Abstract
Hepatitis B virus (HBV) is the primary cause of liver-related malignancies worldwide, and there is no effective cure for chronic HBV infection (CHB) currently. Strong immunological responses induced by T cells are associated with HBV clearance during acute infection; however, the repertoire of epitopes (epi) presented by major histocompatibility complexes (MHCs) to elicit these responses in various African populations is not well understood. In silico approaches were used to map and investigate 15-mers HBV peptides restricted to 9 HLA class II alleles with high population coverage in Botswana. Sequences from 44 HBV genotype A and 48 genotype D surface genes (PreS/S) from Botswana were used. Of the 1819 epi bindings predicted, 20.2% were strong binders (SB), and none of the putative epi bind to all the 9 alleles suggesting that multi-epitope, genotype-based, population-based vaccines will be more effective against HBV infections as opposed to previously proposed broad potency epitope-vaccines which were assumed to work for all alleles. In total, there were 297 unique epi predicted from the 3 proteins and amongst, S regions had the highest number of epi (n = 186). Epitope-densities (Depi) between genotypes A and D were similar. A number of mutations that hindered HLA-peptide binding were observed. We also identified antigenic and genotype-specific peptides with characteristics that are well suited for the development of sensitive diagnostic kits. This study identified candidate peptides that can be used for developing multi-epitope vaccines and highly sensitive diagnostic kits against HBV infection in an African population. Our results suggest that viral variability may hinder HBV peptide-MHC binding, required to initiate a cascade of immunological responses against infection.
Keywords: hepatitis B virus (HBV), HLA class II alleles, T-cell epitopes, in silico, immunoinformatics, candidate multi-epitope vaccines (MEV), escape mutation, Botswana, Africa
1. Background
Hepatitis B virus (HBV), a member of the Hepadnaviradae family, is the major etiology of end stage liver diseases (ESLD), liver cirrhosis (LC) and hepatocellular carcinoma (HCC), and causes up to 887,000 deaths per year [1]. Although more than 90% of healthy adults resolve acute HBV infection within 6 months, there remain over 287 million people who test seropositive for hepatitis B surface antigen (HBsAg) [2] and have chronic HBV infection (CHB). Viral clearance is mediated by cytokines, lymphocytes, and the ability to mount a multi-specific polyclonal and vigorous T cell-mediated response against HBV antigens for a protective immunity [3,4,5]. The quality of these responses is influenced by host genetics, as well as the ability of certain viral variants to escape immune recognition [6,7,8].
The major histocompatibility complexes (MHCs)—known as human leukocytes antigens (HLAs) in humans—are integral components of host genes located at chromosome 6p21. These highly polymorphic proteins serve as mediators of adaptive immune responses by presenting processed antigenic peptides to T cells. The two compatible types of MHCs—class I and class II—present exogenous and endogenous epitopes to CD8+ cytolytic T cells and CD4+ T helper (Th) cells, respectively [9]. The MHC class II alleles (HLA-DR, -DQ and -DP) present epitopes to CD4+ T cells [epi-HLA class II → CD4+ T-cells] that in turn elicit adaptive immune responses against viral infections by facilitating the induction of CD8+ cytotoxic T-lymphocytes (CTLs), production of cytokines crucial for survival, and maturation of B cells [10,11,12,13].
The link between HBV pathogenesis and host immunological profiles is still poorly understood [2]. HBV exhibits a high mutation rate, although only a small number of amino acid substitutions have been characterized functionally due to the costly and time-consuming nature of in vitro assays. Recent approaches have utilized in silico approaches such as machine learning techniques to prioritize candidate peptides for in vitro assays [14,15]. Thus far, the HBV mutations characterized have been associated with sensitivity of immunologic and molecular-based assays and viral escape leading to poor prognosis [16,17,18,19]. However, amino acid variations that influence HBV-MHC binding are poorly understood. In silico mapping of HLA class II binding peptides can be used to identify candidate peptides for in vitro assays to confirm CD4+ T cell epitopes which are crucial for the design of epitope-based vaccines and highly sensitive diagnostic tools that can detect low HBV DNA levels which are frequently missed by diagnostic kits [20,21,22,23].
Sub-Sahara Africa (SSA) and the Western Pacific regions are highly endemic for CHB [24], where the circulating genotypes include A, D, and E for SSA, and B and C for the Western Pacific. HBV genotypes A (subgenotype A1) and D (subgenotype D3) have been reported in Botswana, with genotype E occurring rarely [25,26,27,28,29]. Not only do genotypes show unique geographic distribution, they also differ in treatment response, pathogenesis potential, and prognosis [30,31]. Studies conducted in China have mapped different T cell epitopes that may be eligible for epitope-based vaccines and some were evaluated in vitro [20,32]. However, these findings may be less applicable to African populations whose host genetic pool, circulating genotypes, and immune profiles for HBV (e.g., hepatitis B e antigen [HBeAg] and HBsAg positivity) differ considerably with those of the Chinese population. A prerequisite to determine the epitope(s) for inclusion in epitope-based vaccines include (1) identification of conserved regions of the genome and (2) characterization of those regions that elicit protective immune responses [33]. Although there are hepatitis B vaccines in use currently, vaccine escape does occur; thus, more optimized vaccine candidates may be needed to avoid vaccine failure. In this study, we utilized HLA class II alleles that occur at the highest frequency in Botswana and locally derived HBV strains to identify HLA class II binding peptides which are good candidates for confirmatory in vitro tests of immunogenicity. The present study had three major aims: (1) to determine the repertoires of HLA class II epitopes within HBV envelope sequences of genotypes A and D isolated from different risk groups in Botswana (described in our earlier papers); (2) to compare if the predicted epitopes in genotypes A and D may vary across other HBV genotypes, suggesting that genotype-based multi-epitope vaccines would be more successful than the broad potency vaccines currently in use; (3) to investigate amino acid variations within these epitopes to determine if they may lead to immune escape (i.e., candidate escape mutations).
2. Materials and Methods
2.1. Mapping Peptides from HBV Surface Gene Restricted to HLA-class II Alleles
Three sequence datasets were included in the current analysis. The first database (N1) was used to map epitopes (epi) that bind predominantly to HLA class II alleles in Botswana and consisted of 92 non-recombinant full-length S gene (PreS/S) sequences [25,26,28] retrieved from GenBank (accession numbers MF979142—MF979176, KR139743—KR139748, and MH464807—MH464854). The aligned sequences were sorted by genotype and included A (n = 44) and D (n = 48). The three domains of HBV surface proteins—PreS1, PreS2, and S—were manually extracted from an overlapping Pol/S fragment and were divided into genotypes whose amino acid (aa) sequence alignments were sorted according to column similarities. Nucleotide alignments and sorting were performed using AliView 1.21 software [34]. Each region was then used to create a consensus sequence with the threshold set at 90% for all positions. Variants that did not meet this threshold were investigated independently in post-analyses. To assess if the aa composition of consensus sequences was representative of existing HBV strains, BLAST searches were conducted using the NCBI database, and strains exhibiting 100% similarity and coverage were evaluated further (Supplementary Table S1).
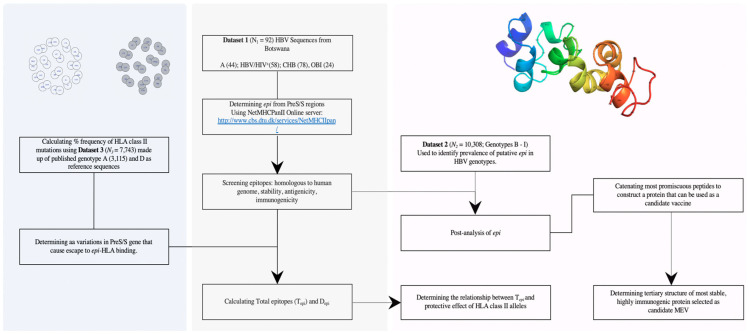
The 15-mer HBV peptides overlapping by 14 aa were tested for binding to 9 HLA class II alleles—HLA-DRB1*0101, DRB1*0301, DRB1*0401, DRB1*0701, DRB1*0802, DRB1*1101, DRB1*1302, DRB1*1501, and DRB5*0101—that have high population coverage in Botswana [35]. The NetMHCIIPan version 3.2 online server (http://www.cbs.dtu.dk/services/NetMHCIIpan/) [36] was used to predict binding peptides, and their binding-affinity scores were categorized based on the Log-transformed binding affinity [1-Log50k (aff)]. The settings were adjusted starting with default [1-Log50k (aff)] of 0.426; 500nM and logAff = 0.638; 50nM for weak and strong binding respectively. Ten previously characterized HBV envelope proteins (PreS1, PreS2, S) epitopes—S: 18–37 epitope ID 51310; S: 70–84 (3966); S: 83–98 (46959); S: 200–211 (66307); S: 201–215 (59353); S: 211–244 (6574); S: 230–247 (47877); S: 363–378 (76458); S: 376–389 (17331); S: 378–386 (37664)—available at (www.iedb.org) [37] were included to calibrate the tool’s settings, and the thresholds showing highest specificity were utilized in the present study. Using percentile rank of eluted ligand prediction score (%Rank_EL), the strong binders (SB) were determined between 2–10% Rank_EL and 10–50% Rank_EL for weak binders (WB). Peptides with binding affinity less than that of WB were deemed pseudo binders (NB). Figure 1 outlines the various analytical steps included in this study, and the NetMHCIIPan results are provided in the Supplementary Table S2.
Figure 1.
Schema illustrating the flow of data analysis used in this study. N = sample size; SB = strong binding peptides; WB = weak biding peptides; Tepi = total predicted epitopes; PreS/S = HBV surface gene; Depi = epitope densities. Sequences were derived from patients with different clinical outcomes: −(HBV/HIV; CHB; OBI)—HIV = human immunodeficiency virus; OBI = occult hepatitis B infection, CHB = chronic hepatitis B infection, HBV/HIV = coinfection. The blue colored segment shows the pipeline used to evaluate the diversity of epi. The grey segment is the pipeline used to determine epi and measure of promiscuity and conservativeness. The pink segment is the pipeline used to determine the best candidate vaccine.
The relationship between the length of the protein and the frequency of binding-peptides were compared using epitope density score (Depi) for all protein domains of S gene (PreS1, PreS2, S) and genotypes (A versus D). Depi score was defined as the proportion of binding peptides epi (where; Iepi = WB + SB) to total predicted epitopes (Tepi) relative to protein size. Tepi represent the total count of all predicted proteins.
2.2. Determining Prevalence of Putative Epitopes in HBV Genotypes (A–I) Except A and D
Putative or promiscuous epi were defined as peptides that exhibit similar binding affinity to 2 or more alleles. The prevalence of predicted putative epi were determined in a second dataset (N2) that included 10,308 PreS/S sequences (genotype B = 2905; C = 5575; E = 1118; F = 477; G = 86; H = 69; I = 78) retrieved from HBV database available at http://hvdr.bioinf.wits.ac.za/alignments/index.html [38]. N2 was also used to determine the overall prevalence of the predicted escape mutations. The sequences used in this analysis are included in the supplementary file provided; Supplementary-Table S3.
2.3. Variations Causing Escape to HLA Class II Binding
Dataset N3 consisted of 7743 HBV sequences (genotype A = 3115; genotype D = 4628) used to determine the frequency of aa variations which were termed HLA escape mutations in other HBV sequences. Sequences used were curated from http://hvdr.bioinf.wits.ac.za/alignments/index.html, partitioned by proteins (PreS1, PreS, S) [38]. Escape mutations were defined as those aa variations within the 15-mer core aa sequence that cause the binding affinity to change from either strong to pseudo binding (SB → NB) or from weak to pseudo binding (WB → NB). Several in-house customized Python pipelines were used to accurately investigate the frequency of escape mutations. The sequences used in this analysis are in the supplementary file provided; Supplementary Table S4.
2.4. Screening Putative Epi and Reconstruction of Tertiary Structure of the Modelled Vaccine
Since the predicted putative epi can be also homologous to human peptides that may (1) cause either autoimmune responses when used as a vaccine or (2) give false results when used as a diagnostic marker, a BLAST search was conducted with the NCBI protein database for all immunogenic epi. Afterwards, the predicted putative epi were catenated using a previously described method [39], and different combinations were used to construct candidate multi-epitope vaccines (MEVs). Physiological and biochemistry proteins such as thermal stability, desirable shelf-life, and pH among other properties are prerequisites during development of an ideal vaccine. The biochemical properties of generated candidate proteins were evaluated using online ProtoParam tool [40]. The proteins exhibiting properties similarly to those of vaccines currently in use were deemed the best candidate. The properties of 3 current HepB vaccines—including VO_0011094, VO_0011095, and VO_0011093—are curated in the DNA vaccine database [41]. Properties predicted include; immunogenicity, antigenicity, instability index, estimated half-life in humans’ molecular weight (mw), aliphatic index (AI), grand average of hydrophobicity (GRAVY), and theoretical (pH).
2.5. Determining the HLA-HBV Association Using Tepi
To test the hypothesis that Tepi could serve as a useful predictor of HLA-HBV associations, Tepi of S genes (A and D) were used to rank the 9 HLA class II alleles in the post epi prediction analyses. The available literature was used to corroborate the analyses.
3. Results
3.1. Predicting T-cell Epitopes Using Consensus from N1 Dataset
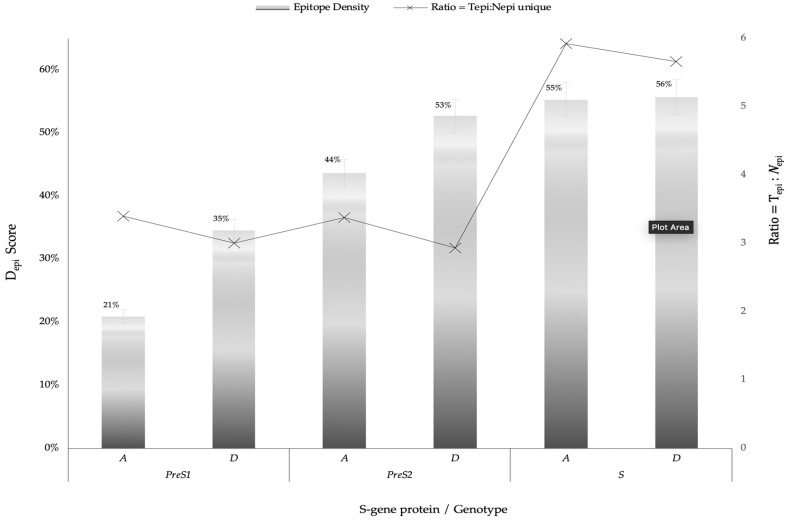
We first generated 6 different consensus sequences from PreS/S sequences of genotypes A and D. Consensus sequences were validated by comparison to the NCBI database and identified 16 pre-existing sequences which exhibit 100% identity to: PreS1 genotype A (PreS1A) consensus sequence; 39 to PreS1D, 6 to PreS2A, 12 to PreS2D, 19 to SA, and 20 to SD, respectively. The consensus sequences, identical sequences, and their country of origin are provided in supplementary file; Supplementary Table S1. Epitope is defined as a peptide that binds either weakly or strongly bind to HLA-DR alleles used, while HLA class II escape mutations were defined as aa variations within the 9-mer core aa sequence that changed the epitope-HLA-DR binding from SB/WB to pseudo binding. In total, there were 1819 total binding predicted (Tepi) from 297 unique epitopes restricted to 9 HLA class II including 20.2% SB. The number of signature aa differentiating HBV genotypes A and D were 31, 13, and 9 for the S, PreS1, and PreS1 regions, respectively (Table 1). S protein had the highest binding peptides constituting 79.9% of the sum of all Tepi (epi), PreS1 constituted 11%, while PreS2 had the least (9.1%) epi. The Depi of the SB and WB among genotypes (A and D) and proteins (PreS1, PreS2, S) are summarized in Figure 2.
Table 1.
Distribution of T cell epitopes restricted to 9 HLA class II alleles with high population coverage in Botswana.
| PreS1 | PreS2 | S | |||||
|---|---|---|---|---|---|---|---|
| A (120 aa) | D (108 aa) | A (55 aa) | D (55 aa) | A (226 aa) | D (226 aa) | ||
| Total bindings (n = 1819) SB (367); WB (1452) (%) | 6; 79 (4.7) | 7; 107 (6.3) | 4; 81 (4.7) | 3; 78 (4.5) | 122; 619 (40.7) | 136; 577 (39.1%) | |
| Unique epi (n = 297) | 25 ¥ | 38 ¥ | 29 ¥ | 24 ¥ | 125 ¥ | 126 ¥ | |
| Ratio = Tepi: Nepi | 3.4 | 3.0 | 3.4 | 2.9 | 5.9 | 5.7 | |
| Most active HLA: DRB * (SB; WB) | *0802; *0101 | *0401; (*0401, *0101) | *0301; (*0401, *0101) | *0301; 5*0101 | *0702; *0401 | *0401; *1501 | |
| Genotype variation: |

|

|

|
||||
| (A|D epi; p-value) | A|D > 0.05 | A|D > 0.05 | A|D > 0.05 | ||||
¥ indicates existence of epi that are common in both genotypes A and D. SB; strong binding peptides. WB; weak binding peptides. aa; amino acids. Web-logo diagrams represent signature aa between consensus sequences of genotype A and D set at a threshold of 100%. HBV; hepatitis B virus. * 0101 means HLA class II allele DRB1*0101 etc. 5*0101 means HLA class II allele DRB5*0101.
Figure 2.
Epitope densities (Depi) of different PreS/S proteins stratified by genotype (A or D) and protein (PreS1, PreS2, S). where can be any protein (PreS/SA versus PreS/SD). PreS1A represent genotype A large Hepatitis B surface antigen (HBsAg); PreS1D represent genotype D large HBsAg; PreS2A represent genotype A middle HBsAg; PreS2D represent genotype D middle HBsAg; SA represent genotype A small HBsAg; SD represent genotype D small HBsAg. Tepi = Total binding peptides (WB + SB). Nepi unique = count of unique binding peptides per each protein.
3.2. Prevalence of Putative Epitopes Across all HBV Genotypes
To assess if the predicted epi may be suitable for vaccine inclusion, the most common epi were compared to sequences other than genotypes A and D. Table 2 shows the number of aa sequences containing the indicted epi. Eight out of 53 epi were found in semi-conserved regions ranked as “++++” and had prevalence > 85% among sequences in N2 dataset. These results suggest that multi-epitope genotype-based vaccines may be better to avoid vaccine escape.
Table 2.
Showing the prevalence of S protein epi in other HBV genotypes (B–I).
| Epitope Sites in S Protein | AA Sequence | B (n = 2905) | C (n = 5575) | E (n = 1118) | F (n = 477) | G (n = 86) | H (n = 69) | I (n = 78) | Prevalence (%) = 1 − *% | Degree of Conservation ↓ (+: Variable) | epi Previously Discussed |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 17–31 | AFGKFLWEWASARFS | E = 998 | C = 32 | F = 1 | 10,0 | + | [42] | ||||
| 180–194 | AGFFLLTRILTIPQS | B = 62 | C = 4627 | E = 5 | F = 2 | G = 75 | 46,3 | ++ | [43] | ||
| 90–104 | CLIFLLVLLDYQGML | B = 2605 | C = 4661 | E = 1034 | F = 442 | G = 84 | H = 65 | I = 70 | 86,9 | ++++ | [44] |
| 69–83 | CPGYRWMCLRRFIIF | B = 2384 | C = 5064 | E = 1027 | F = 465 | G = 84 | H = 67 | I = 65 | 88,8 | ++++ | [42] |
| 19–33 | FFLLTRILTIPQSLD | B = 63 | C = 4717 | E = 5 | F = 2 | G = 76 | 47,2 | ++ | [45,46,47] | ||
| 158–172 | FGKFLWEWASARFSW | E = 996 | C = 31 | F = 1 | 10,0 | + | [48] | ||||
| 20–34 | FLLTRILTIPQSLDS | B = 63 | C = 4709 | E = 5 | F = 5 | G = 78 | 47,1 | ++ | [49] | ||
| 93–107 | FLLVLLDYQGMLPVC | B = 2616 | C = 4720 | E = 1031 | F = 446 | G = 84 | H = 66 | I = 72 | 87,7 | ++++ | [50] |
| 161–175 | FLWEWASARFSWLSL | B = 1 | E = 997 | C = 81 | F = 3 | I = 16 | 10,7 | + | [42] | ||
| 179–193 | FVQWFVGLSPTVWLS | B = 2630 | C = 4698 | E = 76 | G = 85 | I = 75 | 73,4 | +++ | [51] | ||
| 18–32 | GFFLLTRILTIPQSL | B = 63 | C = 4618 | E = 5 | F = 2 | G = 76 | 46,2 | ++ | - | ||
| 159–173 | GKFLWEWASARFSWL | E = 1002 | C = 31 | F = 3 | 10,1 | + | - | ||||
| 202–216 | GPSLYSILSPFLPLL | C = 19 | E = 2 | 0,2 | + | [48] | |||||
| 71–85 | GYRWMCLRRFIIFLF | B = 82 | C = 5065 | E = 1032 | F = 464 | G = 84 | H = 67 | I = 65 | 66,5 | +++ | - |
| 92–106 | IFLLVLLDYQGMLPV | B = 2606 | C = 4659 | E = 1030 | F = 443 | G = 84 | H = 66 | I = 70 | 86,9 | ++++ | - |
| 195–209 | IWMMWYWGPSLYSIL | B = 3 | C = 24 | E = 2 | 0,3 | + | - | ||||
| 160–174 | KFLWEWASARFSWLS | B = 1 | E = 1012 | C = 34 | F = 3 | I = 16 | 10,3 | + | - | ||
| 91–105 | LIFLLVLLDYQGMLP | B = 2611 | C = 4656 | E = 1031 | F = 443 | G = 84 | H = 66 | I = 70 | 86,9 | ++++ | [52] |
| 21–35 | LLTRILTIPQSLDSW | B = 63 | C = 4754 | E = 5 | F = 5 | G = 77 | 47,6 | ++ | [53] | ||
| 94–108 | LLVLLDYQGMLPVCP | B = 2653 | C = 4729 | E = 1032 | F = 445 | G = 84 | H = 66 | I = 72 | 88,1 | ++++ | [54] |
| 15–29 | LQAGFFLLTRILTIP | B = 62 | C = 4735 | E = 5 | F = 2 | G = 76 | 47,3 | ++ | [54] | ||
| 192–206 | LSVIWMMWYWGPSLY | B = 772 | C = 4249 | E = 889 | G = 1 | I = 50 | 57,8 | ++ | [44] | ||
| 95–109 | LVLLDYQGMLPVCPL | B = 2619 | C = 4708 | E = 1022 | F = 444 | G = 84 | H = 66 | I = 73 | 87,5 | ++++ | [55,56] |
| 162–176 | LWEWASARFSWLSLL | B = 7 | E = 1001 | C = 88 | F = 445 | G = 2 | H = 65 | I = 75 | 16,3 | + | [54] |
| 205–219 | LYSILSPFLPLLPIF | C = 21 | E = 2 | 0,2 | + | - | |||||
| 178–192 | PFVQWFVGLSPTVWL | B = 2649 | C = 4723 | E = 76 | G = 85 | I = 74 | 73,8 | +++ | - | ||
| 70–84 | PGYRWMCLRRFIIFL | B = 2375 | C = 5117 | E = 1033 | F = 466 | G = 85 | H = 67 | I = 65 | 89,3 | ++++ | [49] |
| 66–80 | PPTCPGYRWMCLRRF | B = 60 | C = 751 | E = 14 | F = 464 | G = 77 | H = 68 | 13,9 | + | [44] | |
| 203–217 | PSLYSILSPFLPLLP | C = 19 | E = 2 | 0,2 | + | - | |||||
| 67–81 | PTCPGYRWMCLRRFI | B = 60 | C = 750 | E = 14 | F = 464 | G = 77 | H = 68 | 13,9 | + | [57] | |
| 16–30 | QAGFFLLTRILTIPQ | B = 62 | C = 4645 | E = 5 | F = 2 | G = 76 | 46,5 | ++ | [58] | ||
| 181–195 | QWFVGLSPTVWLSVI | B = 2588 | C = 4310 | E = 73 | G = 6 | I = 71 | 68,4 | +++ | [58] | ||
| 38–52 | SLNFLGGTTVCLGQN | B = 5 | C = 2 | E = 2 | 0,1 | + | [55,56,57] | ||||
| 204–218 | SLYSILSPFLPLLPI | C = 19 | E = 2 | 0,2 | + | [44] | |||||
| 193–207 | SVIWMMWYWGPSLYS | B = 3 | C = 24 | E = 2 | 0,3 | + | - | ||||
| 155–169 | SWAFGKFLWEWASAR | E = 998 | C = 31 | F = 1 | 10,0 | + | - | ||||
| 68–82 | TCPGYRWMCLRRFII | B = 63 | C = 757 | E = 17 | F = 466 | G = 77 | H = 68 | 14,0 | + | - | |
| 37–51 | TSLNFLGGTTVCLGQ | B = 5 | C = 2 | E = 2 | 0,1 | + | [59,60] | ||||
| 194–208 | VIWMMWYWGPSLYSI | B = 3 | C = 24 | E = 2 | 0,3 | + | - | ||||
| 14–28 | VLQAGFFLLTRILTI | B = 61 | C = 4676 | E = 5 | F = 2 | G = 76 | 46,8 | ++ | [61]* | ||
| 180–194 | VQWFVGLSPTVWLSV | B = 2636 | C = 4584 | E = 77 | G = 6 | I = 75 | 71,6 | +++ | [62] | ||
| 156–170 | WAFGKFLWEWASARF | E = 1000 | C = 32 | F = 1 | 10,0 | + | [44] | ||||
| 163–177 | WEWASARFSWLSLLV | B = 7 | E = 1002 | C = 87 | F = 445 | G = 2 | H = 64 | I = 75 | 16,3 | + | - |
| 182–196 | WFVGLSPTVWLSVIW | B = 2506 | C = 4125 | E = 73 | G = 6 | I = 71 | 65,8 | +++ | [63] | ||
| 201–215 | WGPSLYSILSPFLPL | C = 19 | E = 2 | 0,2 | + | [52] | |||||
| 196–210 | WMMWYWGPSLYSILS | B = 3 | C = 25 | E = 2 | 0,3 | + | - | ||||
| 36–50 | WTSLNFLGGTTVCLG | B = 5 | C = 2 | E = 2 | 0,1 | + | - | ||||
| 35–49 | WWTSLNFLGGTTVCL | B = 5 | C = 2 | E = 2 | 0,1 | + | - | ||||
| 199–213 | WYWGPSLYSILSPFL | C = 18 | E = 2 | 0,2 | + | - | |||||
| 206–220 | YSILSPFLPLLPIFF | C = 19 | E = 2 | 0,2 | + | - | |||||
| 200–214 | YWGPSLYSILSPFLP | C = 18 | E = 2 | 0,2 | + | - |
+ The degree of conservation. The scale used: if score > = 100, then highly conserved and will be denoted by ‘+++++’. elif score > = 85: then semi conserved = ‘++++’. elif score > = 60: region of mutation and is denoted by ‘+++’. elif score > = 20, then highly variable region = ‘++’. else: high escape mutation = ‘+’. n represents the number of sequences used in the analysis. B represents full-length genotype B sequences, C represents full-length genotype C sequences, etc. 17–31 representing the beginning the position occupied by the 14-mer epitope predicts (e.g., 17 is the first amino acid of the epitope, while 31 is the last amino acid of the epitope).
3.3. Profiles of Strong Binding Epitopes
SB epi of the 3 proteins were sorted by the core aa sequence and analyzed based on genotype. Those found in both genotypes were considered putative when binding to alleles. There were no SB epitopes that were common between sequences of genotypes A and D for PreS1 and PreS2 regions. S protein had 89 out of 230 epi that satisfied the above criteria and were promiscuous for at least 5 alleles. There were 5 unique epi whose core aa were at S protein residues 41–49 (FLGGSPVCL), 14 epi with S protein residues 20–28 (FLLTRILTI), 10 epi with S residues 183–191 (FVGLSPTVW), 9 epi with S residues 22–30 (LTRILTIPQ), 6 epi with S residues 162–170 (LWEWASARF), 6 epi with S residues 184–192 (VGLSPTVWL), 12 epi with S residues 96–104 (VLLDYQGML), 2 epi with S residues 180–188 (VQWFVGLSP), 9 epi with S residues 163–171 (WEWASARFS), 4 epi with S residues 182–190 (WFVGLSPTV), and 12 epi with S residues 72–80 (YRWMCLRRF). Table 3 shows the full profiles of SB epi mapped for the S proteins for genotypes A and D.
Table 3.
List of most promiscuous SB epi mapped from S protein.
| S: Residues | AA Sequence | Geno | Core aa | HLA Class II Alleles | S: Residues | AA Sequence | Geno | Core aa | HLA Class II Alleles | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 69–83 | CPGYRWMCLRRFIIF | A | YRWMCLRRF | *0101 | *0301 | *0401 | *0701 | *0802 | *1302 | 68–82 | TCPGYRWMCLRRFII | D | YRWMCLRRF | *0101 | *0301 | *0401 | *0701 | *0802 | *1302 | *1501 | ||||
| CPGYRWMCLRRFIIF | D | 168–182 | VRFSWLSLLVPFVQW | A | WLSLLVPFV | *0101 | *0401 | *0701 | *0802 | *1101 | *1302 | *1501 | 5*0101 | |||||||||||
| 68–82 | ICPGYRWMCLRRFII | A | 14–28 | VLQAGFFLLTRILTI | A | FLLTRILTI | *0301 | *0401 | *0802 | *1302 | *1501 | 5*0101 | ||||||||||||
| 70–84 | PGYRWMCLRRFIIFL | D | VLQAGFFLLTRILTI | D | ||||||||||||||||||||
| PGYRWMCLRRFIIFL | A | *0101 | *0301 | *1501 | 165–179 | WASARFSWLSLLVPF | D | FSWLSLLVP | *0101 | *0401 | *0802 | *1101 | 5*0101 | |||||||||||
| 71–85 | GYRWMCLRRFIIFLF | A | 174–188 | SLLVPFVQWFVGLSP | A | FVQWFVGLS | *0101 | *0802 | *1101 | *1501 | ||||||||||||||
| GYRWMCLRRFIIFLF | D | SLLVPFVQWFVGLSP | D | |||||||||||||||||||||
| 67–85 | PICPGYRWMCLRRFI | A | 6–20 | SGFLGPLLVLQAGFF | A | LGPLLVLQA | *0101 | *0701 | *0802 | *1101 | *1501 | 5*0101 | ||||||||||||
| 66–84 | PPICPGYRWMCLRRF | A | *0101 | *1501 | 5*0101 | SGFLGPLLVLQAGFF | D | *0701 | ||||||||||||||||
| 67–81 | PTCPGYRWMCLRRFI | D | *1501 | 7–21 | GFLGPLLVLQAGFFL | D | ||||||||||||||||||
| 147–161 | CTCIPIPSSWAFAKY | A | CIPIPSSWA | *0101 | *0401 | *0701 | *0802 | *1501 | GFLGPLLVLQAGFFL | A | ||||||||||||||
| CTCIPIPSSWAFGKF | D | 5–19 | TSGFLGPLLVLQAGF | D | ||||||||||||||||||||
| 145–159 | GNCTCIPIPSSWAFA | A | TSGFLGPLLVLQAGF | A | ||||||||||||||||||||
| 146–160 | NCTCIPIPSSWAFAK | A | 93–107 | FLLVLLDYQGMLPVC | D | LVLLDYQGM | *0101 | *0401 | *0802 | *1101 | *1501 | |||||||||||||
| NCTCIPIPSSWAFGK | D | 5*0101 | FLLVLLDYQGMLPVC | A | *0802 | *1101 | ||||||||||||||||||
| 177–191 | VPFVQWFVGLSPTVW | D | WFVGLSPTV | *0101 | *0401 | *0701 | *0802 | *1101 | 5*0101 | 92–106 | IFLLVLLDYQGMLPV | A | *0802 | *1101 | ||||||||||
| VPFVQWFVGLSPTVW | A | IFLLVLLDYQGMLPV | D | *0802 | *1101 | |||||||||||||||||||
| 168–182 | ARFSWLSLLVPFVQW | D | WLSLLVPFV | *0101 | *0401 | *0701 | *0802 | *1101 | *1501 | 5*0101 | 182–196 | WFVGLSPTVWLSVIW | D | VGLSPTVWL | *0301 | *0401 | *0802 | *1302 | *1501 | |||||
| 169–183 | RFSWLSLLVPFVQWF | D | WFVGLSPTVWLSVIW | A | *0301 | *0401 | *0802 | *1302 | ||||||||||||||||
| RFSWLSLLVPFVQWF | A | 97–111 | LLDYQGMLPVCPLIP | A | YQGMLPVCP | *0101 | *0401 | *0701 | *1101 | *1501 | 5*0101 | |||||||||||||
| 167–181 | SARFSWLSLLVPFVQ | D | LLDYQGMLPVCPLIP | D | *0401 | *0701 | *1101 | |||||||||||||||||
| 176–189 | LVPFVQWFVGLSPTV | A | WFVGLSPTV | *0101 | *0401 | *0701 | *0802 | 5*0101 | 96–110 | VLLDYQGMLPVCPLI | A | *0401 | *0701 | *1101 | ||||||||||
| LVPFVQWFVGLSPTV | D | VLLDYQGMLPVCPLI | D | *0401 | *0701 | *1101 | ||||||||||||||||||
| 166–180 | ASARFSWLSLLVPFV | D | *0101 | *0401 | *0701 | *0802 | 5*0101 | 72–86 | YRWMCLRRFIIFLFI | A | WMCLRRFII | *0701 | *0802 | *1101 | *1501 | 5*0101 | ||||||||
| 170–184 | FSWLSLLVPFVQWFV | A | *1101 | YRWMCLRRFIIFLFI | D | WMCLRRFII | ||||||||||||||||||
| FSWLSLLVPFVQWFV | D | 194–208 | VIWMMWYWGPSLYNI | A | WYWGPSLYN | *0101 | *0401 | *0701 | *0802 | *1101 | 5*0101 | |||||||||||||
| 167–181 | SVRFSWLSLLVPFVQ | A | 5*0101 | |||||||||||||||||||||
PreS1A represent genotype A epi derived from sequences of large Hepatitis B surface antigen (HBsAg); PreS1D represent genotype D epi derived from sequences of large HBsAg; PreS2A represent genotype A epi derived from sequences of middle HBsAg; PreS2D represent genotype D epi derived from sequences of middle HBsAg; SA represent genotype A epi derived from sequences of small HBsAg; SD represent genotype D epi derived from sequences of small HBsAg. *0101 means HLA class II allele DRB1*0101 e.tc. 5*0101 means HLA class II allele DRB5*0101.
3.4. Profiles of Most Promiscuous Epitopes
Since the majority of predicted epi were WB, a strict threshold was applied to select the most the promiscuous epi. Thus, epi were selected if they bind to least 6 alleles or more as shown in Table 4.
Table 4.
Highlighting most promiscuous T cell epitopes restricted to 9 HLA class II alleles.
| Epitope Site | AA Sequence | HBV Protein | Core AA | HLA Class II Alleles | Previously Discussed epi |
|---|---|---|---|---|---|
| 34–48 | PVPNIASHISSISSR | PreS2A | IASHISSIS | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1302, 1*1501, 5*0101 | - |
| 39–53 | ASHISSISSRTGDPA | PreS2A | ISSISSRTG | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 38–52 | IASHISSISSRTGDP | PreS2A | ISSISSRTG | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 37–51 | TTASPLSSIFSRIGD | PreS2D | LSSIFSRIG | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 38–52 | TASPLSSIFSRIGDP | PreS2D | LSSIFSRIG | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 39–53 | ASPLSSIFSRIGDPA | PreS2D | LSSIFSRIG | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 208–222 | ILSPFIPLLPIFFCL | SA | FIPLLPIFF | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 209–223 | LSPFIPLLPIFFCLW | SA | FIPLLPIFF | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 207–221 | NILSPFIPLLPIFFC | SA | FIPLLPIFF | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1302, 5*0101 | - |
| 149–163 | CIPIPSSWAFAKYLW | SA | IPSSWAFAK | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 6–20 | SGFLGPLLVLQAGFF | SA | LGPLLVLQA | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 200–214 | YWGPSLYNILSPFIP | SA | LYNILSPFI | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1302, 1*1501 | - |
| 199–213 | WYWGPSLYNILSPFI | SA | LYNILSPFI | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 164–178 | EWASVRFSWLSLLVP | SA | VRFSWLSLL | 1*0101, 1*0401, 1*0802, 1*1101, 1*1302, 1*1501, 5*0101 | [64] |
| 168–182 | VRFSWLSLLVPFVQW | SA | WLSLLVPFV | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1302, 1*1501, 5*0101 | [44] |
| 169–183 | RFSWLSLLVPFVQWF | SA | WLSLLVPFV | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | [65] |
| 156–170 | WAFAKYLWEWASVRF | SA | YLWEWASVR | 1*0101, 1*0301, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | [49] |
| 208–222 | ILSPFLPLLPIFFCL | SD | FLPLLPIFF | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 209–223 | LSPFLPLLPIFFCLW | SD | FLPLLPIFF | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 155–169 | SWAFGKFLWEWASAR | SD | FLWEWASAR | 1*0101, 1*0301, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501 | - |
| 6–20 | SGFLGPLLVLQAGFF | SD | LGPLLVLQA | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | [44] |
| 199–212 | WYWGPSLYSILSPFL | SD | LYSILSPFL | 1*0101, 1*0401, 1*0802, 1*1101, 1*1302, 1*1501, 5*0101 | - |
| 167–181 | SARFSWLSLLVPFVQ | SD | WLSLLVPFV | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 168–182 | ARFSWLSLLVPFVQW | SD | WLSLLVPFV | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | [44] |
| 169–183 | RFSWLSLLVPFVQWF | SD | WLSLLVPFV | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | [42,49] |
| 197–211 | MMWYWGPSLYSILSP | SD | WYWGPSLYS | 1*0101, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
| 68–82 | TCPGYRWMCLRRFII | SD | YRWMCLRRF | 1*0101, 1*0301, 1*0401, 1*0701, 1*0802, 1*1101, 1*1501, 5*0101 | - |
*0101 means HLA class II allele DRB1*0101 e.tc. 5*0101 means HLA class II allele DRB5*0101.
3.5. Designing and Predicting Structure of Candidate Multi-Epitope Vaccine
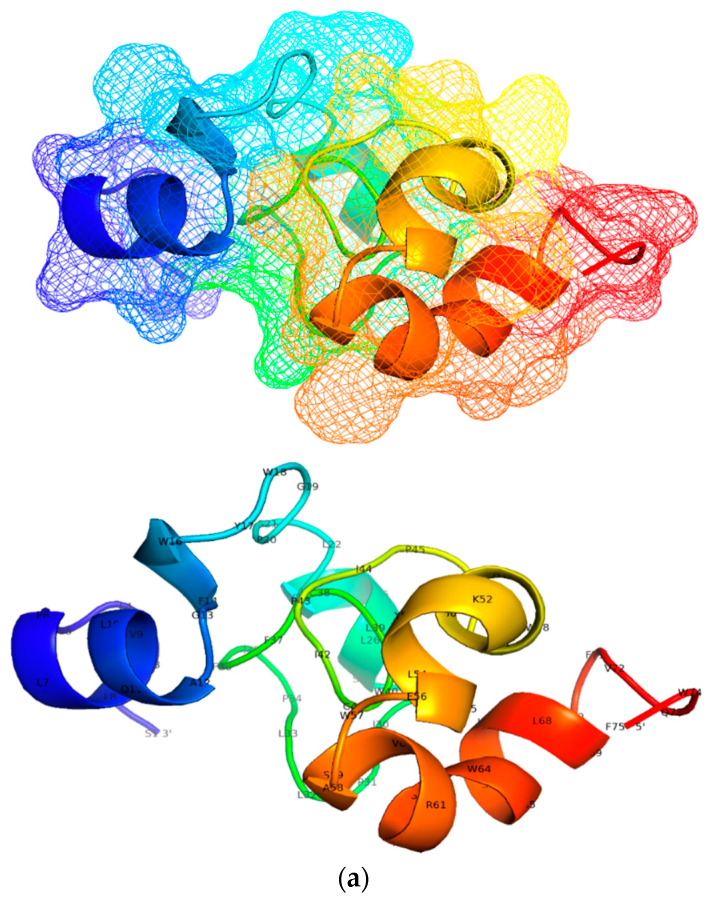
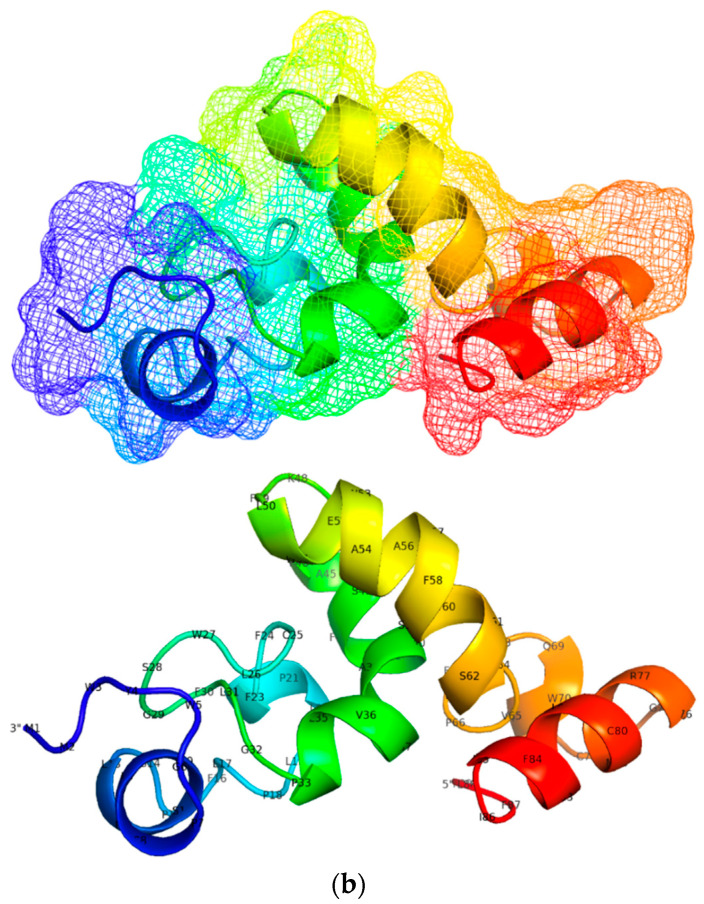
Table 4 shows 27 putative epi binding to at least 6 alleles and were selected to model the tertiary structure of candidate vaccines. The overlapping S region epi (SA = 11, SD = 10) were catenated to form proteins which were used to model a tertiary structure as shown in Figure 3. The overlapping SA epi at S protein residues 6–20 with aa sequence SGFLGPLLVLQAGFF, aa sequence CIPIPSSWAFAKYLWEWASVRFSWLSLLVPFVQWF at S protein residues 155–183, and aa sequence WYWGPSLYNILSPFIPLLPIFFCLW at S protein residues 199–223 yields a 75 amino acid protein of mw = 8878.62. Of the 6 proteins predicted, the most stable SA protein was vacci-SA with amino acid sequence: 5′-SGFLGPLLVLQAGFFWYWGPSLYNILSPFIPLLPIFFCLWCIPIPSSWAFAKYLWEWASVRFSWLSLLVPFVQWF-3′. The overlapping epi in SD occupied residues S: 6–20, S: 68–82, S: 197–223 and S: 155–183. The resulting protein was 85 aa long and had mw = 10,749.99. Of the 24-proteins predicted, the one selected for constructing tertiary structure was vacci-SD with aa sequence: 5′-MMWYWGPSLYSILSPFLPLLPIFFCLWSGFLGPLLVLQAGFFSWAFGKFLWEWASARFSWLSLLVPFVQWFTCPGYRWMCLRRFIIFLF-3′. When a BLAST search was conducted with the NCBI protein database for the 2 protein sequences, results show that both sequences were similar to 2 domains of major surface antigen (vMSA) from hepadnavirus superfamily; accession number pfam00695. A similar approach was used to select proteins from the epi in PreS2 region to determine proteins that can be used to model the 3D tertiary structures of epi in the PreS2 region. In total, there were 6 epi selected 3 for each genotype. The overlapping epi were 20 aa long with mw of 2006.20 and occupying residues PreS2: 34–53 in genotype A sequences. vacci-PreSD was made from overlapping epi occupying residues PreS2: 37–53 and had a mw of 1719.91. All proteins generated using the different epi ordering (permutations and combinations) have been provided under the supplementary file Supplementary Table S5.
Figure 3.
Showing a tertiary structure of candidate vaccines: (a) Tertiary structures of candidate epi modelled using 3Dpro webtool. The SA protein in (a) has the aa composition: 5′-SGFLGPLLVLQAGFFWYWGPSLYNILSPFIPLLPIFFCLWCIPIPSSWAFAKYLWEWASVRFSWLSLLVPFVQWF-3′, and had following theoretical properties: antigenicity (0.04), instability index (II = 47.07), estimated half-life in vitro = 1.9 h, molecular weight (mw) = 8878.62, aliphatic index (AI = 114.40) and grand average of hydrophobicity (GRAVY = 0.995), and theoretical alkalinity (pI = 7,76). Using VaxiJen ver2.0 server set at threshold of 0.4, the overall prediction for the protective antigen was 0.53, displaying it as a plausible antigen [66]. (b) (5′-MMWYWGPSLYSILSPFLPLLPIFFCLWSGFLGPLLVLQAGFFSWAFGKFLWEWASARFSWLSLLVPFVQWFTCPGYRWMCLRRFIIFLF-3′) protein modelled using from SD epi. The protein had following theoretical properties: antigenicity (0.11), instability index (II = 53), estimated half-life in vitro 30 h, molecular weight (mw) = 10749.99, aliphatic index (AI = 101.91) and grand average of hydrophobicity (GRAVY = 0.965), and theoretical alkalinity (pI = 9.42),). The antigenicity score predicted in both candidate vaccine suggests that they are plausible antigens [66].
3.6. Mutations Associated with Escape from Class II HLA Binding
For amino acid variants present in more than 5% of sequences of N1 database, we evaluated the in silico impact on immune recognition. Mutations were labelled relative to the HBV surface gene proteins—PreS11–115, PreS21–42, and S1–226. Mutations that hindered HBV peptide-HLA binding were 86T, 90T, and 94P in PreS1A; 54P, 79E, 84S, and 85Q in PreS1D; 12I, 31I, and 54P in PreS2A; 5F, 22H, 22L, 22P, 32H, 36L, and 42S in PreS2D. The list of escape mutations and their prevalence in 7743 HBV genotype A and D sequences are shown in Table 5 and supplementary file Supplementary Table S5. There were coordinated variations among positions in aa sequence alignments that had an impact on HBV-HLA binding but showed no impact when analyzed individually. These were termed covariance mutations. The combinations of the covariance mutations and their impact on binding potential are shown in Table 5, Table 6 and Table 7 below.
Table 5.
The prevalence of S gene escape mutations.
| Protein | AA Sequence | S Protein AA Residues (epi) | Escape Mutations | Count in A (n = 3115) | Count in D (n = 4628) | Count in Other Genotypes |
|---|---|---|---|---|---|---|
| SA | MENITSGFLGPQLV | 1–14 | L12Q | 13 | 1 | 3 |
| 1–14 | I4T | 13 | 25 | 91∆ | ||
| 1–14 | I4Stop | 10 | - | - | ||
| SA | GPLLVLQAGFFLLTR | 10–24 | L15Stop | 2 | - | 17 |
| SA | LNFPGGSPVCLGQNS | 39–53 | L42P | 13 | 6 | 53 |
| SA | TRILTIPQ *LDSWWT | 23 -37 | S31Stop | 6 | 12 | - |
| SA | DLWWTSLNFLGDPPV | 33–47 | G44D | 4 | - | 16 |
| DSWWTSLNFLGESPV | G44E | 105 | 87 | 826 | ||
| IPIPSSWGFAKYLWE | 150–164 | A157G | 10 | 5 | 3 | |
| SA | IPIPSSWAFVKYLWE | A159V | 20 | 3 | 234 | |
| SA | PPICPGYRWMCQRRF | 66–80 | L77R | 7 | 2 | 43 |
| L77Q | 6 | - | 18 | |||
| SA | PPICPGYRWMCLR *F | R79Stop | 9 | 10 | 20 | |
| R79H | 13 | 58 | 49 | |||
| SA | LIFLLVLLDYQDMLP | 91–105 | G102D | 1 | 2 | 3 |
| SA | CLIFLLVLLDYQGML | 90–104 | D99Stop | 15 | 5 | 8 |
| Y100C | 196 | 12 | 17 | |||
| M103I | 31 | 31 | 29 | |||
| SA | SLLVPFVQWFVGLTP LLVPFVQWFEGLSPT | 174–188 | S187T | 1 | - | - |
| G185E | 6 | 15 | 19 | |||
| V184E | 1 | 2 | 2 | |||
| L186P | 10 | - | 5 | |||
| SA | SPTVWLLAIWMMWYW | 187–193 | S193L | 104 | 257 | 612 |
| A194V | 494 | ∆ * | ||||
| SA | GPSLYNISSPFIPLL | 202–216 | L209S | 4 | 9 | 12 |
| SD | ENITSGCLGPLLVLQAGF | 2–16 | F8C | - | 1 | - |
| SA | YLWEWASVRFSWPSL | 161–175 | L173P | 1 | 3 | 11 |
| SA | SPFIPLLLIFFCLWV | 210–224 | P214L | 11 | 41 | 23 |
| SD | SSWAFGKFLWEWASA | 154–168 208–222 | K160N | 1 | 7 | 9 |
| SD | ILSPYILLLPIFFCI | F212Y + L213I + P214L | -; -; 11 | 40; 202; 41 | 3; ∆ *;23 | |
| NITSGFLGLLLVLQA | 4 -18 | P11L | 2 | 4 | 3 | |
| SD | MENITSGFLGPLLVL | 1–15 | T5P; N3S + I4T + T5A | 2; - | 4; 2 | 4; -29 |
| SD | SWWTSLNFLGETTVC | 34–48 | G44E | 105 | 87 | 826 |
| SD | SWWTSLNFRGGTTVC | L42R | 14 | 8 | 14 | |
| SD | LSVIWMMWYWGPNLY | 192–206 | S204N | 136 | 194 | 848 |
| List of aa Variations within Genotype A and D epi | M1E, M1L, M1K, M1I, M1V, M1T, M1R, E2H, E2Q, E2*, E2V, E2K, E2A, E2G, E2D, N3H, N3E, N3P, N3A, N3C, N3D, N3I, N3R, N3Y, N3K, N3T, N3G, N3S, I4L, I4R, I4Y, I4Q, I4F, I4M, I4H, I4K, I4A, I4P, I4S, I4N, I4V, I4T, T5Y, T5Q, T5L, T5R, T5E, T5K, T5V, T5P, T5I, T5S, T5A, S6G, S6F, S6A, S6P, S6T, S6L, G7C, G7D, G7*, G7A, G7V, G7K, G7E, G7R, F8C, F8A, F8G, F8V, F8Y, F8I, F8H, F8P, F8S, F8L, L9R, L9K, L9V, L9I, L9H, L9Q, L9P, G10D, G10*, G10V, G10Q, G10T, G10A, G10K, G10E, G10R, P11A, P11T, P11H, P11L, L12V, L12E, L12M, L12R, L12P, L12Q, L13Q, L13V, L13I, L13F, L13R, L13H, L13P, V14R, V14L, V14E, V14I, V14M, V14G, V14A, L15E, L15K, L15T, L15*, L15I, L15F, L15V, L15S, Q16K, Q16E, Q16H, Q16L, Q16R, Q16P, A17R, A17T, A17S, A17P, A17V, A17G, A17E, G18W, G18K, G18E, G18A, G18R, G18V, F19I, F19V, F19L, F19S, F19Y, F19C, F20I, F20Y, F20L, F20S, L21V, L21M, L21F, L21*, L21W, L21S, L22Q, L22M, L22V, L22F, L22S, L22*, L22W, T23F, T23R, T23Q, T23S, T23P, T23A, T23I, R24N, R24T, R24Q, R24E, R24I, R24G, R24S, R24K, I25R, I25S, I25F, I25N, I25A, I25T, I25V, L26Y, L26I, L26F, L26Q, L26P, L26H, L26R, T27R, T27P, T27S, T27A, T27K, T27I, I28K, I28L, I28V, I28T, I28M, P29E, P29A, P29F, P29S, P29Q, P29T, P29L, Q30M, Q30S, Q30P, Q30A, Q30L, Q30H, Q30R, Q30K, S31K, S31I, S31D, S31T, S31C, S31G, S31R, S31N, L32V, L32G, L32Q, L32R, L32I, L32P, D33V, D33H, D33Y, D33E, D33N, D33G, S34*, S34A, S34T, S34W, S34P, S34L, W35P, W35C, W35*, W35G, W35R, W35L, W36E, W36G, W36R, W36*, W36L, T37D, T37P, T37L, T37I, T37S, T37N, T37A, S38E, S38A, S38Y, S38F, S38P, L39I, L39V, L39R, L39H, L39F, L39P, N40Q, N40H, N40K, N40I, N40D, N40S, F41Q, F41I, F41Y, F41C, F41L, F41S, L42*, L42S, L42I, L42V, L42Q, L42R, L42P, G43V, G43W, G43K, G43R, G43E, G44Q, G44K, G44R, G44A, G44D, G44V, G44E, S45M, S45D, S45G, S45E, S45H, S45Q, S45R, S45I, S45K, S45N, S45V, S45L, S45P, S45A, S45T, P46N, P46R, P46S, P46I, P46A, P46H, P46L, P46T, V47Q, V47N, V47L, V47P, V47M, V47E, V47R, V47K, V47A, V47G, V47T, C48W, C48L, C48F, C48R, C48G, C48S, C48Y, L49A, L49T, L49C, L49S, L49I, L49V, L49F, L49H, L49R, L49P, G50C, G50W, G50P, G50R, G50D, G50V, G50S, G50A, Q51E, Q51K, Q51H, Q51P, Q51R, Q51L, N52T, N52G, N52H, N52K, N52I, N52D, N52S, S53G, S53M, S53*, S53T, S53P, S53W, S53L, Q54K, Q54*, Q54H, Q54L, Q54P, Q54R, S55T, S55Y, S55A, S55P, S55F, S55C, P56A, P56*, P56S, P56R, P56H, P56L, P56Q, T57S, T57A, T57I, S58Y, S58A, S58L, S58T, S58P, S58F, S58C, N59L, N59I, N59R, N59H, N59D, N59K, N59S, H60D, H60S, H60N, H60Y, H60Q, H60R, H60P, S61T, S61P, S61*, S61L, P62S, P62Q, P62L, T63V, T63F, T63N, T63S, T63A, T63I, S64W, S64P, S64F, S64Y, S64C, C65G, C65F, C65S, C65Y, C65R, P66S, P66L, P66T, P66A, P66Q, P66H, P67T, P67A, P67R, P67S, P67L, P67Q, I68D, I68P, I68V, I68F, I68S, I68N, I68A, I68T, C69F, C69G, C69S, C69R, C69Y, C69W, C69*, P70S, P70H, P70R, P70A, P70T, P70L, G71S, G71D, G71W, G71V, Y72S, Y72D, Y72N, Y72H, Y72F, Y72C, R73L, R73C, R73P, R73H, W74C, W74G, W74R, W74*, W74S, W74L, M75K, M75L, M75R, M75S, M75V, M75T, M75I, C76G, C76*, C76R, C76W, C76S, C76F, C76Y, L77P, L77G, L77M, L77V, L77Q, L77R, R78I, R78G, R78W, R78P, R78L, R78Q, R79P, R79G, R79L, R79C, R79S, R79H, F80G, F80N, F80Y, F80I, F80L, F80S, I81S, I81Y, I81N, I81F, I81M, I81V, I81T, I82F, I82N, I82V, I82M, I82T, I82L, F83Y, F83I, F83L, F83C, F83S, L84F, L84H, L84P, F85Q, F85R, F85P, F85L, F85Y, F85S, F85C, I86M, I86K, I86L, I86F, I86V, I86T, L87M, L87V, L87R, L87Q, L87P, L88R, L88V, L88M, L88Q, L88P, L89V, L89Y, L89T, L89R, L89I, L89Q, L89P, C90G, C90W, C90N, C90I, C90*, C90Y, C90F, C90S, L91G, L91F, L91P, L91R, L91H, I92H, I92N, I92V, I92M, I92L, I92S, I92T, F93R, F93W, F93A, F93Y, F93I, F93L, F93C, F93S, L94G, L94*, L94V, L94F, L94M, L94W, L94S, L95P, L95F, L95M, L95V, L95S, L95*, L95W, V96I, V96E, V96N, V96F, V96L, V96C, V96D, V96A, V96G, L97C, L97R, L97V, L97H, L97F, L97P, V98M, V98W, V98A, V98Q, V98P, V98R, V98L, D99H, D99K, D99V, D99A, D99E, D99N, D99G, Y100P, Y100*, Y100K, Y100D, Y100H, Y100N, Y100L, Y100W, Y100S, Y100F, Y100C, Q101P, Q101L, Q101*, Q101K, Q101H, Q101R, G102N, G102R, G102A, G102D, G102C, G102S, G102V, M103K, M103R, M103L, M103T, M103V, M103I, L104Q, L104M, L104G, L104V, L104W, L104S, L104F, P105T, P105S, P105H, P105A, P105L, P105R, V106L, V106R, V106Q, V106D, V106E, V106G, V106I, V106A, C107A, C107W, C107*, C107G, C107S, C107Y, C107R, P108A, P108T, P108S, P108L, P108H, L109R, L109V, L109I, L109Q, L109M, L109P, I110R, I110H, I110V, I110N, I110F, I110S, I110P, I110T, I110M, I110L, A111N, A111R, A111T, A111Q, A111S, A111L, A111P, G112V, G112K, G112N, G112E, G112A, G112R, S113C, S113R, S113F, S113L, S113Y, S113K, S113P, S113N, S113A, S113T, T114V, T114*, T114L, T114R, T114N, T114M, T114K, T114A, T114P, T114S, T115P, T115S, T115A, T115I, T115N, T116S, T116P, T116A, T116I, T116N, S117Y, S117A, S117V, S117R, S117I, S117G, S117N, S117T, T118W, T118N, T118G, T118I, T118E, T118P, T118S, T118K, T118R, T118M, T118A, T118V, G119S, G119W, G119T, G119*, G119C, G119V, G119E, G119R, P120H, P120N, P120R, P120L, P120A, P120Q, P120S, P120T, C121T, C121G, C121F, C121L, C121Y, C121S, C121W, C121R, K122G, K122V, K122E, K122N, K122T, K122I, K122S, K122Q, K122R, T123G, T123V, T123P, T123S, T123N, T123I, T123A, C124G, C124R, C124W, C124Y, C124S, C124F, T125P, T125I, T125A, T125M, T126P, T126Q, T126K, T126G, T126M, T126L, T126V, T126N, T126A, T126S, T126I, P127F, P127R, P127V, P127H, P127S, P127A, P127I, P127T, P127L, A128T, A128R, A128G, A128V, Q129E, Q129K, Q129L, Q129P, Q129N, Q129H, Q129R, G130V, G130*, G130D, G130K, G130A, G130E, G130S, G130R, G130N, N131Q, N131G, N131D, N131H, N131K, N131S, N131I, N131A, N131P, N131T, S132H, S132Y, S132P, S132C, S132F, M133R, M133G, M133K, M133V, M133Q, M133S, M133I, M133L, M133T, F134K, F134D, F134W, F134T, F134Q, F134C, F134R, F134V, F134H, F134S, F134L, F134N, F134I, F134Y, P135S, P135R, P135T, P135H, P135L, S136T, S136C, S136A, S136L, S136P, S136F, S136Y, C137S, C137R, C137*, C137Y, C137W, C138S, C138*, C138W, C138G, C138R, C138Y, C139S, C139G, C139*, C139Y, C139W, C139R, T140P, T140K, T140M, T140A, T140L, T140I, T140S, K141N, K141Q, K141T, K141I, K141R, K141E, P142T, P142A, P142H, P142R, P142I, P142S, P142L, T143W, T143F, T143A, T143P, T143M, T143L, T143S, D144Y, D144N, D144V, D144A, D144G, D144E, G145D, G145V, G145I, G145S, G145K, G145E, G145A, G145R, N146I, N146T, N146K, N146S, N146D, C147W, C147S, C147R, C147G, C147Y, T148S, T148I, T148A, C149S, C149G, C149W, C149Y, C149R, I150S, I150N, I150M, I150V, I150T, P151S, P151R, P151T, P151L, P151H, I152H, I152F, I152L, I152T, I152V, P153A, P153T, P153L, P153Q, P153S, S154Q, S154A, S154H, S154V, S154T, S154P, S154L, S155T, S155A, S155Y, S155F, S155P, W156S, W156C, W156G, W156R, W156*, W156L, A157S, A157N, A157D, A157P, A157V, A157T, A157G, F158I, F158Y, F158S, F158L, A159P, A159Q, A159L, A159S, A159T, A159R, A159E, A159V, A159G, K160Q, K160T, K160E, K160G, K160S, K160N, K160R, Y161K, Y161C, Y161V, Y161H, Y161I, Y161L, Y161S, Y161F, L162S, L162*, L162R, L162V, L162I, L162P, L162Q, W163G, W163C, W163R, E164*, E164K, E164A, E164V, E164D, E164G, W165Q, W165*, W165G, W165C, W165S, W165R, W165L, A166S, A166D, A166P, A166T, A166V, A166G, S167Y, S167E, S167T, S167G, S167P, S167L, V168L, V168I, V168F, V168G, V168D, V168S, V16887T, V168P, V168A, R169G, R169L, R169S, R169C, R169P, R169H, F170V, F170L, F170S, S171N, S171Y, S171C, S171L, S171P, S171F, W172M, W172F, W172G, W172R, W172S, W172L, W172C, W172*, P173I, P173Y, P173S, P173V, P173F, P173L, S174G, S174K, S174C, S174R, S174T, S174I, S174N, L175I, L175*, L175F, L175S, L176I, L176V, L176Q, L176P, V177P, V177I, V177S, V177G, V177E, V177M, V177L, V177A, P178G, P178R, P178H, P178A, P178L, P178S, P178Q, F179I, F179C, F179L, F179Y, F179S, V180P, V180L, V180E, V180F, V180I, V180A, Q181K, Q181E, Q181L, Q181H, Q181R, W182F, W182G, W182S, W182R, W182C, W182L, W182*, F183V, F183Y, F183I, F183L, F183S, F183C, V184L, V184P, V184D, V184F, V184I, V184E, V184G, V184A, G185K, G185L, G185W, G185R, G185A, G185E, L186M, L186A, L186R, L186C, L186S, L186I, L186V, L186F, L186P, L186H, S187A, S187T, S187C, S187P, S187F, S187L, P188A, P188S, P188R, P188T, P188H, P188L, T189L, T189N, T189A, T189S, T189P, T189I, V190S, V190E, V190P, V190D, V190G, V190I, V190F, V190A, W191K, W191S, W191*, W191G, W191L, W191R, W191C, L192R, L192H, L192V, L192F, L192P, S193*, S193T, S193P, S193F, S193L, A194G, A194D, A194S, A194T, A194F, A194L, A194I, A194V, I195K, I195V, I195L, I195T, I195M, W196G, W196V, W196K, W196R, W196*, W196S, W196L, M197L, M197K, M197R, M197V, M197I, M197T, M198L, M198V, M198R, M198K, M198T, M198I, W199P, W199C, W199R, W199G, W199S, W199*, W199L, Y200H, Y200*, Y200L, Y200N, Y200S, Y200C, Y200F, W201C, W201L, W201G, W201S, W201R, W201*, G202V, G202E, G202R, G202A, P203H, P203T, P203G, P203S, P203A, P203L, P203Q, P203R, S204Q, S204H, S204C, S204D, S204I, S204T, S204G, S204K, S204R, S204N, L205Q, L205R, L205V, L205P, L205M, Y206G, Y206P, Y206Q, Y206*, Y206I, Y206W, Y206V, Y206D, Y206R, Y206L, Y206N, Y206S, Y206F, Y206H, Y206C, N207G, N207K, N207C, N207D, N207P, N207H, N207I, N207T, N207R, N207S, I208L, I208C, I208F, I208S, I208V, I208N, I208T, L209K, L209A, L209F, L209*, L209G, L209M, L209S, L209W, L209V, S210M, S210G, S210I, S210T, S210K, S210R, S210N, P211A, P211T, P211S, P211R, P211L, P211H, F212C, F212S, F212L, F212Y, I213A, I213V, I213S, I213F, I213T, I213M, I213L, P214G, P214R, P214T, P214H, P214S, P214Q, P214L, L215D, L215I, L215V, L215T, L215M, L215Q, L215R, L215P, L216T, L216G, L216C, L216R, L216Y, L216V, L216I, L216S, L216F, L216*, P217A, P217T, P217Q, P217S, P217L, I218P, I218K, I218S, I218F, I218N, I218T, I218L, F219C, F219I, F219L, F219S, F220V, F220I, F220S, F220W, F220Y, F220L, F220C, C221V, C221R, C221W, C221L, C221S, C221G, C221F, C221Y, H222Q, H222V, H222S, H222R, H222F, H222I, H222P, H222L, W223F, W223G, W223C, W223R, W223*, W223L, V224L, V224I, V224E, V224G, V224A, Y225A, Y225*, Y225N, Y225D, Y225L, Y225I, Y225H, Y225C, Y225F, Y225S, I226R, I226H, I226L, I226F, I226N, I226V, I226T, I226M, I226S, | |||||
1R represents the position of the mutation ‘1’ and R is the change in amino acid “candidate escape mutations”. ∆ Genotype C sequences were excluded in the analysis. ∆* genotype B, C, D, E, F, G, H and I sequences were excluded in this analysis. * = Stop codon.
Table 6.
Summary of PreS1 epitopes binding to respective alleles, and mutations that exists in the Botswana HBV sequences.
| Epitope Position | Core AA Sequence | HLA_ DRB*1/5_ Genotype A | Epitopes Position | Core AA Sequence | HLA_ DRB*1/5_ Genotype D | Mutations Relative to Protein: (WT, aa#, Mut) | |
|---|---|---|---|---|---|---|---|
| A | D | ||||||
| 84–92 | ILATVPAVP ¥ | *0802 | 73–81 | ILQTLPANP | I84V, I84T, A86V, A86T, V88M, V88L, A90P, A90V, P92L | I73M, L75H, L77M, L77V, A79E | |
| 85–93 | LATVPAVPP ¥ | *˜0802 | 74–82 | LQTLPANPP | I84V, I84T, A86V, A86T, V88M, V88L, A90P, A90V, P92L | I73M, L75H, L77M, L77V, A79E | |
| 34–42 | FGANSNNPD | 23–30 | FRANTANP ¥ | *0401 | - | R24K | |
| 16–24 | LSVPNPLGF | 5–13 | LSTSNPLGF ¥ | *0401, *1302 | - | - | |
| 24–32 | FFPDHQLDP | 13–21 | FFPDHQLDP | *0401, | F25L | - | |
| 63–71 | FGPGFTPPH | 52–60 | FGLGFTPPH | *0101, *0401, *0701, 5*0101 | F67L | L54M, L54P, F56L | |
| 34–42 | FGANSNNPD | 23–30 | FRANTANPD | *0101, *0401, *0802, *1302 | - | R24K | |
| 67–75 | FTPPHGGVL | 56–64 | FTPPHGGLL | *0101, *0701, | F67L, G73R | - | |
| 28–36 | HQLDPAFGA | 17–25 | HQLDPAFRA | *0301, *0401, | - | R24K | |
| 84–92 | ILATVPAVP | *0101, *0301, *0401, *0701, *0802, *1101, *1302, *1501, 5*0101 | 73–81 | ILQTLPANP | *0101, *0301, *0401, *0701, *0802,*1302, | I84V, I84T, A86V, A86T, V88M, V88L, A90P, A90V, P92L | I73M, L75H, L77M, L77V, A79E |
| 85–93 | LATVPAVPP | *0401, *0802, 5*0101 | 74–82 | LQTLPANPP | *0802, 5*0101 | I84V, I84T, A86V, A86T, V88M, V88L, A90P, A90V, P92L | I73M, L75H, L77M, L77V, A79E |
| 74–82 | LLGWSPQAQ | *1501 | 63–71 | *0101, *0401, *0802, *1501 | W77stop, S78R, P79S, A81S, A81P | S67N, P68S | |
| 16–24 | LSTSNPLGF | 5–13 | *0301, *0701, *1302 | - | - | ||
| 12–21 | MGTNLSVPN | *0401, *0802, *1302 | 1–10 | MGQNLSTSNP | - | G2E | |
| 15–22 | NLSVPNPLG | *0401, | 4–12 | NLSTSNPLGF | - | - | |
| 83–90 | QGILATVPA | *0101, *0802 | 71–79 | QGILQTLPA | *0101, | G83D, I84V, I84K, A86T, A86V, V88L, V88M, A90T, A90V | I73M, L75H, L77M, L77V, A79E |
| 14–22 | TNLSVPNPL | *0101, *0701, *1302 | 3–11 | QNLSTSNPL | *0101, *0401, | - | - |
| 4–12 | WSAKPRKGM | *1101, 5*0101 | - | S5P, A6S, A6T, K10N, K10I | - | ||
| 77–85 | WSPQAQGIL | *0101, *0701, | 66–75 | WSPQAQGIL | *0101, *0701, | W77stop, S78R, P79S, A81P, A81S, G83D, I84V, I84K | P68S, |
*0101 means HLA class II allele DRB1*0101 e.tc. 5*0101 means HLA class II allele DRB5*0101. ¥ indicates SB.
Table 7.
Summary of PreS2 epitopes binding to respective alleles, and mutations that exist within the Botswana HBV sequences.
| Genotype | AA Sequence Core | AA Sequence of epi | Pres2 epi Residues | Count of epi with Core AA Sequence | HLA Class II Alleles | Mutations in the Core Sequence |
|---|---|---|---|---|---|---|
| A | ALQDPRVRG | AFHQALQDPRVRGLYFPA | 7–24 | 7 | *0301∆ | R16K, V17I |
| A | ASHISSISS | VPNTASHISSISSRT | 35–49 | *0401 | A39T, I45S | |
| D | ASPLSSIFS | VPTTASPLSSIFSRIG | 35–50 | 2 | *0101, *0401 | L42I, L42S |
| A | FHQALQDPR | NSTAFHQALQDPRVRG | 4–19 | 2 | *0301, 5*0101 | A11T, R16K |
| D | FHQTLQDPR | NSTTFHQTLQDPRVR | 4–18 | 1 | *0301 | - |
| D | FSRIGDPAL | SPLSSIFSRIGDPALN | 40–55 | 1 | *0101, *0401, *0701, 5*0101 | R48T, P52H, A53V, L54P |
| A | HISSISSRT | VPNTASHISSISSRTG | 35–50 | 4 | *0101, *0401, *0701 | I45S, R48T |
| D | IFSRIGDPA | SPLSSIFSRIGDPALN | 40–55 | 2 | *0802, *1302, *1501 | L42I, L42S |
| A | ISSISSRTG | PNTASHISSISSRTGDPALN | 36–55 | 6 | *0101, *0401, *0701, *0802, *1101, *1501, 5*0101 | I45S, R48T |
| A | LNPVPNTAS | SSSGTLNPVPNTASHISSI | 27–45 | 5 | *0401, *0802 | L32H, L32R, P34L, N37H, N37T, I38T, A38T |
| D | LSSIFSRIG | PTTASPLSSIFSRIGDPALN | 36–55 | 6 | *0101, *0401, *0701, *0802, *1101, *1501, 5*0101 | P52L, A53V |
| A | MQWNSTAFH | MQWNSTAFHQALQDP | 1–15 | 1 | *1302 | Q2I, A7T |
| MQWNSTTFHQTLQDP | - | - | S5F, S5Y, T6A, T7I, T7N | |||
| D | PLSSIFSRI | VPTTASPLSSIFSRI | 35–49 | 1 | *0701 | L42I, L42S |
| A | PVPNTASHI | SSGTLNPVPNTASHISSI | 27–45 | 6 | *1302 | P34L, N37H, N37T |
| D | PVPTTASPL | VNPVPTTASPLSSIF | 32–46 | 2 | *0701 | P34H, P36L, L42I, L42S |
| A | QALQDPRVR | TAFHQALQDPRVRGLYF | 6–22 | 3 | 5*0101 | - |
| D | QTLQDPRVR | STTFHQTLQDPRVRGLYF | 5–22 | 4 | 5*0101 | R22K |
| D | TLQDPRVRG | STTFHQTLQDPRVRGLYFPA | 6 | *0301 | R18K, G19D, G19A | |
| A | VRGLYFPAG | DPRVRGLYFPAGGSSSG | 14–30 | 3 | *0802, *1101, *1501 | V17I, Y21N, Y21S, F22L, F22P, F22T, P23A |
| D | 3 | *0802, *1101, *1501 | R18K, G19D, G19A, F2222Q, F22H, F22P, F22L | |||
| A | WNSTAFHQA | MQWNSTAFHQALQDP | 1–15 | 1 | *0401, *0701 | A7T, A11T |
| D | WNSTTFHQT | MQWNSTTFHQTLQDP | 1 | *0701 | S5F, S5Y, T6A, T7I, T7N | |
| A | YFPAGGSSS | PRVRGLYFPAGGSSSGTLNP | 15–34 | 6 | *0101, *0401, *1101, 5*0101 | Y21N, Y21S, F22L, F22P, F22T, P23A, S29L |
| D | YFPAGGSSS | PRVRGLYFPAGGSSSGTVNP | 6 | *0101, *0401, 5*0101, *1101 | F22Q, F22H, F22P, F22L |
*0101 means HLA class II allele DRB1*0101 e.tc. 5*0101 means HLA class II allele DRB5*0101.
3.7. Assessing the Distribution of HBV-HLA (epi) as a Predictor of HLA Protective Effective
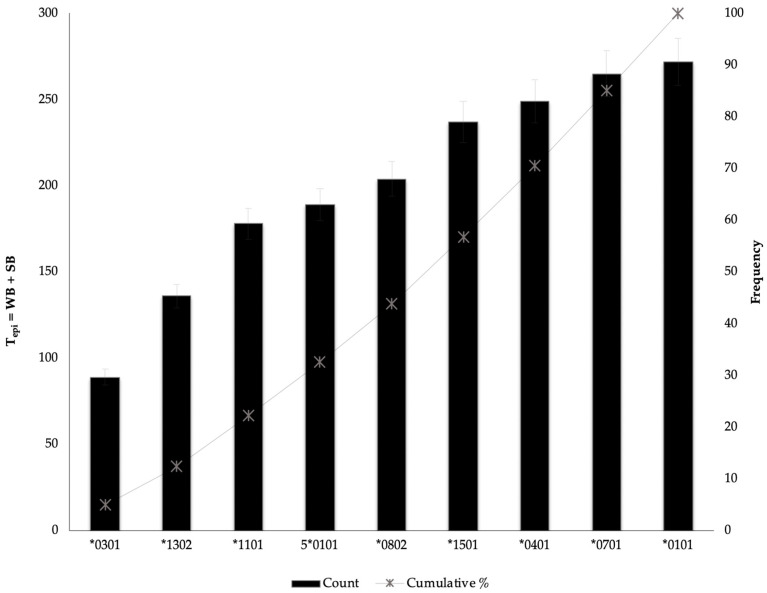
We used existing information on immunological studies for meta-analysis to estimate Tepi as a predictor of the protective effect of MHC class II alleles. Figure 4 shows a Pareto analysis and the 20% threshold indicates the 3 HLA class II alleles—DRB1*0301, 1302, *1101—that are highly likely to be less protective against HBV. We therefore speculate, with caution, that there is a relationship between Tepi and protectiveness and this should be further investigated to establish correlations (p-value < 0.05) with high statistical confidence.
Figure 4.
Pareto Analysis applied to rank the Tepi of alleles against their percentage frequency.
4. Discussion
This detailed HBV immunoinformatics approach outlines the candidate peptides that can be used to develop biologicals against CHB. However, mutations within the T cell epitopes may impair HBV-HLA complex formation, a crucial component responsible for initiating cascade of responses for viral clearance [67]. In this study, we observed that there were no epi that bind to all alleles, and there was a large difference between the epi profiles of genotype A compared to genotype D. This phenomenon may explain previous failures during preclinical trials of candidate vaccines against CHB generated thus far, which were designed for broad potency. [68,69,70,71,72,73,74,75]. This may suggest that a genotype- and population-based, multi-epitope vaccine would be the best candidate to combat HBV. Using the cut-off set in this analysis, 21 epi from S regions and 6 from PreS2 regions were identified and used to construct tertiary structure of candidate vaccine. The candidate vaccines showed high binding in all alleles except for DRB1*0301. Populations with high coverage of DRB1*0301 have been closely associated with high susceptibility to CHB infection and nonresponse vaccination with envelope proteins [53,76,77].
Among the 3 types of proteins (PreS/S) analyzed in this study, S had the highest binding peptides constituting 79.9% of the sum of all Tepi (epi), and genotype D had the most epitopes. However, when Depi were compared between genotypes (A and D) and proteins (PreS/S), it was clear that the length of proteins where independent of the epi but dependent on the aa compositions and the position of the epi within the proteins. While S region was the longest protein (213 aa), it had similar Depi to the smallest protein PreS2D (PreS2 -54 aa). Previous studies that investigated all 7 proteins of HBV and showed that S protein had the most T cell antigenic epitopes [78,79]. However, further investigation should be conducted to determine any relationships between antigenicity and PreS1 protein with least epi.
We also compared the frequency of epi between 2 genotypes, A and D, and observed that genotype D had generally more immunogenic epi than A with exception for PreS2A whose epi were 12% more than those of PreS2D, (Figure 1). Clinically, these trends correspond to data reported from studies that investigated the prognosis of patients infected with HBV genotype A1 strains compared to genotype D3. Others have reported a 10-fold increased progression to HCC among HBV patients infected with genotype A compared to genotype D and that patients infected with genotype A strains were likely to progress to CHB compared to genotype D [8,80]. Most countries in SSA, including Botswana, have low prevalence of HBV genotype D3 among different risk groups than genotype A1 [25,26,27,81].
Using existing information from studies that investigated the impact of alleles on different HBV outcomes to validate our statistical associations [53,82,83,84,85], we observed that out of 9 alleles, HLA-DRB*1301/2 and *0401 alleles—which had most epi—have been associated with spontaneous clearance of HBV infection [18,24,33,68,78,82,85,86,87], and HLA DRB1*0301 that had the least epi in our study has been previously associated with susceptibility to HBV infection, autoimmune hepatitis, chronicity, and non-responsiveness to HBV vaccination across different ethnic groups [9,88,89]. This strongly suggests that Tepi of PreS/S should be explored further as a predictor of the protective effect of HLA class II alleles.
A host immune system can recognize foreign antigens (epi) and clear the infection in some cases [90,91,92]; however, most pathogens including HBV can mutate within epitopes, and this may result in an escape from host immune surveillance leading to persistence of infection [93]. This characteristic is regarded as one of the major hindrances in developing high potency therapeutic drugs. This mechanism of aa changes within epitopes (escape mutations) interferes with both peptide processing reducing the intracellular antigen load and downregulation of MHC expression hence increased risk of developing liver malignancies (HCC, LC) among CHB patients [4,5,94,95,96,97,98]. Studies investigating the role of escape mutations within the T cell epitopes are relatively rare. In this study, we observed coordinated aa variations, which reveal genetic dependencies (i.e., epi that escaped HLA binding when there were two or more mutations); however, some single aa mutations altered the binding potential. These mutations were termed covariance mutations. For instance, in the proportion of PreS1 binding peptides from both genotype A and D against alleles shown in Figure 3, 15-mers with core aa sequence ILATVPAVP84–92 in PreS1A—corresponding to ILQTLPANP73–81 in PreS1D—weakly binds to alleles: *0101; 0401; 0701; 0802; *1101; 1302; *1501; 5*0101 → PreS1A, and *0101; 0401; 0701; 0802; 1302→ PreS1D respectively. The aa Ala in genotype A is replaced by aa Gln for genotype D [A(A) → Q (D)] and [Val(A) → Lue(D)]91 causing PreS1D to pseudo bind to 3 alleles (*1101, 1302, and 5*0101). Additionally, the epitope strongly binds to allele *0802, but the changes in genotype D result in pseudo binding. Table 3, Table 4, Table 5, Table 6 and Table 7 summarize all the core aa of PreS/S epitopes that are restricted to 9 HLAs. We observed that the HBV epitope-HLA is greatly influenced by the position of core aa. For instance PreS1D epi AFGLGFTPPHGGLLG51–65 is a WB to alleles: *0101, *0401 and 5*0101 when using core-aa: FGLGFTPPH52–60 but it can only bind to *0701 when the core aa is FTPPHGGLL57–64. Furthermore, post epi analyses show that there were mutations outside the core aa sequences but had impact on the HBV-HLA blinding. For instance, 2 epi with aa residues S: 41–55—FLGGPPVCLGQNSQS and FLGGSPVCLGQNSQS—and all with core aa sequences FLGGPPVCL show different binding affinities thus NB and WB respectively. The escape mutations defined in the present study were those found within the core aa sequence.
Overall, the present computational study facilitates the development of experimental epitope and escape mutation mapping studies.
5. Conclusions
Vaccines act by inducing strong immunity to counteract viral antigens presented by MHC-epitopes. However, their success is affected by virus evolution (e.g., escape mutations) within known protective epitopes; hence, multi-epitope, population-based vaccine constructs are preferred in order to generate a potent immunologic response against HBV. We demonstrate the quality of T cell epitopes among different HBV genotypes and reconstructed a candidate multi-epitope population-based vaccine. Our results suggest that among aa variations classified as polymorphisms do exit T-cell escape mutations and. In silico studies should be followed up with preclinical assays to validate the novelty of their findings.
Viral hepatitis (VH) is a global burden, and the WHO has put forth an ambitious goal to eliminate VH as a public threat by 2030. HBV contributes a vast majority (77%) of VH cases and there are no therapeutic cures for chronic hepatitis B infections (CHB). We hypothesized that epitope vaccines are a potential CHB treatment because they can induce strong immune systems with ability to achieve hepatitis B virus surface antigen (HBsAg) loss. While several trials have failed to produce effective vaccines against CHB from T cell epitopes, we aimed to investigate the repertoire of T cell epitopes from different HBV genotypes (A and D), MHC class II alleles with high population coverage in Botswana. In silico analyses were used to map promiscuous epitopes (15-mers) using alleles -9 MHC class II alleles-, and PreS/S sequences -genotype A and D- with high population coverage in Botswana. Some epitopes mapped within PreS/S conserved regions, and none were promiscuous to all alleles suggesting that multi-epitope, population-based vaccines (MEPBV) may be more effective candidate vaccines against CHB compared to previously reported broad potency epitope-based candidate vaccines. Highly promiscuous peptides may also be considered as candidate peptides for designing highly sensitive diagnostic chips since current serological kits may fail to detect other HBV clinical phenotypes. The mapped T epitopes exhibited high mean diversity among genotypes and others had coordinated amino acid variations that were genetically dependent on each other in order to escape epitope-HLA binding.
Abbreviations
| HBV | hepatitis B virus |
| HLA class II | human leukocyte antigen class II |
| CHB | chronic hepatitis B infection; |
| epi | epitopes |
| SB | strong binding |
| WB | weak binding |
| MHC | major histocompatibility complex |
| aa | amino acids |
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4915/12/7/731/s1, Table S1: Supplementary_Main, Table S2: Supplementary_NetMHCIIPan_Results, Table S3 Supplementary-10,308-[B-I], Table S4 Supplementary-7743-[A&D], Table S5 Supplementary_vacc-gen-proteins.
Author Contributions
Conceptualization, W.T.C., M.A., B.B.P., T.M., R.M.M., S.M., J.T.B. and S.G.; formal analysis, W.T.C., P.O.K. and S.M.; funding acquisition, S.G.; investigation, W.T.C., K.K.S., T.G.B., J.T.B. and S.G.; methodology, W.T.C., M.A., B.B.P., T.M., R.M.M., S.M., J.T.B. and S.G.; supervision, M.A., E.Z., K.K.S., R.M.M., T.G.B., S.M., J.T.B. and S.G.; validation, P.O.K. and S.G.; writing—original draft, W.T.C.; writing—review and editing, M.A., E.Z., B.B.P., T.M., L.N.B., K.B., K.K.S., T.G.B., S.M., J.T.B. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported through the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative [grant # DEL-15-006]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Welcome Trust [grant # 107752/Z/15/Z] and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Welcome Trust or the UK government.
Conflicts of Interest
All authors declare no conflict of interest.
References
- 1.Ladep N.G., Lesi O.A., Mark P., Lemoine M., Onyekwere C., Afihene M., Crossey M.M., Taylor-Robinson S.D. Problem of hepatocellular carcinoma in West Africa. World J. Hepatol. 2014;6:783–792. doi: 10.4254/wjh.v6.i11.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spearman C.W., Afihene M., Ally R., Apica B., Awuku Y., Cunha L., Dusheiko G., Gogela N., Kassianides C., Kew M., et al. Hepatitis B in sub-Saharan Africa: Strategies to achieve the 2030 elimination targets. Lancet Gastroenterol. Hepatol. 2017;2:900–909. doi: 10.1016/S2468-1253(17)30295-9. [DOI] [PubMed] [Google Scholar]
- 3.Kim J.M., Lee K.W., Sinn D.H., Choi G.S., Yi N.J., Kwon C.H.D., Suh K.S., Joh J.W. Use of direct antiviral agents in liver transplant recipients with hepatitis C virus in Korea: 2-center experience. Ann. Surg. Treat. Res. 2018;95:147–151. doi: 10.4174/astr.2018.95.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chisari F.V., Isogawa M., Wieland S.F. Pathogenesis of hepatitis B virus infection. Pathol. Biol. (Paris) 2010;58:258–266. doi: 10.1016/j.patbio.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg W. Mechanisms of immune escape in viral hepatitis. Gut. 1999;44:759–764. doi: 10.1136/gut.44.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karan M.A., Tascioglu N.E., Ozturk A.O., Palanduz S., Carin M. The role of HLA antigens in chronic hepatitis B virus infection. J. Pak. Med. Assoc. 2002;52:253–256. [PubMed] [Google Scholar]
- 7.Zhang Z.H., Wu C.C., Chen X.W., Li X., Li J., Lu M.J. Genetic variation of hepatitis B virus and its significance for pathogenesis. World J. Gastroenterol. 2016;22:126–144. doi: 10.3748/wjg.v22.i1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y.H. Correlation between hepatitis B virus genotypes and clinical outcomes. Jpn. J. Infect. Dis. 2012;65:476–482. doi: 10.7883/yoken.65.476. [DOI] [PubMed] [Google Scholar]
- 9.Thio C.L., Thomas D.L., Karacki P., Gao X., Marti D., Kaslow R.A., Goedert J.J., Hilgartner M., Strathdee S.A., Duggal P., et al. Comprehensive analysis of class I and class II HLA antigens and chronic hepatitis B virus infection. J. Virol. 2003;77:12083–12087. doi: 10.1128/JVI.77.22.12083-12087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitmire J.K. Induction and function of virus-specific CD4+ T cell responses. Virology. 2011;411:216–228. doi: 10.1016/j.virol.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juno J.A., van Bockel D., Kent S.J., Kelleher A.D., Zaunders J.J., Munier C.M. Cytotoxic CD4 T Cells-Friend or Foe during Viral Infection? Front. Immunol. 2017;8:19. doi: 10.3389/fimmu.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luckheeram R.V., Zhou R., Verma A.D., Xia B. CD4(+)T cells: Differentiation and functions. Clin. Dev. Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaseitsiwe S., Maeurer M.J. Identification of MHC class II binding peptides: Microarray and soluble MHC class II molecules. Methods Mol. Biol. 2009;524:417–426. doi: 10.1007/978-1-59745-450-6_30. [DOI] [PubMed] [Google Scholar]
- 14.Sette A., Fikes J. Epitope-based vaccines: An update on epitope identification, vaccine design and delivery. Curr. Opin. Immunol. 2003;15:461–470. doi: 10.1016/S0952-7915(03)00083-9. [DOI] [PubMed] [Google Scholar]
- 15.Dimitrov I., Flower D.R., Doytchinova I.A. Improving in Silico Prediction of Epitope Vaccine Candidates by Union and Intersection of Single Predictors. World J. Vaccines. 2011;1:8. doi: 10.4236/wjv.2011.12004. [DOI] [Google Scholar]
- 16.Horvat R.T., El Atrouni W., Hammoud K., Hawkinson D., Cowden S. Ribosomal RNA sequence analysis of Brucella infection misidentified as Ochrobactrum anthropi infection. J. Clin. Microbiol. 2011;49:1165–1168. doi: 10.1128/JCM.01131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollicino T., Cacciola I., Saffioti F., Raimondo G. Hepatitis B virus PreS/S gene variants: Pathobiology and clinical implications. J. Hepatol. 2014;61:408–417. doi: 10.1016/j.jhep.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 18.Simon B., Kundi M., Puchhammer E. Analysis of mutations in the S gene of hepatitis B virus strains in patients with chronic infection by online bioinformatics tools. J. Clin. Microbiol. 2013;51:163–168. doi: 10.1128/JCM.01630-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber B. Diagnostic impact of the genetic variability of the hepatitis B virus surface antigen gene. J. Med. Virol. 2006;78(Suppl. 1):S59–S65. doi: 10.1002/jmv.20610. [DOI] [PubMed] [Google Scholar]
- 20.Zheng J., Lin X., Wang X., Zheng L., Lan S., Jin S., Ou Z., Wu J. In Silico Analysis of Epitope-Based Vaccine Candidates against Hepatitis B Virus Polymerase Protein. Viruses. 2017;9:112. doi: 10.3390/v9050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi T.D., Wu Y.Z., Jia Z.C., Zou L.Y., Zhou W. Therapeutic polypeptides based on HBV core 18-27 epitope can induce CD8+ CTL-mediated cytotoxicity in HLA-A2+ human PBMCs. World J. Gastroenterol. 2004;10:1902–1906. doi: 10.3748/wjg.v10.i13.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimitrov I., Garnev P., Flower D.R., Doytchinova I. MHC Class II Binding Prediction-A Little Help from a Friend. J. Biomed. Biotechnol. 2010;2010:705821. doi: 10.1155/2010/705821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosa D.S., Ribeiro S.P., Cunha-Neto E. CD4+ T cell epitope discovery and rational vaccine design. Arch. Immunol. Et Ther. Exp. 2010;58:121–130. doi: 10.1007/s00005-010-0067-0. [DOI] [PubMed] [Google Scholar]
- 24.Schweitzer A., Horn J., Mikolajczyk R.T., Krause G., Ott J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 25.Choga W.T., Anderson M., Zumbika E., Moyo S., Mbangiwa T., Phinius B.B., Melamu P., Kayembe M.K., Kasvosve I., Sebunya T.K., et al. Molecular characterization of hepatitis B virus in blood donors in Botswana. Virus Genes. 2019;55:33–42. doi: 10.1007/s11262-018-1610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson M., Choga W.T., Moyo S., Bell T.G., Mbangiwa T., Phinius B.B., Bhebhe L., Sebunya T.K., Lockman S., Marlink R., et al. Molecular Characterization of Near Full-Length Genomes of Hepatitis B Virus Isolated from Predominantly HIV Infected Individuals in Botswana. Genes (Basel) 2018;9:453. doi: 10.3390/genes9090453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mbangiwa T., Kasvosve I., Anderson M., Thami P.K., Choga W.T., Needleman A., Phinius B.B., Moyo S., Leteane M., Leidner J., et al. Chronic and Occult Hepatitis B Virus Infection in Pregnant Women in Botswana. Genes (Basel) 2018;9:259. doi: 10.3390/genes9050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson M., Gaseitsiwe S., Moyo S., Wessels M.J., Mohammed T., Sebunya T.K., Powell E.A., Makhema J., Blackard J.T., Marlink R., et al. Molecular characterisation of hepatitis B virus in HIV-1 subtype C infected patients in Botswana. Bmc Infect. Dis. 2015;15:335. doi: 10.1186/s12879-015-1096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews P.C., Beloukas A., Malik A., Carlson J.M., Jooste P., Ogwu A., Shapiro R., Riddell L., Chen F., Luzzi G., et al. Prevalence and Characteristics of Hepatitis B Virus (HBV) Coinfection among HIV-Positive Women in South Africa and Botswana. PLoS ONE. 2015;10:e0134037. doi: 10.1371/journal.pone.0134037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wai C.T., Fontana R.J. Clinical significance of hepatitis B virus genotypes, variants, and mutants. Clin. Liver Dis. 2004;8:321–352. doi: 10.1016/j.cld.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Chu C.J., Lok A.S. Clinical significance of hepatitis B virus genotypes. Hepatology. 2002;35:1274–1276. doi: 10.1053/jhep.2002.33161. [DOI] [PubMed] [Google Scholar]
- 32.Zhang T.Y., Guo X.R., Wu Y.T., Kang X.Z., Zheng Q.B., Qi R.Y., Chen B.B., Lan Y., Wei M., Wang S.J., et al. A unique B cell epitope-based particulate vaccine shows effective suppression of hepatitis B surface antigen in mice. Gut. 2020;69:343–354. doi: 10.1136/gutjnl-2018-317725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patronov A., Doytchinova I. T-cell epitope vaccine design by immunoinformatics. Open Biol. 2013;3:120139. doi: 10.1098/rsob.120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsson A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 2014;30:3276–3278. doi: 10.1093/bioinformatics/btu531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ndung’u T., Gaseitsiwe S., Sepako E., Doualla-Bell F., Peter T., Kim S., Thior I., Novitsky V.A., Essex M. Major histocompatibility complex class II (HLA-DRB and -DQB) allele frequencies in Botswana: Association with human immunodeficiency virus type 1 infection. Clin. Diagn. Lab. Immunol. 2005;12:1020–1028. doi: 10.1128/CDLI.12.9.1020-1028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen K.K., Andreatta M., Marcatili P., Buus S., Greenbaum J.A., Yan Z., Sette A., Peters B., Nielsen M. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology. 2018;154:394–406. doi: 10.1111/imm.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vita R., Mahajan S., Overton J.A., Dhanda S.K., Martini S., Cantrell J.R., Wheeler D.K., Sette A., Peters B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019;47:D339–D343. doi: 10.1093/nar/gky1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bell T.G., Yousif M., Kramvis A. Bioinformatic curation and alignment of genotyped hepatitis B virus (HBV) sequence data from the GenBank public database. Springerplus. 2016;5:1896. doi: 10.1186/s40064-016-3312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miruka C.O., Matunda N.C., Ejekwumadu N.J., Mokembo J.N. Design of a Recombinant Hepatitis B Vaccine Based on Stably Binding HLA-I Peptides. J. Biomol. Res. Ther. 2015;4 doi: 10.4172/2167-7956.1000120. [DOI] [Google Scholar]
- 40.Artimo P., Jonnalagedda M., Arnold K., Baratin D., Csardi G., de Castro E., Duvaud S., Flegel V., Fortier A., Gasteiger E., et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012;40:W597–W603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Racz R., Li X., Patel M., Xiang Z., He Y. DNAVaxDB: The first web-based DNA vaccine database and its data analysis. Bmc Bioinform. 2014;15(Suppl. 4):S2. doi: 10.1186/1471-2105-15-S4-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X., Wang J., Lu J., Li R., Zhao S. Correction to: Immunogenicity of adenovirus-vector vaccine targeting hepatitis B virus: Non-clinical safety assessment in non-human primates. Virol. J. 2018;15:137. doi: 10.1186/s12985-018-1049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizukoshi E., Sidney J., Livingston B., Ghany M., Hoofnagle J.H., Sette A., Rehermann B. Cellular immune responses to the hepatitis B virus polymerase. J. Immunol. 2004;173:5863–5871. doi: 10.4049/jimmunol.173.9.5863. [DOI] [PubMed] [Google Scholar]
- 44.Schneider J., Chorlton J., Pearce G., Nicola J., Brown D. Compositions for Inducing an Immune Response against Hepatitis B. Application No. 11/986,294. U.S. Patent. 2008 Oct 30;
- 45.King T.H., Kemmler C.B., Guo Z., Mann D., Lu Y., Coeshott C., Gehring A.J., Bertoletti A., Ho Z.Z., Delaney W., et al. A whole recombinant yeast-based therapeutic vaccine elicits HBV X, S and Core specific T cells in mice and activates human T cells recognizing epitopes linked to viral clearance. PLoS ONE. 2014;9:e101904. doi: 10.1371/journal.pone.0101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeigler D.F., Gage E., Roque R., Clegg C.H. Epitope targeting with self-assembled peptide vaccines. Npj Vaccines. 2019;4:30. doi: 10.1038/s41541-019-0125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falugi F., Petracca R., Mariani M., Luzzi E., Mancianti S., Carinci V., Melli M.L., Finco O., Wack A., Di Tommaso A., et al. Rationally designed strings of promiscuous CD4(+) T cell epitopes provide help to Haemophilus influenzae type b oligosaccharide: A model for new conjugate vaccines. Eur. J. Immunol. 2001;31:3816–3824. doi: 10.1002/1521-4141(200112)31:12<3816::AID-IMMU3816>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 48.Publicover J., Gaggar A., Jespersen J.M., Halac U., Johnson A.J., Goodsell A., Avanesyan L., Nishimura S.L., Holdorf M., Mansfield K.G., et al. An OX40/OX40L interaction directs successful immunity to hepatitis B virus. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aah5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krebber W.J.T.A., Kessler J.H., Melief C.J.M., Kwappenberg K.M.C. Synthetic Long Peptides (slp) for Therapeutic Vaccination Against Hepatitis B Virus Infection. No. 10,376,576. U.S. Patent. 2019 Aug 13;
- 50.Chen L., Zhang Y., Zhang S., Chen Y., Shu X., Lai J., Cao H., Lian Y., Stamataki Z., Huang Y. A novel T-cell epitope in the transmembrane region of the hepatitis B virus envelope protein responds upon dendritic cell expansion. Arch. Virol. 2019;164:483–495. doi: 10.1007/s00705-018-4095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sette A., Sidney J., Southwood S. Identification of Broadly Reactive DR Restricted Epitopes. No. 6,413,517. U.S. Patent. 2002 Jul 2;
- 52.Kent S. Immunomodulating Compositions, Uses Therefore and Processes for Their Production. No. 2007/0248584 A1. U.S. Patent. 2007 Oct 25;
- 53.Godkin A., Davenport M., Hill A.V. Molecular analysis of HLA class II associations with hepatitis B virus clearance and vaccine nonresponsiveness. Hepatology. 2005;41:1383–1390. doi: 10.1002/hep.20716. [DOI] [PubMed] [Google Scholar]
- 54.Shen K., Shen L., Wang J., Jiang Z., Shen B. Understanding Amino Acid Mutations in Hepatitis B Virus Proteins for Rational Design of Vaccines and Drugs. Adv. Protein. Chem. Struct. Biol. 2015;99:131–153. doi: 10.1016/bs.apcsb.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Lerner R.A., Green N., Alexander H., Liu F.T., Sutcliffe J.G., Shinnick T.M. Chemically synthesized peptides predicted from the nucleotide sequence of the hepatitis B virus genome elicit antibodies reactive with the native envelope protein of Dane particles. Proc. Natl. Acad. Sci. USA. 1981;78:3403–3407. doi: 10.1073/pnas.78.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milich D.R., Peterson D.L., Leroux-Roels G.G., Lerner R.A., Chisari F.V. Genetic regulation of the immune response to hepatitis B surface antigen (HBsAg). VI. T cell fine specificity. J. Immunol. 1985;134:4203–4211. [PubMed] [Google Scholar]
- 57.Krchnak V., Mach O., Maly A. Computer prediction of B-cell determinants from protein amino acid sequences based on incidence of beta turns. Methods Enzym. 1989;178:586–611. doi: 10.1016/0076-6879(89)78041-1. [DOI] [PubMed] [Google Scholar]
- 58.Wang H., Luo H., Wan X., Fu X., Mao Q., Xiang X., Zhou Y., He W., Zhang J., Guo Y., et al. TNF-alpha/IFN-gamma profile of HBV-specific CD4 T cells is associated with liver damage and viral clearance in chronic HBV infection. J. Hepatol. 2020;72:45–56. doi: 10.1016/j.jhep.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 59.Pajot A., Michel M.L., Mancini-Bourgine M., Ungeheuer M.N., Ojcius D.M., Deng Q., Lemonnier F.A., Lone Y.C. Identification of novel HLA-DR1-restricted epitopes from the hepatitis B virus envelope protein in mice expressing HLA-DR1 and vaccinated human subjects. Microbes Infect. 2006;8:2783–2790. doi: 10.1016/j.micinf.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 60.Zhou C., Li C., Gong G.Z., Wang S., Zhang J.M., Xu D.Z., Guo L.M., Ren H., Xu M., Xie Q., et al. Analysis of immunological mechanisms exerted by HBsAg-HBIG therapeutic vaccine combined with Adefovir in chronic hepatitis B patients. Hum. Vaccin. Immunother. 2017;13:1989–1996. doi: 10.1080/21645515.2017.1335840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bahrami A.A., Bandehpour M., Khalesi B., Kazemi B. Computational Design and Analysis of a Poly-Epitope Fusion Protein: A New Vaccine Candidate for Hepatitis and Poliovirus. Int. J. Pept. Res. Ther. 2020;26:389–403. doi: 10.1007/s10989-019-09845-z. [DOI] [Google Scholar]
- 62.Sette A., Sidney J., Southwood S., Vitiello M.A., Livingston B.D., Celis E., Kubo R.T., Grey H.M., Robert W. Inducing cellular immune responses to hepatitis B virus using peptide compositions. No. 2005/0063983 A1. U.S. Patent. 2005 Mar 24;
- 63.Paoletti E., Gettig Russell R., Francis J.-C., Cox William I., Pincus Steven E., Johnson Gerard P., Limbach Keith J., Taisne Charles D.E., Riviere M., Norton Elizabeth K., et al. Genetically Engineered Vaccine Strain. AU 1997/010012 A. 1997 Jan 3;
- 64.Shin Y.W., Ryoo K.H., Hong K.W., Chang K.H., Choi J.S., So M., Kim P.K., Park J.Y., Bong K.T., Kim S.H. Human monoclonal antibody against Hepatitis B virus surface antigen (HBsAg) Antivir. Res. 2007;75:113–120. doi: 10.1016/j.antiviral.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 65.Yuan Q., Zhang T., Luo W., Chen Y., Zhang J., Xia N. Polypeptides and antibodies for treating HBV infection and related diseases. No. 9,751,914 B2. U.S Patent. 2017 Sep 5;
- 66.Doytchinova I.A., Flower D.R. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. Bmc Bioinform. 2007;8:4. doi: 10.1186/1471-2105-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klein J., Sato A. The HLA system. First of two parts. N. Engl. J. Med. 2000;343:702–709. doi: 10.1056/NEJM200009073431006. [DOI] [PubMed] [Google Scholar]
- 68.Xu D.Z., Wang X.Y., Shen X.L., Gong G.Z., Ren H., Guo L.M., Sun A.M., Xu M., Li L.J., Guo X.H., et al. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: Experiences and findings. J. Hepatol. 2013;59:450–456. doi: 10.1016/j.jhep.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Kosinska A.D., Bauer T., Protzer U. Therapeutic vaccination for chronic hepatitis B. Curr. Opin. Virol. 2017;23:75–81. doi: 10.1016/j.coviro.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 70.Lobaina Y., Michel M.L. Chronic hepatitis B: Immunological profile and current therapeutic vaccines in clinical trials. Vaccine. 2017;35:2308–2314. doi: 10.1016/j.vaccine.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 71.Boni C., Janssen H.L.A., Rossi M., Yoon S.K., Vecchi A., Barili V., Yoshida E.M., Trinh H., Rodell T.C., Laccabue D., et al. Combined GS-4774 and Tenofovir Therapy Can Improve HBV-Specific T-Cell Responses in Patients With Chronic Hepatitis. Gastroenterology. 2019;157:227–241 e227. doi: 10.1053/j.gastro.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 72.Lok A.S. Hepatitis B: 50 years after the discovery of Australia antigen. J. Viral Hepat. 2016;23:5–14. doi: 10.1111/jvh.12444. [DOI] [PubMed] [Google Scholar]
- 73.Lok A.S., McMahon B.J., Brown R.S., Jr., Wong J.B., Ahmed A.T., Farah W., Almasri J., Alahdab F., Benkhadra K., Mouchli M.A., et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016;63:284–306. doi: 10.1002/hep.28280. [DOI] [PubMed] [Google Scholar]
- 74.Lok A.S., Pan C.Q., Han S.H., Trinh H.N., Fessel W.J., Rodell T., Massetto B., Lin L., Gaggar A., Subramanian G.M., et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J. Hepatol. 2016;65:509–516. doi: 10.1016/j.jhep.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 75.Gaggar A., Coeshott C., Apelian D., Rodell T., Armstrong B.R., Shen G., Subramanian G.M., McHutchison J.G. Safety, tolerability and immunogenicity of GS-4774, a hepatitis B virus-specific therapeutic vaccine, in healthy subjects: A randomized study. Vaccine. 2014;32:4925–4931. doi: 10.1016/j.vaccine.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 76.Jiang Y.G., Wang Y.M., Liu T.H., Liu J. Association between HLA class II gene and susceptibility or resistance to chronic hepatitis B. World J. Gastroenterol. 2003;9:2221–2225. doi: 10.3748/wjg.v9.i10.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thio C.L., Carrington M., Marti D., O’Brien S.J., Vlahov D., Nelson K.E., Astemborski J., Thomas D.L. Class II HLA alleles and hepatitis B virus persistence in African Americans. J. Infect. Dis. 1999;179:1004–1006. doi: 10.1086/314684. [DOI] [PubMed] [Google Scholar]
- 78.Huang C.J., Wu C.F., Lan C.Y., Sung F.Y., Lin C.L., Liu C.J., Liu H.F., Yu M.W. Impact of genetic heterogeneity in polymerase of hepatitis B virus on dynamics of viral load and hepatitis B progression. PLoS ONE. 2013;8:e70169. doi: 10.1371/journal.pone.0070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferrari C., Bertoletti A., Penna A., Cavalli A., Valli A., Missale G., Pilli M., Fowler P., Giuberti T., Chisari F.V., et al. Identification of immunodominant T cell epitopes of the hepatitis B virus nucleocapsid antigen. J. Clin. Investig. 1991;88:214–222. doi: 10.1172/JCI115280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tonetto P.A., Goncales N.S., Fais V.C., Vigani A.G., Goncales E.S., Feltrin A., Goncales F.L., Jr. Hepatitis B virus: Molecular genotypes and HBeAg serological status among HBV-infected patients in the southeast of Brazil. Bmc Infect. Dis. 2009;9:149. doi: 10.1186/1471-2334-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson M., Choga W.T., Moyo S., Bell T.G., Mbangiwa T., Phinius B.B., Bhebhe L., Sebunya T.K., Makhema J., Marlink R., et al. In Silico Analysis of Hepatitis B Virus Occult Associated Mutations in Botswana Using a Novel Algorithm. Genes (Basel) 2018;9:420. doi: 10.3390/genes9090420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tamori A., Kawada N. HLA class II associated with outcomes of hepatitis B and C infections. World J. Gastroenterol. 2013;19:5395–5401. doi: 10.3748/wjg.v19.i33.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thursz M.R., Kwiatkowski D., Allsopp C.E., Greenwood B.M., Thomas H.C., Hill A.V. Association between an MHC class II allele and clearance of hepatitis B virus in the Gambia. N. Engl. J. Med. 1995;332:1065–1069. doi: 10.1056/NEJM199504203321604. [DOI] [PubMed] [Google Scholar]
- 84.Wang L., Zou Z.Q., Wang K. Clinical Relevance of HLA Gene Variants in HBV Infection. J. Immunol. Res. 2016;2016:9069375. doi: 10.1155/2016/9069375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Al-Qahtani A.A., Al-Anazi M.R., Abdo A.A., Sanai F.M., Al-Hamoudi W., Alswat K.A., Al-Ashgar H.I., Khalaf N.Z., Eldali A.M., Viswan N.A., et al. Association between HLA variations and chronic hepatitis B virus infection in Saudi Arabian patients. PLoS ONE. 2014;9:e80445. doi: 10.1371/journal.pone.0080445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yan Z.H., Fan Y., Wang X.H., Mao Q., Deng G.H., Wang Y.M. Relationship between HLA-DR gene polymorphisms and outcomes of hepatitis B viral infections: A meta-analysis. World J. Gastroenterol. 2012;18:3119–3128. doi: 10.3748/wjg.v18.i24.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Z.K., Nie J.J., Li J., Zhuang H. The effect of HLA on immunological response to hepatitis B vaccine in healthy people: A meta-analysis. Vaccine. 2013;31:4355–4361. doi: 10.1016/j.vaccine.2013.06.108. [DOI] [PubMed] [Google Scholar]
- 88.Yang P.M., Sung J.L., Chen D.S. HLA-A, B, C and DR antigens in chronic hepatitis B viral infection. Hepatogastroenterology. 1989;36:363–366. [PubMed] [Google Scholar]
- 89.Czaja A.J., Doherty D.G., Donaldson P.T. Genetic bases of autoimmune hepatitis. Dig. Dis. Sci. 2002;47:2139–2150. doi: 10.1023/A:1020166605016. [DOI] [PubMed] [Google Scholar]
- 90.Parkin J., Cohen B. An overview of the immune system. Lancet. 2001;357:1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- 91.Starzl T.E., Zinkernagel R.M. Antigen localization and migration in immunity and tolerance. N. Engl. J. Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scully C., Georgakopoulou E.A., Hassona Y. The Immune System: Basis of so much Health and Disease: 3. Adaptive Immunity. Dent. Update. 2017;44:322–324, 327. doi: 10.12968/denu.2017.44.4.322. [DOI] [PubMed] [Google Scholar]
- 93.Finlay B.B., McFadden G. Anti-immunology: Evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124:767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 94.Thimme R., Wieland S., Steiger C., Ghrayeb J., Reimann K.A., Purcell R.H., Chisari F.V. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rehermann B. Intrahepatic T cells in hepatitis B: Viral control versus liver cell injury. J. Exp. Med. 2000;191:1263–1268. doi: 10.1084/jem.191.8.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hass M., Hannoun C., Kalinina T., Sommer G., Manegold C., Gunther S. Functional analysis of hepatitis B virus reactivating in hepatitis B surface antigen-negative individuals. Hepatology. 2005;42:93–103. doi: 10.1002/hep.20748. [DOI] [PubMed] [Google Scholar]
- 97.Chang J.J., Lewin S.R. Immunopathogenesis of hepatitis B virus infection. Immunol. Cell Biol. 2007;85:16–23. doi: 10.1038/sj.icb.7100009. [DOI] [PubMed] [Google Scholar]
- 98.Albayrak A., Ertek M., Tasyaran M.A., Pirim I. Role of HLA allele polymorphism in chronic hepatitis B virus infection and HBV vaccine sensitivity in patients from eastern Turkey. Biochem Genet. 2011;49:258–269. doi: 10.1007/s10528-010-9404-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.