Abstract
Growth differentiation factor 11 (GDF11) is a member of the transforming growth factor‐β super family. It has multiple effects on development, physiology and diseases. However, the role of GDF11 in the development of mesenchymal stem cells (MSCs) is not clear. To explore the effects of GDF11 on the differentiation and pro‐angiogenic activities of MSCs, mouse bone marrow–derived MSCs were engineered to overexpress GDF11 (MSCGDF11) and their capacity for differentiation and paracrine actions were examined both in vitro and in vivo. Expression of endothelial markers CD31 and VEGFR2 at the levels of both mRNA and protein was significantly higher in MSCGDF11 than control MSCs (MSCVector) during differentiation. More tube formation was observed in MSCGDF11 as compared with controls. In an in vivo angiogenesis assay with Matrigel plug, MSCGDF11 showed more differentiation into CD31+ endothelial‐like cells and better pro‐angiogenic activity as compared with MSCVector. Mechanistically, the enhanced differentiation by GDF11 involved activation of extracellular‐signal‐related kinase (ERK) and eukaryotic translation initiation factor 4E (EIF4E). Inhibition of either TGF‐β receptor or ERK diminished the effect of GDF11 on MSC differentiation. In summary, our study unveils the function of GDF11 in the pro‐angiogenic activities of MSCs by enhancing endothelial differentiation via the TGFβ‐R/ERK/EIF4E pathway.
Keywords: angiogenesis, angiogenic therapy, differentiation, endothelial cells, GDF11, mesenchymal stem cells
1. INTRODUCTION
Stem cell‐based therapy is a promising method to treat various diseases and has brought new insights into repair and regeneration of organs and tissues. 1 Mesenchymal stem cells (MSCs) isolated from various tissues are the most widely used cells for the therapy, which are also considered as a suitable cell source for clinical treatment of cardiovascular diseases. 2 , 3 However, poor retention and low activity of MSCs in vivo have limited the practical use of MSC‐based therapy for ischaemic diseases. In angiogenic therapy, MSCs display multiple differentiation potentials in vitro, including the ability to differentiate into endothelial cells (ECs) and smooth muscle cells (SMCs) and can secrete various trophic factors to promote cardiovascular regeneration. 4 , 5
Growth differentiation factor 11 (GDF11) is a member of the transforming growth factor‐β (TGF‐β) superfamily. It is also known as bone morphogenetic protein 11 (BMP11). 6 GDF11 is expressed in many tissues, including pancreas, intestine, kidney, skeletal muscle, heart, developing nervous system, olfactory system and retina. 7 GDF11 plays an important role in early embryonic development and regulates the development of many organs. 8 GDF11 signals through binding with activin type II/I receptors (ActRII/I) on cellular membrane and activates the canonical SMAD2/3 signalling pathway 9 to realize its various biological functions. 10 , 11 , 12 The activated SMAD2/3 forms complexes with universal SMAD4, then is transferred to the nucleus and regulates gene transcription. In addition to the canonical Smad signalling pathway, the TGF‐β superfamily members can also activate other non‐Smad signalling pathways. 13 , 14 It has been reported that GDF11 activates p38‐MAPK to regulate the size and function of the nucleolus, affects c‐Jun N‐terminal kinase (JNK) in ECs, as well as cross talking with AMPK and NF‐κB. 8 And the extracellular regulated protein kinases (ERK) pathway was reported to be involved in the differentiation process of multi‐potent adult progenitor cells. 15 Bone marrow‐derived MSCs have been shown to be able to promote angiogenesis by direct differentiation into ECs both in vivo and in vitro. 16 , 17 However, little is known about how GDF11 affects MSC differentiation and weather the effects of GDF11 on MSCs are through TGF‐β/ERK pathway.
Angiogenesis can be modulated by a number of cytokines and growth factors, among which vascular endothelial growth factor (VEGF) and TGF‐β1 play prominent roles. 18 , 19 VEGF and TGF‐β1 are often co‐expressed in tissues in which angiogenesis occurs, notably in a variety of tumours. 20 TGF‐β is a multifunctional growth factor with effects on cell growth, differentiation, fibroblast activation, myofibroblast formation 21 and ECM accumulation. 22 Several recent studies demonstrated that TGF‐β can also induce differentiation of stem cells or progenitor cells towards smooth muscle cells or myofibroblast lineage. 23 The plasma level of GDF11 is closely related to the formation and development of appendage skeleton 24 and has been shown to be involved in cardiovascular disease. 10 A recent study confirmed that higher concentration of GDF11 in the circulation was associated with a lower risk of vascular events and death, indicating that GDF11 may be a protective factor for essential in the setting of vascular events. 25 Other studies have found that GDF11 plays an important role in angiogenesis in different organ system. 26 , 27 , 28 However, there are only a few reports showing the effect of GDF11 in stem cell differentiation 29 , 30 and the role of GDF11 in MSCs remains to be determined.
In this study, we hypothesized that GDF11 can enhance MSC‐mediated angiogenesis by increasing the ability of MSCs to differentiate into endothelial‐like cells, as well as through its anti‐apoptosis and paracrine functions. We found that GDF11 promoted MSC differentiation into endothelial‐like cells and enhanced their pro‐angiogenic activities via activation of TGF‐β receptor and its downstream ERK/EIF4E pathway.
2. MATERIALS AND METHODS
2.1. Culture of mouse BM‐MSCs
Animal protocol was approved by Zhejiang University according to Chinese guidelines for laboratory animal care and use. Bone marrow was isolated from femurs and tibias of 8‐week‐old C57BL/6J male mice, and BM‐MSCs were obtained as described previously 31 and cultured in DMEM medium (Hyclone, USA) supplemented with 10% foetal bovine serum (FBS) (Bioind, USA), 10 U/mL penicillin, and 10 U/mL streptomycin (Hyclone,Los Angeles, CA,USA). Cells were sub‐cultured to 80%‐90% confluence and passed after dissociation with 0.25% Trypsin & 0.02% EDTA (Genom, China) (Figure S1A). For normal oxygen conditions (21% O2, 5% CO2, 37℃), cells were incubated in a standard humidified CO2 incubator. All experiments were performed using cells at passage between 3 and 5.
2.2. Characterization of MSCs
MSCs were characterized by flow cytometric analysis of surface markers and were positive for: CD44, CD105, CD90; negative for: CD31, CD45(Figure S1B). At room temperature, cells were dissociated, re‐suspended in phosphate‐buffered saline (PBS) and incubated with antibodies against following markers in dark for 30 mins: CD44 (Ebioscience, Santiago, USA, Cat #12044181), CD105 (Ebioscience,USA, 550546), CD90 (Ebioscience,553004), CD31(Ebioscience, 561073) and CD45 (Ebioscience, USA, 553098). Non‐specific mouse IgG‐APC and IgG‐APC (Ebioscience,USA) were used as controls. After incubation, the cells were washed twice with PBS and analysed by flow cytometry (BD Biosciences,New Jersey, USA). The data were analysed by Flowjo software.
The ability of MSCs to differentiate into osteocytes and adipocytes was examined by culturing MSCs at passage 3 with osteogenic medium: DMEM supplemented with 10% FBS, 10 mmol/L β‐glycerophosphate, 50 μmol/L ascorbate‐2‐phosphate and 0.1 μmol/L dexamethasone (all from Sigma‐Aldrich), or adipogenic medium: DMEM supplemented with 10%FBS, 5 μg/mL insulin, 5 mmol/L isobutyl methylxanthine, 60 μmol/L indomethacin, and 1 μmol/L dexamethasone (all from Sigma‐Aldrich) for 2 weeks. Medium was changed every 3 days. After 2 weeks, cells were stained with Alizarin Red S (Solarbio, Life science) or Oil red O (Solarbio, Life science) to detect osteocytes and adipocytes, respectively (Figure S1C).
2.3. Lentiviral vector transduction
Lentiviral vectors carrying genes for GDF11 and GFP (LV‐GDF11‐GFP) or control vectors (LV‐GFP) and Luciferase (LV‐ Luc) were prepared by Genechem (Shanghai, China). MSCs were seeded at 1x105 cell per well onto 12‐well plates one day before transduction. Medium was changed with fresh serum‐free DMEM medium (500 μL/well), and viral vectors (~2.5 μL) premixed with 20 μL HiTransG P transfection reagent (Genechem) were added to each well to reach a multiplicity of infection (MOI) at 50 for all transduction. Culture medium was changed 12 hours after transfection with DMEM containing 10% FBS. After 48 hours, cells were observed under fluorescent microscope for GFP+ cells (Figure S2A). Then, the successfully transduced cells were selected by culturing the cells in the presence of purinomycin. Expression of GDF11 at levels of mRNA and protein was detected by RT‐PCR (Figure S2B) and Western blot (Figure S2C), respectively. The cells transducted LV‐GDF11‐GFP/ Luc were abbreviated as MSCGDF11, and control vectors (LV‐GFP/ Luc) were abbreviated as MSCvector. This abbreviation is used the following text.
2.4. Endothelial cell differentiation
MSCs were cultured in 12‐well plates (1 × 105 cells/well) with DMEM medium for 24 hours at 37°C and then cultured in M199 medium (Corning, USA) supplemented with 2% FBS, 50 ng/mL VEGF165 (PEPROTECH, USA, #450‐32‐10UG) for 14 days. Medium was changed every 2 days. Cells were detached for analysis of surface markers CD31 (Ebioscience, 561073), VEGFR2, (Ebioscience, 561993) by flow cytometry as described above, or cellular protein was extracted for Western blot analysis.
For mechanism study, inhibitor of TGF‐β receptor (LY2109761) (SELLECK, USA) or of ERK1/2 (SD5978) (Huaan, China), both in a final concentration of 10 µmol/L, or DMSO(1 μL/ml) was added into the fresh medium for 45 minutes prior to the addition of VEGF165 for differentiation culture for 14 days.
2.5. Western blot assay
Cell lysates were prepared using radioimmunoprecipitation assay (RIPA) buffer (Beyotime, China, P0013B). Total protein was quantified by BCA protein assay (Bio‐Rad, Berkery). Each sample was adjusted to equal amount of protein using 5X loading buffer for loading. The samples were separated by SDS‐PAGE, transferred to a polyvinylidene fluoride membrane, and immunoblotted with the following antibodies: GDF11 (DGDF80, R&D Systems,Emeryville, CA,USA), HGF (ab83760, Abcam, USA), VEGFR1 (ET1605‐11,Huabio,China), CD31 (#77699, Cell Signalling TechnologyBoston,MA, USA), VEGFR2 (#9698, following Abs are all from Cell Signalling Technology,USA), phospho‐p44/42 (Thr202/Try204 phospho‐ERK1/2,#4370), p44/42 MAPK (Erk1/2,#4696), BCL2 (#2827), BAX (#14796), Cleaved Caspase3 (#9661), phospho‐Smad2 (Ser465/Ser467,#18338), phospho‐Smad3 (Ser423/425, # 9520), Smad2(#5339), Smad3(#9523), anti‐β‐actin (#3700), EIF4E (R1512‐8,Huabio, China), Phospho‐eIF4E (S209) (ET1608‐66,Huabio, China) and VEGF ( ER30607,Huabio, China),at 4°C overnight. After incubation of the membranes with peroxidase‐conjugated secondary antibodies (Cell Signalling Technology,USA), bands were visualized using enhanced chemiluminescence reagents (Bio‐Rad,Los Angeles, CA,USA).
2.6. Real‐time RT‐PCR
Total RNA was extracted using Trizol reagent (Invitrogen, USA) according to the manufacturer's protocol. Total RNA (1 μg) was used for reverse transcription to synthesize cDNA using PrimeScript™ 1st Strand cDNA Synthesis Kit (TaKaRa, Dalian, China), and SYBR® Premix Ex TaqTM II (Tli RNaseH Plus) (TaKaRa) was applied for real‐time RT‐PCR process on an ABI PRISM 7500 Fast Detection System (Applied Biosystems, Carlsbad, CA, USA) according to the standard method. Each sample was performed in triplicated and all results were normalized to the expression of normalized to β‐actin (ACTIN). Fold expression relative to the reference ACTIN gene was calculated using the comparative method 2−ΔCt. The sequences of PCR primers were listed in Table S1.
2.7. Immunohistochemistry staining
Cells were washed with PBS containing 3% bovine serum albumin (BSA) and fixed with 4% paraformaldehyde for 15 minutes. The cells were then permeabilized with 0.5% Triton X‐100 for 10 minutes, blocked with 3% BSA in PBS for 30 minutes at room temperature and then incubated with primary antibody CD31 (#77699, Cell Signalling Technology) and VEGFR2 (#9698, Cell Signalling Technology), overnight at 4°C, followed by incubation with secondary antibodies for 1 hour at 37°C. Nuclei were stained with Hoechst (Thermo Fisher, 33342) for 5 minutes. The cells were then washed three times and viewed using a fluorescence microscope (Leica, Germany).
2.8. Tube formation assay
Tube formation assay was performed according to the manufacturer's protocol. Matrigel (50 µL) (Corning, New York, USA, #356231#) was added to each well of a 96‐well plate and allowed to polymerize. GFP‐transduced MSCs (1 × 104 cells) were suspended in culture medium as mentioned above containing 2% FBS and plated on Matrigel. After culture for 2‐12 hours, images were taken using a fluorescence microscope (Leica,Germany). The tube formation was quantified by analysing the total tube length in each well with Image‐Pro Plus (MediaCybernetics, USA).
2.9. Cell viability assay
For in vitro cell viability assay, GDF11‐overexpressed and negative control—MSCs were plated on collagen‐coated 96‐well plates (2 × 103 cells/well) and cultured in serum‐free medium under hypoxia (0.1% O2, 5% CO2) at 37°C for 48 hours. Then, 10 μL of Cell Counting Kit‐8 (CCK‐8, Dojindo, Japan) was added and incubated for 2 hours at 37°C, and the absorbance was determined at a wavelength of 450 nm. MSC viability was evaluated using OD value as described above.
2.10. Matrigel Plug Assay in vivo
Male C57BL/6 mice (8‐week‐old, weighting 22‐25 g) were used. MSCs transduced with GDF11 vector (MSCsGDF11) or with control vector (MSCsVector) (each 5x106 cells) were suspended in 200 µL PBS, mixed with 200 µL Matrigel (Corning, #354262#) in a 1 mL syringe and subcutaneously injected into inguinal region of mice using 26 gauge needle. After 10 days, Matrigel tissues were isolated and half were dissolved with Cell Recovery Solution (Corning, #354253#) and then analysed by Flow Cytometry, and the rest of plug was fixed with 10% formalin overnight and embedded in paraffin for histological analysis.
2.11. Histological analyses
To examine the capillary and arteriole densities, paraffin sections of Matrigel were stained with following antibodies: rat anti‐mouse CD31 (562939, BD Bioscience), rat anti‐rabbit luciferase (ab185924, Abcam) and rat anti‐rabbit GFP (ab290, Abcam). Alexa Fluor 488 or 550 conjugated antibody (Invitrogen) were used for secondary staining. After being mounted with Hochest mounting medium, the samples were analysed using a fluorescence confocal microscope (Leica). For morphometric analysis, sections were stained with haematoxylin and eosin (H & E). Images were taken under 200/400× magnification.
2.12. Transfection of siRNA
Small interfering RNA (siRNA) specific for GDF11 gene expression (siRNAGDF11) and control scrambled siRNA were synthesized by GenePharma Co., Ltd (Shanghai, China). Cells were seeded at 1x105 per cell into 12‐well plates one day before transfection. Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) (2 μL/mL) was mixed with siRNAs (final 50 nmol/L) in reduced serum medium OptiMEM (Invitrogen) and then added into each well (1ml per well). Medium was changed to DMEM containing 10% FBS 8 hours after transfection and cultured for further analysis. Expression of GDF11 at levels of mRNA and protein was detected by RT‐PCR and Western blot, respectively. For the experiment of differentiation that lasted 14 days, the cells were transfected with siRNA again 7 days after the first transfection to maintain GDF11 being knocked down. VEGF165 was added to the culture 24 hours after either transfection to induce differentiation of MSCs to ECs.
2.13. Cell apoptosis analysis
For in vitro cell apoptosis assay, MSCsGDF11 and MSCsVector were plated on 24‐well plates (2 × 104 cells/well) and cultured in serum‐free medium under hypoxia (0.1% O2, 5% CO2) at 37°C for 48 hours. After 48 hours, cell apoptosis was analysed using a TUNEL Cell Apoptosis Assay Kit (Beyotime,Shanghai, China) according to the manufacturer's instructions. Briefly, cells on plates were washed twice with PBS and fixed with 4% paraformaldehyde for 15 minutes, followed with PBS wash twice. Then, cells were incubated with TUNEL reagent for 1 hour. Nuclei were stained with Hoechst for 5 mins. The cells were then washed three times and viewed using a fluorescence microscope.
2.14. Statistics analysis
Results were expressed as means ± SD (standard deviation). Continuous variables were compared by Student's t test, and multiple comparisons were performed by one‐way ANOVA with a Bonferroni correction. Statistical analyses were performed using Prism 6 (GraphPad Software Inc,San Diego, CA, USA). A value of P < 0.05 was accepted as statistically significant.
3. RESULTS
3.1. Mutual effect of GDF11 and VEGF during MSC differentiation
First, we examined gene expression in MSCs under conditions promoting endothelial differentiation. Expression of GDF11 along with other EC markers CD31, VEGFA, VWF, VEGFR2 and PDGFR was significantly increased after MSCs were cultured with VEGF165 for 14 days, while GDF8 and TGF‐β were not changed (Figure 1A,B). On the other hand, when GDF11 was overexpressed in MSCs by transduction with lentiviral vector (MSCGDF11), significantly more VEGF was secreted into supernatant as compared with control MSCVector (Figure 1C), while no significant change in mRNA level was observed (Figure S3A‐B). After MSCGDF11 were induced for endothelial differentiation with VEGF165 for either 7‐ or 14‐days (Figure S4A), higher expression of EC markers was detected as compared with control MSCVector (Figure 1D‐F). These phenomena were further confirmed by immunofluorescent staining (Figure 2A,B and Figure S4B,C) and flow cytometry (Figure 2C,D). More CD31+ and VEGFR2+ cells were observed in MSCGDF11 than MSCVector. Furthermore, such differentiated MSCGDF11 demonstrated higher capability to form tube‐like structures in Matrigel assay than MSCVector (Figure 2E,F). These data indicated that GDF11 was up‐regulated during endothelial differentiation of MSCs and, in turn, that GDF11 enhanced MSC differentiation into endothelial‐like cells.
FIGURE 1.
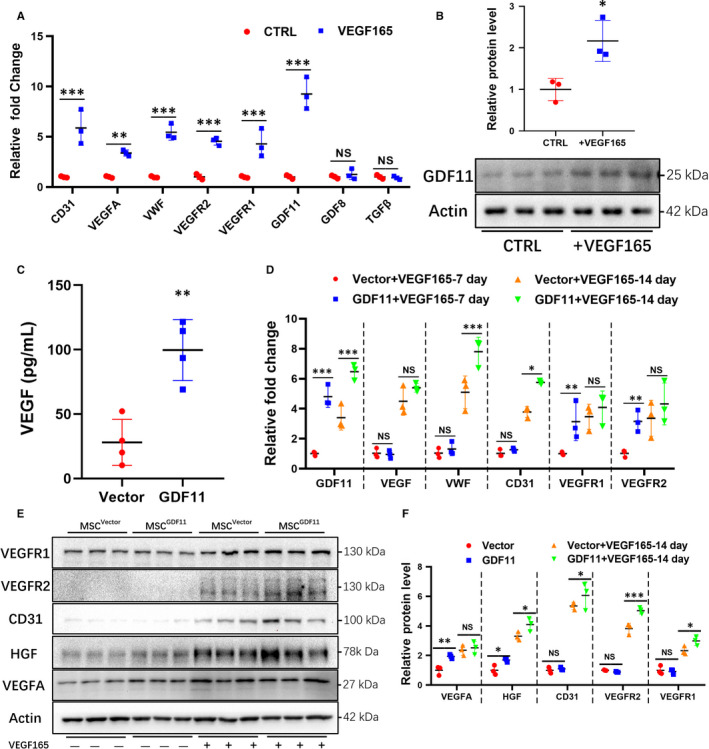
Role of GDF11 in the differentiation of MSCs into endothelial‐like cells. MSCs were cultured in the absence (Control) or presence of VEGF165 (50 ng/mL) for 14 d to induce endothelial differentiation. A, mRNA levels of various genes in MSCs were analysed by RT‐PCR (n = 3). Data are expressed as fold change relative to control. B, GDF11 protein was analysed by Western blot (n = 3). C, Detection of VEGF in the supernatants of MSCVector (Vector)or MSCGDF11(GDF11) after 1‐day culture (n = 4). D, mRNA levels in MSCVector or MSCGDF11 after the cells were cultured for 7‐ or 14 d with or without VEGF165 (n = 3). E, Proteins in MSCVector(Vector) or MSCGDF11(GDF11) were analysed by Western blot before and after culture with VEGF165 for 14 d (n = 3). Molecular weights are indicated next to blots. F, Quantification of proteins in E. Data are presented as the mean ± SD for at least 3 independent experiments and were analysed. *P < 0.05; **P < 0.01 and ***P < 0.001. HGF, hepatocyte growth factor; VEGF, vascular endothelial growth factor; PECAM‐1/CD31, Platelet endothelial cell adhesion molecule‐1; VEGFR1 and VEGFR2, VEGF receptor 1 or 2, respectively
FIGURE 2.
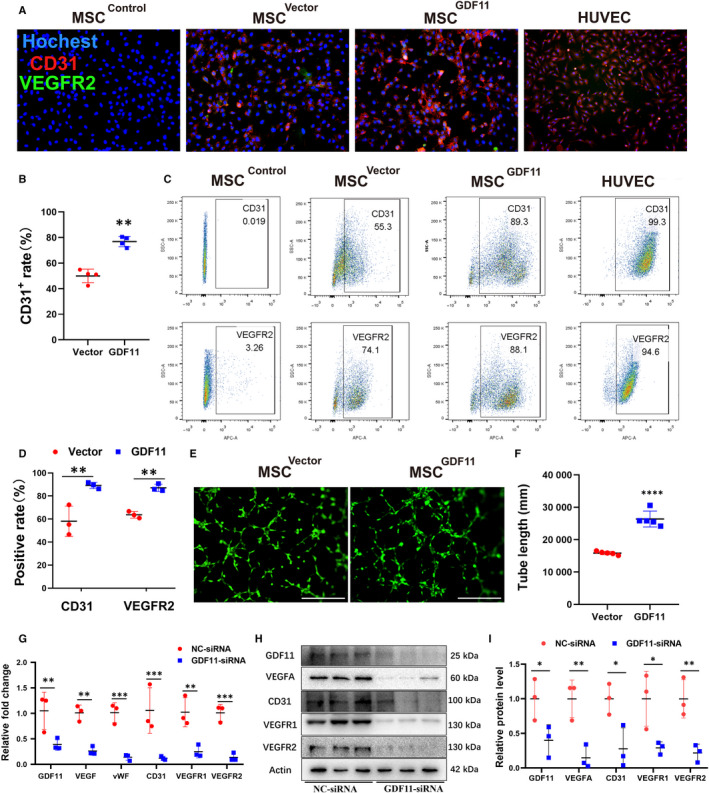
The effect of GDF11 in differentiation of MSCs induced with VEGF165. A, Immunofluorescent staining of MSCVector (2nd raw), MSCGDF11 (3rd raw) with Abs against EC marker CD31 (red) and VEGFR2 (green). Nucleus was stained with DAPI (blue). MSCs were cultured at the presence of VEGF165 for 14 d to induce differentiation. Scale bars = 100 μm. HUVEC was used as positive control (3rd raw), MSCcontrol (1st raw) were cultured without VEGF165, and used as a negative control. B, Quantification of CD31+ cells in MSCVector and MSCGDF11 shown in A. C, Flow cytometry analysis of EC markers (CD31 in upper panel, VEGFR2 in lower panel) on MSCGDF11 and MSCVector. And HUVEC was used as positive control, MSCcontrol without VEGF induction were used as negative control. D, Quantification of positive rates in C (n = 3). E, Representative images showing tube formation of MSCs after differentiation for 14 d on Matrigel for 2 or 8 h. Scale bars: 200 μm. F, Quantification of tube formation in E by measuring branch lengths of formed tube for 8 h. Only length > 200 μm was counted (n = 5 in each group). Data are presented as the mean ± SD for at least 3 independent experiments. G, mRNA levels were examined by RT‐PCR after cultured at the presence of VEGF165 for 14 days to induce differentiation for MSCs either transfected with NC‐siRNA (control) or GDF11‐siRNA (n = 3). H) Western blot assay for detection of proteins: GDF11, VEGFA, CD31, VEGFR1 and VEGFR2 after siRNA transfection and cultured with VEGF165 for 14 days. I, Quantitative analysis of H. *P < 0.05; **P < 0.01 and ***P < 0.001
3.2. Lower expression of GDF11 in MSCs reduces their differentiation into endothelial‐like cells
To confirm the effect of GDF11 on MSCs, siRNA specific for GDF11 gene was transfected into MSCs to knock down GDF11 expression. GDF11 at both mRNA (Figure 2G) and protein levels (Figure 2H,I) in the GDF11‐siRNA‐treated MSCs was lower than that in the NC‐siRNA‐treated control. After MSCs were induced with VEGF165 for endothelial differentiation for 14‐days, lower expression of EC markers was detected in the GDF11‐siRNA‐transfected MSCs as compared with the NC‐siRNA control (Figure 2G‐I; and Figure S5). Along with the down‐regulation of GDF11, the mRNA expressions of genes for VEGFA, vWF, CD31, VEGFR1 and VEGFR2 appeared to be reduced (Figure 2G). The respective proteins in GDF11‐siRNA group were also significantly decreased (Figure 2H,I). These results were further confirmed by flow cytometry analysis (Figure S5A,C) and immunofluorescent staining (Figure S5B,D).
3.3. GDF11 enhances survival rate of MSCs and protects MSCs from hypoxia‐induced apoptosis in vitro
To mimic the ischaemic environment in vivo, MSCs were exposed in hypoxic environment for 48 hours to induce apoptosis in vitro. Higher viability of MSCGDF11 was observed as compared with control MSCVector by using CCK‐8 assay (Figure 3A). Less apoptotic cells in MSCGDF11 than in control MSCVector were detected by flow cytometry (Figure 3B,C) and TUNEL assay (Figure 3D,E) after treatment with hypoxia, which was confirmed with Western Blot analysis of apoptosis‐related proteins (Figure 3F,G).
FIGURE 3.
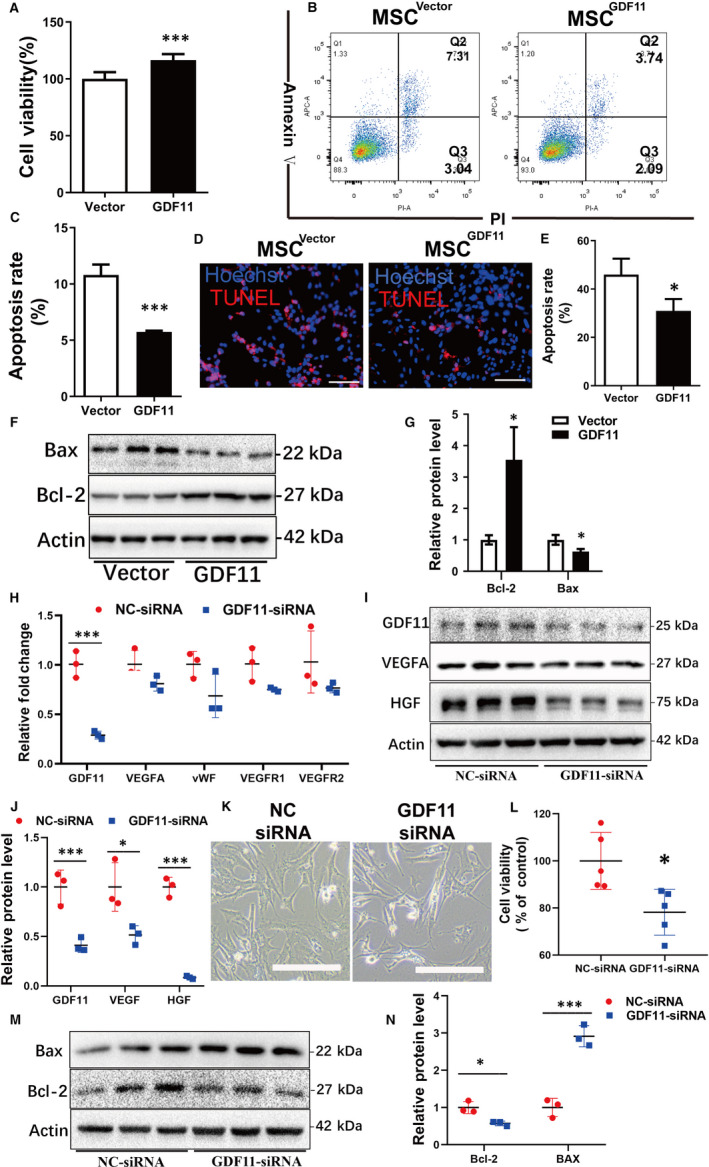
Effects of GDF11 in viability of MSCs in vitro. MSCs were cultured under serum deprivation and hypoxic conditions (0.1% O2) for 48 h to induce apoptosis in vitro. A, Viability of MSCs was examined by cell counting kit‐8 (CCK‐8) assay (n = 5). B, Apoptotic cells were detected with flow cytometry assay after staining with Annexin V & PI. C, Quantifications of apoptotic cells by adding Q2 and Q3 in B. D, TUNEL assay of apoptotic MSCs (pink). Nuclear were stained with Hoechst (blue). E, Quantifications of apoptotic MSCs in D (n = 3 in each group). F, Apoptosis‐related proteins B‐cell lymphoma‐2 protein (BCL‐2) and Bcl2 Associated X Protein (BAX) were detected by Western blot. G, Quantifications of BCL‐2 and BAX related to β‐Actin (n = 3). H, mRNA levels were examined by RT‐PCR for MSCs either transfected with NC‐siRNA (control) or GDF11‐siRNA (n = 3). I, Western blot assay for detection of proteins: GDF11, VEGFA and HGF after siRNA transfection. J, Quantitative analysis of I. K, Representative images of the cell morphology after transfected with specified siRNA for 24 h. Scale bars: 200 μm. L, Viability of MSCs was examined by CCK‐8 assay after knock‐down of GDF11 by siRNA (n = 5). M, Western blot assay for detection of apoptosis‐related proteins BCL‐2 and BAX after siRNA transfection. N, Quantitative analysis of M. Data are presented as mean ± SD for at least 3 independent experiments. *P < 0.05; **P < 0.01 and ***P < 0.001
Likewise, knock‐down of GDF11 expression in MSCs with siRNA reversed the effects of GDF. The mRNA levels of VEGFA, vWF, VEGFR1 genes appeared to be lower in trend in the GDF11‐siRNA‐treated MSCs in comparison with the NC‐siRNA transfected control (Figure 3H). VEGFA and HGF proteins were also significantly decreased in the GDF11‐siRNA‐treated MSCs (Figure 3I,J). The cell morphology became unhealthier as compared with NC‐siRNA transfected cells: more bright detaching cells were observed after the down‐regulation of GDF11 and exposed to hypoxic condition (Figure 3K). Cell viability was also lowered in GDF11‐siRNA transfected MSCs than the control (Figure 3L) The effect of GDF11 on apoptosis‐related proteins Bax and Bcl‐2 was also reversed by the siRNA (Figure 3M,N).
3.4. GDF11 facilitated MSC‐mediate angiogenesis in vivo
In order to verify the pro‐angiogenic activity of GDF11 in vivo, Matrigel plugs containing MSCVector or MSCGDF11 were implanted into mice (Figure 4A). The recovered Matrigel plugs containing MSCGDF11 appeared more reddish indicating more vessel formation in the plug allowing more red blood cells come in as compared with those treated with MSCVector (Figure 4B and Figure S6A). Indeed, more blood vessels were observed in sections of MSCsGDF11 plugs as compared to those from MSCVector controls (Figure 4C,D). In addition, more CD31+ endothelial‐like cells were detected by flow cytometry from MSCGDF11‐plugs (21.40 ± 2.059%) as compared to than that of MSCVector controls (7.478 ± 4.323%, n = 5) (Figure 4E,F). Capillary density as detected by immunostaining for CD31 was also significantly higher in plugs with MSCsGDF11 as compared with MSCVector controls (Figure 4G,H).
FIGURE 4.
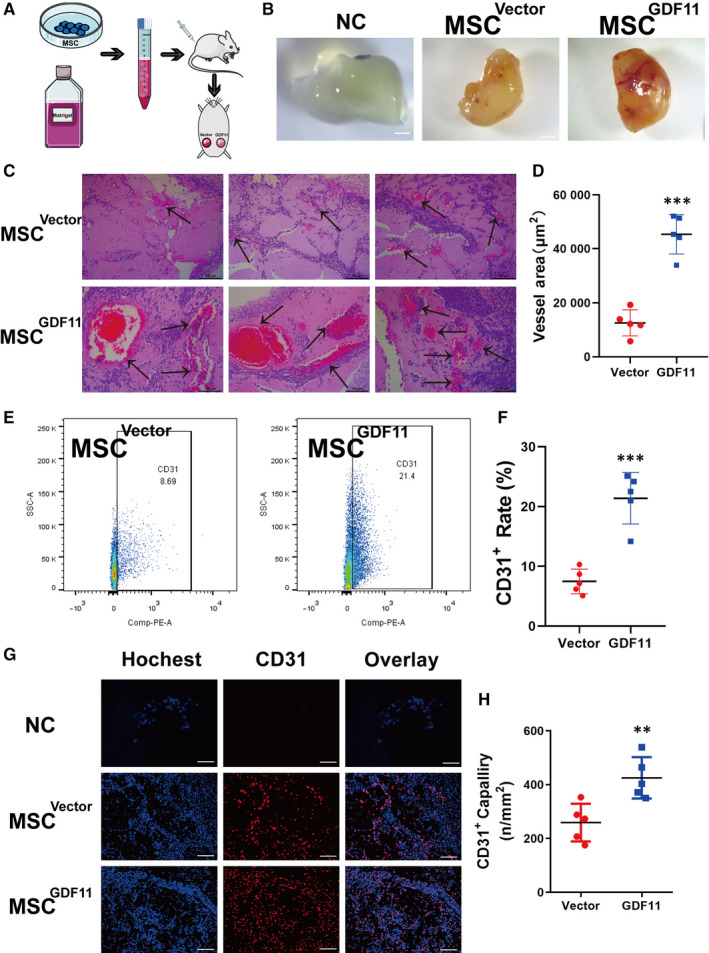
MSCGDF11 promoted angiogenesis in Matrigel plugs in vivo. A, Scheme of angiogenesis assay in vivo using Matrigel plugs. The Matrigel plugs mixed with MSCsVector or MSCsGDF11 were implanted subcutaneously into the left or right side, respectively, of inguinal region of mice for 10 d. B, Photographs of the recovered Matrigel plugs showing gross appearance of angiogenesis (n = 5). NC: PBS with no cells as Negative control, Vector: MSCsVector, GDF11: MSCsGDF11, at 10x magnification. Scale bars: 2 mm. C, Representative images of H & E staining of plug sections. Arrows indicate vessels. Scale bars: 100 μm. D, Quantification of vessel density by measuring the areas. E, Representative pictures of flow cytometry analysis of CD31+ endothelial‐like cells in the recovered Matrigel plugs. F, Quantification of CD31+ endothelial‐like cells in E. G, Representative images of sections of recovered Matrigel plugs immune stained with antibodies against CD31 (red). Nuclei were stained with DAPI (blue). Scale bars: 100μm. H, Quantification of CD31+ EC density in e. Data are presented as the mean ± SD for at 2 independent experiments and were analysed. *P < 0.05, **P < 0.01, ***P < 0.001
3.5. GDF11 promoted MSC differentiation into endothelial‐like cells in vivo
To track the fate of implanted MSCs in the Matrigel plug, both MSCsGDF11 and MSCVector were transduced with genes for GFP or luciferase before implantation. Immunofluorescence co‐localization analysis (Figure 5A) was performed to examine the implanted MSCs in the plugs recovered 10 days after implantation. There were more cells that were positive for both GFP and CD31 in plugs containing MSCsGDF11 as compared to those with MSCVector (Figure 5B). This was further confirmed by co‐localization of luciferase+ with CD31+ cells (Figure S6B,C). And we did the double‐staining with antibodies against GDF11 and CD31. More CD31+ cells in the MSCGDF11 group were observed (Figure 5C). These results indicate that the presence of GDF11 in MSCsGDF11 augments their ability to differentiate into CD31+ endothelial‐like cells.
FIGURE 5.
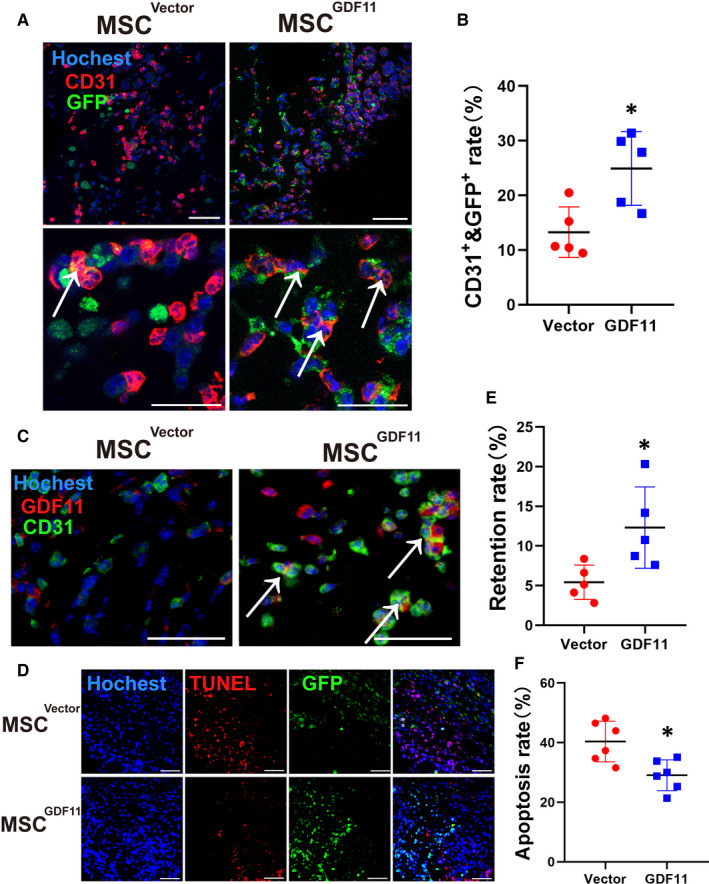
Effect of GDF11 on MSC differentiation, retention and apoptosis in vivo. MSCGDF11 or MSCsVector expressing GFP or luciferase were mixed with Matrigel and implanted into mice. Paraffin sections of the recovered plugs 10 d after implantation were stained with DAPI for nuclei (blue) and specified antibodies (n = 5). A, Antibodies against GFP (green) and CD31 (red) were used for detection of endothelial‐like cells. White arrows point at the MSCs (orange) whose GFP was colocalized with CD31. The pictures in upper panel were taken at 400× magnification and the lower panel are magnified 2×. Scale bars: 50 μm. B, Rate of MSC differentiation into endothelial‐like cells was quantified by dividing number of double positive cells by number of total retained MSCs in an image. C, Antibodies against GDF11 (red) and CD31 (green) for endothelial‐like cells were used. White arrows point at the double positive cells (orange). The pictures were taken at 600× magnification. Scale bars: 50 μm. D, Sections were stained for TUNEL to identify apoptotic cells (red) and retained MSCs (GFP). Scale bars: 100 μm. E, Retention rates were quantified as the number of GFP+ cells out of the total number of cells. F, Apoptosis rate was quantified by the percentage of cells positive for TUNEL staining. Data are presented as the mean ± SD for at 2 independent experiments and were analysed. *P < 0.05; **P < 0.01 and ***P < 0.001
Furthermore, there were more MSCsGDF11 than control MSCsVector in the plugs recovered 10 days after implantation (Figure 5D). The retention rate was higher for MSCsGDF11 than MSCsVector (Figure 5E) concomitant with less apoptosis (Figure 5F). The enhanced survival rate of MSCsGDF11 versus MSCsVector was further confirmed in vitro when MSCs were placed in a hypoxic environment for 48 hours to induce apoptosis (Figure S7A); in addition, fewer TUNEL+ cells were observed in the MSCsGDF11 as compared to MSCsVector (Figure S7B).
3.6. Effects of GDF11 involve TGF‐β receptor‐mediated ERK/EIF4E signalling pathway
To determine the molecular mechanism underlying in the effects of GDF11 on MSCs, we examined ERK1/2 signalling pathway, which was reported to be involved in the differentiation process of progenitor cells. 15 When GDF11 was overexpressed in MSCs, phosphorylation of both ERK and EIF4E was significantly increased (Figure 6A,B). Similarly, when MSCs were cultured under differentiation conditions with VEGF165, both ERK and EIF4E were activated; overexpression of GDF11 further enhanced their phosphorylation. In the meantime, siRNA‐mediated knock‐down of GDF11 in MSCs led to a substantial decrease in phosphorylation of both ERK and EIF4E (Figure 6C,D). To explore whether ERK and EIF4E were essential for GDF11‐induced endothelial‐like cells differentiation, LY2109761, an inhibitor of TGF‐β receptor or SD5978, an inhibitor of ERK1/2 was added to the culture in course of differentiation. Indeed, phosphorylation of both ERK and EIF4E was blocked by either inhibitor (Figure 6E,F). Furthermore, treatment with the inhibitors also blocked the differentiation of MSCs into endothelial‐like cells. GDF11‐induced expressions of EC markers CD31 and VEGFR2 were decreased to the similar level as the control when ERK inhibitor was added into the differentiation medium detected by flow cytometry (Figure 7A,B) and immunofluorescent staining (Figure 7C,D). These were confirmed by Western blot analysis (Figure 7E). The production of VEGF and HGF protein, along with the expression of EC markers CD31 and VEGFR2, was all down‐regulated to the similar level as the control (Figure 7F). Meanwhile, the effect of GDF11 on apoptosis‐related proteins cleaved caspase‐3, Bax and Bcl‐2 was also blocked by the inhibitors, indicating that the function of GDF11 on enhancing resistant of MSCs to apoptosis is also associated with phosphorylation of ERK. As shown in the schematic diagram, these results demonstrate that the TGFβ‐R/ERK/EIF4E signalling pathway plays an important role in GDF11‐induced differentiation of MSCs into ECs. These results demonstrate that the TGFβ‐R/ERK/EIF4E signalling pathway plays an important role in GDF11‐induced differentiation of MSCs into endothelial‐like cells (Figure 7G).
FIGURE 6.
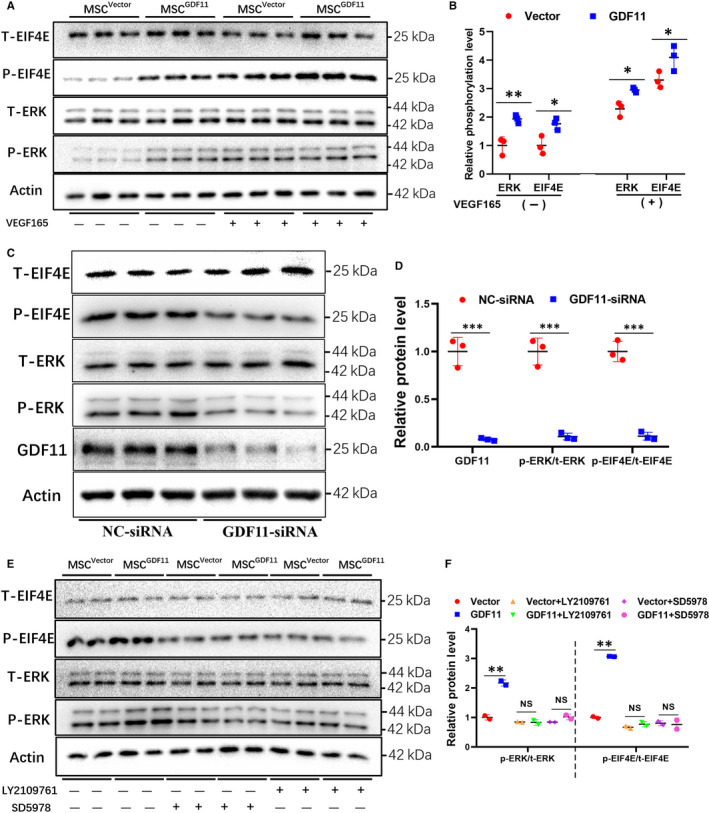
Analysis of the molecular pathways underlying GDF11‐induced MSC differentiation. A, MSCsVector and MSCGDF11 were cultured in the presence or absence of VEGF165 for 14days. Total (T) and phosphorylated (P) ERK and EIF4E were examined by Western blot (n = 3). B, Quantitative analysis of A. The density of each band was calibrated with its corresponding Actin band. Relative phosphorylation levels were quantified by dividing P over T, which was then compared with the level in control MSCsVector to set as 1. C, MSCs were transfected with control (NC)‐ or GDF11‐specific siRNA. Total (T) and phosphorylated (P) ERK and EIF4E were examined by Western blot (n = 3). D, Quantitative analysis of C in the same way as B. E, MSCsVector and MSCGDF11 were cultured with VEGF165 for EC differentiation in the presence or absence of inhibitors for TGF‐β receptor (LY2109761) or for ERK (SD5978) for 14days. T‐ and P‐ ERK and EIF4E were examined by Western blot. F, Quantitative analysis of E. Data are presented as the mean ± SD. *P < 0.05; **P < 0.01 and ***P < 0.001. Each WB was repeated for at least 2 times
FIGURE 7.
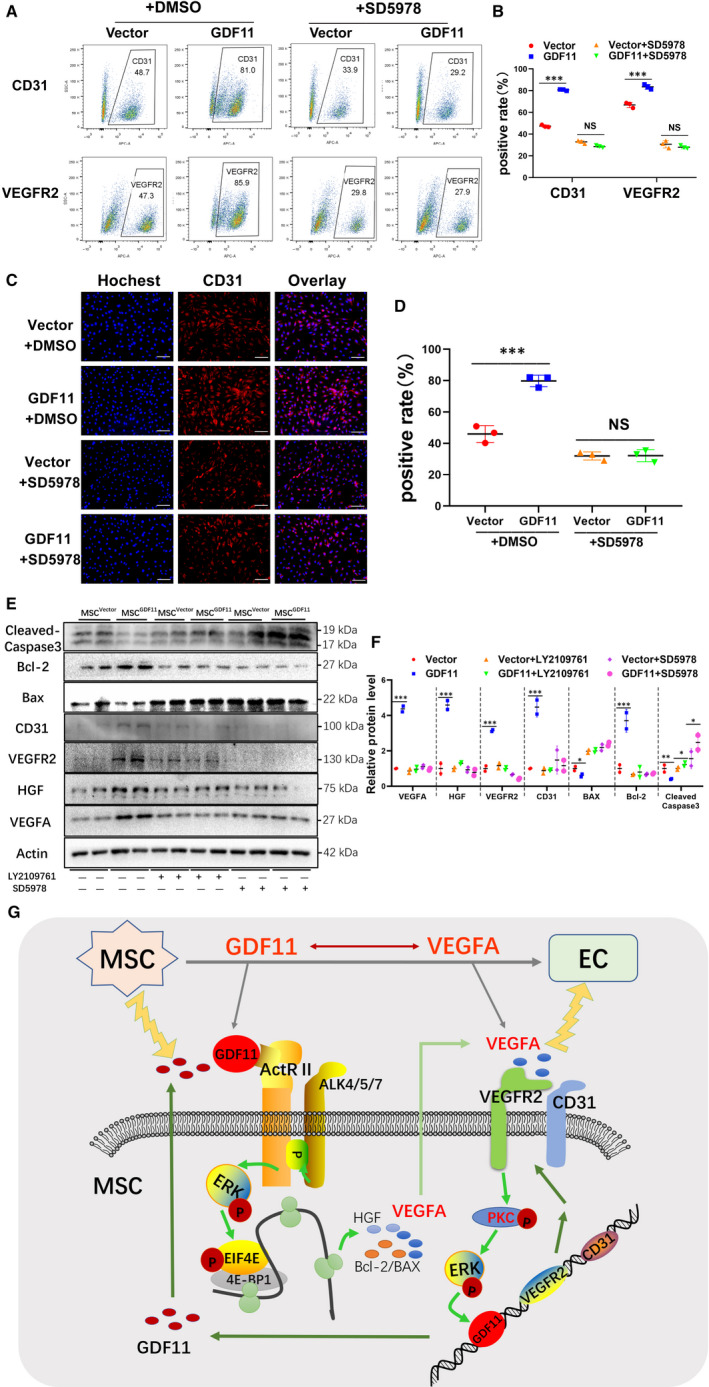
Effect of GDF11 on the differentiation of MSCs into endothelial‐like cells was blocked by ERK inhibitor. MSCVector and MSCGDF11 were treated with inhibitors for ERK (SD5978) or for TGF‐β receptor (LY2109761) or with DMSO as a control during the differentiation of MSCs into endothelial‐like cells for 14 d. A, Representative results of flow cytometry analysis of MSCVector (Vector) and MSCGDF11 (GDF11) for their expression of CD31 and VEGFR2. B, Quantitative analysis of A (n = 3). C, Immunofluorescent staining of MSCVector and MSCGDF11 with antibody against CD31 (red) after culture with or without ERK inhibitor. Nucleus was stained with Hochest. Scale bars = 100 μm. D, Quantitative analysis of C (n = 3). CD31+ rate was obtained by dividing CD31+ cells (red) by total number of cells (blue) in a picture. E, Western blot analysis of proteins in MSCVector and MSCGDF11 in the presence of absence of inhibitors LY2109761 or SD5978during the differentiation of MSCs into endothelial‐like cells. F, Quantitative analysis of E (n = 2). Each WB was repeated for at least 2 times. G, Schematic diagram of the proposed mechanisms by which GDF11 promotes MSC differentiation into endothelial‐like cells. GDF11 interacts with TGF‐β receptor, resulting in activation of the ERK/EIF4E pathway, which enhances the expression of proteins for angiogenesis and anti‐apoptosis, and augment of EC markers during cultured with VEGFA. Data are presented as the mean ± SD. *P < 0.05; **P < 0.01 and ***P < 0.001. BCL‐2 for B‐cell lymphoma‐2 protein; BAX for Bcl2 Associated X Protein
4. DISCUSSION
In this study, we showed that GDF11 and VEGF had mutual effects on differentiation of MSCs into endothelial‐like cells. GDF11 can stimulate the expression of VEGF and vice versa. Both of these molecules promoted MSC differentiation into endothelial‐like cells. Either by overexpressing it in the target cells or providing the protein directly, GDF11 was shown to boost MSC differentiation by enhancing the expression of endothelial‐related markers CD31, VEGFR2 and increasing the viability and retention rate of MSCs following implantation. MSCs overexpressing GDF11 had an enhanced ability of tube formation in vitro and promoted more vascularization in vivo. All these results suggest that GDF11 plays a critical role in differentiation and function of MSCs.
It has been almost two decades since BM‐MSCs were first used to promote angiogenesis for ischaemic diseases. 32 BM‐MSCs were shown to be able to differentiate into ECs both in vitro 16 and in vivo. 33 However, the differentiation rate is low and newly formed capillaries were unstable. 34 There are several factors influencing differentiation of stem cells into ECs. For example, endothelial growth supplements, 35 , 36 shear forces 37 , 38 and composition of extracellular matrix 39 are important factors in EC differentiation. Some members in the TGF‐β family have been reported to promote endothelial differentiation. Bone morphogenetic protein 4 (BMP4) accelerated the commitment of human embryonic stem cells to the endothelial lineage. 40 Treatment of iPSCs with TGF‐β2 can induce EC marker expression and in vitro tube formation. 41 GDF11, as a member of TGF‐β family, has a similar function as other family members, such as TGF‐β1, to promote angiogenesis. 42 , 43 GDF11 was reported to promote migration and sprouting of endothelial progenitor cells 44 and other angiogenic activities. 45
Here, we show for the first time that GDF11 has direct effect on MSC differentiation into endothelial‐like cells. Great efforts have made to improve the efficiency of MSC differentiation 33 , 36 and to augment the therapeutic efficacy of stem cells. 46 , 47 We demonstrated that MSCGDF11 had a greater ability to differentiate into endothelial‐like cells, as evidenced by significantly higher expression of EC‐related markers at both mRNA and protein levels (Figures 1D,F and 2). The in vitro results were confirmed in vivo by implantation of MSC‐containing Matrigel plugs into mice (Figure 4A). When the Matrigel plugs with the MSCs are implanted into the subcutaneous skin of mice, either the implanted MSCs or recruited endothelial cells will form a functional vascular structure which allows blood cells in circulation come into the Matrigel plug. More angiogenesis in the Matrigel, more blood flows in, which turns the Matrigel more reddish. Our Matrigel results verified the role of GDF11 in improving the differentiation efficiency of MSCs (Figures 4 and 5 and Figure S6). Better differentiation efficiency of implanted stem cells could greatly improve the efficacy of stem cell therapy.
However, it is worthy to point out that some of TGF‐β family members may not have similar effect on MSCs. It has been reported that some of TGF‐β family promoted MSCs towards fibrosis, endothelial‐to‐mesenchymal transition (EndMT) 48 , 49 and osteoblastogenesis. 50 As a fact, the role of GDF11 in ageing, cardiovascular diseases and function of ECs is still not fully understood, or sometimes controversial. 6 , 7 , 50 , 51 The cause of contradictory may be due to the difference in materials and methods used, objects of study and genetic background etc. 52 , 53 In our case, further study is needed to confirm that the differentiated EC‐like cells have real function in pro‐angiogenesis in an ischaemic disease model, and the newly formed vessels are stable in long term.
Multiple intracellular signalling pathways have been implicated in stem cell differentiation. These signalling pathways include MAP kinase signalling pathways (such as p38, ERK and JNK), 15 Rho‐like GTPase signalling pathway 46 and phosphatidylinositol‐3‐kinase/AKT signalling pathway. 38 However, the detailed mechanisms for GDF11‐induced differentiation are still not well defined. For the function of GDF11, most studies have shown that GDF11 can activate the canonical Smad2/3 signalling pathway in different tissues or cells through binding with TGF‐β family receptor ActR II/I, leading to the formation of a heteromeric complex of phosphorylated Smad2/3with with Smad4 and then translocation to the nucleus to regulate gene expression. However, we did not observe conclusive Smad2/3 activation in MSCs by GDF11 during endothelial differentiation (Figure S8). This agrees with the previous report that roles of Smad2/3 signalling are stage‐dependent during endothelial differentiation of MSCs induced by TGF‐β1 or BMPs. 54 , 55
Indeed, the downstream pathways of TGF‐β receptors could be either Smad‐dependent or Smad‐independent. 13 , 14 It has been reported that GDF11 activates p38 MAPK to regulate the size and function of the nucleolus, affects JNK in ECs, as well as cross talking with AMPK, eNOS and NF‐κB. 8 On the other hand, we found greater ERK phosphorylation when MSCGDF11 were induced for endothelial differentiation by VEGF165, as compared with the control group (Figure 6). In addition, we also detected more phosphorylated EIF4E, the downstream target of ERK pathway. These activations can be blocked by both inhibitors for TGF‐β receptor and ERK (Figure 6E). It is known that EIF4E is an important component for initiation of translation and its phosphorylation activates the translation of cellular proteins. 56 When EIF4E was knocked down by siRNA, the translation of its downstream targets including c‐Myc, VEGF, CyclinD1 and Bcl‐2 were diminished. 57 Our data show that activation of the ERK/EIF4E pathway was also inhibited when GDF11 was knocked down. The anti‐apoptotic effect of GDF11 on MSCs was blocked by inhibition of TGFβ‐R or ERK (Figure 7E). Together, these results suggest that the TGFβ‐R/ERK/EIF4E pathway is crucial for the actions of GDF11 in this system.
It is well established that most of growth factors usually regulate their effect via activation of the TGFβ‐RAS‐RAF‐MEK1/2‐ERK1/2 signalling cascade. 58 We would infer that GDF11 binds to the TGF‐β receptor and subsequent activates RAS‐RAF‐MEK‐ERK/EIF4E pathway to induce the endothelial differentiation of MSC. Our results are consistent with previous reports showing that the ERK/EIF4E pathway is involved in cell growth, regulating cell cycle and apoptosis. 59
Furthermore, we also showed that GDF11 and VEGF had mutual effects on the expression and differentiation of MSCs into endothelial‐like cells. VEGF binding with its VEGFR receptors can also activate multiple downstream pathways, like PKC‐ERK, which will enhance the expression of genes, for example GDF11, for cell differentiation and proliferation (Figure 7G). However, the specific molecular mechanism of the interaction between GDF11 and VEGFA needs further study to be reviewed.
In summary, we found that GDF11 can significantly enhance the potential of MSCs for endothelial differentiation, increase their viability, and augment the therapeutic efficacy of MSCs to promote angiogenesis. These actions of GDF11 may involve its binding to the TGF‐β receptor and subsequent activation of ERK/EIF4E pathway. This novel function of GDF11 in MSCs could be useful for stem cell therapy for ischaemic cardiovascular diseases.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTION
Chi Zhang: Data curation (lead); Formal analysis (lead); Investigation (lead); Project administration (lead). Yinuo Lin: Conceptualization (lead); Data curation (lead). Qi Liu: Data curation (equal); Methodology (equal). Junhua He: Formal analysis (equal); Software (equal). Pingping Xiang: Investigation (equal). Dianliang Wang: Resources (supporting); Writing‐review & editing (supporting). Xinyang Hu: Resources (equal); Validation (equal). Jinghai Chen: Resources (equal); Visualization (equal); Writing‐original draft (equal). Wei Zhu: Project administration (equal); Writing‐review & editing (equal). Hong Yu: Project administration (lead); Software (equal); Supervision (lead); Writing‐original draft (equal); Writing‐review & editing (lead).
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China Grant (No. 81570251 to HY), National Basic Research Program of China (973 program, number 2014CB965100 to JW and HY) and Wenzhou Municipal Science and Technology Bureau Foundation (Y20190163).
Zhang C, Lin Y, Liu Q, et al. Growth differentiation factor 11 promotes differentiation of MSCs into endothelial‐like cells for angiogenesis. J Cell Mol Med. 2020;24:8703–8717. 10.1111/jcmm.15502
Chi Zhang and Yinuo Lin contributed equally to this article
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Chien KR, Frisen J, Fritsche‐Danielson R, Melton DA, Murry CE, Weissman IL. Regenerating the field of cardiovascular cell therapy. Nat Biotechnol. 2019;37(3):232‐237. [DOI] [PubMed] [Google Scholar]
- 2. Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hare JM, Traverse JH, Henry TD, et al. A randomized, double‐blind, placebo‐controlled, dose‐escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277‐2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143‐147. [DOI] [PubMed] [Google Scholar]
- 6. Loffredo FS, Steinhauser ML, Jay SM, et al. Growth differentiation factor 11 is a circulating factor that reverses age‐related cardiac hypertrophy. Cell. 2013;153(4):828‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harper SC, Brack A, MacDonnell S, et al. Is growth differentiation factor 11 a realistic therapeutic for aging‐dependent muscle defects? Circ Res. 2016;118(7):1143‐1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walker RG, Poggioli T, Katsimpardi L, et al. Biochemistry and biology of GDF11 and myostatin: similarities, differences, and questions for future investigation. Circ Res. 2016;118(7):1125‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296(6):C1258‐C1270. [DOI] [PubMed] [Google Scholar]
- 10. Rochette L, Zeller M, Cottin Y, Vergely C. Growth and differentiation factor 11 (GDF11): functions in the regulation of erythropoiesis and cardiac regeneration. Pharmacol Ther. 2015;156:26‐33. [DOI] [PubMed] [Google Scholar]
- 11. Camici GG, Savarese G, Akhmedov A, Lüscher TF. Molecular mechanism of endothelial and vascular aging: implications for cardiovascular disease. Eur Heart J. 2015;36(48):3392‐3403. [DOI] [PubMed] [Google Scholar]
- 12. Brun Caroline E, Rudnicki MA. GDF11 and the mythical fountain of youth. Cell Metab. 2015;22(1):54‐56. [DOI] [PubMed] [Google Scholar]
- 13. Zhang YE. Non‐Smad pathways in TGF‐beta signaling. Cell Res. 2009;19(1):128‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Demontis F, Patel VK, Swindell WR, Perrimon N. Intertissue control of the nucleolus via a myokine‐dependent longevity pathway. Cell Rep. 2014;7(5):1481‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu J, Liu X, Jiang Y, et al. MAPK/ERK signalling mediates VEGF‐induced bone marrow stem cell differentiation into endothelial cell. J Cell Mol Med. 2008;12(6A):2395‐2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oswald J, Boxberger S, Jørgensen B, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22(3):377‐384. [DOI] [PubMed] [Google Scholar]
- 17. Fernandez‐Alonso R, Martin‐Lopez M, Gonzalez‐Cano L, et al. p73 is required for endothelial cell differentiation, migration and the formation of vascular networks regulating VEGF and TGFbeta signaling. Cell Death Differ. 2015;22(8):1287‐1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581‐611. [DOI] [PubMed] [Google Scholar]
- 19. Chen GJ, Forough R. Fibroblast growth factors, fibroblast growth factor receptors, diseases, and drugs. Recent Pat Cardiovasc Drug Discov. 2006;1(2):211‐224. [DOI] [PubMed] [Google Scholar]
- 20. Pardali K, Moustakas A. Actions of TGF‐β as tumor suppressor and pro‐metastatic factor in human cancer. Biochim Biophys Acta Rev Cancer. 2007;1775(1):21‐62. [DOI] [PubMed] [Google Scholar]
- 21. Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast‐mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10(1):15‐26. [DOI] [PubMed] [Google Scholar]
- 22. Derynck R. TGF‐β‐receptor‐mediated signaling. Trends Biochem Sci. 1994;19(12):548‐553. [DOI] [PubMed] [Google Scholar]
- 23. Guo X, Stice SL, Boyd NL, Chen SY. A novel in vitro model system for smooth muscle differentiation from human embryonic stem cell‐derived mesenchymal cells. Am J Physiol Cell Physiol. 2013;304(4):C289‐C298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsubara Y, Hirasawa T, Egawa S, et al. Anatomical integration of the sacral–hindlimb unit coordinated by GDF11 underlies variation in hindlimb positioning in tetrapods. Nat Ecol Evol. 2017;1(9):1392‐1399. [DOI] [PubMed] [Google Scholar]
- 25. Su HH, Liao JM, Wang YH, et al. Exogenous GDF11 attenuates non‐canonical TGF‐beta signaling to protect the heart from acute myocardial ischemia‐reperfusion injury. Basic Res Cardiol. 2019;114(3):20. [DOI] [PubMed] [Google Scholar]
- 26. Schafer MJ, LeBrasseur NK. The influence of GDF11 on brain fate and function. GeroScience. 2019;41(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang J, Li Y, Li H, et al. GDF11 improves angiogenic function of EPCs in diabetic limb ischemia. Diabetes. 2018;67(10):2084‐2095. [DOI] [PubMed] [Google Scholar]
- 28. Dai Z, Song G, Balakrishnan A, et al. Growth differentiation factor 11 attenuates liver fibrosis via expansion of liver progenitor cells. Gut. 2020;69(6):1104‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Z, Dou M, Liu F, et al. GDF11 induces differentiation and apoptosis and inhibits migration of C17.2 neural stem cells via modulating MAPK signaling pathway. PeerJ. 2018;6:e5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lu Q, Tu M‐L, Li C‐J, et al. GDF11 inhibits bone formation by activating Smad2/3 in bone marrow mesenchymal stem cells. Calcif Tissue Int. 2016;99(5):500‐509. [DOI] [PubMed] [Google Scholar]
- 31. Eslaminejad MB, Nadri S. Murine mesenchymal stem cell isolated and expanded in low and high density culture system: Surface antigen expression and osteogenic culture mineralization. In Vitro Cell Dev Biol Anim. 2009;45(8):451‐459. [DOI] [PubMed] [Google Scholar]
- 32. Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010–2015). Stem Cell Res Ther. 2016;7(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kukumberg M, Yao JY, Neo DJH, Yim EKF. Microlens topography combined with vascular endothelial growth factor induces endothelial differentiation of human mesenchymal stem cells into vasculogenic progenitors. Biomaterials. 2017;131:68‐85. [DOI] [PubMed] [Google Scholar]
- 34. Russo V, Young S, Hamilton A, Amsden BG, Flynn LE. Mesenchymal stem cell delivery strategies to promote cardiac regeneration following ischemic injury. Biomaterials. 2014;35(13):3956‐3974. [DOI] [PubMed] [Google Scholar]
- 35. Wijelath ES, Rahman S, Murray J, Patel Y, Savidge G, Sobel M. Fibronectin promotes VEGF‐induced CD34 cell differentiation into endothelial cells. J Vasc Surg. 2004;39(3):655‐660. [DOI] [PubMed] [Google Scholar]
- 36. Matluobi D, Araghi A, Maragheh BFA, et al. Carvacrol promotes angiogenic paracrine potential and endothelial differentiation of human mesenchymal stem cells at low concentrations. Microvasc Res. 2018;115:20‐27. [DOI] [PubMed] [Google Scholar]
- 37. Hasanzadeh E, Amoabediny G, Haghighipour N, et al. The stability evaluation of mesenchymal stem cells differentiation toward endothelial cells by chemical and mechanical stimulation. In Vitro Cell Dev Biol Anim. 2017;53(9):818‐826. [DOI] [PubMed] [Google Scholar]
- 38. Zeng L, Xiao Q, Margariti A, et al. HDAC3 is crucial in shear‐ and VEGF‐induced stem cell differentiation toward endothelial cells. J Cell Biol. 2006;174(7):1059‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Janeczek Portalska K, Leferink A, Groen N, et al. Endothelial differentiation of mesenchymal stromal cells. PLoS One. 2012;7(10):e46842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goldman O, Feraud O, Boyer‐Di Ponio J, et al. A boost of BMP4 accelerates the commitment of human embryonic stem cells to the endothelial lineage. Stem Cells. 2009;27(8):1750‐1759. [DOI] [PubMed] [Google Scholar]
- 41. Di Bernardini E, Campagnolo P, Margariti A, et al. Endothelial lineage differentiation from induced pluripotent stem cells is regulated by microRNA‐21 and transforming growth factor β2 (TGF‐β2) pathways. J Biol Chem. 2014;289(6):3383‐3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Madri JA, Pratt BM, Tucker AM. Phenotypic modulation of endothelial cells by transforming growth factor‐β depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988;106(4):1375‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cook BD, Ferrari G, Pintucci G, Mignatti P. TGF‐beta1 induces rearrangement of FLK‐1‐VE‐cadherin‐beta‐catenin complex at the adherens junction through VEGF‐mediated signaling. J Cell Biochem. 2008;105(6):1367‐1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Finkenzeller G, Stark GB, Strassburg S. Growth differentiation factor 11 supports migration and sprouting of endothelial progenitor cells. J Surg Res. 2015;198(1):50‐56. [DOI] [PubMed] [Google Scholar]
- 45. Yu X, Chen X, Zheng XD, et al. Growth differentiation factor 11 promotes abnormal proliferation and angiogenesis of pulmonary artery endothelial cells. Hypertension. 2018;71(4):729‐741. [DOI] [PubMed] [Google Scholar]
- 46. Li C, Zhen G, Chai YU, et al. RhoA determines lineage fate of mesenchymal stem cells by modulating CTGF‐VEGF complex in extracellular matrix. Nat Commun. 2016;7(1):11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang J, Zhang CF, Li QL, Chu CH. Cyclic adenosine monophosphate promotes Odonto/Osteogenic differentiation of stem cells from the apical papilla via suppression of transforming growth factor beta 1 signaling. J Endod. 2019;45(2):150‐155. [DOI] [PubMed] [Google Scholar]
- 48. Cooley BC, Nevado J, Mellad J, et al. TGF‐β signaling mediates Endothelial‐to‐Mesenchymal Transition (EndMT) during vein graft remodeling. Sci Transl Med. 2014;6(227):227ra234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pons M, Koniaris LG, Moe SM, Gutierrez JC, Esquela‐Kerscher A, Zimmers TA. GDF11 induces kidney fibrosis, renal cell epithelial‐to‐mesenchymal transition, and kidney dysfunction and failure. Surgery. 2018;164(2):262‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Suh J, Kim N‐K, Lee S‐H, et al. GDF11 promotes osteogenesis as opposed to MSTN, and follistatin, a MSTN/GDF11 inhibitor, increases muscle mass but weakens bone. Proc Natl Acad Sci U S A. 2020;117(9):4910‐4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shen GS, Zhou HB, Zhang H, et al. The GDF11‐FTO‐PPARgamma axis controls the shift of osteoporotic MSC fate to adipocyte and inhibits bone formation during osteoporosis. Biochim Biophys Acta Mol Basis Dis. 2018;1864(12):3644‐3654. [DOI] [PubMed] [Google Scholar]
- 52. Schafer MJ, Atkinson EJ, Vanderboom PM, et al. Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab. 2016;23(6):1207‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rodgers BD. The immateriality of circulating GDF11. Circ Res. 2016;118(10):1472‐1474. [DOI] [PubMed] [Google Scholar]
- 54. Zhao L, Hantash BM. TGF‐beta1 regulates differentiation of bone marrow mesenchymal stem cells. Vitam Horm. 2011;87:127‐141. [DOI] [PubMed] [Google Scholar]
- 55. Ai WJ, Li J, Lin SM, Li W, Liu CZ, Lv WM. R‐Smad signaling‐mediated VEGF expression coordinately regulates endothelial cell differentiation of rat mesenchymal stem cells. Stem Cells Dev. 2015;24(11):1320‐1331. [DOI] [PubMed] [Google Scholar]
- 56. Uttam S, Wong C, Price TJ, Khoutorsky A. eIF4E‐dependent translational control: a central mechanism for regulation of pain plasticity. Front Genet. 2018;9:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen L, Wang H. eIF4E is a critical regulator of human papillomavirus (HPV)‐immortalized cervical epithelial (H8) cell growth induced by nicotine. Toxicology. 2019;419:1‐10. [DOI] [PubMed] [Google Scholar]
- 58. Lu N, Malemud CJ. Extracellular signal‐regulated kinase: a regulator of cell growth, inflammation, chondrocyte and bone cell receptor‐mediated gene expression. Int J Mol Sci. 2019;20(15):3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Boussemart L, Malka‐Mahieu H, Girault I, et al. eIF4F is a nexus of resistance to anti‐BRAF and anti‐MEK cancer therapies. Nature. 2014;513(7516):105‐109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


