Abstract
We report on the MoO3 oxides and their derivatives on microscopic 2H MoS2 flakes oxidized in air and high relative humidity at a moderate temperature range below 410 °C. We combine XPS and AFM measurements such as topography, friction, creation of nanoscale ripples and scratches on the MoS2 flakes deposited on Si substrates. We detect MoO3 oxides mostly by measuring selected nanomechanical properties of the MoO3 layer, such as its compressive mechanical stress at the plastic yield. We discuss basal surface coverage of the single MoS2 flakes by the MoO3 oxides. We discuss conditions for appearance of all possible MoO3 oxide derivatives, such as molybdenum(VI) hydroxyoxides and MoO3 hydrates. Our findings agree with an expected mechanistic switch in thermal oxidation in water vapors vs. air.
Keywords: MoS2, MoO3, surface science, nanoscale ripples, atomic force microscopy, XPS
1. Introduction
Naturally occurring MoS2 crystals, molybdenites, have been widely used as solid lubricants [1]. The most common 2H molybdenite has three other polytypes: 3R, 1T and 1T’, which differ in structure and electronic properties [2,3]. 2H and 3R molybdenites can be easily peeled off mechanically to yield atomically flat MoS2 crystals with thickness down to one monolayer [2]. Easiness of mechanical exfoliation and semiconducting properties of 2H MoS2 crystals have opened up a possibility to use them in a new generation of thin transistors [4]. Today, thin 2H MoS2 crystals contribute tremendously to vigorous growth of flexible nanoelectronics, particularly in sensing, optoelectronics and energy harvesting [2,5,6,7]. In order to exploit all of these applications, the surface reactivity of single 2H MoS2 microscopic flakes needs to be understood. The simplest and most widely applicable surface reactions pertain to their oxidation in ambient conditions and in the presence of water.
It has been confirmed both experimentally and theoretically that bulk MoS2 oxidation is not readily observed at ambient conditions due to high energy barriers for such reactions [8,9,10,11,12,13,14,15]. Nevertheless, all kinds of thin and thick microscopic MoS2 crystals are etched by oxygen within the time scales of minutes when heated to temperatures of at least 320 °C. Such etching progresses according to a following stoichiometry [8,9,10,11,12]:
| 2MoS2 + 7O2 → 2MoO3 + 4SO2(g) | (1) |
Equation (1) states that out of all the possible Mo oxides, only the MoO3 is produced. Exclusive presence of MoO3 on the oxidized MoS2 samples has been observed in many oxidative processes carried out in oxygen or in air. Transparent MoO3 crystals change into yellow and grayish-blue, only when oxygen defects yielding Mo5+ and Mo4+ species, respectively, are purposefully introduced, e.g., via hydrogen adsorption [16]. XPS and XRD experiments on thin Mo films have shown that all defective MoO3 species with Mo4+ and Mo5+ became fully oxidized to MoO3 already at more than 5% of oxygen in reactive gases [17]. The majority of the published studies, mostly using the XPS measurements, detect exclusively Mo6+ oxides—i.e., MoO3, onto thermally oxidized MoS2 samples [12,18,19]. Furthermore, by performing thermodynamic calculations and using enthalpies of bulk reactions, Walter et al. have calculated that for a closed system consisting of the MoS2 crystals in air, Equation (1) is indeed an exclusive reaction pathway, but only till system temperature of 470 °C [12]. Above 470 °C, volatile (MoO3)3 and (MoO3)4 species started to appear in their simulations due to sublimation of the MoO3(s). At 525 °C none of the MoO3 was predicted to stay on the oxidized MoS2 samples.
Somewhat different MoS2 oxidation outcomes, however, are expected in the presence of water or humid air. Ross and Sussman experimented with pulverized bulk MoS2 crystals and showed that water vapors helped in producing substantially more MoO3 than expected from Equation (1). They verified that an additional MoO3 was produced in the course of a following reaction [20]:
| 2MoS2 + 9O2 + 4H2O → 2MoO3 + 4H2SO4 | (2) |
Furthermore, volatile molybdenum(VI) hydroxyoxides, MoO2(OH)2(g), have been predicted to appear above 300 °C in the calculations of Walter et al. (There, partial pressure of water vapors was set to the saturared water vapor pressure at room temperature, or to 100% relative humidity at 25 °C (298K)) [12]. It is not clear whether MoO2(OH)2 originated directly from water-mediated oxidation of the MoS2 crystals or rather from water reacting with the MoO3 adsorbed on the MoS2 crystals, which seemed more likely. Noteworthily, bulk MoO3 has been shown by inorganic chemistry books to dissolve slightly in water. Finally, yet other Mo species, the MoO3 hydrates such as MoO3·H2O, have been recently suggested to appear on the MoS2 crystals after their immersion in water for many hours [21].
Not only is the chemistry of the MoO3 complicated, but its appearance and surface distribution on microscopic 2H MoS2 crystals are elusive too. In the case of very thin MoS2 flakes some loose islands of the MoO3 oxides have been presumably observed directly in the AFM topographs overlaid with AFM-based magnetic force imaging [9]. Therein, MoO3 was reported to be a non-magnetic material. In one of our earlier AFM and micro-Raman studies we pointed out that during initial 10–15 min of heating at temperatures ranging from 320 to 390 °C, in air, a predominantly observed outcome on thick microscopic MoS2 flakes was triangular etch pits within the basal MoS2 surface [11]. Similar triangular etch pits were observed several years earlier on very thin MoS2 samples heated either in oxygen or in oxygen/argon flow or in air [8,9,10]. In all of those studies a typical depth of triangular etch pits was exactly one MoS2 monolayer; i.e., 0.7 nm. Beyond triangular etch pits, very few morphological changes of the MoS2 flakes have been noticed [11]. Since MoO3 crystals are transparent, it is not clear whether MoO3 stayed with those samples at all and/or covered the pits and a surrounding area uniformly. Particularly, almost the same thickness is expected for the MoO3 and MoS2 single layers [22,23,24].
It is not only difficult to detect MoO3 via its physical properties, but according to our knowledge, none of the published results provided a direct chemical proof of the local MoO3 existence on a single microscopic 2H MoS2 flake. Typical XPS measurements are not so local and encompass at least several MoS2 flakes. Raman measurements do not help either. The majority of the published Raman studies show either miniscule shifts of the Raman modes fingerprinting the MoS2 crystals, i.e., the A1g and E12g modes [10,11], or an exclusive presence of the MoO3 crystals on fully oxidized single 2H MoS2 flakes [8]. This is likely due to a very small thickness of the oxide layer. Overall, the MoO3 layers produced during gentle oxidation of single 2H MoS2 crystals are transparent, thin and difficult to be differentiated both physically and chemically from an underlying MoS2 substrate. In addition, such MoO3 layers might contain MoO3 derivatives, such as molybdenum(VI) hydroxyoxides and/or MoO3 hydrates. Clearly, proper identification and surface distribution of the MoO3 species and their derivatives on single 2H MoS2 flakes deserve substantially more attention.
In this study we detected and differentiated the MoO3 oxides and their derivatives from an underlying MoS2 crystal on single and microscopic 2H MoS2 flakes. We used a series of the MoS2 samples, which were oxidized gently, each one at a different temperature between 205 to 410 °C, in dry or humid air. First, using XPS measurements we show how the content of the MoO3 species on the MoS2 samples changes with oxidization temperature. Next, by carefully manipulating AFM tips we created nanoscale ripples within the MoO3 layer and scratched them out from single MoS2 flakes. We related the thickness of the scratched out MoO3 layer to its structure and used a model for formation of nanoscale ripples to obtain its compressive breaking strength. Next, we proved that oxidation on the edges of the MoS2 crystals provides most of the needed MoO3 to cover the MoS2 flakes. Finally, we study sublimation of the MoO3 oxide layer and its formation in high relative humidity conditions.
2. Materials and Methods
Preparation of MoS2 flakes: Laboratory grade 2H molybdenite crystals were bought from SPI Supplies, West Chester, PA, USA, catalogue number #429MM-AB. The MoS2 samples have been mechanically exfoliated using a standard double-sided scotch tape and transferred on fine polished and basically undoped <111> Si crystals with resistivity more than 10,000 Ω cm, bought from ITME, Warsaw, Poland. Prior to a sample transfer, the Si substrates were ultrasonically cleaned with acetone and isopropanol and dried with pure N2. Due to subsequent heating studies at temperatures of more than 300 °C, no annealing nor other cleaning methods have been used to remove any remaining traces of the scotch tape from the Si substrates. For sample heating in air we used a standard hot plate covered with a quartz Petri dish to insure a controlled atmosphere and proper distribution of temperatures within a heating zone. After calibrating the hotplate with a standard thermocouple and a Pt thermometer, we established a temperature error of ± 2K on the sample surface. For experiments in high relative humidity we used a custom-built chemical reactor.
X-ray photoelectron spectroscopy (XPS): XPS was conducted in the multi chamber ultrahigh vacuum system (manufactured by Omicron Nanotechnology, Taunusstein, Germany) equipped with a hemispherical energy analyzer Phoibos 150 (manufactured by SPECS, Berlin, Germany) with a 2D-CCD detector. DAR 400 X-ray lamp with a non-monochromatic radiation of 1253.64 eV (Mg Kα) was used. The spectra for analysis of chemical shifts in Mo 3d core lines were collected with pass energy of 30 eV in “Fixed Analyzer Transmission” mode. For the peak fitting procedure, the CasaXPS (v.2.3, Casa Software Ltd., Teignmouth, UK) software was used. To ensure repeatability of the analysis of all samples, the same set of components with fixed energy separation was used for each of them. The binding energies of the measured spectra were calibrated to the C 1s core line position at 285 eV. For all of the investigated samples, an observed charging effect did not exceed 2 eV and no hardware charge compensation was needed.
Atomic force microscopy (AFM): Non-contact/tapping AFM imaging was conducted at amplitudes of several tens of nanometers with AC-160TSA-R3 cantilevers from Olympus, Tokyo, Japan using Cypher-S AFM manufactured by Asylum Research, Goleta, CA, USA. Contact mode AFM imaging was conducted with MLCT-E and/or MLCT-F cantilevers from Bruker, Santa Barbara, CA, USA and using Dimension Icon AFM manufactured by Bruker. We collected raw topography and lateral force microscopy (LFM) images of the investigated MoS2 flakes. The images were collected with at least 256 points per line and treated with Gwyddion (v.2.51, Czech Metrology Institute, Brno, Czech Republic) software [25]. On topography images we removed glitches and used standard line-by-line first or second-order flattening and three-point plane levelling methods. No other image treatment and/or conditioning was performed.
Lateral spring constant calculations for AFM cantilevers used for measurements of the compressive breaking strength. The value of the lateral stiffness klat for a triangular MLCT-E cantilever used to indent and scratch the oxidized MoS2 crystals was calculated using the Neumeister and Ducker model [26]. Unless otherwise measured from optical microscopy images, we used manufacturer’s specifications for all the relevant variables; i.e., SiNx Young modulus of 304 GPa, Poisson ratio of 0.24, thickness of a cantilever of 0.60 ± 0.05 μm, triangle opening angle of 28 ± 2° (measured), length of cantilever’s arms from the base towards the point they meet of 105 ± 5 μm (measured), distance from the tip to the edge of a cantilever 4 ± 1 μm, width of cantilever arms of 18 ± 1 μm (measured) and tip’s height of 7 ± 1 μm. Those values yielded klat of 54 N/m. Considering the errors of each parameters we obtained a minimum allowable klat of 25 N/m and a maximum of 116 N/m. These values of klat have been calculated using such a combination of parameters (with their errors) to obtain the lowest and the largest values of klat, respectively. Thus, we reported an average value within the errors; i.e., klat = 71 ± 45 N/m. Using the Neumeister and Ducker approach, we also obtained a normal spring constant of 0.097 N/m, which is typical for the MLCT-E levers.
3. Results and Discussion
To investigate the presence of Mo oxides on the MoS2 crystals, we prepared single 2H MoS2 crystals on silicon/silica substrates, as explained in the Materials and Methods. We have studied flakes thicker than 10 nm, i.e., of more than 15 MoS2 monolayers due to known dependencies of several physico-chemical properties of the MoS2 flakes on their thickness, particularly for the flakes thinner than 10 monolayers [27]. First, the MoS2 flakes were localized using light microscopy and AFM; see Figure 1. Next, we selected five samples with MoS2 flakes for the XPS studies of their thermal oxidation. One of the samples served as our reference sample. Four other samples were separately heated on a hot plate for 10–15 min each: first at 320, second at 350, third at 370 and fourth at 410 °C.
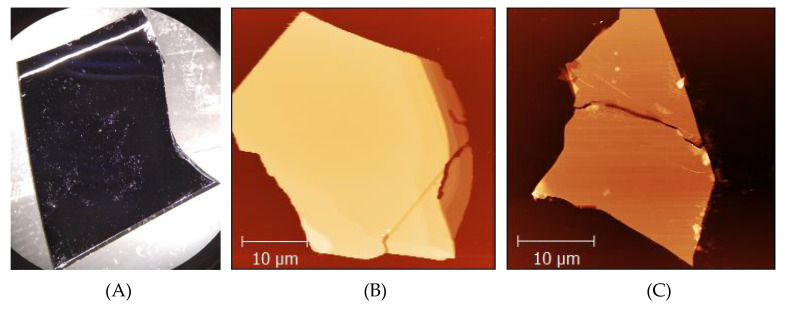
Figure 1.
An exemplary sample containing agglomerates of mostly thick mechanically exfoliated 2H MoS2 crystals on a silicon substrate. (A) An optical microscopy image of a typical sample being ca. 2 cm long and ca. 1.5 cm wide. Visible whitish spots are agglomerates of single MoS2 flakes. Two of such single MoS2 flakes are presented in (B,C). Image (B) is an AFM topograph of a flake with a mean height of a central zone (excluding several lone MoS2 pieces) of ca. 65 nm and a Z-scale of the image of 130 nm. (C) An AFM topograph of another flake with a mean height of a central zone of ca. 25 nm and a Z-scale of the image of ca. 65 nm. For images (B,C) brighter colors represent portions of the image, which are higher (Z-scale).
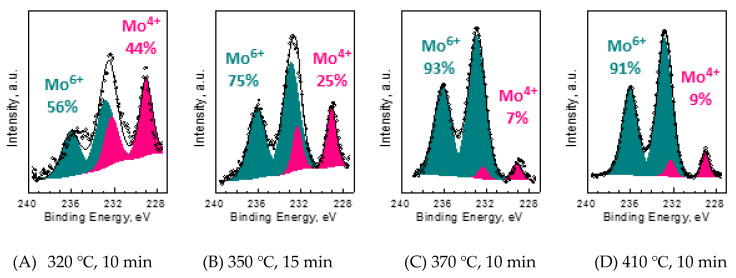
Figure 2 shows the results of our XPS investigations on the samples prepared and oxidized in air. The Mo 3d core line XPS spectra were used in order to probe an oxidation process. Each such XPS spectrum contains four maxima, which have been related to the presence of two different oxidation states of the Mo ions. The applied peak fitting procedure yielded the binding energies of each maxima at 229.0 eV, 232.2 eV, 232.8 eV and 236.0 eV, respectively. The first two maxima were related to the spin-orbit doublet (Mo 3d 5/2 and 3/2) of the Mo4+ oxidation state, which is characteristic for the MoS2 sample. The second two maxima were related to the presence of the Mo6+ oxidation state doublet, which within our reaction conditions has been predicted to be characteristic of the MoO3. During peak fitting procedures, the Gaussian–Lorentzian peak shapes were used and the relative peak areas were fixed to a 2:3 ratios for each set of the respective 3/2 and 5/2 pairs. This procedure and the resulting binding energies are consistent with previous works [12,18,19].
Figure 2.
Our XPS results. (A–D) The XPS Mo 3d core line spectra of MoS2 flakes heated in different temperatures presented together with the charts showing the concentrations of Mo4+ and Mo6+ ions within the surface layer of the flakes. The contributions coming from different oxidation states were fitted to the spectra and are presented in the charts below.
The oxidation of a single MoS2 flake has been suggested to start already above 200 °C, as inferred from electronic density shifts within the MoS2 monolayers via micro-Raman measurements [8]. However, earlier reports have shown through visualization of the obtained triangular etch pits that meaningful MoS2 oxidation occurs in air only above 320 °C [8,9,10,11,28]. Such conclusions agree our XPS analysis in Figure 2. The presented results clearly show that during heating to a certain temperature the concentration of the Mo4+ ions decrease, which is visible when comparing the spectra after heating at 320, 350 and 370 °C; see Figure 2A–C. However, somewhere above 370 °C this trend is stopped or even reversed, as is visible in the XPS spectra obtained after heating the sample in 410 °C; see Figure 2D. The XPS results, however, are averaged over an investigated area, which is typically between several tens to a hundred of µm2. Therefore, we decided to investigate the distribution of the Mo oxides on the surface of MoS2 flakes using local AFM measurements.
After many trials and using high resolution, contact non-contact/tapping AFM imaging, we could not detect any obvious MoO3 islands on and/or in the vicinity of the triangular etch pits on the XPS studied samples till heating them at 370 °C. We did not quit searching for the surface-bound MoO3 layers on the prepared batch of samples; XPS results in Figure 2 clearly show the prevailing presence of oxides on the samples heated between 350 and 410 °C. Furthermore, the XPS results did not change upon sample annealing at 210 °C for 30 min, confirming that the Mo oxide stayed there even after annealing. This is why we decided to locally scratch several samples heated between 350 and 370 °C. Indeed, after several passages of a scanning AFM tip, we managed, at least in several cases, to break through a complete layer of the likely MoO3 oxide after applying normal forces of up to several nN. While continuing the scratching process, we managed to remove any remaining pieces of the scratched layer and to expose a fresh basal plane of the Mo disulfide.
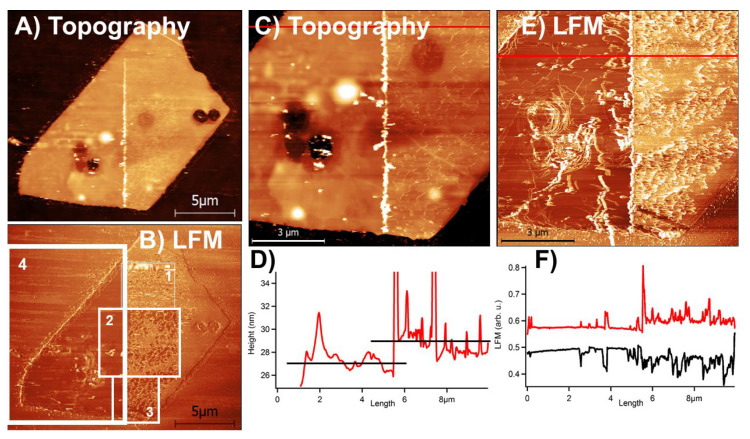
Figure 3 presents the results of removing a surface-bound MoO3 oxide layer with help of an AFM tip. It shows the AFM recorded surface topography and corresponding lateral force microscopy (LFM) signals to visualize changes provoked on the sample during scratching. The investigated MoS2 flake was ca. 30 nm thick. It was oxidized in five cycles of 10-minute heating at 350 °C and subsequent cooling to room temperature. The oxidized flake was continuously scratched in the zones denoted respectively by numbers “1,” “2,” “3” and “4” in Figure 3b. In the zone “1” the MoS2 flake was rastered several times only, but it was enough to break continuity of the top oxide layer and produce lasting and visible indents in this layer. In the zones “2” and “3” the flake was scratched several more times than in “1,” so that visible surface ripples appeared within the oxidized MoS2 surface. In the zone “4” a portion of the MoS2 flake was rescanned more than 10 times, resulting in a complete removal of an oxide layer.
Figure 3.
Removing the surface-bound MoO3 oxide layer on a single MoS2 flake with an AFM tip. The images show AFM recorded topography for (A,C) and uncalibrated friction (LFM signal) for (B,E). (B) shows that the flake was continuously scratched in zones 1, 2, 3 and 4 with a progressively increasing number of rescans between the zones. In the zone “4” the flake was rescanned more than 10 times, which produced complete removal of the oxide layer. (C) shows a close-up on the topography data from (A). (D) presents a topography cross-section line, marked in red in (C), to show that a removed oxide layer was 2 ± 1 nm thick. (E) shows an LFM signal corresponding to (C). (F) shows a friction loop along a cross-sectional line presented in red in (E). A friction loop is an LFM signal recorded in trace (R− > L) and retrace (L− > R) scanning. It corresponds to twice of the uncalibrated friction. For (A,C) brighter colors represent higher portions of the image (Z-scale), while for (B,E) brighter colors represent higher values of the lateral force microscopy (LFM) signal. Z-scale in (A) was 60 nm or (C) 43 nm.
AFM topographs cannot provide direct information about the local chemistry of the investigated samples. Nevertheless, with their help we can discuss the thickness of the scratched out MoO3 layer. To start with, after averaging several cross-sectional lines in local surface topography in Figure 3c, we obtained that a removed oxide layer was 2 ± 1 nm in height; see Figure 3d.
Next, we explain how an obtained thickness of the oxide layer corresponds to its structure. The most thermodynamically stable MoO3 polymorph existing in nature is the α-MoO3 called molybdite. Refined molybdite crystal structure shows a true layered arrangement of the MoO6 octahedra with an orthorhombic unit cell belonging to a Pbnm space group [24]. Any given MoO3 layer is a double layer composed of MoO6 octahedra at two height levels. Within each level the MoO6 octahedra are connected by shared lateral ends only. Connections between a ground level and an upper level are realized by sharing one edge between any two octahedra from ground and upper levels, respectively. Such an arrangement yields an overall height of a single α-MoO3 layer, h, it being 3/2 of the height of a single MoO6 octahedron plus any necessary spacing between the layers. Consequently, we estimate h to be ca. 0.8 nm [24]. On the other hand, we can also estimate the value of h differently. To provide for dense spatial packing within the α-MoO3 structure, a second (upper) α-MoO3 layer is laterally shifted with respect to a first (lower) layer, but a third MoO3 layer is positioned like a first layer. Such an arrangement yields the height of a respective α-MoO3 unit cell stretching from first to third layer to be 1.4 nm. The thickness of an α-MoO3 monolayer atop another α-MoO3 monolayer is half of this value; i.e., 0.7 nm. Again, a slightly larger value is expected for the height of the α-MoO3 monolayer on the 2H MoS2 crystal due to non-matching lateral dimensions between α-MoO3 and 2H MoS2. Thus, the expected thickness of a single MoO3 layer atop the MoS2 basal surface is between 0.7 and 0.8 nm, which makes it extremely close to the thickness of a single MoS2 monolayer of ca. 0.7 nm. Consequently, the detected thickness of the MoO3 layer corresponds to something between one to four MoO3 monolayers.
Another important difference between MoS2 crystals and detected MoO3 oxide layers observed in Figure 3e,f is the substantially larger, ca. 1.5 times larger, friction on the surface containing rippled oxides comparing to a cleaned MoS2 surface. A substantial friction increase was expected on the oxidized vs. pristine MoS2 surface. MoS2 surfaces are known for low friction and their moderately hydrophobic behavior acquired from the pristine MoS2 surface after its quick passivation by hydrocarbons [29]. On the contrary, Mo oxides and their derivatives are strongly hydrophilic, which facilitates formation of water capillary bridges between these surfaces and a scanning AFM tip in ambient conditions. Such capillary bridges are known to account for substantial friction increase in air [30,31,32].
The results are presented in Figure 3, and more of such results together with accompanying high resolution XPS spectra, presented in the Supplementary Materials (Figures S1 and S2), confirm that the Mo oxide layer can be indented and scratched away from MoS2 basal planes of single MoS2 crystals. Let σMoO3 denote the compressive mechanical stress at the plastic yield of that layer. It is also referred to as the compressive breaking strength. The value of σMoO3 can be estimated from our experimental results presented in Figure 3b using the Dugdale model of pressure-induced formation of cracks in solid surfaces [33], which was adopted for conditions of indenting a material with a scanning AFM tip [34]. In brief, the model assumes that a scanning AFM tip performs a stick-and-slip motion between the cracks, which are generated just underneath the very surface of a material and become visible within the indents within the material created by an AFM tip. Any given indent grows when a tip of an AFM cantilever “sticks” to it by exerting a normal load. At the same time the tip shifts laterally in the process of surface scanning. Thus, the tip will “slip” by the lateral distance, Δ, to a new indentation point once mechanical energy stored in the cantilever, due to its increasing lateral bending, exceeds the tip-material surface energy over the contact area. The value of Δ is later measured as the distance between consecutive indents. The lateral length of a given indent within a material is denoted as δt. The local breaking strength of a material, σ, is calculated from a following equation:
| σ = (0.5 · Δ2 · klat)/(1.2 · 2 · π · R · (D + H) · δt) = 0.0663 · Δ2 · klat / (R · (D + H)· δt) | (3) |
In Equation (3), D is the depth of a crack; H is the height of a material pile removed during indentation within a crack; R is the tip curvature radius of an AFM cantilever and klat is its lateral (elastic) stiffness.
After careful examination of the zones “1” and “2” in Figure 3b we have observed that the values of Δ and δt are similar and typically equal several hundreds of nanometers. Following this observation we simplify Equation (3) to yield: σMoO3 = 0.0663(Δ · klat) / (R · (D + H)). The values of (D + H) are limited in our case by a small thickness of an oxide layer. We have estimated (D + H) = 5 ± 2 nm; see Figure 3b. For the used here MLCT-E cantilever, R was estimated as 50 ± 10 nm and klat = 71 ± 45 N/m was obtained, as explained in the Materials and Methods. Consequently, we obtained values of σMoO3 between 0.3 and 7.8 GPa. An upper limit of ca. 8 GPa is likely overestimated, since our values of (D + H) are smaller than in the case of a thick MoO3 layer.
The value of σMoO3 can be explained quite well using some previously published data and our experimental details. We did not find the value of σMoO3 in the literature; however, the MoO6 octahedra within each single MoO3 layer are slightly deformable under pressure along one of the lateral directions [24]. On the contrary, the MoS2 surface exhibits nice honeycomb lattices within each of the respective S and Mo-planes, which are not easy to break through [22,23]. Thus, one expects the value of σMoO3 to be much lower than a respective value of σMoS2. In fact, within our experimental conditions, we did not observe any breaking through the underlying MoS2 layer under the maximum loading forces applied. Those forces were of up to four times of the loads applied to generate scratches presented in Figure 3. Therefore, up to several-times-lower values of σMoO3 than σMoS2 are expected. Several published experimental and theoretical studies reported the values of σMoS2 between 20 and 30 GPa [35,36]. Consequently, our values of σMoO3 might indeed relate to the presence of thin α-MoO3 layers. In conclusion, despite not being easily noticeable, a thin α-MoO3 layer can be differentiated via creating surface ripples and scratching out the oxide layer via repetitive scanning on the MoS2 samples with appropriate AFM cantilevers.
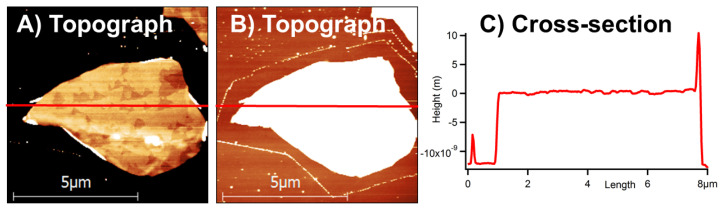
Next, we address the origins of the Mo oxide layer, which deposits on the MoS2 surfaces. Etched triangular pits would account for only a very small amount of the produced oxides and there must be other sources of it. In Figure 4 one can see an example of the MoS2 flake with MoS2 monolayers of thickness between 15 and 20 depending on a position along the flake. The flake was imaged at ambient conditions just after 12 min of its thermal oxidation at 390 °C in air. One can clearly notice triangular etch pits within the basal surface of the flake in Figure 4a, and a clean area surrounding the bottom of the flake in Figure 4b.
Figure 4.
Etching and shrinking of a single MoS2 flake. (A,B) show topography images of the flake, with the Z-scale of several nanometers only in each. (A) is centered on a basal flake surface and (B) is centered on a surface of the silicon substrate. (C) presents a topographical cross-section taken laterally in a middle of the flake, as shown in A and B, in red. The flake is 12 ± 2 nm thick.
The clean area around the MoS2 flake observed in Figure 4b has been freshly produced, since it contains no spots. This should be compared with many small spots visible on the Si substrate due to its prolonged handling in air. Thus, such a clean area must have originated from lateral shrinking of the flake during thermal oxidation. At the same time, not too many triangular etch pits were produced on this flake; see Figure 4a. Consequently, the amount of the Mo oxides produced by volumetric oxidation at edges of the flake must was substantially greater than the amount of oxides produced from its basal surface oxidation. Thus, oxidation on edges of the MoS2 flakes is a primary source of the MoO3, particularly at heating temperatures of 350 °C and above. This result agrees with already published observations made by angle-resolved XPS, but on macroscopic MoS2 crystallites [37]. We have observed similar effects on many other MoS2 flakes, which were not too thick. On thick MoS2 flakes this effect is barely visible due to substantial volume of the MoS2 material enclosed within only slight shrinking of the lateral dimensions of such flakes due to heating.
Consequently, the only way to reconcile our data, i.e., volumetric oxidation, triangular surface etching and formation of an eventual thin Mo oxide layer, is to suggest that freshly-produced MoO3 must escape directly to the gas phase, but some portion of it can deposit back on the surface to form islands and even organized layers under appropriate conditions. In this sense, we have validated microscopically that the MoO3 produced through Equation (1) is initially in the gas form, at least within our experimental conditions.
Following the hypothesis that originally produced MoO3 goes to the gas phase first, we continued to notice that the amount of the MoO3 oxides, which are deposited back on the MoS2 surfaces, decreases as oxidation temperature rises above 370 °C; see Figure 3e. While more “energetic”—i.e., produced at higher temperatures—MoO3 particles in the gas phase have less of a tendency for surface adsorption, such results admit as well a possibility that the MoO3 particles, which have adsorbed on the surface, might quickly sublimate at those conditions. Indeed, high quality MoO3 crystals grown on gold have been showed to sublimate above 400 °C [38] and monolayers of amorphous MoO3 grown on MoS2 via O2 plasma exposure have sublimated completely at 500 °C [39]. Furthermore, thermodynamic calculations of Walter et al. [12] predicted MoO3 sublimation to start above 470 °C. Below, we provide an experimental proof for sublimation of the surface-bound MoO3 layers already at 320 °C.
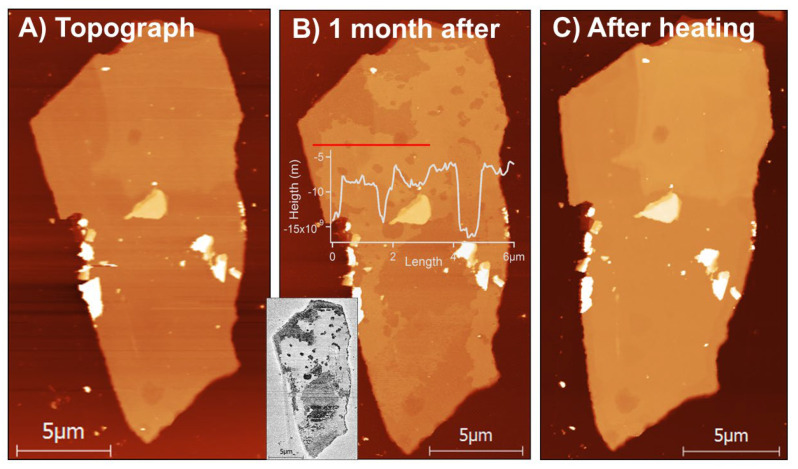
Figure 5 presents an MoS2 flake heated for several minutes at 370 °C to promote oxidation and then left for about a month inside of a humid desiccator. In Figure 5a one can see an initial MoS2 flake. In Figure 5b one observes clearly some additional build-ups, likely MoO3 layers, which have deposited on various portions of the flake after its prolonged stay in desiccator. In Figure 5c one can notice that an entire oxide layer sublimated after the sample was heated at 320 °C for only four minutes. Such a low sublimation temperature does not compare even with lowest sublimation temperatures of 400 °C observed in the aforementioned studies [38]. However, one can quickly realize that the aforementioned MoO3 sublimation studies were conducted under vacuum and/or in highly controlled environments [38,39], whereas our results were obtained in far less clean conditions. In other words, surface adsorbed hydrocarbons and other surface contaminants on the pristine MoS2 surface might have prevented the formation of a well-organized and crystalline MoO3 layer. Alternatively, however, the slowly grown-up MoO3 layer might have contained some MoO3 derivatives, such as molybdenum(VI) hydroxyoxides and MoO2(OH)2, which have been predicted to be formed, when water was present. If fact, the height of the formed MoO3 layer in Figure 5b is ca. 5 nm, which is much larger than thickness of the MoO3 layer obtained in air. This suggests slow formation of either an additional MoO3 in humid air due to Equation (2), or some additional MoO2(OH)2 species crystallizing on the sample. The MoO2(OH)2 species are far more volatile than a MoO3 layer [12] and sublimate at lower temperature than MoO3. We had no means to test for the presence of the hydroxyoxides on the MoS2 flake presented in Figure 5, but we decided to follow up on an unclear role of water in degradation and thermal oxidation of the MoS2 flakes.
Figure 5.
Sublimation of Mo oxide layers. (A) shows an initial MoS2 flake obtained by AFM imaging in non-contact mode. (B) presents additional build-ups, likely MoO3 layers, deposited on various portions of the flake after its short oxidation at 370 °C followed by one month stay in humid desiccator. A marked cross-sectional line (in red) shows that a typical thickness of an oxide layer is ca. 5 nm. Inset: corresponding AFM phase imaging, which seems to differentiate roughly oxidized (white) vs. non-oxidized (gray) portions of the flake, at least on a top part of the flake. (C) shows that the entire oxide layer sublimated after the sample was heated at 320 °C for only 4 minutes. Z-scale in (A) was 132 nm, in (B) 129 nm and in (C) 90 nm.
To start with, we investigated, again by AFM, the microscopic surface topography of several thermally oxidized MoS2 samples after their submergence in water for the times ranging from a few minutes to several hours. We did not observe any topographical changes on those previously oxidized MoS2 flakes. However, we could have incubated them in water for too little time in order to dissolve any MoO3 oxide layers and/or create any etch pits in the MoS2 [21]. Nevertheless, our results suggest that a thin layer of the MoO3 species, which originated on surfaces of the oxidized MoS2 flakes, prevented their further deterioration in water, at least within the time scale of several hours.
In order to start addressing the role of water during thermal oxidation of the single MoS2 crystals from a different perspective, we decided to perform thermal oxidation at high relative humidity. One must acknowledge that our previous thermal oxidation studies above 300 °C, even in very humid air, occurred always at local relative humidity of being almost zero per cent. This is because the saturated water vapor pressure in vicinity of a hot surface, psat(T), is a very strongly rising function of the temperature, so that any partial water pressure, p, in an open container in humid air produces a vanishingly small relative humidity, RH = p / psat(T), above 200 °C. Thus, in order to study an effect of relative humidity in thermal oxidation of single MoS2 flakes, one must utilize an enclosed chemical reactor, which assures the same pressure and relative humidity everywhere on the sample.
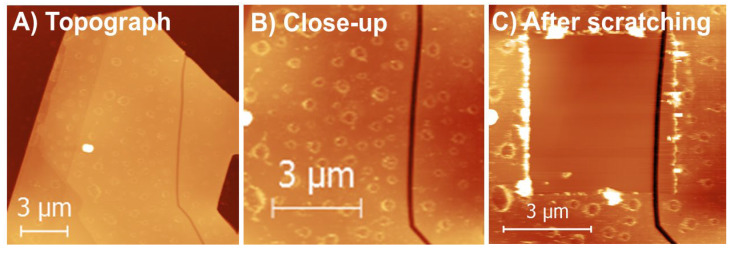
In Figure 6, we present the results of thermal oxidation of single MoS2 crystals at RH = 80 ± 7%. The reaction was conducted within a custom-built chemical reactor. The sample was thermally oxidized for 5 min at mean pressure of 16 bar and mean temperature of 205 °C. It took 14 min to reach the reaction conditions and several more minutes to cool the reactor below 100 °C. A likely oxide layer was formed on the MoS2 samples, because we were able to scratch this layer away (see Figure 6c) at similar forces as for the data presented in Figure 3. Furthermore, the measured thickness of the likely Mo oxide layer was 2.2 ± 0.2 nm, which is also similar to the data presented in Figure 3.
Figure 6.
Thermal oxidation of a single MoS2 flake at high relative humidity. (A,B) show AFM contact mode topography of an oxidized flake and a close-up on a particular spot, which was scratched out later, in (C). Flake thickness depending on a spot was between 90 to 100 nm. The flake was thermally oxidized for 5 min at RH = 80 ± 7% and at a mean temperature of 205 °C. An average thickness of the scratched out layer in (C) was 2.2 ± 0.2 nm. Z-scales: (A) 274 nm; (B) 64 nm; (C) 42 nm.
One can notice that for thermal oxidation in air, when RH was almost zero, the MoO3 oxides were not detected below 320 °C; see Figure 2. Thus, water must substantially accelerate oxidation at high relative humidity, as predicted by Equation (2). If water was acting only like a catalyst in Equation (1), then one would expect some associated triangular etch pits on the oxidized MoS2 surface. However, for several samples and several scratches investigated within this study we observed no triangular etch pits within the oxidized MoS2 layers and the underlying MoS2 surface, i.e., within the scratch. Thus, water does indeed induce an additional oxidation pathway, as in Equation (2). In such a case, however, a thick MoO3 layer would have been observed in Figure 6, which was not the case. Thus, the situation is likely more complicated.
One of the recently published studies suggested that water degrades MoS2 crystals by partially dissolving them to yield pits and to produce non-transparent grains of insoluble crystals of the MoO3 monohydrate, MoO3·H2O [21]. We have indeed observed some discoloration on the oxidized samples. Several MoO3 hydrates have been characterized till now [40,41,42]; in particular, MoO3·nH2O with n = 2, 1, 1/2 and 1/3. Mono and di-hydrates have been found to be most stable structurally [40,41]. The MoO3·2H2O is most stable at room temperature and MoO3·H2O was found to exist between 60 to 140 °C. Above 140 °C the MoO3 hydrates convert to α-MoO3 [41]. We have conducted our experiments above 200 °C, i.e., where any hydrates shall already convert to oxides. The only way for them to appear in our experiments would be to form exclusively at room temperature or during reactor cooling. The MoO3 hydrates, however, are typically produced via crystallization from slowly acidified solutions of molybdate ions [40,41,42]. Thus, in order for the MoO3 hydrates to appear, the molybdate ions, such as MoO42−, need to be produced first. These anions are typically produced from reactions with MoO3 in alkali solutions, which is not the case in our oxidation conditions. Consequently, we are of the opinion that no MoO3 hydrates have been produced on MoS2 in humid air.
Despite a predicted lack of the MoO3 hydrates, we suggest that what it takes place in high relative humidity conditions is an accelerated production of the MoO3 via Equation (2), as in bulk, but together with substantial conversion of the MoO3 layer to volatile MoO2(OH)2 species. Said hypothesis agrees with the following observations. Smolik et al. reported experimental data and thermodynamical calculations on oxidation, volatilization and re-deposition of molybdenum oxide species formed from a certain molybdenum alloy between 400 and 800 °C in flowing air [43]. The Mo oxide species on their Mo alloy underwent volatilization, which was dominated by the appearance of the MoO3 above 550 °C and by the appearance of the MoO2(OH)2, formed from the small ingress of water vapor, at temperatures below 550 °C. Furthermore, within conditions closer to our experimental conditions Walter et al. [12] calculated that MoO2(OH)2 is produced in thermal oxidation of the bulk MoS2 crystals already at 300 °C. This is much earlier than in results of Smolik et al., but similarly to Smolik et al., also for a small ingress of water vapor. Thus, it is expected that at high relative humidity appearance, and fast volatilization of the MoO2(OH)2 species occurs already at much lower temperatures than 300 °C. Consequently, at high relative humidity a crossover is expected from the MoS2 oxidation governed by Equation (1) to oxidation governed by Equation (2), but together with formation and sublimation of the MoO3 hydroxyoxides. In this way, large amounts of MoO3 are produced quickly and a complete basal MoS2 surface is transformed in Mo oxide species very fast, so that no triangular etch pits are observed. At the same time a large portion of the newly produced MoO3 is converted into volatile MoO2(OH)2 species, which leave the reaction environment. Overall, not much of the produced MoO3 stays on the MoS2 surface. However, systematic studies of thermal oxidation as a function of relative humidity and oxidation temperature are needed to address this hypothesis in detail.
Finally, we compare our results with laser-induced oxidation and thinning of MoS2 crystals. To do so, we chose two seminal studies [44,45]. The first study discussed lased induced thinning and oxidation in air [44]. The other study was focused on laser-induced electrochemical thinning of the MoS2 in electrochemical, although aqueous, solutions [45]. In none of these studies, however, have Mo oxides been detected. In [44] the Authors went to great lengths to exclude any MoO3 presence via Raman, photoluminescence and electronic transport studies. Nevertheless, thin MoO3 layers are not necessarily insulating [46] and micro-Raman studies have been notorious for not showing the local presence of MoO3 layers [10,11]. A major reason for difficulty when trying to see any MoO3 on the laser-oxidized MoS2 flakes is certainly that laser-thinning studies are quite local. This means that only a tiny portion of each single MoS2 flake is heated each time and extremely small amounts of the MoO3 are produced. Furthermore, locally obtained temperatures achieved by laser heating of the MoS2 surface are likely much above sublimation temperatures of the MoO3 oxides. This is facilitated by poor (normal) thermal conductivity between subsequently stacked MoS2 layers within a crystal. This effect, however, does not play a major role for globally heated MoS2 samples, as in our case here. Thus, we expect different outcomes between laser-induced heating and thermally induced oxidation, and in particular more of the MoO3 staying on the samples oxidized thermally than in the laser-induced studies. Nevertheless, that all depends on particular experimental conditions. Consequently, it would be extremely interesting to extend the nanomechanical testing for the local presence of the MoO3, such as presented within our paper, to the laser-thinned MoS2 samples.
4. Conclusions
With help of XPS and AFM measurements we reported on microscopic details associated with the oxidation of thick MoS2 flakes deposited on silicon at temperatures between 205 and 410 °C in dry and humid air. We observed for samples oxidized at 350 °C that triangular etch pits started to coexist with MoO3 islands and layers. Above 370 °C the amount of MoO3 detected on the MoS2 surface started to decrease, most likely due to sublimation of the MoO3 layers. The MoO3 layers produced on the MoS2 samples were usually not distinguishable morphologically from an underlying MoS2 surface. However, the MoO3 layers could be scratched away from an underlying MoS2 surface with appropriate AFM cantilevers. We estimated breaking compressive strengths of the MoO3 layer to be between 0.3 and 7.8 GPa. Our results agreed with two important aspects for thermal oxidation of the MoS2 flakes. First, out of the several possible Mo oxides, we detected only the MoO3 species. Second, we suggested that MoO3 species had to be originally produced in the gas phase, and then only some of them adsorbed on the MoS2 surface. MoO3 adsorption has been most effective between 350 and 410 °C.
We also began to address the role of relative humidity in oxidation of the MoS2 crystals. We showed that sublimation of the MoO3 layers occurred already at 320 °C for the MoS2 samples oxidized previously at 370 °C and then left alone to slowly anneal at humid ambient conditions. We suggested that this result could be due to the presence of the MoO3 hydroxyoxides within thick MoO3 layers. Next, we showed the results of thermal oxidization of the MoS2 samples at high relative humidity of 80 ± 7% and mean temperature of only 205 °C. We observed quite dense layers of the likely Mo oxides, similarly to oxidation in air, but with no triangular etch pits within those layers and within an underlying MoS2 surface. We explained such results by substantially faster MoS2 oxidation in the presence of high relative humidity than in air and additional removal of the produced MoO3 oxide due to their conversion into volatile molybdenum(VI) hydroxyoxide species.
Acknowledgments
We are grateful to Wojciech L. Spychalski from Warsaw University of Technology for preparation of technical drawings for a chemical reactor used in high humidity studies and for his invaluable help in construction and testing of this reactor. We thank Krystian Lewkowicz for oxidizing the MoS2 samples at high relative humidity within our chemical reactor and for collecting some AFM data on these samples. We acknowledge Kamil Węgrzyn for assisting in the AFM measurements presented in Figure 4.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/13/14/3067/s1, Figure S1: An example of creating ripples and scratching out an already fragmented oxide layer (due to extensive heating). Left: topography; Right: twice the uncalibrated friction, Figure S2: Progression of the process of removing already loosely bound oxides from the flake in Figure S1. Presented are 6.6 µm by 6.6 µm AFM contact mode topography images.
Author Contributions
Conceptualization, R.S.; methodology, R.S.; validation, R.S., M.R., P.D.; formal analysis, R.S., M.R., P.D.; investigation, R.S., M.R., P.D.; resources, R.S., M.R., P.D.; data curation, R.S., M.R., P.D.; writing—original draft preparation, R.S., M.R., P.D.; writing—review and editing, R.S., M.R., P.D.; supervision, R.S.; project administration, R.S.; funding acquisition, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Center, Poland, grant number 2017/27/B/ST4/00697 (RSz) and grant number 2016/21/B/ST5/00984 (MR, PD), and by the UW Statuary Funds.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lince J.R., Fleischauer P.D. Ch.7. In: Conley P., editor. Space Vehicle Mechanisms: Elements of Successful Design. Wiley-Interscience; New York, NY, USA: 1998. [Google Scholar]
- 2.Wang Q.H., Kalantar-Zadeh K., Kis A., Coleman J.N., Strano M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012;7:699–712. doi: 10.1038/nnano.2012.193. [DOI] [PubMed] [Google Scholar]
- 3.Liu L., Wu J., Wu L., Ye M., Liu X., Wang Q., Hou S., Lu P., Sun L., Zheng J., et al. Phase-selective synthesis of 1T′ MoS2 monolayers and heterophase bilayers. Nat. Mater. 2018;17:1108–1114. doi: 10.1038/s41563-018-0187-1. [DOI] [PubMed] [Google Scholar]
- 4.Radisavljevic B., Radenovic A., Brivio J., Giacometti V., Kis A. Single-layer MoS2 transistor. Nat. Nanotechnol. 2011;6:147–150. doi: 10.1038/nnano.2010.279. [DOI] [PubMed] [Google Scholar]
- 5.Li X., Zhu H. Two-dimensional MoS2: Properties, preparation, and applications. J. Materiomics. 2015;1:33–44. doi: 10.1016/j.jmat.2015.03.003. [DOI] [Google Scholar]
- 6.Gong C., Zhang Y., Chen W., Chu J., Lei T., Pu J., Dai L., Wu C., Cheng Y., Zhai T., et al. Electronic and Optoelectronic Applications Based on 2D Novel Anisotropic Transition Metal Dichalcogenides. Adv. Sci. 2017;4:1700231. doi: 10.1002/advs.201700231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Marin J.F., Unuchek D., Watanabe K., Taniguchi T., Kis A. MoS2 photodetectors integrated with photonic circuits. 2D Mat. Appl. 2019;14:1–7. doi: 10.1038/s41699-019-0096-4. [DOI] [Google Scholar]
- 8.Yamamoto M., Einstein T.L., Fuhrer M.S., Cullen W.G. Anisotropic Etching of Atomically Thin MoS2. J. Phys. Chem. C. 2013;117:25643–25649. doi: 10.1021/jp410893e. [DOI] [Google Scholar]
- 9.Wu J., Li H., Yin Z., Li H., Liu J., Cao X., Zhang Q., Zhang H. Layer thinning and etching of mechanically exfoliated MoS2 nanosheets by thermal annealing in air. Small. 2013;9:3314–3319. doi: 10.1002/smll.201301542. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H., Yu F., Liu Y., Zou X., Cong C., Qiu C., Yu T., Yan Z., Shen X., Sun L., et al. Thickness-dependent patterning of MoS2 sheets with well-oriented triangular pits by heating in air. Nano Res. 2013;6:703–711. doi: 10.1007/s12274-013-0346-2. [DOI] [Google Scholar]
- 11.Ukegbu U., Szoszkiewicz R. Microscopic Kinetics of Heat-Induced Oxidative Etching of Thick MoS2 Crystals. J. Phys. Chem. C. 2019;123:22123–22129. doi: 10.1021/acs.jpcc.9b02739. [DOI] [Google Scholar]
- 12.Walter T.N., Kwok F., Simchi H., Aldosari H.M., Mohney S.E. Oxidation and oxidative vapor-phase etching of few-layer MoS2. J. Vac. Sci. Technol. B. 2017;35:021203. doi: 10.1116/1.4975144. [DOI] [Google Scholar]
- 13.Rao R., Islam A.E., Campbell P.M., Vogel E.M., Maruyama B. In situ thermal oxidation kinetics in few layer MoS2. 2D Mater. 2017;4:025058. doi: 10.1088/2053-1583/aa6532. [DOI] [Google Scholar]
- 14.Wang G., Pandey R., Karna S.P. Physics and chemistry of oxidation of two-dimensional nanomaterials by molecular oxygen. WIREs Comput. Mol. Sci. 2017;7:1–16. doi: 10.1002/wcms.1280. [DOI] [Google Scholar]
- 15.Santosh K., Longo R., Wallace R., Cho K. Surface oxidation energetics and kinetics on MoS2 monolayer. J. Appl. Phys. 2015;117:135301. [Google Scholar]
- 16.Alves de Castro I., Datta R.S., Ou J.Z., Castellanos-Gomez A., Sriram S., Daeneke T., Kalantar-zadeh K. Molybdenum Oxides – From Fundamentals to Functionality. Adv. Mater. 2017;29:1701619. doi: 10.1002/adma.201701619. [DOI] [PubMed] [Google Scholar]
- 17.Bihn J.-H., Park J., Kang Y.-C. Synthesis and Characterization of Mo Films Deposited by RF Sputtering at Various Oxygen Ratios. J. Korean Phys. Soc. 2011;58:509–514. doi: 10.3938/jkps.58.509. [DOI] [Google Scholar]
- 18.Ko T.Y., Jeong A., Kim W., Lee J., Kim Y., Lee Y.E., Ryu G.H., Park K., Kim D., Lee Z., et al. On-stack two-dimensional conversion of MoS2 into MoO3. 2D Mater. 2017;4:014003. doi: 10.1088/2053-1583/4/1/014003. [DOI] [Google Scholar]
- 19.Hussain S., Singh J., Vikraman D., Singh A.K., Iqbal M.Z., Khan M.F., Kumar P., Choi D.-C., Song W., An K.-S., et al. Large-area, continuous and high electrical performances of bilayer to few layers MoS2 fabricated by RF sputtering via post-deposition annealing method. Sci. Rep. 2016;6:30791. doi: 10.1038/srep30791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross S., Sussman A. Surface Oxidation of Molybdenum Disulfide. J. Phys. Chem. 1955;59:889–892. doi: 10.1021/j150531a020. [DOI] [Google Scholar]
- 21.Zhang X., Jia F., Yang B., Song S. Oxidation of Molybdenum Disulfide Sheet in Water under in Situ Atomic Force Microscopy Observation. J. Phys. Chem. C. 2017;121:9938–9943. doi: 10.1021/acs.jpcc.7b01863. [DOI] [Google Scholar]
- 22.Dai Z., Jin W., Grady M., Sadowski J.T., Dadap J.I., Osgood R.M., Jr., Pohl K. Surface structure of bulk 2H-MoS2(0001) and exfoliated suspended monolayer MoS2: A selected area low energy electron diffraction study. Surf. Sci. 2017;660:16–21. doi: 10.1016/j.susc.2017.02.005. [DOI] [Google Scholar]
- 23.Kadantsev E.S., Hawrylak P. Electronic structure of a single MoS2 monolayer. Solid State Comm. 2012;152:909–913. doi: 10.1016/j.ssc.2012.02.005. [DOI] [Google Scholar]
- 24.Asbrink S., Kihlborg L., Malinowski M. High-Pressure Single-Crystal X-ray Diffraction Studies of MoO3. I. Lattice Parameters up to 7.4 GPa. J. Appl. Crystallogr. 1988;21:960–962. doi: 10.1107/S0021889888008271. [DOI] [Google Scholar]
- 25.Nečas D., Klapetek P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012;10:181–188. doi: 10.2478/s11534-011-0096-2. [DOI] [Google Scholar]
- 26.Neumeister J.M., Ducker W.A. Lateral, normal, and longitudinal spring constants of atomic-force microscopy cantilevers. Rev. Sci. Instrum. 1994;65:2527–2531. doi: 10.1063/1.1144646. [DOI] [Google Scholar]
- 27.Ryu Y., Kim W., Koo S., Kang H., Watanabe K., Taniguchi T. Interface-Confined Doubly Anisotropic Oxidation of Two-Dimensional MoS2. Nano Lett. 2017;17:7267–7273. doi: 10.1021/acs.nanolett.7b02621. [DOI] [PubMed] [Google Scholar]
- 28.Spychalski W.L., Pisarek M., Szoszkiewicz R. Microscale Insight into Oxidation of Single MoS2 Crystals in Air. J. Phys. Chem. C. 2017;121:26027–26033. doi: 10.1021/acs.jpcc.7b05405. [DOI] [Google Scholar]
- 29.Kozbial A., Gong X., Liu H., Li L. Understanding the Intrinsic Water Wettability of Molybdenum Disulfide (MoS2) Langmuir. 2015;31:8429–8435. doi: 10.1021/acs.langmuir.5b02057. [DOI] [PubMed] [Google Scholar]
- 30.Lieber C.M., Kim Y. Characterization of the structural, electronic and tribological properties of metal dichalcogenides by scanning probe microscopies. Thin Solid Films. 1991;206:355–359. doi: 10.1016/0040-6090(91)90450-C. [DOI] [Google Scholar]
- 31.Szoszkiewicz R., Riedo E. Nucleation time of nanoscale water bridges. Phys. Rev. Lett. 2005;95:135502. doi: 10.1103/PhysRevLett.95.135502. [DOI] [PubMed] [Google Scholar]
- 32.Szoszkiewicz R., Riedo E. Nanoscopic friction as a probe of local phase transitions. Appl. Phys. Lett. 2005;87:033105. doi: 10.1063/1.1995954. [DOI] [Google Scholar]
- 33.Maugis D. Contact, Adhesion and Rupture of Elastic Solids. Springer-Verlag; Berlin, Germany: 1999. pp. 1–421. [Google Scholar]
- 34.Rice R.H., Mokarian-Tabari P., King W.P., Szoszkiewicz R. Local Thermomechanical Analysis of a Microphase-Separated Thin Lamellar PS b PEO Film. Langmuir. 2012;28:13503–13511. doi: 10.1021/la302565s. [DOI] [PubMed] [Google Scholar]
- 35.Bertolazzi S., Brivio J., Kis A. Stretching and Breaking of Ultrathin MoS2. ACS Nano. 2011;5:9703–9709. doi: 10.1021/nn203879f. [DOI] [PubMed] [Google Scholar]
- 36.Mortazavi B., Ostadhossein A., Rabczuk T., van Duin A.C.T. Mechanical response of all-MoS2 single-layer heterostructures: A ReaxFF investigation. Phys. Chem. Chem. Phys. 2016;18:23695–23701. doi: 10.1039/C6CP03612K. [DOI] [PubMed] [Google Scholar]
- 37.Lince J.R., Frantz P.P. Anisotropic oxidation of MoS2 crystallites studied by angle-resolved X-ray photoelectron spectroscopy. Tribol. Lett. 2000;9:211–218. doi: 10.1023/A:1018869107511. [DOI] [Google Scholar]
- 38.Guimond S., Göbke D., Sturm J.M., Romanyshyn Y., Kuhlenbeck H., Cavalleri M., Freund H.-J. Well-Ordered Molybdenum Oxide Layers on Au(111): Preparation and Properties. J. Phys. Chem. C. 2013;117:8746–8757. doi: 10.1021/jp3113792. [DOI] [Google Scholar]
- 39.Zhu H., Qin X., Cheng L., Azcatl A., Kim J., Wallace R.M. Remote Plasma Oxidation and Atomic Layer Etching of MoS2. ACS Appl. Mater. Interfaces. 2016;8:19119–19126. doi: 10.1021/acsami.6b04719. [DOI] [PubMed] [Google Scholar]
- 40.Seguin L., Figlarz M., Cavagnat R., Lassegues J.-C. Infrared and Raman spectra of MoO3 molybdenum trioxides and MoO3·xH2O molybdenum trioxide hydrates. Spectrochimica Acta Part A. 1995;51:1323–1344. doi: 10.1016/0584-8539(94)00247-9. [DOI] [Google Scholar]
- 41.Kuzmin A., Purans J. Dehydration of the molybdenum trioxide hydrates MoO3·nH2O: In situ x-ray absorption spectroscopy study at the Mo K edge. J. Phys. Cond. Matt. 2000;12:1959–1970. doi: 10.1088/0953-8984/12/9/301. [DOI] [Google Scholar]
- 42.Cruywagen J.J., Heyns J.B.B. Solubility of yellow molybdenum(VI) oxide dihydrate (Mo03·2H20) in 3,0M-sodium perchlorate at 25 °C. S. Afr. J. Chem. 1981;34:118–120. [Google Scholar]
- 43.Smolik G.R., Petti D.A., Schuetz S.T. Oxidation, Volatilization and Redistribution of Molybdenum from TZM Alloy in Air. OSTI; Oak Ridge, TN, USA: 2000. U.S. DOE Report; INEEL/EXT-99-01353. [Google Scholar]
- 44.Castellanos-Gomez A., Barkelid M., Goossens A.M., Calado V.E., van der Zant H.S.J., Steele G.A. Laser-Thinning of MoS2: On Demand Generation of a Single-Layer Semiconductor. Nano Lett. 2012;12:3187–3192. doi: 10.1021/nl301164v. [DOI] [PubMed] [Google Scholar]
- 45.Sunamura K., Page T.R., Yoshida K., Yano T.-A., Hayamizu Y. Laser-induced electrochemical thinning of MoS2. J. Mat. Chem. C. 2016;4:3268. doi: 10.1039/C5TC04409J. [DOI] [Google Scholar]
- 46.Inzani K., Nematollahi M., Vullum-Bruer F., Grande T., Reenaas T.W., Selbach S.M. Electronic properties of reduced molybdenum oxides. Phys. Chem. Chem. Phys. 2017;19:9232. doi: 10.1039/C7CP00644F. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.