Abstract
Protein malfunction is typically caused by abiotic stressors. To ensure cell survival during conditions of stress, it is important for plant cells to maintain proteins in their respective functional conformation. Self-compartmentalizing proteases, such as ATP-dependent Clp proteases and proteasomes are designed to act in the crowded cellular environment, and they are responsible for degradation of misfolded or damaged proteins within the cell. During different types of stress conditions, the levels of misfolded or orphaned proteins that are degraded by the 26S proteasome in the cytosol and nucleus and by the Clp proteases in the mitochondria and chloroplasts increase. This allows cells to uphold feedback regulations to cellular-level signals and adjust to altered environmental conditions. In this review, we summarize recent findings on plant proteolytic complexes with respect to their protective functions against abiotic and biotic stressors.
Keywords: abiotic stresses, Clp protease, defense, pathogen, protease, proteasome
1. Introduction
Plants are immobile organisms and may thus be exposed to dynamically changing environmental conditions including abiotic stress and pathogen invasion [1]. The primary environmental stressors such as high temperature, cold, drought, chemicals, and salinity exert detrimental effects on plants [2]. Plant cells can be damaged by these factors and subsequently experience osmotic and oxidative stresses which are referred to as secondary stresses [2]. Under such conditions, the most crucial function of a plant cell is to induce self-defense against the respective stressor. Such defense mechanisms may include qualitative and quantitative alterations of gene expression which may lead to modulations in certain pathways [3].
Global changes in gene expression in response to stress have been observed in numerous organisms [4]. Under stressful conditions, expression of some genes is upregulated, whereas that of others is downregulated. Some genes known as heat shock (HS) genes are rapidly upregulated during heat stress [5]. HS proteins function in two different ways: (1) as molecular chaperones that counteract adverse protein denaturation and aggregation and (2) as ubiquitination agents that target non-native or orphaned proteins for subsequent degradation [6]. Biotic and abiotic stressors typically cause protein dysfunction, and aberrant proteins represent considerable hazards to cell viability [7]. Various cellular processes are severely affected by aberrant or damaged proteins [8].
In all living cells, proteins are synthesized and may be degenerated within the original cell when the proteins are damaged, misfolded, mislocalized, or when they are no longer required [9]. Protein quality is maintained by degrading abnormally structured proteins stemming from mutations or metabolic damage [10]. It has been reported that protein quality control is crucial in non-dividing cells where accumulation of malfunctioned proteins is detrimental [11]. Furthermore, cells respond to damaged proteins by increasing their proteolytic activity in order to counteract toxic effects of damaged proteins [12]. Removal of undesired or damaged proteins caused by severe environmental stresses is a tightly regulated proteolytic process. Removal of non-functional proteins from cells is crucial for maintaining homeostasis and physiological metabolic activities. Therefore, proteolytic functions of proteases is particularly important during conditions of stress that induce damage or impairment of proteins [13].
Proteolytic enzymes are generally termed proteases, proteinases, or peptidases [14]. Intracellular proteolysis is carried out by two major proteolytic systems: (i) proteases and (ii) proteolytic complexes. Depending on amino acid determinants of catalytic sites or required metal co-factors, proteases are categorized in five classes, namely serine proteases (EC 3.4.21), cysteine proteases (EC 3.4.22), aspartic endopeptidases (EC 3.4.23), metalloproteases (EC 3.4.24), and threonine proteases (EC 3.4.25) [15]. Many of these proteases work independently, however, in proteolytic complexes such as eukaryotic and prokaryotic proteasomes, many proteases and their regulatory proteins form vast quaternary complexes with coordinated functions to effectively degrade undesired proteins [16]. The most common proteolytic complexes of plants are proteasomes in the cytoplasm and the nucleus, and Clp protease complexes in plastids and mitochondria [17].
Accumulating evidence suggests elevated gene expression and increased activities of proteases in response to abiotic and biotic stressors [18]. The protective roles of proteases during environmental stresses and pathogen invasion were reviewed elsewhere [19,20,21]. A number of proteases in plastids are well characterized and were found to be localized in different compartments such as the stroma, thylakoids, and the lumen [22]. ATP-dependent caseinolytic (Clp) protease complexes are multi-subunit complexes that are important for protein degradation in plastids, and plastid proteases appear to be constitutively expressed; however, their expression can be induced in response to certain environmental stressors [23]. Inductive mechanisms during stressful conditions include expression of serine protease due to biotic stress [24]. Plastids can experience severe damage due to environmental and biotic stressors including denaturation of proteins within them [25]; however, a comprehensive review on the effects of Clp protease on stress responses and resistance is lacking so far. Recovery or salvage of denatured plastidal proteins after stress is likely vital for plants to maintain tolerance of numerous environmental stressors including pathogens.
There are vast groups of proteases that are induced by abiotic and biotic stressors, therefore, in this review, we focus on recent findings of plant proteasome complexes with respect to both the prokaryotic and the eukaryotic type. Clp protease complexes in plastids originate from prokaryotes and exert protective roles against abiotic and biotic stressors. 26S proteasomes in the cytoplasm and nucleus also regulate stress responses, thereby increasing resistance. After comparing the structures of these two different types of proteasomes, proteasome functions in response to various abiotic and biotic stressors are explained.
2. 26S Proteasomes in Plants
Proteasomes are highly conserved protein complexes that occur in all eukaryote cells and in archaea [26]. Proteasomes degrade non-native or damaged proteins by proteolysis, thereby regulating the concentration of particular proteins. Such degradation processes result in short peptides of about seven to eight amino acids, and these short peptides are further degraded to amino acids which are used for synthesizing new proteins [27]. Maintaining a tightly coordinated and highly specific system for degradation of individual proteins is crucial for the survival of any organism. In eukaryotes, this is accomplished by tagging target proteins with ubiquitin for subsequent recognition and degradation by the 26S proteasome. The ubiquitin proteasome pathway is predominant in the cytoplasm and nucleus to protect the cell from toxic effects of misfolded proteins [28].
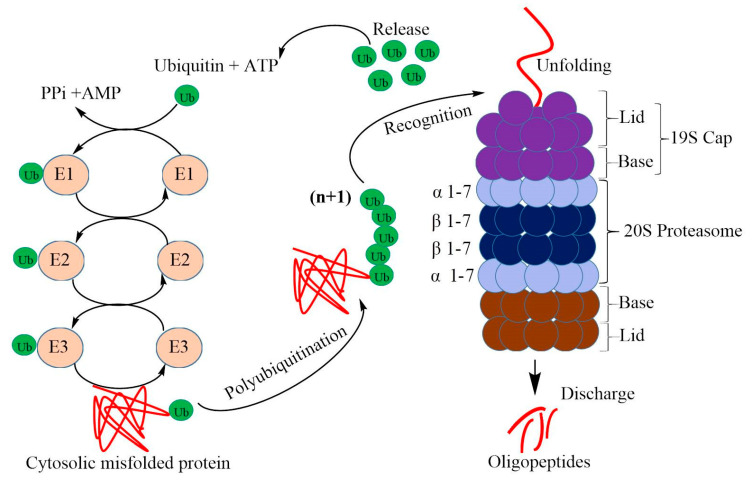
The 26S proteasome contains one 20S core particle and two 19S regulatory caps (Figure 1). The 20S particle is barrel-shaped and consists of four stacked heptagonal rings. The two inner rings are β-catalytic rings (β1-7), and the two outer rings are α rings (α1-7). The α rings serve as an interface for regulatory particle (RP) binding, and the α subunit N-termini form a gate that prevents unregulated access to the interior cavity [29].
Figure 1.
Schematic representation of ATP-powered proteolysis in the cytosol of plant cells. Proteins are tagged with multi-ubiquitin chains by the action of a series of ubiquitin ligases (E1, E2, and E3) and are targeted for degradation by the 26S proteasome. The 26S proteasome consists of the 20S proteasome sandwiched between two 19S regulatory particles. Upon binding of the protein substrate to the 26S proteasome, ubiquitin chains are recycled, and the protein is unfolded and degraded to oligopeptide fragments. E1: ubiquitin-ligase enzyme; E2: ubiquitin-conjugating enzyme; E3: ubiquitin-ligating enzyme.
19S RPs are composed of two sub-complexes. The sub-complex which is proximal to the 20S catalytic particle is termed ‘base’, and the distal sub-complex is termed ‘lid’. The base contains six ATPase subunits (Rpt1 to Rpt6) and four non-ATPase subunits (Rpn1, Rpn2, Rpn10, and Rpn13). The lid contains eight non-ATPase subunits (Rpn3, Rpn5 to Rpn9, Rpn11, and Rpn12) [9,30]. Rpn10 and Rpn13 serve as linkers between lid and base [9]. Orphaned or damaged proteins are recognized and unfolded by the lid and are then fed to the 20S proteolytic core by the base [31]. 26S proteasomes work in a precise manner. To prevent accidental capture of non-substrate proteins, the target proteins are tagged by covalent attachment of polyubiquitin chains (Figure 1). The consecutive action of ubiquitin-activating (E1), -conjugating (E2), and -ligating (E3) enzymes are essential for adequate ubiquitination [28,32].
Non-lysosomal protein degradation which is mediated by the 26S proteasome pathway in the nucleus and cytoplasm is fundamental for regulating diverse cellular processes [33]. The 26S proteasome plays a key role in various cellular processes by disintegrating short- and long-lived proteins. About 80–90% of the bulk of cellular proteins are disintegrated by the 26S proteasome. Even misfolded proteins in the endoplasmic reticulum (ER) go through a retrograde transport from the ER to the cytosol for subsequent degradation by the 26S proteasome [34]. Therefore, inhibition of the 26S proteasome causes severe and rapid loss of universal protein synthesis [35].
3. The Clp Protease System in Plastids
Plant cell plastids comprise an important proteolytic system where proteases play a crucial role in respect of precursor proteins and degradation and removal of undesired or damaged proteins. ATP-dependent proteases combine chaperones with peptidase activity, and chaperone activity is paramount for unfolding protein substrates and feeding them to a protein degradation chamber where peptidolysis occurs [36]. There are three major types of ATP-dependent proteases in plastids and mitochondria of eukaryotic plant cells which were inherited from their eubacterial ancestors, i.e., the ATP-dependent Zn-metalloprotease FtsH family [37,38], the ATP-independent Deg/HtrA family of serine endopeptidases [39,40,41,42], and the ATP-dependent serine-type Clp family [38,43]. FtsH family members are located at the thylakoid or at the inner envelope membranes and, particularly, at plastid thylakoids [44,45,46]. The Deg proteins are ATP-independent serine-type proteases which occur as both lumenal (Deg1, 5, and 8) and stromal compounds (Deg2 and 7) in plastids and play a crucial role in photosystem-II assembly [39,41,42,47]. The Clp family members which are important for protein degradation are confined to the stroma with some occurring at the chloroplast membranes [38,43,45].
The Clp protease system was first identified in Escherichia coli, and it consists of two components, namely the proteolytic subunit (ClpP) and the ATPase subunit. The ATPase subunit belongs to the AAA+ protein family which, consisting of eight subfamilies (ClpA, B, C, D, M, N, X, and Y/HslU), has been identified across various species [48,49,50,51]. Depending on the number of nucleotide binding domains (NBDs), Clp proteins are categorized in two classes [52]: class I proteins (e.g., ClpA, ClpB, ClpC, and ClpD) which are relatively large (68–110 kDa) and have two NBDs and class II proteins (e.g., ClpM, ClpN, ClpX, and ClpY/HslU) which are comparably small (40–50 kDa) and have one NBD.
ClpA has been detected in Gram-negative bacteria, whereas ClpB has been detected in prokaryotes (known as ClpB), yeast (Hsp104), and plants. ClpB in plants occurs in the cytosol (known as ClpB-C), in mitochondria (ClpB-M), and in chloroplasts (ClpB-P) [53]. ClpC occurs in Gram-positive bacteria, cyanobacteria, and in chloroplasts of algae and higher plants, whereas ClpD (also referred to as Erd1) is restricted to the chloroplasts of higher plants. ClpM has been found in Mus musculus and Plasmodium falciparum, ClpN occurs in Pseudomonas aeruginosa, ClpX was found in bacteria, humans, and higher plants, and ClpY occurs in bacteria [54,55].
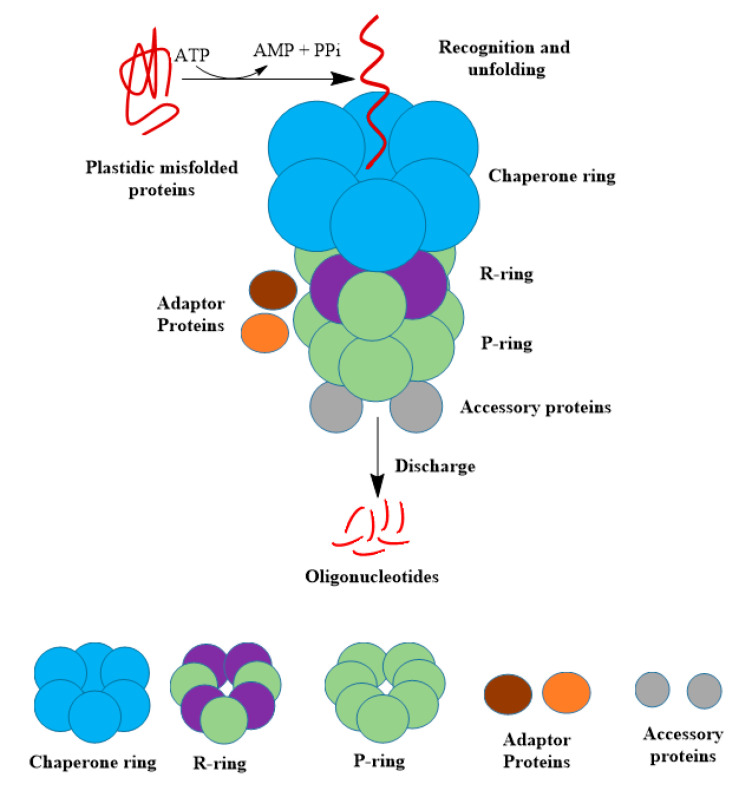
The Clp protease system in higher plants is diverse. In Arabidopsis thaliana, Clp proteases comprises more than 15 proteins with three HSP100 AAA+ chaperones (ClpC1, ClpC2, and ClpD), five serine-type Clp proteolytic subunits (ClpP1, ClpP3, ClpP4, ClpP5, and ClpP6), two adapter proteins (ClpS1 and ClpF) to bind ClpC, four non-proteolytic administrative or regulatory subunits (ClpR1, ClpR2, ClpR3, and ClpR4), and two proteins (ClpT1 and ClpT2) that function as stabilizers of the ClpRP core [17,38,43,56,57,58,59] (Figure 2). All genes encoding Clp protease subunits are part of the nuclear genome, apart from the ClpP1 gene which is part of the plastid genome.
Figure 2.
Schematic representation of ATP-powered proteolysis in plastids of plant cells. Misfolded proteins are recognized, unfolded, and translocated to the proteolytic chamber of the Clp protease complex by ClpC chaperones. The translocated proteins are degraded to oligopeptides that are exported through openings of the proteolytic chamber.
The various proteins of the Clp protease machinery constitute two oligomeric components, namely (i) a tetradecameric barrel-shaped protease core with its catalytic sites within the complex and (ii) an ATP-dependent hexameric ring of chaperones. The chaperone ring recognizes non-native or damaged proteins with or without the help of adaptors and then un-bends these proteins and translocates them into the proteolytic chamber for degradation [43,60]. During and after stress conditions or during normal growth, the fate of any given denatured or misfolded protein in chloroplasts and mitochondria is determined by the Clp chaperone system. Therefore, degradation of orphaned or damaged proteins, stress responses, and gene regulation by proteolysis of transcription factors are the functional role of Clp complexes. 26S proteasome in the cytosol and nucleus and Clp proteases in the plastids and mitochondria show considerable similarities regarding structure and function, and we therefore compared them as shown in Table 1.
Table 1.
Comparison between 26S proteasomes and Clp protease.
| Issues/Characteristics | 26S Proteasomes | Clp Protease |
|---|---|---|
|
Cytoplasm and nucleus | Plastid and mitochondria |
|
19S regulatory particle and 20S core particle | Chaperone subunit and proteolytic subunit |
|
19S regulatory particle, hexameric ring | Chaperone subunit, hexameric ring |
|
20S core particle, heptagonal barrel-shaped ring | Proteolytic subunit, tetradecameric barrel-shaped ring |
|
Regulatory particle consists of base and lid. Base has six ATPase subunits (Rpt1-Rpt6) and two non-ATPase subunits (Rpn1 and Rpn2). Lid contains eight non-ATPase subunits (RPN3, 5–9, 11, and 12) |
ClpC1, ClpC2, and ClpD constitute the chaperone subunit (in Arabidopsis thaliana) |
|
Four ring. Inner two rings are β catalytic rings (β1-7) and outer two rings are α rings (α1-7) | ClpP1, ClpP3, ClpP4, ClpP5, and ClpP6 constitute the proteolytic subunit (in Arabidopsis thaliana) |
|
Rpn10 and Rpn13 serve as linker between lid and base | ClpS1 and ClpF serve as adapter for ClpC (in Arabidopsis thaliana) |
4. Roles of Plant Proteasomes in Response to Stressors and Pathogens
Most of the cytosolic and nuclear proteins are processed by the 26S proteasome system. Ubiquitin binds to a protein at its lysine residue and thereby tags it for degradation by the 26S proteasome system. Misfolded or aberrant proteins and regulators of numerous processes are degraded by the ubiquitin-proteasome system. Ubiquitin/26S proteasome-mediated proteolysis is crucial in numerous cellular responses such as those associated with biotic and abiotic stress tolerance [61], pathogen defense [62], hormone signaling [63], morphogenesis [64], and chromatin modification [65].
Changes in proteasome abundance which are affected by development and environment are important for plant development and survival under adverse conditions [66]. As cell proliferation in plants depends on optimal 26S proteasome activity, stressors that directly affect 26S proteasome activity were suggested to indirectly reduce cell proliferation. Abiotic stress inhibits 26S proteasome activity either by decelerating the turnover rate of other 26S proteasome targets, by increasing the substrate load, or by directly affecting 26S proteasome functions. The substrates are proteins produced due to HS and other stresses that cause protein misfolding. Oxidative stress directly leads to 26S proteasome inhibition [67,68,69].
The ubiquitin-proteasome pathway in plants controls a range of cellular signaling processes, such as those elicited by hormones, sucrose, and light, as well as development and responses to pathogen invasion [2,70]. E3 ubiquitin ligases mediate the final transfer of ubiquitin to target proteins, which is a vital part of the degradation process. Furthermore, numerous studies showed the involvement of E3 ubiquitin ligases in plant defense systems [71]. In the Arabidopsis genome, approximately 1300 genes encode a E3 ubiquitin ligase motif [72]. Microarray screening data of in silico expression analyses on all annotated E3 ubiquitin ligase components revealed that biotic stress caused upregulation of up to 548 E3 ubiquitin ligase components and downregulation of 382 of such components [28]. E3 ubiquitin ligases and associated protein breakdown are vital for signal transduction pathways associated with disease resistance [28,73,74], and they are involved in plant defenses through controlled proteolysis during mechanisms associated with gene-for-gene disease resistance, early-defense response reactions, and late-induced defense responses [19].
Plant cells may evolve more elaborate molecular mechanisms under high-intensity stress and may alter 26S proteasome activity in response to variations in environmental conditions. This type of mechanism depends on the ubiquitin proteasome system [75,76,77]. The hot pepper (Capsicum annuum L.) U-box protein 1 (CaPUB1) and its Arabidopsis thaliana homologues AtPUB22 and AtPUB23 are ubiquitin ligases. During stress caused by abiotic factors such as desiccation, cold, or mechanical wounding, expression of the respective genes CaPUB1, AtPUB22, and AtPUB23 is rapidly induced [75,78]. In C. annuum and A. thaliana, PUBs ubiquitinate specific subunits of the RP lid sub-complex and interfere with functions of the 26S proteasome. CaPUB1 ubiquitinates Rpn6 and destabilizes the RP subunit [78]. Rpn12a is ubiquitinated by AtPUB22 and AtPUB23, which leads to relocation of a portion of Rpn12a to a cytosolic complex reminiscent of the proteasome-related 500-kDa complex (PR500). The PR500 complex contains the subunits of the RP lid and occurs as a stable separate particle in plant cells under physiological conditions (i.e., in unstressed plants). During heat stress and treatments with the amino acid analog canavanine, PR500 is depleted [79]. PR500 is used by plants to accelerate 26S proteasome biogenesis, which is required for ameliorating adverse effects of protein misfolding due to stress, particularly desiccation stress [79].
During drought stress, 26S proteasome levels are reduced due to the effect of AtPUB22/23 action by redirecting a portion of RP subunits to the PR500 particle. A reduction in 26S proteasome activity is detrimental for plant survival because desiccation tolerance depends on the ubiquitin-proteasome system [80]. Overexpression of AtPUB22/23 indicates hypersensitivity to drought stress, whereas loss of function suggests drought tolerance [75,78]. Both ligases are induced during stress, which indicates that they are required by plant cells to ameliorate adverse effects of stress. Loss of function of the 26S proteasome results in decreased root growth [81]. AtPUB22/23 overexpression elicits increased root elongation, which may indicate other functions in addition to AtPUB22/23 effects on 26S proteasome biogenesis.
Proteasomes are involved in plant defenses against pathogen invasion [81,82,83]. The proteasome activity was required in cucumber hypocotyls (Cucumis sativus) for elicitation of defense responses [84]. Tobacco (Nicotiana tabacum) plants treated with cryptogein (a proteinaceous elicitor secreted by a fungal pathogen) upregulated expression of genes encoding b1-tcI 7, α3, and α6. [85,86]. During the induction of systemic acquired resistance and production of reactive oxygen species (ROS), expression of 20S subunits is increased [87]. In tobacco plants treated with cryptogein, production of ROS increases, which is mediated by the NADPH oxidase and elicits accumulation of β1 din 20S subunits [88]. Studies on loss or gain of function of β1 din 20S subunits showed that during elicitation of plant defense reactions, a proteasome consisting of a β1 din 20S subunit acts as a negative regulator of NADPH oxidase and contributes to the regulation of ROS generated during pathogen invasion [88].
Disruption of 26S proteasome function alters the ability of plants to efficiently and effectively respond to and tolerate various environmental stressors. Mutations of RP components affect 26S proteasome functioning, resulting in reduced complex accumulation, reduced rates of ubiquitin-dependent proteolysis, and modifications in responses to abiotic stressors [70,81,89,90]. Arabidopsis rpn1a-4, rpn1a-5, and rpn10-1 mutant plants show limited tolerance to salt stress [89,91], and rpn10-1 mutant plants are also hypersensitive to DNA-damaging agents and UV radiation [89]; rpn1a-4, rpn1a-5, rpn10-1, rpn12a-1, and rpt2a-2 mutants show reduced HS tolerance [81,91], and RPT2a and RPT5a mutant plants are less tolerant to zinc-deficiency [92]. Results of these studies on mutant plants emphasize that the 26S proteasome plays a pivotal role for plant responses to adverse growth conditions.
Ubiquitin and ubiquitin enzymes are also important for plant responses to abiotic stressors. Most ubiquitin genes are expressed during stress [93,94,95]. Overexpression of monoubiquitin or polyubiquitin genes increase tolerance of plants to multiple abiotic stressors including salinity, cold, and drought [80,96]. Expression of E2 enzymes is differentially regulated in response to abiotic stressors. Among 39 E2-encoding genes (OsUBCs) in rice (Oryza sativa), expression of 14 genes was either upregulated or downregulated when plants were subjected to drought and/or salt stress [97]. Overexpression of mung bean (Vigna radiata) VrUBC1, soybean (Glycine max) GmUBC2, and peanut (Arachis hypogaea) AhUBC2 in A. thaliana increased tolerance to drought stress [98,99,100]. Expression of the NtUBC1 gene in tobacco increased in response to cadmium stress [101]. These observations suggest that 26S proteasomes in the cytoplasm and nucleus are critical for modulating the levels of regulatory proteins and for removing orphaned or non-native proteins in response to biotic or abiotic stressors.
5. Roles of Clp Protease Complexes during Stress and Pathogen Defense
In the cytosol and nucleus of plant cells, non-native or damaged proteins are degraded by 26S proteasomes, whereas in chloroplasts and mitochondria, this function is performed by prokaryote-type proteases due to the absence of proteasomes. The major protease in chloroplasts is the ATP-dependent stromal Clp proteolytic complex [17,43,59]. Expression of Clp proteases has been reported during drought, salinity, osmotic shock, pathogen invasion, oxidative stress, heat, and cold [102,103].
5.1. Roles of Clp Protease Complexes in Bacteria during Stress Conditions
Aberrant and denatured proteins are accumulated under stress conditions. Cells respond to this by increasing the synthesis of a set of highly conserved chaperones and proteases which either refold or degrade damaged proteins. In bacteria, ClpP-ClpA proteases are involved in the degradation of misfolded proteins [104,105]. ClpBs resolubilize protein aggregates during HS and other stresses [106]. ClpB is substantially induced in the unicellular Synechococcus sp. strain PCC 7942 during moderate cold stress [107], and the ClpC gene of Bacillus subtilis is induced in response to various stressors including cadmium stress [108]. In Staphylococcus aureus, expression of ClpB, ClpL, and ClpCP increases during heat stress, while ClpXP increases during osmotic stress, oxidative stress, and cold stress [109]. The presence of ClpC, ClpP, or ClpX in the cell is indispensable for stress tolerance, and protein levels of ClpC, ClpP, and ClpX increase during heat stress in B. subtilis [110]. Stress induction of ClpP in E. coli was first shown during heat shock [111], when Clp protease degraded aggregated proteins in vivo [112]. During starvation, ClpP proteases increase their activity which is directed against certain carbon starvation proteins [113]. ClpP proteases play significant roles in stress tolerance by degrading misfolded proteins in Porphyromonas gingivalis [114] and Actinobacillus pleuropneumoniae [115]. Therefore, Clp proteases that degrade misfolded and damaged proteins are likely important for bacteria to survive during adverse environmental conditions.
5.2. Roles of Clp Protease Complexes in Land Plants during Stress Conditions
Clp proteases are constitutively expressed in various plant tissues, and they are most abundant in chloroplasts of green leaves. Molecular chaperones cooperate in vitro as part of a functional network under stress conditions during which chaperones prevent accumulation of misfolded proteins and actively assist in their refolding [116]. ClpB, ClpC, and ClpD subunit proteins work as molecular chaperones that help protect cellular proteins from stress by delivering client proteins to Clp proteases. The A. thaliana organelle ClpB genes in chloroplasts and mitochondria show constitutive expression levels, which increase during high temperatures [117]. Similarly, organelle ClpB genes in rice show low constitutive expression levels which are upregulated during or after heat stress [118]. Chloroplast ClpB genes are also expressed constitutively in lima beans (Phaseolus lunatus), and their expression levels are significantly upregulated at high temperatures [119]. In the clpr2-1 mutant, chloroplast ClpB3 was greatly upregulated in both young and mature leaves [120].
In chloroplasts, constitutive ClpD levels are comparatively low. The ClpD protein is encoded by the gene ERD1 (early responsive to dehydration 1) [121], and its expression increases due to high salinity, dehydration, dark-induced etiolation, cold, and senescence [55,122,123,124,125]. In A. thaliana, long periods of cold increased ClpD protein content in leaves [122,126].
There are some contradictory reports regarding the levels of mRNA and protein of ClpC under different stress conditions or at different developmental stages [126,127]. Following short-term stress, mRNA and proteins levels of ClpC did not change [122], whereas after intensive-light treatments for 2.5 h, the transcript levels increased [128]. In vivo trapping studies for the discovery of the substrates for Clp proteases also revealed that most ClpC components are involved in the stress responses [129].
In rice seedlings, the ATP-binding subunit of ATP-dependent Clp protease responds to cold stress [130]. Proteomics revealed that ClpC levels significantly increased during cadmium stress in tobacco [131]. Protein levels of ClpC were also high in Amaranthus hybridus L. roots under cadmium stress [132], suggesting that ClpC may be important for ameliorating toxic effects in plants.
Co-suppression of ClpC1/C2 in Nicotiana benthamiana produced a phenotype with severe chlorosis, aberrant development, and growth retardation [133]. ClpC1 chaperones unfold proteins for Clp proteases, and their expression is substantially induced during senescence, suggesting altered specificity of this complex [134]. Inactivation of the ClpC1 gene in A. thaliana reduced plant growth and hampered chloroplast development [135,136,137]. When mutants with different variations of Clp protease (clpr1, clps, clpc1, clpc1, clpd, clpt1, and clpt2) were treated with methyl viologen, clpc1 and clpc2 mutants were more resistant to methyl viologen, compared to other mutants [138]. clpc1 and clpc2 mutants show 90% similarity in DNA and amino acid sequences. Due to this sequence similarity, they likely compensate for adverse mutation effects reciprocally to degrade toxic aggregates following UV treatments. Therefore, Clp protease complexes are vital factors of plant survival during various conditions of environmental stress.
In Chlamydomonas reinhardtii, ClpP1 is associated with the deterioration of the thylakoid-bound subunits of cytochrome b6f and photosystem II complex [139,140,141]. The steady-state growth of Cyanobacterium is considerably affected by ClpP1 as it helps cyanobacteria to acclimatize to various environmental conditions. When these cyanobacteria were exposed to extremely intensive light, photoinhibition, or moderate but non-inhibitory lighting, the content of ClpP1 was significantly increased. Inactivation of ClpP1 in cyanobacteria of the genus Synechococcus produced pleiotropic changes during steady-state growth, but slower growth was observed at higher light intensities [142], indicating that protein turnover mediated by ClpP is crucial for cell division. The ClpP1 protein of Synechococcus is analogous to the chloroplast form rather than to bacterial ClpP. ClpP1 expression is strongly induced by UV-B or low-temperature treatments, and loss of ClpP1 substantially affects stress acclimation capacity in Synechococcus [143].
ClpP1 was found to be a prerequisite for shoot development in Nicotiana tabacum [144,145]. Loss of function of the ClpP1 gene regarding the proteolytic subunit of Clp protease increased tolerance of rice seedlings to both ozone [146] and SO2 [147] treatments. Transcriptomics and proteomics revealed that ClpP5 was significantly increased in Nicotiana tabacum under salt stress [148], indicating that Clp proteases may be involved in the defense of plants against stressors.
The abundance of ATP-dependent Clp protease proteolytic subunits in leaves of maize (Zea mays) was increased in response to cold stress [149]. Expression of the proteolytic subunit was also upregulated in wheat (Triticum aestivum) stems during drought-induced senescence [150,151]. In Rhazya stricta, all genes of the proteolytic subunit (ClpP) were upregulated after 12 h of salt stress [152]. Increased mRNA and protein content of several ClpP isomers also occurred in A. thaliana during long-term cold and high-intensity lighting acclimation [122], and proteomics revealed that ClpP levels were significantly increased during cadmium stress in tobacco [131].
ROS are generated under both physiological and stress conditions in plasma membranes, chloroplasts, mitochondria, ERs, peroxisomes, and in the cell wall of plant cells. The major sources of ROS production during light conditions are chloroplasts and peroxisomes, whereas during dark conditions, mitochondria are the predominant producers of ROS in plants [153]. The Clp protease system protects the plants’ chloroplasts from ROS generated in the presence of light and from ROS generated due to environmental stimuli such as excess light, heat, water shortage, or nutrient starvation. Pulido et al. [138] reported that Clp protease systems contribute to plant survival under methyl viologen-triggered oxidative stress.
Clp proteases are upregulated in senescing leaves and participate in the degradation of plastidial photosystem II [154], and they are involved in the degradation of damaged or surplus proteins in plastids [43]. Genes encoding Clp proteases were found to be upregulated during drought stress [155]. Taken together, Clp proteases are crucial for counteracting biotic and abiotic stress by degrading orphaned or non-native proteins.
6. Concluding Remarks
Aggregation of proteins that are damaged owing to stress is frequently a cause of cell death. Clp proteases and proteasomes are important for protecting plant cells under adverse conditions. During normal growth or during and after stress conditions, the fate of a given misfolded, denatured, or non-native protein is determined by the proteasome and/or Clp protease machinery as they help the cell recover from various stresses either by repairing damaged proteins (protein refolding) or by protein degradation. In this way, proteasomes and the Clp protease machinery can restore protein homeostasis and promote cell survival in plants. Comprehensive identification of the ubiquitin proteasome system and of Clp proteases and their substrates regarding their diverse roles in cellular metabolism may be challenging, however, further structural and functional research is needed to fully explore these aspects and their role in diverse cellular pathways such as stress responses and hormone systems. This would provide important insights regarding plant resource utilization and adaption.
Acknowledgments
This work was supported by the 2020 Yeungnam University Research Grant.
Author Contributions
M.S.A. and K.-H.B. conceived and wrote this review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the 2020 Yeungnam University Research Grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Park C.J., Seo Y.S. Heat shock proteins: A review of the molecular chaperones for plant immunity. Plant Pathol. J. 2015;31:323–333. doi: 10.5423/PPJ.RW.08.2015.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu J.K. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kushalappa A.C., Yogendra K.N., Karre S. Plant innate immune response: Qualitative and quantitative resistance. CRC. Crit. Rev. Plant Sci. 2016;35:38–55. doi: 10.1080/07352689.2016.1148980. [DOI] [Google Scholar]
- 4.Haslbeck M., Vierling E. A first line of stress defense: Small heat shock proteins and their function in protein homeostasis. J. Mol. Biol. 2015;427:1537–1548. doi: 10.1016/j.jmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J., Feng L., Li J., He Z. Genetic and epigenetic control of plant heat responses. Front. Plant Sci. 2015;6:267. doi: 10.3389/fpls.2015.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra R.C., Grover A. ClpB/Hsp100 proteins and heat stress tolerance in plants. Crit. Rev. Biotechnol. 2016;36:862–874. doi: 10.3109/07388551.2015.1051942. [DOI] [PubMed] [Google Scholar]
- 7.Houston K., Tucker M.R., Chowdhury J., Shirley N., Little A. The plant cell wall: A complex and dynamic structure as revealed by the responses of genes under stress conditions. Front. Plant Sci. 2016;7:1–18. doi: 10.3389/fpls.2016.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jesenberger V., Jentsch S. Deadly encounter: Ubiquitin meets apoptosis. Nat. Rev. Mol. Cell Biol. 2002;3:112–121. doi: 10.1038/nrm731. [DOI] [PubMed] [Google Scholar]
- 9.Kim H.M., Yu Y., Cheng Y. Structure characterization of the 26S proteasome. Biochim. Biophys. Acta. 2011;1809:67–79. doi: 10.1016/j.bbagrm.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer R.J., Ciechanover A., Rechsteiner M., editors. Protein Degradation. WILEY-VCH Verlag GmbH & Co.; Weinheim, Germany: 2005. [Google Scholar]
- 11.Iyama T., Wilson D.M. DNA repair mechanisms in dividing and non-dividing cells. DNA Repair. 2013;12:620–636. doi: 10.1016/j.dnarep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz-Mendoza M., Velasco-Arroyo B., Santamaria M.E., González-Melendi P., Martinez M., Diaz I. Plant senescence and proteolysis: Two processes with one destiny. Genet. Mol. Biol. 2016;39:329–338. doi: 10.1590/1678-4685-GMB-2016-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Díaz-Villanueva J.F., Díaz-Molina R., García-González V. Protein folding and mechanisms of proteostasis. Int. J. Mol. Sci. 2015;16:17193–17230. doi: 10.3390/ijms160817193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mótyán J.A., Tóth F., Tőzsér J. Research applications of proteolytic enzymes in molecular biology. Biomolecules. 2013;3:923–942. doi: 10.3390/biom3040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett A.J., Rawlings N.D., Woessner J.F., editors. Handbook of Proteolytic Enzymes. Elservier/Academic Press; London, UK: 2004. [Google Scholar]
- 16.Humbard M.A., Maupin-Furlow J.A. Prokaryotic proteasomes: Nanocompartments of degradation. J. Mol. Microbiol. Biotechnol. 2013;23:321–334. doi: 10.1159/000351348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura K., Kato Y., Sakamoto W. Chloroplast proteases: Updates on proteolysis within and across suborganellar compartments. Plant Physiol. 2016;171:2280–2293. doi: 10.1104/pp.16.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres M.A., Dangl J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Baek K.H., Choi D. Roles of plant proteases in pathogen defense. Plant Pathol. J. 2008;24:367–374. doi: 10.5423/PPJ.2008.24.4.367. [DOI] [Google Scholar]
- 20.Figaj D., Ambroziak P., Przepiora T., Skorko-Glonek J. The role of proteases in the virulence of plant pathogenic bacteria. Int. J. Mol. Sci. 2019;20:672. doi: 10.3390/ijms20030672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H., Hu M., Wang Q., Cheng L., Zhang Z. Role of papain-like cysteine proteases in plant development. Front. Plant Sci. 2018;9:1–10. doi: 10.3389/fpls.2018.01717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cline K., Henry R. Import and routing of nucleus-encoded chloroplast proteins. Annu. Rev. Cell Dev. Biol. 1996;12:1–26. doi: 10.1146/annurev.cellbio.12.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan T., Kumar K.R.R., Kirti P.B. Constitutive expression of a trypsin protease inhibitor confers multiple stress tolerance in transgenic tobacco. Plant Cell Physiol. 2009;50:541–553. doi: 10.1093/pcp/pcp014. [DOI] [PubMed] [Google Scholar]
- 24.Clemente M., Corigliano M.G., Pariani S.A., Sánchez-López E.F., Sander V.A., Ramos-Duarte V.A. Plant serine protease inhibitors: Biotechnology application in agriculture and molecular farming. Int. J. Mol. Sci. 2019;20:1345. doi: 10.3390/ijms20061345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leister D., Wang L., Kleine T. Organellar gene expression and acclimation of plants to environmental stress. Front. Plant Sci. 2017;8:387. doi: 10.3389/fpls.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller A.U., Weber-Ban E. The bacterial proteasome at the core of diverse degradation pathways. Front. Mol. Biosci. 2019;6:1–7. doi: 10.3389/fmolb.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lodish H., Berk A., Kaiser C.A., Krieger M., Scott M.P., Bretscher A., Ploegh H., Matsudaira P. Molecular Cell Biology. 8th ed. W.H. Freeman and CO; New York, NY, USA: 2016. pp. 74–88. [Google Scholar]
- 28.Delauré S.L., Van Hemelrijck W., De Bolle M.F.C., Cammue B.P.A., De Coninck B.M.A. Building up plant defenses by breaking down proteins. Plant Sci. 2008;174:375–385. doi: 10.1016/j.plantsci.2008.01.008. [DOI] [Google Scholar]
- 29.Smith D.M., Chang S.C., Park S., Finley D., Cheng Y., Goldberg A.L. Docking of the proteasomal ATPases’ carboxyl termini in the 20S proteasome’s α ring opens the gate for substrate entry. Mol. Cell. 2007;27:731–744. doi: 10.1016/j.molcel.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bai M., Zhao X., Sahara K., Ohte Y., Hirano Y., Kaneko T., Yashiroda H., Murata S. In-depth analysis of the lid subunits assembly mechanism in mammals. Biomolecules. 2019;9:213. doi: 10.3390/biom9060213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raynes R., Pomatto L.C.D., Davies K.J.A. Degradation of oxidized proteins by the proteasome: Distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways. Mol. Aspects Med. 2016;50:41–55. doi: 10.1016/j.mam.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulia R., Sharma R., Bhattacharyya S. A critical role for ubiquitination in the endocytosis of glutamate receptors. J. Biol. Chem. 2017;292:1426–1437. doi: 10.1074/jbc.M116.752105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciechanover A. The unravelling of the ubiquitin system. Nat. Rev. Mol. Cell Biol. 2015;16:322–324. doi: 10.1038/nrm3982. [DOI] [PubMed] [Google Scholar]
- 34.Qi L., Tsai B., Arvan P. New insights into the physiological role of endoplasmic reticulum-associated degradation. Trends Cell Biol. 2017;27:430–440. doi: 10.1016/j.tcb.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins G.A., Goldberg A.L. The logic of the 26S proteasome. Cell. 2017;169:792–806. doi: 10.1016/j.cell.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majeran W., Friso G., van Wijk K.J., Vallon O. The chloroplast ClpP complex in Chlamydomonas reinhardtii contains an unusual high molecular mass subunit with a large apical domain. FEBS J. 2005;272:5558–5571. doi: 10.1111/j.1742-4658.2005.04951.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu X., Yu F., Rodermel S. Arabidopsis chloroplast FtsH, var2 and suppressors of var2 leaf variegation: A review. J. Integr. Plant Biol. 2010;52:750–761. doi: 10.1111/j.1744-7909.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 38.Adam Z., Rudella A., van Wijk K.J. Recent advances in the study of Clp, FtsH and other proteases located in chloroplasts. Curr. Opin. Plant Biol. 2006;9:234–240. doi: 10.1016/j.pbi.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Huesgen P.F., Schuhmann H., Adamska I. Deg/HtrA proteases as components of a network for photosystem II quality control in chloroplasts and cyanobacteria. Res. Microbiol. 2009;160:726–732. doi: 10.1016/j.resmic.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Sun X., Peng L., Guo J., Chi W., Ma J., Lu C., Zhang L. Formation of DEG5 and DEG8 complexes and their involvement in the degradation of photodamaged photosystem II reaction center D1 protein in Arabidopsis. Plant Cell. 2007;19:1347–1361. doi: 10.1105/tpc.106.049510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun X., Ouyang M., Guo J., Ma J., Lu C., Adam Z., Zhang L. The thylakoid protease Deg1 is involved in photosystem-II assembly in Arabidopsis thaliana. Plant J. 2010;62:240–249. doi: 10.1111/j.1365-313X.2010.04140.x. [DOI] [PubMed] [Google Scholar]
- 42.Sun X., Fu T., Chen N., Guo J., Ma J., Zou M., Lu C., Zhang L. The stromal chloroplast Deg7 protease participates in the repair of photosystem II after photoinhibition in Arabidopsis. Plant Physiol. 2010;152:1263–1273. doi: 10.1104/pp.109.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishimura K., van Wijk K.J. Organization, function and substrates of the essential Clp protease system in plastids. Biochim. Biophys. Acta. 2015;1847:915–930. doi: 10.1016/j.bbabio.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Zaltsman A., Ori N., Adam Z. Two types of FtsH protease subunits are required for chloroplast biogenesis and photosystem II repair in Arabidopsis. Plant Cell. 2005;17:2782–2790. doi: 10.1105/tpc.105.035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakamoto W. Protein degradation machineries in plastids. Annu. Rev. Plant Biol. 2006;57:599–621. doi: 10.1146/annurev.arplant.57.032905.105401. [DOI] [PubMed] [Google Scholar]
- 46.Kato Y., Sakamoto W. New insights into the types and function of proteases in plastids. Int. Rev. Cell Mol. Biol. 2010;280:185–218. doi: 10.1016/S1937-6448(10)80004-8. [DOI] [PubMed] [Google Scholar]
- 47.Kapri-Pardes E., Naveh L., Adam Z. The thylakoid lumen protease Deg1 is involved in the repair of photosystem II from photoinhibition in Arabidopsis. Plant Cell. 2007;19:1039–1047. doi: 10.1105/tpc.106.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butler S.M., Festa R.A., Pearce M.J., Darwin K.H. Self-compartmentalized bacterial proteases and pathogenesis. Mol. Microbiol. 2006;60:553–562. doi: 10.1111/j.1365-2958.2006.05128.x. [DOI] [PubMed] [Google Scholar]
- 49.Dougan D.A., Weber-Ban E., Bukau B. Targeted delivery of an ssrA-tagged substrate by the adaptor protein SspB to its cognate AAA+ protein ClpX. Mol. Cell. 2003;12:373–380. doi: 10.1016/j.molcel.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Gottesman S. Proteolysis in bacterial regulatory circuits. Annu. Rev. Cell Dev. Biol. 2003;19:565–587. doi: 10.1146/annurev.cellbio.19.110701.153228. [DOI] [PubMed] [Google Scholar]
- 51.Schlieker C., Zentgraf H., Dersch P., Mogk A. ClpV, a unique Hsp100/Clp member of pathogenic proteobacteria. Biol. Chem. 2005;386:1115–1127. doi: 10.1515/BC.2005.128. [DOI] [PubMed] [Google Scholar]
- 52.Mogk A., Haslberger T., Tessarz P., Bukau B. Common and specific mechanisms of AAA+ proteins involved in protein quality control. Biochem. Soc. Trans. 2008;36:120–125. doi: 10.1042/BST0360120. [DOI] [PubMed] [Google Scholar]
- 53.Mishra R.C., Grover A. Intergenic sequence between Arabidopsis caseinolytic protease B-cytoplasmic/heat shock protein100 and choline kinase genes functions as a heat-inducible bidirectional promoter. Plant Physiol. 2014;166:1646–1658. doi: 10.1104/pp.114.250787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hillier L.D., Lennon G., Becker M., Bonaldo M.F., Chiapelli B., Chissoe S., Dietrich N., DuBuque T., Favello A., Gish W., et al. Generation and analysis of 280,000 human expressed sequence tags. Genome Res. 1996;6:807–828. doi: 10.1101/gr.6.9.807. [DOI] [PubMed] [Google Scholar]
- 55.Shanklin J., DeWitt N.D., Flanagan J.M. The stroma of higher plant plastids contain ClpP and ClpC, functional homologs of Escherichia coli ClpP and ClpA: An archetypal two-component ATP-dependent protease. Plant Cell. 1995;7:1713–1722. doi: 10.1105/tpc.7.10.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peltier J.-B., Ripoll D.R., Friso G., Rudella A., Cai Y., Ytterberg J., Giacomelli L., Pillardy J., van Wijk K.J. Clp protease complexes from photosynthetic and non-photosynthetic plastids and mitochondria of plants, their predicted three-dimensional structures, and functional implications. J. Biol. Chem. 2004;279:4768–4781. doi: 10.1074/jbc.M309212200. [DOI] [PubMed] [Google Scholar]
- 57.Sjögren L.L.E., Clarke A.K. Assembly of the chloroplast ATP-dependent Clp protease in Arabidopsis is regulated by the ClpT accessory proteins. Plant Cell. 2011;23:322–332. doi: 10.1105/tpc.110.082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J., Kimber M.S., Nishimura K., Friso G., Schultz L., Ponnala L., van Wijk K.J. Structures, functions, and interactions of ClpT1 and ClpT2 in the Clp protease system of Arabidopsis chloroplasts. Plant Cell. 2015;27:1477–1496. doi: 10.1105/tpc.15.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishimura K., Kato Y., Sakamoto W. Essentials of proteolytic machineries in chloroplasts. Mol. Plant. 2017;10:4–19. doi: 10.1016/j.molp.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Striebel F., Kress W., Weber-Ban E. Controlled destruction: AAA+ ATPases in protein degradation from bacteria to eukaryotes. Curr. Opin. Struct. Biol. 2009;19:209–217. doi: 10.1016/j.sbi.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Lyzenga W.J., Stone S.L. Abiotic stress tolerance mediated by protein ubiquitination. J. Exp. Bot. 2012;63:599–616. doi: 10.1093/jxb/err310. [DOI] [PubMed] [Google Scholar]
- 62.Luo H., Laluk K., Lai Z., Veronese P., Song F., Mengiste T. The Arabidopsis botrytis susceptible1 interactor defines a subclass of RING E3 ligases that regulate pathogen and stress responses. Plant Physiol. 2010;154:1766–1782. doi: 10.1104/pp.110.163915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X., Garreton V., Chua N.H. The AIP2 E3 ligase acts as a novel negative regulator of ABA signaling by promoting ABI3 degradation. Genes Dev. 2005;19:1532–1543. doi: 10.1101/gad.1318705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang L., Liu Q., Liu Z., Yang H., Wang J., Li X., Yang Y. Arabidopsis C3HC4-RING finger E3 ubiquitin ligase AtAIRP4 positively regulates stress-responsive abscisic acid signaling. J. Integr. Plant Biol. 2016;58:67–80. doi: 10.1111/jipb.12364. [DOI] [PubMed] [Google Scholar]
- 65.Weake V.M., Workman J.L. Histone ubiquitination: Triggering gene activity. Mol. Cell. 2008;29:653–663. doi: 10.1016/j.molcel.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 66.Kurepa J., Wang S., Li Y., Smalle J. Proteasome regulation and stress tolerance. Plant Signal. Behav. 2009;4:924–927. doi: 10.4161/psb.4.10.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hipp M.S., Park S.H., Hartl U.U. Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol. 2014;24:506–514. doi: 10.1016/j.tcb.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Amm I., Sommer T., Wolf D.H. Protein quality control and elimination of protein waste: The role of the ubiquitin-proteasome system. Biochim. Biophys. Acta. 2014;1843:182–196. doi: 10.1016/j.bbamcr.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 69.Chen B., Retzlaff M., Roos T., Frydman J. Cellular strategies of protein quality control. Cold Spring Harb. Perspect. Biol. 2011;3:1–14. doi: 10.1101/cshperspect.a004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smalle J., Vierstra R.D. The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 2004;55:555–590. doi: 10.1146/annurev.arplant.55.031903.141801. [DOI] [PubMed] [Google Scholar]
- 71.Kurepa J., Smalle J.A. Structure, function and regulation of plant proteasomes. Biochimie. 2008;90:324–335. doi: 10.1016/j.biochi.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 72.Kraft E., Stone S.L., Ma L., Su N., Gao Y., Lau O.-S., Deng X.-W., Callis J. Genome analysis and functional characterization of the E2 and RING-type E3 ligase ubiquitination enzymes of Arabidopsis. Plant Physiol. 2005;139:1597–1611. doi: 10.1104/pp.105.067983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dreher K., Callis J. Ubiquitin, hormones and biotic stress in plants. Ann. Bot. 2007;99:787–822. doi: 10.1093/aob/mcl255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Senft D., Ronai Z.A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015;40:141–148. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cho S.K., Ryu M.Y., Song C., Kwak J.M., Kim W.T. Arabidopsis PUB22 and PUB23 are homologous U-Box E3 ubiquitin ligases that play combinatory roles in response to drought stress. Plant Cell. 2008;20:1899–1914. doi: 10.1105/tpc.108.060699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yee D., Goring D.R. The diversity of plant U-box E3 ubiquitin ligases: From upstream activators to downstream target substrates. J. Exp. Bot. 2009;60:1109–1121. doi: 10.1093/jxb/ern369. [DOI] [PubMed] [Google Scholar]
- 77.Qi H.Y., Li L., Ma H. Cellular stress response mechanisms as therapeutic targets of ginsenosides. Med. Res. Rev. 2017;38:625–654. doi: 10.1002/med.21450. [DOI] [PubMed] [Google Scholar]
- 78.Cho S.K., Chung H.S., Ryu M.Y., Park M.J., Lee M.M., Bahk Y.-Y., Kim J., Pai H.S., Kim W.T. Heterologous expression and molecular and cellular characterization of CaPUB1 encoding a hot pepper U-Box E3 ubiquitin ligase homolog. Plant Physiol. 2006;142:1664–1682. doi: 10.1104/pp.106.087965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peng Z., Staub J.M., Serino G., Kwok S.F., Kurepa J., Bruce B.D., Vierstra R.D., Wei N., Deng X.-W. The cellular level of PR500, a protein complex related to the 19S regulatory particle of the proteasome, is regulated in response to stresses in plants. Mol. Biol. Cell. 2001;12:383–392. doi: 10.1091/mbc.12.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo Q., Zhang J., Gao Q., Xing S., Li F., Wang W. Drought tolerance through overexpression of monoubiquitin in transgenic tobacco. J. Plant Physiol. 2008;165:1745–1755. doi: 10.1016/j.jplph.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 81.Kurepa J., Toh-e A., Smalle J.A. 26S proteasome regulatory particle mutants have increased oxidative stress tolerance. Plant J. 2008;53:102–114. doi: 10.1111/j.1365-313X.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- 82.Groll M., Schellenberg B., Bachmann A.S., Archer C.R., Huber R., Powell T.K., Lindow S., Kaiser M., Dudler R. A plant pathogen virulence factor inhibits the eukaryotic proteasome by a novel mechanism. Nature. 2008;452:755–759. doi: 10.1038/nature06782. [DOI] [PubMed] [Google Scholar]
- 83.Zhang L., Du L., Poovaiah B.W. Calcium signaling and biotic defense responses in plants. Plant Signal. Behav. 2014;9:e973818. doi: 10.4161/15592324.2014.973818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Becker J., Kempf R., Jeblick W., Kauss H. Induction of competence for elicitation of defense responses in cucumber hypocotyls requires proteasome activity. Plant J. 2000;21:311–316. doi: 10.1046/j.1365-313x.2000.00677.x. [DOI] [PubMed] [Google Scholar]
- 85.Petitot A.S., Blein J.P., Pugin A., Suty L. Cloning of two plant cDNAs encoding a β-type proteasome subunit and a transformer-2-like SR-related protein: Early induction of the corresponding genes in tobacco cells treated with cryptogein. Plant Mol. Biol. 1997;35:261–269. doi: 10.1023/A:1005833216479. [DOI] [PubMed] [Google Scholar]
- 86.Dahan J., Etienne P., Petitot A.S., Houot V., Blein J.P., Suty L. Cryptogein affects expression of alpha3, alpha6 and beta1 20S proteasome subunits encoding genes in tobacco. J. Exp. Bot. 2001;52:1947–1948. doi: 10.1093/jexbot/52.362.1947. [DOI] [PubMed] [Google Scholar]
- 87.Suty L., Lequeu J., Lançon A., Etienne P., Petitot A.S., Blein J.P. Preferential induction of 20S proteasome subunits during elicitation of plant defense reactions: Towards the characterization of “plant defense proteasomes”. Int. J. Biochem. Cell Biol. 2003;35:637–650. doi: 10.1016/S1357-2725(02)00386-2. [DOI] [PubMed] [Google Scholar]
- 88.Lequeu J., Simon-Plas F., Fromentin J., Etienne P., Petitot A.S., Blein J.P., Suty L. Proteasome comprising a β1 inducible subunit acts as a negative regulator of NADPH oxidase during elicitation of plant defense reactions. FEBS Lett. 2005;579:4879–4886. doi: 10.1016/j.febslet.2005.07.073. [DOI] [PubMed] [Google Scholar]
- 89.Smalle J., Kurepa J., Yang P., Emborg T.J., Babiychuk E., Kushnir S., Vierstra R.D. The pleiotropic role of the 26S proteasome subunit RPN10 in Arabidopsis growth and development supports a substrate-specific function in abscisic acid signaling. Plant Cell. 2003;15:965–980. doi: 10.1105/tpc.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ueda M., Matsui K., Ishiguro S., Sano R., Wada T., Paponov I., Palme K., Okada K. The HALTED ROOT gene encoding the 26S proteasome subunit RPT2a is essential for the maintenance of Arabidopsis meristems. Development. 2004;131:2101–2111. doi: 10.1242/dev.01096. [DOI] [PubMed] [Google Scholar]
- 91.Wang S., Kurepa J., Smalle J.A. The Arabidopsis 26S proteasome subunit RPN1a is required for optimal plant growth and stress responses. Plant Cell Physiol. 2009;50:1721–1725. doi: 10.1093/pcp/pcp105. [DOI] [PubMed] [Google Scholar]
- 92.Sakamoto T., Kamiya T., Sako K., Yamaguchi J., Yamagami M., Fujiwara T. Arabidopsis thaliana 26S proteasome subunits RPT2a and RPT5a are crucial for zinc deficiency-tolerance. Biosci. Biotechnol. Biochem. 2011;75:561–567. doi: 10.1271/bbb.100794. [DOI] [PubMed] [Google Scholar]
- 93.Sun C.W., Callis J. Independent modulation of Arabidopsis thaliana polyubiquitin mRNAs in different organs and in response to environmental changes. Plant J. 1997;11:1017–1027. doi: 10.1046/j.1365-313X.1997.11051017.x. [DOI] [PubMed] [Google Scholar]
- 94.Christensen A.H., Sharrock R.A., Quail P.H. Maize polyubiquitin genes: Structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol. Biol. 1992;18:675–689. doi: 10.1007/BF00020010. [DOI] [PubMed] [Google Scholar]
- 95.Garbarino J.E., Rockhold D.R., Belknap W.R. Expression of stress-responsive ubiquitin genes in potato tubers. Plant Mol. Biol. 1992;20:235–244. doi: 10.1007/BF00014491. [DOI] [PubMed] [Google Scholar]
- 96.Kang H., Zhang M., Zhou S., Guo Q., Chen F., Wu J., Wang W. Overexpression of wheat ubiquitin gene, Ta-Ub2, improves abiotic stress tolerance of Brachypodium distachyon. Plant Sci. 2016;248:102–115. doi: 10.1016/j.plantsci.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 97.Zhiguo E., Yuping Z., Tingting L., Lei W., Heming Z. Characterization of the ubiquitin-conjugating enzyme gene family in rice and evaluation of expression profiles under abiotic stresses and hormone treatments. PLoS ONE. 2015;10:e0122621. doi: 10.1371/journal.pone.0122621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chung E., Cho C.W., So H.A., Kang J.S., Chung Y.S., Lee J.H. Overexpression of VrUBC1, a Mung bean E2 ubiquitin-conjugating enzyme, enhances osmotic stress tolerance in Arabidopsis. PLoS ONE. 2013;8:e66056. doi: 10.1371/journal.pone.0066056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wan X., Mo A., Liu S., Yang L., Li L. Constitutive expression of a peanut ubiquitin-conjugating enzyme gene in Arabidopsis confers improved water-stress tolerance through regulation of stress-responsive gene expression. J. Biosci. Bioeng. 2011;111:478–484. doi: 10.1016/j.jbiosc.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 100.Zhou G.A., Chang R.Z., Qiu L.J. Overexpression of soybean ubiquitin-conjugating enzyme gene GmUBC2 confers enhanced drought and salt tolerance through modulating abiotic stress-responsive gene expression in Arabidopsis. Plant Mol. Biol. 2010;72:357–367. doi: 10.1007/s11103-009-9575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bahmani R., Kim D.G., Lee B.D., Hwang S. Over-expression of tobacco UBC1 encoding a ubiquitin-conjugating enzyme increases cadmium tolerance by activating the 20S/26S proteasome and by decreasing Cd accumulation and oxidative stress in tobacco (Nicotiana tabacum) Plant Mol. Biol. 2017;94:433–451. doi: 10.1007/s11103-017-0616-6. [DOI] [PubMed] [Google Scholar]
- 102.Wang W., Vinocur B., Shoseyov O., Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 103.Muthusamy S.K., Dalal M., Chinnusamy V., Bansal K.C. Differential regulation of genes coding for organelle and cytosolic ClpATPases under biotic and abiotic stresses in Wheat. Front. Plant Sci. 2016;7:1–15. doi: 10.3389/fpls.2016.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gottesman S., Wickner S., Maurizi M.R. Protein quality control: Triage by chaperones and proteases. Genes Dev. 1997;11:815–823. doi: 10.1101/gad.11.7.815. [DOI] [PubMed] [Google Scholar]
- 105.Wawrzynow A., Wojtkowiak D., Marszalek J., Banecki B., Jonsen M., Graves B., Georgopoulos C., Zylicz M. The ClpX heat-shock protein of Escherichia coli, the ATP-dependent substrate specificity component of the ClpP-ClpX protease, is a novel molecular chaperone. EMBO J. 1995;14:1867–1877. doi: 10.1002/j.1460-2075.1995.tb07179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parsell D.A., Lindquist S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- 107.Porankiewicz J., Clarke A.K. Induction of the heat shock protein ClpB affects cold acclimation in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 1997;179:5111–5117. doi: 10.1128/JB.179.16.5111-5117.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kruger E., Volker U., Hecker M. Stress induction of clpC in Bacillus subtilis and its involvement in stress tolerance. J. Bacteriol. 1994;176:3360–3367. doi: 10.1128/JB.176.11.3360-3367.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frees D., Chastanet A., Qazi S., Sørensen K., Hill P., Msadek T., Ingmer H. Clp ATPases are required for stress tolerance, intracellular replication and biofilm formation in Staphylococcus aureus. Mol. Microbiol. 2004;54:1445–1462. doi: 10.1111/j.1365-2958.2004.04368.x. [DOI] [PubMed] [Google Scholar]
- 110.Krüger E., Witt E., Ohlmeier S., Hanschke R., Hecker M. The Clp proteases of Bacillus subtilis are directly involved in degradation of misfolded proteins. J. Bacteriol. 2000;182:3259–3265. doi: 10.1128/JB.182.11.3259-3265.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kroh H.E., Simon L.D. The ClpP component of Clp protease is the sigma32-dependent deat shock protein F21.5. J. Bacteriol. 1990;172:6026–6034. doi: 10.1128/JB.172.10.6026-6034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Laskowska E., Kuczynska-Wisnik D., Skorko-Glonek J., Taylor A. Degradation by proteases Lon, Clp and HtrA, of Escherichia coli proteins aggregated in vivo by heat shock; HtrA protease action in vivo and in vitro. Mol. Microbiol. 1996;22:555–571. doi: 10.1046/j.1365-2958.1996.1231493.x. [DOI] [PubMed] [Google Scholar]
- 113.Damerau K., John A.N.N.C.S.T. The role of Clp protease subunits in degradation of carbon starvation protein in Escherichia coli. J. Bacteriol. 1993;175:53–63. doi: 10.1128/JB.175.1.53-63.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Capestany C.A., Tribble G.D., Maeda K., Demuth D.R., Lamont R.J. Role of the Clp system in stress tolerance, biofilm formation, and intracellular invasion in Porphyromonas gingivalis. J. Bacteriol. 2008;190:1436–1446. doi: 10.1128/JB.01632-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xie F., Zhang Y., Li G., Zhou L., Liu S., Wang C. The ClpP protease is required for the stress tolerance and biofilm formation in Actinobacillus pleuropneumoniae. PLoS ONE. 2013;8:e53600. doi: 10.1371/annotation/fbe62755-866e-4a4e-baf8-4bb40019a8fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cho E.-K., Bae S.-J. ATP-independent thermoprotective activity of Nicotiana tabacum heat shock protein 70 in Escherichia coli. J. Biochem. Mol. Biol. 2007;40:107–112. doi: 10.5483/BMBRep.2007.40.1.107. [DOI] [PubMed] [Google Scholar]
- 117.Lee U., Rioflorido I., Hong S.W., Larkindale J., Waters E.R., Vierling E. The Arabidopsis ClpB/Hsp100 family of proteins: Chaperones for stress and chloroplast development. Plant J. 2006;49:115–127. doi: 10.1111/j.1365-313X.2006.02940.x. [DOI] [PubMed] [Google Scholar]
- 118.Singh A., Singh U., Mittal D., Grover A. Genome-wide analysis of rice ClpB/HSP100, ClpC and ClpD genes. BMC Genomics. 2010;11:1–18. doi: 10.1186/1471-2164-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Keeler S.J., Boettger C.M., Haynes J.G., Kuches K.A., Johnson M.M., Thureen D.L., Keeler C.L., Kitto S.L. Acquired thermotolerance and expression of the HSP100/ClpB genes of lima bean. Plant Physiol. 2000;123:1121–1132. doi: 10.1104/pp.123.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zybailov B., Friso G., Kim J., Rudella A., Rodríguez V.R., Asakura Y., Sun Q., van Wijk K.J. Large scale comparative proteomics of a chloroplast Clp protease mutant reveals folding stress, altered protein homeostasis, and feedback regulation of metabolism. Mol. Cell. Proteomics. 2009;8:1789–1810. doi: 10.1074/mcp.M900104-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kiyosue T., Yamaguchi-Shinozaki K., Shinozaki K. Characterization of cDNA for a dehydration-inducible gene that encodes a CLP A, B-like protein in Arabidopsis thaliana L. Biochem. Biophys. Res. Commun. 1993;196:1214–1220. doi: 10.1006/bbrc.1993.2381. [DOI] [PubMed] [Google Scholar]
- 122.Zheng B., Halperin T., Hruskova-heidingsfeldova O., Adam Z., Clarke A.K. Characterization of chloroplast Clp proteins in Arabidopsis: Localization, tissue specificity and stress responses. Physiol. Plant. 2002;114:92–101. doi: 10.1034/j.1399-3054.2002.1140113.x. [DOI] [PubMed] [Google Scholar]
- 123.Nakashima K., Kiyosue T., Yamaguchi-Shinozaki K., Shinozaki K. A nuclear gene, erd1, encoding a chloroplast-targeted Clp protease regulatory subunit homolog is not only induced by water stress but also developmentally up-regulated during senescence in Arabidopsis thaliana. Plant J. 1997;12:851–861. doi: 10.1046/j.1365-313X.1997.12040851.x. [DOI] [PubMed] [Google Scholar]
- 124.Ostersetzer O., Tabak S., Yarden O., Shapira R., Adam Z. Immunological detection of proteins similar to bacterial proteases in higher plant chloroplasts. Eur. J. Biochem. 1996;236:932–936. doi: 10.1111/j.1432-1033.1996.00932.x. [DOI] [PubMed] [Google Scholar]
- 125.Mishra R.C., Richa. Grover, A Constitutive over-expression of rice ClpD1 protein enhances tolerance to salt and desiccation stresses in transgenic Arabidopsis plants. Plant Sci. 2016;250:69–78. doi: 10.1016/j.plantsci.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 126.Weaver L.M., Froehlich J.E., Amasino R.M. Chloroplast-targeted ERD1 protein declines but its mRNA increases during senescence in Arabidopsis. Plant Physiol. 1999;119:1209–1216. doi: 10.1104/pp.119.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakabayashi K., Ito M., Kiyosue T., Shinozaki K., Watanabe A. Identification of clp genes expressed in senescing Arabidopsis leaves. Plant Cell Physiol. 1999;40:504–514. doi: 10.1093/oxfordjournals.pcp.a029571. [DOI] [PubMed] [Google Scholar]
- 128.Sinvany-Villalobo G., Davydov O., Ben-Ari G., Zaltsman A., Raskind A., Adam Z. Expression in multigene families. Analysis of chloroplast and mitochondrial proteases. Plant Physiol. 2004;135:1336–1345. doi: 10.1104/pp.104.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rei Liao J.Y., van Wijk K.J. Discovery of AAA+ protease substrates through trapping approaches. Trends Biochem. Sci. 2019;44:528–545. doi: 10.1016/j.tibs.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 130.Cui S., Huang F., Wang J., Ma X., Cheng Y., Liu J. A proteomic analysis of cold stress responses in rice seedlings. Proteomics. 2005;5:3162–3172. doi: 10.1002/pmic.200401148. [DOI] [PubMed] [Google Scholar]
- 131.Xie L., He X., Shang S., Zheng W., Liu W., Zhang G., Wu F. Comparative proteomic analysis of two tobacco (Nicotiana tabacum) genotypes differing in Cd tolerance. BioMetals. 2014;27:1277–1289. doi: 10.1007/s10534-014-9789-5. [DOI] [PubMed] [Google Scholar]
- 132.Jin H., Xu M., Chen H., Zhang S., Han X., Tang Z., Sun R. Comparative proteomic analysis of differentially expressed proteins in Amaranthus hybridus L. roots under cadmium stress. Water. Air. Soil Pollut. 2016;227:1–12. doi: 10.1007/s11270-016-2914-z. [DOI] [Google Scholar]
- 133.Ali M.S., Kim K.W., Dhakal R., Choi D., Baek K.-H. Accumulation of high contents of free amino acids in the leaves of Nicotiana benthamiana by the co-suppression of NbClpC1 and NbClpC2 genes. Plant Cell Rep. 2015;1:355–365. doi: 10.1007/s00299-014-1714-4. [DOI] [PubMed] [Google Scholar]
- 134.Lin J.F., Wu S.H. Molecular events in senescing Arabidopsis leaves. Plant J. 2004;39:612–628. doi: 10.1111/j.1365-313X.2004.02160.x. [DOI] [PubMed] [Google Scholar]
- 135.Sjogren L.L.E., MacDonald T.M., Sutinen S., Clarke A.K. Inactivation of the clpC1 gene encoding a chloroplast Hsp100 molecular chaperone causes growth retardation, leaf chlorosis, lower photosynthetic activity, and a specific reduction in photosystem content. Plant Physiol. 2004;136:4114–4126. doi: 10.1104/pp.104.053835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Constan D., Froehlich J.E., Rangarajan S., Keegstra K. A stromal Hsp100 protein is required for normal chloroplast development and function in Arabidopsis. Plant Physiol. 2004;136:3605–3615. doi: 10.1104/pp.104.052928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kovacheva S., Bédard J., Patel R., Dudley P., Twell D., Ríos G., Koncz C., Jarvis P. In vivo studies on the roles of Tic110, Tic40 and Hsp93 during chloroplast protein import. Plant J. 2005;41:412–428. doi: 10.1111/j.1365-313X.2004.02307.x. [DOI] [PubMed] [Google Scholar]
- 138.Pulido P., Llamas E., Rodriguez-Concepcion M. Both Hsp70 chaperone and Clp protease plastidial systems are required for protection against oxidative stress. Plant Signal. Behav. 2017;12:e1290039. doi: 10.1080/15592324.2017.1290039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Majeran W., Wollman F.-A., Vallon O. Evidence for a role of ClpP in the degradation of the chloroplast Cytochrome b6f complex. Plant Cell. 2000;12:137–149. doi: 10.1105/tpc.12.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Majeran W., Olive J., Drapier D., Vallon O., Wollman F.-A. The light sensitivity of ATP synthase mutants of Chlamydomonas reinhardtii. Plant Physiol. 2001;126:421–433. doi: 10.1104/pp.126.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang C., Wang S., Chen L., Lemieux C., Otis C., Turmel M., Liu X.Q. The Chlamydomonas chloroplast clpP gene contains translated large insertion sequences and is essential for cell growth. Mol. Gen. Genet. 1994;244:151–159. doi: 10.1007/BF00283516. [DOI] [PubMed] [Google Scholar]
- 142.Clarke A.K., Schelin J., Porankiewicz J. Inactivation of the clpP1 gene for the proteolytic subunit of the ATP-dependent Clp protease in the cyanobacterium Synechococcus limits growth and light acclimation. Plant Mol. Biol. 1998;37:791–801. doi: 10.1023/A:1006016302074. [DOI] [PubMed] [Google Scholar]
- 143.Porankiewicz J., Schelin J., Clarke A.K. The ATP-dependent Clp protease is essential for acclimation to UV-B and low temperature in the cyanobacterium Synechococcus. Mol. Microbiol. 1998;29:275–283. doi: 10.1046/j.1365-2958.1998.00928.x. [DOI] [PubMed] [Google Scholar]
- 144.Shikanai T., Shimizu K., Ueda K., Nishimura Y., Kuroiwa T., Hashimoto T. The chloroplast clpP gene, encoding a proteolytic subunit of ATP-dependent protease, is indispensable for chloroplast development in tobacco. Plant Cell Physiol. 2001;42:264–273. doi: 10.1093/pcp/pce031. [DOI] [PubMed] [Google Scholar]
- 145.Kuroda H., Maliga P. The plastid clpP1 protease gene is essential for plant development. Nature. 2003;425:86–89. doi: 10.1038/nature01909. [DOI] [PubMed] [Google Scholar]
- 146.Agrawal G.K., Rakwal R., Yonekura M., Kubo A., Saji H. Proteome analysis of differentially displayed proteins as a tool for investigating ozone stress in rice (Oryza sativa L.) seedlings. Proteomics. 2002;2:947–959. doi: 10.1002/1615-9861(200208)2:8<947::AID-PROT947>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 147.Rakwal R., Agrawal G.K., Kubo A., Yonekura M., Tamogami S., Saji H., Iwahashi H. Defense/stress responses elicited in rice seedlings exposed to the gaseous air pollutant sulfur dioxide. Environ. Exp. Bot. 2003;49:223–235. doi: 10.1016/S0098-8472(02)00072-2. [DOI] [Google Scholar]
- 148.Garcia de la Garma J., Fernandez-Garcia N., Bardisi E., Pallol B., Asensio-Rubio J.S., Bru R., Olmos E. New insights into plant salt acclimation: The roles of vesicle trafficking and reactive oxygen species signalling in mitochondria and the endomembrane system. New Phytol. 2015;205:216–239. doi: 10.1111/nph.12997. [DOI] [PubMed] [Google Scholar]
- 149.Wang X., Shan X., Wu Y., Su S., Li S., Liu H., Han J., Xue C., Yuan Y. iTRAQ-based quantitative proteomic analysis reveals new metabolic pathways responding to chilling stress in maize seedlings. J. Proteomics. 2016;146:14–24. doi: 10.1016/j.jprot.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 150.Bazargani M.M., Sarhadi E., Bushehri A.A.S., Matros A., Mock H.P., Naghavi M.R., Hajihoseini V., Mardi M., Hajirezaei M.R., Moradi F., et al. A proteomics view on the role of drought-induced senescence and oxidative stress defense in enhanced stem reserves remobilization in wheat. J. Proteomics. 2011;74:1959–1973. doi: 10.1016/j.jprot.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 151.Zhang W., Zhang H., Ning L., Li B., Bao M. Quantitative proteomic analysis provides novel insights into cold stress responses in petunia seedlings. Front. Plant Sci. 2016;7:1–13. doi: 10.3389/fpls.2016.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hajrah N.H., Obaid A.Y., Atef A., Ramadan A.M., Arasappan D., Nelson C.A., Edris S., Mutwakil M.Z., Alhebshi A., Gadalla N.O., et al. Transcriptomic analysis of salt stress responsive genes in Rhazya stricta. PLoS ONE. 2017;12:e0177589. doi: 10.1371/journal.pone.0177589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Choudhury S., Panda P., Sahoo L., Panda S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013;8:e23681. doi: 10.4161/psb.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Roberts I.N., Caputo C., Criado M.V., Funk C. Senescence-associated proteases in plants. Physiol. Plant. 2012;145:130–139. doi: 10.1111/j.1399-3054.2012.01574.x. [DOI] [PubMed] [Google Scholar]
- 155.Sinha R., Pal A.K., Singh A.K. Physiological, biochemical and molecular responses of lentil (Lens culinaris Medik.) genotypes under drought stress. Indian J. Plant Physiol. 2018;23:772–784. doi: 10.1007/s40502-018-0411-7. [DOI] [Google Scholar]