Abstract
Background
Migraine is associated with altered sensory processing and cortical responsivity that may contribute to susceptibility to attacks by changing brain network excitability dynamics. To gain better insight into cortical responsivity changes in migraine we subjected patients to a short series of light inputs over a broad frequency range (“chirp” stimulation), designed to uncover dynamic features of visual cortex responsivity.
Methods
EEG responses to visual chirp stimulation (10–40 Hz) were measured in controls (n = 24) and patients with migraine with aura (n = 19) or migraine without aura (n = 20). Average EEG responses were assessed at (i) all EEG frequencies between 5 and 125 Hz, (ii) stimulation frequencies, and (iii) harmonic frequencies. We compared average responses in a low (10–18 Hz), medium (19–26 Hz) and high (27–40 Hz) frequency band.
Results
Responses to chirp stimulation were similar in controls and migraine subtypes. Eight measurements (n = 3 migraine with aura; n = 5 without aura) were assigned as “pre-ictal”, based on reported headache within 48 hours after investigation. Pre-ictally, an increased harmonic response to 22–32 Hz stimulation (beta band) was observed (p = 0.001), compared to interictal state measurements.
Conclusions
We found chirp responses to be enhanced in the 48 hours prior to migraine headache onset. Visual chirp stimulation proved a simple and reliable technique with potential to detect changes in cortical responsivity associated with the onset of migraine attacks.
Keywords: Migraine, visual-evoked potential, photic drive, EEG
Introduction
Migraine is a common paroxysmal brain disorder characterized by recurrent disabling attacks of severe headache with associated features such as nausea, vomiting, and enhanced sensitivity to sound and light (1). It remains an enigma exactly why and when attacks strike. It has been suggested that the initiation of an attack may involve variations in cortical responsivity to sensory inputs such as light (2,3), presumably as result of fluctuations in cortical excitability (4). Such dynamics in cortical responsivity may provide functional biomarkers of relevance for attack prediction. There is evidence pointing to the visual cortex as an area of the brain where changes in cortical responsivity in migraine are most apparent. Responsivity to light in migraineurs was particularly enhanced for the visual cortex as assessed in neuroimaging studies (5,6), and in some was reported to be most pronounced for migraine with aura (7,8).
Cortical responsivity to light can be assessed by frequency-specific steady-state stimulation, using a series of flash light stimulation (9). When combined with electroencephalography (EEG), the phenomenon of ‘photic driving’ is observed, which is the frequency-following response measured by EEG at the visual cortex. Photic driving is not only evident as the EEG response in the range of the stimulated frequencies, but also occurs at multiples of these frequencies, the so called higher-order ‘harmonics’ (9). Using steady-state visual stimulation in between attacks, some studies (but not all (10)) reported enhanced photic driving for different stimulation frequencies in migraine patients (6,11–14) and displayed enhanced harmonic activity that could result from altered cortical excitability (15,16).
Changes in photic driving may relate to attack initiation, since frequency-following responses to flash light stimulation at 12 Hz were found to increase prior to the headache phase (10). The use of relatively long stimulation series at different frequencies, however, makes steady-state stimulation less suitable for assessing dynamic changes in frequency-dependent cortical responsivity over the migraine cycle. To this end, we set out to investigate responses in migraine patients to a short ‘visual chirp’ stimulation paradigm, from which the visual cortex EEG response at driving and harmonic frequencies can be assessed within a very short time period. Visual chirp stimulation is a quick and easy-to-apply paradigm to assess photic driving that uses a single, short-duration, flash light stimulation paradigm consisting of increasing stimulation frequencies within a 6-second period (17). When visual chirp stimulation was applied interictally in migraine patients without aura, responses were found to be more pronounced compared to controls, for stimulation frequencies between 18 and 26 Hz (18). Given the association between migraine with aura and visual cortex responsivity (7,8) we here aimed to assess visual chirp responses in the two main migraine subtypes. High-density EEG was used to test the specificity of cortical responses to chirp stimulation by determining the optimal recording location above the visual cortex. In addition, we compared interictal and pre-ictal recordings to investigate whether cortical responsivity to chirp stimulation may change towards an upcoming attack.
Methods
Participants aged 18 to 65 years were recruited from our Leiden University Medical Center Migraine Neuro Analysis (LUMINA) database (19). Pre-screened non-headache controls and patients with migraine with aura or migraine without aura were included in the study. Exclusion criteria for all participants were: (i) psychiatric or neurological disorder (except migraine for participants with migraine); (ii) use of chronic medication (other than oral contraceptives), including migraine prophylactics, in the 4 weeks preceding the measurements; (iii) a history of malignancy. Patients with migraine were diagnosed according to the ICHD-3 beta criteria (1) and were to have an attack frequency of at least one attack per month, for the 6 months prior to the measurement day. Controls, and their first-degree relatives, were not allowed to have migraine or any form of trigeminal autonomic cephalalgia. In addition, controls were not allowed to have any other form of headache on more than 1 day per month. Patients were contacted by telephone interview at least 3 days after the experiment to verify migraine status at the time of measurement. A measurement was considered interictal when the participant was measured at least 3 days after the last migraine attack and 3 days before the next attack. A measurement was a priori defined as pre-ictal (i.e. before the onset of headache) when the measurement was performed within 72 hours before the next migraine attack. In the actual measurements, the pre-ictal group had received EEG recordings between 0.5 and 48 hours prior to the migraine attack. The Medical Ethics Committee of the Leiden University Medical Center approved this study and all participants provided written informed consent.
Experimental protocols
All participants underwent EEG recordings during visual flash stimulation. Two experimental setups (occipital and cortex-wide) were used to record potentials in different experiments. Occipital responses were recorded with seven Ag-AgCl electrodes placed at 10–20 locations; that is, Fz, Cz, C3, C4, Oz, O1 and O2, and online referenced to electrodes at C3 and C4 (EEG-1200; Nihon Kohden, Tokyo, Japan). Data were sampled at 1000 Hz and online band-pass filtered between 0.08 and 300 Hz. Cortex-wide responses were recorded with high-density-EEG cap using 126 Ag/AgCl electrodes (WaveGuard; ANT, Enschede, The Netherlands) arranged according to the 10-5 system. Data were recorded with a common average reference and sampled at 2048 Hz using the 136-channel Refa system (TMSi, Oldenzaal, The Netherlands). A separate ground electrode was placed at the left mastoid, while cap mastoid electrodes at M1 and M2 were left unconnected. All recordings were performed at the Department of Clinical Neurophysiology of the Leiden University Medical Center between 9 am and 5 pm.
Participants lay on a bed with their eyes closed in a darkened room. Spontaneous EEG was recorded for ∼10 minutes before visual stimulation started. Binocular red-light LED goggles (Synergy Plinth; Medelec International, Pleasanton, CA, USA) with a light intensity of 2.64 log cd/m2 (438 lux) at wavelength 654 nm were controlled via custom-written scripts in Matlab (Mathworks, Natick, MA, USA). Goggles were placed on both eyes and taped to the temples on both sides of the head. Chirp stimulation consisted of single-flash stimuli with an increasing frequency between 10 and 40 Hz in 1-Hz incrementing steps, according to Gantenbein et al. (18). At each frequency, four flashes were presented, resulting in 124 flashes and stimulation duration of 5.7 seconds (Figure 1(a)). In total, 10 repetitions were presented at inter-repetition intervals of 10 to 15 seconds. Trigger pulses at the start of each chirp repetition were simultaneously recorded for post-processing.
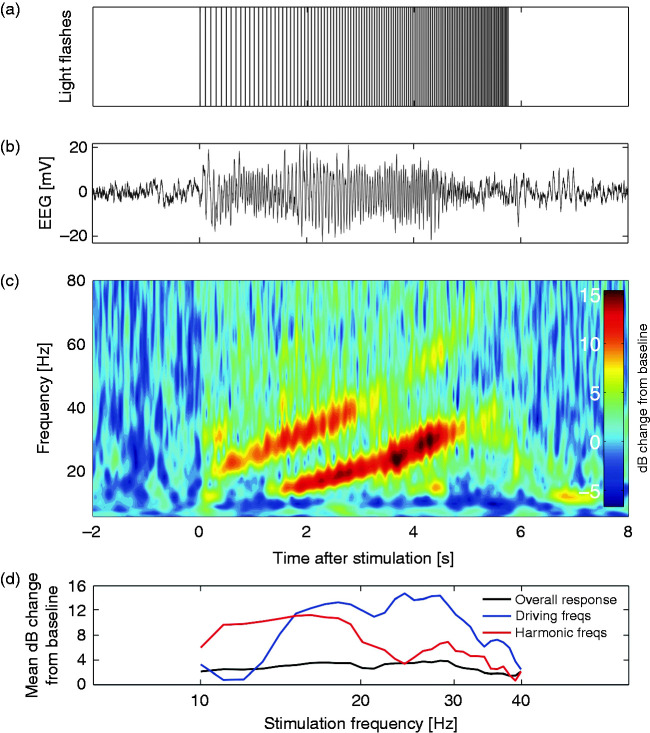
Figure 1.
(a) The chirp stimulus consisting of four light flashes per frequency between 10 and 40 Hz, resulting in a total duration of ∼6 seconds. (b) Example trace of an averaged EEG response (average of 10 responses) at electrode Oz of a control subject. (c) Time-frequency representation of the averaged response with baseline correction, displayed as decibel (dB) change from baseline. Distinct responses at the driving frequency (between 10 and 40 Hz) and at the harmonic frequencies (between 20 and 80 Hz) are present. (d) Example trace of the mean dB change in overall power (response at 5–125 Hz; black line), driving frequencies (response at stimulation frequency; blue line) and harmonic frequencies (responses at twice the stimulation frequency; red line) from baseline per stimulation frequency. Responses are analyzed with respect to EEG power per frequency for the duration of the four flashes plus 100 milliseconds afterwards, for the overall response, driving and harmonic frequencies.
Data pre-processing and analysis
All data analyses were performed in Matlab (version R2013b), performed independently by two researchers (MP and MvdR) who were blinded to group assignment. The EEG response to chirp stimulation was processed per repetition, from 2 seconds before to 8 seconds after stimulation onset. Time-frequency (TF) spectra were calculated using morlet wavelets between 5 and 125 Hz in 1-Hz incrementing steps with wavelet cycles logarithmically increasing between 3 and 10 cycles for the lowest and the highest frequency as time-frequency accuracy trade-off. Spectra were averaged over repetitions and mean baseline power per frequency was calculated between 1.6 and 0.1 seconds before stimulation onset (Figure 1(c)). The stimulation response per participant was dB-converted with respect to mean baseline power. For each stimulation frequency between 10 and 40 Hz (31 frequencies), response power over all frequencies (between 5 and 125 Hz) was averaged in a predefined time window, resulting in 31 total power values per participant (Figure 1(d)). The time window used depended on the stimulation frequency, and consisted of the time period between the starts of the subsequent four flashes plus 100 milliseconds, to take into account possible after-effects. The distinct response components at driving frequencies (EEG responses between 10 and 40 Hz) and harmonic frequencies (responses between 20 and 80 Hz) were analysed separately by averaging the TF response power at the frequencies between −1 and +1 Hz of the driving frequency, and at the stimulation frequency times two (“harmonic frequency”).
Three frequency bands of interest were defined based on previous work (18): (i) stimulation frequencies between 10 and 18 Hz (low frequencies); (ii) frequencies between 19 and 26 Hz (medium frequencies); and (iii) frequencies between 27 and 40 Hz (high frequencies). Averages were calculated within these bands based on overall power (5–125 Hz) and for driving and harmonic frequencies separately.
To determine the electrode showing the strongest response relative to noise level, the signal-to-noise ratio of the high-density EEG recordings was calculated for each of the 126 electrodes. Per electrode, the power between 5 and 45 Hz of the averaged chirp response (calculated by Fast Fourier Transform) was divided by the variance of the frequency domain response, and scaled by the number of repetitions (20) to study the distribution of the overall response power over the cortex. The specific topographic distribution of the response at driving and harmonic frequencies was also studied. For each electrode, the overall response amplitude was calculated separately for the driving frequency and the harmonic frequencies by summation of the photic driving response per frequency.
Statistical analysis
Test-retest reliability was calculated using the intraclass correlation coefficient (ICC; model ICC(2,1)) per outcome variable. Spearman’s correlations examined the shared association between repeated experimental sessions. Between-group differences per outcome variable (mean dB change from baseline, for low, medium and high frequencies) were analyzed using one-way Analyses of Variance (ANOVA) with three groups: (i) controls, migraine with aura (interictal), and migraine without aura (interictal); or (ii) controls, interictal migraine, and pre-ictal migraine. To examine a possible effect of time of day and gender on the results of the two interictal migraine groups (with and without aura) and controls, a three-way ANOVA was conducted additionally, including interactions between the three main factors, time of day (am/pm), gender (male/female) and group (control, migraine with aura interictal, and migraine without aura interictal). As each frequency band was analyzed independently, results were considered significant after compensating for multiple comparisons (p = 0.05/3 = 0.017). Post hoc analyses with respect to specific frequency responses were carried out with Bonferroni correction, with results considered significant at the 5% level (p < 0.05). The relationship between frequency responses – determined using post hoc analyses – and the number of days between the measurement and attack onset was tested using linear regression with four groups: interictal migraine, and three pre-ictal migraine groups (measured either 2 days before, 1 day before, or on the same day as the migraine attack). Statistical analyses were conducted in SPSS version 25 for Windows (IBM, Armonk, NY, US).
Results
EEG responses to visual chirp stimulation (Figure 1(a)) were measured in controls and migraine patients to investigate visual cortex responsivity to light inputs over a broad frequency range. A total of 100 measurements with chirp stimulation were conducted in 63 participants (controls (n = 24), migraine without aura (n = 20), migraine with aura (n = 19)) (Table 1). All participants showed clear EEG photic driving in response to chirp stimulation (see example in Figure 1(b)).
Table 1.
Baseline characteristics of controls and migraine subgroups.
|
7-channel recordings |
126-channel recordings |
|||||
|---|---|---|---|---|---|---|
| Variable | Controls (n = 17) |
Migraine without aura (n = 20) |
Migraine with aura (n = 19) |
Controls (n = 15) |
Migraine without aura (n = 9) |
Migraine with aura (n = 6) |
| Female (n (%)) | 14 (82) | 16 (80) | 15 (75) | 12 (80) | 8 (89) | 5 (83) |
| Age (years) | 38.4 ± 13.7 | 38.9 ± 10.2 | 38.7 ± 12.0 | 42.7 ± 11.3 | 39.3 ± 12.0 | 40.2 ± 12.4 |
| Age at onset of migraine | – | 18.6 ± 6.9 | 16.0 ± 9.0 | – | 18.4 ± 6.5 | 12.7 ± 2.3 |
| Migraine duration (years) | – | 20.4 ± 10.5 | 22.7 ± 14.3 | – | 20.9 ± 12.1 | 27.5 ± 11.8 |
| Migraine attacks per month | – | 2.2 ± 1.7 | 1.5 ± 1.0 | – | 2.1 ± 1.1 | 1.7 ± 1.1 |
| Migraine days per month | – | 3.3 ± 2.3 | 2.1 ± 1.9 | – | 3.7 ± 2.3 | 1.8 ± 1.0 |
| Use of triptans (n (%)) | – | 10 (50) | 9 (47) | – | 6 (67) | 1 (17) |
| Attacks with aura (%) | – | – | 75 ± 34 | – | – | 79 ± 39 |
| Duration of aura (min) | – | – | 49 ± 53 | – | – | 60 ± 33 |
Note: Values are presented as mean with standard deviations, or number with percentage.
Test-retest reproducibility using 7-channel EEG
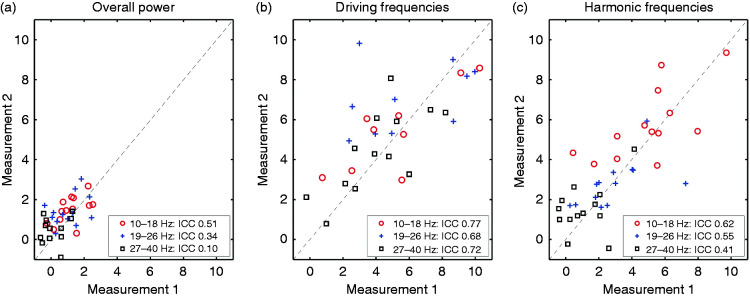
To study reproducibility of the chirp responses we performed retest measurements in 13 participants; that is, controls (n = 7), migraine without aura (n = 3, of whom one was measured in the pre-ictal phase during both measurements), and migraine with aura (n = 3). Retest measurements were conducted 1 to 42 days (median 11 days) after the initial experiment. Repeatability of responses at electrode Oz in the bands of interest was good (ICC ≥0.68, significant rs) for EEG power at the driving frequencies between 10–40 Hz (Table 2). The response at harmonic frequencies showed moderate repeatability (ICC 0.41–0.62), with significant rs for stimulation at low (10–18 Hz) and medium (19–26 Hz) frequencies, but not for stimulation at high frequencies (27–40 Hz) (Figure 2). EEG response power over all frequencies (between 5 and 125 Hz) showed no significant reproducibility, indicating low reliability (all ICC < 0.52, no significant rs).
Table 2.
Test-retest reliability parameters for overall response power, and power at driving and harmonic frequencies, grouped per stimulation band of interest.
|
10–18 Hz |
19–26 Hz |
27–40 Hz |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ICC | rs | p | ICC | rs | p | ICC | rs | p | |
| Overall response | 0.51 | 0.51 | 0.08 | 0.34 | 0.18 | 0.55 | 0.10 | 0.21 | 0.49 |
| Driving frequencies | 0.77 | 0.79 | 0.002 | 0.68 | 0.74 | 0.006 | 0.72 | 0.74 | 0.005 |
| Harmonic frequencies | 0.62 | 0.72 | 0.008 | 0.55 | 0.68 | 0.013 | 0.41 | 0.18 | 0.57 |
ICC: Intraclass correlation coefficient; rs: Spearman’s rho.
Note: Boldfaced values indicate significant association between measurements, with moderate to good repeatability.
Figure 2.
Test-retest reliability of chirp responses (based on 7-channel EEG, electrode Oz) in control and migraine groups is moderate to good at driving and harmonic frequencies. (a) Overall response power in the three bands of interest (red circles: 10–18 Hz; blue crosses: 19–26 Hz; black squares: 27–40 Hz) for measurement 1 and measurement 2 (1 to 42 days after measurement 1). Intraclass correlation coefficients (ICC) are between 0.10 and 0.51, indicating low to moderate reproducibility for the overall power. Dashed line indicates a perfect reproducibility between measurements. (b) Idem for the response at driving frequencies (10–40 Hz), with ICC between 0.68 and 0.77, indicating good reproducibility. (c) Idem for the response at harmonic frequencies (20–80 Hz), with ICC between 0.41 and 0.62, indicating moderate reproducibility.
Interictal occipital recordings of chirp responses in migraine with and migraine without aura
Occipital responses following chirp stimulation were recorded using 7-channel EEG in 56 participants; that is, controls (n = 17), migraine without aura (n = 20), and migraine with aura (n = 19). Eight measurements (five in four migraine without aura patients; three in three migraine with aura patients) were classified as pre-ictal since patients were retrospectively identified to have experienced a migraine headache within 72 hours from the time of investigation. In those cases, the time to the start of the headache ranged between 0.5 to 48 hours (median 24 hours). The other 32 measurements were classified as interictal (16 migraine without aura and 16 migraine with aura patients). No differences with respect to age, gender, migraine years, attack frequency or migraine days were present between interictal and pre-ictal measurements (independent t-tests; all p > 0.05).
To examine the interictal photic driving response between migraine subtypes, we compared interictal chirp responses for migraine without aura, migraine with aura and control groups in the pre-defined frequency bands based on Gantenbein et al. (18). Responses to low (10–18 Hz), medium (19–26 Hz) and high (27–40 Hz) frequency stimulation were not different (Figure 3) for: (i) overall EEG response power (between 5 and 125 Hz; low: F(2,46) = 0.34, p = 0.71; medium: F(2,46) = 0.05, p = 0.95; high: F(2,46) = 0.16, p = 0.85); (ii) EEG power at driving frequencies (low: F(2,46) = 1.78, p = 0.18; medium: F(2,46) = 0.77, p = 0.47; high: F(2,46) = 0.29, p = 0.75); nor (iii) EEG power at harmonic frequencies (low: F(2,46) = 2.08, p = 0.14; medium: F(2,46) = 0.16, p = 0.86; high: F(2,46) = 1.44, p = 0.25).
Figure 3.
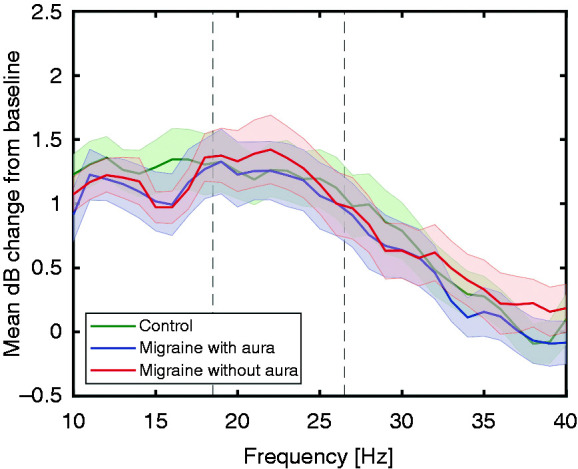
Overall response power (between 5 and 125 Hz), assessed per stimulation frequency as mean (± standard error) decibel (dB) change from baseline, for the different chirp stimulation frequencies. No differences in EEG power at electrode Oz (7-channel EEG) are present between controls and migraine with and without aura subjects, measured interictally, in the three pre-defined bands of interest (10–18 Hz, 19–26 Hz and 27–40 Hz; borders indicated by dashed lines).
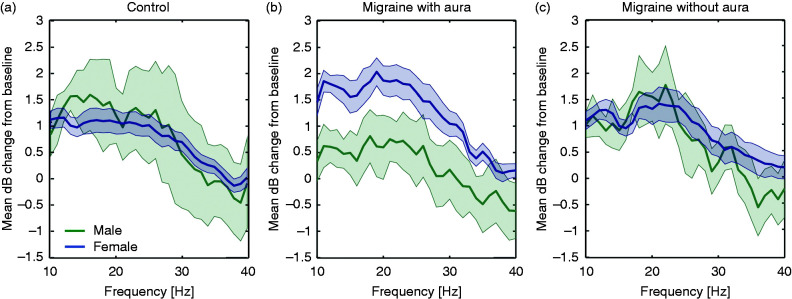
An additional analysis was performed to assess possible effects of gender and the time of day at which the measurements were performed. Gender (controls: n = 3 male, n = 14 female; migraine with aura: n = 5 male, n = 11 female; migraine without aura: n = 3 male, n = 13 female) or time-of-day (controls: n = 9 am, n = 8 pm; migraine with aura: n = 7 am, n = 9 pm; migraine without aura: n = 10 am, n = 6 pm) did not have a significant effect on overall EEG response power, EEG power at driving frequencies, or at harmonic frequencies (main effects for group all p > 0.14, time-of-day all p > 0.23 and gender all p > 0.04; interaction group and time-of-day all p > 0.02, interaction group and gender all p > 0.06, interaction time-of-day and gender all p > 0.11, interaction group, time-of-day and gender all p > 0.07). Female migraine patients with aura showed a tendency to a more pronounced response to chirp stimulation compared to males with respect to overall EEG power, while this distinction was not evident in the migraine without aura and control groups (Figure 4). However, as indicated above, gender differences in response across groups were not statistically significant.
Figure 4.
Gender effect within the overall response power, assessed per stimulation frequency as mean (± standard error) decibel (dB) change from baseline, for controls (a), migraine with aura (b) and migraine without aura (c) subjects. Female migraine patients with aura showed a tendency to more pronounced response to chirp stimulation compared to males. However, this difference did not reach statistical significance (interaction group and gender with respect to low, medium and high frequency windows, all p> 0.06).
Topographic distribution of cortical responses
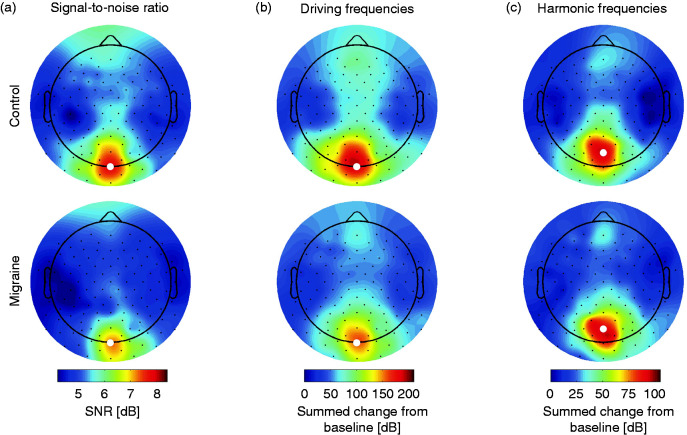
As no interictal differences in the photic driving response to chirp stimulation were found between migraine and control groups, contrary to Gantenbein et al. (18), we assessed the optimal recording location at the visual cortex for measuring responses to chirp light stimulation. Cortex-wide responses were determined using high-density 126-channel EEG in a number of participants from the various groups. Chirp stimulation was performed in 30 participants; that is, in controls (n = 15), of which seven did not undergo the occipital recordings; migraine without aura (n = 9), and migraine with aura (n = 6), who all underwent the occipital recordings. Nine frontal electrodes (i.e. channels Fp1, Fpz, Fp2, AF7, AF8, FT9, FT10, AFp3h and AFp4h) were discarded from further analyses due to excessive noise in most participants. Cortical activation patterns (topoplots in Figure 5) did not show differences between the migraine and control groups, neither in signal-to-noise (SNR) ratio over the complete chirp response, nor in location of driving or harmonics responses. The response pattern was clustered at the occipital lobe, with highest SNR for both groups at Oz and POz. Maximum response amplitude showed a slight parietal shift for harmonic (maximum at POz) compared to driving responses (maximum at Oz). Responses at Oz to low, medium and high frequency bands were not different between combined migraine (with and without aura) and control groups for these recordings, comparable to the interictal recordings with seven electrodes.
Figure 5.
Topographical distribution of signal-to-noise ratio (SNR) of 126-channel EEG responses between 5 and 45 Hz (a) and summed responses at driving (b) and harmonic frequencies (c) as change from baseline in decibel (dB). Highlighted channel (white dot) indicates the channel with maximum response per group and parameter (Oz for SNR and driving frequencies, POz for harmonic frequencies).
Photic driving response in the pre-ictal phase
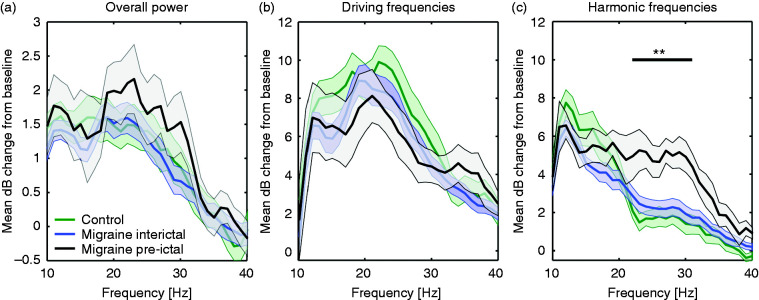
To assess the photic driving response to chirp stimulation in the pre-ictal phase, comparisons were made between the migraine group (n = 8 pre-ictal and n = 32 interictal) and control group (n = 17). Because we found no interictal difference between migraine subtypes, the interictal data from migraine with and without aura patients were combined. Overall EEG response power was not different between controls, interictal and pre-ictal migraine patients (low: F(2,54) = 0.36, p = 0.70; medium: F(2,54) = 0.56, p = 0.57; high: F(2,54) = 0.38, p = 0.68; Figure 6(a)), neither was the power at driving frequencies (low: F(2,54) = 1.10, p = 0.34; medium: F(2,54) = 0.74, p = 0.48; high: F(2,54) = 0.34, p = 0.72; Figure 6(b)). Instead, response power between groups was divergent for the harmonics of the high stimulation frequencies (F(2,54) = 5.74, p = 0.005). The difference in harmonic response power for medium stimulation frequencies just failed to reach significance after compensating for multiple comparisons (F(2,54) = 4.33, p = 0.02). The harmonic responses to the low stimulation frequencies did not differ between groups (F(2,54) = 2.17, p = 0.12). Post hoc analyses for the high frequency response harmonics revealed higher power in the pre-ictal compared to the interictal state in migraine patients as well as to controls (all p < 0.02, Bonferroni corrected; Figure 6(c)). The most pronounced increase in power in the pre-ictal period was found for the harmonics of stimulation frequencies between 22 and 32 Hz. An additional one-way ANOVA for this 22–32 Hz frequency band revealed a significant effect of the group (F(2,54) = 7.37, p = 0.001), with post hoc analysis demonstrating a statistically significant difference between pre-ictal measurements and both the interictal and control measurements (all p < 0.004, Bonferroni corrected). Harmonic response power in this frequency band increased from interictal responses to pre-ictal responses as the time (in days) to the next migraine attack onset decreased (R2 = 0.21, F(1,38) = 10.58, p = 0.002).
Figure 6.
Response at electrode Oz (7-channel EEG) for the different chirp stimulation frequencies for control, interictal and pre-ictal migraine groups, showing an increase of EEG power for the harmonics of the stimulation frequencies between 22–32 Hz during the pre-ictal phase. (a) Overall response power (between 5 and 125 Hz), assessed per stimulation frequency as mean decibel (dB) change from baseline, was not different between groups for the defined EEG bands of interest. (b) Similarly, EEG responses at driving frequencies showed no differences between groups. (c) For the harmonics of the stimulation frequencies, an increased power was present for the pre-ictal group for the high frequency band (27–40 Hz). A one-way ANOVA confirmed group differences across frequency bands (between 22–32 Hz), with post hoc analyses showing an increase in power in the pre-ictal measurement compared to the interictal and control measurements (significance indicated: **p < 0.004 for all frequencies, Bonferroni corrected). Shown per line: mean ± standard error. Note the different y-axis scaling for panels b and c compared to panel a.
Discussion
Here we used visual ‘chirp’ stimulation as a tool to measure the photic driving response and to assess cortical responsivity dynamics in migraine patients with and without aura compared to controls. Chirp responses showed good test-retest reliability over days within participants and could be measured with a few scalp electrodes over the occipital cortex. Interictally, no differences in cortical responses were observed between migraine patients, regardless of migraine subtype, and controls. However, in a group of migraine patients that were measured in a pre-ictal time window, 1 to 48 hours before an attack, the harmonic EEG response to stimulation in the higher beta band (22–32 Hz) was enhanced compared to measurements outside an attack or compared to controls.
Our high-density EEG recordings indicated the specificity of visual cortex activation by chirp light stimulation. This result is in line with earlier visual chirp recordings performed in healthy controls using 32-channel EEG (17). Using 7-channel EEG, we demonstrated in the present study that interictal chirp-induced photic driving responses in subgroups of migraine patients with or without aura were not different from responses in controls. This contrasts with a previous report using chirp stimulation interictally in migraine patients without aura showing an increased overall response power between 18 and 26 Hz (18), as well as the enhanced ‘H-response’ between 18 and 24 Hz reported interictally for migraine with and without aura (13,14). An enhanced response in the 18–24 Hz range has not been a consistent finding, as migraine patients were also shown to have attenuated EEG responses in this frequency window (10,12). In earlier studies into the H-response, controls seemed to have a lack of EEG response instead of an attenuated response compared to migraineurs (13,14), while healthy subjects have been reported to be able to respond to flashing light stimulation up to 100 Hz (9). It thus remains unclear if the responses of controls in the present study are particularly enhanced. Differences in migraine attack frequency between studies may contribute to this discrepancy, as we only included patients with at least one headache per month, or it could be due to variations in stimulation paradigm in, for example, length, waveform and device used in those studies. With respect to the use of chirp stimulation, there is also a methodological difference as we used red light, whereas Gantenbein et al. (18) used white light for chirp stimulation. However, as the color of flash light stimulation was shown to have little effect on visual evoked potentials in migraine patients (21) we would not expect the color difference to explain the absence of an enhanced interictal chirp response in our study. Although the visual cortex was suggested to show particularly enhanced excitability in migraine with aura patients (7,8), our data did not reveal differences in the chirp response between migraine with and without aura in the interictal phase.
In patients with migraine, in a pre-ictal time window less than 48 hours prior to reported headache, we observed increased power of the harmonic EEG responses to chirp stimulation. Based on previous literature (18), initial analysis was performed with respect to three stimulation frequency bands (10–18 Hz, 19–26 Hz and 27–40 Hz). Only the harmonic responses to stimulation in the highest frequency window showed a statistically significant difference. Harmonic responses to the medium stimulation frequencies just failed to reach significance, possibly due to the small number of pre-ictal measurements, and inherent variance between measurements as well as within the frequency bands. The pre-ictal increase of harmonic EEG responses was largest for stimulation in the higher beta band, for frequencies between 22–32 Hz, and increased when the number of days to the next attack onset decreased. This frequency band overlaps and extends the 18–26 Hz frequency band reported in relation to interictal hyperresponsivity of the visual cortex (13,14,18). A longitudinal EEG study in migraine with and without aura patients was the first to report enhanced pre-ictal photic driving responses within 72 hours before the migraine attack, showing an increased response to steady-state stimulation at 12 Hz, but not at beta band frequencies (10). Discrepancy between enhanced H-responses reported interictally in earlier studies and changes at 12 Hz in pre-ictal patients was attributed to possible inclusion of pre-ictal patients in the interictal studies (10).
Enhanced cortical responsivity towards a migraine attack as observed in our chirp data is suggestive of cortical hyperexcitability underlying attack initiation, a concept largely supported by preclinical findings (22). In transgenic models of familial hemiplegic migraine type 1 (FHM1), in which cortical excitation-inhibition balance is disturbed (23–25), susceptibility to cortical spreading depolarization (CSD, the correlate of the migraine aura) is enhanced (26–28). Our (preliminary) observation that overall EEG responsivity in between attacks appeared larger for females than males in the migraine with aura group is of interest given the female preponderance of migraine (29) and in line with data from FHM type 1 mutant mice that show most pronounced CSD susceptibility in females (28). Photic driving to flash light stimulation was reported to be variably enhanced for female migraineurs (30). As we did not design our study to investigate gender differences, a follow-up study with more participants of both genders should assess whether visual responsivity towards an attack may indeed be more pronounced in females.
The chirp visual stimulation paradigm was quickly applicable within an experimental timeframe of less than three minutes. This will reduce bias that may be caused by habituation to long-duration steady-state visual stimulation paradigms (31,32), which is of particular relevance when comparing migraine patients for whom habituation to visual stimulation has been reported to be abnormal (2). Our test-retest measurements indicated that predominantly responses at driving frequencies and harmonic responses, but not the overall EEG power, were reproducible over days to weeks. Responses to steady-state visual stimulation are mainly expressed at the driving and harmonic frequencies (9,15) and not at other frequencies. Therefore, to increase the reproducibility of the visual chirp response, outcome measures based on responses at driving and harmonic frequencies are preferential over the overall EEG response.
Our results are supportive of the hypothesis that in migraine patients, cyclic changes in cortical excitability result in higher harmonic frequency output before an attack (33). Our dataset did not allow for a pair-wise comparison between interictal and pre-ictal phases. As a next step, within-patient longitudinal studies should substantiate whether the chirp-induced photic driving response can be a suitable marker of an impending migraine attack. The reliable chirp readouts from repeated measurements on different days support implementation of visual chirp stimulation in patients to assess day-to-day fluctuations in photic driving response over the migraine cycle. With a short-duration paradigm like chirp stimulation and using a minimum of two occipital EEG electrodes, longitudinal tests of visual cortex responsivity seem feasible and may eventually lead to a predictive measure of an impending migraine attack.
Clinical implications
In the 48 hours prior to the migraine headache phase, harmonic EEG responses to visual ‘chirp’ stimulation were enhanced in the beta band.
The response to visual chirp stimulation in between attacks in migraine patients with and without aura was similar to controls.
Visual chirp stimulation seems suitable and practical to assess dynamic changes in cortical responsivity linked to migraine attacks.
Acknowledgements
The authors thank Professor Dr JG van Dijk, P van Someren and colleagues of our clinical neurophysiology group for their help in setting up EEG recordings, and Professor Dr JJ Goeman for advice on statistical analyses.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants of the Netherlands Organization for Scientific Research (NWO; Dutch national science prize, Spinoza Award 2009 to MDF), Medical Delta program “Medical NeuroDelta: Ambulant Neuromonitoring for Prevention and Treatment of Brain Disease” (to AMJMvdM), Leiden University profile area “Brain Function and Dysfunction Across the Lifespan” (grant no. 2406303915 to MvdR and EAT) and European Community (within the European Union’s Seventh Framework programme “EUROHEADPAIN”, grant agreement no. 602633, to MDF and AMJMvdM).
ORCID iD
Else A Tolner https://orcid.org/0000-0002-6501-9971
References
- 1.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 2.de Tommaso M, Ambrosini A, Brighina F, et al. Altered processing of sensory stimuli in patients with migraine. Nat Rev Neurol 2014; 10: 144–155. [DOI] [PubMed] [Google Scholar]
- 3.Coppola G, Pierelli F, Schoenen J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia 2007; 27: 1429–1439. [DOI] [PubMed] [Google Scholar]
- 4.Cosentino G, Fierro B, Brighina F. From different neurophysiological methods to conflicting pathophysiological views in migraine: A critical review of literature. Clin Neurophysiol 2014; 125: 1721–1730. [DOI] [PubMed] [Google Scholar]
- 5.Boulloche N, Denuelle M, Payoux P, et al. Photophobia in migraine: An interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry 2010; 81: 978–984. [DOI] [PubMed] [Google Scholar]
- 6.Mehnert J, Bader D, Nolte G, et al. Visual input drives increased occipital responsiveness and harmonized oscillations in multiple cortical areas in migraineurs. NeuroImage Clin 2019; 23: 101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datta R, Aguirre GK, Hu S, et al. Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia 2013; 33: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brighina F, Bolognini N, Cosentino G, et al. Visual cortex hyperexcitability in migraine in response to sound-induced flash illusions. Neurology 2015; 84: 2057–2061. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann CS. Human EEG responses to 1–100 Hz flicker: Resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Exp Brain Res 2001; 137: 346–353. [DOI] [PubMed] [Google Scholar]
- 10.Bjørk M, Hagen K, Stovner L, et al. Photic EEG-driving responses related to ictal phases and trigger sensitivity in migraine: A longitudinal, controlled study. Cephalalgia 2011; 31: 444–455. [DOI] [PubMed] [Google Scholar]
- 11.de Tommaso M, Sciruicchio V, Guido M, et al. Steady-state visual-evoked potentials in headache: Diagnostic value in migraine and tension-type headache patients. Cephalalgia 1999; 19: 23–26; discussion 1. [DOI] [PubMed] [Google Scholar]
- 12.Nyrke T, Kangasniemi P, Lang a. H. Difference of steady-state visual evoked potentials in classic and common migraine. Electroencephalogr Clin Neurophysiol 1989; 73: 285–294. [DOI] [PubMed] [Google Scholar]
- 13.Chorlton P, Kane N. Investigation of the cerebral response to flicker stimulation in patients with headache. Clin Electroencephalogr 2000; 31: 83–87. [DOI] [PubMed] [Google Scholar]
- 14.Golla FL, Winter AL. Analysis of cerebral responses to flicker in patients complaining of episodic headache. Electroencephalogr Clin Neurophysiol 1959; 11: 539–549. [DOI] [PubMed] [Google Scholar]
- 15.Shibata K, Yamane K, Otuka K, et al. Abnormal visual processing in migraine with aura: A study of steady-state visual evoked potentials. J Neurol Sci 2008; 271: 119–126. [DOI] [PubMed] [Google Scholar]
- 16.Shibata K, Yamane K, Nishimura Y, et al. Spatial frequency differentially affects habituation in migraineurs: A steady-state visual-evoked potential study. Doc Ophthalmol 2011; 123: 65–73. [DOI] [PubMed] [Google Scholar]
- 17.Tu T, Xin Y, Gao X, et al. Chirp-modulated visual evoked potential as a generalization of steady state visual evoked potential. J Neural Eng 2012; 9: 016008. [DOI] [PubMed] [Google Scholar]
- 18.Gantenbein AR, Sandor PS, Goadsby PJ, et al. Chirp stimulation: H-response short and dynamic. Cephalalgia 2014; 34: 554–558. [DOI] [PubMed] [Google Scholar]
- 19.van Oosterhout WPJ, Weller CM, Stam AH, et al. Validation of the web-based LUMINA questionnaire for recruiting large cohorts of migraineurs. Cephalalgia 2011; 31: 1359–1367. [DOI] [PubMed] [Google Scholar]
- 20.Vlaar MP, Solis-Escalante T, Vardy AN, et al. Quantifying nonlinear contributions to cortical responses evoked by continuous wrist manipulation. IEEE Trans Neural Syst Rehabil Eng 2017; 25: 481–491. [DOI] [PubMed] [Google Scholar]
- 21.Noseda R, Bernstein CA, Nir RR, et al. Migraine photophobia originating in cone-driven retinal pathways. Brain 2016; 139: 1971–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolner EA, Chen SP, Eikermann-Haerter K. Current understanding of cortical structure and function in migraine. Cephalalgia 2019; 39: 1683–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tottene A, Conti R, Fabbro A, et al. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in CaV2.1 knockin migraine mice. Neuron 2009; 61: 762–773. [DOI] [PubMed] [Google Scholar]
- 24.Vecchia D, Tottene A, van den Maagdenberg AMJM, et al. Mechanism underlying unaltered cortical inhibitory synaptic transmission in contrast with enhanced excitatory transmission in CaV2.1 knockin migraine mice. Neurobiol Dis 2014; 69: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vecchia D, Tottene A, van den Maagdenberg AMJM, et al. Abnormal cortical synaptic transmission in CaV2.1 knockin mice with the S218L missense mutation which causes a severe familial hemiplegic migraine syndrome in humans. Front Cell Neurosci 2015; 9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Maagdenberg AMJM, Pietrobon D, Pizzorusso T, et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron 2004; 41: 701–710. [DOI] [PubMed] [Google Scholar]
- 27.van den Maagdenberg AMJM, Pizzorusso T, Kaja S, et al. High cortical spreading depression susceptibility and migraine-associated symptoms in Ca(v)2.1 S218L mice. Ann Neurol 2010; 67: 85–98. [DOI] [PubMed] [Google Scholar]
- 28.Eikermann-Haerter K, Dileköz E, Kudo C, et al. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest 2009; 119: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart WF, Wood C, Reed ML, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia 2008; 28: 1170–1178. [DOI] [PubMed] [Google Scholar]
- 30.Lehtonen J, Hyyppä MT, Kaihola H‐L, et al. Visual evoked potentials in menstrual migraine. Headache 1979; 19: 63–70. [DOI] [PubMed] [Google Scholar]
- 31.Bergholz R, Lehmann TN, Fritz G, et al. Fourier transformed steady-state flash evoked potentials for continuous monitoring of visual pathway function. Doc Ophthalmol 2008; 116: 217–229. [DOI] [PubMed] [Google Scholar]
- 32.Rankin CH, Abrams T, Barry RJ, et al. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem 2009; 92: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cosentino G, Fierro B, Vigneri S, et al. Cyclical changes of cortical excitability and metaplasticity in migraine: Evidence from a repetitive transcranial magnetic stimulation study. Pain 2014; 155: 1070–1078. [DOI] [PubMed] [Google Scholar]