Abstract
Background:
Epilepsy is a chronic, complex, unprovoked, and recurrent disorder of the nervous system that affected several people worldwide. Phyllanthus amarus (PA) has been documented to have neuroprotective potential.
Aim:
To evaluate the potential of standardized extract of PA and its possible mechanism of action against the Pentylenetetrazol (PTZ)-induced convulsion and kindling associated post-ictal depression in experimental mice.
Materials and Methods:
Phyllathin was isolated from methanolic extract of PA and well-characterized using HPTLC, ESI-MS/MS, and LC/MS. Phyllathin containing a standardized extract of PA (50, 100, and 200 mg/kg) was administered in convulsed and kindled mice, followed by an assessment of various parameters.
Results:
The spectral analysis confirmed the molecular formula and weight of phyllanthin as C24H34O6 and 418.2342 Da. PA (100 and 200 mg/kg) significantly ameliorated PTZ-induced (p < 0.05) duration, onset of tonic-clonic convulsion, and mortality in mice. It also significantly attenuated (p < 0.05) PTZ-induced kindling in mice. Alteration in brain GABA, dopamine, and glutamate, Na+K+ATPase, Ca+2-ATPase activities, and oxido-nitrosative stress in kindled mice was significantly restored (p < 0.05) by PA treatment. It also significantly (p < 0.05) down-regulated brain mRNA expressions of NF-κB, TNF-α, IL-1β, COX-2, and TLR-4. Histological aberrations induced by PTZ in the brain of a kindled rat was significantly (p < 0.05) ameliorated by PA.
Conclusion:
Phyllanthin containing a standardized extract of PA exerts its antiepileptic potential via balancing excitatory (glutamate) and inhibitory (GABA) brain monoamines, voltage-gated ion channels (Na+K+/Ca+2-ATPase) and inhibition of NF-κB/TLR-4 pathway to ameliorate neuroinflammation (TNF-α, IL-1β, and COX-2) in experimental mice.
Keywords: COX-2, epilepsy, kindling, NF-κB, pentylenetetrazol, phyllanthin, Phyllanthus amarus, TLR-4
Introduction
Epilepsy is a chronic, second most common disorder of the nervous system, mainly characterized by recurrent spontaneous seizures.1 This unprovoked seizure is a major health issue affecting 1 to 2% population of all ages and genders around the globe.2 Despite significant development in epilepsy research, approximately one-third of epileptic patients still suffer from uncontrolled seizures and thus need an effective treatment. An abnormal, spontaneous, and hypersynchronous neuronal activity resulted in abnormal electrical discharges and an imbalance of complex neurotransmitter systems in the brain, which leads to ictogenesis.3 Based on the involvement of neuronal activities in the various regions of the brain (localize or complete), epilepsy mainly categories into partial seizures and generalized seizures, which further subcategories as tonic-clonic seizures and absence seizures.2
According to the WHO (World Health Organization) report, the estimated incidence rate of epilepsy is approximately 50 per hundred million in the developed country, whereas 100 per hundred million in developing.4 A report based on a population-based survey suggested that depression is one of the most frequent comorbidities in almost 29% of patients with epilepsy.5 In such cases, the depression remains untreated because of inevitable opinion on antidepressant treatment that may worsen the epileptic condition due to its potential of lower seizures threshold.6 Thus, the treatment of depression during epilepsy remains challenging.
Numerous researcher has provided fundamentals of epileptogenesis where development of the chronic phase of epilepsy occurred with spontaneously recurrent seizures.1 A meta-analysis suggested the involvement of various pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukins (ILs) in the development of and maintenance of epileptic seizures in patients with major depressive disorders.7 Additionally, evidence suggested the vital role of neuroinflammatory markers, including Cyclooxygenase-2 (COX-2) in the pathophysiology of epilepsy.8 The other researchers reported manifestation implicated in experimental and human epilepsy includes impairment in membrane-bound inorganic phosphate ion channels such as Na+K+ATPase, voltage- and receptor-gated ion channels impairment, impaired GABA (gamma-Aminobutyric acid), glutamatergic and cholinergic transmission.1,7 Furthermore, the researcher has well established the link between elevated c-fos expression and voltage-gated ion channels modulated convulsions.9 Moreover, the susceptibility of neuronal tissue toward ROS (reactive oxygen species) indicated the major contribution of oxidative stress in an array of neurodegenerative disorders, including Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, and epilepsy.10
Currently, the management of epilepsy has been oriented mainly toward the multi-targeted approach, including amelioration of neuroinflammation, oxidative stress, correction in an imbalance in voltage-gated ion channels, and neurotransmitters. Nowadays, an effective anti-epileptic drug (AEDs) used in the treatment of seizures is Gabapentin, Carbamazepine, Lamotrigine, Phenobarbital, Phenytoin, Pregabalin, etc.6 However, most of these regimens associated with cognitive impairment and oxidative damage.6,11 Thus, there is a need to develop better, safe, and effective therapies with multi-targeted epileptogenesis. A growing body of evidence has suggested that various chemical-induced seizure models have been routinely implicated for the development of various moieties with antiepileptic potential.12 Pentylenetetrazol (PTZ) which is a GABA receptor antagonist responsible for inducing convulsion via downstream blocking of chloride ionophore complex with GABA-A receptor.13 It has been well documented that the intraperitoneal administration of PTZ affects various neurotransmitters, including GABA and glutamate, which imitates clinicopathological features of clonic-tonic seizures.1 PTZ has an ability to induced oxidative damage, blocked membrane-bound inorganic phosphate enzyme activities, activation of protein kinase C (PKC), NF-kβ (nuclear factor kappa beta), and inflammatory response that resulted in mitochondria dysfunction and neuroinflammation which in turn caused DNA fragmentation and neuronal apoptosis.13
It has been estimated that in the developing countries majority of the population depends on the herbal remedies as a safe and alternative option for the management of an array of maladies including epilepsy, arthritis, diabetes, etc.14 Thus, significant progress has been made for the identification of such therapeutic moieties from the plant origin based on different traditional systems of medicine. Phyllanthus amarus, a widely used medicinal herb native to various Asian counties and has a long history of therapeutic use for the management of neuronal diseases. Studies have shown that P. amarus contains a wide range of pharmacological activity, including anti-inflammatory, antioxidant, antidiabetic, anti-hyperlipidemic, anti-cancer, cardioprotective, hepatoprotective, and nephroprotective effects.15-17 Phytochemical analysis of P. amarus suggests that it contain tannins, flavonoids, lignans, triterpenes, alkaloids, sterols, and volatile oils.18,19 Its rich source of lignans suggested to comprised of phyllanthin and gallic acid, a vital phytoconstituents responsible for its antioxidant, anti-inflammatory, antiulcer, colonoprotective potential.20 A researcher reported that P. amarus exerts its anti-inflammatory effect via modulation of endogenous biomarkers such as COX-2, iNOS (inducible NO synthase), and NF-kB.17 Furthermore, Kandhare et al. (2013) reported that phyllanthin and hypophyllanthin from the standardized extract of P. amarus inhibited DNA damage and elevated levels of TNF-α, thus produced antiulcer potential in a murine model of ulcerative colitis.16 Furthermore, a recent study demonstrated the antiasthmatic potential of phyllanthin from P. amarus through inhibition of IgE (Immunoglobulin E), iNOs, HO-1 (Heme oxygenase-1), TNF-α, IL’s (interleukins) and TGF-β (Transforming growth factor beta).19 However, no significant data available to support antiepileptic efficacy of P. amarus in various available literature. Thus, the present investigation was undertaken to evaluate the potential of standardized extract of P. amarus and its possible mechanism of action against the PTZ-induced convulsion and kindling associated post-ictal depression in experimental mice.
Materials and Methods
Chemicals and Reagents
Pentylenetetrazole was purchased from Sigma Chemical Co. (St Louis, MO, USA). Total RNA Extraction kit and qRT-PCR (quantitative real time polymerase chain reaction) kit was purchased from MP Biomedicals India Private Limited, India. Microliter syringe (Hamilton, Bonaduz, Switzerland) was obtained from Anchrome Enterprises (I) Pvt. Ltd (Mumbai, India). Methanol, ethyl acetate, toluene, formic acid, precoated thin layer chromatographic (TLC) silica gel plates (Kieselgel 60, F-254, 0.2 mm) was purchased from Merck Life Science Pvt Ltd, India. High-performance thin-layer chromatography (HPTLC) spectra were recorded on a Linomat V (Camag, Muttenz, Switzerland).
Preparation, Isolation, and Characterization of an Isolated Molecule From Methanolic Extract of P. Amarus
It was carried out according to a previously reported method.16 Briefly, weighed quantity (500 g) of air-dried powder (Mesh size-16) of the aerial parts of P. amarus. was macerated with distilled methanol at room temperature by soaking it with eventual stirring for 7 days and filtered. The filtrate was dried in a tray dryer maintained at 40°C. Semisolid methanolic extract of P. amarus (PAME) was obtained to which a colloidal silicon dioxide was added and dried in a vacuum tube dryer. The phytochemical analysis of PAME was performed for the identification of phyllanthin content by HPLC (High-performance liquid chromatography).
HPLC conditions
Analyses were carried out using an HPLC system (Camag, Muttenz, Switzerland) with a column of RP C18, 5 µ, 250 X 4.6 mm, and a flow rate of 1.5 ml/min. The mobile phase for isolation and detection was Acetonitrile: Buffer (40:60). The buffer consists of 0.136 g of potassium hydrogen phosphate and 0.5 ml of o-phosphoric acid. The optimum injection volume was 20 μL, and the detection wavelength of the detector was set at 230 nm. The autosampler temperature was maintained at 10°C, and the pressure of the system was 1000 psi. The chemical structure of the isolated compound was elucidated by HPLC-ESI-QTOF-MS/MS (High-Performance Liquid Chromatography coupled to Electrospray Ionization and quadrupole time-of-flight Mass Spectrometry) and LC-MS (Liquid Chromatography-Mass Spectrometry) spectroscopy.
HPLC-ESI-MS/MS conditions
Analyses were carried out using an Agilent 1200 HPLC system interfaced with Agilent 6520 hybrid quadrupole time of flight mass spectrometer (Agilent Technologies, USA). 1200 HPLC system was equipped with a quaternary pump (G1311A), online vacuum degasser (G1322A), autosampler (G1329A), column compartment (G1316C) and diode-array detector (G1315D).
Mass spectrometric condition
Mass spectrometer was operated in negative electrospray ionization mode, and spectra were recorded by scanning the mass range from m/z 50 to 1500 in both MS and MS/MS modes. Nitrogen was used as drying, nebulizing, and collision gas. The drying gas flow rate was 12 L/min. The heated capillary temperature was set at 350°C and nebulizer pressure at 45 psi. The source parameters such as capillary voltage (VCap), fragmentor, skimmer, and octapole voltages were set at 3500 V, 175 V, 65 V, and 750 V, respectively. For the MS/MS analysis, collision energies were set at 15, 20, 25, 30, 35, and 40 eV. The accurate mass data of the molecular ions were processed through the Mass Hunter Workstation (version B 04.00) software.
Experimental Animals
Adult male Swiss albino mice (weight: 18-22 g, Age: 6 weeks) were obtained from the Laboratory Animal Centre of Affiliated Hospital of North Sichuan Medical College (China). They were maintained at 24°C ± 1°C with a relative humidity of 45 to 55% and normal dark/light cycle. The animals had free access to standard pellet chow and water throughout the experimental protocol. All the experimental protocol number (AE2019WT09) was approved by the animal care and use committee of the North Sichuan Medical College (China). All experiments were carried out between 09:00 and 17:00 h. The experiments were carried out in accordance with the guidelines and approved by the animal care and use committee of the North Sichuan Medical College. Animals were brought to the testing laboratory 1 h before the experimentation for adaptation purposes. The experimentation was carried out in the noise-free area.
Experimental Design
Pentylenetetrazole-induced convulsions
A previously reported protocol was followed to induced convulsion using PTZ (Sigma Chemical Co., St Louis, MO, USA).21 Mice were randomly divided various groups (n = 12) viz., normal (distilled water (DW), 200 mg/kg, p.o.), PTZ control (DW, 10 mg/kg, p.o.), diazepam (5 mg/kg, i.p.) and P. amarus (50, 100 and 200 mg/kg, i.p.). All animals (except normal received saline (10 mg/kg, i.p.)) received PTZ (90 mg/kg, i.p.) 45 min. after administration treatment. Immediately after PTZ administration mice were observed for the next 30 min for symptoms such as the onset of convulsion, duration of clonic convulsion, duration of tonic convulsion, incidence (number of mice showing convulsions), and mortality.
Pentylenetetrazole-induced kindling and post-ictal depression
Kindling was induced in mice by administration of PTZ (an initial sub convulsive dose of 30 mg/kg, s.c.) on alternate days at the same time (thrice a week), and the animals were observed for the appearance of seizure activity for 30 min.22 The evaluation of seizure activity was done as follows: unresponsiveness = 0, mild contractions = 1, clonic seizures = 2, tonic seizures = 3 (forelimb and then hindlimb rigidly extended to rear) and death = 4.
To study the effect of P. amarus and diazepam on the development of kindling, mice were received either diazepam (5 mg/kg, i.p.), P. amarus (50, 100 and 200 mg/kg, i.p.)16 or DW (200 mg/kg, p.o) in various groups (n = 12). All animals (except normal received saline (10 mg/kg, s.s.)) received PTZ (30 mg/kg, s.c.) 30 min after the treatment.
After cessation of seizures, the locomotor activity of the animals was accessed using an actophotometer (INCO, India) according to the previously reported method.21 Animals were individually placed in the actophotometer, and total activity count was registered for 5 min. The locomotor activity was expressed in terms of total photo beam interruption counts/5 min. After that, the animals were subjected to forced swimming test (FST) to assess the depressive behavior. Briefly, the animals were placed individually in a glass cylinder (25 × 12 × 25 cm3), containing water at 25°C (±3°C) up to a level 15 cm for 5 min and total immobility period (seconds) was recorded. The animals were judged to be immobile when they ceased struggling and remained floating motionless in the water, making only those movements necessary to keep their head above water.
Brain GABA, dopamine, glutamate, oxido-nitrosative stress, Na-K-ATPase, and Ca+2-ATPase activity estimation in mice brain hippocampus
Mice were sacrificed soon after completion of behavioral analysis, and the brain was isolated immediately. Brain GABA, dopamine, glutamate, superoxide dismutase (SOD), reduced glutathione (GSH), lipid peroxidation (MDA content), nitric oxide (NO content), Na+K+ATPase and Ca+2-ATPase as described previously.9
Determination of NF-κB, TNF-α, IL-1β, COX-2, and TLR-4 by RT-PCR in mice brain hippocampus
The levels of NF-κB, TNF-α, IL-1β, COX-2, and TLR-4 (Toll-like receptor 4) messenger RNA (mRNA) expressions were analyzed in brain hippocampus tissue using quantitative reverse transcription-polymerase chain reaction (qRT–PCR) using CFX Real-Time PCR detection systems paired with CFX MaestroTM analysis software (v 1.1) following the manufacturer’s instructions. PCR was performed using 1 X forward and reverse primers, and 2.5 U Taq polymerase (MP Biomedicals India Private Limited). Amplification of β-actin served as a control for sample loading and integrity.
Histopathology of mice brain hippocampus
The brain hippocampus was fixed in 10% (v/v) neutral buffered formalin for 24 h for histopathological studies. It was processed for 12 h using isopropyl alcohol, xylene, and paraffin-embedded for light microscopic study (Nikon E200, Japan). Paraffin-embedded tissue sections cut at 5µm thickness were prepared and stained after deparaffination using hematoxylin and eosin stain (H & E) to verify morphological assessment. Photomicrographs were captured at a magnification of 40X.
Statistical Analysis
Data were expressed as mean ± standard error mean (SEM) and analyzed by using GraphPad Prism 5.0 (GraphPad, San Diego, USA). The p < 0.05 was considered statistically significant.
Results
Isolation, and Characterization of Phyllanthin From Methanolic Extract of P. Amarus
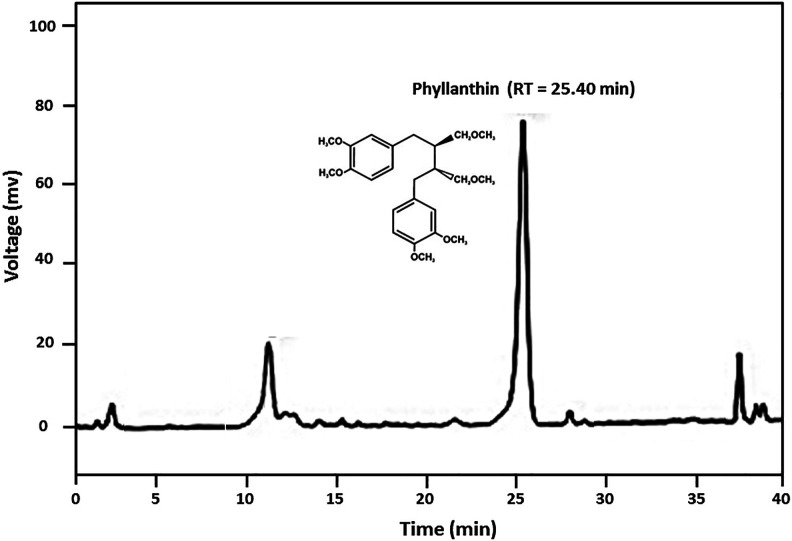
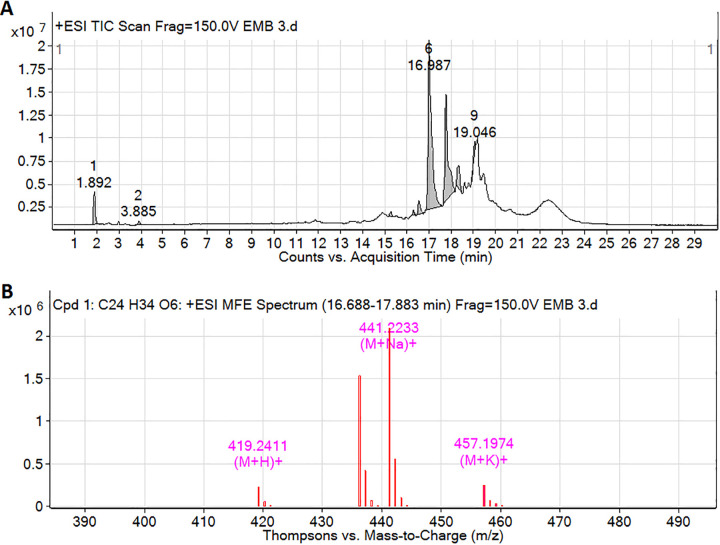
The percent yield of methanolic extract of P. amarus extract was 47.12% w/w, and it showed the presence of lignins, glycosides, tannins, steroids, and phenolic compounds. The retention time (RT) for phyllanthin on the HPLC column was 25.40 min in a total run time of 40 min, with a percent area of 72.15 (Figure 1). The compound was identified by spectroscopic analysis using UV spectra (Supplementary File 1) and ESI-MS (Figure 2A). LC/MS spectra isolated compound showed the molecular ion peak fragments at m/z 419.2411 (M + H)+, 441.2233 (M + Na)+ and 457.1974 (M + K)+ indicating the molecular formula C24H34O6 and average mass of 418.2342 Da (Figure 2B). The chemical structure was identified by comparing the spectral data with those of reported by previous researcher as 4-[(2 S,3 S)-3-[(3,4-dimethoxyphenyl)methyl]-4-methoxy-2-(methoxymethyl)butyl]-1,2-dimethoxybenzene (i.e., Phyllanthin).18,20
Figure 1.
HPLC chromatogram showing the peak of phyllanthin (RT = 25.40 min).
Figure 2.
The base peak chromatograms of P. amarus in ESITIC mode (A) and LC/MS spectra of phyllanthin (B).
Effects of P. Amarus on PTZ-Induced Alteration in Duration and Onset of Tonic-Clonic Convulsion as well as Mortality in Mice
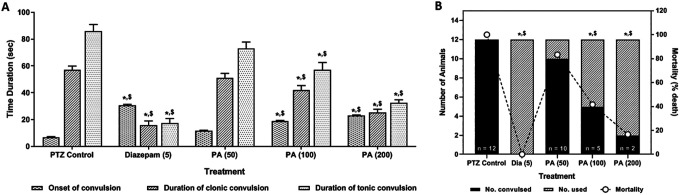
Intraperitoneal administration of PTZ caused significant induction of convulsion reflected by a significant number of convulsed mice. Administration of diazepam (5 mg/kg) significantly (p < 0.05) inhibited the number of animals convulsed, delayed the onset of convulsion, and decreased the duration of clonic-tonic convulsion as compared to PTZ control mice. Treatment with PA (100 and 200 mg/kg) also significantly (p < 0.05) attenuated PTZ induced convulsion reflected by decreased in a number of animal convulsed and duration of clonic-tonic convulsion along with the delayed onset of convulsion when compared with PTZ control mice. However, the administration of diazepam showed more significant (p < 0.05) attenuation toward decreased PTZ induced convulsion as compared to PA treatment. The percent mortality was significantly reduced (p < 0.05) by treatment with diazepam (0%) and PA (100 and 200 mg/kg, 41%, and 16%) as compared to PTZZ control mice (100%) (Figure 3).
Figure 3.
Effects of P. amarus on PTZ-induced duration and onset of tonic-clonic convulsions (A) and number of animals convulsed with percent mortality in mice (B). Data are expressed as mean ± S.E.M. n = 6 to 12 in each group. Data of duration, the onset of tonic-clonic convulsions were analyzed by one-way ANOVA followed by Tukey’s multiple range test. Data on “the incidence of convulsion” and “mortality” was analyzed by the Chi2 test. *p < 0.05 as compared to PTZ treated mice, $ p < 0.05 as compared to one another (Diazepam and P. amarus). DZP: Diazepam; PA: P. amarus; PTZ: Pentylenetetrazol. Figure in parenthesis represents the dose in mg/kg.
Effects of P. Amarus on PTZ-Induced Kindling in Mice
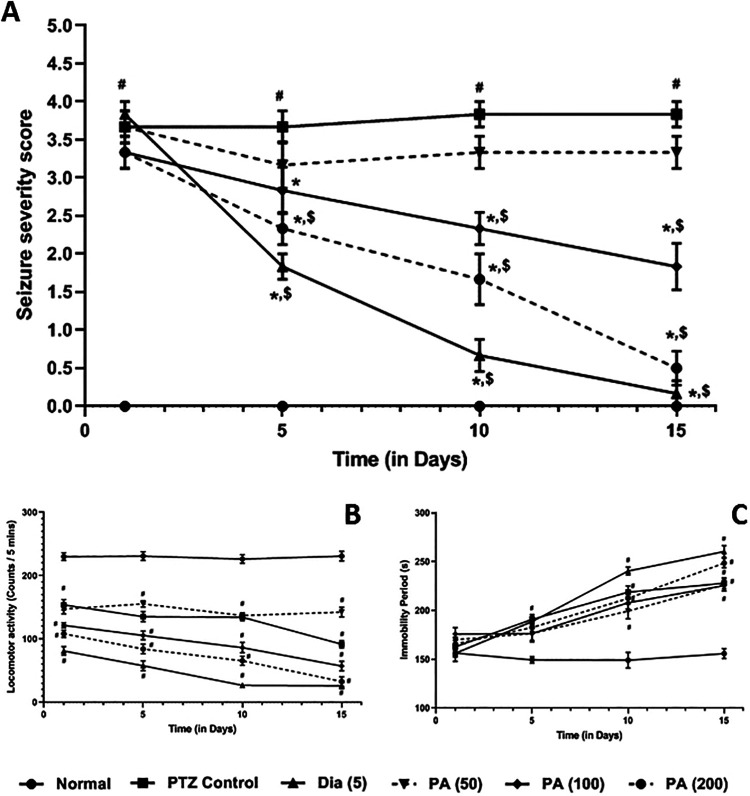
Chronic administration of PTZ significantly (p < 0.05) induced kindling in the mice reflected by elevated seizure severity score as compared to normal mice. Administration of diazepam (5 mg/kg) significantly (p < 0.05) decreased PTZ induced kindling in the mice as compared to PTZ control mice. PA (100 and 200 mg/kg) treatment also significantly (p < 0.05) reduced seizure severity score over a duration of 15 days as compared to PTZ control mice. When compared with PA treated mice, administration of diazepam showed more significant (p < 0.05) attenuation in PTZ-induced kindling in mice (Figure 4A).
Figure 4.
Effects of P. amarus on PTZ-induced kindling (mean seizure score) (A) locomotor activity (B) and immobility period (C) in kindled mice. Data are expressed as mean ± S.E.M. n = 6 in each group. Data of mean seizure score was analyzed by one way ANOVA followed by the Kruskal-Wallis test was applied for post hoc analysis. Data on locomotor activity and immobility period was analyzed by one-way ANOVA followed by Tukey’s multiple range test. # p < 0.05 as compared to normal mice, *p < 0.05 as compared to PTZ treated mice, $ p < 0.05 as compared to one another (Diazepam and P. amarus). DZP: Diazepam; PA: P. amarus; PTZ: Pentylenetetrazol. Figure in parenthesis represents the dose in mg/kg.
Effects of P. Amarus on Alteration in Locomotor Activity and Immobility Period in Kindled Mice
The locomotor activity was decreased significantly (p < 0.05), whereas and duration of the immobility period increased significantly (p < 0.05) in PTZ control, as well as diazepam (5 mg/kg) and PA, treated mice as compared to normal mice. Treatment with diazepam (5 mg/kg) and PA further decreased locomotor activity and increased immobility period significantly (p < 0.05) over a duration of 15 days as compared to normal mice (Figure 4B and C).
Effect of P. Amarus on PTZ-Induced Alteration in Brain GABA, Dopamine, and Glutamate Levels in Kindled Mice
Chronic administration of PTZ significantly (p < 0.05) decreased the levels of brain monoamines, i.e. GABA and dopamine, whereas the level of glutamine increased significantly (p < 0.05) as compared to normal mice. Administration of diazepam (5 mg/kg) significantly (p < 0.05) inhibited PTZ-induced alteration in brain GABA, dopamine, and glutamate levels as compared to PTZ control mice. When compared with PTZ control mice, PA (100 and 200 mg/kg) administration significantly (p < 0.05) increased the levels of brain GABA and dopamine as well as decreased glutamine levels. However, diazepam treatment showed more significant (p < 0.05) attenuation in PTZ induced alternation in altered brain GABA, dopamine, and glutamate levels as compared to PA treatment (Table 1).
Table 1.
Effects of P. amarus and Diazepam on PTZ-Induced Duration Alterations in GABA, Dopamine, Glutamate Levels as Well as Na+K+-ATPase and Ca+2-ATPase Activities in Kindled Mice.
| Treatment | GABA (ng/g of brain tissue) | DA (ng/g of brain tissue) | Glutamate (ng/g of brain tissue) | Na+K+ATPase (µmol/mg of protein) | Ca+2ATPase (μmol/mg of protein) |
|---|---|---|---|---|---|
| Normal | 62.03 ± 2.26 | 75.31 ± 2.73 | 51.66 ± 3.71 | 20.14 ± 1.54 | 8.81 ± 0.51 |
| PTZ Control | 19.34 ± 3.09# | 42.91 ± 3.49# | 131.2 ± 3.74# | 8.32 ± 1.23# | 4.30 ± 0.37# |
| DZP (5) | 51.00 ± 2.33*,$ | 72.39 ± 2.82*,$ | 75.51 ± 2.30*,$ | 20.10 ± 1.38*,$ | 7.99 ± 0.33*,$ |
| PA (50) | 18.82 ± 0.96 | 43.17 ± 3.97 | 124.2 ± 3.14 | 10.05 ± 0.81 | 4.55 ± 0.32 |
| PA (100) | 30.68 ± 1.31*,$ | 60.47 ± 3.06*,$ | 96.51 ± 3.69*,$ | 14.73 ± 1.46*,$ | 7.21 ± 0.35*,$ |
| PA (200) | 44.54 ± 2.02*,$ | 70.98 ± 2.26*,$ | 80.79 ± 3.94*,$ | 17.74 ± 0.89*,$ | 8.07 ± 0.43*,$ |
Data are expressed as mean ± S.E.M. n = 5 to 6 in each group. Data were analyzed by one way ANOVA followed by Tukey’s multiple range test. *p < 0.05 as compared to PTZ control group, # p < 0.05 as compared to normal group and $ p < 0.05 as compared to one another (Diazepam and P. amarus). DZP: Diazepam; DA: Dopamine; GABA: Gamma Amino Butyric Acid; PA: P. amarus; PTZ: Pentylenetetrazol. Figure in parenthesis represents the dose in mg/kg.
Effect of P. Amarus on PTZ-Induced Alteration in Brain Na+K+ATPase and Ca+2-ATPase Activities in Kindled Mice
There was a significant (p < 0.05) decreased in brain Na+K+ATPase and Ca+2-ATPase activities in PTZ control mice as compared to normal mice. Treatment with diazepam (5 mg/kg) significantly (p < 0.05) increased the activities of membrane-bound inorganic phosphate enzymes, i.e. Na+K+ATPase and Ca+2-ATPase as compared to PTZ control mice. Treatment with PA (100 and 200 mg/kg) also significantly (p < 0.05) increased Na+K+ATPase and Ca+2-ATPase activities when compared with PTZ control mice. Administration of PA (200 mg/kg) more significantly (p < 0.05) increased Ca+2-ATPase activity as compared to diazepam treatment (Table 1).
Effect of P. Amarus on PTZ-Induced Alteration in Oxido-Nitrosative Stress in Kindled Mice
Chronic administration of PTZ significantly (p < 0.05) elevated oxido-nitrosative stress in PTZ control mice as compared to normal mice. Administration of diazepam (5 mg/kg) significantly (p < 0.05) increased SOD and GSH levels, whereas significantly (p < 0.05) decreased MDA and NO levels as compared to PTZ control mice. When compared with PTZ control mice, treatment with PA (100 and 200 mg/kg) significantly (p < 0.05) attenuated PTZ-induced elevated oxido-nitrosative stress. When compared with diazepam treated mice, the administration of PA (200 mg/kg) showed more significant (p < 0.05) decreased in elevated MDA and NO levels (Table 2).
Table 2.
Effects of P. amarus and Diazepam on PTZ-Induced Alteration in Brain Oxido-Nitrosative Stress in Kindled Mice.
| Treatment | SOD (U/mg of protein) | GSH (µg/mg of protein) | MDA (nM/ mg of protein) | NO (mg/mL) |
|---|---|---|---|---|
| Normal | 12.64 ± 1.05 | 2.37 ± 0.13 | 3.88 ± 0.26 | 0.16 ± 0.01 |
| PTZ Control | 2.81 ± 0.75# | 0.86 ± 0.17# | 8.98 ± 0.33# | 0.25 ± 0.01# |
| DZP (5) | 11.22 ± 0.61*,$ | 2.34 ± 0.10*,$ | 4.88 ± 0.20*,$ | 0.19 ± 0.01*,$ |
| PA (50) | 4.51 ± 0.75 | 1.41 ± 0.20 | 8.21 ± 0.25 | 0.25 ± 0.01 |
| PA (100) | 5.58 ± 0.78*,$ | 1.74 ± 0.13*,$ | 6.81 ± 0.33*,$ | 0.20 ± 0.01*,$ |
| PA (200) | 10.00 ± 1.15*,$ | 2.22 ± 0.23*,$ | 4.62 ± 0.40*,$ | 0.18 ± 0.01*,$ |
Data are expressed as mean ± S.E.M. n = 5 to 6 in each group. Data were analyzed by one way ANOVA followed by Tukey’s multiple range test. *p < 0.05 as compared to PTZ control group, # p < 0.05 as compared to normal group and $ p < 0.05 as compared to one another (Diazepam and P. amarus). DZP: Diazepam; SOD: Superoxide Dismutase; GSH: Reduced Glutathione; MDA: Malondialdehyde; NO: Nitric Oxide; PA: P. amarus; PTZ: Pentylenetetrazol. Figure in parenthesis represents the dose in mg/kg.
Effect of P. Amarus on PTZ-Induced Alteration in Brain NF-κB, TNF-α, IL-1β, COX-2, and TLR-4 mRNA Expressions in Kindled Mice
There was a significant (p < 0.05) up-regulation in the brain mRNA expressions of NF-κB, TNF-α, IL-1β, COX-2 and TLR-4 in PTZ control mice after administration of PTZ as compared to normal mice. Administration of diazepam (5 mg/kg) significantly (p < 0.05) inhibited PTZ-induced up-regulated NF-κB, TNF-α, IL-1β, COX-2, and TLR-4 brain mRNA expressions as compared to PTZ control mice. Treatment with PA (100 and 200 mg/kg) also significantly (p < 0.05) down-regulated brain NF-κB, TNF-α, IL-1β, COX-2, and TLR-4 mRNA expressions when compared with PTZ control mice (Table 3).
Table 3.
Effects of P. amarus and Diazepam on PTZ-Induced Alteration in Brain NF-κB, TNF-α, IL-1β, COX-2 and TLR-4 mRNA Expressions in Kindled Mice.
| Treatment | NF-κB/β-actin ratio | TNF-α/β-actin ratio | IL-1β/β-actin ratio | COX-2/β-actin ratio | TLR-4/β-actin ratio |
|---|---|---|---|---|---|
| Normal | 0.46 ± 0.06 | 0.95 ± 0.09 | 1.05 ± 0.13 | 0.11 ± 0.02 | 1.06 ± 0.10 |
| PTZ Control | 1.22 ± 0.06# | 1.72 ± 0.11# | 2.40 ± 0.06# | 0.52 ± 0.02# | 2.33 ± 0.05# |
| DZP (5) | 0.61 ± 0.03*,$ | 1.01 ± 0.11*,$ | 1.34 ± 0.08*,$ | 0.16 ± 0.02*,$ | 1.34 ± 0.10*,$ |
| PA (50) | 1.07 ± 0.06 | 1.67 ± 0.07 | 2.24 ± 0.14 | 0.46 ± 0.02 | 2.20 ± 0.08 |
| PA (100) | 0.86 ± 0.05*,$ | 1.40 ± 0.08*,$ | 1.57 ± 0.09*,$ | 0.36 ± 0.01*,$ | 1.92 ± 0.15*,$ |
| PA (200) | 0.72 ± 0.07*,$ | 1.13 ± 0.03*,$ | 1.37 ± 0.08*,$ | 0.20 ± 0.03*,$ | 1.45 ± 0.10*,$ |
Data are expressed as mean ± S.E.M. n = 4 in each group. Data were analyzed by one way ANOVA followed by Tukey’s multiple range test. *p < 0.05 as compared to PTZ control group, # p < 0.05 as compared to normal group and $ p < 0.05 as compared to one another (Diazepam and P. amarus). COX-2: Cyclooxygenase-2; DZP: Diazepam; IL-1β: Interleukin 1beta; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; PA: P. amarus; PTZ: Pentylenetetrazol; TLR-4: Toll-like receptor 4; TNF-α: Tumor necrosis factor-α. Figure in parenthesis represents the dose in mg/kg.
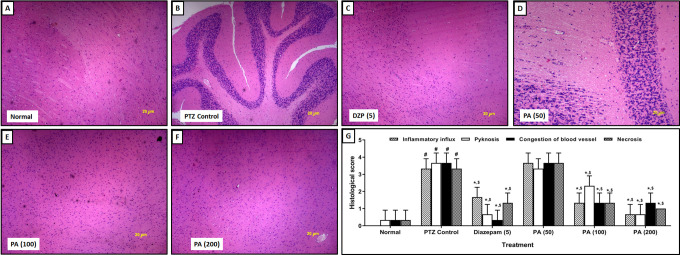
Effect of P. amarus on PTZ-Induced Histological Alteration in Kindled Mice Brain
Chronic administration of PTZ induces histological aberration in the brain hippocampus reflected by significantly (p < 0.05) elevated inflammatory infiltration, necrosis, pyknosis, and congestion in PTZ control mice (Figure 5B) as compared to normal mice (Figure 5A). Histological analysis of the hippocampal region of brain tissue from normal mice did not show any pyknosis with the presence of mild inflammatory infiltration and necrosis (Figure 5A). Diazepam (5 mg/kg) treatment significantly (p < 0.05) inhibited PTZ-induced histological aberrations in the hippocampal region, evident by decreased inflammatory infiltration, necrosis pyknosis and congestion (Figure 5C) as compared to PTZ control mice. Treatment with PA (50 mg/kg) did not produce any significant protection against PTZ-induced histological aberrations in the hippocampal region (Figure 5D). However, the administration of PA (100 and 200 mg/kg) significantly (p < 0.05) attenuated elevated inflammatory infiltration, necrosis, pyknosis, and congestion in the hippocampal region (Figure 5E and 5F) when compared with PTZ control mice (Figure 5G).
Figure 5.
Effect of P. amarus on PTZ induced alterations in brain histopathology in kindled mice. Representative images of sections of brain of normal (A), PTZ control (B), Diazepam (5 mg/kg) (C), PA (50 mg/kg) (D), PA (100 mg/kg) (E) and PA (200 mg/kg) (F) treated group. Images at 40X. The quantitative representation of P. amarus on PTZ-induced alterations in brain histopathology in mice (G). Data are expressed as mean ± S.E.M. n = 3 in each group. Data were analyzed by one-way ANOVA followed by a Kruskal-Wallis test. # p < 0.05 as compared to normal mice, *p < 0.05 as compared to PTZ treated mice, $ p < 0.05 as compared to one another (Diazepam and P. amarus). DZP: Diazepam; PA: P. amarus; PTZ: Pentylenetetrazol. Figure in parenthesis represents the dose in mg/kg.
Discussion
Epilepsy is a chronic, complex, unprovoked, and recurrent neurological disorder affecting more than 70 million people worldwide, and its estimated diagnosis is approximately 2.4 million people per year.4 An array of comorbidities has been associated with epilepsy, which includes anxiety, depression, autism, Alzheimer’s, learning, and memory deficits.2,7 Due to its significant economic burden and worsen quality of life, it becomes a major public health issue worldwide.23 The existing antiepileptic drugs (AEDs) such as lamotrigine, gabapentin, phenobarbital, and valproic acid associated with poor response rate, long-term remission, and considerable adverse events including depression, gastrointestinal discomfort, osteoporosis and impaired cognition in a number of patients.6,11 Thus, these unmet medical needs can be achieved by a safe and effective treatment strategy from the herbal origin. In this view, we have investigated the potential of standardized extract of P. amarus in PTZ-induced convulsion and kindling associated post-ictal depression in mice. Findings of present study suggested that phyllanthin isolated from P. amarus mainly responsible for its anticonvulsant potential. This antiepileptic potential of P. amarus mediated through the balancing excitatory (glutamate) and inhibitory (GABA) brain monoamines, voltage gated ion channels (Na+K+/Ca+2-ATPase) and inhibition of NF-κB/TLR-4 pathway to ameliorate neuroinflammation (TNF-α, IL-1β and COX-2).
Numerous researchers have evaluated qualitative and quantitative analyses of P. amarus using HPLC based on its RT and UV profiling.18,20 Studies suggested that HPLC-ESI-LC/MS is an excellent tool for the structural identification and characterization of various compounds based on its molecular formula.18,20 Furthermore, HPLC-ESI-MS provides detailed structural elucidation with targeted fragmentation of an ion. In the present investigation, the structural characterization of isolated moiety from P. amarus was performed using HPLC-ESI-LC/MS, and findings suggest the molecular formula of a compound as C24H34O6 with an average mass of 418.2342 Da which depicted the Phyllanthin molecule. Recently, researchers have identified various moieties from P. amarus using LC-MS/MS analysis suggested the presence of Phyllanthin as an important structural compound. Thus, structural elucidation of isolated moiety from P. amarus in the present investigation is in accordance with the findings of previous researchers.18,20
It has been well documented that the intraperitoneal administration of PTZ resulted in the production of behavioral manifestations such as myoclonic jerking movements, clonic convulsions, or forelimbs/hind limbs tonic extensor.21 These effects produced by PTZ by competitive blocking at the GABAA receptor for the picrotoxin-sensitive site.13,24 Studies evident that increased response to PTZ is a hallmark of network excitability, and continuous response to PTZ results in hyperexcitability, which leads to the development of spontaneous seizures and behavioral abnormalities.13 The researcher suggested that therapeutic moiety, which provides a significant effect in PTZ-induced tonic-clonic convulsion, can be considered as a potential treatment for clinical myoclonic and absence seizure.25 In the present investigation, single intraperitoneal administration of PTZ (90 mg/kg) induces clonic-tonic convulsion whereas subcutaneous administration of a subconvulsant dose of PTZ (30 mg/kg) induces persistent kindling which is in line with findings of the previous researcher.25 Administration of standardized extract of P. amarus significantly attenuated PTZ-induced convulsion as well as kindling in the mice.
It has been reported that epilepsy is associated with learning and psychiatric comorbidities such as cognitive deficit and depression, which leads to increased socioeconomic burden and poor quality of life.26 The researcher reported that seizures-induced imbalance between inhibitory and excitatory neurotransmission is responsible for these debilitating comorbidities of epilepsy.1 Significant locomotor activity is a reflection of a hallmark of alertness, and a decrease in such activity suggested the sedation, which might be due to an increase in inhibitory neurotransmitters such as GABA and a decrease in excitatory neurotransmitter such as dopamine.1 Several antiepileptic drugs, including benzodiazepine and levetiracetam, are the potential anticonvulsant; however, they associated with worsen depression.27 Additionally, the previous investigator suggests that repetitive seizures induce depression and decrease locomotor activity in a post-ictal state.22 Clinically also, it has been proven that comorbidities situation of epilepsy, such as depression, depends upon the frequency and severity of convulsions.28 In the present investigation also repeated administration of PTZ in mice induces learning and memory deficits reflected by decreased locomotor activity and increased duration of immobility. However, the administration of diazepam as well as P. amarus did not show any correction in cognitive deficit and depression.
Studies have well established the role of various neurotransmitters (NTs) amino acids in the initiation and maintenance of epileptic seizures.1,13 The pathophysiological mechanisms involved in epileptogenesis suggest the imbalance between excitatory and inhibitory NTs pathways, including GABA, dopamine, glutamate, noradrenaline, and serotonin in the brain.1 The significant changes in the levels of various NTs followed by epileptic seizures include an increase in the excitatory NT level, such as glutamate and aspartate, as well as a decrease in inhibitory NT level, including dopamine and GABA.29 During epileptic seizure GABAA receptor plays a significant role; thus, PTZ, which is a GABAA receptor antagonist, has been widely used to induce severe convulsions and kindling by administrating repetitive sub convulsive doses in various murine models.13,22 It has been reported that a decrease in GABAergic transmission leads to hyperexcitability and neuronal discharges, which leads to the induction of clonic and tonic seizures.22 An attempt has been made to ameliorate epilepsy by inducing the opening of GABA associated channels for the influx of Cl- ions in the neuronal membrane. In line with previous literature, in the present study, the administration of PTZ induces a decreased in GABA and dopamine levels with an increase in glutamate levels.22 However, the administration of P. amarus induces antiepileptogenic and antidepressant effects via modulation of PTZ-induced alteration of these excitatory and inhibitory NTs.
Studies reported that ATPases cause coupling of ATP hydrolysis with energy processes and thus play a significant role in the maintenance of ionic gradient.30 ATP modulates the release of various NTs via its receptors or acting on NTs receptors; thus, it serves as a neuromodulator and neurotransmitter.31 Na+K+ATPase, a membrane-bound inorganic phosphatase enzyme, plays a central dogma role in the maintenance of cellular electrochemical gradient of Na+ and K+ ions in the cell membrane in CNS.32 Furthermore, the uniform distribution of Na+K+ATPase on the axolemma is essential to perform their functions in the generation and transmission of bioelectricity.32 Accumulation of Na results in a negative charge; thereby, action potential may not be generated. Researchers documented that decreased activity of this membrane-bound phosphatase enzyme facilitates neuronal hyperexcitability leads to excitatory potential, which results in convulsions.33 It has been previously shown that membrane potential and depolarization of the action potential of neurons was affected by the slow and fast K+ channels and slow Na+ currents.33 Similarly, studies demonstrated that the decrease in the activity of other voltage-gated channels such as Ca2+-ATPase associated with long-term plasticity changes during epileptogenesis.34 In the present investigation administration of P. amarus ameliorated PTZ-induced kindling may be via elevated activity of ATPases enzymes.
Numerous scientific evidence also well-established the relationship between epilepsy and reactive oxygen species (ROS) hat implicated in neurodegeneration.35 A researcher has suggested that PTZ-induced seizures are associated with increases in oxido-nitrosative stress.15,35 Thus, efforts have been taken to alleviate PTZ-induced convulsion by implicating the various antioxidant compounds to show its neuroprotective potential against the seizures.22,36 In the current study, PTZ-induced seizures associated with an increase in MDA and NO levels as well as decreased in SOD and GSH levels. Whereas pre-treatment with P. amarus prevented these reactive oxygen species and reactive nitrogen species induced lipid peroxidation indicated by a decrease in MDA levels; thus, it provides neuroprotection against PTZ-induced seizures. The results of the present investigation provide a credential to findings of the previous investigator where the administration of phyllanthin from the standardized extract of P. amarus attenuates the elevated brain oxido-nitrosative stress. Our results are in accordance with the findings of previous researchers where phyllanthin exerts its protective efficacy via amelioration of oxidative stress.17,19
Neuroinflammation is thought to be an essential pathophysiological pathway for the induction of epileptogenesis.37 An array of stimuli initiate various responses such as activation of endothelial cells astrocytes, microglia, peripheral immune cells, and inflammatory influx, which contributes to the pathophysiology of epilepsy.38 Increasing evidence suggests that elevated expressions of various chemokines and pro-inflammatory cytokines caused structural alteration in the blood–brain barrier and thus cause neuronal hyperexcitability and elevated susceptibility to epileptic seizures.39 The important role of pro-inflammatory cytokines also has been evident during the induction and propagation of seizures clinically.38 Furthermore, elevated levels of these pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and Interleukin-1β (IL-1β) has been identified in the cerebrospinal fluid and serum of epileptic patients.40 IL-1β has been identified as an essential acute pro-inflammatory mediator which release immediately after injury, which further initiates and maintains the seizure activity.41 Numerous researcher have strengthened the role of neuroinflammatory mediators such as NF-κB, TNF-α, and ILs in the epileptogenesis through experimental and clinical studies.1,42 In the present study, also the administration of PTZ associated with an elevated inflammatory response in the brain region of mice, which was inhibited by the administration of P. amarus. Previous researchers suggested that presence of phyllanthin in P. amarus contributes to its anti-inflammatory potential via inhibition of TNF-α, and IL’s16,19 and the results of present investigation corroborates with the findings of previous researchers.19
Cyclooxygenase-2 (COX-2) is another pro-inflammatory enzyme besides cytokines, which play a key role in the epileptic seizures during the kindling.8 A researcher has well established the crosstalk between the COX-2 signaling and development of epilepsy.8,43 The elevated response of IL-1β via activated microglia in CNS induces COX-2 synthesis in astrocytes and neuroblastoma cells clinically.43 Additionally, TNF-α also causes induction of COX-2 expression brain capillary endothelial cells, which in turn increases vascular permeability.8 Moreover, a TLR-4, which is a toll-like receptor family member, also modulates the intracellular signaling of COX-2 via activation of NF-κB and pro-inflammatory cytokine production pathway.44 Thus, COX-2 acts as a vital molecule in various downstream processes of neuroinflammation. In this view, a significant attempt has been made to ameliorate the seizure induction via the implementation of selective COX-2 inhibitors such as celecoxib or aspirin.8 The result of the present investigation also suggests that the administration of P. amarus attenuated PTZ-induced convulsion and kindling via inhibition of COX-2 and TLR-4 expressions, which is line with findings of the previous researcher.17
Management of epileptogenesis with the use of herbal therapies provide a ray of hope due to its less side effect as compared to existing antiepileptic drugs (AEDs). A double-blind study conducted in epileptic patients treated with Nigella sativa showed a marked reduction in seizure frequency, and it was well tolerated.36,45 A recent study conducted by George et al. (2019) showed that a standardized extract of P. amarus (PHYLLPRO™) containing phyllanthin and hypophyllanthin as an active phytoconstituents ameliorates inflammation and intoxication via inhibition of pro-inflammatory cytokines in hangover populations without serious adverse events.15 Thus, P. amarus can be considered as a potential herbal therapeutic remedy for the management of epileptic seizures.
Conclusion
The findings of the present investigation suggest that the administration of phyllanthin from the standardized extract of P. amarus ameliorates PTZ-induced convulsion and kindling associated post-ictal depression in mice. P. amarus inhibited epileptogenesis via balancing excitatory (glutamate) and inhibitory (GABA) brain monoamines, voltage-gated ion channels (Na+K+/Ca+2-ATPase) and inhibition of NF-κB/TLR-4 pathway to inhibit neuroinflammation (TNF-α, IL-1β, and COX-2) and ameliorate the seizure severity in kindled mice.
Supplemental Material
Supplementary_file for Phyllathin From Phyllanthus Amarus Ameliorates Epileptic Convulsion and Kindling Associated Post-Ictal Depression in Mice via Inhibition of NF-κB/TLR-4 Pathway by Zhang Tao, Hu Chun-Yan, Peng Hua, Yang Bin-Bin and Tang Xiaoping in Dose-Response
Footnotes
Authors’ Note: Zhang Tao and Hu Chun-Yan contributed equally.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Tang Xiaoping  https://orcid.org/0000-0001-8795-4293
https://orcid.org/0000-0001-8795-4293
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kobylarek D, Iwanowski P, Lewandowska Z, et al. Advances in the potential biomarkers of epilepsy. Front Neurol. 2019;10(685):685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393(10172):689–701. [DOI] [PubMed] [Google Scholar]
- 3. Bertini G, Bramanti P, Constantin G, et al. New players in the neurovascular unit: insights from experimental and clinical epilepsy. Neurochem Int. 2013;63(7):652–659. [DOI] [PubMed] [Google Scholar]
- 4. Fiest KM, Sauro KM, Wiebe S, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seminario NA, Farias ST, Jorgensen J, Bourgeois JA, Seyal M. Determination of prevalence of depression in an epilepsy clinic using a brief DSM-IV-based self-report questionnaire. Epilepsy Behav. 2009;15(3):362–366. [DOI] [PubMed] [Google Scholar]
- 6. Singh SP, Sankaraneni R, Antony AR. Evidence-based guidelines for the management of epilepsy. Neurol India. 2017;65(supplement):S6–S11. [DOI] [PubMed] [Google Scholar]
- 7. Kwon A, Kwak BO, Kim K, et al. Cytokine levels in febrile seizure patients: a systematic review and meta-analysis. Seizure. 2018;59:5–10. [DOI] [PubMed] [Google Scholar]
- 8. Rawat C, Kukal S, Dahiya UR, Kukreti R. Cyclooxygenase-2 (COX-2) inhibitors: future therapeutic strategies for epilepsy management. J Neuroinflammation. 2019;16(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang L, Wu T, Kandhare A, et al. Elucidation of the molecular mechanism of tempol in pentylenetetrazol-induced epilepsy in mice: role of gamma-aminobutyric acid, tumor necrosis factor-alpha, interleukin-1β and c-Fos. Phcog Mag. 2018;14(59):520–527. [Google Scholar]
- 10. Shekh-Ahmad T, Kovac S, Abramov AY, Walker MC. Reactive oxygen species in status epilepticus. Epilepsy Behav. 2019;101(Pt B):106410. [DOI] [PubMed] [Google Scholar]
- 11. Reeta KH, Mehla J, Gupta YK. Curcumin is protective against phenytoin-induced cognitive impairment and oxidative stress in rats. Brain Res. 2009;1301:52–60. [DOI] [PubMed] [Google Scholar]
- 12. Loscher W. Animal models of seizures and epilepsy: past, present, and future role for the discovery of antiseizure drugs. Neurochem Res. 2017;42(7):1873–1888. [DOI] [PubMed] [Google Scholar]
- 13. Shimada T, Yamagata K. Pentylenetetrazole-induced kindling mouse model. J Vis Exp. 2018;136:56573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tugume P, Nyakoojo C. Ethno-pharmacological survey of herbal remedies used in the treatment of paediatric diseases in Buhunga parish, Rukungiri District, Uganda. BMC Complement Altern Med. 2019;19(1):353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. George A, Udani JK, Yusof A. Effects of Phyllanthus amarus PHYLLPRO(TM) leaves on hangover symptoms: a randomized, double-blind, placebo-controlled crossover study. Pharm Biol. 2019;57(1):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kandhare AD, Ghosh P, Ghule AE, Zambare GN, Bodhankar SL. Protective effect of Phyllanthus amarus by modulation of endogenous biomarkers and DNA damage in acetic acid induced ulcerative colitis: role of phyllanthin and hypophyllanthin. Apollo Medicine. 2013;10(1):87–97. [Google Scholar]
- 17. Kiemer AK, Hartung T, Huber C, Vollmar AM. Phyllanthus amarus has anti-inflammatory potential by inhibition of iNOS, COX-2, and cytokines via the NF-kappaB pathway. J Hepatol. 2003;38(3):289–297. [DOI] [PubMed] [Google Scholar]
- 18. Kumar S, Singh A, Kumar B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J Pharm Anal. 2017;7(4):214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu W, Li Y, Jiao Z, Zhang L, Wang X, Qin R. Phyllanthin and hypophyllanthin from Phyllanthus amarus ameliorates immune-inflammatory response in ovalbumin-induced asthma: role of IgE, Nrf2, iNOs, TNF-alpha, and IL’s. Immunopharmacol Immunotoxicol. 2019;41(1):55–67. [DOI] [PubMed] [Google Scholar]
- 20. Kumar S, Chandra P, Bajpai V, et al. Rapid qualitative and quantitative analysis of bioactive compounds from Phyllanthus amarus using LC/MS/MS techniques. Ind Crops Prod. 2015;69:143–152. [Google Scholar]
- 21. Patil MVK, Kandhare AD, Ghosh P, Bhise S. Determination of role of GABA and nitric oxide in anticonvulsant activity of Fragaria vesca L. ethanolic extract in chemically induced epilepsy in laboratory animals. Orient Pharm Exp Med. 2012;12(4):255–264. [Google Scholar]
- 22. Reddy AJ, Dubey AK, Handu SS, et al. Anticonvulsant and antioxidant effects of musa sapientum stem extract on acute and chronic experimental models of epilepsy. Pharmacognosy Res. 2018;10(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmidt D, Sillanpaa M. Evidence-based review on the natural history of the epilepsies. Curr Opin Neurol. 2012;25(2):159–163. [DOI] [PubMed] [Google Scholar]
- 24. Kandhare A, Raygude K, Ghosh P, Gosavi TP, Bodhankar SL. Patentability of animal models: India and the globe. Int J Pharm Biol Arc. 2011;2(4):1024–1032. [Google Scholar]
- 25. Sun ZQ, Meng FH, Tu LX, Sun L. Myricetin attenuates the severity of seizures and neuroapoptosis in pentylenetetrazole kindled mice by regulating the of BDNF-TrkB signaling pathway and modulating matrix metalloproteinase-9 and GABAA. Exp Ther Med. 2019;17(4):3083–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patel RS, Elmaadawi A, Mansuri Z, et al. Psychiatric comorbidities and outcomes in epilepsy patients: an insight from a nationwide inpatient analysis in the United States. Cureus. 2017;9(9):e1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaufman KR, Bisen V, Zimmerman A, Tobia A, Mani R, Wong S. Apparent dose-dependent levetiracetam-induced de novo major depression with suicidal behavior. Epilepsy Behav Case Rep. 2013;1:110–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwon OY, Park SP. Depression and anxiety in people with epilepsy. J Clin Neurol. 2014;10(3):175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Svob Strac D, Pivac N, Smolders IJ, Fogel WA, De Deurwaerdere P, Di Giovanni G. Monoaminergic mechanisms in epilepsy may offer innovative therapeutic opportunity for monoaminergic multi-target drugs. Front Neurosci. 2016;10(492):492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tiwari S, Sharma V, Mujawar M, Mishra YK, Kaushik A, Ghosal A. Biosensors for epilepsy management: state-of-art and future aspects. Sensors (Basel). 2019;19(7):1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu T, Yu X, Deng J, et al. CXCR7 regulates epileptic seizures by controlling the synaptic activity of hippocampal granule cells. Cell Death Dis. 2019;10(11):825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Staley K. Molecular mechanisms of epilepsy. Nat Neurosci. 2015;18(3):367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rho JM, Shao LR, Stafstrom CE. 2-Deoxyglucose and beta-hydroxybutyrate: metabolic agents for seizure control. Front Cell Neurosci. 2019;13:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tiwari MN, Mohan S, Biala Y, Yaari Y. Protein kinase A-mediated suppression of the slow after hyperpolarizing KCa3.1 current in temporal lobe epilepsy. J Neurosci. 2019;39(50):9914–9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Militao GC, Ferreira PM, de Freitas RM. Effects of lipoic acid on oxidative stress in rat striatum after pilocarpine-induced seizures. Neurochem Int. 2010;56(1):16–20. [DOI] [PubMed] [Google Scholar]
- 36. Shawki M, El Wakeel L, Shatla R, El-Saeed G, Ibrahim S, Badary O. The clinical outcome of adjuvant therapy with black seed oil on intractable paediatric seizures: a pilot study. Epileptic Disord. 2013;15(3):295–301. [DOI] [PubMed] [Google Scholar]
- 37. Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 2019;15(8):459–472. [DOI] [PubMed] [Google Scholar]
- 38. Webster KM, Sun M, Crack P, O’Brien TJ, Shultz SR, Semple BD. Inflammation in epileptogenesis after traumatic brain injury. J Neuroinflammation. 2017;14(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gorter JA, Aronica E, van Vliet EA. The roof is leaking and a storm is raging: repairing the blood-brain barrier in the fight against epilepsy. Epilepsy Curr. 2019;19(3):177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shi LM, Chen RJ, Zhang H, Jiang C-M, Gong J. Cerebrospinal fluid neuron specific enolase, interleukin-1beta and erythropoietin concentrations in children after seizures. Childs Nerv Syst. 2017;33(5):805–811. [DOI] [PubMed] [Google Scholar]
- 41. Wang Y, Wang D, Guo D. Interictal cytokine levels were correlated to seizure severity of epileptic patients: a retrospective study on 1218 epileptic patients. J Transl Med. 2015;13(1):378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qu Z, Jia L, Xie T, et al. (-)-Epigallocatechin-3-gallate protects against lithium-pilocarpine-induced epilepsy by inhibiting the toll-like receptor 4 (TLR4)/nuclear factor-kappaB (NF-kappaB) signaling pathway. Med Sci Monit. 2019;25:1749–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu Y, Zhang S, Shen M, et al. Anticonvulsant effects of dingxian pill in pentylenetetrazol-kindled rats. Evid Based Complement Alternat Med. 2019;2019:4534167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharma R, Leung WL, Zamani A, O’Brien TJ, Casillas Espinosa PM, Semple BD. Neuroinflammation in post-traumatic epilepsy: pathophysiology and tractable therapeutic targets. Brain Sci. 2019;9(11):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Akhondian J, Parsa A, Rakhshande H. The effect of Nigella sativa L. (black cumin seed) on intractable pediatric seizures. Med Sci Monit. 2007;13(12):CR555–559. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_file for Phyllathin From Phyllanthus Amarus Ameliorates Epileptic Convulsion and Kindling Associated Post-Ictal Depression in Mice via Inhibition of NF-κB/TLR-4 Pathway by Zhang Tao, Hu Chun-Yan, Peng Hua, Yang Bin-Bin and Tang Xiaoping in Dose-Response