Abstract
A hallmark of HIV-1 infection is chronic inflammation, which plays a significant role in disease pathogenesis. Acute HIV infection induces robust inflammatory responses, which are insufficient to prevent or eliminate virus in mucosal tissues. While establishment of viral set-point is coincident with downregulation of acute innate responses, systemic inflammatory responses persist during the course of chronic HIV infection. Since the introduction of combination antiviral therapy (cART), most HIV-1+ individuals can suppress viremia under detection levels for decades. However, chronic immune activation persists and has been postulated to cause HIV associated non-AIDS complications (HANA). Importantly, inflammatory cytokines and activation markers associated with macrophages are strongly and selectively correlated with the incidence of HIV-associated neurocognitive disorder (HAND), cardiovascular dysfunctions (CVD) and other HANA conditions. In this review, we discuss the roles of macrophages in facilitating viral persistence and contributing to generation of persistent inflammatory responses.
Keywords: HIV, macrophage, PAMPs, persistent viral RNA expression, chronic innate immune activation, intron-containing RNA
1. HIV Infection in Macrophages
Tissue-resident macrophages such as those in the lungs (alveolar macrophages), the central nervous system (CNS, microglia), the bones (osteoclasts) and the spleen (splenic macrophages) (reviewed in [1]) have been hypothesized to play an important role in HIV-1 pathogenesis [2]. Earlier studies in cART-naive individuals have shown that HIV-infected macrophages are frequently found in lymph nodes [3,4]. These studies reported on detection of HIV RNA and mature virions by immunohistochemistry and electron microscopy in lymphoid tissue-associated macrophages from HIV-1+ infected individuals with opportunistic infections. HIV-infected multinucleated macrophages have also been found in the brain from HIV+ individuals with encephalopathy [5]. Since it is difficult to obtain primary tissue-resident macrophages from humans, animal models of HIV infection such as SIV/SHIV infection of non-human primates (NHPs) and humanized mice have been widely used to study roles of macrophages in viral infection. Using NHP–SIV/SHIV models, it has been shown that macrophages play a predominant role as a source of viral replication and as a cause of tissue damage at late stages of disease progression when CD4+ T cells, the major target of HIV infection, are depleted [6,7,8]. A recent study in humanized mouse model of HIV-1 infection utilized electron tomography to reveal the presence of budding virions from infected bone-marrow resident macrophages [9]. In contrast to these studies, the role of macrophage infection in vivo has been challenged by the findings that transmitted/founder (T/F) viruses are unable to efficiently infect monocyte-derived macrophages (MDMs) [10,11,12,13]. The inability of T/F viruses, as well as some viruses isolated from chronically-infected patients, to establish MDM infections is correlated primarily to low cell surface CD4 expression in MDMs [11]. Interestingly, Calantone et al. claimed that SIV+ macrophages found in experimentally infected NHPs are not productively infected, but rather SIV antigen positivity might be attributed to phagocytosis of infected CD4+ T cells by macrophages [14]. While in vitro findings also suggest that macrophages can phagocytose or fuse with HIV-infected T cells [15,16], uptake of or fusion with infected T cells can lead to establishment of productive macrophage infection [15,16], suggesting that phagocytic or fusion-mediated delivery of infected cells can be an alternative route of HIV infection in macrophages [17,18]. These in vitro studies coupled with ex vivo findings that primary tissue-resident macrophages are susceptible to HIV infection [19,20,21] implicate that tissue-resident macrophages as virus reservoirs, regardless of the route of infection.
Additional support for macrophages as tissue reservoirs comes from studies of experimental SIV-infections of NHPs. For instance, macrophages isolated from various tissues of SIV-infected animals harbor replication competent viruses, as determined by the quantitative viral outgrowth assay (QVOA) [22,23]. Utilizing TCR beta as a marker of T cell contamination in macrophage preparations, these authors concluded that contribution of contaminated or phagocytosed infected T cells in the QVOA is negligible [22,23]. Macrophage tropic HIV-1 that can infect cells with low CD4 expression has been isolated from cerebrospinal fluid (CSF) of a patient on suppressive cART, suggesting production of HIV particles from replicating CNS reservoirs, that are most likely macrophages/microglia [24]. Persistent HIV infection of macrophages infection is further supported by the findings in a unique humanized mouse model of HIV-1 infection [25]. This mouse (myeloid-only mice, MoM) has engrafted human myeloid cells (monocytes, macrophages and dendritic cells) but is devoid of human T cells. Infection of MoM with HIV has resulted in persistent infection of human macrophages and, importantly, rebound of viremia after interruption of cART [25]. These studies suggest that while the route of infection and extent of virus production from infected macrophages would vary depending on anatomical locations and course of infection, tissue-resident macrophages contribute to HIV replication and persistence in vivo.
2. HIV Persistence in Tissue-Resident Macrophages
HIV-infected macrophages can serve as long-term tissue reservoirs of virus, particularly in the CNS. In experimental SIV infection of NHPs, SIV replication in brain macrophages could be detected as early as four days post virus inoculation [26]. Similarly, HIV infection can also be rapidly established in the brain soon after detection of peripheral viremia in blood within 15 days after infection with HIV [27]. Furthermore, HIV-infected macrophages can be found in patients, even on suppressive cART in diverse tissue sites (reviewed in [2,28]), although the mechanisms that account for long-term persistence of HIV+ macrophages remain unclear. While CD14+ macrophages such as dermal macrophages derived from circulating monocytes have a relatively short life (<6 days) [29], tissue-resident macrophages such as alveolar macrophages have a longer lifespan (>2 months) [30]. In contrast, microglia, the CNS-resident macrophages, are derived from yolk sac and are maintained in the brain for the entire life of an individual due to their self-renewal capacity [31,32,33,34,35]. In the human brain, 28% of microglia renew every year, and microglia age is about four years on average [36]. Thus, it remains formally possible that HIV-infected microglia might persist for the lifespan of the infected individual. In fact, recent QVOA studies optimized for myeloid cells demonstrated the presence of replication competent infectious viruses in microglia from SIV-infected cART-suppressed macaques [22,23,37]. Although macrophages have been postulated to harbor HIV-1 as transcriptionally silent provirus or unintegrated DNA (see the review in [38]), frequency and mode of HIV-1 latency in tissue-resident macrophages in patients on therapy are largely unknown. HIV latency has been hypothesized to be reactivated by multiple mechanisms such as co-infections and cytokines [39,40]. Whether latently infected microglia in HIV+ individuals are reactivated with such stimuli and to what extent HIV production from microglia can contribute to systemic viremia remain to be determined.
Macrophages in other anatomical locations are also persistently infected with HIV or SIV (reviewed in [2]). In the liver, HIV positive Kupffer cells have been found from HIV+ individuals on cART [41,42]. However, recovery of replication competent HIV ex vivo from liver-resident macrophages was unsuccessful [42]. Alveolar macrophages in the lung are HIV DNA/RNA positive [43], although whether infected alveolar macrophages can produce infectious progeny virions remains unclear. In SIV-infected NHPs on suppressive cART, tissue-resident macrophages from the lung and the spleen were viral DNA positive, expressed SIV gag RNA, and produced infectious particles upon stimulation [22,23,44]. Interestingly, urethral macrophages from SIV-infected monkeys express high levels of SIV RNA even during suppressive cART [45]. Recently, Ganor et al. reported that urethral macrophages from HIV+ patients on long-term (>3 years) cART harbor HIV DNA, RNA and proteins [46]. Importantly, HIV-infected urethral macrophages were induced to produce infectious particles upon ex-vivo stimulation with LPS, demonstrating urethral macrophages are one of the anatomical reservoirs of replication competent HIV in cART-suppressed patients [46]. Whether production of infectious HIV from these macrophages contributes to person-to-person transmission remains to be determined.
3. Pro-Inflammatory Responses in Tissue (CNS)-Resident Macrophages
A wide range of macrophage-associated immune activation markers have been linked to CVD [47,48], HAND, frailty [49], cancer [50] and pneumonia [51]. For example, elevated monocyte activation markers such as MCP-1, sCD14 and sCD163 have been repeatedly linked to CVD events or subclinical onset [52,53,54,55,56,57] and interestingly are independent predictors of all-cause mortality in virally suppressed cohorts [58,59,60]. Among tissue-resident macrophages, the contribution of HIV infected microglia to neuroinflammation has been most studied. Although cART has greatly reduced the severity of HAND, up to 50% of HIV+ individuals still suffer from neurocognitive disorders [61]. While the definitive cellular etiology of neurocognitive disorders in cART-suppressed HIV+ individual remains to be elucidated, chronic inflammation is postulated to be the chief driver of neuronal damage [61,62,63]. The primary virus positive cells in the CNS include brain perivascular macrophages and parenchymal microglia [64,65]. Microglia are unique among tissue-resident macrophages in that microglia can self-renew to maintain their population in the brain for the entire life [31,32,33] and are not re-populated by myeloid derived monocytes [31,32,33]. These unique features of microglia might shed light on the role of microglia in HIV persistence and neuroinflammation in the brain of HIV+ individuals on cART.
Although prolonged cART can suppress plasma viremia in HIV+ individuals under the detection limit for decades, viral RNA is detected in the CSF [66,67,68,69], suggestive of ongoing HIV transcription in CNS-resident cells including microglia and/or infiltrating cells. It has been reported that HIV infection of microglia can result in innate immune activation and neuronal injury (reviewed in [70,71,72]), although the molecular mechanisms underlying microglia activation and neuronal damage caused by HIV infection remain inconclusive. An obstacle to studying HIV infection in primary human microglia is the limited access to primary microglia, which restricts rigorous experimental strategies to elucidate molecular mechanisms of microglia infection and HIV-infection-induced immune activation. To overcome these limitations, many protocols have been developed to generate microglia from human induced pluripotent stem cells (iPSCs) [73,74,75,76,77,78]. iPSCs might provide an inexhaustible and reproducible source of cells, and they are amenable to gene-editing strategies such as CRISPR/CAS9 [79,80]. Additional advantages include the feasibility of establishing iPSC lines from patients with diverse genetic backgrounds, such as those with microglia-associated neurodegenerative diseases [81] or those with higher CNS HIV reservoirs, which might reveal pathways that could be harnessed to suppress disease states. A recent study has used commercially available iPSC-derived microglia and demonstrated that neuronal status (healthy or damaged) affects HIV replication in microglia, indicating interplay between microglia and neurons [40]. Further studies are warranted to use microglia and neurons from the same iPSC line and to include other CNS-resident cells such as astrocytes to form self-organizing three-dimensional organoid cell cultures to recapitulate cell-to-cell interactions and model brain structures in HIV-infected states.
4. HIV PAMPs and Induction of Persistent Immune Activation in Macrophages
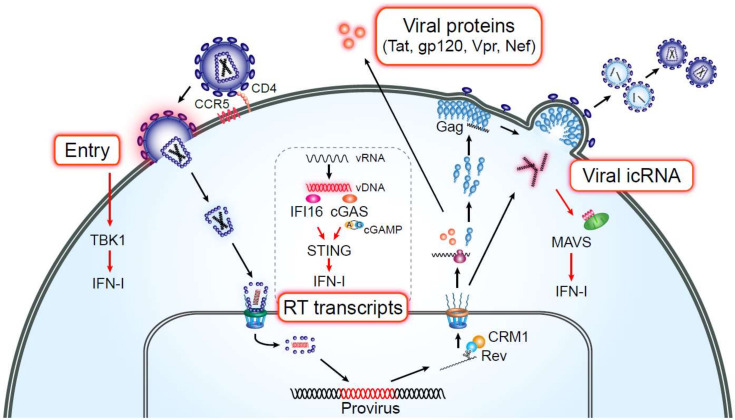
Inflammatory markers associated with myeloid cell activation are strongly and selectively predictive of HAND and HANA [65,82,83,84]. Since access to tissue-resident macrophages is limited, MDMs have been used as a relevant in vitro model to study the role of macrophages in HIV-infection-induced inflammation. MDMs are equipped with multiple innate immune sensors that detect foreign pathogens to induce innate immune responses (Figure 1). Toll-like receptors are well-characterized sensors detecting viral and bacterial pathogens and detect HIV-associated viral RNA in some cell types (reviewed in [85,86]). However, HIV-mediated macrophage activation has been shown to be TLR-independent [87]. Decalf and colleagues demonstrated that HIV-1 fusion and entry into MDMs can trigger interferon-stimulated gene (ISG) expression in a TBK-1-dependent manner [88]. In contrast to HIV-1 entry, cytosolic DNA sensors (reviewed in [89]) have been hypothesized to sense the viral reverse transcription step. HIV RT products can be recognized by a cytosolic enzyme cGAS, which generates circular GMP-AMP dinucleotide (cGAMP), and cGAMP binds to STING to induce IFN-I responses via TBK1–IRF3 axis in MDMs [90]. Another study has shown that HIV RT intermediates trigger IFN-I production in MDMs in an IFI16–STING-dependent manner [91]. It should be noted that both studies utilized strategies to deliver SIVmac Vpx via virus-like particles (VLPs) into the host cell cytoplasm to inactivate SAMHD1-mediated restriction of reverse transcription. SAMHD1, a pyrophosphatase that limits availability of dNTPs for reverse transcription in resting cells and terminally differentiated cells such as MDMs [92,93], is degraded by Vpx/Vpr alleles derived from several primate lentiviral lineages, including SIVsm/SIVmac/HIV-2 in a CUL4A dependent manner [94,95], thus facilitating robust reverse transcription and infection of non-dividing cells including dendritic cells and macrophages [92,93,96]. As a consequence of SAMHD1 restriction, the kinetics of HIV-1 RT reaction as well as the amount of HIV-1 RT products are limited in MDMs, when infections are initiated in the absence of SAMHD1 antagonism [97]. Not surprisingly, HIV-1 RT products in MDMs and dendritic cells in the absence of co-infection with SIVmac Vpx containing VLPs fail to activate cGAS–STING-dependent innate immune sensing pathway [98,99,100,101,102].
Figure 1.
HIV PAMPs in macrophages. Multiple steps of the HIV-1 life cycle are detected by pathogen sensing mechanisms in macrophages. HIV-1 fusion and entry [88], over-exuberant expression of HIV-1 RT transcripts (upon SAMHD1 antagonism) [90,91] and de novo expression and Rev–CRM1-dependent nuclear export of HIV icRNA to the cytosol [98] can all lead to induction of ISG expression and IFN-I responses. Exposure to soluble viral proteins can activate tissue-resident macrophages, such as microglia (reviewed in [72]), resulting in secretion of pro-inflammatory responses.
It has long been appreciated that HIV-1 capsid remains associated with the viral cDNA and has been hypothesized to shield RT products from cytosolic nucleic acid sensing pathways (reviewed in [103,104]). In addition, host cells are equipped with cytosolic exonucleases, such as TREX1, that degrade excess amount of dsDNA in the cytosol to prevent constitutive activation of cytosolic DNA sensors [105]. Additional support for this hypothesis comes from recent findings that suggest disassembly of viral capsid occurs predominantly in the nucleus [106,107], thus further restricting cytosolic nucleic acid sensor(s) access to RT products and prevent host detection of the early steps of the viral life cycle. While cytosolic sequestration and shielding of viral nucleic acids by HIV-1 capsid is an attractive hypothesis, recent studies have described nuclear localization of cGAS and IFI16 [108,109,110], suggesting the existence of additional yet-to-be-defined viral mechanisms to disable nuclear-resident innate immune sensing mechanisms.
Other inducers of macrophage activation are viral proteins which are mostly characterized in microglia with regard to HIV-associated neurocognitive disorders. To date, HIV-1 proteins, Tat, gp120, Nef and Vpr have all been shown to activate microglia and affect their functions and neighboring neuronal health (reviewed in [72]). Although these findings are important, most of the studies have used overexpression of recombinant viral proteins or transgenic animals, and whether the concentration of these viral proteins used in these studies is physiologically relevant needs to be carefully considered. Johnson et al. detected Tat proteins by immunohistochemistry in infiltrating mononuclear cells, whereas HIV p24Gag is not detected in brain biopsy samples from HIV+ individuals on suppressive cART [111]. In addition, Tat proteins were detectable by ELISA in three out of eight CSF samples from HIV+ individuals with undetectable plasma and CSF viremia [111]. Interestingly, one donor expressed over 30 ng/mL of Tat in the CSF, levels high enough to induce IL-6 secretion from fetal microglia in vitro [112]. Since the efficacy of certain cART regimens may be reduced in peripheral tissues such as the brain, allowing for the possibility of residual low-level viral replication and transcription [113], the role of viral proteins on chronic immune activation observed in cART-suppressed patients requires further analysis.
5. HIV Infection of Macrophages as a Driver of Chronic Inflammation
Many groups have shown that HIV-1 infection of MDMs induces pro-inflammatory cytokine production [98,114,115,116] and ISG expression [98,117,118,119], although robust IFN-I production has not been detected. In contrast, other studies have failed to observe robust innate immune activation in HIV-1 infected MDMs [99,100]. These differential findings may partially stem from differences in experimental setup such as differentiation protocols. For instance, supplementation of MDM culture media with M-CSF induces expression of phosphorylated SAMHD1 that does not have anti-viral activity [120,121], while addition of GM-CSF induces expression of G1/S-specific cyclin D2 and dephosphorylation of SAMHD1, thus limiting HIV infection [122]. Use of bovine serum instead of human serum is also known to alter activation status of MDMs and expression level of cell cycle-associated proteins including MCM2 and cyclins A, E and D1/D3 in M-CSF-differentiated macrophages [123]. Alternatively, the time post infection at which MDM activation was analyzed might also contribute to differences in reported infection-associated MDM activation outcomes. Recently, detailed quantitative analysis has revealed that completion of HIV reverse transcription and integration in MDMs takes 2–3 days post initiation of infection [97]. Therefore, it is highly likely that early stages of HIV replication (prior to integration) in MDMs are not subject to sensing by host nucleic sensors, but establishment of productive viral infection and especially late steps in the viral life cycle in MDMs induce pro-inflammatory responses.
6. Sensing of HIV RNA in Macrophages
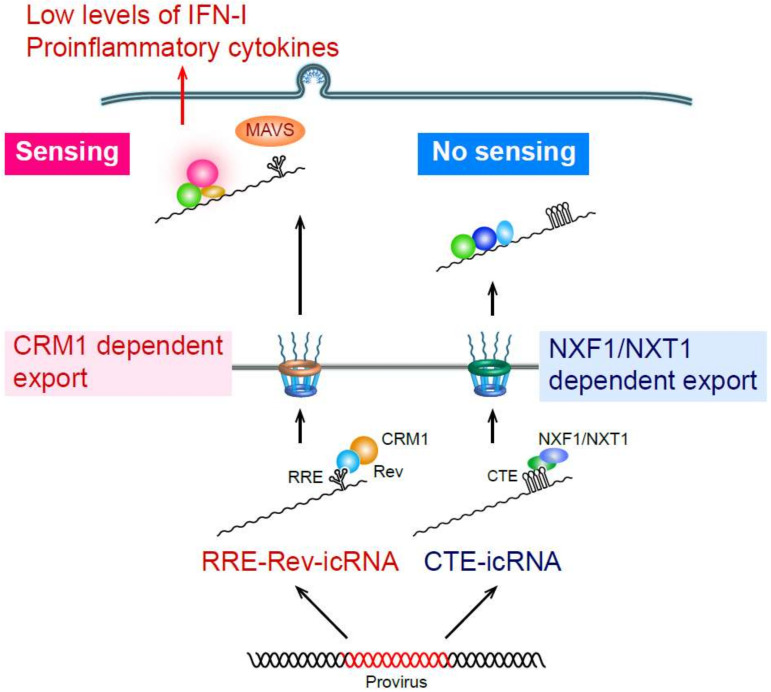
Cells are equipped with numerous nucleic acid sensors to detect cytosolic viral RNAs. Well-characterized sensors include retinoic acid-inducible gene I (RIG-I)-like receptors (RLR) family members, RIG-I and MDA5 (reviewed in [124,125]), which recognize non-self single- and double-stranded RNA with an uncapped triphosphate group at the 5′ end and elicit robust IFN-I responses by inducing MAVS activation. While transfection of purified HIV RNA can induce RIG-I-dependent IFN-I responses [126,127], whether RIG-I can sense de novo transcribed HIV RNA upon infection remains unclear. We and others demonstrated that post-transcriptional HIV replication steps trigger innate immune responses in macrophages [98,117]. In particular, we showed that HIV RNA, and specifically intron-containing RNA (icRNA), induces MAVS-dependent IFN-I responses in MDMs, resulting in pro-inflammatory cytokine production and ISG upregulation (Figure 2) [98]. HIV RNA is transcribed as a ~9-kB fragment and undergoes splicing events similar to cellular mRNA, leading to synthesis of early viral protein products, Tat, Rev and Nef. Upon accumulation of Rev, icRNA (unspliced RNA or singly-spliced RNA) can be exported into cytosol in a Rev-RRE-CRM1-dependent manner (reviewed in [128]). Recent studies by us and others suggest that inhibition of HIV icRNA nuclear export alone attenuates induction of pro-inflammatory responses in HIV-infected MDMs [98] and dendritic cells [129]. Interestingly our studies also suggest that route of HIV icRNA nuclear export, specifically CRM-1 dependent pathway, is important for triggering cytosolic nucleic acid sensing mechanisms, since CTE (constitutive transporting element from Mason–Pfizer monkey virus [130])-dependent alternative nuclear export pathway (NXF1/NXT1-dependent pathway) failed to induce pro-inflammatory responses in MDMs. Importantly, cytosolic expression of HIV icRNA induced innate immune responses via MAVS, but MAVS activation was independent of RIG-I or MDA5 [98]. Increasing evidence suggests a non-redundant role for host RNA surveillance and degradation mechanisms such as nonsense-mediated decay and RNA exosomes in controlling foreign RNA and aberrant host-derived RNA (reviewed in [131]). Future studies are needed to elucidate molecular mechanisms of how host RNA surveillance mechanisms distinguish foreign RNAs such as HIV-1 icRNA from host-derived RNAs and identify the cytosolic RNA sensor that detects HIV icRNA in myeloid cells.
Figure 2.
HIV icRNA-induced inflammation in macrophages. HIV icRNA exported from nucleus to cytosol by the Rev–CRM1-dependent pathway (RRE–Rev-icRNA) is sensed by a yet-to-be-identified RNA sensor, triggering ISG and IFN-I expression and pro-inflammatory cytokine production via MAVS. In contrast, cytosolic HIV icRNA exported by the NXF1/NXT1-dependent pathway (CTE-icRNA) does not result in ISG expression and IFN-I production.
The current cART regimen in clinical practice includes entry, RT, integrase and protease inhibitors. Hence, once a provirus is established, none of the current ART can inhibit HIV proviral transcription or cytosolic HIV RNA export from nucleus. In fact, in HIV+ individuals or SIV-infected NHPs on suppressive cART, viral gag RNAs (i.e., icRNA) are still detectable in various tissues [46,132,133,134,135,136]. Although the majority of integrated proviruses are defective, containing large internal deletions and/or hyper mutations [137,138], these defective proviruses remain transcriptionally active and can lead to expression of icRNA [139,140]. It is conceivable that HIV icRNA from intact or defective proviruses are transcribed even in the presence of the current ART drugs, leading to chronic immune activation. Since tissue-resident macrophages are long-lived [30,31,32,33,34] and resistant to the cytopathic effects of HIV [141], continuous cytosolic HIV icRNA expression in tissue-resident macrophages may perpetuate chronic inflammation even in cART-suppressed HIV patients.
7. Pathological Consequences of Macrophage Inflammation by HIV-1 Infection
Macrophage activation and secretion of proinflammatory cytokines and chemokines, such as IP-10, caused by HIV infection might have a significant impact on HIV pathogenesis. For instance, IP-10 is one of the most abundant chemokines produced from HIV-infected macrophages [98,115]. IP-10 levels are highly associated with HIV disease progression, and IP-10 is known to suppress immune cell functions and facilitate HIV replication and dissemination (reviewed in [142]). IP-10 has also been shown to induce HIV latency in resting CD4+ T cells by altering actin structures [143], implying the role for activated macrophages in promoting HIV latency in secondary lymph nodes. Exposure of latently infected CD4+ T cells to immune activation stimuli can also result in spontaneous reactivation of viral transcription, which may lead to localized replication of HIV in tissues and contributing to blips of viremia. IFN-I is involved in activation of CD4+ T cell-derived HIV transcription [144], suggesting HIV-infected MDMs may contribute to viral transcription in latently infected CD4+ T cells in vitro. Moreover, we showed that HIV icRNA-expressing MDMs promote an immune exhaustion phenotype in co-cultured CD4+ and CD8+ T cells in an IFN-I-dependent manner [98]. In chronic HIV-1 infections, persistent immune activation can result in T cell exhaustion (reviewed in [145]). In particular, chronic exposure to IFN-I has been indicated as the driver of T cell activation [146,147], resulting in loss of immunological control of HIV-infected cells, thus contributing to HIV reactivation and persistence. Whether HIV icRNA expression in infected myeloid cells in vivo such as gut-resident macrophages in cART-suppressed animals or patients induce T cell exhaustion and promote virus persistence remains to be determined. It is also plausible that HIV-infection induced cytokines skew tissue-resident macrophages towards certain phenotypes [148]. Macrophages, while historically have been classified as either “pro-inflammatory” or “anti-inflammatory” cell subsets based on cytokines present in the culture conditions in vitro, are now known to be not mutually exclusive in function and are thought to exhibit diverse functional phenotypes [149]. Future studies are needed to define the diversity of functional phenotypes exhibited by HIV-infected tissue-resident macrophages and their contribution to tissue pathology.
The role of microglia activation caused by HIV infection in neuronal toxicity has been intensively studied, as described above. Microglia activation by HIV-1 infection induces pro-inflammatory cytokines which may directly affect neuronal viability, and viral proteins released from infected microglia may cause microglial activation, dysfunction and neuronal death (reviewed in [70,71,72]). Another important consequence of microglia activation by HIV infection is that it leads to dysfunction of microglia including reduced phagocytic activity [72]. Clearance of neurotoxins such as Tau proteins or fibrillar amyloid beta is an important homeostatic function of microglia, and persistently activated microglia have reduced capacity for phagocytosis of these potential neurotoxins [150]. It is plausible that HIV induced microglia activation leads to poor clearance of debris/neurotoxins that affects neuronal health. Elevated levels of activated microglia-derived neurotoxic metabolites, such as glutamate, arachidonic acid and quinolinic acid, have been reported in the CSF of HIV-1 infected individuals with HAND [151,152]. In addition, HIV+ individuals have a high risk of CVD including atherosclerosis. Markers of macrophage activation sCD14 and sCD163 are associated with progression of carotid plaque development [153]. Thus, it is likely that macrophages activated directly by infection or indirectly by cytokines increase risks of cardiovascular diseases in HIV+ individuals. Further studies on primary tissue-resident macrophages are warranted to investigate the role of macrophage infection and inflammatory responses on HIV-associated comorbidities.
8. Concluding Remarks
Resting CD4+ T cells are thought to be the major HIV reservoir in cART-suppressed patients. Recent findings have demonstrated that sequences of HIV-1 recovered from latently-infected resting CD4+ T cells in blood by in vitro stimulation are frequently different from those of rebound HIV-1 upon interruption of cART or latency reversing agent treatment [154,155,156]. These findings suggest the importance of studying HIV reservoirs in tissues. As discussed above, accumulating evidence suggests that tissue-resident macrophages are persistently infected, and thus it is highly plausible that these tissue macrophages also contribute to long-term viral reservoirs. In addition, pro-inflammatory cytokines produced from HIV infected tissue-resident macrophages may contribute to HIV pathogenesis and/or HANA conditions including HAND. Although our knowledge on the role of tissue-resident macrophages has been increasing, there remain numerous open questions. What are the molecular mechanisms that allow HIV persistence in tissue-resident myeloid cells? Do persistently infected macrophages contribute to systemic HIV dissemination? What strategies are needed to reduce viral reservoirs in tissue-resident macrophage reservoirs in addition to T cells? Does HIV infection in tissue-resident macrophages induce pro-inflammatory responses? What are the molecular mechanisms that contribute to myeloid cell-activation induced immunopathology? To date, most of the studies have utilized MDMs as a model of tissue-resident macrophages due to the limited access to these cell types from tissues. Although iPSC-derived microglia have been the focus of numerous recent studies as an in vitro model of CNS-resident macrophages [73,74,75,76,77,78], development of other tissue-resident macrophages from iPSC-lines, such as alveolar macrophages, Langerhans cells and Kupffer cells, has also been attempted (reviewed in [157]). Recent advances in generating tissue environment niches using iPSC-derived organoids [158,159,160,161] might provide a platform to terminally differentiate iPSC-derived macrophage progenitors to tissue-resident macrophages specialized in unique tissue environments [157]. Use of these stem cell-derived, self-organizing three-dimensional cell culture model systems might provide unique in vitro tools for performing rigorous studies on mechanisms of HIV persistence and chronic immune activation in tissue-resident macrophages, and provide unique insights into the role of macrophages in HIV-1 pathogenesis.
Author Contributions
H.A. and S.G. wrote the manuscript. All authors have read and agree to the published version of the manuscript.
Funding
This work was supported by NIH grants R01AI064099 (S.G.), R01HD083111 (S.G.), R01AG060890 (S.G.), R21NS105837 (S.G.) and P30AI042853 (S.G. and H.A.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruize Z., Kootstra N.A. The Role of Macrophages in HIV-1 Persistence and Pathogenesis. Front. Microbiol. 2019;10:2828. doi: 10.3389/fmicb.2019.02828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Embretson J., Zupancic M., Ribas J.L., Burke A., Racz P., Tenner-Racz K., Haase A.T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 4.Orenstein J.M., Fox C., Wahl S.M. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 5.Koenig S., Gendelman H.E., Orenstein J.M., Dal Canto M.C., Pezeshkpour G.H., Yungbluth M., Janotta F., Aksamit A., Martin M.A., Fauci A.S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 6.Micci L., Alvarez X., Iriele R.I., Ortiz A.M., Ryan E.S., McGary C.S., Deleage C., McAtee B.B., He T., Apetrei C., et al. CD4 depletion in SIV-infected macaques results in macrophage and microglia infection with rapid turnover of infected cells. PLoS Pathog. 2014;10:e1004467. doi: 10.1371/journal.ppat.1004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igarashi T., Brown C.R., Endo Y., Buckler-White A., Plishka R., Bischofberger N., Hirsch V., Martin M.A. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): Implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA. 2001;98:658–663. doi: 10.1073/pnas.98.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Y., Sugimoto C., Liu D.X., Midkiff C.C., Alvarez X., Lackner A.A., Kim W.K., Didier E.S., Kuroda M.J. Increased monocyte turnover is associated with interstitial macrophage accumulation and pulmonary tissue damage in SIV-infected rhesus macaques. J. Leukoc. Biol. 2015;97:1147–1153. doi: 10.1189/jlb.4A0914-441R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladinsky M.S., Khamaikawin W., Jung Y., Lin S., Lam J., An D.S., Bjorkman P.J., Kieffer C. Mechanisms of virus dissemination in bone marrow of HIV-1-infected humanized BLT mice. eLife. 2019;8:e46916. doi: 10.7554/eLife.46916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salazar-Gonzalez J.F., Salazar M.G., Keele B.F., Learn G.H., Giorgi E.E., Li H., Decker J.M., Wang S., Baalwa J., Kraus M.H., et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 2009;206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph S.B., Arrildt K.T., Swanstrom A.E., Schnell G., Lee B., Hoxie J.A., Swanstrom R. Quantification of entry phenotypes of macrophage-tropic HIV-1 across a wide range of CD4 densities. J. Virol. 2014;88:1858–1869. doi: 10.1128/JVI.02477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaacman-Beck J., Hermann E.A., Yi Y., Ratcliffe S.J., Mulenga J., Allen S., Hunter E., Derdeyn C.A., Collman R.G. Heterosexual transmission of human immunodeficiency virus type 1 subtype C: Macrophage tropism, alternative coreceptor use, and the molecular anatomy of CCR5 utilization. J. Virol. 2009;83:8208–8220. doi: 10.1128/JVI.00296-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochsenbauer C., Edmonds T.G., Ding H., Keele B.F., Decker J., Salazar M.G., Salazar-Gonzalez J.F., Shattock R., Haynes B.F., Shaw G.M., et al. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J. Virol. 2012;86:2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calantone N., Wu F., Klase Z., Deleage C., Perkins M., Matsuda K., Thompson E.A., Ortiz A.M., Vinton C.L., Ourmanov I., et al. Tissue myeloid cells in SIV-infected primates acquire viral DNA through phagocytosis of infected T cells. Immunity. 2014;41:493–502. doi: 10.1016/j.immuni.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baxter A.E., Russell R.A., Duncan C.J., Moore M.D., Willberg C.B., Pablos J.L., Finzi A., Kaufmann D.E., Ochsenbauer C., Kappes J.C., et al. Macrophage infection via selective capture of HIV-1-infected CD4+ T cells. Cell Host Microbe. 2014;16:711–721. doi: 10.1016/j.chom.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bracq L., Xie M., Lambele M., Vu L.T., Matz J., Schmitt A., Delon J., Zhou P., Randriamampita C., Bouchet J., et al. T Cell-Macrophage Fusion Triggers Multinucleated Giant Cell Formation for HIV-1 Spreading. J. Virol. 2017;91 doi: 10.1128/JVI.01237-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sattentau Q.J., Stevenson M. Macrophages and HIV-1: An Unhealthy Constellation. Cell Host Microbe. 2016;19:304–310. doi: 10.1016/j.chom.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupont M., Sattentau Q.J. Macrophage Cell-Cell Interactions Promoting HIV-1 Infection. Viruses. 2020;12:492. doi: 10.3390/v12050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen R., Richter H.E., Clements R.H., Novak L., Huff K., Bimczok D., Sankaran-Walters S., Dandekar S., Clapham P.R., Smythies L.E., et al. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J. Virol. 2009;83:3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jambo K.C., Banda D.H., Kankwatira A.M., Sukumar N., Allain T.J., Heyderman R.S., Russell D.G., Mwandumba H.C. Small alveolar macrophages are infected preferentially by HIV and exhibit impaired phagocytic function. Mucosal Immunol. 2014;7:1116–1126. doi: 10.1038/mi.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cenker J.J., Stultz R.D., McDonald D. Brain Microglial Cells Are Highly Susceptible to HIV-1 Infection and Spread. AIDS Res. Hum. Retrovir. 2017;33:1155–1165. doi: 10.1089/aid.2017.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avalos C.R., Price S.L., Forsyth E.R., Pin J.N., Shirk E.N., Bullock B.T., Queen S.E., Li M., Gellerup D., O’Connor S.L., et al. Quantitation of Productively Infected Monocytes and Macrophages of Simian Immunodeficiency Virus-Infected Macaques. J. Virol. 2016;90:5643–5656. doi: 10.1128/JVI.00290-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abreu C.M., Veenhuis R.T., Avalos C.R., Graham S., Parrilla D.R., Ferreira E.A., Queen S.E., Shirk E.N., Bullock B.T., Li M., et al. Myeloid and CD4 T Cells Comprise the Latent Reservoir in Antiretroviral Therapy-Suppressed SIVmac251-Infected Macaques. mBio. 2019;10:e01659-19. doi: 10.1128/mBio.01659-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph S.B., Kincer L.P., Bowman N.M., Evans C., Vinikoor M.J., Lippincott C.K., Gisslen M., Spudich S., Menezes P., Robertson K., et al. Human Immunodeficiency Virus Type 1 RNA Detected in the Central Nervous System (CNS) After Years of Suppressive Antiretroviral Therapy Can Originate from a Replicating CNS Reservoir or Clonally Expanded Cells. Clin. Infect. Dis. 2019;69:1345–1352. doi: 10.1093/cid/ciy1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honeycutt J.B., Thayer W.O., Baker C.E., Ribeiro R.M., Lada S.M., Cao Y., Cleary R.A., Hudgens M.G., Richman D.D., Garcia J.V. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat. Med. 2017;23:638–643. doi: 10.1038/nm.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witwer K.W., Gama L., Li M., Bartizal C.M., Queen S.E., Varrone J.J., Brice A.K., Graham D.R., Tarwater P.M., Mankowski J.L., et al. Coordinated regulation of SIV replication and immune responses in the CNS. PLoS ONE. 2009;4:e8129. doi: 10.1371/journal.pone.0008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis L.E., Hjelle B.L., Miller V.E., Palmer D.L., Llewellyn A.L., Merlin T.L., Young S.A., Mills R.G., Wachsman W., Wiley C.A. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/WNL.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 28.Wong M.E., Jaworowski A., Hearps A.C. The HIV Reservoir in Monocytes and Macrophages. Front. Immunol. 2019;10:1435. doi: 10.3389/fimmu.2019.01435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGovern N., Schlitzer A., Gunawan M., Jardine L., Shin A., Poyner E., Green K., Dickinson R., Wang X.N., Low D., et al. Human dermal CD14(+) cells are a transient population of monocyte-derived macrophages. Immunity. 2014;41:465–477. doi: 10.1016/j.immuni.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakata K., Gotoh H., Watanabe J., Uetake T., Komuro I., Yuasa K., Watanabe S., Ieki R., Sakamaki H., Akiyama H., et al. Augmented proliferation of human alveolar macrophages after allogeneic bone marrow transplantation. Blood. 1999;93:667–673. doi: 10.1182/blood.V93.2.667. [DOI] [PubMed] [Google Scholar]
- 31.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R., et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ajami B., Bennett J.L., Krieger C., Tetzlaff W., Rossi F.M. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto D., Chow A., Noizat C., Teo P., Beasley M.B., Leboeuf M., Becker C.D., See P., Price J., Lucas D., et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Utz S.G., See P., Mildenberger W., Thion M.S., Silvin A., Lutz M., Ingelfinger F., Rayan N.A., Lelios I., Buttgereit A., et al. Early Fate Defines Microglia and Non-parenchymal Brain Macrophage Development. Cell. 2020;181:557–573. doi: 10.1016/j.cell.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Bian Z., Gong Y., Huang T., Lee C.Z.W., Bian L., Bai Z., Shi H., Zeng Y., Liu C., He J., et al. Deciphering human macrophage development at single-cell resolution. Nature. 2020;582:571–576. doi: 10.1038/s41586-020-2316-7. [DOI] [PubMed] [Google Scholar]
- 36.Reu P., Khosravi A., Bernard S., Mold J.E., Salehpour M., Alkass K., Perl S., Tisdale J., Possnert G., Druid H., et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017;20:779–784. doi: 10.1016/j.celrep.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avalos C.R., Abreu C.M., Queen S.E., Li M., Price S., Shirk E.N., Engle E.L., Forsyth E., Bullock B.T., Mac Gabhann F., et al. Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: A Functional Latent Reservoir. mBio. 2017;8:e01186-17. doi: 10.1128/mBio.01186-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar A., Abbas W., Herbein G. HIV-1 latency in monocytes/macrophages. Viruses. 2014;6:1837–1860. doi: 10.3390/v6041837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caselli E., Galvan M., Cassai E., Caruso A., Sighinolfi L., Di Luca D. Human herpesvirus 8 enhances human immunodeficiency virus replication in acutely infected cells and induces reactivation in latently infected cells. Blood. 2005;106:2790–2797. doi: 10.1182/blood-2005-04-1390. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez-Carbonell D., Ye F., Ramanath N., Garcia-Mesa Y., Knapp P.E., Hauser K.F., Karn J. Cross-talk between microglia and neurons regulates HIV latency. PLoS Pathog. 2019;15:e1008249. doi: 10.1371/journal.ppat.1008249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosoian A., Zhang L., Hong F., Cunyat F., Rahman A., Bhalla R., Panchal A., Saiman Y., Fiel M.I., Florman S., et al. Frontline Science: HIV infection of Kupffer cells results in an amplified proinflammatory response to LPS. J. Leukoc. Biol. 2017;101:1083–1090. doi: 10.1189/jlb.3HI0516-242R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kandathil A.J., Sugawara S., Goyal A., Durand C.M., Quinn J., Sachithanandham J., Cameron A.M., Bailey J.R., Perelson A.S., Balagopal A. No recovery of replication-competent HIV-1 from human liver macrophages. J. Clin. Investig. 2018;128:4501–4509. doi: 10.1172/JCI121678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cribbs S.K., Lennox J., Caliendo A.M., Brown L.A., Guidot D.M. Healthy HIV-1-infected individuals on highly active antiretroviral therapy harbor HIV-1 in their alveolar macrophages. AIDS Res. Hum. Retrovir. 2015;31:64–70. doi: 10.1089/aid.2014.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abreu C.M., Veenhuis R.T., Avalos C.R., Graham S., Queen S.E., Shirk E.N., Bullock B.T., Li M., Metcalf Pate K.A., Beck S.E., et al. Infectious Virus Persists in CD4(+) T Cells and Macrophages in Antiretroviral Therapy-Suppressed Simian Immunodeficiency Virus-Infected Macaques. J. Virol. 2019;93:e00065-19. doi: 10.1128/JVI.00065-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matusali G., Dereuddre-Bosquet N., Le Tortorec A., Moreau M., Satie A.P., Mahe D., Roumaud P., Bourry O., Sylla N., Bernard-Stoecklin S., et al. Detection of Simian Immunodeficiency Virus in Semen, Urethra, and Male Reproductive Organs during Efficient Highly Active Antiretroviral Therapy. J. Virol. 2015;89:5772–5787. doi: 10.1128/JVI.03628-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ganor Y., Real F., Sennepin A., Dutertre C.A., Prevedel L., Xu L., Tudor D., Charmeteau B., Couedel-Courteille A., Marion S., et al. HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nat. Microbiol. 2019;4:633–644. doi: 10.1038/s41564-018-0335-z. [DOI] [PubMed] [Google Scholar]
- 47.Duprez D.A., Neuhaus J., Kuller L.H., Tracy R., Belloso W., De Wit S., Drummond F., Lane H.C., Ledergerber B., Lundgren J., et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS ONE. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ford E.S., Greenwald J.H., Richterman A.G., Rupert A., Dutcher L., Badralmaa Y., Natarajan V., Rehm C., Hadigan C., Sereti I. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. Aids. 2010;24:1509–1517. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erlandson K.M., Allshouse A.A., Jankowski C.M., Lee E.J., Rufner K.M., Palmer B.E., Wilson C.C., MaWhinney S., Kohrt W.M., Campbell T.B. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J. Infect. Dis. 2013;208:249–259. doi: 10.1093/infdis/jit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borges A.H., Silverberg M.J., Wentworth D., Grulich A.E., Fatkenheuer G., Mitsuyasu R., Tambussi G., Sabin C.A., Neaton J.D., Lundgren J.D., et al. Predicting risk of cancer during HIV infection: The role of inflammatory and coagulation biomarkers. Aids. 2013;27:1433–1441. doi: 10.1097/QAD.0b013e32835f6b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bjerk S.M., Baker J.V., Emery S., Neuhaus J., Angus B., Gordin F.M., Pett S.L., Stephan C., Kunisaki K.M., Group I.S.S. Biomarkers and bacterial pneumonia risk in patients with treated HIV infection: A case-control study. PLoS ONE. 2013;8:e56249. doi: 10.1371/journal.pone.0056249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shikuma C.M., Barbour J.D., Ndhlovu L.C., Keating S.M., Norris P.J., Budoff M., Parikh N., Seto T., Gangcuangco L.M., Ogata-Arakaki D., et al. Plasma monocyte chemoattractant protein-1 and tumor necrosis factor-alpha levels predict the presence of coronary artery calcium in HIV-infected individuals independent of traditional cardiovascular risk factors. AIDS Res. Hum. Retrovir. 2014;30:142–146. doi: 10.1089/aid.2013.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koethe J.R., Grome H., Jenkins C.A., Kalams S.A., Sterling T.R. The metabolic and cardiovascular consequences of obesity in persons with HIV on long-term antiretroviral therapy. Aids. 2016;30:83–91. doi: 10.1097/QAD.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chow D.C., Kagihara J.M., Zhang G., Souza S.A., Hodis H.N., Li Y., Mitchell B.I., Nakamoto B.K., Kallianpur K.J., Keating S.M., et al. Non-classical monocytes predict progression of carotid artery bifurcation intima-media thickness in HIV-infected individuals on stable antiretroviral therapy. HIV Clin. Trials. 2016;17:114–122. doi: 10.1080/15284336.2016.1162386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Looby S.E., Fitch K.V., Srinivasa S., Lo J., Rafferty D., Martin A., Currier J.C., Grinspoon S., Zanni M.V. Reduced ovarian reserve relates to monocyte activation and subclinical coronary atherosclerotic plaque in women with HIV. Aids. 2016;30:383–393. doi: 10.1097/QAD.0000000000000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McKibben R.A., Margolick J.B., Grinspoon S., Li X., Palella F.J., Jr., Kingsley L.A., Witt M.D., George R.T., Jacobson L.P., Budoff M., et al. Elevated Levels of Monocyte Activation Markers Are Associated With Subclinical Atherosclerosis in Men With and Those Without HIV Infection. J. Infect. Dis. 2014;211:1219–1228. doi: 10.1093/infdis/jiu594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Halloran J.A., Dunne E., Gurwith M., Lambert J.S., Sheehan G.J., Feeney E.R., Pozniak A., Reiss P., Kenny D., Mallon P. The effect of initiation of antiretroviral therapy on monocyte, endothelial and platelet function in HIV-1 infection. HIV Med. 2015;16:608–619. doi: 10.1111/hiv.12270. [DOI] [PubMed] [Google Scholar]
- 58.Burdo T.H., Lo J., Abbara S., Wei J., DeLelys M.E., Preffer F., Rosenberg E.S., Williams K.C., Grinspoon S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J. Infect. Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelesidis T., Kendall M.A., Yang O.O., Hodis H.N., Currier J.S. Biomarkers of microbial translocation and macrophage activation: Association with progression of subclinical atherosclerosis in HIV-1 infection. J. Infect. Dis. 2012;206:1558–1567. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandler N.G., Wand H., Roque A., Law M., Nason M.C., Nixon D.E., Pedersen C., Ruxrungtham K., Lewin S.R., Emery S., et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saylor D., Dickens A.M., Sacktor N., Haughey N., Slusher B., Pletnikov M., Mankowski J.L., Brown A., Volsky D.J., McArthur J.C. HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016;12:234–248. doi: 10.1038/nrneurol.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klatt N.R., Chomont N., Douek D.C., Deeks S.G. Immune activation and HIV persistence: Implications for curative approaches to HIV infection. Immunol. Rev. 2013;254:326–342. doi: 10.1111/imr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phillips A.N., Neaton J., Lundgren J.D. The role of HIV in serious diseases other than AIDS. Aids. 2008;22:2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez-Scarano F., Martin-Garcia J. The neuropathogenesis of AIDS. Nat. Rev. Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 65.Spudich S., Gonzalez-Scarano F. HIV-1-related central nervous system disease: Current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harb Perspect. Med. 2012;2:a007120. doi: 10.1101/cshperspect.a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Canestri A., Lescure F.X., Jaureguiberry S., Moulignier A., Amiel C., Marcelin A.G., Peytavin G., Tubiana R., Pialoux G., Katlama C. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin. Infect. Dis. 2010;50:773–778. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 67.Dahl V., Peterson J., Fuchs D., Gisslen M., Palmer S., Price R.W. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. Aids. 2014;28:2251–2258. doi: 10.1097/QAD.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garvey L.J., Everitt A., Winston A., Mackie N.E., Benzie A. Detectable cerebrospinal fluid HIV RNA with associated neurological deficits, despite suppression of HIV replication in the plasma compartment. Aids. 2009;23:1443–1444. doi: 10.1097/QAD.0b013e32832d077c. [DOI] [PubMed] [Google Scholar]
- 69.Peluso M.J., Ferretti F., Peterson J., Lee E., Fuchs D., Boschini A., Gisslen M., Angoff N., Price R.W., Cinque P., et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. Aids. 2012;26:1765–1774. doi: 10.1097/QAD.0b013e328355e6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown A. Understanding the MIND phenotype: Macrophage/microglia inflammation in neurocognitive disorders related to human immunodeficiency virus infection. Clin. Transl. Med. 2015;4:7. doi: 10.1186/s40169-015-0049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rappaport J., Volsky D.J. Role of the macrophage in HIV-associated neurocognitive disorders and other comorbidities in patients on effective antiretroviral treatment. J. Neurovirol. 2015;21:235–241. doi: 10.1007/s13365-015-0346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen N.C., Partridge A.T., Sell C., Torres C., Martin-Garcia J. Fate of microglia during HIV-1 infection: From activation to senescence? Glia. 2017;65:431–446. doi: 10.1002/glia.23081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haenseler W., Sansom S.N., Buchrieser J., Newey S.E., Moore C.S., Nicholls F.J., Chintawar S., Schnell C., Antel J.P., Allen N.D., et al. A Highly Efficient Human Pluripotent Stem Cell Microglia Model Displays a Neuronal-Co-culture-Specific Expression Profile and Inflammatory Response. Stem Cell Rep. 2017;8:1727–1742. doi: 10.1016/j.stemcr.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Douvaras P., Sun B., Wang M., Kruglikov I., Lallos G., Zimmer M., Terrenoire C., Zhang B., Gandy S., Schadt E., et al. Directed Differentiation of Human Pluripotent Stem Cells to Microglia. Stem Cell Rep. 2017;8:1516–1524. doi: 10.1016/j.stemcr.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pandya H., Shen M.J., Ichikawa D.M., Sedlock A.B., Choi Y., Johnson K.R., Kim G., Brown M.A., Elkahloun A.G., Maric D., et al. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat. Neurosci. 2017;20:753–759. doi: 10.1038/nn.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takata K., Kozaki T., Lee C.Z.W., Thion M.S., Otsuka M., Lim S., Utami K.H., Fidan K., Park D.S., Malleret B., et al. Induced-Pluripotent-Stem-Cell-Derived Primitive Macrophages Provide a Platform for Modeling Tissue-Resident Macrophage Differentiation and Function. Immunity. 2017;47:183–198.e6. doi: 10.1016/j.immuni.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 77.Muffat J., Li Y., Yuan B., Mitalipova M., Omer A., Corcoran S., Bakiasi G., Tsai L.H., Aubourg P., Ransohoff R.M., et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 2016;22:1358–1367. doi: 10.1038/nm.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abud E.M., Ramirez R.N., Martinez E.S., Healy L.M., Nguyen C.H.H., Newman S.A., Yeromin A.V., Scarfone V.M., Marsh S.E., Fimbres C., et al. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron. 2017;94:278–293.e9. doi: 10.1016/j.neuron.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin Y.T., Seo J., Gao F., Feldman H.M., Wen H.L., Penney J., Cam H.P., Gjoneska E., Raja W.K., Cheng J., et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron. 2018;98:1141–1154.e7. doi: 10.1016/j.neuron.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hockemeyer D., Jaenisch R. Induced Pluripotent Stem Cells Meet Genome Editing. Cell Stem Cell. 2016;18:573–586. doi: 10.1016/j.stem.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krasemann S., Madore C., Cialic R., Baufeld C., Calcagno N., El Fatimy R., Beckers L., O’Loughlin E., Xu Y., Fanek Z., et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity. 2017;47:566–581.e9. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burdo T.H., Soulas C., Orzechowski K., Button J., Krishnan A., Sugimoto C., Alvarez X., Kuroda M.J., Williams K.C. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pulliam L., Gascon R., Stubblebine M., McGuire D., McGrath M.S. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 84.Deeks S.G., Tracy R., Douek D.C. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iwasaki A. Innate immune recognition of HIV-1. Immunity. 2012;37:389–398. doi: 10.1016/j.immuni.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 87.Brown J.N., Kohler J.J., Coberley C.R., Sleasman J.W., Goodenow M.M. HIV-1 activates macrophages independent of Toll-like receptors. PLoS ONE. 2008;3:e3664. doi: 10.1371/journal.pone.0003664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Decalf J., Desdouits M., Rodrigues V., Gobert F.X., Gentili M., Marques-Ladeira S., Chamontin C., Mougel M., Cunha de Alencar B., Benaroch P. Sensing of HIV-1 Entry Triggers a Type I Interferon Response in Human Primary Macrophages. J. Virol. 2017;91 doi: 10.1128/JVI.00147-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jakobsen M.R., Olagnier D., Hiscott J. Innate immune sensing of HIV-1 infection. Curr. Opin. HIV AIDS. 2015;10:96–102. doi: 10.1097/COH.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 90.Gao D., Wu J., Wu Y.T., Du F., Aroh C., Yan N., Sun L., Chen Z.J. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jonsson K.L., Laustsen A., Krapp C., Skipper K.A., Thavachelvam K., Hotter D., Egedal J.H., Kjolby M., Mohammadi P., Prabakaran T., et al. IFI16 is required for DNA sensing in human macrophages by promoting production and function of cGAMP. Nat. Commun. 2017;8:14391. doi: 10.1038/ncomms14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hrecka K., Hao C., Gierszewska M., Swanson S.K., Kesik-Brodacka M., Srivastava S., Florens L., Washburn M.P., Skowronski J. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Laguette N., Sobhian B., Casartelli N., Ringeard M., Chable-Bessia C., Segeral E., Yatim A., Emiliani S., Schwartz O., Benkirane M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bergamaschi A., Ayinde D., David A., Le Rouzic E., Morel M., Collin G., Descamps D., Damond F., Brun-Vezinet F., Nisole S., et al. The human immunodeficiency virus type 2 Vpx protein usurps the CUL4A-DDB1 DCAF1 ubiquitin ligase to overcome a postentry block in macrophage infection. J. Virol. 2009;83:4854–4860. doi: 10.1128/JVI.00187-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahn J., Hao C., Yan J., DeLucia M., Mehrens J., Wang C., Gronenborn A.M., Skowronski J. HIV/simian immunodeficiency virus (SIV) accessory virulence factor Vpx loads the host cell restriction factor SAMHD1 onto the E3 ubiquitin ligase complex CRL4DCAF1. J. Biol. Chem. 2012;287:12550–12558. doi: 10.1074/jbc.M112.340711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goujon C., Jarrosson-Wuilleme L., Bernaud J., Rigal D., Darlix J.L., Cimarelli A. With a little help from a friend: Increasing HIV transduction of monocyte-derived dendritic cells with virion-like particles of SIV(MAC) Gene Ther. 2006;13:991–994. doi: 10.1038/sj.gt.3302753. [DOI] [PubMed] [Google Scholar]
- 97.Bejarano D.A., Puertas M.C., Borner K., Martinez-Picado J., Muller B., Krausslich H.G. Detailed Characterization of Early HIV-1 Replication Dynamics in Primary Human Macrophages. Viruses. 2018;10:620. doi: 10.3390/v10110620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Akiyama H., Miller C.M., Ettinger C.R., Belkina A.C., Snyder-Cappione J.E., Gummuluru S. HIV-1 intron-containing RNA expression induces innate immune activation and T cell dysfunction. Nat. Commun. 2018;9:3450. doi: 10.1038/s41467-018-05899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsang J., Chain B.M., Miller R.F., Webb B.L., Barclay W., Towers G.J., Katz D.R., Noursadeghi M. HIV-1 infection of macrophages is dependent on evasion of innate immune cellular activation. Aids. 2009;23:2255–2263. doi: 10.1097/QAD.0b013e328331a4ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rasaiyaah J., Tan C.P., Fletcher A.J., Price A.J., Blondeau C., Hilditch L., Jacques D.A., Selwood D.L., James L.C., Noursadeghi M., et al. HIV-1 evades innate immune recognition through specific cofactor recruitment. Nature. 2013;503:402–405. doi: 10.1038/nature12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Granelli-Piperno A., Golebiowska A., Trumpfheller C., Siegal F.P., Steinman R.M. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc. Natl. Acad. Sci. USA. 2004;101:7669–7674. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gringhuis S.I., Hertoghs N., Kaptein T.M., Zijlstra-Willems E.M., Sarrami-Forooshani R., Sprokholt J.K., van Teijlingen N.H., Kootstra N.A., Booiman T., van Dort K.A., et al. HIV-1 blocks the signaling adaptor MAVS to evade antiviral host defense after sensing of abortive HIV-1 RNA by the host helicase DDX3. Nat. Immunol. 2017;18:225–235. doi: 10.1038/ni.3647. [DOI] [PubMed] [Google Scholar]
- 103.Campbell E.M., Hope T.J. HIV-1 capsid: The multifaceted key player in HIV-1 infection. Nat. Rev. Microbiol. 2015;13:471–483. doi: 10.1038/nrmicro3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamashita M., Engelman A.N. Capsid-Dependent Host Factors in HIV-1 Infection. Trends Microbiol. 2017;25:741–755. doi: 10.1016/j.tim.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yan N., Regalado-Magdos A.D., Stiggelbout B., Lee-Kirsch M.A., Lieberman J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat. Immunol. 2010;11:1005–1013. doi: 10.1038/ni.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burdick R.C., Li C., Munshi M., Rawson J.M.O., Nagashima K., Hu W.S., Pathak V.K. HIV-1 uncoats in the nucleus near sites of integration. Proc. Natl. Acad. Sci. USA. 2020;117:5486–5493. doi: 10.1073/pnas.1920631117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dharan A., Bachmann N., Talley S., Zwikelmaier V., Campbell E.M. Nuclear pore blockade reveals that HIV-1 completes reverse transcription and uncoating in the nucleus. Nat. Microbiol. 2020 doi: 10.1038/s41564-020-0735-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Orzalli M.H., Broekema N.M., Diner B.A., Hancks D.C., Elde N.C., Cristea I.M., Knipe D.M. cGAS-mediated stabilization of IFI16 promotes innate signaling during herpes simplex virus infection. Proc. Natl. Acad. Sci. USA. 2015;112:E1773–E1781. doi: 10.1073/pnas.1424637112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li T., Diner B.A., Chen J., Cristea I.M. Acetylation modulates cellular distribution and DNA sensing ability of interferon-inducible protein IFI16. Proc. Natl. Acad. Sci. USA. 2012;109:10558–10563. doi: 10.1073/pnas.1203447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gentili M., Lahaye X., Nadalin F., Nader G.P.F., Puig Lombardi E., Herve S., De Silva N.S., Rookhuizen D.C., Zueva E., Goudot C., et al. The N-Terminal Domain of cGAS Determines Preferential Association with Centromeric DNA and Innate Immune Activation in the Nucleus. Cell Rep. 2019;26:2377–2393.e13. doi: 10.1016/j.celrep.2019.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johnson T.P., Patel K., Johnson K.R., Maric D., Calabresi P.A., Hasbun R., Nath A. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc. Natl. Acad. Sci. USA. 2013;110:13588–13593. doi: 10.1073/pnas.1308673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sheng W.S., Hu S., Hegg C.C., Thayer S.A., Peterson P.K. Activation of human microglial cells by HIV-1 gp41 and Tat proteins. Clin. Immunol. 2000;96:243–251. doi: 10.1006/clim.2000.4905. [DOI] [PubMed] [Google Scholar]
- 113.Asahchop E.L., Meziane O., Mamik M.K., Chan W.F., Branton W.G., Resch L., Gill M.J., Haddad E., Guimond J.V., Wainberg M.A., et al. Reduced antiretroviral drug efficacy and concentration in HIV-infected microglia contributes to viral persistence in brain. Retrovirology. 2017;14:47. doi: 10.1186/s12977-017-0370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Poluektova L., Moran T., Zelivyanskaya M., Swindells S., Gendelman H.E., Persidsky Y. The regulation of alpha chemokines during HIV-1 infection and leukocyte activation: Relevance for HIV-1-associated dementia. J. Neuroimmunol. 2001;120:112–128. doi: 10.1016/S0165-5728(01)00413-1. [DOI] [PubMed] [Google Scholar]
- 115.Foley J.F., Yu C.R., Solow R., Yacobucci M., Peden K.W., Farber J.M. Roles for CXC chemokine ligands 10 and 11 in recruiting CD4+ T cells to HIV-1-infected monocyte-derived macrophages, dendritic cells, and lymph nodes. J. Immunol. 2005;174:4892–4900. doi: 10.4049/jimmunol.174.8.4892. [DOI] [PubMed] [Google Scholar]
- 116.Porcheray F., Samah B., Leone C., Dereuddre-Bosquet N., Gras G. Macrophage activation and human immunodeficiency virus infection: HIV replication directs macrophages towards a pro-inflammatory phenotype while previous activation modulates macrophage susceptibility to infection and viral production. Virology. 2006;349:112–120. doi: 10.1016/j.virol.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 117.Nasr N., Alshehri A.A., Wright T.K., Shahid M., Heiner B.M., Harman A.N., Botting R.A., Helbig K.J., Beard M.R., Suzuki K., et al. Mechanism of Interferon-Stimulated Gene Induction in HIV-1-Infected Macrophages. J. Virol. 2017;91 doi: 10.1128/JVI.00744-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nasr N., Maddocks S., Turville S.G., Harman A.N., Woolger N., Helbig K.J., Wilkinson J., Bye C.R., Wright T.K., Rambukwelle D., et al. HIV-1 infection of human macrophages directly induces viperin which inhibits viral production. Blood. 2012;120:778–788. doi: 10.1182/blood-2012-01-407395. [DOI] [PubMed] [Google Scholar]
- 119.Pujantell M., Badia R., Ramirez C., Puig T., Clotet B., Ballana E., Este J.A., Riveira-Munoz E. Long-term HIV-1 infection induces an antiviral state in primary macrophages. Antivir. Res. 2016;133:145–155. doi: 10.1016/j.antiviral.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 120.Cribier A., Descours B., Valadao A.L., Laguette N., Benkirane M. Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep. 2013;3:1036–1043. doi: 10.1016/j.celrep.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 121.White T.E., Brandariz-Nunez A., Valle-Casuso J.C., Amie S., Nguyen L.A., Kim B., Tuzova M., Diaz-Griffero F. The retroviral restriction ability of SAMHD1, but not its deoxynucleotide triphosphohydrolase activity, is regulated by phosphorylation. Cell Host Microbe. 2013;13:441–451. doi: 10.1016/j.chom.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Badia R., Pujantell M., Riveira-Munoz E., Puig T., Torres-Torronteras J., Marti R., Clotet B., Ampudia R.M., Vives-Pi M., Este J.A., et al. The G1/S Specific Cyclin D2 Is a Regulator of HIV-1 Restriction in Non-proliferating Cells. PLoS Pathog. 2016;12:e1005829. doi: 10.1371/journal.ppat.1005829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mlcochova P., Sutherland K.A., Watters S.A., Bertoli C., de Bruin R.A., Rehwinkel J., Neil S.J., Lenzi G.M., Kim B., Khwaja A., et al. A G1-like state allows HIV-1 to bypass SAMHD1 restriction in macrophages. EMBO J. 2017;36:604–616. doi: 10.15252/embj.201696025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Takeuchi O., Akira S. MDA5/RIG-I and virus recognition. Curr. Opin. Immunol. 2008;20:17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 125.Loo Y.M., Gale M., Jr. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Berg R.K., Melchjorsen J., Rintahaka J., Diget E., Soby S., Horan K.A., Gorelick R.J., Matikainen S., Larsen C.S., Ostergaard L., et al. Genomic HIV RNA induces innate immune responses through RIG-I-dependent sensing of secondary-structured RNA. PLoS ONE. 2012;7:e29291. doi: 10.1371/journal.pone.0029291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Solis M., Nakhaei P., Jalalirad M., Lacoste J., Douville R., Arguello M., Zhao T., Laughrea M., Wainberg M.A., Hiscott J. RIG-I-mediated antiviral signaling is inhibited in HIV-1 infection by a protease-mediated sequestration of RIG-I. J. Virol. 2011;85:1224–1236. doi: 10.1128/JVI.01635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jeang K.T. Multi-Faceted Post-Transcriptional Functions of HIV-1 Rev. Biology. 2012;1:165–174. doi: 10.3390/biology1020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McCauley S.M., Kim K., Nowosielska A., Dauphin A., Yurkovetskiy L., Diehl W.E., Luban J. Intron-containing RNA from the HIV-1 provirus activates type I interferon and inflammatory cytokines. Nat. Commun. 2018;9:5305. doi: 10.1038/s41467-018-07753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wodrich H., Schambach A., Krausslich H.G. Multiple copies of the Mason-Pfizer monkey virus constitutive RNA transport element lead to enhanced HIV-1 Gag expression in a context-dependent manner. Nucleic Acids Res. 2000;28:901–910. doi: 10.1093/nar/28.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rigby R.E., Rehwinkel J. RNA degradation in antiviral immunity and autoimmunity. Trends Immunol. 2015;36:179–188. doi: 10.1016/j.it.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Estes J.D., Kityo C., Ssali F., Swainson L., Makamdop K.N., Del Prete G.Q., Deeks S.G., Luciw P.A., Chipman J.G., Beilman G.J., et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat. Med. 2017;23:1271–1276. doi: 10.1038/nm.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mavigner M., Habib J., Deleage C., Rosen E., Mattingly C., Bricker K., Kashuba A., Amblard F., Schinazi R.F., Lawson B., et al. Simian Immunodeficiency Virus Persistence in Cellular and Anatomic Reservoirs in Antiretroviral Therapy-Suppressed Infant Rhesus Macaques. J. Virol. 2018;92 doi: 10.1128/JVI.00562-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ko A., Kang G., Hattler J.B., Galadima H.I., Zhang J., Li Q., Kim W.K. Macrophages but not Astrocytes Harbor HIV DNA in the Brains of HIV-1-Infected Aviremic Individuals on Suppressive Antiretroviral Therapy. J. Neuroimmune Pharm. 2019;14:110–119. doi: 10.1007/s11481-018-9809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lamers S.L., Rose R., Ndhlovu L.C., Nolan D.J., Salemi M., Maidji E., Stoddart C.A., McGrath M.S. The meningeal lymphatic system: A route for HIV brain migration? J. Neurovirol. 2016;22:275–281. doi: 10.1007/s13365-015-0399-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wong J.K., Yukl S.A. Tissue reservoirs of HIV. Curr. Opin. HIV AIDS. 2016;11:362–370. doi: 10.1097/COH.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ho Y.C., Shan L., Hosmane N.N., Wang J., Laskey S.B., Rosenbloom D.I., Lai J., Blankson J.N., Siliciano J.D., Siliciano R.F. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bruner K.M., Murray A.J., Pollack R.A., Soliman M.G., Laskey S.B., Capoferri A.A., Lai J., Strain M.C., Lada S.M., Hoh R., et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 2016;22:1043–1049. doi: 10.1038/nm.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pollack R.A., Jones R.B., Pertea M., Bruner K.M., Martin A.R., Thomas A.S., Capoferri A.A., Beg S.A., Huang S.H., Karandish S., et al. Defective HIV-1 Proviruses Are Expressed and Can Be Recognized by Cytotoxic T Lymphocytes, which Shape the Proviral Landscape. Cell Host Microbe. 2017;21:494–506.e4. doi: 10.1016/j.chom.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Imamichi H., Smith M., Adelsberger J.W., Izumi T., Scrimieri F., Sherman B.T., Rehm C.A., Imamichi T., Pau A., Catalfamo M., et al. Defective HIV-1 proviruses produce viral proteins. Proc. Natl. Acad. Sci. USA. 2020;117:3704–3710. doi: 10.1073/pnas.1917876117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gendelman H.E., Orenstein J.M., Martin M.A., Ferrua C., Mitra R., Phipps T., Wahl L.A., Lane H.C., Fauci A.S., Burke D.S., et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J. Exp. Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lei J., Yin X., Shang H., Jiang Y. IP-10 is highly involved in HIV infection. Cytokine. 2019;115:97–103. doi: 10.1016/j.cyto.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 143.Cameron P.U., Saleh S., Sallmann G., Solomon A., Wightman F., Evans V.A., Boucher G., Haddad E.K., Sekaly R.P., Harman A.N., et al. Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc. Natl. Acad. Sci. USA. 2010;107:16934–16939. doi: 10.1073/pnas.1002894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Van der Sluis R.M., Zerbato J.M., Rhodes J.W., Pascoe R.D., Solomon A., Kumar N.A., Dantanarayana A.I., Tennakoon S., Dufloo J., McMahon J., et al. Diverse effects of interferon alpha on the establishment and reversal of HIV latency. PLoS Pathog. 2020;16:e1008151. doi: 10.1371/journal.ppat.1008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wherry E.J. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 146.Zhen A., Rezek V., Youn C., Lam B., Chang N., Rick J., Carrillo M., Martin H., Kasparian S., Syed P., et al. Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J. Clin. Investig. 2017;127:260–268. doi: 10.1172/JCI89488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheng L., Ma J., Li J., Li D., Li G., Li F., Zhang Q., Yu H., Yasui F., Ye C., et al. Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. J. Clin. Investig. 2017;127:269–279. doi: 10.1172/JCI90745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Burdo T.H., Walker J., Williams K.C. Macrophage Polarization in AIDS: Dynamic Interface between Anti-Viral and Anti-Inflammatory Macrophages during Acute and Chronic Infection. J. Clin. Cell Immunol. 2015;6:333. [PMC free article] [PubMed] [Google Scholar]
- 149.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bolos M., Llorens-Martin M., Perea J.R., Jurado-Arjona J., Rabano A., Hernandez F., Avila J. Absence of CX3CR1 impairs the internalization of Tau by microglia. Mol. Neurodegener. 2017;12:59. doi: 10.1186/s13024-017-0200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cassol E., Misra V., Dutta A., Morgello S., Gabuzda D. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. Aids. 2014;28:1579–1591. doi: 10.1097/QAD.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Valle M., Price R.W., Nilsson A., Heyes M., Verotta D. CSF quinolinic acid levels are determined by local HIV infection: Cross-sectional analysis and modelling of dynamics following antiretroviral therapy. Brain. 2004;127:1047–1060. doi: 10.1093/brain/awh130. [DOI] [PubMed] [Google Scholar]
- 153.Hanna D.B., Lin J., Post W.S., Hodis H.N., Xue X., Anastos K., Cohen M.H., Gange S.J., Haberlen S.A., Heath S.L., et al. Association of Macrophage Inflammation Biomarkers With Progression of Subclinical Carotid Artery Atherosclerosis in HIV-Infected Women and Men. J. Infect. Dis. 2017;215:1352–1361. doi: 10.1093/infdis/jix082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lu C.L., Pai J.A., Nogueira L., Mendoza P., Gruell H., Oliveira T.Y., Barton J., Lorenzi J.C.C., Cohen Y.Z., Cohn L.B., et al. Relationship between intact HIV-1 proviruses in circulating CD4(+) T cells and rebound viruses emerging during treatment interruption. Proc. Natl. Acad. Sci. USA. 2018;115:E11341–E11348. doi: 10.1073/pnas.1813512115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Salantes D.B., Zheng Y., Mampe F., Srivastava T., Beg S., Lai J., Li J.Z., Tressler R.L., Koup R.A., Hoxie J., et al. HIV-1 latent reservoir size and diversity are stable following brief treatment interruption. J. Clin. Investig. 2018;128:3102–3115. doi: 10.1172/JCI120194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vibholm L.K., Lorenzi J.C.C., Pai J.A., Cohen Y.Z., Oliveira T.Y., Barton J.P., Garcia Noceda M., Lu C.L., Ablanedo-Terrazas Y., Del Rio Estrada P.M., et al. Characterization of Intact Proviruses in Blood and Lymph Node from HIV-Infected Individuals Undergoing Analytical Treatment Interruption. J. Virol. 2019;93 doi: 10.1128/JVI.01920-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee C.Z.W., Kozaki T., Ginhoux F. Studying tissue macrophages in vitro: Are iPSC-derived cells the answer? Nat. Rev. Immunol. 2018;18:716–725. doi: 10.1038/s41577-018-0054-y. [DOI] [PubMed] [Google Scholar]
- 158.Takasato M., Er P.X., Chiu H.S., Maier B., Baillie G.J., Ferguson C., Parton R.G., Wolvetang E.J., Roost M.S., Lopes S.M., et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2016;536:238. doi: 10.1038/nature17982. [DOI] [PubMed] [Google Scholar]
- 159.Marton R.M., Pasca S.P. Organoid and Assembloid Technologies for Investigating Cellular Crosstalk in Human Brain Development and Disease. Trends Cell Biol. 2019;30:133–143. doi: 10.1016/j.tcb.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 160.Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., Zhang R.R., Ueno Y., Zheng Y.W., Koike N., et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 161.Spence J.R., Mayhew C.N., Rankin S.A., Kuhar M.F., Vallance J.E., Tolle K., Hoskins E.E., Kalinichenko V.V., Wells S.I., Zorn A.M., et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]