Abstract
Objective:
Restless legs syndrome (RLS) is a sensorimotor disorder with alterations in somatosensory processing in association with a dysfunctional cerebral network, involving the basal ganglia, limbic network, and sensorimotor pathways. Resting state functional magnetic resonance imaging (MRI) is a powerful tool to provide in vivo insight into functional processing and as such is of special interest in RLS considering the widespread pattern of networks involved in this disorder. In this meta-analysis of resting state functional MRI studies, we analyzed the preponderance of functional connectivity changes associated with RLS and discussed possible links to sensorimotor dysfunction and somatosensory processing.
Methods:
A systematic research using the online library PubMed was conducted and a total of seven studies passed the inclusion criteria of the meta-analysis. The results of these studies were merged and a statistical probability map was generated that indicated the likelihood of functional connectivity changes within the combined cohort, both for increased and decreased connectivity.
Results:
The meta-analysis demonstrated decreased functional connectivity within the dopaminergic network in participants with RLS compared with healthy controls, including the nigrostriatal, mesolimbic, and mesocortical pathways. Increased functional connectivity was observed bilaterally in the thalamus, including its ventral lateral, ventral anterior, and ventral posterior lateral nuclei, and the pulvinar.
Discussion:
Sensorimotor dysfunction in RLS seems to be reflected by decreased functional connectivity within the dopaminergic pathways. Network extension in the thalamus can be regarded as an adaptation to somatosensory dysfunction in RLS. This differential functional connectivity pattern extends prior findings on cerebral somatosensory processing in RLS and offers an explanation for the efficacy of dopaminergic treatment.
Keywords: magnetic resonance imaging (MRI), networks, restless legs syndrome, resting state, thalamus
Introduction
Restless legs syndrome (RLS, Willis–Ekbom disease) is a common neurological movement and sleep disorder, with a prevalence of up to 5% in the adult general population.1 RLS is defined by clinical diagnostic criteria, that is, the urge to move the legs, predominantly during episodes of rest and in the evening, which is accompanied by discomforting sensations and alleviated by moving the legs.2,3 Idiopathic RLS is often described as a sensorimotor disorder, given that both sensory and motor circuits and their interplay in sensorimotor integration are under descending control from monoaminergic clusters in the brain.4 Altered dopaminergic modulation of neuronal excitability is generally thought to be one main underlying pathophysiological mechanism of RLS.5 Neuroimaging studies in RLS indicate a disease-specific dysfunctional cerebral network, involving the basal ganglia, the limbic network, and the sensorimotor system.6,7 Abnormal iron metabolism in the brain is believed to cause dopaminergic dysfunction in the mesolimbic and nigrostriatal pathways, which in turn could cause the sensorimotor network dysfunction.8–11 At network level, dopaminergic, glutamatergic, GABA-mediated, and adenosinergic dysfunctions are considered to be associated with subsequent cortical hyperexcitability or disinhibition. Thus, RLS might be regarded as a multitransmitter neurochemical disorder, globally with enhanced excitability and decreased inhibition.12 In this context, electrophysiological data suggest that in RLS, both cortical, subcortical, spinal cord, and peripheral nerve generators are involved in the network disorder, resulting in an enhanced excitability and/or decreased inhibition.13
Resting state functional magnetic resonance imaging (rs-fMRI) is a powerful tool to provide in vivo insight into functional processing in a task-free condition, beyond the structural level.14,15 Prior rs-fMRI studies indicated that patients with RLS may have deficits in controlling and managing sensory information, supporting the hypothesis that RLS could be a disorder of somatosensory processing.7 Given the broad array of networks that exhibit functional connectivity changes in RLS, we asked where the preponderance of changes is located and if it is specifically associated with somatosensory processing. To this end, we conducted a meta-analysis including all available data on idiopathic RLS and rs-fMRI.
Methods
Search strategy and study selection
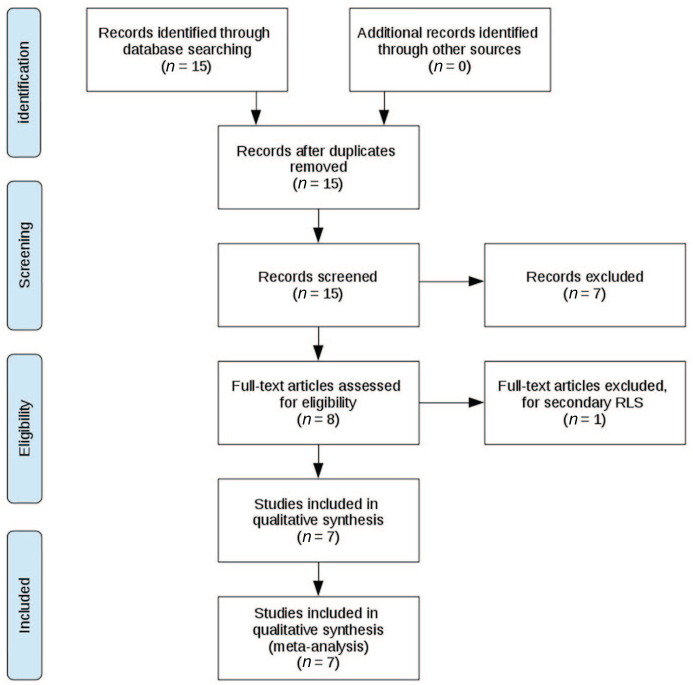
The literature review and study inclusion process was conducted in accordance with the PRISMA guidelines.16 In a systematic search conducted in February 2020, data were collected from the online library PubMed (https://www.ncbi.nlm.nih.gov/pubmed/). The search queries and keywords were (a) “resting state” AND “restless legs”, (b) “resting state” AND “RLS”, (c) “resting-state” AND “RLS”, and (d) “resting-state” AND “restless legs”. All studies up until February 2020 were included. In total, this search yielded 15 results. These 15 studies were probed for the following criteria: the studies had to be published in the English language in peer-reviewed journals; only human studies that used rs-fMRI including a whole-brain analysis were included; both participants with RLS and a control group of similar size like the RLS group, matched for age and gender, had to be present; only studies were included for which it was stated that the participants met diagnostic criteria according to common diagnostic guidelines for RLS;2 only studies on idiopathic RLS were included. Patients had to be either drug-naïve or asked to stop medication before the trial, that is, cessation of medication had to be performed prior to the study for at least 3-fold its biological half-life. From the 15 studies in question, only 8 conducted rs-fMRI with either seed-based or independent component analysis, and only these studies were included since other methods were not considered comparable. From these eight studies, one was excluded due to secondary RLS as the condition under investigation, as opposed to idiopathic RLS. In one study, both treated and drug-naïve participants were present.17 Here, only the analyses of the drug-naïve subjects were included. The references in each of the 15 studies were carefully studied for further candidate studies, but this cross-reference search did not yield additional inclusions. In total, seven studies were included in the meta-analysis. For a summary of the literature review and study inclusion process, see Figure 1.
Figure 1.
PRISMA flow diagram depicting the literature review, exclusion and inclusion process.
This analysis is a meta-analysis without acquisition of new data. All contributing studies reported that they were conducted in human subjects according to the current World Medical Association Declaration of Helsinki.
Imaging data processing
The seven included studies were screened for significant differences in functional connectivity between participants with idiopathic RLS and healthy controls.17–23 For each study, the number of contributing subjects was noted for later statistical weighting to prevent bias from small studies. Only clusters that were significant after correction for multiple comparisons (at least p < 0.05) were included. In two studies,17,18 no correction for multiple comparisons was reported; here, all clusters that passed an uncorrected significance threshold of p < 0.005 were included, which we considered equivalent. Clusters had to be reported in a common stereotaxic space, that is, Montreal Neurological Institute (MNI) coordinates (n = 7). From each study, all significant clusters were extracted including cluster size, peak MNI coordinates, and associated t-value (or related parameters). Each cluster was then reshaped to a spherical volume around the peak coordinate for the subsequent statistical analysis resulting in a total of 60 clusters for the inclusion into the further analysis.
The Tensor Imaging and Fiber Tracking (TIFT) software package was used for statistical analyses, as described in a previous meta-analysis with diffusion-weighted MRI data.24,25 The statistical process was transferred to the present rs-fMRI data set: each of the 60 coordinates was placed as Gaussian shaped result-spheres with its corresponding size, weighted with the square-root of contributing subjects of the respective study. The signal from overlaying clusters was added, leading to high signal strength in areas in which several clusters converged. In order to spatially homogenize the input information, the resulting 3-D data set was spatially smoothed with an isotropic Gaussian kernel of full-width-at-half-maximum size of 18 mm. This filter size was regarded as a good balance between sensitivity and specificity, as it allowed eliminating small isolated result clusters, while collimating neighboring clusters from different studies. In order to account for this lenient smoothing, we decided to discard voxels with signal strength of less than half of the global maximum. Then, a standard clustering procedure was applied; in order to discard false-positive results, clusters below a cluster size threshold of 512 voxels (mm³) were excluded.26 The entire procedure was done separately for clusters with increased and decreased functional connectivity, respectively.
Results
Qualitative synthesis
Each of the studies included in the meta-analysis used a seed-based approach to resting state fMRI data analysis. Common seeds were the default mode network,20,21 the thalamus,17,18,22 the putamen,23 or a combination of several networks.19 While the integration of these results may seem challenging, a rather coherent picture emerged from the synthesis. Among the areas most affected by functional connectivity changes were the basal ganglia, the frontal lobe, and limbic structures. Each of the following areas was reported in at least two of the analyzed studies: Increased functional connectivity was most prominent in the thalamus, but also found in parahippocampal gyrus and the superior parietal lobe. Decreased functional connectivity was observed in the basal ganglia (most notably the caudate nucleus and the putamen), the cingulate and paracingulate gyrus, the orbito-frontal cortex, as well as the medial and superior frontal gyrus. For a detailed list of the studies and their respective results, see Table 1.
Table 1.
Studies included in the meta-analysis, ordered by time of publication.
| Study | Subjects RLS/controls |
Seed Regions | Functional connectivity | IRLS | |
|---|---|---|---|---|---|
| Increase | Decrease | ||||
| Ku et al.18 | 25/25 | ventral posterior lateral nucleus of the thalamus | right parahippocampal gyrus, right precuneus, right precentral gyrus, left/right lingual gyrus | right superior temporal gyrus, left/right middle temporal gyrus, right medial frontal gyrus | 26 ± 7 |
| Gorges et al.19 | 26/26 | motor/sensorimotor network | − | − | 27 ± 6 |
| sensory thalamic network | left caudate nucleus, right putamen, left/right thalamus, right parahippocampal cortex, | ||||
| ventral attention network | left/right thalamus, right parahippocampal cortex | ||||
| dorsal attention network | right medial prefrontal cortex, right cingulate cortex, right supplementary eye field | ||||
| basal ganglia-thalamic network | left/right medial prefrontal cortex, left caudate nucleus, left putamen, left thalamus, | ||||
| cingulate network | left cingulate cortex | ||||
| brainstem network | − | − | |||
| Ku et al.20 | 16/16 | default Mode Network | right superior parietal lobule, right supplementary motor area, left thalamus | left posterior cingulate cortex, right orbito-frontal gyrus, left precuneus, right subcallosal gyrus | 26 ± 7 |
| Ku et al.21 | 15/15 | default mode network (morning) | Left/right thalamus, right superior parietal lobule | right medial frontal gyrus, left posterior cingulate, left precuneus, right subcallosal gyrus, right parahippocampal gyrus | 26 ± 7 |
| default mode network (evening) | left declive, left cuneus, right middle occipital gyrus | right caudate tail, right caudate head, left anterior mid-cingulate gyrus, left caudate head | |||
| Liu et al.22 | 16/26 | cuneus | left medial frontal gyrus/paracentral lobule | 23 ± 6 | |
| superior frontal gyrus | right medial prefrontal cortex | ||||
| thalamus | left cerebellum posterior lobe, right middle temporal gyrus | ||||
| Li et al.23 | 20/18 | right putamen | cingulate gyrus | 23 ± 7 | |
| left dorsal rostral putamen | left/right putamen, cingulate gyrus | ||||
| left dorsal caudal putamen | left putamen, left frontal pole, right superior frontal gyrus | ||||
| left ventral rostral putamen | left/right putamen, left frontal pole, right paracingulate gyrus | ||||
| right dorsal rostral putamen | right cerebellum | left putamen, right caudate, left frontal pole, right paracingulate gyrus | |||
| right dorsal caudal putamen | left putamen | ||||
| right ventral rostral putamen | left caudate, right putamen, left superior cingulate gyrus | ||||
| Lee et al.17 | 16/16 | ventral posterior lateral nucleus of the thalamus | right temporal gyrus | left/right lingual gyrus | 25 ± 8 |
| Total | 134/142 | ||||
IRLS, international RLS rating scale ± standard deviation, RLS, restless legs syndrome.
Quantitative synthesis
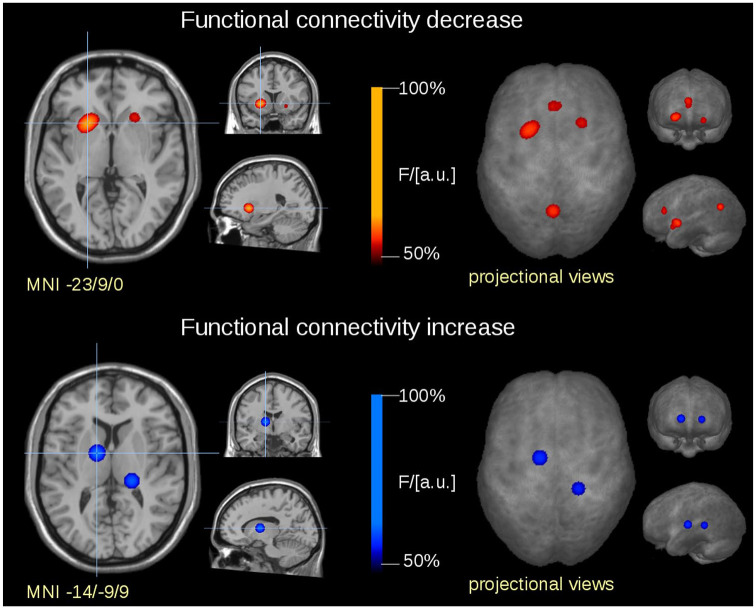
In total, six significant clusters emerged from the analysis. The majority of functional connectivity changes was located in the thalamus and in areas belonging to the dopaminergic pathways, the former showing increased and the latter showing decreased connectivity (Figure 2). Increased functional connectivity in the thalamus involved the ventral lateral, ventral anterior, and ventral posterior lateral nuclei in the right hemisphere and the pulvinar in the left hemisphere. Decreased functional connectivity in the dopaminergic pathways was observed bilaterally in the putamen and the nucleus accumbens, the left caudate head, and the left medial frontal gyrus. In addition to these findings, decreased functional connectivity was observed in the anterior cingulate gyrus and the precuneus. For a detailed list of the results clusters from this meta-analysis, reported separately for increased and decreased connectivity, see Table 2.
Figure 2.
Frequency of occurrence depicted in transversal, coronal, and sagittal slices at the level of the global maximum (left side) and in projectional view (right side). Clusters represent areas which were commonly affected in participants with RLS in this meta-analysis. The heat map indicates frequency of occurrence (F) relative to the global maximum (= 100% F/arbitrary units). Clusters with increased functional connectivity are depicted in the upper panel (warm color map), clusters with decreased functional connectivity are depicted in the lower panel (cold color map).
MNI, Montreal Neurological Institute.
Table 2.
Significant clusters resulting from the meta-analysis (increased and decreased connectivity reported separately, clusters ordered by size). Anatomical brain regions are reported in order of proximity to the peak coordinate, according to the MNI Atlas.
| Increased connectivity | |||
|---|---|---|---|
| Cluster | Cluster size (mm³) | Peak MNI coordinate (X, Y, Z) | Anatomical brain regions |
| #1 | 2523 | (–14, –9, 9) | right: ventral lateral, ventral anterior, and ventral posterior lateral nucleus of the thalamus. lateral globus pallidus, putamen |
| #2 | 1922 | (20, –36, 8) | left: thalamus: pulvinar |
| Decreased connectivity | |||
| Cluster | Cluster size (mm³) | Peak MNI coordinate (X, Y, Z) | Anatomical brain regions |
| #1 | 3722 | (–23, 9, 0) | right: putamen, caudate head, nucleus accumbens |
| #2 | 2541 | (–1, 30, 19) | left/right: anterior cingulate gyrus (BA 24, 32), right: medial frontal gyrus (BA 6, 9) |
| #3 | 2019 | (–2, –62, 26) | left/right: cingulate gyrus (BA 23, 31), precuneus (BA 7, 31) |
| #4 | 1626 | (23, 15, –6) | left: putamen, ncl. accumbens, subcallosal gyrus (BA 47) |
MNI, Montreal Neurological Institute; BA, Brodmann area.
Discussion
This rs-fMRI meta-analysis demonstrated differential functional connectivity in thalamic and dopaminergic pathways in participants with RLS compared with controls, with increased connectivity in the thalamus and decreased connectivity in the dopaminergic pathways. Within the dopaminergic system, all of the major components, that is, nigrostriatal, mesolimbic, and mesocortical pathways, were shown to be altered.
Previous rs-fMRI studies in RLS reported connectivity changes in brain regions related to the thalamus, indicating that the thalamic regions may be associated with altered sensory monitoring and perception processing in RLS.18 The thalamus was previously observed to show functional connectivity changes within its associated sensory thalamic circuits.19 In addition, diurnal functional connectivity changes in the thalamus were reported, from hyperconnectivity in the morning to hypoconnectivity in the evening, reflecting the circadian presentation of RLS symptomatology.21 With respect to these pathoanatomical concepts, increased functional connectivity in the current meta-analysis was indeed observed bilaterally in the thalamus, including its anterior, ventral lateral, ventral anterior, and ventral posterior lateral nuclei in the left hemisphere and the pulvinar in the right hemisphere. These results are in general accordance with prior interpretations of rs-fMRI studies that RLS patients may have deficits in controlling and managing sensory information.27
The finding of decreased functional connectivity within the dopaminergic system adds new aspects to this concept of RLS-associated alterations of somatosensory processing. Our results are supported by recent studies that emphasized the importance of the putamen in the pathophysiology of RLS. In a morphometric analysis of subcortical gray matter, Li and colleagues found abnormalities in the putamen that correlated with disease duration of RLS.23 In addition, diurnal changes that reflect circadian characteristics of RLS were not only reported to occur in the thalamus, but also in the striatum.21 Interestingly, our meta-analytic findings were not confined to the nigrostriatal pathway, but also included functional connectivity alterations in the mesolimbic and mesocortical pathways. This constellation can be regarded as a representation of a distributed dysfunction of dopamine metabolism in association with RLS as it was previously shown by positron emission tomography analyses, which indicated dysfunction not only the nigrostriatal but also mesolimbic pathways.6,7,9,10 The broad spectrum of RLS symptomatology could relate to each of these pathways, that is, motor symptoms to the nigrostriatal pathway and discomfort and pain to the mesolimbic/limbic system.2,3 A recent rs-fMRI study demonstrated specific effects of dopaminergic treatment in the mesocortical pathways: when compared with drug-naïve RLS patients, dopamine intake increased functional connectivity in the mesocortical pathway.17 It is possible that this increase might compensate for otherwise insufficient mesocortical functional connectivity, offering an explanation for its therapeutic effect. In general, this finding is not at odds with pathophysiological concepts of RLS as a hyperdopaminergic state,3 given that RLS seems to constitute a condition with cerebral dopamine dysfunction.
Cerebral networks involved in pain bear a striking resemblance to the functional connectivity changes observed in this meta-analysis. Sensory input from the spinal cord to both thalamic and limbic structures is considered to convey affective information related to nociceptive sensations. Together with the sensory information from thalamocortical pathways, these pathways converge in the anterior cingulate, where the information is integrated.28 Given that pain is among the most commonly reported symptoms of RLS, both altered thalamic functional connectivity and decreased connectivity in the limbic system, specifically the anterior cingulate gyrus, may reflect involvement of the nociceptive system in RLS symptomatology.29,30 The multiple neurotransmission dysfunction may also constitute a link between RLS and other chronic pain disorders.12
The current meta-analysis was not without limitations. A relatively low number of seven studies (with a total of 134 participants with RLS) that met the inclusion criteria could be included, indicating a limited application of the rs-fMRI approach to RLS yet. In general, the number of advanced MRI studies in this common disorder is rather limited, perhaps due to the patients’ restlessness and inability to lie down in the scanner without moving. All of the studies included in this meta-analysis followed a seed-based approach to rs-fMRI; this, however, might have biased the analysis as it is possible that functional connectivity changes within networks that are not covered by a specific seed are not registered. As a further limitation, no weighting and no correlation analyses were performed between regional connectivity strength and measures of disease severity (e.g. international RLS rating scale), respectively, given that the mean scores were almost identical in the contributing studies with relatively high standard deviations (Table 1). The functional connectivity changes observed in association with RLS might be attributed to structural abnormalities. A well-accepted hypothesis is that abnormal iron metabolism in the putamen is associated with dopaminergic and sensorimotor dysfunction in RLS.6,7,27 Decreased functional connectivity within the dopaminergic pathways as found in this meta-analysis could be interpreted as a result of these deficits. In addition, macro- and microstructural abnormalities in the spinothalamic fibers, the brainstem, the midbrain, and the thalamus are considered to provoke functional and metabolic changes within the sensorimotor system and specifically the thalamus.3,5,31–33 Increased functional connectivity in the thalamus could thus be regarded as an adaptive consequence to compensate for these structural deficits.34 Given that both the thalamus and the dopaminergic pathways are interwoven within the sensorimotor system, such a combination of these pathoanatomical concepts could explain somatosensory processing deficits and sensorimotor dysfunction in RLS. A straightforward integration of our results in current clinical neurophysiology data in RLS is possible; neurophysiological studies using transcranial magnetic stimulation (TMS) reported altered motor cortex excitability and dysfunctional sensorimotor integration in RLS.35 In addition, RLS patients exhibited an impairment of the long-term depression-like mechanisms induced by inhibitory repetitive TMS compared with healthy subjects, indicating abnormal cortical plasticity.36 Modification of these networks via repetitive TMS-mediated modulation of cortical excitability within the sensorimotor network has shown to alleviate the sensorimotor symptoms in RLS.37 These findings support the abnormal somatosensory processing function and the altered functional connectivity observed in RLS.
However, it remains yet unresolved how the functional and structural brain abnormalities in RLS relate to each other. As a perspective, a future longitudinal study with rs-fMRI, perhaps together with structural imaging modalities like diffusion tensor imaging, might demonstrate how these abnormalities develop over time and how they relate to the course of symptom severity. An ‘ideal’ candidate study should monitor iron content within the brain, micro- and macrostructural changes, as well as rs-fMRI in newly diagnosed RLS over an interval of several years and correlate the MRI data with clinical and neuropsychological scores. In addition, a longitudinal observation of the effects of pharmaceutical treatment could help explain efficacy and long-term side effects, such as augmentation. This way it might be possible to further unravel the relationship between structural, functional, and clinical findings in RLS.
In conclusion, the current meta-analysis of rs-MRI data demonstrated differential functional connectivity in thalamic and dopaminergic pathways in participants with RLS compared with controls, with decreased connectivity in the dopaminergic system and increased connectivity in the thalamus. Increased functional connectivity in the thalamus can be interpreted as network extension, adaptive to somatosensory dysfunction. Decreased functional connectivity in the dopaminergic system could reflect sensorimotor dysfunction. The broad affection of the dopaminergic system (nigrostriatal, mesolimbic, and mesocortical pathways) may partially explain RLS symptomatology, including discomfort, sleep, and motor symptoms. The present rs-fMRI meta-analysis supports the hypothesis of somatosensory processing deficits in RLS and contributes to the framework around RLS pathophysiology and the efficacy of dopaminergic treatment. In highlighting the importance of sensorimotor integration in RLS, our data also support other pharmacological and non-pharmacological treatments,3,38 including non-invasive neuromodulation.37 By this contribution to the understanding of the pathophysiology, this meta-analysis might assist in the development of new treatment options in RLS that will specifically target the dysfunction of somatosensory processing.
Footnotes
Author contributions: TDK: design of the study, drafting the article, analysis and interpretation of data, approved the version to be published.
HPM: analysis and interpretation of data, critical revision of the article, approved the version to be published.
JK: design of the study and interpretation of data, critical revision for important intellectual content, approved the version to be published.
All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jan Kassubek  https://orcid.org/0000-0002-7106-9270
https://orcid.org/0000-0002-7106-9270
Contributor Information
Thomas Derya Kocar, Department of Neurology, University of Ulm, Ulm, Germany.
Hans-Peter Müller, Department of Neurology, University of Ulm, Ulm, Germany.
Jan Kassubek, Department of Neurology, University of Ulm, Oberer Eselsberg 45, Ulm, 89081, Germany.
References
- 1. Trenkwalder C, Winkelmann J, Inoue Y, et al. Restless legs syndrome-current therapies and management of augmentation. Nat Rev Neurol 2015; 11: 434–445. [DOI] [PubMed] [Google Scholar]
- 2. Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated international restless legs syndrome study group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med 2014; 15: 860–873. [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez-Latapi P, Malkani R. Update on restless legs syndrome: from mechanisms to treatment. Curr Neurol Neurosci Rep 2019; 19: 54. [DOI] [PubMed] [Google Scholar]
- 4. Trenkwalder C, Allen R, Högl B, et al. Comorbidities, treatment, and pathophysiology in restless legs syndrome. Lancet Neurol 2018; 17: 994–1005. [DOI] [PubMed] [Google Scholar]
- 5. Trenkwalder C, Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol 2010; 6: 337–346. [DOI] [PubMed] [Google Scholar]
- 6. Rizzo G, Tonon C, Manners D, et al. Imaging brain functional and metabolic changes in restless legs syndrome. Curr Neurol Neurosci Rep 2013; 13: 372. [DOI] [PubMed] [Google Scholar]
- 7. Rizzo G, Li X, Galantucci S, et al. Brain imaging and networks in restless legs syndrome. Sleep Med 2017; 31: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Godau J, Klose U, Di Santo A, et al. Multiregional brain iron deficiency in restless legs syndrome. Mov Disord 2008; 23: 1184–1187. [DOI] [PubMed] [Google Scholar]
- 9. Oboshi Y, Ouchi Y, Yagi S, et al. In vivo mesolimbic D2/3 receptor binding predicts posttherapeutic clinical responses in restless legs syndrome: a positron emission tomography study. J Cereb Blood Flow Metab 2012; 32: 654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Earley CJ, Kuwabara H, Wong DF, et al. Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep 2013; 36: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Margariti PN, Astrakas LG, Tsouli SG, et al. Investigation of unmedicated early onset restless legs syndrome by voxel-based morphometry, T2 relaxometry, and functional MR imaging during the night-time hours. AJNR Am J Neuroradiol 2012; 33: 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lanza G, Ferri A. The neurophysiology of hyperarousal in restless legs syndrome: hints for a role of glutamate/GABA. Adv Pharmacol 2019; 84: 101–119. [DOI] [PubMed] [Google Scholar]
- 13. Lanza G, Bachmann CG, Ghorayeb I, et al. Central and peripheral nervous system excitability in restless legs syndrome. Sleep Med 2017; 31: 49–60. [DOI] [PubMed] [Google Scholar]
- 14. Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A 2009; 106: 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol 2010; 20: 519–534. [DOI] [PubMed] [Google Scholar]
- 16. Panic N, Leoncini E, de Belvis G, et al. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One 2013; 8: e83138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee YS, Ku J, Kim KT, et al. Resting-state connectivity and the effects of treatment in restless legs syndrome. Sleep Med 2020; 67: 33–38. [DOI] [PubMed] [Google Scholar]
- 18. Ku J, Cho YW, Lee YS, et al. Functional connectivity alternation of the thalamus in restless legs syndrome patients during the asymptomatic period: a resting-state connectivity study using functional magnetic resonance imaging. Sleep Med 2014; 15: 289–294. [DOI] [PubMed] [Google Scholar]
- 19. Gorges M, Rosskopf J, Müller HP, et al. Patterns of increased intrinsic functional connectivity in patients with restless legs syndrome are associated with attentional control of sensory inputs. Neurosci Lett 2016; 617: 264–269. [DOI] [PubMed] [Google Scholar]
- 20. Ku J, Lee YS, Chang H, et al. Default mode network disturbances in restless legs syndrome/Willis-Ekbom disease. Sleep Med 2016; 23: 6–11. [DOI] [PubMed] [Google Scholar]
- 21. Ku J, Lee YS, Chang HW, et al. Diurnal variation of default mode network in patients with restless legs syndrome. Sleep Med 2018; 41: 1–8. [DOI] [PubMed] [Google Scholar]
- 22. Liu C, Wang J, Hou Y, et al. Mapping the changed hubs and corresponding functional connectivity in idiopathic restless legs syndrome. Sleep Med 2018; 45: 132–139. [DOI] [PubMed] [Google Scholar]
- 23. Li T, Liu C, Lyu H, et al. Alterations of sub-cortical gray matter volume and their associations with disease duration in patients with restless legs syndrome. Front Neurol 2018; 9: 1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Müller HP, Unrath A, Ludolph AC, et al. Preservation of diffusion tensor properties during spatial normalization by use of tensor imaging and fibre tracking on a normal brain database. Phys Med Biol 2007; 52: N99–N109. [DOI] [PubMed] [Google Scholar]
- 25. Gorges M, Del Tredici K, Dreyhaupt J, et al. Corticoefferent pathology distribution in amyotrophic lateral sclerosis: in vivo evidence from a meta-analysis of diffusion tensor imaging data. Sci Rep 2018; 8: 15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Müller HP, Gorges M, Grön G, et al. Motor network structure and function are associated with motor performance in Huntington’s disease. J Neurol. 2016; 263: 539–549. [DOI] [PubMed] [Google Scholar]
- 27. Rizzo G, Plazzi G. Neuroimaging applications in restless legs syndrome. Int Rev Neurobiol 2018; 143: 31–64. [DOI] [PubMed] [Google Scholar]
- 28. Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 2000; 288: 1769–1772. [DOI] [PubMed] [Google Scholar]
- 29. Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med 2005; 165: 1286–1292. [DOI] [PubMed] [Google Scholar]
- 30. Karroum EG, Golmard JL, Leu-Semenescu S, et al. Sensations in restless legs syndrome. Sleep Med 2012; 13: 402–408. [DOI] [PubMed] [Google Scholar]
- 31. Lindemann K, Müller HP, Ludolph AC, et al. Microstructure of the midbrain and cervical spinal cord in idiopathic restless legs syndrome: a diffusion tensor imaging study. Sleep 2016; 39: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Etgen T, Draganski B, Ilg C, et al. Bilateral thalamic gray matter changes in patients with restless legs syndrome. Neuroimage 2005; 24: 1242–1247. [DOI] [PubMed] [Google Scholar]
- 33. Rizzo G, Tonon C, Testa C, et al. Abnormal medial thalamic metabolism in patients with idiopathic restless legs syndrome. Brain 2012; 135: 3712–3720. [DOI] [PubMed] [Google Scholar]
- 34. Hillary FG, Grafman JH. Injured brains and adaptive networks: the benefits and costs of hyperconnectivity. Trends Cogn Sci 2017; 21: 385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lanza G, Cantone M, Lanuzza B, et al. Distinctive patterns of cortical excitability to transcranial magnetic stimulation in obstructive sleep apnea syndrome, restless legs syndrome, insomnia, and sleep deprivation. Sleep Med Rev 2015; 19: 39–50. [DOI] [PubMed] [Google Scholar]
- 36. Lanza G, Lanuzza B, Aricó B, et al. Impaired short-term plasticity in restless legs syndrome: a pilot rTMS study. Sleep Med 2018; 46: 1–4. [DOI] [PubMed] [Google Scholar]
- 37. Lanza G, Cantone M, Aricó B, et al. Clinical and electrophysiological impact of repetitive low-frequency transcranial magnetic stimulation on the sensory-motor network in patients with restless legs syndrome. Ther Adv Neurol Disord 2018; 11: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anguelova GV, Vlak MHM, Kurvers AGY, et al. Pharmacologic and nonpharmacologic treatment of restless legs syndrome. Sleep Med Clin 2020; 15: 277–288. [DOI] [PubMed] [Google Scholar]