Abstract
Purpose
Recently developed implantable microdevices can perform multi-drug response assessment of cancer drugs in-vivo, with potential to develop highly optimized personalized cancer treatment strategies. However, minimally invasive/interventional image-guided methods of in-vivo microdevice implantation, securement, and retrieval are needed for broad clinical translation. Here we demonstrate proof-of-concept of an interventional microdevice implantation and retrieval method for personalized drug response assessment, using ex-vivo phantom, ex-vivo tissue, and in-vivo murine models.
Methods
A method for minimally-invasive microdevice implantation and retrieval was developed, by which a custom-prototyped 6 mm retrievable microdevice can be implanted into a live tumor, deliver drugs into 10 discrete regions of adjacent tissue, and retrieved along with the adjacent drug-exposed tissue with a custom-prototyped retrieval needle device to allow in-vivo multi-drug response assessment. Computed tomography (CT) and ultrasound (US)-guided minimally invasive microdevice implantation and retrieval were tested in ex-vivo phantom and tissue models. Successful retrieval was defined as retrieval of the microdevice and adjacent core phantom/tissue sample containing at least 4/10 drug delivery sites. Subsequently, 10 implantation and retrieval trials in phantom models were performed using bi-axial and tri-axial retrieval needles; success rates were calculated and compared using a two-proportion z-test and the number of successfully retrieved drug release sites per microdevice was calculated and compared using a one-tailed independent t-test. Finally, five microdevices, each containing ten reservoirs preloaded with chemotherapy agent Doxorubicin, were implanted into mouse tumors in-vivo, secured for 24-h during drug release, and microdevice/tissue retrieval was performed under ultrasound guidance. Fluorescence microscopy of the retrieved tissue was used to confirm drug delivery and apoptosis staining assessed in-vivo tissue response; correlation of drug release and apoptosis staining were used to assess in-vivo drug efficacy.
Results
Image-guided microdevice implantation and retrieval were successful in ex-vivo phantom and tissue models with both US and CT guidance. Bi-axial retrieval success rate was significantly higher than triaxial retrieval in ex-vivo phantom trials (90% vs 50%, z = 1.95, P = 0.026), and had nonsignificantly higher number of retrieved drug-release sites per microdevice (8.3 vs 7.0, t = 1.37, P = 0.097). Bi-axial retrieval was successful in all five in-vivo mouse tumor models, and allowed in-vivo drug response assessment at up to ten discrete drug delivery sites per microdevice. An average of 6.8/10 discrete tumor sites containing micro-doses of delivered drug were retrieved per in-vivo attempt (min 5, max 10, std 1.93). Tissue regions of drug delivery, as assessed with fluorescent Doxorubicin drug signal, correlated with regions of apoptosis staining in all in-vivo models, indicating drug efficacy. No bleeding, microdevice migration, or other complications were noted during implantation, 24-h observation, or retrieval.
Conclusions
The demonstrated image-guided minimally invasive microdevice implantation and retrieval method is similar to routine outpatient biopsy procedures, obviates the need for surgery, and can be performed at varying depths under CT and/or US guidance. There is potential for this method to enable clinical translation of in-vivo personalized drug response assessment/prediction in a much larger number of patients than currently possible. © 2019 American Association of Physicists in Medicine [https://doi.org/10.1002/mp.13803]
Keywords: implantable microdevice, interventional oncology, in-vivo drug screening, personalized medicine
1. INTRODUCTION
Minimally invasive image-guided tumor biopsy is a routine clinical procedure in which a biopsy needle is placed into a tumor under image guidance [e.g., ultrasound, computed tomography (CT), Magnetic resonance imaging (MRI)] and a small sample of tissue is retrieved for ex-vivo analysis. Tissue biopsy and subsequent analysis has an important role in the development and optimization of individualized cancer treatments, particularly with the advent of targeted chemotherapy agents and personalized treatment strategies.1–4 However, recent studies have suggested that biopsy samples may not always accurately represent the properties of the entire tumor and retrieved tissue can be insufficient to predict a patient’s response to a chemotherapy agent.5–7 Even when biopsy samples are of high quality, they may not account for complex in-vivo factors including tumor microenvironment, vascularity, and immune function, which have a significant impact on individual treatment response.8–10 Follow-up imaging or laboratory tests to determine drug efficacy, performed weeks to months after initiation of systemic chemotherapy, can be inadequate in quantifying drug efficacy.7,11 This limitation can prolong ineffective treatment, worsen patient outcomes, and create unnecessary healthcare costs. Patient-derived xenografts, in which tumor tissue is removed from the patient and implanted into mice,12–14 allow in-vivo drug response assessment and increasingly intricate in-vitro biomimetic models are being developed to simulate the tumoral microenvironment.15 However, these methods do not completely capture the patient’s complex tumor environment. In addition, they are prohibitively costly and time consuming for large scale clinical translation.
An implantable microdevice was recently developed to overcome these limitations.16 This device can be placed in a patient to allow high-throughput in-vivo cancer drug screening and optimize systemic treatment selection for each individual patient. It can be implanted into native tumor tissue in-vivo (e.g., into the patient directly) and contains multiple drug reservoirs that release tiny doses of drugs into spatially discrete regions of adjacent tissue. The device and surrounding tissue are removed after 24 h and analyzed to determine the efficacy of each delivered drug, without exposing the patient to systemically toxic quantities of the drugs. Assessing local tissue response in this manner has been shown to be safe in animal models and to predict systemic drug response in multiple tumor models.16–18
However, the microdevice and adjacent tissue currently have to be removed surgically. In tissues other than very superficial areas such as the breast or skin, surgical retrieval would require a large operation with high risk of complications and prolonged inpatient admission; this would not be a safe or practical option for most patients. Therefore, in order for this technology to be widely clinically translated for patient use, there is a need for a safe and practical method to implant and retrieve microdevices with adjacent drug-exposed tissue without requiring invasive surgery.
We have developed a minimally invasive interventional method that is similar to routinely performed outpatient biopsy procedures, wherein the microdevice is implanted and retrieved over a tracking guidewire using custom prototyped needles under image guidance with a single small needle puncture. Here, we describe this method and demonstrate proof-of-concept for in-vivo personalized cancer drug evaluation.
2. MATERIALS AND METHODS
2.A. Interventional microdevice implantation, drug release, and retrieval concept
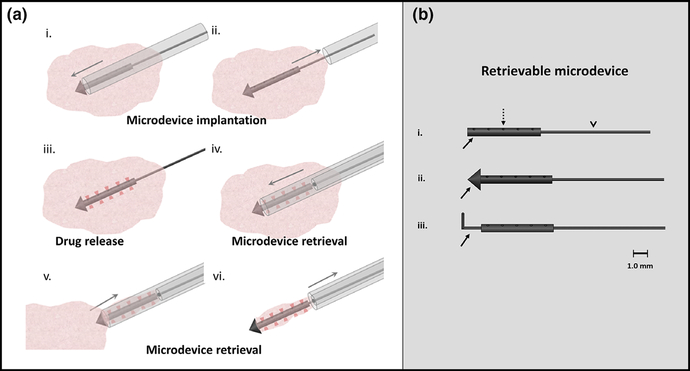
Figure 1(a) illustrates our concept and method of minimally invasive microdevice implantation, drug release, and subsequent tissue and microdevice retrieval for in-vivo drug response assessment [Fig. 1(a)]. The microdevice is placed into in-vivo tissue using an implantation needle under image guidance; microscopic drug release occurs from 10 tiny spatially distinct sites over 24 h while the microdevice is secured within the tumor; the microdevice and adjacent tissue is retrieved using a custom over-the-wire retrieval needle and technique under image guidance; the retrieved tissue is analyzed to assess drug efficacy at each drug release site.
FIG. 1.
(a) Percutaneous microdevice implantation and retrieval schematic. An implantation needle delivers a microdevice into in-vivo tumor tissue (i) and (ii). The microdevice releases drug into ten spatially discrete sites over 24 h (red shaded regions, (iii). This tissue and the microdevice is percutaneously retrieved intact using an over-the-wire retrieval device to assess drug response (iv)—(vi). Up to ten drug combinations can be simultaneously assessed with a single microdevice. (b) (i) Retrievable microdevice with body (solid arrow) that contains ten distinct drug release reservoirs/sites (dashed arrow) preloaded with micro-doses of drug compound and released into adjacent tissue. A nitinol wire (arrowhead, v) attached to the back end of the microdevice body allows precise alignment and localization during retrieval. (ii) A “tophat” conical distal end (arrow) prevents microdevice migration after tissue implantation. (iii) A nitinol wire ‘anchor’ (arrow) can also be used to secure the microdevice in place.
2.B. Retrievable microdevice development and optimization
Drug delivery microdevices were CNC machined from medical grade delrin acetyl-resin blocks (DuPont) [Fig. 1(b)]. The microdevice body, which sits in the tumor and delivers drug micro-doses into the adjacent tissue, is a 6.0 mm long, 625 μm diameteg cylinder [Figs. 1(b)—1(i), solid arrow]. It has 10 discrete reservoirs, each 200-μm deep and 200 μm in diameter that are prefilled with drugs and allow controlled drug release along the outer surface [Figs. 1(b)—1(i), dashed arrow]. Adjacent reservoirs are spaced 1.1 mm apart along the long axis and 180° along the circumference to ensure drug release into spatially distinct tumoral regions. Reservoir size and spacing was chosen to avoid drug overlap between delivery sites, informed by previously published data that has shown that at 24 h, Doxorubicin diffuses passively within 450 μm into adjacent tissues in all directions (axial and radial).16
A 0.008" diameter nitinol wire (Malin Co.) is attached to one end of the microdevice and can be externalized outside the tissue/patient after implantation [Figs. 1(b)—1(i), arrow-head]. A retrieval needle passes over this wire to align with the microdevice during retrieval (discussed below).
Drugs are delivered into precise sub-millimeter spatial regions from multiple reservoirs; therefore, it is important that the microdevices do not move within the tissue for the 24-h period between implantation and retrieval. Two designs were implemented to secure the device inside the tumor and prevent microdevice migration. In the first design (“tophat”), a conical edge was machined as part of the distal end of the microdevice, with a 1.7 mm diameter base and 1.0 mm height [Fig. 1(b), ii]. In the second design (“anchor”), a prebent nitinol wire anchor extends from the distal end of the body [Fig. 1(b), iii].
2.C. Microdevice retrieval tool prototype development
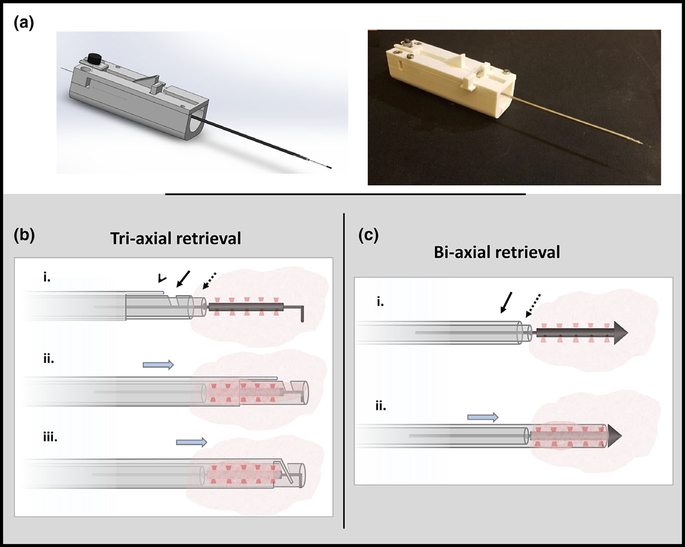
A custom needle prototype was developed with the aid of three-dimensional (3D) printing (Stratasys), using ABS thermoplastic material for minimally invasive microdevice retrieval (Fig. 2). Two models were developed, a tri-axial system containing an end cutting mechanism for retrieval of the anchor microdevices and a bi-axial system for retrieval of the tophat microdevices. The end-cutting prototype used during anchor microdevice retrieval is a tri-axial system with three concentric layers that completely enclose and separate the microdevice and surrounding tissue [Fig. 2(b); Fig. S1]. The tophat microdevices do not require an end cutting mechanism because the tissue is completely severed when the core needle advances to the conical edge of the microdevice. Therefore a second bi-axial version of the prototype was created [Fig. 2(c)], which does not have the end-cutting needle. The outermost largest needle in both designs is 14-gauge.
FIG. 2.
Microdevice retrieval prototype. (a) CAD model and actual prototype of a device allowing precise retrieval of the microdevice and a small adjacent cylindrical tissue sample. (b) Tri-axial retrieval device has an inner stylet (dotted arrow) that passes over the wire, a notched coring needle (solid arrow), and an outer end-cutting needle (arrow head). The retrieval device is advanced over the guidewire to the proximal end of the microdevice (row i), the coring and end cutting needles are advanced around the microdevice to cut and enclose the surrounding tissue (row ii) and the end cutting needle is advanced through the notch to completely sever the distal end of the tissue (row iii). (c) Bi-axial retrieval device has an inner stylet (dotted arrow) and outer coring needle (solid arrow). This device is advanced over the wire to the proximal end of the microdevice (row i) and the coring needle is advanced to cut/enclose and simultaneously sever the surrounding tissue (row ii).
2.D. Retrieval success definition/quantification
Successful microdevice delivery for the following studies discussed below was defined as retrieval of the microdevice with surrounding phantom/tissue material covering at least 4/ 10 drug release reservoirs. Retrieval of 4/10 reservoirs would allow treatment assessment of 2 candidate drugs (retrieval of at least one reservoir site containing each drug) in 98% of cases with 3 implanted microdevices, 93% with 2 microdevices, and 73% with 1 microdevice. With 3 possible candidate drug treatments, treatment assessment of all 3 drugs would be achieved in 82%, 69%, and 44% with 3, 2 and 1 microdevices, respectively. Given the large potential benefit of identifying optimal treatments with relatively small projected risk of a minimally invasive procedure (similar to biopsies), it was determined that a 4/10 reservoir retrieval rate would represent a promising result and justify further investigation.
2.E. Phantom and ex-vivo tissue studies
2.E.1. Ultrasound and CT-guided retrieval
A tophat retrievable microdevice was implanted in an ex-vivo tissue mimicking gel phantom (Parker, Aquaflex) at a depth of 3 cm and ultrasound-guided over-the-wire retrieval was performed using a linear high-frequency ultrasound transducer (Philips Lumify, L12–4) (Video S1). A custom 16G needle, similar to routinely used brachytherapy and fiducial marker deployment needles, was used for implantation and the 14G custom bi-axial retrieval needle was used for retrieval. Ultrasound-guided implantation and retrieval was also performed in ex-vivo liver tissue using an implantation depth of 7 cm (Fig. 3; Fig. S2).
FIG. 3.
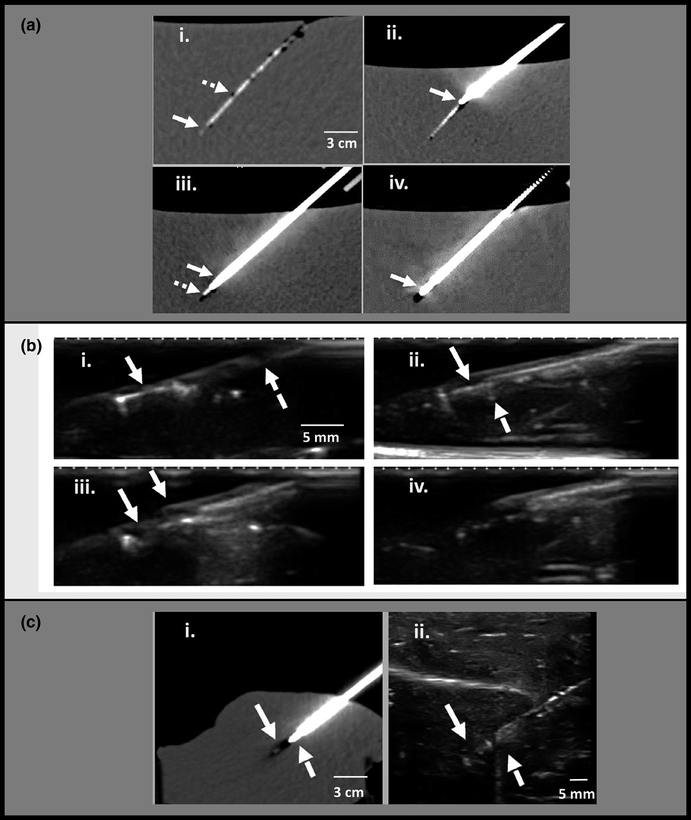
Computed tomography (CT) and ultrasound guided microdevice retrieval in phantom and ex-vivo liver tissue. (a) CT-guided retrieval in tissue-mimicking phantom. (i) Microdevice is implanted at a depth of 12 cm below the surface of a tissue-mimicking phantom. The microdevice (solid arrow) and nitinol wire (dashed arrow) are faintly seen. (ii) The retrieval tool is inserted into the tissue over the wire with a mild bend at the tip (solid arrow) indicating slight axis misalignment. (iii) Retrieval tool trajectory is corrected and advanced to the edge of the microdevice. The thicker coring needle (solid arrow) and thinner inner stylet (dashed arrow) are well seen. (iv) Coring needle advanced around the microdevice (arrow). (b) Ultrasound guided retrieval in tissue-mimicking phantom. (i) Microdevice is well visualized (solid arrow), and the wire is faintly seen (dashed arrow). (ii) retrieval tool is advanced and ultrasound confirms that its tip (dashed arrow) is sitting against the microdevice (solid arrow) with good alignment. (iii) the outer coring needle (dashed arrow) has been advanced halfway over the microdevice to cut the surrounding tissue. (iv) after tissue cutting, the entire retrieval tool with enclosed tissue is retracted and removed. (c) Ex-vivo animal tissue experiments demonstrate similar imaging findings as the phantom. (i) retrieval needle (dashed arrow) advanced to the edge of the microdevice (solid arrow) under CT guidance to depth of 10 cm. (ii) ultrasound image during tissue retrieval also shows the retrieval needle (dashed arrow) located at the edge of the microdevice (solid arrow) with good alignment.
CT-guided implantation and retrieval were performed using a clinical CT scanner (Siemens) in gel phantom and liver tissue models at implantation depths of 12 and 10 cm, respectively (Fig. 3). Axial CT images with 600 μm slices were obtained and 3D postprocessing allowed image reconstruction along the retrieval tool and nitinol wire axis.
2.E.2. Comparison of anchor and tophat microdevice design retrieval efficacy
Under ultrasound guidance, ten tophat microdevices were placed in tissue mimicking gel phantoms at depths of 4–7 cm, and ultrasound-guided retrieval was attempted for each microdevice using the bi-axial retrieval device. Ten ultrasound-guided implantation and retrieval attempts were then made in an identical fashion using a tri-axial retrieval device prototype to extract the anchor microdevices.
Success rates were calculated for the bi-axial and tri-axial approaches; in the successfully retrieved samples, the average (mean) retrieved drug release sites, minimum, maximum, and standard deviation were calculated. A two-proportion one-tailed z-test was used to determine if the bi-axial success rate was significantly higher compared to tri-axial retrieval, and one-tailed independent t-test was used to assess if the mean number of retrieved reservoirs was significantly higher.
2.F. In-vivo animal proof-of-concept study
Institutional animal care and use committee (IACUC) approval was obtained (Protocol #2017N00003).
2.F.1. Drug preparation and loading
All microdevices implanted in-vivo were preloaded with the chemotherapeutic agent, Doxorubicin. Doxorubicin was purchased in powder form (Selleck Chem) and diluted to 25% concentration (w/w) in PEG1450 (Polysciences). The protocol was optimized to ensure local tissue drug release at similar concentrations as would be observed for systemic treatment, as described in detail previously.16 However, 10 drug release reservoirs were preloaded for each implanted microdevice.
2.F.2. Microdevice implantation and retrieval
In-vivo minimally invasive microdevice implantation and retrieval was performed as a proof-of-concept study with five mice flank tumors, including three MC38 colon adenocarcinoma and two A375 melanoma tumors. Approximately 100 ul cell suspension was injected into each flank under 1%—3% isoflurane anesthesia, and tumors were allowed to grow to 2 cm maximal diameter.
Microdevices were implanted under US guidance into the tumors. The mice were observed for 24 h without sedation to allow drug delivery and evaluate for distress or discomfort. US images of the microdevice position after implantation were compared with images immediately prior to retrieval to assess for migration. The length of the externalized portion of the tracking wire was also measured immediately after implantation and 24 h later immediately before retrieval. This served as a quantitative assessment of migration, since any shift in microdevice position would result in retraction or advancement of the attached wire. Ultrasound-guided over-the-wire microdevice and adjacent core tissue retrieval (Fig. 4) was then performed.
FIG. 4.
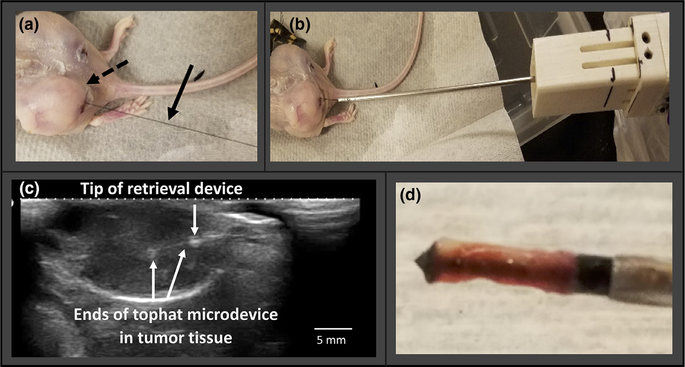
Ultrasound-guided in-vivo microdevice retrieval, A375 mouse tumor model. (a) microdevice implanted into a flank tumor with externalized wire (arrow). (b) retrieval tool advanced over the wire into the tumor. (c) ultrasound shows the microdevice within the tumor, and retrieval tool position and alignment in real time. (d) retrieved tissue sample around the microdevice.
3. RESULTS
3.A. Phantom and ex-vivo tissue studies
3.A.1. Ultrasound and CT-guided retrieval
Ultrasound-guided implantation and retrieval were successful in phantom and ex-vivo tissue models at implantation depths of 3 and 7 cm, respectively. Ultrasound allowed real-time visualization and tracking during microdevice placement and retrieval (Video S1). The microdevice, guidewire, and retrieval device were well visualized with the linear high frequency probe at the tested depths.
2.F.3. Tissue processing and analysis
Retrieved core tissue samples were formalin fixed, paraffin processed, and sectioned along with the microdevices for fluorescence imaging, immunohistochemistry and histopathology. 10–20 μm frozen sections from the retrieved tissue samples were cryo-sectioned (CM1950 cryostat, Leica) from optimal-cutting temperature blocks at five discrete levels containing drug delivery reservoirs, with two reservoirs spaced 180° apart at each level. The sections were cut perpendicular to the long axis of the retrieved sample as shown in Fig. 5(a). This enabled assessment of up to ten spatially discrete regions of tissue, each exposed to a microdose of released drug.
FIG. 5.
Retrieval sample processing, in-vivo MC38 tumor sample. (a) The microdevice and adjacent tissue specimen is retrieved together as shown in the schematic (left) and actual sample image (right). Here, 10–20 lm thick slices are acquired at 5 discrete cross-sectional levels (as numbered), corresponding to ten distinct areas of drug release. (b) Representative microscopic analysis at three different levels. Fluorescence microscopy shows doxorubicin drug distribution (white arrows) 180° apart into spatially discrete tissue regions, and the corresponding cleaved caspase 3 (CC3) stained slides demonstrate apoptotic tissue response (black arrows). Tissue regions not exposed to drug remain viable and do not demonstrate fluorescent signal or apoptosis (asterisks). Spatial correlation between the drug release and apoptotic response at all levels indicates therapeutic efficacy.
CT-guided implantation and retrieval were successful in phantom and ex-vivo tissues at implantation depths of 10 and 12 cm, respectively. The microdevice was faintly seen, as the 600 μm CT slice thickness was similar to the microdevice diameter (625 μm). CT allowed sufficient visualization for over-the-wire retrieval needle tracking and alignment [Figs. 3(a) and 3(c)]. Real-time imaging during the actual phantom/tissue cutting process was not possible (similar to CT/CT-fluoroscopic needle biopsies).
At each section/level, fluorescence imaging (Echo Revolve) was performed with a 555 nm excitation and 590 nm emission red-fluorescent-protein (RFP) filter to assess the spatial distribution of Doxorubicin. Sections were stained with cleaved-caspase-3 (CC3) to evaluate for apoptosis. Spatial correlation of the fluorescent drug release signal and apoptotic tissue response was used to evaluate drug efficacy [Fig. 5(b)].
3.A.2. Comparison of anchor and tophat microdevice design retrieval efficacy
90% (9/10) of the tophat microdevices were successfully retrieved in the phantom model using the bi-axial retrieval device. Out of the successfully retrieved microdevices, an average of 8.33 drug delivery reservoirs was covered by phantom tissue per microdevice (minimum 6, maximum 10, std 1.66).
Successful retrieval was assessed as defined in section D, with drug release sites confirmed by fluorescent imaging. The average (mean) number of retrieved drug release sites, minimum, maximum, and standard deviation were calculated per retrieved microdevice. Tissue thickness was measured at all drug release sites from microscopic images of the sectioned tissue and average (mean), minimum, maximum, and standard deviation thickness were calculated.
Here, 50% (5/10) of the anchor microdevices were successfully retrieved using the tri-axial retrieval device. Out of the successfully retrieved microdevices, an average of 7.0 reservoirs was covered by phantom tissue (minimum 5, maximum 10, std 1.87).
Success rate was significantly higher (z = 1.95, P = 0.026) with bi-axial retrieval. The mean number of retrieved reservoirs among the successfully retrieved samples was also higher for bi-axial retrieval but did not reach statistical significance (t = 1.37, P = 0.097). The bi-axial retrieval system was less technically challenging and required less needle manipulation, which likely accounted for the higher success rate. Based on these results, the tophat microdevice design with bi-axial system was selected for subsequent in-vivo studies.
3.B. In-vivo animal proof-of-concept study
3.B.1. Microdevice implantation and retrieval
Ultrasound-guided microdevice implantation and retrieval was successful in the five in-vivo tumors. The mice resumed baseline activity and ambulation within 15 min after implantation without restraint or sedation; all mice maintained their baseline level of activity and ambulation at 24 h. Following 24 h, repeat ultrasound imaging confirmed stable positioning of the microdevices in all cases without migration. External wire lengths measured immediately after implantation and immediately prior to retrieval were within 0.5 mm in all cases, also confirming 24-h microdevice stability. Ultrasound allowed real-time visualization of the retrieval device, nitinol wire, and microdevices during retrieval. No tumoral bleeding or infection at the guidewire exit site was seen after implantation, before retrieval, or after retrieval.
3.B.2. Retrieved tissue sample processing and quality
Localization of drug release and tissue analysis for drug response assessment was successful in retrieved tissue samples from all five tumors (Figs. 5 and 6). 4/5 retrieved samples remained adherent to the microdevice throughout processing. One of the tissue specimens separated from the microdevice during placement in formalin, but this did not prevent drug response assessment.
FIG. 6.
Retrieval specimen analysis from five in-vivo tumor samples. Sufficient tissue was retrieved for analysis in all five samples. The average number of drug sites assessed for each sample was 6.8 (minimum 5, maximum 10) and average tissue thickness at the drug delivery sites was 553.3 um (minimum 218.2, maximum 938.1). Drug delivery to apoptotic response spatial correlation was seen in all tumors, indicating therapeutic efficacy in the tested A375 and MC38 animal models. *Specimen 5 separated from the microdevice during processing; drug delivery location was still able to be assessed by identifying the area of fluorescent doxorubicin signal.
Optimal drug release was seen between 100 and 400 μm radially from the drug release reservoirs, with no overlap seen on fluorescent imaging between the adjacent sites. An average of 6.8/10 spatially distinct tumor sites containing released drug were successfully retrieved for each implanted microdevice (min 5, max 10, std 1.93). Of note, this retrieval rate would ensure treatment response assessment of 2 candidate drugs in >99%, >99%, and 95% of cases and 3 candidate drugs at 99%, 95%, and 79% of cases with 3, 2, and 1 implanted microdevices respectively. Average tissue thickness at the drug delivery sites was 553 μm (min 218 μm, max 938 μm, std 241).
3.B.3. Drug response assessment
The spatial distribution of Doxorubicin release on fluorescence imaging correlated with the distribution of CC3 apoptosis staining in all five tumors and at each individual drug delivery site, indicating therapeutic effect with homogenous drug response [Figs. 5(b) and 6 column 3]. Adjacent tissue not exposed to drug showed viable tumor without significant apoptotic staining or necrosis. This confirmed that the apoptotic tissue changes were drug effects rather than tissue damage from microdevice implantation, retrieval, or processing.
4. DISCUSSION
We have demonstrated a minimally invasive wire-guided microdevice delivery method to evaluate drug efficacy in live tumors without requiring surgery. We previously found minimally invasive delivery, using a thin needle to implant and extract only the microdevice and adjacent tissue, to be too technically challenging to be consistent or practical. In particular, precise alignment of the biopsy needle with the microdevice axis during retrieval was nearly impossible, despite image guidance. Tiny variation in the microdevice orientation or any minimal misalignment of the needle resulted in retrieval failure. Even with good alignment on imaging, tiny movements of the microdevice or needle during the cutting/coring process again resulted in retrieval failure. In our current method, a guidewire attached to the microdevice enables precise needle tracking and axis alignment, while mechanical anchoring methods keep the microdevice and needle stable during retrieval. With these modifications and custom-prototyped microdevice and delivery/retrieval needles, we were able to consistently retrieve the microdevice and surrounding tissue in a minimally invasive manner. To our knowledge, this is the first minimally invasive method for retrieval of implanted foreign objects from solid tissues.
Microdevice implantation and retrieval was possible at varying depths using CT and US guidance. Image guidance selection is typically based on target site (e.g., tumor) visualization, radiation considerations, and user expertise/preference. If tumors and interventional devices are well seen on ultrasound, this is often preferred as it is usually faster and does not have the radiation exposure associated with CT.19 Conversely, some tumors are not well seen on US and procedures must be performed under CT guidance. Therefore, the ability to visualize and perform procedures under both CT and US guidance, as demonstrated here, will allow greater operator flexibility and should enable in-vivo drug response assessment in more patients and tumor sites.
This method can assess efficacy of multiple different drugs or drug combinations in-vivo (in our study, up to 10 discrete drug delivery sites were assessed for each implanted microdevice). Multiple microdevices can be placed into a single tumor or more drug delivery reservoirs can be added to each microdevice to simultaneously evaluate a larger number of drugs. In addition, single drug response can be assessed at multiple spatially discrete regions (as in the current study) to partially account for intratumoral heterogeneity, wherein different regions of the same tumor can have varying genetic and phenotypic properties and treatment responses.5,20,21 Since implantation and retrieval requires only a single thin needle puncture, there is potential for this approach to be widely used in patients without requiring morbid surgical techniques.
Our approach should have similar low bleeding risk as current routine minimally invasive procedures. The 16-gauge implantation needles are similar to clinically used brachytherapy and fiducial marker placement needles.22–24 The externalized guidewire is thinner than drainage catheters and other devices that are routinely left externalized for longer duration.25,26 The nitinol guidewire material used here is biocompatible and routinely used in stents, vascular filters, and other implanted devices.27,28 In our in-vivo studies, all mice tolerated wire externalization well with no evidence of bleeding and resumed baseline activity within minutes following implantation. The retrieval technique is similar to routine biopsies. We found 14-gauge cutting needles to be optimal. Although larger than more conventional 18-gauge biopsy needles, 14-gauge needles have been used for deep tissue biopsies,29,30 and similar sized needles are used for ablation and other interventional procedures in deep organs.31 Furthermore, our custom retrieval needle traverses a shorter distance (~6 mm) and cuts a smaller volume of tissue (~14 mm3) than standard 18-gauge needles (~20 mm distance and ~18 mm3 volume).
Although we have focused on cancer drug evaluation, minimally invasive in-vivo drug delivery and tissue response has potential applications in other disease areas. Autoimmune and other inflammatory-mediated conditions can impact essentially every organ system and standard treatment strategies often have significant risks and side effects.32 Although genetic and biomarker testing are being explored to optimize drug selection for many of these conditions, they have not been clinically validated and individual treatment response to anti-inflammatory agents remains highly variable.33,34 Many of these patients could benefit from microscopic in-vivo drug efficacy confirmation prior to systemic delivery. New classes of novel gene editing molecules and cellular delivery mechanisms are being developed for various diseases; however, unintended off-target effects and host immune response can be unpredictable and potentially dangerous.35 Localized microscale delivery of these agents using retrievable microdevices placed into specific tissues/organs, with genetic analysis and microscopic immune response assessment of the retrieved tissue may help predict these effects in preclinical and clinical models and avoid systemic toxicity.
This minimally invasive implantation and retrieval technique may facilitate translation of other miniaturized sensors and microdevices for various applications, including controlled drug release, tissue microenvironmental monitoring and regulation, and early disease detection.36–39 Precise implantation and retrieval in a safe, minimally invasive nature will be essential for clinical translation of many of these devices and has not been previously addressed, to our knowledge. In addition, the externalized wire could enable microdevice communication and/or monitoring. For example, it could be modified to transmit electronic data, allow reloading of drug into a drug-delivery microdevice, or contain optical imaging probes for visualizing real-time microscopic tissue changes. These are potential areas for future development.
There are several limitations of the study. This was a proof-of-concept study with a small sample size; inferences on population success rates will require larger powered in-vivo trials. Also, although local tissue response from microdevice drug release has predicted systemic response in animal models,16–18 this has not been validated in humans. Several clinical trials are underway to address this. Solid homogenous tumors were used in our studies, which facilitated retrieval of high quality tissue samples; implantation and retrieval from soft, heterogeneous, and/or partially necrotic tissues may result in poor/fragmented samples, precluding drug response assessment. Finally, although our ex-vivo studies were successful at implantation depths up to 12 cm, in-vivo depths were limited to superficial (<4 cm) flank tumors; deeper implantation in larger animals or human clinical trials would be helpful to ensure feasibility in deeper tissues and are planned.
5. CONCLUSION
In conclusion, this study demonstrates an interventional image-guided method by which a microdevice is implanted into a live tumor, releases microscopic quantities of drug into adjacent tissues, and the tissue and microdevice is retrieved in a manner similar to needle biopsies to assess drug efficacy. The approach presented here could allow clinicians to test and identify which cancer treatments will work for an individual patient prior to delivery of potentially toxic and/or ineffective systemic treatments. Since the method is similar to routinely performed interventional procedures such as image-guided needle biopsies, it would be applicable to many patients and has potential for broad clinical translation.
Supplementary Material
Fig. S1: Tri-axial microdevice retrieval prototype mechanism. The retrieval tool is advanced over the nitinol wire (row 1) until it reaches the microdevice (row 2). A notched coring needle is advanced to cut/enclose the tissue surrounding the microdevice (row 3). An additional end-cutting needle separates the distal tissue from the surrounding tumor (row 4). This step is not required for the tophat design as the tissue is completely separated in step 3. The retrieval device is pulled out of the patient/tissue with tissue sample enclosed; needle is retracted to expose the microdevice and small specimen sample (row 5).
Fig. S2: Microdevice retrieval in ex-vivo liver tissue. (a) Microdevice has been implanted to a depth of 7 cm, with the nitinol wire (arrows) extending out of the tissue sample. (b) Retrieval needle with inner hollow stylet (dashed arrow) is passed over the wire (solid arrow) to maintain alignment. (c) Retrieval device is advanced and extracts the microdevice under ultrasound guidance (ultrasound images not shown). (d) Retrieved microdevice with adjacent cylindrical tissue sample.
Video S1: Ultrasound-guided microdevice placement and retrieval in a phantom.
Footnotes
CONFLICTS OF INTEREST
Dr. Cima is a director and consultant at T2 Biosystems, Taris Biomedical, MicroChips Biotech, and Elute Inc. Dr. Jonas has a financial interest in Kibur Medical. Dr. Jonas’s interests were reviewed and are managed by BWH and Partners HealthCare in accordance with their conflict of interest policies.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Contributor Information
Sharath K. Bhagavatula, Department of Radiology, Brigham and Women's Hospital, 75 Francis Street, Boston, MA 02115, USA
Kunj Upadhyaya, Department of Radiology, Brigham and Women's Hospital, 75 Francis Street, Boston, MA 02115, USA.
Brendyn J. Miller, Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, 500 Main Street, Boston, MA 02139, USA
Patrick Bursch, Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, 500 Main Street, Boston, MA 02139, USA.
Alex Lammers, Department of Radiology, Brigham and Women's Hospital, 75 Francis Street, Boston, MA 02115, USA.
Michael J. Cima, Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, 500 Main Street, Boston, MA 02139, USA
Stuart G. Silverman, Department of Radiology, Brigham and Women's Hospital, 75 Francis Street, Boston, MA 02115, USA
Oliver Jonas, Department of Radiology, Brigham and Women's Hospital, 75 Francis Street, Boston, MA 02115, USA; Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, 500 Main Street, Boston, MA 02139, USA.
REFERENCES
- 1.Marshall D, LaBerge JM, Firetag B, Miller T, Kerlan RK. The changing face of percutaneous image-guided biopsy: molecular profiling and genomic analysis in current practice. J Vasc Interv Radiol. 2013;24:1094–1103. [DOI] [PubMed] [Google Scholar]
- 2.Tam AL, Lim HJ, Wistuba II, et al. Image-guided biopsy in the era of personalized cancer care: proceedings from the society of interventional radiology research consensus panel. J Vasc Interv Radiol. 2016;27:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziv E, Durack JC, Solomon SB. The importance of biopsy in the era of molecular medicine. Cancer J. 2016;22:418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dancey JE, Bedard PL, Onetto N, Hudson TJ. The genetic basis for cancer treatment decisions. Cell. 2012;148:409–420. [DOI] [PubMed] [Google Scholar]
- 5.Gerlinger M, Rowan AJ, Horswell S, et al. intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlinger M, Horswell S, Larkin J, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014;46:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meric-Bernstam F, Mills GB. Overcoming implementation challenges of personalized cancer therapy. Nat Rev Clin Oncol. 2012;9:542–548. [DOI] [PubMed] [Google Scholar]
- 8.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson JI, Decker S, Zaharevitz D, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yankeelov TE, Mankoff DA, Schwartz LH, et al. Quantitative imaging in cancer clinical trials. Clin Cancer Res. 2016;22:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel VC, Marchionni L, Hierman JS, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69:3364–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9:338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garralda E, Paz K, Lopez-Casas PP,et al. Integratednext-generation sequencing and avatar mouse models for personalized cancer treatment. Clin Cancer Res. 2014;20:2476–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradhan S, Smith AM, Garson CJ, et al. A microvascularized tumor-mimetic platform for assessing anti-cancer drug efficacy. Sci Rep. 2018;8:3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas O, Landry HM, Fuller JE, et al. An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Sci Transl Med. 2015;7:284ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonas O, Calligaris D, Methuku KR, et al. First in vivo testing of compounds targeting group 3 medulloblastomas using an implantable microdevice as a new paradigm for drug development. J Biomed Nanotechnol. 2016;12:1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonas O, Oudin MJ, Kosciuk T, et al. Parallel in-vivo assessment of drug phenotypes at various time points during systemic BRAF inhibition reveals tumor adaptation and altered treatment vulnerabilities. Clin Cancer Res. 2016;22:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang K, Ganguli S, DeLorenzo MC, Zheng H, Li X, Liu B. Procedure-specific CT dose and utilization factors for CT-guided interventional procedures. Radiology. 2018;289:150–157. [DOI] [PubMed] [Google Scholar]
- 20.Jamal-Hanjani M, Quezada SA, Larkin J, Swanton C. Translational implications of tumor heterogeneity CME staff planners’ disclosures. Clin Cancer Res. 2015;21:1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta - Rev Cancer. 2010;1805:105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trumm CG, Häußler SM, Muacevic A, et al. CT fluoroscopy-guided percutaneous fiducial marker placement for cyberknife stereotactic radiosurgery: technical results and complications in 222 consecutive procedures. J Vasc Interv Radiol. 2014;25:760–768. [DOI] [PubMed] [Google Scholar]
- 23.Kulkarni NM, Hong TS, Kambadakone A, Arellano RS. CT-guided implantation of intrahepatic fiducial markers for proton beam therapy of liver lesions: assessment of success rate and complications. AJR Am J Roentgenol. 2015;204:W207–W213. [DOI] [PubMed] [Google Scholar]
- 24.Kothary N, Heit JJ, Louie JD, et al. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J Vasc Interv Radiol. 2009;20:235–239. [DOI] [PubMed] [Google Scholar]
- 25.Funaki B. Central venous access: a primer for the diagnostic radiologist. Am J Roentgenol. 2002;179:309–318. [DOI] [PubMed] [Google Scholar]
- 26.Gerzof S, Robbins A, Birkett D, Johnson W, Pugatch R, Vincent M. Percutaneous catheter drainage of abdominal abscesses guided by ultrasound and computed tomography. Am J Roentgenol. 1979;133:1–8. [DOI] [PubMed] [Google Scholar]
- 27.Rabkin DJ, Lang EV, Brophy DP. Nitinol properties affecting uses in interventional radiology. J Vasc Interv Radiol. 2000;11:343–350. [DOI] [PubMed] [Google Scholar]
- 28.Duerig T, Pelton A, Stöckel D. An overview of nitinol medical applications. Mater Sci Eng A. 1999;5093:149–160. [Google Scholar]
- 29.Oeak S, Duplaquet F, Jamart J, et al. Diagnostic accuracy and safety of CT-guided percutaneous transthoracic needle biopsies: 14-gauge versus 22-gauge needles. J Vasc Interv Radiol. 2016;27:674–681. [DOI] [PubMed] [Google Scholar]
- 30.Tung KT, Downes MO, O’Donnell PJ. Renal biopsy in diffuse renal disease — experience with a 14-gauge automated biopsy gun. Clin Radiol. 1992;46:111–113. [DOI] [PubMed] [Google Scholar]
- 31.Saldanha DF, Khiatani VL, Carrillo TC, et al. Current tumor ablation technologies: basic science and device review. Semin Intervent Radiol. 2010;27:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho JH, Gregersen PK. Genomics and the multifactorial nature of human autoimmune disease. Feero WG, Guttmacher AE, eds. N Engl J Med. 2011;365:1612–1623. [DOI] [PubMed] [Google Scholar]
- 34.Tavakolpour S, Darvishi M, Ghasemiadl M. Pharmacogenetics: a strategy for personalized medicine for autoimmune diseases. Clin Genet. 2018;93:481–497. [DOI] [PubMed] [Google Scholar]
- 35.Maeder ML, Gersbach CA. Genome-editing technologies for gene and cell therapy. Mol Ther. 2016;24:430–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farra R, Sheppard NF, McCabe L, et al. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci Transl Med. 2012;4:122ra21. [DOI] [PubMed] [Google Scholar]
- 37.Ring A, Sorg H, Weltin A, et al. In-vivo monitoring of infection via implantable microsensors: a pilot study. Biomed Eng/Biomed Tech. 2018;63:421–426. [DOI] [PubMed] [Google Scholar]
- 38.Chu MKL, Chen J, Gordijo CR, et al. In vitro and in vivo testing of glucose-responsive insulin-delivery microdevices in diabetic rats. Lab Chip. 2012;12:2533. [DOI] [PubMed] [Google Scholar]
- 39.Gurman P, Miranda OR, Clayton K, Rosen Y, Elman NM. Clinical applications of biomedical microdevices for controlled drug delivery. Mayo Clin Proc. 2015;90:93–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1: Tri-axial microdevice retrieval prototype mechanism. The retrieval tool is advanced over the nitinol wire (row 1) until it reaches the microdevice (row 2). A notched coring needle is advanced to cut/enclose the tissue surrounding the microdevice (row 3). An additional end-cutting needle separates the distal tissue from the surrounding tumor (row 4). This step is not required for the tophat design as the tissue is completely separated in step 3. The retrieval device is pulled out of the patient/tissue with tissue sample enclosed; needle is retracted to expose the microdevice and small specimen sample (row 5).
Fig. S2: Microdevice retrieval in ex-vivo liver tissue. (a) Microdevice has been implanted to a depth of 7 cm, with the nitinol wire (arrows) extending out of the tissue sample. (b) Retrieval needle with inner hollow stylet (dashed arrow) is passed over the wire (solid arrow) to maintain alignment. (c) Retrieval device is advanced and extracts the microdevice under ultrasound guidance (ultrasound images not shown). (d) Retrieved microdevice with adjacent cylindrical tissue sample.
Video S1: Ultrasound-guided microdevice placement and retrieval in a phantom.