Abstract
The human zona pellucida (ZP) is a highly organized glycoprotein matrix that encircles oocytes and plays an essential role in successful reproduction. Previous studies have reported that mutations in human ZP1, ZP2 and ZP3 influence their functions and result in a lack of ZP or in an abnormal oocytes and empty follicle syndrome, which leads to female infertility. Here, we performed whole‐exome sequencing in two probands with primary infertility whose oocytes lacked a ZP, and we identified a heterozygous mutation in ZP1 (NM_207341:c.326G>A p.Arg109His), which is situated in the N‐terminus, and a heterozygous mutation in ZP3 (NM_001110354:c.400G>A p.Ala134Thr), which is situated in the ZP domain. The effects of the mutations were investigated through structure prediction and in vitro studies in HeLa cells. The results, which were in line with the phenotype, suggested that these mutations might impede the function of cross‐linking and secretion of ZP proteins. Our study showed that the two mutations in ZP1 and ZP3 influenced the formation of the ZP, causing female infertility. Meanwhile, these data highlight the importance of the ZP1 N‐terminus in addition to the conserved domains for ZP1 function and ZP formation. Additionally, the patient with the ZP1 mutation delivered a baby following intracytoplasmic sperm injection (ICSI); thus, we suggest the targeted genetic diagnosis of ZP genes to choose appropriate fertilization methods and improve the success rate of assisted reproductive technology (ART) treatments.
Keywords: ART, female infertility, mutation, zona pellucida
1. INTRODUCTION
The zona pellucida (ZP) is an extracellular glycoprotein matrix that surrounds mammalian oocytes and embryos until the early stage of blastocyst development. ZP proteins have been detected in the oocyte and granulosa cells during the primordial follicle stage, and they increased with follicular development. 1 , 2 The ZP is involved in process critical for reproductive development, such as oogenesis, fertilization and preimplantation embryo development. 3 During mammalian fertilization, the ZP regulates sperm‐oocyte binding, induces the acrosome reaction and engages in sperm‐egg interactions to avoid polyspermy. 4 , 5 Subsequently, the ZP protects the embryo during preimplantation development.
ZP proteins contain several conservative structural features, such as an N‐terminal signal sequence, a ZP domain (ZPD), a C‐terminal propeptide with a consensus furin cleavage site (CFCS) and a transmembrane domain (TMD). ZP1 and ZP4 also contain a trefoil domain. 6 , 7 The ZP of mice contains ZP1, ZP2 and ZP3. 8 Mice lacking ZP3 and ZP2, which act as sperm receptors, produce oocytes without a ZP, and the female mice are completely sterile. 9 , 10 mZp1−/− females have eggs with abnormal ZPs, and the mice are subfertile due to early embryonic loss. 11 The structures of murine and human ZPs are similar; both have long heterodimeric ZP2 and ZP3 filaments that are linked ZP1 homodimers. Another human protein, ZP4, was later discovered as another ZP protein. 12 In clinical ART, morphological assessment of the zona pellucida is an important way to determine the quality of oocytes. ZP dysmorphology can include extracellular abnormalities such as a dark ZP, an irregularly shaped ZP or the absence of a ZP. Therefore, understanding the genetic mechanism behind abnormal zona pellucida formation and maintenance is significant for the success rate in IVF. Recent studies have shown that several mutations in ZP1, ZP2 and ZP3 cause abnormal zona pellucida formation. 13 , 14 , 15 , 16 , 17
In this study, we identified a mutation within ZP3, c.400G>A p.A134T in a sterile female patient, and the mutation was further confirmed in her family characterized by a dominant inheritance pattern; further, we investigated a novel heritable mutation in ZP1 (NM_207341:c.326G>A) that was found in a primary infertile female patient from whom 5 degenerated oocytes and three mature oocytes with no ZP were retrieved in an ICSI attempt. Surprisingly, the eggs of patient with ZP1 mutation fertilized successfully and developed to the blastocyst stage in vitro in the absence of ZP to ensure its integrity. We further investigated the effect of these missense mutations by structure prediction and in vitro studies.
2. MATERIALS AND METHODS
2.1. Human subjects and ethics approval
Infertility patients were recruited from the Reproductive Medicine Center of the Affiliated Obstetrics and Gynecology Hospital of Nanjing Medical University. All blood samples from donors were obtained with informed consent. This study was approved by the Ethics Committee of the Nanjing Medical University (2018/651) updated in October 2018.
2.2. Genomic DNA extraction
Genomic DNA was extracted from peripheral blood with a RelaxGene Blood DNA System (Tiangen, DP319), and a NanoDrop 2000 spectrophotometer (Thermo Scientific) was used to determine the DNA concentration and quality.
2.3. Genetic analysis
Whole‐exome sequencing was used to identify candidate variants. Whole‐exome capture was performed using the Agilent SureSelect Human All Exon V6 (Agilent), and sequencing was carried out on the Illumina NovaSeq 6000 platform (Illumina) by Microanaly Genetech Co., Ltd (Anhui). We selected candidate variants with the following criteria: (a) present in the patient and her father, (b) absent in her mother and (c) had not been reported previously or had under 1% frequency in public databases (such as the 1000 Genomes database, the genome Aggregation Database (gnomAD) and the Exome Aggregation Consortium (ExAC) Browser). Subsequently, the candidate variant was validated by Sanger sequencing, and PCR amplification was performed using 2× Rapid Taq Master Mix (Vazyme, P222). The PCR products were sequenced by GenScript.
2.4. Vector construction
Wild‐type human ZP1, mutant ZP1 (p.Arg109His), wild‐type human ZP2, wild‐type human ZP3 and mutant ZP3 (p.Ala134Thr) were constructed and then recombined with the eukaryotic expression vector pcDNA3.1. An HA‐tag, a FLAG‐tag and a MYC‐tag were fused at both N‐terminus and C‐terminus of ZP1, ZP2 and ZP3, respectively. The vectors were constructed by GenScript.
2.5. Cell culture and transfection
HeLa cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Life Technologies/Gibco, #11995073) supplemented with 10% foetal bovine serum (FBS) (Life Technologies/Gibco, #10270106), 100 U/mL penicillin, and 100 mg/mL streptomycin (Beyotime Biotechnology, C0222), and the cells were kept at 37°C with 5% CO2. Cells were transiently transfected for 6 hours using Lipofectamine 2000 reagent (Invitrogen, #11668019); then, cells were washed twice with PBS and maintained in serum‐free medium for 48 hours before harvesting.
2.6. Western blots
Cell culture supernatants were collected and concentrated with Amicon Ultra0.5 centrifugal filter devices (Millipore, UFC5010BK) according to the manufacturer's protocol. Cell lysates and concentrated supernatants were prepared with RIPA cell lysis buffer (Beyotime Biotechnology, P0013C). Protein concentrations were determined with a BCA Protein Assay (Beyotime Biotechnology, P0012). Sixty micrograms of protein was separated by 10% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis before being transferred to polyvinylidene fluoride membranes (Millipore, IPVH00010). Non‐specific binding sites were blocked for 2 hours at room temperature with 5% non‐fat milk in Tris‐buffered saline containing 0.05% Tween‐20. Membranes were incubated overnight at 4°C with a dilution of the following antibodies: GAPDH (Abclonal, AC002), vinculin (Proteintech, #66305‐1‐lg), MYC (Cell Signaling Technology, #2278), FLAG (SIGMA‐ALDRICH, F7425) and HA (MBL, #561). After incubation with an anti‐immunoglobin horseradish peroxidase‐linked antibody (Invitrogen, #31430 and #31460) for 1 hour, the immune complexes were detected by enhanced chemiluminescence (FDBIO, FD8020). For densitometric analyses, protein bands on the blots were measured by ImageJ software.
2.7. Coimmunoprecipitation (Co‐IP) assays
Cells were harvested and lysed with lysis buffer on ice for 30 minutes. Then, the cells were centrifuged at 138000 g for 30 minutes at 4°C. Protein lysates were incubated with 80 µL of Protein A/G Magnetic Beads (Bimake, B23202) after preclearing the beads. Then, 8 µg of anti‐HA antibody (MBL, M180‐3) or 8 µg anti‐MYC antibody (Abcam, ab32) was added, and mix was subjected to gentle rotation overnight at 4°C. Western blots were performed after the beads were washed six times with lysis buffer.
2.8. ELISA
To detect the levels of secreted ZP1 and ZP3, ELISAs were used according to the manufacturer's instructions. We tested the ZP1 or ZP3 levels using 20 samples of medium from non‐transfected cells as negative samples. After the ZP1/ZP3 levels were calculated, the cut‐off value was calculated as the mean value of the negative samples plus 2 × SD. The specificities of the ELISA kits were determined as true negative rate. The cut‐off values of ZP1 and ZP3 in this study were 37.07 and 30.09 pg/mL. Cells were plated at 1 × 106 per 100 cm2 dish, and cell culture medium was collected and centrifuged. ELISA reagents from Nanjing SenBeiJia Biotech Co., Ltd. (H2263 and H2304) were used to detect secreted ZP1 and ZP3 in supernatants. All assays were performed in duplicate and were repeated three times.
2.9. Homology modelling and structure prediction
Human and chicken ZP1 proteins share high sequence similarities; thus, the crystal structures of ZP1 (PDB: 6GF6) were employed as the template for homology modelling. The structure of the N‐terminal ZP‐N domain of human ZP1 was generated in the Swiss‐Model server using default parameters. 18
2.10. Statistical analyses
Mean comparisons among groups were performed by 1‐way analysis of variance, and a multiple range least significant difference analysis was used for intergroup comparisons. All experiments in this study were conducted in triplicate. Derived values are presented as the means ± SEM. P values < .05 were considered statistically significant. All statistical analyses were performed with SPSS 16.0.
3. RESULTS
3.1. Clinical characterization
The patient (family 1 Ⅱ‐2) had not conceived after 4 years of attempts without contraception, and she was diagnosed with primary infertility at the age of 29. She had regular menstrual cycles and normal sex hormone concentrations (Table 1). In the ICSI treatment attempt, a gonadotropin‐releasing hormone (GnRH) agonist protocol was performed. After an hCG trigger for 36 hours, oocytes were retrieved, and 8 cumulus‐oocyte complexes (COCs) were retrieved. Five oocytes degenerated after the cumulus cells were removed, and the other three oocytes developed to maturity but lacked a ZP. Subsequently, two of the mature oocytes were successfully fertilized and developed to the blastocyst state (Figure 1); however, one egg showed degradation following ICSI.
Table 1.
Clinical features of the patients
| Patient | Age | Primary infertility history | BMI | Basal sexual hormone | AMH (ng/mL) | Cycle | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FSHa (IU/L) | LHb (IU/L) | Ec (pg/mL) | Testo (ng/dL) | No. of follicles on trigger day | No. of oocytes retrieved | No. of ZP‐free oocytes | No. of degenerated oocytes | |||||
| Family 1 Ⅱ‐2 | 29 | 4 | 19.6 | 9.06 | 10.14 | 50 | 0.03 | 3.59 | 11 | 8 | 3 | 5 |
| Family 2 Ⅲ‐6 | 28 | 5 | 18.4 | 7.13 | 3.08 | 61 | 0.66 | 2.02 | 8 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | |||||||||
| 9 | 4 | 2 | 2 | |||||||||
FIGURE 1.
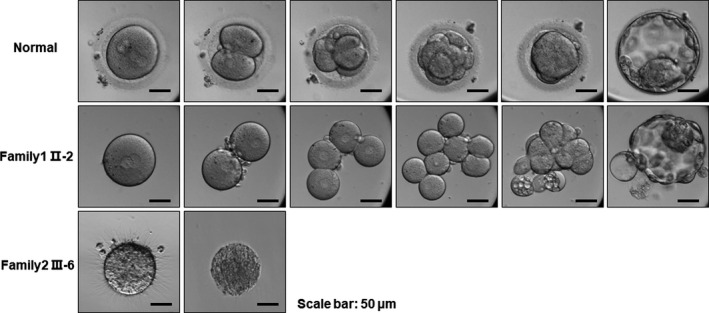
Clinical characterization. The oocytes retrieved from family 1 Ⅱ‐2 lacked a ZP, but they fertilized successfully and developed to the state of blastocyst in vitro during the ICSI treatment. The processes of early embryonic development were observed by time‐lapse microscopy. The oocytes retrieved from family 2 III‐6 lacked a ZP and degenerated the following day
The proband of family 2 (family 2 III‐6) had been diagnosed with infertility 5 years earlier; she had regular menstrual cycles and normal sex hormone concentrations, and she had undergone three cycles of IVF. No oocytes were obtained in the first two cycles, and 4 oocytes without a ZP were obtained from the 9 cumulus‐oocyte complexes in the third cycle, of which 2 degenerated shortly after retrieval and the other 2 oocytes died the following day (Table 1; Figure 1).
3.2. Heterozygous mutations in ZP1 and ZP3
To determine whether paternally transmitted mutations were involved in infertility, we performed whole‐exome sequencing of individuals in the family. We identified two mutations responsible for the phenotypes of these two patients. The variant of family 1 Ⅱ‐2, a heterozygous missense mutation c.326G>A (p.Arg109His) of ZP1 (NM_207341) (Figure 2A, Table 2), could influence protein function since it is located in exon 3. Sanger sequencing was then used to validate the mutation (Figure 2A), and the patient (family 1 Ⅱ‐2) and her father (family 1 Ⅰ‐1) carried the heterozygous ZP1 mutation, indicating a dominant pattern of inheritance, which may be involved in the ZP‐defective phenotype. The mutation was located in the N‐terminus of ZP1 before the trefoil domain (Figure 2C). The mutation in family 2 Ⅲ‐6, c.400G>A (p.Ala134Thr) in exon 2 of ZP3, had been identified in patients in a previous study. 16 In addition to the previous study, we identified the mutation in a family instead of in sporadic individual cases. Sanger sequencing validated that the patient's father (family 2 Ⅱ‐6) and infertile aunt (family 2 Ⅱ‐4) carried the heterozygous missense ZP3 mutation, whereas her fertile aunt (family 2 Ⅱ‐3) did not, indicating a dominant pattern of inheritance (Figure 2B). The altered amino acid substitution was located in the conserved ZP domain of ZP3 (Figure 2C). Moreover, the positions where these two mutations in ZP1 and ZP3 occur are highly conserved in most species (Figure 2D).
FIGURE 2.
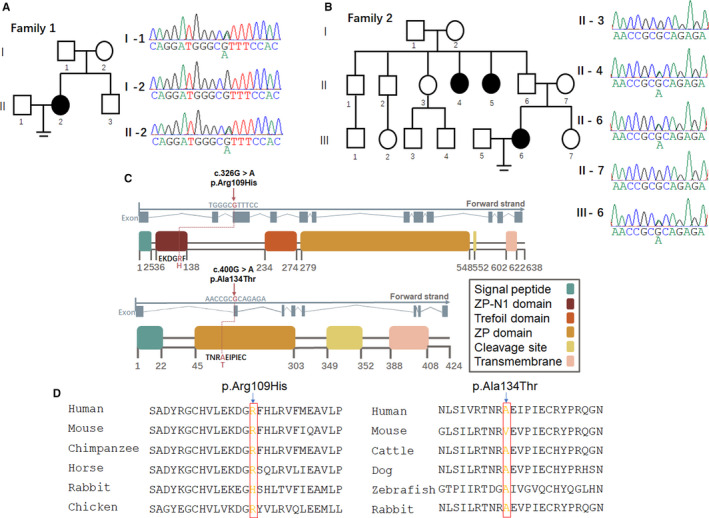
Heterozygous mutations in ZP genes. A, B, Left panel, pedigree of the patients. Male family members are represented by squares, and female family members are represented by circles; solid indicates affected members, and equal signs denote infertility. Right panel, Sanger sequencing results of the heterozygous missense mutation site in the patient (family 1 II‐2), her father (family 1 I‐1) and her mother (family 1 I‐2), and in the patient (family 2 III‐6), her fertile aunt (family 2 II‐3), her infertile aunt (family 2 II‐4), her father (family 2 II‐6) and her mother (family 2 II‐7). C, Location of the mutation site in ZP1 and ZP3. The positions of mutations and functional domains are indicated in the gene structures. The ZP1 missense mutation c.326G>A is in exon 3 of ZP1, causing the 109th amino acid, which is an R amino acid, to be replaced by an H amino acid. The ZP3 missense mutation c.400G>A is in exon 2 of ZP3, causing the 134th amino acid, which is an A amino acid, to be replaced by a T amino acid. D, Conservation of mutated sites in ZP1 and ZP3 between species
Table 2.
Overview of mutations identified in the patients
| Patient | Mutation gene | Genomic position (bp) | cDNA change | Protein change | Mutation type | Genotype | Mutation Assessor a | RefSNP ID | 1KG_EAS b | ExAC_EAS b |
|---|---|---|---|---|---|---|---|---|---|---|
| Family 1 Ⅱ‐2 | ZP1 | chr19:60637017 | c.G326A | p.R109H | Missense Mutation | Heterozygous | Possibly_damaging | rs369565345 | NA | NA |
| Family2 Ⅲ‐6 | ZP3 | chr7:76058919 | c.G400A | p.A134T | Missense Mutation | Heterozygous | Damaging | rs1554625334 | NA | NA |
Abbreviation: NA, Not Available.
Mutation pathogenicity prediction website: http://mutationassessor.org/.
Allele frequency in East Asian population of 1000 Genomes (1KG) and ExAC Browser.
3.3. R109H mutation in the predicted structure
The A134T mutation caused altered structure of ZP3. 16 In addition, to predict the molecular consequences of the novel mutation R109H in human ZP1, we performed homology modelling using the crystal structure of chicken ZP1. Not surprisingly, the overall structure of human ZP1‐N1 is similar to those of mouse ZP2‐N1 and ZP3‐N. Arg109 is located in strand H. The guanidino group of Arg109 would make hydrogen bonds with Pro136, thus stabilizing the protein structure. However, there would be a clash between the R109H substitution and neighbouring residues, including Asp107, Gly108 and Pro136, suggesting that the R109H mutation may affect protein stability or conformation (Figure 3).
FIGURE 3.
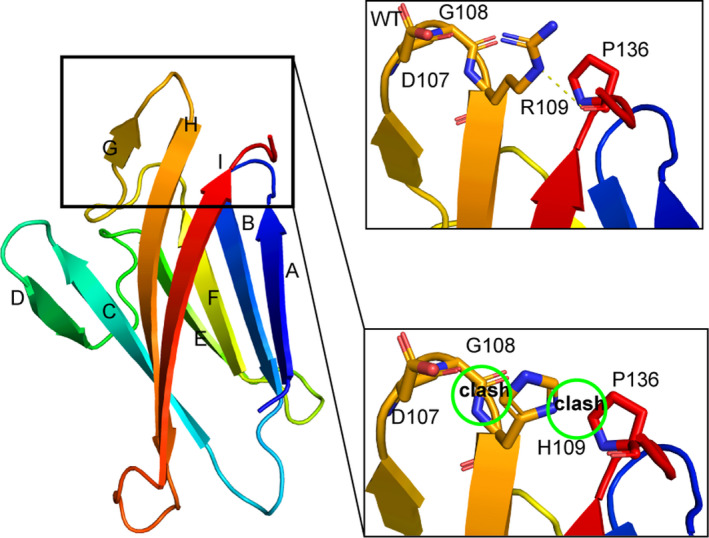
Structure prediction of the ZP1 mutation. Left panel: ribbon representation of the human ZP1‐N1 domain in rainbow coloration; right panel: close‐up view of residue 109 and its neighbouring residues
3.4. Mutations in ZP1 and ZP3 affect interactions between ZP glycoproteins
To elucidate how these missense mutations led to ZP loss, wild‐type and mutant expression plasmids encoding human ZP1, ZP2, ZP3 proteins (HA‐tagged wild‐type human ZP1, HA‐tagged human mutant ZP1 (p.Arg109His), FLAG‐tagged human ZP2, MYC‐tagged human wild‐type ZP3, MYC‐tagged human mutant ZP3 (p.Ala134Thr) were co‐transfected into HeLa cells. We performed coimmunoprecipitation (Co‐IP) analysis to detect interactions between the mutant ZP1 and the other two ZP glycoproteins. When HA‐ZP1R109H was co‐transfected with HA‐ZP1WT, FLAG‐ZP2 or MYC‐ZP3, imitating heterozygous mutations in the patient (family 1 II‐2), the expression of global ZP1 production was not affected when the mutated form is transfected, as shown in the input panel. Remarkably, the largely decreased interaction between ZP1 and ZP3 and slightly decreased interaction between ZP1 and ZP2 occurred when ZP1R109H was transfected. As expected, ZP1R109H diminished the interaction between ZP1 and ZP2 and ZP3 (Figure 4A–D).
FIGURE 4.
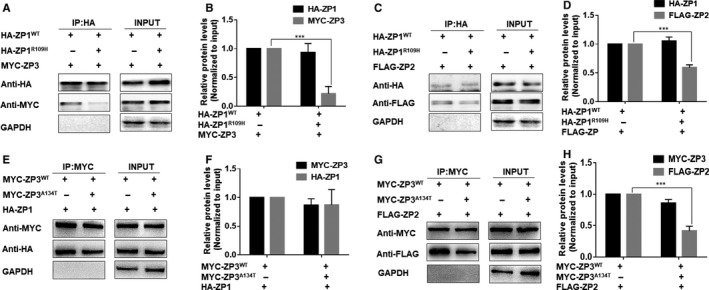
Interactions between ZP glycoproteins with mutated ZP1 and ZP3. A, B, Interaction of ZP1WT/ZP1R109H with ZP3 or (C, D) ZP2 was evaluated by co‐IP with an anti‐HA antibody. E, F, Interaction of ZP3WT/ZP3A134T with ZP1 or (G, H) ZP2 was evaluated by co‐IP with an anti‐HA antibody. Data are represented as the mean ± SEM; ***P < .001
When MYC‐ZP3A134T was co‐transfected with HA‐ZP1, FLAG‐ZP2 or MYC‐ZP3 WT, imitating heterozygous mutations in the patient (family 2 III‐6), the expression of global ZP3 production was not affected when the mutated form was transfected, as shown in the input panel. Remarkably, the largely decreased interaction between ZP3 and ZP2 occurred when ZP3A134T was transfected (Figure 4E,F), which was in accordance with a previous study. 16
3.5. ZP1 and ZP3 mutations disrupted secretion
We next asked whether mutations influenced the secretory function. To answer this question, we co‐transfected HA‐ZP1WT or ZP1R109H with FLAG‐ZP2 and MYC‐ZP3 to determine the influence of the ZP3 missense mutations on secretion. We co‐transfected MYC‐ZP3WT or ZP3A134T with FLAG‐ZP2 and HA‐ZP1 into HeLa cells and also had a group transfected with double mutant ZP1 or ZP3 to simulate homozygous mutations (Figure 5A).
FIGURE 5.
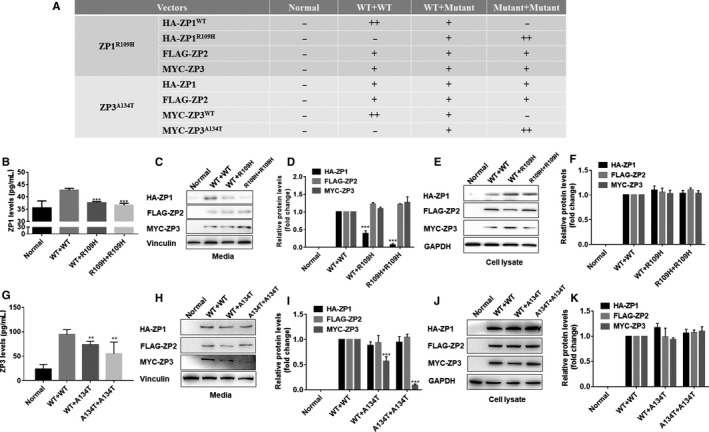
Effect of ZP1 and ZP3 mutations on ZP secretion. A, Transfection of vectors in different groups of cells. B, The ZP1 levels in the medium were measured by ELISA. C, D, ZP1, ZP2 and ZP3 protein levels in the medium were determined by Western blot. E, F, ZP1, ZP2 and ZP3 protein levels in cell lysates were determined by Western blot. G, The levels of ZP1 present in the medium were measured by ELISA. H, I, ZP1, ZP2 and ZP3 protein levels in the medium were determined. J, K ZP1, ZP2 and ZP3 protein levels in cell lysates were determined. Data are represented as the mean ± SEM; **P < .01 and***P < .001
Then, we collected the cell culture supernatants for assessment of secreted factors by a human ZP1 ELISA kit and a human ZP3 ELISA kit. We found that ZP1 and ZP3 levels were significantly decreased in the presence of mutant ZP1 and ZP3 compared to the levels of the control group (Figure 5B and G). Additionally, we used Western blotting to detect the expression level of ZP1, ZP2 and ZP3 protein in the cell lysate, and we detected their secretion levels in cell culture supernatants after co‐transfection. The results revealed that the secretion levels of ZP2 and ZP3 were comparable upon coexpression with either wild‐type ZP1 or mutant ZP1, whereas the levels of ZP1 in cell culture supernatants significantly decreased in the groups transfected with mutant ZP1 (Figure 5C and D), and ZP proteins were expressed normally in transfected cell lysates (Figure 5E and F). The bands indicated similar results when mutant ZP3 was co‐transfected (Figure 5H–K). Thus, these results showed that ZP1 with the p.Arg109His mutation and ZP3 with the p.Ala134Thr mutation interfered with the secretion of ZP proteins.
4. DISCUSSION
The ZP ensures the integrity of structure and participates in processes involved with fertilization, such as sperm‐oocyte recognition, binding and fusing. 19 With the increase in ART application, oocyte quality and embryo development are being increasingly observed and investigated. The zona pellucida is clinically used as one of the indicators of oocyte quality. ZP dysmorphology, such as dark zona pellucida or the lack of a zona pellucida, has an incidence of 2%‐5% of all oocytes. 20 , 21 ZP dysmorphology has been reported to be associated with a significant reduction in pregnancy rates and implantation rates in IVF. 22 However, the mechanism of ZP dysmorphology is rarely reported in the literature. In this study, we identified two infertile patients who had oocytes retrieved for ART treatments, and clinical observation revealed that the oocytes lacked a ZP, suggesting that the loss of the zona pellucida was the cause of their infertility.
As the ZP is quite important in fertilization, mutations in ZP genes may affect the zona pellucida and cause infertility. Recent studies have reported several cases of primary infertility caused by abnormalities in the zona pellucida. 13 , 14 , 15 , 16 , 17 , 23 A mutation of ZP1 (I390fs404X) causing the protein to be truncated was detected in an infertile patient whose oocytes lacked a zona pellucida. 14 Another study identified a heterozygous mutation in ZP2 (NM_003460.2:c.2092C>T) and another in ZP3 (NM_001110354.1:c.1045_1046insT) in a patient diagnosed with primary infertility, and her retrieved oocytes had a thin or absent ZP. 15 A recent study reported that two homozygous mutations in ZP2 (c.1695‐2A>G, and c.1691_1694dup) led to a thin ZP and influenced sperm‐binding. 17 Recently, several mutations in ZP1 (three homozygous mutations: c.1708G>A, p.Val570Met; c.1228C>T, p.Arg410Trp; c.507del, p.His170Ilefs*52, and two compound heterozygous mutations: c.1430+1G>T, p.Cys478X and c.1775‐8T>C, p.Asp592Glyfs*29), ZP2 (c.1115G>C, p.Cys372Ser) and in ZP3 (c.763C>G, p.Arg255Gly) were characterized in patients with similar phenotypes. 13 Our study identified a heritable heterozygous mutation in ZP1 (c.326G>A p.Arg109His) from the patient (family 1 Ⅱ‐2) and her father (family 1 Ⅰ‐1), suggesting that this ZP1 mutation is associated with ZP loss and infertility in this patient. In addition, we recruited a family with female primary infertility and speculated that it was due to dominant inheritance. Then, we identified a previously reported ZP3 mutation (c.400G>A p.Ala134Thr) that was associated with ZP‐free oocytes and degeneration.
Many studies have been carried out on the secretion and assembly of ZP glycoproteins using mouse models to date. The mouse ZP proteins are ZP1, ZP2 and ZP3. The human ZP proteins are ZP1, ZP2, ZP3 and ZP4. All four zona pellucida proteins present high homology and have common structural features such as signal peptide, ZP domain, CFCS, transmembrane‐like domain and short cytoplasmic tail. ZP1 and ZP4 also contain the trefoil domain. These domains have been extensively investigated; for example, the signal peptide and CFCS are associated with secretion. Additionally, the ZP domain is known to be responsible for regulating the combination of ZP2 and ZP3 with ZP filaments, and it has a functional activity and plays a role during fertilization. 24 It is worth noting that the reported ZP mutations mentioned above are mostly located in the ZP domain and ZP‐C‐terminal; instead, in our study and another recent study the mutation of ZP1 lies in the ZP‐N1 domain of hZP1, and the ZP‐N1 domain lies in the N‐terminal of ZP1, which may harbour its cross‐linking function. 25 According to Jovine's work, it was not the trefoil or ZP module region of human ZP1 that formed cross‐links; it was human ZP1‐N1. ZP1‐N1 was essential for the assembly of the human ZP and fertility. In line with this, our results indicated that ZP1 with the p.Arg109His mutation exhibited structural instability or conformational changes that affect cross‐linking and impede the formation of the ZP. Thus, it is suggested that mutations to ZP1‐N1 may also participate in the ZP formation, thus affecting the function of the zona pellucida and resulting in infertility.
In human ZP assembly, ZP1 cross‐links three other ZP proteins to form long filaments. 26 , 27 This finding was consistent with the phenotype of a mouse model in which mZp2−/− and mZp3−/− females produced oocytes completely lacking a ZP, and oocytes from mZp1−/− females were surrounded by a loosely organized and abnormal ZP. 11 , 28 , 29 As reported, the ZP3 mutation (c.400G>A p.Ala134Thr) could influence the link between ZP3 and ZP2, and the interruption of their connection could destroy ZP assembly, as confirmed by the results in our research. 16 In the present study, we analysed the ZP1 mutation through structural prediction. The substitution of amino acids caused by the mutation resulted in conversion of an arginine residue to a histidine. We found that the alteration is likely to be significant because it causes structural instability to affect the cross‐linking or binding function of proteins. Here, in vitro experiments in HeLa cells showed that mutant ZP1 reduced the interaction with ZP2 and ZP3. Taken together, we propose that this mutation influences the cross‐linking function of ZP1 and impedes the formation of ZP.
Zona pellucida is a glycoproteinaceous matrix that is synthesized in oocytes and secreted during follicular development. 30 ZP proteins are known to be synthesized and secrete independently, and then ZP2‐ZP4 heterodimers are formed via cross‐linking by ZP1 homodimers. 24 , 27 It is thought that if the ZP proteins are not secreted appropriately, then the formation of the zona pellucida will be affected. It was reported that mutant ZP2 and ZP3 accumulated in the endomembrane system and could not bind to the cell membranes, resulting in a traffic barrier and the absence or thinness of the ZP. 15 Hence, in our study, we used ELISA and Western blot experiments to detect the secreted levels of ZP1 and ZP3, both of which after introducing mutant forms of the proteins, and they also presented a mutation dosage‐dependent effect. The results indicate that the mutations are involved in the reduced secretion of ZP1 and ZP3, and leading to connection failure of the ZP filaments in vitro. The data suggest a potential that the mutations may be involved in the lacking ZP phenotype, which need to be further investigated in vivo.
Although the patient (family 1 Ⅱ‐2) had retrieved oocytes lacking a ZP, after undergoing intracytoplasmic sperm injection (ICSI) with a mature in vitro culture system, she successfully delivered a baby with an Apgar score of 10. This case suggests that ZP1 is an essential element of the zona matrix. Fertility is a complex process, and ZP1 plays important roles in fertilization, but the mechanism underlying this remains largely undiscovered. In the clinic, when encountering infertility patients who seek ART for help, we suggest that the ZP genes sequencing should be advised if a ZP anomaly is visible. If the sequencing result reveals a mutation in ZP1, the method of ICSI could be preferentially chosen instead of the traditional IVF method, which would greatly improve the probability of successful pregnancy via ART in infertility patients who have a ZP problem.
In conclusion, our study identified a ZP1 mutant situated in the ZP1‐N1 segment upstream of the trefoil domain, ZP domain, cleavage site and so on. The mutation in this region impeded the cross‐linking and secretion function and may account for the absence of the ZP. It also suggested that human ZP1‐N1 also engages in the formation of ZP in addition to the domains of known functions such as the ZP domain. Moreover, the confirmation of a previously identified ZP3 mutation emphasized its pathogenicity and phenotypic consistency. Our work increases the understanding of the pathogenesis of ZP1 and ZP3 gene mutations and recommends selecting a rational fertilization method of ART in combination with ZP target gene diagnosis for infertility patients in the clinic carrying ZP1 mutations and ZP‐free oocytes.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
AUTHOR CONTRIBUTION
Qiqi Cao: Conceptualization (lead); Data curation (equal); Investigation (lead); Methodology (lead); Validation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Chun Zhao: Conceptualization (equal); Data curation (equal); Investigation (equal); Writing‐original draft (equal). Xiaolan Zhang: Conceptualization (equal); Formal analysis (equal); Resources (equal); Validation (equal); Visualization (equal); Writing‐original draft (equal). Heng Zhang: Software (equal); Visualization (equal). Qianneng Lu: Investigation (equal); Methodology (equal). Congjing Wang: Methodology (equal); Validation (equal); Writing‐review & editing (equal). Yue Hu: Formal analysis (equal); Investigation (equal). Xiufeng Ling: Project administration (equal); Resources (equal); Supervision (equal). Junqiang Zhang: Project administration (equal); Resources (equal); Supervision (equal). Ran Huo: Funding acquisition (lead); Project administration (lead); Supervision (lead); Writing‐original draft (equal); Writing‐review & editing (equal).
ACKNOWLEDGEMENTS
This work was supported by the National Key Research and Development Program of China (2018YFC1004002) and National Nature Science Foundation of China (31671556, 81871210, 81471457, 81971386).
Cao Q, Zhao C, Zhang X, et al. Heterozygous mutations in ZP1 and ZP3 cause formation disorder of ZP and female infertility in human. J Cell Mol Med. 2020;24:8557–8566. 10.1111/jcmm.15482
Qiqi Cao, Chun Zhao and Xiaolan Zhang contributed equally.
Contributor Information
Junqiang Zhang, Email: zhangjunqiang_njfy@163.com.
Ran Huo, Email: huoran@njmu.edu.cn.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Gook DA, Edgar DH, Borg J, Martic M. Detection of zona pellucida proteins during human folliculogenesis. Hum Reprod. 2008;23:394‐402. [DOI] [PubMed] [Google Scholar]
- 2. Wolgemuth DJ, Celenza J, Bundman DS, Dunbar BS. Formation of the rabbit zona pellucida and its relationship to ovarian follicular development. Dev Biol. 1984;106:1‐14. [DOI] [PubMed] [Google Scholar]
- 3. Wassarman PM. Zona pellucida glycoproteins. J Biol Chem. 2008;283:24285‐24289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wassarman PM, Jovine L, Litscher ES. A profile of fertilization in mammals. Nat Cell Biol. 2001;3:E59‐64. [DOI] [PubMed] [Google Scholar]
- 5. Gupta SK. Role of zona pellucida glycoproteins during fertilization in humans. J Reprod Immunol. 2015;108:90‐97. [DOI] [PubMed] [Google Scholar]
- 6. Jovine L, Qi H, Williams Z, Litscher ES, Wassarman PM. A duplicated motif controls assembly of zona pellucida domain proteins. Proc Natl Acad Sci USA. 2004;101:5922‐5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jovine L, Qi H, Williams Z, Litscher E, Wassarman PM. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat Cell Biol. 2002;4:457‐461. [DOI] [PubMed] [Google Scholar]
- 8. Wassarman PM, Litscher ES. Influence of the zona pellucida of the mouse egg on folliculogenesis and fertility. Int J Dev Biol. 2012;56:833‐839. [DOI] [PubMed] [Google Scholar]
- 9. Wassarman PM. Fertilization: Welcome to the fold. Nature. 2008;456:586‐587. [DOI] [PubMed] [Google Scholar]
- 10. Monne M, Han L, Schwend T, Burendahl S, Jovine L. Crystal structure of the ZP‐N domain of ZP3 reveals the core fold of animal egg coats. Nature. 2008;456:653‐657. [DOI] [PubMed] [Google Scholar]
- 11. Rankin T, Talbot P, Lee E, Dean J. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development. 1999;126:3847‐3855. [DOI] [PubMed] [Google Scholar]
- 12. Lefievre L, Conner SJ, Salpekar A, et al. Four zona pellucida glycoproteins are expressed in the human. Hum Reprod. 2004;19:1580‐1586. [DOI] [PubMed] [Google Scholar]
- 13. Zhou Z, Ni C, Wu L, et al. Novel mutations in ZP1, ZP2, and ZP3 cause female infertility due to abnormal zona pellucida formation. Hum Genet. 2019;138:327‐337. [DOI] [PubMed] [Google Scholar]
- 14. Huang HL, Lv C, Zhao YC, et al. Mutant ZP1 in familial infertility. N Engl J Med. 2014;370:1220‐1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu W, Li K, Bai D, et al. Dosage effects of ZP2 and ZP3 heterozygous mutations cause human infertility. Hum Genet. 2017;136:975‐985. [DOI] [PubMed] [Google Scholar]
- 16. Chen T, Bian Y, Liu X, et al. A Recurrent Missense Mutation in ZP3 Causes Empty Follicle Syndrome and Female Infertility. Am J Hum Genet. 2017;101:459‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dai C, Hu L, Gong F, et al. ZP2 pathogenic variants cause in vitro fertilization failure and female infertility. Genet Med. 2019;21:431‐440. [DOI] [PubMed] [Google Scholar]
- 18. Biasini M, Bienert S, Waterhouse A, et al. SWISS‐MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252‐W258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta SK, Bhandari B, Shrestha A, et al. Mammalian zona pellucida glycoproteins: structure and function during fertilization. Cell Tissue Res. 2012;349:665‐678. [DOI] [PubMed] [Google Scholar]
- 20. Rienzi L, Vajta G, Ubaldi F. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update. 2011;17:34‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Oocyte morphology does not affect fertilization rate, embryo quality and implantation rate after intracytoplasmic sperm injection. Hum Reprod. 1998;13:3431‐3433. [DOI] [PubMed] [Google Scholar]
- 22. Sauerbrun‐Cutler MT, Vega M, Breborowicz A, et al. Oocyte zona pellucida dysmorphology is associated with diminished in‐vitro fertilization success. J Ovarian Res. 2015;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dai C, Chen Y, Hu L, et al. ZP1 mutations are associated with empty follicle syndrome: evidence for the existence of an intact oocyte and a zona pellucida in follicles up to the early antral stage. A case report. Hum Reprod. 2019;34:2201‐2207. [DOI] [PubMed] [Google Scholar]
- 24. Qi H, Williams Z, Wassarman PM. Secretion and assembly of zona pellucida glycoproteins by growing mouse oocytes microinjected with epitope‐tagged cDNAs for mZP2 and mZP3. Mol Biol Cell. 2002;13:530‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishimura K, Dioguardi E, Nishio S, et al. Molecular basis of egg coat cross‐linking sheds light on ZP1‐associated female infertility. Nat Commun. 2019;10:3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Green DP. Three‐dimensional structure of the zona pellucida. Rev Reprod. 1997;2:147‐156. [DOI] [PubMed] [Google Scholar]
- 27. Monne M, Jovine L. A structural view of egg coat architecture and function in fertilization. Biol Reprod. 2011;85:661‐669. [DOI] [PubMed] [Google Scholar]
- 28. Rankin TL, O'Brien M, Lee E, Wigglesworth K, Eppig J, Dean J. Defective zonae pellucidae in Zp2‐null mice disrupt folliculogenesis, fertility and development. Development. 2001;128:1119‐1126. [DOI] [PubMed] [Google Scholar]
- 29. Rankin T, Familari M, Lee E, et al. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development. 1996;122:2903‐2910. [DOI] [PubMed] [Google Scholar]
- 30. Phillips DM, Shalgi RM. Surface properties of the zona pellucida. J Exp Zool. 1980;213:1‐8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


