Abstract
The oleoresin essential oils of Liquidambar formosana have potential therapeutic benefits. However, current research on L. formosana oleoresin essential oil is still in its early stages, and its chemotypic characterization is undefined. For better leveraging of plant resources and application of the essential oil, we collected 25 L. formosana oleoresin essential oil samples of individual trees from different geographical areas of Southern China. The essential oils were obtained by hydrodistillation and analyzed by gas chromatography–mass spectrometry (GC–MS) and gas chromatography–flame ionization detection (GC–FID). The major components of the essential oils were (E)-caryophyllene (3.3%-64.4%), α-pinene (0.6%-34.5%), β-pinene (0.6%-26.0%), camphene (0.3%-17.3%), and limonene (0.2%-7.9%). A chiral GC–MS analysis was carried out on the essential oil samples and (–)-α-Pinene, (–)-β-pinene, (–)-camphene, and (–)-limonene were the dominant enantiomers in L. formosana essential oil. The chemical categories of L. formosana oleoresin essential oils were clarified by agglomerative hierarchical cluster analysis (AHC) and principal component analysis (PCA). The multivariate analyses demonstrated that a total of four chemical groups can be delineated for L. formosana. The L. formosana essential oils were screened for antimicrobial activity against a panel of potentially pathogenic bacteria and fungi and showed promising antimicrobial activities with minimum inhibitory concentration (MIC) ≤ 625 μg/mL. These results highlight the economic value of L. formosana oleoresin essential oil, the importance of L. formosana sustainability, and the potential therapeutic benefits of its oleoresin essential oils.
Keywords: sweetgum, chemical composition, (E)-caryophyllene, α-pinene, β-pinene, camphene, enantiomeric distribution, hierarchical cluster analysis, principal component analysis, antibacterial, antifungal
1. Introduction
In recent years, essential oils or resins from aromatic plants have been widely applied in the food, cosmetic, and medicinal industries. Research and the related applications of aromatic plants play a more and more important role in preserving biodiversity, encouraging agroecology, and helping social and environmental development. The genus Liquidambar L. is one category of aromatic plants; it includes five species, in which two species and one variety are found in China. Liquidambar formosana Hance is one of the species in the Hamamelidaceae family [1,2]. Known for its bright orange autumn leaves, L. formosana is a large, flowering, deciduous tree.
The fruit of L. formosana (Chinese name LuLuTong) has been used as a traditional Chinese medicine for thousands of years [3]; the pharmacology and phytochemistry of L. formosana has been reviewed [4]. The fruit extract of L. formosana has shown anti-inflammatory activity [5]. The fruits of L. formosana have yielded lupane and oleanane triterpenoids [6]. The fruit essential oil has shown antifungal activity and was dominated by α-pinene (16.8%), (E)-caryophyllene (10.1%), τ-muurolol (8.3%), τ-cadinol (7.6%), β-pinene (6.7%), and sabinene (5.7%) [7].
The leaves of L. formosana are a source of lignan glycosides and flavonoid glycosides as well as hydrolysable tannins [6,8], which is consistent with the antioxidant activity of L. formosana leaf extracts [9,10]. The leaf essential oil of L. formosana from Taiwan has shown α-pinene (40.9%), β-pinene (24.8%), limonene (18.3%), α-phellandrene (8.9%), terpinen-4-ol (8.1%), and sabinene (5.6%) as the major components [11]. In contrast, another leaf essential oil sample from Taiwan had terpinen-4-ol (32.0%), β-pinene (18.0%), γ-terpinene (13.8%), and α-terpinene (9.7%) as the predominant compounds [12].
The trunk of L. formosana can secrete an oleoresin due to insects, artificial tapping, or other mechanical damage. L. formosana oleoresin is made up of a solid resin component and a volatile component. The essential oil from the oleoresin can be obtained by hydrodistillation. L. formosana resin has numerous medical applications in Asian folk medicine, such as being a promoter of blood circulation, an alleviator of blood stasis, an analgesic, and an anti-inflammatory and wound-healing agent [3,4]. L. formosana oleoresin has been the source of several abietane diterpenoids [13], oleanane and lupane triterpenoids with cytotoxic [14], antifungal [15], glycogen phosphorylase inhibitory [16], and nuclear factor of activated T cell (NFAT) transcription factor inhibitory [17] activities. Chen et al. have reported the toxicity and antibacterial efficacy of L. formosana [18]. The main components of essential oil are the monoterpenes α-pinene (22.4–34.1%), β-pinene (15.7–23.8%), myrcene (up to 10.9%), and limonene (3.4–8.4%); and the sesquiterpene (E)-caryophyllene (7.8–26.5%) [15,19]. These components suggest that L. formosana oleoresin essential oil also has potential therapeutic benefits for human health.
Liquidambar formosana is widely distributed in China, mainly in the Yellow River Basin and further south. However, there is a lack of fundamental research on the development of L. formosana as a source of non-timber forest products (NTFP), which has limited the comprehensive utilization of this species. This has resulted in little production of L. formosana oleoresin and low oleoresin product quality. Currently, many L. formosana trees have been harvested for timber or for pulp [20], or they are cleared to allow for grazing [21], which is detrimental to the environment. Although they are an important part of the primary forest, according to our field observations, some old L. formosana trees have been cut down and their growing areas converted into cultivated land. As a renewable NTFP, the oleoresin collection from L. formosana represents a more eco-friendly practice by preserving old-growth forests while still providing economic incentives for local people. For the sustainable development of L. formosana plant resources, efforts have been made to develop methods that not only protect the ecology but also increase the oleoresin yield [22]. The few comprehensive analyses of the potential chemotypes of L. formosana oleoresin essential oils have not revealed any information about the quality of the oleoresin oils or explained whether the oils had been adulterated. In this work, we present the chemical characterization of L. formosana oleoresin essential oils, multivariate analyses to define potential chemotypes, and the screening of the oleoresin essential oils for activity against bacteria and fungi of dermatological or pulmonary importance.
2. Results and Discussion
2.1. Essential Oil Chemical Composition
Liquidambar formosana oleoresins were collected from 25 individual trees from several locations in Southern China (i.e., Leye, Longsheng, and Wangmo). These areas are geologically the same; they consist of limestone mountains with karst formations. In general, secondary growth, dominated by younger even-aged trees with low biodiversity, were found in the Wangmo area, whereas the Leye area had old growth trees in naturally biodiverse remnant forest patches. The amount of resin collected from each tree is given in Table 1. The oleoresin samples were hydrodistilled to give essential oils in yields ranging from 1.54% to 30.2% (Table 1). Each of the essential oils was analyzed by gas chromatography–mass spectrometry (GC–MS) and gas chromatography–flame ionization detection (GC–FID) (three replicates per essential oil sample) (Table 2). A total of 191 compounds were identified in the essential oils, accounting for 98.1–99.9% of the compositions. The major components in the essential oils were (E)-caryophyllene (3.3–64.4%), α-pinene (0.6–34.5%), β-pinene (0.6–26.0%), camphene (0.3–17.3%), and limonene (0.2–7.9%).
Table 1.
Liquidambar formosana oleoresin collection details.
| Sample Code | Collection Site | dbh, cm | GPS Coordinates | Collection Date | Oleoresin Mass (g) | Essential Oil Yield (%) |
|---|---|---|---|---|---|---|
| RE190401A | Longsheng (tree A) | 170 | 25°41′51.70”N, 110° 9′8.80”E, elev 1204 m | 3/16/2019 | 23.10 | 11.43 |
| RE190401D | Wangmo (tree C) | 105 | 25°22′35.22”N, 106° 2′6.54”E, elev 573 m | 3/17/2019 | 173.60 | 13.61 |
| RE190401E | Wangmo (tree F) | 87 | 25°24′32.88”N, 105°58′23.16”E, elev 780 m | 3/17/2019 | 92.00 | 18.00 |
| LD190910C | 4 Leye | 200 | 24°37′36.23”N, 106°33′25.27”E, elev 921 m | 8/27/2019 | 55.10 | 23.96 |
| LD190910D | 5 Leye | 303 | 24°37′36.23”N, 106°33′25.26”E, elev 924 m | 8/27/2019 | 41.20 | 14.20 |
| LD190910E | 6 Leye | 150 | 24°52′56.10”N, 106°26′20.36”E, elev 1111 m | 8/27/2019 | 49.40 | 19.98 |
| LD190910F | 7 Leye | 190 | 24°52′56.10”N, 106°26′20.36”E, elev 1111 m | 8/27/2019 | 40.54 | 30.17 |
| LD190910G | 8 Leye | 158 | 24°52′58.85”N, 106°25′52.28”E, elev 1213 m | 8/27/2019 | 52.80 | 17.92 |
| LD190910H | 9 Leye | 144 | 24°52′58.85”N, 106°25′52.28”E, elev 1213 m | 8/27/2019 | 40.00 | 17.53 |
| LD190910I | 10 Leye | 187 | 24°52′59.58”N, 106°26′19.95”E, elev 1127 m | 8/27/2019 | 38.80 | 23.30 |
| LD190910J | 11 Leye | 94 | 24°47′20.39”N, 106°34′28.89”E, elev 1118 m | 8/27/2019 | 55.34 | 17.96 |
| LD190910K | 12 Leye | 244 | 24°47′20.47”N, 106°34′30.28”E, elev 1130 m | 8/27/2019 | 41.60 | 25.84 |
| LD190910L | 13 Leye | 116 | 24°47′3.38”N, 106°34′35.56”E, elev 1136 m | 8/27/2019 | 25.93 | 7.71 |
| LD190910M | 14 Leye | 92 | 24°47′2.77”N, 106°34′35.08”E, elev 1142 m | 8/27/2019 | 49.36 | 15.80 |
| LD190910N | 15 Leye | 265 | 24°38′56.00”N, 106°39′0.70”E, elev 883 m | 8/27/2019 | 45.87 | 20.58 |
| LD190910O | 16 Wangmo | 120 | 25°14′59.65”N, 106°28′7.61”E, elev 717 m | 8/31/2019 | 60.50 | 13.59 |
| LD190910P | 17 Wangmo | 106 | 25°14′56.69”N, 106°28′8.62”E, elev 731 m | 8/31/2019 | 38.10 | 20.29 |
| LD190910Q | 18 Wangmo | 110 | 25°14′56.11”N, 106°28′9.10”E, elev 739 m | 8/31/2019 | 52.40 | 14.56 |
| LD190910R | 19 Wangmo | 70 | 25°14′40.42”N, 106°28′17.92”E, elev 761 m | 8/31/2019 | 44.40 | 9.28 |
| LD190910S | 20 Wangmo | 72 | 25°14′40.50”N, 106°28′18.04”E, elev 764 m | 8/31/2019 | 49.60 | 15.73 |
| LD190910T | 21 Wangmo | 85 | 25°14′40.90”N, 106°28′17.84”E, elev 765 m | 8/31/2019 | 43.25 | 21.32 |
| LD190910U | 22 Wangmo | 107 | 25°14′40.89”N, 106°28′17.08”E, elev 757 m | 8/31/2019 | 47.60 | 21.32 |
| LD190910V | 23 Wangmo | 145 | 25°13′48.24”N, 106° 7′44.64”E, elev 991 m | 8/31/2019 | 61.40 | 17.51 |
| LD190910W | 24 Wangmo | 80 | 25°13′47.85”N, 106° 7′44.85”E, elev 993 m | 8/31/2019 | 36.65 | 14.05 |
| LD190910X | 25 Wangmo | 105 | 25°13′50.79”N, 106° 7′44.79”E, elev 992 m | 8/31/2019 | 3.96 | 15.04 |
dbh = Diameter at breast height, by convention measured at 1.3 m from the ground.
Table 2.
Percentage compositions of the major components in Liquidambar formosana oleoresin essential oils.
| Compound | Group #1 | Group #2 | Group #3 | Group #4 | Overall |
|---|---|---|---|---|---|
| Average (Range) | Average (Range) | Average (Range) | Average (Range) | Average (Range) | |
| (E)-Caryophyllene | 14.2 (8.9–18.5) | 26.0 (19.9–34.8) | 5.0 (3.3–6.5) | 49.0 (42.0–64.4) | 23.5 (3.3–64.4) |
| α-Pinene | 23.4 (18.7–27.8) | 19.3 (15.3–25.3) | 31.7 (29.1–34.5) | 6.3 (0.6–11.8) | 20.0 (0.6–34.5) |
| β-Pinene | 16.9 (12.0–20.7) | 14.2 (10.9–18.7) | 23.3 (20.6–26.0) | 5.4 (0.6–9.2) | 14.8 (0.6–26.0) |
| Camphene | 12.3 (9.2–15.3) | 8.6 (6.0–10.9) | 14.5 (11.0–17.3) | 2.7 (0.3–5.2) | 9.6 (0.3–17.3) |
| Limonene | 5.8 (3.9–7.9) | 3.6 (2.5–5.6) | 6.7 (6.4–7.3) | 0.9 (0.2–1.9) | 4.3 (0.2–7.9) |
| Germacrene D | 2.2 (0.0–6.5) | 2.9 (0.0–9.9) | 0.9 (0.2–1.8) | 2.3 (0.2–5.5) | 2.2 (0.0–9.9) |
| Camphor | 3.3 (0.3–7.9) | 1.6 (0.2–2.8) | 1.2 (0.0–3.6) | 1.0 (0.0–2.6) | 1.9 (0.0–7.9) |
| β-Copaene | 0.9 (0.3–1.5) | 2.1 (0.4–5.3) | 0.3 (0.1–0.6) | 3.7 (2.7–4.9) | 1.8 (0.1–5.3) |
| Bornyl acetate | 1.7 (0.0–8.7) | 1.3 (0.2–6.1) | 3.1 (1.0–6.3) | 1.3 (0.2–3.0) | 1.7 (0.0–8.7) |
| α-Muurolol | 1.1 (0.3–2.0) | 1.8 (0.4–2.9) | 0.3 (0.1–0.6) | 3.7 (2.5–5.7) | 1.7 (0.1–5.7) |
| p-Cymene | 3.7 (0.1–13.6) | 0.7 (0.1–1.9) | 1.1 (0.2–3.8) | 0.4 (0.0–0.7) | 1.7 (0.0–13.6) |
| Sabinene | 1.8 (0.4–3.1) | 1.3 (0.4–1.9) | 3.0 (2.4–3.6) | 0.4 (0.0–1.1) | 1.5 (0.0–3.6) |
| β-Cubebene | 0.7 (0.2–1.2) | 1.1 (0.4–1.8) | 0.3 (0.1–0.6) | 2.4 (0.1–3.4) | 1.1 (0.1–3.4) |
| Caryophyllene oxide | 0.8 (0.3–2.5) | 1.0 (0.5–1.5) | 0.3 (0.2–0.5) | 1.6 (0.7–4.4) | 0.9 (0.2–4.4) |
| α-Muurolene | 0.4 (0.1–0.6) | 0.9 (0.2–2.1) | 0.1 (0.0–0.2) | 1.9 (1.4–2.4) | 0.8 (0.0–2.4) |
| Myrcene | 0.7 (0.0–1.6) | 0.4 (0.1–1.3) | 2.2 (0.1–5.1) | 0.4 (0.1–1.3) | 0.8 (0.0–5.1) |
| (2E,4E)-3,7-Dimethylocta-2,4-diene | 1.3 (0.3–3.9) | 0.5 (0.1–1.0) | 0.5 (0.1–0.9) | 0.3 (0.0–0.6) | 0.7 (0.0–3.9) |
| δ-Cadinene | 0.3 (0.1–0.5) | 0.7 (0.3–1.9) | 0.1 (0.1–0.1) | 1.5 (0.7–2.2) | 0.7 (0.1–2.2) |
| γ-Muurolene | 0.3 (0.1–0.5) | 0.7 (0.2–1.7) | 0.1 (0.0–0.2) | 1.5 (1.0–1.9) | 0.6 (0.0–1.9) |
| α-Humulene | 0.3 (0.2–0.4) | 0.7 (0.5–0.9) | 0.1 (0.1–0.1) | 1.4 (1.1–2.0) | 0.6 (0.1–2.0) |
| Cubebol | 0.3 (0.1–0.7) | 0.6 (0.1–1.3) | 0.1 (0.0–0.2) | 1.2 (0.7–2.1) | 0.5 (0.0–2.1) |
| trans-β-Elemene | 0.1 (0.0–0.1) | 1.3 (0.0–5.3) | 0.2 (0.0–0.9) | 0.2 (0.0–0.3) | 0.5 (0.0–5.3) |
| Bornyl cinnamate | 0.5 (0.1–1.2) | 0.5 (0.1–0.8) | 0.5 (0.3–0.8) | 0.4 (0.2–1.0) | 0.5 (0.1–1.2) |
| Borneol | 0.4 (0.1–0.8) | 0.6 (0.3–1.0) | 0.5 (0.2–0.7) | 0.4 (0.0–0.8) | 0.5 (0.0–1.0) |
| α-Cubebene | 0.1 (0.0–0.3) | 0.6 (0.1–2.5) | 0.0 (0.0–0.1) | 0.8 (0.4–1.4) | 0.4 (0.0–2.5) |
The results from the present study (Supplementary Table S1) are in qualitative agreement with previous studies on the oleoresin essential oil. Liu and Chen reported α-pinene (24.9%), β-pinene (23.6%), (E)-caryophyllene (19.6%), and camphene (8.8%) as the major components in the oleoresin essential oil [23]. Similarly, Chien and co-workers carried out a solid-phase microextraction (SPME) and found the oleoresin volatiles to have α-pinene (23.3%), (E)-caryophyllene (22.7%), β-pinene (19.6%), myrcene (10.9%), and limonene (8.4%) [15]. Song and Zeng obtained oleoresin essential oils from three different sites (Jiangxi, Guangxi, and Guizhou provinces) [19]. These workers found the oleoresin essential oils to be composed largely of α-pinene (34.1%, 33.5%, 22.4%), β-pinene (21.4%, 23.8%, 15.7%), camphene (14.1%, 7.7%, 7.3%), limonene (7.9%, 4.1%, 3.4%), and (E)-caryophyllene (7.8%, 8.9%, 26.5%). The present work, however, is much expanded compared to previous examinations of L. formosana oleoresin essential oil. In this work, the diameter at breast height (dbh), global positioning system (gps) coordinates, and elevation for 25 individual trees were measured and the oleoresins obtained from each tree. The essential oils were obtained and a more thorough chemical analysis carried out, revealing a total of 191 compounds identified in the essential oils, in order to assess any correlation between these data and the essential oils’ chemical profiles.
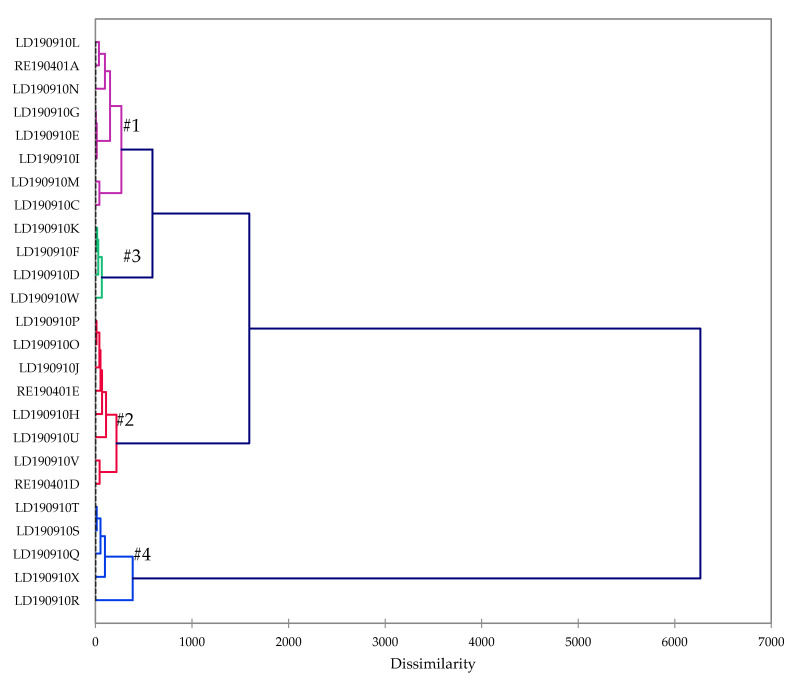
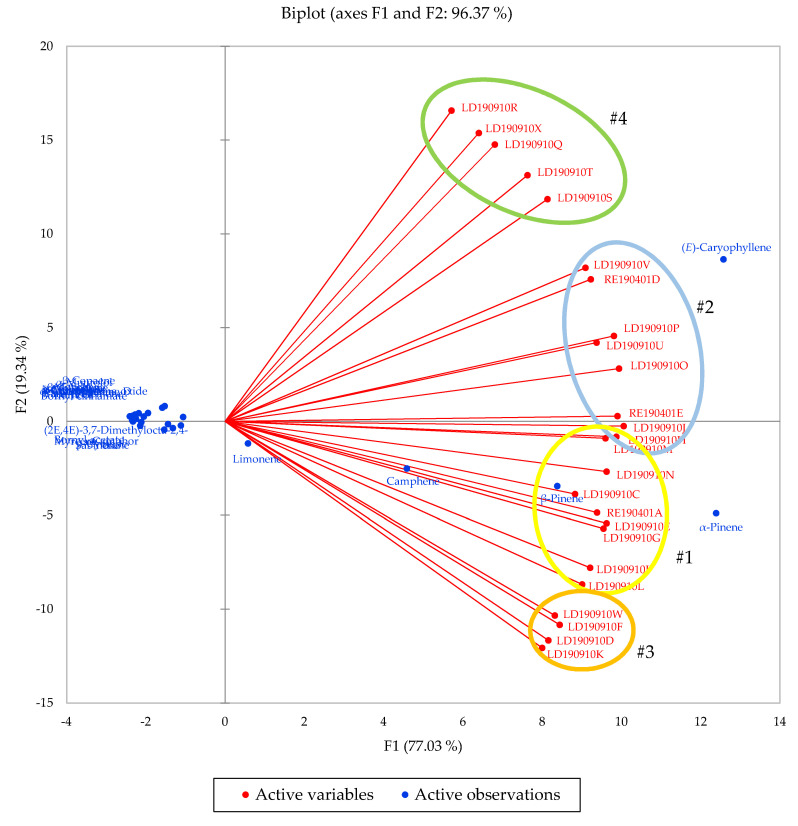
Agglomerative hierarchical cluster (AHC) analysis revealed four clearly defined groups (Figure 1, Table 2). Group #1 was dominated by α-pinene (18.8–27.8%), β-pinene (12.0–20.8%), (E)-caryophyllene (8.9–18.5%), and camphene (9.2–15.3%). Group #2 had the same major components in a different order of concentration: (E)-caryophyllene (19.9–34.8%), α-pinene (15.3–25.4%), β-pinene (10.9–18.7%), and camphene (6.0–10.9%). Group #3 was rich in α-pinene (29.1–34.5%), β-pinene (20.6–26.0%), and camphene (11.0–17.3%), while group #4 was dominated by (E)-caryophyllene (42.0–64.4%). A biplot from the principal component analysis (Figure 2) shows the associations between the clusters and the major components.
Figure 1.
Dendrogram obtained from the agglomerative hierarchical cluster analysis of 25 Liquidambar formosana oleoresin essential oil compositions.
Figure 2.
Principal component biplot of PC1 and PC2 scores and loadings indicating the chemical groupings of Liquidambar formosana based on compositions of the oleoresin essential oils.
There is no obvious correlation between the oleoresin essential oil chemistry from the cluster analysis and the geographical location of the collections, but there are some trends. Most of the trees from the Wangmo collections were dominated by (E)-caryophyllene (groups #2 and #4) and most of the trees from the Leye collection sites were dominated by pinenes (groups #1 and #3). Interestingly, however, adjacent trees (Leye 8 and Leye 9) fell into different clusters (#1 and #2, respectively). Likewise, adjacent trees Leye 6 and Leye 7 were also in different groups (#1 and #3), although both of these groups were dominated by pinenes. Three trees, Wangmo 23, 24, and 25, were collected from the same general area and all showed different chemistries; Wangmo 23 fell into group #2, Wangmo 24 into group #3, and Wangmo 25 into group #4. Furthermore, there does not seem to be a correlation between the time of year (March vs. August) in the observed oleoresin essential oil composition. Thus, for example, samples RE190401D and RE190401E, both collected from Wangmo in March 2019, fell into group #2, along with four Wangmo samples collected in August 2019 (LD190910O, LD190910P, LD190910U, and LD190910V). Similarly, there is little correlation between tree size and oleoresin essential oil composition. Oleoresin essential oils from the largest trees (i.e., LD190910D, LD190910K, and LD190910N) were either in group #1 or group #3 (pinene-rich groups), but one of the smallest trees (LD190910W) also yielded a pinene-rich essential oil (group #3). This suggests that there are no major differences in regard to collection site, size of tree, or collection time of year.
A chiral GC–MS analysis was carried out on the L. formosana oleoresin essential oils (Supplementary Table S2). For both α-pinene and β-pinene, the (–)-enantiomer predominated (65.1–95.6% and 56.7–97.6%, respectively). Limonene was exclusively (–)-limonene while camphor was exclusively (+)-camphor. The (+)-enantiomers also dominated α-thujene and sabinene, while the (–)-enantiomers dominated camphene, borneol, and bornyl acetate. There are no obvious correlations between the enantiomeric distributions of monoterpenoids and the geographical location, size of tree, or time of year of the collection.
2.2. Antibacterial and Antifungal Activity
The L. formosana essential oils were screened for antimicrobial activity against a panel of potential dermal and pulmonary pathogenic bacteria (Bacillus cereus, Cutibacterium acnes, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Pseudomonas aeruginosa, and Serratia marcescens) (Table 3) and fungi (Aspergillus fumigatus, Aspergillus niger, Microsporum canis, Microsporum gypseum, Trichophyton mentagrophytes, Trichophyton rubrum, and Candida albicans) (Table 4). All of the tested essential oil samples demonstrated similar antibacterial and antifungal profiles, which is not surprising considering the similarity of the essential oil compositions. Sartoratto and co-workers have suggested that essential oils showing minimum inhibitory concentration (MIC) values < 500 μg/mL are strong inhibitors, while those with MIC values of 600–1500 μg/mL are moderate inhibitors [24]. Based upon these criteria, the L. formosana oleoresin essential oils showed strong antimicrobial activity against all organisms except for S. marcescens (MIC = 625 μg/mL). In particular, the oil of L. formosana showed excellent antibacterial activity against S. epidermidis (MIC = 78 μg/mL) and strong antifungal activity against A. niger (MIC = 78–313 μg/mL). The mechanisms for antimicrobial activities of essential oils are not completely understood. However, it has been suggested that the lipophilic essential oil components serve to disrupt and penetrate the lipid structure of the cell wall, increasing membrane fluidity and causing the leakage of H3O+ and K+ ions, ultimately leading to cell lysis and death [25,26].
Table 3.
Antibacterial activities, MIC a (μg/mL), of oleoresin essential oils of Liquidambar formosana and major essential oil components.
| Essential Oil Sample | Gram-Positive Bacteria | Gram-Negative Bacteria | |||||
|---|---|---|---|---|---|---|---|
| Bacillus Cereus | Cutibacterium Acnes b | Staphylococcus Aureus | Staphylococcus Epidermidis | Streptococcus Pyogenes | Pseudomonas Aeruginosa | Serratia Marcescens | |
| LD190910C | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910D | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910E | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910F | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910G | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910H | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910I | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910J | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910K | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910L | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910M | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910N | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910O | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910P | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910Q | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910R | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910S | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910T | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910U | 156 | 78 | 156 | 78 | 156 | 313 | 625 |
| LD190910V | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910W | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| LD190910X | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| Re190401A | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| Re190401D | 78 | 156 | 156 | 78 | 156 | 313 | 625 |
| Re190401E | 156 | 156 | 156 | 78 | 156 | 313 | 625 |
| Pure Compounds | |||||||
| (E)-Caryophyllene | 313 | 625 | 313 | 313 | 313 | 313 | 313 |
| (+)-α-Pinene | 313 | 625 | 625 | 313 | 625 | 313 | 313 |
| (–)-α-Pinene | 313 | 625 | 313 | 313 | 313 | 313 | 313 |
| (–)-β-Pinene | 313 | 313 | 156 | 313 | 625 | 313 | 313 |
| Camphene | 313 | 625 | 313 | 313 | 313 | 313 | 313 |
| (+)-Limonene | 313 | 625 | 313 | 313 | 313 | 313 | 625 |
| (–)-Limonene | 313 | 39 | 313 | 78 | 625 | 313 | 313 |
| Gentamicin | <19.5 | <19.5 | <19.5 | <19.5 | <19.5 | <19.5 | <19.5 |
a Minimum inhibitory concentration. b Formerly Propionibacterium acnes.
Table 4.
Antifungal activities, MIC a (μg/mL), of oleoresin essential oils of Liquidambar formosana and major essential oil components.
| Essential Oil Sample | Molds | Yeast | |||||
|---|---|---|---|---|---|---|---|
| Aspergillus Fumigatus | Aspergillus Niger | Microsporum Canis | Microsporum Gypseum | Trichophyton Mentagrophytes | Trichophyton Rubrum | Candida Albicans | |
| LD190910C | 156 | 313 | 313 | 313 | 156 | 313 | 313 |
| LD190910D | 156 | 313 | 156 | 313 | 156 | 313 | 313 |
| LD190910E | 156 | 156 | 156 | 313 | 156 | 313 | 313 |
| LD190910F | 156 | 313 | 313 | 313 | 156 | 313 | 313 |
| LD190910G | 156 | 313 | 313 | 313 | 156 | 313 | 313 |
| LD190910H | 156 | 78 | 313 | 313 | 156 | 313 | 313 |
| LD190910I | 156 | 313 | 313 | 313 | 156 | 313 | 313 |
| LD190910J | 156 | 156 | 313 | 313 | 156 | 313 | 313 |
| LD190910K | 156 | 156 | 313 | 313 | 156 | 313 | 313 |
| LD190910L | 156 | 313 | 313 | 313 | 156 | 313 | 313 |
| LD190910M | 156 | 156 | 313 | 313 | 156 | 313 | 313 |
| LD190910N | 156 | 78 | 156 | 313 | 156 | 313 | 313 |
| LD190910O | 156 | 78 | 156 | 313 | 156 | 313 | 313 |
| LD190910P | 156 | 156 | 156 | 313 | 156 | 313 | 313 |
| LD190910Q | 156 | 313 | 156 | 313 | 156 | 313 | 313 |
| LD190910R | 156 | 313 | 156 | 313 | 156 | 313 | 313 |
| LD190910S | 156 | 313 | 156 | 313 | 156 | 313 | 313 |
| LD190910T | 156 | 313 | 156 | 313 | 156 | 313 | 313 |
| LD190910U | 156 | 156 | 156 | 313 | 156 | 313 | 313 |
| LD190910V | 156 | 156 | 156 | 313 | 156 | 313 | 313 |
| LD190910W | 156 | 313 | 156 | 313 | 156 | 313 | 313 |
| LD190910X | 156 | 313 | 156 | 313 | 156 | 313 | 313 |
| Re190401A | 156 | 313 | 156 | 313 | 156 | 313 | 313 |
| Re190401D | 156 | 313 | 156 | 313 | 156 | 313 | 313 |
| Re190401E | 156 | 313 | 156 | 313 | 156 | 313 | 313 |
| Pure Compounds | |||||||
| (E)-Caryophyllene | 156 | 1250 | 313 | 313 | 625 | 313 | 156 |
| (+)-α-Pinene | 156 | 78 | 313 | 156 | 156 | 313 | 156 |
| (–)-α-Pinene | 313 | 156 | 313 | 313 | 313 | 313 | 156 |
| (–)-β-Pinene | 156 | 78 | 313 | 313 | 156 | 313 | 156 |
| Camphene | 313 | 156 | 313 | 313 | 625 | 313 | 156 |
| (+)-Limonene | 156 | 156 | 313 | 313 | 313 | 313 | 156 |
| (–)-Limonene | 156 | 156 | 313 | 156 | 156 | 313 | 156 |
| Amphotericin B | <19.5 | <19.5 | <19.5 | <19.5 | <19.5 | <19.5 | <19.5 |
a Minimum inhibitory concentration.
In general, the oleoresin essential oils showed better antibacterial activity against Gram-positive organisms over Gram-negative organisms. Except for S. epidermidis (MIC = 78 μg/mL), the antibacterial activity of L. formosana oleoresin essential oils against Gram-positive organisms was 156 μg/mL, whereas the MIC values against Gram-negative P. aeruginosa were 313 μg/mL and 625 μg/mL against S. marcescens. It has often been observed that Gram-negative bacteria are less susceptible to the inhibitory effects of essential oils than Gram-positive bacteria [27,28,29]. This phenomenon has been attributed to the presence of cell wall lipopolysaccharides in the Gram-negative organisms, which can inhibit the lipophilic essential oil components from diffusing into the cells [30].
The antibacterial activity observed for the L. formosana oleoresin essential oils against S. epidermidis cannot be attributed solely to the activities of the individual major components (Table 3). Thus, for example, (E)-caryophyllene, α-pinene, β-pinene, camphene, and (+)-limonene have MIC values of 313 μg/mL against S. epidermidis, and only (–)-limonene has an MIC value of 78 μg/mL. Similarly, the MIC value for L. formosana oleoresin essential oils against S. pyogenes was 156 μg/mL, but the MIC values for the major components ranged from 313 μg/mL to 625 μg/mL. It is likely that the synergistic effects of the major components, possibly involving minor components, are responsible for the antimicrobial activity [31,32]. Crevelin and co-workers observed synergistic antimicrobial effects in a combination of α-pinene, β-pinene, (E)-caryophyllene, and caryophyllene oxide [33]. Both α-pinene and β-pinene showed good antifungal activity against A. niger, and these components may be responsible for the activity of L. formosana essential oil against this fungal organism. (E)-Caryophyllene, on the other hand, was inactive against A. niger.
3. Materials and Methods
3.1. Oleoresin Collection
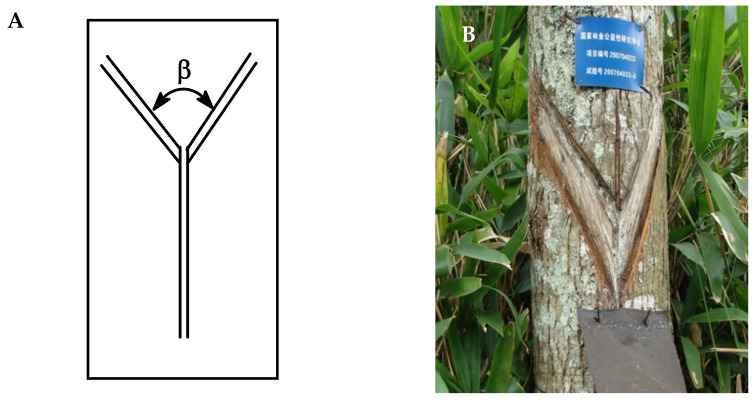
Liquidambar formosana oleoresins were collected from 25 individual trees from several locations in Southern China (Table 1). The oleoresin tapping practice that was utilized in this work not only increases resin tapping efficiency but also ensures the sustainability of the trees and the ecosystem. It was invented by Zeng and co-workers and named the “downward tapping method of V-shaped” [22]. More specifically, mature L. formosana trees with DBH (diameter at breast height) ≥ 60 cm, without pest infection, qualified for oleoresin tapping. The oleoresin tapping was carried out by local farmers at average temperatures above 15 °C. The tapping surface was on the sun-facing side of the trunk. An area of the trunk was shaved of bark, a medial groove was cut in the center of the shaved area, and a V-shaped ditch was cut from top to bottom along the vertical direction of the trunk. The V-shaped angle (β) was between 60° and 80° (Figure 3) and was cut to the first or second annual ring of the tree. A minimum 10-cm space was preserved between each V-shaped ditch to ensure the health of the trees. The oleoresin aggregations were located at the bottom of the V-shaped ditch, which guided the oleoresin into a container. Immediately after the resin was collected, it was transferred to amber-colored glass bottles and stored at 4 °C until distillation.
Figure 3.
Illustration of the V-shaped downward tapping method. A: Schematic diagram, B: Photograph of appropriately tapped L. formosana.
3.2. Oleoresin Hydrodistillation
The hydrodistillation of the samples of L. formosana oleoresin was performed with an all-glass Clevenger apparatus for 7 h. The water and resin were mixed in a ratio of 6:1 and the hydrodistillation was carried out with constant stirring of the mixture. The rate of hydrodistillation was around 2 mL/min. The isolated oil had a strong resinous aroma with floral, pine, and spicy notes. Oleoresin masses and essential oil yields are summarized in Table 1. The L. formosana oleoresin essential oils were stored in sealed amber vials at 4 °C until chromatographic analysis and bioactivity screening.
3.3. Gas Chromatographic–Mass Spectral Analysis
The oleoresin essential oils from L. formosana were subjected to gas chromatographic–mass spectral (GC–MS) analysis, as previously reported [34]: Shimadzu GCMS-QP2010 Ultra, electron impact (EI) mode with electron energy = 70 eV, scan range = 40–400 atomic mass units, scan rate = 3.0 scans/s, and Shimadzu GC-MS solution software v. 4.45 (Shimadzu Scientific Instruments, Columbia, MD, USA); ZB-5ms fused silica capillary GC column Phenomenex, Torrance, CA, USA; (5% phenyl)-polymethylsiloxane stationary phase, 0.25 μm film thickness; helium carrier gas, column head pressure = 552 kPa, flow rate = 1.37 mL/min; injector temperature = 260 °C, ion source temperature = 260 °C; GC oven temperature program: initial temperature = 50 °C, temperature increased 2 °C/min to 260 °C. For each sample, a 5% w/v solution in CH2Cl2 was prepared, and 0.1 μL was injected using a split ratio of 30:1. Identification of the individual components of the essential oils was determined by comparison of the Kovats retention indices, determined using a series of n-alkanes, in addition to comparison of the mass spectral fragmentation patterns with those found in the MS databases [35,36,37,38], using the LabSolutions GCMS solution software version 4.45 (Shimadzu Scientific Instruments, Columbia, MD, USA) and with matching factors >90%.
3.4. Gas Chromatographic–Flame Ionization Detection
Quantitative analysis of the L. formosana essential oils was carried out by GC–FID, as previously reported [34]: Shimadzu GC 2010 equipped with FID, a split/splitless injector, and Shimadzu autosampler AOC-20i (Shimadzu Scientific Instruments, Columbia, MD, USA), with a ZB-5 capillary column (Phenomenex, Torrance, CA, USA). The GC–FID measurements were carried out using the same oven temperature program as that for GC–MS. Injector temperature = 250 °C, detector temperature = 280 °C, and nitrogen was the carrier gas, with a flow rate of 1.0 mL/min. The concentrations of the oleoresin essential oil components were calculated from raw peak areas, normalized to 100%, without standardization.
3.5. Chiral Gas Chromatography–Mass Spectrometry
Chiral GC–MS of the L. formosana oleoresin essential oils was carried out, as reported previously [34]: Shimadzu GCMS-QP2010S (Shimadzu Scientific Instruments, Columbia, MD, USA), electron impact (EI) mode, electron energy = 70 eV; scan range = 40–400 amu, scan rate = 3.0 scans/s; Restek B-Dex 325 chiral capillary GC column (Restek Corp., Bellefonte, PA, USA) (30 m × 0.25 mm ID × 0.25 μm film thickness). Oven temperature program: starting temperature = 50 °C, temperature increased 1.5 °C/min to 120 °C, then 2 °C/min to 200 °C, and kept at 200 °C for an additional 5 min; carrier gas was helium, flow rate = 1.8 mL/min. For each essential oil sample, a 3% w/v solution in CH2Cl2 was prepared, and 0.1 μL was injected using a split ratio of 1:45. The enantiomers of the monoterpenoids were identified by comparison of retention times with authentic samples obtained from Sigma-Aldrich (Milwaukee, WI, USA). The enantiomer percentages were determined from peak areas.
3.6. Antimicrobial Screening
The essential oils were screened for antibacterial activity against Gram-positive bacteria (Bacillus cereus (ATCC No. 14579), Cutibacterium acnes (ATCC No. 11827), Staphylococcus aureus (ATCC No. 29213), Staphylococcus epidermidis (ATCC No. 12228), and Streptococcus pyogenes (ATCC No. 19615)) and Gram-negative bacteria (Pseudomonas aeruginosa (ATCC No. 27853) and Serratia marcescens (ATCC No. 14756)), and for antifungal activity against molds (Aspergillus niger (ATCC No. 16888), Aspergillus fumigatus (ATCC No. 96918), Microsporum canis (ATCC No. 11621), Microsporum gypseum (ATCC No. 24102), and Trichophyton mentagrophytes (ATCC No. 18748)), and one yeast (Candida albicans (ATCC No. 18804)) using the microbroth dilution technique [39,40], as previously reported [41,42]. Individual essential oil components, ((E)-caryophyllene, (+)-α-pinene, (–)-α-pinene, (–)-β-pinene, camphene, (+)-limonene, and (–)-limonene, were obtained from Sigma-Aldrich (St. Louis, MO) and were used as received, without additional purification.
All bacteria were cultured on tryptic soy agar, except for C. acnes, which was grown on tryptic soy agar supplemented with 7% (v/v) defibrinated whole sheep blood (Cleveland Scientific, Bath, Ohio, USA), under micro-aerophilic conditions [43]. All fungi were cultured on yeast malt agar (Sigma-Aldrich, St. Louis, MO). For the bacteria and fungi, 50 μL of 1% (w/v) solution of the samples in dimethyl sulfoxide (DMSO) was diluted in 50 μL of cation-adjusted Mueller Hinton broth (CAMHB) (Sigma-Aldrich, St. Louis, MO). The sample solutions were then serially diluted (1:1) in fresh CAMHB to obtain concentrations of 2500, 1250, 625, 313, 156, 78, 39, and 20 μg/mL. The microbes were harvested from a fresh culture and added to each well at a concentration of approximately 1.5 × 108 CFU/mL for bacteria and 7.5 × 107 CFU/mL for fungi, and the 96-well microdilution plates for bacteria were incubated at 37 °C and the fungi were incubated at 35 °C for 24 h. The minimum inhibitory concentration (MIC) was determined as the lowest concentration with no turbidity. Gentamicin (Sigma-Aldrich, St. Louis, MO) was used as a positive antibiotic control and DMSO was used as the negative control (50 μL DMSO diluted in 50 μL broth medium and then serially diluted, as above). For fungi, the above-mentioned method was implemented using a yeast-nitrogen base growth medium (Sigma-Aldrich, St. Louis, MO, USA) and amphotericin B (Sigma-Aldrich, St. Louis, MO, USA) as a positive antifungal control.
3.7. Statistical Analysis
Multivariate analyses were carried out on the essential oil compositions of the L. formosana oleoresins. The chemical compositions of the 25 essential oils were treated as operational taxonomic units (OTUs), and the percentage compositions of 25 major components were used to ascertain the associations between the oleoresin essential oil compositions using agglomerative hierarchical cluster (AHC) analysis, using XLSTAT Premium, version 2018.5.53172 (Addinsoft, Paris, France). The dissimilarity of the samples was evaluated using Euclidean distance, and the clusters were defined using Ward’s method [44]. The principal component analysis (PCA) was carried out using the 25 major chemical components as variables, with a Pearson correlation matrix, using XLSTAT Premium, version 2018.1.1.60987 (Addinsoft, Paris, France). In all cases, 625 data (25 samples × 25 variables) were utilized for the principal component analysis.
4. Conclusions
L. formosana trees and their essential oils are important non-timber forest product (NTFP) resources. Due to the lack of research on L. formosana, the resources are under-utilized or even being destroyed. The oleoresin essential oils collected from 25 different L. formosana trees from different regions of Southern China showed very little variation in either chemical composition or enantiomeric distribution. The essential oil yields ranged from 7.7% to 30.2%. The oleoresin essential oils showed promising antibacterial efficacy against Gram-positive bacteria and antifungal activity. The biological potency, coupled with improved tree-tapping methods, promoted L. formosana oleoresin essential oil in terms of its economic potential as well as its therapeutic benefits. This is an innovative research work that extends our understanding of the phytochemistry of this tree as well as providing scientific and practical support for the development and utilization of L. formosana tree resources. As a new NTFP product, the oleoresin essential oils also improve awareness of ecosystem protection.
Acknowledgments
We are grateful to Lei He and Zhujie Huang and to the local farmers for all of their assistance in L. formosana oleoresin collection. This work was carried out as part of the activities of the Aromatic Plant Research Center (APRC, https://aromaticplant.org/).
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/7/822/s1, Table S1: Chemical compositions of Liquidambar formosana resin essential oils from southern China, Table S2: Enantiomeric distribution, (+)-enantiomer%: (–)-enantiomer%, of monoterpenoids in Liquidambar formosana resin essential oils.
Author Contributions
Conceptualization, A.D. and T.Z.; methodology, T.Z. and W.N.S.; validation, A.D. and W.N.S.; formal analysis, N.S.D., P.S., and W.N.S.; investigation, A.D., T.Z., N.S.D., P.S., and W.N.S.; resources, A.D.; data curation, W.N.S.; writing—original draft preparation, A.D., T.Z., N.S.D., and W.N.S.; writing—review and editing, A.D., T.Z., N.S.D., and W.N.S.; supervision, A.D. and T.Z.; project administration, A.D. and T.Z.; funding acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by dōTERRA International (https://www.doterra.com/US/en) and the APC was funded by dōTERRA International.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Hatano T., Kira R., Yoshizaki M., Okuda T. Seasonal changes in the tannins of Liquidambar formosana reflecting their biogenesis. Phytochemistry. 1986;25:2787–2789. doi: 10.1016/S0031-9422(00)83742-5. [DOI] [Google Scholar]
- 2.Yoshizaki M., Shingu T., Okuda T., Hatano T., Kaneda T. Liquidambin, an ellagitannin from Liquidambar formosana. Phytochemistry. 1987;26:2053–2055. doi: 10.1016/S0031-9422(00)81757-4. [DOI] [Google Scholar]
- 3.Feng Y., Liu S., Liu G., Yao J. Facile and fast removal of oil through porous carbon spheres derived from the fruit of Liquidambar formosana. Chemosphere. 2017;170:68–74. doi: 10.1016/j.chemosphere.2016.11.166. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang X., Yi S., Lu H., Wu S., Zhao H. Liquidambar formosana Hance: A mini-review of chemical constituents and pharmacology. Eur. J. Med. Plants. 2016;17:1–11. doi: 10.9734/EJMP/2016/29440. [DOI] [Google Scholar]
- 5.Ma H., Wang F., Jiang J., Cheng L., Zhang H., Zhang G. In vivo anti-inflammatory activity of Liquidambar formosana Hance infructescence extract. Trop. J. Pharm. Res. 2017;16:2403–2410. [Google Scholar]
- 6.Dictionary of Natural Products on DVD. CRC Press; Boca Raton, FL, USA: 2019. version 28.2. [Google Scholar]
- 7.Su Y.C., Ho C.L. Composition, in vitro cytotoxicity, anti-mildew and anti-wood-decay fungal activities of the fruit essential oil of Liquidambar formosana from Taiwan. Nat. Prod. Commun. 2017;12:287–290. doi: 10.1177/1934578X1701200237. [DOI] [PubMed] [Google Scholar]
- 8.Hatano T., Kira R., Yasuhara T., Okuda T. Tannins of hamamelidaceous plants. III. Isorugosins A, B and D, new ellagitannins from Liquidambar formosana. Chem. Pharm. Bull. 1988;36:3920–3927. doi: 10.1248/cpb.36.3920. [DOI] [Google Scholar]
- 9.Wang K., Pan Y., Wang H., Zhang Y., Lei Q., Zhu Z., Li H., Liang M. Antioxidant activities of Liquidambar formosana Hance leaf extracts. Med. Chem. Res. 2010;19:166–176. doi: 10.1007/s00044-009-9181-0. [DOI] [Google Scholar]
- 10.Zhang L., Zhu M.F., Tu Z.C., Zhao Y., Wang H., Li G.J., Wang H., Sha X.M. α-Glucosidase inhibition, anti-glycation and antioxidant activities of Liquidambar formosana Hance leaf, and identification of phytochemical profile. South Afr. J. Bot. 2017;113:239–247. doi: 10.1016/j.sajb.2017.08.010. [DOI] [Google Scholar]
- 11.Chen C.-J., Chu F.-H., Chien S.-C., Tsao N.-W., Wang S.-Y. Comparative analysis of phytoncides released frm Liqudambar formosana Hance trees and seedlings. J. Agric. For. 2013;62:137–144. [Google Scholar]
- 12.Hua K.-F., Yang T.-J., Chiu H.-W., Ho C.-L. Essential oil from leaves of Liquidambar formosana ameliorates inflammatory response in lipopolysaccharide-activated mouse macrophages. Nat. Prod. Commun. 2014;9:869–872. doi: 10.1177/1934578X1400900638. [DOI] [PubMed] [Google Scholar]
- 13.Shang H.-J., Li D.-Y., Wang W.-J., Li Z.-L., Hua H.-M. Three new diterpenoids from the resin of Liquidambar formosana. Nat. Prod. Res. 2014;28:1–6. doi: 10.1080/14786419.2013.825915. [DOI] [PubMed] [Google Scholar]
- 14.Yang N.-Y., Chen J.-H., Zhou G.-S., Tang Y.-P., Duan J.-A., Tian L.-J., Liu X.-H. Pentacyclic triterpenes from the resin of Liquidambar formosana. Fitoterapia. 2011;82:927–931. doi: 10.1016/j.fitote.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Chien S.-C., Xiao J.-H., Tseng Y.-H., Kuo Y.-H., Wang S.-Y. Composition and antifungal activity of balsam from Liquidambar formosana Hance. Holzforschung. 2013;67:345–351. doi: 10.1515/hf-2012-0086. [DOI] [Google Scholar]
- 16.Liu J., Zhang H., Zhu P., Wu X., Yao H., Ye W., Jiang J., Xu J. Synthesis and biological evaluation of ambradiolic acid as an inhibitor of glycogen phosphorylase. Fitoterapia. 2015;100:50–55. doi: 10.1016/j.fitote.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Dat N.T., Lee I.S., Cai X.F., Shen G., Kim Y.H. Oleanane triterpenoids with inhibitory activity against NFAT transcription factor from Liquidambar formosana. Biol. Pharm. Bull. 2004;27:426–428. doi: 10.1248/bpb.27.426. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z., Chen W., Zeng T., Shen X. Toxicity and antibacterial activity of Chinese sweetgum resin. Nat. Prod. Res. Dev. 2015;27:1651–1656. [Google Scholar]
- 19.Song X., Zeng T. Analysis on chemical components of essential oil of Chinese sweetgum resin. Chem. Ind. For. Prod. 2010;30:40–44. [Google Scholar]
- 20.Yi L.P., Wen S.Z., Wang Z.Z., Yang L.L. Biomass and productivity of Liquidambar formosana Hance. J. Cent. South Univ. For. Technol. 2018;2:50–53. [Google Scholar]
- 21.Hunter I., von Hahn C.-G., Zhu Z., Zhou Y. Stabilising forest margins by growing non-timber forest products: A novel example from Hainan Island, China. Land Use Policy. 2003;20:225–230. doi: 10.1016/S0264-8377(03)00029-2. [DOI] [Google Scholar]
- 22.Zeng T., Song X., Zeng C., Luo J., Yang X. Method for Collecting Resin of Sweetgum 2012. CN 102090300A. China patent. 2012 Jan 4;
- 23.Liu H., Chen M.Y. Studies on the chemical constituents of Liquidambar formosana resin. Chem. Ind. For. Prod. 1995;15:61–66. [Google Scholar]
- 24.Sartoratto A., Machado A.L.M., Delarmelina C., Figueira G.M., Duarte M.C.T., Rehder V.L.G. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz. J. Microbiol. 2004;35:275–280. doi: 10.1590/S1517-83822004000300001. [DOI] [Google Scholar]
- 25.Fisher K., Phillips C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008;19:156–164. doi: 10.1016/j.tifs.2007.11.006. [DOI] [Google Scholar]
- 26.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 27.Smith-Palmer A., Stewart J., Fyfe L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 1998;26:118–122. doi: 10.1046/j.1472-765X.1998.00303.x. [DOI] [PubMed] [Google Scholar]
- 28.Izadi Z., Esna-Ashari M., Piri K., Davoodi P. Chemical composition and antimicrobial activity of feverfew (Tanacetum parthenium) essential oil. Int. J. Agric. Biol. 2010;12:759–763. [Google Scholar]
- 29.Djihane B., Wafa N., Elkhamssa S., Pedro D.H.J., Maria A.E., Mohamed Mihoub Z. Chemical constituents of Helichrysum italicum (Roth) G. Don essential oil and their antimicrobial activity against Gram-positive and Gram-negative bacteria, filamentous fungi and Candida albicans. Saudi Pharm. J. 2017;25:780–787. doi: 10.1016/j.jsps.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inouye S., Takizawa T., Yamaguchi H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J. Antimicrob. Chemother. 2001;47:565–573. doi: 10.1093/jac/47.5.565. [DOI] [PubMed] [Google Scholar]
- 31.Herman A., Tambor K., Herman A. Linalool affects the antimicrobial efficacy of essential oils. Curr. Microbiol. 2016;72:165–172. doi: 10.1007/s00284-015-0933-4. [DOI] [PubMed] [Google Scholar]
- 32.Requena R., Vargas M., Chiralt A. Study of the potential synergistic antibacterial activity of essential oil components using the thiazolyl blue tetrazolium bromide (MTT) assay. LWT Food Sci. Technol. 2019;101:183–190. doi: 10.1016/j.lwt.2018.10.093. [DOI] [Google Scholar]
- 33.Crevelin E.J., Caixeta S.C., Dias H.J., Groppo M., Cunha W.R., Martins C.H.G., Crotti A.E.M. Antimicrobial activity of the essential oil of Plectranthus neochilus against cariogenic bacteria. Evid. Based Complement. Altern. Med. 2015;2015:102317. doi: 10.1155/2015/102317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeCarlo A., Johnson S., Okeke-Agulu K.I., Dosoky N.S., Wax S.J., Owolabi M.S., Setzer W.N. Compositional analysis of the essential oil of Boswellia dalzielii frankincense from West Africa reveals two major chemotypes. Phytochemistry. 2019;164:24–32. doi: 10.1016/j.phytochem.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing; Carol Stream, IL, USA: 2007. [Google Scholar]
- 36.Mondello L. FFNSC 3. Shimadzu Scientific Instruments; Columbia, MD, USA: 2016. [Google Scholar]
- 37.National Institute of Standards and Technology . NIST17. National Institute of Standards and Technology; Gaithersburg, MD, USA: 2017. [Google Scholar]
- 38.Satyal P. Ph.D. Dissertation. University of Alabama in Huntsville; Huntsville, AL, USA: 2015. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. [Google Scholar]
- 39.Sahm D.H., Washington J.A. Antibacterial susceptibility tests: Dilution methods. In: Balows A., Hausler W.J., Herrmann K.L., Isenberg H.D., Shamody H.J., editors. Manual of Clinical Microbiology. American Society for Microbiology; Washington, DC, USA: 1991. [Google Scholar]
- 40.EUCAST Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003;9:ix–xv. doi: 10.1046/j.1469-0691.2003.00790.x. [DOI] [PubMed] [Google Scholar]
- 41.Setzer M.C., Setzer W.N., Jackes B.R., Gentry G.A., Moriarity D.M. The medicinal value of tropical rainforest plants from Paluma, North Queensland, Australia. Pharm. Biol. 2001;39:67–78. doi: 10.1076/phbi.39.1.67.5944. [DOI] [Google Scholar]
- 42.Satyal P., Paudel P., Poudel A., Dosoky N.S., Pokharel K.K., Setzer W.N. Bioactivities and compositional analyses of Cinnamomum essential oils from Nepal: C. camphora, C. tamala, and C. glaucescens. Nat. Prod. Commun. 2013;8:1777–1784. doi: 10.1177/1934578X1300801232. [DOI] [PubMed] [Google Scholar]
- 43.Blanchard T.G., Nedrud J.G. Laboratory maintenance of Helicobacter species. Curr. Protoc. Microbiol. 2006;1:8B-1. doi: 10.1002/9780471729259.mc08b01s24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963;58:236–244. doi: 10.1080/01621459.1963.10500845. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.