Abstract
Breast cancer is a complex disease posing a serious threat to the female population worldwide. A complex molecular landscape and tumor heterogeneity render breast cancer cells resistant to drugs and able to promote metastasis and invasiveness. Despite the recent advancements in diagnostics and drug discovery, finding an effective cure for breast cancer is still a major challenge. Positive and negative regulation of apoptosis has been a subject of extensive study over the years. Numerous studies have shed light on the mechanisms that impede the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) signaling cascade. Long non-coding RNAs (lncRNAs) have been implicated in the orchestration, development, proliferation, differentiation and metastasis of breast cancer. However, the roles of lncRNAs in fine-tuning apoptosis regulating machinery in breast cancer remain to be elucidated. The present review illuminates the roles of these molecules in the regulation of breast cancer and the interplay between lncRNA and TRAIL in breast cancer. The present review also attempts to reveal their role in the regulation of apoptosis in breast cancer appears a promising approach for the development of new diagnostic and therapeutic regimens.
Keywords: long non-coding RNA, tumor necrosis factor-related apoptosis-inducing ligand, diagnosis, therapeutics, breast cancer
1. Introduction
In recent years, there has been an upsurge in cancer burden worldwide, and cancer has become the leading cause of death, following cardiovascular diseases, in both men and women globally (1). There are nearly 18 million cases of cancer registered worldwide; among them, 268,600 are breast cancer patients (2). Among the different cancer types, breast cancer is one of the major causes of death in the female population (3). Compelling evidence suggests that specific genetic, epigenetic and environmental factors play a critical role in the development of breast cancer. The prevalence of breast cancer is caused by many factors, including unhealthy lifestyle, excessive consumption of red meat, alcohol, smoking and genetics (4). Nowadays, high-throughput technologies, such as next-generation sequencing have begun to elucidate tumor heterogeneity and has brought us closer towards devising new diagnostic and therapeutic strategies (5). Advanced experimental methodologies have started to categorize proteome into sub-classes of pro-apoptotic and anti-apoptotic proteins (5). This has led to characterization of tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) sub-proteomes (5). Alterations in the TRAIL-mediated signaling pathway are associated with the proliferation of breast cancer (6). Translation and functional studies have clarified the underlying mechanisms and biomolecular signatures responsible for impeding cancer treatment (7,8) Thus, the search for better diagnostic and management of breast cancer is needed.
Positive and negative regulation of apoptosis has been a subject of extensive study over the past decades (9). There has been an increase in new regulators of apoptosis that have deepened our understanding of the process (10). A number of studies have investigated the mechanisms that impede the TRAIL signaling cascade (11–13). Knowledge of the association between different pro-survival and cell death pathways in cancer is vital for devising therapeutic strategies for cancer. TRAIL belongs to a small subset of pro-apoptotic protein ligands in the TNF superfamily, which also includes TNF and cluster of differentiation (CD)95L (FasL/APO-1L) (14). TRAIL has been investigated since 1997, when it was observed that TRAIL-mediated apoptosis was responsible for death in cancer cells, leaving normal cells intact (15). This was followed by a number of studies documenting the molecular characteristics of TRAIL-mediated apoptosis in various cancer types, such as breast (16), thyroid (17), colorectal (18), renal (19), bladder, prostate (20) and ovarian cancer (21). Parallel studies revealed that in cancer cells, TRAIL was underexpressed, leading to loss of TRAIL-induced apoptosis (22–24). TRAIL-induced apoptosis is triggered through the activation of death receptors (DRs), specifically DR4 and DR5 (25). This interaction in turn facilitates the attachment of the apoptosis antigen 1 (Fas)-associated death domain containing protein (FADD) (26). FADD attachment results in the recruitment of adapter proteins to the cytoplasmic domain of DR (26). Recruitment of adapter proteins facilitates the activation of pro-caspases 8 and 10, which then trigger the activation of caspase 3 (27). Activation of caspase 3 in turn leads to activation of either the extrinsic pathway (caspase 8-mediated) or the intrinsic pathway, which involves the release of cytochrome c (28). Cytochrome c-mediated activation of procaspase 9 to caspase 9 promotes activation of the intrinsic pathway, which involves the translocation of the BH3 interacting-domain death agonist to the mitochondria (29). This facilitates recruitment of Bax/Bak, which aid in the transportation of cytochrome c and second mitochondria-derived activator of caspases/Diablo homolog through the formation of the mitochondrial pore (30,31).
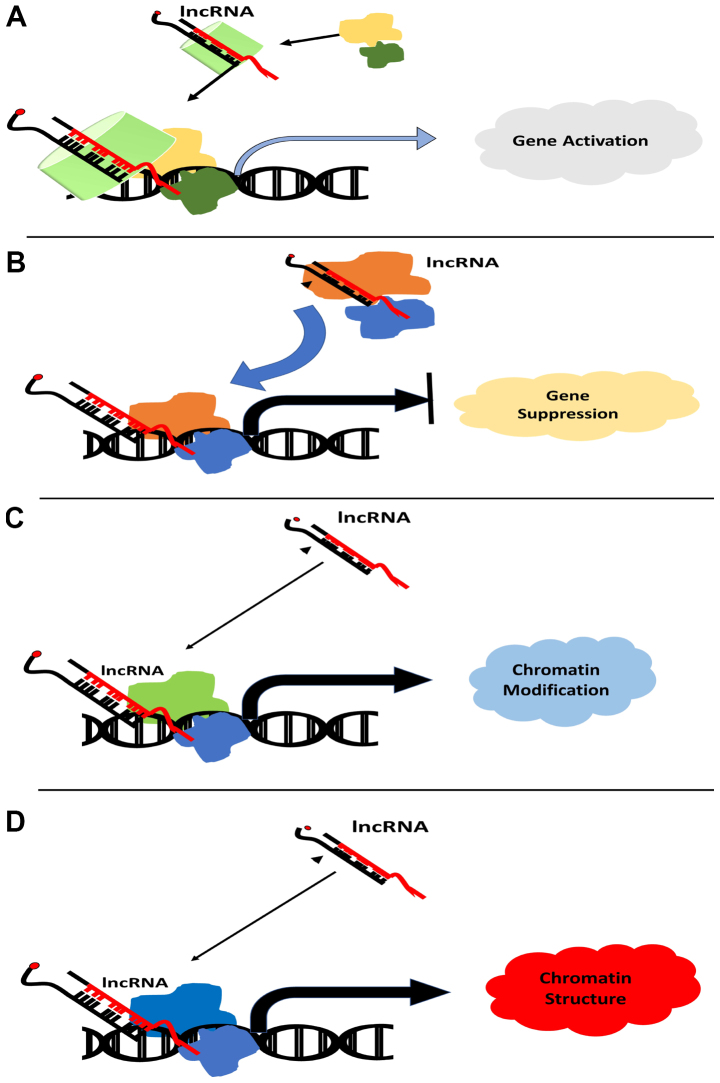
Long non-coding RNAs (lncRNAs) are RNA molecules in the range of 200–2,000 bp (32). lncRNAs have been found to play a crucial role in the development of various cancer types, including breast (33), thyroid (34), renal (35), colorectal (36), prostate (37) and ovarian cancer (38), and have been reported to interact with various molecules during transcription, chromosome remodeling, cellular trafficking and translation (39). In addition, lncRNAs serve regulatory roles during transcription, mRNA processing, maturation of mRNAs, modification of histone complexes and DNA methyltransferase modifications that occur during epigenetic regulation (Fig. 1) (40). Mutations in the signaling cascades responsible for growth arrest and apoptosis are predominant in most breast cancers. lncRNA-mediated regulation of apoptosis machinery in breast cancer remains to be elucidated. Nevertheless, various studies have reported the regulatory role of lncRNAs in apoptosis and growth arrest (41,42). Exploring the role of lncRNA in the regulation of the apoptosis and growth arrest in breast cancer appears a promising approach, which may aid in the development of lncRNA-based therapeutics, as well as being a biomarker for disease diagnosis.
Figure 1.
lncRNA-based modulation of gene expression via epigenetic modification. lncRNA can induce (A) gene activation or (B) suppression by inducing histone demethylation and histone methylation, respectively. Histone modification further allows recruitment of transcription factors or transcription repressor to the promoter region according to the fate of the gene. (C) and (D) lncRNAs also induce chromatin modification for silencing or promoting expression of gene. lncRNA, long non-coding RNA.
2. Role of lncRNA in breast cancer
Bioinformatics studies and RNA sequencing have been used to delineate the role of lncRNA in breast cancer (43). Genetic heterogeneity of the individual tumor is a crucial factor that triggers activation of certain lncRNAs (44).
X inactive specific transcript (XIST) is an oncogenic lncRNA that plays a significant role in the progression of breast cancer. XIST RNA directs transcriptional changes by binding to poly comb repressive complex 2 (PRC2). Deregulated XIST promotes tumor progression (45). XIST activation has been reported to accelerate tumor growth of breast cancer gene 1-deficient ovarian cell lines (46). Accumulation of XIST promotes the expression of X-linked oncogenes, including the V-RAF murine sarcoma 3611 oncogene homolog 1 and member of ETS oncogene family, which triggers the growth of tumor cells (47). Several factors are prerequisite for triggering XIST. A recent study has reported that scaffold attachment factor A, also known as heterogenous ribonucleoprotein U, aids lncRNA attachment to the X chromosome. This promotes activation of SMART/histone deacetylase (HDAC)1-associated repressor protein, which recruits HDAC C3 and PRC2 components to formulate histone repressive complex (48). In addition, high-throughput sequencing has revealed that several XIST interactors serve a role in the activation of XIST. In a recent study, lower expression of XIST was reported in triple-negative breast cancer (TNBC). The restored expression of XIST reduces the epithelial-mesenchymal transition (EMT) property of cancer cells and cell proliferation, and induces apoptosis (49). XIST in TNBC functions by inhibiting microRNA (miR)-454 (49). XIST expression is also reported to be downregulated in estrogen receptor-negative (ER−) and progesterone receptor-negative (PR−) breast cancer (50). However, XIST is highly expressed in human epidermal growth factor receptor 2 (HER2)-positive breast cancer (51).
HOX antisense intergenic RNA (HOTAIR) is another lncRNA that has been reported to facilitate cancer progression (52). Despite its location at chromosome 12, HOTAIR has been reported to activate distant genes. Functional studies have shed light on the important role of HOTAIR in metastasis and invasion. Hepatocyte nuclear factor 4-α (HNF4-α), an initiator of epithelial differentiation, represses the transcription of HOTAIR (53). HNF4-α promotes the release of the chromatin loop on the HOTAIR regulatory element and a decrease in the expression levels of homeobox D cluster-targeted genes (54). PRC2 and lysine-specific demethylase 1 (LSD1) are the two regulators of chromatin dynamics that interact with HOTAIR (55). HOTAIR interacts with either LSD1 or PRC2 via various mechanisms; its interaction with PRC2 is through its 5′ end, which enhances repression of PRC2 target loci (56). By contrast, HOTAIR interacts with LSD1 through its 3′ end to regulate gene silencing (57). HOTAIR is highly overexpressed in various cancer types, such as hepatocellular carcinoma (58), lung (59) and breast cancer (60) and has been reported to serve a decisive role in tumor proliferation, invasion and metastasis (61). Thus, HOTAIR could be considered as a plasma based biomarker for breast cancer and various tumors. Furthermore, the consistent overexpression of HOTAIR is observed in ER+/PR+ breast cancer (62). Overexpression of HOTAIR increases invasiveness in metastatic breast cancer (63). This has led to the conclusion that HOTAIR is a valid biomarker for breast cancer (64).
NEAT1 is an oncogenic lncRNA that promotes proliferation and metastasis in breast cancer (65). NEAT1 lncRNA is highly expressed in breast cancer tissues, and its expression correlates with tumor size and metastatic potential. NEAT1 interacts with the FOXN3, SIN3 and SIN3A repressor complex. This has been brought to light by RNA immunoprecipitation and high-throughput sequencing. Together, this trio forms a nucleoprotein complex that facilitates EMT, invasion and metastasis in ER+ cells via inhibition of GATA binding protein 3 (GATA3), a transcription factor (66). In addition, overexpression of NEAT1 and FOXN3 decreases overall survival rate in breast cancer patients (66). Elevated NEAT1 expression has also been reported in TNBC, and its inhibition via short hairpin (sh)NEAT1 in TNBC cells leads to sensitization to chemotherapy and reduced cancer stemness (67). NEAT1 overexpression is directly associated with enhanced tumor growth, proliferation and metastasis (68,69). Tests, including MTT and wound healing assays, on BC MDA-MB-468 and MCF-7 cell lines revealed that the suppression of NEAT1 expression via small interfering (si)RNA, not only reduces cell proliferation and inhibits metastasis, but also prompts apoptosis via the activation of caspase 3 (65). The expression of NEAT1 is modulated by miR-548ar. Overexpression of miR-548ar significantly reduced NEAT1 expression levels in MCF-7 and MDA-MB-231 human breast cell lines and also facilitated the induction of cellular apoptosis (70). The role NEAT1 plays in breast cancer proliferation, invasiveness and chemo-resistance makes it a potential diagnostic biomarker and a therapeutic target for this cancer (69).
BCAR4 is another lncRNA that has been demonstrated to confer tamoxifen resistance independently of ER1 expression (71). Ectopic expression of BCAR4 in MCF7 and ZR-75-1 cell lines was able to increase proliferation in estrogen-free media (72). Furthermore, BCAR4 overexpression was shown to promote growth and metastasis in primary breast tumor cells. In xenograft models, BCAR4 is a potent proliferative agent; its expression is tissue-specific, thus making it a suitable target for treating anti-estrogen resistance in breast cancer (72). BCAR4 promotes the expression of GLI-2 via activation of the non-canonical hedgehog-Gli pathway (73). This activation, in turn, promotes metastasis, migration and invasiveness. BCAR4 also promotes the activation of phosphatase 1 (PP1) via Smad nuclear interacting protein 1 (SNIP1), thus inhibiting p300-mediated histone acetylation (74). PP1 interaction with SNIP1 also promotes the dephosphorylation of pol II ser5, which promotes activation of GLI2 target genes (75).
DSCAM-AS1 is another oncogenic lncRNA whose expression is regulated by ER (76). DSCAM-AS1 has been reported as a downstream effector of ER and its upregulation has been observed in ER+ and ER− breast tumors (76). Strong estrogen induction in MCF7 and T47D cells can promote overexpression of DSCAM-AS1 (77). Knockdown of DSCAM-AS1 results in growth arrest and decreased migration and invasiveness, suggesting that DSCAM-AS1 functions downstream of ER (76). These findings shed light on the use of DSCAM-AS1 as a potential biomarker for the detection of breast cancer.
Few studies have been performed to indicate breast cancer subtype-specific expression of lncRNAs (78); however, the underlying mechanism for the tumorigenicity in breast cancer remains to be elucidated.
lncRNA in metastatic breast cancer
The contribution of lncRNAs to the growth, proliferation and survival of different types of cancer has been studied (36,46,79–83) Several studies have emphasized the potential of lncRNAs in promoting metastasis in breast cancer cell lines and tissues (84,85). Dysregulation of lncRNA inhibiting proliferation and metastasis (NLIPMT) has been reported to enhance growth and metastasis in breast cancer tissue. Restoration of NLIPMT expression in the breast cancer MDA-MB-231 cell line inhibits cellular proliferation by suppressing glycogen synthase kinase 3β phosphorylation (86). Some lncRNAs are overexpressed in breast cancer cells, which facilitates tumor growth, spread and survival by targeting the transcription of proteins. Such lncRNAs are associated with cell growth suppression and apoptosis (87). High expression of lncRNA FOXD3-AS1 in cancer tissues has a direct correlation with tumor size increase and distant metastasis (88). A high level of lncRNA AWPPH in patients' plasma is associated with enhanced cell growth in early stage TNBC (89). Overexpression of lncRNA AWPPH causes resistance to carboplatin treatment (89).
Dysregulation of some lncRNAs is also associated with the potential of breast tumor cells to metastasize to different organ sites (90). The majority of studies have reported an lncRNA role in the metastasis of breast cancer to the lungs (91,92). LINC00478-associated cytoplasmic RNA (lacRNA) is a cleaved version of lncRNA LINC00478. LINC00478 is significantly downregulated in metastatic breast tumors and promotes active transcription of MYC proto-oncogene (MYC)-activated genes (93). lncRNA overexpression suppresses the metastatic and invasive potential of breast cancer cells by stabilizing prohibitin-2 (PHB2) protein. PHB2 then brings about transcriptional inhibition of MYC target genes (93). Furthermore, its overexpression inhibits lung metastasis in mouse models (93). lncRNA HOXA11-AS is also reported to be associated with breast cancer metastasis to the lungs; it modulates EMT by downregulating E-cadherin and vimentin expression. In mouse models treated with shHOXA11-AS, the expression of HOXA11-AS is decreased in both primary and secondary tumors (94). lncRNA ANCR is downregulated in breast tumor cells and induces metastasis via active signal transduction through the TGF-β pathway (95). Upon introduction of ANCR-deficient MDA-MB-231-ANCR cells into BABL/c nude mice, these cells metastasize to the lungs (95).
The role of lncRNAs in promoting metastasis in breast cancer subtypes with different molecular signatures, such as luminal A, luminal B, HER2-type, normal-like and triple-negative, has yet to be properly studied. This indicates the need for further studies in the area to better understand the role of lncRNAs in breast cancer according to the different subtypes. This may be helpful in designing more effective therapeutics for this disease.
3. Interplay of lncRNA and TRAIL in breast cancer
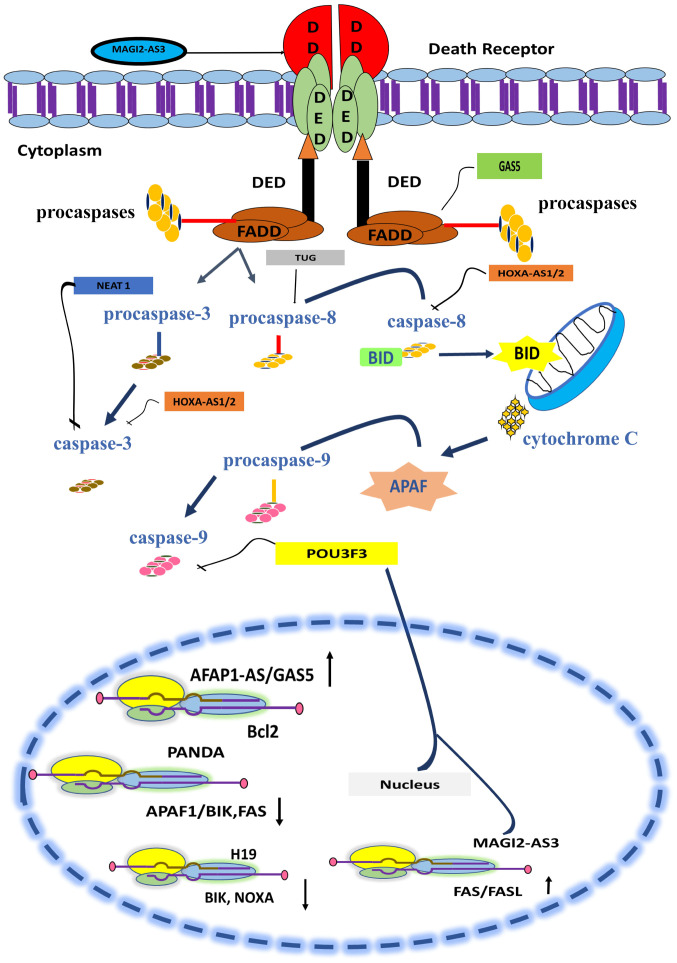
lncRNAs have a dual role in cellular homeostasis. Depending on their interactive molecular landscape they can either favor survival of the cancer cells or apoptosis (84). TRAIL-mediated apoptosis is one such pathway and the alteration in the expression of its members shifts the balance of the cell in favor of survival (96,97). Recent advances in biomolecular studies have hinted towards the association of the interplay of TRAIL and lncRNA with breast cancer development (77). The activity of caspases is a chief factor that is modulated by most lncRNAs in breast cancer to ensure the rapid multiplication and growth of cancerous cells (98). Table I contains a list of lncRNAs whose dysregulation in breast cancer disrupts the TRAIL-induced apoptosis pathway by modulating the activity of caspases. The modulatory role of lncRNA in the extrinsic pathway is illustrated in Fig. 2.
Table I.
lncRNAs whose expression modulates caspase activity.
| Author, year | lncRNA | Affected caspase | lncRNA role | (Refs.) |
|---|---|---|---|---|
| Yang et al, 2019 | POU3F3 | Caspase 9 | Enhances proteolytic activation | (99) |
| Zhang et al, 2019 | NEAT1 | Caspase 3 | Inhibits activity | (65) |
| Gooding et al, 2017 | BORG | Caspase 3, 7, 8 | Inhibits activity | (107) |
| Wang et al, 2018 | Z38 | Caspase 3, 9 | Inhibits activity | (110) |
| Li et al, 2017 | TUG1 | Caspase 3, 9 | Inhibits activity | (98) |
| Ma et al, 2019 | AFAP1-AS1 | Caspase 3 | Inhibits activity | (113) |
lncRNAs, long non-coding RNAs.
Figure 2.
Schematic description of TRAIL signaling cascade and interaction of lncRNA. lncRNAs modulate the apoptotic pathway at different levels. A few lncRNAs promote apoptosis; for instance, MAGI2-AS3 enhances Fas ligand expression and APAF1-AS/GAS5 upregulates Bcl-2 expression. A few lncRNAs halt apoptosis and promote cell survival and growth; PANDA downregulates pro-apoptotic BIK protein, APAF-1 and Fas expression, and H19 downregulates BIK expression. NEAT-1 and HOXA-AS1/2 inhibit caspase 3 activity, while POU3F3 causes inhibition of caspase 9. HOXA-AS1/2 and TUG suppress activation of caspase 8. TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; lncRNA, long non-coding RNA; BIK, Bcl2 interacting killer; APAF, apoptotic protease-activating factor-1; Fas, apoptosis antigen 1.
lncRNA-mediated regulation of the extrinsic apoptotic pathway in breast cancer
POU3F3 lncRNA modulates the TRAIL pathway by modulating caspase activation (99). Data have suggested a positive correlation between tumor proliferation in TNBC and POU3F3 (99). POU3F3 inhibits the proteolytic cleavage-mediated activation of caspase 9 (99). High POU3F3 expression in tumor tissues and in the blood plasma of patients suggests its use as a diagnostic marker for TNBC (99).
In addition, in vitro knockdown of POU3F3 leads to enhanced cleavage of caspase 9, restoring the intrinsic apoptotic pathway, triggering growth arrest, and inhibiting tumor migration and invasiveness (100). A previous study showed that exogenous induction of procaspase 9 cleavage brings about attenuation in the oncogenic effects of overexpressed POU3F3 and leads to cell apoptosis (99). These findings suggest that POU3F3 as a potential therapeutic target for TNBC.
The extrinsic and intrinsic apoptotic pathways are both regulated by the various lncRNAs (101). Death receptor triggers the activation of caspases. Several lncRNAs serve pivotal roles in the regulation of caspase activity (101). HOXAS1/2 is involved in inhibition of caspase 8 and 3 (102). NEAT1 inhibits the activity of caspase 3 (65) and TUG promotes the activity of caspase 8. Signals from caspases are transferred to mitochondria and lead to apoptosis. lncRNAs such as GAS5/AFAP1-AS and MAG12-AS3 promote the upregulation of BCL-2 and FAS genes and facilitate apoptosis (103–105). lncRNA PANDA (p21-associated ncRNA DNA damage activated) downregulates the expression of proapoptotic proteins such as the Fas cell surface death receptor (FAS)/BCL-2 interacting killer (BIK) and apoptotic protease activating factor (APAF)1, thus inhibiting apoptosis and promoting cell growth in breast cancer cells (106).
BORG (BMP/OP Responsive Gene) is another highly expressed oncogenic lncRNA in breast cancer that affects caspases activity in order stop the apoptotic pathway of cells and promote aggressive tumor proliferation (107). High expression of BORG has been reported in TNBC and is also associated with chemoresistance and high cancer cell growth (108). The activity of caspase 3, 7 and 8 significantly reduces the expression of BORG in BORG-expressing cell lines (107).
Suppression subtractive hybridization in combination with reverse dot-blotting suggests the correlation between high expression of lncRNA Z38 and tumorigenesis in breast cancer cells. Suppression of Z38 expression via shRNA causes inhibition of in vivo tumorigenesis and a reduction in cell viability. In addition, the TUNEL assay performed after administration of Z38 siRNA reveals induction of apoptosis in cancerous cells (109). This study indicated that the administration of Z38 siRNA mechanistically activates the intrinsic apoptotic pathway. Knockdown of Z38 negatively influences cell proliferative rate together with the induction of apoptosis in gastric cancer in a similar way in breast cancer (109). Z38 acts through the activation of caspase 3 and 9 to initiate the apoptotic pathway (110). High expression of Z38 and its oncogenic influence makes it prognostically significant in cases of breast cancer (111).
AFAP1-AS1 is also among the lncRNAs whose aberrant expression in breast cancer leads to the inactivity of various caspases. AFAP-AS1 is mapped on chromosome 4 in humans and its transcription occurs in an anti-sense direction from the AFAP1 gene (112). Reverse transcription-quantitative PCR (RT-qPCR) confirms that AFAP1-AS1 overexpression is observed in breast cancer tissues and MCF-7, SK-BR3, MDA-MB-231 and MDA-MB-436 breast cancer cell lines. Caspase 3 activity assay, cell cycle analysis, and Bax and Bcl-2 expression analyses demonstrate that the rate of apoptosis is increased in AFAP1-AS1 siRNA-transfected cell lines due to the restored activity of caspase 3 and increased Bcl-2 expression (113).
The malignant role of lncRNA TUG1 is controversial. However, recent data has reported the association of TUG1 high expression with malignancy and increased invasiveness in breast cancer (98). TUG1 overexpression is observed in malignant breast cancer cell lines such as MDA-MB-231, MDA-MB-436, MCF7 and T47D. TUG1 overexpression is reported at the highest levels in the breast cancer highly invasive MDA-MB-231 and MDA-MB-436 cell lines (98). TUG1 promotes cell proliferation by inhibiting caspase 3 and caspase 9 activities. The knockdown of TUG1 results in augmented activity of both caspases, which leads to a reduction in metastasis and increased apoptosis (98). Conversely, TUG1 expression induced in MDA-MB-231 and MCF7 by transfecting them with pCDNA-TUG suggests that the overexpression of TUG1 has tumor-suppressive effect on cancer cells where it modulates cell growth by suppressing the expression of cyclin D1 and CDK4, and promotes cell apoptosis and retards cancer cell growth (114). The tumor-suppressive role of TUG1 is also demonstrated in TNBC. A recent study has reported that lower expression of TUG1 induces chemo-therapy resistance and promotes cell proliferation, but whether its high expression activates TRAIL-induced apoptosis is not demonstrated (115).
Cancer cells manage to grow and survive after hijacking TRAIL-mediated apoptosis (116). Tumor cells use Fas receptor as a reserve route to initiate activation of caspase 8 via proteolytic cleavage and hence induce apoptosis (117,118). In breast cancer, the expression levels of Fas and FasL are also downregulated, eliminating all apoptotic threats for cancerous cells and making their proliferation possible (119). In breast cancer tissue, the expression of Fas and FasL is reported to be positively correlated with the expression of lncRNA MAGI2-AS3 (105). Using transcript transfection and lentiviral approaches, Yang et al (120) reported that MAGI2-AS3 expression facilitates the upregulation of Fas and FasL expression in MDA-MB-231 and MCF-7 cell lines. CCK-8 assay and flow cytometry further demonstrated that the lentivirus-induced expression of MAGI2-AS3 reduces cell viability and promotes cell death via activation of the Fas/FasL-induced apoptotic pathway (120).
lncRNA-mediated regulation of the intrinsic apoptotic pathway in breast cancer
A few identified lncRNAs negatively modulate the TRAIL-induced apoptotic pathway by affecting the transcription of pro-apoptotic proteins whose expression is triggered by TRAIL signaling (Table II). Among them; overexpression of H19 is reported in ERα+ breast cancer cells, where it halts apoptotic signaling of the cell by suppressing transcription of BIK and NOXA genes (121). Due to its aberrant levels in ERα+ breast cancer tissues and patients' plasma, it has the potential to be used as a diagnostic marker for this breast cancer type (122–124). lncRNA H19, with the help of epigenetic modification, brings about the silencing of the BIK gene; it blocks the promoter region of BIK by facilitating the recruitment of EZH2, which then induces trimethylation of histone H3 at lysine 27 (121). A recent development has revealed that the expression of lncRNA H19 is modulated by lncRNA PTCSC3 in TNBC (77). The high H19 level in TNBC tumor tissues is inversely correlated with PTCSC3 expression. Wang et al (121,122) transfected the BT-549 and HCC70 cell lines with PTCSC3 vectors and reported that overexpression of PTCSC3 attenuates the expression of lncRNA H19 and consequently suppresses cancer cell proliferation. Considering the role of lncRNA H19 in the rapid proliferation and chemo-resistance of breast cancer (125), treatment with PTCSC3 could be a potential strategy to counter the oncogenic effects of H19 in breast cancer.
Table II.
TRAIL-specific lncRNAs involved in breast cancer and their targets.
| Author, year | lncRNA | Expression | Target | Mechanism | (Refs.) |
|---|---|---|---|---|---|
| Si et al, 2016 | H19 | High | BIK | Blocking promoter region | (121) |
| Hung et al, 2011, Zhang et al, 2014 | PANDA | High | APAF1, BIK, FAS | Interact with NF-YA | (127,128) |
| He et al, 2015 | GAS-5 | Low | BIK | Expression activation via epigenetic modification | (133) |
| Zhang et al, 2019 | CASC-2 | Low | Smad-2 | Direct inhibition | (137) |
| Si et al, 2016 | HOXA-AS2 | High | TGFBR2 | Expression facilitation via directly inhibiting miR-520c-3p | (121) |
BIK, Bcl2 interacting killer; APAF, apoptotic protease-activating factor-1; CASC-2, cancer susceptibility 2; FAS, apoptosis antigen 1; GAS, growth arrest-specific 5; HOXA-AS2, HOXA cluster antisense 2 RNA; NF-YA, nuclear transcription factor Y; PANDA, p21-associated ncRNA DNA damage activated; TGFBR2, tumor growth factor β receptor 2.
lncRNA PANDA is also highly expressed in breast cancer (126). The expression of PANDA in primary breast cancer cells is induced in response to DNA damage to suppress apoptosis; its expression is reported in cells that do not contain p53 mutations, but PANDA elevated expression has no effect on p53 expression. Instead, it exerts its oncogenic influence in breast cancer cells by hindering the expression of pro-apoptotic proteins, including apoptotic protease-activating factor 1 (APAF1), BIK and FAS (126). Mechanistically, it first interacts with nuclear transcription factor NF-YA, restraining the expression of pro-apoptotic activators. Suppression of PANDA expression promotes apoptosis by upregulating the expression of APAF-1, FAS and BIK gene (127,128).
It has been demonstrated that the expression of lncRNA GAS5 induces apoptosis in breast cancer cells (129). Low GAS5 expression in breast cancer is associated with tumor progression and suppression of the apoptotic pathway (130). It has been found through use of lncRNA RT-PCR arrays that GAS5 expression in breast cancer is modulated by the high expression of miR-21. The exon 4 of GAS5 possesses a binding site for miR-21, and abolition of that site markedly reduces miR-21 affinity for GAS5 and also attenuates suppression of apoptosis in MDA-MB-231 cells (131). In breast cancer, miR-21 negatively regulates expression of the pro-apoptotic protein Bcl-2 (132). It is reported that the ectopic expression of GAS5 downregulates miR-21, which negatively affects the growth of tumor cells and enhances cellular death (131). More data on the tumor-suppressive capability of GAS5 have been provided by Pickard and Williams (129). The study reported that GAS5 contains HREM sequences through which it interacts with the DNA-binding domain of the glucocorticoid receptor, halting cellular growth and promoting apoptosis. The study further demonstrated that HREM oligonucleotides alone also have the capability to induce apoptosis in the absence of endogenous GAS5 expression in resistant breast cancer cells. Unfortunately, the mechanism that is triggered for inducing apoptosis upon employment of HREM sequences is not known.
GAS5 also gives rise to the small RNA pi-sno75, which has direct correlation with enhanced TRAIL expression in breast cancer cells; it utilizes the tool of epigenetic modification to enhance the expression of TRAIL ligand. Mechanistically, pi-sno75 binds with PIWIL1/4 protein. The pair then interact with WD repeat domain 5, which brings about recruitment of human complex of proteins associated with Set 1-like complexes comprising MLL3 and UTX at the promoter region of TRAIL, which causes H3K4 methylation and H3K27 demethylation, hence facilitating activation of TRAIL transcription (133). This finding emphasizes the therapeutic significance of GAS5 and pi-sno75, the exogenous administration of which could promote apoptosis and reduce cellular viability by initiating the TRAIL-induced apoptotic pathway in breast cancer cells.
A few more involvements of lncRNA in breast cancer have been demonstrated to modulate the TRAIL-mediated apoptotic pathway by regulating downstream factors of the TGF-β signaling pathway. It has been well established by various studies that TGF-β induces TRAIL expression, which is necessary for preventing cancerous cell growth (28,134) By contrast, the tumor-suppressive role of TGF-β reverses in advanced types of cancer, including in breast cancer, where it promotes cancer advancement and metastasis by downregulating the expression of TRAIL (135). Long intergenic non-protein coding RNA regulator of reprograming (linc-ROR) lncRNA plays a crucial role in the upregulation of TGF-β expression in advanced stages of cancer (136); it is highly expressed in tumor tissue and also in the highly invasive breast cancer MCF-7 and MDA-MB-231 cell lines. Knockdown of linc-ROR through siRNA in MCF-7 and MDA-MB-231 cells showed that linc-ROR silencing negatively regulates TGF-β and the expression of its downstream factors, which consequently attenuates aggressive tumor growth (136). Unlike lncRNA linc-ROR, the expression of lncRNA CASC2 is downregulated, which facilitates TGF-β pathway activation in advanced breast cancer (137). Induced expression of CASC2 in MCF-7 and LCC-9 cell lines via transfection with pcDNA-CASC2 results in CASC2 overexpression in these cell lines. Furthermore, CASC2 inhibits cell metastasis and promotes cell death by targeting smad-2 (a downstream factor of the TGF-β pathway) and triggering TRAIL apoptosis (137).
TGF-β needs to halt the apoptotic pathway in order to ensure tumor proliferation and metastasis (138). Through the application of northern blotting and qPCR, it has been determined in several mouse breast cancer cell lines, that to prompt suppression of the apoptotic pathway, TGF-β induces the expression of a ~3-kb long transcript of lncRNA Smad7 (139). The results from TUNEL staining and RT-qPCR have established that lncRNA Smad7 functions as a downstream anti-apoptotic factor of TGF-β signaling, the overexpression of which halts apoptosis by inhibiting Bim expression and upregulating anti-apoptotic protein differentiated embryonic chondrocyte-expressed gene 1 expression in invasive breast cancer cell lines (139,140).
In TNBC, the elevated expression of lncRNA ANRIL has also been reported (141). ANRIL uses the TGF-β signaling pathway for tumor exponential growth and suppression of the apoptotic pathway (142). CCK-8 assays in MDA-MB-231 and MDA-MB-468 cell lines have revealed that knocking down ANRIL enhances the rate of apoptosis and reduces cellular proliferation (141). RNA immunoprecipitation and luciferase reporter assays have further demonstrated that ANRIL exerts its oncogenic influence in TNBC cell lines by sponging tumor-suppressive miR-199a, which is reported to downregulate the expression of TGF-β in TNBC (141,143–146). These findings indicate the prognostic significance of ANRIL, whose knockdown in xenografted mice not only attenuates tumor proliferation, but also promotes cell apoptosis (94,141).
Elevated lncRNA HOXA-AS2 expression in tissues and cell lines of breast cancer has direct regulatory control over TGF-β signaling via upregulation of the expression of transforming growth factor β receptor 2 (TGFBR2), which causes tumor proliferation and invasiveness (145). HOXA-AS2 promotes TGFBR2 expression by negatively modulating expression of miR-520c-3p (146). The silencing of HOXA-AS2 causes an elevation in miR-520c-3p levels, which in turn induces suppression of TGFBR2 expression (146) The silencing of HOXA-AS2 in model mice by subcutaneously administrating siRNA-HOXA-AS2-transfected MCF-7 cells leads to a reciprocal increase in miR-520c-3p expression, which by targeting TGFBR2 induces tumor growth inhibition (146). Although this finding emphasizes that HOXA-AS2 could be implemented as a therapeutic target for breast cancer, how miR-520c-3p inhibits TGF-β signaling and activates TRAIL-mediated apoptosis currently needs to be explored.
4. Conclusion
Breast cancer is a highly complex disease involving a number of types and genetics. Thus, an efficient and precise therapeutic regimen for breast cancer patients can only be achieved by rapid and comprehensive prognosis and diagnosis. lncRNAs have crucial implementations in different cancer types; they have established themselves as important regulators of transcription, as well as activators of various signaling cascades. These non-coding RNA molecules are tissue-specific and have the potential to serve as biomarkers for breast cancer. However, few studies have elucidated the involvement of these micromanagers in regulating apoptosis and even fewer have addressed their interplay with TRAIL-mediated apoptosis. Technological advances in bioinformatics, sequencing and mass spectrometry have, to some extent, delineated the role of lncRNA in tumor biology. Identifying lncRNA as non-invasive biomarkers that can be robustly detected in liquid biopsies could revolutionize the way breast cancer is detected. Unearthing the many functions of ncRNAs in cancer development delves into the genomic complexity of cancer and further highlights the extensive interplay between various genetic elements in the cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Authors' contributions
ZJ, KK and BS wrote the manuscript. MI, QR, TA, BS and SR revised the review. HS, JR and WC conceptualized the study and revised it critically. All authors have read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 4.Zur Hausen H, Bund T, de Villiers EM. Specific nutritional infections early in life as risk factors for human colon and breast cancers several decades later. Int J Cancer. 2019;144:1574–1583. doi: 10.1002/ijc.31882. [DOI] [PubMed] [Google Scholar]
- 5.Naoum GE, Buchsbaum DJ, Tawadros F, Farooqi A, Arafat WO. Journey of TRAIL from bench to bedside and its potential role in immuno-oncology. Oncol Rev. 2017;11:332. doi: 10.4081/oncol.2017.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 7.Shi X, Li Y, Sun Y, Zhao X, Sun X, Gong T, Liang Z, Ma Y, Zhang X. Genome-wide analysis of lncRNAs, miRNAs, and mRNAs forming a prognostic scoring system in esophageal squamous cell carcinoma. PeerJ. 2020;8:e8368. doi: 10.7717/peerj.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tirosh I, Suvà ML. Deciphering human tumor biology by single-cell expression profiling. Ann Rev Cancer Biol. 2019;3:151–166. doi: 10.1146/annurev-cancerbio-030518-055609. [DOI] [Google Scholar]
- 9.Farooqi AA, Mukhtar S, Riaz AM, Waseem S, Minhaj S, Dilawar BA, Malik BA, Nawaz A, Bhatti S. Wnt and SHH in prostate cancer: Trouble mongers occupy the TRAIL towards apoptosis. Cell Prolif. 2011;44:508–515. doi: 10.1111/j.1365-2184.2011.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walensky LD. Cheating death: New molecules block BAX. Trends Mol Med. 2019;25:259–261. doi: 10.1016/j.molmed.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazurek N, Byrd JC, Sun Y, Hafley M, Ramirez K, Burks J, Bresalier RS. Cell-surface galectin-3 confers resistance to TRAIL by impeding trafficking of death receptors in metastatic colon adenocarcinoma cells. Cell Death Differ. 2012;19:523–533. doi: 10.1038/cdd.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seyrek K, Richter M, Lavrik IN. Decoding the sweet regulation of apoptosis: The role of glycosylation and galectins in apoptotic signaling pathways. Cell Death Differ. 2019;26:981–993. doi: 10.1038/s41418-019-0317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanova S, Polajnar M, Narbona-Perez AJ, Hernandez-Alvarez MI, Frager P, Slobodnyuk K, Plana N, Nebreda AR, Palacin M, Gomis RR, et al. Regulation of death receptor signaling by the autophagy protein TP53INP2. EMBO J. 2019;38:e99300. doi: 10.15252/embj.201899300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann T, Strasser A, Jost PJ. Fas death receptor signalling: Roles of Bid and XIAP. Cell Death Differ. 2012;19:42–50. doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, et al. TRAIL-R2: A novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahman M, Davis SR, Pumphrey JG, Bao J, Nau MM, Meltzer PS, Lipkowitz S. TRAIL induces apoptosis in triple-negative breast cancer cells with a mesenchymal phenotype. Br Cancer Res Treat. 2009;113:217–230. doi: 10.1007/s10549-008-9924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad M, Shi Y. TRAIL-induced apoptosis of thyroid cancer cells: Potential for therapeutic intervention. Oncogene. 2000;19:3363–3371. doi: 10.1038/sj.onc.1203679. [DOI] [PubMed] [Google Scholar]
- 18.Zhao L, Dong A, Gu J, Liu Z, Zhang Y, Zhang W, Wang Y, He L, Qian C, Qian Q, Liu X. The antitumor activity of TRAIL and IL-24 with replicating oncolytic adenovirus in colorectal cancer. Cancer Gene Ther. 2006;13:1011–1022. doi: 10.1038/sj.cgt.7700969. [DOI] [PubMed] [Google Scholar]
- 19.Brooks AD, Sayers TJ. Reduction of the antiapoptotic protein cFLIP enhances the susceptibility of human renal cancer cells to TRAIL apoptosis. Cancer Immunol Immunother. 2005;54:499–505. doi: 10.1007/s00262-004-0595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voelkel-Johnson C. TRAIL-mediated signaling in prostate, bladder and renal cancer. Nat Rev Urol. 2011;8:417–427. doi: 10.1038/nrurol.2011.81. [DOI] [PubMed] [Google Scholar]
- 21.Cuello M, Ettenberg SA, Nau MM, Lipkowitz S. Synergistic induction of apoptosis by the combination of trail and chemotherapy in chemoresistant ovarian cancer cells. Gynecol Oncol. 2001;81:380–390. doi: 10.1006/gyno.2001.6194. [DOI] [PubMed] [Google Scholar]
- 22.Finnberg NK, El-Deiry WS. TRAIL death receptors as tumor suppressors and drug targets. Cell Cycle. 2008;7:1525–1528. doi: 10.4161/cc.7.11.5975. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Zhang B. TRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4 and 5. Mol Cancer Res. 2008;6:1861–1871. doi: 10.1158/1541-7786.MCR-08-0313. [DOI] [PubMed] [Google Scholar]
- 24.Tollefson AE, Toth K, Doronin K, Kuppuswamy M, Doronina OA, Lichtenstein DL, Hermiston TW, Smith CA, Wold WS. Inhibition of TRAIL-induced apoptosis and forced internalization of TRAIL receptor 1 by adenovirus proteins. J Virol. 2001;75:8875–8887. doi: 10.1128/JVI.75.19.8875-8887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suliman A, Lam A, Datta R, Srivastava RK. Intracellular mechanisms of TRAIL: Apoptosis through mitochondrial-dependent and-independent pathways. Oncogene. 2001;20:2122–2133. doi: 10.1038/sj.onc.1204282. [DOI] [PubMed] [Google Scholar]
- 26.Screaton RA, Kiessling S, Sansom OJ, Millar CB, Maddison K, Bird A, Clarke AR, Frisch SM. Fas-associated death domain protein interacts with methyl-CpG binding domain protein 4: A potential link between genome surveillance and apoptosis. Proc Natl Acad Sci USA. 2003;100:5211–5216. doi: 10.1073/pnas.0431215100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aggarwal BB, Bhardwaj U, Takada Y. Regulation of TRAIL-induced apoptosis by ectopic expression of antiapoptotic factors. Vitamins & Hormones Elsevier. 2004:453–483. doi: 10.1016/S0083-6729(04)67023-3. [DOI] [PubMed] [Google Scholar]
- 28.Jang CW, Chen CH, Chen CC, Chen JY, Su YH, Chen RH. TGF-Beta induces apoptosis through Smad-mediated expression of DAP-kinase. Nat Cell Biol. 2002;4:51–58. doi: 10.1038/ncb731. [DOI] [PubMed] [Google Scholar]
- 29.Kruidering M, Evan GI. Caspase-8 in apoptosis: The beginning of ‘the end’? IUBMB Life. 2000;50:85–90. doi: 10.1080/713803693. [DOI] [PubMed] [Google Scholar]
- 30.Farooqi AA, De Rosa G. TRAIL and microRNAs in the treatment of prostate cancer: Therapeutic potential and role of nanotechnology. Appl Microbiol Biotechnol. 2013;97:8849–8857. doi: 10.1007/s00253-013-5227-9. [DOI] [PubMed] [Google Scholar]
- 31.Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: Decisions between life and death. Int J Biochem Cell Biol. 2007;39:1462–1475. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Han Li C, Chen Y. Small and long non-coding RNAs: Novel targets in perspective cancer therapy. Curr Genomics. 2015;16:319–326. doi: 10.2174/1389202916666150707155851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller V, Oliveira-Ferrer L, Steinbach B, Pantel K, Schwarzenbach H. Interplay of lncRNA H19/miR-675 and lncRNA NEAT1/miR-204 in breast cancer. Mol Oncol. 2019;13:1137–1149. doi: 10.1002/1878-0261.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Javed Z, Ahmed Shah F, Rajabi S, Raza Q, Iqbal Z, Ullah M, Ahmad T, Salehi B, Sharifi-Rad M, Pezzani R, et al. LncRNAs as potential therapeutic targets in thyroid cancer. Asian Pac J Cancer Prev. 2020;21:281–287. doi: 10.31557/APJCP.2020.21.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang A, Bao Y, Wu Z, Zhao T, Wang D, Shi J, Liu B, Sun S, Yang F, Wang L, Qu L. Long noncoding RNA EGFR-AS1 promotes cell growth and metastasis via affecting HuR mediated mRNA stability of EGFR in renal cancer. Cell Death Dis. 2019;10:154. doi: 10.1038/s41419-019-1331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo H, Xu C, Le W, Ge B, Wang T. lncRNA CASC11 promotes cancer cell proliferation in bladder cancer through miRNA-150. J Cell Biochem. 2019;120:13487–13493. doi: 10.1002/jcb.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo J, Wang K, Yeh S, Sun Y, Liang L, Xiao Y, Xu W, Niu Y, Cheng L, Maity SN, et al. LncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat Commun. 2019;10:2571. doi: 10.1038/s41467-019-09784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Ruan F. LncRNA LEF1-AS1 promotes ovarian cancer development through interacting with miR-1285-3p. Cancer Manag Res. 2020;12:687–694. doi: 10.2147/CMAR.S227652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He RZ, Luo DX, Mo YY. Emerging roles of lncRNAs in the post-transcriptional regulation in cancer. Genes Dis. 2019;6:6. doi: 10.1016/j.gendis.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lau E. Non-coding RNA: Zooming in on lncRNA functions. Nat Rev Genet. 2014;15:574–575. doi: 10.1038/nrg3795. [DOI] [PubMed] [Google Scholar]
- 41.van Leeuwen S, Mikkers H. Long non-coding RNAs: Guardians of development. Differentiation. 2010;80:175–183. doi: 10.1016/j.diff.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Ye N, Wang B, Quan ZF, Cao SJ, Wen XT, Huang Y, Huang XB, Wu R, Ma XP, Yan QG, et al. Functional roles of long non-coding RNA in human breast cancer. Asian Pac J Cancer Prev. 2014;15:5993–5997. doi: 10.7314/APJCP.2014.15.15.5993. [DOI] [PubMed] [Google Scholar]
- 43.Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47:199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheetham S, Gruhl F, Mattick J, Dinger M. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108:2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JT, Bartolomei MS. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013;152:1308–1323. doi: 10.1016/j.cell.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 46.Zhang TH, Liang LZ, Liu XL, Wu JN, Su K, Chen JY, Zheng QY. LncRNA UCA1/miR-124 axis modulates TGFβ1-induced epithelial-mesenchymal transition and invasion of tongue cancer cells through JAG1/Notch signaling. J Cell Biochem. 2019;120:10495–10504. doi: 10.1002/jcb.28334. [DOI] [PubMed] [Google Scholar]
- 47.Kawakami T, Zhang C, Taniguchi T, Kim CJ, Okada Y, Sugihara H, Hattori T, Reeve AE, Ogawa O, Okamoto K. Characterization of loss-of-inactive X in Klinefelter syndrome and female-derived cancer cells. Oncogene. 2004;23:6163–6169. doi: 10.1038/sj.onc.1207808. [DOI] [PubMed] [Google Scholar]
- 48.Postlmayr A, Wutz A. Insights into the establishment of chromatin states in pluripotent cells from studies of X inactivation. J Mol Biol. 2017;429:1521–1531. doi: 10.1016/j.jmb.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Hou L, Yin L, Zhao S. LncRNA XIST interacts with miR-454 to inhibit cells proliferation, epithelial mesenchymal transition and induces apoptosis in triple-negative breast cancer. J Biosci. 2020;45:45. doi: 10.1007/s12038-020-9999-7. [DOI] [PubMed] [Google Scholar]
- 50.Zheng R, Lin S, Guan L, Yuan H, Liu K, Liu C, Ye W, Liao Y, Jia J, Zhang R. Long non-coding RNA XIST inhibited breast cancer cell growth, migration, and invasion via miR-155/CDX1 axis. Biochem Biophys Res Commun. 2018;498:1002–1008. doi: 10.1016/j.bbrc.2018.03.104. [DOI] [PubMed] [Google Scholar]
- 51.Zhao L, Zhao Y, He Y, Li Q, Mao Y. The functional pathway analysis and clinical significance of miR-20a and its related lncRNAs in breast cancer. Cell Signal. 2018;51:152–165. doi: 10.1016/j.cellsig.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Li CH. Novel therapeutic targets for hepatocellular carcinoma treatment. Hepatocellular Carcinoma Basic Res. 2012:35. doi: 10.5772/28894. [Google Scholar]
- 54.Battistelli C, Sabarese G, Santangelo L, Montaldo C, Gonzalez FJ, Tripodi M, Cicchini C. The lncRNA HOTAIR transcription is controlled by HNF4α-induced chromatin topology modulation. Cell Death Differ. 2019;26:890–901. doi: 10.1038/s41418-018-0170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qu X, Alsager S, Zhuo Y, Shan B. HOX transcript antisense RNA (HOTAIR) in cancer. Cancer Lett. 2019;454:90–97. doi: 10.1016/j.canlet.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 56.Bhan A, Mandal SS. LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim Biophys Acta. 2015;1856:151–164. doi: 10.1016/j.bbcan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai B, Song X, Cai J, Zhang S. HOTAIR: A cancer-related long non-coding RNA. Neoplasma. 2014;61:379–391. doi: 10.4149/neo_2014_075. [DOI] [PubMed] [Google Scholar]
- 58.Yang Z, Zhou L, Wu LM, Lai MC, Xie HY, Zhang F, Zheng SS. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol. 2011;18:1243–1250. doi: 10.1245/s10434-011-1581-y. [DOI] [PubMed] [Google Scholar]
- 59.Hajjari M, Salavaty A. HOTAIR: An oncogenic long non-coding RNA in different cancers. Cancer Bio Med. 2015;12:1–9. doi: 10.7497/j.issn.2095-3941.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhan A, Hussain I, Ansari KI, Kasiri S, Bashyal A, Mandal SS. Antisense transcript long noncoding RNA (lncRNA) HOTAIR is transcriptionally induced by estradiol. J Mol Biol. 2013;425:3707–3722. doi: 10.1016/j.jmb.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amândio AR, Necsulea A, Joye E, Mascrez B, Duboule D. Hotair is dispensible for mouse development. PLoS Genet. 2016;12:e1006232. doi: 10.1371/journal.pgen.1006232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sørensen KP, Thomassen M, Tan Q, Bak M, Cold S, Burton M, Larsen MJ, Kruse TA. Long non-coding RNA HOTAIR is an independent prognostic marker of metastasis in estrogen receptor-positive primary breast cancer. Breast cancer Res Treat. 2013;142:529–536. doi: 10.1007/s10549-013-2776-7. [DOI] [PubMed] [Google Scholar]
- 63.Tao S, He H, Chen Q. Estradiol induces HOTAIR levels via GPER-mediated miR-148a inhibition in breast cancer. J Transl Med. 2015;13:131. doi: 10.1186/s12967-015-0489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lv R, Zhang J, Zhang W, Huang Y, Wang N, Zhang Q, Qu S. Circulating HOTAIR expression predicts the clinical response to neoadjuvant chemotherapy in patients with breast cancer. Cancer Biomark. 2018;22:249–256. doi: 10.3233/CBM-170874. [DOI] [PubMed] [Google Scholar]
- 65.Zhang M, Wu WB, Wang ZW, Wang XH. lncRNA NEAT1 is closely related with progression of breast cancer via promoting proliferation and EMT. Eur Rev Med Pharmacol Sci. 2017;21:1020–1026. [PubMed] [Google Scholar]
- 66.Li W, Zhang Z, Liu X, Cheng X, Zhang Y, Han X, Zhang Y, Liu S, Yang J, Xu B, et al. The FOXN3-NEAT1-SIN3A repressor complex promotes progression of hormonally responsive breast cancer. J Clin Invest. 2017;127:3421–3440. doi: 10.1172/JCI94233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shin VY, Chen J, Cheuk IW, Siu MT, Ho CW, Wang X, Jin H, Kwong A. Long non-coding RNA NEAT1 confers oncogenic role in triple-negative breast cancer through modulating chemoresistance and cancer stemness. Cell Death Dis. 2019;10:270. doi: 10.1038/s41419-019-1513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qian K, Liu G, Tang Z, Hu Y, Fang Y, Chen Z, Xu X. The long non-coding RNA NEAT1 interacted with miR-101 modulates breast cancer growth by targeting EZH2. Arch Biochem Biophys. 2017;615:1–9. doi: 10.1016/j.abb.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Li X, Wang S, Li Z, Long X, Guo Z, Zhang G, Zu J, Chen Y, Wen L. The lncRNA NEAT1 facilitates cell growth and invasion via the miR-211/HMGA2 axis in breast cancer. Int J Biol Macromol. 2017;105:346–353. doi: 10.1016/j.ijbiomac.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 70.Ke H, Zhao L, Feng X, Xu H, Zou L, Yang Q, Su X, Peng L, Jiao B. NEAT1 is required for survival of breast cancer cells through FUS and miR-548. Gene Regul Syst Bio. 2016;10(Suppl 1):S11–S17. doi: 10.4137/GRSB.S29414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Godinho M, Meijer D, Setyono-Han B, Dorssers LC, van Agthoven T. Characterization of BCAR4, a novel oncogene causing endocrine resistance in human breast cancer cells. J Cell Physiol. 2011;226:1741–1749. doi: 10.1002/jcp.22503. [DOI] [PubMed] [Google Scholar]
- 72.Godinho MF, Wulfkuhle JD, Look MP, Sieuwerts AM, Sleijfer S, Foekens JA, Petricoin EF, III, Dorssers LC, van Agthoven T. BCAR4 induces antioestrogen resistance but sensitises breast cancer to lapatinib. Br J Cancer. 2012;107:947–955. doi: 10.1038/bjc.2012.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xing Z, Park PK, Lin C, Yang L. LncRNA BCAR4 wires up signaling transduction in breast cancer. RNA Biol. 2015;12:681–689. doi: 10.1080/15476286.2015.1053687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Q, Hao Q, Prasanth KV. Nuclear long noncoding RNAs: Key regulators of gene expression. Trends Genet. 2018;34:142–157. doi: 10.1016/j.tig.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Godinho MF, Sieuwerts AM, Look MP, Meijer D, Foekens JA, Dorssers LC, van Agthoven T. Relevance of BCAR4 in tamoxifen resistance and tumour aggressiveness of human breast cancer. Br J Cancer. 2010;103:1284–1291. doi: 10.1038/sj.bjc.6605884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Niknafs YS, Han S, Ma T, Speers C, Zhang C, Wilder-Romans K, Iyer MK, Pitchiaya S, Malik R, Hosono Y, et al. The lncRNA landscape of breast cancer reveals a role for DSCAM-AS1 in breast cancer progression. Nat Commun. 2016;7:12791. doi: 10.1038/ncomms12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Z, Zöller M. Exosomes, metastases, and the miracle of cancer stem cell markers. Cancer Metastasis Rev. 2019;38:259–295. doi: 10.1007/s10555-019-09793-6. [DOI] [PubMed] [Google Scholar]
- 78.Ouyang D, Su J, Huang P, Li M, Li Q, Zhao P, Chen Q, Zou Q, Feng X, Qian K, et al. Identification of lncRNAs via microarray analysis for predicting HER2-negative breast cancer response to neoadjuvant chemotherapy. Int J Clin Exp Pathol. 2018;11:2621–2628. [PMC free article] [PubMed] [Google Scholar]
- 79.Chen YK, Yen Y. The ambivalent role of lncRNA Xist in carcinogenesis. Stem Cell Rev Rep. 2019;15:314–323. doi: 10.1007/s12015-019-9871-z. [DOI] [PubMed] [Google Scholar]
- 80.Mazor G, Levin L, Picard D, Ahmadov U, Carén H, Borkhardt A, Reifenberger G, Leprivier G, Remke M, Rotblat B. The lncRNA TP73-AS1 is linked to aggressiveness in glioblastoma and promotes temozolomide resistance in glioblastoma cancer stem cells. Cell Death Dis. 2019;10:246. doi: 10.1038/s41419-019-1477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y, Yang L, Chen T, Liu X, Guo Y, Zhu Q, Tong X, Yang W, Xu Q, Huang D, Tu K. A novel lncRNA MCM3AP-AS1 promotes the growth of hepatocellular carcinoma by targeting miR-194-5p/FOXA1 axis. Mol Cancer. 2019;18:28. doi: 10.1186/s12943-019-0957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang CL, Qi B, Cai QQ, Fu LS, Yang Y, Tang C, Zhu P, Chen QW, Pan J, Chen MH, Wu XZ. LncRNA AY promotes hepatocellular carcinoma metastasis by stimulating ITGAV transcription. Theranostics. 2019;9:4421–4436. doi: 10.7150/thno.32854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang H, Zhu M, Du Y, Zhang H, Zhang Q, Liu Q, Huang Z, Zhang L, Li H, Xu L, et al. A panel of 12-lncRNA signature predicts survival of pancreatic adenocarcinoma. J Cancer. 2019;10:1550–1559. doi: 10.7150/jca.27823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang G, Lu X, Yuan L. LncRNA: A link between RNA and cancer. Biochim Biophys Acta. 2014;1839:1097–1109. doi: 10.1016/j.bbagrm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 85.Farooqi AA, Attar R, Qureshi MZ, Fayyaz S, Sohail MI, Sabitaliyevich UY, Nurmurzayevich SB, Yelekenova A, Yaylim I, Alaaeddine N. Interplay of long non-coding RNAs and TGF/SMAD signaling in different cancers. Cell Mol Biol (Noisy-le-Grand) 2018;64:1–6. doi: 10.14715/cmb/2017.64.15.1. [DOI] [PubMed] [Google Scholar]
- 86.Jiang Y, Lin L, Zhong S, Cai Y, Zhang F, Wang X, Miao R, Zhang B, Gao S, Hu X. Overexpression of novel lncRNA NLIPMT inhibits metastasis by reducing phosphorylated glycogen synthase kinase 3β in breast cancer. J Cell Physiol. 2019;234:10698–10708. doi: 10.1002/jcp.27738. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y, Sharma S, Watabe K. Roles of lncRNA in breast cancer. Front Biosci (Schol Ed) 2015;7:94–108. doi: 10.2741/s427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guan Y, Bhandari A, Xia E, Yang F, Xiang J, Wang O. lncRNA FOXD3-AS1 is associated with clinical progression and regulates cell migration and invasion in breast cancer. Cell Biochem Funct. 2019;37:239–244. doi: 10.1002/cbf.3393. [DOI] [PubMed] [Google Scholar]
- 89.Liu AN, Qu HJ, Gong WJ, Xiang JY, Yang MM, Zhang W. LncRNA AWPPH and miRNA-21 regulates cancer cell proliferation and chemosensitivity in triple-negative breast cancer by interacting with each other. J Cell Biochem. 2019;120:14860–14866. doi: 10.1002/jcb.28747. [DOI] [PubMed] [Google Scholar]
- 90.Shi SJ, Wang LJ, Yu B, Li YH, Jin Y, Bai XZ. LncRNA-ATB promotes trastuzumab resistance and invasion-metastasis cascade in breast cancer. Oncotarget. 2015;6:11652–11663. doi: 10.18632/oncotarget.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Augoff K, McCue B, Plow EF, Sossey-Alaoui K. miR-31 and its host gene lncRNA LOC554202 are regulated by promoter hypermethylation in triple-negative breast cancer. Mol Cancer. 2012;11:5. doi: 10.1186/1476-4598-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Z, Hou P, Fan D, Dong M, Ma M, Li H, Yao R, Li Y, Wang G, Geng P, et al. The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 2017;24:59–71. doi: 10.1038/cdd.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo R, Su Y, Xue J, Si J, Chi Y, Wu J. Abstract P6-05-01: A novel cleaved cytoplasmic lncRNA LacRNA interacts with PHB2 and suppresses breast cancer metastasis via repressing MYC targets. Cancer Res. 2019:79. doi: 10.1158/1538-7445. [Google Scholar]
- 94.Li W, Jia G, Qu Y, Du Q, Liu B, Liu B. Long non-coding RNA (LncRNA) HOXA11-AS promotes breast cancer invasion and metastasis by regulating epithelial-mesenchymal transition. Med Sci Monit. 2017;23:3393–3403. doi: 10.12659/MSM.904892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Z, Dong M, Fan D, Hou P, Li H, Liu L, Lin C, Liu J, Su L, Wu L, et al. LncRNA ANCR down-regulation promotes TGF-β-induced EMT and metastasis in breast cancer. Oncotarget. 2017;8:67329–67343. doi: 10.18632/oncotarget.18622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Naval J, de Miguel D, Gallego-Lleyda A, Anel A, Martinez-Lostao L. Importance of TRAIL molecular anatomy in receptor oligomerization and signaling. Implications for Cancer Therapy. Cancers (Basel) 2019;11:444. doi: 10.3390/cancers11040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mert U, Sanlioglu AD. Intracellular localization of DR5 and related regulatory pathways as a mechanism of resistance to TRAIL in cancer. Cell Mol Life Sci. 2017;74:245–255. doi: 10.1007/s00018-016-2321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li T, Liu Y, Xiao H, Xu G. Long non-coding RNA TUG1 promotes cell proliferation and metastasis in human breast cancer. Breast Cancer. 2017;24:535–543. doi: 10.1007/s12282-016-0736-x. [DOI] [PubMed] [Google Scholar]
- 99.Yang J, Meng X, Yu Y, Pan L, Zheng Q, Lin W. LncRNA POU3F3 promotes proliferation and inhibits apoptosis of cancer cells in triple-negative breast cancer by inactivating caspase 9. Biosci Biotechnol Biochem. 2019;83:1117–1123. doi: 10.1080/09168451.2019.1588097. [DOI] [PubMed] [Google Scholar]
- 100.Shan TD, Xu JH, Yu T, Li JY, Zhao LN, Ouyang H, Luo S, Lu XJ, Huang CZ, Lan QS, et al. Knockdown of linc-POU3F3 suppresses the proliferation, apoptosis, and migration resistance of colorectal cancer. Oncotarget. 2016;7:961–975. doi: 10.18632/oncotarget.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rossi MN, Antonangeli F. LncRNAs: New players in apoptosis control. Int J Cell Biol. 2014;2014:473857. doi: 10.1155/2014/473857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qu Y, Wang Y, Wang P, Lin N, Yan X, Li Y. Overexpression of long noncoding RNA HOXA-AS2 predicts an adverse prognosis and promotes tumorigenesis via SOX4/PI3K/AKT pathway in acute myeloid leukemia. Cell Biol Int. 2020 May 5; doi: 10.1002/cbin.11370. doi: 10.1002/cbin.11370 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 103.Awasthee N, Rai V, Verma SS, Francis KS, Nair MS, Gupta SC. Anti-cancer activities of Bharangin against breast cancer: Evidence for the role of NF-κB and lncRNAs. Biochim Biophys Acta Gen Subj. 2018;1862:2738–2749. doi: 10.1016/j.bbagen.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 104.Dianatpour A, Faramarzi S, Geranpayeh L, Mirfakhraie R, Motevaseli E, Ghafouri-Fard S. Expression analysis of AFAP1-AS1 and AFAP1 in breast cancer. Cancer Biomark. 2018;22:49–54. doi: 10.3233/CBM-170831. [DOI] [PubMed] [Google Scholar]
- 105.Zhang H, Lu B. microRNAs as biomarkers of ovarian cancer. Expert Rev Anticancer Ther. 2020;20:373–385. doi: 10.1080/14737140.2020.1760095. [DOI] [PubMed] [Google Scholar]
- 106.Huang YS, Chang CC, Lee SS, Jou YS, Shih HM. Xist reduction in breast cancer upregulates AKT phosphorylation via HDAC3-mediated repression of PHLPP1 expression. Oncotarget. 2016;7:43256. doi: 10.18632/oncotarget.9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gooding AJ, Zhang B, Jahanbani FK, Gilmore HL, Chang JC, Valadkhan S, Schiemann WP. The lncRNA BORG drives breast cancer metastasis and disease recurrence. Sci Rep. 2017;7:12698. doi: 10.1038/s41598-017-12716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gooding AJ, Zhang B, Gunawardane L, Beard A, Valadkhan S, Schiemann WP. The lncRNA BORG facilitates the survival and chemoresistance of triple-negative breast cancers. Oncogene. 2019;38:2020. doi: 10.1038/s41388-018-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deng R, Liu B, Wang Y, Yan F, Hu S, Wang H, Wang T, Li B, Deng X, Xiang S, Yang Y, Zhang J. High expression of the newly found long noncoding RNA Z38 promotes cell proliferation and oncogenic activity in breast cancer. J Cancer. 2016;7:576–578. doi: 10.7150/jca.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y, Zheng C, Li T, Zhang R, Wang Y, Zhang J, He Q, Sun Z, Wang X. Long noncoding RNA Z38 promotes cell proliferation and metastasis and inhibits cell apoptosis in human gastric cancer. Oncolo Lett. 2018;16:6051–6058. doi: 10.3892/ol.2018.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nie ZL, Wang YS, Mei YP, Lin X, Zhang GX, Sun HL, Wang YL, Xia YX, Wang SK. Prognostic significance of long noncoding RNA Z38 as a candidate biomarker in breast cancer. J Clin Lab Anal. 2018;32:e22193. doi: 10.1002/jcla.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang F, Li J, Xiao H, Zou Y, Liu Y, Huang W. AFAP1-AS1: A novel oncogenic long non-coding RNA in human cancers. Cell Proliferation. 2018;51:e12397. doi: 10.1111/cpr.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma D, Chen C, Wu J, Wang H, Wu D. Up-regulated lncRNA AFAP1-AS1 indicates a poor prognosis and promotes carcinogenesis of breast cancer. Breast Cancer. 2019;26:74–83. doi: 10.1007/s12282-018-0891-3. [DOI] [PubMed] [Google Scholar]
- 114.Fan S, Yang Z, Ke Z, Huang K, Liu N, Fang X, Wang K. Downregulation of the long non-coding RNA TUG1 is associated with cell proliferation, migration, and invasion in breast cancer. Biomed Pharmacother. 2017;95:1636–1643. doi: 10.1016/j.biopha.2017.09.076. [DOI] [PubMed] [Google Scholar]
- 115.Tang T, Cheng Y, She Q, Jiang Y, Chen Y, Yang W, Li Y. Long non-coding RNA TUG1 sponges miR-197 to enhance cisplatin sensitivity in triple negative breast cancer. Biomed Pharmacother. 2018;107:338–346. doi: 10.1016/j.biopha.2018.07.076. [DOI] [PubMed] [Google Scholar]
- 116.Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, Los M. Apoptosis and cancer: Mutations within caspase genes. J Med Genet. 2009;46:497–510. doi: 10.1136/jmg.2009.066944. [DOI] [PubMed] [Google Scholar]
- 117.Rossin A, Miloro G, Hueber AO. TRAIL and FasL functions in cancer and autoimmune diseases: Towards an increasing complexity. Cancers. 2019;11:639. doi: 10.3390/cancers11050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eberle J. Countering TRAIL resistance in melanoma. Cancers. 2019;11:656. doi: 10.3390/cancers11050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kolben T, Jeschke U, Reimer T, Karsten N, Schmoeckel E, Semmlinger A, Mahner S, Harbeck N, Kolben TM. Induction of apoptosis in breast cancer cells in vitro by Fas ligand reverse signaling. J Cancer Res Clin Oncol. 2018;144:249–256. doi: 10.1007/s00432-017-2551-y. [DOI] [PubMed] [Google Scholar]
- 120.Yang Y, Yang H, Xu M, Zhang H, Sun M, Mu P, Dong T, Du S, Liu K. Long non-coding RNA (lncRNA) MAGI2-AS3 inhibits breast cancer cell growth by targeting the Fas/FasL signalling pathway. Hum Cell. 2018;31:232–241. doi: 10.1007/s13577-018-0206-1. [DOI] [PubMed] [Google Scholar]
- 121.Si X, Zang R, Zhang E, Liu Y, Shi X, Zhang E, Shao L, Li A, Yang N, Han X, et al. LncRNA H19 confers chemoresistance in ERα-positive breast cancer through epigenetic silencing of the pro-apoptotic gene BIK. Oncotarget. 2016;7:81452–81462. doi: 10.18632/oncotarget.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun H, Wang G, Peng Y, Zeng Y, Zhu QN, Li TL, Cai JQ, Zhou HH, Zhu YS. H19 lncRNA mediates 17β-estradiol-induced cell proliferation in MCF-7 breast cancer cells. Oncol Rep. 2015;33:3045–3052. doi: 10.3892/or.2015.3899. [DOI] [PubMed] [Google Scholar]
- 123.Zhang K, Luo Z, Zhang Y, Zhang L, Wu L, Liu L, Yang J, Song X, Liu J. Circulating lncRNA H19 in plasma as a novel biomarker for breast cancer. Cancer Biomark. 2016;17:187–194. doi: 10.3233/CBM-160630. [DOI] [PubMed] [Google Scholar]
- 124.Lin Y, Tao H. Diagnostic value of plasma exosomal lncRNA H19 for breast cancer. Chin J Clin Laboratory Sci. 2018;36:99–101. [Google Scholar]
- 125.Han J, Han B, Wu X, Hao J, Dong X, Shen Q, Pang H. Knockdown of lncRNA H19 restores chemo-sensitivity in paclitaxel-resistant triple-negative breast cancer through triggering apoptosis and regulating Akt signaling pathway. Toxicol Appl Pharmacol. 2018;359:55–61. doi: 10.1016/j.taap.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 126.Li J, Tian H, Yang J, Gong Z. Long noncoding RNAs regulate cell growth, proliferation, and apoptosis. DNA Cell Biol. 2016;35:459–470. doi: 10.1089/dna.2015.3187. [DOI] [PubMed] [Google Scholar]
- 127.Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P, et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011;43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang A, Xu M, Mo YY. Role of the lncRNA-p53 regulatory network in cancer. J Mol Cell Biol. 2014;6:181–191. doi: 10.1093/jmcb/mju013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pickard MR, Williams GT. The hormone response element mimic sequence of GAS5 lncRNA is sufficient to induce apoptosis in breast cancer cells. Oncotarget. 2016;7:10104. doi: 10.18632/oncotarget.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zong Y, Zhang Y, Sun X, Xu T, Cheng X, Qin Y. miR-221/222 promote tumor growth and suppress apoptosis by targeting lncRNA GAS5 in breast cancer. Biosci Rep. 2019;39:BSR20181859. doi: 10.1042/BSR20181859. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 131.Zhang Z, Zhu Z, Watabe K, Zhang X, Bai C, Xu M, Wu F, Mo YY. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013;20:1558–1568. doi: 10.1038/cdd.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wickramasinghe NS, Manavalan TT, Dougherty SM, Riggs KA, Li Y, Klinge CM. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009;37:2584–2595. doi: 10.1093/nar/gkp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.He X, Chen X, Zhang X, Duan X, Pan T, Hu Q, Zhang Y, Zhong F, Liu J, Zhang H, et al. An Lnc RNA (GAS5)/SnoRNA-derived piRNA induces activation of TRAIL gene by site-specifically recruiting MLL/COMPASS-like complexes. Nucleic Acids Res. 2015;43:3712–3725. doi: 10.1093/nar/gkv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Y, Chu J, Yi P, Dong W, Saultz J, Wang Y, Wang H, Scoville S, Zhang J, Wu LC, et al. SMAD4 promotes TGF-β-independent NK cell homeostasis and maturation and antitumor immunity. J Clin Invest. 2018;128:5123–5136. doi: 10.1172/JCI121227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cano-González A, López-Rivas A. Opposing roles of TGF-β and EGF in the regulation of TRAIL-induced apoptosis in human breast epithelial cells. Biochim Biophys Acta. 2016;1863:2104–2114. doi: 10.1016/j.bbamcr.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 136.Hou L, Tu J, Cheng F, Yang H, Yu F, Wang M, Liu J, Fan J, Zhou G. Long noncoding RNA ROR promotes breast cancer by regulating the TGF-β pathway. Cancer Cell Int. 2018;18:142. doi: 10.1186/s12935-018-0638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Y, Zhu M, Sun Y, Li W, Wang Y, Yu W. Upregulation of lncRNA CASC2 suppresses cell proliferation and metastasis of breast cancer via inactivation of the TGF-β signaling pathway. Oncol Res. 2019;27:379–387. doi: 10.3727/096504018X15199531937158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arase M, Horiguchi K, Ehata S, Morikawa M, Tsutsumi S, Aburatani H, Miyazono K, Koinuma D. Transforming growth factor-β-induced lnc RNA-Smad7 inhibits apoptosis of mouse breast cancer JygMC(A) cells. Cancer Sci. 2014;105:974–982. doi: 10.1111/cas.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hoshino Y, Katsuno Y, Ehata S, Miyazono K. Autocrine TGF-β protects breast cancer cells from apoptosis through reduction of BH3-only protein, Bim. J Biochem. 2011;149:55–65. doi: 10.1093/jb/mvq114. [DOI] [PubMed] [Google Scholar]
- 141.Xu ST, Xu JH, Zheng ZR, Zhao QQ, Zeng XS, Cheng SX, Liang YH, Hu QF. Long non-coding RNA ANRIL promotes carcinogenesis via sponging miR-199a in triple-negative breast cancer. Biomed Pharmacother. 2017;96:14–21. doi: 10.1016/j.biopha.2017.09.107. [DOI] [PubMed] [Google Scholar]
- 142.Zhao JJ, Hao S, Wang LL, Hu CY, Zhang S, Guo LJ, Zhang G, Gao B, Jiang Y, Tian WG, Luo DL. Long non-coding RNA ANRIL promotes the invasion and metastasis of thyroid cancer cells through TGF-β/Smad signaling pathway. Oncotarget. 2016;7:57903–57918. doi: 10.18632/oncotarget.11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen J, Shin VY, Siu MT, Ho JC, Cheuk I, Kwong A. miR-199a-5p confers tumor-suppressive role in triple-negative breast cancer. BMC Cancer. 2016;16:887. doi: 10.1186/s12885-016-2916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang Y, Fan KJ, Sun Q, Chen AZ, Shen WL, Zhao ZH, Zheng XF, Yang X. Functional screening for miRNAs targeting Smad4 identified miR-199a as a negative regulator of TGF-β signalling pathway. Nucleic Acids Res. 2012;40:9286–9297. doi: 10.1093/nar/gks667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang J, Su Z, Lu S, Fu W, Liu Z, Jiang X, Tai S. LncRNA HOXA-AS2 and its molecular mechanisms in human cancer. Clin Chim Acta. 2018;485:229–233. doi: 10.1016/j.cca.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 146.Fang Y, Wang J, Wu F, Song Y, Zhao S, Zhang Q. Long non-coding RNA HOXA-AS2 promotes proliferation and invasion of breast cancer by acting as a miR-520c-3p sponge. Oncotarget. 2017;8:46090. doi: 10.18632/oncotarget.17552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.