Abstract
Purpose
Inspired by the American Society of Clinical Oncology’s recommendations to strengthen the evidence base for older adults with cancer, the purpose of this systematic review is to identify the reporting of treatment efficacy and adverse events specific to older adults with cancer in Phase III chemo-therapeutic clinical trials. This review also investigates the frequency with which these data points were reported in the literature to identify gaps inreporting and opportunities to expandthe knowledge base on clinica loutcomes for older adults with cancer.
Methods
Chemo-therapeutic clinical trial data published from July 1, 2016 to June 30, 2017 was reviewed. Manuscripts (n = 929) were identified based on keyword searches of EMBASE and PubMed. After removal of duplicates (n = 116) and articles that did not meet this study’s inclusion criteria (n = 654), 159 articles were identified for review.
Results
Reviewed papers were published in 36 different scientific journals and included twenty-five different cancer types. Of the 159 articles, 117 (73.6%) reported age-specific medians and 75 (47.2%) included stratifications of data by age. Treatment efficacy was reported in 96.2% of the articles with 39.9% reporting effectiveness of treatment by age. Reporting of adverse events was included in 84.9% of the articles with only 8.9% reporting these events stratified by age.
Conclusion
Results suggest inadequate reporting of treatment efficacy and adverse events as well as basic descriptive statistics about the age distribution of study subjects. Conscious efforts are needed to address these deficiencies at every level of planning and conducting clinical trials as wells as reporting outcomes stratified by age. Ultimately, standardized reporting could lead to improved treatment decisions and outcomes for older adults with cancer.
Keywords: Geriatric oncology, Older adults, Phase III clinical trials, Systematic review
1. Introduction
Older adults, defined as those aged 65 years and older, constitute the largest percentage of the cancer patient and survivor population [1–22]. However, this growing group is not adequately represented in clinical trials [2]. The 2013 Institute of Medicine report, Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis, highlighted this important and ongoing issue, concluding that lack of an evidence base for this special population is detrimental to the quality of cancer care in older adults [3]. Recommendations from this report can be summarized as follows: first, more patients reflecting the population and actual disease experience need to be enrolled in clinical trials and second, more data need to be collected, analyzed, and reported regarding this underrepresented population.
In 2015, the American Society of Clinical Oncology (ASCO) published recommendations to strengthen the literature regarding care for older adults with cancer, Improving the Evidence Base for Treating Older Adults with Cancer [4]. This white paper included several suggestions to expand the body of data used to treat older adults with cancer. Journal editors were urged to develop policies to improve the inclusion and reporting of clinical trial data specific to older adults. An important action step from this recommendation was:
“Require authors to submit and report the detailed age distribution (by decade) of the population included in the study, not just the age ranges of the population, and data analyses that could potentially yield valuable age-related information, including age-based analyses of response, and toxicity.” (p. 3831) [4]
In November 2017, ASCO and the US Food and Drug Administration convened a conference to discuss this issue. From this joint workshop came action items to shift the landscape of clinical trial enrollment and reporting. This meeting reiterated the charge to move the 2015 ASCO recommendations into practice, including the directive to change data reporting practices for older adult study participants.
Our research group conducted a systematic review to identify the reporting status of Phase III chemo-therapeutic clinical trial treatment efficacy and adverse events in older adults with cancer. We sought to investigate the frequency with which these data points were reported in the literature as a means to identify gaps in reporting and opportunities to expand evidence about outcomes in older adults with cancer.
2. Methods
The data used in this systematic review were from articles published online or only in print between July 1, 2016 and June 30, 2017, based on PubMed and EMBASE searches. This time interval was selected to capture articles that would have been unlikely to be affected by the 2015 ASCO statement. Inclusion criteria were articles that reported findings from chemo-therapeutic Phase III cancer clinical trials in adults. This included articles that were secondary analyses of data pertaining to health-related quality of life as well as long-term follow-up of select patients from the original Phase III cancer clinical trials. The exclusion criteria were: 1) all Phase I, Phase II, and aggregated data from Phase II/III studies; 2) Phase III studies that focused upon radiation and/or surgical treatment interventions; 3) case studies; 4) cross-sectional studies; and 5) qualitative studies.
A keyword search strategy was developed by medical librarians (See Box 1). The initial search, conducted in early January 2017, covered journals published from July 1, 2016. Subsequent searches were performed automatically every Monday to cover publications through July 31, 2017. All results were entered into RefWorks, a reference management system. The title and abstract of all articles entered into RefWorks were reviewed by research group member KBS to determine if the inclusion criteria were met.
Box 1. Key Word Search for PubMed and Embase.
PubMed
(((“Clinical Trial, Phase III”[Publication Type] OR “Clinical Trials, Phase III as Topic”[Mesh] OR “phase 3 trial”[All Fields] OR “phase III trial”[All Fields]) AND (“Neoplasms”[Mesh] OR “cancer”[All Fields] OR “cancers”[All Fields] OR “cancerous”[All Fields] OR “tumor”[All Fields] OR “tumors”[All Fields] OR “tumour”[All Fields] OR “tumours”[All Fields] OR “neoplasm”[All Fields] OR “neoplasms”[All Fields] OR “neoplastic”[All Fields] OR “malignant”[All Fields] OR “malignancy”[All Fields] OR “malignancies”[All Fields] OR “metastatic”[All Fields] OR “metastasis”[All Fields] OR “metastases”[All Fields])) NOT (“case reports”[Publication Type] OR “guideline”[Publication Type] OR “practice guideline”[Publication Type] OR “review”[Publication Type] OR “meta analysis”[Publication Type] OR systematic[sb])) Filters: Publication date from 2016/07/01 to 2017/06/30; English; Adult: 19+ years
Embase
(‘phase 3 clinical trial’/de OR ‘phase 3 clinical trial’:it OR ‘phase 3 clinical trial (topic)’/de OR ‘phase 3 trial’ OR ‘phase iii trial’) AND (‘neoplasm’/exp. OR ‘cancer’ OR ‘cancers’ OR ‘cancerous’ OR ‘tumor’ OR ‘tumors’ OR ‘tumour’ OR ‘tumours’ OR ‘neoplasm’ OR ‘neoplasms’ OR ‘neoplastic’ OR ‘malignant’ OR ‘malignancy’ OR ‘malignancies’ OR ‘metastatic’ OR ‘metastasis’ OR ‘metastases’) NOT (‘case reports’:it OR ‘guideline’:it OR ‘practice guideline’:it OR ‘review’:it OR ‘meta analysis’:it OR ‘systematic’:it OR ‘conference abstract’:it OR ‘conference paper’:it OR ‘letter’:it OR ‘note’:it OR ‘editorial’:it OR ‘surgery’:ab,ti OR ‘surgeries’:ab,ti OR ‘surgical’:ab,ti) AND [embase]/lim NOT ([embase]/lim AND [medline]/ lim) AND ([adult]/lim OR [young adult]/lim) AND [english]/lim AND [2016–2017]:py
2.1. Sample
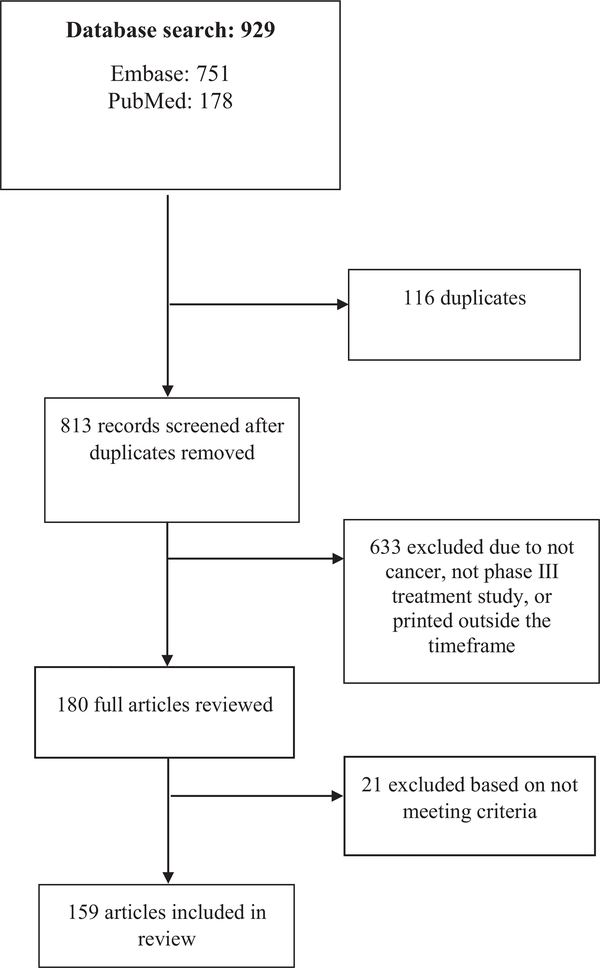
There were 929 articles captured by the keyword searches, 178 articles through PubMed and 751 through EMBASE. There were 116 duplicares. Six-hundred and thirty-three (68.1%) articles were excluded due to not meeting the inclusion criteria in terms of disease, being a phase III treatment study, and/or not having a publishing date in the designated time frame. An additional 21 (2.2%) of the articles were found not to meet the inclusion criteria after full article review by members of the research group. The final sample included 159 articles (See Fig. 1).
Fig. 1.
Flow Chart according to PRISMA Framework”. Footnote: Modified From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6 (7): e1000097. doi:https://doi.org/10.1371/journal.pmed100009
2.2. Data Collection
A data extraction survey was developed through an iterative process by five members of the Cancer and Aging Research Group (CARG) Advocacy Group. At two points in the survey development process, other members of CARG reviewed and provided comments during regularly scheduled conference calls. The data extraction survey was built in REDCap [5] and pilot tested using 10 articles,after which modifications were made and the final survey established (See Appendix 1). The data extraction survey included DOI, journal title, article title, cancer type, and specific questions focusing on the data in the methods, results, and discussion sections.
To evaluate interrater reliability, 15 articles were reviewed by three to six reviewers duringthe early stage of the investigation. The resulting agreement rate was 85%, ranging from 71% to 100%. The remaining articles were evaluated independently by at least two reviewers. Responses were downloaded from REDCap into SPSS version 23 [6] and evaluated for inter-reviewer differences. If extracted data differed between reviewers of the same article, a research assistant reviewed the article and resolved discrepancies. These revisions were reviewed for accuracy by the first author. The data was then corrected in the dataset resulting in 159 articles (Appendix 2) with extracted data that was congruent across reviewers.
3. Results
The 159 articles were published in 36 different scientific journals. One-third of the articles were published in 14 of the journals (range: 3–19 articles per journal). The investigations focused on twenty-five different cancer types. Four articles reported findings on four or more cancers, and one article did not name the cancer(s) of interest. Approximately 78% of the articles included ten of the twenty-five cancer types (Table 1).
Table 1.
Journals and cancer sites included in the systematic review (n = 159 articles).
| Variables | N | % |
|---|---|---|
| Journal titles | ||
| Lancet Oncology | 19 | 11.9 |
| New England Journal of Medicine | 19 | 11.9 |
| Journal of Clinical Oncology | 18 | 11.3 |
| Annals of Oncology | 7 | 4.4 |
| European Journal of Cancer | 6 | 3.8 |
| Lancet Hematology | 5 | 3.1 |
| Lancet | 5 | 3.1 |
| British Journal of Cancer | 5 | 3.1 |
| Lung Cancer | 3 | 1.9 |
| British Journal of Hematology | 3 | 1.9 |
| BMC Cancer | 3 | 1.9 |
| Haematologica | 3 | 1.9 |
| Othera | 63 | 39.6 |
| Cancer site | ||
| Breast | 25 | 15.7 |
| Lung | 17 | 10.7 |
| Colorectal | 16 | 10.1 |
| Multiple Myeloma | 14 | 8.8 |
| Leukemia | 12 | 7.5 |
| Prostate | 9 | 5.7 |
| Lymphoma | 9 | 5.7 |
| Melanoma | 8 | 5.0 |
| Gynecologic | 7 | 4.4 |
| Pancreas | 6 | 3.8 |
| Other | 36 | 22.6 |
Other joumals include: Cancer, The Oncologist, Tumori Joumal, The Prostate, The Journal of Community and Supportive Oncology, Targeted Oncology, Surgery Today, Supportive Care in Cancer, Sarcoma, Research and Reports in Urology, Quality of Life research, Pancreatology, Oncortargets and Therapy, Neoplasia, medicine, Leukemia and Lymphoma, Leukemia, Lancet Gastroenterology and Hepatology, Journal of Translational Medicine, Journal of Thoracic Oncology, JAMA, Journal of Hematology and Oncology, Journal of Geriatric Oncology, Journal of Gastroenterology, Journal of Clinical Endocrine Metabolism, Journal of Cancer Research and Therapeutics, Journal of Cancer Research and Clinical Oncology, JAMA Oncology, International Journal of Hematology, Gynecological Oncology, European Urology, Clinics, Clinical Translational Oncology, Clinical Pharmacology and Therapeutics, Clinical Colorectal Cancer, Clinical Breast Cancer, Chinese Clinical Oncology, Cancer Research and Treatment, British Journal of Clinical Pharmacology, Breast Cancer Research and Treatment, Blood Cancer, Biomedicine and Pharmacotherapy, Asian Journal of Urology, Anticancer Drugs, Annals of Hematology, American Journal of Hematology, Acta Oncologica, American Journal of Clinical Oncology: Cancer Clinical Trials.
One-hundred and seventeen of the eligible articles (117/159, 74.6%) listed age-specific medians compared to mean ages in their respective demographic sections. Of these 117 articles, 1) 63 (53%) reported study populations with median ages ≥60; 2) 29 (24%) reported study populations with median ages ≥65; and 3) 6 (5%) reported study populations withmedian ages ≥70. Information regarding theages of the participants, presented as demographics in the Results sections of the articles, included the following: median and range 61.0%; age categories (e.g., 40 to 64, 65 to 75, ≥ 75) 27.7%; median and interquartile range 12.6%; mean and standard deviation 11.3%; age range 6.3%; and mean and range 5.7% (Table 2). Finally, 75 (47.2%) articles included stratifications of data by age.
Table 2.
Results on extracted data (n = 159 articles).
| Variables | N | % |
|---|---|---|
| Methods section | ||
| Inclusion Criteria Included | 152 | 95.6 |
| Upper Age Cut-off in Inclusion/Exclusion Criteria | 29 | 19.1 |
| Results section | ||
| Age presentation in demographics | ||
| Age categories | 44 | 27.7 |
| Age Range | 10 | 6.3 |
| Mean/Standard Deviation | 18 | 11.3 |
| Mean/Range | 9 | 5.7 |
| Median/Range | 97 | 61.0 |
| Median/Interquartile Range | 20 | 12.6 |
| Other | 14 | 8.8 |
| Stratified by age in any section of Results | 75 | 47.2 |
| Effectiveness of Cancer Treatment | 153 | 96.2 |
| Effectiveness of Cancer Treatment Stratified by Age | 61 | 39.9 |
| Adverse Events/Complications | 135 | 84.9 |
| Adverse Events/Complications Stratified by Age | 12 | 8.9 |
| Discussion section | ||
| Effectiveness of Treatment Based on Age | 22 | 13.8 |
| Adverse Events/Complications Based on Age | 9 | 5.7 |
| Limitations | ||
| Effectiveness of Treatment Based on Age | 1 | 0.7 |
| Adverse Events/Complications Based on Age | 1 | 0.7 |
| Lack of Other Age-Related Issues | 6 | 3.8 |
Of the articles, 95.6% reported inclusion criteria and of those, 19.1% reported an upper age cut-off. Treatment efficacy was reported in 96.2% of the articles, with 39.9% of these including efficacy stratified by age. The reporting of adverse events or complications among all participants was included in 84.9% of the articles with 8.9% reporting these events stratified by age (Table 2).
In the Discussion sections, treatment efficacy based on age was mentioned in 13.8% of the articles and age stratification of adverse events or complications was only mentioned in 5.7% of the articles. Lastly, 3.8% of the articles reported age-related issues as study limitations (Table 2).
4. Discussion
This systematic review identifies the reporting status of Phase III chemo-therapeutic cancer clinical trials in terms of efficacy and adverse events specific to older adults. In addition, this study investigates the frequency with which these data points were reported in the literature in order to identify gaps in reporting and opportunities to expand the evidence base for older adults with cancer. Despite ASCO and FDA recommendations [7] pertaining to age distribution and health risk profiles of clinical trial research participants, this systematic review found a dearth in reporting regarding treatment efficacy and adverse events specific to older adults with cancer. When outcomes pertaining to older adults were reported, the results were inconsistent as evidenced by the array of age distributions and varying categorization of “older adults”. Seventy-five percent of the articles in this review reported age-specific medians. Median age is important as it summarizes the age distribution of a study’s population and facilitates the reporting of age stratification. Despite older adults being the majority of patients with cancer [8], reporting of treatment efficacy and adverse events by age was observed in <40% and 9% of the studies, respectively, in this review. This gap in evidence severely limits informed clinical management of older adults with cancer.
Solutions to address this deficiency in reporting include 1) a conscious effort at every level of clinical trial planning to identify and report age-specific issues and 2) the reporting of outcomes stratified by age unless appropriately not relevant. Funding from federal and private entites should not only require the planned and actual recruitment and participation of diverse, older patients, but also the reporting of treatment efficacy, adverse events, and age-related issues pertaining to treatments and outcomes for this population. Protocols for clinical trials should avoid, for example, exclusion criteria related to functional and cognitive status, and also be designed for a priori reporting of subgroup effects by age at study entry [9,10].
We agree that increasing enrollment of older adults in clinical trials is of the utmost importance. Evidence demonstrates that older adults are willing to enroll in trials [11] and have similar treatment-related toxicity profiles compared to that of younger patients [12–14]. Yet, as noted by Levit and colleagues [ 15],increasing enrollment of older adults in trials alone will not solve the evidence gap. Thus, expanded representation and reporting of older adult-specific research is imperative. One strategy might be the inclusion of broader, more age-specific endpoints, such as the impact of treatment on function and cognition, as well as utilizing innovative trial designs [7,16,17]. Similarly, in addition to the typical outcomes of hospitalization and death, the recognition and reporting of adverse events in a geriatric-specific context is needed. These events include 1) need for skilled nursing facility for rehabilitation; 2) admission to nursing home or need for a higher level of care (e.g., moving to an assisted living facility); 3) caregiver burden; 4) domains of health and functioning (e.g., cognitive, social, and physical); and 5) quality of life. These outcomes are at least equally meaningful to older patients as treatment efficacy and disease survival [18,19]. These outcomes are also provide critical information for the health care team to provide optimal care. The multi-faceted nature of geriatric oncology must be considered. Older adult-specific research efforts need to incorporate biopsychosocial approaches [20] in reporting outcomes.
Levit and colleagues [15] concluded that academic journals should incentivize researchers to report more detailed age distributions and health risk profiles of research participants. We, as members of the Advocacy Committee of the Cancer and Aging Research Group, believe that this reporting should be standard practice and a customary component of the reviewing rubric adhered to by journal review boards and editors. We challenge Geriatric, Geriatric Oncology, and Oncology-focused journals to be the first to implement these criteria. In addition, as Witham and colleagues [9] have encouraged, the outcomes of older adult study participants should be reported and discussed within the main paper and not solely as supplementary data.
In summary, the enrollment of older patients with cancer in clinical trials and the reporting of results are in need of change through conscious efforts that can ultimately improve the treatment decisions and clinical outcomes for this burgeoning yet underserved population.
Based on our findings, we propose the following recommendations:
| 1. Conduct clinical trials targeting all patients, regardless of age, if they meet other eligibility criteria. |
| 2. Report the median age of study participants. |
| 3. Utilize age stratification reporting methods automatically when ≥25% of the study cohort is ≥65 years of age. |
| 4. Utilize age stratification reporting methods automatically when the median age of the study participants is ≥60 years of age. |
| 5. Stratify the following by age: 1) demographic profile; 2) intervention results; 3) existence of adverse events; and 4) any other relevant results |
| 6. Report results by age stratification preferably in the main section of manuscripts, if not possible due to word count and page limits as supplementary data. |
| 7. Discuss the implications of the findings (e.g., treatment efficacy, adverse events or complications) specific to older adults. |
| 8. Describe age-related issues as study limitations when applicable. |
These recommendations extend those put forth in the 2013 IOM [3], 2015 ACSO [2], and 2017 ASCO, the US Food and Drug Administration [7] reports, the CONSORT statement 2010 [ 21],and and the European Organisation for Research and Treatment of Cancer–Alliance for Clinical Trials in Oncology–International Society of Geriatric Oncology 2013 position article [22]. We provide specific recommendations to facilitate funders, journal editors, and cancer researchers in identifying important factors required in translating evidence into clinical practice.
Many of the articles included in this review presented data demographics and adverse events in tables. Few presented data segregated by age group (younger adults, older adults) in these tables. In an attempt to assist in implementing the recommendations pertaining to the stratification of data by age group, two templates are offered (See Tables 3 and 4).
Table 3.
Template 1: Demographic data comparing two treatment modalities.
| Characteristics | Treatment 1 (N = xxx) |
Treatment 2 (N = xxx) |
||
|---|---|---|---|---|
| Youngera | Older | Younger | Older | |
| Gender | ||||
| Female | n (%) | n (%) | n (%) | n (%) |
| Male | n (%) | n (%) | n (%) | n (%) |
| Age (years) | ||||
| Median (IQR) | n (%) | n (%) | n (%) | n (%) |
| Disease | ||||
| Type of cancer | n (%) | n (%) | n (%) | n (%) |
| Type of cancer | n (%) | n (%) | n (%) | n (%) |
| ECOG | ||||
| 0 | n (%) | n (%) | n (%) | n (%) |
| 1 | n (%) | n (%) | n (%) | n (%) |
| 2 | n (%) | n (%) | n (%) | n (%) |
Replace with age range of group.
Table 4.
Template 2: Adverse events by grade.
| Adverse Event |
Grade |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
≥ 4 |
|||||
| Youngera | Older | Younger | Older | Younger | Older | Younger | Older | |
| Anemia | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Anorexia | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Diarrhea | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Fatigue | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Increased creatinine | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Neutropenia | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Thrombocytopenia | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
Note: Include adverse events stratified by age and treatment arm.
Replace with age range of group.
4.1. Limitations
There are limitations to our analysis. The reviewed articles included articles published online or only in print between July 1, 2016 to June 30, 2017, which limits the application of findings published before or after this time frame. The review focused on Phase III pharmaceutical trials and excluded Phase I, Phase II, and Phase II/III (aggregated data) studies, treatments that included radiation and/or surgery, case studies, cross-sectional studies, and qualitative studies. If all studies were included our results might be more generalizable, but they would be limited in their scientific rigor and/or accuracy in comparing different study designs.
5. Conclusion
This systematic review identifies the reporting of Phase III chemo-therapeutic cancer clinical trial results of efficacy and adverse events in older patients. This review also investigates the frequency with which these data points were reported in the literature as a means to identify gaps in reporting and opportunities to expand the knowledge base related to geriatric oncology. Results of our systematic review suggest that there is inadequate reporting of treatment efficacy and adverse events as well as discrepancies as to how older age is defined, considered, and reported. This sparse and varied reporting critically limits the evidence base for treating older patients with cancer. There is a significant and timely need to design all clinical trials to include older adults and utilize a broad array of geriatric-specific outcomes. Incorporating these geriatric-specific outcomes as well as reporting the age-stratified data in a standardized and comprehensive manner can lead to better-informed treatment strategies for older adults with cancer.
Appendix A. Data Entry of Phase III Cancer Treatment Trial Literature
A.1. Reviewer and Article Information
| doi only | |
| Reviewer name | |
| Article title | |
| Date of publication | |
| Journal | British Journal of Cancer |
| Cancer | |
| European Journal of Cancer | |
| Lancet | |
| Lancet Oncology | |
| Annals of Oncology | |
| NEJM | |
| Journal of Clinical Oncology | |
| CA: A Cancer Journal for | |
| Clinicians | |
| Journal of the NCI | |
| Cancer Research | |
| International Journal of Cancer | |
| Neuro-Oncology | |
| Cancer Treatment Reviews | |
| Journal of the National | |
| Comprehensive Cancer | |
| Network | |
| Advances in Cancer Research | |
| Other | |
| Other journal title | |
| Cancer site (select all that apply) | Breast |
| Prostate | |
| Lung | |
| Colorectal | |
| Gynecologic | |
| Bladder | |
| Kidney | |
| Leukemia | |
| Lymphoma | |
| Melanoma | |
| Pancreas | |
| Thyroid | |
| Head & Neck | |
| Brain | |
| Four or more | |
| Other GI | |
| Other | |
| Please specify the other type of GI cancer | |
| Please specify other type of cancer | |
| Methods Section (Full Text) | |
| Are any “inclusion/exclusion criteria” included in the methods section? | Yes |
| No | |
| Do the inclusion/exclusion criteria include an “upper age cut-off point”? | Yes |
| No | |
| Results Section (Full Text) | |
| How is “age” presented in the demographics | Age categories |
| Age range | |
| Mean/Standard Deviation | |
| Median/Standard Deviation | |
| Mean/Range | |
| Median/Range | |
| Other | |
| Please specify | |
| Is there a component of the Results Section that “stratified by age”? (presents data for different age groups such as 45 to 64 and 65 and older) | Yes |
| No | |
| What are the “age stratification categories”? | Yes |
| No | |
| Is effectiveness of “cancer treatment” presented in the Results Section? | Yes |
| No | |
| Is there an age stratification subset analysis of the effectiveness of the cancer treatment? | Yes |
| No | |
| Are “adverse events/complications” presented in the Results Section? | Yes |
| No | |
| Is there an age stratification analysis for adverse events/complications? | Yes |
| No | |
| Discussion (Full Text) | |
| Is the effective of cancer treatment based on age discussed? | Yes |
| No | |
| Is the lack of effectiveness of cancer treatment results based on age listed as a study limitation? | Yes |
| No | |
| Are age stratification “adverse events/complications” discussed”? | Yes |
| No | |
| Is the lack of data on adverse events/complications based on age listed as a study limitation? | Yes |
| Is there lack of other age-related issues/items mentioned as a study limitation? | Yes |
| No |
Appendix B. List of Articles in Systematic Review
- Aapro M, Karthaus M, Schwartzberg L, et al. NEPA, a fixed oral combination of netupitant and palonosetron, improves control of chemotherapy-induced nausea and vomiting (CINV) over multiple cycles of chemotherapy: Results of a randomized, double-blind, phase 3 trial versus oral palonosetron. Supportive Care Cancer. 2017;25 (4):1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwala SS, Lee SJ, Yip W, et al. Phase III randomized study of 4 weeks of high-dose interferon-alpha-2b in stage T2bNO, T3a-bNO, T4a-bNO, and T1–4N1a-2a (microscopic) melanoma: A trial of the eastern cooperative oncology group-american college of radiology imaging network cancer research group (E1697). J Clin Oncol. 2017;35 (8):885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascierto PA, Del Vecchio M, Robert C, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: A randomized, double-blind, multicentre, phase 3 trial. Lancent Oncol. 2017;18 (5):611–622. [DOI] [PubMed] [Google Scholar]
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Oncol. 2016;17 (9):1248–1260. [DOI] [PubMed] [Google Scholar]
- Attal M, Lauwers-cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. New Engl J Med. 2017;376 (14):1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggstrom MQ, Socinski MA, Wang XF, et al. Maintenance sunitinib following initial platinum-based combination chemotherapy in advanced-stage IIIB/IV Non–Small cell lung cancer: A Randomized, double-blind, placebo-controlled phase III Study—CALGB 30607 (alliance). J Thorac Oncol. 2017;12 (5):843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Brown J, Parmar M, et al. Final efficacy and updated safety resilts of the randomized phase III BEATRICE trial evaluating adjuvant bevacizumab-containing therapy in triple-negative early breast cancer. Ann Oncol. 2017;28 (4):754–760. [DOI] [PubMed] [Google Scholar]
- Bellmunt J, De Wit R, Vaughn DJ, et al. Pembrolizumab assecond-line therapy for advanced urothelial carcinoma. New Engl J Med. 2017;376 (11):1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biran N, Jacobus S, Vesole DH, et al. Outcome with lenalidomide plus dexamethasone followed by early autologous stem cell transplantation in patients with newly diagnosed multiple myeloma on the ECOG-ACRIN E4A03 randomized clinical trial: Long-term follow-up. Blood Cancer J. 2016;6 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradstock KF, Link E, Iulio JD, et al. Idarubicin dose escalation during consolidation therapy for adult acute myeloid leukemia. J Clin Oncol. 2017;35 (15):1678–1685. [DOI] [PubMed] [Google Scholar]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. [DOI] [PubMed] [Google Scholar]
- Cardoso F, vanť Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. The New England journal of medicine. 2016;375 (8):717–729. 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- Casali PG, Fumagalli E, Gronchi A, et al. Ten-year progression-free and overall survival in patients with unresectable or metastatic GI stromal tumors: Long-term analysis of the European organisation for research and treatment of cancer, Italian sarcoma group, and Australasian gastrointestinal trials group intergroup phase III randomized trial on imatinib at two dose levels. J Clin Oncol. 2017;35 (15):1713–1720. [DOI] [PubMed] [Google Scholar]
- Casas F, Henriquez I, Bejar A, et al. Intermittent versus continuous androgen deprivation therapy to biochemical recurrence after external beam radiotherapy: A phase 3 GICOR study. Clin Transl Oncol.2017;19 (3):373–378. [DOI] [PubMed] [Google Scholar]
- Castagnetti F, Breccia M, Gugliotta G, et al. Nilotinib 300 mg twice daily: An academic single-arm study of newly diagnosed chronic phase chronic myeloid leukemia patients. Haematologica. 2016;101 (10):1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Hu C-, Chen X-, et al. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: Long-term results of a phase 3 multicentre randomized controlled trial. Eur J Cancer, 2017;75:150–158. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Trask PC, Gribben JG, et al. Health-related quality of life and symptoms in patients with rituximab-refractory indolent non-hodgkin lymphoma treated in the phase III GADOLIN study with obinutuzumab plus bendamustine versus bendamustine alone. Ann Hematol. 2017;96 (2):253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi KN, Higano CS, Blumenstein B, et al. Custirsen in combination with docetaxel and prednisone for patients with metastatic castration-resistantprostatecancer(SYNERGYtrial):Aphase3, multicentre, open-label, randomized trial. Lancet Oncol. 2017;18 (4):473–485. [DOI] [PubMed] [Google Scholar]
- Cicènas S, Geater SL, Petrov P, et al. Maintenance erlotinib versus erlotinib at disease progression in patients with advanced non-small-cell lung cancer who have not progressed following platinum-based chemotherapy (IUNO study). Lung Cancer. 2016;102:30–37. [DOI] [PubMed] [Google Scholar]
- Coens C, Suciu S, Chiarion-Sileni V, et al. Health-related quality of life with adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): Secondary outcomes of a multinational, randomized, double-blind, phase 3 trial. Lancet Oncol. 2017;18 (3):393–403. 10.1016/S1470-2045(17)30015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G, Ashcroft AJ, Cairns DA, et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF myeloma X relapse [intensive]): A randomized, open-label, phase 3 trial. Lancet Haematol. 2016;3 (7):e34–e351. [DOI] [PubMed] [Google Scholar]
- Cortelazzo S, Tarella C, Gianni AM, et al. Randomized trial comparing R-CHOP versus high-dose sequential chemotherapy in high-risk patients with diffuse large B-cell lymphomas. J Clin Oncol. 2016;34 (33):4015–4022. [DOI] [PubMed] [Google Scholar]
- Cortes JE, De Souza CA, Ayala M, et al. Switching to nilotinib versus imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase with suboptimal response to imatinib (LASOR): A randomized, open-label trial. Lancet Haematol. 2016;3 (12):e58–e591. [DOI] [PubMed] [Google Scholar]
- Davies A, Merli F, Mihaljevic B, et al. Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): A randomized, open-label, phase 3 trial. Lancet Haematol. 2017;4 (6):e27–e282. [DOI] [PubMed] [Google Scholar]
- de la Cruz-Merino L, Di Guardo L, Grob J-, et al. Clinical features of serous retinopathy observed with cobimetinib in patients with BRAF-mutated melanoma treated in the randomized coBRIM study. J Transl Med. 2017;15 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Morrée ES, Vogelzang NJ, Petrylak DP, et al. Association of survival benefit with docetaxel in prostate cancer and total number of cycles administered: A post hoc analysis of the mainsail study. JAMA Oncology. 2017;3 (1):68–75. 10.1001/jamaoncol.2016.3000. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Stewart AK, Masszi T, et al. Carfilzomib, lenalidomide, and dexamethasone in patients with relapsed multiple myeloma categorised by age: Secondary analysis from the phase 3 ASPIRE study. Br J Haematol.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos MA, Trotman J, Tedeschi A, et al. Ibrutinib for patients with rituximab-refractory waldenstrom’s macroglobulinaemia (iNNOVATE): An open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. 2017;18 (2):241–250. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375 (14):1319–1331. 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): A descriptive analysis of final overall survival results from a randomized, open-label, phase 3 trial. Lancet Oncol. 2017;18 (6):732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer R, Schadendorf D, Ascierto PA, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): A multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2017;18 (4):435–445. 10.1016/S1470-2045(17)30180-8. [DOI] [PubMed] [Google Scholar]
- Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomized, open-label, phase 3 trial. Lancet. 2017;389(10068): 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl HM, Hiller L, Howard HC, et al. Addition of gemcitabine to paclitaxel, epirubicin, and cyclophosphamide adjuvant chemotherapy for women with early-stage breast cancer (tAnGo): Final 10-year follow-up of an open-label, randomized, phase 3 trial. Lancet Oncol. 2017;18 (6):755–769. [DOI] [PubMed] [Google Scholar]
- Eggermont AMM, Chiarion-sileni V, Grob J-, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. New Engl J Med. 2016;375 (19):1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Sharma P, McDermott DF, et al. CheckMate 025 randomized phase 3 study: Outcomes by key baseline factors and prior therapy for nivolumab versus everolimus in advanced renal cell carcinoma. Eur Urol. 2017. [DOI] [PubMed]
- Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. New Engl J Med. 2016;375 (19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. New Engl J Med. 2016;375 (20):1925–1936. [DOI] [PubMed] [Google Scholar]
- Foukakis T, Von Minckwitz G, Bengtsson N-, et al. Effect of tailored dose-dense chemotherapy vs standard 3-weekly adjuvant chemotherapy on recurrence-free survival amongwomen with high-risk early breast cancer: A randomized clinical trial. JAMA. 2016;316 (18):1888–1896. [DOI] [PubMed] [Google Scholar]
- Gentile M, Magarotto V, Offidani M, et al. Lenalidomide and low-dose dexamethasone (rd) versus bortezomib, melphalan, prednisone (VMP) in elderly newly diagnosed multiple myeloma patients: A comparsion of two prospective trials. Am J Hematol. 2017;92 (3):244–250. 10.1002/ajh.24621. [DOI] [PubMed] [Google Scholar]
- Gill S, Ko Y-, Cripps C, et al. PANCREOX: A randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol. 2016;34 (32):3914–3920. [DOI] [PubMed] [Google Scholar]
- Gleeson M, Hawkes EA, Cunningham D, et al. Rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) in the management of primary mediastinal B-cell lymphoma: A subgroup analysis of the UK NCRI R-CHOP 14 versus 21 trial. Br J Haematol. 2016;175 (4):668–672. [DOI] [PubMed] [Google Scholar]
- Goossens ME, Zeegers MP, van Poppel H, et al. Phase III randomized chemoprevention study with selenium on the recurrence of non-invasive urothelial carcinoma. The SELEnium and BLAdder cancer trial. Eur J Cancer. 2016;69:9–18. [DOI] [PubMed] [Google Scholar]
- Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375 (3):209–219. 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): An international, open-label, randomized, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18 (6):812–822. [DOI] [PubMed] [Google Scholar]
- Guren TK, Thomsen M, Kure EH, et al. Cetuximab in treatment of metastatic colorectal cancer: Final survival analyses and extended RAS data from the NORDIC-VII study. Br J Cancer. 2017;116 (10):1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna NH, Kaiser R, Sullivan RN, et al. Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non-small cell lung cancer (LUME-lung 2): A randomized, double-blind, phase III trial. Lung Cancer. 2016;102:65–73. [DOI] [PubMed] [Google Scholar]
- Harrington KJ, Andtbacka RHI, Collichio F, et al. Efficacy and safety of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in patients with stage IIIB/C and IVMIa melanoma: Subanalysis of the phase III OPTiM trial. OncoTargets Ther. 2016;9:7081–7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegg R, Mattar A, Matos-Neto JN d, et al. A phase III, randomized, non-inferiority study comparing the efficacy and safety of biosimilar filgrastim versus originator filgrastim for chemotherapy-induced neutropenia in breast cancer patients. Clinics (Sao Paulo). 2016;71 (10):586–592. 10.6061/clinics/2016(10)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickish T, Andre T, Wyrwicz L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: A randomized, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2017;18 (2):192–201. 10.1016/S1470-2045(17)30006-2. [DOI] [PubMed] [Google Scholar]
- Hillmen P, Janssens A, Babu KG, et al. Health-related quality of life and patient-reported outcomes of ofatumumab plus chlorambucil versus chlorambucil monotherapy in the COMPLEMENT 1 trial of patients with previously untreated CLL. Acta Oncol. 2016;55 (9, 10):1115–1120. [DOI] [PubMed] [Google Scholar]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375 (18):1738–1748 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- Hudgens S, Forsythe A, Kontoudis I, D’adamo D, Bird A, Gelderblom H. Evaluation of quality of life at progression in patients with soft tissue sarcoma. Sarcoma. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata H, Masuda N, Yamamoto D, et al. Circulating tumor cells as a prognostic marker for efficacy in the randomized phase III JO21095 trial in Japanese patients with HER2-negative metastatic breast cancer. Breast Cancer Res Treat. 2017;162 (3):501–510. [DOI] [PubMed] [Google Scholar]
- Jethava YS, Mitchell A, Epstein J, et al. Adverse metaphase cytogenetics can be overcome by adding bortezomib and thalidomide to fractionated melphalan transplants. Clin Cancer Res. 2017;23 (11):2665–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JA, Robak T, Brown JR, et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukemia: An open-label, randomized phase 3 trial. Lancet Haematol. 2017;4 (3):e11–e126. [DOI] [PubMed] [Google Scholar]
- Kamba T, Kamoto T, Maruo S, et al. A phase III multicenter, randomized, controlled study of combined androgen blockade with versus without zoledronic acid in prostate cancer patients with metastatic bone disease: Results of the ZAPCA trial. Int J Clin Oncol. 2017;22 (1):166–173. 10.1007/s10147-016-1037-2. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375 (8):740–753. 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376 (9):836–847. 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karampeazis A, Vamvakas L, Kotsakis A, et al. Docetaxel plus gemcitabine versus gemcitabine in elderly patients with advanced non-small cell lung cancer and use of a geriatric assessment: Lessons from a prematurely closed hellenic oncology research group randomized phase III study. J Geriatr Oncol. 2017;8 (1):23–30. [DOI] [PubMed] [Google Scholar]
- Kerr RS, Love S, Segelov E, et al. Adjuvant capecitabine plus bevacizumab versus capecitabine alone in patients with colorectal cancer (QUASAR 2): An open-label, randomized phase 3 trial. Lancet Oncol. 2016;17 (11):1543–1557. 10.1016/S1470-2045(16)30172-3. [DOI] [PubMed] [Google Scholar]
- Kim TW, Elme A, Kusic Z, et al. A phase 3 trial evaluating panitumumab plus best supportive care vs best supportive care in chemorefractory wild-type KRAS or RAS metastatic colorectal cancer. Br J Cancer. 2016;115 (10):1206–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D-, Nam D-, Kang S-, et al. Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in Korea. Oncotarget. 2017;8 (4):7003–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krop IE, Kim S-, Martin AG, et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): Final overall survival results from a randomized open-label phase 3 trial. Lancet Oncol. 2017;18 (6):743–754. [DOI] [PubMed] [Google Scholar]
- Kudo M, Hatano E, Ohkawa S, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma: Japanese subgroup analysis of the REACH trial. J Gastroenterol. 2017;52 (4):494–503. [DOI] [PubMed] [Google Scholar]
- Kusano M, Honda M, Okabayashi K, et al. Randomized controlled phase III study comparing hepatic arterial infusion with systemic chemotherapy after curative resection for liver metastasis of colorectal carcinoma: JFMC 29–0003. J Cancer Res Ther. 2017;13 (1):84–90. [DOI] [PubMed] [Google Scholar]
- Kwakman JJM, Simkens LHJ, van Rooijen JM,et al. Randomized phase III trial of S-1 versus capecitabine in the first-line treatment of metastatic colorectal cancer: SALTO study by the Dutch colorectal cancer group. Ann Oncol. 2017;28 (6):1288–1293. [DOI] [PubMed] [Google Scholar]
- Langer CJ, Socinski MA, Patel JD, et al. Isolating the role of bevacizumab in elderly patients with previously untreated nonsquamous non-small cell lung cancer. Am J Clin Oncol Cancer Clin Trials. 2016;39 (5):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Chung MJ, Park JY, et al. A randomized, multicenter, phase III study of gemcitabine combined with capecitabine versus gemcitabine alone as first-line chemotherapy for advanced pancreatic cancer in south Korea. Medicine (Baltimore). 10.1097/MD.0000000000005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Logan B, Westervelt P, et al. Comparison of patient-reported outcomes in 5-year survivors who received bone marrow vs peripheral blood unrelated donor transplantation long-term follow-up of a randomized clinical trial. JAMA Oncol. 2016;2 (12):1583–1589. 10.1001/jamaoncol.2016.2520.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinert E, Singer S, Janni W, et al. The impact of age on quality of life in breast cancer patients receiving adjuvant chemotherapy: A comparative analysis from the prospective multicenter randomized ADEBAR trial. Clin Breast Cancer. 2017;17 (2):100–106. 10.1016/j.clbc.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Liguigli W, Tomasello G, Toppo L, et al. Safety and efficacy of dose-dense chemotherapy with TCF regimen in elderly patients with locally advanced or metastatic gastric cancer. Tumori. 2017;103 (1):93–100. 10.5301/tj.5000556. [DOI] [PubMed] [Google Scholar]
- Lindemann K, Gibbs E, Åvall-Lundqvist E, et al. Chemotherapy vs tamoxifen in platinum-resistant ovarian cancer: A phase III, randomized, multicentre trial (ovaresist). Br J Cancer. 2017;116 (4):455–463. 10.1038/bjc.2016.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonardi S, Sobrero A, Rosati G, et al. Phase III trial comparing 36 months of adjuvant FOLFOX4/XELOX in stage II-III colon cancer: Safety and compliance in the TOSCA trial. Ann Oncol. 2016;27 (11):2074–2081. [DOI] [PubMed] [Google Scholar]
- Longo-Muñoz F, Argiles G, Tabernero J, et al. Efficacy of trifluridine and tipiracil (TAS-102) versus placebo, with supportive care, in a randomized, controlled trial of patients with metastatic colorectal cancer from Spain: Results of a subgroup analysis of the phase 3 RECOURSE trial. Clin Transl Oncol. 2017;19 (2):227–235 10.1007/s12094-016-1528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loriot Y, Fizazi K, de Bono JS, Forer D, Hirmand M, Scher HI. Enzalutamide in castration-resistant prostate cancer patients with visceral disease in the liver and/or lung: Outcomes from the randomized controlled phase 3 AFFIRM trial. Cancer. 2017;123 (2):253–262. 10.1002/cncr.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Lee JH, Huang S, et al. Continuous treatment with lenalidomide and low-dose dexamethasone in transplant-ineligible patients with newly diagnosed multiple myeloma in Asia: Subanalysis of the FIRST trial. Br J Haematol. 2017;176 (5):743–749 10.1111/bjh.14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth C, Vergote I, Scambia G, et al. ENGOT-ov-6/TRINOVA-2: Randomized, double-blind, phase 3 study of pegylated liposomal doxorubcin plus trebananib or placebo in women with recurrent partially platinum-sensitive or resistant ovarian cancer. Eur J Cancer. 2017;70:111–121. [DOI] [PubMed] [Google Scholar]
- Masuda N, Lee S, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376 (22):2147–2159. 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- Mateos M-, Hernández M-, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): Long-term follow-up of a randomized, controlled, phase 3 trial. Lancet Oncol. 2016;17 (8):1127–1136. [DOI] [PubMed] [Google Scholar]
- Mavroudis D, Matikas A, Malamos N, et al. Dose-dense FEC followed by docetaxel versus docetaxel plus cyclophosphamide as adjuvant chemotherapy in women with HER2-negative, axillary lymph node-positive early breast cancer: A multicenter randomized study by the Hellenic oncplogy research group (HORG). Ann Oncol. 2016;27 (10):1–6. [DOI] [PubMed] [Google Scholar]
- Mesa R, Verstovsek S, Kiladjian J, et al. Changes in quality of life and disease-related symptoms in patients with polycythemia vera receiving ruxolitinib or standard therapy. Eur J Haematol. 2016;97 (2):192–200. 10.1111/ejh.12707. [DOI] [PubMed] [Google Scholar]
- Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375 (22):2154–2164. 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Nishimura J, Kato T, et al. Phase III trial comparing UFT + PSK to UFT + LV in stage IIB, III colorectal cancer (MCSGOCCTG). Surg Today. 2017:1–7. [DOI] [PubMed] [Google Scholar]
- Mok TS, Wu Y, Ahn M, et al. Osimertinib or platinum-pemetrexed in EGFR T790 M-positive lung cancer. N Engl J Med. 2017;376 (7):629–640. 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk BJ, Poveda A, Vergote I, et al. Final results of a phase 3 study of trebananib plus weekly paclitaxel in recurrent ovarian cancer (TRINOVA-1): Long-term survival, impact of ascites, and progression-free survival-2. Gynecol Oncol. 2016;143 (1):27–34. 10.1016/j.ygyno.2016.07.112. [DOI] [PubMed] [Google Scholar]
- Morabito A, Daniele G, Costanzo R, et al. A multicenter, randomized, phase 3 trial comparing fixed dose versus toxicity-adjusted dose of cisplatin + etoposide in extensive small-cell lung cancer (SCLC) patients: The small-cell-lung cancer toxicity adjusted dosing (STAD-1) trial. Lung Cancer. 2017;108:15–21. [DOI] [PubMed] [Google Scholar]
- Nakamae H, Fujisawa S, Ogura M, et al. Dasatinib versus imatinib in japanese patients with newly diagnosed chronic phase chronic myeloid leukemia: A subanalysis of the DASISION 5-year final report. Int J Hematol. 2017;105 (6):792–804. 10.1007/s12185017-2208-2. [DOI] [PubMed] [Google Scholar]
- Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375 (2):134–142. 10.1056/NEJMoa1515725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouhaud F, Rigaud J, Saint F, et al. Final results of the phase III URO-BCG 4 multicenter study: Efficacy and tolerance of one-third dose BCG maintenance in nonmuscle invasive bladder cancer. Anti-Cancer Drugs. 2017;28 (3):335–340. 10.1097/CAD.0000000000000456. [DOI] [PubMed] [Google Scholar]
- Oh I-, Kim K-, Park C-, et al. Belotecan/cisplatin versus etoposide/cisplantin in previously untreated patients with extensive-stage small cell lung carcinoma: A multi-center randomized phase III trial. BMC Cancer. 2016;16 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusaka T, Miyakawa H, Fujii H, et al. Updated results from GEST study: A randomized, three-arm phase III study for advanced pancreatic cancer. J Cancer Res Clin Oncol. 2017;143 (6):1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani O, Klingbiel D, Ruhstaller T, et al. Do all patients with advanced HER2 positive breast cancer need upfront-chemo when receiving trastuzumab? randomized phase III trial SAKK 22/99. Ann Oncol. 2017;28 (2):305–312. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375 (8):754 –766. 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Griesshammer M, Palandri, et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): A randomized, open-label, phase 3b study. Lancet Oncology, The. 2016;18 (1):88–99. 10.1016/S1470-2045(16)30558-7. [DOI] [PubMed] [Google Scholar]
- Pavel ME, Becerra C, Grosch K, Cheung W, Hasskarl J, Yao JC. Effect of everolimus on the pharmacokinetics of octreotide long-acting repeatable in patients with advanced neuroendocrine tumors: An analysis of the randomized phase III RADIANT-2 trial. Clin Pharmacol Ther. 2017;101 (4):462–468. 10.1002/cpt.559. [DOI] [PubMed] [Google Scholar]
- Pavel M, Unger N, Borbath I, et al. Safety and QOL in patients with advanced NET in a phase 3b expanded access study of everolimus. Targeted Oncol. 2016;11 (5):667–675. [DOI] [PubMed] [Google Scholar]
- Platzbecker U, Avvisati G, Cicconi L, et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapyin non-high-riskacute promyelocytic leukemia:Final results of the randomized Italian-German APL0406 trial. J Clin Oncol. 2017;35 (6):605–612. [DOI] [PubMed] [Google Scholar]
- Pugh SA, Bowers M, Ball A, et al. Patterns of progression,treatmentof progressive disease and post-progression survival in the new EPOC study. Br J Cancer. 2016;115 (4):420–424. 10.1038/bjc.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravaud A, Motzer RJ,Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. New Engl J Med. 2016;375 (23):2246–2254. [DOI] [PubMed] [Google Scholar]
- Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740–3748. [DOI] [PubMed] [Google Scholar]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New Engl J Med. 2016;375 (19):1823–1833. [DOI] [PubMed] [Google Scholar]
- Rini BI, Stenzl A, Zdrojowy R, et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): A multicentre, open-label, randomized, controlled, phase 3 trial. Lancet Oncol. 2016;17 (11):1599–1611. [DOI] [PubMed] [Google Scholar]
- Robak T, Warzocha K, Govind Babu K, et al. Ofatumumab plus fludarabine and cyclophosphamide in relapsed chronic lymphocytic leukemia: Results from the COMPLEMENT 2 trial. Leukemia & Lymphoma. 2017;58 (5):1084–1093. 10.1080/10428194.2016.1233536. [DOI] [PubMed] [Google Scholar]
- Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): An international, randomized, double-blind, phase 3 trial. Lancet, The. 2016;388(10063):2997–3005. 10.1016/S0140-6736(16)32389-3. [DOI] [PubMed] [Google Scholar]
- Robinson B, Schlumberger M, Wirth LJ, et al. Characterization of tumor size changes over time from the phase 3 study of lenvatinib in thyroid cancer. J Clin Endocrinol Metab. 2016;101 (11):4103–4109. 10.1210/jc.2015-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlitz C, Bigler M, von Moos R, et al. SAKK 24/09: Safety and tolerability of bevacizumab plus paclitaxel vs. bevacizumab plus metronomic cyclophosphamide and capecitabine as first-line therapy in patients with HER2-negative advanced stage breast cancer - a multicenter, randomized phase III trial. BMC Cancer.2016;16(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosiñol L, Oriol A, Teruel AI, et al. Bortezomib and thalidomide maintenance after stem cell transplantation for multiple myeloma: A PETHEMA/GEM trial. Leukemia. 2017. [DOI] [PubMed] [Google Scholar]
- Rugo HS, Barve A, Waller CF, et al. Effect of a proposed trastuzumab biosimilar compared with trastuzumab on overall response rate in patients with ERBB2 (HER2)-positive metastatic breast cancer: A randomized clinical trial. JAMA. 2017;317(1):37–47. [DOI] [PubMed] [Google Scholar]
- Rummel M, Kim TM, Aversa F, et al. Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20+ diffuse large B-cell lymphoma or follicular lymphoma: Results from a prospective, randomized, open-label, crossover study (PrefMab). Ann Oncol. 2017;28 (4):836–842. 10.1093/annonc/mdw685. [DOI] [PubMed] [Google Scholar]
- Ryan CW, Merimsky O, Agulnik M, et al. PICASSO III: A phase III, placebo-controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol. 2016;34 (32):3898–3905. [DOI] [PubMed] [Google Scholar]
- San-Miguel JF, Hungria VTM, Yoon S-, et al. Overall survival of patients with relapsed multiple myeloma treated with panobinostat or placebo plus bortezomib and dexamethasone (the PANORAMA 1 trial): A randomized, placebo-controlled, phase 3 trial. Lancet Haematol. 2016;3 (11):e50–e515. [DOI] [PubMed] [Google Scholar]
- Sartor O, Hoskin P, Coleman RE, et al. Chemotherapy following radium-223 dichloride treatment in ALSYMPCA. Prostate. 2016;76 (10):905–916. 10.1002/pros.23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg L, Mosier M, Geller RB, Klepper MJ, Schnadig I, Vogelzang NJ. APF530 for nausea and vomiting prevention following cisplatin: Phase 3 MAGIC trial analysis. J Community Supportive Oncol. 2017;15 (2):82–88. [Google Scholar]
- Scott BL, Pasquini MC, Logan BR, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol. 2017;35 (11):1154–1161. 10.1200/JCO.2016.70.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl MJ, Ottensmeier CH, Cullen M, et al. Multicenter, phase III, randomized, double-blind, placebo-controlled trial of pravastatin added to first-linestandardchemotherapy in small-cell lungcancer (LUNGSTAR). J Clin Oncol. 2017;35 (14):1506–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehouli J, Tomè O, Dimitrova D, et al. A phase III, open label, randomized multicenter controlled trial of oral versus intravenous treosulfan in heavily pretreated recurrent ovarian cancer: A study of the northeastern German society of gynecological oncology (NOGGO). J Cancer Res Clin Oncol. 2017;143 (3):541–550. 10.1007/s00432-016-2307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamash J, Sarker S-, Huddart R, et al. A randomized phase III study of 72 h infusional versus bolus bleomycin in BEP (bleomycin, etoposide and cisplatin) chemotherapy to treat IGCCCG good prognosis metastatic germ cell tumors (TE-3). Ann Oncol. 2017;28 (6):1333–1338. [DOI] [PubMed] [Google Scholar]
- Shiroiwa T, Fukuda T, Shimozuma K, et al. Long-term health status as measured by EQ-5D among patients with metastatic breast cancer: Comparison of first-line oral S-1 and taxane therapies in the randomized phase III SELECT BC trial. Qual Life Res. 2017;26 (2):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shitara K, Takashima A, Fujitani K, et al. Nab-paclitaxel versus solvent-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE): An open-label, randomized, non-inferiority, phase 3 trial. The Lancet Gastroenterology & Hepatology. 2017;2 (4):277–287 10.1016/S2468-1253(16)30219-9. [DOI] [PubMed] [Google Scholar]
- Singh PP, Shi Q, Foster NR, et al. Relationship between metformin use and recurrence and survival in patients with resected stage III colon cancer receiving adjuvant chemotherapy: Results from north central cancer treatment group N0147 (alliance). Oncologist. 2016;21 (12):1509–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene T, Crump M, Savage KJ, et al. Salvage chemotherapy and autologous stem cell transplantation for peripheral T-cell lymphoma: A subset analysis of the Canadian cancer trials group LY.12 randomized phase 3 study. Leuk Lymphoma. 2017;58 (10):2319–2327. [DOI] [PubMed] [Google Scholar]
- Smith I, Yardley D, Burris H, et al. Comparative efficacy and safety of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor-positive, node-positive early breast cancer: Final resultsof therandomized phase III femara versus anastrozole clinical evaluation (FACE) trial. J Clin Oncol. 2017;35 (10):1041–1048. [DOI] [PubMed] [Google Scholar]
- Smith M, De Bono J, Sternberg C, et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J Clin Oncol. 2016;34(25):3005–3013. 10.1200/JCO.2015.65.5597. [DOI] [PubMed] [Google Scholar]
- Snoeren N, van Hillegersberg R, Schouten SB, et al. Randomized phase III study to assess efficacy and safety of adjuvant CAPOX with or without bevacizumab in patients after resection of colorectal liver metastases: HEPATICA study. Neoplasia. 2017;19 (2):93–99. 10.1016/j.neo.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son MK, Ryu M-, Park JO, et al. Efficacy and safety of regorafenib in Korean patients with advanced gastrointestinal stromal tumor after failure of imatinib and sunitinib: A multicenter study based on the management access program. Cancer Res Treat. 2017;49 (2):350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria J-, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomized, open-label, phase 3 study. Lancet. 2017;389(10072):917–929. [DOI] [PubMed] [Google Scholar]
- Sorio R, Roemer-Becuwe C, Hilpert F, et al. Safety and efficacy of single-agent bevacizumab-containing therapy in elderly patients with platinum-resistant recurrent ovarian cancer: Subgroup analysis of the randomized phase III AURELIA trial. Gynecol Oncol. 2017;144 (1):65–71. 10.1016/j.ygyno.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Spitz A, Gittelman M, Karsh LI, et al. Intramuscular depot formulations of leuprolide acetate suppress testosterone levels below a 20 ng/ dL threshold: A retrospective analysis of two phase III studies. Res Reports Urology. 2016;8:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AK, Dimopoulos MA, Masszi T, et al. Health-related quality-of-life results from the open-label, randomized, phase III ASPIRE trial evaluating carfilzomib, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone in patients with relapsed multiple myeloma. J Clin Oncol. 2016;34(32):3921–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzing S, Modest DP, Rossius L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE3): A post-hoc analysis of tumor dynamics in the final RAS wild-type subgroup of this randomized open-label phase 3 trial. Lancet Oncol. 2016;17 (10):1426–1434. [DOI] [PubMed] [Google Scholar]
- Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of (177)ludotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376 (2):125–135. 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Okamoto A, Enomoto T, et al. Randomized phase III trial of irinotecan plus cisplatin compared with paclitaxel plus carboplatin as first-line chemotherapy for ovarian clear cell carcinoma: JGOG3017/ GCIG trial. J Clin Oncol. 2016;34(24):2881–2887. [DOI] [PubMed] [Google Scholar]
- Suto T, Ishiguro M, Hamada C, et al. Preplanned safety analysis of the JFMC37–0801 trial: A randomized phase III study of six months versus twelve months of capecitabine as adjuvant chemotherapy for stage III colon cancer. Int J Clin Oncol. 2017;22 (3):494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A, Al-Ali HK, Barosi G, et al. A randomized study of pomalidomide vs placebo in persons with myeloproliferative neoplasm-associated myelofibrosis and RBC-transfusion dependence. Leukemia. 2017;31 (4):896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiseo M, Boni L, Ambrosio F, et al. Italian, multicenter, phase III, randomized study of cisplatin plus etoposide with or without bevacizumab as first-line treatment in extensive-disease small-cell lung cancer: The GOIRC-AIFA FARM6PMFJM trial. J Clin Oncol. 2017;35 (12):1281–1287. [DOI] [PubMed] [Google Scholar]
- Twelves C, Cortes J, O’Shaughnessy J, et al. Health-related quality of life in patients with locally recurrent or metastatic breast cancer treated with etirinotecan pegol versus treatment of physician’s choice: Results from the randomized phase III BEACON trial. Eur J Cancer. 2017;76:205–215. [DOI] [PubMed] [Google Scholar]
- Törlén J, Ringdén O, Garming-Legert K, et al. A prospective randomized trial comparing cyclosporine/methotrexate and tacrolimus/ sirolimus as graft-versus-host disease prophylaxis after allogeneic hematopoietic stem cell transplantation. Haematologica. 2016;101 (11):1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef G, Robak T, Huang H, et al. Association between quality of response and outcomes in patients with newly diagnosed mantle cell lymphoma receiving VR-CAP versus R-CHOP in the phase 3 LYM-3002 study. Haematologica. 2017;102 (5):895–902. 10.3324/haematol.2016.152496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Bartlett CH, Schnell P, et al. Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: Detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist. 2016;21 (10):1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers MM, Lee C, Tu D, et al. Significance of baseline and change in quality of life scores in predicting clinical outcomes in an international phase III trial of advanced pancreatic cancer: NCIC CTG PA.3. Pancreatology. 2016;16 (6):1106–1112. [DOI] [PubMed] [Google Scholar]
- Vogel A, Römmler-Zehrer J, Li JS, McGovern D, Romano A, Stahl M. Efficacy and safety profile of nab-paclitaxel plus gemcitabine in patients with metastatic pancreatic cancer treated to disease progression: A subanalysis from a phase 3 trial (MPACT). BMC Cancer. 2016;16 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volovat C, Bondarenko I, Gladkov O, et al. Efficacy and safety of lipegfilgrastim compared with placebo in patients with non-small cell lung cancer receiving chemotherapy: Post hoc analysis of elderly versus younger patients. Supportive Care Cancer. 2016;24 (12):4913–4920. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang H, Jiang Y, Zhang Y, Wang X. A randomized phase III study of combining erlotinib with bevacizumab and panitumumab versus erlotinib alone as second-line therapy for Chinese patients with non-small-cell lung cancer. Biomed Pharmacother. 2017;89:875–879. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kuranami M, InoueK,et al. Comparison of an AC-taxane versus AC-free regimen and paclitaxel versus docetaxel in patients with lymph node-positive breast cancer: Final results of the national surgical adjuvant study of breast cancer 02 trial, a randomized comparative phase 3 study. Cancer. 2017;123 (5):759–768. 10.1002/cncr.30421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K,Ando M, Ooki A, et al. Quality of life analysis in patients with RAS wild-type metastatic colorectal cancer treated with first-line cetuximab plus chemotherapy. Clin Colorectal Cancer. 2017;16 (2):e2–e37.. [DOI] [PubMed] [Google Scholar]
- Yang JJ, Zhou Q, Yan HH, et al. A phase III randomized controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer. 2017;116 (5):568–574. 10.1038/bjc.2016.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L-,Sun X-, Qin S-, et al. Transdermal granisetronfor theprevention of nausea and vomiting following moderately or highly emetogenic chemotherapy in Chinese patients: A randomized, double-blind, phase III study. Chin Clin Oncol. 2016;5 (6). [DOI] [PubMed] [Google Scholar]
- Yao JC, Moran C, Guthrie KA, et al. Phase III prospective randomized comparison trial of depot octreotide plus interferon alfa-2b versus depot octreotide plus bevacizumab in patients with advanced carcinoid tumors: SWOG S0518. J Clin Oncol. 2017;35 (15):1695–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JC, Pavel M, Lombard-Bohas C, et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: Overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 study. J Clin Oncol. 2016;34(32):3906–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Huang Y, Zhou F, et al. A phase 3, double-blind, randomized placebo-controlled efficacy and safety study of abiraterone acetate in chemotherapy-naive patients with mCRPC in China, Malaysia, Thailand and Russia. Asian J Urol.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino S, Nishikawa K, Morita S,et al. Randomized phase III studyof S-1 alone versus S-1 plus lentinan for unresectable or recurrent gastric cancer (JFMC36–0701). Eur J Cancer. 2016;65:164–171. [DOI] [PubMed] [Google Scholar]
- Yoshino T, Obermannova R, Bodoky G, et al. Baseline carcinoembryonic antigen as a predictive factor of ramucirumab efficacy in RAISE, a second-line metastatic colorectal carcinoma phase III trial. Eur J Cancer. 2017;78:61–69. [DOI] [PubMed] [Google Scholar]
- Zelenetz AD, Barrientos JC, Brown JR, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukemia: Interim results from a phase 3, randomized, double-blind, placebo-controlled trial. Lancet Oncol. 2017;18 (3):297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: A multicentre, randomized, open-label, phase 3 trial. Lancet. 2016;388(10054):1883–1892. [DOI] [PubMed] [Google Scholar]
- Zhang P, Sun T, Zhang Q, et al. Utidelone plus capecitabine versus capecitabine alone for heavily pretreated metastatic breast cancer refractory to anthracyclines and taxanes: A multicentre, open-label, superiority, phase 3, randomized controlled trial. Lancet Oncol. 2017;18 (3):371–383. 10.1016/S1470-2045(17)30088-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kuchimanchi M, Zhu M, Doshi S, Hoang T, Kasichayanula S. Assessment of pharmacokinetic interaction between rilotumumab and epirubicin, cisplatin and capecitabine (ECX) in a phase 3 study in gastric cancer. Br J Clin Pharmacol. 2017;83 (5):1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Yang J-, Chen Z-, et al. Serial cfDNA assessment of response and resistance to EGFR-TKI for patients with EGFR-L858R mutant lung cancer from a prospective clinical trial. J Hematol Oncol. 2016;9 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski C, Láng I, Inbar M, et al. Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer (TURANDOT): Primary endpoint results of a randomized, open-label, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17 (9):1230–1239. [DOI] [PubMed] [Google Scholar]
- Zucca E, Conconi A, Martinelli G, et al. Final results of the IELSG-19 randomized trial of mucosa-associated lymphoid tissue lymphoma: Improved event-free and progression-free survival with rituximab plus chlorambucil versus either chlorambucil or rituximab monotherapy. J Clin Oncol. 2017;35 (17):1905–1912. [DOI] [PubMed] [Google Scholar]
Footnotes
Declaration of Competing Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest.
References
- [1].American Cancer Society. Cancer facts & figures, 2019Atlanta, GA: American Cancer Society; 2019. [Google Scholar]
- [2].Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol 2004;22(22):4626–31. [DOI] [PubMed] [Google Scholar]
- [3].Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in Crisis. Washington, D.C: Institute of Medicine; 2013. [PubMed] [Google Scholar]
- [4].Hurria A, Levit LA, Dale W, et al. Improving the evidence base for treating older adults with Cancer: American Society of Clinical Oncology statement. J Clin Oncol 2015;33(32):3826–33. [DOI] [PubMed] [Google Scholar]
- [5].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2): 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].IBM Corp. IBM SPSS statistics for windows. IBM Corp: Armonk, NY; 2019. [Google Scholar]
- [7].Singh H, Hurria A, Klepin HD. Progress through collaboration: an ASCO and U.S. Food and Drug Administration workshop to improve the evidence base for treating older adults with cancer. American Society of Clinical Oncology educational book American Society of Clinical Oncology Annual Meeting, 38; 2018. p. 392–9. [DOI] [PubMed] [Google Scholar]
- [8].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69(1): 7–34. [DOI] [PubMed] [Google Scholar]
- [9].Witham MD, George J. Clinical trial design for older people–time for a rethink. QJM 2014;107(1):15–6. [DOI] [PubMed] [Google Scholar]
- [10].Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 2005;23(13):3112–24. [DOI] [PubMed] [Google Scholar]
- [11].Ayodele O, Akhtar M, Konenko A, et al. Comparing attitudes of younger and older patients towards cancer clinical trials. J Geriatr Oncol 2016;7(3):162–8. [DOI] [PubMed] [Google Scholar]
- [12].Buechel M, McGinnis A, Vesely SK, Wade KS, Moore KN, Gunderson CC. Consideration of older patients for enrollment in phase 1 clinical trials: exploring treatment related toxicities and outcomes. Gynecol Oncol 2018;149(1):28–32. [DOI] [PubMed] [Google Scholar]
- [13].Khan KH, Yap TA, Ring A, et al. Phase I trial outcomes in older patients with advanced solid tumours. Br J Cancer 2016;114(3):262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Subbiah IM, Tang C, Rao A, et al. Older adults in phase 1 clinical trials: a comparative analysis of participation and clinical benefit rate among older adults versus middle age and AYA patients on phase 1 clinical trials with VEGF/VEGFR inhibitors. Oncotarget 2018;9(48):28842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Levit LA, Singh H, Klepin HD, Hurria A. Expanding the evidence base in geriatric oncology: action items from an FDA-ASCO workshop. J Natl Cancer Inst 2018;110(11): 1163–70. [DOI] [PubMed] [Google Scholar]
- [16].Hurria A, Dale W, Mooney M, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 2014;32 (24):2587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Soto-Perez-De-Celis E, Lichtman SM. Considerations for clinical trial design in older adults with cancer. Expert Opin Investig Drugs 2017;26(10):1099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med 2002;346(14):1061–6. [DOI] [PubMed] [Google Scholar]
- [19].Ruiter R, Burggraaf J, Rissmann R. Under-representation of elderly in clinical trials: an analysis of the initial approval documents in the Food and Drug Administration database. Br J Clin Pharmacol 2019;85(4):838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Treating vulnerable populations of cancer survivors: A biopsychosocial approach. AG Switzerland: Spring international publishing; 2016. [Google Scholar]
- [21].Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010;63(8):e1–37. [DOI] [PubMed] [Google Scholar]
- [22].Wildiers H, Mauer M, Pallis A, et al. End points and trial design in geriatric oncology research: a joint European organisation for research and treatment of cancer– Alliance for clinical trials in oncology–international society of geriatric oncology position article. J Clin Oncol 2013;31(29):3711–8. [DOI] [PubMed] [Google Scholar]