Abstract
Endoplasmic reticulum (ER) stress is recognized as a contributing factor to various ocular neurovascular pathologies including retinitis pigmentosa, glaucoma, and diabetic retinopathy (DR). ER stress in particular is implicated in the development of DR, which is significantly influenced by inflammation driven retinal vascular degeneration and dysfunction. Ultimately, loss of vision occurs if left untreated. However, the identity of the target cells and their temporal involvement in diabetes-mediated dysfunction need further investigation. Early diabetes-induced stress in photoreceptor cells is proposed as the driver of inflammatory mediated neurovascular changes during diabetes. Although tunicamycin induced ER stress results in photoreceptor loss, its consequences for retinal vascular degeneration and retinal ganglion (RGC) and pigment epithelium (RPE) cell loss remains unclear. Here we show intravitreal delivery of tunicamycin primarily induced ER stress in photoreceptor cells resulting in their loss by apoptosis. This was concomitant with induced expression of the unfolded protein response marker CHOP in these cells. We also demonstrated significant degeneration of retinal capillaries following the loss of photoreceptor cells with minimal impact on loss of RGC and RPE cells. However, activation of retinal microglial and Muller cells were noticeable. Thus, our data support the notion that ER stress mediated dysfunction and/or loss of photoreceptor cells in response to inflammation and oxidative stress could precede retinal vascular and neuronal dysfunction and degeneration.
Keywords: Diabetic retinopathy, Retinal vasculature, Inflammation, Retinal degeneration
1. Introduction
Ocular complications of diabetes including diabetic retinopathy (DR) are associated with vision impairment and blindness in middle-aged people (Duh et al., 2017; Gardner et al., 2002). Although DR is a major consequence of chronic hyperglycemia, the pathogenesis of DR remains poorly understood. The majority of investigations have focused on diabetes-mediated vascular dysfunction, including endoplasmic reticulum (ER) stress and inflammation, as the primary insult in the onset and progression of DR. However, recent studies indicate a vital role for changes in the neuroretina, mainly retinal ganglion cells (RGC), that could contribute to and/or precede detected vascular damage during diabetes (Barber et al., 2011). How these stress mediated cellular changes are brought about and in what order these changes occur remains unclear and is the focus of current study.
Specialized retinal neuronal cells, namely photoreceptors, have been recently implicated as the primary source of inflammation and oxidative stress in the onset and advancement of diabetes ocular complications (Kern and Berkowitz, 2015), as well as proper retinal vascularization (Fu et al., 2018). Although diabetes induced degeneration of retinal capillaries in wild type mice, in mice lacking photoreceptors diabetes did not result in capillary degeneration (Du et al., 2013). These data suggest that the diabetes impact on retinal vasculature is indirect, and could occur through metabolic dysfunction and/or loss of photoreceptor cells (Fu et al., 2018; Liu et al., 2016; Tonade et al., 2016). Furthermore, a survey of patients with diabetes, who also had retinitis pigmentosa (lacked photoreceptors), found that these patients had less retinopathy than the diabetic patients without retinitis pigmentosa (Chen et al., 2012). Thus, there is great interest in determining the contribution of these neurovascular alterations to the pathogenesis of DR and susceptibility to retinopathy of prematurity.
A number of studies have demonstrated a role for ER stress in retinal neurovascular changes during diabetes (Elmasry et al., 2018; Kroeger et al., 2018; Li et al., 2011; Ma et al., 2014; Oshitari et al., 2008; Yang et al., 2017; Zhang et al., 2015). However, the major cellular source of this ER stress and its contribution to neurovascular dysfunction and retinal vascular degeneration is not well characterized. ER stress is observed in the retinal blood vessels and its cellular components during diabetes (Adachi et al., 2011; Li et al., 2011; Li et al., 2012; Li et al., 2009), and in human photoreceptor diseases retinitis pigmentosa and achromatopsia (Chan et al., 2016). However, whether ER stress mediated loss of photoreceptor cells drives retinal neurovascular dysfunction or the reported ER stress in the retinal neurovasculature drives loss of photoreceptor cells and retinal neurons remains unknown.
Here we investigated whether ER stress could contribute to retinal neurovascular changes, and whether toxicity in retinal neuronal cells or ER stress drive retinal capillary degeneration or the other way around. As previously demonstrated, we showed tunicamycin causes ER stress in the retina, more specifically in the photoreceptor cells, which was followed by the degeneration of retinal capillaries. These changes mimic the early non-proliferative changes observed during diabetes including the formation of acellular capillaries, and were concomitant with activation of microglial and Muller cells. Tunicamycin treatment resulted in retinal vascular degeneration without a significant effect on RGC and RPE cell integrity. We observed a significant loss of photoreceptor cells with tunicamycin treatment prior to retinal vascular degeneration. These findings support a model whereby retinal photoreceptor stress drives retinal capillary degeneration, and is in line with the recently suggested role of hyperglycemia in photoreceptor cell oxidative stress, inflammation, and neurovascular dysfunction.
2. Material and methods
2.1. Animals
All animal studies were performed following the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and approved by the Animal Care and Use Committee of the University of Wisconsin School of Medicine and Public Health (Assurance number D16–00239). Six-week-old C57BL/6j mice were used in these studies and housed on a 12-hour light–dark cycle, without food and water restrictions. C57BL/6J and CX3CR1gfp/gfp knock in mice were obtained from Jackson Laboratories and a colony was established in our laboratory. All the mice were on C57BL/6J background, and free of retinal degeneration mutations. Both male and female mice were used. Each group was consisted of at least five mice.
2.2. NMDA and tunicamycin induced retinal damage
N-methyl-D-aspartic acid (NMDA) or tunicamycin mediated retinal damage was produced by intravitreal injection of these compounds. Briefly, mice were anesthetized with ketamine hydrochloride (100 mg/kg) and xylazine (10 mg/Kg), Body temperature was maintained between 37.0 and 37.5°C with the aid of a heating pad. For NMDA (Cat# M3262; Sigma, St. Louis, MO) injections, 2 µl of a 40 mM solution of NMDA prepared in balanced salt solution (BSS; Alcon, Fort Worth, TX) was delivered by intravitreal injection into both eyes. Control animals received the vehicle only, BSS. Tunicamycin (T7765; Sigma) was prepared in dimethyl sulfoxide (1 µg/µl; D8418; Sigma). For tunicamycin injections, the stack solution was further diluted into 0.025 µg/µl or 0.05 µg/µl in BSS and 2 µl of solution was injected into both eyes. Vehicle control group receive a BSS-DMSO mixture (2 µl) representing the higher tunicamycin dose (0.05 µg/µl). Intravitreal injections were carried out using a Harvard pump (Harvard Apparatus, Holliston, MA) and pulled-glass capillaries. Each glass capillary was calibrated to deliver 2 µL of vehicle or test compound using a foot switch. The pupils of anesthetized mice were dilated using a drop of tropicamide (1%). For intravitreal injections, the sharpened tip of the glass capillary was passed through the sclera, just behind the limbus, into the vitreous cavity. The foot switch was then depressed to inject the test compounds. The mice were sacrificed at different time points post injection and the eyes were enucleated for different analysis as described below.
2.3. Trypsin-digest preparations
Eyes were fixed in 4% paraformaldehyde for at least 24 h, and bisected equatorially to remove the entire retina. Retinas were washed in distilled water overnight, and incubated in 3% trypsin (Trypsin 250, Cat# 215240; Difco, Fisher Scientific, Maple Grove, IL) for approximately 1–1.5 h at 37°C. The trypsin solution was prepared by dissolving the desired amount of trypsin in 0.1 M Tris, 0.1 M maleic acid, pH 7.8 containing 0.2 M sodium fluoride (NaF; Cat# 201154; Sigma). Following digestion, retinal vessels were flattened by four radial cuts, mounted on glass slides, and subjected to Periodic Acid-Schiff (PAS) and hematoxylin staining.
2.4. Quantification of degenerated blood vessels
The slides with retinal blood vessels prepared above were used for these quantifications. Degenerated capillaries were quantified in the whole retina (x200 magnification) in a masked fashion. Degenerated capillaries were identified as capillary-sized ghost vessels having no nuclei anywhere along their length. Ghost vessels with a diameter <20% of the diameter of adjacent capillaries were identified as strands and were not counted.
2.5. Histological analysis
Eyes from mice with different treatments were fixed in 10% formalin for 24 h, and paraffin embedded for sectioning. Serial sections (6 µm), separated by at least 40 µm, were obtained from the vicinity of the optic nerve. The hematoxylin and eosin (H&E) stained sections were evaluated in masked fashion for structural organization and alterations with various treatments.
2.6. Retinal wholemount immunostaining
Mice treated with tunicamycin or NMDA, and untreated controls were sacrificed, eyes were removed, and fixed in 4% paraformaldehyde, 4 min on ice. This was followed by fixing in methanol for at least 24 h at −20°C. Following fixation, retinas were removed in phosphate buffered saline (PBS; Cat# D1408; Sigma) and washed with PBS three times, 10 min each. The retinas were then incubated in a blocking buffer (50% fetal calf serum and 20% normal goat serum in PBS) for 1 h. Following blocking, the retinas were incubated with rabbit anti-mouse collagen IV antibody (Cat# AB756P; Chemicon, Temecula, CA; diluted 1:500 in blocking buffer) at 4°C overnight. Retinas were then washed three times with PBS, 10 min each, and incubated with secondary antibody Alexa Fluor 594 goat-anti-rabbit (Cat # A-11037; Invitrogen, Carlsbad, CA; diluted 1:500 in blocking buffer) for 2 h at room temperature. Following incubation, retinas were washed three times with PBS, 10 min each, and mounted on a slide with PBS/glycerol (2 vol/1 vol). Retinas were examined using a Zeiss fluorescence microscope and images were captured in digital format (Zeiss, Chester, VA).
2.6. RPE ZO-1 wholemount immunostaining
Mice treated with tunicamycin (0.05 µg) or solvent control were sacrificed after 3 days of injection, eyes were removed, and fixed in 4% paraformaldehyde (1.5 h on ice). The anterior segment and retina were dissected under a dissecting microscope. During the removal of retina special attention was paid to avoid damaging the underlying RPE. The remaining RPE-choroid-sclera complex was relaxed by four radial incisions, and was washed with PBS three times, 10 min each. Following incubation in a blocking buffer (see above) for 1 h, the samples were incubated with rabbit anti-mouse ZO-1 antibody (Cat # 61–7300; Invitrogen; diluted 1:500 in blocking buffer) at 4°C overnight. Samples were then washed three times with PBS, 10 min each, and incubated with the secondary antibody Alexa Fluor 594 goat-anti-rabbit (Cat # A-11037; Invitrogen; diluted 1:500 in blocking buffer) for 2 h at room temperature. Following incubation, samples were washed three times with PBS, 10 min each, and mounted on a slide using PBS/glycerol (2 vol/1 vol). RPE cells were viewed using a Zeiss fluorescence microscopy and images were captured in digital format using (Zeiss, Chester, VA).
2.7. TUNEL staining
To detect retinal cell apoptosis induced by tunicamycin, TUNEL staining was conducted using the DeadEnd™ Fluorometric TUNEL System according to the manufacturer’s protocol (Cat# G3250; Promega, Madison, WI USA). Mice were sacrificed at desired times after intravitreal injection of tunicamycin or solvent control, eyes were enucleated, fixed overnight in 4% paraformaldehyde, embedded in paraffin, sectioned, and paraffin embedded tissue slides stored at room temperature. For TUNEL staining, the tissue was first treated with decreasing concentrations of ethanol to remove paraffin (100%, 95%, 85%, 70%, and 50%). The slide samples were incubated with 20 µg/ml Proteinase K solution at room temperature (RT), washed with PBS, and incubated with TdT reaction mix for 60 min at 37°C in a humidified chamber. The reaction was stopped by adding 2X SSC, and followed by adding ProLong™ Gold Antifade Mounting medium with DAPI (Cat# P36935; Invitrogen) to slides. The slide were examined using a confocal fluorescence microscop (Nikon Eclipse 50i fluorescence microscope) for detection of green fluorescent apoptotic cells and DAPI-stained nuclei.
2.8. Double antibody and TUNEL staining
Paraffin was removed from embedded eye sections on slides as described above. The slides were stained with TUNEL first as above, without adding the mounting medium. The slides were then blocked with 5% goat serum for 2 h at RT, incubated with 1:100 diluted primary antibody overnight at 4°C, washed with PBS and incubated with florescence labeled goat secondary antibody for 1 h at RT. The specimens were washed in PBS, and mounted using ProLong™ Gold Antifade Mounting medium with DAPI (Invitrogen). The antibodies used were mouse-anti-CHOP (Cat#2895, Cell Signaling, Danvers, MA), and rabbit anti-RBPMS (Cat# ABN1362, Sigma). The secondary Ab was goat anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor 594 (Cat# A11037, Invitrogen). Images were obtained in digital format using a Nikon Eclipse 50i fluorescence microscope.
2.9. Immunostaining of frozen sections
Eyes from animals treated with various test compound or solvent control were embedded in Optimal Cutting Temperature (OCT) compound at −80°C. Eyes were sectioned (9 µm) using a cryostat, sections were placed on glass slides, and allowed to dry for 2 h. For immunostaining, sections were fixed in cold acetone (4°C) on ice for 10 min, followed by three washes in PBS, 5 minutes each. Slides were incubated in blocker solution (1% BSA, 0.2% skim milk, and 0.3% Triton X-100 in PBS) for 15 minutes at room temperature, and then incubated with rabbit anti-mouse-GFAP (Cat#: Z0334; DAKO, Denmark; 1:500 dilution) overnight at 4°C in humid environment. After three washes in PBS, 5 min each, samples were incubated with appropriate secondary antibodies for 2 h at room temperature (Jackson Immunoresearch, West Grove, PA; 1:500 dilution). As a control, some sections were incubated only with secondary antibody. Sections were washed three times in PBS, covered with PBS: glycerol (2 vol/1 vol), and mounted with a coverslip. Retina sections were examined using a Zeiss fluorescence microscope and images were captured in digital format.
2.10. Statistical analysis
Data in figures represent means ± SE from animals with n ≥ 5 (as indicated in figure legends). Student’s unpaired t-test (2-tailed) or one-way ANOVA with post-Bonferroni test for multiple comparisons were used for statistical evaluations between control and treated samples. P ≤ 0.05 was considered significant. All data analysis was done in GraphPad Prism or Microsoft Excel.
3. Results
3.1. Tunicamycin mediated ER stress promotes retinal capillary degeneration in a dose dependent manner
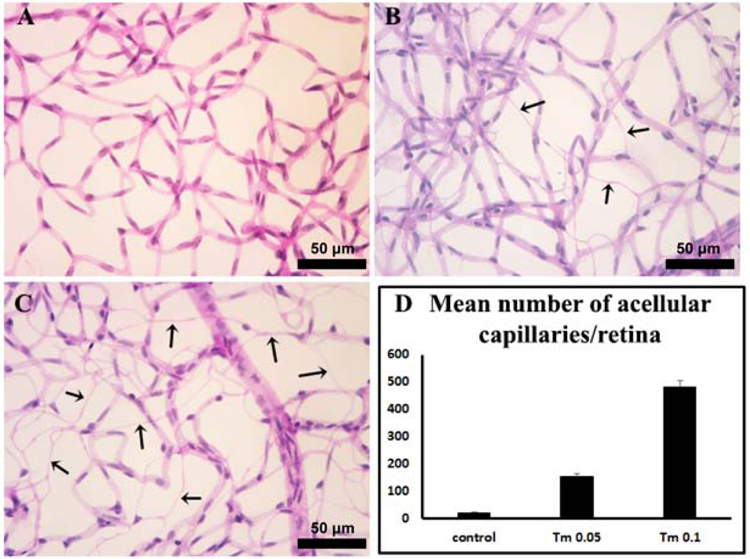
An increase in the number of degenerating capillaries are noted in the retinal vasculature with diabetes progression. Available rodent diabetes models, however, show limited levels of acellular capillaries, especially with short duration of diabetes. This makes the detailed study of underlying mechanisms challenging. ER stress, as a risk factor, is known to contribute to retinal vascular degeneration during diabetes (Elmasry et al., 2018; Li et al., 2011; Li et al., 2012). Here to investigate ER stress mediated capillary degeneration, various doses of tunicamycin, a known ER stress inducer, were delivered by intravitreal injection. We assessed the formation of acellular capillaries two weeks after tunicamycin injection by preparing retinal trypsin digests. A significant increase in the number of degenerating capillaries was noted in both tunicamycin treatment groups compared with vehicle controls (Fig. 1). More degenerating capillaries were observed with increasing the dose of tunicamycin from 0.05 mg to 0.1 mg. Thus, the degree of capillary degeneration with tunicamycin was dose-dependent.
Fig. 1. ER stress induces vascular degeneration in the retina.
Representative images of retinal vasculatures 14 days after intravitreal injection of vehicle (A), 0.05 µg tunicamycin (B) and 0.1 µg tunicamycin (C). Quantitative results of retinas treated with different doses of tunicamycin are shown in (D) (n = 6 per group). Arrows indicate acellular capillaries. **P < 0.01 compared to vehicle retinas or 0.5 µg tunicamycin-injected retinas. Scale bar, 50 µm.
3.2. Thinning of the photoreceptor layer, but not RGC layer, following tunicamycin treatment
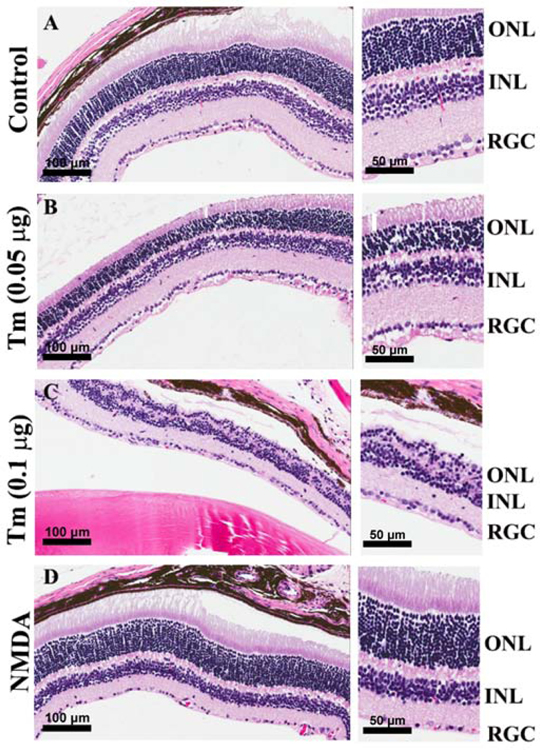
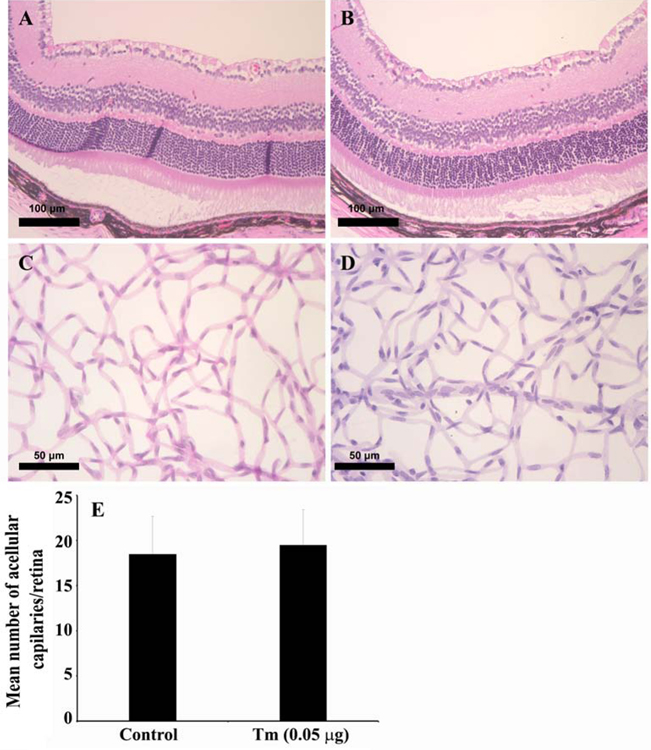
In diabetic retinopathy studies accumulating data suggest a new role for photoreceptor cell dysfunction and/or loss in the outer retina and the initiation of the degenerative vascular lesions associated with the early stages of DR (Du et al., 2013). We next determined the integrity and organization of the photoreceptors in the tunicamycin induced ER stress mice. Tunicamycin has previously been shown to specifically induce photoreceptor loss (Fliesler et al., 1984). The retinal damage induced by tunicamycin was evaluated by H&E staining of histological sections prepared from mice receiving vehicle and those receiving tunicamycin. A dramatic loss of photoreceptors in eyes injected with tunicamycin was observed compared with vehicle control, which was dose dependent. In the 0.1 µg tunicamycin group, nearly 80% of photoreceptors were uniformly lost throughout the retina. Interestingly, no morphological evidence of disruption was observed in other retinal layers. More specifically, tunicamycin did not cause RGC loss. Thus, photoreceptor loss and capillary degeneration are closely linked in mice receiving tunicamycin (Fig. 2), without significantly affecting RGC. These observations are consistent with the resistance of RGC to adverse effects of photoreceptor loss in retinal degeneration models (Lin and Peng, 2013; Mazzoni et al., 2008).
Fig. 2. Photoreceptor loss in tunicamycin injected mouse eyes.
BSS injected control (A), 0.05 µg (B), 0.1 µg of Tunicamycin (C), and NMDA injected mice (D). Eyes were enucleated 2 weeks post injection, and tissue sections examined by H&E staining. The photoreceptor cells loss was dose dependent. In 0.1 µg tunicamycin, nearly 80% of photoreceptors were lost throughout the retina. In contrast, the RGC were minimally affected. The RGC numbers in control and tunicamycin treated groups were similar, while that of NMDA treated eyes was dramatically decreased as shown in histological sections. Scale bars, left panels 100 µm and right panels 50 µm.
To further explore the potential contribution of RGC loss to retinal capillary degeneration, mice received NMDA (40 µM) through intravitreal injection, a known inducer of RGC loss. Fig. 2 shows that the RGC layer was intact in tunicamycin treated eyes and did not show a decrease in number of RGC nuclei with the doses of tunicamycin used here. However, as expected in the NMDA treated eyes, the RGC layer was dramatically and uniformly affected and showed loss of RGC throughout the retina. Thus, RGC are more resistant to tunicamycin mediated ER stress compared with photoreceptor cells.
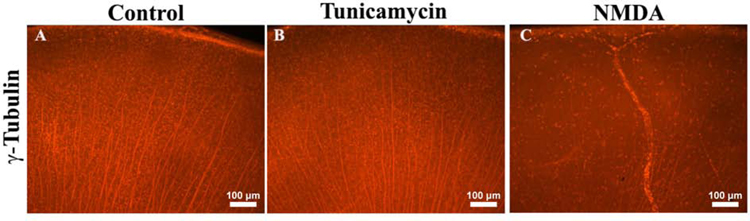
To confirm the status of RGC that was observed by H&E staining, we prepared flatmounts of the eyes from various treatment groups and stained with anti-γ-tubulin to assess the integrity of RGC. Tubulin positive cell density and organization did not differ between vehicle or tunicamycin treated mouse eyes, in striking contrast to the disruption observed in the NMDA treated mice (Fig. 3). These results further confirmed that tunicamycin did not induce RGC loss, while NMDA dramatically decreased the density of tubulin positive RGC.
Fig. 3. Minimal RGC loss in the tunicamycin treated wholemount retina.
Wholemount preparations of the eyes treated with various compounds were stained with a neuron specific marker, γ-tubulin. The tubulin positive cells in control (BSS) (A) and 0.1 µg tunicamycin injected (B) showed no significant differences in terms of organization and number of RGC 2 weeks post injection. In contrast, the NMDA treated eyes showed dramatic disruption in tubulin staining patterns and numbers of RGC (C). Scale bar, 100 µm.
3.3. Tunicamycin treatment induces apoptosis in the photoreceptors but not RGC
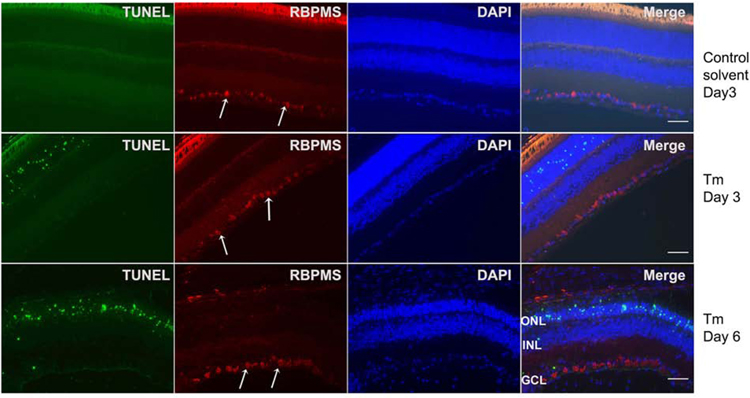
The results described above suggested that tunicamycin spared the RGC layer. To confirm which cells were being lost and whether apoptosis was involved, we examined retinas at earlier time points after tunicamycin treatment by TUNEL staining, along with an antibody to RNA-binding protein with multiple splicing (RBPMS), a RGC marker (Kwong et al., 2010; Rodriguez et al., 2014) (Fig. 4). At 3 and 6 days post injection, no TUNEL positive cells were detected among RGC. The RGC were identified by positive staining for RBPMS. In addition, the staining with RBPMS antibody showed uniform expression in RGC under all conditions with s modest variation in staining intensity among RGC.
Fig. 4. Tunicamycin induces apoptosis in photoreceptor cells but not RGC.
Sections from eyes subjected to solvent control, tunicamycin (0.1 µg) treatments on day 3 and day 6 post-injection were double stained with TUNEL and RBPMS. TUNEL positive cells were located in the photoreceptor layer, not in the GCL. GCL neurons did not show apoptosis. The white arrows are pointing to RBPMS positive RGC in the GCL. Please note all RGC uniformly stained with RBPMS antibody. No TUNEL staining was noted in sections from control eyes. (GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer). Scale bar, 100 µm.
In contrast to RGC, a significant number of TUNEL positive cells were uniformly present among photoreceptor cells throughout the retinal ONL. The TUNEL positive cells were not detected in the control solvent treated samples. Thus, these results indicated that tunicamycin did not induce RGC apoptosis, but induced apoptosis in photoreceptor layer neurons.
To determine whether the loss of photoreceptors occurred prior to retinal vasculature degeneration, we prepared trypsin digests from retinas subjected to tunicamycin for three days. At this time, photoreceptor loss is minimal, but apoptosis is well underway (Fig. 4). We observed no significant changes in the number of degenerating capillaries in eyes from mice that received intravitreal tunicamycin after three days compared with solvent control (Fig. 5). Thus, degeneration of retinal vasculature occurs following the initiation of apoptosis in photoreceptor cells and their loss.
Fig. 5. Tunicamycin treatment minimally affects the organization of retinal layers and acellular capillary formation.
In histological examination of retinas, the organization of retinal layers grossly appeared similar in control and treated (0.1 µg tunicamycin, 3 days) mice, despite the presence of apoptotic cell at this stage (Fig. 4). However, no significant changes in number of acellular capillaries were observed (P > 0.05; n =6). Scale bars, top panels 100 µm and lower panes 50 µm.
3.4. Increased CHOP expression in the apoptotic photoreceptor layer
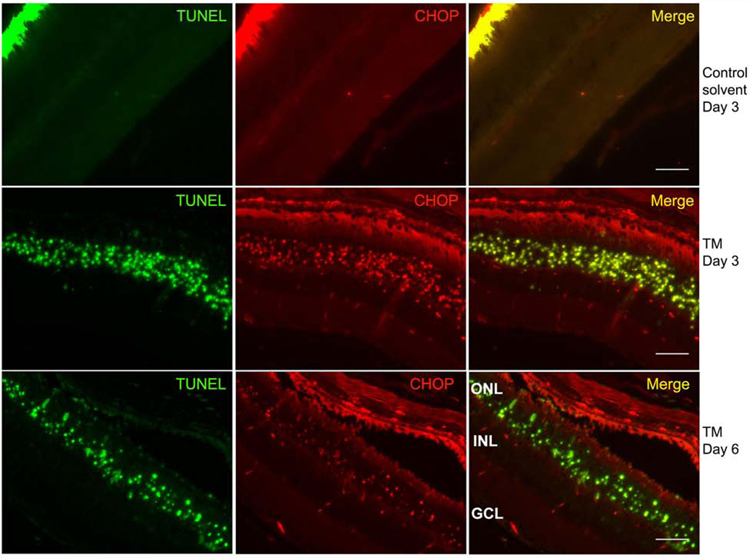
The unfolded protein response (UPR) is a homeostatic response that allows cells to tolerate ER stress. However, chronic or severe ER stress results in the induction of the pro-apoptotic UPR target gene CHOP (CCAAT-enhancer-binding protein homologous protein). To determine if the apoptosis in the photoreceptor cell layer reflects a proapoptotic UPR induction, the retina samples from 3 days and 6 days post tunicamycin injection were TUNEL and anti-CHOP stained. Fig. 6 shows that 3 days post injection, the majority of TUNEL positive (apoptotic) cells in the photoreceptor layer were also CHOP positive. There were no TUNEL staining or CHOP positive cells in the RGC layer as expected. By day 6, there were fewer TUNEL positive cells and much lower CHOP expression (Fig. 4 and 6), possibly reflecting cell loss and/or CHOP downregulation at this time point. Please also note the co-localization seen in the ONL is not due to bleeding from one channel to the other. The only co-localization seen is in the ONL indicating TUNEL positive cells, which are also positive for CHOP. Thus, photoreceptor cells demonstrate CHOP positive apoptotic loss, which precedes the degeneration of the retinal vasculature.
Fig. 6. CHOP expression in photoreceptor cells undergoing apoptosis.
Eye section from mice subjected to solvent control, tunicamycin treatments (0.1 µg) for 3 or 6 days were double stained with TUNEL (green) and CHOP (red). Tunicamycin treatment at day 3 (middle row) and day 6 (lower row) showed the apoptotic (TUNEL positive) and ER stress positive (CHOP positive) cells, which are located in the ONL. No staining was observed in sections from solvent control eyes (top row). Significant overlap staining is only seen in the ONL. (GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer). Scale bar, 100 µm.
3.5. Activation of ER stress results in activation of microglial and Muller cells with minimal effect on RPE cell integrity
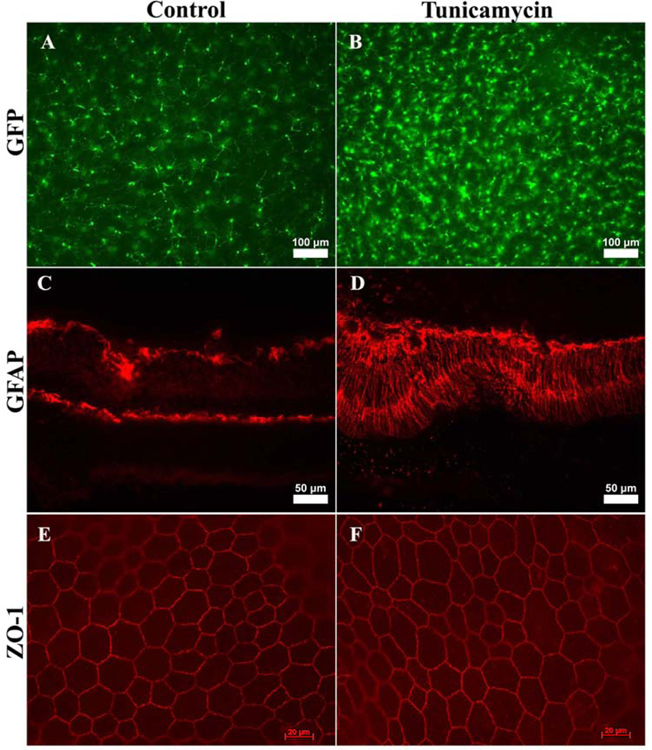
Since cell death can cause gliosis (Subirada et al., 2018; Winkler et al., 2000), we next investigated whether tunicamycin induced ER stress results in activation of microglial cells, as seen with degenerative retinal diseases with an inflammatory component (Al-Shabrawey et al., 2008; Arroba and Valverde, 2017; Brucklacher et al., 2008; Devi et al., 2012; Ibrahim et al., 2010; Nguyen et al., 2012). Using CX3CR1gfp/gfp knock in mice, a mouse prone to inflammation and retinal degeneration (Eandi et al., 2016; Lavalette et al., 2011a), we found an increase in the number of microglial cells that was concomitant with morphologic changes (Fig. 7A, B). Microglial cells exhibit a ramified morphology with long processes during the resting state. These processes appeared to retract, resulting in an amoeboid morphology indicating their activation in tunicamycin-injected retinas 14 days post treatment.
Fig. 7. Increased microglial and Muller cell activation in retinas from tunicamycin injected mice.
Wholemount retinas prepared from CX3CR1gfp/gfp mice treated with vehicle or tunicamycin (0.05 µg, 2 weeks post injection) were evaluated for microglial cells (A, B). GFAP staining of the retinal sections from control and tunicamycin injected mice (C, D). RPE/scleral flatmounts from mice treated with control or tunicamycin stained for ZO-1 (E, F). Please note the increase in microglial cell number and morphological changes indicting activation. Enhanced GFAP expression in treated mice compared to control mice demonstrate Muller glial cell activation. The smooth junctional localization and similar shape of cells demonstrated by ZO-1 staining indicates minimal changes in RPE cells of eyes injected with tunicamycin. These experiments were repeated with eyes from 5 mice with similar results. Scale bars, top panels 100 µm, middle panels 50 µm, and lower panels 20 µm.
We next explored the GFAP expression changes in the eyes from tunicamycin treated mice. Retinas from tunicamycin treated mice had activated Muller cells, as evident by enhanced GFAP staining observed across the whole retinal layers. These results are consistent with an ocular inflammatory environment and gliosis (Fig. 7C, D). To determine whether ER stress affects RPE cell integrity and function, we prepared wholemount scleral/RPE flat mounts and stained them with anti-ZO-1. Normally ZO-1 staining exhibits a smooth and uniform localization at the cell-cell junction of RPE cells and can be used to assess changes in size and shape of the cells and function (Georgiadis et al., 2010; Kleinman et al., 2008). The disruption of this staining pattern is an indicator of RPE dysfunction and stress (Ambati et al., 2003). We noted the RPE cells from eyes injected with tunicamycin retained their classic hexagonal packing and were similar in shape and size to those of control eyes (Fig. 7E, F). Thus, tunicamycin had minimal impact on the RPE cells in the studies presented here.
4. Discussion
The tunicamycin induced ER stress model described here mimics the non-proliferative stage of DR and capillary degeneration in a relatively short time, 2 weeks. The induction of ER stress was concomitant with a dose dependent loss of photoreceptors and accompanied by capillary degeneration. Our data support the hypothesis that photoreceptor dysfunction reported during diabetes plays a vital role in the diabetes-mediated neuroinflammation, retinal capillary degeneration, and ischemia. Potential release of cytokines, in response to UPR and ER stress activation, as well as hyperglycemia, by retinal cells including Muller cells, astrocytes, and photoreceptor cells has been previously reported (Dharmarajan et al., 2017; Fu et al., 2018; Lu et al., 2017; Rana et al., 2017; Roche et al., 2016; Yang et al., 2008; Yang et al., 2007). Photoreceptor and glial cells express cytokines and chemokines including CX3CL1, MCP-1, Rantes, Il-1β, and TNF-α in response to light injury, an activator of the UPR, and to LPS treatment (Rana et al., 2017; Rutar et al., 2011; Singh and Kumar, 2015; Yang et al., 2007; Zhang et al., 2012). Incubation of photoreceptor 661W cells with tunicamycin induces the production of Il-1β and Il-6, which is dependent on an intact ER stress/UPR system, including CHOP and ATF-4. In addition, proinflammatory factor Il-1β can induce retinal photoreceptor and vascular degeneration (Eandi et al., 2016; Hu et al., 2015; Lavalette et al., 2011b; Palenski et al., 2013; Rana et al., 2014; Rivera et al., 2013). These observations are consistent with the activation of microglial and Muller cells noted here.
We did not observe a major impact on the RGC with tunicamycin treatment. Thus, loss of RGC was not associated with the retinal vascular degeneration induced by tunicamycin-mediated ER stress. Our results support the findings of previous studies showing RGC number are not affected by retinal degeneration with the loss of photoreceptors (Lin and Peng, 2013; Mazzoni et al., 2008). However, loss of RGC by intravitreal injection of NMDA was accompanied by retinal vascular degeneration (not shown). Thus, the integrity of RGC could also affect the stability and function of retinal vasculature. Unfortunately, the details of these neurovascular interactions, the factors involved, and the cell death pathways activated require further investigation (Awai et al., 2006; Fahrenthold et al., 2018; Syc-Mazurek et al., 2017).
Animal models have been useful in investigating the RGC death mechanisms in glaucoma (Doh et al., 2010), ischemia (Binet et al., 2013), or optic nerve injury (Lindsey et al., 2015). Although several reports indicated intravitreal injections of higher doses of tunicamycin induced RGC death in vivo (Inokuchi et al., 2009; Ito et al., 2006; Shimazawa et al., 2007a; Tsuruma et al., 2012), our results showed mouse RGC were resistant to the doses of tunicamycin used here, without any sign of apoptosis. In contrast, the number of photoreceptors in the outer nuclear layer uniformly decreased and apoptosis appeared almost exclusively throughout this layer. These apoptotic cells showed increased CHOP expression, a marker of ER stress, consistent with terminal UPR signaling. The activation/increased CHOP levels have also been reported in stressed vascular cells including retinal endothelial cells (Shao et al., 2017), pericytes (Ikesugi et al., 2006; Zhong et al., 2012), and RPE cells (Chen et al., 2014; Matsui et al., 2015; Roybal et al., 2004), and its adverse effects are attenuated in CHOP deficient mice (Yang et al., 2017; Zhang et al., 2015; Zhang et al., 2014). Here we observed minimal expression of CHOP in these cells at the time points examined.
In the absence of a stress signal, lack of CHOP expression has a minimal impact on cellular phenotype (Yang et al., 2017). However, under stressed conditions such as oxidative stress/inflammation as occur in diabetes or ischemia/reperfusion injury, CHOP activation/expression could show both positive and negative effects (Choi et al., 2016; Loinard et al., 2012; Nashine et al., 2014). This likely contributed to the existence of a threshold level for ER stress (Zhang et al., 2015). CHOP can promote cell survival through modulation of autophagy during early stages/limited ER stress, before ER stress becomes irreversible (Yang et al., 2017). CHOP deficient mice used in studies for retinal degeneration in T17M or P23H Rho transgenic mice or excitotoxicity in RGC cells showed minimal protection for photoreceptor degeneration (Adekeye et al., 2014; Chiang et al., 2016a; Nashine et al., 2013) or excitotoxic RGC death (Fahrenthold et al., 2018). Thus, the cell death pathways activated in these cells under various conditions, and the role CHOP plays in these activities, need further elucidation.
Although changes in CHOP levels do not play a significant role in photoreceptor degeneration associated with retinitis pigmentosa (Adekeye et al., 2014; Chiang et al., 2016b; Nashine et al., 2013), we observed significant CHOP expression in photoreceptors of tunicamycin treated mice. These observations suggest the mechanisms of photoreceptor loss might be different from those seen here, and could likely be through ferroptosis, an iron mediated lipid peroxidation cell death modality (Hong et al., 2017; Shimada et al., 2016; Yang and Stockwell, 2016). Iron chelator deferiprone is shown to attenuate tunicamycin induced photoreceptor degeneration (Shirai et al., 2015). This could be attributed, at least in part, to the ability of CHOP to modulate the production of iron regulatory hormone hepcidin (Mueller et al., 2013), which deserves further exploration.
There are several possible reasons for the marked departure in outcome from previous reports regarding the loss of RGC with ER stress. Commonly, RGC-5, which was first isolated and characterized as being from a “postnatal day 1 rat” in 2001 (Krishnamoorthy et al., 2001) is the only ganglion cell line used in cell culture experiments. Most of the RGC death mechanisms that have been studied have used these cells (Inokuchi et al., 2009; Shimazawa et al., 2007a; Shimazawa et al., 2007b; Uchibayashi et al., 2011). In vitro experiments using this cell line have shown that tunicamycin was able to induce RGC-5 death or apoptosis in vitro (Shimazawa et al., 2007a; Shimazawa et al., 2007b). In 2009, a group in Australia disputed the source of the RGC-5 cells (Van Bergen et al., 2009). They found these cells were contaminated with mouse mitochondrial DNA, and lacked RGC-specific protein markers. Another group from the University of North Texas Health Science Center (UNTHSC), where the RGC-5 cells were originally isolated, confirmed the species of currently used RGC-5 cells as mouse. Krishnamoorthy concluded that the RGC-5 was 661W (Krishnamoorthy et al., 2013), a mouse photoreceptor cell line developed by Al-Ubaidi et al. in the early 1990’s (al-Ubaidi et al., 1992), since RGC-5 demonstrated many similar properties, such as the presence of SV40 large T-antigen, which is expected for 661W but not for RGC-5 cells. Given the confusion over the origin of these cells, it is difficult to draw any relevant conclusions about RGC death in response to ER stress using these cells.
A second possible explanation for the difference in our results reflects the dose of ER stressor utilized. For in vitro experiments, tunicamycin is generally used at 10 µg/ml (Liu et al., 2012). For in vivo experiments, tunicamycin is injected intraperitoneally at the concentration of 1 µg/g bodyweight (Zhang and Kaufman, 2008). In intravitreal injections, one study used 2 doses, 1 µg/eye and 0.1 µg/eye. In our experiments, 1 µg/eye was too strong of a stimulation, and the whole retina was lost within 2 weeks. Here we used 0.05 µg and 0.1 µg per eye, which is consistent with a recently published report examining ER stress in the retina (Alavi et al., 2015). Based on our results, it appears that there is a difference between the sensitivity of RGC and photoreceptors to the ER stressor tunicamycin at these lower doses. The reason why RGC are more tolerant of tunicamycin than photoreceptor cells needs to be further investigated. However, the significant inhibition of membrane morphogenesis in photoreceptor outer segments and its proper development, including incorporation of opsin, by tunicamycin may be involved (Anderson et al., 1988; Fliesler and Basinger, 1985; Fliesler et al., 1984; Fliesler et al., 1985). It will be interesting to determine if RGC and photoreceptors show differences in susceptibility to other types of ER stress, such as that stemming from hyperglycemia. Collectively, our studies suggest degeneration of retinal vasculature, as seen with NMDA, is not sufficient to drive loss of photoreceptor cells, and support an important role for affected photoreceptor cells in retinal vascular dysfunction with alterations in glucose metabolism (Kanow et al., 2017).
Here we show that the selective sensitivity of photoreceptor cells to tunicamycin induced ER stress leads to activation of microglial and Muller cells, and retinal vascular degeneration with minimal effect on retinal ganglion and pigment epithelium cells.
Acknowledgements
The work presented here was supported by an award from RPB to the Department of Ophthalmology and Visual Sciences, Retina Research Foundation, P30 EY016665, P30 CA014520, EPA 83573701, EY022883, and EY026078. CMS is supported by the RRF/Daniel M. Albert Chair. NS is a recipient of RPB Stein Innovation Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi T, Yasuda H, Nakamura S, Kamiya T, Hara H, Hara H, Ikeda T, 2011. Endoplasmic reticulum stress induces retinal endothelial permeability of extracellular-superoxide dismutase. Free Radic. Res 45, 1083–1092. [DOI] [PubMed] [Google Scholar]
- Adekeye A, Haeri M, Solessio E, Knox BE, 2014. Ablation of the proapoptotic genes CHOP or Ask1 does not prevent or delay loss of visual function in a P23H transgenic mouse model of retinitis pigmentosa. PLoS One 9, e83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shabrawey M, Rojas M, Sanders T, Behzadian A, El-Remessy A, Bartoli M, Parpia AK, Liou G, Caldwell RB, 2008. Role of NADPH Oxidase in Retinal Vascular Inflammation. Invest. Ophthalmol. Vis. Sci 49, 3239–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Ubaidi MR, Font RL, Quiambao AB, Keener MJ, Liou GI, Overbeek PA, Baehr W, 1992. Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. The Journal of cell biology 119, 1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi MV, Chiang WC, Kroeger H, Yasumura D, Matthes MT, Iwawaki T, LaVail MM, Gould DB, Lin JH, 2015. In Vivo Visualization of Endoplasmic Reticulum Stress in the Retina Using the ERAI Reporter Mouse. Investigative ophthalmology & visual science 56, 6961–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK, 2003. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat. Med 9, 1390–1397. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Williams DS, Neitz J, Fariss RN, Fliesler SJ, 1988. Tunicamycin-induced degeneration in cone photoreceptors. Vis. Neurosci 1, 153–158. [DOI] [PubMed] [Google Scholar]
- Arroba AI, Valverde AM, 2017. Modulation of microglia in the retina: new insights into diabetic retinopathy. Acta Diabetol 54, 527–533. [DOI] [PubMed] [Google Scholar]
- Awai M, Koga T, Inomata Y, Oyadomari S, Gotoh T, Mori M, Tanihara H, 2006. NMDA-induced retinal injury is mediated by an endoplasmic reticulum stress-related protein, CHOP/GADD153. J. Neurochem 96, 43–52. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Gardner TW, Abcouwer SF, 2011. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci 52, 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet F, Mawambo G, Sitaras N, Tetreault N, Lapalme E, Favret S, Cerani A, Leboeuf D, Tremblay S, Rezende F, Juan AM, Stahl A, Joyal JS, Milot E, Kaufman RJ, Guimond M, Kennedy TE, Sapieha P, 2013. Neuronal ER stress impedes myeloid-cell-induced vascular regeneration through IRE1alpha degradation of netrin-1. Cell metabolism 17, 353–371. [DOI] [PubMed] [Google Scholar]
- Brucklacher RM, Patel KM, VanGuilder HD, Bixler GV, Barber AJ, Antonetti DA, Lin CM, LaNoue KF, Gardner TW, Bronson SK, Freeman WM, 2008. Whole genome assessment of the retinal response to diabetes reveals a progressive neurovascular inflammatory response. BMC Med Genomics 1, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P, Stolz J, Kohl S, Chiang WC, Lin JH, 2016. Endoplasmic reticulum stress in human photoreceptor diseases. Brain Res 1648, 538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Cano M, Wang JJ, Li J, Huang C, Yu Q, Herbert TP, Handa JT, Zhang SX, 2014. Role of unfolded protein response dysregulation in oxidative injury of retinal pigment epithelial cells. Antioxid Redox Signal 20, 2091–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Chen HY, Lin CC, Chen MS, Chen PC, Wang IJ, 2012. Retinitis pigmentosa reduces the risk of proliferative diabetic retinopathy: a nationwide population-based cohort study. PLoS One 7, e45189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang W-C, Joseph V, Yasumura D, Matthes MT, Lewin AS, Gorbatyuk MS, Ahern K, LaVail MM, Lin JH, 2016a. Ablation of Chop Transiently Enhances Photoreceptor Survival but Does Not Prevent Retinal Degeneration in Transgenic Mice Expressing Human P23H Rhodopsin Springer International Publishing, Cham, pp. 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang WC, Joseph V, Yasumura D, Matthes MT, Lewin AS, Gorbatyuk MS, Ahern K, LaVail MM, Lin JH, 2016b. Ablation of Chop Transiently Enhances Photoreceptor Survival but Does Not Prevent Retinal Degeneration in Transgenic Mice Expressing Human P23H Rhodopsin. Adv. Exp. Med. Biol 854, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YY, Kim S, Han JH, Nam DH, Park KM, Kim SY, Woo CH, 2016. CHOP deficiency inhibits methylglyoxal-induced endothelial dysfunction. Biochem. Biophys. Res. Commun 480, 362–368. [DOI] [PubMed] [Google Scholar]
- Devi TS, Lee I, Huttemann M, Kumar A, Nantwi KD, Singh LP, 2012. TXNIP links innate host defense mechanisms to oxidative stress and inflammation in retinal Muller glia under chronic hyperglycemia: implications for diabetic retinopathy. Exp Diabetes Res 2012, 438238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmarajan S, Fisk DL, Sorenson CM, Sheibani N, Belecky-Adams TL, 2017. Microglia activation is essential for BMP7-mediated retinal reactive gliosis. Journal of neuroinflammation 14, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doh SH, Kim JH, Lee KM, Park HY, Park CK, 2010. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Res 1308, 158–166. [DOI] [PubMed] [Google Scholar]
- Du Y, Veenstra A, Palczewski K, Kern TS, 2013. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc. Natl. Acad. Sci. U. S. A 110, 16586–16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duh EJ, Sun JK, Stitt AW, 2017. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eandi CM, Charles Messance H, Augustin S, Dominguez E, Lavalette S, Forster V, Hu SJ, Siquieros L, Craft CM, Sahel JA, Tadayoni R, Paques M, Guillonneau X, Sennlaub F, 2016. Subretinal mononuclear phagocytes induce cone segment loss via IL-1beta. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmasry K, Ibrahim AS, Saleh H, Elsherbiny N, Elshafey S, Hussein KA, Al-Shabrawey M, Mavlyutov TA, Guo LW, 2018. Role of endoplasmic reticulum stress in 12/15-lipoxygenase-induced retinal microvascular dysfunction in a mouse model of diabetic retinopathy Peeking into Sigma-1 Receptor Functions Through the Retina. Diabetologia 61, 1220–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenthold BK, Fernandes KA, Libby RT, 2018. Assessment of intrinsic and extrinsic signaling pathway in excitotoxic retinal ganglion cell death. Scientific reports 8, 4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, Basinger SF, 1985. Tunicamycin blocks the incorporation of opsin into retinal rod outer segment membranes. Proc. Natl. Acad. Sci. U. S. A 82, 1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, Rapp LM, Hollyfield JG, 1984. Photoreceptor-specific degeneration caused by tunicamycin. Nature 311, 575–577. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Rayborn ME, Hollyfield JG, 1985. Membrane morphogenesis in retinal rod outer segments: inhibition by tunicamycin. J. Cell Biol 100, 574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Löfqvist CA, Liegl R, Wang Z, Sun Y, Gong Y, Liu CH, Meng SS, Burnim SB, Arellano I, Chouinard MT, Duran R, Poblete A, Cho SS, Akula JD, Kinter M, Ley D, Pupp IH, Talukdar S, Hellström A, Smith LE, 2018. Photoreceptor glucose metabolism determines normal retinal vascular growth. EMBO molecular medicine 10, 76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW, 2002. Diabetic retinopathy: more than meets the eye. Surv. Ophthalmol 47 Suppl 2, S253–262. [DOI] [PubMed] [Google Scholar]
- Georgiadis A, Tschernutter M, Bainbridge JW, Balaggan KS, Mowat F, West EL, Munro PM, Thrasher AJ, Matter K, Balda MS, Ali RR, 2010. The tight junction associated signalling proteins ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in mice. PLoS One 5, e15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SH, Lee D-H, Lee Y-S, Jo MJ, Jeong YA, Kwon WT, Choudry HA, Bartlett DL, Lee YJ, 2017. Molecular crosstalk between ferroptosis and apoptosis: emerging role of ER stress-induced p53-independent PUMA expression. Oncotarget 8, 115164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SJ, Calippe B, Lavalette S, Roubeix C, Montassar F, Housset M, Levy O, Delarasse C, Paques M, Sahel JA, Sennlaub F, Guillonneau X, 2015. Upregulation of P2RX7 in Cx3cr1-Deficient Mononuclear Phagocytes Leads to Increased Interleukin-1beta Secretion and Photoreceptor Neurodegeneration. J. Neurosci 35, 6987–6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, El-Shishtawy MM, Pena A Jr., Liou GI, 2010. Genistein attenuates retinal inflammation associated with diabetes by targeting of microglial activation. Molecular Vision 16, 2033–2042. [PMC free article] [PubMed] [Google Scholar]
- Ikesugi K, Mulhern ML, Madson CJ, Hosoya K, Terasaki T, Kador PF, Shinohara T, 2006. Induction of endoplasmic reticulum stress in retinal pericytes by glucose deprivation. Curr. Eye Res 31, 947–953. [DOI] [PubMed] [Google Scholar]
- Inokuchi Y, Nakajima Y, Shimazawa M, Kurita T, Kubo M, Saito A, Sajiki H, Kudo T, Aihara M, Imaizumi K, Araie M, Hara H, 2009. Effect of an inducer of BiP, a molecular chaperone, on endoplasmic reticulum (ER) stress-induced retinal cell death. Invest. Ophthalmol. Vis. Sci 50, 334–344. [DOI] [PubMed] [Google Scholar]
- Ito Y, Shimazawa M, Akao Y, Nakajima Y, Seki N, Nozawa Y, Hara H, 2006. Lig-8, a bioactive lignophenol derivative from bamboo lignin, protects against neuronal damage in vitro and in vivo. Journal of pharmacological sciences 102, 196–204. [DOI] [PubMed] [Google Scholar]
- Kanow MA, Giarmarco MM, Jankowski CS, Tsantilas K, Engel AL, Du J, Linton JD, Farnsworth CC, Sloat SR, Rountree A, Sweet IR, Lindsay KJ, Parker ED, Brockerhoff SE, Sadilek M, Chao JR, Hurley JB, 2017. Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern TS, Berkowitz BA, 2015. Photoreceptors in diabetic retinopathy. J Diabetes Investig 6, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Kariko K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J, 2008. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 452, 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy RR, Agarwal P, Prasanna G, Vopat K, Lambert W, Sheedlo HJ, Pang IH, Shade D, Wordinger RJ, Yorio T, Clark AF, Agarwal N, 2001. Characterization of a transformed rat retinal ganglion cell line. Brain research. Molecular brain research 86, 1–12. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy RR, Clark AF, Daudt D, Vishwanatha JK, Yorio T, 2013. A forensic path to RGC-5 cell line identification: lessons learned. Investigative ophthalmology & visual science 54, 5712–5719. [DOI] [PubMed] [Google Scholar]
- Kroeger H, Chiang WC, Felden J, Nguyen A, Lin JH, Yang L, Li S, Miao L, Huang H, Liang F, Teng X, Xu L, Wang Q, Xiao W, Ridder WH 3rd, Ferguson TA, Chen DF, Kaufman RJ, Hu Y, 2018. ER stress and unfolded protein response in ocular health and disease Rescue of Glaucomatous Neurodegeneration by Differentially Modulating Neuronal Endoplasmic Reticulum Stress Molecules. Febs j 36, 5891–5903. [Google Scholar]
- Kwong JM, Caprioli J, Piri N, 2010. RNA binding protein with multiple splicing: a new marker for retinal ganglion cells. Invest. Ophthalmol. Vis. Sci 51, 1052–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavalette S, Raoul W, Houssier M, Camelo S, Levy O, Calippe B, Jonet L, Behar-Cohen F, Chemtob S, Guillonneau X, Combadiere C, Sennlaub F, 2011a. Interleukin-1beta inhibition prevents choroidal neovascularization and does not exacerbate photoreceptor degeneration. Am J Pathol 178, 2416–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavalette S, Raoul W, Houssier M, Camelo S, Levy O, Calippe B, Jonet L, Behar-Cohen F, Chemtob S, Guillonneau X, Combadière C, Sennlaub F, 2011b. Interleukin-1β Inhibition Prevents Choroidal Neovascularization and Does Not Exacerbate Photoreceptor Degeneration. The American journal of pathology 178, 2416–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Wang HS, Li GG, Zhao MJ, Zhao MH, 2011. The role of endoplasmic reticulum stress in the early stage of diabetic retinopathy. Acta Diabetol 48, 103–111. [DOI] [PubMed] [Google Scholar]
- Li C, Wang L, Huang K, Zheng L, 2012. Endoplasmic reticulum stress in retinal vascular degeneration: protective role of resveratrol. Invest. Ophthalmol. Vis. Sci 53, 3241–3249. [DOI] [PubMed] [Google Scholar]
- Li J, Wang JJ, Yu Q, Wang M, Zhang SX, 2009. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett 583, 1521–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Peng EB, 2013. Retinal ganglion cells are resistant to photoreceptor loss in retinal degeneration. PLoS One 8, e68084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey JD, Duong-Polk KX, Hammond D, Leung CK, Weinreb RN, 2015. Protection of injured retinal ganglion cell dendrites and unfolded protein response resolution after long-term dietary resveratrol. Neurobiology of aging 36, 1969–1981. [DOI] [PubMed] [Google Scholar]
- Liu H, Tang J, Du Y, Saadane A, Tonade D, Samuels I, Veenstra A, Palczewski K, Kern TS, 2016. Photoreceptor Cells Influence Retinal Vascular Degeneration in Mouse Models of Retinal Degeneration and Diabetes. Invest. Ophthalmol. Vis. Sci 57, 4272–4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YP, Zeng L, Tian A, Bomkamp A, Rivera D, Gutman D, Barber GN, Olson JK, Smith JA, 2012. Endoplasmic reticulum stress regulates the innate immunity critical transcription factor IRF3. Journal of immunology 189, 4630–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loinard C, Zouggari Y, Rueda P, Ramkhelawon B, Cochain C, Vilar J, Recalde A, Richart A, Charue D, Duriez M, Mori M, Arenzana-Seisdedos F, Levy BI, Heymes C, Silvestre JS, 2012. C/EBP homologous protein-10 (CHOP-10) limits postnatal neovascularization through control of endothelial nitric oxide synthase gene expression. Circulation 125, 1014–1026. [DOI] [PubMed] [Google Scholar]
- Lu YZ, Natoli R, Madigan M, Fernando N, Saxena K, Aggio-Bruce R, Jiao H, Provis J, Valter K, 2017. Photobiomodulation with 670 nm light ameliorates Muller cell-mediated activation of microglia and macrophages in retinal degeneration. Exp. Eye Res 165, 78–89. [DOI] [PubMed] [Google Scholar]
- Ma JH, Wang JJ, Zhang SX, 2014. The unfolded protein response and diabetic retinopathy. J Diabetes Res 2014, 160140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Kaneko H, Kachi S, Ye F, Hwang SJ, Takayama K, Nagasaka Y, Sugita T, Terasaki H, 2015. Expression of Vascular Endothelial Growth Factor by Retinal Pigment Epithelial Cells Induced by Amyloid-beta Is Depressed by an Endoplasmic Reticulum Stress Inhibitor. Ophthalmic Res 55, 37–44. [DOI] [PubMed] [Google Scholar]
- Mazzoni F, Novelli E, Strettoi E, 2008. Retinal ganglion cells survive and maintain normal dendritic morphology in a mouse model of inherited photoreceptor degeneration. J. Neurosci 28, 14282–14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Sunami Y, Stuetzle M, Guldiken N, Kucukoglu O, Mueller S, Kulaksiz H, Schwarz P, Strnad P, 2013. CHOP-mediated hepcidin suppression modulates hepatic iron load. J. Pathol 231, 532–542. [DOI] [PubMed] [Google Scholar]
- Nashine S, Bhootada Y, Lewin AS, Gorbatyuk M, 2013. Ablation of C/EBP Homologous Protein Does Not Protect T17M RHO Mice from Retinal Degeneration. PLOS ONE 8, e63205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashine S, Liu Y, Kim BJ, Clark AF, Pang IH, 2014. Role of C/EBP homologous protein in retinal ganglion cell death after ischemia/reperfusion injury. Invest. Ophthalmol. Vis. Sci 56, 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DV, Shaw LC, Grant MB, 2012. Inflammation in the pathogenesis of microvascular complications in diabetes. Front Endocrinol (Lausanne) 3, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshitari T, Hata N, Yamamoto S, 2008. Endoplasmic reticulum stress and diabetic retinopathy. Vascular health and risk management 4, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palenski TL, Sorenson CM, Sheibani N, 2013. Inflammatory cytokine-specific alterations in retinal endothelial cell function. Microvasc. Res 89, 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana T, Kotla P, Fullard R, Gorbatyuk M, 2017. TNFa knockdown in the retina promotes cone survival in a mouse model of autosomal dominant retinitis pigmentosa. Biochimica et biophysica acta. Molecular basis of disease 1863, 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana T, Shinde VM, Starr CR, Kruglov AA, Boitet ER, Kotla P, Zolotukhin S, Gross AK, Gorbatyuk MS, 2014. An activated unfolded protein response promotes retinal degeneration and triggers an inflammatory response in the mouse retina. Cell Death Dis 5, e1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera JC, Sitaras N, Noueihed B, Hamel D, Madaan A, Zhou T, Honore JC, Quiniou C, Joyal JS, Hardy P, Sennlaub F, Lubell W, Chemtob S, 2013. Microglia and interleukin-1beta in ischemic retinopathy elicit microvascular degeneration through neuronal semaphorin-3A. Arterioscler Thromb Vasc Biol 33, 1881–1891. [DOI] [PubMed] [Google Scholar]
- Roche SL, Wyse-Jackson AC, Gomez-Vicente V, Lax P, Ruiz-Lopez AM, Byrne AM, Cuenca N, Cotter TG, 2016. Progesterone Attenuates Microglial-Driven Retinal Degeneration and Stimulates Protective Fractalkine-CX3CR1 Signaling. PLoS One 11, e0165197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AR, de Sevilla Muller LP, Brecha NC, 2014. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J. Comp. Neurol 522, 1411–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybal CN, Yang S, Sun CW, Hurtado D, Vander Jagt DL, Townes TM, Abcouwer SF, 2004. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J. Biol. Chem 279, 14844–14852. [DOI] [PubMed] [Google Scholar]
- Rutar M, Natoli R, Valter K, Provis JM, 2011. Early focal expression of the chemokine Ccl2 by Muller cells during exposure to damage-inducing bright continuous light. Invest. Ophthalmol. Vis. Sci 52, 2379–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Yin Y, Yin X, Ji L, Xin Y, Zou J, Yao Y, 2017. Transthyretin Exerts Pro-Apoptotic Effects in Human Retinal Microvascular Endothelial Cells Through a GRP78-Dependent Pathway in Diabetic Retinopathy. Cell. Physiol. Biochem 43, 788–800. [DOI] [PubMed] [Google Scholar]
- Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ, Brown LM, Valenzuela CA, Wolpaw AJ, Stockwell BR, 2016. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nature chemical biology 12, 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazawa M, Inokuchi Y, Ito Y, Murata H, Aihara M, Miura M, Araie M, Hara H, 2007a. Involvement of ER stress in retinal cell death. Mol. Vis 13, 578–587. [PMC free article] [PubMed] [Google Scholar]
- Shimazawa M, Ito Y, Inokuchi Y, Hara H, 2007b. Involvement of double-stranded RNA-dependent protein kinase in ER stress-induced retinal neuron damage. Invest. Ophthalmol. Vis. Sci 48, 3729–3736. [DOI] [PubMed] [Google Scholar]
- Shirai Y, Mori A, Nakahara T, Sakamoto K, Ishii K, 2015. Deferiprone Protects against Photoreceptor Degeneration Induced by Tunicamycin in the Rat Retina. Biol. Pharm. Bull 38, 1076–1080. [DOI] [PubMed] [Google Scholar]
- Singh PK, Kumar A, 2015. Retinal photoreceptor expresses toll-like receptors (TLRs) and elicits innate responses following TLR ligand and bacterial challenge. PLoS One 10, e0119541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subirada PV, Paz MC, Ridano ME, Lorenc VE, Vaglienti MV, Barcelona PF, Luna JD, Sánchez MC, 2018. A journey into the retina: Müller glia commanding survival and death. Eur. J. Neurosci 47, 1429–1443. [DOI] [PubMed] [Google Scholar]
- Syc-Mazurek SB, Fernandes KA, Wilson MP, Shrager P, Libby RT, 2017. Together JUN and DDIT3 (CHOP) control retinal ganglion cell death after axonal injury. Molecular Neurodegeneration 12, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonade D, Liu H, Kern TS, 2016. Photoreceptor Cells Produce Inflammatory Mediators That Contribute to Endothelial Cell Death in Diabetes. Invest. Ophthalmol. Vis. Sci 57, 4264–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruma K, Shimazaki H, Nakashima K, Yamauchi M, Sugitani S, Shimazawa M, Iinuma M, Hara H, 2012. Annatto prevents retinal degeneration induced by endoplasmic reticulum stress in vitro and in vivo. Molecular nutrition & food research 56, 713–724. [DOI] [PubMed] [Google Scholar]
- Uchibayashi R, Tsuruma K, Inokuchi Y, Shimazawa M, Hara H, 2011. Involvement of Bid and caspase-2 in endoplasmic reticulum stress- and oxidative stress-induced retinal ganglion cell death. Journal of neuroscience research 89, 1783–1794. [DOI] [PubMed] [Google Scholar]
- Van Bergen NJ, Wood JP, Chidlow G, Trounce IA, Casson RJ, Ju WK, Weinreb RN, Crowston JG, 2009. Recharacterization of the RGC-5 retinal ganglion cell line. Investigative ophthalmology & visual science 50, 4267–4272. [DOI] [PubMed] [Google Scholar]
- Winkler BS, Arnold MJ, Brassell MA, Puro DG, 2000. Energy metabolism in human retinal Muller cells. Invest. Ophthalmol. Vis. Sci 41, 3183–3190. [PMC free article] [PubMed] [Google Scholar]
- Yang LP, Wu LM, Guo XJ, Li Y, Tso MO, 2008. Endoplasmic reticulum stress is activated in light-induced retinal degeneration. J. Neurosci. Res 86, 910–919. [DOI] [PubMed] [Google Scholar]
- Yang LP, Zhu XA, Tso MO, 2007. A possible mechanism of microglia-photoreceptor crosstalk. Mol. Vis 13, 2048–2057. [PubMed] [Google Scholar]
- Yang WS, Stockwell BR, 2016. Ferroptosis: death by lipid peroxidation. Trends Cell Biol 26, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu L, Naik I, Braunstein Z, Zhong J, Ren B, 2017. Transcription Factor C/EBP Homologous Protein in Health and Diseases. Frontiers in immunology 8, 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ, 2008. Identification and characterization of endoplasmic reticulum stress-induced apoptosis in vivo. Methods in enzymology 442, 395–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Xu G, Liu W, Ni Y, Zhou W, 2012. Role of fractalkine/CX3CR1 interaction in light-induced photoreceptor degeneration through regulating retinal microglial activation and migration. PLoS One 7, e35446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Ma JH, Bhatta M, Fliesler SJ, Wang JJ, 2015. The unfolded protein response in retinal vascular diseases: implications and therapeutic potential beyond protein folding. Prog. Retin. Eye Res 45, 111–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Sanders E, Fliesler SJ, Wang JJ, 2014. Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration. Exp. Eye Res 125, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Wang JJ, Zhang SX, 2012. Intermittent but not constant high glucose induces ER stress and inflammation in human retinal pericytes. Adv. Exp. Med. Biol 723, 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]