Abstract
Mixed chimerism and thymic tissue transplantation strategies have achieved xenogeneic tolerance in pig-to-mouse models, and both have been extended to pig-to-baboon models. A mixed chimerism strategy has shown promise toward inducing tolerance in allogeneic models in mice, pigs, nonhuman primates (NHP), humans, and a rat-to-mouse small animal xeno-model. However, even though α−1,3-galactosyltransferase gene knockout (GalTKO) pigs have been used as bone marrow (BM) donors, direct intravenous injection of porcine BM cells was detected for only up to 4 days (peripheral macro-chimerism) in one case, and the rest lost chimerism within 2 days.
Recent data in allogeneic models demonstrated that direct injection of donor BM cells into recipient BM spaces (intra-bone bone marrow transplantation: IBBMTx) produces rapid reconstitution and a higher survival rate compared to i.v. injection. In order to minimize the loss of injected porcine BM peripherally before reaching the BM space, Yamada developed a xeno-specific regimen including IBBMTx coated with a collagen gel matrix in a preclinical pig-to-baboon model (Yamada IBBMTx). This strategy aims to achieve improved, persistent macro-chimerism as well as engraftment of BM across a xenogeneic barrier. The initial study published in 2015 demonstrated that this IBBMTx strategy leads to markedly prolonged peripheral macro-chimerism detectable for up to 23 days. Furthermore, a more recent study using human CD47-transgenic (Tg) GalTKO pigs as xeno-donors achieved long-lasting macro-chimerism >60 days with evidence of reduction of anti-pig natural antibodies (nAb). This is the longest macro-chimerism that has ever been achieved in a preclinical large animal xenotransplant model to date. In this chapter, we introduce a brief summary of our achievements in regard to successful tolerance induction by utilizing our novel strategy of IBBMTx as well as describe the step-by-step methodology of surgical and in vitro procedures that are required for this project.
Keywords: Xenotransplantation, Intra-bone bone marrow transplantation, Human CD47 transgenic, Pig-to-baboon model, Tolerance
1. Introduction
Xenotransplantation is becoming a more realistic strategy for solving the organ shortage crisis. However, even with high-dose anti-CD40 or anti-CD154 mAb-based immunosuppression combined with multiple immunosuppressive drugs, recipients typically succumb to infection associated with chronic immunosuppression or graft rejection [1–8]. These data are consistent with previous reports [9, 10] indicating that the human-anti-porcine T-cell response is similar or stronger than the response seen across allogeneic barriers. Because of the strength of both innate and adaptive immunity in xenotransplantation, the level of continuous immunosuppression needed to control these immune responses and prolong xenograft survival has been associated with prohibitive morbidity and mortality [7, 11, 6]. These results provide compelling rationale to pursue a clinically applicable strategy for the induction of tolerance.
Despite the great immunologic differences between differing species, both mixed chimerism and thymic transplantation strategies have demonstrated efficacy in inducing tolerance of human T cells to porcine xenografts in mouse models [12–16]. However, these strategies require fundamental modifications when applied to pig-to-primate xenograft tolerance. Details of our strategy for thymic transplantation in a pig-to-baboon model are described in Chap. 11 [17–19].
Approaches toward achieving mixed chimerism by infusion of donor BM have successfully induced tolerance in clinical renal transplantation [20, 21]. However, because of the activation of T cells, which in turn activate B cells and NK cells sequentially [22–25], durable chimerism is likely required to induce stable T/B/NK-cell tolerance across xenogeneic barriers. In support of this, previous studies have shown that (1) mouse anti-rat natural antibodies, which have undefined specificities, disappear when non-myeloablative mixed chimerism is induced [25, 26] and (2) human NK cells are rendered tolerant by porcine mixed chimerism. This was reflected in some cases by specific unresponsiveness with otherwise preserved NK-cell function and, in others, global hyporesponsiveness in the pig-to-human mixed chimerism model in humanized mice. Even though specific humoral unresponsiveness was induced in some recipients [27], most porcine cells were cleared within 24–48 h post-GalTKO BM infusion [27–29]. These data indicate that in order to achieve durable chimerism across xenogeneic barriers for the induction of tolerance in pig-to-nonhuman primates or humans, further innovative strategies are required.
1.1. Our Strategy of IBBMTx Has Achieved >60 Days Durable Chimerism
1.1.1. IBBMTx Using GalTKO Pigs Facilitates the Prolongation of Macro-chimerism from Days to Up to 3 Weeks
A previous study performed in mice showed a smaller proportion of donor BM cells (1–2%) localized to the BM in recipients that were preconditioned by irradiation [30], indicating that the localization of donor BM cells at the host BM is a critical component of both BM engraftment and the reconstitution of host hematopoiesis. Therefore, we hypothesized that direct injection of collagen-coated porcine BM cells would not only minimize the loss of BM cells peripherally, but also the collagen coating would facilitate that porcine BM cells stay inside the host BM. This would result in the persistence of macro-chimerism and engraftment of porcine BM cells. Our results demonstrate that our new strategy involving IBBMTx (Yamada IBBMTx, detailed below) leads to (1) a high percentage of durable macro-chimerism for up to 23 days and (2) a high incidence (four of six animals) of BM engraftment with hyporesponsiveness across xenogeneic barriers in our pig-to-baboon model [31].
1.1.2. IBBMTx Using hCD47-Tg GalTKO Pigs Achieves Long-Lasting Chimerism >6 Months, Which Is the Longest Ever Attained in a Large Animal Xenotransplant Model
Recent work to circumvent the rapid clearance of BM cells has focused on innate immunity and macrophage-associated mechanisms. Specifically, attention is on SIRP-alpha, a transmembrane protein with intracellular tyrosine kinase activity that is present on macrophages, dendritic cells, and neutrophils. The ligand of this receptor is CD47, and recognition of CD47 by SIRP-alpha downregulates phagocytosis by macrophages [32, 33]. In a small animal study in which human hematopoietic stem cells were transplanted into severely immunocompromised mice to allow for human hematopoiesis, the authors found significant improvement in hematopoiesis and engraftment in mice transgenic for human SIRP-alpha [34]. These data suggest that expression of human CD47 on porcine BM cells may minimize the loss of circulating porcine BM cells in nonhuman primates. Hawley et al. first attempted to infuse hCD47/hCD55 Tg GalTKO BM cells intravenously into baboons. Contrary to high expectations, injected hCD47/hCD55 Tg GalTKO BM cells disappeared within 7 days.
Durable mixed xenogeneic chimerism is required for the induction of tolerance of human T-cell-independent B cells producing anti-pig Abs [35], which potentially results in elimination or minimization of non-Gal nAb-related renal graft injury as well as innate or T-cell rejection. Therefore, we recently combined our strategy of IBBMTx with hCD47 transgenic (Tg) donors in order to achieve further durable mixed xenogeneic chimerism [36]. Briefly, we have achieved long-lasting macro-chimerism (longer than 8 weeks) following hCD47/hCD55-Tg GalTKO IBBMTx in baboons, while neither GalTKO alone or human complement regulatory genes without hCD47 Tg maintained macro-chimerism for longer than 5 weeks. In addition, preformed nAb markedly decreased in recipients of hCD47-Tg IBBMTx, especially after 2 months following IBBMTx. Our data has been partially presented at the TTS 2019, Madrid, Spain (Watanabe H), and our original article describing the details of our results is in preparation (Yamada K et al). The following section describes our methods for IBBMTx, in addition to helpful tips and pitfalls to avoid for this procedure.
2. Materials
2.1. Catheter Placement
A lightweight spandex/lycra jacket (Lomir Biomedical Inc.,Malone, NY).
A rigid swivel that allows the animal to move freely in the cage without disturbing line connections.
A 1.0–1.5-m aluminum tether that attaches the swivel to the jacket.
Catheters, generally 1.5–2.0 m flexible, clear Tygon microbore tubing (Norton Performance Plastics, Akron, Ohio).
2.2. Splenectomy
Recipient baboons.
Surgical telescopes (preferably ×3.5 or greater) for the primary assistant and the primary surgeon.
Surgical draping and surgical instruments including Weitlaner retractors, electrocautery, as well as suture materials (silk ties).
Anesthesia and mechanical ventilator.
2.3. Irradiation for NHP
Small container for animal transport.
Irradiation machine.
2.4. Bone Marrow Transplantation
Collagen gel culturing kit (Wako Chemicals, USA).
Bone marrow injection gun (PerSys Medical, USA).
Surgical draping and infusion set.
0.9% NaCl (NS).
2.5. Flow Cytometry to Assess T-Cell and B-Cell Depletion and Macro-chimerism
Antihuman (cross-reactive to baboons) antibodies for CD3 (polyclonal rabbit antihuman CD3, A0452; Dako North America, Inc., Carpinteria, California, USA), CD4 (mouse antihuman CD4 mAb, 1F6; Invitrogen, Carlsbad, California, USA), CD8 (mouse antihuman CD8; BD Biosciences, Franklin Lakes, New Jersey, USA), CD20 (mouse antihuman CD20; BD Biosciences), CD25 (mouse antihuman, BD Biosciences), and CD45 (mouse anti-NHP; BD Biosciences). All conjugated to a fluorochrome.
Anti-pig monoclonal antibodies (mAb, available in our research center): Anti-porcine CD1, CD2, CD3, CD4, CD8, CD25, CD45, Class I, Class II, Pan Pig (produced internally). All conjugated to fluorochrome.
Conjugated anti-mouse IgG1 and anti-mouse IgG2a to be used as controls.
Cold FACS media: 1 L of Hanks’ balanced salt solution, 1 g of bovine serum albumin (BSA), 1 g of sodium azide.
FACS tubes.
2.6. CFU Assay
Reagents: fetal bovine serum (FBS), porcine stem cell factor, porcine GM-CSF, porcine IL-3, recombinant human erythropoietin (epoetin alfa), and IMDM MethoCult H4230 (STEM-CELL Technologies) for CFU medium.
Cell-culture dish (Fisher Scientific).
40-micron filter.
DNA extraction kit (Qiagen).
3. Methods
3.1. Catheter Placement
At the authors’ institution, kidney xenotransplantation is conducted in life-supporting models, and a bilateral native nephrectomy is typically performed at the time of transplantation. Thus, daily laboratory values are required to monitor the animal’s health and graft function. For these reasons, the placement of central venous and arterial catheters or “lines” is required for animal care during the induction, peri-transplantation, and post-transplantation periods. Lines provide the caregiver with the ability to both deliver drug therapy and draw blood for diagnostic testing. This is of particular importance in baboons or monkeys because it is difficult to perform a physical examination beyond simple observation. Although central catheters confer a risk of infection, there is greater risk associated with sedation for daily or twice-daily blood draws and drug-administration, especially in potentially fragile post-xenotransplantation recipients.
The lines are utilized for (1) continuous blood pressure monitoring; (2) blood samples for CBC, blood chemistry, and drug levels; and (3) the number of drugs administered (continuous and/or intermittent) and use of different lines due to drug compatibility. We used to place three lines (two venous, one arterial) 1 week prior to transplantation. The arterial line is used primarily for blood draws, and the two venous lines are used for drug delivery. However, we have recently started placing only two venous lines in order to lessen the risk of infection by minimizing indwelling lines with success. We have found that continuous blood pressure monitoring is generally not needed once the animal is recovered from anesthesia, and blood draws are able to be performed from one of the two venous lines. Importantly, soluble MMF must be placed on its own dedicated D5%W carrier line and not mixed with normal saline (see Fig. 1). The following is our procedure for placing two lines into the jugular veins (the procedure for placing three lines is described in the Chap. 11.
Fig. 1.
(a) A picture of the line system used for baboon recipients of xenografting procedures. The mesh jacket is rigidly attached to an aluminum tether through which 1–3 catheters are placed. The tethers are 4–6 ft. in length. (b) A close-up view of the swivel shown in (a), which has been unattached from the aluminum tether. As many as three catheters are securely affixed to each of three ports on the swivel. This allows the animal to move within the cage without twisting the lines. (c) A close-up view of the connection between the jacket and the tether. In this image, the jacket has been detached from the tether, showing the tape-wrapped catheters traveling through the jacket and into the tethering system
Generally, central lines are placed in the great vessels of the neck, on the left side.
Make a 3-cm transverse incision sharply, one fingerbreadth above the clavicle, starting from the midline and extending across the anterior border of the sternocleidomastoid (SCM).
Electrocautery and blunt dissection should be used to transect the platysma and isolate the external jugular (EJ) vein and the internal jugular (IJ) vein.
Make a small incision in the animal’s back (between shoulder blades), and tunnel the lines through the subcutaneous tissue and into the open neck wound.
Dissect the sternocleidomastoid and retract medially or laterally for access to the IJ followed by the EJ.
Place a small bulldog clamp on the vessels or tie the vessels, 1 cm proximally.
Create a venotomy using tenotomy scissors and then cannulate each vessel.
Release clamps and advance the venous line (if clamped).
Close the skin with buried subcuticular sutures.
Place a protective line jacket on the animal in standard fashion.
3.2. Splenectomy
A left abdominal incision is made from the left subcostal margin to the middle of the abdomen and is carried down through the peritoneum.
Gently retract the spleen and carefully dissect the pancreatic tail from the spleen. Rotate the spleen to release the splenocolonic and retroperitoneum attachments.
Expose the spleen and isolate the splenic vein and artery.
Transect splenic vein and artery between ligature ties.
Expose short gastric arteries and transect between ligature ties.
After removing the spleen and ensuring hemostasis, close the abdomen in 3–4 layers. Our practice is to close the peritoneum with absorbable suture, followed by closure of the fascia with a running permanent suture, and then closing the superficial fascia and skin with a running absorbable suture.
Wean off the ventilator and replace the jacket. Once recovered from anesthesia, the animal should be perched within 2 h of extubation. See Note 13 for animal care recommendations.
3.3. Irradiation for NHP
Animal is first sedated and placed in a transport cage for traveling to the irradiation center.
All animals receive whole body irradiation and thymic irradiation (thymic area is marked and other areas are shielded).
After the irradiation, animals are immediately brought back to the cage. Animals should be perched within 2 h after recovery from anesthesia.
3.4. Bone Marrow Transplantation
3.4.1. Intra-bone Bone Marrow Transplantation (IBBMTx. Yamada Method [9])
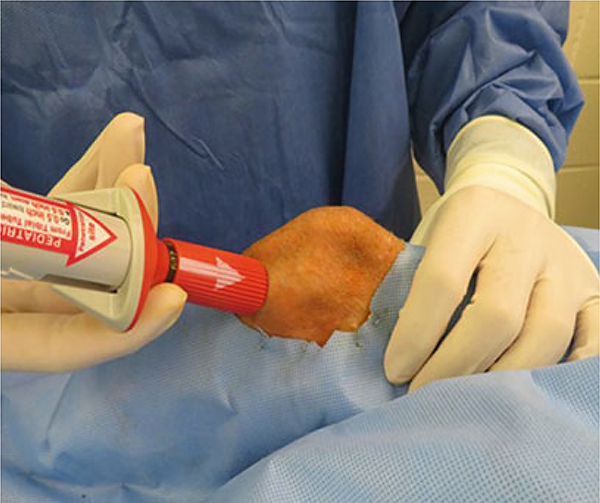
The animal is first brought to the OR and intubated. The animal is placed in the supine position, and the legs are prepped and draped in the usual sterile fashion. It is our practice to clip the hair from the mid thigh distal to the mid tibia and prep the entire leg. Once draped, the tibial tuberosity is palpated (Fig. 2).
It is important to ensure the legs are stable for the procedure. This can be aided by first tying the legs to the OR table in slight flexion using soft restraints. Grasp the leg firmly, and discharge the biopsy needle gun flush against the medial tibia, approximately one fingerbreadth beneath the tibial tuberosity. Remove the cannula and draw back 5–10 mL of BM.
Inject donor BM through the injection spike, which has been pre-mixed with collagen solution. At this time, it is extremely important to inject the BM slowly as well as communicate with the anesthesiologist. It is very common for the animal to display an anaphylactic reaction to the IBBMTx, and it is good practice to premedicate with steroids and diphenhydramine. Anticipate that the animal may require vasopressor support during the procedure, which is not uncommon.
Remove injection spike and hold firm compression over the wound for approximately 30 min to prevent hematoma formation.
Fig. 2.
A picture of the proper positioning for IBBM injection. Rt. tibial tuberosity was used for IBBM injection
3.4.2. IBBMTx
Peripheral line insertion is performed with an 18–22G angio-catheter before the procedure begins.
Flush the line with normal saline.
Slowly inject diluted BM through the line.
After BM injection, flush the line again with normal saline.
If the animal displays anaphylaxis to the IBBMTx, the peripheral line can be used for emergency drug injection.
3.5. Flow Cytometry to Assess T-Cell and B-Cell Depletion and Macro-chimerism
Stain 75 μL of whole blood with anti-Pan pig and anti-pig CD45 antibodies. Incubate for 30 min at room temperature in the dark. Use conjugated anti-mouse IgG1 and anti-mouse IgG2a as controls for nonspecific binding.
Add 2 mL of FACS Lysing Buffer to each tube and vortex well.
Incubate for 5 min at room temperature in the dark.
Vortex each tube once more.
Centrifuge 5 min at 260 × g at room temperature.
Add 10 μL of secondary reagent (i.e., PE-avidin), if necessary.
Vortex gently.
Incubate for 15–30 min at room temperature in the dark.
Wash twice with FACS buffer.
Acquire cells using flow cytometer.
3.6. CFU Assay
Obtain a BM sample and drain away most of the liquid, and then place into a medium Petri dish.
Filter through a 40-micron filter into a 50-mL conical tube.
Centrifuge 10 min at 350 × g at room temperature.
Discard supernatant and resuspend pellet in 5 mL of PBS.
Count cells using standard cell counting technique.
Dilute with PBS to make a suspension of 2.5 × 105 cells/mL.
Take 200 μL (5 × 104 cells/mL) and add to each 3-mL aliquot of methylcellulose media.
Incubate the plate in the CO2 incubator for 2 weeks at 37 °C.
Pick colonies under an inverted microscope using a pipette tip.
Spin down at highest speed (at least 16k) on a standard tabletop microcentrifuge for 10 min.
Extract DNA using the blood DNA extraction kit.
PCR assay to amplify the porcine cytochrome b gene.
4. Notes
For animal use in the OR, ensure all equipment is sterilized before animal sedation. Surgeons should scrub and maintain sterile technique throughout the procedure.
The EJ should be wrapped with a vessel loop. This is done because the vein is very small and frequently experiences vasospasm. To prevent visual obstruction, the EJ catheter is placed after the IJ vein and arterial catheters.
The arterial catheter should not be advanced more than 6 cm in a 6-kg baboon.
-
The IJ and EJ are low pressure vessels. After distal ligation, a proximal clamp is typically unnecessary prior to venotomy. Because, anatomically, the EJ communicates with the subclavian vein at an acute angle, the EJ line is typically only advanced 1–2 cm. The catheters are tacked to the SCM, deep to the reapproximated platysma. This may help to avoid contamination if a wound infection (rare) occurs.
Catheter-related issues: if line infection occurs or the lines thrombose, they must be removed. In order to do so, the animal is taken to the OR for line removal through the same incision used for placement. Expect dense scar tissue around the lines, and extreme care must be taken to avoid puncture or damaging the lines during dissection, as this would introduce a potentially fatal air embolus or hemorrhage. If after removal additional lines are required, the femoral vessels can be used. In doing so, the femoral artery will need to be tied distally, which is tolerated remarkably well in baboons [37]. Both ipsilateral femoral vessels can be used during a single line placement. In some cases, it may be necessary to subcutaneously tunnel a second venous line across the animal’s anterior pelvis. Two and sometimes three lines can be tunneled safely through the subcutaneous tissues over the animal’s back (at the level of L1). The longitudinal groin incisions, despite not being covered by the jacket, are rarely a problem. If needed, all four vessels (two femoral arteries and two femoral veins) can be ligated without causing clinically significant morbidity.
The tail of the pancreas usually overlies the splenic hilum, and the surgeon should take great care in avoiding injury to the pancreas during splenectomy.
All splenic vessels including the short gastrics should be identified, clipped, or ligated between silk sutures.
If BM biopsy is performed in the correct position on the tibia, BM is easily aspirated back through the injection spike. If BM does not aspirate easily, researchers should remove the spike and hold pressure for 30 min. Attempt biopsy from a different site.
It is of tantamount importance to communicate with the anesthesiologist and inject BM slowly. Note the blood pressure, heart rate, and respiratory rate before and after BM injection. Acute anaphylaxis commonly occurs during injection.
To avoid or minimize anaphylaxis, BM should be injected slowly. Ideally, the BM should be injected 1 mL at a time, with pauses between each mL to monitor the animal’s condition.
Compression of needle incision site should be held for 30 min. If bleeding is still noted, additional compression is recommended to avoid hematoma.
For intravenous BM injection, BM should be diluted in NS and slowly injected through the peripheral line.
Animal care: following line placement and prior to transplantation, the animals undergo a brief but stringent induction using T-cell and B-cell depleting agents. These are generally monoclonal or polyclonal antibodies that are commercially available. Administration of any cellular depleting antibody should be preempted by flow-cytometric T- and B-cell subset phenotyping. T-cell depleting agents have been tested at multiple doses and at multiple time points; however, the general goal is to achieve near-complete T-cell depletion (50–150 cells/μL) for the first 2 weeks which protects the transplanted BM cells in the induction period.
Acknowledgments
We thank Ms. Haruna Shimizu for her editorial assistance. This research was supported by NIH grant (NIAID 5P01AI045897). All procedures and animal care were performed in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by Columbia University Medical Center.
References
- 1.Shin JS, Kim JM, Kim JS et al. (2015) Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant 15(11):2837–2850. 10.1111/ajt.13345 [DOI] [PubMed] [Google Scholar]
- 2.Shin JS, Min BH, Kim JM et al. (2016) Failure of transplantation tolerance induction by autologous regulatory T cells in the pig-to-nonhuman primate islet xenotransplantation model. Xenotransplantation 23(4):300–309. 10.1111/xen.12246 [DOI] [PubMed] [Google Scholar]
- 3.Higginbotham L, Mathews D, Breeden CA et al. (2015) Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation 22(3):221–230. 10.1111/xen.12166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwase H, Hara H, Ezzelarab M et al. (2017) Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation 24(2):e12293 10.1111/xen.12293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohiuddin MM, Singh AK, Corcoran PC et al. (2016) Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun 7:11138 10.1038/ncomms11138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwase H, Liu H, Wijkstrom M et al. (2015) Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation 22(4):302–309. 10.1111/xen.12174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hering BJ, Wijkstrom M, Graham ML et al. (2006) Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med 12(3):301–303. 10.1038/nm1369 [DOI] [PubMed] [Google Scholar]
- 8.Cardona K, Korbutt GS, Milas Z et al. (2006) Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med 12(3):304–306. 10.1038/nm1375 [DOI] [PubMed] [Google Scholar]
- 9.Yamada K, Sachs DH, DerSimonian H (1995) Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol 155(11):5249–5256 [PubMed] [Google Scholar]
- 10.Murray AG, Khodadoust MM, Pober JS, Bothwell AL (1994) Porcine aortic endothelial cells activate human T cells: direct presentation of MHC antigens and costimulation by ligands for human CD2 and CD28. Immunity 1 (1):57–63 [DOI] [PubMed] [Google Scholar]
- 11.Yamada K, Yazawa K, Shimizu A et al. (2005) Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med 11(1):32–34. 10.1038/nm1172 [DOI] [PubMed] [Google Scholar]
- 12.Nikolic B, Gardner JP, Scadden DT, Arn JS, Sachs DH, Sykes M (1999) Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. J Immunol 162(6):3402–3407 [PubMed] [Google Scholar]
- 13.Kalscheuer H, Onoe T, Dahmani A et al. (2014) Xenograft tolerance and immune function of human T cells developing in pig thymus xenografts. J Immunol 192(7):3442–3450. 10.4049/jimmunol.1302886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu I, Fudaba Y, Shimizu A, Yang YG, Sykes M (2008) Comparison of human T cell repertoire generated in xenogeneic porcine and human thymus grafts. Transplantation 86 (4):601–610. 10.1097/TP.0b013e318182d47a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Swenson K, Sergio JJ, Arn JS, Sachs DH, Sykes M (1996) Skin graft tolerance across a discordant xenogeneic barrier. Nat Med 2(11):1211–1216 [DOI] [PubMed] [Google Scholar]
- 16.Lee LA, Gritsch HA, Sergio JJ et al. (1994) Specific tolerance across a discordant xenogeneic transplantation barrier. Proc Natl Acad Sci U S A 91(23):10864–10867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada K, Shimizu A, Utsugi R et al. (2000) Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J Immunol 164(6):3079–3086 [DOI] [PubMed] [Google Scholar]
- 18.Kamano C, Vagefi PA, Kumagai N et al. (2004) Vascularized thymic lobe transplantation in miniature swine: thymopoiesis and tolerance induction across fully MHC-mismatched barriers. Proc Natl Acad Sci U S A 101 (11):3827–3832. 10.1073/pnas.0306666101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivard CJ, Tanabe T, Lanaspa MA et al. (2018) Upregulation of CD80 on glomerular podocytes plays an important role in development of proteinuria following pig-to-baboon xenorenal transplantation - an experimental study. Transplant international : official journal of the European Society for Organ Transplantation 31(10):1164–1177. 10.1111/tri.13273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DM Jr, Sachs DH (2000) Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest 105 (2):173–181. 10.1172/JCI7913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai T, Cosimi AB, Colvin RB et al. (1995) Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation 59(2):256–262 [PubMed] [Google Scholar]
- 22.Cretin N, Bracy J, Hanson K, Iacomini J (2002) The role of T cell help in the production of antibodies specific for gal alpha 1–3Gal. J Immunol 168(3):1479–1483 [DOI] [PubMed] [Google Scholar]
- 23.Tonomura N, Shimizu A, Wang S et al. (2008) Pig islet xenograft rejection in a mouse model with an established human immune system. Xenotransplantation 15(2):129–135. 10.1111/j.1399-3089.2008.00450.x [DOI] [PubMed] [Google Scholar]
- 24.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG (2006) Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 108(2):487–492. 10.1182/blood-2005-11-4388 [DOI] [PubMed] [Google Scholar]
- 25.Aksentijevich I, Sachs DH, Sykes M (1992) Humoral tolerance in xenogeneic BMT recipients conditioned by a nonmyeloablative regimen. Transplantation 53(5):1108–1114 [DOI] [PubMed] [Google Scholar]
- 26.Sharabi Y, Aksentijevich I, Sundt TM 3rd, Sachs DH, Sykes M (1990) Specific tolerance induction across a xenogeneic barrier: production of mixed rat/mouse lymphohematopoietic chimeras using a nonlethal preparative regimen. J Exp Med 172 (1):195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griesemer A, Liang F, Hirakata A et al. (2010) Occurrence of specific humoral non-responsiveness to swine antigens following administration of GalT-KO bone marrow to baboons. Xenotransplantation 17 (4):300–312. 10.1111/j.1399-3089.2010.00600.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng YL, Dor FJ, Kuwaki K et al. (2004) Bone marrow transplantation from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Xenotransplantation 11 (4):361–370. 10.1111/j.1399-3089.2004.00151.x [DOI] [PubMed] [Google Scholar]
- 29.Liang F, Wamala I, Scalea J et al. (2013) Increased levels of anti-non-gal IgG following pig-to-baboon bone marrow transplantation correlate with failure of engraftment. Xenotransplantation 20(6):458–468. 10.1111/xen.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui J, Wahl RL, Shen T et al. (1999) Bone marrow cell trafficking following intravenous administration. Br J Haematol 107 (4):895–902 [DOI] [PubMed] [Google Scholar]
- 31.Tasaki M, Wamala I, Tena A et al. (2015) High incidence of xenogenic bone marrow engraftment in pig-to-baboon intra-bone bone marrow transplantation. Am J Transplant 15 (4):974–983. 10.1111/ajt.13070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ide K, Wang H, Tahara H et al. (2007) Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A 104(12):5062–5066. 10.1073/pnas.0609661104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, VerHalen J, Madariaga ML et al. (2007) Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood 109 (2):836–842. 10.1182/blood-2006-04-019794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Wang H, Ide K et al. (2011) Human CD47 expression permits survival of porcine cells in immunodeficient mice that express SIRPalpha capable of binding to human CD47. Cell Transplant 20(11–12):1915–1920. 10.3727/096368911X566253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang YG, deGoma E, Ohdan H et al. (1998) Tolerization of anti-Galalpha1–3Gal natural antibody-forming B cells by induction of mixed chimerism. J Exp Med 187 (8):1335–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tena AA, Sachs DH, Mallard C et al. (2017) Prolonged survival of pig skin on baboons after Administration of pig Cells Expressing Human CD47. Transplantation 101(2):316–321. 10.1097/TP.0000000000001267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sengupta D, Harper M, Jennett B (1974) Effect of carotid ligation on cerebral blood flow in baboons. 2. Response to hypoxia and haemorrhagic hypertension. J Neurol Neurosurg Psychiatry 37(5):578–584 [DOI] [PMC free article] [PubMed] [Google Scholar]