Abstract
Long-chain non-coding RNAs (lncRNAs) are RNA molecules with a length greater than 200 nt and no function of encoding proteins. lncRNAs play a precise regulatory function at different levels of transcription and post-transcription, and they interact with various regulatory factors to regulate gene expression, and then participate in cell growth, differentiation, apoptosis, and other life processes. In recent years, studies have shown that the abnormal expression of lncRNAs is closely related to the occurrence and development of tumors, which is expected to become an effective biomarker in tumor diagnosis. The sequencing analysis of mutations in the whole tumor genome suggests that mutations in non-coding regions may play an important role in the occurrence and development of tumors. Therefore, in-depth study of lncRNAs is helpful to clarify the molecular mechanism of tumor occurrence and development and to provide new targets for tumor diagnosis and treatment. This review introduces the molecular mechanism and clinical application prospect of lncRNAs affecting tumor development from the perspective of gene expression and regulation.
Keywords: lncRNA, tumor, gene regulation, diagnostic marker, therapeutic target
Graphical Abstract
lncRNAs play a precise regulatory function at different levels of transcription and post-transcription and participate in cell growth, differentiation, and apoptosis, and are related to the occurrence and development of tumors, which is expected to become an effective biomarker in tumor diagnosis and treatment.
Main Text
Cancer is fundamentally a disease of genotype.1, 2, 3 The discovery of protein genetic codon mutations is a breakthrough to understanding the mechanism of these mutations driving tumor development, so as to establish scientific principles for targeted treatment of malignant tumors.4, 5, 6 The human genome project found that less than 3% of the genes in the human genome can be encoded into proteins, which means that the non-coding part has greater potential to drive the characterization of tumors, and there is evidence that if the non-coding region changes, it can affect the expression and regulation of genes, leading to the formation of tumors.7, 8, 9 In recent years, with the deepening of cancer research, the mutation of non-coding genes, the change of epigenetic structures, and the change of genome structures can drive the generation of tumors.10, 11, 12, 13 From the point of view of gene expression and the regulation process, the gene expression and regulation of tumor cells are different from those of normal cells, so that they have the ability of infinite proliferation, even invasion and metastasis.14
At present, it has been confirmed that long-chain non-coding RNAs (lncRNAs) not only affect the growth and development of embryos, participate in the maintenance of organ and tissue functions, regulate the stability of the immune system, and protect the integrity of telomere structure, but they also are related to the occurrence and development of tumors.15, 16, 17, 18 The abnormal expression of lncRNAs often plays a role in promoting or inhibiting tumor development.19 lncRNAs participate in the regulation of gene expression and are involved in the biological mechanism of tumor development.20 They are expected to become biomarkers for early diagnosis, treatment, and prognosis of tumors.21 The expression of lncRNAs in tumor cells has certain specificity.22 Its expression level is affected by many factors. Abnormal epigenetic modification is one of the important factors that cause lncRNA expression disorder and disease.23 In this review, the biological behavior of lncRNAs in tumors is described, and the role of lncRNAs in tumor occurrence and development, as well as the potential significance of lncRNAs in early clinical diagnosis, prognosis, and treatment target, are explained.
Discovery of lncRNAs
A lncRNA is a kind of nucleic acid molecule with a length of more than 200 nt, lack of a complete specific open reading frame, and no function of a coding protein.24 Generally speaking, lncRNA refers in the narrow sense to lncRNA excluding rRNA, which can be transcribed into more than 200,000 kinds.25 Although there are many kinds of lncRNAs, most of the copies in cells are relatively low; some, even with several cells, contain one copy.26 Most of the annotated lncRNAs are expressed in specific cell types, and usually at lower levels than the protein coding genes.27
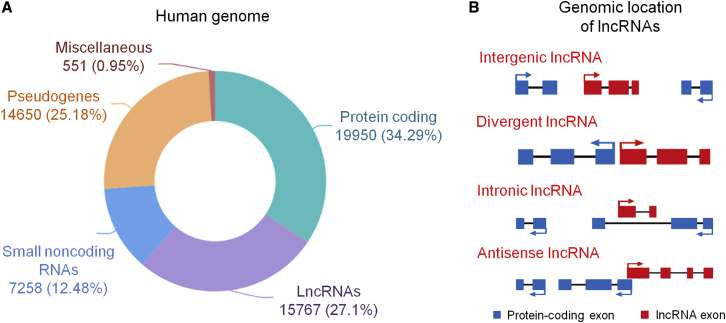
lncRNAs can be transcribed from the antisense strand, promoter region, intron region, and intergenic region of mRNAs.28, 29, 30 That is, lncRNAs can be transcribed from anywhere in the genome. According to the location of lncRNAs in the genome, lncRNAs can be divided into three categories: long gene non-coding RNAs (lincRNAs), natural antisense transcripts (NATs), and intron lncRNAs.31, 32, 33 In addition to the lincRNAs located between the two protein coding genes, most of the lncRNAs and the adjacent protein coding genes have a certain degree of gene sequence overlap.29,34,35 For example, intron lncRNAs are transcribed from the intron of the protein coding gene, whereas NATs are transcribed from the opposite (complementary) chain of the protein coding gene.36 Antisense lncRNA is especially common in mice.37 Up to 72% of the genomic sites show that differential transcription leads to the production of antisense lncRNA38 (Figure 1).
Figure 1.
The Abundance and Classification of lncRNAs
(A) The abundance of protein-coding and non-protein-coding genes in the human genome. The data represent GENCODE v25 estimates. (B) Classification of lncRNAs based on their genomic location with respect to nearby protein-coding genes.
Most lncRNAs are transcribed from RNA polymerase II, which are spliced and matured.39 Similar to mRNAs, most lncRNAs are blocked, polyadenylated, and spliced.40 Their primary structures are poorly conserved, but their secondary structures and splicing patterns are functionally conserved with tissue or cell specificity.41 The classification of lncRNAs is based on the idea that RNAs with a base number greater than 200 nt can form various complex high-level structures, which can be distinguished from microRNAs (miRNAs).42 In fact, however, when the base number of an RNA is 50–70 nt, some complex structures can be formed.43 In addition to the linear structure, lncRNAs also have circular RNAs, which may be affected by the structure. The half-life of circular RNAs is longer and more stable than that of linear lncRNAs.44 They also have tissue-specific expression in a specific period, affect the growth and development process, and cause diseases such as tumors.45
lncRNAs Participate in Gene Expression and the Regulation Mechanism
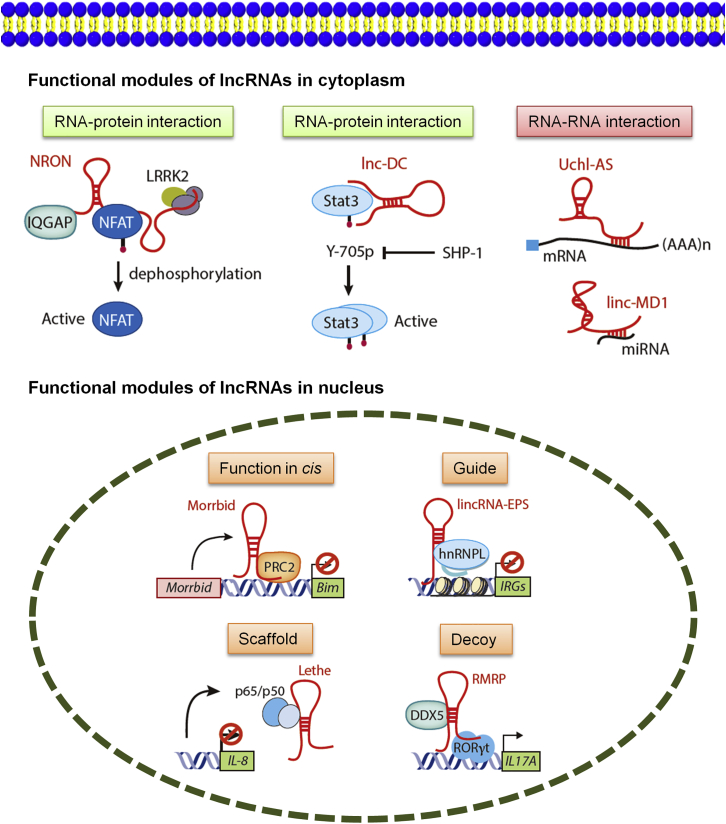
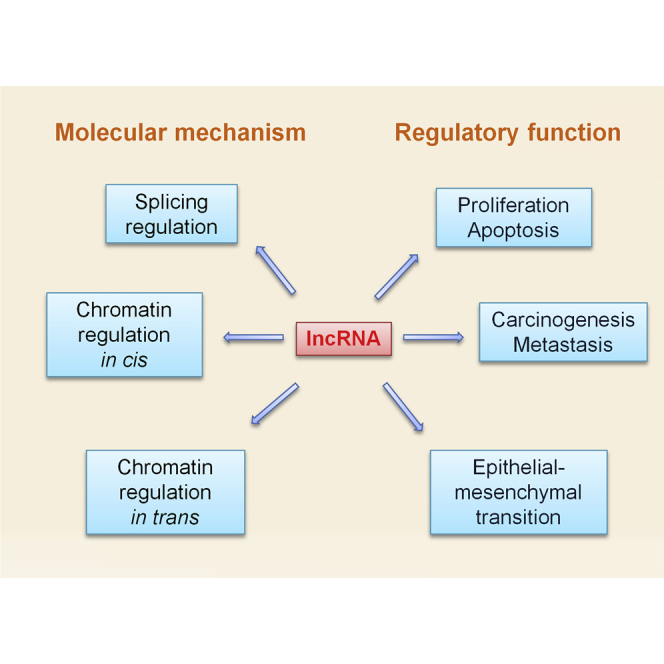
lncRNAs can interact through RNA-protein, RNA-RNA, or RNA-DNA interactions (Figure 2). lncRNAs can target all levels of gene regulation, including transcription, mRNA stability, and translation. In cytosol, lncRNAs are known to interact with RNA or protein to achieve their molecular functions. For some base pairs of lncRNAs and mRNAs, this interaction can lead to changes in the level of these mRNAs. lincRNA linc-MD1 acts as a competitive endogenous lncRNA to inhibit miR-133.35 Antisense lncRNA UCHL1 promotes the translation of UCHL1 mRNA by enhancing the binding of UCHL1 mRNA to the polymer.22 On the contrary, some lncRNAs, such as lincRNA-p21, are paired with the target mRNA to inhibit their translation. However, a more common pattern of lncRNA interaction involves interaction with one or more specific proteins. In the nucleus, lncRNAs regulate gene expression through various mechanisms. lncRNAs, as modular guidance and scaffolds for proteins, can recruit proteins or RNAs.8 These complexes successively assemble high-order protein/RNA complexes in cells. These proteins cooperate with each other to deposit inhibitory histone markers to silence gene expression of target gene sites. Because lncRNAs guide proteins to specific genomic sites or act as molecular scaffolds to stabilize protein complexes, lncRNAs may also contribute to the functional diversity of DNA-binding proteins.
Figure 2.
lncRNA Participates in Gene Expression and the Regulation Mechanism
lncRNAs mediate their molecular functions through a multitude of mechanisms in the cytoplasm or the nucleus. In the cytoplasm, lncRNAs act through RNA-protein (e.g., NRON and lnc-DC) or RNA-RNA (e.g., Uchl1-AS and linc-MD1) interactions. In the nucleus, lncRNAs can act in cis or trans. A lncRNA can interact with its protein partner as a guide (e.g., lincRNA-EPS:hnRNPL), scaffold (e.g., RMRP interaction with DDX5), or decoy molecule (e.g., Lethe: NF-κB p65) to mediate its molecular functions.
Gene Regulation in the Chromatin Level
The regulation of lncRNA on chromatin mainly affects the structure of chromatin, thus changing gene expression. For example, it interacts with the chromosomal remodeling factor complex, which causes chromatin remodeling, activates or silences gene expression, and affects the occurrence of disease.46 By reducing the location of nucleosome in the gene promoter region, the inhibition of gene expression is maintained, leading to disease through epigenetics, and the chromosome can be methylated to inhibit gene expression.13 These genes are usually tumor suppressor proteins, because when their expression is inhibited the occurrence of tumors is promoted.
There are two modes of lncRNA regulation at the chromatin level: cis regulation and trans regulation. cis regulation generally refers to when lncRNA plays a regulatory role in the genes adjacent to its transcription region.47 The most classic example is X-inactive-specific transcript (XIST). The Pasteurella in the nucleus of female mammals is actually a condensed X chromosome, which is formed by the random inactivation of X chromosome due to the metrological compensation effect of cells.48 Inactivation of the X chromosome is mainly accomplished by the cis function of lncRNA XIST. XIST is a 17- to 20-kb RNA transcribed from the X chromosome. It begins to wrap the X chromosome at the initial stage of inactivation.49 By binding with combed protein inhibitor complex 2, H3K27me3 occurs at the position of histone H3K27, which affects the transcription of the chromosome and silences it.50 For another example, INK4 protein is a tumor suppressor, and the expression of INK4α/INK4β is also regulated by the antisense chain of its locus, which is transcribed from lncRNA ANRIL (antisense non-coding RNA in the INK4 locus).51 lncRNA ANRIL can bind to chromo domain homolog 7 (CBX7). CBX7 is a member of the PRC1 and PRC2 complex. The combination of lncRNA ANRIL and CBX7 makes the histone of this gene site methylated and inhibits the expression of INK4β.52 lncRNA ANRIL was initially found to be absent in hereditary tumors of the nervous system. It was found that this RNA was also expressed abnormally in hereditary melanoma of the skin.53
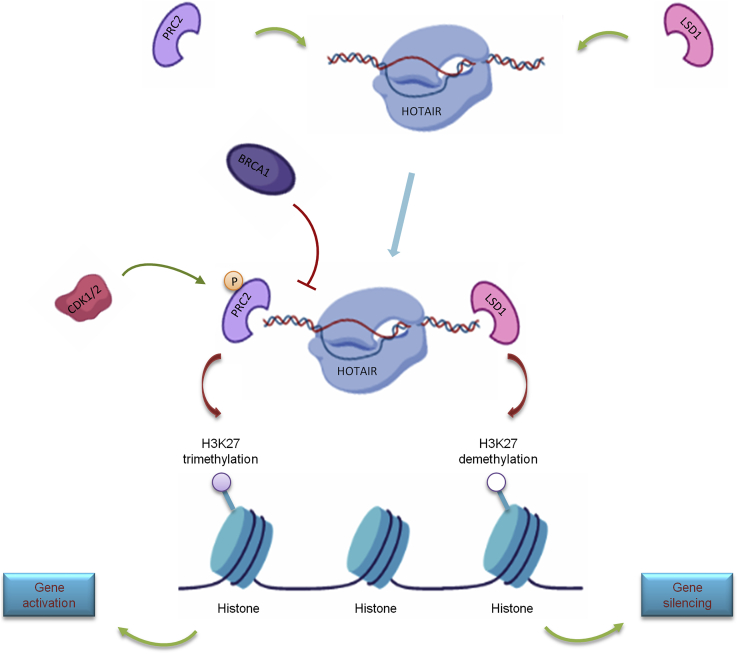
The trans regulatory RNA is different from the cis regulatory RNA, and the RNA and the regulated gene are often located on different chromosomes or both on the same chromosome but far apart.54 One of the most famous examples is lncRNA HOTAIR (Hox script antisense RNA). It is a 2.1-kb-long lncRNA transcribed from the HoxC locus on chromosome 12. Its 5′ end can combine with the polycombin complex PRC2, which makes the histone in the HoxD locus region on chromosome 2 undergo epigenetic modification and H3K27me3, resulting in the silencing of HoxD gene expression.55 In lung cancer, the expression of HOTAIR is high, which is to make H3K27me3 on the tumor suppressor gene by combining with the PRC2 complex, and then silence the expression.56 However, the mechanism of HOTAIR and PRC2 combination is not clear at present, and a thorough understanding of the mechanism of their combination may become a new strategy for cancer treatment in the future17 (Figure 3).
Figure 3.
Gene Silencing Mechanism Induced by HOTAIR
HOTAIR interacts with PRC2 and LSD1 complexes to recruit them to target genes. PRC2 trimethylated histone H3K27 and LSD1 induced H3K4 demethylation, which silenced the target gene. BRCA1 can compete with HOTAIR to combine PRC2 and inhibit the combination of HOTAIR and PRC2. CDK1/2 mediates the phosphorylation of PRC2 and promotes HOTAIR activation.
Of course, there are also some non-coding RNAs that can be either cis regulated or trans regulated to play different functions, such as the repetitive repeat-containing RNAs produced by telomere transcription.57 Telomere is located at both ends of linear chromatin and, if abnormal, it will lead to aging or cancer and other diseases. Previous studies have pointed out that lncRNA TERRA, together with telomere protein, forms a cap-like structure at the end of chromatin to protect the integrity of chromosomes.16,58,59 A recent study pointed out that TERRA can not only cis act on adjacent telomeres, regulating the activity of telomerase, but also trans act on other genes, antagonizing each other with RNA helicase ATRX, thus affecting the expression of its target gene.60
Transcriptional-Level Regulation
The effect of lncRNA on gene transcription is mainly realized by transcription factors. Mouse retrotransposon VL30 can change the conformation of PSF (polypyrimidine track-binding protein-associated splicing factor), which should be the first non-coding RNA that can directly bind to protein.61 PSF protein can inhibit the expression of many proto-oncogenes, thus inhibiting the proliferation and migration of tumor cells; PSF protein has two RNA-binding domains (RBDs) and one DNA-binding domain (DBD) in structure.62 Under normal circumstances, the DNA-binding region of the PSF protein can be bound to the promoter of the target gene to inhibit the expression of the target gene. However, when VL30 exists, the RNA-binding region of PSF protein binds to VL30, so as to change the conformation of the PSF protein, so that it can no longer be bound to the promoter of the gene, and the target gene can be expressed.63 One of the characteristics of solid tumors is hypoxia. Malignant tumors will accelerate growth and metastasis under a hypoxic environment. Hypoxia inducible factor-1 (HIF-1), which is composed of one α subunit and one β subunit, is the regulator of cells in response to hypoxia.64 In a hypoxic environment, HIF-1 will be located in the nucleus, on the promoter of its target gene, and activate the transcription of the target gene.65 The target gene of HIF-1 is related to the occurrence and development of tumors, such as glycolysis, energy metabolism, and cell migration, among others.66 lncHIFCAR (long non-coding HIF-1α-coactivating RNA) seems to reveal the mechanism of HIF-1 in activating downstream genes. The expression of lncHIFCAR in hypoxia is twice as much as that in the normal condition. It can be combined with HIF-1α to locate on the promoter of the target gene. Meanwhile, HIF-1 and transcription cofactor p300 are recruited to work together to activate the expression of downstream genes.67
Posttranscriptional-Level Regulation
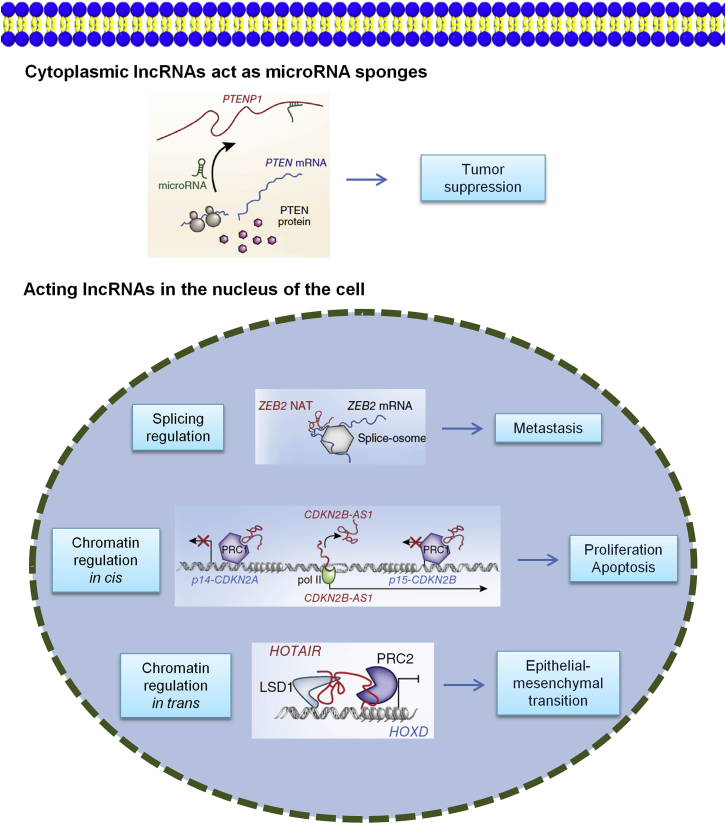
The regulation of lncRNA on the post-transcriptional level of genes mainly affects the variable RNA cutting, RNA stability, and translation (Figure 4).
Figure 4.
Diverse Mechanisms of Cancer-Related lncRNAs
Acting in the nucleus of the cell, some lncRNAs affect the expression of proximally located genes, such as ANRIL (CDKN2B-AS1), which mediates the epigenetic silencing of two genes on the same locus, CDKN2A and CDKN2B, inducing cell proliferation. HOTAIR promotes metastasis in breast cancer by targeting distant genes, such as those in the HOXD cluster, for epigenetic silencing by the PRC2 complex. Other nuclear lncRNAs act post-transcriptionally, such as the NAT of ZEB2 mRNA, ZEB2. The ZEB2 NAT blocks splicing of ZEB2 mRNA, promoting the use of an internal ribosome entry site for translation initiation and delivering high ZEB2 protein levels, which induces epithelial-to-mesenchymal transition. In contrast, a number of cytoplasmic lncRNAs may act as microRNA sponges. For instance PTENP1 binds to microRNAs that otherwise bind to the 3′ untranslated region of PTEN mRNA, reducing its expression and tumor suppressor activity.
Regulation of Variable Cutting
After eukaryotic genes are transcribed into precursor (pre-)mRNA, their introns will be cut off, and their exons will be spliced in different ways, called variable splicing. Therefore, a gene can be encoded into different proteins. More than 95% of the genes in human cells have variable cleavage, which makes the same gene express different proteins in different cells and tissues,68 including small nuclear ribonucleoproteins (snRNPs), the serine/arginine-rich (SR) family of nuclear phosphoproteins (SR proteins) and their related egg whites, as well as heterogeneous nuclear ribonucleoproteins (hnRNPs).69 Among them, the SR protein family regulates variable shear by its self-phosphorylation state.70 Lung cancer-associated transcription 1 (MALAT1) is a lncRNA with a length of about 8.7 kb. It was originally screened from tumor cells of lung cancer patients. It is highly conserved and exists in the paraspots in the nucleus. Early studies suggested that MALAT1 was associated with tumor cell metastasis.71 Later, it was found that MALAT1 was involved in variable cutting of genes in normal cells. MALAT1 can locate the phosphorylated SR protein in the paramacula and nucleoplasm, recruit and regulate pre-mRNA, and complete variable shearing; if MALAT1 is knocked out in the cell, the total amount of SR protein in the nucleus will increase, but the phosphorylated SR protein will decrease, and the original shearing site will be changed, so the gene expression will change.72
Regulation of mRNA Stability
lncRNA can also regulate the stability of mRNA, such as GADD7 (growth-arrested DNA damage-induced gene 7), which is a 754-nt-long lncRNA. It was found that the expression of GADD7 would increase when the cells were damaged by UV rays and in other ways, and the combination of GADD7 and TDP-43 protein (TAR DNA-binding protein 43) would be interrupted, and the combination of the original TDP-43 protein and cyclin-dependent kinase 6 (Cdk6) mRNA would be interrupted, and thus the stability of the mRNA would be reduced and the degradation would be accelerated.73
Regulation of mRNA Translation
The influence of lncRNAs on mRNA translation is mainly realized by a changing nucleosome, such as lncRNA lincRNA-p21 between genes. The first discovery of lncRNA is that it can regulate p53 protein and inhibit gene expression through the p53 pathway.74 Later, it was found that lincRNA-p21 also affects the translation of mRNA. HUR protein is a RNA-binding protein that can bind to mRNA and participate in various cell responses, inflammatory reactions, and tumor formation through the phosphatidylinositol 3-kinase (PI3K)-AKT-nuclear factor κB (NF-κB) signaling pathway.75 A recent study showed that the HuR protein is related to the occurrence, invasion, and metastasis of colorectal cancer, gastric cancer, breast cancer, and other tumors.76 When there is HuR protein in cells, lincRNA-p21 will become unstable. HuR protein binds to mRNA (such as CTNNB1 mRNA and Jun mRNA) so that ribosomes can smoothly bind to this mRNA and facilitate translation.77 If there is no HuR protein in cells, lincRNA-p21 will become stable and increase in number, and then bind to mRNA through base complementary pairing, so as to bind to ribosomes. Site reduction inhibited the translation of this mRNA.78 The lncRNAs that play a regulatory role in the process of translation are called translational regulatory lncRNAs (treRNAs), which were first discovered through bioinformatics.79 In clinical breast cancer samples with lymph node metastasis, treRNA overexpression promotes tumor cell metastasis and invasion.80
The epithelial-mesenchymal transition (EMT) is one of the markers of malignant tumor metastasis. Low expression of E-cadherin can induce the EMT. Although the mRNA level of calmodulin has not changed in malignant tumor cells, the expression of calmodulin is significantly lower than that in normal cells, because the translation-regulated lncRNA affects the translation of calmodulin.81 lincRNA-p21 affects the translation of mRNA through complementary base pairing between RNA and RNA, which is totally different from the mechanism of translation-regulated lncRNA.82
Other Regulatory Roles
Some lncRNAs have more than one regulatory role. For example, XIST is not only involved in the regulation of X chromosome inactivation. Recent studies have found that it can also interact with miRNA and affect the formation of tumors.83 As mentioned earlier, lncRNA lincRNA-p21 can not only affect the translation of mRNA, but it also interacts with the heterogeneous protein K in the nucleus, cis regulates the expression of p21 protein, and affects the cell cycle change.84 In addition to transcriptional regulation of the sense chain, some lncRNAs, which are transcribed from the antisense chain of the locus, have their own functions in other aspects. The tumor suppressor DIRAS3 is related to the occurrence and development of breast cancer and ovarian cancer.85 An antisense RNA, lncRNA GNG12-AS1, transcribed from its locus is closely related to tumor metastasis and invasion at the transcription level and post-transcription level, respectively.86 At the transcription level, knockdown of the first exon of lncRNA GNG12-AS1 will reduce the transcription level and increase the expression of DIRAS3, thus regulating the cell cycle and inhibiting tumor development.87 At the post-transcriptional level, knockdown of the seventh exon of lncRNA GNG12-AS1 will not affect the transcription of the RNA or the expression of DIRAS3, but at this time, the amount of lncRNA GNG12-AS1 will decrease. The epithelial-mesenchymal transformation is enhanced, and the cells will undergo metastasis and invasion.88
Biological Role of lncRNAs in Tumorigenesis and Development
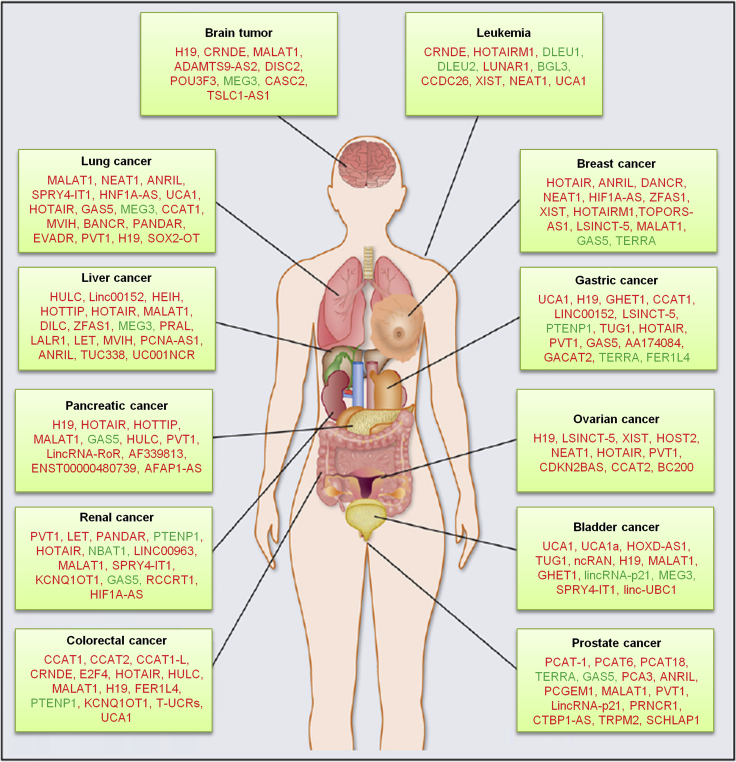
At first, it was thought that lncRNAs were the “noise” of genome transcription and the byproduct of RNA polymerase II transcription, which had no biological function.89 In recent years, more and more studies have shown that lncRNAs are widely involved in DNA methylation, histone modification, chromatin remodeling, and other biological processes in vivo, which can directly interact with transcription factors, functional RNA molecules, and chromatin remodeling modifiers, and regulate the expression of target genes at the epigenetic, transcribed, and post-transcribed levels.90, 91, 92, 93 In vivo, lncRNAs are mainly used as signaling molecules, bait molecules, guiding molecules, and scaffold molecules to perform biological functions.94 In addition, some lncRNAs have diversity in the mode of action and can participate in gene expression regulation in a variety of ways at the same time.95 lncRNAs are widely involved in the physiological and pathological processes of the body, play an important role in the occurrence and development of tumors, and have guiding significance in the diagnosis and treatment of diseases (Figure 5).
Figure 5.
lncRNAs Associated with Various Types of Cancer
`Genome-wide association studies of tumor samples have identified a large number of lncRNAs associated with various types of cancer. Alterations in lncRNA expression and their mutations promote tumorigenesis and metastasis. lncRNAs may exhibit tumor-suppressive (green) and tumor-promoting (red) functions. Because of their genome-wide expression patterns in a variety of tissues and their tissue-specific expression characteristics, lncRNAs hold strong promise as novel biomarkers and therapeutic targets for cancer.
lncRNAs and the Growth of Tumor Cells
Tumor cells can secrete some growth factors to promote their growth.96 In T cell lymphocytic acute leukemia, Notch-1 protein activates the transcription of lncRNA LUNAR1, and the combination of the two enhances the expression of insulin-like growth factor 1 and its signaling pathway, and it promotes the growth of tumor cells.97 There is a base region lacking transcription activity in the 8q24 region of the human chromosome.98 In many malignant tumor cells, the copy number of DNA in this region is increased abnormally, accompanied by the rapid amplification of the proto-oncogene Myc in this region.99, 100, 101 Now there is evidence that lncRNA can participate in Myc oncogenesis. Some lncRNAs regulated by Myc transcription can also cause cell cycle changes and cancer cell proliferation.102
In human Burkitt’s lymphoma, the PVT1 gene located in this chromosome region was heterozygous.103 The product of the PVT1 gene is a lncRNA PVT1, which is homologous with mouse transcripts. In the mouse model of Myc tumor formation, the amplification of the Myc gene alone is not enough to promote tumor formation. Only when multiple genes including Myc and PVT1 are amplified at the same time can tumors occur.104 The lncRNA PCGEM1 specifically expressed in prostate tissue is also located in the 8q24 region of the chromosome, which can bind with Myc protein, enhance the transcription of downstream genes by Myc, and promote the proliferation of prostate cancer cells.105
lncRNAs and Apoptosis of Tumor Cells
The occurrence and development of tumors are not only due to the rapid proliferation of cells, but are also related to the decline of cell mortality.106 In malignant tumor cells, apoptosis is inhibited to ensure the rapid growth of tumor cells. p53 protein, as a tumor suppressor, plays an important role in cell monitoring.107 Once the cell is damaged, p53 protein can change the cell cycle or induce apoptosis by repairing DNA.108 There was early evidence that p53 protein can bind to a variety of RNAs. Now it has been confirmed that many lncRNAs when combined with p53 can regulate apoptosis through the p53 pathway.109 For example, lncRNA PANDA is induced by DNA damage and regulated by p53, and it can combine with transcription factor NF-YA so that it can no longer promote the expression of apoptosis-promoting factors, thus inhibiting cell apoptosis.110 Under the environmental pressure of lack of nutrition, tumor cells have growth advantages compared with normal cells.111 When the nutrition is insufficient or the growth factor is reduced in the environment, it will induce the formation of lncRNA GAS5, which competently binds to the glucocorticoid receptor with the glucocorticoid response element, thus inhibiting cell apoptosis.112 In epithelial ovarian cancer, the expression of GAS5 is lower than that in the adjacent tissues, and GAS5 inhibits DDP resistance and tumor progression of epithelial ovarian cancer via the GAS5-E2F4-PARP1-mitogen-activated protein kinase (MAPK) axis.113 Therefore, inhibiting the expression of GAS5 in breast cancer cells can improve the their survival rate in a barren environment, suggesting that increasing the expression of GAS5 can be used to treat breast cancer.
lncRNAs and Metastasis of Tumor Cells
Many tumor-related lncRNAs can regulate the invasion and metastasis of tumor cells.114, 115, 116 There is evidence that in tumor cells, MALAT1 can affect the genes of the cell differentiation and the tumor metastasis signaling pathway through variable shear. Knockdown of MALAT1 resulted in increased adhesion and decreased migration of tumor cells.117 Most cancer-related deaths are related to tumor cell migration induced by transforming growth factor β (TGF-β).118 In hepatoma cells, TGF-β can activate the expression of lncRNA ATB, promote the EMT transformation of cells, and acquire the invasion ability and metastasis.119 In breast cancer cells, lncRNA BCAR4 induced by chemokines can combine with transcription factors SNIP1 and PNUTS to respond to CCL21, activate the atypical GLI2 signaling pathway in cells, and promote tumor cell migration.120
lncRNAs and Chromosome Stability of Tumor Cells
lncRNAs can also be involved in maintaining chromosome stability. Most cancer cells are characterized by unstable chromosome numbers.121 p53 protein can interact with a variety of lncRNAs, maintain cell chromosome stability, and regulate cell fate.122 Similarly, p53 protein can be regulated by lncRNAs. When the cell DNA is damaged, it will induce the formation of lncRNA DINO, which can directly bind with p53 protein and promote the transcription of the target gene downstream of p53, and determine the cell fate.123 The genomic instability caused by the deletion of p53 may make tumor cells accumulate more cancer drivers, thus accelerating carcinogenesis, tumor metastasis, and drug resistance.124 In 2016, NORAD, the lncRNA transcribed from the NORAD gene, was found to help cells maintain the normal number of chromosomes. The lncRNA NORAD can control the separation of chromosomes by the protein PUMILIO in the interphase of cell division, so as to maintain the stability of chromosome number.125
The Application of lncRNAs in Tumor Diagnosis and Treatment
The expression of lncRNAs is tissue-specific and has an important impact on the occurrence and development of tumors.44,126 Therefore, some lncRNAs with expression characteristics can be used as clinical diagnosis markers and treatment targets, such as high expression of lncHIFCAR in tumor cells of patients with oral cancer, which can be used as clinical detection markers and treatment targets for the disease.127 In patients with gastric cancer, lncRNA AA174084 is in a downregulated state, so it can be used as a marker for the early diagnosis of gastric cancer;128 in patients with colorectal cancer, if lncRNA CCAT1 and CCAT2 are highly expressed, it means that the survival rate and recurrence rate of the disease are low, so it can also be used as a clinical examination test marker.129 In 2017, lncRNAs were found in exosomes.130, 131, 132, 133 In normal cells, these lncRNAs can interact with RNA-binding proteins to neutralize their own effects. In tumor cells, the expression of lncRNAs in exosomes increases, which promotes the occurrence of tumors.134
Outlook
In the process of gene expression, RNA is involved in almost all aspects, and non-coding RNA controls the fate of cells. Even with the occurrence and development of tumors, lncRNA is also inextricably linked with it. However, for many tumors, although we can find some specific high expression or low expression, or even no expression, through high-throughput sequencing, we still do not understand the molecular mechanism thoroughly, and thus we need to do further research in clinical diagnosis and treatment. Generally speaking, although the number of lncRNAs is huge, the mechanism of lncRNA is not clear, and there are some lncRNAs with more than one function, so there is still a large gap in the field of lncRNAs to be understood.
Author Contributions
Y.-S.M. and D.F. designed and supervised research. All authors interpreted the data and contributed to the final version of the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We would like to give our sincere appreciation to the reviewers for their helpful comments on this article. This work was supported partly by grants from the National Natural Science Foundation of China (81972214, 81772932, 81472202, 81201535, 81302065, 81671716, 81301993, 81372175, and 81472209); The Fundamental Research Funds for the Central Universities (22120170212 and 22120170117); the Shanghai Natural Science Foundation (12ZR1436000 and 16ZR1428900); the Shanghai Municipal Commission of Health and Family Planning (201540228 and 201440398); the Scientific Research Fund Project of Anhui Medical University (2018xkj058); the Construction of the Clinical Medical Center for Tumor Biological Samples in Nantong (HS2016004); and by the Jiangsu 333 Program (BRA2017205).
Contributor Information
Da Fu, Email: fu800da900@126.com.
Yu-Shui Ma, Email: mayushui2006@126.com.
References
- 1.Su R., Cao S., Ma J., Liu Y., Liu X., Zheng J., Chen J., Liu L., Cai H., Li Z. Knockdown of SOX2OT inhibits the malignant biological behaviors of glioblastoma stem cells via up-regulating the expression of miR-194-5p and miR-122. Mol. Cancer. 2017;16:171. doi: 10.1186/s12943-017-0737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y., Wang C., Becker S.A., Hurst K., Nogueira L.M., Findlay V.J., Camp E.R. miR-145 antagonizes SNAI1-mediated stemness and radiation resistance in colorectal cancer. Mol. Ther. 2018;26:744–754. doi: 10.1016/j.ymthe.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai X., Ni J., Beretov J., Graham P., Li Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat. Rev. 2018;69:152–163. doi: 10.1016/j.ctrv.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Laissue P. The forkhead-box family of transcription factors: key molecular players in colorectal cancer pathogenesis. Mol. Cancer. 2019;18:5. doi: 10.1186/s12943-019-0938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z., Lin Y., Zhang J., Zhang Y., Li Y., Liu Z., Li Q., Luo M., Liang R., Ye J. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019;38:447. doi: 10.1186/s13046-019-1412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y.S., Huang T., Zhong X.M., Zhang H.W., Cong X.L., Xu H., Lu G.X., Yu F., Xue S.B., Lv Z.W., Fu D. Proteogenomic characterization and comprehensive integrative genomic analysis of human colorectal cancer liver metastasis. Mol. Cancer. 2018;17:139. doi: 10.1186/s12943-018-0890-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh M.K., Chakraborty D., Sarkar S., Bhowmik A., Basu M. The interrelationship between cerebral ischemic stroke and glioma: a comprehensive study of recent reports. Signal Transduct. Target. Ther. 2019;4:42. doi: 10.1038/s41392-019-0075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cristóbal I., Sanz-Alvarez M., Torrejón B., Santos A., Luque M., Rojo F., García-Foncillas J. Potential therapeutic impact of miR-145 deregulation in colorectal cancer. Mol. Ther. 2018;26:1399–1400. doi: 10.1016/j.ymthe.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X., Chen Z., Liu Y. RNAi-mediated control of CRISPR functions. Theranostics. 2020;10:6661–6673. doi: 10.7150/thno.44880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Z., Sun L., Xie S., Zhang S., Fan S., Li Q., Chen W., Pan G., Wang W., Weng B. Chemotherapy-induced long non-coding RNA 1 promotes metastasis and chemo-resistance of TSCC via the Wnt/β-catenin signaling pathway. Mol. Ther. 2018;26:1494–1508. doi: 10.1016/j.ymthe.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrera M., Llorens C., Rodríguez M., Herrera A., Ramos R., Gil B., Candia A., Larriba M.J., Garre P., Earl J. Differential distribution and enrichment of non-coding RNAs in exosomes from normal and cancer-associated fibroblasts in colorectal cancer. Mol. Cancer. 2018;17:114. doi: 10.1186/s12943-018-0863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warner S.G., Kim S.I., Chaurasiya S., O’Leary M.P., Lu J., Sivanandam V., Woo Y., Chen N.G., Fong Y. A novel chimeric poxvirus encoding hNIS is tumor-tropic, imageable, and synergistic with radioiodine to sustain colon cancer regression. Mol. Ther. Oncolytics. 2019;13:82–92. doi: 10.1016/j.omto.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yue B., Liu C., Sun H., Liu M., Song C., Cui R., Qiu S., Zhong M. A positive feed-forward loop between lncRNA-CYTOR and Wnt/β-catenin signaling promotes metastasis of colon cancer. Mol. Ther. 2018;26:1287–1298. doi: 10.1016/j.ymthe.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu T., Han Z., Li H., Zhu Y., Sun Z., Zhu A. lncRNA DLEU1 contributes to colorectal cancer progression via activation of KPNA3. Mol. Cancer. 2018;17:118. doi: 10.1186/s12943-018-0873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J., Han Z., Sun Z., Wang Y., Zheng M., Song C. lncRNA SLCO4A1-AS1 facilitates growth and metastasis of colorectal cancer through β-catenin-dependent Wnt pathway. J. Exp. Clin. Cancer Res. 2018;37:222. doi: 10.1186/s13046-018-0896-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M.H., Zhao L., Wang L., Ou-Yang W., Hu S.S., Li W.L., Ai M.L., Wang Y.Q., Han Y., Li T.T. Nuclear lncRNA HOXD-AS1 suppresses colorectal carcinoma growth and metastasis via inhibiting HOXD3-induced integrin β3 transcriptional activating and MAPK/AKT signalling. Mol. Cancer. 2019;18:31. doi: 10.1186/s12943-019-0955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu K.P., Ma X.L., Zhang C.L. lncRNA ODRUL contributes to osteosarcoma progression through the miR-3182/MMP2 axis. Mol. Ther. 2017;25:2383–2393. doi: 10.1016/j.ymthe.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao M., Yang Q., Zhu W., Jin H., Wang J., Song J., Kong Y., Lv X. lncHOXA10 drives liver TICs self-renewal and tumorigenesis via HOXA10 transcription activation. Mol. Cancer. 2018;17:173. doi: 10.1186/s12943-018-0921-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimassa L., Danesi R., Pressiani T., Merle P. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat. Rev. 2019;77:20–28. doi: 10.1016/j.ctrv.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Cruz L., György B., Cheah P.S., Kleinstiver B.P., Eimer W.A., Garcia S.P., Sharma N., Ozelius L.J., Bragg D.C., Joung J.K. Mutant allele-specific CRISPR disruption in DYT1 dystonia fibroblasts restores cell function. Mol. Ther. Nucleic Acids. 2020;21:1–12. doi: 10.1016/j.omtn.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elsedawy N.B., Nace R.A., Russell S.J., Schulze A.J. Oncolytic activity of targeted picornaviruses formulated as synthetic infectious RNA. Mol. Ther. Oncolytics. 2020;17:484–495. doi: 10.1016/j.omto.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou H., Zhu P., Wang J., Toan S., Ren J. DNA-PKcs promotes alcohol-related liver disease by activating Drp1-related mitochondrial fission and repressing FUNDC1-required mitophagy. Signal Transduct. Target. Ther. 2019;4:56. doi: 10.1038/s41392-019-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai K.W., Lo Y.H., Liu H., Yeh C.Y., Chen Y.Z., Hsu C.W., Chen W.S., Wang J.H. Linc00659, a long noncoding RNA, acts as novel oncogene in regulating cancer cell growth in colorectal cancer. Mol. Cancer. 2018;17:72. doi: 10.1186/s12943-018-0821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta S.C., Awasthee N., Rai V., Chava S., Gunda V., Challagundla K.B. Long non-coding RNAs and nuclear factor-κB crosstalk in cancer and other human diseases. Biochim. Biophys. Acta Rev. Cancer. 2020;1873:188316. doi: 10.1016/j.bbcan.2019.188316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X., Yu H., Sun W., Kong J., Zhang L., Tang J., Wang J., Xu E., Lai M., Zhang H. The long non-coding RNA CYTOR drives colorectal cancer progression by interacting with NCL and Sam68. Mol. Cancer. 2018;17:110. doi: 10.1186/s12943-018-0860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu G., Ma Z., Cheng Y., Hu W., Deng C., Jiang S., Li T., Chen F., Yang Y. Targeting Gas6/TAM in cancer cells and tumor microenvironment. Mol. Cancer. 2018;17:20. doi: 10.1186/s12943-018-0769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrzejewska A., Dabrowska S., Nowak B., Walczak P., Lukomska B., Janowski M. Mesenchymal stem cells injected into carotid artery to target focal brain injury home to perivascular space. Theranostics. 2020;10:6615–6628. doi: 10.7150/thno.43169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma M., Zhang Y., Weng M., Hu Y., Xuan Y., Hu Y., Lv K. lncRNA GCAWKR promotes gastric cancer development by scaffolding the chromatin modification factors WDR5 and KAT2A. Mol. Ther. 2018;26:2658–2668. doi: 10.1016/j.ymthe.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei L., Wang X., Lv L., Liu J., Xing H., Song Y., Xie M., Lei T., Zhang N., Yang M. The emerging role of microRNAs and long noncoding RNAs in drug resistance of hepatocellular carcinoma. Mol. Cancer. 2019;18:147. doi: 10.1186/s12943-019-1086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie B., Bai B., Xu Y., Liu Y., Lv Y., Gao X., Wu F., Fang Z., Lou Y., Pan H., Han W. Tumor-suppressive function and mechanism of HOXB13 in right-sided colon cancer. Signal Transduct. Target. Ther. 2019;4:51. doi: 10.1038/s41392-019-0086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y., Liu Y., Lin L., Huang Q., He W., Zhang S., Dong S., Wen Z., Rao J., Liao W., Shi M. The lncRNA MACC1-AS1 promotes gastric cancer cell metabolic plasticity via AMPK/Lin28 mediated mRNA stability of MACC1. Mol. Cancer. 2018;17:69. doi: 10.1186/s12943-018-0820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X.L., Wang B.B., Wang Y., Wang Y.X., Yang C.H., Tan C., Zhang X., He Q.J., Ding J., Meng L.H. Unbiased screening reveals that blocking exportin 1 overcomes resistance to PI3Kα inhibition in breast cancer. Signal Transduct. Target. Ther. 2019;4:49. doi: 10.1038/s41392-019-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding C.H., Yin C., Chen S.J., Wen L.Z., Ding K., Lei S.J., Liu J.P., Wang J., Chen K.X., Jiang H.L. The HNF1α-regulated lncRNA HNF1A-AS1 reverses the malignancy of hepatocellular carcinoma by enhancing the phosphatase activity of SHP-1. Mol. Cancer. 2018;17:63. doi: 10.1186/s12943-018-0813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C., Liao Y., Liu P., Du Q., Liang Y., Ooi S., Qin S., He S., Yao S., Wang W. FABP5 promotes lymph node metastasis in cervical cancer by reprogramming fatty acid metabolism. Theranostics. 2020;10:6561–6580. doi: 10.7150/thno.44868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawamura E., Maruyama M., Abe J., Sudo A., Takeda A., Takada S., Yokota T., Kinugawa S., Harashima H., Yamada Y. Validation of gene therapy for mutant mitochondria by delivering mitochondrial RNA using a MITO-Porter. Mol. Ther. Nucleic Acids. 2020;20:687–698. doi: 10.1016/j.omtn.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Grand M., Mukha A., Püschel J., Valli E., Kamili A., Vittorio O., Dubrovska A., Kavallaris M. Interplay between MycN and c-Myc regulates radioresistance and cancer stem cell phenotype in neuroblastoma upon glutamine deprivation. Theranostics. 2020;10:6411–6429. doi: 10.7150/thno.42602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J., Zhu S., Meng N., He Y., Lu R., Yan G.R. ncRNA-encoded peptides or proteins and cancer. Mol. Ther. 2019;27:1718–1725. doi: 10.1016/j.ymthe.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hewes A.M., Sansbury B.M., Barth S., Tarcic G., Kmiec E.B. gRNA sequence heterology tolerance catalyzed by CRISPR/Cas in an in vitro homology-directed repair reaction. Mol. Ther. Nucleic Acids. 2020;20:568–579. doi: 10.1016/j.omtn.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X., Wang M., Xiang R. Clonal replacement of novel T cells: a new phenomenon in the tumor microenvironment following PD-1 blockade. Signal Transduct. Target. Ther. 2019;4:43. doi: 10.1038/s41392-019-0077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L., Meng X., Zhu X.W., Yang D.C., Chen R., Jiang Y., Xu T. Long non-coding RNAs in oral squamous cell carcinoma: biologic function, mechanisms and clinical implications. Mol. Cancer. 2019;18:102. doi: 10.1186/s12943-019-1021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J., Zhu Y., Wang H., Ji X. Targeting long noncoding RNA in glioma: a pathway perspective. Mol. Ther. Nucleic Acids. 2018;13:431–441. doi: 10.1016/j.omtn.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horita K., Kurosaki H., Nakatake M., Kuwano N., Oishi T., Itamochi H., Sato S., Kono H., Ito M., Hasegawa K. lncRNA UCA1-mediated Cdc42 signaling promotes oncolytic vaccinia virus cell-to-cell spread in ovarian cancer. Mol. Ther. Oncolytics. 2019;13:35–48. doi: 10.1016/j.omto.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan J., Xu Y., Wen X., Ge S., Jia R., Zhang H., Fan X. A cohesin-mediated intrachromosomal loop drives oncogenic ROR lncRNA to accelerate tumorigenesis. Mol. Ther. 2019;27:2182–2194. doi: 10.1016/j.ymthe.2019.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng Z., Liu C., Wu M. New insights into long noncoding RNAs and their roles in glioma. Mol. Cancer. 2018;17:61. doi: 10.1186/s12943-018-0812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie S., Yu X., Li Y., Ma H., Fan S., Chen W., Pan G., Wang W., Zhang H., Li J., Lin Z. Upregulation of lncRNA ADAMTS9-AS2 promotes salivary adenoid cystic carcinoma metastasis via PI3K/Akt and MEK/Erk signaling. Mol. Ther. 2018;26:2766–2778. doi: 10.1016/j.ymthe.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao X., Sun J., Chen Y., Su W., Shan H., Li Y., Wang Y., Zheng N., Shan H., Liang H. lncRNA PFAR promotes lung fibroblast activation and fibrosis by targeting miR-138 to regulate the YAP1-twist axis. Mol. Ther. 2018;26:2206–2217. doi: 10.1016/j.ymthe.2018.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu M., Xu X., Pan B., Chen X., Lin K., Zeng K., Liu X., Xu T., Sun L., Qin J. lncRNA SATB2-AS1 inhibits tumor metastasis and affects the tumor immune cell microenvironment in colorectal cancer by regulating SATB2. Mol. Cancer. 2019;18:135. doi: 10.1186/s12943-019-1063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J., Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct. Target. Ther. 2019;4:34. doi: 10.1038/s41392-019-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li T., Li Z., Wan H., Tang X., Wang H., Chai F., Zhang M., Wang B. Recurrence-associated long non-coding RNA LNAPPCC facilitates colon cancer progression via forming a positive feedback loop with PCDH7. Mol. Ther. Nucleic Acids. 2020;20:545–557. doi: 10.1016/j.omtn.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S., Gilbreath C., Kollipara R.K., Sonavane R., Huo X., Yenerall P., Das A., Ma S., Raj G.V., Kittler R. Mithramycin suppresses DNA damage repair via targeting androgen receptor in prostate cancer. Cancer Lett. 2020;488:40–49. doi: 10.1016/j.canlet.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gan Q.X., Wang J., Hu J., Lou G.H., Xiong H.J., Peng C.Y., Huang Q.W. Modulation of apoptosis by plant polysaccharides for exerting anti-cancer effects: a review. Front. Pharmacol. 2020;11:792. doi: 10.3389/fphar.2020.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrer A., Roser C.T., El-Far M.H., Savanur V.H., Eljarrah A., Gergues M., Kra J.A., Etchegaray J.P., Rameshwar P. Hypoxia-mediated changes in bone marrow microenvironment in breast cancer dormancy. Cancer Lett. 2020;488:9–17. doi: 10.1016/j.canlet.2020.05.026. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L., Niu H., Ma J., Yuan B.Y., Chen Y.H., Zhuang Y., Chen G.W., Zeng Z.C., Xiang Z.L. The molecular mechanism of LncRNA34a-mediated regulation of bone metastasis in hepatocellular carcinoma. Mol. Cancer. 2019;18:120. doi: 10.1186/s12943-019-1044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C.J., Zhu C.C., Xu J., Wang M., Zhao W.Y., Liu Q., Zhao G., Zhang Z.Z. The lncRNA UCA1 promotes proliferation, migration, immune escape and inhibits apoptosis in gastric cancer by sponging anti-tumor miRNAs. Mol. Cancer. 2019;18:115. doi: 10.1186/s12943-019-1032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y., Ren Y., Wang Y., Tan Y., Wang Q., Cai J., Zhou J., Yang C., Zhao K., Yi K. A compound AC1Q3QWB selectively disrupts HOTAIR-mediated recruitment of PRC2 and enhances cancer therapy of DZNep. Theranostics. 2019;9:4608–4623. doi: 10.7150/thno.35188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh A.P., Biswas A., Shukla A., Maiti P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019;4:33. doi: 10.1038/s41392-019-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gu P., Chen X., Xie R., Xie W., Huang L., Dong W., Han J., Liu X., Shen J., Huang J., Lin T. A novel AR translational regulator lncRNA LBCS inhibits castration resistance of prostate cancer. Mol. Cancer. 2019;18:109. doi: 10.1186/s12943-019-1037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L., Wang Y., Zhang L., Xia X., Chao Y., He R., Han C., Zhao W. ZBTB7A, a miR-663a target gene, protects osteosarcoma from endoplasmic reticulum stress-induced apoptosis by suppressing lncRNA GAS5 expression. Cancer Lett. 2019;448:105–116. doi: 10.1016/j.canlet.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 59.Huang Y., Xiang B., Liu Y., Wang Y., Kan H. lncRNA CDKN2B-AS1 promotes tumor growth and metastasis of human hepatocellular carcinoma by targeting let-7c-5p/NAP1L1 axis. Cancer Lett. 2018;437:56–66. doi: 10.1016/j.canlet.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 60.Tellez-Gabriel M., Heymann M.F., Heymann D. Circulating tumor cells as a tool for assessing tumor heterogeneity. Theranostics. 2019;9:4580–4594. doi: 10.7150/thno.34337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y., Yang L., Chen T., Liu X., Guo Y., Zhu Q., Tong X., Yang W., Xu Q., Huang D., Tu K. A novel lncRNA MCM3AP-AS1 promotes the growth of hepatocellular carcinoma by targeting miR-194-5p/FOXA1 axis. Mol. Cancer. 2019;18:28. doi: 10.1186/s12943-019-0957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu J., Chen Z., Bao L., Zhou L., Hou Y., Liu L., Xiong M., Zhang Y., Wang B., Tao Z. Single-cell transcriptome analysis reveals intratumoral heterogeneity in ccRCC, which results in different clinical outcomes. Mol. Ther. 2020;28:1658–1672. doi: 10.1016/j.ymthe.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui S., Zhang L. circ_001653 silencing promotes the proliferation and ECM synthesis of NPCs in IDD by downregulating miR-486-3p-mediated CEMIP. Mol. Ther. Nucleic Acids. 2020;20:385–399. doi: 10.1016/j.omtn.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Wang R., Ma Z., Feng L., Yang Y., Tan C., Shi Q., Lian M., He S., Ma H., Fang J. lncRNA MIR31HG targets HIF1A and p21 to facilitate head and neck cancer cell proliferation and tumorigenesis by promoting cell-cycle progression. Mol. Cancer. 2018;17:162. doi: 10.1186/s12943-018-0916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pulakat L., Chen H.H. Pro-senescence and anti-senescence mechanisms of cardiovascular aging: cardiac microRNA regulation of longevity drug-induced autophagy. Front. Pharmacol. 2020;11:774. doi: 10.3389/fphar.2020.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu K., Zhan Y., Yuan Z., Qiu Y., Wang H., Fan G., Wang J., Li W., Cao Y., Shen X. Hypoxia induces drug resistance in colorectal cancer through the HIF-1α/miR-338-5p/IL-6 feedback loop. Mol. Ther. 2019;27:1810–1824. doi: 10.1016/j.ymthe.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu J., Dong Y., Ding L., Dong Y., Wu Z., Wang W., Shen M., Duan Y. Local delivery of arsenic trioxide nanoparticles for hepatocellular carcinoma treatment. Signal Transduct. Target. Ther. 2019;4:28. doi: 10.1038/s41392-019-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X., Yang J., Guo G., Feng R., Chen K., Liao Y., Zhang L., Sun L., Huang S., Chen J.L. Novel lncRNA-IUR suppresses Bcr-Abl-induced tumorigenesis through regulation of STAT5-CD71 pathway. Mol. Cancer. 2019;18:84. doi: 10.1186/s12943-019-1013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song X., Xu P., Meng C., Song C., Blackwell T.S., Li R., Li H., Zhang J., Lv C. lncITPF promotes pulmonary fibrosis by targeting hnRNP-L depending on its host gene ITGBL1. Mol. Ther. 2019;27:380–393. doi: 10.1016/j.ymthe.2018.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhai W., Zhu R., Ma J., Gong D., Zhang H., Zhang J., Chen Y., Huang Y., Zheng J., Xue W. A positive feed-forward loop between lncRNA-URRCC and EGFL7/P-AKT/FOXO3 signaling promotes proliferation and metastasis of clear cell renal cell carcinoma. Mol. Cancer. 2019;18:81. doi: 10.1186/s12943-019-0998-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei L., Li J., Han Z., Chen Z., Zhang Q. Silencing of lncRNA MALAT1 prevents inflammatory injury after lung transplant ischemia-reperfusion by downregulation of IL-8 via p300. Mol. Ther. Nucleic Acids. 2019;18:285–297. doi: 10.1016/j.omtn.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu X.Q., Duan L.S., Chen Y.Q., Jin X.J., Zhu N.N., Zhou X., Wei H.W., Yin L., Guo J.R. lncRNA MALAT1 accelerates wound healing of diabetic mice transfused with modified autologous blood via the HIF-1α signaling pathway. Mol. Ther. Nucleic Acids. 2019;17:504–515. doi: 10.1016/j.omtn.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Viennois E., Chassaing B., Tahsin A., Pujada A., Wang L., Gewirtz A.T., Merlin D. Host-derived fecal microRNAs can indicate gut microbiota healthiness and ability to induce inflammation. Theranostics. 2019;9:4542–4557. doi: 10.7150/thno.35282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin S., Yang X., Li J., Yang W., Ma H., Zhang Z. p53-targeted lincRNA-p21 acts as a tumor suppressor by inhibiting JAK2/STAT3 signaling pathways in head and neck squamous cell carcinoma. Mol. Cancer. 2019;18:38. doi: 10.1186/s12943-019-0993-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shen W., Zhang W., Ye W., Wang H., Zhang Q., Shen J., Hong Q., Li X., Wen G., Wei T., Zhang J. SR9009 induces a REV-ERB dependent anti-small-cell lung cancer effect through inhibition of autophagy. Theranostics. 2020;10:4466–4480. doi: 10.7150/thno.42478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu Y.P., Jin Y.P., Wu X.S., Yang Y., Li Y.S., Li H.F., Xiang S.S., Song X.L., Jiang L., Zhang Y.J. lncRNA-HGBC stabilized by HuR promotes gallbladder cancer progression by regulating miR-502-3p/SET/AKT axis. Mol. Cancer. 2019;18:167. doi: 10.1186/s12943-019-1097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang F., Hu A., Li D., Wang J., Guo Y., Liu Y., Li H., Chen Y., Wang X., Huang K. circ-HuR suppresses HuR expression and gastric cancer progression by inhibiting CNBP transactivation. Mol. Cancer. 2019;18:158. doi: 10.1186/s12943-019-1094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang F., Chen W., Peng J., Li Y., Zhuang Y., Zhu Z., Shao C., Yang W., Yao H., Zhang S. lncRNA PVT1 triggers cyto-protective autophagy and promotes pancreatic ductal adenocarcinoma development via the miR-20a-5p/ULK1 axis. Mol. Cancer. 2018;17:98. doi: 10.1186/s12943-018-0845-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Zhang G., Li S., Lu J., Ge Y., Wang Q., Ma G., Zhao Q., Wu D., Gong W., Du M. lncRNA MT1JP functions as a ceRNA in regulating FBXW7 through competitively binding to miR-92a-3p in gastric cancer. Mol. Cancer. 2018;17:87. doi: 10.1186/s12943-018-0829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang Y., Cao W., Wu K., Qin X., Wang X., Li Y., Yu B., Zhang Z., Wang X., Yan M. lncRNA LINC00460 promotes EMT in head and neck squamous cell carcinoma by facilitating peroxiredoxin-1 into the nucleus. J. Exp. Clin. Cancer Res. 2019;38:365. doi: 10.1186/s13046-019-1364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mai H., Zhou B., Liu L., Yang F., Conran C., Ji Y., Hou J., Jiang D. Molecular pattern of lncRNAs in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019;38:198. doi: 10.1186/s13046-019-1213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buckley A., Hagler S.B., Lettry V., Bagó J.R., Maingi S.M., Khagi S., Ewend M.G., Miller C.R., Hingtgen S.D. Generation and profiling of tumor-homing induced neural stem cells from the skin of cancer patients. Mol. Ther. 2020;28:1614–1627. doi: 10.1016/j.ymthe.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen J.F., Wu P., Xia R., Yang J., Huo X.Y., Gu D.Y., Tang C.J., De W., Yang F. STAT3-induced lncRNA HAGLROS overexpression contributes to the malignant progression of gastric cancer cells via mTOR signal-mediated inhibition of autophagy. Mol. Cancer. 2018;17:6. doi: 10.1186/s12943-017-0756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schenkwein D., Afzal S., Nousiainen A., Schmidt M., Ylä-Herttuala S. Efficient nuclease-directed integration of lentivirus vectors into the human ribosomal DNA locus. Mol. Ther. 2020 doi: 10.1016/j.ymthe.2020.05.019. Published online May 22, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xue Z., Zhang Y., Liu Y., Zhang C., Shen X.D., Gao F., Busuttil R.W., Zheng S., Kupiec-Weglinski J.W., Ji H. PACAP neuropeptide promotes hepatocellular protection via CREB-KLF4 dependent autophagy in mouse liver ischemia reperfusion injury. Theranostics. 2020;10:4453–4465. doi: 10.7150/thno.42354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stojic L., Niemczyk M., Orjalo A., Ito Y., Ruijter A.E., Uribe-Lewis S., Joseph N., Weston S., Menon S., Odom D.T. Transcriptional silencing of long noncoding RNA GNG12-AS1 uncouples its transcriptional and product-related functions. Nat. Commun. 2016;7:10406. doi: 10.1038/ncomms10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi H., Li H., Zhen T., Dong Y., Pei X., Zhang X. The potential therapeutic role of exosomal microRNA-520b derived from normal fibroblasts in pancreatic cancer. Mol. Ther. Nucleic Acids. 2020;20:373–384. doi: 10.1016/j.omtn.2019.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Wu Z.R., Yan L., Liu Y.T., Cao L., Guo Y.H., Zhang Y., Yao H., Cai L., Shang H.B., Rui W.W. Inhibition of mTORC1 by lncRNA H19 via disrupting 4E-BP1/Raptor interaction in pituitary tumours. Nat. Commun. 2018;9:4624. doi: 10.1038/s41467-018-06853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Z., Zhang J., Zheng H., Li C., Xiong J., Wang W., Bao H., Jin H., Liang P. Modulating lncRNA SNHG15/CDK6/miR-627 circuit by palbociclib, overcomes temozolomide resistance and reduces M2-polarization of glioma associated microglia in glioblastoma multiforme. J. Exp. Clin. Cancer Res. 2019;38:380. doi: 10.1186/s13046-019-1371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Y., Li Y., Sheng J., Wu F., Li K., Huang R., Wang X., Jiao T., Guan X., Lu Y. P53-R273H mutation enhances colorectal cancer stemness through regulating specific lncRNAs. J. Exp. Clin. Cancer Res. 2019;38:379. doi: 10.1186/s13046-019-1375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma Y., Zhang J., Wen L., Lin A. Membrane-lipid associated lncRNA: a new regulator in cancer signaling. Cancer Lett. 2018;419:27–29. doi: 10.1016/j.canlet.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 92.Huang M.S., Zhu T., Li L., Xie P., Li X., Zhou H.H., Liu Z.Q. lncRNAs and circRNAs from the same gene: masterpieces of RNA splicing. Cancer Lett. 2018;415:49–57. doi: 10.1016/j.canlet.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 93.Fu Z., Chen C., Zhou Q., Wang Y., Zhao Y., Zhao X., Li W., Zheng S., Ye H., Wang L. lncRNA HOTTIP modulates cancer stem cell properties in human pancreatic cancer by regulating HOXA9. Cancer Lett. 2017;410:68–81. doi: 10.1016/j.canlet.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 94.Shi D., Wu F., Mu S., Hu B., Zhong B., Gao F., Qing X., Liu J., Zhang Z., Shao Z. lncRNA AFAP1-AS1 promotes tumorigenesis and epithelial-mesenchymal transition of osteosarcoma through RhoC/ROCK1/p38MAPK/Twist1 signaling pathway. J. Exp. Clin. Cancer Res. 2019;38:375. doi: 10.1186/s13046-019-1363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luo J., Wang K., Yeh S., Sun Y., Liang L., Xiao Y., Xu W., Niu Y., Cheng L., Maity S.N. lncRNA-p21 alters the antiandrogen enzalutamide-induced prostate cancer neuroendocrine differentiation via modulating the EZH2/STAT3 signaling. Nat. Commun. 2019;10:2571. doi: 10.1038/s41467-019-09784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yan H., Li H., Silva M.A., Guan Y., Yang L., Zhu L., Zhang Z., Li G., Ren C. lncRNA FLVCR1-AS1 mediates miR-513/YAP1 signaling to promote cell progression, migration, invasion and EMT process in ovarian cancer. J. Exp. Clin. Cancer Res. 2019;38:356. doi: 10.1186/s13046-019-1356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu X., Mao D., Deng G., Song Y., Zhang F., Yang S., Li G., Liu F., Cao W., Zhu X. Nondestructive analysis of tumor-associated membrane protein MUC1 in living cells based on dual-terminal amplification of a DNA ternary complex. Theranostics. 2020;10:4410–4421. doi: 10.7150/thno.42951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu D.M., Zheng Z.H., Zhang Y.B., Fan S.H., Zhang Z.F., Wang Y.J., Zheng Y.L., Lu J. Down-regulated lncRNA DLX6-AS1 inhibits tumorigenesis through STAT3 signaling pathway by suppressing CADM1 promoter methylation in liver cancer stem cells. J. Exp. Clin. Cancer Res. 2019;38:237. doi: 10.1186/s13046-019-1239-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Liu H., Deng H., Zhao Y., Li C., Liang Y. lncRNA XIST/miR-34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET-PI3K-AKT signaling. J. Exp. Clin. Cancer Res. 2018;37:279. doi: 10.1186/s13046-018-0950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Q., Dong C., Cui J., Wang Y., Hong X. Over-expressed lncRNA HOTAIRM1 promotes tumor growth and invasion through up-regulating HOXA1 and sequestering G9a/EZH2/Dnmts away from the HOXA1 gene in glioblastoma multiforme. J. Exp. Clin. Cancer Res. 2018;37:265. doi: 10.1186/s13046-018-0941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rossi M., Bucci G., Rizzotto D., Bordo D., Marzi M.J., Puppo M., Flinois A., Spadaro D., Citi S., Emionite L. lncRNA EPR controls epithelial proliferation by coordinating Cdkn1a transcription and mRNA decay response to TGF-β. Nat. Commun. 2019;10:1969. doi: 10.1038/s41467-019-09754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dong H., Wang W., Mo S., Chen R., Zou K., Han J., Zhang F., Hu J. SP1-induced lncRNA AGAP2-AS1 expression promotes chemoresistance of breast cancer by epigenetic regulation of MyD88. J. Exp. Clin. Cancer Res. 2018;37:202. doi: 10.1186/s13046-018-0875-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Casciello F., Al-Ejeh F., Miranda M., Kelly G., Baxter E., Windloch K., Gannon F., Lee J.S. G9a-mediated repression of CDH10 in hypoxia enhances breast tumour cell motility and associates with poor survival outcome. Theranostics. 2020;10:4515–4529. doi: 10.7150/thno.41453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu J., Hu S., Zhang L., Xin J., Sun C., Wang L., Ding K., Wang B. Tumor circulome in the liquid biopsies for cancer diagnosis and prognosis. Theranostics. 2020;10:4544–4556. doi: 10.7150/thno.40532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xin X., Lu Y., Xie S., Chen Y., Jiang X., Song S., Wang L., Pu H., Gui X., Li T. miR-155 accelerates the growth of human liver cancer cells by activating CDK2 via targeting H3F3A. Mol. Ther. Oncolytics. 2020;17:471–483. doi: 10.1016/j.omto.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu Y., Yao Y., Jiang X., Zhong X., Wang Z., Li C., Kang P., Leng K., Ji D., Li Z. SP1-induced upregulation of lncRNA SPRY4-IT1 exerts oncogenic properties by scaffolding EZH2/LSD1/DNMT1 and sponging miR-101-3p in cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2018;37:81. doi: 10.1186/s13046-018-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang Z.W., Jia Y.X., Zhang W.J., Song L.J., Gao M., Li M.J., Zhao R.H., Li J., Zhong Y.L., Sun Q.Z., Qin Y.R. lncRNA-TUSC7/miR-224 affected chemotherapy resistance of esophageal squamous cell carcinoma by competitively regulating DESC1. J. Exp. Clin. Cancer Res. 2018;37:56. doi: 10.1186/s13046-018-0724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang Y., Zhang J., Hou L., Wang G., Liu H., Zhang R., Chen X., Zhu J. lncRNA AK023391 promotes tumorigenesis and invasion of gastric cancer through activation of the PI3K/Akt signaling pathway. J. Exp. Clin. Cancer Res. 2017;36:194. doi: 10.1186/s13046-017-0666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feng S., Liu W., Bai X., Pan W., Jia Z., Zhang S., Zhu Y., Tan W. lncRNA-CTS promotes metastasis and epithelial-to-mesenchymal transition through regulating miR-505/ZEB2 axis in cervical cancer. Cancer Lett. 2019;465:105–117. doi: 10.1016/j.canlet.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 110.Song Z., Xie Y., Guo Z., Han Y., Guan H., Liu X., Ma T., Zhou P.-k. Genome-wide identification of DNA-PKcs-associated RNAs by RIP-Seq. Signal Transduct. Target. Ther. 2019;4:22. doi: 10.1038/s41392-019-0057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang J., Li J., Li Y., Lu Z., Che Y., Mao S., Lei Y., Zang R., Zheng S., Liu C. Interferon-inducible lncRNA IRF1-AS represses esophageal squamous cell carcinoma by promoting interferon response. Cancer Lett. 2019;459:86–99. doi: 10.1016/j.canlet.2019.05.038. [DOI] [PubMed] [Google Scholar]
- 112.Huo X., Han S., Wu G., Latchoumanin O., Zhou G., Hebbard L., George J., Qiao L. Dysregulated long noncoding RNAs (lncRNAs) in hepatocellular carcinoma: implications for tumorigenesis, disease progression, and liver cancer stem cells. Mol. Cancer. 2017;16:165. doi: 10.1186/s12943-017-0734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gu J., Wang Y., Wang X., Zhou D., Shao C., Zhou M., He Z. Downregulation of lncRNA GAS5 confers tamoxifen resistance by activating miR-222 in breast cancer. Cancer Lett. 2018;434:1–10. doi: 10.1016/j.canlet.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 114.Fan C., Tang Y., Wang J., Xiong F., Guo C., Wang Y., Zhang S., Gong Z., Wei F., Yang L. Role of long non-coding RNAs in glucose metabolism in cancer. Mol. Cancer. 2017;16:130. doi: 10.1186/s12943-017-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu Z., Li Y., Che Y., Huang J., Sun S., Mao S., Lei Y., Li N., Sun N., He J. The TGFβ-induced lncRNA TBILA promotes non-small cell lung cancer progression in vitro and in vivo via cis-regulating HGAL and activating S100A7/JAB1 signaling. Cancer Lett. 2018;432:156–168. doi: 10.1016/j.canlet.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 116.Fan H., Lv P., Mu T., Zhao X., Liu Y., Feng Y., Lv J., Liu M., Tang H. lncRNA n335586/miR-924/CKMT1A axis contributes to cell migration and invasion in hepatocellular carcinoma cells. Cancer Lett. 2018;429:89–99. doi: 10.1016/j.canlet.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 117.Luo X., Qiu Y., Jiang Y., Chen F., Jiang L., Zhou Y., Dan H., Zeng X., Lei Y.L., Chen Q. Long non-coding RNA implicated in the invasion and metastasis of head and neck cancer: possible function and mechanisms. Mol. Cancer. 2018;17:14. doi: 10.1186/s12943-018-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li W., Yang F.Q., Sun C.M., Huang J.H., Zhang H.M., Li X., Wang G.C., Zhang N., Che J.P., Zhang W.T. circPRRC2A promotes angiogenesis and metastasis through epithelial-mesenchymal transition and upregulates TRPM3 in renal cell carcinoma. Theranostics. 2020;10:4395–4409. doi: 10.7150/thno.43239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hosseini E.S., Meryet-Figuiere M., Sabzalipoor H., Kashani H.H., Nikzad H., Asemi Z. Dysregulated expression of long noncoding RNAs in gynecologic cancers. Mol. Cancer. 2017;16:107. doi: 10.1186/s12943-017-0671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alame M., Cornillot E., Cacheux V., Tosato G., Four M., De Oliveira L., Gofflot S., Delvenne P., Turtoi E., Cabello-Aguilar S. The molecular landscape and microenvironment of salivary duct carcinoma reveal new therapeutic opportunities. Theranostics. 2020;10:4383–4394. doi: 10.7150/thno.42986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ye H., Liu Y., Zhan L., Liu Y., Qin Z. Signal amplification and quantification on lateral flow assays by laser excitation of plasmonic nanomaterials. Theranostics. 2020;10:4359–4373. doi: 10.7150/thno.44298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang Y.T., Lin T.P., Tang J.T., Campbell M., Luo Y.L., Lu S.Y., Yang C.P., Cheng T.Y., Chang C.H., Liu T.T. HOTAIR is a REST-regulated lncRNA that promotes neuroendocrine differentiation in castration resistant prostate cancer. Cancer Lett. 2018;433:43–52. doi: 10.1016/j.canlet.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 123.Sohretoglu D., Zhang C., Luo J., Huang S. ReishiMax inhibits mTORC1/2 by activating AMPK and inhibiting IGFR/PI3K/Rheb in tumor cells. Signal Transduct. Target. Ther. 2019;4:21. doi: 10.1038/s41392-019-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kanwal R., Plaga A.R., Liu X., Shukla G.C., Gupta S. MicroRNAs in prostate cancer: functional role as biomarkers. Cancer Lett. 2017;407:9–20. doi: 10.1016/j.canlet.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 125.Tichon A., Gil N., Lubelsky Y., Havkin Solomon T., Lemze D., Itzkovitz S., Stern-Ginossar N., Ulitsky I. A conserved abundant cytoplasmic long noncoding RNA modulates repression by Pumilio proteins in human cells. Nat. Commun. 2016;7:12209. doi: 10.1038/ncomms12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang N., Wang X., Xie X., Liao Y., Liu N., Liu J., Miao N., Shen J., Peng T. lncRNA DANCR promotes tumor progression and cancer stemness features in osteosarcoma by upregulating AXL via miR-33a-5p inhibition. Cancer Lett. 2017;405:46–55. doi: 10.1016/j.canlet.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 127.Shih J.W., Chiang W.F., Wu A.T.H., Wu M.H., Wang L.Y., Yu Y.L., Hung Y.W., Wang W.C., Chu C.Y., Hung C.L. Long noncoding RNA LncHIFCAR/MIR31HG is a HIF-1α co-activator driving oral cancer progression. Nat. Commun. 2017;8:15874. doi: 10.1038/ncomms15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nepstad I., Hatfield K.J., Grønningsæter I.S., Aasebø E., Hernandez-Valladares M., Hagen K.M., Rye K.P., Berven F.S., Selheim F., Reikvam H., Bruserud Ø. Effects of insulin and pathway inhibitors on the PI3K-Akt-mTOR phosphorylation profile in acute myeloid leukemia cells. Signal Transduct. Target. Ther. 2019;4:20. doi: 10.1038/s41392-019-0050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Selvaraj S., Dhoke N.R., Kiley J., Mateos-Aierdi A.J., Tungtur S., Mondragon-Gonzalez R., Killeen G., Oliveira V.K.P., López de Munain A., Perlingeiro R.C.R. Gene correction of LGMD2A patient-specific iPSCs for the development of targeted autologous cell therapy. Mol. Ther. 2019;27:2147–2157. doi: 10.1016/j.ymthe.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim T., Viard M., Afonin K.A., Gupta K., Popov M., Salotti J., Johnson P.F., Linder C., Heldman E., Shapiro B.A. Characterization of cationic bolaamphiphile vesicles for siRNA delivery into tumors and brain. Mol. Ther. Nucleic Acids. 2020;20:359–372. doi: 10.1016/j.omtn.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu F., Zhu J., Lin L., Zhang C., Sun W., Fan Y., Yin F., van Hest J.C.M., Wang H., Du L., Shi X. Multifunctional PVCL nanogels with redox-responsiveness enable enhanced MR imaging and ultrasound-promoted tumor chemotherapy. Theranostics. 2020;10:4349–4358. doi: 10.7150/thno.43402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu C., Zhang T., Chen L., Chen Y. The choice of anti-tumor strategies based on micromolecules or drug loading function of biomaterials. Cancer Lett. 2020;487:45–52. doi: 10.1016/j.canlet.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 133.Shen G., Ren H., Shang Q., Zhang Z., Zhao W., Yu X., Tang J., Yang Z., Liang D., Jiang X. miR-128 plays a critical role in murine osteoclastogenesis and estrogen deficiency-induced bone loss. Theranostics. 2020;10:4334–4348. doi: 10.7150/thno.42982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.von Knethen A., Brüne B. PD-L1 in the palm of your hand: palmitoylation as a target for immuno-oncology. Signal Transduct. Target. Ther. 2019;4:18. doi: 10.1038/s41392-019-0053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]