Abstract
The first documented case of COVID-19 in the United States occurred on January 30th, 2020. Soon after, a global pandemic was declared in March 2020 with each state issuing stay at home orders based on population, risk for community transmission and current number of positive cases. A priority for each region was to develop efficient systems for testing large patient volumes in a safe manner to reduce the risk of community transmission. A community based United States health care system in the upper mid-west implemented a drive through testing site in an attempt to divert suspected cases of COVID-19 away from larger patient areas while protecting staff and patients. This commentary outlines the planning, work flow and challenges of implementing this drive through testing site in a rural community setting.
Keywords: COVID-19, testing, rural health, respiratory virus, pandemic
Background
In early 2020, an outbreak of a novel coronavirus (COVID-19) that originated in Wuhan, China began to emerge within the United States (US) with the first documented case occurring in Snohomish County, Washington on January 30, 2020.1 The novel virus is classified as a respiratory virus with a complex pathology, impacting multiple organ systems and has the potential to result in an excessive immune response, known as a cytokine storm.2 A global pandemic was declared by the World Health Organization on March 11, 2020. In response, each state instituted certain social distancing measures and stay at home orders on a state by state basis, depending on the number of documented cases, population size and potential for community transmission of the virus. Each regional health system was challenged with developing safe, efficient and effective protocols for testing, isolation and contact tracing measures for their patients and surrounding community members. Several health systems even opted to defer all elective procedures and out-patient visit services to minimize patient and staff exposure while reallocating resources towards the development of infrastructure to combat a potential surge in the event of widespread community transmission of COVID-19.
The first documented case in southwest Wisconsin (SWWI) occurred on March 18, 2020. A “Stay-At-Home” order soon went into effect on March 25, 2020 for the entire state of Wisconsin, which was later overturned by the state supreme court on May 13, 2020. The SWWI region is classified as a rural community health care setting and therefore a primary focus was to protect rural community residents and minimize community transmission. Epidemiologists highlighted testing as an essential cornerstone of containment strategies to prevent widespread community transmission.3 Unfortunately, early struggles in testing procedures and availability of resources slowed the progress of disease surveillance across the US.4 Through the use of a real time polymerase chain reaction (RT-PCR) assay developed by Mayo Clinic, the current community based health care system employed this key resource and developed a drive-through screening system on March 16, 2020 in an attempt to reduce the risk of exposure to staff and patients while allowing for the screening of a high patient volume in a safe and isolated manner. The development of the drive-through testing site also created an efficient screening system to protect the community at large by helping to collect positive case count data to help the local county health department make informed decisions regarding policies and to assist with contact tracing measures. A precedent for drive-through testing had been set in a larger health care setting at Mayo Clinic in Rochester, MN for which the development of the current testing site was modeled after.5
Site Planning and Development
The week prior to March 16, 2020 a multidisciplinary team was brought together which included physicians, nursing, administrators, infection prevention and control (IPAC), facilities, laboratory services, security, public affairs, registration, and information technology to develop the required procedures and work flow for the drive-through testing site. Local administrators consulted with clinicians and administrators at Mayo Clinic in Rochester, MN on best practices for drive-through testing implementation and work flow. Local community partners (county health department, emergency medical services, and police) were updated on the recent developments of the drive-through testing site and were instrumental in informing community members and assisting with the coordination of traffic flow into the testing site. On March 15th, 2020 an industrial tent was constructed with the necessary lighting, environmental controls and appropriate signage for the development of the drive-through testing site. The tent was located in a parking lot adjacent to a decommissioned health system building that was reconfigured and outfitted with the necessary equipment to function as a satellite testing site command center (ie, computers, telecommunications, personal protective equipment, testing kits and refrigeration).
Work Flow
In an attempt to preserve limited testing resources, early testing protocols established conservative criteria for testing, which included potential exposure to previously documented case of COVID-19, recent travel to a “hot spot” of known cases, fever and/or cough. The drive-through testing was open to all current patients and non-patients with no age restrictions. All patients suspected of a potential exposure to COVID-19, or if symptomatic with any signs and symptoms of a fever and respiratory illness were instructed to first contact their primary care physician’s office or call the COVID-19 triage line to determine if criteria for testing had been met. If testing criteria was met, as determined by evolving algorithms, patients were directed to the drive-through testing site. An email was subsequently sent to the centralized registration team on site and the patient was then directed to the drive-through testing site. Upon arrival, a nurse greeted the patient in their vehicle to collect the patient identification number, brief travel history and document if the patient was an emergency medical service or healthcare worker. The nurse then communicated to the on-site registration staff via hand-held communication to create a patient encounter and testing orders were placed by a registered nurse on-site, under a designated physician. Patients that presented to testing site without pre-registering were registered on site after an initial screening of symptoms or previous exposure.
A patient label and testing kit was then utilized by nursing staff wearing personal protective equipment, including a gown, gloves, mask and face shield to collect a nasopharyngeal swab for RT-PCR testing while the patient remained in their vehicle. The specimen was initially kept on-site prior to being transported to local lab facilities for relabeling and preparation. Samples were later transported to a centralized laboratory center for processing every 2 hours and test results were typically returned within 24-hours. All positive cases were contacted by an infectious diseases specialist and also further monitored by a local Physician for follow up care and to ensure that appropriate information and recommendations were being followed. Patients were also contacted by the local county health department with patient education materials on symptom management, instructions to self-quarantine and initiate contact tracing procedures to reduce the risk of potential community transmission in accordance with county and state-wide guidelines. In the event the patient was acutely ill and in need of emergency care, the patient was given a mask and referred immediately to the emergency department across the street with an appropriate hand off for follow up care. COVID-19 undetectable tests were communicated to patients via centralized routing process coordinated by nursing staff. All negative tests were organized by nursing colleagues through a central routing process. Positive cases were also contacted for future plasma donation as part of the multi-site convalescent plasma clinical trial.
A secondary drive-through testing site was established in a small rural community approximately 30 miles from the original site in an attempt to further protect patients and staff in the surrounding rural communities from exposure to suspected cases of COVID-19 which led to an increase in testing volumes. Additionally, on May 1, 2020 the current health system began to re-initiate elective procedures and outpatient visits. Therefore, any patient scheduled for a procedure was initially directed to the drive-through testing site for pre-procedure testing for COVID-19, also contributing to further increases in testing volumes. Later, these pre-procedural tests were collected on site in the hospital. As resources and testing capabilities improved, along with a better understanding of new symptoms of COVID-19 infection, testing criteria was modified and more widespread testing ensued. At the time of article submission, there were a total of 267 confirmed cases in La Crosse County with a total of 8998 total negative test results. Across the entire state of Wisconsin, there have been 24 819 positive and 460 334 negative test results with 3220 hospitalizations and 744 deaths as of June 22, 2020.
Discussion
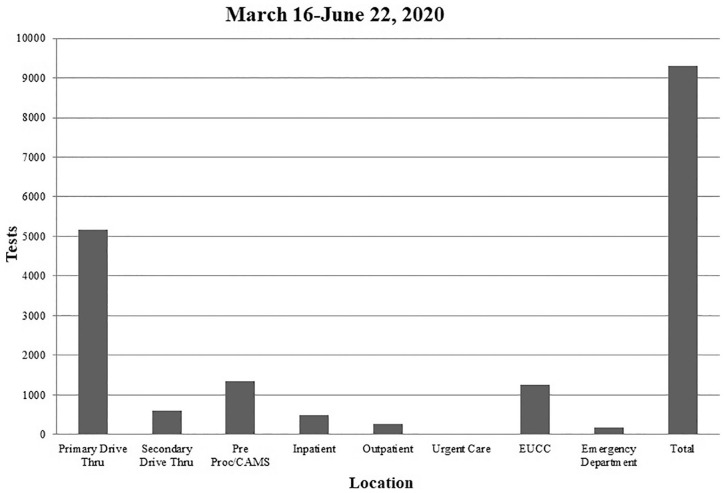
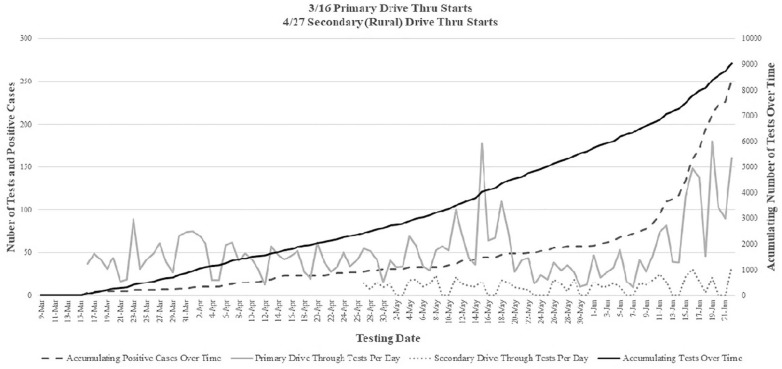
At the onset of the pandemic within the US, representatives nationwide were brought together to discuss, advise and help set up testing recommendations. Administrators have been, and continue to be, involved with the ongoing conversations regarding best practices for screening, treatment and clinical research addressing the COVID-19 pandemic. The implementation of the drive-through screening for COVID-19 discussed in the current manuscript and previously5 required a multidisciplinary coordination of effort from administration, physicians, nursing, security, facilities, IPAC, registration, and information technology. However, it is the opinion of the authors that the rapid installation of the satellite drive-through testing sites and respiratory clinics, helped to reduce the community spread throughout the SWWI region as numbers of confirmed cases have been and continue to be low. By testing patients at remote locations, it helped keep most of the symptomatic COVID-19 carriers out of the primary care facilities, and even some asymptomatic carriers. The drive-through testing site also created an efficient screening system to protect the entire community at large and support the efforts of the local county health department. The majority of the testing performed across the current health system in SWWI region were tested at the primary drive-through site as can be seen in Figures 1 and 2. A contributor to the success of the drive-through testing was the collaboration between the current health system and the local county health department, municipal organizations, and neighboring health systems. Constant lines of communication remained open and daily briefings were provided on updates for patients being tested, the number of confirmed cases and potential community transmission. If high risk of direct exposure was suspected, patients were directed to the drive-through testing site. In 1 instance, it was reported that a local business had 2 confirmed cases which resulted in a widespread testing of the remaining staff and residents, resulting in 1 of the largest single day testing volumes on May 15th as seen in Figure 2. Out of the 230 tests performed, only 2 additional positive cases were found.
Figure 1.
COVID-19 tests collected by location.
Abbreviations: EUCC, emergency room urgent care; Pre Proc/CAMS, pre-procedural screening / center for advanced medicine.
Figure 2.
COVID-19 Total tests completed per day at the primary and secondary drive through testing sites and the total accumulating number of COVID-19 tests completed and positive case counts over time.
The selection of the testing sites provided convenient and centralized testing locations in 2 counties within the SWWI region for rural patient populations to access. Each specific parking lot was also strategically located as both were adjacent to larger hospital facilities which provided convenient solutions for staffing, resources and follow up care if needed. However, although close in proximity to hospital campuses, each drive through site did provide a segregated site for sample collection and created an isolated option for testing that was located away from primary patient care areas in order to minimize the potential risk for COVID-19 exposure. The development of the RT-PCR assay out of the Mayo Clinic was also key in facilitating the rapid turnaround of positive test results. This reduced wait times from 3 to 5 days to a turnaround time of less than 24 hours and in-turn, allowed for the immediate notification of patients to initiate self-quarantine measures and contact tracing procedures to help mitigate community transmission. At the outset of the drive-through testing site implementation, funding and resources were provided solely by the current health system with future reimbursement possible. The implementation of the drive-through testing sites were deemed a top priority of local administration and key resources, including essential personnel, were reallocated to the testing site.
A valuable lesson learned throughout this process was the importance of maintaining open lines of communication with local community partners (ie, civic organizations, municipalities, county health departments, etc.) to provide up-to-date information and to provide a unified effort to provide optimal care to community members. Another important lesson was the importance of bringing a multidisciplinary team together as representation across each division was essential and provided alternative points of view to function as a team and optimize an efficient work flow. This also required constant communication and daily meetings, which allowed for rapid reactions, the identification of short-comings and problem solving. An initial challenge of the drive-through testing was screening patients without a preexisting relationship with the current health system. Additional steps were required, often on-site, to get the patient registered and enter the prerequisite information. Further, efforts were also made to identify these patients prior to testing, with an emphasis placed on the importance of pre-registering via COVID-19 triage line. In addition, an internal email inbox was created for staff to create a patient identification number in advance of testing with the appropriate patient demographics. It also proved to be a challenge to report results and any needed follow-up care for patients outside of the health system as their primary physician was in another health system. As a result, emphasis was placed on getting patients to download and use a customized portal web-based application tool in order to obtain results in a timely fashion. Another challenge was the development of an efficient process for providing patients with proper return to work paperwork following screening and if any self-quarantine measures were deemed necessary. It was difficult for staff on-site to provide the appropriate documentation to be sent to the employer of the patient. This process was eventually deferred to the county health department and in conjunction with neighboring health care systems, a custom algorithmic tool was developed to define appropriate length of quarantine measures and any additional precautionary measures for self-care. The lessons learned and challenges resulting from the current drive-through testing site development mirror those reported from other health care systems tasked with developing an efficient model for widespread community testing in larger population areas and in different patient population sub-groups.5,6 Specifically, open lines of communication across departments and administrators in addition to being flexible while adapting to new challenges seem to be common themes. The use of drive-through testing is 1 of several strategies being utilized across the world to efficiently track trends in infection rates. Other such strategies such as population surveys,7 artificial intelligence,8 internet search trends9 and wearable technologies,10 while useful in monitoring large trends across specific regions, lack the specificity of in-person testing for confirmed positive case counts and do not provide the ability for contact tracing measures to be employed locally. The global efforts against the COVID-19 pandemic will likely require an assortment of various testing, tracing and predictive modeling strategies to effectively contain and mitigate largescale community transmission.
The drive-through testing sites continue to be active screening centers with the anticipation of increasing cases since the recent termination of the stay-at-home order across the state and as businesses begin to open back up. As a result, the recent increases in testing and confirmed positive cases seen in Figure 2 are likely an indication of increasing community transmission. Further, as patient volumes and elective procedures continue to ramp up, there is an increasing need for pre-procedural screenings. As can be seen in Figure 2, both of these changes have resulted in continued increases in the amount of daily testing performed at the drive-through testing site with the number of total tests performed continuing to increase over time (Figure 2). To help accommodate the continued need for testing resources, a labor pool was generated to allow administrators to select key staff members to be relocated for testing purposes. An emphasis was placed on keeping similar staffing groups together to help enhance work efficiency and flow. Initial challenges in securing appropriate testing kits and PPE eventually subsided as equipment inventories across the health system were centralized to testing sites and other areas of need. It is the opinion of the authors that the implementation of the drive through testing site, in collaboration with the local county health department’s continued efforts regarding increased awareness and recommendations, played an integral role in keeping the number of confirmed positive cases low as seen in Figure 2 with a total of 251 positive cases detected at the various testing sites. This makes up a high percentage of the total positive cases across the entire county (267) at the time of article submission.
Conclusion
The rapid implementation of satellite drive-through testing sites are feasible with the coordination of efforts through multiple departments and local community partners. At the time of article submission, although no local stay at home orders or travel restrictions are currently in place it continues to be a rapidly evolving situation and local administrators are monitoring positive case numbers in the event of a second wave; warranting the need for further testing measures. This rapid communication serves as a model of care and template of implementation across all rural health system sites. Further work is needed to address best practices regarding widespread testing for vulnerable populations, pediatric populations and disabled individuals who make not be able to access drive-through testing sites.
Acknowledgments
The authors would like to thank all members of the nursing staff, information technology, facilities, registration, laboratory services and infection prevention and control for their efforts in implementing a drive through testing site. The authors would also like to thank members of the local county health department for their collaborative efforts in helping to combat the COVID-19 pandemic locally.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Andrew R. Jagim  https://orcid.org/0000-0002-6651-5096
https://orcid.org/0000-0002-6651-5096
References
- 1. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020;80:607-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walensky RP, Del Rio C. From mitigation to containment of the COVID-19 pandemic: putting the SARS-CoV-2 genie back in the bottle. JAMA. 2020;323:1889-1890. [DOI] [PubMed] [Google Scholar]
- 4. Gupta P. Why is SARS-CoV-2 testing not possible in every medical laboratory? Indian J Pathol Microbiol. 2020;63:173-174. [DOI] [PubMed] [Google Scholar]
- 5. Shah A, Challener, Douglas, et al. Drive-through testing: a unique, efficient method of collecting large volume of specimens during the SARS-CoV-2 (COVID-19) pandemic. Mayo Clin Proc. 2020;95:1420-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flynn EF, Kuhn E, Shaik M, Tarr E, Scattolini N, Ballantine A. Drive-through COVID-19 testing during the 2020 pandemic: a safe, efficient, and scalable model for pediatric patients and health care workers [published online May 28, 2020]. Acad Pediatr. doi: 10.1016/j.acap.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rossman H, Keshet A, Shilo S, et al. A framework for identifying regional outbreak and spread of COVID-19 from one-minute population-wide surveys. Nat Med. 2020;26:634-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu D, Clemente L, Poirier C, et al. A machine learning methodology for real-time forecasting of the 2019-2020 COVID-19 outbreak using Internet searches, news alerts, and estimates from mechanistic models. ArXiv. 2020. [Google Scholar]
- 9. Walker A, Hopkins C, Surda P. Use of google trends to investigate loss-of-smell-related searches during the COVID-19 outbreak. Int Forum Allergy Rhinol. 2020;10:839-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sayre M. WVU Rockefeller Neuroscience Institute and Oura Health unveil study to predict the outbreak of COVID-19 in healthcare professionals. West Virginia University Today 2020. https://wvutoday.wvu.edu/stories/2020/04/08/wvu-rockefeller-neuroscience-institute-and-oura-health-unveil-study-to-predict-the-outbreak-of-covid-19-in-healthcare-professionals. Accessed July 14, 2020.