Abstract
Early life social experiences are critical to behavioral and cognitive development, and can have a tremendous influence on developing social phenotypes. Most work has focused on outcomes of experiences at a single stage of development (e.g. perinatal or post-weaning). Few studies have assessed the impact of social experience at multiple developmental stages and across sex. Oxytocin and vasopressin are profoundly important for modulating social behavior and these nonapeptide systems are highly sensitive to developmental social experience, particularly in brain areas important for social behavior. We investigated whether oxytocin receptor (OTR) and vasopressin receptor (V1aR) distributions of prairie voles (Microtus ochrogaster) change as a function of parental composition within the natal nest or social composition after weaning. We raised pups either in the presence or absence of their fathers. At weaning, offspring were housed either individually or with a same-sex sibling. We also examined whether changes in receptor distributions are sexually dimorphic because the impact of the developmental environment on the nonapeptide system could be sex-dependent. We found that differences in nonapeptide receptor expression were region-specific, sex-specific and rearing condition-specific, indicating a high level of complexity in the ways that early life experiences shape the social brain. We found many more differences in V1aR density compared to OTR density, indicating that nonapeptide receptors demonstrate differential levels of neural plasticity and sensitivity to environmental and biological variables. Our data highlight that critical factors including biological sex and multiple experiences across the developmental continuum interact in complex ways to shape the social brain.
Keywords: development of social behavior, oxytocin and vasopressin receptors, parental and paternal care, sex differences, social isolation and housing
INTRODUCTION
An organism’s biological sex shapes and constrains the ecological and social context, and can extensively impact social behavior. Many social behaviors of interest, such as parental care or courtship, are inherently tied to the sex of an animal due to morphological or physiological requirements necessary to express specific behaviors (i.e. nursing and secondary sexual characteristics). Interest in studying behavioral sex differences across species has logically led to studying the underlying endocrinological and neural mechanisms that give rise to sexual dimorphism in behavior (Dulac & Kimchi 2007). This focus has in part helped to reveal the functional roles that the neuropeptides oxytocin (OT) and vasopressin (VP) play in shaping behavioral sex differences. OT and VP act as neuromodulators when bound to their receptors (OTR and V1aR, respectively), which function to regulate widespread physiological and behavioral processes (Landgraf & Neumann 2004). The distributions of OTR, V1aR and nonapeptide-containing neurons are specific to species, age and sex, and, thus, have functional implications for the display of social behaviors, including pair-bonding, parental care, social recognition, gregariousness and courtship (Goodson 2008; Dumais & Veenema 2016).
The impact of early life social experiences, such as variation in parental caregiving, shapes the social brain. For example, the density of nonapeptide receptors across regions of the forebrain are sensitive to environmental experiences in perinatal life (Champagne et al. 2001; Curley et al. 2012; Prounis et al. 2015). Decades of research on maternal separation and caregiving in rodents has revealed the critical role that mothers play in shaping offspring brains and behaviors in relation to stress regulation, cognitive processing and sociality (Meaney 2001). Variation in the quality and quantity of maternal care given in the first few weeks of life has lasting consequences for offspring, such that lower levels of maternal care can lead to higher levels of anxiety-like behavior, elevations in glucocorticoid responses to stress, dysregulation of the hypothalamic–pituitary–adrenal axis, and even modifications to the epigenome that subsequently facilitate the intergenerational transmission of parental caregiving (Liu et al. 1997; Caldji et al. 1998; Meaney 2001).
The emphasis on the developmental impact of maternal care over paternal care has likely been driven by the rarity of paternal care in mammals; approximately 5% of mammalian species exhibit biparental care (Clutton-Brock 1991). In biparental mammals such as the socially monogamous prairie vole (Microtus orchrogaster), pups receive care from both their mothers and their fathers (Getz & Carter 1996). This characteristic makes the prairie vole a suitable species in which to ask questions regarding the role of paternal care in shaping offspring behavioral phenotypes. Variation in parental composition (such as manipulating the presence or absence of fathers during the rearing period) has been shown to alter the rate at which prairie vole pups develop (Wang & Novak 1992). This variation also impacts social affiliation towards conspecifics in adulthood (Tabbaa et al. 2017) and the species-typical pair-bonding behavior of adult animals (Ahern & Young 2009).
In addition to variation in social environments resulting from different experience with parental caregivers, the dynamics of post-wean social experiences influence brain development. Juvenile exposure to social experiences, such as aggressive encounters (Delville et al. 1998), opportunities to engage in social play behaviors (Van Den Berg et al. 1999) and housing conditions (Kaiser et al. 2007) all have significant consequences for adult rodent social behavior, stress physiology and neuroendocrinology (Sachser et al. 2013).
Despite ongoing interest in how early life social experiences impact the development of the social brain, a comprehensive understanding of the ways in which complex social experiences might differentially impact the development of the male and female brain remains underdeveloped. To this end, the current study asks how the distributions of nonapeptide receptors vary as a function of sex, and how interactions across postnatal social conditions subsequently impact receptor expression in both males and females. We accomplish this task by investigating prairie vole sex differences in nonapeptide receptor profiles after manipulating the presence and absence of the father during pre-weaning, and by varying social housing conditions after weaning.
MATERIALS AND METHODS
Subjects and rearing conditions
Animals were housed in polycarbonate rodent cages (29 × 18 × 13 cm) under a 14:10 light-dark cycle (lights on at 0600 hours). Animals had ad libitum access to water and Rodent Chow 5000 (Harlan Teklad, Madison, WI, USA). Primiparous breeding pairs were formed in opposite-sex pairs (n = 41 pairs), using colony offspring derived from wild-caught prairie voles we trapped in Champaign County, Illinois, USA. Breeders were monitored closely for the birth of pup litters. When pups were born, the number of pups per litter was recorded (mean = 3.8 ± 1, range of 2–6). Fathers in the Father-absent condition were then removed from the home cage. Fathers in the Father-present condition remained with the litter. All pups were weaned and sexed on post-natal day (PND) 21. Upon weaning, animals from the Father-present and Father-absent groups were each sub-divided into 2 groups: Single-housed or Pair-housed (housed with a same-sex sibling). Thus, the final design was a 2 × 2 × 2 factorial design (Fig. 1) with 2 levels of Pre-wean condition (Father-absent vs Father-present), 2 levels of Post-wean condition (Single-housed vs Pair-housed) and 2 levels of Sex (Male vs Female). All procedures were approved by the Institutional Animal Care and Use Committee of Cornell University (2013–0102).
Figure 1.
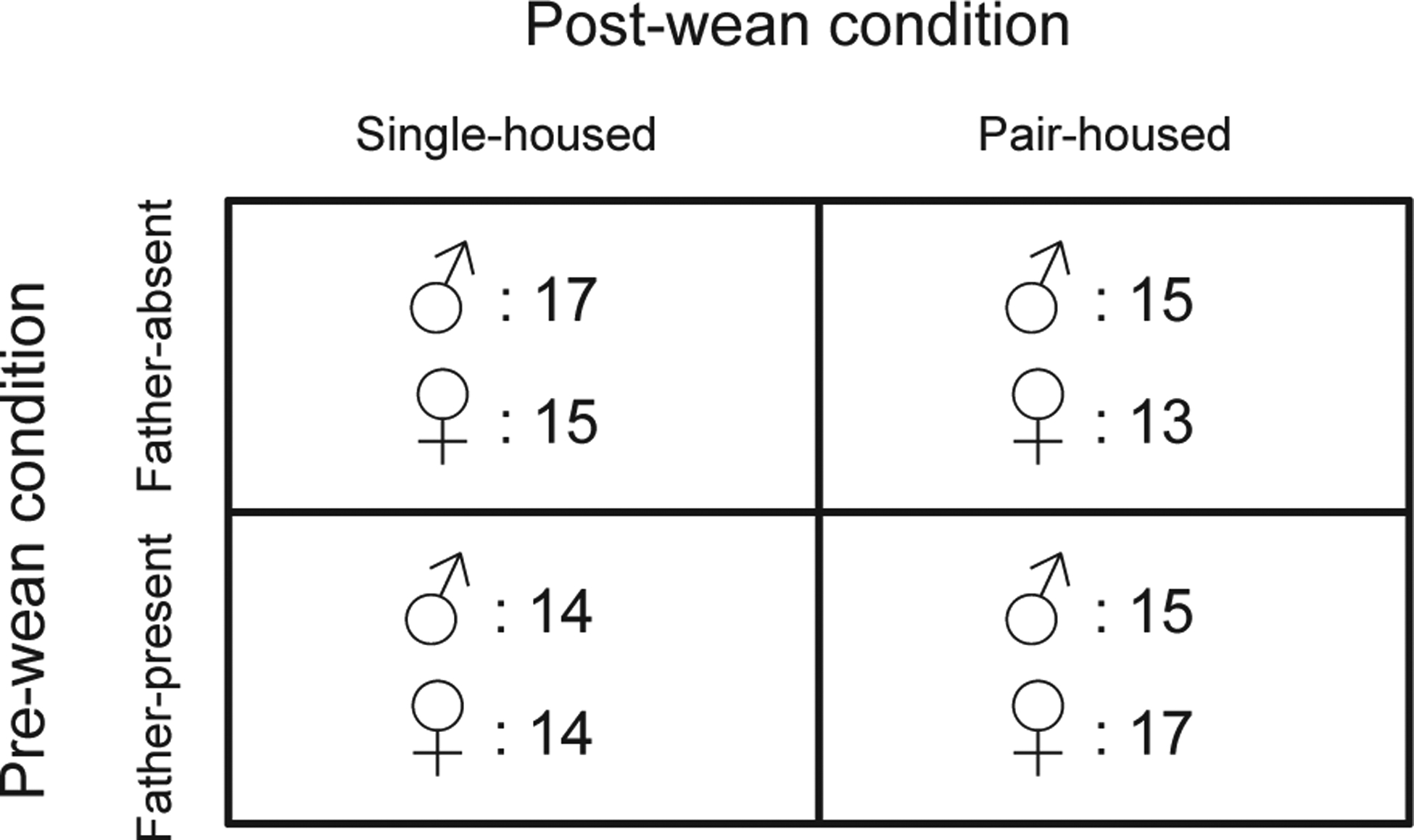
Experimental design. Sample sizes for each group are indicated within the cells next to the symbols for female (♀) and male (♂).
Histology and autoradiography
Subjects were killed between PND37 and 45 by CO2 inhalation. Brains were immediately extracted, snap-frozen on powdered dry ice, and stored at −80 °C. Brains were coronally cryosectioned at 20-μm thickness onto 4 sets on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA, USA) at 100-μm intervals. The mounted slides were stored at −80 °C until autoradiographic labeling. Two sets of slides were labeled with 125I radioligands to visualize oxytocin receptor (ornithine vasotocin analogue ([125I]-OVTA); NEX254, PerkinElmer, Waltham, MA, USA) and vasopressin 1a receptor (vasopressin (Linear), V-1S antagonist (Phenylacetyl1,0-Me-D-Tyr2,[125I-Arg6]-); NEX 310, PerkinElmer), as previously described (Ophir et al. 2013).
Digital imaging and analysis
The radiolabeled slides and 125I radiographic microscales (American Radiolabeled Chemicals, St Louis, MO, USA) were stored in film cassettes and exposed to storage phosphoreimaging screens (Fujifilm, Tokyo, Japan) for 23 h. The screens were removed from the cassettes under dark light, and positioned in a Typhoon FLA 7000 laser scanner (GE Healthcare, Marlborough, MA, USA). Screens were scanned using the Typhoon FLA 7000 control software version 1.3 (GE Healthcare, Marlborough, MA, USA) and analyzed in ImageQuant TL Toolbox Version 8.1 (GE Healthcare, Marlborough, MA, USA). Brain areas of interest were measured for densitometry analysis in 3 sequential slices of tissue by encircling each region of interest bilaterally. Mean values of intensity were calculated for each region and automatically adjusted for background by the ImageQuant program. 125I-labeled radiographic microscales were used to create decay formulas, which transformed mean intensity measures to standardized values of disintegrations per minute (dpm) adjusted for tissue equivalence (TE; for 1 mg in rat brain). Transformed mean non-specific binding measurements from cortex taken from the same brain sections at each region of interest were then subtracted from these values to calculate a final value for mean receptor density (units dpm/mg TE). V1aR was measured in the main olfactory bulb (MOB), accessory olfactory bulb (AOB), lateral septum (LS), ventral pallidum (VPall), lateral bed nucleus of the stria terminalis (BSTl), medial bed nucleus of the stria terminalis (BSTm), ventral bed nucleus of the stria terminalis (BSTv), anterior hypothalamus (AH), paraventricular nucleus of the hypothalamus (PVN), medio-dorsal nucleus of the thalamus (MDTh), latero-dorsal nucleus of the thalamus (LDTh), ventro-posterior nucleus of the thalamus (VPTh), central amygdala (CeA), medial amygdala (MeA), retrosplenial cortex (RSC) and ventromedial hypothalamus (VMH). OTR was measured in the basolateral amygdala (BLA), caudate-putamen (CPu), hippocampus (HPC), anterior portion of the insular cortex (ICa), medial portion of the insular cortex (ICm), intermediodorsal thalamic nucleus (IMD), LS, nucleus accumbens (NAcc), prefrontal cortex (PFC) and septo-hippocampal nucleus (SHi).
Statistical analysis
Mean receptor density data were analyzed using linear mixed models (LMM) in R v.3.2.1 (R Core Team 2016) using the R package lme4 (Bates et al. 2015). For each receptor type (OTR, V1aR), we included Pre-wean condition, Post-wean condition and Sex as fixed factors. Phosphoreimaging screen, Autoradiography chamber, and Litter were included as factored random effects to control for potential variation across histological processing and genetic profiles. A model was run for each brain region of interest. When the omnibus ANOVA revealed a significant effect, we conducted paired contrasts using the R package lsmeans (Lenth 2016). Post-hoc comparisons were adjusted using Tukey corrections, and we considered a 0.05 α-level threshold for statistical significance.
RESULTS
Our analyses revealed that there were significant effects of sex and early life experiences that shaped V1aR and OTR expression in the brains of developing prairie voles (Tables 1 and 2). Below we detail these results, first exploring main effects and then moving on to interaction effects.
Table 1.
Vasopressin 1a receptor (V1aR) results from the omnibus linear-mixed model ANOVAs for each structure analyzed
| Structure | Main effects | Two-way interactions | Three-way interaction | |||||
|---|---|---|---|---|---|---|---|---|
| Sex | Pre-wean | Post-wean | Sex × Pre-wean | Sex × Post-wean | Pre-wean × Post-wean | |||
| Vasopressin receptor (V1aR) | MOB |
F1, 77.5 = 8.6, P < 0.005 |
F1, 31.0 = 2.8, P = 0.1 |
F1, 81.1 = 3.3, P = 0.07 |
F1, 76.1 = 0.27, P = 0.6 |
F1, 81.5 = 0.45, P = 0.5 |
F1, 85.2 = 0.039, P = 0.8 |
F1, 83.6 = 0.3, P = 0.6 |
| AOB |
F1, 80.4 = 6.1, P< 0.05 |
F1, 33.7 = 2.5, P = 0.1 |
F1, 82.4 = 0.69, P = 0.4 |
F1, 79.3 = 0.65, P = 0.4 |
F1, 85.1 = 3.1, P = 0.08 |
F1, 88.4 = 0.35, P = 0.6 |
F1, 86.4 = 0.63, P = 0.4 |
|
| LS |
F1, 94.1 = 7.2,
P< 0.01 |
F1, 30.8 = 0.56, P = 0.5 |
F1, 87.9 = 3.1 P = 0.08 |
F1, 89.6 = 0.017, P = 0.9 |
F1, 93.2 = 0.09, P = 0.8 |
F1, 98.2 < 0.01, P = 0.9 |
F1, 93.4 = 2.3, P = 0.1 |
|
| VPall |
F1, 80.3 = 0.82, P = 0.4 |
F1, 30.0 = 0.27, P = 0.6 |
F1, 80.2 = 2.2, P = 0.1 |
F1, 75.1 = 2.0, P = 0.2 |
F1, 79.0 = 0.2, P = 0.7 |
F1, 88.2 = 0.054, P = 0.8 |
F1, 81.1 = 0.13, P = 0.7 |
|
| BSTl |
F11, 100.5 = 11.0, P < 0.005 |
F1, 99.8 = 0.61, P = 0.4 |
F1, 100.0 = 0.15, P = 0.7 |
F1, 99.8 = 0.081, P = 0.8 |
F1, 100.0 < 0.01, P = 1 |
F1, 100.1 = 0.32, P = 0.6 |
F1, 100.6 = 1.9, P = 0.2 |
|
| BSTm |
F1, 98.8 = 4.6,
P< 0.05 |
F1, 23.8 = 1.2, P = 0.3 |
F1, 100.0 < 0.01, P = 1 |
F1, 97.4 = 0.91, P = 0.3 |
F1, 99.8 = 0.84, P = 0.4 |
F1, 99.6 = 0.3, P = 0.6 |
F1, 100.9 = 1.0, P = 0.3 |
|
| BSTv |
F1, 100.3 = 079, P = 0.4 |
F1, 99.7 = 0.37, P = 0.5 |
F1, 9.99 = 0.18, P = 0.7 |
F1, 9.97 = 0.011, P = 0.9 |
F1, 9.99 = 0.08, P = 0.8 |
F1, 100.0 = 0.012, P = 0.9 |
F1, 100.5 = 0.5, P = 0.5 |
|
| AH |
F1, 98.4 = 2.9, P = 0.09 |
F1, 20.7 = 1.0, P = 0.3 |
F1, 98 = 3.9, P = 0.05 |
F1, 97.1 = 0.022, P = 0.9 |
F1, 97.9 = 0.95, P = 0.3 |
F1, 96.2 = 0.16, P = 0.7 |
F1, 98.9 = 0.3, P = 0.6 |
|
| PVN |
F1, 98.7 = 078, P = 0.4 |
F1, 30.6 = 4.0, P = 0.05 |
F1, 98.0 = 0.58, P = 0.4 |
F1, 96.0 = 0.35, P = 0.6 |
F1, 97.7 = 0.24, P = 0.6 |
F1, 98.9 = 0.69, P = 0.4 |
F1, 99.6 = 068, P = 0.4 |
|
| MDTh |
F1, 99.1 = 017, P = 0.7 |
F1, 35.5 = 1.8, P = 0.2 |
F1, 101.6 = 0.76, P = 0.4 |
F1, 95.2 = 1.1, P = 0.3 |
F1, 98.9 = 0.02, P = 0.9 |
F1, 102.4 = 0.13, P = 0.7 |
F1, 102.1 = 0.5, P = 0.5 |
|
| LDTh |
F1, 97.0 = 1.7, P = 0.2 |
F1, 32.2 < 0.01, P = 0.9 |
F1, 99.4 = 2.1, P = 0.1 |
F1, 93.7 < 0.01, P = 0.9 |
F1, 9.72 = 0.26, P = 0.6 |
F1, 100.5 = 0.84, P = 0.4 |
F1, 9.99 = 0.07, P = 0.8 |
|
| VPTh |
F1, 94.0 = 0.74, P = 0.4 |
F1, 31.0 = 1.9, P = 0.2 |
F1, 90.9 = 3.4, P = 0.07 |
F1, 90.6 = 0.022, P = 0.9 |
F1, 94.5 = 0.07, P = 0.8 |
F1, 99.1 = 0.01, P = 0.9 |
F1, 93.8 = 0.18, P = 0.7 |
|
| CeA |
F1, 92.2 = 3.9 P = 0.05 |
F1, 30.1 = 0.24, P = 0.6 |
F1, 9.49 = 0.31, P = 0.6 |
F1, 89.4 < 0.01, P = 1 |
F1, 9.44 = 0.62, P = 0.4 |
F1, 97.9 = 3.2, P = 0.08 |
F1, 97.9 = 3.3, P = 0.07 |
|
| MeA |
F1, 90.4 = 0.78, P = 0.4 |
F1, 23.7 = 0.02, P = 0.9 |
F1, 88.3 = 0.42, P = 0.5 |
F1, 87.2 = 0.13, P = 0.7 |
F1, 90.3 = 2.7, P = 0.1 |
F1, 94.6 < 0.01, P = 1 |
F1, 90.4 = 0.6, P = 0.4 |
|
| RSC |
F1, 84.4 = 0.85, P = 0.4 |
F1, 36.4 = 0.96, P = 0.3 |
F1, 87.1 = 2.6, P = 0.1 |
F1, 81.9 = 2.4, P = 0.1 |
F1, 86.6 = 0.01, P = 0.9 |
F1, 915 = 0.058, P = 0.8 |
F1, 88.3 = 0.25, P = 0.6 |
|
| VMH |
F1, 99.2 = 0.41, P = 0.5 |
F1, 31.7 = 0.54, P = 0.5 |
F1, 9.95 = 0.03, P = 0.9 |
F1, 97.6 = 4.6, P< 0.05 |
F1, 99.3 = 14 P = 0.2 |
F1, 100.3 = 0.92, P = 0.3 |
F1, 100.6 < 0.01, P = 0.9 |
|
Significant P-values (after corrections for multiple comparisons) are highlighted in bold and italic font. Values presented in each cell are F-values (degrees of freedom); P-values. Abbreviations for neural structures are: AOB, accessory olfactory bulb; AH, anterior hypothalamus; BSTl, lateral bed nucleus of the stria terminalis; BSTm, medial bed nucleus of the stria terminalis; BSTv, ventral bed nucleus of the stria terminalis; CeA, central amygdala; LDTh, latero-dorsal nucleus of the thalamus; LS, lateral septum; MDTh, medio-dorsal nucleus of the thalamus; MeA, medial amygdala; MOB, main olfactory bulb; PVN, paraventricular nucleus of the hypothalamus; RSC, retrosplenial cortex; VMH, ventromedial hypothalamus; VPall, ventral pallidum; VPTh, ventro-posterior nucleus of the thalamus.
Table 2.
Oxytocin receptor (OTR) results from the omnibus linear-mixed model ANOVAs for each structure analyzed
| Structure | Main effects | Two-way interactions | Three-way interaction | |||||
|---|---|---|---|---|---|---|---|---|
| Sex | Pre-wean | Post-wean | Sex × Pre-wean | Sex × Post-wean | Pre-wean × Post-wean | |||
| Oxytocin receptor (OTR) | BLA |
F1, 94.6 = 0.12, P = 0.7 |
F1, 99.6 = 3.4 P = 0.07 |
F1, 88.8 = 0.42, P = 0.5 |
F1, 97.7 = 2.5 P = 0.1 |
F1, 97.9 = 12 P = 0.3 |
F1, 103.3 = 0.55, P = 0.5 |
F1, 100.6 = 0.21, P = 0.6 |
| CeA |
F1, 92.5 = 2.1, P = 0.1 |
F1, 97.4 = 1.9, P = 0.2 |
F1, 87.4 = 1.6, P = 0.2 |
F1, 95.7 = 2.0, P = 0.2 |
F1, 95.7 = 0.12, P = 0.7 |
F1, 101.8 = 0.18, P = 0.7 |
F1, 99.2 = 0.18, P = 0.7 |
|
| CPu |
F1, 103.4 = 2.9, P = 0.09 |
F1, 102.2 = 3.0, P = 0.08 |
F1, 100.5 = 0.44, P = 0.5 |
F1, 101.1 = 0.3, P = 0.6 |
F1, 102.1 <0.01, P = 0.9 |
F1, 106.1 = 0.16, P = 0.7 |
F1, 108.5 = 3.1, P = 0.08 |
|
| HPC |
F1, 90.9 = 0.29, P = 0.6 |
F1, 28.2 = 16, P = 0.2 |
F1, 81.0 = 0.032, P = 0.9 |
F1, 91.9 = 15 P = 0.2 |
F1, 92.3 = 0.13, P = 0.7 |
F1, 100.5 = 0.23, P = 0.6 |
F1, 98.5 = 13 P = 0.3 |
|
| ICa |
F1, 100.9 = 0.17, P = 0.7 |
F1, 99.7 < 0.01, P = 1 |
F1, 98.4 = 2.8, P = 0.1 |
F1, 98.7 = 0.077, P = 0.8 |
F1, 99.7 = 0.4, P = 0.5 |
F1, 103.2 <0.01, P = 1 |
F1, 105.4 = 0.03, P = 0.9 |
|
| ICm |
F1, 90.3 = 0.085, P = 0.8 |
F1, 95.9 = 1.7, P = 0.2 |
F1, 84.9 = 0.1, P = 0.7 |
F1, 95.7 = 1.6, P = 0.2 |
F1, 94.4 = 2.0, P = 0.2 |
F1, 100.3 = 0.54, P = 0.5 |
F1, 98.7 = 0.3, P = 0.6 |
|
| IMD |
F1, 91.8 = 0.28, P = 0.6 |
F1, 17.7 = 0.02, P = 0.9 |
F1, 92.6 = 0.056, P = 0.8 |
F1, 94.5 = 0.85, P = 0.4 |
F1, 101.2 = 0.02, P = 0.9 |
F1, 108.4 = 0.2, P = 0.7 |
F1, 100.8 = 0.2, P = 0.7 |
|
| LS |
F1, 108.3 = 0.71, P = 0.4 |
F1, 106.3 = 0.45, P = 0.5 |
F1, 102.7 = 0.72, P = 0.4 |
F1, 104.0 = 06, P = 0.4 |
F1, 105.9 = 0.83, P = 0.4 |
F1, 111.0 = 0.11, P = 0.7 |
F1, 109.6 = 3.8, P = 0.05 |
|
| NAcc |
F1, 103.4 = 0.2, P = 0.7 |
F1, 102.3 = 6.8, P = 0.01 |
F1, 100.5 = 3.4, P = 0.07 |
F1, 101.1 = 0.82, P = 0.4 |
F1, 102.1 = 0.48, P = 0.5 |
F1, 106.3 = 0.039, P = 0.8 |
F1, 108.7 = 0.01, P = 0.9 |
|
| PFC |
F1, 103.5 = 1.2, P = 0.3 |
F1, 102.5 = 0.32, P = 0.6 |
F1, 100.1 = 1.1, P = 0.3 |
F1, 100.7 = 0.28, P = 0.6 |
F1, 102.2 = 0.25, P = 0.6 |
F1, 107.2 = 0.058, P = 0.8 |
F1, 108.9 = 0.02, P = 0.9 |
|
| SHi |
F1, 94.7 = 0.16, P = 0.7 |
F1.37.0 = 0.11, P = 0.7 |
F1, 86.0 = 0.072, P = 0.8 |
F1, 96.4 = 0.47, P = 0.5 |
F1, 96.7 < 0.01, P = 1 |
F1, 103.6 = 9.4, P < 0.005 |
F1, 98.5 = 0.11, P = 0.7 |
|
Significant P-values (after corrections for multiple comparisons) are highlighted in bold and italic font. Values presented in each cell are F-values (degrees of freedom); P-values. Abbreviations for neural structures are: BLA, basolateral amygdala; CeA, central amygdala; CPu, caudate-putamen; HPC, hippocampus; ICa, anterior portion of the insular cortex; ICm, medial portion of the insular cortex; IMD, intermediodorsal thalamic nucleus; LS, lateral septum; NAcc, nucleus accumbens; PFC, prefrontal cortex; SHi, septo-hippocampal nucleus.
Main effect of sex
We found a main effect of sex on V1aR expression in the main olfactory bulbs (MOB; F1,77.5 = 8.6, P < 0.005), accessory olfactory bulbs (AOB; F1,80.4 = 6.1, P < 0.05), lateral septum (LS; F1,94.1 = 7.2, P < 0.01), bed nucleus of the stria terminalis lateral subdivision (BSTl; F1,100.5 = 11.0, P < 0.005), bed nucleus of the stria terminalis medial subdivision (BSTm; F1,98.8 = 4.6, P < 0.05), and central amygdala (CeA; F1,92.2 = 3.9, P = 0.05). Post-hoc contrasts revealed that for both the MOB (P = 0.005) and the AOB (P = 0.02), males had a significantly greater density of V1aR compared to females (Fig. 2). Conversely, we found that females had significantly higher V1aR density compared to males in the LS (P < 0.01), BSTl (P < 0.01) and the BSTm (P < 0.05; Fig. 2). There were no main effects of sex on OTR expression in any of the regions analyzed (all P > 0.05).
Figure 2.
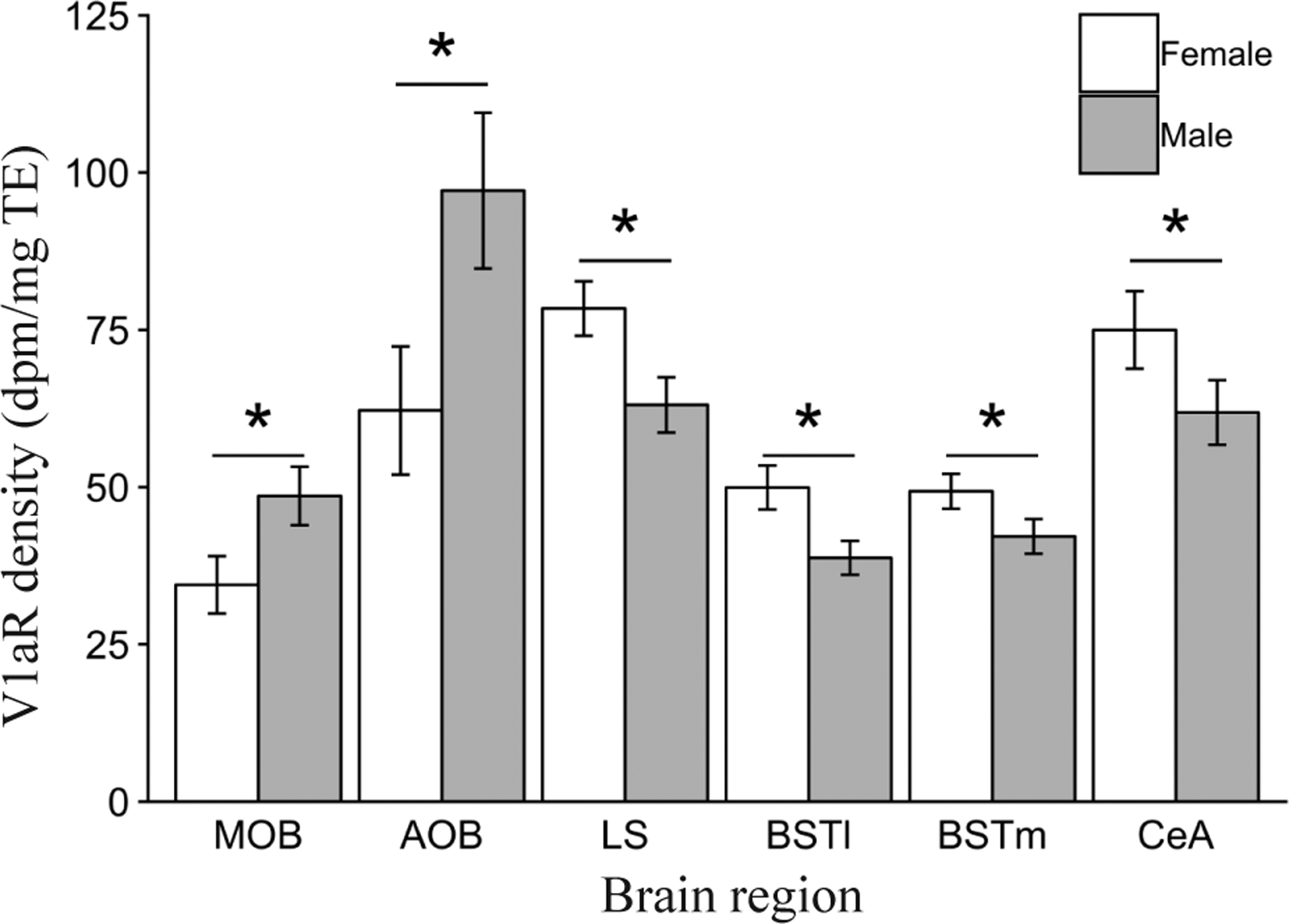
Main effects of Sex on mean vasopressin 1a receptor (V1aR) binding density (dpm/mg TE) ± standard error bars for the main olfactory bulbs (MOB), accessory olfactory bulbs (AOB), lateral septum (LS), lateral bed nucleus of the stria terminalis (BSTl) and medial bed nucleus of the stria terminalis (BSTm). *P ≤ 0.05.
Main effect of pre-wean social experience
We found a main effect of Pre-wean social experience on V1aR density in the paraventricular nucleus of the hypothalamus (PVN; F1,30.6 = 4.0, P = 0.05). A post-hoc contrast showed that offspring reared in the Father-present condition had significantly greater V1aR density compared to offspring reared in the Father-absent condition (P = 0.05, Fig. 3). No other main effects of Pre-wean on V1aR density were found for any of the other areas analyzed (all P > 0.05).
Figure 3.
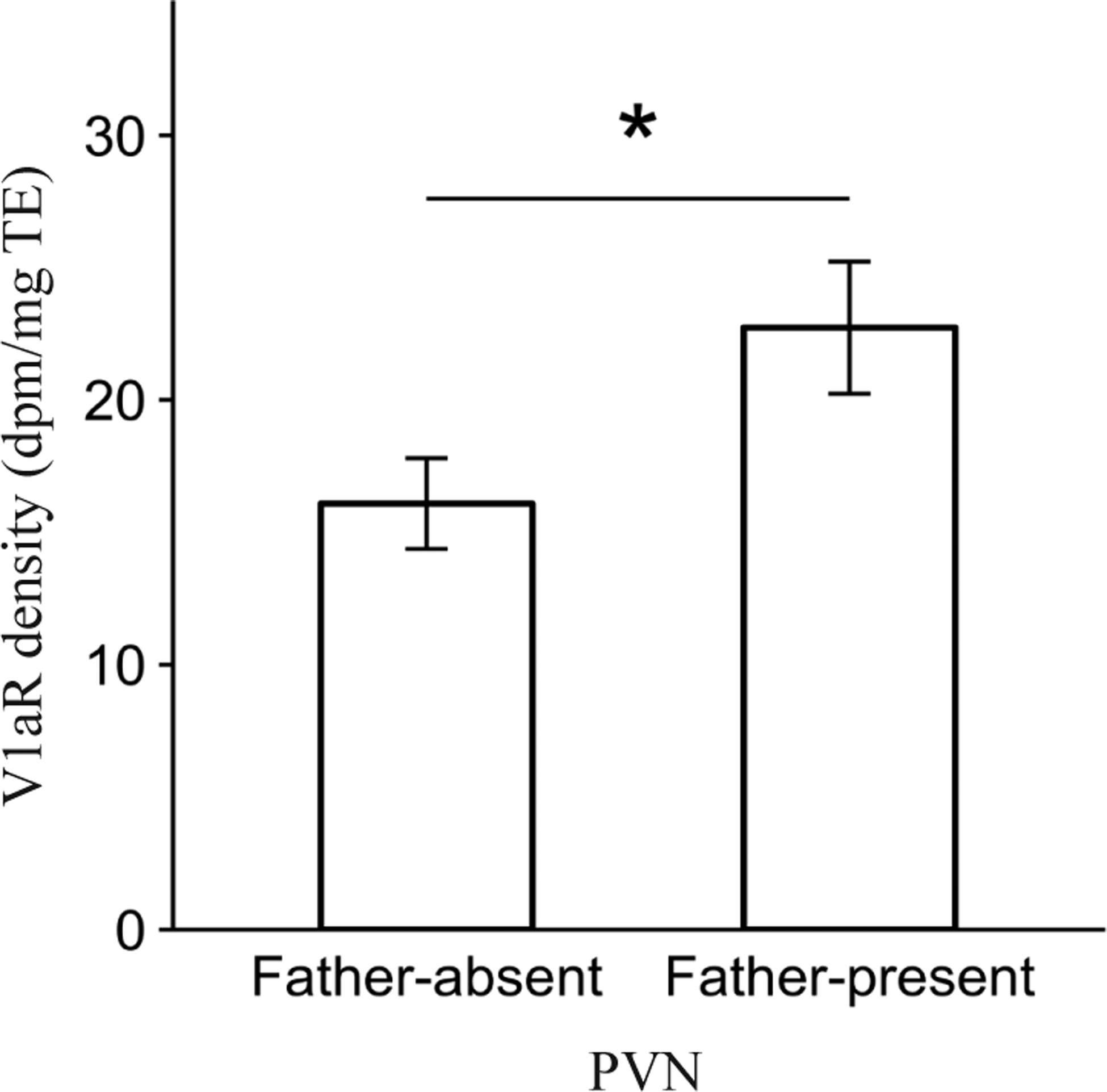
Main effect of Pre-wean condition on mean vasopressin 1a receptor (V1aR) binding density (dpm/mg TE) ± standard error bars for the paraventricular nucleus of the hypothalamus (PVN). *P ≤ 0.05.
Pre-wean social experience impacted OTR density in the nucleus accumbens (NAcc; F1,102.3 = 6.8, P = 0.01). A post-hoc contrast revealed that offspring in the Father-absent condition expressed significantly greater OTR density than offspring in the Father-present condition (P = 0.01, Fig. 4). There were no main effects of Pre-wean condition on OTR density within any of the other regions analyzed (all P > 0.05).
Figure 4.
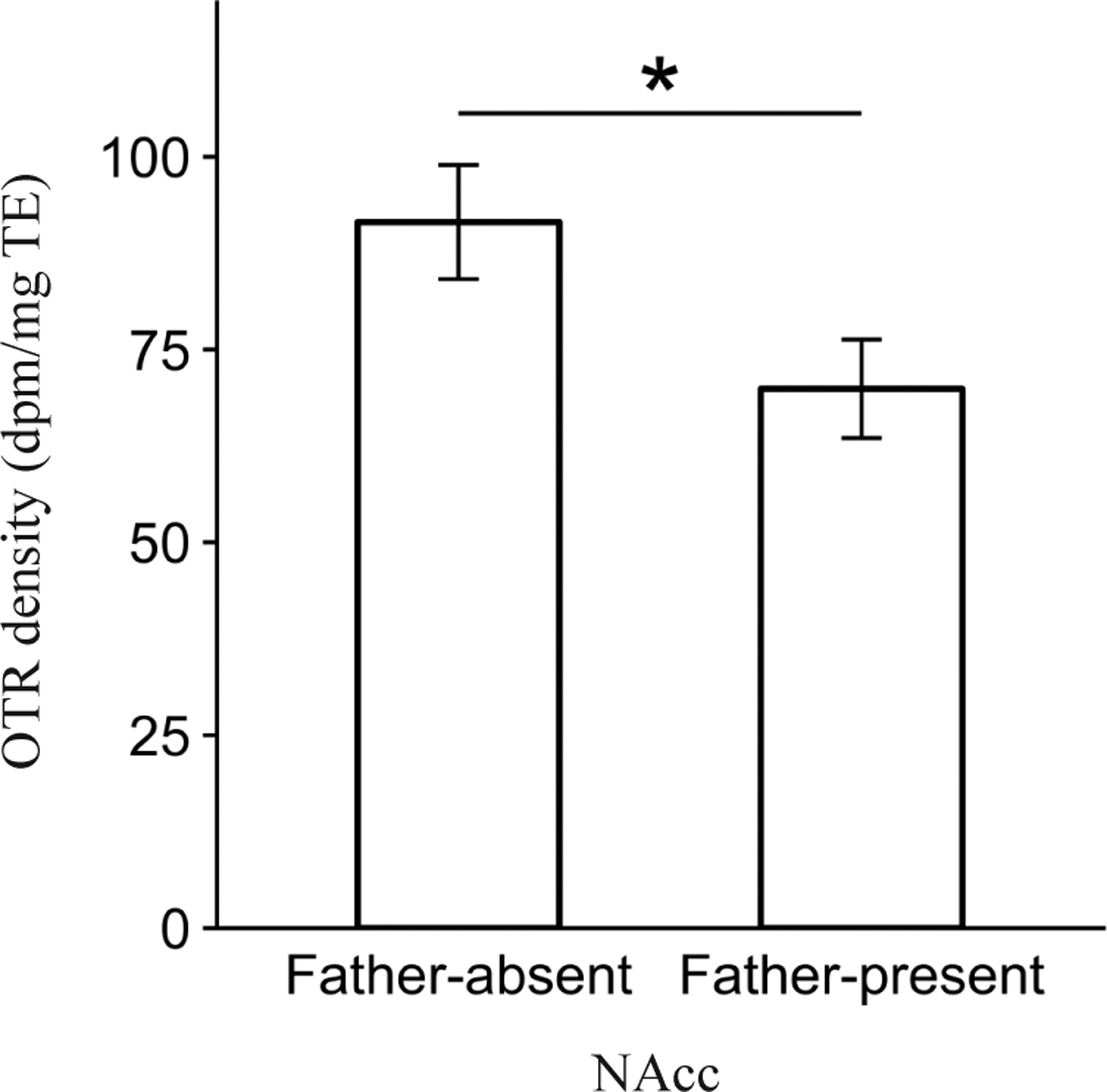
Main effect of Pre-wean condition on mean oxytocin receptor (OTR) binding density (dpm/mg TE) ± standard error bars for the nucleus accumbens (NAcc). *P ≤ 0.05.
Main effect of post-wean social experience
Post-wean experience affected V1aR density in the anterior hypothalamus (AH; F1,97.9 = 3.9, P = 0.05). A post-hoc contrast revealed that offspring in the Single-housed condition had significantly greater V1aR density compared to animals in the Pair-housed condition (P < 0.05, Fig. 5). There were no significant main effects of Post-wean experience on V1aR density in other brain areas or on OTR density in any of the OTR-expressing regions analyzed (all P > 0.05)
Figure 5.
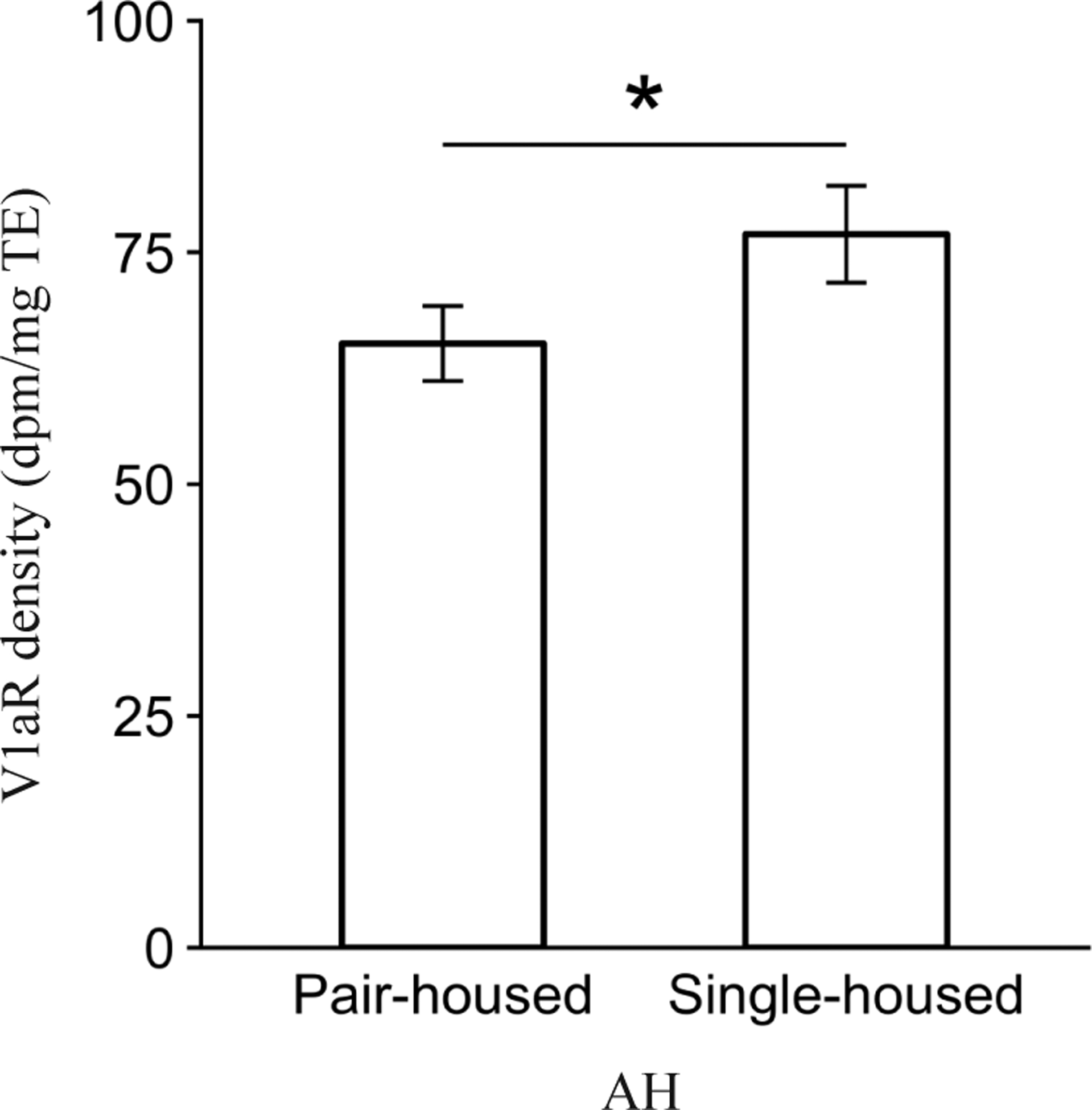
Main effect of Post-wean condition on mean vasopressin 1a receptor (V1aR) binding density (dpm/mg TE) ± standard error bars for the anterior hypothalamus (AH). *P ≤ 0.05.
Two-way interaction effects
Beyond the main effects reported above, we found only 1 significant 2-way interaction for V1aR density in any brain area. Specifically, the ventromedial hypothalamus (VMH) demonstrated a significant Sex by Pre-wean interaction (F1,97.6 = 4.6, P < 0.05). Post-hoc contrasts showed that female, but not male, offspring in the Father-present condition had significantly higher V1aR density compared to females in the Father-absent condition (P = 0.05, Fig. 6). In addition, females had higher V1aR density compared to males (P = 0.05) within the Father-present condition, but not the Father-absent condition.
Figure 6.
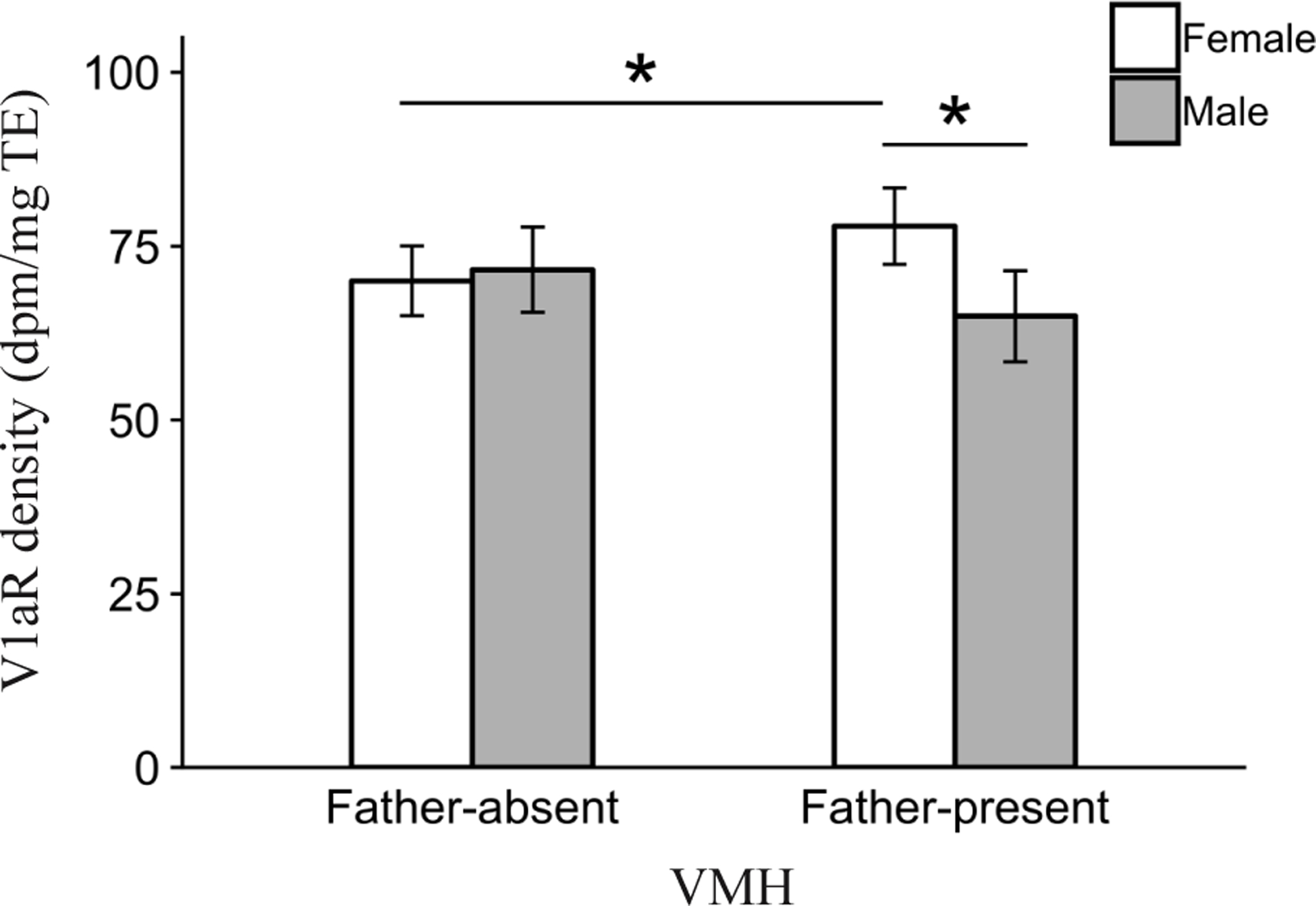
Interaction effect of Pre-wean condition and Sex on mean vasopressin 1a receptor (V1aR) binding density (dpm/mg TE) ± standard error bars for the ventromedial hypothalamus (VMH). *P ≤ 0.05.
We did not find any significant interactions of Sex by Pre-wean conditions, or significant interactions of Sex by Post-wean conditions on OTR density (all P > 0.05).
We found a significant Pre-wean by Post-wean interaction in the septohippocampal nucleus (SHi; F1,103.6 = 9.4, P < 0.005) for OTR density (Fig. 7). Post-hoc contrasts showed that Pair-housed animals had significantly greater OTR density compared to Single-housed animals (P < 0.05) in the Father-absent condition. Conversely, Single-housed animals had significantly greater OTR density compared to Pair-housed animals (P < 0.05) in the Father-present condition. Finally, we found that Single-housed offspring in the Father-present condition had higher levels of OTR density compared to Single-housed offspring in the Father-absent condition (P < 0.05).
Figure 7.
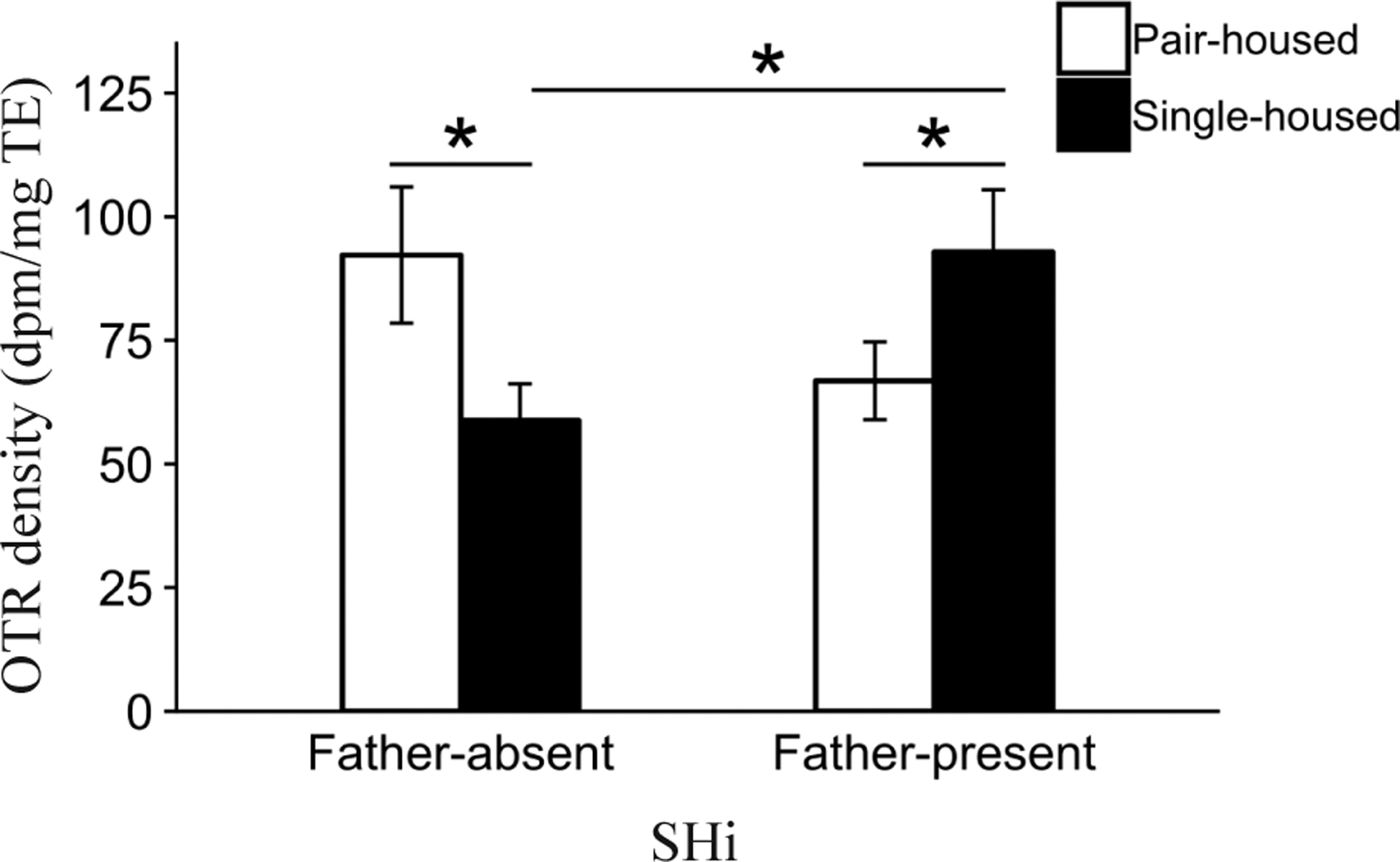
Interaction effect of Pre-wean condition and Post-wean condition on mean oxytocin receptor (OTR) binding density (dpm/mg TE) ± standard error bars for the septohippocampal nucleus (SHi). *P ≤ 0.05.
No other significant 2-way interactions were found on OTR density in any of the regions analyzed (all P > 0.05).
Three-way interaction effects
We observed only 1 significant 3-way interaction of Sex by Pre-wean by Post-wean condition. This interaction effect was found for OTR expression in the LS (F1,109.6 = 3.8, P = 0.05). Post-hoc contrasts revealed that males had significantly greater OTR density compared to females within Single-housed and Father-present animals (P < 0.05, Fig. 8). No 3-way interaction effects were found for any other OTR expressing brain area or for V1aR expression in any of the regions analyzed (all P > 0.05).
Figure 8.
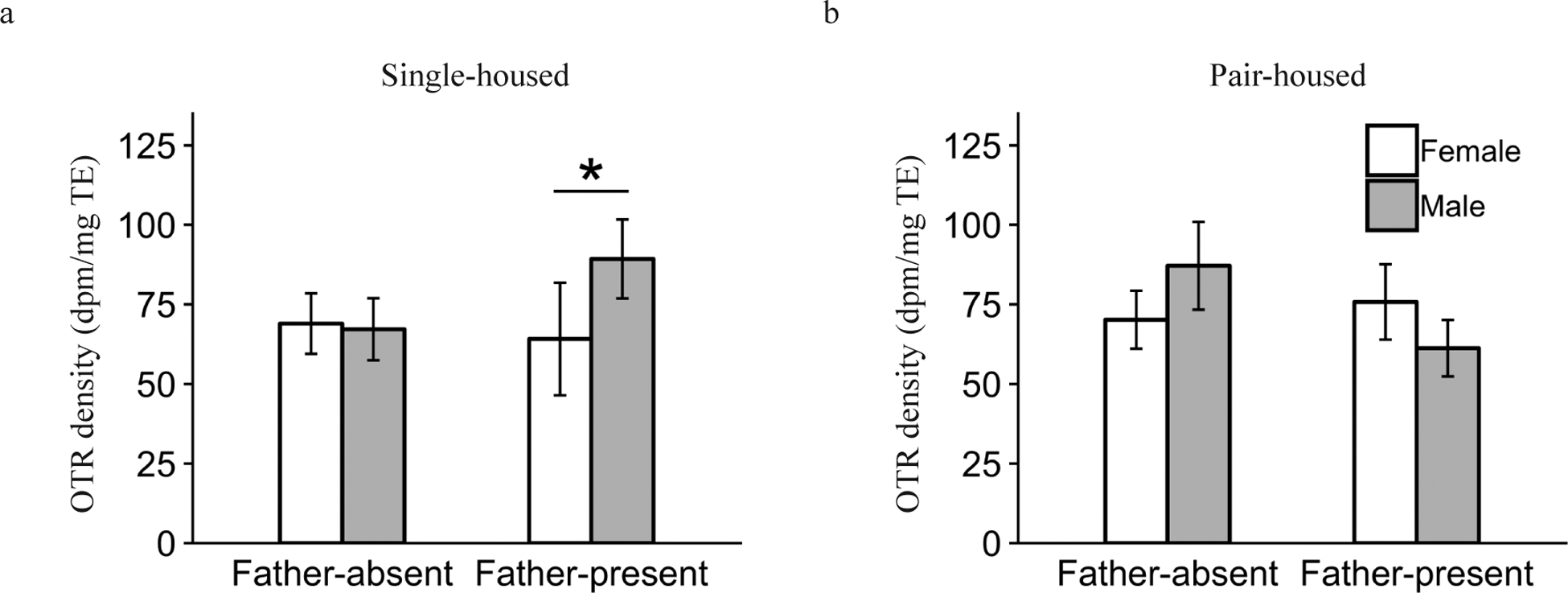
Interaction effect of Pre-wean condition, Post-wean condition and Sex on mean oxytocin receptor (OTR) binding density (dpm/mg TE) ± standard error bars for the lateral septum (LS). (a) shows the relationship within the Single-housed animals, and (b) shows the relationship within the Pair-housed subjects. *P ≤ 0.05.
DISCUSSION
Sex differences in vasopressin receptor expression
Our data revealed several areas of the brain in which there were significant sex differences in the expression of V1aR in prairie voles. These included the main and accessory olfactory bulbs (MOB and AOB), the lateral septum (LS), and the lateral and medial divisions of the bed nucleus of the stria termialis (BSTl and BSTm). Intriguingly, these regions are structurally interconnected and work in concert to facilitate social chemosensory processing via the vasopressin system. VP activity in both the MOB and AOB is critical in rodent social recognition, which is facilitated by processing the scent signature of a conspecific. Administering a V1a receptor antagonist or using siRNA to target V1aR in the MOB impairs social recognition performance in rats (Tobin et al. 2010). Similarly, V1aR in the LS is both necessary and sufficient for facilitating social recognition in rats and mice (Bielsky et al. 2005; Gabor et al. 2012). Although we are unaware of any portion of the BST being involved in social recognition per se, this region is known to respond to sociosexual olfactory cues in hamsters (Fiber et al. 1993), rats (Bressler & Baum 1996), mice (Veyrac et al. 2011) and mandarin voles (He et al. 2014). The BST also happens to be one of the densest extra-hypothalamic regions that produces central VP in rats (de Vries et al. 1985). Moreover, the BST is structurally connected with both the olfactory system and the LS. For example, the AOB projects to the BST, and the LS is densely innervated by VP neurons that originate in the BST (de Vries & Buijs 1983). Sex differences in V1aR binding within these regions are not well characterized. However, Veenema et al. (2012) found that female rats express significantly greater LS V1aR binding than males, a result that is consistent with our study. Furthermore, the sex differences we report are very interesting considering that the innervation of VP fibers within the LS is sexually dimorphic; male prairie voles, meadow voles and rats possess greater VP fiber density in the LS compared to females (De Vries et al. 1981; Bamshad et al. 1993). This distinction may partially underlie behavioral sex differences in social recognition. Female rats retain social memories for longer intervals compared to males (Bluthé & Dantzer 1990). Furthermore, peripheral blockade of V1aR impairs recognition performance in male but not in female rats (Bluthé & Dantzer 1990). However, subsequent work has demonstrated that both adult male and female rats exhibit impaired social recognition after LS V1aR antagonism (Veenema et al. 2012). Despite some mixed results, the sex differences in V1aR density we report support the established interpretation that VP facilitates social recognition in a sex-specific manner, and our data provide evidence of developmental-based sexual dimorphisms of receptor expression within brain areas upstream in the social chemosensory pathway.
Despite the various sex differences observed in V1aR expression, we did not find any sex differences in OTR density in any of the brain regions analyzed. This distinction falls in line with a more general trend in the rodent nonapeptide literature, in which cross-species comparisons have established more sex differences in the VP/V1aR systems compared to the OT/OTR systems (Dumais & Veenema 2016). The reason for this phenomenon could be due to a general lack of comprehensive sex comparisons of OT/OTR systems in the literature (Dumais & Veenema 2016), an artifact of selecting particular OTR-expressing regions of interest, or a fundamental difference in the plasticity of OTR and V1aR expression (see below).
Effect of pre-wean post-natal social experiences on nonapeptide receptor expression
An extraordinary number of studies have highlighted the ways in which early life social experiences with a mother can shape the social behaviors of offspring. By utilizing the biparental prairie vole, we demonstrated that the absence of a father yields a drastic drop in V1aR expression within the PVN. Early life social experiences, such as variation in maternal care, handling or social deprivation, have been known to impact both OT and VP immunoreactivity (ir) in the PVN. However, few studies have reported corresponding changes in PVN nonapeptide receptor expression as a function of early social experiences (Veenema 2012). Our study provides evidence that paternal presence is a significant factor for V1aR expression in the hypothalamus of a biparental mammal. Studies in rats have demonstrated that antagonism of V1aR within the PVN impairs anxiety-like and maternal behavior (Bayerl et al. 2016). In addition, levels of V1aR expression in the PVN of rat dams are highest at parturition compared to the days following parturition (Caughey et al. 2011), which suggests that V1aR in the PVN might be linked to rodent maternal behavior. The functional implications of upregulation or down-regulation of V1a receptor expression in the PVN, however, remain unclear without corresponding behavioral measures for our subjects. Nevertheless, these data provide a promising avenue of research for understanding how paternal presence during development may impact the mechanisms underlying maternal care.
Paternal presence at the nest shaped offspring neural phenotype, such that the absence of fathers resulted in greater OTR density in the NAcc. The NAcc is heavily involved in processing the reinforcing effects of both pharmacological and natural rewards across species, including those elicited from social stimuli (McBride et al. 1999; Young et al. 2001). Prairie voles densely express OTR within the NAcc compared to their socially promiscuous congener, the montane voles (M. montanus), and this phenotype is believed to contribute heavily to the species-specific propensity to form opposite sex pair-bonds (Insel et al. 1992). Decades of follow-up studies have contributed to this interpretation. For example, mating-induced partner preference formation is blocked in female prairie voles after NAcc infusion of an OTR antagonist (Young et al. 2001) or RNAi knockdown of OTR (Keebaugh et al. 2015), whereas overexpressing NAcc OTR via viral vector gene transfer subsequently enhances partner preference formation in females (Ross et al. 2009; Keebaugh & Young 2011). Furthermore, differences in NAcc OTR are highly likely to relate to differences in natural motivation to engage in bonding behavior. For instance, of all the neural structures that laboratory studies have shown to be necessary and sufficient for establishing a pair-bond, pair-bonded male prairie voles living freely in outdoor enclosures differed from single males in only NAcc OTR density, with bonded males expressing more OTR than single males (Ophir et al. 2008, 2012).
Collectively, the aforementioned studies demonstrate a causal link between more OT/OTR activation in the NAcc translating into increased bonding. Under this context, our results demonstrating that Father-absent offspring had more NAcc OTR would indicate that single-raised animals should also be more likely to form bonds. Unfortunately, we did not assess differences in any behavior, including general or pair-bond-specific motivated behaviors.
Interestingly, Ahern and Young (2009) found that single mother-reared prairie voles show a delayed onset of partner preference formation compared to biparentally reared offspring, a result that is the opposite of what our data would have predicted. However, Ahern and Young (2009) also found no differences in NAcc OTR. Such inconsistencies are particularly intriguing and highlight 2 important points that must be kept in mind for any studies interested in aligning brain and behavioral data, particularly within a developmental context. First, there are mechanisms beyond the activation of OTR within the NAcc that have functional implications for selective affiliation in adulthood. Second, putatively inconsistent outcomes of studies that result from different perturbations over development must be placed in a broader context. Different manipulations over the course of development can create different forces that operate on a suite of neural mechanisms in distinct ways. These differences can collectively affect each component of a behavioral system, such as propensity to form bonds in dramatically different ways depending on the type of developmental experience that occurred. In other words, a simple developmental alteration (i.e. 1 variable) of an operational network (built on the interactions of several different mechanisms operating on cell populations across a neural circuit) could result in neural and behavioral outcomes of one form, whereas complex (multivariate) manipulations of that same network are very likely to result in neural and behavioral phenotypes of another form. This is because more interdependent components of a network are potentially impacted in many more and several different ways when multiple factors that are certain to interact are manipulated in tandem. More simply stated, manipulating one important aspect of development might have caused a behavioral change (altered bonding), which could be potentially attributed to mechanisms other than NAcc OTR (e.g. CRF; see Ahern & Young 2009), whereas manipulating multiple aspects of development might induce changes in a broader set of neural areas (e.g. NAcc OTR among other potential mechanisms; see results) that collectively have the potential to negate or reverse the behavioral outcomes that animals are likely to produce. As mentioned above, our neural results would predict that single-raised animals would be more likely to form bonds, despite the contrary results of Ahern and Young (2009). Nevertheless, whatever the direction it might take, it is clear that early life social experiences hold great potential to impact motivated behaviors by operating on the OT system within the NAcc.
Effect of post-wean post-natal social experiences on nonapeptide receptor expression
We found that post-wean experience significantly impacts V1aR expression in the AH, such that Single-housed animals had higher V1aR expression compared to Pair-housed animals. The AH is a region closely associated with rodent aggression (Ferris et al. 1997; Albers 2012). Pair-bonded male prairie voles display aggression toward novel females (Winslow et al. 1993), and this selective aggression is mediated by VP activation of V1aR in the AH (Gobrogge et al. 2009). In addition, pair-bonded males show higher levels of AH V1aR compared to sexually naïve males. Furthermore, naïve males show increased levels of aggression toward novel females after AH V1aR agonism, and overexpression of V1aR in the AH via viral vector-mediated gene transfer. Our data indicate that Single-housed animals have a neural profile that reflects particularly aggressive behavioral phenotypes in this species, such that postnatal social experience may shape adult aggression.
Indeed, a wealth of literature on rodent social isolation and adult levels of aggression supports the interpretation that early experiences impact aggression. In rats and mice, social isolation experienced during development or adulthood have both been found to lead to increased levels of adult aggression (Valzelli 1973; Wongwitdecha & Marsden 1996). In prairie voles, adult females that were isolated for 4 weeks showed increased pup-directed aggression, and a greater likelihood of attacking an adult intruder, compared to non-isolated females (Grippo et al. 2008; Scotti et al. 2015). Sexually-naïve male prairie voles reared by low-contact parents exhibit more aggression towards a novel male compared to males reared by high-contact parents, providing further evidence that low levels of social exposure (either by social isolation or diminished parental care) during development can impact adult levels of aggression in this species (Perkeybile & Bales 2015). Although the neural and endocrinological mechanisms that underlie the connection between social isolation and increased adult aggression in rodents remain unclear, our neural data suggest a potential role for developmental plasticity of AH V1aR in adult aggression. Further work that explores the interface between AH V1aR and early-life social experiences at a developmental stage that is roughly equivalent with adolescence (Schneider 2013) is likely to provide important insight into the roots and labile nature of aggressive social behavior.
Nonapeptide receptor expression is influenced by developmental interactions
Unsurprisingly, the complexity of the social brain is obscured by the ways in which experiences and biology can interact to shape neural phenotypes across development. We found that males and females appear differentially susceptible to the influence of paternal presence. Specifically, we found that V1aR density in the VMH was greater in females than males, but only for offspring reared with a father present. In addition, females with fathers present had greater V1aR density in the VMH than females reared by mothers alone, whereas males were not impacted by paternal presence when compared to each other in this region. The VMH is a sexually dimorphic structure (Matsumoto & Arai 2008) that is sensitive to the organizational effects of sex steroid exposure during development (Matsumoto & Arai 1983), and is critical in rodent sexual behavior (Pfaff & Sakuma 1979; Wersinger et al. 1993).
Unfortunately, the role of V1aR in the VMH is not well characterized, largely because V1aR does not appear to be expressed in rat VMH. The majority of what we do know regarding V1aR VMH expression has been identified in hamsters. For example, male Siberian hamsters have higher VMH V1aR density than female Siberian hamsters. Furthermore, castrated males have reduced V1aR density in the VMH compared to intact males, and testosterone implants rescue this reduction, indicating that V1aR expression in the VMH is modulated by gonadal steroids in this species (Dubois-Dauphin et al. 1994). In addition, dominant male Syrian hamsters have increased V1aR density in the VMH compared to subordinate males (Cooper et al. 2005), indicating a potential role of VMH V1aR density in aggression and social dominance status. Considering the seemingly opposite direction of sex effects found between Siberian hamsters (where males have higher VMH V1aR density compared to females) and Prairie voles (where females have greater VMH V1aR density compared to males in the Father-present condition), it remains possible that V1aR has species-specific functions within the VMH, which require behavioral data for further elucidation.
Considering the roles that both the VMH and V1aR play in rodent reproductive behavior, we speculate that the presence of a father may shape the mechanisms that underlie adult sociosexual behaviors in a sex-specific manner. This result also highlights the need to include sex as an important biological variable. Without explicitly including both sexes as subjects, the nuanced heterogeneity of the ways in which the male and female social brains are differentially shaped by experience cannot be understood.
The pre-wean experience also interacts with social environments in later postnatal life. Oxytocin receptor density in the SHi (a region important for memory functioning: see Ophir 2017) varied across levels of both paternal presence and post-wean housing. Our lab previously demonstrated that OTR SHi density is susceptible to the interaction of pre-wean and post-wean social environments (Prounis et al. 2015). It is important to note that the current study included both sexes as a factor, whereas Prounis et al. (2015) focused on just males but incorporated a behavioral study as well. It is unclear why the results from these 2 studies were inconsistent; however, the methodological and design differences between them might explain why animals without fathers in the current study demonstrated the opposite pattern of OTR expression from those in Prounis et al. (2015). Nevertheless, the results from both studies clearly indicate that OTR in the SHi is particularly sensitive to the interactive effects of social experiences in early and late development.
More broadly, our study reaffirms that nonapeptide systems that are shaped by social experiences in the natal nest can be pronounced or offset by social experiences later in development. It is imperative to consider developmental experiences along a continuum, in which there are multiple sensitive periods that may be both independently and/or synergistically influential in neural scaffolding (Fox et al. 2010). This point is amplified when sex is considered as a variable. For example, OTR expression in the LS showed a significant 3-way interaction such that only males that were raised with fathers but that were housed singly after weaning showed an increase in septal OTR density compared to females. Considering the broad involvement of the LS and oxytocin in social behavior, social memory and other behaviors that are relevant to social functioning (Francis et al. 2001; Guzmán et al. 2014), this unique sensitivity of males to a particular series of early life experiences might help explain how differences in complex behavioral phenotypes develop in some animals or sub-populations of animals and not others. We suggest that sexual dimorphisms in neurobiology and physiology may open the potential for offspring to be differentially sensitive to subsequent neural shaping by interacting perinatal experiences (Curley & Champagne 2016; Moore & Depue 2016).
Stability and variability among the nonapeptide systems
Childhood and adolescence are periods of time in development with obvious implications for shaping the adult phenotype. Our study is a relatively exhaustive foray into the potential impact of early life experience at the rodent equivalent of these 2 distinct developmental time points (see Spear 2000 and references therein) on forebrain nonapeptide receptor phenotype. Based on our results, we highlight 2 important overall patterns: (i) the discrepancy in the distribution of effects found in V1aR and OTR; and (ii) the apparent robustness of these systems despite diversity in sex and social environments across development.
The first point speaks to the comparatively larger number of effects found for V1aR expression compared to OTR expression in our results. Interestingly, all 3 variables analyzed yield the main effects on V1aR expression, and all the main effects of sex reported are in V1aR expression. This pattern of results draws attention to the contrast between the plasticity of V1aR compared to the consistency of OTR expression across our variables. Other studies have shown a similar pattern, in which OTR expression appears to be less malleable compared to that of V1aR (Ophir et al. 2013; Prounis et al. 2018). For example, a comparison of OTR and V1aR densities across the forebrain of pregnant female prairie voles showed that females only differed in V1aR expression within the ventral pallidum and the PVN, a contrast to the striking stability of OTR forebrain pattering in the wake of large hormonal fluctuations across pregnancy (Ophir et al. 2013). A comparison of OTR and V1aR within the social decision-making network (O’Connell & Hofmann 2012) of female prairie voles living freely in outdoor enclosures showed that most structures expressing V1aR differed by the reproductive mating tactic, reproductive success or the interaction therein, whereas this was only true of 2 OTR expressing brain areas (Zheng et al. 2013). Indeed, a thorough analysis of the extant literature (for which there are fewer studies targeting OT/OTR) reveals many more sex differences in VP/V1aR systems compared to OT/OTR differences (Dumais & Veenema 2016). A systematic cross-species characterization of OT-ir and VP-ir across developmental time and between the sexes exhibited plentiful differences in VP-ir in the social behavior network (Newman 1999; Goodson 2005), but none on OT-ir in rats (DiBenedictis et al. 2017). Taken together, there is substantial evidence indicating that the vasopressin system varies more than that of oxytocin, particularly as a function of sex and developmental experiences. Why the VP system is relatively more plastic than the OT system is a pressing and extremely interesting question, but currently remains unresolved. Still, this collection of evidence leads to testable predictions that differences in both plasticity and functional roles between the 2 nonapeptide systems should exist.
Our second point, in contrast, emphasizes how relatively few differences were found in either OTR or V1aR expression overall, despite the number of potential interactions between variables and the numerous regions we analyzed. Although we found clear effects of sex, pre-wean experiences and post-wean experiences on receptor density, a remarkable number of regions exhibited no differences in receptor expression. Thus, our data reveal a robust consistency in receptor profiles despite profound biological and environmental variation. These results fall in line with the paucity of structural sex differences found in monogamous species more generally. Considering the known changes in adult behavior that occur as a function of similar early life experiences and sex (Ahern & Young 2009), how do we reconcile this receptor stability with divergences in behavioral outcomes? One explanation for these results could be that early social experiences and sex have more substantial impacts on the density of nonapeptide-producing neurons or fiber innervation than on receptor turnover. If true, this explanation would have important implications for the modulation of social behavior. In any case, it is critical to bear in mind that these differences in receptor profiles or immunoreactivity may either drive sexual dimorphisms in behavior, or compensate for other structural and physiological sex differences to facilitate behavioral equifinality (De Vries 2004). This hypothesis cannot be tested without directly manipulating receptors within specific cell groups and systematically measuring behavioral outcomes (Kelly & Goodson 2014).
CONCLUSIONS
Our study demonstrates that both OTR and V1aR binding densities are sensitive to early life social experiences, biological sex, and the interactions between these variables. The direction of the effects is region-specific and receptor-specific; instances of low social exposure (Father-absent and/or Single-housed) or high social exposure (Father-present and/or Pair-housed) did not have uniform effects on nonapeptide expression across the social brain. Instead, regulation of receptor density as a function of experience was either enhanced or dampened in a manner specific to each area of interest, which likely has functional implications for the neuromodulatory effects of OT/VP systems in response to social stimuli. As such, sweeping generalizations regarding how experiential factors impact the development of adult sociality and neural functioning as they relate to the nonapeptides are ineffectual in working towards a systems-level understanding of the social brain. Our data reaffirm prior work that has demonstrated sex-specific effects on the impacts that interacting early life experiences have on nonapeptide systems (Perkeybile et al. 2015). To further elucidate these functional relationships, we should strive to incorporate factors across developmental time and biology to better reflect the complexity of the real world.
ACKNOWLEDGMENTS
The authors would like to thank Johnna Graham, Ruth Witmer, Marcos Moreno, Lauren Foley, Asad Rehman and George Prounis for their contributions to the data collection. The authors acknowledge the support from the National Institutes of Health (Eunice Kennedy Shriver National Institute of Child Health and Human Development HD079573 to A.G.O.) and the National Science Foundation Graduate Research Fellowship Program (under DGE-1650441 to L.C.H.).
REFERENCES
- Ahern TH, Young LJ (2009). The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster). Frontiers in Behavioral Neuroscience 3, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE (2012). The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Hormones and Behavior 61, 283–92. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ (1993). Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, microtus ochrogaster and meadow voles, Microtus pennsylvanicus. Journal of Neuroendocrinology 5, 247–55. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48. [Google Scholar]
- Bayerl DS, Hönig JN, Bosch OJ (2016). Vasopressin V1a, but not V1b, receptors within the PVN of lactating rats mediate maternal care and anxiety-related behaviour. Behavioural Brain Research 305, 18–22. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ (2005). The V1a vasopressin receptor is necessary and sufficient for normal social recognition: A gene replacement study. Neuron 47, 503–13. [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Dantzer R (1990). Social recognition does not involve vasopressinergic neurotransmission in female rats. Brain Research 535, 301–4. [DOI] [PubMed] [Google Scholar]
- Bressler SC, Baum MJ (1996). Sex comparison of neuronal fos immunoreactivity in the rat vomeronasal projection circuit after chemosensory stimulation. Neuroscience 71, 1063–72. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. PNAS 95, 5335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey SD, Klampfl SM, Bishop VR et al. (2011). Changes in the intensity of maternal aggression and central oxytocin and vasopressin V1a receptors across the peripartum period in the rat. Journal of Neuroendocrinology 23, 1113–24. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ (2001). Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. PNAS 98, 12736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH (1991). The Evolution of Parental Care. Princeton University Press, Princeton, NJ. [Google Scholar]
- Cooper MA, Karom M, Huhman KL, Albers HE (2005). Repeated agonistic encounters in hamsters modulate AVP V1a receptor binding. Hormones and Behavior 48, 545–51. [DOI] [PubMed] [Google Scholar]
- Curley JP, Champagne FA (2016). Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Frontiers in Neuroendocrinology 40, 52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Jensen CL, Franks B, Champagne FA (2012). Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Hormones and Behavior 61, 454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville Y, Melloni RH, Ferris CF (1998). Behavioral and neurobiological consequences of social subjugation during puberty in golden hamsters. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 18, 2667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ (2004). Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145, 1063–8. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buds RM, Swaab DF (1981). Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain-presence of a sex difference in the lateral septum. Brain Research 218, 67–78. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RMM (1983). The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Research 273, 307–17. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, van Leeuwen FW, Caffé AR, Swaab DF (1985). The vasopressinergic innervation of the brain in normal and castrated rats. Journal of Comparative Neurology 233, 236–54. [DOI] [PubMed] [Google Scholar]
- DiBenedictis BT, Nussbaum ER, Cheung HK, Veenema AH (2017). Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. Journal of Comparative Neurology 525, 2549–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Theler JM, Ouarour A, Pévet P, Barberis C, Dreifuss JJ (1994). Regional differences in testosterone effects on vasopressin receptors and on vasopressin immunoreactivity in intact and castrated Siberian hamsters. Brain Research 638, 267–76. [DOI] [PubMed] [Google Scholar]
- Dulac C, Kimchi T (2007). Neural mechanisms underlying sex-specific behaviors in vertebrates. Current Opinion in Neurobiology 17, 675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH (2016). Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Frontiers in Neuroendocrinology 40, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH Jr, Koppel G, Perry KW, Fuller RW, Delville Y (1997). Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. The Journal of neuroscience: The Official Journal of the Society for Neuroscience 17, 4331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiber JM, Adames P, Swann JM (1993). Pheromones induce c-fos in limbic areas regulating male hamster mating behavior. Neuroreport 4, 871–4. [DOI] [PubMed] [Google Scholar]
- Fox SE, Levitt P, Nelson CA (2010). How the timing and quality of early experiences influence the development of brain architecture. Child Development 81, 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ (2001). Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. Journal of Neuroendocrinology 12, 1145–48. [DOI] [PubMed] [Google Scholar]
- Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E (2012). Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behavioral Neuroscience 126, 97–109. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS (1996). Prairie–vole partnerships. American Scientist 84, 55–62. [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z (2009). Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. PNAS 106, 19144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL (2005). The vertebrate social behavior network: Evolutionary themes and variations. Hormones and Behavior 48, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL (2008). Nonapeptides and the evolutionary patterning of sociality. Progress in Brain Research 170, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS (2008). Social isolation in prairie voles induces behaviors relevant to negative affect: Toward the development of a rodent model focused on co-occurring depression and anxiety. Depression and Anxiety 25, E17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán YF, Tronson NC, Sato K et al. (2014). Role of oxytocin receptors in modulation of fear by social memory. Psychopharmacology 231, 2097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Wu R, Yu P (2014). Study of Fos, androgen receptor and testosterone expression in the sub-regions of medial amygdala, bed nucleus of stria terminalis and medial preoptic area in male mandarin voles in response to chemosensory stimulation. Behavioural Brain Research 258, 65–74. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE, Insel TR (1992). Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. PNAS 89, 5981–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S, Harderthauer S, Sachser N, Hennessy MB (2007). Social housing conditions around puberty determine later changes in plasma cortisol levels and behavior. Physiology and Behavior 90, 405–11. [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Young LJ (2011). Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Hormones and Behavior 60, 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ (2015). RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Social Neuroscience 10, 561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL (2014). Social functions of individual vasopressin-oxytocin cell groups in vertebrates: What do we really know? Frontiers in Neuroendocrinology 35, 512–29. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID (2004). Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology 25, 150–76. [DOI] [PubMed] [Google Scholar]
- Lenth RV (2016). Least-Squares means: The R package lsmeans. Journal of Statistical Software 69, 1–33. [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C et al. (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277, 1659–62. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y (1983). Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinologia japonica 30, 277–80. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y (2008). Male–female difference in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendocrinology 42, 232–6. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S (1999). Localization of brain reinforcement mechanisms: Intracranial self-administration and intracranial place-conditioning studies. Behavioural Brain Research 101, 129–52. [DOI] [PubMed] [Google Scholar]
- Meaney MJ (2001). Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience 24, 1161–92. [DOI] [PubMed] [Google Scholar]
- Moore SR, Depue RA (2016). Neurobehavioral foundation of environmental reactivity. Psychological Bulletin 142, 107–64. [DOI] [PubMed] [Google Scholar]
- Newman SW (1999). The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Annals of the New York Academy of Sciences 877, 242–57. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA (2012). Evolution of a vertebrate social decision-making network. Science 336, 1154–7. [DOI] [PubMed] [Google Scholar]
- Ophir AG (2017). Navigating monogamy: Nonapeptide sensitivity in a memory neural circuit may shape social behavior and mating decisions. Frontiers in Neuroscience 11, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng DJ, Phelps SM (2012). Oxytocin receptor density is associated with male mating tactics and social monogamy. Hormones and Behavior 61, 445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Sorochman G, Evans BL, Prounis GS (2013). Stability and dynamics of forebrain vasopressin receptor and oxytocin receptor during pregnancy in prairie voles. Journal of Neuroendocrinology 25, 719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, Bales KL (2015). Early rearing experience is related to altered aggression and vasopressin production following chronic social isolation in the prairie vole. Behavioural Brain Research 283, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, Delaney-Busch N, Hartman S, Grimm KJ, Bales KL (2015). Intergenerational transmission of alloparental behavior and oxytocin and vasopressin receptor distribution in the prairie vole. Frontiers in Behavioral Neuroscience 9, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y (1979). Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. The Journal of Physiology 288, 203–10. [PMC free article] [PubMed] [Google Scholar]
- Prounis GS, Foley L, Rehman A, Ophir AG (2015). Perinatal and juvenile social environments interact to shape cognitive behaviour and neural phenotype in prairie voles. Proceedings of the Royal Society B 282, 20152236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prounis GS, Thomas K, Ophir AG (2018). Developmental trajectories and influences of environmental complexity on oxytocin receptor and vasopressin 1a receptor expression in male and female prairie voles. Journal of Comparative Neurology 526, 1820–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2016). R: A language and environment for statistical computing R Foundation for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ (2009). Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. Journal of Neuroscience 29, 1312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachser N, Kaiser S, Hennessy MB (2013). Behavioural profiles are shaped by social experience: When, how and why. Philosophical Transactions of the Royal Society B 368, 20120344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M (2013). Adolescence as a vulnerable period to alter rodent behavior. Cell and Tissue Research 354, 99–106. [DOI] [PubMed] [Google Scholar]
- Scotti M-AL, Carlton ED, Demas GE, Grippo AJ (2015). Social isolation disrupts innate immune responses in both male and female prairie voles and enhances agonistic behavior in female prairie voles (Microtus ochrogaster). Hormones and Behavior 70, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews 24, 417–63. [DOI] [PubMed] [Google Scholar]
- Tabbaa M, Lei K, Liu Y, Wang Z (2017). Paternal deprivation affects social behaviors and neurochemical systems in the offspring of socially monogamous prairie voles. Neuroscience 343, 284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin VA, Hashimoto H, Wacker DW et al. (2010). An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature 464, 413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valzelli L (1973). The “isolation syndrome” in mice. Psychopharmacologia 31, 305–20. [DOI] [PubMed] [Google Scholar]
- Van Den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM (1999). Play is indispensable for an adequate development of coping with social challenges in the rat. Developmental Psychobiology 34, 129–38. [PubMed] [Google Scholar]
- Veenema AH (2012). Toward understanding how early-life social experiences alter oxytocin- and vasopressin-regulated social behaviors. Hormones and Behavior 61, 304–12. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ (2012). Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Hormones and Behavior 61, 50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyrac A, Wang G, Baum MJ, Bakker J (2011). The main and accessory olfactory systems of female mice are activated differentially by dominant versus subordinate male urinary odors. Brain Research 1402, 20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Novak MA (1992). Influence of the social environment on parental behavior and pup development of meadow voles (Microtus pennsylvanicus) and prairie voles (M. ochrogaster). Journal of Comparative Psychology 106, 163–71. [Google Scholar]
- Wersinger SR, Baum MJ, Erskine MS (1993). Mating-induced FOS-like immunoreactivity in the rat forebrain: A sex comparison and a dimorphic effect of pelvic nerve transection. Journal of Neuroendocrinology 5, 557–68. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR (1993). A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–8. [DOI] [PubMed] [Google Scholar]
- Wongwitdecha N, Marsden CA (1996). Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behavioural Brain Research 75, 27–32. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR (2001). Cellular mechanisms of social attachment. Hormones and Behavior 40, 133–8. [DOI] [PubMed] [Google Scholar]
- Zheng D-J, Larsson B, Phelps SM, Ophir AG (2013). Female alternative mating tactics, reproductive success and nonapeptide receptor expression in the social decision-making network. Behavioural Brain Research 246, 139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]


