Abstract
A work group from the American Physical Therapy Association (APTA) Academy of Oncologic Physical Therapy developed a clinical practice guideline to aid clinicians in identifying interventions for people with breast cancer–related lymphedema, targeting volume reduction, beginning at breast cancer diagnosis and continuing through cancer treatments and survivorship. Following a systematic review of published studies and a structured appraisal process, recommendations were developed to guide physical therapists and other health care clinicians in their intervention selection. Overall, clinical practice recommendations were formulated based on the evidence for each intervention and were assigned a grade based on the strength of the evidence. The evidence for each specific intervention was synthesized and appraised by lymphedema stage, when the information was available. In an effort to make recommendations clinically applicable, they were presented by modality throughout the care trajectory. Methodology and research populations varied significantly across studies, and it will be important for future research to use standardized definitions for participant characteristics, diagnostic criteria, and interventions.
Breast cancer–related lymphedema (BCRL) significantly lowers an individual’s quality of life (QOL) due to impairments affecting participation at home and in the community.1 The overall incidence rate varies because of differences in diagnostic measures and oncologic management. DiSipio et al2 reported an overall BCRL incidence rate of 16.6% (95% CI = 13.6–20.2) in individuals 3 months to 20 years after diagnosis. People who are post–axillary lymph node dissection (ALND) have an increased incidence at 19.9% (95% CI = 13.5–28.2). This incidence rate suggests that 1 in 5 survivors may develop BCRL.2 In 87.1% to 89% of individuals who develop BCRL, diagnosis occurs within 2 or 3 years postsurgery.3,4 Individuals receiving sentinel lymph node biopsy, a more recent management technique, demonstrated a BCRL incidence from 0% to 63.4% at 6 to 12 months, indicating that BCRL continues to be an issue.5
Interventions for cancer-related lymphedema are needed at various time points along the clinical trajectory, beginning at diagnosis of breast cancer and continuing through cancer treatments and survivorship. Evidence-based recommendations, based on an individual’s clinical presentation, are needed to guide the clinician’s decision when recommending interventions. (See Table 1 for overview of recommendations.) The lymphedema staging model used for this clinical practice guideline (CPG) is an adaptation of the International Society of Lymphology (ISL) staging criteria (Tab. 2).6 For this CPG, a diagnosis of lymphedema was considered when present at a time point greater than 3 months postsurgical management to differentiate from postoperative swelling.7 Although this CPG was initially intended to address all upper quadrant lymphedema, a lack of clinical trials in populations other than breast cancer required the limitation of this CPG to BCRL. Specifically, this CPG identified interventions targeting volume reduction as a direct impact on upper extremity BCRL due to limitations in diagnostic measurements of the trunk and chest.
Table 1.
Overview of Practice Recommendationsa
| Interventions by Stage | Practice Recommendations |
|---|---|
| Early Postoperative Care/Early Preventive Intervention | Postoperative exercise and resumption of activity should be coordinated with the interprofessional team and an individualized exercise program should be gradually increased while monitoring for adverse events. (Best Practice) Individually tailored exercises should be included postoperatively and gradually progressed. (Grade B) In individuals who have undergone axillary lymph node dissection: • The addition of therapist-provided manual lymphatic drainage (MLD) to the postoperative care plan may not reduce the risk of developing BCRL. (Grade C) • Provision of a fitted compression garment to patients at high risk of developing lymphedema, when paired with upper extremity exercise and diaphragmatic breathing, may reduce development of lymphedema. (Grade B) |
| Prospective Surveillance Model and Identified Subclinical (ISL Stage 0) Lymphedema | Early identification of subclinical lymphedema in high-risk groups through prospective surveillance may improve outcomes. (Grade C) • Monitoring with bioelectric impedance spectroscopy or volume measures may begin with a preoperative assessment, repeated every 3 months for the first year postoperatively, and then biannually for up to 5 years. (Grade C) Intervention for subclinical lymphedema may include education, self-massage, and use of compression garments. (Grade C) If early subclinical lymphedema persists or progresses after initial conservative intervention, individuals may benefit from more intensive interventions, such as complete decongestive therapy (CDT). (Grade C) |
| Exercise for Individuals at Risk for or With Subclinical (ISL Stage 0) BCRL | Progressive resistance training is safe when an individualized program is supervised beginning at least 1 month postsurgery. (Grade A) Individualized aerobic exercise programs should be provided. (Grade A) Monitoring for exercise tolerance and adverse effects should initially occur at least weekly and then taper according to clinical presentation. (Grade A) |
| Interventions Recommended for Individuals Diagnosed With BCRL |
Early Lymphedema (ISL Stage I): If early signs and/or symptoms of lymphedema are noted, the patient should be individually fitted with a compression garment, instructed in an exercise program, and provided education as first-line treatment. (Grade A) • If first-line treatment is not successful for early lymphedema, then CDT may be recommended. (Grade B) • Compression (garment or bandaging) should be tailored for the individual’s lymphedema stage and impairments, in consultation with the patient. (Grade A) Moderate and Late Lymphedema (ISL Stages II and III): CDT should be used to reduce limb volume in those diagnosed with moderate and late BCRL. (Grade B) • Compression bandaging and exercise are key components of CDT and should be used. (Grade A) • Modifying CDT, specifically shortening or omitting the MLD component, may yield similar results on long-term volume reduction. (Grade B) • In all treatment phases, compression interventions should be tailored for the individual’s lymphedema stage, impairments, and preferences. (Grade A) • Kinesiotape may reduce volume but cannot be recommended to replace short-stretch compression bandaging in stage II and III BCRL. (Grade B) If kinesiotape is used in BCRL, clinicians should closely monitor for adverse events. (Grade B) • Once a stable volume reduction is achieved with phase I clinical treatment, a program of home care including self-MLD, individually fitted compression garment, appropriate nightly compression if indicated, and exercise should be recommended. (Grade B) • Use of a standard or advanced intermittent pneumatic compression device may be considered in phase II home care treatment. (Grade C) • Monitoring for volume changes with follow-up care may be an important component for optimal long-term volume reduction. (Grade C) Low-level laser therapy may be considered either in combination with compression or CDT in patients with established lymphedema of the upper extremity. (Grade B) For All Stages (ISL Stages 0–III) in Relation to Other Therapeutic Modalities: The addition of myofascial therapy to stretching, exercise, and scar massage may be safe in patients greater than 3 months post–radiation therapy who are at risk for BCRL. (Grade C) Acupuncture has insufficient evidence to support use for volume reduction. (Grade C) |
a BCRL = breast cancer-related lymphedema; ISL = International Lymphology Society.
Table 2.
Lymphedema Staging Model
| Patient Presentation From the Clinical Practice Guideline Recommendations 6 | Stages From International Society of Lymphology (ISL) 5 | Description of Stages |
|---|---|---|
| At Risk | NA | Individuals with insult to the lymphatic system but without symptoms or signs of lymphatic transport impairment. |
| Subclinical | Stage 0 | Subclinical state where swelling is not visible, but lymphatic transport is impaired by clinical measures. Symptoms and subtle tissue changes may be noted. |
| Early Lymphedema | Stage I | Early onset of swelling that is visible and subsides with elevation. Pitting may be present. |
| Moderate Lymphedema | Stage II | Consistent volume change with pitting present. Elevation rarely reduces the swelling and progressive tissue fibrosis occurs. |
| Late Lymphedema | Stage III | Skin changes such as thickening, hyperpigmentation, increased skin folds, fat deposits, and warty overgrowths occur. Tissue is very fibrotic and pitting is absent. |
The aim of this CPG was to identify interventions targeting increased interstitial fluid and volume of the upper extremity as a direct impact on BCRL. Although many other impairments, activity limitations, and participation restrictions can occur in individuals impacted by BCRL, this CPG was constructed to identify interventions that impact the core impairment of increased interstitial fluid and overall limb volume. In concordance with a CPG for diagnostic measures,7 volume measures or bioelectric impedance spectroscopy (BIS) were determined as the current best standard for diagnosing and measuring the effectiveness of lymphedema treatments on increased interstitial fluid. Other impairments and activity restrictions related to BCRL, such as pain and decreased QOL, exist and should be addressed by clinicians. Clinicians should consider the evidence provided in this guideline along with the individual’s clinical presentation, patient preferences, and goals when determining an appropriate plan of care.
Methods
In accordance with the American Physical Therapy Association (APTA) manual for development of CPGs,8 a literature search and review was conducted by the BCRL guideline development group (GDG) with the assistance of academic librarians from Saint Louis University and the University of Southern California, using the time frame of January 2000 through March 2019. The GDG consisted of 4 physical therapists certified in lymphedema management, with more than 90 combined years of experience treating people with cancer, and 1 academic physical therapist who coordinated the process. The following databases were utilized: PubMed, CINAHL Plus with full text, Cochrane Reviews, Agency for Healthcare Research and Quality (AHRQ), National Guideline Clearing House, SCOPUS, Sports Discus with full text, Physiotherapy Evidence Database (PEDRo), and Occupational Therapy Systematic Evaluation of Evidence (OTseekr). The final search terms included: Lymphedema, Elephantiasis, and truncated text words lymphedema*, lymphoedema*, elephantiasis. Articles were excluded if they included the terms filariasis, parasites, congenital, hereditary, as well as editorial, letter, and comment.
Titles and abstracts were reviewed by 1 GDG member for meeting inclusion criteria of investigating BCRL. The most frequent reasons for exclusion were: case reports, disease other than cancer, lower extremity lymphedema, pelvic or genital lymphedema, or animal studies (Figure). Articles that were literature reviews, but not systematic reviews, were also excluded. Systematic reviews were examined to ensure that relevant studies were included in the review of literature. Full articles (n = 1517) that included interventions for BCRL, within the scope of rehabilitation therapist practice, were included. Pharmacologic and surgical interventions were excluded for this review. Overall, 209 articles on interventions were included in this review (Suppl. Tab. 1).
Figure.
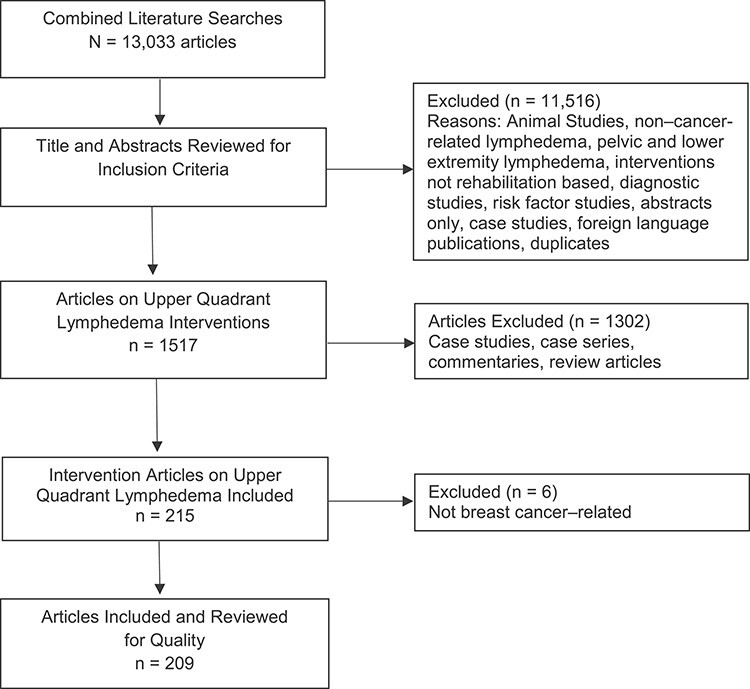
Evidence flow chart showing literature inclusion and exclusion.
Critical Appraisal Process
APTA’s Critical Appraisal Tool for Experimental Intervention Studies (CAT-EI) review tool was used to assess study quality (Tab. 3).8 Additional physical therapists with experience in lymphedema management, listed in the acknowledgments, were trained to appraise studies using this tool. Reviewers initially appraised 3 articles collaboratively to establish agreement on ratings. A single article was then reviewed individually and verified to have ≥90% agreement on quality criteria with the GDG’s rating before a reviewer was included. Two reviewers then independently appraised each article, and, if consensus on a quality rating could not be achieved, a third was used. All articles were reviewed by at least 1 GDG member. The CAT-EI, which assesses the risk of bias in clinical research, was used to assign a quality rating based on the APTA CPG manual.8 Number of articles by quality rating are presented in Table 4, and the quality rating for each article is reported in the parentheses after its introduction throughout this report.8 For a full listing of articles by quality rating and intervention type, see Supplementary Table 1.
Table 3.
Quality Rating Scale for Individual Articles7
| Level | Criteriaa |
|---|---|
| I | High-quality randomized controlled trials: met all 8 essential scoring items on Critical Appraisal Tool for Experimental Intervention Studies (CAT-EI), including: randomized controlled trial of appropriate patient population and sample size, blinding of assessment, reliable and valid outcome measure, adequate follow-up, and appropriate statistical analysis. |
| II | Acceptable quality: evidence obtained from lesser quality clinical trials and met 6 of 8 quality indicators (eg, no blinding, short follow-up), high-quality prospective cohort studies or outcomes research. |
| III | Low quality: case-controlled studies, retrospective cohort studies, or other low-quality trials; met between 2 and 5 of essential scoring items on CAT-EI. |
| Unacceptable: met 0 or 1 of the essential scoring items on CAT-EI. |
a See Supplementary Data for full details.
Table 4.
Numbers of Articles by Quality Rating
| Intervention | High Quality | Acceptable Quality | Low Quality | Unacceptable Quality |
|---|---|---|---|---|
| Early intervention | 0 | 3 | 5 | 0 |
| Prospective surveillance model | 0 | 0 | 8 | 0 |
| Complete decongestive therapy | 1 | 6 | 38 | 10 |
| Compression garments | 0 | 1 | 12 | 2 |
| Compression devices | 0 | 0 | 10 | 6 |
| Exercise | 5 | 9 | 39 | 6 |
| Laser | 0 | 1 | 11 | 0 |
| Kinesiotape | 0 | 1 | 6 | 1 |
| Manual therapy | 0 | 1 | 1 | 0 |
| Complementary and alternative medicine/ acupuncture | 0 | 3 | 5 | 2 |
| Yoga | 0 | 0 | 4 | 0 |
| Other | 0 | 0 | 8 | 4 |
| Totals | 6 | 25 | 147 | 31 |
The evidence for each specific intervention was synthesized and appraised per lymphedema stage. Evidence grades (Tab. 5) were assigned for each intervention based on an overall appraisal by the GDG. Recommendations were generated from high-quality and acceptable-quality studies when available. If no high-quality or acceptable-quality studies were available, low-quality studies and expert opinion were used.
Table 5.
Evidence Grades Based on the Quality of Evidence8
| Grade | Recommendation | Criteria and Strength of Recommendation |
|---|---|---|
| A | Strong | High-quality studies (level I) with moderate to substantial benefit/harm—“must/should” or “must not/should not” |
| B | Moderate | High-quality studies (level I) with slight benefit/harm OR acceptable-quality studies (level II) for moderate benefit/harm—“should” or “should not” |
| C | Weak | Acceptable-quality studies (level II) for slight benefit/harm OR low-quality studies (level III) for substantial benefit/harm—“may” or “may not” |
| Best Practice | Best Practice | Based on current clinical norms or expert opinion |
Recommendations are written to reflect the level of clinician obligation (should vs may) and are based on the strength of evidence, intervention effect, and value judgments of benefits or harms in accordance with the Institute of Medicine standards.9
Practice recommendations were presented for further review and revision. The first public presentation occurred at the APTA Combined Sections Meeting in Washington, DC, 2019. Review by external health care providers and representatives from multiple professional groups occurred by public invitation. In addition, there was a public comment period on the Academy of Oncologic Physical Therapy website. Feedback from the public comment period and solicited reviews were then considered for incorporation by the GDG. Reviewers of the CPG are listed in the acknowledgment section.
Early Postoperative Care/Early Preventive Intervention
Prior to the initiation of an intervention, the therapist should perform a thorough examination to identify impairments and activity and participation restrictions.10 Due to the complexity of each person’s medical history and oncologic treatment plan, it is imperative that the therapist constructs the plan of care in collaboration with the interprofessional team. Therapists should perform a systems review due to the multisystem side effects from cancer-related treatments and variations in surgical approaches, comorbid conditions, and oncologic management. These side effects may require variances from the recommendations due to their impact on exercise tolerance. The therapist is a critical member of the interprofessional care team and should monitor the initiation and progression of an exercise program.10
It is recommended that:
Postoperative exercise and resumption of activity should be coordinated with the interprofessional team, and an individualized exercise program should be gradually increased while monitoring for adverse events. (Best Practice)
Individually tailored exercises should be included postoperatively and gradually progressed. (Grade B)
-
In individuals who have undergone axillary lymph node dissection:
The addition of therapist-provided manual lymphatic drainage (MLD) to the postoperative care plan may not reduce the risk of developing BCRL. (Grade C)
Provision of a fitted compression garment to patients at high risk of developing lymphedema, when paired with upper extremity exercise and diaphragmatic breathing, may reduce development of lymphedema. (Grade B)
Evidence Summary
In people at risk for BCRL, early individualized and supervised exercise postsurgery—with gradual resumption of activity with monitoring for adverse events—is reported to be safe in consultation with the interprofessional team. Bendz et al11 (II) investigated early versus late shoulder exercise in individuals post–radical mastectomy or quadrant resection with ALND. An early shoulder exercise program, initiated 1 or 2 days postsurgery, was compared to delayed exercise that began 14 days postoperatively. The early postoperative exercise program was gradually increased and supervised. On day 1 or 2, the exercise program included intermittent hand grasps with a ball, elbow flexion and extension progressing to 90 degrees of shoulder flexion, and abduction with bent elbow on day 3. From days 8 to 14, the exercise progressed to 90 degrees of shoulder flexion and abduction with elbow extended. This study supported the safety of early, progressive shoulder exercise with no significant increase in lymphedema onset at 2 years. Sagen et al12 (I) compared supervised low resistive exercise (0.5 kg) initiated 2 days postsurgery to usual care with activity restrictions in patients at risk for BCRL. The individualized, progressive resistive exercises were slowly increased with the goal of enhancing muscular strength and endurance. In both groups, there was an increase in BCRL over the 2-year follow-up period, but there were no differences between groups (effect size = −0.18), supporting gradual return to activity in a supervised setting.12
One level III study reported increased drainage when exercise was initiated within 48 hours postsurgery,13 reinforcing the need for clinicians to monitor patient response to intervention. However, Oliveria et al14 (III) also supported the safety of exercise and MLD initiated at 48 hours postoperatively. Insufficient evidence exists to determine whether unsupervised early exercise is safe at this time for individuals at risk for BCRL. Based on the current evidence, early activity and exercise, when progressed gradually and slowly under supervision, appears to be safe with the added benefit of increasing endurance, strength, and range of motion. Inadequate evidence exists on the needed level of monitoring for adverse events; therefore, clinicians need to use their clinical judgment in determining the frequency and mode necessary for each individual.
The impact of early administration of interventions to individuals at risk for BCRL was investigated by Lacomba et al15 (II), Devoogdt et al16 (II), and Ochalek et al17 (II). Lacomba et al15 (II) demonstrated that an early (3–5 days post–hospital discharge) MLD (Leduc), exercise, and education program is better than education alone. Because of the study design, the benefit of exercise versus MLD could not be determined. Devoogdt et al16 (II) investigated if the addition of MLD (Vodder or LeDuc) post–drain removal to a program consisting of exercise, mobilization, stretching, scapular stabilization, and scar massage improved outcomes. MLD did not provide additional benefit to their control intervention, as lymphedema incidence was not significantly different between groups at 60 months (relative risk = 1.08; 95% CI = 0.74–1.58). Ochalek et al17 (II) randomly assigned women post-ALND to compression (fitted, circular knit, 15–21 mmHg) or no compression postoperatively. Both groups were instructed in upper extremity exercise with deep diaphragmatic breathing to be performed daily for 15 minutes. The authors reported significantly less edema (P < .001) at 12 months in the compression group. When selecting preventative interventions for BCRL, clinicians should consider multiple factors including the presence of comorbid conditions, risk factors, and the impact of cancer-related treatments.
Prospective Surveillance Model and Identified Subclinical (ISL Stage 0) Lymphedema
It is recommended that:
-
Early identification of subclinical lymphedema in high risk groups through prospective surveillance may improve outcomes. (Grade C)
Monitoring with BIS or volume measures may begin with a preoperative assessment, repeated every 3 months for the first year postoperatively, and then biannually for up to 5 years. (Grade C)
Intervention for subclinical lymphedema may include education, self-massage, and use of compression garments. (Grade C)
If early subclinical lymphedema persists or progresses after initial conservative intervention, individuals may benefit from more intensive interventions, such as complete decongestive therapy (CDT). (Grade C)
Evidence Summary
Although high-quality evidence regarding the impact of prospective surveillance models (PSMs) has not yet been published, a number of centers have provided evidence to support this practice (Suppl. Tab. 2).18–25 Instead of risk reduction interventions, such as providing compression garments to all patients, PSMs follow patients over time to detect subclinical lymphedema and intervene when specific thresholds are met. Most studies reported completing a preoperative measure, followed by postoperative measurement every 3 months until 1 year and then biannually for up to 5 years.18–25 In the included literature investigating the PSM, all studies were rated as low quality either due to a retrospective chart review study design or due to lack of randomization influencing bias ratings. All studies, except for Kaufman et al22 (III), investigated patient groups where most or all participants had ALND and thus were at higher risk. Other high-risk groups included populations receiving axillary node radiation and taxane chemotherapy.22,23 In all PSM studies, interventions were initiated when subclinical lymphedema was detected either by BIS L-Dex scores of ≥1022–25 or a 3% increase from preoperative volume using perometry (Suppl. Tab. 2).19 Studies used a compression garment and education for subclinical lymphedema and reserved CDT for those with lymphedema that persisted or progressed. Other interventions for subclinical lymphedema in these trials included self-massage and short-term “physical therapy.”
All included trials supported PSM (Suppl. Tab. 2). For example, Kilgore et al23 (III) surveilled a high-risk group using BIS beginning 3 months postoperatively. In addition, the investigators provided comprehensive education to all participants. When an individual had a BIS L-Dex score of >10 units consistent with subclinical lymphedema, self-massage and compression garments were initiated. If lymphedema persisted for more than 4 weeks or progressed, further intervention was recommended. Using this model of care, 34% of the population was found to have subclinical BCRL, of which 82% could be managed with this conservative treatment alone. According to Koelmeyer et al,24 identification of BCRL with BIS occurred significantly earlier in a PSM group than a traditional referral group. Although they found no reduction in the number of treatment sessions between groups when BCRL did occur, the traditional referral group was more likely to be diagnosed with lymphedema of greater severity (stages II and III, 24% traditional vs 4% PSM). Similarly, Stout Gergich et al19 (III) described a PSM where women were fitted with a compression garment (ready-made, fitted, 20–30 mmHg) if a >3% limb volume change occurred. The duration of the intervention was 4.4 weeks on average. At follow-up, which occurred at a mean of 4.8 months, a 4.1% volume decrease was maintained. In a separate study in 2012, this same author found a significant cost savings per individual of $2488.73 if lymphedema was identified early through the PSM.26
Exercise Recommended for Individuals At Risk for or With Subclinical (ISL Stage 0) BCRL
It is recommended that:
Progressive resistance training is safe when an individualized program is supervised beginning at least 1 month postsurgery. (Grade A)
Individualized aerobic exercise programs should be provided. (Grade A)
Monitoring for exercise tolerance and adverse effects should initially occur at least weekly and then taper according to clinical presentation. (Grade A)
Evidence Summary
Exercise in the later postoperative period, starting at 4 to 6 weeks postsurgery, is reported to be safe. Kilbreath et al27 (I) initiated individualized resistance training 4 to 6 weeks post–breast cancer surgery in at-risk groups (sentinel node biopsy or ALND) as compared with no exercise or advice. No difference in lymphedema onset at 6 months (BIS: P = .35; calculated volume: P = .5) was reported.27 Hayes et al28 (II) compared women 6 weeks postsurgery who received individualized aerobic and strength exercise in person or via phone or usual care with no exercise advice. At 12-month follow-up, there was no statistical or clinical difference in lymphedema incidence between groups (13.1%, 12.9%, and 16.4%, respectively). The authors reported improvements in QOL, aerobic fitness, and decline in fatigue in the face-to-face or phone exercise instruction groups but not in the usual care group. Retention was high (93%–94%) in both groups at 12 months, with adherence slightly higher in the face-to-face instruction (88%) when compared with phone-based intervention (81%). Both groups received skilled intervention indicating the benefits of an individualized and monitored program no matter the method of delivery.28 Monitoring for exercise tolerance and adverse effects of interventions were initially monitored weekly during the early interventions phase and then tapered.
Schmitz et al29 (I) compared a weightlifting intervention in breast cancer survivors (1–5 years posttreatment) with at least 2 lymph nodes removed to a group with no change in activity level and reported no increased risk of BCRL (cumulative incidence ratio = 0.64; range = 0.28–1.45). The authors described a reduced risk in a subgroup of women who had 5 of more lymph nodes removed with weightlifting.29 Ammitzbøll et al30 (I) reported that progressive resistance exercise is safe for women in the first year following breast cancer surgery. Women initiated supervised exercises at 3 weeks postsurgery for 20 weeks, followed by 30 weeks of self-administered resistance exercises. There were no adverse events or mean group differences in arm volume (0.3%).30 In both studies,29,30 exercises were supervised by professionals with knowledge of complications from surgery and cancer-related treatments. Exercises were gradually increased to enhance muscle strength and endurance.
Historically, there have been concerns with exercise during chemotherapy treatment. Courneya et al31 (III) randomly assigned women undergoing adjuvant chemotherapy to usual care, supervised resistance, or aerobic exercise. The authors reported supervised exercise during chemotherapy did not cause lymphedema (P = .38) or other adverse events. The authors reported that aerobic exercise compared to usual care improved aerobic fitness (P = .01) and that resistance exercise improved strength (P < .001), lean body mass (P = .02), and chemotherapy completion rate (P = .03). Both modes of exercise improved self-esteem (resistance: P = .02; aerobic: P = .02). The American College of Sports Medicine (ACSM) Roundtable recently recommended a supervised resistance exercise program with the principle to “start low, progress slow” to minimize the number of lymphedema related adverse events.32 Additionally, the ACSM Roundtable reported aerobic exercise as safe with no significant increase in lymphedema-related adverse events.32
Interventions Recommended for Individuals Diagnosed With BCRL
Early Lymphedema (ISL Stage I)
It is recommended that:
-
If early signs and/or symptoms of lymphedema are noted, the patient should be individually fitted with a compression garment, instructed in an exercise program, and provided education as first-line treatment. (Grade A)
If first-line treatment is not successful for early lymphedema, then CDT may be recommended. (Grade B)
Compression (garment or bandaging) should be tailored for the individual’s lymphedema stage and impairments, in consultation with the patient. (Grade A)
Moderate and Late Lymphedema (ISL Stages II and III)
It is recommended that:
-
CDT should be used to reduce limb volume in those diagnosed with moderate and late BCRL. (Grade B)
Compression bandaging and exercise are key components of CDT and should be used. (Grade A)
Modifying CDT, specifically shortening or omitting the MLD component, may yield similar results on long-term volume reduction. (Grade B)
In all treatment phases, compression interventions should be tailored for the individual’s lymphedema stage, impairments, and preferences. (Grade A)
-
Kinesiotape may reduce volume but cannot be recommended to replace short-stretch compression bandaging in stage II and III BCRL. (Grade B)
If kinesiotape is used in BCRL, clinicians shouldclosely monitor for adverse events. (Grade B)
Once a stable volume reduction is achieved with phase I clinical treatment, a program of home care including self-MLD, individually fitted compression garment, appropriate nightly compression if indicated, and exercise should be recommended. (Grade B)
Use of a standard or advanced intermittent pneumatic compression device may be considered in phase II home care treatment. (Grade C)
Monitoring for volume changes with follow-up care may be an important component for optimal long-term volume reduction. (Grade C)
Low-level laser therapy may be considered either in combination with compression or CDT in patients with established lymphedema of the upper extremity. (Grade B)
The evidence for the recommendations pertaining to individuals with BCRL is presented by modality, referencing important differences between stages when information is available.
Evidence summary: CDT
CDT consists of 2 phases: phase I clinical and phase II home care. The clinical phase I includes: MLD, multilayer short-stretch compression bandage, exercise, and skin care 5 times per week based on the individual’s clinical presentation and needs.33 Phase II is initiated once volume reduction of the upper extremity has stabilized.33 Phase II consists of a home care program to maintain the reduction achieved during phase I.33 In phase II, care typically includes: skin care, exercises, self-MLD, and use of compression therapy.33 In the studies reviewed, there were variations in CDT components, such as different MLD approaches and compression systems. Therefore, the term “modified CDT” (mCDT) will be used if the intervention did not follow the phase I and II definition above. Methods of the interventions are provided as reported by the study authors. Due to variation in dosage of treatment in the available studies, it is difficult to provide a recommendation for the most appropriate frequency and duration of each of the intensive intervention components. However, 2 level III studies suggested that at least 3 weeks of clinical phase I CDT are needed for significant lymphedema volume reduction.34,35 Therapists should use clinical reasoning based on the patient presentation and treatment response.
In individuals with early stage lymphedema, the literature supports the initiation of a compression garment instead of CDT. Dayes et al36 (I) assigned women with a history of BCRL (stage I–III) to CDT or a compression garment of 30 to 40 mmHg, worn 12 hours per day. The individuals in the experimental group received CDT 5 times per week for 4 weeks followed by fitting of a similar garment as the compression group. Therapists trained in lymphedema interventions performed standardized CDT treatments including daily MLD and short-stretch bandaging 23 hours per day. Women at baseline in the CDT group had higher arm volumes, body mass index, and age. At 1-year follow-up, there was no statistically significant difference between groups (P = .34), and the mean arm volume reduction was initially higher in the CDT group (29.0% vs 22.6%).
Multiple studies reporting on early intervention using a PSM propose referral for CDT if conservative interventions are not successful.18,21–23,25 For example, Kaufman et al22 (III) initiated treatment with over-the-counter compression sleeve for 4 weeks if an individual at high risk for BCRL (ALND, obesity, radiation, taxane chemotherapy) demonstrated an elevated L-Dex score > 10 from baseline. Individuals who did not have resolution as evidenced by a decline in the L-Dex score were referred to CDT. Similarly, Whitworth et al25 (III) reported individuals with ALND were more likely to develop an elevated (>10) L-Dex score (P < .001). These individuals were provided an over-the-counter compression sleeve for 4 weeks. If the L-Dex score did not decline, individuals were referred for CDT.
Although most of the studies reviewed did not focus on one specific stage of lymphedema, Gradalski et al37 (II) compared compression bandaging and exercises to compression bandaging, exercises, and 30 minutes of MLD in individuals with stage II BCRL. During clinical phase I, both groups performed deep diaphragmatic breathing and active/self-assistive exercises beginning with the proximal musculature for 15 minutes per day. In phase II home care, both groups wore custom-made, flat knit compression garments with the addition of aerobic exercise to the previous program prescribed. Both groups reduced volume (47.2% vs 47.4%) in phase I and maintained in phase II. At 1 year, there was no volume difference between groups. In both studies, exercises were supervised, and compression garments/bandages were customized by the therapist.36,37 Gradalski et al37 reported large volume changes in subjects with stage II BCRL, even when the CDT program was modified and did not include MLD.
In the context of moderate and late stage lymphedema, Dayes et al36 noted that although the study was not powered to complete subgroup analysis, individuals with a greater than 1-year diagnosis of BCRL benefitted from CDT more than those recently diagnosed (<1 year). Tambour et al38 (II) investigated mCDT in individuals with stage II and III BCRL, with and without MLD, using a different type of compression system, 2 times per week for 3 weeks during clinical phase I. Both groups received physical activity, skin care, and bandaging with Coban 2 Lite multilayer compression (20–30 mmHg). The intervention group additionally received 30 minutes of MLD. At 4 weeks or when participants achieved 2 consecutive stable volume measures, an individualized compression sleeve was applied. Both groups demonstrated volume reduction, but no significant difference was found between groups (−6.8% vs −1.0%; P = .54). Although the compression systems used by Gradalski37 and Tambour38 differ, they both provided similar pressure gradients. Together, these studies suggest that 30 minutes of MLD as a component of clinical phase I mCDT may not improve efficacy. Clearly, more research is needed to determine the contributions of each component of CDT.
Pujol-Blaya et al39 (II) investigated the CircAid Reduction system (CircAid Medical Products Inc, Whitsett, North Carolina, USA) as an alternative to self-bandaging as a component of mCDT. Although increased adverse events were reported in the CircAid Reduction group, comparable volume reduction to multilayer bandaging was achieved (−133.8 ± 232.1 vs −106.2 ± 148.6 mL). Adverse events (n = 42) reported using the CircAid Reduction system included paresthesia (n = 3), paresthesia and pain (n = 1), pain (n = 3), pruritus (n = 3), and skin issues (n = 6). The control group also reported adverse events of sweating (n = 1), paresthesia (n = 1), pain (n = 3), pruritus (n = 4), and skin problems (n = 5). The choice of either self-bandaging or CircAid Reduction system should, therefore, be decided based on individual needs or preference.
Once stable volume reduction is achieved, it is important to initiate and maintain phase II home care interventions. Ligabue et al40 (II) investigated the impact of group intervention sessions in phase II of women with BCRL stages II and III. Individuals who attended 4 weeks of group sessions in self-care continued to decrease in volume (−232 mL), whereas those receiving only paper-based education had volume increases (+41 mL). Participants were provided a compression sleeve to wear during the day. During phase II, Ochalek et al41 (II) investigated the impact of self-care and continued lymphedema monitoring by a health care provider. At 5 years, individuals who were adherent with self-care and 6-month follow-up visits maintained the volume reduction as compared with those who were nonadherent (+53.6 vs +399 mL). Adherence to phase II compression therapy is also supported by Vignes et al42 (III).
At this time, no high-quality or acceptable quality studies have investigated IPC as a stand-alone intervention. Two low-quality articles investigated adding standard IPC (sIPC) to clinical phase I CDT in BCRL. Uzkeser et al43 (III) applied IPC (MARK III Plus, model MK400; 40 mmHg, 45 minutes) (Whitsett, NC, USA) following MLD. Although significant volume reduction occurred in both groups (CDT: P = .01; CDT + IPC: P = .02), the addition of sIPC did not significantly improve outcomes. However, Szuba et al44 (III) reported the addition of sIPC (Sequential Circulator 2004, 30 minutes) (Daesung Maref Co Ltd, Gyeonggi-do, Republic of Korea) to CDT yielded an additional volume reduction (45.3% vs 26%; P < .05). Two low-quality studies investigated reducing MLD time and replacing with sIPC. Haghighat et al45 (III) compared CDT to mCDT with reduced MLD and sIPC (40 mmHg, 30 minutes) (BioCompression Systems Inc, Moonachie, NJ, USA). Both groups demonstrated reduced volume (CDT: −43.1%; mCDT+ IPC: −37.5%), but CDT alone showed a greater reduction (P = .04). Szolnoky et al46 (III) compared CDT to mCDT with reduced MLD and sIPC (brand not provided) (Lympha Mat, 30 minutes, 50 mmHg). A significant volume reduction (CDT: −2.9% to −3.6%; mCDT + sIPC: −7.9% to −9.6%) occurred in both groups, but the addition of sIPC appeared to improve the results (P ≤ .05). Based on conflicting results between low-quality studies, the addition of sIPC to clinical phase I CDT is not recommended for additional volume reduction. Likewise, insufficient evidence exists to support sIPC in place of the MLD component of phase I CDT.
In phase II home care CDT, IPC may be considered as 5 low-quality articles44,47–50 reported benefits. Szuba et al44 (III) investigated the addition of 1 hour of sIPC (40–50 mmHg, Sequential Circulator 2004) (Bosl Medizintechnik, Aschen, Germany) to self-MLD and a class II compression garment. The addition of sIPC resulted in greater volume reduction (IPC: −89.5 ± 195.5 mL; non-IPC: 32.7 ± 115.2 mL; P < .05) without adverse events. Fife et al47 (III) compared sIPC (BioCompression 2004 Sequential Circulator PCD) (BioCompression Systems Inc) to advanced pneumatic compression (APC) (Flexitouch system; Tactile Medical, Minneapolis, MN, USA). The APC group improved as compared with the sIPC group (−29% ± 44% vs 16% ± 63%; P = .18) with fewer adverse events (APC: 1 “possibly” device-related; sIPC: 3 “definitely” device-related). Wilburn et al49 (III) compared self-management, including garment use, with either APC or self-MLD. The APC group reduced volume (−208 ± 157 mL; P = .002), whereas the self-MLD group increased volume (+52 ± 106 mL; P > .05). Ridner et al48 (III) evaluated the use of APC for self-management of BCRL with truncal edema, and, although no significant change in volume was attained (−2.51 ± 5.77 cm), improvement in subjective symptoms occurred (P = .02). In a subsequent study, Ridner et al50 evaluated the use of APC for management of individuals with stage II BCRL without truncal edema. The authors compared APC to the trunk, chest, and arm versus the arm only. Both groups experienced a statistically significant volume reduction (P = .02) with no adverse events. However, there was no difference in volume reduction between groups (P = .48). The authors concluded there may be no advantage to treating the trunk with APC in the absence of truncal edema.
Tsai et al51 (II) compared kinesiotape (KT) to short stretch compression bandaging in individuals with stage II and III BCRL. Both groups received routine treatment consisting of skin care, 1 hour of IPC, 30 minutes of MLD, and exercise. Although there was greater limb volume reduction in the bandaging group, it was not significantly different (bandaging: 81.4 mL; KT: 51.3 mL; P > .05). Although there was no description, the authors reported increased frequency of “wounds” in the KT group as compared with the compression group (bandaging: 0.05 ± 0.22; KT: 0.55 ± 0.83; P = .01).51 This increased risk may be attributed to the individual’s self-removal of the KT. Six lower-quality studies52–57 in stage II and III BCRL reported skin allergies, but no other adverse events. Across all studies reviewed, KT demonstrated volume reduction, but it was not significantly better than other interventions.
Evidence summary: laser therapy
Laser therapy has been proposed to be used in BCRL due to the potential to reduce edema, improve lymph vessel angiogenesis, and treat fibrosis.58 The ability to appraise the evidence for laser therapy in BCRL is limited by the wide array of laser types, frequency, and dosage used in the current studies. For example, there was only 1 level II study by Storz et al59 on cluster low-level laser therapy (LLLT). The authors compared cluster LLLT to a placebo in women with a 3-month history of unilateral BCRL. A cluster of 16 continuous-wave diodes (980 nm, 640 mW) (TIMELAS Vital, Schwa-medico, Medizinische Apparate Vertriebsgesellschaft mbH, Ehringshausen, Germany) was applied to one area in the axilla in a noncontact mode 2 times weekly for 4 weeks. Both groups were advised to continue daily limb exercises and skin care. There were no significant intergroup differences for limb volume (P = .13). As no adverse events occurred, the authors concluded noncontact, cluster laser is a safe modality for women with BCRL.
Several low-quality studies supported LLLT in conjunction with other interventions. Khalaf et al60 (III) compared CDT with either LLLT (Helium neon) (no laser unit manufacturer listed) or placebo in individuals with BRCL 3 times per week for 6 months. A greater volume reduction occurred with the addition of LLLT to CDT (−285.2 vs −158.1 mL; P < .001). Ridner et al61 (III) compared MLD, LLLT (904 nm) (RianCorp LTU, Elettronica Pagani IR27/4, Richmond, South Australia, Australia) or MLD + LLLT in individuals with stage I and II BCRL. All participants received compression bandaging. No difference between interventions for volume reduction was seen at 6 months (P = .42). Ahmed Omar et al62 (III) compared LLLT (904 nm) (RianCorp LTU) applied to the axilla and antecubital fossa to placebo 3 times per week for 12 weeks. Both groups were instructed in skin care and exercise and advised to wear a 40 to 60 mmHg compression garment for 20 hours daily. The LLLT group had a greater limb circumference reduction at all time points (P < .05). Kozanoglu et al63 (III) compared LLLT (Ga-As 904 nm) (Elettronica Pagani IR27/4, Richmond, South Australia, Australia) to 2 hours of IPC (MJS Healthcare Ltd, Luton, Bedfordshire, United Kingdom) in addition to skin care and exercises. Although both groups had a significant circumferential reduction, LLLT had better results at 12 months (P = .02). Lau et al64 (III) compared LLLT (Comby 3 Terza Serie Model D; Asa S.r.l., Vicenza, Italy) to education only. Both groups received an education session on skin care, self-MLD, and upper limb exercises. At 8 weeks, the LLLT group had greater volume reduction (P = .04). None of these studies reported safety concerns. Currently, LLLT is not recommended as a stand-alone treatment65,66; however, it may be effective when used in conjunction with CDT and may provide other benefits.61–63,65,66
All Stages (ISL Stage 0–III) in Relation to Exercise
It is recommended that:
-
Individualized programs of aerobic and resistance exercise should be provided for those who have BCRL (stages 0–III). (Grade A)
Resistance exercise should be initiated at low level intensity and progressed slowly. (Best Practice)
Individuals with comorbidities or complications due to cancer-related treatments should be referred to a specialist for evaluation and exercise prescription. (Best Practice)
Sequential proximal to distal exercises incorporating diaphragmatic breathing should be used to improve volume reduction. (Grade B)
Compression use with exercise may have benefit. (Grade B)
Yoga may be a safe form of exercise but does not show evidence of effectiveness for lymphedema volume reduction. (Grade C)
Aerobic and resistive exercises should be incorporated as an intervention in individuals with and without BCRL for health benefits such as improved fitness, QOL, increased lean muscle mass, and bone mineral density.29,67 Exercise programs should be initially supervised and individualized and gradually increased. It should be noted that most exercise trials in BCRL were developed for safety, instead of lymphedema efficacy, as the investigated outcome. As recommended by the ACSM Roundtable,32 individuals with complications due to cancer-related treatments or multiple comorbidities should obtain a pre-exercise medical evaluation and referral to trained personnel for modifying the exercise prescription based on the individual’s needs.
Evidence summary
Five articles of high-quality or acceptable quality reported that aerobic and resistive exercise was safe in individuals with BCRL. Zhang et al67 (II) reported individuals with BCRL (stages 0–III) who performed 13 weeks of supervised, progressive weight-lifting exercise followed by 39 weeks of unsupervised exercise had no changes in arm volume (P = .60). Additional benefits included improved lean mass (P = .01) and bone mineral density (P = .02). Cormie et al68 (II) compared high-load versus low-load upper extremity resistance exercise in individuals with long-term BCRL and reported no significant volume increase (+29.1 mL vs −6.4 mL) at 72 hours. Hayes et al69 (l) compared supervised resistive and aerobic exercises to habitual activities in individuals with BCRL. Aerobic and resistive exercises did not exacerbate BCRL at 3-month follow-up (BIA: P = .88; perometry: P = .53).69 Buchan et al70 (ll) compared individuals with stage I and II BCRL performing either supervised resistive or aerobic exercise for 150 minutes for 12 weeks. The authors reported no differences between groups for volume changes (P = .48).70 Therefore, supervised aerobic as well as low- and high-load resistive exercise appears safe for individuals with BCRL without risk of exacerbation.
When instructing individuals in upper extremity resistive exercises, clinicians should consider sequencing from proximal to distal, incorporating diaphragmatic breathing. Bracha et al71 (II) compared sequencing of exercises in individuals with BCRL. Proximal arm exercise (−34 mL) and a combination of proximal and distal exercises (−29 mL) yielded significant (P ≤ .01) immediate decrease in arm volume when compared with distal exercise (−20 mL) without carryover from session to session.71 Jönsson and Johansson72 (II) compared pole walking for 30 to 60 minutes, 3–5 times per week for 8 weeks to a 2-week period of normal activity. Twenty-four hours after pole walking, individuals with BCRL experienced a significant reduction in total arm volume (−51 mL; P = .01). Although some individuals reported adverse events of arm tightness (1.3%), muscle aches (2.6%), and worsening of hand edema (1.3%), pole walking improved fitness without significant exacerbation.72 Tidhar et al73 (II) compared aquatic therapy to usual care in individuals with BCRL. No infections or exacerbations of limb volume occurred. Individuals performed 45 minutes of proximal to distal exercise while incorporating diaphragmatic breathing and self-massage, 1 time per week in a 1.2-m pool at 32°C to 33°C. A minor reduction initially occurred (−92.8 mL; P = .02), but no long-term effect was seen at 12-week follow-up (P = .51).73 Therefore, pole walking and aquatic exercise appear to be safe, should be monitored for side effects, and may provide some short-term reduction.
Health care professionals should discuss the benefits of using compression garments during exercise for individuals with BCRL. Three level I/II studies reported compression wear was determined by the participant during the interventions,68–70 and 1 fitted the individuals with compression for use during weightlifting sessions.67 Zhang et al67 (ll) fitted all participants (stages 0–III) with a custom-fitted compression garment during 13 weeks of supervised, progressive weightlifting exercise followed by 39 weeks of unsupervised exercise. No changes in arm volume occurred (P = .60), indicating that weightlifting while wearing custom-fitted compression garments is safe for individuals with BCRL. Only 1 study directly assessed compression garment use during exercise. Singh et al74 (lI) compared individuals with BCRL performing an episode of moderate load resistance exercise with or without compression (23–32 mmHg). No significant difference (P = .89) in limb volume was found in either group at 24-hour follow-up. Because all level I/II articles reviewed involved some level of compression wear during aerobic and resistive exercise, compression garments may be worn to mitigate risk of exacerbation. According to Singh et al,74 if an individual did not wear a compression garment while performing moderate load resistive exercise, the risk for short-term exacerbation was likely minimal.
Several low-quality studies support yoga as a safe form of exercise for patients at risk for or with BCRL.75–78 Mazor et al75 (III) enrolled women at risk for BCRL in a weekly Ashtanga yoga program for 8 weeks. Individuals were instructed in poses to emphasize upper body strength and flexibility while minimizing dependent positions. Volume reduction occurred but was not statistically significant (P = .40). Lai et al76 (III) reported insignificant volume differences (P = .76) following a 12-week aerobic yoga program 3 times per week for 60 minutes in individuals at risk for BCRL. Douglas et al77 (III) reported that 90-minute weekly sessions of Satayanda yoga was safe for individuals with BCRL and yielded a slight volume reduction (BIS: 14.3%; perometry: 9.8%). The sessions incorporated modified poses, breathing exercises, meditation, and restricted static positions. Loudon et al78 (lII) measured the effects of Satayanda yoga as compared to usual self-care in women with BCRL. After 8 weeks, the yoga group had less tissue induration (P = .05), although no volume changes were reported between groups. At 1-month follow-up, volume increased more in the control versus the yoga group (P = .03). None of the studies investigated compression garment use during yoga.
All Stages (ISL Stages 0–III) in Relation to Other Therapeutic Modalities
It is recommended that:
The addition of myofascial therapy (MFT) to stretching, exercise, and scar massage may be safe in patients greater than 3 months post–radiation therapy who are at risk for BCRL. (Grade C)
Acupuncture has insufficient evidence to support use for volume reduction. (Grade C)
Evidence summary: MFT
DeGrof et al79 (II) reported on the effect of MFT to the upper body and arm in addition to standard physical therapy in breast cancer survivors 3 months post–radiation therapy. Standard physical therapy included stretching, active and passive range of motion, scar tissue massage, and exercise to improve flexibility, strength and endurance. The control group received standard physical therapy with placebo MFT. Significant improvement in long-term physical function was achieved in both groups at 6- and 12-month follow-ups (P = .02), without significant volume difference between groups (P = .39). Therefore, MFT with standard physical therapy appears to be safe.
Evidence summary: acupuncture
Bao et al80 (II) applied acupuncture to individuals with stage II and III BCRL. The intervention group received acupuncture to 8 points, 2 times per week for 6 weeks, whereas the control group received no treatment. The acupuncture group did reduce limb volume postintervention; however, no lasting reduction was observed at 3-month follow-up (P = .4). Although there were no serious adverse events, bruises (58%), pain (2.6%), skin infection (1.3%), and hematoma (2.6%) occurred. Although acupuncture appears safe, the evidence is insufficient to recommend it as a stand-alone treatment in reducing arm volume.81,82
At this time, there is insufficient evidence involving nutrition/diet,83 reflexology,84 aromatherapy,83 moxibustion,85 extracorporeal shock wave therapy,86 and relaxation/mediation techniques87 in individuals with BCRL. Although no significant volume reduction occurred with these interventions, there may be other benefits such as decreased anxiety, decreased depression,87 and increased overall well-being.
Limitations
As a result of limited space, this CPG cannot provide all details regarding study methodologies. Readers are encouraged to refer to the references and read the specific manuscripts regarding interventions and outcomes. Due to the lack of studies in populations other than breast cancer, this CPG is limited to individuals with BCRL. Because the aim of this CPG was to identify interventions that impact the core impairment of increased interstitial fluid and overall limb volume, other intervention studies that investigated QOL, function, and pain were not included. Additionally, articles may have been published outside of the review time frame or in languages other than English and, therefore, were not included in this CPG. Clinicians need to remain aware that newly published literature could change the state of the evidence body.
When reconciling the findings of this CPG with other systematic reviews, it is important to note that quality ratings and the ability to separate upper and lower extremity lymphedema outcomes influenced the recommendations. For example, the Smoot et al88 systematic review on laser included evidence from studies using point circumference measures that were excluded in this study, and therefore our recommendations differ. Many of the intervention articles were pilot studies and therefore lacked sufficient sample sizes, blinding, and follow-up.
Other limitations to the CPG involved stakeholders. The CPG was sent to multiple stakeholders and was available for public review; however, in the time frame allocated, only a few individuals representing disciplines other than physical therapy provided feedback. This lack of response is a limitation to the study. Also, a patient representative was not involved in the CPG process including barrier analysis to inform drafting or reviewing the recommendations, which limits the recommendations.
Future Research Needs
Methodology and research populations varied significantly across studies included in this CPG; therefore, it is important for future studies to use standardized definitions for participant characteristics, diagnostic criteria, and interventions. For example, many studies included individuals with all stages of BCRL, and it is critical to identify appropriate interventions for specific stages and to determine appropriate dosing. Future studies are recommended to include larger sample sizes with longer follow-up times. Additional research needs include:
Preventive interventions for individuals at high risk
Higher-quality trials of PSM and each intervention
Early postoperative interventions, including type and dosage
Timing and dosage of interventions based on stage or risk
Impact of qualification/training of the provider, patient age, socioeconomic status, geographic, and insurance coverage on treatment response
Upper quadrant lymphedema from cancers other than breast cancer
Comparison of various compression types, including foam, devices, bandages, and garments
Impact of exercise on the lymphatic system
Cost effectiveness of interventions
Impact of adjunctive treatments to CDT
Translating the research to practice, including adherence and dose response
Impact of lymphedema interventions on QOL, function, and disability besides volume reduction
Supplementary Material
Contributor Information
Claire Davies, C. Davies, PT, PhD, Nursing and Allied Health Research Office, Baptist Health Lexington, 1740 Nicholasville Rd, Lexington, KY 40503 (USA.).
Kimberly Levenhagen, K. Levenhagen, PT, DPT, Department of Physical Therapy & Athletic Training, Saint Louis University, St Louis, Missouri. Dr Levenhagen is a certified lymphedema therapist.
Kathryn Ryans, K. Ryans, PT, DPT, Doctor of Physical Therapy Program, Mercy College, Dobbs Ferry, New York. Dr Ryans is a board-certified clinical specialist in oncologic physical therapy and a certified lymphedema therapist-Lymphology Association of North America.
Author Contributions and Acknowledgments
Concept/idea/research design: C. Davies, K. Levenhagen, K. Ryans, M. Perdomo, L. Gilchrist.
Writing: C. Davies, K. Levenhagen, K. Ryans, M. Perdomo, L. Gilchrist.
Data collection: C. Davies, K. Levenhagen, K. Ryans, M. Perdomo, L. Gilchrist.
Data analysis: C. Davies, K. Ryans, M. Perdomo, L. Gilchrist.
Project management: L. Gilchrist.
Fund procurement: L. Gilchrist.
Providing institutional liaisons: K. Levenhagen, M. Perdomo.
Consultation (including review of manuscript before submitting): K. Ryans, M. Perdomo.
The following people were involved in quality reviews of the literature: Kathy Bartley, Christine Beuthin, PT, DPT, GCS, CLT; Linda Boyle, PT, CLT-LANA; Jennifer Brooks, PT, DPT, CLT-LANA; Barbara Feltman, PT, DHS, CLT-LANA; Amy Flinn, PT, CLT-LANA; Brandi Johnson, PT, DPT, CLT-LANA; Meagan Kaley, PT, DPT, CLT-LANA; Jean Kastner, PT, DPT, CLT; Kiersten Kilczewski, PT, DPT, CLT-LANA; Linda Koehler, PT, PhD, CLT-LANA; Vince Lepak III, PT, DPT, CWS; Anne Lehman, PT, CLT-LANA; Vicki Naugler, PT; Lisa O’Block, PT, DPT; Nancy Potter; Kristin Ryan, PT, DPT, CLT-LANA; and Christina Wright, PT, DPT, CLT/CES.
The following people provided feedback on initial drafts of the CPG: Connie Brenna, RN, BSN; Cheryl Brunelle, PT, MS, CCS, CLT; Carmela Claypool, PT, CLT-LANA; Diane Galvin, PT; Nancy Hutchison, MD; Leslen Keith, OTD, CLT-LANA; Guenter Klose, CLT-LANA; Linda Koehler, PT, PhD, CLT-LANA; Jenette Lee, PT, PhD, CLT, CSCS; Patricia O’Brien, MD, PT; Lucinda Pfalzer, PT, PhD; Antionette Sanders, PT, DPT; Betty Smoot, PT, DPTSc; Bryan Spinelli, PT, PhD; Nicole Stout, PT, DPT, CLT-LANA; Linda Tripp, PT, DPT; Nadia Van Diepen, PT, DPT, CLT-LANA, WCC; Megan Webster, PT; Jan Weiss, PT, CLT-LANA; Jodi Winicour, PT, CLT-LANA.
Funding
This CPG was supported by a grant from the American Physical Therapy Association and by the American Physical Therapy Association Academy of Oncologic Physical Therapy.
Financial Disclosure and Conflicts of Interest
Each of the guideline development group members were asked to disclose any existing or potential conflicts of interest—including financial relationships with pharmaceutical, medical device, or biotechnology companies—prior to being included in the group. The guideline development work group declared no conflicts of interest. The authors also completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest. L. Gilchrist received a grant to cover travel expenses related to this study’s production.
References
- 1. Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women's health study. J Clin Oncol. 2008;26:5689–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Disipio TRS, Newman B, Hayes S. Incidence of unilateral arm lymphedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. [DOI] [PubMed] [Google Scholar]
- 3. Rupp J, Hadamitzky C, Henkenberens C, et al. Frequency and risk factors for arm lymphedema after multimodal breast-conserving treatment of nodal positive breast cancer–A long-term observation. Radiat Oncol. 2019;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Norman SA, Localio AR, Potashnik SL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gebruers N VH, De Vrieze T, Coeck D, Tjalma W. Incidence and time path of lymphedema in sentinel node negative breast cancer patients: a systematic review. Arch Phys Med Rehabil. 2015;96:1131–1139. [DOI] [PubMed] [Google Scholar]
- 6. International Society of Lymphology . The diagnosis and treatment of peripheral lymphedema: 2016 consensus document of the International Society of Lymphology. Lymphology. 2016;49:170–184. [PubMed] [Google Scholar]
- 7. Levenhagen K, Davies C, Perdomo M, Ryans K, Gilchrist L. Diagnosis of upper quadrant lymphedema secondary to cancer: clinical practice guideline from the oncology section of the American Physical Therapy Association. Phys Ther. 2017;97:729–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Physical Therapy Association . APTA Clinical Practice Guideline Process Manual. VA: Alexandria; 2018. [Google Scholar]
- 9. Institute of Medicine . Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 10. American Physical Therapy Association . Guide to physical therapy practice 3.0. http://guidetoptpractice.apta.org/. Published 2014. Accessed 2 February, 2019.
- 11. Bendz I, Fagevik Olsen M. Evaluation of immediate versus delayed shoulder exercises after breast cancer surgery including lymph node dissection--a randomised controlled trial. Breast. 2002;11:241–248. [DOI] [PubMed] [Google Scholar]
- 12. Sagen A, Karesen R, Risberg MA. Physical activity for the affected limb and arm lymphedema after breast cancer surgery. A prospective, randomized controlled trial with two years follow-up. Acta Oncol. 2009;48:1102–1110. [DOI] [PubMed] [Google Scholar]
- 13. Todd J Ea. a randomised controlled trial of two programmes of shoulder exercise following axillary node dissection for invasive breast cancer. Physiotherapy. 2008;94:265–273. [Google Scholar]
- 14. Oliveira MMF, Gurgel MSC, Amorim BJ, et al. Long term effects of manual lymphatic drainage and active exercises on physical morbidities, lymphoscintigraphy parameters and lymphedema formation in patients operated due to breast cancer: a clinical trial. PLoS One. 2018;13:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torres Lacomba M, Yuste Sanchez MJ, Zapico Goni A, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial [with consumer summary]. BMJ. 2010;340:b5396 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Devoogdt N, Geraerts I, Van Kampen M, et al. Manual lymph drainage may not have a preventive effect on the development of breast cancer-related lymphoedema in the long term: a randomised trial. J Physiother. 2018;64:245–254. [DOI] [PubMed] [Google Scholar]
- 17. Ochalek K GT, Partsch H. Preventing early postoperative arm swelling and lymphedema manifestation by compression sleeves after axillary lymph node interventions in breast cancer patients: a randomized controlled trial. Journal of pain and symptom management. 2017;54:346–354. [DOI] [PubMed] [Google Scholar]
- 18. Box RC, Reul-Hirche HM, Bullock-Saxton JE, Furnival CM. Physiotherapy after breast cancer surgery: results of a randomised controlled study to minimise lymphoedema. Breast Cancer Res Treat. 2002;75:51–64. [DOI] [PubMed] [Google Scholar]
- 19. Stout Gergich NL, Pfalzer LA, McGarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–2819. [DOI] [PubMed] [Google Scholar]
- 20. Soran A, Ozmen T, McGuire KP, et al. The importance of detection of subclinical lymphedema for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection; a prospective observational study. Lymphat Res Biol. 2014;12:289–294. [DOI] [PubMed] [Google Scholar]
- 21. Yang EJ, Ahn S, Kim EK, et al. Use of a prospective surveillance model to prevent breast cancer treatment-related lymphedema: a single-center experience. Breast Cancer Res Treat. 2016;160:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaufman DI, Shah C, Vicini FA, Rizzi M. Utilization of bioimpedance spectroscopy in the prevention of chronic breast cancer-related lymphedema. Breast Cancer Res Treat. 2017;166:809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kilgore LJ, Korentager SS, Hangge AN, et al. Reducing breast cancer-related lymphedema (BCRL) through prospective surveillance monitoring using bioimpedance spectroscopy (BIS) and patient directed self-interventions. Ann Surg Oncol. 2018;25:2948–2952. [DOI] [PubMed] [Google Scholar]
- 24. Koelmeyer LA, Borotkanics RJ, Alcorso J, et al. Early surveillance is associated with less incidence and severity of breast cancer-related lymphedema compared with a traditional referral model of care. Cancer. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whitworth PW, Cooper A. Reducing chronic breast cancer-related lymphedema utilizing a program of prospective surveillance with bioimpedance spectroscopy. Breast J. 2018;24:62–65. [DOI] [PubMed] [Google Scholar]
- 26. Stout NL, Pfalzer LA, Springer B, et al. Breast cancer-related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther. 2012;92:152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kilbreath S, Refshauge K, Beith J, Lee M. Resistance and stretching shoulder exercises early following axillary surgery for breast cancer. Rehabilitation Oncology. 2006;24:9–14. [Google Scholar]
- 28. Hayes SC, Rye S, Disipio T, et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Research & Treatment. 2013;137:175–186. [DOI] [PubMed] [Google Scholar]
- 29. Schmitz KH, Ahmed RL, Troxel AB, et al. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA. 2010;304:2699–2705. [DOI] [PubMed] [Google Scholar]
- 30. Ammitzboll G, Johansen C, Lanng C, et al. Progressive resistance training to prevent arm lymphedema in the first year after breast cancer surgery: results of a randomized controlled trial. Cancer. 2019;125:1683–1692. [DOI] [PubMed] [Google Scholar]
- 31. Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. [DOI] [PubMed] [Google Scholar]
- 32. Campbell KL, Winters-Stone K, Wiskermann J, et al. Exercise guidelines for cancer survivors: consensus statement from the international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51:2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Földi M FE, Strößenreuther C, Kubik S. Földi’s textbook of lymphology. For physicians and lymphedema therapists . 3rd Edition ed: Urban & Fische; r; 2012. [Google Scholar]
- 34. Sezgin Ozcan D, Dalyan M, Unsal Delialioglu S, et al. Complex decongestive therapy enhances upper limb functions in patients with breast cancer-related lymphedema. Lymphat Res Biol. 2018;16:446–452. [DOI] [PubMed] [Google Scholar]
- 35. Mobarakeh ZS, Mokhtari-Hesari P. Lotfi-Tokaldany M, et al. combined decongestive therapy and reduction of pain and heaviness in patients with breast cancer-related lymphedema. Support Care Cancer. 2019. [DOI] [PubMed] [Google Scholar]
- 36. Dayes IS, Whelan TJ, Julian JA, et al. Randomized trial of decongestive lymphatic therapy for the treatment of lymphedema in women with breast cancer. J Clin Oncol. 2013;31:3758–3763. [DOI] [PubMed] [Google Scholar]
- 37. Gradalski T, Ochalek K, Kurpiewska J. Complex decongestive lymphatic therapy with or without Vodder II manual lymph drainage in more severe chronic Postmastectomy upper limb lymphedema: a randomized noninferiority prospective study. J Pain Symptom Manage. 2015;50:750–757. [DOI] [PubMed] [Google Scholar]
- 38. Tambour M, Holt M, Speyer A, Christensen R, Gram B. Manual lymphatic drainage adds no further volume reduction to complete decongestive therapy on breast cancer-related lymphoedema: a multicentre, randomised, single-blind trial. Br J Cancer. 2018;119:1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pujol-Blaya V, Salinas-Huertas S, Catasús ML, Pascual T, Belmonte R. Effectiveness of a precast adjustable compression system compared to multilayered compression bandages in the treatment of breast cancer-related lymphoedema: a randomized, single-blind clinical trial. Clin Rehabil. 2019;33:631–641. [DOI] [PubMed] [Google Scholar]
- 40. Ligabue MB, Campanini I, Veroni P, et al. Efficacy of self-administered complex decongestive therapy on breast cancer-related lymphedema: a single-blind randomized controlled trial. Breast Cancer Res Treat. 2019;175:191–201. [DOI] [PubMed] [Google Scholar]
- 41. Ochalek K, Gradalski T, Szygula Z. Five-year assessment of maintenance combined physical therapy in postmastectomy lymphedema. Lymphat Res Biol. 2015;13:54–58. [DOI] [PubMed] [Google Scholar]
- 42. Vignes S, Porcher R, Arrault M, Dupuy A. Long-term management of breast cancer-related lymphedema after intensive decongestive physiotherapy. Breast Cancer Res Treat. 2007;101:285–290. [DOI] [PubMed] [Google Scholar]
- 43. Uzkeser H, Karatay S, Erdemci B, Koc M, Senel K. Efficacy of manual lymphatic drainage and intermittent pneumatic compression pump use in the treatment of lymphedema after mastectomy: a randomized controlled trial. Breast Cancer. 2015;22:300–307. [DOI] [PubMed] [Google Scholar]
- 44. Szuba A, Achalu R, Rockson SG. Decongestive lymphatic therapy for patients with breast carcinoma-associated lymphedema. A randomized, prospective study of a role for adjunctive intermittent pneumatic compression. Cancer. 2002;95:2260–2267. [DOI] [PubMed] [Google Scholar]
- 45. Haghighat S, Lotfi-Tokaldany M, Yunesian M, et al. Comparing two treatment methods for post mastectomy lymphedema: complex decongestive therapy alone and in combination with intermittent pneumatic compression. Lymphology. 2010;43:25–33. [PubMed] [Google Scholar]
- 46. Szolnoky G, Lakatos B, Keskeny T, et al. Intermittent pneumatic compression acts synergistically with manual lymphatic drainage in complex decongestive physiotherapy for breast cancer treatment-related lymphedema. Lymphology. 2009;42:188–194. [PubMed] [Google Scholar]
- 47. Fife CE, Davey S, Maus EA, Guilliod R, Mayrovitz HN. A randomized controlled trial comparing two types of pneumatic compression for breast cancer-related lymphedema treatment in the home. Support Care Cancer. 2012;20:3279–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ridner SH, Murphy B, Deng J, et al. Advanced pneumatic therapy in self-care of chronic lymphedema of the trunk. Lymphat Res Biol. 2010;8:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilburn O, Wilburn P, Rockson SG. A pilot, prospective evaluation of a novel alternative for maintenance therapy of breast cancer-associated lymphedema [ISRCTN76522412]. BMC Cancer. 2006;6:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ridner SH, Murphy B, Deng J, et al. A randomized clinical trial comparing advanced pneumatic truncal, chest, and arm treatment to arm treatment only in self-care of arm lymphedema. Breast Cancer Res Treat. 2012;131:147–158. [DOI] [PubMed] [Google Scholar]
- 51. Tsai HJ, Hung HC, Yang JL, Huang CS, Tsauo JY. Could Kinesio tape replace the bandage in decongestive lymphatic therapy for breast-cancer-related lymphedema? A pilot study. Support Care Cancer. 2009;17:1353–1360. [DOI] [PubMed] [Google Scholar]
- 52. Malicka I, Rosseger A, Hanuszkiewicz J, Woźniewski M. Kinesiology taping reduces lymphedema of the upper extremity in women after breast cancer treatment: a pilot study. Przeglad Menopauzalny. 2014;13:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pop TB, Karczmarek-Borowska B, Tymczak M, Halas I, Banas J. The influence of kinesiology taping on the reduction of lymphoedema among women after mastectomy - preliminary study. Contemp Oncol (Pozn). 2014;18:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smykla A, Walewicz K, Trybulski R, et al. Effect of kinesiology taping on breast cancer-related lymphedema: a randomized single-blind controlled pilot study. Biomed Res Int. 2013;2013:767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Melgaard D. What is the effect of treating secondary lymphedema after breast cancer with complete decongestive physiotherapy when the bandage is replaced with Kinesio Textape?–A pilot study. Physiotherapy Theory & Practice. 2016;32:446–451. [DOI] [PubMed] [Google Scholar]
- 56. Taradaj J, Halski T, Rosinczuk J, et al. The influence of kinesiology taping on the volume of lymphoedema and manual dexterity of the upper limb in women after breast cancer treatment. Eur J Cancer Care (Engl). 2016;25:647–660. [DOI] [PubMed] [Google Scholar]
- 57. Collins S, Bradley N, Fitzgibbon S, McVeigh JG. Kinesiology taping for breast lymphoedema after breast cancer treatment: a feasibility randomised controlled trial. Physiotherapy Practice & Research. 2018;39:107–116. [Google Scholar]
- 58. Assis L MA, Abrahao TB, de Souza HP, Hamblin MR,Parizotto NA. Low-level laser therapy (808nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis ante-rior muscle after cryolesion. Lasers Med Sci. 2013;28:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Storz MA, Gronwald B, Gottschling S, et al. Photobiomodulation therapy in breast cancer-related lymphedema: a randomized placebo-controlled trial. Photodermatol Photoimmunol Photomed. 2017;33:32–40. [DOI] [PubMed] [Google Scholar]
- 60. Khalaf MM, Hassan MA, Ibrahim ZM. Helium neon laser therapy for post mastectomy lymphedema and shoulder mobility. Egyptian Journal of Medical Human Genetics. 2013;14:195–199. [Google Scholar]
- 61. Ridner SH, Poage-Hooper E, Kanar C, et al. A pilot randomized trial evaluating low-level laser therapy as an alternative treatment to manual lymphatic drainage for breast cancer-related lymphedema. Oncol Nurs Forum. 2013;40:383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ahmed Omar MT, Abd-El-Gayed Ebid A, El Morsy AM. Treatment of post-mastectomy lymphedema with laser therapy: double blind placebo control randomized study. J Surg Res. 2011;165:82–90. [DOI] [PubMed] [Google Scholar]
- 63. Kozanoglu E, Basaran S, Paydas S, Sarpel T. Efficacy of pneumatic compression and low-level laser therapy in the treatment of postmastectomy lymphoedema: a randomized controlled trial. Clin Rehabil. 2009;23:117–124. [DOI] [PubMed] [Google Scholar]
- 64. Lau RW, Cheing GL. Managing postmastectomy lymphedema with low-level laser therapy. Photomed Laser Surg. 2009;27:763–769. [DOI] [PubMed] [Google Scholar]
- 65. Carati CJ, Anderson SN, Gannon BJ, Piller NB. Treatment of postmastectomy lymphedema with low-level laser therapy: a double blind, placebo-controlled trial. Cancer. 2003;98:1114–1122. [DOI] [PubMed] [Google Scholar]
- 66. Kaviani A, Fateh M, Yousefi Nooraie R, Alinagi-zadeh MR, Ataie-Fashtami L. Low-level laser therapy in management of postmastectomy lymphedema. Lasers Med Sci. 2006;21:90–94. [DOI] [PubMed] [Google Scholar]
- 67. Zhang X, Brown JC, Paskett ED, et al. Changes in arm tissue composition with slowly progressive weight-lifting among women with breast cancer-related lymphedema. Breast Cancer Res Treat. 2017;164:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cormie P, Galvao DA, Spry N, Newton RU. Neither heavy nor light load resistance exercise acutely exacerbates lymphedema in breast cancer survivor. Integr Cancer Ther. 2013. [DOI] [PubMed] [Google Scholar]
- 69. Hayes SC, Reul-Hirche H, Turner J. Exercise and secondary lymphedema: safety, potential benefits, and research issues. Med Sci Sports Exerc. 2009;41:483–489. [DOI] [PubMed] [Google Scholar]
- 70. Buchan J, Janda M, Box R, Schmitz K, Hayes S. A randomized trial on the effect of exercise mode on breast cancer-related lymphedema. Medicine and Science in Sports and Exercise 2016;48:1866–1874. 2016. [DOI] [PubMed] [Google Scholar]
- 71. Bracha J, Katz-Leurer M. The immediate effect of upper arm exercise compared with lower or combined upper and lower arms exercise on arm volume reduction in women with breasts cancer related lymphedema: a randomize prelimanry study. Rehabilitation Oncology. 2012;30:3–8. [Google Scholar]
- 72. Jonsson C, Johansson K. Pole walking for patients with breast cancer-related arm lymphedema. Physiother Theory Pract. 2009;25:165–173. [DOI] [PubMed] [Google Scholar]
- 73. Tidhar D, Katz-Leurer M. Aqua lymphatic therapy in women who suffer from breast cancer treatment-related lymphedema: a randomized controlled study. Support Care Cancer. 2010;18:383–392. [DOI] [PubMed] [Google Scholar]
- 74. Singh B, Buchan J, Box R, et al. Compression use during an exercise intervention and associated changes in breast cancer–related lymphedema. Asia-Pacific Journal of Clinical Oncology. 2016;12:216–224. [DOI] [PubMed] [Google Scholar]
- 75. Mazor M, Lee JQ, Peled A, et al. The effect of yoga on arm volume, strength, and range of motion in women at risk for breast cancer-related lymphedema. J Altern Complement Med. 2018;24:154–160. [DOI] [PubMed] [Google Scholar]
- 76. Lai YT, Hsieh CC, Huang LS, et al. The effects of upper limb exercise through yoga on limb swelling in Chinese breast cancer survivors - a pilot study. Rehabil Nurs. 2017;42:46–54. [DOI] [PubMed] [Google Scholar]
- 77. Douglass J, Immink M, Piller N, Ullah S. Yoga for women with breast cancer-related Lymphoedema: a preliminary 6-month study. Journal of Lymphoedema. 2012;7:30–38. [Google Scholar]
- 78. Loudon A, Barnett T, Piller N, Immink MA, Williams AD. Yoga management of breast cancer-related lymphoedema: a randomised controlled pilot-trial. BMC Complement Altern Med. 2014;14:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. De Groef A, Van Kampen M, Verlvoesem N, et al. Effect of myofascial techniques for treatment of upper limb dysfunctions in breast cancer survivors: randomized controlled trial. Support Care Cancer. 2017;25:2119–2127. [DOI] [PubMed] [Google Scholar]
- 80. Bao T, Iris Zhi W, Vertosick EA, et al. Acupuncture for breast cancer-related lymphedema: a randomized controlled trial. Breast Cancer Res Treat. 2018;170:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cassileth BR, Van Zee KJ, Chan Y, et al. A safety and efficacy pilot study of acupuncture for the treatment of chronic lymphoedema. Acupunct Med. 2011;29:170–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Smith CA, Pirotta M, Kilbreath S. A feasibility study to examine the role of acupuncture to reduce symptoms of lymphoedema after breast cancer: a randomised controlled trial. Acupunct Med. 2014;32:387–393. [DOI] [PubMed] [Google Scholar]
- 83. Oliveira J, Cesar TB. Influence of complex descongestive physical therapy associated with intake of medium-chain triglycerides for treating upper-limb lymphedema. Brazilian Journal of Physical Therapy / Revista Brasileira de Fisioterapia. 2008;12:31–36. [Google Scholar]
- 84. Whatley J, Street R, Kay S, Harris PE. Use of reflexology in managing secondary lymphoedema for patients affected by treatments for breast cancer: a feasibility study. Complement Ther Clin Pract. 2016;23:1–8. [DOI] [PubMed] [Google Scholar]
- 85. Yao C, Xu Y, Chen L, et al. Effects of warm acupuncture on breast cancer–related chronic lymphedema: a randomized controlled trial. Current Oncology. 2016;23:e27-e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bae H, Kim HJ. Clinical outcomes of extracorporeal shock wave therapy in patients with secondary lymphedema: a pilot study. Ann Rehabil Med. 2013;37:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Abbasi B, Mirzakhany N, Angooti Oshnari L, et al. The effect of relaxation techniques on edema, anxiety and depression in post-mastectomy lymphedema patients undergoing comprehensive decongestive therapy: a clinical trial. PLoS One. 2018;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Smoot B, Chiavola-Larson L, Lee J, Manibusan H, Allen DD. Effect of low-level laser therapy on pain and swelling in women with breast cancer-related lymphedema: a systematic review and meta-analysis. J Cancer Surviv. 2015;9:287–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


