Abstract
Several previous studies have shown that mutations in B-Raf proto-oncogene (BRAF) and telomerase reverse transcriptase (TERT) can be used for the diagnosis and prognosis of papillary thyroid carcinoma (PTC). However, whether mutations in BRAF and the TERT promoter may improve the accurate identification and risk stratification of high-risk patients in the early stage of PTC remains unclear and requires further investigation. In the present study, mutations in BRAF and the TERT promoter were examined in 205 patients using PCR and Sanger DNA sequencing. The potential association between mutations in these two genes and the clinicopathological characteristics of patients with PTC was then analyzed. BRAF mutations were identified in 169/205 (82.4%) patients, whereas only 8/205 (3.9%) patients presented mutations in the TERT promoter, seven patients exhibited a C228T mutation, and the remaining one had a C250T mutation. There were 6/205 (2.9%) patients with mutations in both BRAF and the TERT promoter. Importantly, compared with patients with no mutations, patients with mutations in BRAF were more likely to exhibit mutations in the TERT promoter. A significant difference in lymph node metastasis was found between the BRAF V600E mutation group and the group without mutations in BRAF. Mutations in the TERT promoter were significantly correlated with older age, extrathyroidal invasion, tumor multifocality and advanced tumor/node/metastasis stage, which are associated with the aggressiveness of PTC. Moreover, compared with patients exhibiting mutations in BRAF, mutations in the TERT promoter were found to be significantly associated with aggressive clinicopathological features and higher risk of recurrence or distant metastasis. Collectively, mutations in the TERT promoter were not frequent, but were significantly correlated with more aggressive clinicopathological features of PTC. Therefore, mutations in the TERT promoter may be an important factor in the genetic background of PTC, and detection of such mutations may help the accurate identification and management of high-risk patients with recurrent or distant metastasis.
Keywords: mutations in telomerase reverse transcriptase promoter, clinicopathological features of papillary thyroid carcinoma, papillary thyroid carcinoma, B-Raf proto-oncogene mutation
Introduction
Papillary thyroid carcinoma (PTC) is a common endocrine malignant tumor, that has a high incidence worldwide (1,2). PTC usually develops slowly, and most patients with PTC have a high overall survival (3). However, ~10% of PTC cases are characterized by aggressive characteristics and high mortality rates (4–6). Recently, various studies have emphasized the importance of risk stratification in order to design individualized treatments for patients with aggressive PTC (7–9). Therefore, it is important to identify novel molecular biomarkers to improve the accurate identification of high-risk patients with early-stage PTC.
B-Raf proto-oncogene (BRAF), a major human oncogene, has been identified in various cancers, including thyroid carcinoma (10,11). The mitogen-activated protein kinase (MAPK) signaling pathway may be activated by the BRAF V600E mutation and subsequently contribute to the tumorigenesis of thyroid cancer (12). Mutations in BRAF occur in ~50% of patients with PTC and have been reported to be associated with the aggressiveness-associated features of PTC, including older age, lymph node metastasis, larger tumor size and advanced tumor stage (13–16). However, contrasting results have been reported, and no significant associations between BRAF mutations and high-risk PTC characteristics were detected in several studies (17–19).
Human telomerase reverse transcriptase (TERT) is an important gene involved in the maintenance of chromosomal integrity and genome stability (20). TERT encodes the catalytic reverse transcriptase subunit of the telomerase enzyme (20). In total, two common mutations in the TERT promoter are located at positions −124 and −146 bp upstream of its translation start site, and are characterized by a C>T mutation at position 1,295,228 (C228T) and 1,295,250 of chromosome 5 (C250T), respectively. Mutations in the TERT promoter lead to TERT overexpression by creating an extra E26 binding motif, thus facilitating cancer growth (21,22). This novel genetic alteration occurs in PTC with a prevalence of 7.5-27% (23–26). Interestingly, mutations in BRAF and the TERT promoter could co-exist in PTC (25). Several studies have reported that mutations in the TERT promoter are associated with aggressive clinicopathological characteristics, especially when BRAF V600E mutations were also identified (27–29). However, another study found contrasting results (30). Therefore, the significance of TERT promoter mutations in predicting the aggressiveness of PTC is inconclusive and requires further investigation.
In the present study, the incidence and clinicopathological significance of BRAF and TERT promoter mutations were analyzed in patients with PTC. Additional studies on TERT and BRAF mutations may clarify whether these molecular factors could be used as biomarkers for the diagnosis and/or prognosis of patients with PTC.
Materials and methods
Patients and tissue samples
In the present study, 205 patients with PTC were enrolled at The Affiliated Yantai Yuhuangding Hospital of Qingdao University from January 2015 to December 2016. The surgical procedures for patients with PTC, including conventional papillary thyroid carcinoma (CPTC) and papillary thyroid microcarcinoma (PTMC), were based on the Guidelines for the diagnosis and treatment of thyroid nodules and differentiated thyroid cancer (31). According to the surgical procedure recommended in the guidelines, patients with thyroid papillary carcinoma underwent total thyroidectomy or thyroid gland combined with isthmus resection, and routine central lymph node dissection at the tumor site. Based on the preoperative and intraoperative conditions, it was decided whether to perform lymph node dissection of the contralateral neck area. In addition, the selection of routine use of radioactive iodine after surgery was also based on the aforementioned guidelines. According to the World Health Organization classification criteria, 118 patients were diagnosed with CPTC and 87 patients with PTMC. The American Joint Committee on Cancer staging system (32) was used for the classification of the TNM stage. After institutional review board approval and informed patient consent, thyroid tumor specimens were obtained for genetic analysis and clinicopathological data was retrospectively collected. All mutational analyses were performed after surgery, and the results had no influence on the surgical procedures. Patients who declined genetic testing or lacked clinicopathological data were excluded from the study. The clinicopathological data of the patients enrolled in the present study are presented in Table I.
Table I.
Association between BRAF V600E or TERT promoter mutations and clinicopathological characteristics in patients with papillary thyroid carcinoma.
| BRAF V600E | TERT promoter mutation | |||||||
|---|---|---|---|---|---|---|---|---|
| Clinicopathological features | Mutated, n=169 | Wild type, n=36 | OR (95% CI) | P-value | Mutated, n=8 | Wild type, n=197 | OR (95% CI) | P-value |
| Sex | ||||||||
| Female | 133 | 28 | 0.947 | 0.903 | 5 | 156 | 2.283 | 0.373 |
| Male | 36 | 8 | (0.398-2.256) | 3 | 41 | (0.524-9.950) | ||
| Age at diagnosis (years) | ||||||||
| ≤45 | 90 | 16 | 0.702 | 0.337 | 1 | 105 | 7.989 | 0.030a |
| >45 | 79 | 20 | (0.341-1.448) | 7 | 92 | (1.001-66.154) | ||
| Tumor size (mm) | ||||||||
| ≤10 | 74 | 12 | 0.642 | 0.248 | 1 | 85 | 5.312 | 0.142 |
| >10 | 95 | 24 | (0.301-1.368) | 7 | 112 | (0.641-44.002) | ||
| Extrathyroidal invasion | ||||||||
| No | 87 | 24 | 1.885 | 0.097 | 1 | 110 | 8.851 | 0.025a |
| Yes | 82 | 12 | (0.885-4.014) | 7 | 87 | (1.069-73.300) | ||
| Multifocality | ||||||||
| Single | 85 | 24 | 1.976 | 0.074 | 1 | 108 | 8.494 | 0.027a |
| Multifocal | 84 | 12 | (0.928-4.208) | 7 | 89 | (1.026-70.344) | ||
| Lymph node metastasis | ||||||||
| No | 69 | 22 | 2.277 | 0.026a | 2 | 89 | 2.472 | 0.305 |
| Yes | 100 | 14 | (1.090-4.759) | 6 | 108 | (0.487-12.551) | ||
| TNM stage | ||||||||
| I–II | 100 | 23 | 1.221 | 0.600 | 1 | 122 | 11.387 | 0.007a |
| III–IV | 69 | 13 | (0.579-2.574) | 7 | 75 | (1.374-94.385) | ||
P<0.05. BRAF, B-Raf proto-oncogene; TERT, telomerase reverse transcriptase; OR, odds ratio; TNM, tumor/node/metastasis.
Genomic DNA isolation
Genomic DNA in the formalin-fixed and paraffin-embedded specimens was extracted using a DNA Extraction kit (Promega Corporation) according to the manufacturer's instructions.
Mutational analysis of BRAF V600E and the TERT promoter
A human BRAF mutant gene detection kit (Amoy Diagnostics Co., Ltd.) was used for the detection of BRAF V600E mutation, as previously described (33). DNA was further analyzed using an ABI7500 real-time PCR thermocycler (Promega Corporation). The 5-carboxyfuorescein (FAM) and 5-hexachloro-fuorescein (HEX) contained in the BRAF mutant gene detection kit was used. The thermocycling conditions were as follows: 95°C For 5 min, 15 cycles of 95°C for 25 sec, 64°C for 20 sec, 72°C for 20 sec, and then 31 cycles of 93°C for 25 sec, 60°C for 35 sec, 72°C for 20 sec. The primers used were the following: Forward, 5′-TCATAATGCTTGCTCTGATAGGA-3′ and reverse, 5′-GGCCAAAAATTTAATCAGTGGA-3′. The mutation plot was determined by the cycle threshold values of FAM according to the manufacturer's instructions. The quality of the extracted DNA was verified by the amplification of a housekeeping gene, which was reported in the HEX channel. PCR was used to amplify the TERT promoter containing the C228T and C250T mutation hotpots, and the PCR products were sequenced for the detection of TERT promoter mutations, as previously reported (34,35). Taq polymerase was used and purchased from Kapa Biosystems; Roche Diagnostics. The primers for TERT promoter region were the following 5′-AGTGGATTCGCGGGCACAGA-3′ (sense) and 5′-CAGCGCTGCCTGAAACTC-3′ (antisense) and the PCR conditions were 95°C for 3 min, followed by 10 cycles of 95°C for 30 sec, 55°C for 30 sec and 68°C for 1 min. This was then followed by 30 cycles of the same settings except for elongation for an additional 5 sec in each cycle. The PCR was completed with a final elongation step at 68°C for 7 min.
Statistical analysis
Statistical analysis was performed using SPSS (version 19.0; IBM Corp.). Fisher's exact test and χ2 tests were used for analyzing the relationship between mutations and clinicopathological features of patients with PTC. P<0.05 was considered to indicate a statistically significant difference.
Results
Prevalence of mutations in BRAF and the TERT promoter in patients with PTC
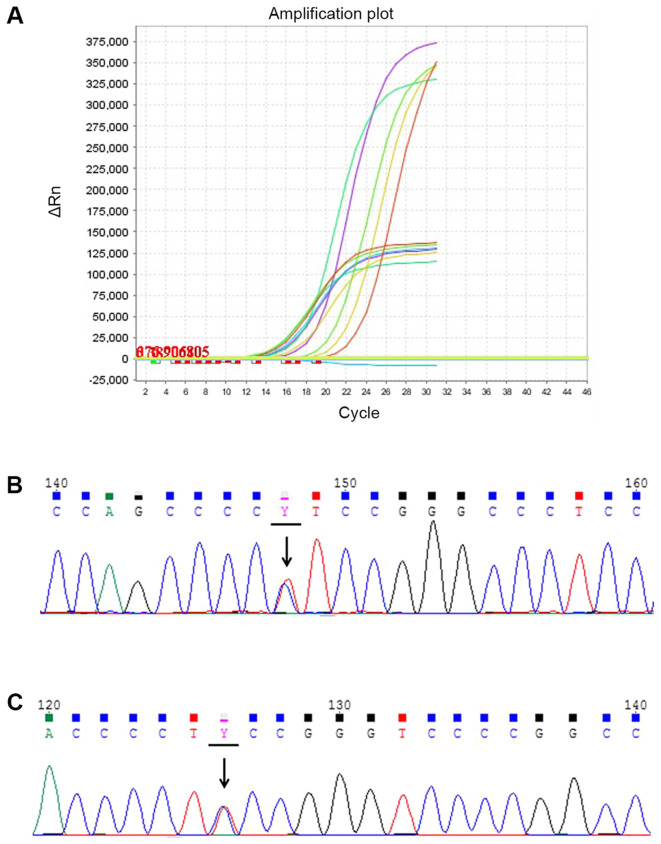
Mutations in BRAF and the TERT promoter were investigated in 205 PTC patients (161 females and 44 males). Mutations in BRAF and the TERT promoter were analyzed with quantitative PCR and Sanger DNA sequencing, respectively (Fig. 1). In total, 169 patients exhibited BRAF V600E mutations, accounting for 82.4% (169/205) of patients with PTCs. Mutations of the TERT promoter were found in eight patients, with a prevalence of 3.9% (8/205; Table I). Of the eight cases analyzed, seven presented a C228T mutation (7/8) and the remaining patient exhibited a C250T mutation. According to previous studies, only one type of TERT promoter mutation is commonly found (34). No cases of simultaneous mutations (C228T and C250T) were found in the present study. In addition, among the eight patients with TERT promoter mutations, six presented with the BRAF V600E mutation (6/8). In total, 118 patients with CPTC and 87 patients with PTMC was involved in the present study. The mutation prevalence of BRAF V600E and TERT promoter was different in these two histological subtypes. In patients with CPTC, the mutation rate of BRAF V600E and TERT promoter was 79.66 and 5.93%, while in patients with PTMC the mutation rate was 86.21 and 1.15% (data not shown).
Figure 1.
BRAF V600E and two common TERT promoter mutations in patients with PTC. (A) Quantitative PCR amplification plot of PTC with BRAF V600E mutation. The different colored lines are different samples. The mutation is indicated by the higher amplification plot and the housekeeping gene is indicated by the lower amplification plot. Positive control is indicated in red. Negative control is indicated in blue. (B) Sequencing chromatogram of the C228T TERT promoter mutation in a case of PTC. (C) Sequencing chromatogram of the C250T TERT promoter mutation in a case of PTC. Arrows indicate the mutation. PTC, papillary thyroid carcinoma; BRAF, B-Raf proto-oncogene; TERT, telomerase reverse transcriptase.
Correlation between mutations in BRAF or the TERT promoter and clinicopathological features of PTC
In the present study, the association between mutations in BRAF or the TERT promoter and the clinicopathological parameters of PTC was investigated. As shown in Table I, a significant difference in lymph node metastasis was detected between patients with the BRAF V600E mutation and patients without BRAF mutations (P=0.026). However, no significant associations were observed between the BRAF V600E mutation and patient sex, age at diagnosis, tumor multifocality, extrathyroidal invasion or tumor/node/metastasis (TNM) stage. Compared with the group without mutations in the TERT promoter, mutations of the TERT promoter were significantly associated with an older age at diagnosis, tumor multifocality, extrathyroidal invasion and advanced TNM stage (P=0.03, P=0.027, P=0.025 and P=0.007, respectively), but not with tumor size, sex or lymph node metastasis.
In order to determine the significance of mutations in BRAF and the TERT promoter in risk stratification, patients with PTC were divided into the following three subgroups: i) Negative for mutations in both BRAF and the TERT promoter (BRAF−/TERT−); ii) only positive for the BRAF V600E mutation, (BRAF+/TERT−); and iii) with or without the BRAF V600E mutation and positive for TERT promoter mutations, (BRAF+/−/TERT+). Compared with the BRAF−/TERT−group, the BRAF+/TERT− group was significantly associated with tumor multifocality (P=0.042) and lymph node metastasis (P=0.012), while the BRAF+/−/TERT+ group showed a significant association with extrathyroidal invasion (P=0.004) and advanced TNM stage (P=0.013), and tumor multifocality (P=0.004), and trend towards an increase in lymph node metastasis (P=0.05; Table II). Interestingly, the BRAF+/−/TERT+ group showed significant association with extrathyroidal invasion (P=0.032) and TNM stage (P=0.009) in comparison with the BRAF+/TERT− group (Table II). Importantly, the BRAF+/−/TERT+ group had a higher incidence of recurrence and distant metastasis compared with both BRAF−/TERT− and BRAF+/TERT− groups (Table III). Collectively, these two molecular biomarkers, and in particular, mutations in the TERT promoter, may be useful for the identification and management of patients with poor outcome.
Table II.
Association of BRAF V600E/TERT promoter mutation status with clinicopathological characteristics in patients with papillary thyroid carcinoma.
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinicopathological features | BRAF −/TERT − (1) n=34 | BRAF +/TERT − (2) n=163 | BRAF +/−/TERT + (3) n=8 | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Sex | ||||||||||
| Female | 26 | 130 | 5 | 0.484 | 0.825 (0.342-1.988) | 0.668 | 1.950 (0.380-10.013) | 0.412 | 2.364 (0.537-10.399) | 0.367 |
| Male | 8 | 33 | 3 | |||||||
| Age at diagnosis (years) | ||||||||||
| ≤45 | 16 | 89 | 1 | 0.056 | 0.739 (0.352-1.550) | 0.423 | 6.222 (0.689-56.203) | 0.114 | 8.419 (1.013-69.989) | 0.028a |
| >45 | 18 | 74 | 7 | |||||||
| Tumor size (mm) | ||||||||||
| ≤10 | 12 | 73 | 1 | 0.135 | 0.672 (0.312-1.450) | 0.309 | 3.818 (0.419-34.812) | 0.398 | 5.678 (0.683-47.204) | 0.140 |
| >10 | 22 | 90 | 7 | |||||||
| Extrathyroidal invasion | ||||||||||
| No | 24 | 86 | 1 | 0.009a | 2.149 (0.966-4.779) | 0.057 | 16.800 (1.822-154.894) | 0.004a | 7.818 (1.041-64.987) | 0.032a |
| Yes | 10 | 77 | 7 | |||||||
| Multifocality | ||||||||||
| Single | 24 | 84 | 1 | 0.008a | 2.257 (1.015-5.019) | 0.042a | 16.800 (1.822-154.894) | 0.004a | 7.443 (0.895-61.866) | 0.064 |
| Multifocal | 10 | 79 | 7 | |||||||
| Lymph node metastasis | ||||||||||
| No | 22 | 67 | 2 | 0.022a | 2.627 (1.217-5.670) | 0.012a | 5.500 (1.021-31.589) | 0.050 | 2.094 (0.410-10.691) | 0.303 |
| Yes | 12 | 96 | 6 | |||||||
| TNM stage | ||||||||||
| I–II | 23 | 99 | 1 | 0.015a | 1.352 (0.617-2.961) | 0.450 | 14.636 (1.598-134.097) | 0.013a | 10.828 (1.301-90.097) | 0.009a |
| III–IV | 11 | 64 | 7 | |||||||
P<0.05. BRAF, B-Raf proto-oncogene; TERT, telomerase reverse transcriptase; OR, odds ratio; TNM, tumor/node/metastasis.
Table III.
Association between BRAF V600E/TERT promoter mutation status and recurrences or distant metastasis in patients with papillary thyroid carcinoma.
| 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Mutation status | No. of recurrences or distant metastasis/no. patients | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| BRAF−/TERT− (1) | 1/34 | |||||||
| BRAF+/TERT− (2) | 4/169 | 0.800 (0.087-7.388) | 1.000 | 19.800 (1.707-129.643) | 0.018a | 24.750 (4.338-141.205) | 0.002a | |
| BRAF+/−/TERT+ (3) | 3/8 | |||||||
P<0.05. BRAF, B-Raf proto-oncogene; TERT, telomerase reverse transcriptase; OR, odds ratio.
Discussion
In the present study, mutations in the TERT promoter showed a greater association with the aggressive clinicopathological features of PTC compared with the BRAF V600E mutation. Moreover, patients with TERT promoter mutations had a poorer outcome, as assessed by recurrence and distant metastasis rates.
The prevalence of the BRAF V600E mutation in the present study was 82.4%, which was relatively high compared with the average worldwide prevalence of ~45%. Several studies have shown that the prevalence of the BRAF V600E mutation in patients from Asian countries, including Japan, South Korea and China, is higher than that of Western countries (16,27,36,37). The present study found that the frequency of mutations in the TERT promoter was lower than that of BRAF mutations. These differences in the mutation frequency can be caused by various factors, including iodide intake, endocrine disruptors, analysis of ethnically diverse groups and environmental factors in certain geographical areas, such as radiation and increased exposure to asbestos amphibole fluoroedenite in the volcanic areas (38–41). Moreover, it has been reported that the prevalence of the BRAF V600E mutation is increasing in China (27). Another important reason is that the distribution of BRAF mutation is associated with the distinct histological subtypes of PTC (40,41). The present study included 118 patients with CPTC and 87 patients with PTMC. In a prior study, the distribution of mutations in BRAF and the TERT promoter displayed a clear subtype-related pattern (25). In the present study, the mutation prevalence of BRAF V600E in patients with CPTC and PTMC was 79.66 and 86.21%, respectively. In addition, the mutation rate of the TERT promoter was 5.93 and 1.15% in patients with CPTC and PTMC, respectively.
It has been reported in several previous studies that the BRAF V600E mutation is associated with high-risk clinicopathological characteristics (15,42–45). However, some studies have shown no significant association between BRAF V600E mutations and any clinicopathological features of PTC (17,18,46–48). A modest association between BRAF V600E mutations and PTC clinicopathological features was reported in the present study. Due to the high prevalence of BRAF V600E, it is difficult to use this marker to improve the risk stratification and identify high-risk patients with poor outcome. Therefore, in addition to BRAF V600E, additional studies are required to identify novel gene mutations associated with aggressive PTC phenotypes.
In the present study, mutations in the TERT promoter were significantly associated with aggressive clinicopathological features compared with patients without mutations or the BRAF V600E mutation alone, such as the presence of extrathyroidal invasion and advanced TNM stage. Furthermore, patients harboring TERT promoter mutations showed a higher possibility of recurrence and distant metastasis. The present data suggested that mutations in the TERT promoter may be a promising genetic molecular biomarker associated with aggressive PTC. The present findings are in line with previous studies, and suggest that mutations in the TERT promoter may enhance the aggressiveness of PTC (24,29,49). Mutations in BRAF and the TERT promoter co-existed in 6/8 patients with BRAF V600E in the present study. The BRAF V600E mutation may upregulate the expression of TERT by activating the MAPK pathway (25). Whether mutations in BRAF and TERT are directly related to PTC oncogenesis, and if these gene mutations have synergistic or additive effects on PTC, will require further investigation.
Genetic testing of thyroid cancer is of great significance for the diagnosis and prognosis assessment of patients with PTC and may facilitate follow-up treatments. Importantly, genetic testing can be used as an auxiliary means for pre-operative fine needle aspiration biopsy to diagnose unidentified thyroid nodules, thereby improving the accuracy of diagnosis (50). BRAF V600E plays an important role as a driving mutation in the early stage of tumorigenesis and has become an ideal biomarker for thyroid cancer (51). Moreover, BRAF V600E has been used as one of the prognostic indicators in patients with PTC in the 2015 edition of the American Thyroid Association (ATA) Guidelines (52). Previous studies have shown that TERT promoter mutation is involved in the pathogenesis of tumors and is associated with tumor aggressiveness (20). However, whether TERT promoter mutations could be used as a prognostic indicator for patients with PTC remains unclear, and the present study has contributed towards further understanding of this. The detection of TERT promoter mutations may be helpful to improve the ATA risk stratification system and guide clinicians to select appropriate treatments for patients with PTC.
One of the main limitations of the present study is that no follow-up data was obtained for the patients exhibiting these mutations, partly due to the short time period after the diagnosis and the better 5-year survival rate of PTC patients. In future studies, it would be useful to investigate whether TERT promoter mutations were of complementary value to the ATA risk stratification system, and could help identify the high-risk patients and guide appropriate treatments of them. In addition, the findings of the present study need to be further confirmed with a larger sample size, as the prevalence of TERT mutations was relatively low. Moreover, although TERT promoter mutations may be a promising molecular biomarker for identifying aggressive PTC, the therapeutic potential of simultaneous mutations in BRAF and the TERT promoter requires further investigation.
In conclusion, mutations in the TERT promoter may have a low prevalence, but a high value in improving the risk stratification system and management of patients with aggressive PTC. The aggressiveness of PTC may be cooperatively driven by TERT promoter and other gene mutations, and the implication of TERT promoter mutations for the prognosis and treatment of patients with PTC should be further investigated.
Acknowledgements
Not applicable.
Funding
The present work was supported by the Natural Science Fund of Shandong Province, China (grant no. ZR2016HL37).
Availability of data and materials
The data used and/or analyzed in the present study are available from the corresponding author on reasonable request.
Authors' contributions
RL, YuL and CS conceived the study. RL, YuL, WC, JC, ZZ, LM, LC, HX, YZ, YoL, YX, QY and XY designed the experiments, provided reagents and collected data. ZZ conducted the experiments. YuL performed data analysis and wrote the manuscript. All authors read and approved the final version of the paper.
Ethics approval and consent to participate
The study protocol was approved by the ethics committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao University. Written informed consent was provided by each patient. When the patient was <18 years of age, informed consent was provided by the patient's legal guardian.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 2.La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E. Thyroid cancer mortality and incidence: A global overview. Int J Cancer. 2015;136:2187–2195. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- 3.Ito Y, Kudo T, Kihara M, Takamura Y, Kobayashi K, Miya A, Miyauchi A. Improvement of lymph node recurrence rate, but not distant recurrence and carcinoma death rates, in patients with papillary thyroid carcinoma after disease-free survival for 5 years. Endocr J. 2012;59:895–901. doi: 10.1507/endocrj.EJ12-0176. [DOI] [PubMed] [Google Scholar]
- 4.Brown RL, de Souza JA, Cohen EE. Thyroid cancer: Burden of illness and management of disease. J Cancer. 2011;2:193–199. doi: 10.7150/jca.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 6.Durante C, Montesano T, Torlontano M, Attard M, Monzani F, Tumino S, Costante G, Meringolo D, Bruno R, Trulli F, et al. Papillary thyroid cancer: Time course of recurrences during postsurgery surveillance. J Clin Endocrinol Metab. 2013;98:636–642. doi: 10.1210/jc.2012-3401. [DOI] [PubMed] [Google Scholar]
- 7.Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381:1058–1069. doi: 10.1016/S0140-6736(13)60109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn D, Park JS, Sohn JH, Kim JH, Park SK, Seo AN, Park JY. BRAFV600E mutation does not serve as a prognostic factor in Korean patients with papillary thyroid carcinoma. Auris Nasus Larynx. 2012;39:198–203. doi: 10.1016/j.anl.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 10.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 11.Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, Beller U, Westra WH, Ladenson PW, Sidransky D. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 12.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Lee KC, Schneider EB, Zeiger MA. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: A meta-analysis. J Clin Endocrinol Metab. 2012;97:4559–4570. doi: 10.1210/jc.2012-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 15.Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, Carson KA, Vasko V, Larin A, Tallini G, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 16.Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–1501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brzezianska E, Pastuszak-Lewandoska D, Wojciechowska K, Migdalska-Sek M, Cyniak-Magierska A, Nawrot E, Lewiński A. Investigation of V600E BRAF mutation in papillary thyroid carcinoma in the Polish population. Neuro Endocrinol Lett. 2007;28:351–359. [PubMed] [Google Scholar]
- 18.Henke LE, Pfeifer JD, Ma C, Perkins SM, DeWees T, El-Mofty S, Moley JF, Nussenbaum B, Haughey BH, Baranski TJ, et al. BRAF mutation is not predictive of long-term outcome in papillary thyroid carcinoma. Cancer Med. 2015;4:791–799. doi: 10.1002/cam4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasirden A, Saito T, Fukumura Y, Hara K, Akaike K, Kurisaki-Arakawa A, Asahina M, Yamashita A, Tomomasa R, Hayashi T, et al. In Japanese patients with papillary thyroid carcinoma, TERT promoter mutation is associated with poor prognosis, in contrast to BRAF (V600E) mutation. Virchows Arch. 2016;469:687–696. doi: 10.1007/s00428-016-2027-5. [DOI] [PubMed] [Google Scholar]
- 20.Alzahrani AS, Alsaadi R, Murugan AK, Sadiq BB. TERT promoter mutations in thyroid cancer. Horm Cancer. 2016;7:165–177. doi: 10.1007/s12672-016-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 22.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, Sun H, El-Naggar AK, Xing M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20:603–610. doi: 10.1530/ERC-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Wang N, Cao J, Sofiadis A, Dinets A, Zedenius J, Larsson C, Xu D. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene. 2014;33:4978–4984. doi: 10.1038/onc.2013.446. [DOI] [PubMed] [Google Scholar]
- 25.Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer. 2016;23:R143–R155. doi: 10.1530/ERC-15-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marques IJ, Moura MM, Cabrera R, Pinto AE, Simoes-Pereira J, Santos C, Menezes FD, Montezuma D, Henrique R, Rodrigues Teixeira M, et al. Identification of somatic TERT promoter mutations in familial nonmedullary thyroid carcinomas. Clin Endocrinol (Oxf) 2017;87:394–399. doi: 10.1111/cen.13375. [DOI] [PubMed] [Google Scholar]
- 27.Jin L, Chen E, Dong S, Cai Y, Zhang X, Zhou Y, Zeng R, Yang F, Pan C, Liu Y, et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: A study of 653 patients. Oncotarget. 2016;7:18346–18355. doi: 10.18632/oncotarget.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Qu S, Liu R, Sheng C, Shi X, Zhu G, Murugan AK, Guan H, Yu H, Wang Y, et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J Clin Endocrinol Metab. 2014;99:E1130–E1136. doi: 10.1210/jc.2013-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, Pai S, Bishop J. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32:2718–2726. doi: 10.1200/JCO.2014.55.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Biase D, Gandolfi G, Ragazzi M, Eszlinger M, Sancisi V, Gugnoni M, Visani M, Pession A, Casadei G, Durante C, et al. TERT promoter mutations in papillary thyroid microcarcinomas. Thyroid. 2015;25:1013–1019. doi: 10.1089/thy.2015.0101. [DOI] [PubMed] [Google Scholar]
- 31.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, corp-author. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 32.Amin MB ES, Greene FL, Byrd DR, Brookland RK, Washington MK. 8th. New York: Springer International; 2017. AJCC Cancer Staging Manual. [DOI] [Google Scholar]
- 33.Liu R, Hao S, Zhang H, Ma J, Liu X, Xu J, Liu X, Ning J, Sun Y, Jiang L, et al. Correlation of thyroid stimulating hormone receptor mRNA expression levels in peripheral blood with undesirable clinicopathological features in papillary thyroid carcinoma patients. Oncotarget. 2017;8:74129–74138. doi: 10.18632/oncotarget.18273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren H, Shen Y, Hu D, He W, Zhou J, Cao Y, Mao Y, Dou Y, Xiong W, Xiao Q, et al. Co-existence of BRAF(V600E) and TERT promoter mutations in papillary thyroid carcinoma is associated with tumor aggressiveness, but not with lymph node metastasis. Cancer Manag Res. 2018;10:1005–1013. doi: 10.2147/CMAR.S159583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun J, Zhang J, Lu J, Gao J, Ren X, Teng L, Duan H, Lin Y, Li X, Zhang B, Liang Z. BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Carcinoma in Chinese Patients. PLoS One. 2016;11:e0153319. doi: 10.1371/journal.pone.0153319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song YS, Lim JA, Choi H, Won JK, Moon JH, Cho SW, Lee KE, Park YJ, Yi KH, Park DJ, Seo JS. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer. 2016;122:1370–1379. doi: 10.1002/cncr.29934. [DOI] [PubMed] [Google Scholar]
- 37.Matsuse M, Yabuta T, Saenko V, Hirokawa M, Nishihara E, Suzuki K, Yamashita S, Miyauchi A, Mitsutake N. TERT promoter mutations and Ki-67 labeling index as a prognostic marker of papillary thyroid carcinomas: Combination of two independent factors. Sci Rep. 2017;7:41752. doi: 10.1038/srep41752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Argyropoulou M, Veskoukis AS, Karanatsiou PM, Manolakelli A, Kostoglou-Athanassiou I, Vilaras G, Karameris A, Liadaki K. Low Prevalence of TERT Promoter, BRAF and RAS Mutations in Papillary Thyroid Cancer in the Greek Population. Pathol Oncol Res. 2020;26:347–354. doi: 10.1007/s12253-018-0497-2. [DOI] [PubMed] [Google Scholar]
- 39.Kim HJ, Park HK, Byun DW, Suh K, Yoo MH, Min YK, Kim SW, Chung JH. Iodine intake as a risk factor for BRAF mutations in papillary thyroid cancer patients from an iodine-replete area. Eur J Nutr. 2018;57:809–815. doi: 10.1007/s00394-016-1370-2. [DOI] [PubMed] [Google Scholar]
- 40.Frasca F, Nucera C, Pellegriti G, Gangemi P, Attard M, Stella M, Loda M, Vella V, Giordano C, Trimarchi F, et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr Relat Cancer. 2008;15:191–205. doi: 10.1677/ERC-07-0212. [DOI] [PubMed] [Google Scholar]
- 41.Schulten HJ, Salama S, Al-Mansouri Z, Alotibi R, AlGhamdi K, Al-Hamour OA, Sayadi H, Al-Aradati H, Al-Johari A, Huwait E, et al. BRAF mutations in thyroid tumors from an ethnically diverse group. Hered Cancer Clin Pract. 2012;10:10. doi: 10.1186/1897-4287-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, Romei C, Miccoli P, Pinchera A, Basolo F. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 43.Oler G, Cerutti JM. High prevalence of BRAF mutation in a Brazilian cohort of patients with sporadic papillary thyroid carcinomas: Correlation with more aggressive phenotype and decreased expression of iodide-metabolizing genes. Cancer. 2009;115:972–980. doi: 10.1002/cncr.24118. [DOI] [PubMed] [Google Scholar]
- 44.Elisei R, Viola D, Torregrossa L, Giannini R, Romei C, Ugolini C, Molinaro E, Agate L, Biagini A, Lupi C, et al. The BRAF(V600E) mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: Single-institution results from a large cohort study. J Clin Endocrinol Metab. 2012;97:4390–4398. doi: 10.1210/jc.2012-1775. [DOI] [PubMed] [Google Scholar]
- 45.Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, Elisei R, Bendlová B, Yip L, Mian C, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33:42–50. doi: 10.1200/JCO.2014.56.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito Y, Yoshida H, Maruo R, Morita S, Takano T, Hirokawa M, Yabuta T, Fukushima M, Inoue H, Tomoda C, et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: Its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J. 2009;56:89–97. doi: 10.1507/endocrj.K08E-208. [DOI] [PubMed] [Google Scholar]
- 47.Nam JK, Jung CK, Song BJ, Lim DJ, Chae BJ, Lee NS, Park WC, Kim JS, Jung SS, Bae JS. Is the BRAF(V600E) mutation useful as a predictor of preoperative risk in papillary thyroid cancer? Am J Surg. 2012;203:436–441. doi: 10.1016/j.amjsurg.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Fugazzola L, Puxeddu E, Avenia N, Romei C, Cirello V, Cavaliere A, Faviana P, Mannavola D, Moretti S, Rossi S, et al. Correlation between B-RAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: Data from a multicentric Italian study and review of the literature. Endocr Relat Cancer. 2006;13:455–464. doi: 10.1677/erc.1.01086. [DOI] [PubMed] [Google Scholar]
- 49.Muzza M, Colombo C, Rossi S, Tosi D, Cirello V, Perrino M, De Leo S, Magnani E, Pignatti E, Vigo B, et al. Telomerase in differentiated thyroid cancer: Promoter mutations, expression and localization. Mol Cell Endocrinol. 2015;399:288–295. doi: 10.1016/j.mce.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 50.Yin L, Tang Y, Yu S, Wang C, Xiao M, Wang Y, Liu SJ, Gao L, Huang K, Jin L. The role of BRAF V600E in reducing AUS/FLUS diagnosis in thyroid fine needle aspiration. Endocr Pathol. 2019;30:312–317. doi: 10.1007/s12022-019-09591-4. [DOI] [PubMed] [Google Scholar]
- 51.Aubhishek Z, Wei W, Trever GB. Targeting Oncogenic BRAF: Past, Present, and Future. Cancers (Basel) 2019;11:1197. doi: 10.3390/cancers11081197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li XY, Zhang B, Lin YS. The Interpretation of 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;52:309–315. doi: 10.3760/cma.j.issn.1673-0860.2017.04.018. (In Chinese) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and/or analyzed in the present study are available from the corresponding author on reasonable request.