Abstract
As liver biopsy in children poses inherent risks, noninvasive measures of liver fibrosis are needed. This was a cross-sectional, liver biopsy validation pilot study of 16 participants evaluating the ability of shear wave elastography, aspartate transaminase to platelet ratio index (APRI), fibrosis index based on the 4 factors, and novel serum biomarkers to stage liver fibrosis in children with chronic hepatitis B or C. There was very high intrasegmental shear wave speed variation in our participants and little correlation with fibrosis. APRI and monocyte chemoattractant protein (MCP-1) were higher in fibrosis stage F2-3 versus F0-1 (P = .02, P = .06, respectively). Soluble Fas (sFas) was lower in F2-3 versus F0-1 (P = .046). A logistic regression analysis calculated by (APRI × MCP-1)/sFas demonstrated an area under the receiver operating characteristic curve of 0.92 (P < .001), suggesting that this combination can differentiate fibrosis stage F0-1 from F2-3 in children with chronic viral hepatitis.
Keywords: Shear wave elastography, APRI, pediatric chronic viral hepatitis, hepatitis B, hepatitis C, liver stiffness, MCP-1, sFas
Introduction
Hepatitis B virus (HBV) and hepatitis C virus (HCV) continue to have a significant global impact, infecting approximately 400 million and 170 million people worldwide, respectively.1-3 Both lead to chronic liver disease and cirrhosis, accounting for 80% of hepatocellular carcinoma worldwide with high mortality rates.4-6 At higher rates than adults, 90% and 60% of infants infected with HBV and HCV, respectively, will develop chronic infection.3,7 Unfortunately, most children remain asymptomatic with minimal to no abnormal clinical and laboratory findings even with advanced fibrosis, making it difficult to monitor disease progression. In addition, children are at higher risk for progression to cirrhosis and hepatocellular carcinoma given their long exposure to these viruses.3,8,9 Close monitoring of fibrosis progression in children with viral hepatitis is important for implementing early therapeutic interventions to slow progression or reverse fibrosis with antiviral therapy.10,11 Liver biopsy has long been the gold standard for staging liver fibrosis in children, but is an invasive and costly procedure with limitations and risks including sampling error, interobserver variability, pain, hemorrhage, anesthesia complications, and infection.12-15
Real-time shear wave elastography (SWE) is a readily available noninvasive ultrasound technique to evaluate the elasticity of tissue measured in kilopascals or meters/second (m/s), though less well studied in children. SWE has been studied in adult populations with chronic HBV and HCV, showing potential to replace liver biopsy as the gold standard for staging liver fibrosis, but there is limited biopsy-validated data in pediatric populations with chronic viral hepatitis.16-18
Noninvasive fibrosis scores including aspartate transaminase to platelet ratio index (APRI) and fibrosis index based on the 4 factors (FIB-4) utilize a combination of standard of care laboratory tests and have been studied extensively in both adults and children with good accuracy in staging liver fibrosis.19-26 Furthermore, many novel serum biomarkers are involved in extracellular matrix formation or degradation, inflammation, or hepatocellular damage. Studies of these biomarkers in pediatric patients with biliary atresia, nonalcoholic fatty liver disease, cystic fibrosis–associated liver disease, and adults with chronic viral hepatitis show promise for accurately staging liver fibrosis.27-43 It is unclear if these markers are age-dependent or affected by growth. In this study, we evaluate the utility of 3 noninvasive modalities to stage liver fibrosis in children with chronic viral hepatitis validated by liver biopsy using SWE, fibrosis scores, and a panel of 10 novel serum biomarkers.
Methods
Study Population
In this single-center, prospective study, participants were enrolled based on the following inclusion criteria: 2 to 17 years of age; diagnosis of chronic HBV or HCV based on the Centers for Disease Control and Prevention, American Association for the Study of Liver Diseases, or North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition guidelines; elevated transaminases for >6 months; and planned, clinically indicated liver biopsy. Exclusion criteria included the following: known concomitant fibrosing liver disease (ie, primary sclerosing cholangitis, autoimmune, metabolic, biliary atresia, or other hepatic infection) and/or any medical contraindication to sedation or anesthesia.
Serum was collected and SWE was performed ±7 days of liver biopsy. Each biopsy was staged for fibrosis using the Metavir classification (F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = portal fibrosis with few septa, F3 = numerous septa without cirrhosis, and F4 = cirrhosis).44 SWE was performed by 2 experienced sonographers trained in SWE using an iU22 ElastPQ (Philips Healthcare) with the C5-1 PureWave transducer. All patients were fasted overnight and studies were performed in a supine position with the right arm elevated. Images were obtained after a small breath hold when feasible and with free breathing in younger patients. Scans were obtained using an intercostal approach, with sampling greater than 1 cm from the liver surface and away from major vessels. The standard machine region of interest (ROI) was used. At least 2 measurements were obtained from each of segments 5 to 8 of the right lobe of the liver. Images were interpreted by a single, blinded senior radiologist.
Using Young’s modulus, a liver stiffness measurement (LSM) in kilopascals can be calculated using SWE generated shear waves and measuring the shear wave speed (SWS) in meters/second through the liver.45 There is a direct correlation between SWS and LSM. Using an aspartate transaminase (AST) upper limit of normal (ULN) of 40 U/L, APRI was calculated (AST/AST ULN)/platelet count and FIB-4 was calculated (age × AST)/(platelet count × √ALT). The serum biomarker panel included tissue inhibitor of metalloproteinase 1 (TIMP-1), prolyl hydroxylase (PH), hyaluronic acid (HA), monocyte chemoattractant protein 1 (MCP-1), collagen IV, interleukin 8 (IL-8), platelet-derived growth factor (PDGF-BB), soluble Fas (sFas), soluble intercellular adhesion molecule (sICAM), and lysyl hydroxylase (LH) assayed via sandwich enzyme linked immunoassays.
Data and Statistical Analysis
Data was grouped by liver biopsy fibrosis stage F0-1 versus F2-3 for analysis. Wilcoxon rank sum tests were used for comparing continuous variables between the 2 cohorts. Fisher’s exact test was used to compare nominal variables. A multivariable logistic regression analysis and receiver operating characteristic (ROC) curve analysis were performed for APRI, sFas, and MCP-1 to predict liver fibrosis stage F2-3. The area under the ROC (AUROC) curves was estimated with 95% confidence intervals.
Ethical Approval and Informed Consent
This study was approved by the Baylor College of Medicine Institutional Review Board (approval protocol number H-30472). Written informed consent was obtained prior to enrollment in the study from parents/legal guardians and assent from children of appropriate age. Consent was obtained for the addition of SWE to routine liver ultrasound, collection of extra blood for serum biomarkers at the time of routine blood sample collection, and use of aggregated data (demographics, clinical characteristics, SWE, biopsy, and laboratory values) in this research study.
Results
A total of 17 participants were screened, and all 17 were enrolled in the study although one was later excluded due to lack of data collection. Data from 16 participants were used in the analysis. The median age of the 16 study participants was 12.6 years, and 62.5% (n = 10) were female (Table 1). Fifty percent of participants with a liver biopsy had stage 2 fibrosis and none had stage 4 fibrosis (F0 = 4, F1 = 2, F2 = 8, and F3 = 2). Thirteen (13/15; 86.7%) participants had a gray scale ultrasound score of 1 (heterogeneous echogenicity). Participants with F2-3 fibrosis tended to be younger than those with F0-1 (9 years vs 13 years, P = .64).
Table 1.
Clinical Characteristics of Participants.
| Clinical characteristics | All fibrosis stages (min, max) | F0-1 (min, max) | F2-3 (min, max) |
|---|---|---|---|
| N | 16 | 6 | 10 |
| Median age at enrollment, years | 12.6 (3.4, 17.9) | 13.0 (4.4, 17.1) | 8.9 (3.4, 17.9) |
| Median BMI, kg/m2 | 18.4 (14.6, 22.5) | 19.2 (14.6, 21.2) | 18.1 (15.4, 22.5) |
| Median BMI Z score | 0.1 (−0.9, 2.9) | 0 (−0.9, 2.9) | 0.6 (−0.9, 2.0) |
| Female, n (%) | 10 (62.5) | 3 (50) | 7 (70) |
| Hepatitis B, n (%) | 9 (56.3) | 5 (83.3) | 4 (40) |
| Hepatitis C, n (%) | 7 (43.8) | 1 (16.7) | 6 (60) |
| Ethnicity, n (%) | |||
| Asian | 10 (62.5) | 5 (83.3) | 5 (50) |
| White | 5 (31.3) | 1 (16.7) | 4 (40) |
| Other | 1 (6.3) | 0 (0) | 1 (10) |
Abbreviation: BMI, body mass index.
There was poor reproducibility of SWE within segments, and no significant difference in the SWS between F0-1 and F2-3 in all segments (1.18 m/s vs 1.14 m/s, P = .46). Seventy percent of the variance in SWS was intrasegmental, 17% was intersegmental, and 13% due to variation between participants. Segments 6 and 7 demonstrated higher SWS in F0-1 than F2-3. Only segment 8 had higher SWS trends in F2-3 versus F0-1 (1.30 m/s vs 1.19 m/s, P = .57; Table 2).
Table 2.
Median Shear Wave Speed (m/s) per Segment for F0-1 Versus F2-3.
| Liver segments | N | All fibrosis stages, (min, max) | N | F0-1 (min, max) | N | F2-3 (min, max) | P |
|---|---|---|---|---|---|---|---|
| All segments | 15 | 1.17 (0.87, 1.69) | 6 | 1.18 (1.13, 1.43) | 9 | 1.14 (0.87, 1.69) | .46 |
| Segment 5 | 15 | 1.26 (0.20, 1.46) | 6 | 1.28 (1.18, 1.36) | 9 | 1.23 (0.20, 1.46) | .75 |
| Segment 6 | 15 | 1.09 (0.81, 2.14) | 6 | 1.28 (0.81, 1.50) | 9 | 1.06 (0.83, 2.14) | .69 |
| Segment 7 | 15 | 1.14 (0.46, 1.89) | 6 | 1.38 (1.05, 1.67) | 9 | 1.06 (0.46, 1.89) | .09 |
| Segment 8 | 14 | 1.24 (0.22, 1.64) | 6 | 1.19 (0.22, 1.62) | 8 | 1.30 (0.85, 1.64) | .57 |
APRI was significantly higher in F2-3 versus F0-1 (0.5 vs 0.3, P = .02). SFas level was significantly lower in F2-3 versus F0-1 (5.6 vs 6.9, P = .046). MCP-1 was higher in F2-3 versus F0-1 (751.5 vs 559.5, P = .06). There were no significant differences in FIB-4, HA, TIMP-1, PH, collagen IV, IL-8, PDGF-BB, sICAM, or LH between F0-1 and F2-3 (Table 3). There were no significant differences in serum biomarker levels when divided by age groups (0-4, 5-9, ≥10 years), but there were differences in AST, alanine aminotransferase (ALT), and platelets by age (P = .01, P = .03, P = .003, respectively; Table 4). There was only 1 participant in the 5- to 9-year age group. AST and ALT were lowest in participants >10 years of age, and platelets decreased with increasing age.
Table 3.
Median Biomarker Scores of F0-1 Versus F2-3.
| Biomarker | N | All fibrosis stages (min, max) | N | F0-1 (min, max) | N | F2-3 (min, max) | P |
|---|---|---|---|---|---|---|---|
| APRI | 16 | 0.5 (0.2, 0.8) | 6 | 0.3 (0.2, 0.5) | 10 | 0.5 (0.3, 0.8) | .02 |
| FIB-4 | 16 | 0.3 (0.1, 0.5) | 6 | 0.3 (0.1, 0.4) | 10 | 0.3 (0.1, 0.5) | .71 |
| TIMP-1 | 14 | 79.5 (49.8, 171.8) | 6 | 84.8 (53.7, 161.2) | 8 | 79.5 (49.8, 171.8) | .85 |
| PH | 14 | 5.3 (1.7, 12.7) | 6 | 3.6 (1.9, 10.3) | 8 | 5.8 (1.7, 12.7) | .18 |
| HA | 14 | 35.1 (11.0, 72.2) | 6 | 38.6 (18.0, 72.2) | 8 | 34.6 (11.0, 52.6) | .76 |
| MCP-1 | 14 | 650.7 (470.5, 1095.0) | 6 | 559.5 (470.5, 675.8) | 8 | 751.5 (472.8, 1095.0) | .06 |
| Collagen IV | 14 | 692.4 (445.5, 1939.5) | 6 | 692.4 (458.3, 863.3) | 8 | 751.5 (445.5, 1939.5) | .66 |
| IL-8 | 14 | 7.5 (5.1, 14.0) | 6 | 8.1 (5.3, 14.0) | 8 | 7.5 (5.1, 10.3) | .66 |
| PDGF-BB | 14 | 70.0 (38.1, 83.7) | 6 | 59.2 (38.1, 77.6) | 8 | 73.6 (41.3, 83.7) | .23 |
| SFas | 14 | 6.1 (3.3, 7.9) | 6 | 6.9 (4.9, 7.9) | 8 | 5.6 (3.3, 6.3) | .046 |
| sICAM | 14 | 217.4 (171.2, 348.2) | 6 | 204.1 (189.9, 348.2) | 8 | 242.0 (171.2, 300.4) | .66 |
| LH | 14 | 104.7 (40.5, 174.7) | 6 | 88.8 (40.5, 172.5) | 8 | 123.3 (55.1, 174.7) | .66 |
Abbreviations: APRI, aspartate transaminase to platelet ratio index; FIB-4, fibrosis index based on 4 factors; TIMP-1, tissue inhibitor of metalloproteinase 1; PH, prolyl hydroxylase; HA, hyaluronic acid; MCP-1, monocyte chemoattractant protein 1; IL-8, interleukin 8; PDGF-BB, platelet-derived growth factor; SFas, soluble Fas; sICAM, soluble intercellular adhesion molecule; LH, lysyl hydroxylase.
Table 4.
Median Biomarker and Noninvasive Fibrosis Scores Among Different Age Groups.
| Biomarker | N | 0-4 years (min, max) | N | 5-9 years (min, max) | N | ≥10 years (min, max) | P |
|---|---|---|---|---|---|---|---|
| APRI | 6 | 0.5 (0.3, 0.8) | 1 | 0.7 (0.7, 0.7) | 9 | 0.5 (0.2, 0.7) | .29 |
| FIB-4 | 6 | 0.1 (0.1, 0.1) | 1 | 0.1 (0.1, 0.1) | 9 | 0.4 (0.3, 0.5) | .003 |
| AST | 6 | 66.0 (50.0, 85.0) | 1 | 76.0 (76.0, 76.0) | 9 | 37.0 (14.0, 62.0) | .01 |
| ALT | 6 | 77.5 (52.0, 112.0) | 1 | 113.0 (113.0, 113.0) | 9 | 42.0 (17.0, 78.0) | .03 |
| Platelets | 6 | 322.0 (269.0, 536.0) | 1 | 276.0 (276.0, 276.0) | 9 | 201.0 (171.0, 238.0) | .003 |
| TIMP-1 | 5 | 91.1 (49.8, 171.8) | 1 | 76.5 (76.5, 76.5) | 8 | 77.8 (53.7, 171.8) | .98 |
| PH | 5 | 5.5 (3.0, 12.7) | 1 | 4.6 (4.6, 4.6) | 8 | 4.9 (1.7, 11.6) | .67 |
| HA | 5 | 33.3 (18.0, 52.6) | 1 | 29.0 (29.0, 29.0) | 8 | 39.0 (11.0, 72.2) | .81 |
| MCP-1 | 5 | 659.2 (480.5, 927.8) | 1 | 1066.6 (1066.6, 1066.6) | 8 | 630.0 (470.5, 1095.0) | .37 |
| Collagen IV | 5 | 837.0 (458.3, 890.3) | 1 | 543.8 (543.8, 543.8) | 8 | 692.4 (445.5, 1939.5) | .82 |
| IL-8 | 5 | 10.0 (5.4, 14.0) | 1 | 9.5 (9.5, 9.5) | 8 | 6.0 (5.1, 11.8) | .36 |
| PDGF-BB | 5 | 73.9 (44.1, 77.6) | 1 | 68.9 (68.9, 68.9) | 8 | 66.9 (38.1, 83.7) | .94 |
| SFas | 5 | 4.9 (3.3, 7.5) | 1 | 6.0 (6.0, 6.0) | 8 | 6.2 (5.0, 7.9) | .11 |
| sICAM | 5 | 258.2 (184.1, 348.2) | 1 | 299.6 (299.6, 299.6) | 8 | 199.7 (171.2, 342.5) | .50 |
| LH | 5 | 129.2 (55.1, 167.9) | 1 | 60.5 (60.5, 60.5) | 8 | 104.7 (40.5, 174.7) | .81 |
Abbreviations: APRI, aspartate transaminase to platelet ratio index; FIB-4, fibrosis index based on 4 factors; TIMP-1, tissue inhibitor of metalloproteinase 1; PH, prolyl hydroxylase; HA, hyaluronic acid; MCP-1, monocyte chemoattractant protein 1; IL-8, interleukin 8; PDGF-BB, platelet-derived growth factor; SFas, soluble Fas; sICAM, soluble intercellular adhesion molecule; LH, lysyl hydroxylase.
A multiple logistic regression model predicting odds of F2-3 included APRI, sFas, and MCP-1 as predictors (N = 14). A 0.1-unit increase in APRI score was associated with a 1.86-fold (95% CI = 0.48-7.25; P = .37) increased odds of a participant having F2-3. A 1-unit increase in sFas was associated with a 0.37-fold (95% CI = 0.07-1.98; P = .24) decreased odds of a participant having F2-3. A 1-unit increase in MCP-1 was associated with a 1.01-fold (95% CI = 0.99-1.02; P = .37) increased odds of a participant having F2-3. The AUROC curve for simple logistic regression models for APRI, sFas, and MCP-1 to predict F2-3 separately were 0.88 (95% CI = 0.68-1.00), 0.82 (95% CI = 0.57-1.00), and 0.81 (95% CI = 0.57-1.00), respectively (Figure 1). A logistic regression analysis combining APRI, sFas, and MCP-1 as calculated by (APRI × MCP-1)/sFas was performed (N = 14). A 1-unit increase in the combined measure was associated with a 1.1-fold increased odds of F2-3 (95% CI = 0.98-1.22; P = .10). AUROC curve for this model was 0.92 (P < .001, 95% CI = 0.75-1.00; Figure 2). A cutoff value of 49.8 for the calculated value provided a sensitivity of 87.5% (95% CI = 47-100) and specificity of 100% (95% CI = 54-100).
Figure 1.
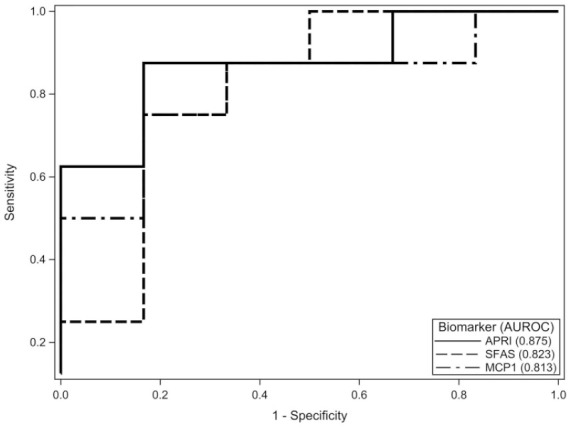
Individual AUROC of APRI, sFas, and MCP-1. AUROC, area under the receiver operating characteristic curve; APRI, aspartate transaminase to platelet ratio index; sFas, soluble Fas; MCP-1, monocyte chemoattractant protein 1.
Figure 2.
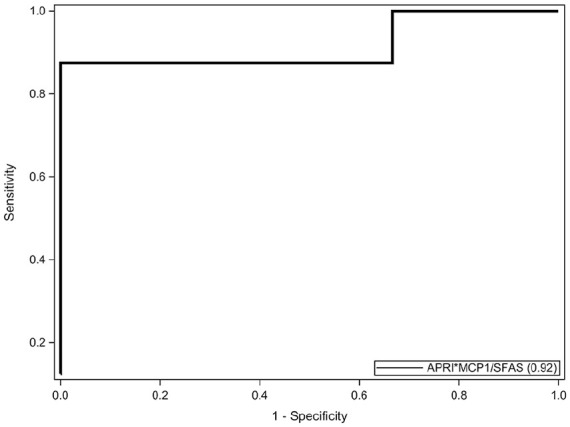
AUROC of multiple logistic regression analysis combining (APRI × MCP-1)/sFas. AUROC, area under the receiver operating characteristic curve; APRI, aspartate transaminase to platelet ratio index; sFas, soluble Fas; MCP-1, monocyte chemoattractant protein 1.
Discussion
Progressive liver fibrosis and cirrhosis are serious complications of chronic pediatric viral hepatitis.6 The ability to accurately monitor the progression of fibrosis noninvasively would better inform the timing of initiating highly effective nucleos(t)ide analogues or direct-acting antivirals.3,10 While some studies have shown greater correlation between SWE and fibrosis stage in the right upper lobe, the optimal segment for reproducibility within the right upper lobe has not been established in children.46,47 In our study, measurements were taken in multiple segments of the right liver lobe, in order to compare reproducibility and identify if one provided more consistent measurements correlating with liver biopsy fibrosis stage. In our cross-sectional liver biopsy validation study, SWE demonstrated poor reproducibility within segments of the right upper lobe and poor correlation with fibrosis among pediatric participants with chronic viral hepatitis, as seen in previous studies.46,48,49 Factors such as steatosis and inflammation early in the process of viral hepatitis may have confounded SWS.50 Only segment 8 consistently demonstrated a positive correlation between fibrosis stage and SWS as a surrogate for LSM, as reported by adult studies. We identified very high intrasegmental SWS variation in our participants, suggesting either poor compliance during acquisition of measurements or operator inconsistency. Variation between segments in a single participant may reflect heterogeneous fibrosis and/or inflammation. SWE does not appear as reliable, reproducible, or standardized as transient elastography (ie, Fibroscan) in children with viral hepatitis.51-53
While the primary limitation to this study is its sample size, liver biopsy validation was performed for each participant. Collection of 20% and 33% of serum samples and SWE, respectively, were performed >3 months from the time of biopsy; however, significant change in liver remodeling or fibrosis during this time is unlikely. None of the participants had stage 4 fibrosis, eliminating an important population for SWE measurement analysis. However, stage 4 fibrosis (cirrhosis) from hepatitis B and C in children is uncommon with an estimated prevalence of 0.2% to 3.8% and 2%, respectively.54-57 Since the median age of our study cohort was only 12.6 years, the likelihood of identifying a study participant with F4 or cirrhosis was very small.
Most liver biomarker analyses typically compare difference between F0-2 (not severe) versus F3-4 (severe); however, given the lack of F4 in our participants, we compared F0-1 (minimal to none) versus F2-3 (mild to moderate fibrosis).58,59 These findings are clinically important for providers as patients with F2-3 are often prioritized for earlier antiviral treatment. Importantly, our finding suggests that a few of the novel serum biomarkers, namely, sFas and MCP-1 both individually and in combination with APRI can differentiate between minimal (F0-1) and moderate fibrosis (F2-3), which we believe is even more clinically useful as it may inform sooner clinical management before progression to F3-4 (severe fibrosis), which may be harder to reverse. In particular, APRI and MCP-1 were increased in F2-3 versus F0-1. MCP-1 is an inflammatory chemokine, which attracts monocytes and leads to increased inflammation and later fibrosis.35-37 Interestingly, sFas, known to trigger apoptosis leading to hepatocyte damage, was lower in F2-3 versus F0-1. While the literature in adults suggests sFas concentrations are higher in more advanced fibrosis, we hypothesize that our finding of low sFas levels among F2-3 may reflect immunoregulation of inflammation once moderate fibrosis has formed.60-62 Our biomarker concentrations were not affected by age, which would be an important confounder for which to control, as markers associated with collagen may be influenced by growth. The logistic regression analysis combining APRI, sFas, and MCP-1 was able to predict F2-F3 fibrosis with high sensitivity. When examining the AUROC for each of the variables individually, they all had a significant AUROC, which shows that the combined regression analysis is not driven by one single factor, but the combined impact of them all.
Conclusion
In this pilot study, we conclude that APRI, sFas, and MCP-1 show promise as noninvasive markers of the progression of liver fibrosis in chronic pediatric viral hepatitis. Further studies of APRI and serum biomarkers in a larger cohort of children with viral hepatitis may inform thresholds predicting specific stages of fibrosis. Our SWE data highlight the importance of reliability and reproducibility of this testing modality, which may be more difficult in a pediatric population.
Footnotes
Author Contributions: Rebecca Mercedes contributed to analysis and interpretation of the data and drafting of the manuscript. Jameisha Brown contributed to the conception and design of the project and acquisition of the data. Charles Minard contributed to design of the project, analysis and interpretation of the data, and drafting of the manuscript. Cynthia M. Tsai contributed to acquisition of data. Sridevi Devaraj contributed to conception and design of the project and analysis and interpretation of the data. Marthe Munden contributed to conception and design of the project, acquisition, analysis and interpretation of the data. Daniel Leung contributed to conception and design of the project, acquisition, analysis and interpretation of the data, and drafting of the manuscript. All authors critically revised the manuscript for important intellectual content.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant funded by the Texas Children’s Hospital Pediatric Pilot Research Fund.
ORCID iD: Rebecca Mercedes  https://orcid.org/0000-0002-0855-090X
https://orcid.org/0000-0002-0855-090X
References
- 1. Global Burden of Hepatitis C Working Group. Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44:20-29. doi: 10.1177/0091270003258669 [DOI] [PubMed] [Google Scholar]
- 2. Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500. doi: 10.1056/NEJMra0801644 [DOI] [PubMed] [Google Scholar]
- 3. Karnsakul W, Schwarz KB. Hepatitis B and C. Pediatr Clin North Am. 2017;64:641-658. doi: 10.1016/j.pcl.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. National Center for HIV/AIDS, viral hepatitis, STD, and TB prevention. Annual Report 2015. Published 2015. Accessed June 16, 2020 https://www.cdc.gov/nchhstp/publications/docs/NCHHSTP-Annual-Report-2015.pdf
- 5. Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42(suppl 3):S206-S214. doi: 10.1016/S1590-8658(10)60507-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perz JF, Armstrong GL, Farrington LA, Hutin YJF, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529-538. doi: 10.1016/j.jhep.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 7. MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med. 2015;5:a021410. doi: 10.1101/cshperspect.a021410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haber BA, Block JM, Jonas MM, et al. ; Hepatitis B Foundation. Recommendations for screening, monitoring, and referral of pediatric chronic hepatitis B. Pediatrics. 2009;124:e1007-e1013. doi: 10.1542/peds.2009-0567 [DOI] [PubMed] [Google Scholar]
- 9. Mohamed MA, Majda E, Mohamed K, et al. Do prothrombin time, transaminases, and platelet count predict the severity of fibrosis in viral liver diseases? Tunis Med. 2005;83:400-403. [PubMed] [Google Scholar]
- 10. Bonis PA, Friedman SL, Kaplan MM. Is liver fibrosis reversible? N Engl J Med. 2001;344:452-454. doi: 10.1056/NEJM200102083440610 [DOI] [PubMed] [Google Scholar]
- 11. Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J Gastroenterol Hepatol. 2006;21(suppl 3):S84-S87. doi: 10.1111/j.1440-1746.2006.04584.x [DOI] [PubMed] [Google Scholar]
- 12. Zhou K, Lu LG. Assessment of fibrosis in chronic liver diseases. J Dig Dis. 2009;10:7-14. doi: 10.1111/j.1751-2980.2008.00356.x [DOI] [PubMed] [Google Scholar]
- 13. Castera L. Assessing liver fibrosis. Expert Rev Gastroenterol Hepatol. 2008;2:541-552. doi: 10.1586/17474124.2.4.541 [DOI] [PubMed] [Google Scholar]
- 14. Huang J, Hsieh M, Dai C, et al. The incidence and risks of liver biopsy in non-cirrhotic patients: an evaluation of 3806 biopsies. Gut. 2007;56:736-737. doi: 10.1136/gut.2006.115410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westheim BH, Østensen AB, Aagenæs I, Sanengen T, Almaas R. Evaluation of risk factors for bleeding after liver biopsy in children. J Pediatr Gastroenterol Nutr. 2012;55:82-87. doi: 10.1097/MPG.0b013e318249c12a [DOI] [PubMed] [Google Scholar]
- 16. Grgurevic I, Puljiz Z, Brnic D, et al. Liver and spleen stiffness and their ratio assessed by real-time two dimensional-shear wave elastography in patients with liver fibrosis and cirrhosis due to chronic viral hepatitis. Eur Radiol. 2015;25:3214-3221. doi: 10.1007/s00330-015-3728-x [DOI] [PubMed] [Google Scholar]
- 17. Nitta Y, Kawabe N, Hashimoto S, et al. Liver stiffness measured by transient elastography correlates with fibrosis area in liver biopsy in patients with chronic hepatitis C. Hepatol Res. 2009;39:675-684. doi: 10.1111/j.1872-034X.2009.00500.x [DOI] [PubMed] [Google Scholar]
- 18. Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C, Liver Fibrosis Study Group. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125-2133. doi: 10.1002/hep.25936 [DOI] [PubMed] [Google Scholar]
- 19. Liu DP, Lu W, Zhang ZQ, et al. Comparative evaluation of GPR versus APRI and FIB-4 in predicting different levels of liver fibrosis of chronic hepatitis B. J Viral Hepat. 2018;25:581-589. doi: 10.1111/jvh.12842 [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization, Global Hepatitis Programme. Guidelines for the Prevention, Care, and Treatment of Persons with Chronic Hepatitis B Infection. World Health Organization; 2015. [PubMed] [Google Scholar]
- 21. Liu J, Zhao J, Zhang Y, et al. Noninvasive assessment of liver fibrosis stage using ultrasound-based shear wave velocity measurements and serum algorithms in patients with viral hepatitis B: a retrospective cohort study: noninvasive assessment of liver fibrosis. J Ultrasound Med. 2017;36:285-293. doi: 10.7863/ultra.16.01069 [DOI] [PubMed] [Google Scholar]
- 22. Jin W, Lin Z, Xin Y, Jiang X, Dong Q, Xuan S. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis B-related fibrosis: a leading meta-analysis. BMC Gastroenterol. 2012;12:14. doi: 10.1186/1471-230X-12-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726-736. doi: 10.1002/hep.24105 [DOI] [PubMed] [Google Scholar]
- 24. Leung DH, Khan M, Minard CG, et al. Aspartate aminotransferase to platelet ratio and fibrosis-4 as biomarkers in biopsy-validated pediatric cystic fibrosis liver disease. Hepatology. 2015;62:1576-1583. doi: 10.1002/hep.28016 [DOI] [PubMed] [Google Scholar]
- 25. Shah I, Madgum N. Correlation of APRI Index with Metavir Index in children with neonatal cholestasis without biliary atresia. Ann Hepatol. 2018;17:592-595. doi: 10.5604/01.3001.0012.0924 [DOI] [PubMed] [Google Scholar]
- 26. Grieve A, Makin E, Davenport M. Aspartate aminotransferase-to-platelet ratio index (APRi) in infants with biliary atresia: prognostic value at presentation. J Pediatr Surg. 2013;48:789-795. doi: 10.1016/j.jpedsurg.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 27. Nobili V, Alisi A, Torre G, et al. Hyaluronic acid predicts hepatic fibrosis in children with nonalcoholic fatty liver disease. Transl Res. 2010;156:229-234. doi: 10.1016/j.trsl.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 28. Pereira TN, Lewindon PJ, Smith JL, et al. Serum markers of hepatic fibrogenesis in cystic fibrosis liver disease. J Hepatol. 2004;41:576-583. doi: 10.1016/j.jhep.2004.06.032 [DOI] [PubMed] [Google Scholar]
- 29. Nobili V, Marcellini M, Giovannelli L, et al. Association of serum interleukin-8 levels with the degree of fibrosis in infants with chronic liver disease. J Pediatr Gastroenterol Nutr. 2004;39:540-544. [DOI] [PubMed] [Google Scholar]
- 30. Rath T, Menendez KM, Kügler M, et al. TIMP-1/-2 and transient elastography allow non invasive diagnosis of cystic fibrosis associated liver disease. Dig Liver Dis. 2012;44:780-787. doi: 10.1016/j.dld.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 31. Kobayashi H, Tamura T, Yamataka A, Lane GJ, Miyano T. Is serum prolyl 4-hydroxylase useful as noninvasive marker of liver fibrosis in patients with biliary atresia? J Pediatr Surg. 2005;40:1366-1367. doi: 10.1016/j.jpedsurg.2005.05.020 [DOI] [PubMed] [Google Scholar]
- 32. Leonardi S, Giambusso F, Sciuto C, Castiglione S, Castiglione N, La Rosa M. Are serum type III procollagen and prolyl hydroxylase useful as noninvasive markers of liver disease in patients with cystic fibrosis? J Pediatr Gastroenterol Nutr. 1998;27:603-605. [DOI] [PubMed] [Google Scholar]
- 33. de Avila RE, Carmo RA, Farah K, et al. Hyaluronic acid in the evaluation of liver fibrosis in patients with hepatitis C on haemodialysis. Braz J Infect Dis. 2010;14:335-341. [PubMed] [Google Scholar]
- 34. Moghaddam FM, Arrbabi H, Khajedaloei M. Determination of the relationship of serum hyaluronic acid levels to the degree of liver fibrosis in biopsies of patients with chronic viral hepatitis B and C. Hepat Mon. 2010;10:168-172. [PMC free article] [PubMed] [Google Scholar]
- 35. Baeck C, Wehr A, Karlmark KR, et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61:416-426. doi: 10.1136/gutjnl-2011-300304 [DOI] [PubMed] [Google Scholar]
- 36. Mühlbauer M, Bosserhoff AK, Hartmann A, et al. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology. 2003;125:1085-1093. doi: 10.1016/S0016-5085(03)01213-7 [DOI] [PubMed] [Google Scholar]
- 37. Zamara E, Galastri S, Aleffi S, et al. Prevention of severe toxic liver injury and oxidative stress in MCP-1-deficient mice. J Hepatol. 2007;46:230-238. doi: 10.1016/j.jhep.2006.09.007 [DOI] [PubMed] [Google Scholar]
- 38. Zimmermann HW, Seidler S, Gassler N, et al. Interleukin-8 is activated in patients with chronic liver diseases and associated with hepatic macrophage accumulation in human liver fibrosis. PLoS One. 2011;6:e21381. doi: 10.1371/journal.pone.0021381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen SW, Zhang XR, Wang CZ, Chen WZ, Xie WF, Chen YX. RNA interference targeting the platelet-derived growth factor receptor β subunit ameliorates experimental hepatic fibrosis in rats. Liver Int. 2008;28:1446-1457. doi: 10.1111/j.1478-3231.2008.01759.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang BB, Cai WM, Weng HL, et al. Diagnostic value of platelet derived growth factor-BB, transforming growth factor-β1, matrix metalloproteinase-1, and tissue inhibitor of matrix metalloproteinase-1 in serum and peripheral blood mononuclear cells for hepatic fibrosis. World J Gastroenterol. 2003;9:2490-2496. doi: 10.3748/wjg.v9.i11.2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen SW, Chen YX, Zhang XR, Qian H, Chen WZ, Xie WF. Targeted inhibition of platelet-derived growth factor receptor-beta subunit in hepatic stellate cells ameliorates hepatic fibrosis in rats. Gene Ther. 2008;15:1424-1435. [DOI] [PubMed] [Google Scholar]
- 42. Shteyer E, Ramm GA, Xu C, White FV, Shepherd RW. Outcome after portoenterostomy in biliary atresia: pivotal role of degree of liver fibrosis and intensity of stellate cell activation. J Pediatr Gastroenterol Nutr. 2006;42:93-99. [DOI] [PubMed] [Google Scholar]
- 43. Busk TM, Bendtsen F, Nielsen HJ, Jensen V, Brünner N, Møller S. TIMP-1 in patients with cirrhosis: relation to liver dysfunction, portal hypertension, and hemodynamic changes. Scand J Gastroenterol. 2014;49:1103-1110. doi: 10.3109/00365521.2014.934910 [DOI] [PubMed] [Google Scholar]
- 44. Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598-607. doi: 10.1016/j.jhep.2007.07.006 [DOI] [PubMed] [Google Scholar]
- 45. GE Healthcare. LOGIQ E9 Shear Wave Elastography. Accessed June 16, 2020 https://www3.gehealthcare.com/~/media/rsna-2016-press-kit-assets/press%20releases/ultrasound/global%20shear%20wave%20whitepaper_october%202014.pdf?Parent=%7B1FFF8A39-2AC6-4EA6-9119-7458819CB4F7%7D
- 46. Samir AE, Dhyani M, Vij A, et al. Shear-wave elastography for the estimation of liver fibrosis in chronic liver disease: determining accuracy and ideal site for measurement. Radiology. 2014;274:888-896. doi: 10.1148/radiol.14140839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barr RG, Ferraioli G, Palmeri ML, et al. Elastography assessment of liver fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2015;276:845-861. doi: 10.1148/radiol.2015150619 [DOI] [PubMed] [Google Scholar]
- 48. Ryu H, Ahn SJ, Yoon JH, Lee JM. Inter-platform reproducibility of liver stiffness measured with two different point shear wave elastography techniques and 2-dimensional shear wave elastography using the comb-push technique. Ultrasonography. 2019;38:345-354. doi: 10.14366/usg.19001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sporea I, Bota S, Jurchis A, et al. Acoustic radiation force impulse and supersonic shear imaging versus transient elastography for liver fibrosis assessment. Ultrasound Med Biol. 2013;39:1933-1941. doi: 10.1016/j.ultrasmedbio.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 50. Ferraioli G, Parekh P, Levitov AB, Filice C. Shear wave elastography for evaluation of liver fibrosis. J Ultrasound Med. 2014;33:197-203. doi: 10.7863/ultra.33.2.197 [DOI] [PubMed] [Google Scholar]
- 51. Behairy BES, Sira MM, Zalata KR, Salama ESE, Abd-Allah MA. Transient elastography compared to liver biopsy and morphometry for predicting fibrosis in pediatric chronic liver disease: does etiology matter? World J Gastroenterol. 2016;22:4238-4249. doi: 10.3748/wjg.v22.i16.4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu JF, Song SH, Lee CS, et al. Clinical predictors of liver fibrosis in patients with chronic hepatitis B virus infection from children to adults. J Infect Dis. 2018;217:1408-1416. doi: 10.1093/infdis/jiy048 [DOI] [PubMed] [Google Scholar]
- 53. Raizner A, Shillingford N, Mitchell PD, et al. Hepatic inflammation may influence liver stiffness measurements by transient elastography in children and young adults. J Pediatr Gastroenterol Nutr. 2017;64:512-517. doi: 10.1097/MPG.0000000000001376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bortolotti F, Calzia R, Cadrobbi P, et al. Liver cirrhosis associated with chronic hepatitis B virus infection in childhood. J Pediatr. 1986;108:224-227. doi: 10.1016/s0022-3476(86)80987-8 [DOI] [PubMed] [Google Scholar]
- 55. Komatsu H, Inui A, Fujisawa T. Pediatric hepatitis B treatment. Ann Transl Med. 2017;5:37. doi: 10.21037/atm.2016.11.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goodman ZD, Makhlouf HR, Liu L, et al. Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds-C Trial. Hepatology. 2008;47:836-843. doi: 10.1002/hep.22094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Squires JE, Balistreri WF. Hepatitis C virus infection in children and adolescents. Hepatol Commun. 2017;1:87-98. doi: 10.1002/hep4.1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li Q, Lu C, Li W, Huang Y, Chen L. Impact of age on the diagnostic performances and cut-offs of APRI and FIB-4 for significant fibrosis and cirrhosis in chronic hepatitis B. Oncotarget. 2017;8:45768-45776. doi: 10.18632/oncotarget.17470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McGoogan KE, Smith PB, Choi SS, Berman W, Jhaveri R. Performance of the AST to platelet ratio index (APRI) as a noninvasive marker of fibrosis in pediatric patients with chronic viral hepatitis. J Pediatr Gastroenterol Nutr. 2010;50:344-346. doi: 10.1097/MPG.0b013e3181aed725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iio S, Hayashi N, Mita E, et al. Serum levels of soluble Fas antigen in chronic hepatitis C patients. J Hepatol. 1998;29:517-523. [DOI] [PubMed] [Google Scholar]
- 61. Song LH, Binh VQ, Duy DN, et al. Variations in the serum concentrations of soluble Fas and soluble Fas ligand in Vietnamese patients infected with hepatitis B virus. J Med Virol. 2004;73:244-249. doi: 10.1002/jmv.20082 [DOI] [PubMed] [Google Scholar]
- 62. Raghuraman S, Abraham P, Daniel HD, Ramakrishna BS, Sridharan G. Characterization of soluble FAS, FAS ligand and tumour necrosis factor-alpha in patients with chronic HCV infection. J Clin Virol. 2005;34:63-70. doi: 10.1016/j.jcv.2005.01.009 [DOI] [PubMed] [Google Scholar]


