Abstract
With the increasing use of endovascular technology in the treatment of various conditions, there has been an inevitable rise in cases with extravasation of contrast medium. We present a case in which extravasation of a large quantity of contrast medium resulted in acute communicating hydrocephalus. A 78-year-old woman came to the hospital because of abnormal right-sided limb movement, and was diagnosed with occlusion of the left internal carotid artery. The patient underwent timely intra-arterial mechanical thrombectomy. Postoperative computed tomography (CT) examination revealed a high-density shadow of the left basal ganglia and left frontal lobe. Twelve hours later, the patient fell into a coma. Repeat head CT indicated acute hydrocephalus with no evidence of obstruction. The patient underwent emergency external ventricular drainage, and the drainage fluid was observed to be clear. The patient regained consciousness after the procedure and the drainage tube was removed 4 days later. The patient had no recurrence of hydrocephalus. The mechanisms and treatment of this condition are discussed.
Keywords: Cerebrospinal fluid, communicating hydrocephalus, hydrocephalus, intra-arterial mechanical thrombectomy, endovascular technology, extravasation of contrast medium
Introduction
In recent years, increasing numbers of intracranial aneurysm and large vessel occlusion cases have been treated using endovascular technology,1–3 because of the rapid development of this technology. As a consequence, extravasation of contrast media often occurs.4 However, a case of acute communicating hydrocephalus caused by extravasation of contrast media has not previously been reported.
Case report
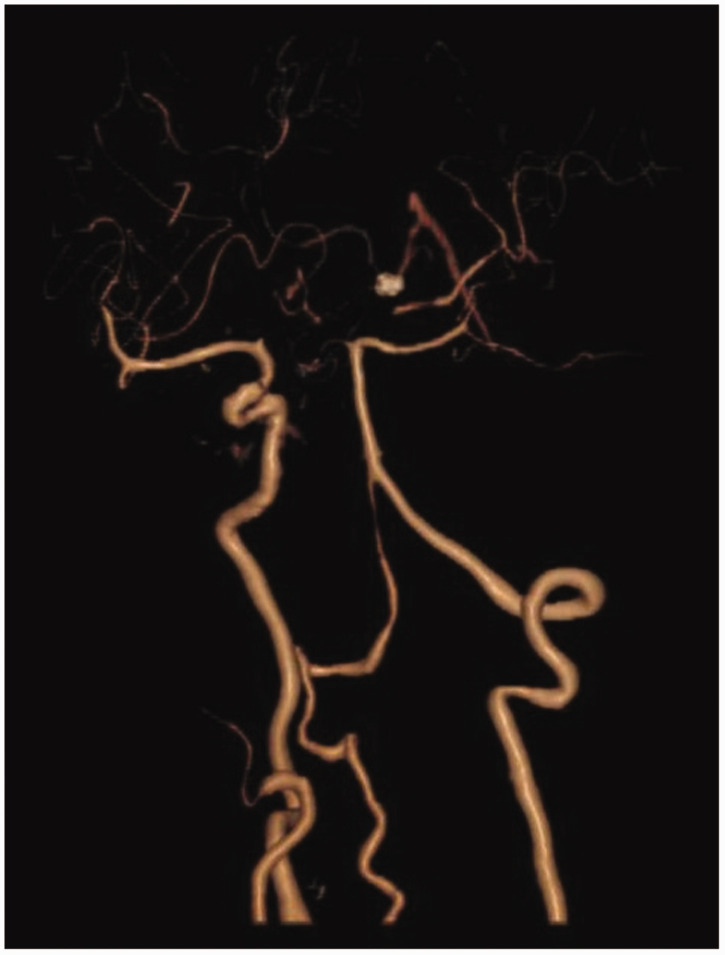
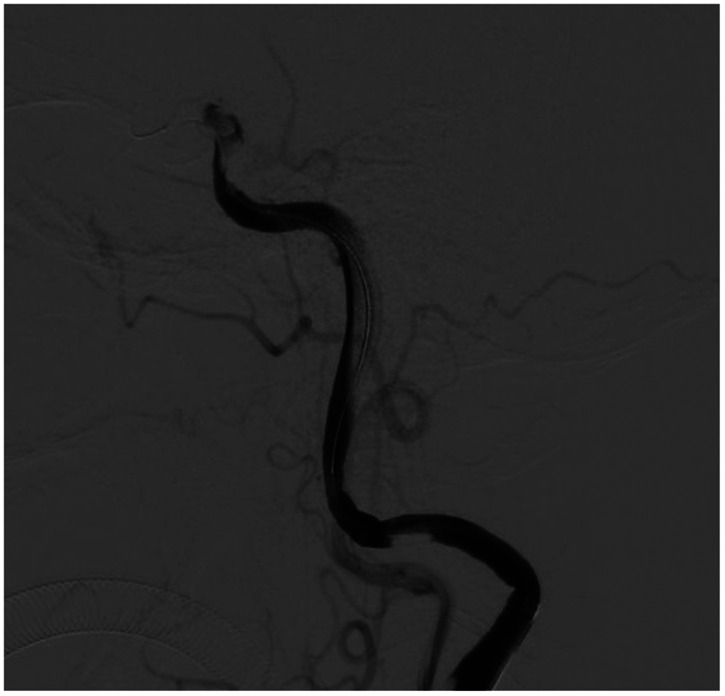
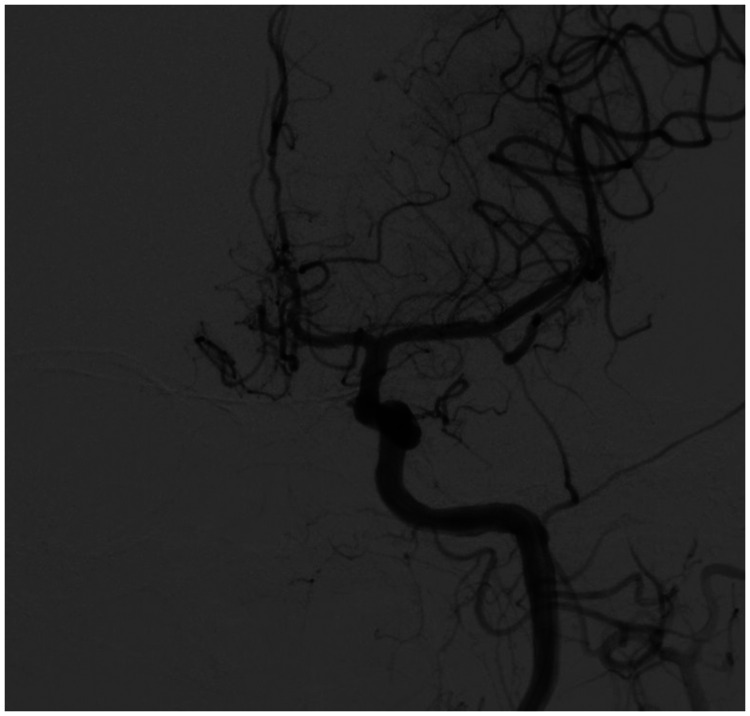
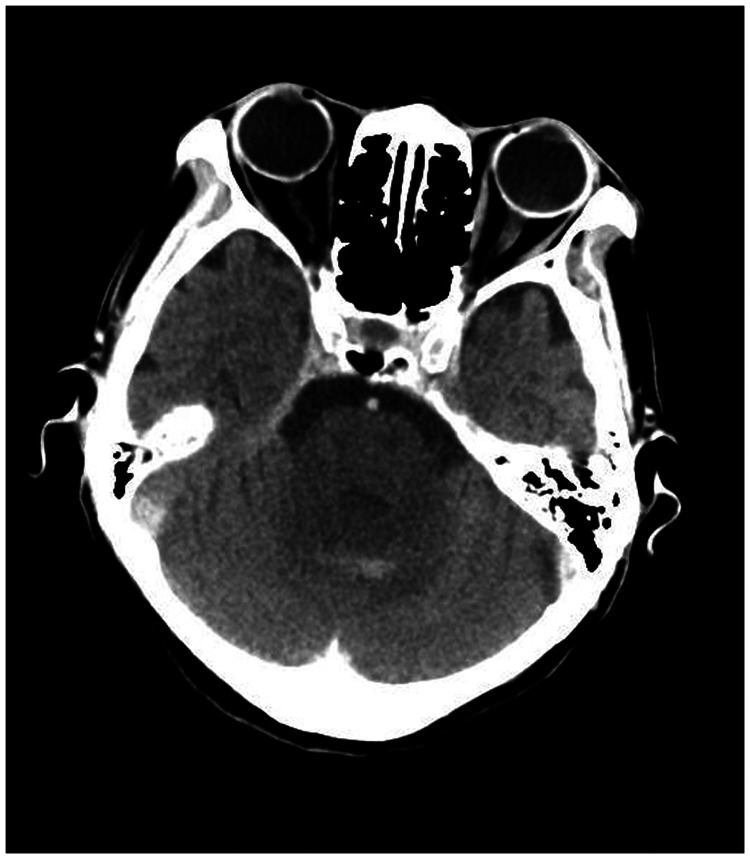
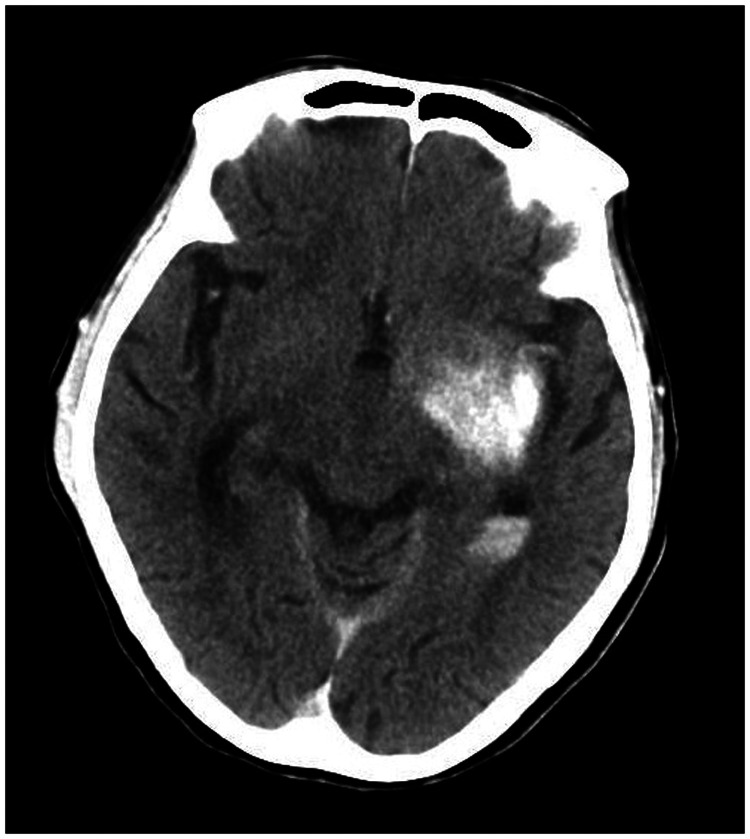
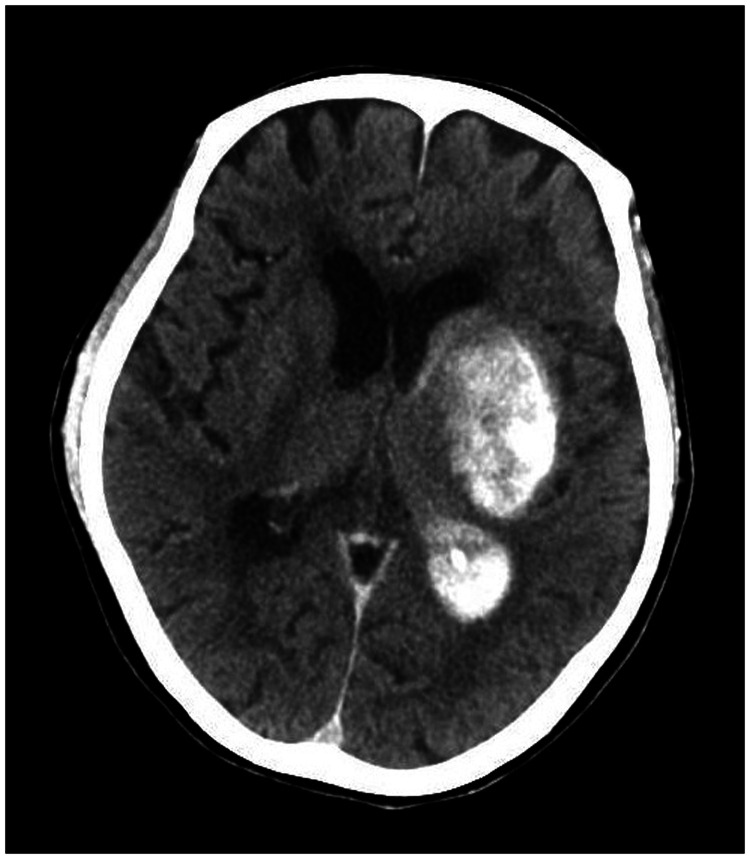
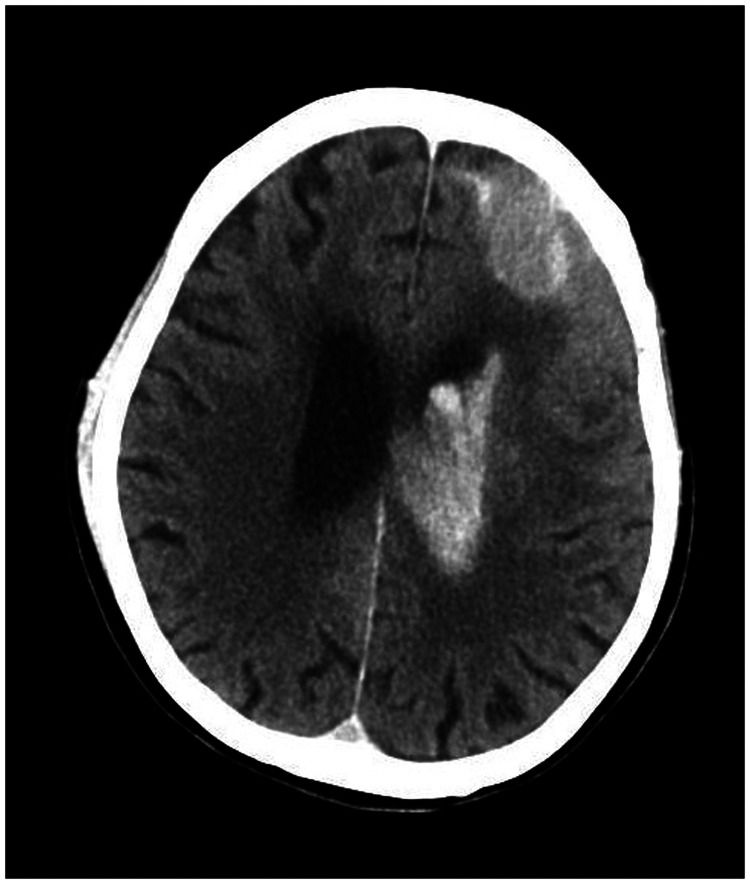
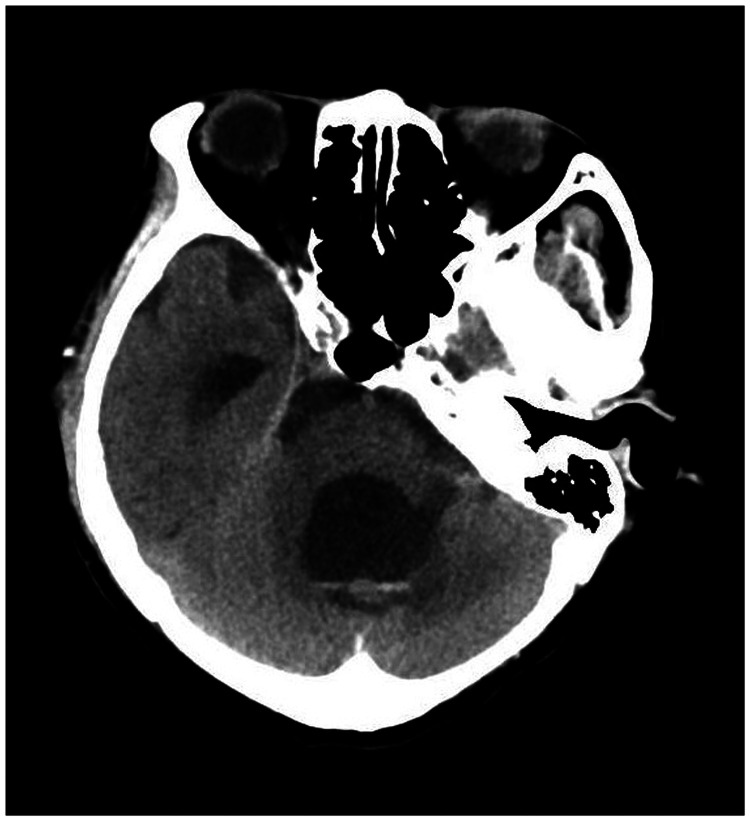
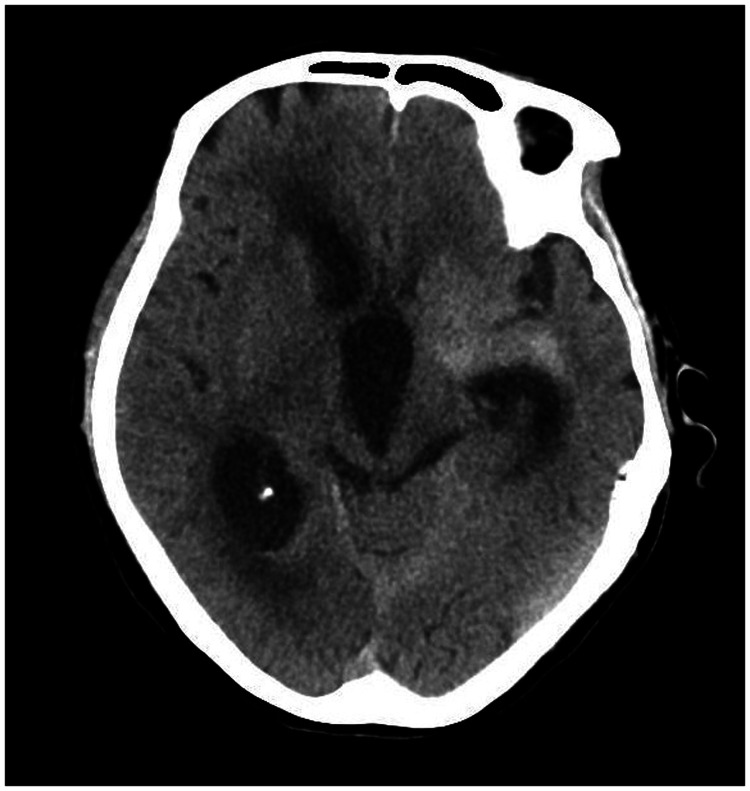
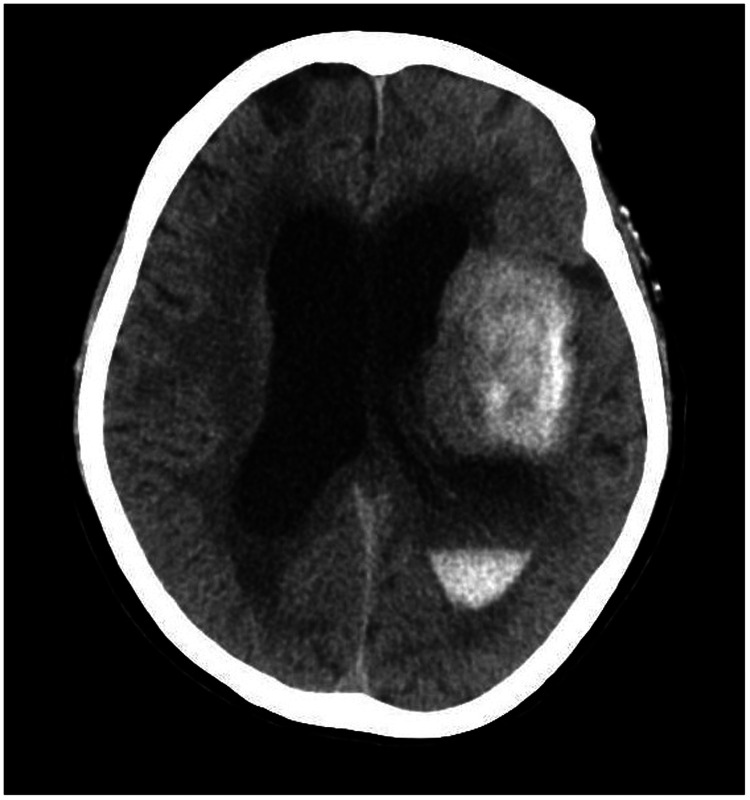
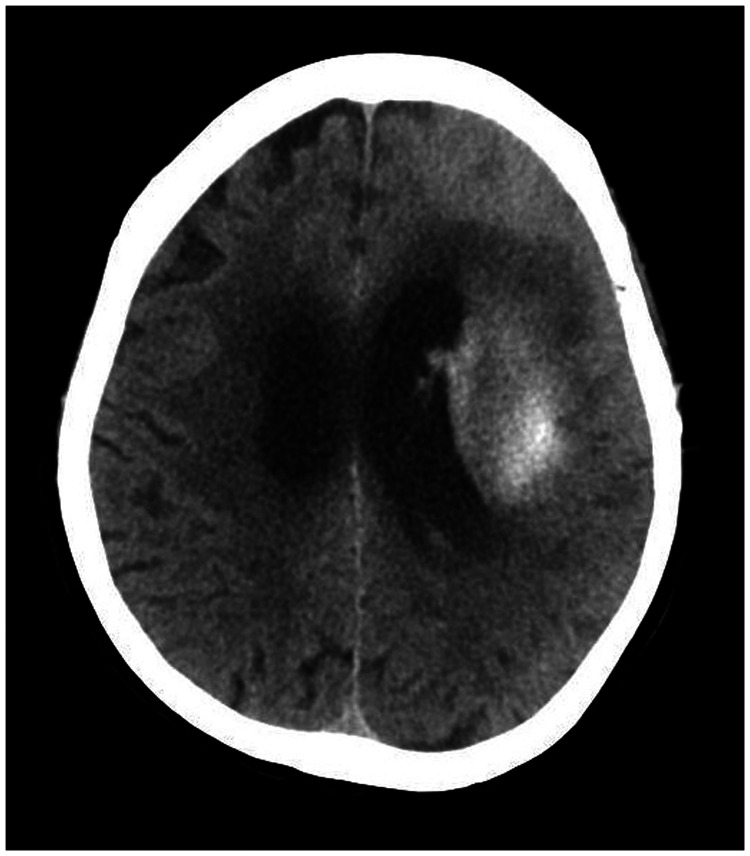
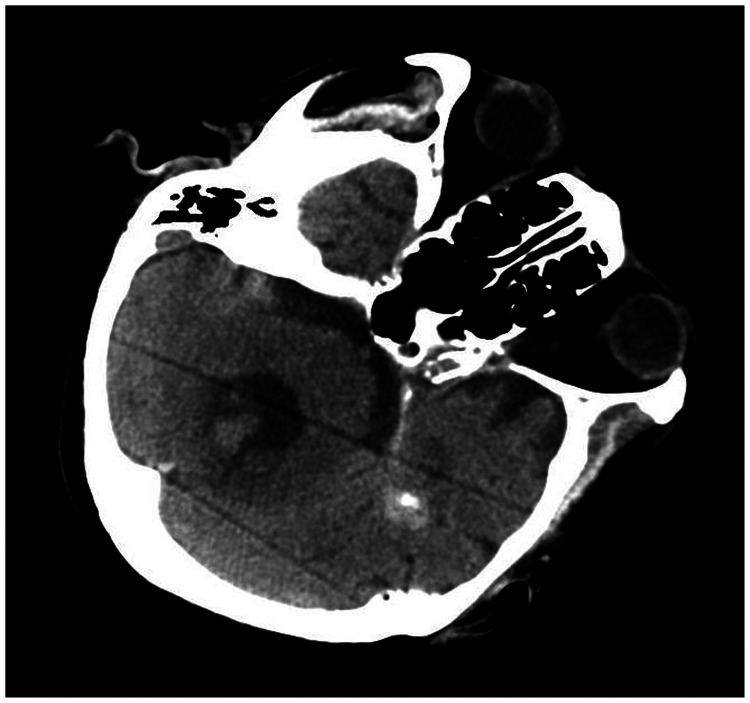
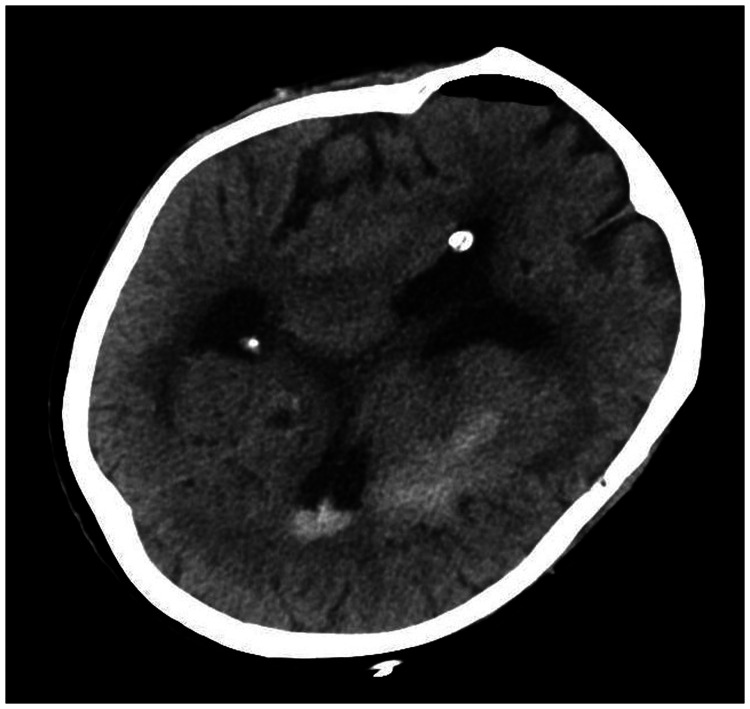
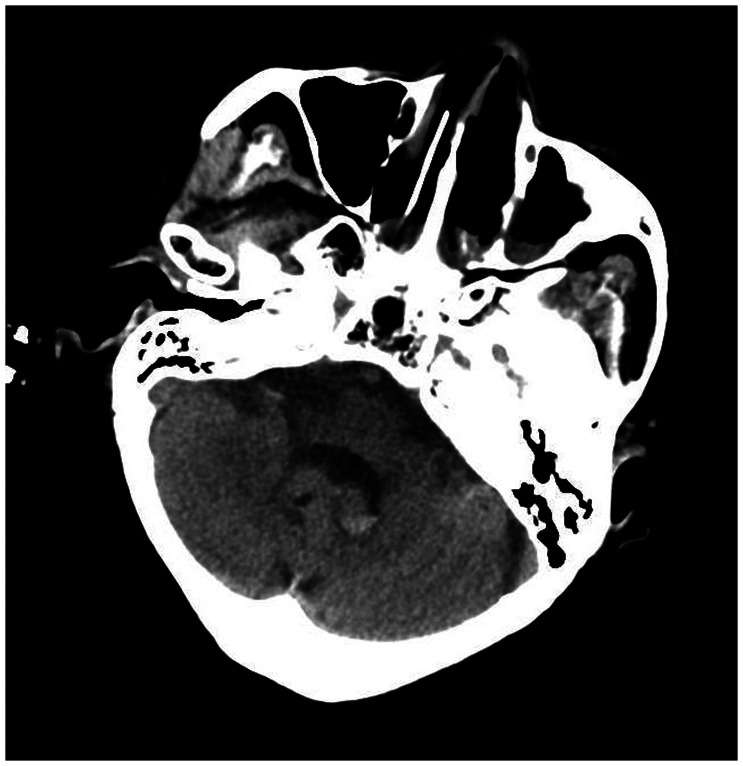
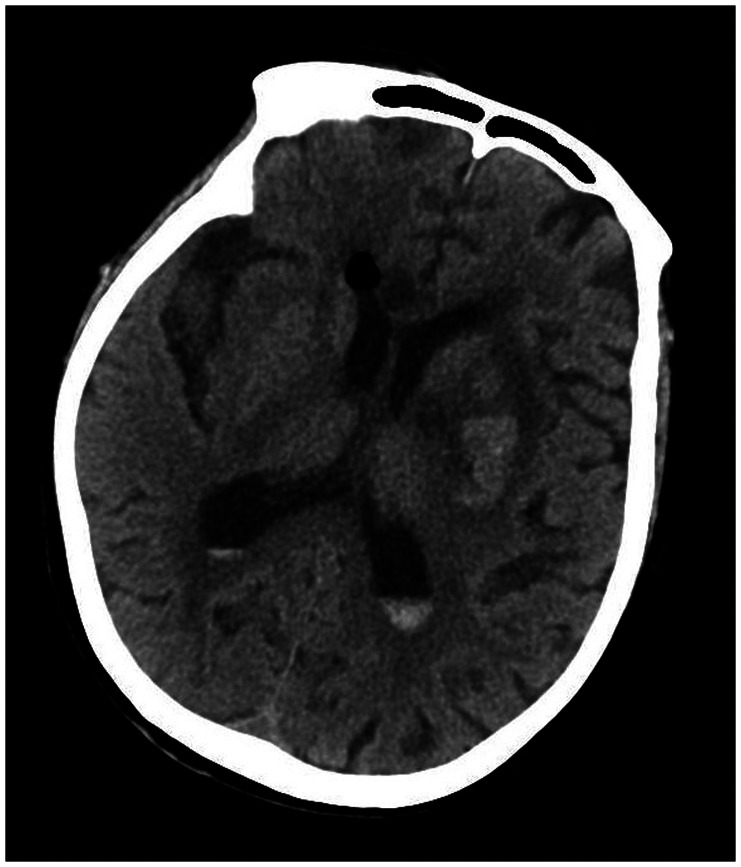
A 78-year-old woman came to the hospital because of sudden right-sided limb weakness for 4.5 hours. The muscle strength of the right limb was determined by physical examination to be grade 1/5. Her National Institutes of Health Stroke Scale (NIHSS) score was 15. A head computed tomography angiography was performed, which indicated occlusion of the left internal carotid artery (Figure 1). This was followed by emergency digital subtraction angiography (DSA), which revealed occlusion of the C4 (intracavernous) portion of the left internal carotid artery (Figure 2). Next, an intra-arterial mechanical thrombectomy procedure was performed (using a Solitaire FR, Medtronic, Inc., Fridley, MN, USA). Postoperative imaging of the left internal carotid artery revealed unobstructed restoration of blood flow, and the thrombolysis in cerebral infarction (TICI) was assessed to be grade 3 (Figure 3). However, immediately after the operation, head computed tomography (CT) revealed high-density shadows in the left basal ganglia, occipital horn of the left lateral ventricle, and left frontal lobe (Figures 4–7). After 12 hours, the patient fell into a coma. Repeat head CT indicated acute hydrocephalus with significant enlargement of the fourth ventricle, third ventricle, and bilateral lateral ventricles (Figures 8–11). Concurrently, the high-density shadow in the basal ganglia, occipital horn of the left lateral ventricle, and frontal lobe became much lighter (Figures 8–11). Emergency cerebral drainage was performed. The drainage fluid was clear, with scant red blood cells found in routine cerebrospinal fluid (CSF) examination (CSF routine examination: red blood cell count 100/μL). The high-density shadow on the CT image had become even lighter by 1 day after the operation (Figures 12, 13), indicating extravasation of a considerable amount of contrast medium. The patient regained consciousness after the procedure, and the drainage tube was removed 4 days later. There was no recurrence of hydrocephalus (Figures 14, 15). The patient’s condition improved gradually, and after 1 month the patient was transferred to a rehabilitation hospital for further treatment.
Figure 1.
Computed tomography angiography indicating occlusion of the left internal carotid artery.
Figure 2.
Digital subtraction angiography suggesting occlusion of the C4 (intracavernous) portion of the left internal carotid artery.
Figure 3.
Postoperative restoration of blood flow in the left internal carotid artery.
Figure 4.
Postoperative head computed tomography demonstrating a high-density shadow in the left basal ganglia, occipital horn of the left lateral ventricle, and left frontal lobe.
Figure 5.
Postoperative head computed tomography demonstrating a high-density shadow in the left basal ganglia, occipital horn of the left lateral ventricle, and left frontal lobe.
Figure 6.
Postoperative head computed tomography demonstrating a high-density shadow in the left basal ganglia, occipital horn of the left lateral ventricle, and left frontal lobe.
Figure 7.
Postoperative head computed tomography demonstrating a high-density shadow in the left basal ganglia, occipital horn of the left lateral ventricle, and left frontal lobe.
Figure 8.
Twelve hours after the scan in Figures 4–7, repeat head computed tomography indicated acute hydrocephalus with significant enlargement of the fourth ventricle, third ventricle, and bilateral lateral ventricles. The high-density shadow in the basal ganglia, occipital horn of the left lateral ventricle, and frontal lobe had also become lighter.
Figure 9.
Twelve hours after the scan in Figures 4–7, repeat head computed tomography indicated acute hydrocephalus with significant enlargement of the fourth ventricle, third ventricle, and bilateral lateral ventricles. The high-density shadow in the basal ganglia, occipital horn of the left lateral ventricle, and frontal lobe had also become lighter.
Figure 10.
Twelve hours after the scan in Figures 4–7, repeat head computed tomography indicated acute hydrocephalus with significant enlargement of the fourth ventricle, third ventricle, and bilateral lateral ventricles. The high-density shadow in the basal ganglia, occipital horn of the left lateral ventricle, and frontal lobe had also become lighter.
Figure 11.
Twelve hours after the scan in Figures 4–7, repeat head computed tomography indicated acute hydrocephalus with significant enlargement of the fourth ventricle, third ventricle, and bilateral lateral ventricles. The high-density shadow in the basal ganglia, occipital horn of the left lateral ventricle, and frontal lobe had also become lighter.
Figure 12.
The high-density shadow on the computed tomography image was reduced by 1 day after the operation (36 hours after the digital subtraction angiography).
Figure 13.
The high-density shadow on the computed tomography image was reduced by 1 day after the operation (36 hours after the digital subtraction angiography).
Figure 14.
Removal of the drainage tube at 4 days after the extraventricular drainage procedure. No recurrence of hydrocephalus was recorded during follow-up.
Figure 15.
Removal of the drainage tube at 4 days after the extraventricular drainage procedure. No recurrence of hydrocephalus was recorded during follow-up.
Discussion
There are two kinds of hydrocephalus: communicating hydrocephalus and non-communicating hydrocephalus (formerly known as obstructive hydrocephalus). Communicating hydrocephalus occurs when there is increased resistance to CSF outflow after it exits the ventricles, exhibited by the expansion of the whole ventricle. One kind of communicating hydrocephalus is caused by absorption disorders, such as subarachnoid hemorrhage, meningitis, tumor, and brain trauma. The other kind is caused by excessive production disorders, such as normal pressure hydrocephalus and overproduction of CSF, and is not related to absorption defects.
Although a large number of studies have investigated the causes of communicating hydrocephalus, the underlying mechanisms remain unclear. The most widely accepted mechanism is blockage of the arachnoid granulations, with impaired CSF absorption resulting in dilatation of the entire ventricular system.5 Meningitis can lead to communicating hydrocephalus, the two best described mechanisms are blockage of the arachnoid granulations by exudates and high protein levels in the CSF, which both prevent CSF absorption.6 In addition, there is a vast amount of literature demonstrating that elevated CSF protein levels may lead to CSF malabsorption at the level of arachnoid granulations.7–9
Hydrocephalus presents in approximately 20% to 30% of patients after subarachnoid hemorrhage,10,11 and is often of the communicating variety. Patients can be treated with ventriculoperitoneal shunts. Certain tumors (both benign and malignant) can also present with communicating hydrocephalus, such as vestibular schwannomas (in 13 of 291 patients, or 4.46%)12 and high-grade gliomas (in 8 of 278 patients, or 3%).13 These patients can be treated with a ventriculoperitoneal shunt or endoscopic third ventriculostomy.
Acute hydrocephalus, whether communicating or non-communicating, is often fatal and requires urgent treatment. Acute non-communicating hydrocephalus caused by cerebellar hemorrhage is common, and external ventricular drainage is an effective treatment. Recent reports of acute communicating hydrocephalus include a case of intracranial endodermal cyst that presented with acute non-obstructive hydrocephalus,14 a case of neuromyelitis optica causing acute communicating hydrocephalus,15 and a case of acute communicating hydrocephalus after lumbar lipomyelocele.16
Extravasation rates are 0.045% for gadolinium-based contrast agents and nearly six-fold higher, at 0.26%, for iodinated contrast agents.17 Extravasation is considered to be caused by disruption of the blood–brain barrier or blood–CSF barrier.18,19 Most extravasations are well-tolerated and resolve without surgical intervention.17 In the present case, a differential diagnosis of hemorrhage was able to be eliminated with the following five points: (1) the high-density shadow on CT scans had rapid dissolution; (2) there was drainage of clear fluid; (3) the structure of the left basal ganglia remained intact after the high-density shadow disappeared; (4) a scant amount of red blood cells were found in the routine CSF examination; and (5) the HU of the high-density shadow on CT was markedly higher than that of a hematoma (approximately 90 HU, on average). The contrast agent that was used in our patient was composed of iodixanol (Visipaque), which is relatively viscous. After injection, it is primarily eliminated in its original form through the kidneys. We therefore concluded that the patient’s acute communicating hydrocephalus was caused by the malabsorption of CSF.
Conclusion
Extravasation of contrast medium is a rare complication of DSA that can result in acute communicating hydrocephalus. A prompt CT scan should always be performed when patients experience neurological worsening following these procedures, to avoid the fatal evolution of such complications.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Ethics and informed consent
The family of the patient provided informed consent for the publication of this case. This report was approved by the Ethical Committee in Zhejiang Hospital.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Xiao-Yong Shi https://orcid.org/0000-0003-2466-9211
References
- 1.Pierot L, Biondi A. Endovascular techniques for the management of wide-neck intracranial bifurcation aneurysms: a critical review of the literature. J Neuroradiol 2016; 43: 167–175. [DOI] [PubMed] [Google Scholar]
- 2. Benaissa A, Barbe C, Pierot L. Analysis of recanalization after endovascular treatment of intracranial aneurysm (ARETA trial): presentation of a prospective multicenter study. J Neuroradiol 2015; 42: 80–85. [DOI] [PubMed] [Google Scholar]
- 3.Ilyas A, Chen CJ, Ding D, et al. Endovascular mechanical thrombectomy for acute ischemic stroke under general anesthesia versus conscious sedation: a systematic review and meta-analysis. Surg Neurol 2018; 112: e355–e367. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Zhang Y, Su Y, et al. Contrast extravasation is predictive of poor clinical outcomes in patients undergoing endovascular therapy for acute ischemic stroke in the anterior circulation. J Stroke Cerebrovasc Dis 2020; 29: 104494. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Bathla G, Kanekar S. Imaging of communicating hydrocephalus. Semin Ultrasound CT MR 2016; 37: 100–108. [DOI] [PubMed] [Google Scholar]
- 6.Wang KW, Chang WN, Chang HW, et al. Clinical relevance of hydrocephalus in bacterial meningitis in adults. Surg Neurol 2005; 64: 61–65. [DOI] [PubMed] [Google Scholar]
- 7.Hayhurst C, Dhir J, Dias PS. Stereotactic radiosurgery and vestibular schwannoma: hydrocephalus associated with the development of a secondary arachnoid cyst: a report of two cases and review of the literature. Br J Neurosurg 2005; 19: 178–181. [DOI] [PubMed] [Google Scholar]
- 8.Bloch J, Vernet O, Aube M, et al. Non-obstructive hydrocephalus associated with intracranial schwannomas: hyperproteinorrhachia as an etiopathological factor? Acta Neurochir (Wien) 2003; 145: 73–78. [DOI] [PubMed] [Google Scholar]
- 9.Sawamura Y, Shirato H, Sakamoto T, et al. Management of vestibular schwannoma by fractionated stereotactic radiotherapy and associated cerebrospinal fluid malabsorption. J Neurosurg 2003; 99: 685–692. [DOI] [PubMed] [Google Scholar]
- 10.Germanwala AV, Huang J, Tamargo RJ. Hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurg Clin North Am 2010; 21: 263–270. [DOI] [PubMed] [Google Scholar]
- 11.Van Asch CJ, Van Schaaf IC, Rinkel GJ. Acute hydrocephalus and cerebral perfusion after aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 2010; 31: 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon CJ, Kong DS, Nam DH, et al. Communicating hydrocephalus associated with surgery or radiosurgery for vestibular schwannoma. J Clin Neurosci 2010; 17: 862–864. [DOI] [PubMed] [Google Scholar]
- 13.Beez T, Burgula S, Kamp M, et al. Symptomatic communicating hydrocephalus in a contemporary cohort of high grade glioma patients. Br J Neurosurg 2018; 32: 68–72. [DOI] [PubMed] [Google Scholar]
- 14.Fujii Y, Nagaishi M, Nakae R, et al. Intracranial endodermal cyst presenting with nonobstructive hydrocephalus: a case report. Medicine (Baltimore) 2019; 98: e14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Close LN, Zanaty M, Kirby P, et al. Acute hydrocephalus resulting from neuromyelitis optica: a case report and review of the literature. World Neurosurg 2019; 129: 367–371. [DOI] [PubMed] [Google Scholar]
- 16.Prior A, Severino M, Rossi A, et al. Acute communicating hydrocephalus as spinal cord surgery complication in patient with lumbar lipomyelocele. World Neurosurg 2018; 115: 468–472. [DOI] [PubMed] [Google Scholar]
- 17.Heshmatzadeh Behzadi A, Farooq Z, Newhouse JH, et al. MRI and CT contrast media extravasation: a systematic review. Medicine (Baltimore) 2018; 97: e0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bretscheider T, Troidl N, Strotzer M, et al. Extravasation of contrast media in brain parenchyma and cerebrospinal fluid space after CT or DSA of thorax or abdomen. Rofo 2001; 173: 497–501. [DOI] [PubMed] [Google Scholar]
- 19.Foltys H, Krings T, Bloc F. Cerebral contrast medium extravasation after coronary angioplasty. Nervenarzt 2003; 74: 892–895. [DOI] [PubMed] [Google Scholar]