55:15486–15502. doi: 10.1002/anie.201604615. [DOI] [PubMed] [Google Scholar]
- 3.Cole D.C., Lennox W.J., Lombardi S., Ellingboe J.W., Bernotas R.C., Tawa G.J., Mazandarani H., Smith D.L., Zhang G., Coupet J., et al. Discovery of 5-arylsulfonamido-3-(pyrrolidin-2-ylmethyl)-1H-indole derivatives as potent, selective 5-HT6 receptor agonists and antagonists. J. Med. Chem. 2005;48:353–356. doi: 10.1021/jm049243i. [DOI] [PubMed] [Google Scholar]
- 4.Gao G.-Y., Colvin A.J., Chen Y., Zhang X.P. Synthesis of meso-Arylsulfanyl- and Alkylsulfanyl-Substituted Porphyrins via Palladium-Mediated C-S Bond Formation. J. Org. Chem. 2004;69:8886–8892. doi: 10.1021/jo048552d. [DOI] [PubMed] [Google Scholar]
- 5.Liu J., Yang J., Yang Q., Wang G., Li Y. Hydrothermally Stable Thioether-Bridged Mesoporous Materials with Void Defects in the Pore Walls. Adv. Funct. Mater. 2005;15:1297–1302. doi: 10.1002/adfm.200500122. [DOI] [Google Scholar]
- 6.Petracca R., Bowen K.A., McSweeney L., O’Flaherty S., Genna V., Twamley B., Devocelle M., Scanlan E.M. Chemoselective Synthesis of N-Terminal Cysteinyl Thioesters via β,γ-C,S Thiol-Michael Addition. Org. Lett. 2019;21:3281–3285. doi: 10.1021/acs.orglett.9b01013. [DOI] [PubMed] [Google Scholar]
- 7.Ravasco J.M.J.M., Faustino H., Trindade A., Gois P.M.P. Bioconjugation with Maleimides: A Useful Tool for Chemical Biology. Chem. Eur. J. 2019;25:43–59. doi: 10.1002/chem.201803174. [DOI] [PubMed] [Google Scholar]
- 8.Asmus K.D. Sulfur-centered free radicals. Methods Enzymol. 1990;186:168–180. doi: 10.1016/0076-6879(90)86107-7. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand M.P., Ferreri C. Sulfur-Centered Radicals. In: Renaud P., Sibi M.P., editors. Radicals in Organic Synthesis. Wiley-VCH; Weinheim, Germany: 2001. pp. 485–504. [Google Scholar]
- 10.Chauhan P., Mahajan S., Enders D. Organocatalytic carbon-sulfur bond-forming reactions. Chem. Rev. 2014;114:8807–8864. doi: 10.1021/cr500235v. [DOI] [PubMed] [Google Scholar]
- 11.Glass R.S. Sulfur Radicals and Their Application. Top. Curr. Chem. 2018;376:1–42. doi: 10.1007/s41061-018-0197-0. [DOI] [PubMed] [Google Scholar]
- 12.Guo W., Tao K., Tan W., Zhao M., Zheng L., Fan X. Recent advances in photocatalytic C-S/P-S bond formation: Via the generation of sulfur centered radicals and functionalization. Inorg. Chem. Front. 2019;6:2048–2066. doi: 10.1039/C8QO01353E. [DOI] [Google Scholar]
- 13.Makarov S.V., Horváth A.K., Makarova A.S. Reactivity of Small Oxoacids of Sulfur. Molecules. 2019;24:2768. doi: 10.3390/molecules24152768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mampuys P., McElroy C.R., Clark J.H., Orru R.V.A., Maes B.U.W. Thiosulfonates as Emerging Reactants: Synthesis and Applications. Adv. Synth. Catal. 2020;362:3–64. doi: 10.1002/adsc.201900864. [DOI] [Google Scholar]
- 15.McCourt R.O., Scanlan E.M. 5-exo versus 6-endo Thiyl-Radical Cyclizations in Organic Synthesis. Helv. Chim. Acta. 2019;102:e1900162. doi: 10.1002/hlca.201900162. [DOI] [Google Scholar]
- 16.Postigo A. Synthetically useful carbon-carbon and carbon-sulphur bond construction mediated by carbon- and sulphur-centred radicals in water and aqueous media. RSC Adv. 2011;1:14–32. doi: 10.1039/c1ra00372k. [DOI] [Google Scholar]
- 17.Taniguchi T. Recent Advances in Reactions of Heteroatom-Centered Radicals. Synthesis. 2017;49:3511–3534. doi: 10.1055/s-0036-1588481. [DOI] [Google Scholar]
- 18.Luo Y.R. Comprehensive Handbook of Chemical Bond Energies. 1st ed. CRC Press; Boca Raton, FL, USA: 2007. pp. 1–1656. [Google Scholar]
- 19.Adams G.E., Armstrong R.C., Charlesby A., Michael B.D., Willson R.L. Pulse radiolysis of sulphur compounds. Part 3.—Repair by hydrogen transfer of a macromolecule irradiated in aqueous solution. Trans. Faraday Soc. 1969;65:732–742. doi: 10.1039/TF9696500732. [DOI] [Google Scholar]
- 20.Mu X.-J., Zou J.-P., Zeng R.-S., Wu J.-C. Mn(III)-Promoted cyclization of substituted thioformanilides under microwave irradiation: A new reagent for 2-substituted benzothiazoles. Tetrahedron Lett. 2005;46:4345–4347. doi: 10.1016/j.tetlet.2005.04.090. [DOI] [Google Scholar]
- 21.Nguyen V.-H., Nishino H., Kajikawa S., Kurosawa K. Mn(III)-based reactions of alkenes and alkynes with thiols. An approach toward substituted 2,3-dihydro-1,4-oxathiins and simple route to (E)-vinyl sulfides. Tetrahedron. 1998;54:11445–11460. doi: 10.1016/S0040-4020(98)00707-8. [DOI] [Google Scholar]
- 22.Tyson E.L., Ament M.S., Yoon T.P. Transition Metal Photoredox Catalysis of Radical Thiol-Ene Reactions. J. Org. Chem. 2013;78:2046–2050. doi: 10.1021/jo3020825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amaral G.A., Ausfelder F., Izquierdo J.G., Rubio-Lago L., Bañares L. Imaging the photodissociation of CH3SH in the first and second absorption bands: The CH3(X2A1)+SH(X2Π) channel. J. Chem. Phys. 2007;126:24301. doi: 10.1063/1.2409925. [DOI] [PubMed] [Google Scholar]
- 24.Dénès F., Pichowicz M., Povie G., Renaud P. Thiyl Radicals in Organic Synthesis. Chem. Rev. 2014;114:2587–2693. doi: 10.1021/cr400441m. [DOI] [PubMed] [Google Scholar]
- 25.Hoyle C.E., Bowman C.E. Thiol–Ene Click Chemistry. Angew. Chem. Int. Ed. 2010;49:1540–1573. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- 26.Kade M.J., Burke D.J., Hawker C.J. The power of thiol-ene chemistry. J. Polym. Sci. Part A Polym. Chem. 2010;48:743–750. doi: 10.1002/pola.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Killops K.L., Campos L.M., Hawker C.J. Robust, Efficient, and Orthogonal Synthesis of Dendrimers via Thiol-ene “Click” Chemistry. J. Am. Chem. Soc. 2008;130:5062–5064. doi: 10.1021/ja8006325. [DOI] [PubMed] [Google Scholar]
- 28.McCourt R.O., Scanlan E.M. A Sequential Acyl Thiol-Ene and Thiolactonization Approach for the Synthesis of δ-Thiolactones. Org. Lett. 2019;21:3460–3464. doi: 10.1021/acs.orglett.9b01271. [DOI] [PubMed] [Google Scholar]
- 29.Pramanik M., Choudhuri K., Chakraborty S., Ghosh A., Mal P. (Z)-Selective anti-Markovnikov or Markovnikov thiol–yne-click reactions of an internal alkyne by amide hydrogen bond control. Chem. Commun. 2020;56:2991–2994. doi: 10.1039/D0CC00702A. [DOI] [PubMed] [Google Scholar]
- 30.Kuehne M.E., Damon R.E. Thiyl Radical Induced Cyclizations of Dienes. Cyclization of α-Acoradiene, α-Bulnesene, and Geranyl Acetate to Cedrane, Patchulane, and Cyclogeranyl Acetate Products. J. Org. Chem. 1977;42:1825–1832. doi: 10.1021/jo00431a001. [DOI] [Google Scholar]
- 31.Padwa A., Nimmesgern H., Wong G.S.K. Synthesis of the Pyrrolidine Ring System by Radical Cyclization. J. Org. Chem. 1985;50:5620–5627. doi: 10.1021/jo00350a038. [DOI] [Google Scholar]
- 32.Beckwith A.L.J., Ingold K.U. Essay 4—Free-Radical Rearrangements. In: de Mayo P., editor. Organic Chemistry: A Series of Monographs. Volume 42. Academic Press; San Diego, CA, USA: 1980. pp. 161–310. [Google Scholar]
- 33.Beckwith A.L.J., Blair I., Phillipou G. Preferential cis cyclization of 6-hepten-2-yl and related radicals. Example of orbital symmetry control. J. Am. Chem. Soc. 1974;96:1613–1614. doi: 10.1021/ja00812a066. [DOI] [Google Scholar]
- 34.Naim A., Mills G., Shevlin P.B. Cyclization of 1,6-heptadienes by α-hydroxy radicals. Tetrahedron Lett. 1992;33:6779–6782. doi: 10.1016/S0040-4039(00)61774-4. [DOI] [Google Scholar]
- 35.Agrawal A.R., Kumar N.R., Debnath S., Das S., Kumar C., Zade S.S. Radical-Cascade Avenue for 3,4-Fused-Ring-Substituted Thiophenes. Org. Lett. 2018;20:4728–4731. doi: 10.1021/acs.orglett.8b01577. [DOI] [PubMed] [Google Scholar]
- 36.Naito T., Honda Y., Miyata O., Ninomiya I. Total Synthesis of (±)-Anantine and (±)-Isoanantine via Thiyl Radical Addition-Cyclization Reaction. Chem. Pharm. Bull. 1993;41:217–219. doi: 10.1248/cpb.41.217. [DOI] [Google Scholar]
- 37.Maat L., Beyerman H.C. Chapter 5: The Imidazole Alkaloids. In: Brossi A., editor. The Alkaloids: Chemistry and Pharmacology. Volume 22. Academic Press; San Diego, CA, USA: 1984. pp. 281–333. [Google Scholar]
- 38.Naito T., Honda Y., Miyata O., Ninomiya I. Radical cyclisation in heterocycle synthesis. Part 1. Sulfanyl radical addition–cyclisation of dienylamides for lactam synthesis. J. Chem. Soc. Perkin Trans. 1. 1995;32:19–26. doi: 10.1039/P19950000019. [DOI] [Google Scholar]
- 39.Laird E.R., Jorgensen W.L. Computer-assisted mechanistic evaluation of organic reactions. 17. Free-radical chain reactions. J. Org. Chem. 1990;55:9–27. doi: 10.1021/jo00288a005. [DOI] [PubMed] [Google Scholar]
- 40.Wagner P.J., Sedon J.H., Lindstrom M.J. Rates of radical β cleavage in photogenerated diradicals. J. Am. Chem. Soc. 1978;100:2579–2580. doi: 10.1021/ja00476a068. [DOI] [Google Scholar]
- 41.Walling C., Helmreich W. Reactivity and Reversibility in the Reaction of Thiyl Radicals with Olefins. J. Am. Chem. Soc. 1959;81:1144–1148. doi: 10.1021/ja01514a032. [DOI] [Google Scholar]
- 42.Harrowven D.C., Lucas M.C., Howes P.D. Total syntheses of aplysin and debromoaplysin using a diastereoselective, sulfur mediated radical cyclisation strategy. Tetrahedron Lett. 1999;40:4443–4444. doi: 10.1016/S0040-4039(99)00768-6. [DOI] [Google Scholar]
- 43.Harrowven D.C., Hannam J.C., Lucas M.C., Newman N.A., Howes P.D. A thiyl radical mediated cascade sequence for the co-cyclisation of 1,6-hexadienes with sulfur atom transfer. Tetrahedron Lett. 2000;41:9345–9349. doi: 10.1016/S0040-4039(00)01701-9. [DOI] [Google Scholar]
- 44.Beckwith A.L.J., Easton C.J., Lawrence T., Serelis A.K. Reactions of methyl-substituted Hex-5-enyl and Pent-4-enyl radicals. Aust. J. Chem. 1983;36:545–556. doi: 10.1071/CH9830545. [DOI] [Google Scholar]
- 45.Beckwith A.L.J., Schiesser C.H. Regio- and stereo-selectivity of alkenyl radical ring closure: A theoretical study. Tetrahedron. 1985;41:3925–3941. doi: 10.1016/S0040-4020(01)97174-1. [DOI] [Google Scholar]
- 46.Harrowven D.C., May P.J., Bradley M. Sulfur-mediated radical cyclisation reactions on solid support. Tetrahedron Lett. 2003;44:503–506. doi: 10.1016/S0040-4039(02)02594-7. [DOI] [Google Scholar]
- 47.Bennett W.D., Hamaker L.K., Peterson M.L., Rhodes M.R., Saneii H.H. Handbook of Combinatorial and Solid Phase Organic Chemistry. Advanced Chemtech; Louisville, KY, USA: 1998. p. 399. [Google Scholar]
- 48.Du X., Armstrong R.W. SmI2-mediated sequential radical cyclization/anionic capture of aryl iodides on solid support. Tetrahedron Lett. 1998;39:2281–2284. doi: 10.1016/S0040-4039(98)00292-5. [DOI] [Google Scholar]
- 49.Miyabe H., Fujii K., Tanaka H., Naito T. Solid-phase tandem radical addition–cyclisation reaction of oxime ethers. Chem. Commun. 2001;2:831–832. doi: 10.1039/b101074n. [DOI] [PubMed] [Google Scholar]
- 50.Yim A.-M., Vidal Y., Viallefont P., Martinez J. Solid-phase synthesis of α-amino acids by radical addition to adehydroalanine derivative. Tetrahedron Lett. 1999;40:4535–4538. doi: 10.1016/S0040-4039(99)00799-6. [DOI] [Google Scholar]
- 51.Barrero A.F., Arseniyadis S., Herrador M.M., Quílez Del Moral J.F., Arteaga J.F., Sánchez E.M. Sulfanyl radical-induced cyclization of linalyl acetate to the iridane skeleton: A short synthesis of (±)-dehydroiridomyrmecin. Synlett. 2005;4:591–594. doi: 10.1055/s-2005-863729. [DOI] [Google Scholar]
- 52.Miyata O., Nishiguchi A., Ninomiya I., Aoe K., Okamura K., Naito T. Radical cyclization in heterocycle synthesis. 11. A novel synthesis of α,β-disubstituted γ-lactones via sulfanyl radical addition-cyclization using hydroximates as a tether. J. Org. Chem. 2000;65:6922–6931. doi: 10.1021/jo000472w. [DOI] [PubMed] [Google Scholar]
- 53.Parsons A.F. Recent developments in kainoid amino acid chemistry. Tetrahedron. 1996;52:4149–4174. doi: 10.1016/0040-4020(95)01022-X. [DOI] [Google Scholar]
- 54.Miyata O., Nakajima E., Naito T. Radical cyclization in heterocycle synthesis. 12. Sulfanyl radical addition-addition-cyclization (SRAAC) of unbranched diynes and its application to the synthesis of A-ring fragment of 1 α,25-dihydroxyvitamin d3. Chem. Pharm. Bull. 2001;49:213–224. doi: 10.1248/cpb.49.213. [DOI] [PubMed] [Google Scholar]
- 55.Miyata O., Ozawa Y., Ninomiya I., Naito T. Radical cyclization in heterocycle synthesis. Part 10: A concise synthesis of (-)-kainic acid via sulfanyl radical addition-cyclization-elimination reaction. Tetrahedron. 2000;56:6199–6207. doi: 10.1016/S0040-4020(00)00579-2. [DOI] [Google Scholar]
- 56.Pedrosa R., Andrés C., Duque-Soladana J.P., Maestro A., Nieto J. Regio- and diastereoselective tandem addition-carbocyclization promoted by sulfanyl radicals on chiral perhydro-1,3-benzoxazines. Tetrahedron Asymmetry. 2003;14:2985–2990. doi: 10.1016/j.tetasy.2003.06.003. [DOI] [Google Scholar]
- 57.James P., Schenk K., Landais Y. Radical-Mediated 5-Exo-Trig Cyclizations of 3-Silylhepta-1,6-dienes. The J. Org. Chem. 2006;71:3630–3633. doi: 10.1021/jo060024+. [DOI] [PubMed] [Google Scholar]
- 58.Kamimura A., Kawakami Y. A radical cascade reaction triggered by thiyl radical; an approach toward synthesis of tricyclic structure of platensimycin. Phosphorus Sulfur Silicon Relat. Elem. 2015;191:259–262. doi: 10.1080/10426507.2015.1064922. [DOI] [Google Scholar]
- 59.Wang F.X., Tian S.K. Cyclization of N-Arylacrylamides via Radical Arylsulfenylation of Carbon-Carbon Double Bonds with Sulfonyl Hydrazides. J. Org. Chem. 2015;80:12697–12703. doi: 10.1021/acs.joc.5b02322. [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto T., Kawamata Y., Maruoka K. An organic thiyl radical catalyst for enantioselective cyclization. Nat. Chem. 2014;6:702–705. doi: 10.1038/nchem.1998. [DOI] [PubMed] [Google Scholar]
- 61.Giacalone F., Gruttadauria M., Agrigento P., Noto R. Low-loading asymmetric organocatalysis. Chem. Soc. Rev. 2012;41:2406–2447. doi: 10.1039/C1CS15206H. [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto T., Takino K., Hato K., Maruoka K. A Bulky Thiyl-Radical Catalyst for the [3+2] Cyclization of N-Tosyl Vinylaziridines and Alkenes. Angew. Chem. Int. Ed. 2016;55:8081–8085. doi: 10.1002/anie.201602723. [DOI] [PubMed] [Google Scholar]
- 63.Ryss J.M., Turek A.K., Miller S.J. Disulfide-Bridged Peptides That Mediate Enantioselective Cycloadditions through Thiyl Radical Catalysis. Org. Lett. 2018;20:1621–1625. doi: 10.1021/acs.orglett.8b00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dutta S., Mallick R.K., Prasad R., Gandon V., Sahoo A.K. Alkyne Versus Ynamide Reactivity: Regioselective Radical Cyclization of Yne-Ynamides. Angew. Chem. Int. Ed. 2019;58:2289–2294. doi: 10.1002/anie.201811947. [DOI] [PubMed] [Google Scholar]
- 65.Wang S.C., Tantillo D.J. Viability of dodecahedrane-forming radical polycyclizations. Org. Biomol. Chem. 2017;15:1976–1979. doi: 10.1039/C6OB02300B. [DOI] [PubMed] [Google Scholar]
- 66.Hopf H. Classics in Hydrocarbon Chemistry. Wiley-VCH; Weinheim, Germany: 2000. p. 560. [Google Scholar]
- 67.Woodward R.B., Fukunaga T., Kelly R.C. Triquinacene. J. Am. Chem. Soc. 1964;86:3162–3164. doi: 10.1021/ja01069a046. [DOI] [Google Scholar]
- 68.Ternansky R.J., Balogh D.W., Paquette L.A. Dodecahedrane. J. Am. Chem. Soc. 1982;104:4503–4504. doi: 10.1021/ja00380a040. [DOI] [Google Scholar]
- 69.Broka C.A., Reichert D.E.C. Thiophenol promoted cyclization of enynes. Tetrahedron Lett. 1987;28:1503–1505. doi: 10.1016/S0040-4039(01)81027-3. [DOI] [Google Scholar]
- 70.Ichinose Y., Wakamatsu K., Nozaki K., Birbaum J.-L., Oshima K., Utimoto K. Et3B Induced Radical Addition of Thiols to Acetylenes. Chem. Lett. 1987;16:1647–1650. doi: 10.1246/cl.1987.1647. [DOI] [Google Scholar]
- 71.El Kaim L., Gacon A., Perroux A. Thiophenol promoted radical cyclisation of hydrazones and oxime ethers. Tetrahedron Lett. 1998;39:371–374. doi: 10.1016/S0040-4039(97)10592-5. [DOI] [Google Scholar]
- 72.Alcaide B., Almendros P., Aragoncillo C. Straightforward asymmetric entry to highly functionalized medium-sized rings fused to β-lactams via chemo- and stereocontrolled divergent radical cyclization of Baylis-Hillman adducts derived from 4-oxoazetidine-2-carbaldehydes. J. Org. Chem. 2001;66:1612–1620. doi: 10.1021/jo005715z. [DOI] [PubMed] [Google Scholar]
- 73.Lamberto M., Corbett D.F., Kilburn J.D. Microwave assisted free radical cyclisation of alkenyl and alkynyl isocyanides with thiols. Tetrahedron Lett. 2003;44:1347–1349. doi: 10.1016/S0040-4039(02)02888-5. [DOI] [Google Scholar]
- 74.Majumdar K.C., Mondal S., Ghosh D. An easy access to pyrimidine-fused azocine derivatives by thiophenol-mediated radical cyclization via 8-endo-trig mode. Tetrahedron Lett. 2010;51:348–350. doi: 10.1016/j.tetlet.2009.11.020. [DOI] [Google Scholar]
- 75.Majumdar K.C., Debnath P., Maji P.K. Thiophenol-catalyzed Claisen rearrangement and radical cyclization: Formation of furo- and pyrano-coumarin derivatives. Tetrahedron Lett. 2007;48:5265–5268. doi: 10.1016/j.tetlet.2007.05.133. [DOI] [Google Scholar]
- 76.Majumdar K.C., Mondal S., Ghosh D. Thiophenol mediated radical cyclization: An expedient approach to 2H-pyrrolo[3,2-d]pyrimidines (9-deazaxanthine analogs) Tetrahedron Lett. 2010;51:5273–5276. doi: 10.1016/j.tetlet.2010.07.163. [DOI] [Google Scholar]
- 77.Majumdar K.C., Ray K., Debnath P., Maji P.K., Kundu N. Thiophenol-mediated intramolecular radical cyclization: An efficient method for the synthesis of benzoxocine derivatives. Tetrahedron Lett. 2008;49:5597–5600. doi: 10.1016/j.tetlet.2008.07.030. [DOI] [Google Scholar]
- 78.Majumdar K.C., Taher A. Wittig olefination and thiol mediated 7-endo-trig radical cyclization: Novel synthesis of oxepin derivatives. Tetrahedron Lett. 2009;50:228–231. doi: 10.1016/j.tetlet.2008.10.134. [DOI] [Google Scholar]
- 79.Montevecchi P.C., Navacchia M.L., Spagnolo P. Sulfanyl radical addition to alkynyl azides: An insight into vinyl radical cyclization onto the azido function. Tetrahedron Lett. 1997;38:7913–7916. doi: 10.1016/S0040-4039(97)10045-4. [DOI] [Google Scholar]
- 80.Montevecchi P.C., Navacchia M.L., Spagnolo P. Attempts at vinyl radical carbonylation through cyclization onto carbonyl and cyano groups. Tetrahedron. 1998;54:8207–8216. doi: 10.1016/S0040-4020(98)00458-X. [DOI] [Google Scholar]
- 81.Melandri D., Montevecchi P.C., Navacchia M.L. Thiol radical addition to alkynes. Sulfanyl radical addition and hydrogen atom abstraction relative reaction rates. Tetrahedron. 1999;55:12227–12236. doi: 10.1016/S0040-4020(99)00699-7. [DOI] [Google Scholar]
- 82.Fernández M., Alonso R. Tandem radical addition and cyclization of ε-substituted δ-Yne ketimines. Org. Lett. 2005;7:11–14. doi: 10.1021/ol048104s. [DOI] [PubMed] [Google Scholar]
- 83.Friestad G.K., Jiang T., Fioroni G.M. Tandem thiyl radical addition and cyclization of chiral hydrazones using a silicon-tethered alkyne. Tetrahedron Asymmetry. 2003;14:2853–2856. doi: 10.1016/S0957-4166(03)00540-8. [DOI] [Google Scholar]
- 84.Friestad G.K., Massari S.E. Diastereoselective Vinyl Addition to Chiral Hydrazones via Tandem Thiyl Radical Addition and Silicon-Tethered Cyclization. Org. Lett. 2000;2:4237–4240. doi: 10.1021/ol0067991. [DOI] [PubMed] [Google Scholar]
- 85.Friestad G.K., Massari S.E. A Silicon Tether Approach for Addition of Functionalized Radicals to Chiral α-Hydroxyhydrazones: Diastereoselective Additions of Hydroxymethyl and Vinyl Synthons. J. Org. Chem. 2004;69:863–875. doi: 10.1021/jo035405r. [DOI] [PubMed] [Google Scholar]
- 86.Benati L., Leardini R., Minozzi M., Nanni D., Scialpi R., Spagnolo P., Zanardi G. Radical chain reaction of benzenethiol with pentynylthiol esters: Production of aldehydes under stannane/silane-free conditions. Synlett. 2004;6:987–990. doi: 10.1055/s-2004-822884. [DOI] [Google Scholar]
- 87.Tojino M., Otsuka N., Fukuyama T., Matsubara H., Schiesser C.H., Kuriyama H., Miyazato H., Minakata S., Komatsu M., Ryu I. Cyclizative radical carbonylations of azaenynes by TTMSS and hexanethiol leading to α-silyl- and thiomethylene lactams. Insights into the E/Z stereoselectivities. Org. Biomol. Chem. 2003;1:4262–4267. doi: 10.1039/B309944J. [DOI] [PubMed] [Google Scholar]
- 88.Ryu I., Miyazato H., Kuriyama H., Matsu K., Tojino M., Fukuyama T., Minakata S., Komatsu M. Broad-Spectrum Radical Cyclizations Boosted by Polarity Matching. Carbonylative Access to α-Stannylmethylene Lactams from Azaenynes and CO. J. Am. Chem. Soc. 2003;125:5632–5633. doi: 10.1021/ja034896u. [DOI] [PubMed] [Google Scholar]
- 89.Miyata O., Kajisa S., Ueda M., Yamauchi M., Naito T. Radical cyclization in heterocycle synthesis 15. Relationship between a radical species and radical acceptors of three different types of double bond in radical addition-cyclization. Chem. Pharm. Bull. 2005;53:995–1002. doi: 10.1248/cpb.53.995. [DOI] [PubMed] [Google Scholar]
- 90.Nanni D., Calestani G., Leardini R., Zanardi G. On the Regioselectivity of Imidoyl Radical Cyclisations. European J. Org. Chem. 2000;2000:707–711. doi: 10.1002/(SICI)1099-0690(200003)2000:5<707::AID-EJOC707>3.0.CO;2-I. [DOI] [Google Scholar]
- 91.Camaggi C.M., Leardini R., Nanni D., Zanardi G. Radical annulations with nitriles: Novel cascade reactions of cyano-substituted alkyl and sulfanyl radicals with isonitriles. Tetrahedron. 1998;54:5587–5598. doi: 10.1016/S0040-4020(98)00230-0. [DOI] [Google Scholar]
- 92.Rainier J.D., Kennedy A.R. Cascades to substituted indoles. J. Org. Chem. 2000;65:6213–6216. doi: 10.1021/jo000831n. [DOI] [PubMed] [Google Scholar]
- 93.Mitamura T., Tsuboi Y., Iwata K., Tsuchii K., Nomoto A., Sonoda M., Ogawa A. Photoinduced thiotelluration of isocyanides by using a (PhS)2-(PhTe)2 mixed system, and its application to bisthiolation via radical cyclization. Tetrahedron Lett. 2007;48:5953–5957. doi: 10.1016/j.tetlet.2007.06.126. [DOI] [Google Scholar]
- 94.Ito O. Kinetic study for reactions of phenylseleno radical with vinyl monomers. J. Am. Chem. Soc. 1983;105:850–853. doi: 10.1021/ja00342a034. [DOI] [Google Scholar]
- 95.Perkins M.J., Turner E.S. SH2 reactions of diphenyl diselenide; preparation and reactions of bridgehead selenides. J. Chem. Soc. Chem. Commun. 1981;174:139–140. doi: 10.1039/c39810000139. [DOI] [Google Scholar]
- 96.Russell G.A., Ngoviwatchai P., Tashtoush H.I., Pla-Dalmau A., Khanna R.K. Reactions of alkylmercurials with heteroatom-centered acceptor radicals. J. Am. Chem. Soc. 1988;110:3530–3538. doi: 10.1021/ja00219a030. [DOI] [Google Scholar]
- 97.Russell G.A., Tashtoush H. Free-radical chain-substitution reactions of alkylmercury halides. J. Am. Chem. Soc. 1983;105:1398–1399. doi: 10.1021/ja00343a069. [DOI] [Google Scholar]
- 98.Minozzi M., Nanni D., Zanardi G., Calestani G. Multi-step radical spiro-cyclization of an alkynylaryl isothiocyanate. ARKIVOC. 2006;2006:6–14. doi: 10.3998/ark.5550190.0007.602. [DOI] [Google Scholar]
- 99.Wang C.-H. Redox Cleavage of S-S Linkage in Diaryl and Cyclic Alkyl Disulphides. Nature. 1964;203:75–76. doi: 10.1038/203075a0. [DOI] [Google Scholar]
- 100.Taniguchi T., Fujii T., Ldota A., Lshibashi H. Reductive addition of the benzenethiyl radical to alkynes by amine-mediated single electron transfer reaction to diphenyl disulfide. Org. Lett. 2009;11:3298–3301. doi: 10.1021/ol901084k. [DOI] [PubMed] [Google Scholar]
- 101.Crick P.J., Simpkins N.S., Highton A. Synthesis of the asperparaline core by a radical cascade. Org. Lett. 2011;13:6472–6475. doi: 10.1021/ol202769f. [DOI] [PubMed] [Google Scholar]
- 102.Hirata K., Kataoka S., Furutani S., Hayashi H., Matsuda K. A Fungal Metabolite Asperparaline A Strongly and Selectively Blocks Insect Nicotinic Acetylcholine Receptors: The First Report on the Mode of Action. PLoS ONE. 2011;6:e18354. doi: 10.1371/journal.pone.0018354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kamimura A., Miyazaki K., Yamane Y., Yo R., Ishikawa S., Uno H., Sumimoto M. A radical cascade cyclization to prepare dihydrothiophenes induced by thiyl radicals as sulfur biradical equivalents. J. Org. Chem. 2013;78:7816–7822. doi: 10.1021/jo400975t. [DOI] [PubMed] [Google Scholar]
- 104.Patel B., Saviolaki G., Ayats C., Garcia M.A.E., Kapadia T., Hilton S.T. Tuneable radical cyclisations: A tin-free approach towards tricyclic and spirocyclic heterocycles via a common precursor. RSC Adv. 2014;4:18930–18932. doi: 10.1039/C4RA02420F. [DOI] [Google Scholar]
- 105.Qiu Y.F., Zhu X.Y., Li Y.X., He Y.T., Yang F., Wang J., Hua H.L., Zheng L., Wang L.C., Liu X.Y., et al. AgSCF3-Mediated Trifluoromethylthiolation/Radical Cascade Cyclization of 1,6-Enynes. Org. Lett. 2015;17:3694–3697. doi: 10.1021/acs.orglett.5b01657. [DOI] [PubMed] [Google Scholar]
- 106.Chen C., Xiong Q., Wei J. Synthesis of 2-sulfenylindenones by visible-light-mediated addition of sulfur-centered radicals to 1,3-diarylpropynones. Synth. Commun. 2019;49:869–877. doi: 10.1080/00397911.2019.1580744. [DOI] [Google Scholar]
- 107.Gharpure S.J., Shelke Y.G. Cascade Radical Cyclization of N-Propargylindoles: Substituents Dictate Stereoselective Formation of N-Fused Indolines versus Indoles. Org. Lett. 2017;19:5022–5025. doi: 10.1021/acs.orglett.7b02005. [DOI] [PubMed] [Google Scholar]
- 108.Fernandez L.S., Buchanan M.S., Carroll A.R., Feng Y.J., Quinn R.J., Avery V.M. Flinderoles A–C: Antimalarial Bis-indole Alkaloids from Flindersia Species. Org. Lett. 2009;11:329–332. doi: 10.1021/ol802506n. [DOI] [PubMed] [Google Scholar]
- 109.Bradner W.T. Mitomycin C: A clinical update. Cancer Treat. Rev. 2001;27:35–50. doi: 10.1053/ctrv.2000.0202. [DOI] [PubMed] [Google Scholar]
- 110.Vepsäläinen J.J., Auriola S., Tukiainen M., Ropponen N., Callaway J.C. Isolation and Characterization of Yuremamine, a New Phytoindole. Planta Med. 2005;71:1053–1057. doi: 10.1055/s-2005-873131. [DOI] [PubMed] [Google Scholar]
- 111.Shibata N., Tsuchiya T., Hashimoto Y., Morita N., Ban S., Tamura O. Thiyl radical-mediated cyclization of omega-alkynyl O-tert-butyldiphenylsilyloximes. Org. Biomol. Chem. 2017;15:3025–3034. doi: 10.1039/C7OB00279C. [DOI] [PubMed] [Google Scholar]
- 112.Floyd R.A., Hensley K., Forster M.J., Kelleher-Anderson J.A., Wood P.L. Nitrones as Neuroprotectants and Antiaging Drugs. Ann. N. Y. Acad. Sci. 2002;959:321–329. doi: 10.1111/j.1749-6632.2002.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 113.Xie X., Li P., Shi Q., Wang L. Visible-light-induced tandem cyclization of 2-alkynylanilines with disulfides: A convenient method for accessing benzothiophenes under transition-metal-free and photocatalyst-free conditions. Org. Biomol. Chem. 2017;15:7678–7684. doi: 10.1039/C7OB01747B. [DOI] [PubMed] [Google Scholar]
- 114.Ye L.M., Qian L., Chen Y.Y., Zhang X.J., Yan M. A practical synthesis of benzothiophenes via visible-light-promoted cyclization of disulfides and alkynes. Org. Biomol. Chem. 2017;15:550–554. doi: 10.1039/C6OB02461K. [DOI] [PubMed] [Google Scholar]
- 115.Yuan Y.Q., Kumar P.S., Zhang C.N., Yang M.H., Guo S.R. Aerobic thiyl radical addition/cyclization of N-methacryloyl benzamides for the synthesis of isoquinoline-1,3(2H,4H)-dione derivatives. Org. Biomol. Chem. 2017;15:7330–7338. doi: 10.1039/C7OB01552F. [DOI] [PubMed] [Google Scholar]
- 116.Nair A.M., Kumar S., Volla C.M.R. Visible Light Mediated Sulfenylation-Annulation Cascade of Alkyne Tethered Cyclohexadienones. Adv. Synth. Catal. 2019;361:4983–4988. doi: 10.1002/adsc.201900874. [DOI] [Google Scholar]
- 117.Mallick R.K., Dutta S., Vanjari R., Voituriez A., Sahoo A.K. Thioarylative Radical Cyclization of Yne-Dienone. J. Org. Chem. 2019;84:10509–10517. doi: 10.1021/acs.joc.9b01445. [DOI] [PubMed] [Google Scholar]
- 118.Li D., Lei J. Thio radical-induced denitrogenative annulation of 1-azido-2-isocyanoarenes to construct 2-thiolated benzimidazoles. Org. Biomol. Chem. 2019;17:9666–9671. doi: 10.1039/C9OB02165E. [DOI] [PubMed] [Google Scholar]
- 119.Xu B., Chen Y., Shi M. The reactions of thiols and diphenyldisulfide with terminally substituted methylenecyclopropanes. Tetrahedron Lett. 2002;43:2781–2784. doi: 10.1016/S0040-4039(02)00384-2. [DOI] [Google Scholar]
- 120.Saegusa T., Kobayashi S., Ito Y. Radical reaction of isocyanide with thiol. J. Org. Chem. 1970;35:2118–2121. doi: 10.1021/jo00832a003. [DOI] [Google Scholar]
- 121.Gharpure S.J., Padmaja, Prasath V., Shelke Y.G. Cascade Radical Cyclization on Alkynyl Vinylogous Carbonates for the Divergent Synthesis of Tetrasubstituted Furans and Dihydrofurans. Org. Lett. 2019;21:223–227. doi: 10.1021/acs.orglett.8b03668. [DOI] [PubMed] [Google Scholar]
- 122.Kim K., Choi H., Kang D., Hong S. Visible-Light Excitation of Quinolinone-Containing Substrates Enables Divergent Radical Cyclizations. Org. Lett. 2019;21:3417–3421. doi: 10.1021/acs.orglett.9b01155. [DOI] [PubMed] [Google Scholar]
- 123.Ho H.E., Pagano A., Rossi-Ashton J.A., Donald J.R., Epton R.G., Churchill J.C., James M.J., O’Brien P., Taylor R.J.K., Unsworth W.P. Visible-light-induced intramolecular charge transfer in the radical spirocyclisation of indole-tethered ynones. Chem. Sci. 2020;11:1353–1360. doi: 10.1039/C9SC05311E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cao Z.-Y., Ghosh T., Melchiorre P. Enantioselective radical conjugate additions driven by a photoactive intramolecular iminium-ion-based EDA complex. Nat. Commun. 2018;9:3274. doi: 10.1038/s41467-018-05375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]