Abstract
The Measles & Rubella Initiative (M&RI) identified five key strategies to achieve measles and rubella elimination, including research and innovation to support cost-effective operations and improve vaccination and diagnostic tools. In 2016, the M&RI Research and Innovation Working Group (R&IWG) completed a research prioritization process to identify key research questions and update the global research agenda. The R&IWG reviewed meeting reports and strategic planning documents and solicited programmatic inputs from vaccination experts at the program operational level through a web survey, to identify previous research priorities and new research questions. The R&IWG then convened a meeting of experts to prioritize the identified research questions in four strategic areas: (1) epidemiology and economics, (2) surveillance and laboratory, (3) immunization strategies, and (4) demand creation and communications. The experts identified 19 priority research questions in the four strategic areas to address key areas of work necessary to further progress toward elimination. Future commitments from partners will be needed to develop a platform for improved coordination with adequate and predictable resources for research implementation and innovation to address these identified priorities.
Keywords: Measles, Rubella, Eradication, Research, Immunization, Vaccines
1. Introduction
The Measles & Rubella Initiative1 (M&RI) holds a vision of achieving a world free of measles and rubella [1]. Significant progress has been made toward achieving this vision through focused efforts by partners and countries. In 2010, an expert advisory panel convened by the World Health Organization (WHO) concluded that measles can and should be eradicated [2]; and the WHO Strategic Advisory Group of Experts (SAGE) on Immunization endorsed these conclusions. In January 2011, the World Health Assembly (WHA) Executive Board endorsed the SAGE recommendations. In 2012, the WHA subsequently endorsed the Global Vaccine Action Plan (GVAP) that set a target to eliminate measles and rubella (MR) in five of the six WHO regions by 2020 [3]. In addition to this global goal for MR elimination, countries in all six regions have established regional goals for measles elimination and three have set regional goals for rubella elimination by 2020 or earlier [4].
The Global Measles and Rubella Strategic Plan, 2012–2020, was developed by the M&RI with targets that are aligned with GVAP[5]. The five key strategies for measles and rubella elimination are: (1) high population immunity through vaccination with two doses of measles- and rubella-containing vaccine; (2) effective surveillance, monitoring and evaluation; (3) outbreak prepared-ness and response, ensuring case management; (4) communication to build public confidence and demand for immunization; and (5) research and development to support cost-effective operations and improve vaccination and diagnostic tools. In 2015, the M&RI established the Research and Innovation Working Group (R&IWG) to facilitate implementation of the research strategy, including prioritizing and cataloguing research projects. R&IWG collaborates with the WHO SAGE to develop a robust research agenda.
During 2000–2016, the number of estimated annual measles deaths decreased 84%, from 550,021 to 89,663, and an estimated 20.4 million deaths were prevented [4]. Measles and rubella elimination has been achieved in the Region of the Americas; however, the remaining regions are not on track to meet elimination goals for measles or rubella by 2020 [4,6]. Measles remains a major cause of child mortality, and rubella is the leading cause of birth defects among all infectious diseases globally, despite the fact that both are vaccine-preventable [7].
In 2016, the Midterm Review of the Measles and Rubella Elimination Strategic Plan concluded that developing new technologies and making better use of data are necessary to ensure further progress toward measles and rubella elimination [8,9]. Research findings have provided critical evidence for establishing policy, strategies, and key innovations for disease eradication initiatives [10–12] to accelerate the progress toward the goals. Research activities for measles and rubella elimination have led to effective innovations and tools to enhance the core elimination strategies, including disease surveillance, immunization delivery activities, and communications [13]. In 2016, building on previous efforts to identify and prioritize research needs, the R&IWG initiated a prioritization process, to expand the evidence base for strategies and policies to achieve global and regional measles and rubella elimination. This manuscript describes the R&IWG prioritization process and the research questions that were identified as priorities for measles and rubella elimination and eradication. The full meeting report and comprehensive list of all identified research questions can be found on the M&RI website at https://measlesrubellainitiative.org/research-innovation-meeting-2016/.
2. Methods
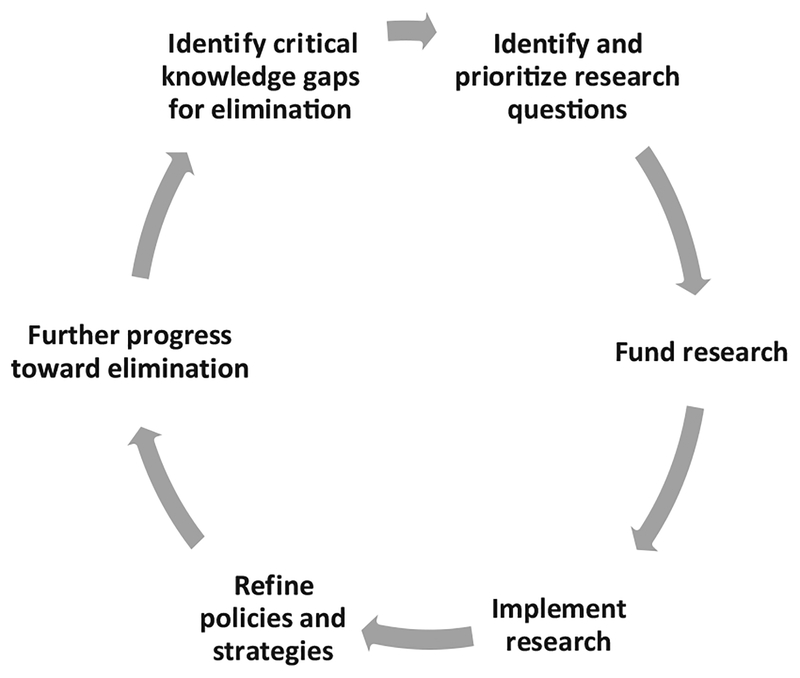
A research development cycle was used as a conceptual framework for outlining stages of research and innovation to accelerate elimination activities (Fig. 1). To prioritize research needed to achieve elimination, the R&IWG designed a prioritization process that focused on operational research questions directly related to strategy implementation. The primary outcome of the prioritization process was to identify priority research questions to address critical knowledge and evidence gaps needed to reach, maintain and verify measles and rubella elimination. In addition, the identified research questions needed to be answerable with a feasible study design or approach and have potential impact by addressing a significant bottleneck to achieve elimination.
Fig. 1.
The research cycle for driving program improvements to accelerate elimination activities for measles and rubella, Measles & Rubella Initiative research prioritization process, 2016.
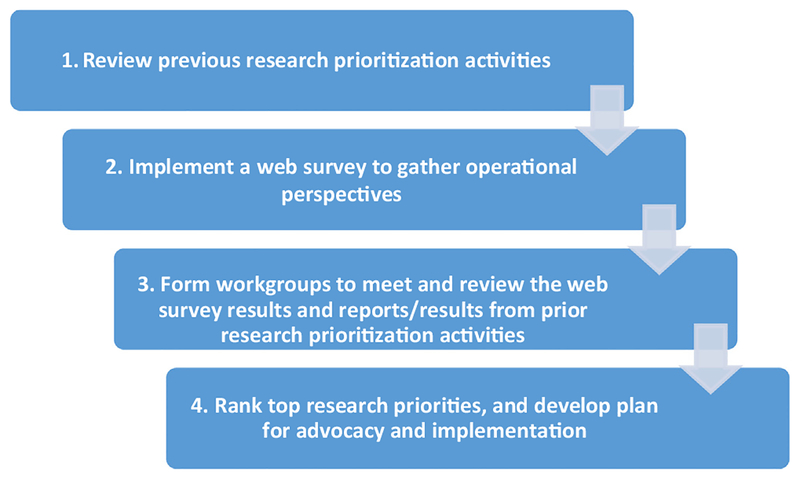
A four-step process to identify and prioritize research questions was used by the R&IWG (Fig. 2). First, previous research prioritization activities and meeting reports were reviewed, to develop a foundation of potential current research questions. The previous meetings and reports included a WHO steering committee meeting in 2007 [14], a Global Measles and Rubella Research External Peer Review meeting in 2008 [15], a WHO Technical Consultation to Assess the Feasibility of Measles Eradication in 2010 [2], a WHO Global Measles and Rubella Research Meeting in 2011 [13], the SAGE Measles Rubella survey in 2015 [16], and the Measles and Rubella Elimination Strategy 2012–2020 Midterm Review in 2015 [8,9].
Fig. 2.
The four-step process for the Measles & Rubella Initiative research prioritization process, 2016.
Second, the findings were compiled and formulated into research questions for a cross-sectional web-based survey of scientific and programmatic experts, with a major aim to obtain input for the process from a broad group of experts from all operational levels and from all six WHO regions [17].
Third, the R&IWG formed workgroups to address four identified strategic areas: (1) epidemiology and economic analysis; (2) surveillance and laboratory; (3) immunization strategies; and (4) demand creation and communications. Each workgroup was comprised of experts for each of the strategic areas and led by one or two global experts on the subject area to identify four to five priority research questions. Experts were selected so that each group would have a mix of participants from academia, national-level programs, and global organizations. Data from the web-based survey were summarized by the R&IWG for review by the work-groups, along with findings outlined in previous research prioritization meetings and reports. Individual workgroups reviewed open-ended responses from the web-based survey to identify new research questions and questions on implementation challenges to achieving elimination goals.
Fourth, to rank top research priorities and develop plans for advocacy and implementation, a consultative meeting of the R&IWG and workgroups was convened during November 29–30, 2016 at the Pan-American Health Organization (PAHO) headquarters in Washington, D.C., with the support of the M&RI, Sabin Vaccine Institute, and PAHO. The primary meeting objectives were to:(1) identify the critical knowledge and evidence gaps, and (2) develop a list of the highest priority research questions within the four strategic areas for achieving, verifying, and maintaining measles and rubella elimination. To provide an accurate report of the results of this process, the prioritized research questions from each workgroup were reported without rearrangement or consolidation of topics.
3. Results
Table 1 presents the high-priority research questions in rank order from each workgroup’s process of prioritization by topic area. The sections that follow provide background on the strategic areas and describe the priority research questions identified by each workgroup.
Table 1.
Priority research questions to achieve measles and rubella elimination. Questions were rank ordered by four topic workgroups convened as part of the Measles & Rubella Initiative Research Prioritization Process, 2016.
| Epidemiology and economics |
|
| Surveillance and laboratory |
|
| Immunization strategies |
|
| Demand creation and communications |
|
Abbreviations – CRS: “congenital rubella syndrome”, MCV1: “first routine dose of measles-containing vaccine”, MCV2: “second routine dose of measles-containing vaccine”, SIA: “supplemental immunization activity”.
3.1. Epidemiology and economics workgroup
This workgroup considered broad topics of epidemiology and economics of measles, rubella and congenital rubella syndrome (CRS). Research on the epidemiology and economics of measles, rubella, and CRS is critical to support cost-effective operations, improve vaccination and diagnostic tools, and support the four core programmatic strategies of the Measles and Rubella Elimination Strategic Plan 2012–2020 [18]. Epidemiologic and economic questions that could be addressed using analytical methods, including mathematical modelling methods, were considered. The priority questions ranged across a spectrum of issues, from improving epidemiologic analysis by improving data quality; to using epidemiologic data to target at-risk populations, interrupt chains of transmission, and determine country-specific impact of vaccination; and to assessing the economic benefits of measles and rubella interventions.
1. How can programmatic data be used to better identify susceptible populations to target interventions?
The fundamental challenge for measles and rubella elimination is the need to increase population immunity and reduce the proportion of susceptible individuals to a level below the level needed to sustain virus transmission. Susceptible individuals are generally invisible to the program until an outbreak occurs; therefore, methods to identify susceptible populations before an outbreak could help prevent outbreaks and accelerate progress toward elimination [19,20]. Ideally, existing programmatic data, including surveillance data, could be used to identify susceptible subpopulations [21–24]; however, while such data are available, poor data quality often impedes accurate prediction. Knowing when the available data are of sufficient quality and detail, and when other sources of data such as serologic surveys are needed, is fundamental to achieving and maintaining measles, rubella and CRS elimination by identifying and targeting susceptible populations with vaccination activities.
2. What are common reasons for under-diagnosing and under-reporting measles, rubella, and CRS cases, and what strategies should be adopted to improve case ascertainment and reporting in different transmission settings?
Sensitive surveillance systems are necessary to monitor progress toward and achievement of elimination [25,26]. Improving accurate case detection and reporting in various settings is necessary to measure the burden of measles, rubella, and CRS at the national and the subnational levels, to understand virus transmission pathways, design and implement effective elimination strategies, and track progress toward achieving and maintaining elimination [4,27].
3. What are the incremental costs and benefits of prevention, surveillance and outbreak response for measles, rubella and CRS, and the financial resources required to achieve measles and rubella elimination?
Estimated costs, and health and economic benefits achieved from measles and rubella elimination, compared with current high control, are critical for advocacy and resource mobilization at country, regional, and global levels [28,29]. To make such a comparison, a better understanding of the costs of surveillance and outbreak response for measles and rubella is needed [30]. Understanding the return on investment value of elimination efforts, including measles and rubella vaccination, surveillance and out-break response, is needed to secure funds necessary to support and expand elimination activities [28,31–34]. Some data are available, although limited and additional data are necessary.
4. What is the estimated public health impact of measles and rubella vaccination at the national level?
Ensuring government ownership and public support for measles and rubella elimination targets as well as other components of national immunization programs requires accurate estimates of the public health benefits of measles and rubella vaccination [7]. This is of particular importance in low-burden settings where morbidity and mortality of measles and rubella, including CRS, are no longer common and misunderstandings of the need to sustain high vaccination coverage arise. Accurate estimates of the number of cases, hospitalizations and deaths averted by vaccination are critical for advocacy efforts to ensure sustained commitment to measles and rubella elimination.
5. How can data on population characteristics, susceptibility profiles and virus genotypes be best used to identify transmission pathways and predict areas and populations at risk for measles and rubella outbreaks?
In endemic settings, uncertainty about the spatial distribution of target populations and heterogeneity of susceptibility among those populations are critical information gaps limiting effective targeting of vaccination activities to eliminate measles, rubella, and CRS. In addition to better use of programmatic data to identify and target susceptible populations, as described in the first priority question identified by this work group, uncertainty about population movements, contact patterns, as well as susceptibility among special populations were identified as information gaps in understanding potential virus transmission pathways and the risk of out-breaks, particularly in post-elimination settings. Knowledge of both susceptibility patterns and transmission pathways is critical to assess risk, to anticipate potential measles and rubella out-breaks, and to intervene preemptively [35]. Increasingly, detailed data on population movements and settlements, contact patterns, susceptibility profiles and virus genotypes are available, but they need to be integrated and used by programs to target interventions to prevent measles and rubella outbreaks [19,20].
3.2. Surveillance and laboratory workgroup
The surveillance and laboratory workgroup discussed questions related to improving surveillance quality, data reporting, and surveillance indicators. In addition, the workgroup also considered research issues related to novel vaccine delivery technologies currently under development.
1. Can vaccine safety, effectiveness, and/or coverage be improved by developing more thermo-stable vaccines and by alternative delivery methods (e.g. microarray patches)?
A potentially significant way to increase measles and rubella immunization coverage during SIAs would be to improve vaccine delivery, such as by instituting house-to-house vaccination; however, the current needle-and-syringe technology and cold-chain requirements of licensed measles-rubella vaccines make house-to-house vaccination very difficult [36], requiring the development of alternative vaccine delivery methods. The alternative delivery method to deliver of medicines and vaccines that is most advanced in development is the microarray patch [37,38]. Challenges related to adverse events associated with the current vaccine delivery method includes contamination, use of incorrect diluents, and incorrect injection techniques. These safety issues are key concerns when considering taking the vaccine out of the clinic for house-to-house campaigns with measles or MR vaccines. These concerns could be overcome by using microarray patches. These ther-mostable patches do not require a cold chain, skilled individuals to deliver vaccine, or injection equipment; therefore, easily used in house-to-house vaccination. As of 2017, influenza microarray patches have completed phase I studies with ongoing field accept-ability studies [37,38]. A measles-rubella microarray patch is currently in pre-clinical development, as a proof of concept for this alternative delivery method [39,40]. Along with device development, there are critical knowledge gaps that need to be addressed to bring the device to market, including optimal immunogenicity and thermal stability; safety and reactogenicity, minimal required wear time, and possible dose sparing. Vaccine-specific clinical trials are needed to demonstrate safety, reactogenicity, immunogenicity, and non-inferiority of microneedle patches compared with sub-cutaneous delivery by needle and syringe. A demand forecast and implementation strategy will be needed to define the value proposition for this technology and support the potential transition away from delivery of measles and rubella vaccine by subcutaneous injections, possibly as early as 2025–2030. The programmatic impact of using microneedle patches to improve vaccine delivery also needs to be evaluated (see Immunization Strategies, #5).
2. How can point-of-care tests be optimized to have the maximum impact to improve surveillance?
Surveillance for measles and rubella share an identical platform. The surveillance system currently utilizes national or sub-national laboratories to test specimens, creating significant specimen transport costs and delays in test results. A point-of-care test (POCT) for measles and rubella could improve the timeliness and completeness of laboratory investigation of suspected cases, particularly in settings where specimen transport to a national laboratory is logistically challenging. A POCT could facilitate prompt case confirmation at the location of the suspected case, minimize specimen handling, and obtain a specimen suitable for sending to a laboratory for molecular analysis [41]. A POCT test would be beneficial in low-incidence settings where serologic testing has added cost due to testing kits expiring relatively quickly; although challenges such as the impact of false positives and false negative test results need to be evaluated. An inexpensive POCT has been developed to detect measles-specific immunoglobulin M antibody that has excellent sensitivity and specificity; relative ease of use with serum, whole blood or oral fluid specimens; and residual sample that can be used for virus detection and characterization by PCR. Additional work is needed to develop a rubella POCT. One of the challenges facing the widespread use of the measles POCT is the need for a licensing and manufacturing agreement. Translational research will be required to optimize POCT so that they can be used effectively in the field and integrated into existing case-based surveillance systems.
3. What innovations are needed to strengthen measles and rubella molecular epidemiology to demonstrate the success of vaccination programs?
Molecular epidemiology uses viral sequences to evaluate virus transmission dynamics and define sources of importation [42]. In countries with endemic measles or rubella virus circulation, molecular epidemiology analysis can establish a baseline of virus genotypes that can be referenced prospectively to determine whether newly identified chains of transmission are endemic or import-linked, and monitor the effectiveness of disease control programs.
For measles and rubella, molecular epidemiology can provide essential data required for the verification of elimination. The global distribution of measles virus genotypes is well established, with the exception of the African region [26]. Because of elimination efforts, the genetic diversity in circulating measles viruses is decreasing, exposing the limitations of current sequencing protocols. While genotyping transmission chains is useful, innovative methods are needed to further differentiate chains within genotypes. Improved differentiation can facilitate improved identification of multiple, simultaneous importation events and multiple transmission chains of the same genotype in a population. Such approaches include whole genome sequencing or sequencing other specific windows of the measles virus genome to provide higher resolution of the genomic variation among strains [43,44]. Knowledge about the global distribution of circulating rubella viruses remains limited, so collection of more rubella specimens for genotyping is greatly needed [26]. This research addresses complementary issues to those identified on susceptibility profiles and transmission pathways discussed in a previous question (Epidemiology and Economics #5).
4. What are innovative methods and corresponding costs for CRS surveillance in areas with limited human and/or financial resources?
Elimination of rubella requires high-quality surveillance, but surveillance for rubella is often insensitive because of the high percentage of infections that are asymptomatic or result in mild disease and because of a measles-focused suspected case definition. The workgroup noted that detection of CRS cases is more likely than rubella because of the more severe manifestations of CRS (e.g., cataracts, cardiac defects, etc.). However, surveillance for CRS does not exist or is inadequate in many countries, in part, because CRS surveillance is resource intensive. A critical knowledge gap identified by the workgroup was the explanation for weak CRS surveillance. Identifying these causes could inform efforts to strengthen CRS surveillance by improving methods and ensuring their cost-effectiveness.
3.3. Immunization strategies workgroup
This workgroup was responsible for identifying priority research questions on routine immunization against measles, rubella and other diseases, and on outbreak response immunization (ORI), including measles and rubella ORI. A variety of approaches to improving routine delivery of measles, rubella and other vaccines, and the strategies can be categorized as enhancing access to vaccination services, increasing community demand for vaccinations, or improving provider- or system-based issues. The Global Routine Immunization Strategies and Practices manual, published in 2016, provides a useful description and approach to implement these strategies and practices for routine immunization[45]. For outbreak response activities, the questions focused on effective strategies that will have an optimal impact, including the timing and scope of response immunization.
1. What is the cost-effectiveness of interventions to increase coverage with routine first and second dose of measles-containing vaccine from 80% to 95%?
Routine immunization is a key strategy for measles and rubella elimination; the target for elimination strategies is ≥95% coverage with the routine first (MCV1) and second (MCV2) dose of measles-containing vaccine. In many countries, however, MCV1 and MCV2 coverage have remained 80–85% for several years [46]. Multiple strategies to improve coverage have been identified [45], but have not been evaluated to determine which are the most appropriate, effective (including cost-effective) and context-relevant for countries to achieve elimination. These strategies include interventions to (1) reduce missed opportunities for vaccination, (2) improve defaulter tracing, (3) improve coverage through use of a 5-dose measles vial, and (4) improve coverage using school entry checks.
2. What are best strategies to reach geographically and socio-culturally hard-to-reach populations with two doses of measles-containing vaccine?
In order to achieve the high population immunity necessary to interrupt measles virus transmission, it is crucial to be able to identify measles-susceptible sub-populations and close population immunity gaps [47]. Achieving homogenous high coverage across all districts and age groups in identified target populations reduces immunity gaps and decreases measles virus transmission [19]. Microplanning methods and appropriate immunization strategies to vaccinate hard-to-reach, urban/peri-urban, transient and other under-vaccinated populations need to be evaluated and improved upon.
3. What factors determine the appropriate target age range, geo-graphic scope (national versus subnational), and frequency of preventive supplementary immunization activities (SIAs) to achieve rubella/CRS and measles elimination?
SIAs need to be conducted to prevent outbreaks and increase population immunity to the level needed to interrupt endemic virus transmission, given suboptimal routine measles immunization coverage in some countries.. Currently, guidelines for the timing and scope of planned preventive campaigns is based on expert opinion but with limited data [48]. A better understanding of how to evaluate existing data to identify the appropriate target population, geographic scope, and frequency of preventive measles and rubella SIAs is needed so that resources can be better utilized to achieve elimination. These approaches include both traditional epidemiologic analysis, as well as mathematical modelling from surveillance and other data sources. This research addresses complementary issues to those identified for routine immunization discussed in a previous question (see Epidemiology and Economics #1).
4. What indicators are needed to guide extent and timeliness of outbreak response immunization?
Similar to preventive SIAs, the evidence on effective methods for conducting ORI activities is limited [49]. In recent years, many ORI campaigns have failed to interrupt transmission, often because they have been implemented long after the outbreak started [50]. At the country level, determining the need, timing and scope of ORI activities is a common decision-making challenge, with substantial financial and policy-related implications [51,52]. There is a need for better understanding of the indicators needed to guide ORI extent and timeliness, along with improved methods for planning, implementing, monitoring and evaluating ORI campaigns.
5. What is the potential programmatic impact of microarray patches to increase vaccination coverage?
The microarray patch (described in detail in the surveillance and laboratory workgroup section) has the potential to overcome key challenges to MR vaccine delivery, including cold chain requirements and medical waste associated with needle-and-syringe injectable vaccines [36,39]. However, little is known about whether use of a patch could contribute to higher levels of vaccination coverage and whether the costs would be acceptable for wide-scale use [53]. As part of the development of the microarray patch, an investment case and estimates of cost-effectiveness are needed. Additionally, when suitable, field trials of the patch should incorporate evaluation of both costs and the impact of the patch on measles vaccination coverage and timeliness (see Surveillance and Laboratory, #1).
3.4. Demand creation and communications workgroup
Despite overwhelming evidence of the safety of measles and rubella vaccines, misinformation, rumors, and concerns exist among parents, politicians, policy makers, and even some health care providers [54,55]. This mistrust has led to decreasing rates of vaccination in many developed and developing countries (either nationally or in specific communities) and, in turn, has contributed to multiple measles outbreaks [56,57]. Vaccine hesitancy tends to be higher in settings where the immediate risk of a vaccine-preventable disease is minimal or absent and where decisions are based upon personal stories instead of data [58,59]. This work-group discussed social mobilization, communication strategies, and evidence-based demand generation as potential methods to curb and address vaccine refusal.
1. How can service delivery be altered to create and increase vaccine acceptance and demand?
Community vaccine acceptance and demand can be influenced by contacts with the health services delivery system. For example, a positive clinic experience is an important driver for demand; healthcare providers are trusted sources of vaccine information. A positive contact with the health services delivery system minimizes missed opportunities for routine immunization by positively affecting parents’ perceptions of the importance that clinic staff place on vaccination. Leveraging SIAs to catch-up children with missed routine doses sends another positive message about the need to receive all vaccinations. Service delivery improvements may affect vaccine acceptance variably in different settings, such as endemic, near elimination/re-introduction and post-elimination settings, and they have not been rigorously evaluated in low-income countries.
2. What is the effectiveness of social mobilization as a tool for vaccine demand creation for SIAs, and how can it be adapted for routine immunization?
During SIAs, social mobilization is the most widely used tool for increasing community awareness and acceptance of the vaccines being offered in the campaign. However, the impact of social mobilization on SIAs has not been rigorously evaluated. In addition, social mobilization has not been systematically adapted for routine immunization, making this an important research topic area. Such research could also address the effectiveness and cost-effectiveness of different types of social mobilization, which has not been previously assessed.
3. Are news and social media (e.g., WhatsApp, Facebook) effective tools for vaccine demand generation?
Younger people and new parents are increasingly engaged in social media applications such as Instagram, SnapChat, Twitter and WhatsApp. The media landscape is evolving and shifting from mass media sources into segmented new media sources such as these platforms. There is substantial evidence regarding the usefulness of short messaging service (SMS) messages as reminders [60]; however, there is a knowledge gap related to the operational aspects of these reminders (e.g., one-way vs. two-way messaging, location-based messaging, etc.). There are also few data on the utility of social media and news media platforms in generating vaccine demand and acceptance. Examining the use and effectiveness of these media sources could help public health officials better understand and reach younger populations and new parents with positive messages about vaccines and vaccinations.
4. How to design laws and regulations that result in an increase in vaccine coverage?
In a few middle- and high-income country settings, vaccine mandates have been effective in increasing vaccination coverage [61,62]. While a vaccine mandate is a promising tool to increase vaccine acceptance and use, it is unclear what forms of mandates are most effective in different cultural contexts (i.e., draconian measures versus behavioral nudges through legislation). In addition, there is a risk of community backlash and inadequate vaccination coverage if vaccination mandates or laws are not effectively implemented or are abused, particularly among vulnerable and marginalized populations. Vaccination mandates, legislation, and regulatory tools for increasing vaccine acceptance and coverage with minimal negative consequences need evaluation in a variety of settings.
5. What is an appropriate surveillance framework for measles and rubella vaccine behavioral demand and acceptance?
The ability to understand the attitudes and practices of a community with regard to vaccine demand and acceptance could be invaluable in planning elimination efforts. Currently, there are no tools available to track vaccine attitudes over time in a variety of communities. A validated tool to track vaccine attitudes longitudinally would help monitor vaccine demand; and could be used to track attitudes toward vaccines in future eradication efforts [63]. It could also complement vaccine-preventable disease surveillance or response activities to reported adverse events following immunization.
4. Discussion
During the research prioritization process, 19 high-priority research questions were identified to address key strategic areas to move toward MR elimination. The wording of each of the research questions is broad, and each will require several research activities with specific study objectives to answer various aspects of each question. For example, the MR microarray patch, identified as a potential game changer, will require addressing questions from strategic areas to advance the technology to licensure and use. A point-of-care test for improved surveillance and outbreak response was identified as another priority innovation and a potential game changer for MR elimination. Other important priority research questions addressed key areas, often overlapping, for elimination, including: (1) improving vaccine delivery, (2) developing innovative planning tools and implementation methods to identify target populations and characterize chains of transmission, (3) strengthening surveillance to better monitor progress towards elimination,(4) generating evidence for country decision-making, and (5) developing tools to better use data for advocacy and decision-making.
The interpretation of these results is subject to at least three limitations. First, the a priori frame of four strategic areas for this process, limited the scope of research that could be considered, potentially excluding important research priorities that fell outside of the process framework. A second limitation was that groups prioritized areas independent of each other, resulting in similar research topics being prioritized by multiple workgroups, such as microarray patches, though being identified by more than one workgroup emphasized the question as a high priority. Third, while the process made efforts to reflect the practical research needs and input from the operational level, particularly through the web-based survey, the final participants of the workgroups might have biased results toward their own areas of knowledge and interest. Future prioritization processes may require newer techniques being developed to improve the process [64,65].
The current research priorities evolved from the results from previous prioritization activities completed in 2011 and 2015 [13,16]. However, some previous research topics have remained a priority because they remained unresolved; topics that continued to be priorities included development of a microarray patch, identification of effective strategies to increase routine immunization coverage, and improvement of laboratory surveillance tools. Compared with previously identified priorities, there was a shift toward research and innovation for better use of data for decision-making at the national level, especially to identify susceptible populations, and less emphasis on understanding transmission pathways in specific settings. The current research prioritization process focused on the four programmatic elimination strategies of the M&RI, resulting in an increased emphasis on research to advance demand creation and communication techniques.
In addition to establishing a research agenda, a research program with funding mechanisms is needed for new research activities and for developing and testing innovations, as was emphasized by the M&RI mid-term review [8]. To achieve this, approaches such as establishing a M&RI research funding mechanism, including a ‘small grants’ fund, is needed. This would ensure that key priority research receives funding in a coordinated manner to generate evidence and new tools needed to support policy and strategy. Establishing a research program would enable M&RI partners to continue to set priorities, fund high-priority research activities, and provide a forum where current and future research proposals can be discussed to advance efforts to achieve global and regional elimination goals. A similar platform exists for polio eradication with committed annual support for the Polio Research Committee that provides coordination for setting research priorities, funding research, and providing a forum to discuss current and future research activities [66]. This approach has dramatically influenced strategies and progress towards polio eradication [67]; for example, research leading the availability of type-specific monovalent and bivalent oral polio vaccines was a “game-changer”, resulting in the deployment of monovalent vaccines in 2005 and bivalent vaccine in 2009 [12]. Currently, measles and rubella research has limited capacity and funding from partners, foundations, and donor agencies [9,68]. Future commitments from partners will be needed to build capacity and develop a plat-form for improved coordination with adequate and predictable resources for research and innovation to address these identified priorities.
Acknowledgements
Other contributors who attended the Global Measles and Rubella Research Meeting in Washington, D.C. in November 2016: Mary Agocs, Jon Andrus, Salah Al Awaidy, Bettina Bankamp, Pamela Bravo, David Brown, Subhash Chandir, Reinaldo de Menezes Martins, Matthew Ferrari, Deepa Gamage, Birgitte Giersing, Alan Hinman, Joseph Icenogle, Najwa Khouri-Bulos, Elesha Kingshott, Justin Lessler, Karen Mah, Lisa Menning, James Noe, Sarah Pallas, Mark Papania, Desiree Pastor, Minal Patel, Susan Reef, Peter Strebel, Makoto Takeda, Kimberly Thompson, Lauren Vul-vanovic, Aaron Wallace and Brent Wolff. We also are grateful to all of those who participated in the development of this report, and Natasha McCall (CDC) and Brian Shaw (Sabin) for their administrative support and meeting planning. Also, many thanks to PAHO for hosting the meeting of experts. Thank you to Rachael Porter (Emory University) for help in compiling and drafting the demand creation and communications section of this report, and to James P. Alexander for his thoughtful review of the manuscript, and to those who provided inputs through the research prioritization web-based survey.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the World Health Organization or the U.S. Centers for Disease Control and Prevention.
The Measles & Rubella Initiative was established in 2001 as the Measles Initiative.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Strebel PM, Cochi SL, Hoekstra E, Rota PA, Featherstone D, Bellini WJ, et al. A world without measles. J Infect Dis 2011;204(suppl 1):S1–3. [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization. Proceedings of the Global Technical Consultation to assess the feasibility of measles eradication, 28–30 July 2010. J Infect Dis 2011;204(Suppl 1). S4–13. [DOI] [PubMed] [Google Scholar]
- [3].Global Vaccine Action Plan. Decade of vaccine collaboration. Vaccine 2013;31 (Suppl 2):B5–B31. [DOI] [PubMed] [Google Scholar]
- [4].Dabbagh A, Patel MK, Dumolard L, Gacic-Dobo M, Mulders MN, Okwo-Bele JM, et al. Progress toward regional measles elimination – Worldwide, 2000–2016. MMWR Morb Mortal Wkly Rep 2017;66(42):1148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].World Health Organization. Global measles and rubella strategic plan: 2012. Geneva: World Health Organization; 2012. p. 42. [Google Scholar]
- [6].Grant G, Reef S, Patel M, Knapp J, Dabbagh A. Progress in rubella and congenital rubella syndrome control and elimination - Worldwide, 2000–2016. MMWR Morb Mortal Wkly Rep 2017;66(45):1256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Global Burden of Disease 2015 Healthcare Access and Quality Collaborators. Healthcare Access and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990–2015: a novel analysis from the Global Burden of Disease Study 2015. Lancet; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Orenstein WA, Cairns L, Hinman A, Nkowane B, Olive JM, Reingold AL. Measles and rubella global strategic plan 2012–2020 midterm review report: background and summary. Vaccine 2018;36(Suppl 1):A35–42. [DOI] [PubMed] [Google Scholar]
- [9].Orenstein WA, Hinman A, Nkowane B, Olive JM, Reingold A. Measles and rubella global strategic plan 2012–2020 midterm review. Vaccine 2018;36 (Suppl 1):A1–A34. [DOI] [PubMed] [Google Scholar]
- [10].Henderson DA. The eradication of smallpox–an overview of the past, present, and future. Vaccine 2011;29(Suppl 4):D7–9. [DOI] [PubMed] [Google Scholar]
- [11].Breman JG, de Quadros CA, Dowdle WR, Foege WH, Henderson DA, John TJ, et al. The role of research in viral disease eradication and elimination programs: lessons for malaria eradication. PLoS Med 2011;8(1):e1000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rutter PD, Donaldson LJ. Oversight role of the independent monitoring board of the global polio eradication initiative. J Infect Dis 2014;210(Suppl 1): S16–22. [DOI] [PubMed] [Google Scholar]
- [13].Goodson JL, Chu SY, Rota PA, Moss WJ, Featherstone DA, Vijayaraghavan M, et al. Research priorities for global measles and rubella control and eradication. Vaccine 2012;30(32):4709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Muller CP, Kremer JR, Best JM, Dourado I, Triki H, Reef S, et al. Reducing global disease burden of measles and rubella: report of the WHO Steering Committee on research related to measles and rubella vaccines and vaccination, 2005. Vaccine 2007;25(1):1–9. [DOI] [PubMed] [Google Scholar]
- [15].National Center for Immunization and Respiratory Disease CfDCaP. Global measles and rubella research external peer review; 2009.
- [16].Ford AQ, Touchette N, Hall BF, Hwang A, Hombach J. Global vaccine and immunization research forum: opportunities and challenges in vaccine discovery, development, and delivery. Vaccine 2016;34(13):1489–95. [DOI] [PubMed] [Google Scholar]
- [17].Kriss JL, Grant GB, Moss WJ, Durrheim DN, Cutts FT, Shefer A, et al. Research priorities for accelerating progress toward measles and rubella elimination identified by a cross-sectional web-based survey. Vaccine 2019;37(38):5745–53. 10.1016/j.vaccine.2019.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].World Health Organization. Global Measles and Rubella Strategic Plan 2012–2020; 2012. http://www.who.int/immunization/newsroom/Measles_Rubella_StrategicPlan_2012_2020.pdf [accessed March 7, 2013].
- [19].Takahashi S, Metcalf CJE, Ferrari MJ, Tatem AJ, Lessler J. The geography of measles vaccination in the African Great Lakes region. Nat Commun 2017;8:15585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li S, Ma C, Hao L, Su Q, An Z, Ma F, et al. Demographic transition and the dynamics of measles in six provinces in China: a modeling study. PLoS Med 2017;14(4). e1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kriss JL, Stanescu A, Pistol A, Butu C, Goodson JL. The World Health Organization Measles Programmatic Risk Assessment Tool-Romania, 2015. Risk analysis: an official publication of the Society for Risk Analysis; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Goel K, Naithani S, Bhatt D, Khera A, Sharapov UM, Kriss JL, et al. The World Health Organization Measles Programmatic Risk Assessment Tool-Pilot Testing in India, 2014. Risk analysis: an official publication of the Society for Risk Analysis; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kriss JL, De Wee RJ, Lam E, Kaiser R, Shibeshi ME, Ndevaetela EE, et al. Development of the World Health Organization Measles Programmatic Risk Assessment Tool Using Experience from the 2009 Measles Outbreak in Namibia Risk analysis: an official publication of the Society for Risk Analysis; 2016. [DOI] [PubMed] [Google Scholar]
- [24].Lam E, Schluter WW, Masresha BG, Teleb N, Bravo-Alcantara P, Shefer A, et al. Development of a district-level programmatic assessment tool for risk of measles virus transmission. Risk Anal: an official publication of the Society for Risk Analysis 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mulders MN, Serhan F, Goodson JL, Icenogle J, Johnson BW, Rota PA. Expansion of surveillance for vaccine-preventable diseases: building on the global polio laboratory network and the global measles and rubella laboratory network platforms. J Infect Dis 2017;216(suppl_1):S324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mulders MN, Rota PA, Icenogle JP, Brown KE, Takeda M, Rey GJ, et al. Global measles and rubella laboratory network support for elimination goals, 2010–2015. MMWR Morb Mortal Wkly Rep 2016;65(17):438–42. [DOI] [PubMed] [Google Scholar]
- [27].Simons E, Ferrari M, Fricks J, Wannemuehler K, Anand A, Burton A, et al. Assessment of the 2010 global measles mortality reduction goal: results from a model of surveillance data. Lancet (London, England). 2012;379 (9832):2173–8. [DOI] [PubMed] [Google Scholar]
- [28].Ozawa S, Clark S, Portnoy A, Grewal S, Brenzel L, Walker D. Return on investment from childhood immunization in low- and middle-income countries, 2011–20. Health Aff 2016;35(2):199–207. [DOI] [PubMed] [Google Scholar]
- [29].Lee LA, Franzel L, Atwell J, Datta SD, Friberg IK, Goldie SJ, et al. The estimated mortality impact of vaccinations forecast to be administered during 2011–2020 in 73 countries supported by the GAVI Alliance. Vaccine 2013;31(Suppl2):B61–72. [DOI] [PubMed] [Google Scholar]
- [30].Wallace A, Masresha B, Grant G, Goodson J, Birhane H, Abraham M, et al. Evaluation of economic costs of a measles outbreak and outbreak response activities in Keffa Zone, Ethiopia. Vaccine 2014;32(35):4505–14. [DOI] [PubMed] [Google Scholar]
- [31].Durrheim DN, Crowcroft NS. The price of delaying measles eradication. Lancet Public Health 2017;2(3):e130–1. [DOI] [PubMed] [Google Scholar]
- [32].Thompson KM, Odahowski CL. Systematic review of health economic analyses of measles and rubella immunization interventions. Risk Anal: an official publication of the Society for Risk Analysis 2016;36(7):1297–314. [DOI] [PubMed] [Google Scholar]
- [33].Thompson KM, Odahowski CL, Goodson JL, Reef SE, Perry RT. Synthesis of evidence to characterize national measles and rubella exposure and immunization histories. Risk Anal: an official publication of the Society for Risk Analysis 2016;36(7):1427–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ozawa S, Mirelman A, Stack ML, Walker DG, Levine OS. Cost-effectiveness and economic benefits of vaccines in low- and middle-income countries: a systematic review. Vaccine 2012;31(1):96–108. [DOI] [PubMed] [Google Scholar]
- [35].Bharti N, Djibo A, Tatem AJ, Grenfell BT, Ferrari MJ. Measuring populations to improve vaccination coverage. Sci Rep 2016;5:34541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Durrheim DN, Goodson JL. Time for an immunisation paradigm shift. Trans R Soc Trop Med Hyg 2017;111(2):41–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rouphael NG, Paine M, Mosley R, Henry S, McAllister DV, Kalluri H, et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. The Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Arya J, Henry S, Kalluri H, McAllister D, Pewin W, Prausnitz M. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials 2017;128:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Edens C, Collins ML, Goodson JL, Rota PA, Prausnitz MR. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine 2015;33(37):4712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Joyce JC, Carroll TD, Collins ML, Chen MH, Fritts L, Dutra JC, Rourke TL, Goodson JL, McChesney MB, Prausnitz MR, Rota PA. A Microneedle Patch for Measles and Rubella Vaccination Is Immunogenic and Protective in Infant Rhesus Macaques. J Infect Dis 2018;218(1):124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shonhai A, Warrener L, Mangwanya D, Slibinskas R, Brown K, Brown D, et al. Investigation of a measles outbreak in Zimbabwe, 2010: potential of a point of care test to replace laboratory confirmation of suspected cases. Epidemiol Infect 2015;143(16):3442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].World Health Organiazation. The role of extended and whole genome sequencing for tracking transmission of measles and rubella viruses: report from the Global Measles and Rubella Laboratory Network meeting, 2017. Wkly Epidemiol Rec 2018;93(6):55–9. [PubMed] [Google Scholar]
- [43].Rota P, Bankamp B. Whole-genome sequencing during measles outbreaks. J Infect Dis 2015;212(10):1529–30. [DOI] [PubMed] [Google Scholar]
- [44].The role of extended and whole genome sequencing for tracking transmission of measles and rubella viruses: report from the Global Measles and Rubella Laboratory Network meeting, 2017. Releve epidemiologique hebdomadaire/Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record/Health Section of the Secretariat of the League of Nations 2018;93(6):55–9. [PubMed] [Google Scholar]
- [45].World Health Organiazation. Global Routine Immunization Strategies and Practices (GRISP): a companion document to the Global Vaccine Action Plan (GVAP); 2016.
- [46].Casey R, Dumolard L, Danovaro Holliday MC, Gacic Dobo M, Diallo M, Hampton L, et al. Global routine vaccination coverage, 2015. MMWR Morb Mortal Wkly Rep 2016;65(45):1270–3. [DOI] [PubMed] [Google Scholar]
- [47].Rota PA, Moss WJ, Takeda M, de Swart RL, Thompson KM, Goodson JL. Measles. Nat Rev Dis Primers 2016;2:16049. [DOI] [PubMed] [Google Scholar]
- [48].World Health Organization. Planning and implementing high-quality supplementary immunization activities for injectable vaccines using an example of measles and rubella vaccines: field guide. http://www.who.int/immunization/diseases/measles/SIA-Field-Guide.pdf: WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland; 2016. [Google Scholar]
- [49].World Health Organiazation. Response to measles outbreaks in measles mortality reduction settings. Geneva: Immunization, Vaccines and Biologicals, World Health Organization; 2009. [PubMed] [Google Scholar]
- [50].Hagan JE, Greiner A, Luvsansharav UO, Lake J, Lee C, Pastore R, et al. Use of a diagonal approach to health system strengthening and measles elimination after a large nationwide outbreak in Mongolia. Emerg Infect Dis 2017;23(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fonnesbeck C, Shea K, Carran S, Cassio de Moraes J, Gregory C, Goodson J, et al. Measles outbreak response decision-making under uncertainty: a retrospective analysis. J Roy Soc Interface 2018;15(140). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gastañaduy PA, Funk S, Paul P, Tatham L, Fisher N, Budd J, et al. Impact of public health responses during a measles outbreak in an Amish community in Ohio: modelling the dynamics of transmission. Am J Epidemiol 2018. kwy082-kwy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Adhikari B, Goodson JL, Chu S, Rota PA, Meltzer M. Assessing the potential cost-effectiveness of microneedle patches in childhood measles vaccination programs: the case for further research and development. Drugs R&D 2016;16(4):327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Saint-Victor DS, Omer SB. Vaccine refusal and the endgame: walking the last mile first. Philos Trans R Soc Lond B Biol Sci 2013;368(1623):20120148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Godlee F, Smith J, Marcovitch H. Wakefield’s article linking MMR vaccine and autism was fraudulent. BMJ 2011;342:c7452. [DOI] [PubMed] [Google Scholar]
- [56].Chatterjee A, O’Keefe C. Current controversies in the USA regarding vaccine safety. Expert Rev Vaccines 2010;9(5):497–502. [DOI] [PubMed] [Google Scholar]
- [57].On the ups and downs of measles Surveillance Report. Stockholm, Sweeden: European Centers for Disease Control; 2012. 21 February, 2012. [Google Scholar]
- [58].UNICEF. Communications for Development 2016 [updated February 16, 2016; cited February 16, 2016. Available from: https://www.unicef.org/cbsc/index_42150.html.
- [59].Bryant KA, Wesley GC, Wood JA, Hines C, Marshall GS. Use of standardized patients to examine physicians’ communication strategies when addressing vaccine refusal: a pilot study. Vaccine 2009;27(27):3616–9. [DOI] [PubMed] [Google Scholar]
- [60].Manakongtreecheep K. SMS-reminder for vaccination in Africa: research from published, unpublished and grey literature. Pan Afr Med J 2017;27(Suppl3):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Buttenheim AM, Sethuraman K, Omer SB, Hanlon AL, Levy MZ, Salmon D. MMR vaccination status of children exempted from school-entry immunization mandates. Vaccine 2015;33(46):6250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Opel DJ, Omer SB. Measles, mandates, and making vaccination the default option. JAMA Pediatrics 2015;169(4):303–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Omer SB, Orenstein WA, Koplan JP. Go big and go fast–vaccine refusal and disease eradication. New Engl J Med 2013;368(15):1374–6. [DOI] [PubMed] [Google Scholar]
- [64].Yoshida S. Approaches, tools and methods used for setting priorities in health research in the 21(st) century. J Global Health 2016;6(1):010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chalmers I, Glasziou P. Systematic reviews and research waste. Lancet 2016;387(10014):122–3. [DOI] [PubMed] [Google Scholar]
- [66].Global Polio Eradication Initiative. Polio Research Committee. Accessed May 23, 2017 at http://polioeradication.org/tools-and-library/research-innovation/polio-research-committee/; 2008.
- [67].Cochi SL, Freeman A, Guirguis S, Jafari H, Aylward B. Global polio eradication initiative: lessons learned and legacy. J Infect Dis 2014;210(Suppl 1):S540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Goodson JL, Alexander JP, Linkins RW, Orenstein WA. Measles and rubella elimination: learning from polio eradication and moving forward with a diagonal approach. Expert Rev Vaccines 2017;16(12):1203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]